- 1Agricultural Innovation and Technology Transfer Center (AITTC), Mohammed VI Polytechnic University, Benguerir, Morocco
- 2OCP Group, OCP Sustainability Platform, Casablanca, Morocco
Salinity and sodicity are the most agricultural challenges in arid and semi-arid regions. A pot experiment was undertaken, to evaluate the effect of Phosphogypsum (PG) and Gypsum (G), to remove salts, nutrients and trace elements in leached water from saline and saline-sodic soils. In order to determine the efficiency and safety of these amendments, as an affordable strategy, for overcoming salinity and sodicity stress. The PG at 0, 15, 30 and 45 t/ha and G at 15 t/ha were mixed with the upper 9 cm soil in the pot before being leached. The soils were collected from Sed El Masjoune and Sidi El Mokhtar areas of morocco with ECe of 140.6 mS/cm and 11.7 mS/cm respectively. The highest doses of PG (≥30 t/ha) removed significant amount of salts and nutrients. Calcium sulfate supplies calcium ions to replace salt ions (sodium, especially). The replaced salts are leached from the soil. The PG was more efficient compared to G in terms of salts leaching. Quantities of trace elements in the leachate, for most analyzed elements, were below the recommended limits of drinking and irrigation water. Because the experiment’s alkaline conditions (basic water and soil) reduce the solubility and mobility of trace elements. The amendment application did not affect saturation index (SI) of the main minerals. However, water passing through the soil increased the SI. which could result in groundwater mineral precipitation.
Introduction
Soils are fundamental to life on Earth but human and environmental pressures on soil resources are reaching critical limits. Soil is one of the most important natural resources which can be subject to different forms of degradation (salinity, acidity, erosion...). Salinity and sodicity are among the ten threats to soils identified by FAO (FAO, 1999). Salinity is increasingly threatening agricultural production, especially in arid and semi-arid areas. The human-induced salinity through irrigating soils with salty (brackish/saline) water (2ndry salinity) is great threat to irrigated agriculture, whereby, we are losing 2,000 ha daily of farm land due to only soil salinization (UNU, 2014). Based on the electrical conductivity of saturated soil paste (ECe). The soil is classified as no-saline (0 < Ece (dS/m)≤2) to very strongly saline (Ece (dS/m) > 16) (Figure 1) (Lech et al., 2016). While sodic soil has a percentage of exchangeable sodium (ESP) higher 15% (FAO, 1988). The area of total land impacted by salts is about one billion hectares, and the trend is significantly increasing (Ivushkin et al., 2019). In Morocco, salinization affects 5% of agricultural soils (Antipolis, 2003). Which corresponds to 435,000 ha. In addition to saline soils, most of the groundwater is also saline in fact, 25% of Moroccan groundwater is characterized by a salts content of 1–2 g/L, and 27.5% has a salt concentration greater than 2 g/L (Hssaisoune et al., 2020).

FIGURE 1. Soil salinization classification standards (Lech et al., 2016).
Prolonged drought periods with low and erratic rainfall distribution and extreme temperatures, due to climate changes, cause, the increase of saline soils in arid, semi-arid, and coastal agricultural areas (Corwin, 2021). On the other hand, anthropogenic activities, especially irrigation management and low water quality induce significant soil salinization (Moharana et al., 2019). Salt affected soils usually generate physical, chemical and physiological disorders in soil-plant- water system. The presence of salts leads to the development of osmotic potential of soil water, which reduces plant transpiration thereby affecting crop yield (Lamsal et al., 1999). Whereas sodicity degrades soil structure and destroy its stability (El hasini et al., 2019) which subsequently affect water and air movement and root development. The reclamation of salt affected soils has been subject of numerous studies around the world. Many approaches were evaluated to mitigate soil salinity for example phytoremediation by using halophyte plants which have a capacity to tolerate saline conditions (Okur et al., 2020). Mu et al. (2021) reported that halophytes plants (Sedum aizoon L. and Sesbania cannabina Pers.) performed better at saline soil remediation. Devi et al. (2016) characterized Suaeda fruticosa and Atriplex lentiformis as salt hyperaccumulator species. Hirich et al. (2021) reported that the introduction of alternative crops, such as blue panicum, quinoa and sesbania, reduces the impacts of soil and water salinity. Moreover, the reclamation of saline and sodic soils can be achieved using integrated soil reclamation approach including physical, chemical, hydrological and biological methods, based on the scientific diagnostics of both the salinity and sodicity, such as PG (Smaoui-Jardak et al., 2017; El Mejahed et al., 2020), and G (Makoi and Verplancke, 2010; Zaman et al., 2018). The PG is a byproduct of phosphate industry according to the following reaction.
The world annual production of PG is about 300 Mt (Cuadri et al., 2021). However, only 15% of PG produced at the global level is recycled. The PG has been used in construction industry (Manal et al., 2012) and in agriculture as fertilizer and as amendment for reclamation of degraded soils such as saline, sodic, acidic and alkaline soils (Mesić et al., 2016).
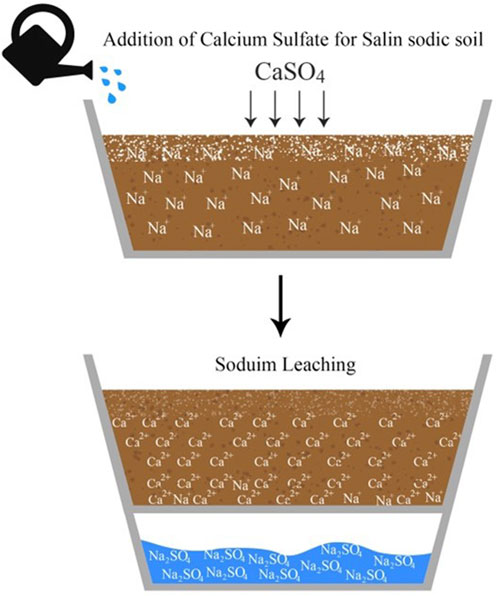
Calcium sulfate can readily furnish soluble calcium (Ca) to substitute exchangeable sodium (Na), the reaction is as follows (FAO, 1988):
Reclamation process is illustrated in Figure 2.
The use of 40 t/ha of PG for reclamation of a very strongly saline soil (ECe = 52 ds/m and ESP = 35.7%) using moderate saline water (EC = 2.2 ds/m and Sodium adsorption ratio (SAR) = 4.1 Meq/l) increased the relative removal of sodium (Na/Na0), which was 0.80 in the treated soil leachate compared to 0.42 in the control test (Gharaibeh et al., 2010).
The PG showed its desalinization and desodification abilities by reducing soil EC and exchangeable sodium percentage (ESP) (Abdel-Fattah and EL-Naka, 2015). The application of PG reclaimed the soil which passes from saline-sodic situation to normal (Diop et al., 2019). On the other hand, Makoi and Verplancke (2010) indicated that leaching after gypsum application reduced exchangeable Na, Sodium adsorption ratio (SAR) and subsequently the ESP of the soil.
However PG contains several heavy metals and radioactive elements (Lütke et al., 2020; Qamouche et al., 2020). The agricultural PG use should examine the transferability of these elements to the soil, plant, and water systems. Gypsum has relatively low solubility, especially in saline water (EC > 2.2 dS/m), resulting in its precipitation (Porta, 1998).
Groundwater, a natural water resource, is mainly used for domestic and irrigation purposes (Jiang et al., 2009; Nagarajan et al., 2012). Groundwater quality is very much affected by anthropogenic activities, especially agriculture, which may be considered as one of the most important sources of groundwater contamination, mainly by nitrate (NO3) and trace elements leaching (Agostini, et al., 2010; Zhao et al., 2011; Adimalla and Qian, 2019), for these reasons, the evaluation of leached water quality is highly important. The use of PG for saline soils shows the tendency of sodium to be leached from the soil, and the accumulation of phosphorus and rare-earth elements in saline soils (Gorbunov et al., 1992). Because of alkaline conditions that decrease the leachability and mobility of the majority of heavy metals (Al-Masri et al., 2004). Several authors (Ismail et al., 2015; Walawalkar et al., 2016; Mashifana et al., 2019) have used different acids, such as nitric, hydrochloric, sulfuric and citric acids to leach rare elements from PG. In addition, Motalane and Strydom (2004) reported that the acidic nature of Phosphogypsum can increase heavy metal mobility and leachability.
