
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 16 September 2022
Sec. Freshwater Science
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.998246
This article is part of the Research Topic Freshwater Science in the Tropical Anthropocene View all 8 articles
Freshwater ecosystems are considered amongst the most imperiled on earth, since rivers, lakes, wetlands, and other surface waters receive most of the impacts from unsustainable human activities. This has had measurable impacts on freshwater species, and more specifically on freshwater fishes, as data from the Red List show that 23.5% of the 11,937 freshwater fish species evaluated so far, are classified as threatened. Mexico is not exempt from this situation, as a recent report demonstrates that 39.9% of Mexican freshwater fishes are threatened, and there are 21 lost species (extinct + extinct in the wild), the highest number for any country or region of the world. Here we develop a Theory of Change (ToC) to guide management interventions when seeking to prevent further freshwater fish extinctions in Mexico and reversing the current extinction crisis. We describe four thematic areas of intervention: (1) restoration and reintroduction aimed at eight extinct in the wild and four regionally extinct species, (2) conservation management prioritizing 39 critically endangered species, distinguishing between those inhabiting protected areas, water parks, and those with no management nor protection, (3) explorations to find eight possibly extinct species, and (4) communication and outreach to gain support for conservation interventions. The framework has been developed as a tool for conservation advocates and policymakers to implement and monitor change that prevents extinctions, but also to seek and attract funding. It is also meant to guide different levels of government in setting priorities for conservation interventions.
The Anthropocene is characterized by an unparalleled human impact on the global environment, leading to dramatic declines in biodiversity and potentially the first mass extinctions brought on by a single species (Geldman et al., 2019). Nowhere is the biodiversity crisis more acute than in freshwater ecosystems (Tickner et al., 2020). Even though freshwater covers less than 1% of the planet’s surface, freshwater ecosystems support 11% of all animal species and 5% of all plant species (Román-Palacios et al., 2022), and they provide important global ecosystem services that contribute to human welfare and livelihoods (Acreman et al., 2020).
To date 11,937 freshwater fish species (65.2%) out of about 18,290 valid species (Eschmeyer 2022), have been assessed using IUCN Red List criteria, there are 74 extinct and 10 Extinct in the Wild species, 2,576 species are regarded as threatened (23.5%) (Red List 2022).
The same pattern applies to Mexican freshwater ecosystems, as they are possibly the most affected by destructive human activities. Consequently rivers, lakes, lagoons, and other surface waters receive most of the pollutants from large cities, industrial parks, and from livestock production and agricultural activities. These stressors have had measurable impacts on freshwater species (Dirzo et al., 2009), and more specifically on freshwater fishes (Contreras-Balderas et al., 2008; Díaz-Pardo et al., 2016). A recent report (Lyons et al., 2020) demonstrates that the main threats to freshwater fish biodiversity are those from dams and water management/use (including the conversion of spring ecosystems into touristic swimming facilities known as water parks, or “Balnearios” in Spanish), agricultural and forestry effluents, and invasive alien species.
One of the most alarming indicators of the conservation condition of Mexican freshwater fishes, is related to extinct species, as Mexico has 21 extinct freshwater fish species (13 Extinct and 8 extinct in the wild) (Red List 2022). The magnitude of the freshwater fish extinction crisis in Mexico, is evident by comparing extinctions from the IUCN regions, where clearly Mesoamerica stands out with 21 recorded extinctions (all Mexican species), followed by North America (18), South and Southeast Asia (17), Europe (12), West and Central Asia (9), Sub-Saharan Africa (5), East Asia (3), North Asia (2), Oceania (1) and North Africa (1).
Without a formal freshwater fish conservation initiative in Mexico, things could get worse in the short term, as there are four species that are now regionally extinct, and eight that are regarded as possibly extinct. Moreover, there a currently 44 critically endangered (CR), 71 endangered (EN) and 50 vulnerable (VU) species that could add to the extinction crises (Lyons et al., 2020).
Trying to go beyond Red List assessments, IUCN’s Species Survival Commission (SSC) has adopted an “Assess–Plan–Act cycle” and a goal that every species that needs conservation attention is covered by an effective plan of action’ (Lees et al., 2020), this motivated the development of the current planning process, that was created mainly to protect critically endangered (CR) and extinct in the wild (EW) Mexican freshwater fish species.
In selecting species for the present study, we started with the 536 freshwater fish species native to Mexico, that have been evaluated IUCN Red List criteria (Lyons, et al., 2020) and from these, only those considered as threatened by IUCN Red List categories were selected (IUCN 2012). This led us to consider 173 species, however, due to the relatively high number of species, and because the goal is to avoid possible extinctions, it was decided to focus only on those species with the highest risk of extinction, thus critically endangered species (47) as well as those extinct in the wild (8) were selected, so the final number was reduced to 55 species.
As a second step, we reviewed the information from the Red List Databases for each of the 55 species, to identify their distribution, direct threats, as well as the conservation efforts that exist for some of them. As a result, these were divided into three groups: (1) extinct in nature, with eight species, (2) possibly extinct, with eight species and (3) critically threatened, with 39 species, which in turn were subdivided into three groups. based on the level of protection and/or management they currently have, (a) species inhabiting protected areas, 11 species (b) species inhabiting water parks, four species, and (c) species with no management nor protection, 22 species.
The intervention model for this proposal, is based on the “Theory of Change” (ToC), which is a structured approach to the planning process, that includes the definition of the expected results, the establishment of actions and goals. The ToC is flexible, which is appropriate, given the complexities that are present in these types of interventions, this makes it easier to achieve the expected results (see www.theoryofchange.org). Using this model also makes the implementation and evaluation processes transparent (Balfour et al., 2019). This model has been successful in other interventions associated with biodiversity conservation (Biggs et al., 2017; Rice et al., 2020) and in projects for species restoration, management, and conservation (Balfour et al., 2019; van Eeden et al., 2021).
The ToC describes four thematic intervention streams or pathways to impact (Biggs et al., 2017). They are (1) restoration and reintroduction, (2) conservation management, (3) explorations to search for possibly extinct species, and (4) communication and outreach (Figure 1). For didactic purposes they are presented as a series of parallel processes, but in reality, the intervention streams are networked and developments in one stream can have an influence on others. Reversing the current Mexican freshwater fish extinction crisis will require a series of enabling conditions, including suitable habitat (water), multi-institutional arrangements (governance), conservation actions and monitoring (management), money (finance), and legality (policy and procedures).
