- 1Department of Hepatobiliary Disease, The 900th Hospital of Joint Logistics Support Force, Fuzhou, China
- 2Department of Hepatobiliary Disease, Fuzong Clinical Medical College of Fujian Medical University, Fuzhou, China
- 3Department of Hepatobiliary Disease, Cangshan District of the 900th Hospital of Joint Logistics Support Force, Fuzhou, China
Lead is one of the most important toxic heavy metals in the environment; however, the relationship between blood lead levels and liver fibrosis in individuals without chronic liver diseases (CLD) is unclear. This study used data obtained from the National Health and Nutrition Examination Survey (NHANES) 2017–2020. Multivariate logistic regression was used to explore the relationship between the blood lead level and significant liver fibrosis. A total of 1962 cases without underlying CLD were included, 77 (3.9%) of whom were diagnosed with advanced liver fibrosis by transient elastography. The proportion of advanced fibrosis significantly increased with the blood lead level (p < 0.001), and the blood lead level was higher in the fibrosis group than in the non-fibrosis group (1.2 μg/dl vs 0.9 μg/dl, p < 0.001). After adjusting for relevant confounding factors, the blood lead level was found to be independently associated with advanced liver fibrosis (OR = 1.168; 95% CI, 1.006–1.356; p = 0.041). The blood lead level remained an independent risk factor for advanced liver fibrosis (OR = 1.249; 95% CI, 1.048–1.489; p = 0.013) after controlling for age and sex through propensity score matching. In conclusion, the blood lead level is associated with liver fibrosis in individuals without known CLD.
Introduction
Liver fibrosis/cirrhosis is defined as the excessive accumulation of extracellular matrix proteins in liver tissue in response to chronic liver injury (Guo and Lu, 2020; Cheng et al., 2021; Ginès et al., 2021). Chronic viral hepatitis, fatty liver disease, and alcoholic liver disease are the three leading causes of fibrosis. However, for a small group of patients, the cause of fibrosis is difficult to identify (Nalbantoglu and Jain, 2019; Ginès et al., 2021).
Lead is one of the most important toxic heavy metals that are released from exhaust gas, paints, and industrial wastes. In the United States, the sources of lead include paint, batteries, and the industrial legacy of lead exposure (Obeng-Gyasi, 2019). Due to the historically widespread use of lead in gasoline and paint and the persistence of lead in the environment, lead exposure is common in the modern society, and the legacy of lead exposure remains a major health problem worldwide (Meyer et al., 2008).
Lead can affect nearly all organs and systems of the human body. A high blood lead concentration is a causative factor of renal impairment, anemia, cognitive dysfunction, and neurological diseases (Boskabady et al., 2018; El-Naggar et al., 2018). The importance of liver lead toxicity has been acknowledged recently (Kasperczyk et al., 2013), and the association between lead level and liver fibrosis has been reported in two survey-based studies from the United States and Korea, respectively (Reja et al., 2020; Kim et al., 2021). However, these two studies did not exclude patients with chronic liver diseases, especially non-alcoholic fatty liver disease (NAFLD), which is a known cause of liver fibrosis (Lin et al., 2021). Previous studies have suggested that blood lead level is also significantly associated with higher prevalence of NAFLD (Chung et al., 2020; Reja et al., 2020; Betanzos-Robledo et al., 2021). As preexisting chronic liver disease, especially NAFLD, might act as a mediating factor, it is difficult to conclude that lead exposure directly increases the risk of fibrosis. Moreover, in previous studies, fibrosis was assessed using noninvasive fibrosis score, which is not as accurate as liver elasticity (Xiao et al., 2017; Staufer et al., 2019; Chuah et al., 2020).
Therefore, the relationship between lead level and liver fibrosis requires further clarification. In this context, this study aimed to evaluate the relationship between blood lead level and liver fibrosis assessed by liver elasticity based on data from the NHANES 2017–2020 database.
Methods
Study population
The study dataset was obtained from the National Health and Nutrition Examination Survey (NHANES) 2017–2020 database. NHANES is a national survey conducted by the Centers for Disease Control and Prevention of the United States. It is designed to assess the health and nutrition status of the non-institutionalized population in the United States. The inclusion criterion for this study was a blood lead test. The exclusion criteria were as follows: age <18 years; hepatic steatosis assessed based on the controlled attenuation parameter (CAP); hepatitis B or C virus infection; excessive alcohol consumption; history of chronic liver disease; ineligibility to liver fibrosis test; and with missing key data.
Assessment of liver steatosis and fibrosis
Liver steatosis and liver fibrosis were assessed based on the CAP and liver stiffness measurement (LSM), respectively, which were obtained using FibroScan®. Liver steatosis was defined as CAP ≥248 dB/m (Karlas et al., 2017), and significant liver fibrosis was defined as LSM ≥8.2 kPa (Cassinotto et al., 2016).