Leaching of PG with distilled water shows that lead (Pb), selenium (Se), silver (Ag), zinc (Zn) and copper (Cu) were retained in soil surface and their contents in leached water were bellow recommended limits for drinking water, while arsenic (As), cadmium (Cd), chromium (Cr) and nickel (Ni) could present transfer risk to groundwater (Hassoune et al., 2017). Al-Hwaiti et al. (2010) concluded that As, Cd, Cr, Cu, uranium (U) and Zn were not leached from the Phosphogypsum in significant amounts, and they were not transferred easily to the aquatic environment. The PG leaching tests showed that Ca, fluoride (F), phosphorus (P), sulfur (S), silicon (Si), As, barium (Ba), Cr, Cu, nickel (Ni), Pb, strontium (Sr), Zn contents in leachate did not exceed the threshold limits. However, Zn, Cd and Se were above the allowed limits (Zmemla et al., 2020).
Soil nutrients losses, such as Ca, magnesium (Mg), potassium (K), and nitrogen (N) has been released by PG (Brien and Sumner, 2008) and gypsum (Alva and Gascho, 1991) applications.
Salinity is a growing threat to food security in arid and semi-arid regions. Phosphogypsum is an affordable saline soil amendment. In fact, Moroccan phosphate industry generates huge quantities of PG (around 25 MT/year) (Harrou et al., 2020). However, limited number of papers focus on the valorization of Moroccan PG in agriculture. The objectives of this study is to evaluate salts, nutrients, and trace elements removal in leachate of salt affected soils treated with G and different rates of PG, and to investigate the environmental impacts of G and PG on groundwater.
Materials and methods
Soil sampling and analysis
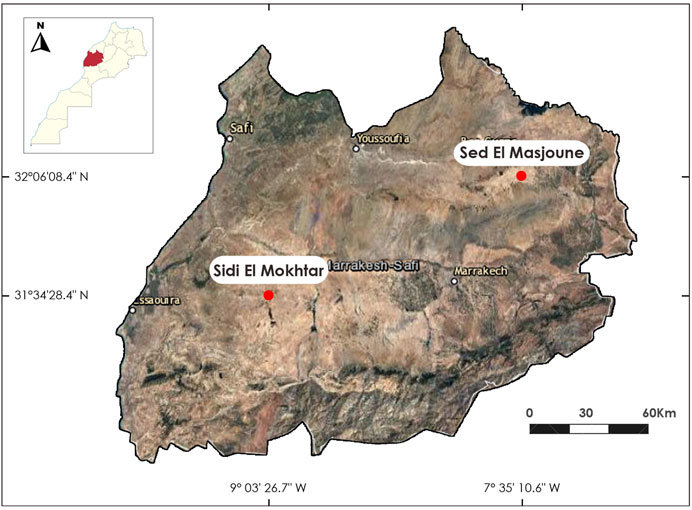
The bulk soil samples were collected at 0–20 cm soil depth from Sed El Masjoune (32°06′08.4″N 7°35′10.6″W), a well-known salt-affected region in Morocco (El hasini et al., 2019) and Sidi El Mokhtar (31°34′28.4″N 9°03′26.7 “W) region of Morocco (Figure 3).
Sed el Masjoune is a seasonal lake, and more than 10,000 ha of saline land surround this lake. It corresponds to the final valley, where water runoff stored on the lake’s high plateaus evaporates during the hot seasons, causing the soil salinization (El hasini et al., 2019). It has a semiarid climate with an average annual precipitation of 200 mm/year and temperature ranges as min/max values of −3.6°C in winter and 48°C in summer (Zouahri et al., 2018).
Sidi El Mokhtar area is part of the large basin of the Tensift wadi. It is a semi-arid region with annual average precipitation of 180 mm and temperatures ranging from 15 to 20°C (Fathallah et al., 2021).
Soil samples were air dried, ground and passed through a 2 mm sieve to collect fine-earth fraction (<2 mm). Soil pH was measured in the 1:5 soil: water extract (ISO 10390). Soil salinity was determined by measuring EC of the extract (ECe) from saturated soil paste (Richards, 1954) and presented as mS/cm. Phosphorus content was determined by Olsen method (Olsen et al., 1954). Spectrophotometry (Agilent Technologies. Cary 60 UV-Vis) was used to determine, sulfate, ammonium (NH4), chlorine (Cl) and nitrate contents. The sodium, potassium, calcium and magnesium contents were determined by atomic absorption spectroscopy (Agilent Technologies. 200 Series AA). Total lime was analyzed using the method of Allison. (1960). Heavy metals were determined by ICP (Agilent Technologies. 5110 ICP-OES). The ESP was calculated using standard equation (Qadir et al., 2007)
with all the cations expressed as cmolc kg.−1
Soils, PG, G, water used for drainage and leached water analysis were carried out at the soil, plant, and water laboratory of the Agriculture Innovation Transfer Technololgy Center of the University Mohammed six Polytechnic at Ben Guerir, Morocco.
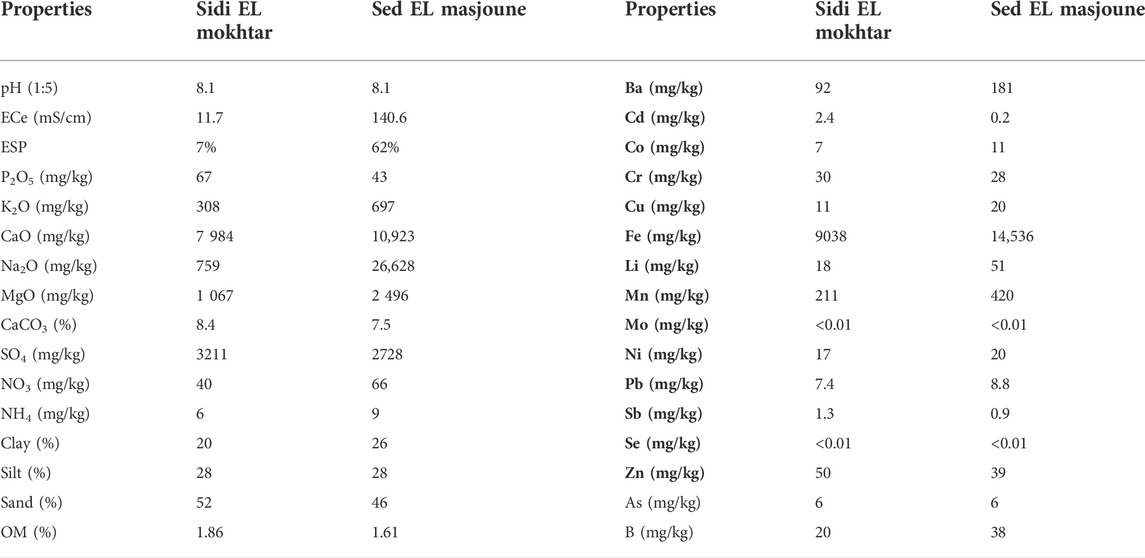
Based on the values of ECe and ESP (Table 1), the soil of Sidi El Mokhtar is classified as moderately saline soil and Sed El Masjoune soil is strongly saline-sodic soil (Qadir et al., 2007; Lech et al., 2016). Both the soils are categorized as moderately alkaline with respect to soil pH. Chemical characteristics of the soils used in this experiment are presented in Table 1.
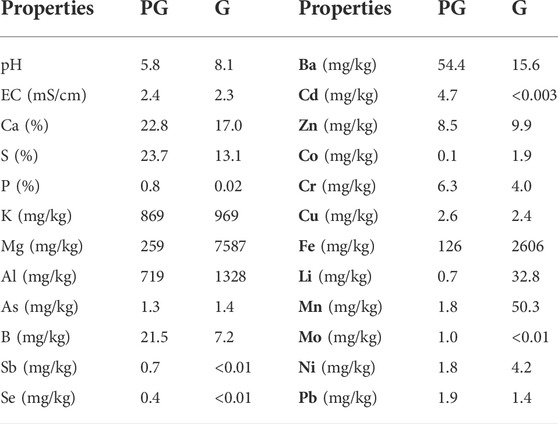
The pH and EC of amendments were measured in the 1:5 PG or G: water extracts. Nutrients and heavy metals contents were quantified by ICP-OES. The results show (Table 2) that PG is acidic and richer on Ca, S and P than natural gypsum. However, the G is richer in Mg and K relative to PG. In terms of heavy metals content, PG is more charged with Ba, Cd, Cr, Cu, boron (B), molybdenum (Mo), antimony (Sb) and Se, while Gypsum contains more of Zn, cobalt (Co), Iron (Fe), aluminium (Al), lithium (Li), As, manganese (Mn) and Ni.