The ToC is intended to serve as a high-level conservation “blueprint” that can be used by policy makers at different levels of government, conservation practitioners, and researchers interested in conserving one or several Mexican threatened freshwater fish species, as has been done for fishes in other regions (Hammer et al., 2009; Lees et al., 2020). When deciding on a specific conservation intervention, other specific and more detailed planning tools must be applied, such as the Open Standards for the Practice of Conservation (CMP 2020), the Guidelines for Reintroductions and Other Conservation Translocations (IUCN/SSC 2013), or the Guidelines for Species Conservation Planning (IUCN–SSC Species Conservation Planning Sub-Committee, 2017), among many others.
Fortunately, stakeholder analysis demonstrates that Mexico has an institutional framework that could facilitate the implementation of such a strategy and ensure its success, since there is a solid national system of protected natural areas, which is complemented by protected natural areas of a state level, and the National Biodiversity Commission (CONABIO), that produces and oversees programs such as this. It also has academic institutions that have demonstrated capacity for the development of successful conservation projects related to freshwater fish species; there are strong Mexican and International NGOs and Aquariums that are interested and have been involved in the conservation of these organisms, in addition to these, there is an increasingly informed population on environmental issues and especially indigenous communities and social groups interested in conservation (Table 1).
Based on the author’s experience in freshwater fish conservation interventions, pathways to impact were further developed, by integrating result chains, where hexagons represent actions, dotted boxes partial results and boxes results or outcomes (Figure 2).
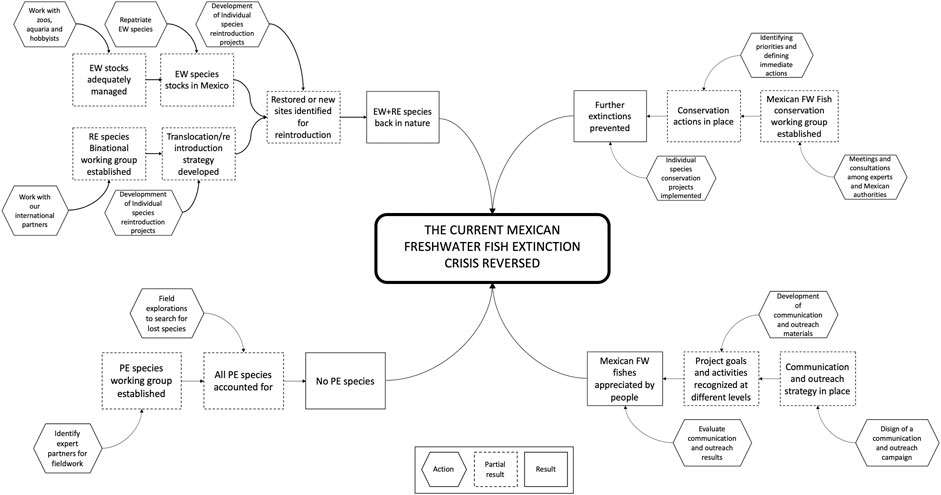
FIGURE 2. Theory of change (ToC) for reversing the current Mexican freshwater fish extinction crises.
Due to the complexity of the diagram, each thematic intervention, their actions, partial results, and the expected result are described in detail in the next sections.
Reintroduction plays a vital role in conservation for many endangered species (Cheng et al., 2021), especially those extinct in the wild, as it is clear that keeping them in captivity for extended periods of time is unsustainable (Trask et al., 2020). We are in the United Nations decade of restoration, that among other things is a call to action that has the purpose of recognizing the need to massively accelerate global restoration of degraded ecosystems (Waltham et al., 2020). Mexico has fallen far behind in the issue of the effective restoration of aquatic ecosystems, even though this is one of the most significant issues to address if we want to stop or avoid future extinctions of Mexican freshwater fish, since it is intimately related to extinct in the wild species as well as those that are regionally extinct.
As shown in Figure 2, due to the differences in the conservation status of extinct in the wild (EW) and regionally extinct (RE) species these two groups are treated separately, however they converge at the end of the partial result chain, with which it is expected that some of them will be reintroduced back to nature. The details of both groups are described below.
There are currently 8 species of Mexican freshwater fish extinct in the wild (Cyprinodon alvarezi, Cyprinodon longidorsalis, Cyprinodon veronicae, Allotoca goslinei, Skiffia francesae, Notropis amecae, Xiphophorus couchianus and Xiphophorus meyeri) (Red List 2022). It is important to recognize the role that researchers, aquarists, zoos, and aquariums have had in the ex situ conservation of these species, because it has been thanks to their efforts that they have managed to survive despite the threats they faced and in many cases the destruction of their natural habitat (Lascuráin et al., 2009; Grist 2010; Maceda-Veiga et al., 2016). However, there is an urgent need to implement a coordinated strategy for the long-term conservation of these species. This becomes evident with cases such as the recent extinction of the Catarina pupfish (Megupsilon aporus), that despite having been kept in captivity in several aquaria for many years, due to the lack of a consistent coordinated effort, it ended up becoming extinct (González et al., 2020). To prevent this from happening again, it is necessary to work with zoos, aquariums, academic institutions, the aquarium trade, and hobbyists, to implement a strategy that leads to the proper management of stocks in captivity, something already considered in the “Turning the Tide” conservation strategy, developed by the World Association of Zoos and Aquariums (Penning et al., 2009; da Silva et al., 2019).
Part of the ex situ stock management strategy will require, especially if the goal is to return them to nature, to have stocks of these species in Mexico. But to achieve this, the installed capacity of Mexican institutions, in this case universities, zoos and aquariums, must be strengthened to properly manage these stocks, since at present the only facility recognized with this capacity is the “Laboratorio de Ecología Acuática at the Universidad Michoacana de San Nicolás de Hidalgo”, that has led the Fish Ark project, for nearly 20 years (Domínguez 2010). So, the second activity indicated in this component of the project has to do with the repatriation of extinct in the wild species to Mexico, to have sufficient fish stocks in country available for reintroduction.