Definition
The following demographic variables were obtained from the original database: age, sex, race, body mass index (BMI), waist circumference, and history of hypertension and diabetes. BMI was calculated as weight (in kilograms) divided by the square of the height (in meters). Hypertension was defined as resting blood pressure persistently at or above 140/90 mmHg according to the international standard (Gabb et al., 2016). Diabetes was defined as a fasting blood glucose (FBG) level ≥7.0 mmol/L, a glycosylated hemoglobin (HbA1c) level ≥6.5%, a previous diagnosis of diabetes, or treatment with oral hypoglycemic drugs or insulin (American Diabetes Association, 2020). The estimated glomerular filtration rate (eGFR) was calculated according to the 2009 CKD-EPI eGFR formula (Levey et al., 2009). A smoker was defined as having smoked at least 100 cigarettes in their entire life (Horne et al., 2012). Fibrosis-4 score (FIB-4) and NAFLD fibrosis score (NFS) were used for noninvasive assessment of fibrosis. The cutoff values of FIB-4 and NFS to diagnose advanced fibrosis were 2.670 and 0.676, respectively (Kabbany et al., 2017).
Laboratory parameters
Whole blood specimens were processed, stored, and shipped to the National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA for analysis. All biochemical parameter assessments were performed using standard methods with automated techniques (Roche Calibrator for Automated Systems), including those for FBG, total cholesterol (TCHO), total triglycerides (TG), aspartate aminotransferase (AST), alanine transaminase (ALT), serum creatinine, blood urea nitrogen (BUN), and uric acid. The blood lead concentration was detected using inductively coupled plasma mass spectrometry with a dynamic reaction cell. Quality controls were applied throughout all these assays. For a detailed description of the laboratory methods used, please see ‘https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/P_BIOPRO.htm’.