The pH and EC of water for drainage and leached water were measured by using calibrated pH (InoLab pH 7310) and conductivity meters (Mettler Toledo. SevenCompact) respectively. The Sulfate, Ammonium, Chlorine and Nitrate contents were quantified by Spectrophotometry. ICP-OES was used to determine the Phosphorus, Sodium, Potassium, Calcium, Magnesium and Heavy metals contents.
The SAR is calculated from the following equation (Qadir et al., 2007):
Na, Ca and Mg expressed as meq/l.
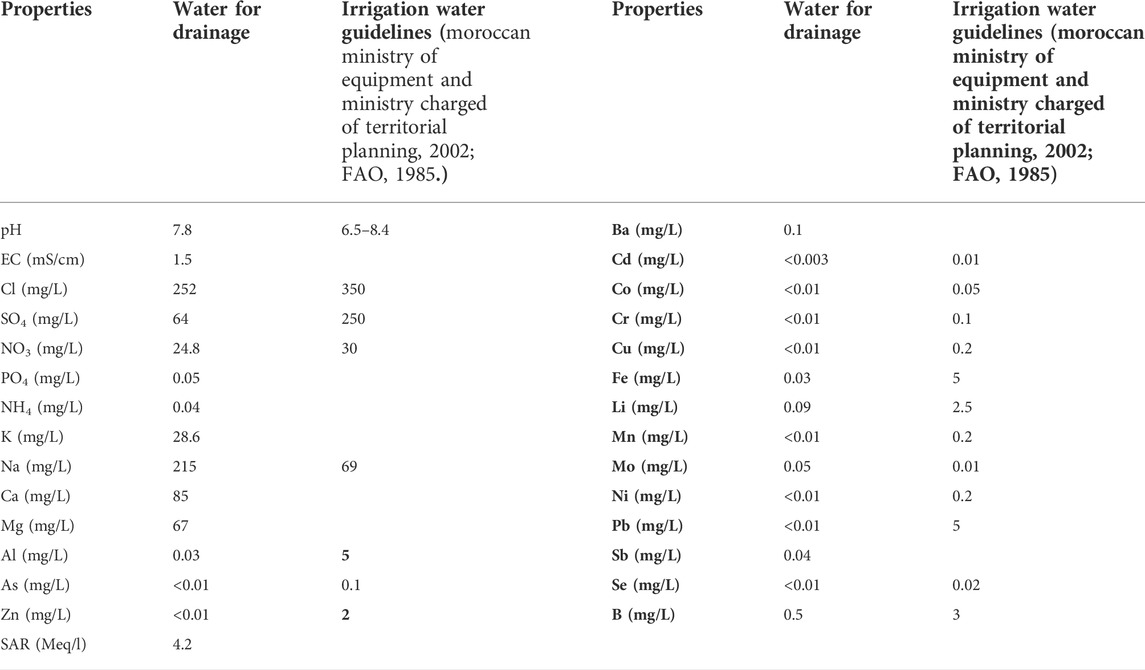
Nutrients and heavy metals contents of the water used for drainage are below the recommended limits for irrigation water, excluding sodium and molybdenum contents (Table 3).
According to (Suarez et al., 2006), Irrigation water with values of SAR higher than 4 Meq/l, represents sodicity risks for the soils. For this reason, we used the PG also for the soil of Sidi El Mokhtar even if it was not initially sodic (sodicity problem prediction).
The saturation index is one of the crucial criteria that affect the thermodynamic stability of groundwater. Saturation index of mineral is calculated by using the following equation (Garrels and Mackenzie, 1967):
KIAP is the ion activity product. It is determined based on the chemical analysis of the water.
KSP is the mineral’s solubility product
SI was calculated using the PHREEQC software (Parkhurst and Appelo, 1999).
Trials installation
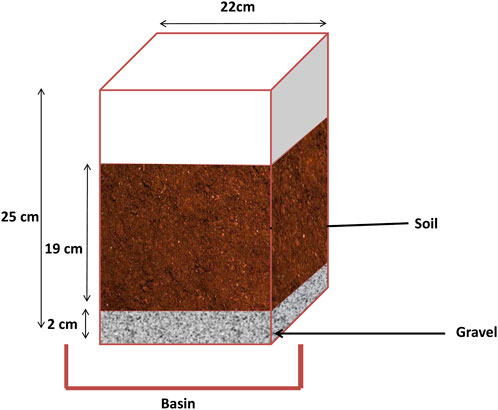
The pot experiment was conducted at the experimental farm of Mohamed six Polytechnic University (UM6P) in Ben Guerir Morocco. Moroccan PG is the first amendment used. The second one was is the natural Gypsum of agriculture grade commonly used to reclaim sodic and saline-sodic soils in agriculture fields. Different experimental treatments [Control (T1), 15 t/ha of G (T2), 15 (T3), 30 (T4) and 45 (T5) t/ha of PG] were used. Each pot was filled with 10 kg of the processed soil (<2 mm). The PG and G were mixed with the upper 9 cm of the soil. The base of pots was perforated and covered with stones to avoid soil leakage and to ensure water drainage; the leached water was collected in small basins under the pots for analyses.
The thickness and the diameter of the soil column were 19 and 22 cm, respectively (Figure 4). The water replenishment was intermittent.
Statistical analysis
The experiment was conducted in a complete randomized design (CRD), in which the treatments are entirely assigned randomly; indeed, each experimental unit has an equal probability of obtaining any treatment (FAO, 1999; Alkutubi 2012), with seven replications for Sidi El Mokhtar soil and eight replications for Sed El Masjoune soil. Data were analyzed statistically using IMB SPSS (Version 20, IBM SPSS Inc., Chicago, IL, USA). . One-way analysis of variance (ANOVA), tests were performed to relieve the effect of treatments on pH, Ec, salts, nutrients, heavy metals contents in leached water. If the differences between the treatment were significant (p < 0.05), the Student-Newman-keuls Test was used as a post hoc mean separation test to detect mean differences (Kucuk et al., 2016). All data are presented as mean ± standard deviation.
Results and discussion
pH of leached water
The amendment application did not significantly affect the pH of leached water (Figure 5). Several authors confirmed that PG (Gharaibeh et al., 2011) and G (Chaganti et al., 2015) did not influence soil pH after leaching.
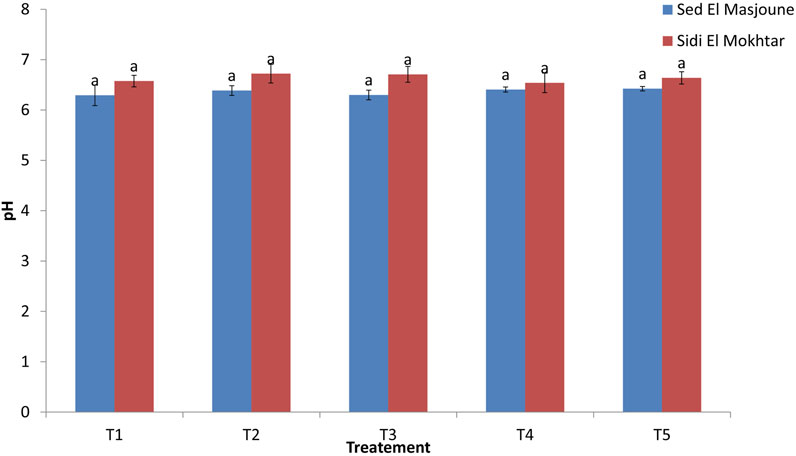
FIGURE 5. pH of leached water for different treatments. In a column series, same letters indicate no significant differences among treatments.
Salts and nutrients leaching
Results of salts, and nutrients contents of leachate of Sidi El Mokhtar soil showed that PG application removed significant amounts of Na and Cl. The same trend was observed for EC of leached water. Indeed, the increase in salts concentrations in leachate results in an increase of its EC. Compared to the control, EC of leached water was increased by 23 and 28% with T4 and T5 respectively, and correspondingly, Cl content increased by 27 and 33%, and sodium content by 36 and 33% (Table 4). The increased salts in leachate can be reflected by soil salinity reduction. Diop et al. (2019) concluded that PG offered significant soil salinity reduction that is up to 45%.