The second line of action within the component relates to the four regionally extinct species (Xyrauchen texanus, Gila elegans, Hybognathus amarus and Ptychocheilus lucius), that have disappeared from Mexican waters but still exist in bodies of water in the United States. All these species have longstanding conservation projects in the US (Minckley et al., 2003; Marsh et al., 2005; Propst et al., 2018), so the strategy will be to work with our North American colleagues to establish a joint effort for the development of specific projects for each of the species and thus achieve their translocation and reintroduction to Mexico. In this case there is already an important project developed by the Centro Intercultural de Estudios de Desiertos y Océanos (CEDO), who is currently, through a collaboration with the USFWS, working to protect two threatened species (Cyprinodon eremus and Agosia crysogaster) from the Rio Sonoyta in northern Mexico (Duncan 2021), to which the species indicated in the section above could be added.
The two lines of action described above will lead to the development of individual species reintroduction projects, for which the original sites will have to be restored or, where appropriate, new suitable sites will be identified, something that has been developed for various conservation projects in other countries (Cochran-Biederman et al., 2015; Esquivel-Muelbert et al., 2018) and in Mexico (Contreras-MacBeath et al., 2016; Domínguez et al., 2018). So, the expected result will be to return to nature some extinct in the wild and regionally extinct species. This result alone, could help achieve the general goal of reversing the current freshwater fish extinction trend in Mexico.
The most immediate way to avoid extinctions is to prioritize conservation actions related to the 39 species evaluated as Critically Endangered (CR) by the Red List. But to achieve this, there must be greater involvement from the Mexican environmental authorities, who have the responsibility of the conservation of species in Mexico (Figure 2), as marked by the national environmental law (LGEEPA 2022). So, the first action of this component will be to hold a series of meetings and consultations with the Mexican environmental authorities at the Federal level, to establish a working group who will be responsible for developing, in collaboration with academics and civil society organizations, an intervention model in which conservation actions will be proposed for individual species and/or sites.
To facilitate the identification of priorities and relevant actors, as well as definition of conservation interventions (second action) critically endangered species were grouped in three intervention categories, based on the level of protection and/or management they currently have, (1) species inhabiting protected areas, (2) species inhabiting water parks, and (3) species with no management nor protection, these are described below:
Protected areas (PAs) have historically been the global cornerstone of biodiversity conservation and restoration (Vimal et al., 2021) to an extent that currently one-sixth of the earths terrestrial surface falls within protected areas (Geldman et al., 2019). Mexico has followed this global tendency and now has 184 federal protected areas that cover an area of 90,956,124 ha (CONANP 2021), an area roughly four times the size of the United Kingdom. Unfortunately, most of these PAs have been established seeking the conservation of terrestrial ecosystems and/or species, so their impact on the conservation of freshwater organisms has not been very significant, as freshwater biodiversity continues to decline (Hermoso et al., 2016), even though a recent systematic review that analyzed 75 case studies suggests that 51% of protected areas were effective in protecting freshwater biodiversity (Acreman et al., 2020).
Our findings show that there are 11 CR species that are found in 8 Protected Areas, which also include three endangered and one vulnerable species (Table 2).
At the Federal level of protection there is the “Área de Protección de Flora y Fauna Cuatro Ciénegas” in the state of Coahuila (INE 1999), where Etheostoma lugoi is critically endangered and Cyprinella xanthicara, and Xiphophorus gordoni are endangered. The critically endangered Rhamdia reddelli is present in the Tehuacán-Cuicatlán UNESCO-MAB Biosphere Reserve (Arroyave 2019). In the Ramsar site Humedales del Lago de Pátzcuaro, Michoacán threre are three CR species Allotoca diazi, Chirostoma patzcuaro y Algansea lacustris. While the Ramsar site Manantiales Geotermales de Julimes, Chihuahua, is home for Cyprinodon julimes.
Regarding PAs at the state level, we have the “Zona Sujeta a Preservación Ecológica Laguna de Zacapu y su Ribera”, of Michoacán State, also a Ramsar site, where there are two CR species Allotoca zacapuensis y Hubbsina turneri and the threatened Notropis grandis; the “Zona Sujeta a Conservación Ecológica del Estado de Nuevo León, Baño de San Ignacio”, also a Ramsar site, home to the CR Fundulus philpisteri, and the VU Cyprinodon bobmilleri; the “Área Natural Protegida Parque Estatal Manantial de la Media Luna”, State of San Luis Potosí inhabited by Tampichthys dichromus; and lastly the “Zona Protectora Forestal Bosque de Aldama” in Chihuahua, where Cyprinodon pachycephalus is found.
Of all the PAs mentioned above, the only two that have clear conservation actions aimed at protecting their freshwater fish species, are the “Área de Protección de Flora y Fauna Cuatro Ciénegas” (INE 1999). The other, is the Ramsar site “Manantiales Geotermales de Julimes” from Chihuahua, that has many activities aimed at protecting Cyprinodon julimes (De la Maza-Benignos et al., 2012).
All the other PAs have failed to recognize their critically endangered freshwater fish species and to act accordingly. Schleicher et al. (2019) stress the importance of management in achieving conservation results by protected areas, in this sense, our immediate action will be to work with the Mexican Commission on Protected Areas (CONANP), to define specific conservation activities to be implemented by their park rangers in each of the protected areas managed by them. The same will be done with the four State Environmental Ministries in charge of the protected areas under their management.
One of the most common uses given to large springs in Mexico is related to the construction and operation of water parks, most of which are not managed sustainably, in consequence water is normally conduced into traditional swimming pools, but in some cases the original spring and the resulting stream are relatively unaffected, a situation that has turned these into sanctuaries for critically endangered fish species. This is the case of the four springs presented in Table 3, that constitute the remaining sites for four CR species.
There is one species in the northern state of Chihuahua, Gambusia hurtadoi in “Balneario ejidal Ojo de Hacienda Dolores” (Lozano-Vilano and De la Maza-Benignos 2017). There are two species of Goodeidae Ameca splendens CR and Zoogoneticus tequila EN in the “Balneario el Rincón de Tehuchitlán” in Jalisco, this last species has recently been reintroduced into the wild (Domínguez et al., 2018). Astyanax salvatoris is endemic to the Balsas rive basin, and it has a very restricted range as it only occurs at the “Ojo de agua” spring, within the natural springs of Tamazulapan in Oaxaca, in the Pacific slope of Mexico (Schmitter-Soto 2019). In the sulphidic waters of “Balneario El Azufre”, in the locality of Teapa in the southern state of Tabasco there are two species of Poeciliidae Gambusia eurystoma and Poecilia sulphuraria (Tobler et al., 2008).