Statistical analysis
Continuous variables were expressed as means ± standard deviation (SD), and c categorical variables were expressed as percentages. The Student t-test (for normally distributed variables), Mann-Whitney U test (for non-normally distributed variables) and chi-squared test (for categorical variables) were used to investigate the differences between groups. Propensity score matching (PSM) was applied to match the age and sex between two groups. Multivariate logistic regression was used to explore the independent factors for significant liver fibrosis. All tests were two-tailed, and results with a p-value < 0.05 were considered statistically significant. All analyses were conducted using R 3.6.2 (https://www.r-project.org/).
Results
Baseline characteristics of the study subjects
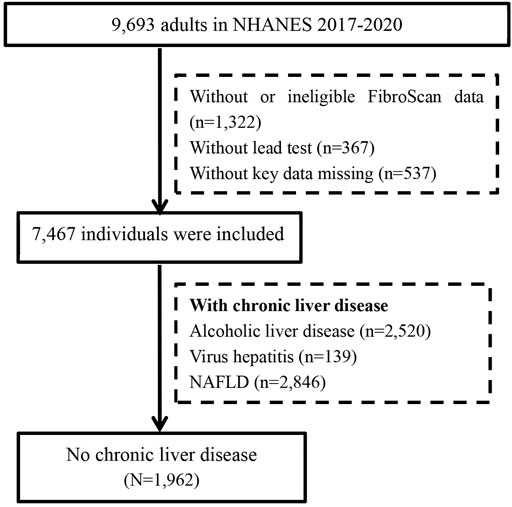
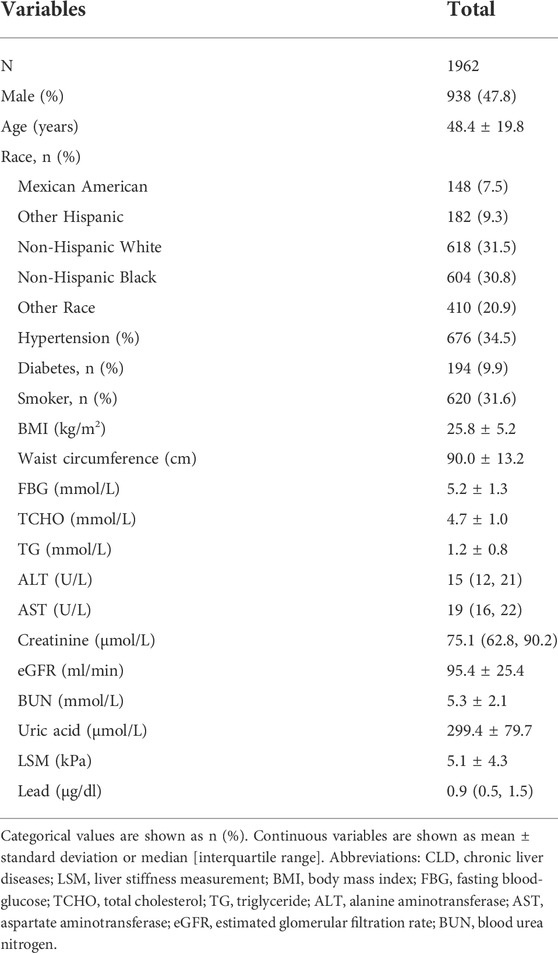
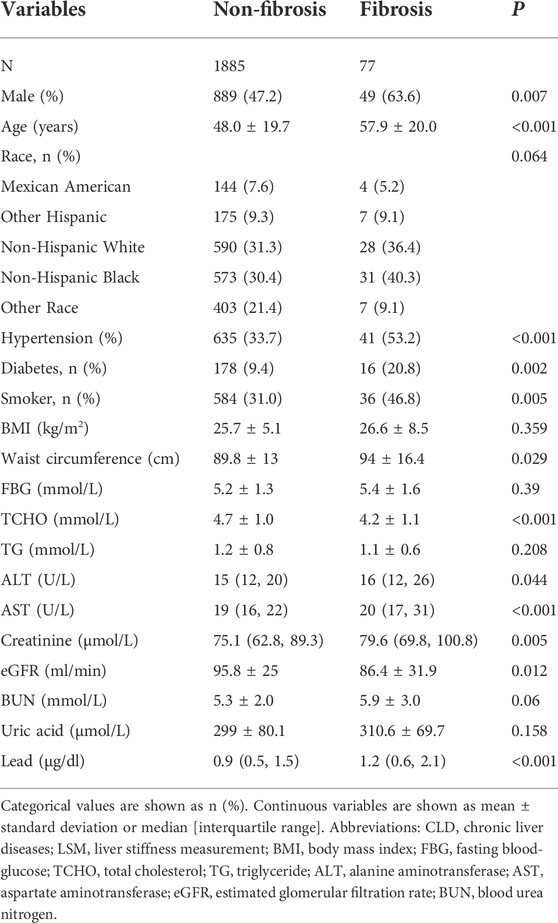
A total of 9,693 adults participated the survey from 2017 to 2020. After excluding 2,226 cases with ineligible data and 5,505 cases with chronic liver diseases, 1962 patients without underlying liver disease were included in the final analysis (Figure 1). The proportion of men was 47.8% (938/1962), and the mean age was 48.4 ± 19.8 years. Overall, 9.9% of cases had diabetes, and 34.5% had hypertension. The liver enzyme (ALT and AST) levels of the study population were within normal range. The mean LSM was 5.1 ± 4.3 kPa. A total of 77 (3.9%) participants were diagnosed with advanced liver fibrosis by LSM. The other details are shown in Table 1.