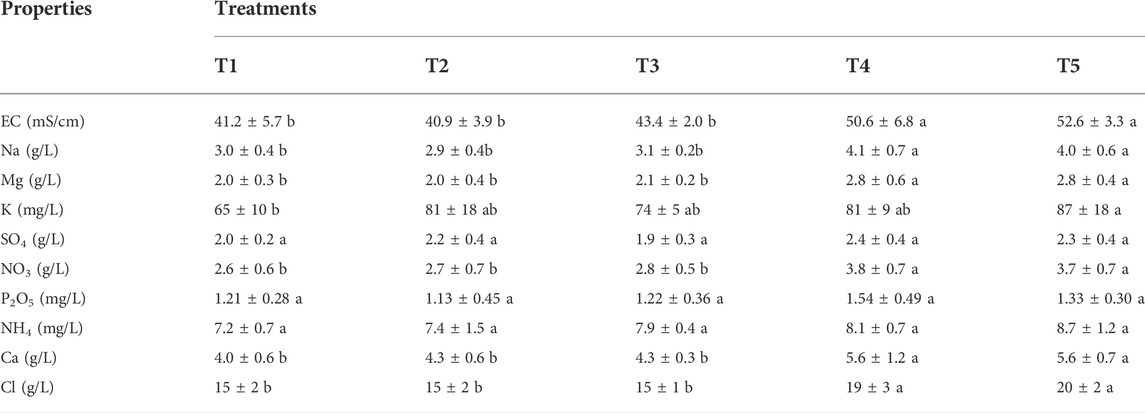
TABLE 4. EC, salts, and nutrients contents of leachate of Sidi El Mokhtar soil for different treatments. In a line series, same letters indicate no significant differences among treatments (p < 0.05, S-N-K ‘s test).
In addition to the removal of salts, the PG application contributed to substantial nutrients losses, such as Ca, Mg, K, and N. These elements play key role in plant development and soil fertility. Similar results were reported by (Brien and Sumner, 2008) who affirmed that the use of PG has improved Ca, Mg, K composition of the leachate. Shainberg et al. (1989) reported that the use of PG renders Mg and K prone to leaching and proposed post drainage fertilization to restore balanced nutrition. Because the saline and sodic soils fertility is typically low, the use of PG must be reasoned, mainly if it is applied frequently.
Even though the effect of 15 t/ha of natural gypsum and PG were not statistically different, the latter increased the EC of the leachate by 6% compared to the former. The same trend was observed for Cl, Na and Mg, this finding can be explained by the fact that PG was more acidic than G (Table 2). The acidic conditions favor salts solubilization and ameliorate their leaching (Phung et al., 1979).
The NH4, P2O5 and SO4 contents of the leachate were not statistically significant. The ammonium is known as a low mobile element in the soil (Li et al., 2003)and its initial soil content was low (6 ppm). Phosphorus has a limited mobility in the soil and tends to precipitate, particularly, in soils with alkaline pH range (Llanderal et al., 2021). Additionally, P has a high soil matrix fixation rate (Shen et al., 2011) Hence, low concentrations of ammonium and phosphorus in the leached water were recorded.
The EC, salts, and nutrients contents of the leachate from Sed El Masjoune soil are presented in Table 5. All amendments application rates significantly increased sodium concentration in the drainage water. The most significant rise of Na was recorded with T4 and T5 treatments with subsequent increase of EC of the leachates. Calcium sulfate furnishes soluble calcium to substitute exchangeable sodium, which results in sodium leaching from the saline-sodic soil (FAO, 1988). Increasing the PG rate induces the enhancement of soluble calcium in the soil. As a result, a large amount of sodium was substituted.
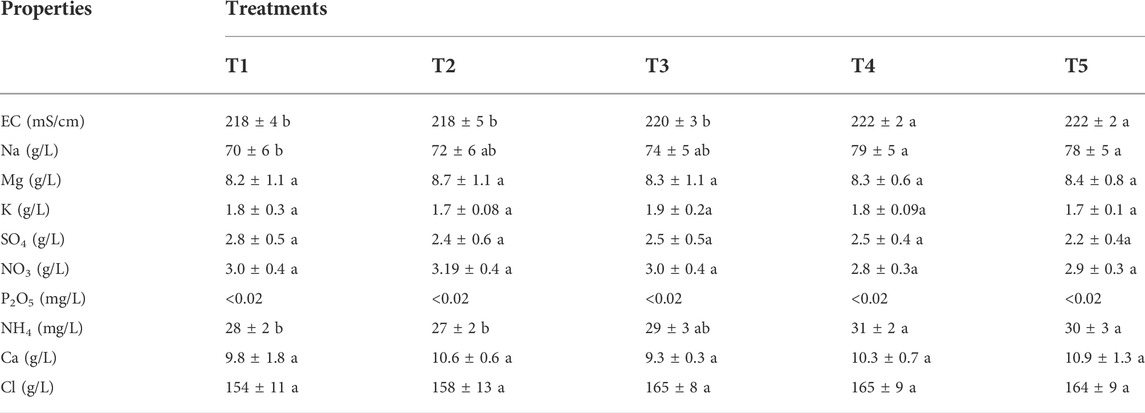
TABLE 5. EC, salts, and nutrients contents of leachate of Sed El Masjoune soil for different treatments. In a line series, same letters indicate no significant differences among treatments (p < 0.05, S-N-K ‘s test).
The same trend was observed for NH4 content.
The amendments (PG and G) did not produce significant effect in terms of removing Cl, SO4, NO3, Mg, Ca, K and P2O5. These findings can be explained by the fact that one time drainage was not enough to produce significant effect, especially when the soil is more charged with salts. Sed El Masjoune’s soil is strongly saline-sodic, with an ESP of 62%, whereas Sidi El Mokhtar’s soil is saline with an ESP of 7% (Table 1). Goncalo Filho et al. (2020) Reported that Calcium ions must eliminate a significant portion of exchangeable sodium to improve sodic soil. Which explains that the PG used rates were more effective for the soil of Sidi El Mokhtar than Sed El Masjoune.
Abdel-Fattah (2012) reported that total soluble salts removal in the leachate was related to increased number of leaching performed. Moreover, Gharaibeh et al. (2010) affirmed that most Na removed was detected after three and four drainage sessions.
For both soils, the highest doses of PG (30 and 45 t/ha) are more efficient for removing the salts. A Similar finding was reported by Gharaibeh et al. (2014) who indicated that effluent sodium levels were higher with rising PG rates.
Heavy metals leaching
For the leachate of Sidi El Mokhtar soil. The amendment application did not result in significant differences on leachate concentrations of B, Co, Li, Ni, Zn, Sb and Mo (Table 6). Zn is a very mobile element in PG (Al-Masri et al., 2004; Al-Hwaiti et al., 2019) but it is affected by pH, indeed under alkaline conditions, Zn can be almost undetected (Zmemla et al., 2016). Arsenic, chromium, lead, and selenium leachate values were <0.01 mg/L regardless of treatments. Hassoune et al. (2017) Indicated that Phosphogypsum leachate had low values of Se and Pb. However, Zmemla et al. (2016) reported a potential release of Se and Cr from PG. The T4 and T5 have caused an increase of Al, Cd and Mn contents in the leached water. In addition, all amendments rates (G and PG) significantly affected Ba content mainly with T4 and T5 treatments. Copper and iron levels in leachate were significantly higher with T4 compared to the other treatments. Al-Hwaiti et al. (2005) reported that Cu had a highest mobility during the PG leaching experiments. While, Al and Fe have shown limited removal (Zmemla et al., 2016).
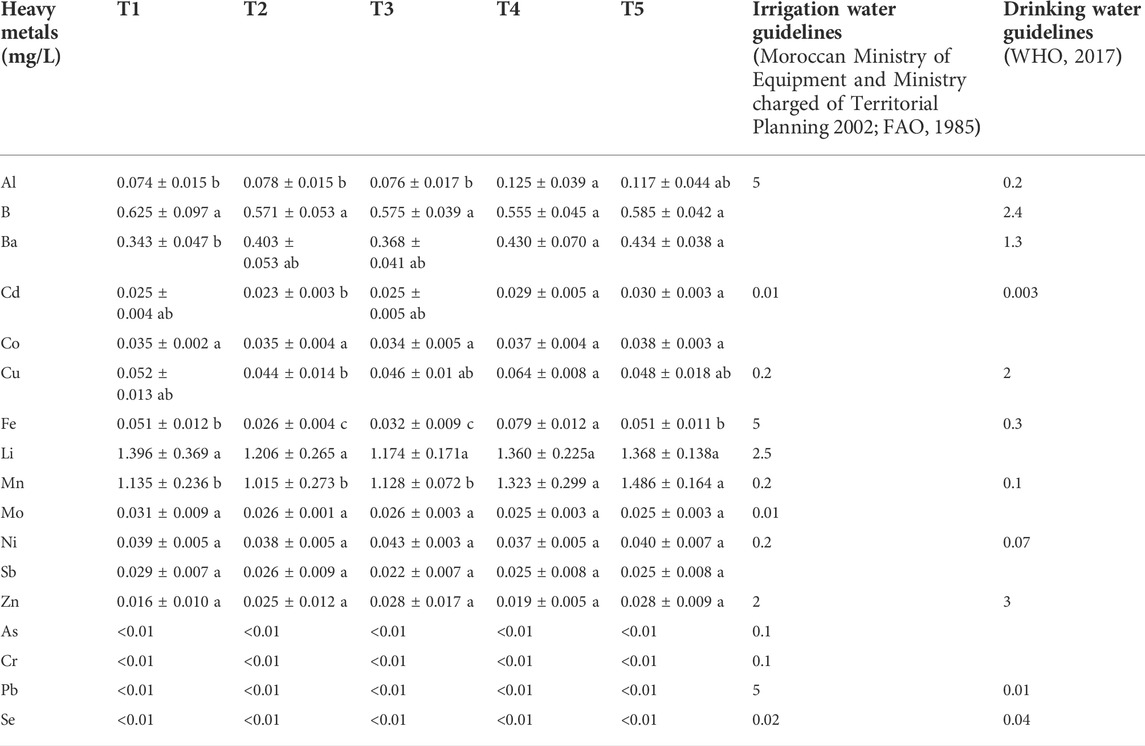
TABLE 6. Heavy metals contents of leachate of Sidi El Mokhtar soil for different treatments. Same letters in a line series indicate no significant differences among treatments (p < 0.05, S-N-K’s test).