The strategy here will be a “mainstreaming” approach (Rivera 2017), with entitles going beyond the environmental sector, and to work with the Mexican tourism ministry, trying to access non-environmental funds, and to get formal recognition towards these water parks as sustainable touristic conservation entities. This approach has proven to be relatively effective in several projects in the Mexican State of Morelos (Contreras-MacBeath 2020).
In the 22 sites with no management nor protection, there are 22 critically endangered species (Table 4), distributed by family as follows: Goodeidae (8), Atherinopsidae (6), Leuciscidae (4), Salmonidae (3), and Percidae (1). Sites in this category were grouped by State, because most are found in small areas, consequently apart from Federal protection, it is feasible that each Mexican State could “adopt” its species, as priorities in their governmental programs, something that had relatively good results for the State of Morelos (Contreras-MacBeath et al., 2020).
The state of Michoacán has five CR species, Allotoca catarinae, Allotoca meeki, Neoophorus regalis, Chirostoma melanoccus and Notropis calientis, this last one shared with the State of Mexico (Lyons et al., 2019). In Jalisco there are four species, all goodeids: Allotoca maculata, Allodontichthys polylepis, Xenotoca doadrioi and Xenotoca lyonsi (Koeck 2019). Puebla also has four species all Atherinopsidae and belonging to the genus Poblana (Poblana alchichica, P. letholepis, P. squamata and P. ferdebueni) which are distributed in three crater lakes of the Cuenca Oriental and a small lake (Lira-Guerrero et al., 2008; Alcocer et al., 2010). In the State of Mexico there are three species Chirostoma riojai, Notropis calientis (shared with Michoacan), and a vestigial population of Algansea Barbata, that is difficult to find, but occasionally some specimens end up in a fish farm in the locality of “Los Reyes” (Figueroa-Lucero & Ontiveros-López 2000). In the Northern state of Durango there are three species, the goodeid Characodon lateralis, and two undescribed species of trout Oncorhynchus sp. nov. “Baluarte Trout” and Oncorhynchus sp. nov. “Acaponeta Trout” (Lyons et al., 2020). In State of Chihuahua there are two species Cyprinella bocagrande from Ojo Solo spring, and another undescribed trout Oncorhynchus sp. nov. “Northern Conchos Trout”. While Coahuila and San Luis Potosí have one species each, Etheostoma segrex and Notropis calabazas, respectively.
These sites need urgent protection and/or species conservation plans must be developed and implemented (third action), and as mentioned above, the main strategy will be to work with regional and/or local governments to establish protection and conservation measures, using conservation planning tools mentioned above. The overall goal of this thematic intervention is to prevent further extinctions.
One of the issues that has been most sought with the refinement of the red list, is to avoid subjectivity and uncertainty, either due to lack of information or information errors (Rodrigues et al., 2006; Mace et al., 2008; Duenas et al., 2021). Going forwards in the development of a Mexican freshwater fish conservation strategy a fundamental issue is precisely to reduce uncertainty. Because of this, a series of explorations are proposed to search for Mexican lost fishes, that will lead us once and for all to know the real situation of the eight CR species (Tetrapleurodon spadiceus, Chirostoma bartoni, Cyprinodon latifasciatus, Chapalichthys pardalis, Gobiesox juniperoserrai, Chirostoma charari, Chirostoma aculeatum and Notropis marhabatiensis) listed as possibly extinct in the Red List (Red List 2022).
This type of exploration is not new, in 2010 Conservation International launched a globe-spanning search for amphibians (Moseman 2010), and Re: Wild has an ongoing initiative to search for species not seen in decades (GWC 2017). Consequently, the main objective of this intervention will be to clarify the conservation status of each of the species cited above, for which field explorations will be carried out in their historical distribution areas to confirm if they are extinct, or extant.
Work will consist of establishing a task force in charge of developing the initiative. To achieve this, experts who have worked with each species will be included. Special attention will be paid to establishing contact with local researchers as well as with fishermen or members of the communities of each of the basins where the species to be studied are known to exist.
As a first step, the historical records of each of the species will be analyzed to develop a field strategy directed towards the most likely sites in which we can find them, as well as potential sites where they could be present. This will allow us to organize and schedule the field expeditions in such a way as to ensure success.
In field explorations each of the species will be intensively searched for by means of sampling using different fishing gear such as trawl and casting nets, and electrofishing gear. Where possible, underwater observations will also be employed. In each of the field expeditions we will be accompanied by regional experts, as well as professional photographers and videographers from the Mexican Alliance of Conservation Photography, to record in detail each of the explorations and generate material to disseminate our results.
At the end of the explorations, we hope to know in detail the situation of each of the species and be able to reduce uncertainty and consequently we will have a more definitive number of freshwater fish species of extinct in Mexico.
Reduced availability of nature, along with the rise of urban lifestyles, has alienated people from nature in what is referred to as the “extinction of experience”, this is considered as one of the greatest causes of the biodiversity crisis (Schuttler et al., 2018). To implement in practice nature conservation activities, in some cases neither the deficit of experts, nor scientists are the problem, but the conflict of interest between local people, policy-makers and conservationists (Szabó and Macalik 2020). Thus, communication and outreach are a fundamental part of any conservation endeavor (Sutherland 2008), as they are ways to o cultivate a broad public understanding of the diverse benefits that biodiversity provides and promote engagement in actions that may prevent its decline (Cooper et al., 2019). This is especially true when dealing with what some people consider as “non-charismatic” species, a concept well established in the conservation literature (Ducarme et al., 2013). In this sense, much of the problem faced by Mexico’s freshwater fish species, has its origin in the fact that most people do not know either the species or their conservation status, so outside of a fraction of the specialized academic field, very few people know of the extinction crisis happening in the country. In this sense, having a communication and outreach campaign (fist action) will be essential to be able to gain support for the conservation interventions that will be implemented.
The communication strategy will be designed following the methodology proposed by “the biodiversity project (Elder et al., 1998) and based on the principles of the Conservation Optimism (2020) Toolkit. It will be developed with the help of UAEM’s Media Lab, this institution has participated in several of our conservation projects (Contreras-MacBeath et al., 2016; Viveros 2019; Ramírez 2021) and has developed communication materials for IUCN/SSC’s Freshwater Fish Specialist Group (FFSG), Freshwater Conservation Committee (FCC) and for the Alliance for Freshwater Life. The main goal of the communication strategy will be to inform people on what we are doing, and to get them involved in Mexican freshwater fish conservation.