Characteristics of individuals with different blood lead levels (quartiles 1–4)
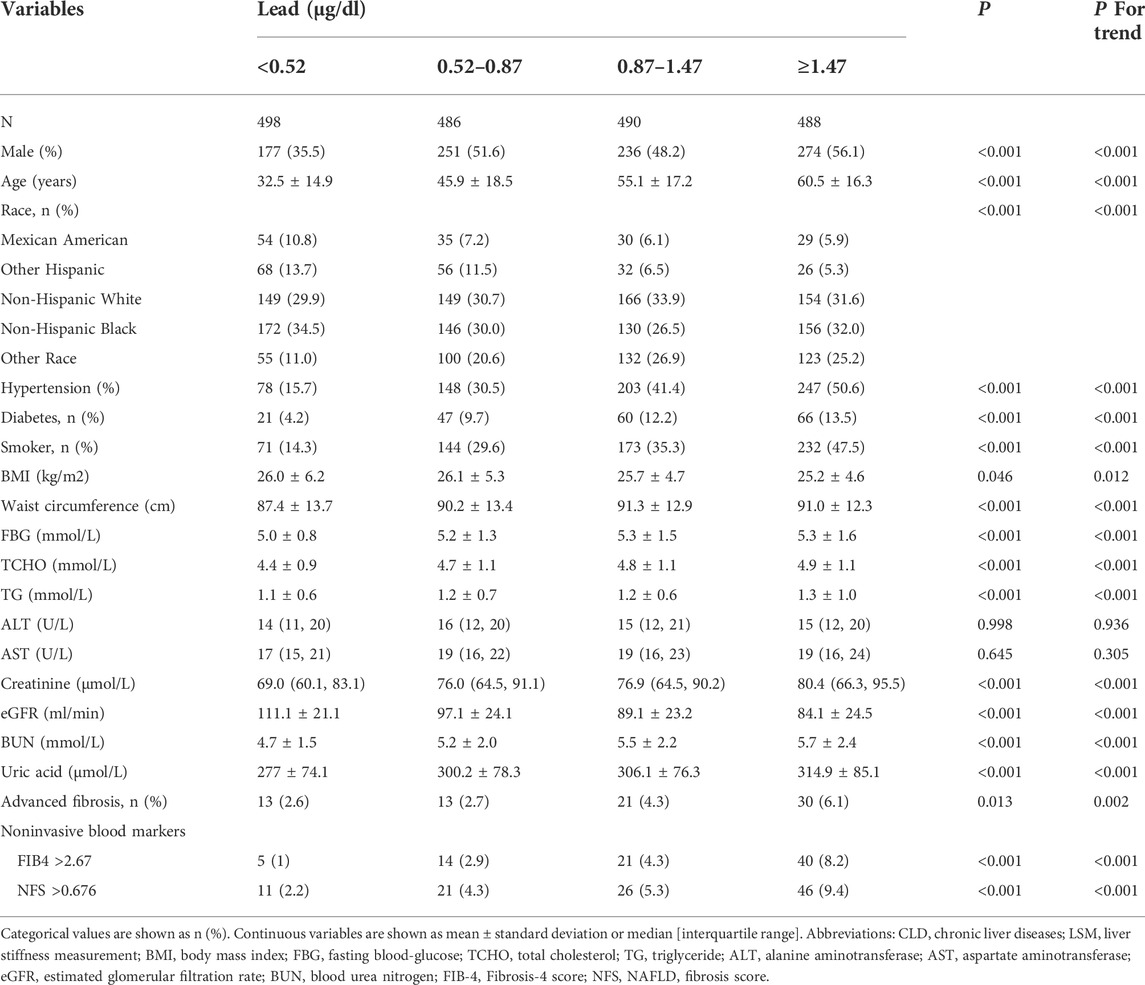
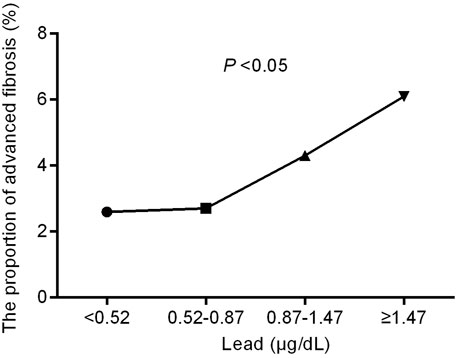
The study subjects were divided into four groups according to the quartile of blood lead level (<0.52, 0.52–0.87, 0.87–1.47, and ≥1.47 μg/dl). The comparisons of the four groups are shown in Table 2. Higher lead levels were associated with higher age and more metabolic derangements such as hypertension, diabetes, obesity and hyperlipidemia (all with a P for trend value < 0.05). Conversely, the eGFR decreased with the increase in lead concentration (p < 0.05). The proportion of advanced fibrosis assessed by LSM also significantly increased with blood lead levels (Figure 2 and Table 2).
Characteristics of individual with and without advanced liver fibrosis
Overall, 77 (3.9%) cases had an LSM ≥8.2 kPa and were diagnosed with advanced liver fibrosis. The comparison between advanced liver fibrosis (LSM ≥8.2 kPa) and non-fibrosis (LSM <8.2 kPa) is shown in Table 3. Patients with fibrosis were relatively older than those without (57.9 ± 20.0 vs 48.0 ± 19.7, p < 0.001). The fibrosis group had a higher proportion of men (63.6% vs 47.2%, p = 0.007) and significantly higher proportions of hypertension, diabetes, and smoking history. Liver injury indicators, including ALT and AST levels, were higher in the fibrotic individuals (both with a p-value < 0.05). There was no statistical difference in BMI and FBG and TG levels between the two groups (all with a p-value > 0.05); however, the blood lead level was higher in the fibrosis group than in the non-fibrosis group (1.2 μg/dl vs 0.9 μg/dl, p < 0.001).