All heavy metals contents in the leachate of Sed El Masjoune soil have not shown any significant differences between the treatments (Table 7). Al-Masri et al. (2004) attributed the low removal of trace elements to alkaline conditions which strongly decrease their mobility and solubility.
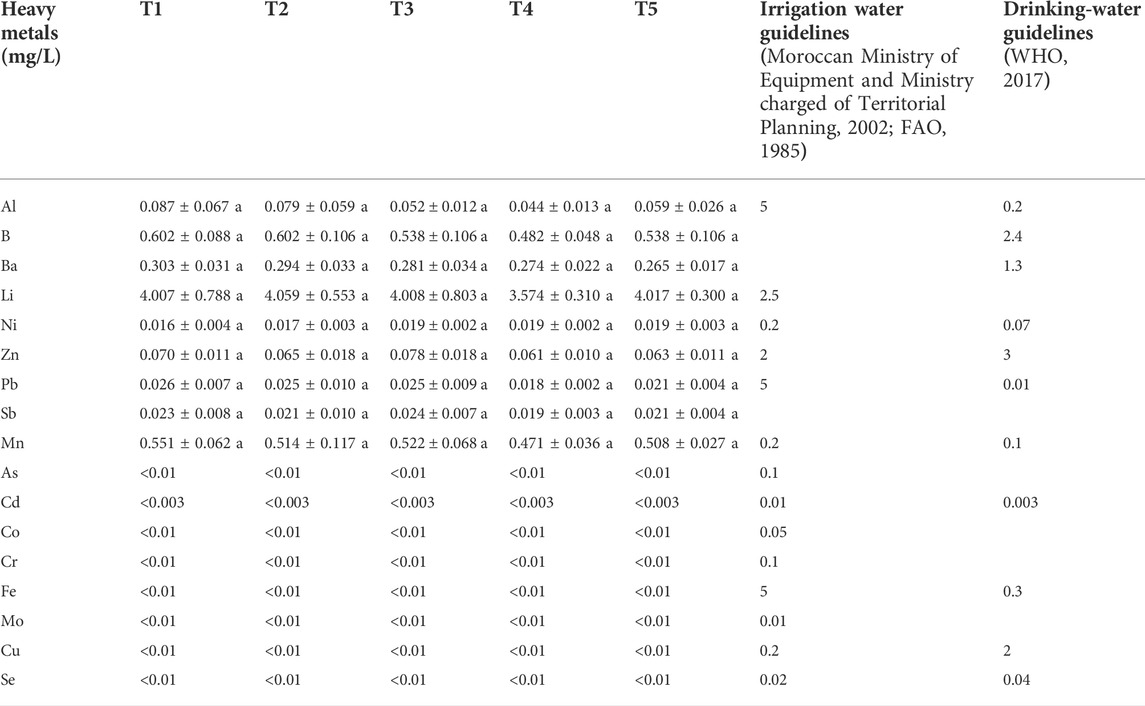
TABLE 7. Heavy metals contents of leachate of Sed El Masjoune soil for different treatments. Same letters in a line series indicate no significant differences among treatments (p < 0.05, S-N-K’s test).
The comparison of leachate trace element contents with irrigation water and drinking water guidelines standards is necessary to reveal the effects of PG and G applications on the environment. For all treatments, trace elements were conformed to irrigation water standards, except Mn, Cd and Mo for Sidi El Mokhtar and Li and Mn for Sed El Masjoune leachates (Tables 6 and 7). When comparing the drainage water with drinking water guidelines, the results showed that for Sidi El Mokhtar soil Cd and Mn and for Sed El Masjoune Pb and Mn contents exceeded the limits regardless of the treatments.
In the light of findings from the present study, for most heavy metals analyzed, the obtained values remained below the recommended limits of drinking and irrigation waters. Therefore, leachate of soils may not negatively affect groundwater quality. In fact, the alkaline conditions in Morocco for irrigation water and soils (Carle, 1930; El Oumlouki et al., 2014; Touhtouh et al., 2014) could lead to the safe use of PG and G in Morocco, considering, that heavy metals solubility and leachability decrease at high pH.
Saturation index
The saturation index determines the degree of chemical equilibrium between water and mineral in the aquifer matrix, and it identifies the dissolution or precipitation process regarding the water-rock interaction (Aghazadeh et al., 2017).
The mineral is in an equilibrium state when SI = 0. When SI exceeds zero, the mineral is oversaturated and tends to precipitate in order to attain equilibrium. SI less than zero indicates the undersaturation and the mineral will dissolve until the balance achievement (Reyes-Santiago et al., 2021).
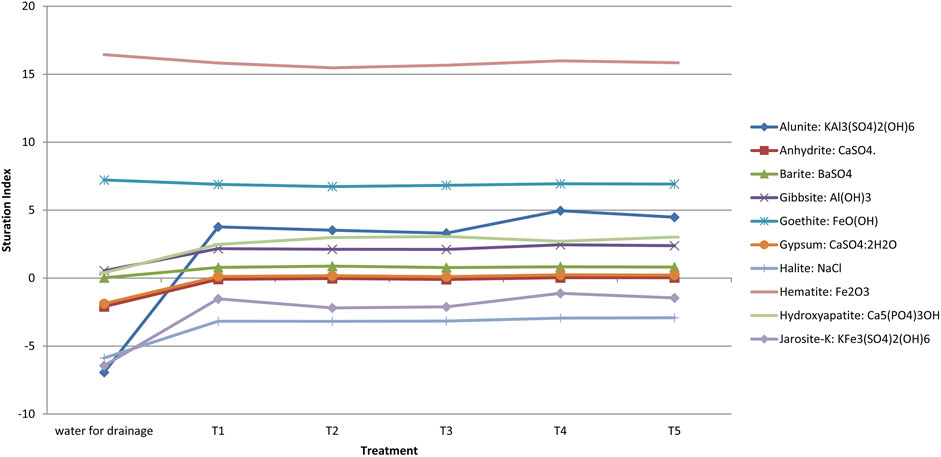
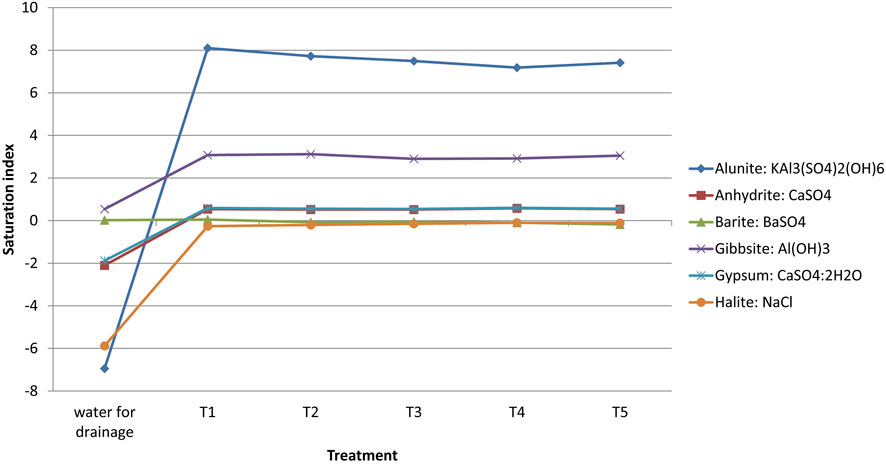
In this study, the SI of minerals reveals three distinct groups in the initial water stat (Figures 6, 7):
Group A: Alunite, jarosite-K, halite, anhydrite, and gypsum were undersaturated.