Once the communication strategy has been developed, the second activity will be to produce the communication and outreach materials needed for its implementation. The result will be a widespread knowledge among important stakeholders of the situation that Mexican freshwater fish species are facing, what we are doing to protect them, and how they can get involved in the solutions, in other words Mexican freshwater fished appreciated by people.
In recent years, Mexico has achieved mixed results with regards to its biodiversity commitments to the Convention of Biological Diversity (CBD), with some positives related to raising awareness to biodiversity values, invasive alien species, protected areas, and preventing extinctions (CONABIO and UNDP 2019), but as highlighted in this document, this has not positively impacted freshwater species, nor their ecosystems. To cite an example, the Programme for the Conservation of Species at Risk (PROCER) (SEMARNAT 2020), coordinated by the National Commission of Protected Areas (CONANP), has developed, and implemented to date 51 Action Programs for Species Conservation (PACE), but unfortunately at present there is not a single PACE focused on the conservation of any Mexican freshwater fish species. Considering that the Mexican species Protection List (SEMARNAT 2010) recognizes 2606 Mexican species at risk and that the existing 51 PACE only cover 10% of the Mexican species at risk. If things remain as they are, no foreseeable progress will be made in the development of PACE for freshwater fish species that are in urgent need of conservation actions.
In response to this, we propose an initial overarching ToC, that as stated by Rice et al. (2020) can serve as a pathway useful in identifying potential weaknesses in the intervention’s design. We recognize limitations to this proposal, mostly related to enabling conditions, including the availability of suitable habitat (water), as Mexico deals with severe problems in water availability and pollution, as well as increased drought and flooding. The most overexploited aquifers are situated near the biggest cities, or at the north, where most arid areas occur (Ortiz-Partida et al., 2020) (Otazo-Sánchez and Navarro-Frómeta 2020), and this condition is expected to worsen due to climate change, which will increase the pressure on the already highly threatened freshwater ecosystems in Mexican arid lands (Contreras-MacBeath et al., 2014). Another limitation that relates to the reintroduction component of this proposal, is the availability of suitable species founder stocks, with adequate genetic lineages, to avoid hybridization, or other detrimental genetic consequences. With this in mind, strict conservation intervention procedures are suggested be followed, such as the Guidelines for Reintroductions and Other Conservation Translocations (IUCN/SSC 2013).
There may also be limited support by important stakeholders or lack of political will to promote changes via any of the four proposed mechanisms. For example, we are not certain that federal and local authorities will agree to get involved and allocate funds and personnel towards planning and conservation actions, as there has been a negative trend in environmental spending in Mexico since 2016 (UNDP Mexico 2021), which has limited the capacity of many environmental Institutions such as CONANP and CONABIO to achieve their goals.
If negative Anthropogenic impact results from previous conscious decision making by humans, so it follows, that conscious decisions can also steer the planetary future away from the existential risks to shape a positive outcome for the Anthropocene (Thomson and Newman 2016). By means of a simple, but innovative planning process, our ToC seeks to take advantage of the knowledge we now have on the conservation status of freshwater fish species (Lyons et al., 2020), the robust Mexican environmental institutional framework, the interest of many academic and private institutions (mainly zoos and aquariums) and a public eager to participate in species conservation interventions, to shape a positive future for freshwater fish species in Mexico.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.iucnredlist.org/.
All three authors (TC-M, HM, and JR) participated in the review of the Red List database to identify priority species, in the development of the conceptual intervention model, as well as in the identification of examples and in the description of each of the thematic elements that are part of the ToC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acreman, M., Hughes, K. A., Arthington, A. H., Tickner, D., and Dueñas, M. A. (2020). Protected areas and freshwater biodiversity: A novel systematic review distils eight lessons for effective conservation. Conserv. Lett. 13 (1), e12684. doi:10.1111/conl.12684 |
Alcocer, J., Arce, E., Zambrano, L., and Chiappa-Carrara, X. (2010). Poblana alchichica: A threatened silverside species? Verh. Internat. Verein. Limnol. 30 (9), 1429–1432. doi:10.1080/03680770.2009.11902347 |
Arroyave, J. (2019). Rhamdia reddelli. The IUCN red list of threatened species 2019: e.T19455A2349297. Available at: https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T19455A2349297.en. (Accessed on May 31, 2022).
Balfour, D., Barichievy, C., Gordon, C., and Brett, R. (2019). A theory of change to grow numbers of African rhino at a conservation site. Conservation Sci. Pract. 1 (6), e40. doi:10.1111/csp2.40 |
Biggs, D., Cooney, R., Roe, D., Dublin, H. T., Allan, J. R., Challender, D. W. S., et al. (2017). Developing a theory of change for a community-based response to illegal wildlife trade. Conserv. Biol. 31, 5–12. doi:10.1111/cobi.12796 | |
Cheng, Z., Tian, X., Zhong, Z., Li, P., Sun, D., Bai, J., et al. (2021). Reintroduction, distribution, population dynamics and conservation of a species formerly extinct in the wild: A review of thirty-five years of successful milu (Elaphurus davidianus) reintroduction in China. Glob. Ecol. Conservation 31, e01860. doi:10.1016/j.gecco.2021.e01860 |
CMP (2020). The open standards for the practice of conservation. Bethesda, MD: Conservation Measures Partnership. Retrieved from http://cmp-openstandards.org/download-os.
Cochran-Biederman, J. L., Wyman, K. E., French, W. E., and Loppnow, G. L. (2015). Identifying correlates of success and failure of native freshwater fish reintroductions. Conserv. Biol. 29 (1), 175–186. doi:10.1111/cobi.12374 | |
CONABIO and UNDP (2019). Sixth national report of Mexico to the convention on biological diversity: Summary for policy makers. México: CONABIO/PNUD.
CONANP (2021). Áreas naturales protegidas. Comisión nacional de Áreas naturales protegidas. Available at https://www.gob.mx/conanp/documentos/areas-naturales-protegidas-278226 (Accessed on May 12, 2022).