Binary logistic regression analysis of the association between lead level and fibrosis
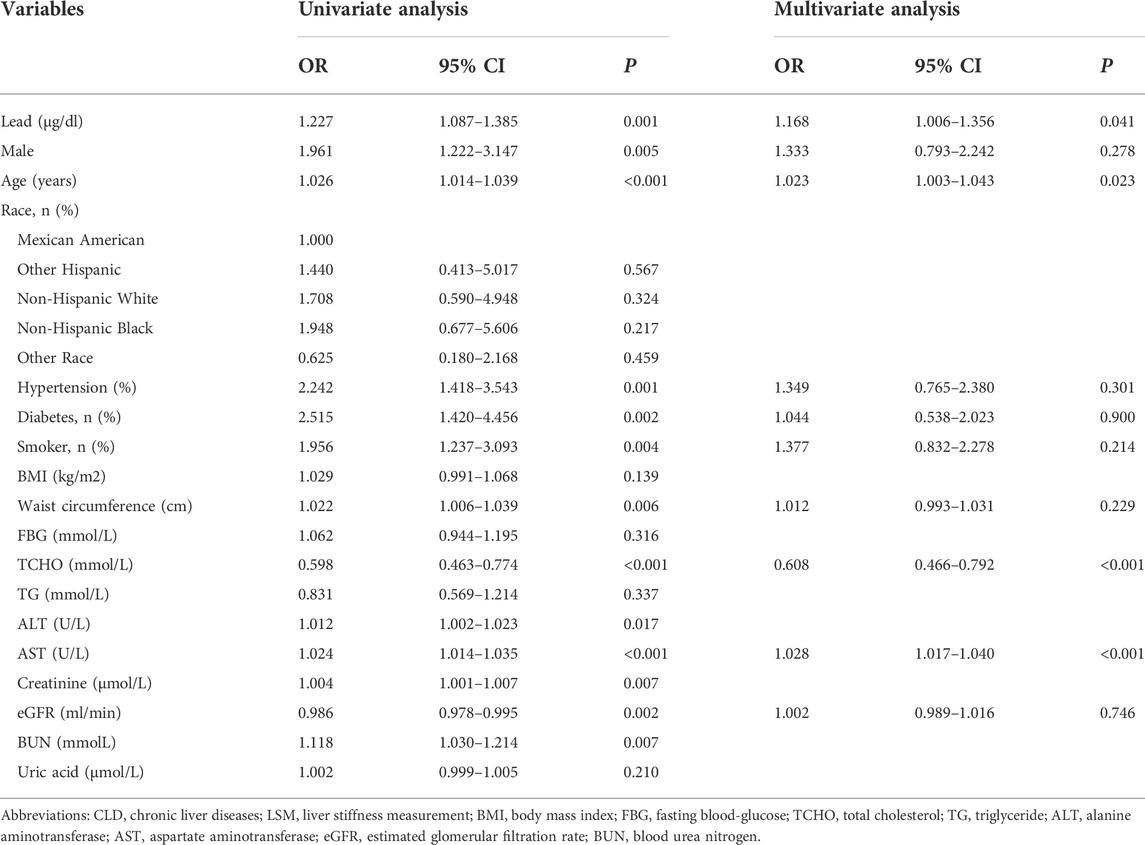
Univariate logistic regression analysis showed that blood lead level was a risk factor for advanced liver fibrosis (OR = 1.227; 95% CI, 1.087–1.385, p = 0.001, Table 4). The factors with a p-value < 0.05 in the univariate analysis were set as candidate parameters for the multivariate regression. A co-linearity test revealed that ALT and AST, creatinine, eGFR, and BUN showed collinearity. We selected the parameters with the largest area under the receiver operating characteristic curve for multivariate regression. After adjusting for sex, age, smoking history, presence of metabolic profiles (diabetes, hypertension, waist circumstance, TCHO), AST, and eGFR, the blood lead level was still independently associated with advanced liver fibrosis (OR = 1.168; 95% CI, 1.006–1.356; p = 0.041).
Results of the age- and sex-matched cohort
As the risk of liver fibrosis increases with age, we used PSM to match age and sex between the fibrosis and non-fibrosis groups. The comparison after PSM is summarized in Supplementary Table S1. No difference in age and sex was detected between individuals with and without advanced liver fibrosis after PSM. The blood lead levels in the fibrosis group were significantly higher than those of the non-fibrosis group (1.2 μg/dl vs 1.0 μg/dl, p < 0.001). Univariate logistic regression analysis showed that blood lead level was a risk factor for advanced liver fibrosis (OR = 1.273; 95% CI, 1.079–1.502, p = 0.004). After adjusting for hypertension, diabetes, TCHO, ALT and AST, the blood lead level remained as an independent risk factor for advanced liver fibrosis (OR = 1.249; 95% CI, 1.048–1.489, p = 0.013) (Supplementary Table S2).
Discussion
Utilizing the data from NHANES 2017–2020, this study showed that higher blood lead was independently associated with the risk of liver fibrosis in adults without underlying chronic liver disease.
In the present study, liver fibrosis was assessed as the liver elasticity measured using FibroScan. This method was completely different from those used in previous studies, in which a noninvasive model was used to diagnose fibrosis. For example, (Reja et al., 2020) used the NAFLD Fibrosis Score (NFS) and (Chung et al., 2020) used the fibrosis-4 index (FIB-4) to diagnose fibrosis. The accuracy of liver elasticity via FibroScan has been consistently reported to be better than those of noninvasive models (Ragazzo et al., 2017; Petta et al., 2019; Staufer et al., 2019).