(Bernis), 2015 reported similar findings, in fact halite, anhydrite, and gypsum were undersaturated in the alkaline water.
Group B: Hematite and goethite were oversaturated and tended to precipitate.
Group C: Barite, gibbsite and hydroxyapatite were close to the balanced state.
For Sidi El Mokhtar soil leached (Figure 6), except for hematite, and goethite, soil leaching generates an increase on the mineral’s saturation index for all treatment compared to the initial stat of water. Alunite was passed from undersaturation state (SI = -6.94) to saturation stat after drainage applying, especially with 30 and 45 t/ha of PG, respectively SI = 4.96 and 4.48. Anhydrite and gypsum moved from the undersaturated to equilibrium state due to drainage application. In addition, because of leaching, the saturation index of barite increased slightly, but it remains close to the balance state. Hydroxyapatite and gibbsite were transferred from the equilibrium to the oversaturation state. Although, Halite and Jarosite-K saturation indices increased after drainage, they remained in the undersaturation range. For goethite and hematite, a slight decrease in SI was observed when drainage was applied.
Halite, gypsum, and anhydrite were moved from an undersaturation, shape in the initial water, to a steady state in the leached water of Sed El Masjoune soil. The gibbsite was converted from equilibrium to oversaturation state whatever treatment. The application of leaching caused an increase in the saturation index of alunite and did not affect the barite SI (Figure 7).
The leaching of the component elements (Ca, SO4, K, Al, P, Na, Cl, Ba and Fe) of the studied minerals from the soil into the leached water results in minerals precipitation or saturation, which explains the augmentation of the SI (Maia, 2018). Water pH impacts significantly the saturation index (Gitari et al., 2011). We noticed a reduction in the leached water’s pH compared to the initial pH (Figure 5). Nordstrom and Ball (1989), reported the gibbsite index saturation increasing following the pH reduction from eight to 6.
The saturation index was not affected by the amendment application. Water passing through the soil has increased the saturation index of the main minerals, which will result in groundwater mineral precipitation (Zhang et al., 2020).
Conclusion
It was concluded that PG application to soil has increased salts significantly in the leachate. Compared to the control, two treatments (T4 and T5) have removed 36 and 33% of sodium in leachate of Sidi El Mokhtar soil, and 13 and 11% in leachate of Sed El Masjoune respectively. The PG application induced losses of beneficial nutrients from the soil, thus fertilization after leaching is recommended. The PG had high ability to leach salts compared to G. The majority of analyzed trace elements in the leachates were below the recommended limits for drinking and irrigation water quality. The saturation index was increased after applying drainage without being affected by amendment application. The present work suggested that PG can be used for reclamation of saline and saline-sodic soils with no negative environmental impacts.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation. Data can be provided upon request from the corresponding author.
Author contributions
MBO: contributed to the study conception and design, investigation, analyzed the data and original draft writing; RC-A: language editing and substantial correction; MEG: co-supervisor and contributed to the study conception and design; KEO: contributed to the study conception and design; AS: contributed to the chemical analysis; KEM: supervisor, contributed to the study conception and design, language editing and substantial correction. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the OCP sustainability platform under the specific agreement “AS 49—P1”.
Acknowledgments
Authors would like to impressively acknowledge the AITTC Soil, Water and Plant Analysis Laboratory, the AITTC experimental farm for providing the facilities and OCP for financial support to carry out the present investigation.
Conflict of interest
KEO are employed by the company OCP Group manufacturing company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Fattah, M., and El-Naka, E. S. (2015). Empirical approach of leaching curves for determining the efficiency of reclaiming saline-sodic soils in sahl el-Tina, sinai, Egypt. Ijpss 8, 1–9. doi:10.9734/ijpss/2015/18606
Abdel-Fattah, M. K. (2012). Role of gypsum and compost in reclaiming saline-sodic soils. Iosrjavs 1, 30–38. doi:10.9790/2380-0133038
Adimalla, N., and Qian, H. (2019). Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 176, 153–161. doi:10.1016/j.ecoenv.2019.03.066
Aghazadeh, N., Chitsazan, M., and Golestan, Y. (2017). Hydrochemistry and quality assessment of groundwater in the ardabil area, Iran. Appl. Water Sci. 7, 3599–3616. doi:10.1007/s13201-016-0498-9
Agostini, F. T., Tei, M., Silgram, M., Farneselli, P., Benincasa, M. F., and Aller, M. F. (2010). “Decreasing nitrate leaching in vegetable crops with better N management,” in Genetic engineering, biofertilisation, soil quality and organic farming (E. Lichtfouse), 147–200. doi:10.1007/978-90-481-8741-610.1007/978-90-481-8741-6_6
Al-Hwaiti, M., Ibrahim, K. A., and Harrara, M. (2019). Removal of heavy metals from waste phosphogypsum materials using polyethylene glycol and polyvinyl alcohol polymers. Arabian J. Chem. 12 (8), 3141–3150. doi:10.1016/j.arabjc.2015.08.006
Al-Hwaiti, M., Carney, V., Ranville, J. F., and Ross, P. E. (2005). Heavy metal assessment of phosphogypsum waste stockpile material from Jordan. Asmr 2005, 1–22. doi:10.21000/jasmr05010001
Al-Hwaiti, M. S., Ranville, J. F., and Ross, P. E. (2010). Bioavailability and mobility of trace metals in phosphogypsum from Aqaba and Eshidiya, Jordan. Geochemistry 70 (3), 283–291. doi:10.1016/j.chemer.2010.03.001
Al-Masri, M. S., Amin, Y., Ibrahim, S., and Al-Bich, F. (2004). Distribution of some trace metals in Syrian phosphogypsum. Appl. Geochem. 19 (5), 747–753. doi:10.1016/j.apgeochem.2003.09.014
Alkutubi, H. (2012). Completely random design and least significant differences for breast cancer in Al-najaf city ( 2005-2009 ). Int. Res. J. Appl. Basic Sci. 3 (6), 1178–1182.
Allison, L. E. (1960). Wet-combustion apparatus and procedure for organic and inorganic carbon in soil. Soil Sci. Soc. Am. J. 24 (1), 36–40. doi:10.2136/sssaj1960.03615995002400010018x
Alva, A. K., and Gascho, G. J. (1991). Differential leaching of cations and sulfate in gypsum amended soils. Commun. Soil Sci. Plant Analysis 22 (11–12), 1195–1206. doi:10.1080/00103629109368484
Antipolis, S. (2003). Les menaces sur les sols dans les pays mediterraneens. Plan Bleu, 80. Bernis, NAnalyse Statistique Et Geochimique De La Dynamique Des Parametres Physico-Chimiques Des Eaux Souterraines Du Synclinal De Ghassira Algerie Orientale Brinis. Larhyss J. 22, 123–137.
Chaganti, V. N., Crohn, D. M., and Šimůnek, J. (2015). Leaching and reclamation of a biochar and compost amended saline-sodic soil with moderate SAR reclaimed water. Agric. Water Manag. 158, 255–265. doi:10.1016/j.agwat.2015.05.016
Corwin, D. L. (2021). Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 72 (2), 842–862. doi:10.1111/ejss.13010
Cuadri, A. A., Pérez-Moreno, S., Altamar, C. L., Navarro, F. J., and Bolívar, J. P. (2021). Phosphogypsum as additive for foamed bitumen manufacturing used in asphalt paving. J. Clean. Prod. 283, 124661. doi:10.1016/j.jclepro.2020.124661
Devi, S., Nandwal, A. S., Angrish, R., Arya, S. S., Kumar, N., and Sharma, S. K. (2016). Phytoremediation potential of some halophytic species for soil salinity. Int. J. Phytoremediation 18 (7), 693–696. doi:10.1080/15226514.2015.1131229
Diop, T., Ndiaye, R., Sow, S. A., and Ba, D. D. (2019). Analyse des effets du phophogypse et du fumier sur la salinité de la cuvette de Ndiol dans le Delta du fleuve Sénégal Résumé. Afrique Sci. 15 (4), 71–80.