Conservation Optimism (2020). The conservation optimism positive communication toolkit. Available at https://conservationoptimism.org/portfolio-items/positive-communication-toolkit/(Accessed on June 09, 2022).
Contreras-Balderas, S., Ruiz-Campos, G., Schmitter-Soto, J. J., Díaz-Pardo, E., Contreras-MacBeath, T., Medina-Soto, M., et al. (2008). Freshwater fishes and water status in méxico: A country-wide appraisal. Aquatic Ecosyst. Health & Manag. 11 (3), 246–256. doi:10.1080/14634980802319986 |
Contreras-MacBeath, T. (2020). “Esfuerzos intersectoriales para la gestión ambiental enfocada en la biodiversidad (mainstreaming),” in En: La biodiversidad en Morelos. Estudio de Estado 2. (México: CONABIO), 201–207.
Contreras-MacBeath, T., González-Flores, L., and Fuentes Vargas, L. (2020). “Estrategia para la conservación de especies prioritarias,” in En: La biodiversidad en Morelos. Estudio de Estado 2. (México: CONABIO), 307–315.
Contreras-MacBeath, T., Mejia Mojica, H., Rivas González, M., and Preciado Chino, I. (2016). “Re-Introduction of the Morelos minnow in the "barranca de Chapultepec" protected area, cuernavaca, Morelos, Mexico,” in Global Re-introduction perspectives: 2016. Case-Studies from around the globe. Editor P. S. En Soorae (Gland, Switzerland: IUCN/SSC Reintroduction Specialist Group and Abu Dhabi, UAE: Environment Agency-Abu Dhabi).
Contreras-MacBeath, T., Rodríguez, M. B., Sorani, V., Goldspink, C., and Reid, G. M. (2014). Richness and endemism of the freshwater fishes of Mexico. J. Threat. Taxa 6 (2), 5421–5433. doi:10.11609/jott.o3633.5421-33 |
Cooper, M. W., Di Minin, E., Hausmann, A., Qin, S., Schwartz, A. J., and Correia, R. A. (2019). Developing a global indicator for Aichi Target 1 by merging online data sources to measure biodiversity awareness and engagement. Biol. Conserv. 230, 29–36. doi:10.1016/j.biocon.2018.12.004 |
da Silva, R., Pearce-Kelly, P., Zimmerman, B., Knott, M., Foden, W., and Conde, D. A. (2019). Assessing the conservation potential of fish and corals in aquariums globally. J. Nat. Conservation 48, 1–11. doi:10.1016/j.jnc.2018.12.001 |
De la Maza-Benignos, M., Rodriguez-Pineda, J.A., De la Mora-Covarrubias, A., Carson, E.W., Quiñonez-Martínez, M., Lavín-Murcio, P., Vela-Valladares, L., Lozano-Vilano, M.L., Parra-Gallo, H., Macías-Duarte, A., Lebgue-Keleng, T., Pando-Pando, E., Pando-Pando, M., Andazola-González, M., Anchondo-Najera, A., Quintana-Martínez, G., Banda-Villanueva, I.A., Ibarrola-Reyes, H.J., and Zapata-López, J. (2012). “Planes de Manejo y Programa de Monitoreo de Signos Vitales para las Áreas de Manantiales de la UMA El Pandeño; y San Diego de Alcalá en el Desierto Colombiano,” in Pronatura Noreste, A.C. (Amigos del Pandeño, A.C), 162.
Diaz-Pardo, E., Campos, M., Contreras-MacBeath, O., Mejía, G., and Gerardo Ceballos, E. S. G. (2016). “PARTE 2. Situación actual y conservación,” in En: Los peces dulceacuícolas de México en peligro de extinción. Editors G. Ceballos, E. Díaz-Pardo, L. Martínez, and H. Espinoza (Mexico City: Ediciones Científicas Universitarias. Fondo de Cultura Económica), 31–45.
Dirzo, R., González Montagut, R., and March, I. J. (2009). “Estado de conservación del capital natural de México: Retos y perspectivas,” in En Capital natural de México, vol. II: Estado de conservación y tendencias de cambio (México: Conabio), 805–809.
Domínguez, O. (2010). “Conservación de Goodeidos, familia en alto riesgo,” in Patrimonio natural de México. Cien casos de éxito. México, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Editor J Carabias (Mexico City: New Life Publications), 48–49.
Domínguez, O. D., Morales, R. H., Nava, M. M., Diego, Y. H., Venegas, D. T., Jiménez, A. L. E., et al. (2018). “Progress in the reintroduction program of the tequila splitfin in the springs of teuchitlán, Jalisco, Mexico. Global reintroduction perspectives: 2018,” in Case studies from around the globe (Gland, Switzerland: IUCN/SSC Reintroduction Specialist Group and Abu Dhabi, UAE: Environment Agency-Abu Dhabi), 38.
Ducarme, F., Luque, G. M., and Courchamp, F. (2013). What are “charismatic species” for conservation biologists. Biosci. Master Rev. 10, 1–8. doi:10.1371/journal.pbio.2003997 |
Duenas, M. A., Hemming, D. J., Roberts, A., and Diaz-Soltero, H. (2021). The threat of invasive species to IUCN-listed critically endangered species: A systematic review. Glob. Ecol. Conservation 26, e01476. doi:10.1016/j.gecco.2021.e01476 |
Duncan, D. (2021). Native fishes of the Rio Sonoyta. CEDO Blog Post Available at: https://www.cedo.org/read/cedo-en/native-fishes-of-the-rio-sonoyta/ (Accessed May 11, 2022).
Elder, J., Coffin, C., and Farrior, M. (1998). Engaging the public on biodiversity–a road map for education and communication strategies. Madison, Wisconsin: The Biodiversity Project.
Eschmeyer, W. (2022). Catalog of fishes. California Academy of Sciences. Available at: https://www.calacademy.org/scientists/projects/eschmeyers-catalog-of-fishes (Accessed April 30, 2022). Online publication.
Esquivel-Muelbert, J. R., Fontoura, L., Zardo, É., Streit, D. P., Esquivel-Muelbert, A., and Garcia, J. R. (2018). Assessing the viability of reintroduction of locally extinct migratory fish Brycon orbignyanus: Successful growth, dispersal and maturation. Fishes 3 (4), 39. doi:10.3390/fishes3040039 |
Figueroa-Lucero, G., and Ontiveros-López, G. (2000). Algansea barbata (pisces: Cyprinidae) in the state of Mexico, Mexico. Rev. Biol. Trop. 48 (1), 34–56.