In line with many previous studies, which have shown that long-term lead exposure was significantly associated with liver injury (Can et al., 2008; Chen et al., 2019; Nakhaee et al., 2019) and fibrosis (Chung et al., 2020; Reja et al., 2020) in both the overall and NAFLD populations, we also demonstrated that the proportion of advanced liver fibrosis was related to blood lead level. In contrast to the published literature, we ruled out cases known to have an increased risk of chronic liver disease, such as those with viral hepatitis, alcoholic liver disease, or fatty liver disease. By excluding patients with preexisting chronic liver disease, we minimized the fibrogenetic effect of known etiology. The results of this study further confirmed the association between blood lead concentration and risk of liver fibrosis.
The exact pathogenesis of chronic blood lead exposure and liver fibrosis remains unclear. Previous studies have shown that blood lead could cause hepatic damage by increasing the inflammatory response and oxidative stress (Berrahal et al., 2011). Moreover, lead is a divalent cation, which can replace calcium at multiple levels in the human body and therefore interfere with cell signaling pathways (Mitra et al., 2017). Furthermore, the molecular level of lead exposure resulting in various systemic effects is being extensively explored (Qian et al., 2020).
The suggestive safe blood lead level has declined dramatically from 60 μg/dl in 1960 to 5 μg/dl in 2012 (Ruckart et al., 2021). In 2021, this number was 5 μg/dl, as recommended by the National Center for Environmental Health/Agency for Toxic Substances and Disease Registry of the United States (Ruckart et al., 2021). In the present study, the median blood lead level was 0.9 μg/dl, which was much lower than the suggestive “safe” level. The results indicated that even a very low dose exposure might cause liver injury. This also raised concerns regarding the “real safe” blood lead level. As indicated by a previous study, there may in fact be no safe blood lead level for humans (Lanphear et al., 2018).
To the best of our knowledge, this is the first study to use liver elasticity to explore the relationship between liver fibrosis and blood lead level. The diagnostic accuracy for fibrosis has been greatly improved by using this new method. However, the current study should be interpreted in light of several limitations. First, as it was a cross-sectional study, we could not draw a causal relationship between lead exposure and liver fibrosis. Second, we were not able to rule out cases with undiagnosed liver disease as no data was provided in the database. However, the liver enzyme levels of this study population were within the normal range, and it has been comprehensively shown that normal liver enzymes are uncommon in patients with chronic liver disease. Therefore, although we may have missed some cases with undiagnosed chronic liver disease, this would not be common and therefore should not have greatly influenced the conclusion of this study. Nevertheless, the results of this study need to be confirmed in prospective studies.
In conclusion, this study demonstrated an association between blood lead level and liver fibrosis in individuals without known chronic liver disease. However, the causal relationship and underlying mechanism between lead and fibrosis requires further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ZZh, ZL, HL conducted the formal statistical analysis and data management. ZZe and ZL drafted the paper. DL and JH edited and revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Startup Fund for scientific research of Fujian Medical University (No. 2020QH1252).
Acknowledgments
The authors thank the National Health and Nutrition Examination Survey program for providing the data for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.995795/full#supplementary-material
Abbreviations
NHANES, National Health and Nutrition Examination Surveys; NAFLD, nonalcoholic fatty liver disease; LSM, liver stiffness measurement; CAP, controlled attenuation parameter; BMI, body mass index; FBG, fasting blood-glucose; TCHO, total cholesterol; TG, triglyceride; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; PSM, propensity score matching; FIB-4, Fibrosis-4 index; NFS, NAFLD fibrosis score.