El hasini, S., Iben. HalimaHalima, O., El. Azzouzi, M., Douaik, A., Azim, K., and Zouahri, A. (2019). Organic and inorganic remediation of soils affected by salinity in the sebkha of sed el mesjoune - marrakech (Morocco). Soil Tillage Res. 193, 153–160. doi:10.1016/j.still.2019.06.003
El Mejahed, K., Zeroual, Y., and Hiltonno, J. (2020). “Valorisation of phosphogypsum as amendment for the reclamation of saline/sodic soils: Agronomic field trials - Morocco,” in Phosphogypsum leadership innovation partnership (IFA Paris), 60–69.
El Oumlouki, K., Moussadek, R., Zouahri, A., Dakak, H., Chati, M., and El Amrani, M. (2014). Étude de la qualité physico-chimique des eaux et des sols de la région Souss Massa, (Cas de périmètre Issen), Maroc (Study of physic-chemical quality of water and soil in the region Souss Massa (Case perimeter Issen), Morocco). J. Mat. Environ. Sci. 5 (S2), 2365–2374.
FAO (1999). “A statistical manual for forestry research. Forestry research support programme,” in Status of the world’s soil resources (SWSR) - main report, food and agriculture organization of the united nations and intergovernmental technical panel on soils (Rome, Italy: FAO and ITPS). Available at: https://www.fao.org/3/x6831e/X6831E00.htm#TOC.
FAO (1988). Salt-affected soils and their management. Available at: http://www.fao.org/3/x5871e/x5871e00.htm.
FAO (1985). Water quality for agriculture. Available at: https://www.fao.org/3/T0234E/T0234E01.htm.
Fathallah, F. E., Algouti, A., and Algouti, A. (2021). Normalized difference vegetation index (NDVI) of the chichaoua watershed-Morocco: Comparison and evolution. J. Anal. 3, 65–73. doi:10.48402/IMIST.PRSM/jasab-v3i1.28247
Garrels, R., and Mackenzie, F. (1967). “Origin of the chemical compositions of some springs and lakes,” in Equilibrium concepts in natural water systems. Editor R. F. Ground (Washington: American Chemical Society Publications). doi:10.1021/ba-1967-0067.ch010
Gharaibeh, M. A., Eltaif, N. I., and Albalasmeh, A. A. (2011). Reclamation of highly calcareous saline sodic soil using Atriplex halimus and by-product gypsum. Int. J. Phytoremediation 13 (9), 873–883. doi:10.1080/15226514.2011.573821
Gharaibeh, M. A., Eltaif, N. I., and Shra’ah, S. H. (2010). Reclamation of a calcareous saline-sodic soil using phosphoric acid and by-product gypsum. Soil Use Manag. 26 (2), 141–148. doi:10.1111/j.1475-2743.2010.00260.x
Gharaibeh, M. A., Rusan, M. J., Eltaif, N. I., and Shunnar, O. F. (2014). Reclamation of highly calcareous saline-sodic soil using low quality water and phosphogypsum. Appl. Water Sci. 4, 223–230. doi:10.1007/s13201-014-0189-3
Gitari, W. M., Leslie, F. P., Key., David L., and Charles, O. (2011). Interaction of acid mine drainage with ordinary portland cement blended solid residues generated from active treatment of acid mine drainage with coal fly ash. J. Environ. Sci. Health 46, 37–117. doi:10.1080/10934529.2011.532423
Gonçalo Filho, F. G., da Silva Dias, N., Suddarth, S. R. P., Ferreira, J. F. S., Anderson, R. G., and dos Santos Fernandes, C. (2020). Reclaiming tropical saline-sodic soils with gypsum and cow manure. WaterSwitzerl. 12 (1), 57. doi:10.3390/w12010057
Gorbunov, A. V., Frontasyeva, M. V., Gundorina, S. F., Onischenko, T. L., Maksjuta, B. B., and PalSen, C. (1992). Effect of agricultural use of phosphogypsum on trace elements in soils and vegetation. Sci. Total Environ. 122 (3), 337–346. doi:10.1016/0048-9697(92)90051-S
Harrou, A., Gharibi, E. K., Taha, Y., Fagel, N., and El Ouahabi, M. (2020). Phosphogypsum and black steel slag as additives for ecological bentonite-based materials: Microstructure and characterization. Minerals 10 (12), 1–16. doi:10.3390/min10121067
Hassoune, H., Lahhit, M., Khalid, A., and Lachehab, A. (2017). Application of leaching tests on phosphogypsum by infiltration-percolation. Water Sci. Technol. 76 (7), 1844–1851. doi:10.2166/wst.2017.368
Hirich, A., Choukr-Allah, R., Ezzaiar, R., Shabbir, S. A., and Lyamani, A. (2021). Introduction of alternative crops as a solution to groundwater and soil salinization in the Laayoune area, South Morocco. Euro-Mediterranean J. Environ. Integration 6 (2), 1–13. doi:10.1007/s41207-021-00262-7
Hssaisoune, M., Bouchaou, L., Sifeddine, A., Bouimetarhan, I., and Chehbouni, A. (2020). Moroccan groundwater resources and evolution with global climate changes. Geosci. Switz. 10 (2), 81. doi:10.3390/geosciences10020081
Ismail, Z. H., Elgoud, E. M. A., Hai, F. A., Ali, I. O., and Aly, H. F. (2015). Leaching of some lanthanides from phosphogypsum fertilizers by mineral acids. Arab J. Nucl. Sci. Appl. 48 (2), 37–50.
Ivushkin, K., Bartholomeus, H., Bregt, A. K., Pulatov, A., Kempen, B., and de Sousa, L. (2019). Global mapping of soil salinity change. Remote Sens. Environ. 231, 111260. doi:10.1016/j.rse.2019.111260
Jiang, Y., Wu, Y., Groves, C., Yuan, D., and Kambesis, P. (2009). Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J. Contam. Hydrology 109, 49–61. doi:10.1016/j.jconhyd.2009.08.001
Kucuk, U., Eyuboglu, M., Kucuk, H. O., and Degirmencioglu, G. (2016). Importance of using proper post hoc test with ANOVA. Int. J. Cardiol. 209, 346. doi:10.1016/j.ijcard.2015.11.061
Lamsal, K., Paudyal, G. N., and Saeed, M. (1999). Model for assessing impact of salinity on soil water availability and crop yield. Agric. Water Manag. 41 (1), 57–70. doi:10.1016/S0378-3774(98)00116-4
Lech, M., Fronczyk, J., Radziemska, M., Sieczka, A., Garbulewski, K., Koda, E., et al. (2016). Monitoring of total dissolved solids on agricultural lands using electrical conductivity measurements. Appl. Ecol. Environ. Res. 14 (4), 285–295. doi:10.15666/aeer/1404_285295
Li, J., Zhang, J., and Ren, L. (2003). Water and nitrogen distribution as affected by fertigation of ammonium nitrate from a point source. Irrigation Sci. 22 (1), 19–30. doi:10.1007/s00271-003-0064-8
Llanderal, A., Garcia-Caparros, P., Contreras, J. I., Lao, M. T., and Segura, M. L. (2021). Spatial distribution and mobility of nutrients on sand mulching soil for fertigated green bean crops under greenhouse conditions in southern Spain: (I) macronutrients. Agronomy 11 (5), 842. doi:10.3390/agronomy11050842
Lütke, S. F., Oliveira, M. L. S., Silva, L. F. O., Cadaval, T. R. S., and Dotto, G. L. (2020). Nanominerals assemblages and hazardous elements assessment in phosphogypsum from an abandoned phosphate fertilizer industry. Chemosphere 256, 127138. doi:10.1016/j.chemosphere.2020.127138
Maia, F. M. S. (2018). Impact of increasing the temperature up to 80 ºC on the behav- iour of radionuclides in the callovo-oxfordian formation: Appli- cation to uranium. Report.
Makoi, J. H. J. R., and Verplancke, H. (2010). Effect of gypsum placement on the physical chemical properties of a saline sandy loam soil. Aust. J. Crop Sci. 4 (7), 556–563.
Manal, N., Samdi, A., Elabassi, K., Gomina, M., and Moussa, R. (2012). Recyclage de déchets industriels, phosphogypse et cendres volantes, dans des matériaux de construction. MATEC Web Conf. 2, 15. doi:10.1051/matecconf/20120201015
Mashifana, T., Ntuli, F., and Okonta, F. (2019). Leaching kinetics on the removal of phosphorus from waste phosphogypsum by application of shrinking core model. South Afr. J. Chem. Eng. 27, 1–6. doi:10.1016/j.sajce.2018.11.001
Mesić, M., Brezinščak, L., Zgorelec, Z., Perčin, A., Sestak, I., Bilandžija, D., et al. (2016). The application of phosphogypsum in agriculture. acs 81, 7–13.