Geldmann, J., Manica, A., Burgess, N. D., Coad, L., and Balmford, A. (2019). A global-level assessment of the effectiveness of protected areas at resisting anthropogenic pressures. Proc. Natl. Acad. Sci. U. S. A. 116 (46), 23209–23215. doi:10.1073/pnas.1908221116 | |
González, A. V., Estévez, L. M., Villeda, M. E. Á., and Ceballos, G. (2020). The extinction of the Catarina pupfish Megupsilon aporus and the implications for the conservation of freshwater fish in Mexico. Oryx 54 (2), 154–160. doi:10.1017/s003060531800056x |
Grist, C. (2010). “Viviparous fish programs at chester zoo,” in Viviparous fishes II. Editors M. C. Uribe, and H. J. Grier (Mexico City: New Life Publications), 423–430.
GWC (2017). The search for lost species: Global wildlife conservation to launch most extensive worldwide quest for species not seen in decades. Available at: https://www.rewild.org/press/the-search-for-lost-species-global-wildlife-conservation-to-launch-most-extensive-worldwide-quest-for-species-not-seen-in-decades (Accessed April 30, 2022).
Hammer, M., Eedderburn, S., and van Weenen, J. (2009). “Action plan for south Australian freshwater fishes,” in Native fish Australia (Adelaide: SA Inc.), 232.
Hermoso, V., Abell, R., Linke, S., and Boon, P. (2016). The role of protected areas for freshwater biodiversity conservation: Challenges and opportunities in a rapidly changing world. Aquat. Conserv. 26, 3–11. doi:10.1002/aqc.2681 |
INE (1999). Programa de Manejo del Área de Protección de Flora y Fauna Cuatrociénegas, México. Mexico City: Instituto Nacional de Ecología. Secretaría de Medio Ambiente Recursos Naturales y Pesca, 166.
IUCN (2012). IUCN red list categories and criteria: Version 3.1. Second edition. Gland, Switzerland and Cambridge, UK: IUCN. Available at: https://portals.iucn.org/library/node/10315.
IUCN/SSC (2013). Guidelines for reintroductions and other conservation translocations. Gland, Switzerland: IUCN Species Survival Commission, 57.
IUCN–SSC Species Conservation Planning Sub-Committee (2017). Guidelines for species conservation planning. Version 1.0. Gland, Switzerland: IUCN, xiv+114.
Koeck, M. (2019). Allotoca maculata. The IUCN red list of threatened species 2019. Available at: https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T881A3147389.en. (Accessed on May 9, 2022).
Lascuráin, M., List, R., Barraza, L., Díaz Pardo, E., Gual Sill, F., Maunder, M., et al. (2009). “Conservación de especies ex situ,” in Capital natural de México: Estado de conservación y tendencias de cambio (Mexico: CONABIO, Ciudad de México), 517–544.
C. Lees, C. Gibson, Z. Jaafar, H. H. Ng, H. H. Tan, K. W. J. Chuaet al. (Editors) (2020). Assessing to Plan: Next steps towards conservation action for threatened freshwater fishes of the Sunda region (Mnusa: IUCN Conservation Planning Specialist Group, Apple Valley).
LGEEPA, (2022). Ley General del Equilibrio Ecológico y la Protección al Ambiente. Ciudad de México: Congreso de la Unión. SEMARNAT. (ültima versión DOF 11-04-2022).
Lira-Guerrero, G., García-prieto, L., and Pérez-Ponce de León, G. (2008). Helminth parasites of atherinopsid freshwater fishes (osteichthyes: Atheriniformes) from central Mexico. Rev. Mex. Biodiv. 70, 325–331.
Lozano-Vilano, M. L., and De la Maza-Benignos, M. (2017). Diversity and status of Mexican killifishes. J. Fish. Biol. 90 (1), 3–38. doi:10.1111/jfb.13186 | |
Lyons, J., Piller, K. R., Artigas-Azas, J. M., Dominguez-Dominguez, O., Gesundheit, P., Köck, M., et al. (2019). Distribution and current conservation status of the Mexican Goodeidae (actinopterygii, cyprinodontiformes). ZooKeys 885, 115–158. doi:10.3897/zookeys.885.38152 | |
Lyons, T. J., Máiz-Tomé, L., Tognelli, M., Daniels, A., Meredith, C., Bullock, R., et al. (2020). The status and distribution of freshwater fishes in Mexico. Cambridge, UK and Albuquerque, New Mexico, USA: IUCN and ABQ BioPark.
Mace, G. M., Collar, N. J., Gaston, K. J., Hilton-Taylor, C. R. A. I. G., Akçakaya, H. R., Leader Williams, N. I. G. E. L., et al. (2008). Quantification of extinction risk: IUCN's system for classifying threatened species. Conserv. Biol. 22 (6), 1424–1442. doi:10.1111/j.1523-1739.2008.01044.x | |
Maceda-Veiga, A., Domínguez-Domínguez, O., Escribano-Alacid, J., and Lyons, J. (2016). The aquarium hobby: Can sinners become saints in freshwater fish conservation? Fish. Fish. (Oxf). 17 (3), 860–874. doi:10.1111/faf.12097 |
Marsh, P. C., Kesner, B. R., and Pacey, C. A. (2005). Repatriation as a management strategy to conserve a critically imperiled fish species. North Am. J. Fish. Manag. 25 (2), 547–556. doi:10.1577/m04-123.1 |
Minckley, W. L., Marsh, P. C., Deacon, J. E., Dowling, T. E., Hedrick, P. W., Matthews, W. J., et al. (2003). A conservation plan for native fishes of the lower Colorado River. BioScience 53 (3), 219–234. doi:10.1641/0006-3568(2003)053[0219:acpfnf]2.0.co;2 |
Moseman, A. (2010). Search for long-lost Amphibians finds its first three. Planet earth. Discover Magazine. Available at: https://www.discovermagazine.com/planet-earth/search-for-long-lost-amphibians-finds-its-first-three (Accessed April 30, 2022).