References
American Diabetes Association (2020). Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care 43, S14–s31. doi:10.2337/dc20-S002
Berrahal, A. A., Lasram, M., El Elj, N., Kerkeni, A., Gharbi, N., and El-Fazâa, S. (2011). Effect of age-dependent exposure to lead on hepatotoxicity and nephrotoxicity in male rats. Environ. Toxicol. 26 (1), 68–78. doi:10.1002/tox.20530
Betanzos-Robledo, L., Cantoral, A., Peterson, K. E., Hu, H., Hernández-Ávila, M., Perng, W., et al. (2021). Association between cumulative childhood blood lead exposure and hepatic steatosis in young Mexican adults. Environ. Res. 196, 110980. doi:10.1016/j.envres.2021.110980
Boskabady, M., Marefati, N., Farkhondeh, T., Shakeri, F., Farshbaf, A., and Boskabady, M. H. (2018). The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 120, 404–420. doi:10.1016/j.envint.2018.08.013
Can, S., Bağci, C., Ozaslan, M., Bozkurt, A., Cengiz, B., Cakmak, E. A., et al. (2008). Occupational lead exposure effect on liver functions and biochemical parameters. Acta Physiol. hung. 95 (4), 395–403. doi:10.1556/APhysiol.95.2008.4.6
Cassinotto, C., Boursier, J., de Lédinghen, V., Lebigot, J., Lapuyade, B., Cales, P., et al. (2016). Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and arfi with liver biopsy. Hepatology 63 (6), 1817–1827. doi:10.1002/hep.28394
Chen, Y., Xu, X., Zeng, Z., Lin, X., Qin, Q., and Huo, X. (2019). Blood lead and cadmium levels associated with hematological and hepatic functions in patients from an e-waste-polluted area. Chemosphere 220, 531–538. doi:10.1016/j.chemosphere.2018.12.129
Cheng, D., Chai, J., Wang, H., Fu, L., Peng, S., and Ni, X. (2021). Hepatic macrophages: Key players in the development and progression of liver fibrosis. Liver Int. 41 (10), 2279–2294. doi:10.1111/liv.14940
Chuah, K. H., Lai, L. L., Vethakkan, S. R., Nik Mustapha, N. R., Mahadeva, S., and Chan, W. K. (2020). Liver stiffness measurement in non-alcoholic fatty liver disease: Two is better than one. J. Gastroenterol. Hepatol. 35 (8), 1404–1411. doi:10.1111/jgh.14978
Chung, S. M., Moon, J. S., Yoon, J. S., Won, K. C., and Lee, H. W. (2020). The sex-specific effects of blood lead, mercury, and cadmium levels on hepatic steatosis and fibrosis: Korean nationwide cross-sectional study. J. Trace Elem. Med. Biol. 62, 126601. doi:10.1016/j.jtemb.2020.126601
El-Naggar, N. E., Hamouda, R. A., Mousa, I. E., Abdel-Hamid, M. S., and Rabei, N. H. (2018). Biosorption optimization, characterization, immobilization and application of Gelidium amansii biomass for complete Pb(2+) removal from aqueous solutions. Sci. Rep. 8 (1), 13456. doi:10.1038/s41598-018-31660-7
Gabb, G. M., Mangoni, A. A., Anderson, C. S., Cowley, D., Dowden, J. S., Golledge, J., et al. (2016). Guideline for the diagnosis and management of hypertension in adults - 2016. Med. J. Aust. 205 (2), 85–89. doi:10.5694/mja16.00526
Ginès, P., Krag, A., Abraldes, J. G., Solà, E., Fabrellas, N., and Kamath, P. S. (2021). Liver cirrhosis. Lancet 398 (10308), 1359–1376. doi:10.1016/s0140-6736(21)01374-x
Guo, Y. C., and Lu, L. G. (2020). Antihepatic fibrosis drugs in clinical trials. J. Clin. Transl. Hepatol. 8 (3), 1–9. doi:10.14218/jcth.2020.00023
Horne, D. J., Campo, M., Ortiz, J. R., Oren, E., Arentz, M., Crothers, K., et al. (2012). Association between smoking and latent tuberculosis in the U.S. Population: An analysis of the national health and nutrition examination survey. PLoS One 7 (11), e49050. doi:10.1371/journal.pone.0049050
Kabbany, M. N., Conjeevaram Selvakumar, P. K., Watt, K., Lopez, R., Akras, Z., Zein, N., et al. (2017). Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: An analysis of national health and nutrition examination survey data. Am. J. Gastroenterol. 112 (4), 581–587. doi:10.1038/ajg.2017.5
Karlas, T., Petroff, D., Sasso, M., Fan, J. G., Mi, Y. Q., de Lédinghen, V., et al. (2017). Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatology 66 (5), 1022–1030. doi:10.1016/j.jhep.2016.12.022
Kasperczyk, A., Dziwisz, M., Ostałowska, A., Swietochowska, E., and Birkner, E. (2013). Function of the liver and bile ducts in humans exposed to lead. Hum. Exp. Toxicol. 32 (8), 787–796. doi:10.1177/0960327112468177
Kim, D. W., Ock, J., Moon, K. W., and Park, C. H. (2021). Association between Pb, Cd, and Hg exposure and liver injury among Korean adults. Int. J. Environ. Res. Public Health 18 (13), 6783. doi:10.3390/ijerph18136783