Moharana, P. C., Singh, R. S., Singh, S. K., Tailor, B. L., Jena, R. K., and Meena, M. D. (2019). Development of secondary salinity and salt migration in the irrigated landscape of hot arid India. Environ. Earth Sci. 78 (15), 4. doi:10.1007/s12665-019-8460-4
Moroccan Ministry of Equipment and Ministry charged of Territorial Planning (2002). Normes de qualité des eaux destinées à l ’ irrigation. Bulletin officiel n° 5062 du 30 ramadan 1423.
Motalane, M. P., and Strydom, C. A. (2004). Potential groundwater contamination by fluoride from two South African phosphogypsums. Water sa. 30 (4), 465–468. doi:10.4314/wsa.v30i4.5098
Mu, Y., Tang, D., Mao, L., Zhang, D., Zhou, P., Zhi, Y., et al. (2021). Phytoremediation of secondary saline soil by halophytes with the enhancement of γ-polyglutamic acid. Chemosphere 285, 131450. doi:10.1016/j.chemosphere.2021.131450
Nagarajan, R., Thirumalaisamy, S., and Lakshumanan, E. (2012). Impact of leachate on groundwater pollution due to non-engineered municipal solid waste landfill sites of erode city, Tamil Nadu, India. J. Environ. Health Sci. Eng. 9 (1), 1–12. doi:10.1186/1735-2746-9-35
Nordstrom, D. K., and Ball, J,B. (1989). Mineral Saturation States in Natural Waters and Their Sensitivity to Thermodynamic and Analytic Errors. Etat de Saturation Des Minéraux Dans Les Eaux Naturelles et Sensibilité Des Tests Aux Données Thermodynamiques et Aux Erreurs Analytiques. Sci. Géologiques. Bull. 42, 80–269. doi:10.3406/sgeol.1989.1828
O'Brien, L. O. O., and Sumner, M. E. (2008). Effects of phosphogypsum on leachate and soil chemical composition. Commun. Soil Sci. Plant Analysis 19, 1319–1329. doi:10.1080/00103628809368015
Okur, B ., and Örçen, N. (2020). “Soil salinization and climate change,” in Climate change and soil interactions (International Journal of Agriculture, Forestry and Plantation), 331–350. doi:10.1016/b978-0-12-818032-7.00012-6
Olsen, S. R., Cole, C. V., Watanabe, F. S., and Dean, L. A. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ, 19. No. 939.
Parkhurst, D. L., and Appelo, C. A. J. (1999). User’s guide to PHREEQC (ver. 2): A computer program for speciation batch-reaction one- dimensional transport and inverse geochemical calculations. U. S. Geo Surv. Water Resour. Invest. Rept 99, 4259.
Phung, H. T., Lam, L. H., Page, A. L., and Lund, L. J. (1979). The practice of leaching boron and soluble salts from fly ash-amended soils. Water, Air, AndSoilPollution 12, 247–254. doi:10.1007/bf01047127
Qadir, M., Oster, J. D., Schubert, S., Noble, A. D., and Sahrawat, K. L. (2007). Phytoremediation of sodic and saline-sodic soils. Adv. Agron. 96, 197–247. doi:10.1016/S0065-2113(07)96006-X
Qamouche, K., Chetaine, A., Elyahyaoui, A., Moussaif, A., Touzani, R., Benkdad, E., et al. (2020). Radiological characterization of phosphate rocks, phosphogypsum, phosphoric acid and phosphate fertilizers in Morocco: An assessment of the radiological hazard impact on the environment. Mater. Today Proc. 27, 3234–3242. doi:10.1016/j.matpr.2020.04.703
Reyes-Santiago, J. R., García-Villanueva, L. A., Fernández-Villagómez, G., and Guzmán-Guadarrama, P. (2021). Geochemical characterization and saturation index (Si) in the montebello lagunar system liquidamber lagoon, chiapas Mexico. Nat. Environ. Pollut. Technol. 20, 1415–1425. doi:10.46488/NEPT.2021.v20i04.004
Richards, L. A. (1954). Diagnosis and improvement of saline and alkaline soils. Soil Sci. Soc. Am. J. 18 (3), 348. doi:10.2136/sssaj1954.03615995001800030032x
Shainberg, I., Sumner, M. E., Miller, W. P., Farina, M. P. W., Pavan, M. A., and Fey, M. V. (1989). Use of gypsum on soils: A review. January 9, 1–111. doi:10.1007/978-1-4612-3532-3_1
Shen, J., Yuan, L., Zhang, J., Li, H., Bai, Z., Chen, X., et al. (2011). Phosphorus dynamics: From soil to plant. Plant Physiol. 156 (3), 997–1005. doi:10.1104/pp.111.175232
Smaoui-Jardak, M., Kriaa, W., Maalej, M., Zouari, M., Kamoun, L., Trabelsi, M., et al. (2017). Effect of the phosphogypsum amendment of saline and agricultural soils on growth, productivity and antioxidant enzyme activities of tomato (Solanum lycopersicum L.). Ecotoxicology 26 (8), 1089–1104. doi:10.1007/s10646-017-1836-x
Suarez, D. L., Wood, J. D., and Lesch, S. M. (2006). Effect of SAR on water infiltration under a sequential rain-irrigation management system. Agric. Water Manag. 86 (1–2), 150–164. doi:10.1016/j.agwat.2006.07.010
Touhtouh, D., Elfaleh, E. M., and Moujahid, Y. (2014). Caractérisations physico-chimiques et minéralogiques des sols du Saïs, Maroc (Physicochemical and mineralogical characterizations of soils of Saïs, Morocco). Environ. Sci. 5, 2534–2539.
UNU (2014). World losing 2,000 hectares of farm soil daily to salt-induced degradation. Available at: https://unu.edu/media-relations/releases/world-losing-2000-hectares-of-farm-soil-daily-to-salt-induced-degradation.html.
Walawalkar, M., Nichol, C. K., and Azimi, G. (2016). Process investigation of the acid leaching of rare Earth elements from phosphogypsum using HCl, HNO3, and H2SO4. Hydrometallurgy 166, 195–204. doi:10.1016/j.hydromet.2016.06.008
WHO (2017). Guidelines for drinking-water quality. Fourth edition. Incorporating the first addendum.
Zaman, M., Shahid, S. A., and Heng, L. (2018). “Introduction to soil salinity, sodicity and diagnostics techniques,” in Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques (International Atomic Energy Agency), 1–38. doi:10.1007/978-3-319-96190-3
Zhao, B. Q., Li, X. Y., Liu, H., Wang, B. R., Zhu, P., Huang, S. M., et al. (2011). Results from long-term fertilizer experiments in China: The risk of groundwater pollution by nitrate. NJAS - Wageningen J. Life Sci. 58 (3–4), 177–183. doi:10.1016/j.njas.2011.09.004
Zmemla, R., Chaurand, P., Benjdidia, M., Elleuch, B., and Yves, J. (2016). Characterization and pH dependent leaching behavior of Tunisian phosphogypsum. Am. Sci. Res. J. Eng. Technol. Sci. 24 (1), 230–244.
Zmemla, R., Sdiri, A., Naifar, I., Benjdidia, M., and Elleuch, B. (2020). Tunisian phosphogypsum tailings: Assessment of leaching behavior for an integrated management approach. Environ. Eng. Res. 25 (3), 345–355. doi:10.4491/eer.2019.046
Keywords: soil salinity and sodicity, phosphogypsum, gypsum, leached water, plant nutrients, trace elements, saturation index
Citation: Outbakat MB, Choukr-Allah R, EL Gharous M, EL Omari K, Soulaimani A and EL Mejahed K (2022) Does phosphogypsum application affect salts, nutrients, and trace elements displacement from saline soils?. Front. Environ. Sci. 10:964698. doi: 10.3389/fenvs.2022.964698
Received: 08 June 2022; Accepted: 25 July 2022;
Published: 08 September 2022.
Edited by:
Thiru Selvan, Tripura University, IndiaReviewed by:
Manuel Canovas, Catholic University of the North, ChileQingguang Li, Guizhou University, China
Copyright © 2022 Outbakat, Choukr-Allah, EL Gharous, EL Omari, Soulaimani and EL Mejahed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khalil EL Mejahed, a2hhbGlsLmVsbWVqYWhlZEB1bTZwLm1h
 M Barka Outbakat
M Barka Outbakat Redouane Choukr-Allah1
Redouane Choukr-Allah1 Khalil EL Mejahed
Khalil EL Mejahed