Ortiz-Partida, J. P., Sandoval-Solis, S., Arellano-Gonzalez, J., Medellín-Azuara, J., and Taylor, J. E. (2020). Managing water differently: Integrated Water Resources Management as a framework for adaptation to climate change in Mexico. Integrated Water Resource Management, 59–72. |
Otazo-Sánchez, E. M., and Navarro-Frómeta, A. E. (2020). “Water at a glance in Mexico,” in Water availability and management in Mexico (Cham: Springer), 1–13.
M. Penning, G. McG. Reid, H. Koldewey, G. Dick, B. Andrews, K. Araiet al. (Editors) (2009). Turning the Tide: A global aquarium strategy for conservation and sustainability (Switzerland: World Association of Zoos and Aquariums, Bern).
Propst, D. L., Bixby, K., and Center, S. E. (2018). Conserving native Rio grande fishes in southern New Mexico and west Texas: A conceptual approach.
Ramírez, N. L. (2021). “Creación de un santuario para Poeciliopsis balsas en el Estado de Morelos, México. Aprobada por Unanimidad. Maestría en Recursos Naturales,” in Centro de Investigaciones biológicas (UAE) (Cuernavaca, Mexico: UAEM), 62.
Red List (2022). The IUCN red list of threatened Species™ (2000–2021; version 2021-3). Available at: https://www.iucnredlist.org/(Accessed April 28, 2022).
Rice, W. S., Sowman, M. R., and Bavinck, M. (2020). Using theory of change to improve post-2020 conservation: A proposed framework and recommendations for use. Conserv. Sci. Pract. 2 (12), e301. doi:10.1111/csp2.301 |
Rodrigues, A. S., Pilgrim, J. D., Lamoreux, J. F., Hoffmann, M., and Brooks, T. M. (2006). The value of the IUCN Red List for conservation. Trends Ecol. Evol. 21 (2), 71–76. doi:10.1016/j.tree.2005.10.010 | |
Román-Palacios, C., Moraga-López, D., and Wiens, J. J. (2022). The origins of global biodiversity on land, sea and freshwater. Ecol. Lett. 25, 1376–1386. doi:10.1111/ele.13999 | |
Schleicher, J., Peres, C. A., and Leader-Williams, N. (2019). Conservation performance of tropical protected areas: How important is management? Conserv. Lett. 12 (5), e12650. doi:10.1111/conl.12650 |
Schmitter-Soto, J. (2019). Astyanax salvatoris. The IUCN red list of threatened species 2019. Available at: https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T881A3147389.en. Accessed on 06 June 2022.
Schuttler, S. G., Sorensen, A. E., Jordan, R. C., Cooper, C., and Shwartz, A. (2018). Bridging the nature gap: Can citizen science reverse the extinction of experience? Front. Ecol. Environ. 16 (7), 405–411. doi:10.1002/fee.1826 |
SEMARNAT (2020). Programa de Acción para la Conservación de Especies en Riesgo (PROCER). Available at: https://www.gob.mx/conanp/acciones-y-programas/programa-de-conservacion-de-especies-en-riesgo (Accessed on June 10, 2022).
SEMARNAT (2010). Norma Oficial Mexicana NOM-059-ECOL-2001, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación Segunda Sección. (Mexico City: Secretaría de Medio Ambiente y Recursos Naturales)
Sutherland, W. J. (2008). The conservation handbook: Research, management, and policy. Norwich, UK: John Wiley & Sons.
Szabó, C., and Macalik, K. (2020). Publications, target groups, methods and applications in the communication of biodiversity. J. Appl. Tech. Educ. Sci. 10 (2), 61–91. doi:10.24368/jates.v10i2.171 |
Thomson, G., and Newman, P. (2016). Geoengineering in the anthropocene through regenerative urbanism. Geosciences 6 (4), 46. doi:10.3390/geosciences6040046 |
Tickner, D., Opperman, J. J., Abell, R., Acreman, M., Arthington, A. H., Bunn, S. E., et al. (2020). Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience 70 (4), 330–342. doi:10.1093/biosci/biaa002 | |
Tobler, M., Riesch, R., Garcia de Leon, F. J., Schlupp, I., and Plath, M. (2008). Two endemic and endangered fishes, Poecilia sulphuraria (Alvarez, 1948) and Gambusia eurystoma Miller, 1975 (Poeciliidae, Teleostei) as only survivors in a small sulphidic habitat. J. Fish. Biol. 72, 523–533. doi:10.1111/j.1095-8649.2007.01716.x |
Trask, A., Canessa, S., Moehrenschlager, A., Newland, S., Medina, S., and Ewen, J. (2020). Extinct-in-the-wild species' last stand. Science 369 (6503), 516. doi:10.1126/science.abd4560 | |
UNDP Mexico (2021). “Biodiversity expenditure review in Mexico 2019,” in Biodiversity finance initiative BIOFIN Mexico. (Mexico City: United Nations Development Program)
van Eeden, L., Dickman, C., Crowther, M., and Newsome, T. (2021). A Theory of Change for promoting coexistence between dingoes and livestock production. Conserv. Sci. Pract. 3 (3), e304. doi:10.1111/csp2.304 |
Vimal, R., Navarro, L. M., Jones, Y., Wolf, F., Le Moguédec, G., and Réjou-Méchain, M. (2021). The global distribution of protected areas management strategies and their complementarity for biodiversity conservation. Biol. Conserv. 256, 109014. doi:10.1016/j.biocon.2021.109014 |
Viveros, G. D. (2019). “Estrategia de conservación de Pseudothelphusa dugesi (Rathbun, 1893) Aprobado por Unanimidad,” in Maestría en Recursos Naturales (UAE: Centro de Investigaciones Biológicas), 54.
Keywords: theory of change, freshwater fishes, extinction, conservation planning, Mexico
Citation: Contreras-MacBeath T, Mejia Mojica H and Rivas González JM (2022) A theory of change to reverse the current Mexican freshwater fish extinction crisis. Front. Environ. Sci. 10:998246. doi: 10.3389/fenvs.2022.998246
Received: 19 July 2022; Accepted: 26 August 2022;
Published: 16 September 2022.
Edited by:
Tatenda Dalu, University of Mpumalanga, South AfricaReviewed by:
Ryan Wasserman, Rhodes University, South AfricaCopyright © 2022 Contreras-MacBeath, Mejia Mojica and Rivas González. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Topiltzin Contreras-MacBeath, dG9waXNAdWFlbS5teA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.