Lanphear, B. P., Rauch, S., Auinger, P., Allen, R. W., and Hornung, R. W. (2018). Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 3 (4), e177–e184. doi:10.1016/s2468-2667(18)30025-2
Levey, A. S., Stevens, L. A., Schmid, C. H., Zhang, Y. L., Castro, A. F., Feldman, H. I., et al. (2009). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150 (9), 604–612. doi:10.7326/0003-4819-150-9-200905050-00006
Lin, H., Zhang, X., Li, G., Wong, G. L., and Wong, V. W. (2021). Epidemiology and clinical outcomes of metabolic (Dysfunction)-associated fatty liver disease. J. Clin. Transl. Hepatol. 9 (6), 000–982. doi:10.14218/jcth.2021.00201
Meyer, P. A., Brown, M. J., and Falk, H. (2008). Global approach to reducing lead exposure and poisoning. Mutat. Research/Reviews Mutat. Res. 659 (1-2), 166–175. doi:10.1016/j.mrrev.2008.03.003
Mitra, P., Sharma, S., Purohit, P., and Sharma, P. (2017). Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 54 (7-8), 506–528. doi:10.1080/10408363.2017.1408562
Nakhaee, S., Amirabadizadeh, A., Brent, J., and Mehrpour, O. (2019). Impact of chronic lead exposure on liver and kidney function and haematologic parameters. Basic Clin. Pharmacol. Toxicol. 124 (5), 621–628. doi:10.1111/bcpt.13179
Nalbantoglu, I., and Jain, D. (2019). Cryptogenic cirrhosis: Old and new perspectives in the era of molecular and genomic medicine. Semin. Diagn. Pathol. 36 (6), 389–394. doi:10.1053/j.semdp.2019.07.003
Obeng-Gyasi, E. (2019). Sources of lead exposure in various countries. Rev. Environ. Health 34 (1), 25–34. doi:10.1515/reveh-2018-0037
Petta, S., Wai-Sun Wong, V., Bugianesi, E., Fracanzani, A. L., Cammà, C., Hiriart, J. B., et al. (2019). Impact of obesity and alanine aminotransferase levels on the diagnostic accuracy for advanced liver fibrosis of noninvasive tools in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 114 (6), 916–928. doi:10.14309/ajg.0000000000000153
Qian, B., Xue, L., Qi, X., Bai, Y., and Wu, Y. (2020). Gene networks and toxicity/detoxification pathways in juvenile largemouth bass (Micropterus salmoides) liver induced by acute lead stress. Genomics 112 (1), 20–31. doi:10.1016/j.ygeno.2019.06.023
Ragazzo, T. G., Paranagua-Vezozzo, D., Lima, F. R., de Campos Mazo, D. F., Pessoa, M. G., Oliveira, C. P., et al. (2017). Accuracy of transient elastography-FibroScan®, acoustic radiation force impulse (ARFI) imaging, the enhanced liver fibrosis (ELF) test, APRI, and the FIB-4 index compared with liver biopsy in patients with chronic hepatitis C. Clin. (Sao Paulo) 72 (9), 516–525. doi:10.6061/clinics/2017(09)01
Reja, D., Makar, M., Visaria, A., Karanfilian, B., and Rustgi, V. (2020). Blood lead level is associated with advanced liver fibrosis in patients with non-alcoholic fatty liver disease: A nationwide survey (NHANES 2011-2016). Ann. Hepatol. 19 (4), 404–410. doi:10.1016/j.aohep.2020.03.006
Ruckart, P. Z., Jones, R. L., Courtney, J. G., LeBlanc, T. T., Jackson, W., Karwowski, M. P., et al. (2021). Update of the blood lead reference value - United States, 2021. MMWR. Morb. Mortal. Wkly. Rep. 70 (43), 1509–1512. doi:10.15585/mmwr.mm7043a4
Staufer, K., Halilbasic, E., Spindelboeck, W., Eilenberg, M., Prager, G., Stadlbauer, V., et al. (2019). Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United Eur. Gastroenterol. J. 7 (8), 1113–1123. doi:10.1177/2050640619865133
Keywords: lead, fibrosis, environment, liver, NHANES
Citation: Zhang Z, Li Z, Lin H, Zeng Z, Huang J and Li D (2022) Lead exposure was associated with liver fibrosis in subjects without known chronic liver disease: An analysis of NHANES 2017–2020. Front. Environ. Sci. 10:995795. doi: 10.3389/fenvs.2022.995795
Received: 16 July 2022; Accepted: 20 September 2022;
Published: 17 October 2022.
Edited by:
Liu Zhirong, East China University of Technology, ChinaReviewed by:
Mohamed Helal, National Institute of Oceanography and Fisheries, EgyptAugusta Chinyere Nsonwu-Anyanwu, University of Calabar, Nigeria
Copyright © 2022 Zhang, Li, Lin, Zeng, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongliang Li, bGRsaWFuZzkwMEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
‡ORCID: Zhiqiang Zhang, https://orcid.org/0000-0002-2278-7778; Zhangping Li, https://orcid.org/0000-0002-1133-8088; Haiyan Lin, https://orcid.org/0000-0002-3009-0516; Zhiyu Zeng, https://orcid.org/0000-0002-2403-8957; Jiaofeng Huang, https://orcid.org/0000-0003-1383-6897
 Zhiqiang Zhang1,2†‡
Zhiqiang Zhang1,2†‡ Dongliang Li
Dongliang Li