
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 06 October 2022
Sec. Toxicology, Pollution and the Environment
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.955279
Background: Evidence regarding the association between particulate matter with aerodynamic diameter ≤2.5 μm (PM2.5) and blood lipid levels is insufficient in the rural areas of developing countries. Few studies have estimated the role of PM2.5 in blood lipid levels. We investigated the relationship between long-term exposure to PM2.5, blood lipids, and dyslipidaemia in rural Chinese adults.
Methods: Baseline data of 15,802 participants (aged 35–74 years) in the China Northwest Cohort-Ningxia Project were used in this study. PM2.5 levels were assessed using satellite remote sensing data in accordance with each participant’s home address. Personally exposed PM2.5 was defined as the 3-year mean concentration prior to the baseline survey. Logistic and linear models were utilised to quantify the associations of PM2.5 with the prevalence of dyslipidaemia and with blood lipids, including total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
Results: The 3-year mean level of PM2.5 was 35.36 ± 4.21 μg/m3. Every 1-μg/m3 increase in PM2.5 was related to an increase of 0.04% (95% CI: −0.44–0.53%) in TG and decreases of 0.37% (95% CI: 0.16–0.90%) in TC, 5.76% (95% CI: 5.32–6.21%) in LDL-C, and 0.89% (95% CI: 0.72–1.05%) in HDL-C. Every 1-μg/m3 increment in PM2.5 was related with a 4% (95% CI:3–5%) and 18% (95% CI:16–20%) higher risk of dyslipidaemia and hypoalphalipoproteinemia, respectively, and a decrease of 11% (95% CI:10–13%) in hyperbetalipoproteinemia. Sex, age, and BMI were adjusted for the relationships between PM2.5, blood lipids, and dyslipidaemia.
Conclusion: Greater PM2.5 exposure was related to harmful changes in blood lipids and dyslipidaemia. Male, elderly, and overweight individuals may be more vulnerable to the negative effects of PM2.5.
Dyslipidaemia refers to rising triglyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels, and a decrease in high-density lipoprotein cholesterol (HDL-C) levels. It is an essential but controllable risk factor for the prevalence of cardiovascular disease (CVD) outcomes (Toth, 2008; Franssen et al., 2011; Lee et al., 2012) and poses a serious threat to health in the general population. Evidence suggests that in 2012, the prevalence of hypercholesterolaemia, hypertriglyceridaemia, hypoalphalipoproteinemia, and hyperbetalipoproteinemia in China was significantly higher than 10 years earlier (Zhang M. et al., 2018). Air pollution poses a severe threat to China. The Global Burden of Disease Study showed nearly 1.1 million deaths from PM2.5 exposure in China, in comparison with 88,400 deaths in the US (Cohen et al., 2017).
Ambient particulate matter (PM) is a serious public health problem worldwide. PM2.5 has an aerodynamic diameter of ≤2.5 μm and is a crucial component of air pollution. Epidemiological studies have demonstrated that exposure to long-term PM2.5 can cross the blood-brain barrier and directly enter the pulmonary alveoli and bloodstream, posing a severe health hazard (Feigin et al., 2016; Xu et al., 2016; Zhang M. et al., 2018; Xu et al., 2018).
Evidence from PM2.5 and oxidative stress (Miller et al., 2012) may explain the relationship with an elevated risk of CVD events (Araujo, 2011). A previous study showed that lipids associated with PM10 exposure contribute to the development of CVD (Chuang et al., 2010). In another study, mice exposed to PM2.5 displayed decreased plasma HDL and increased LDL oxidation and TG (Li et al., 2013). However, the present knowledge of the relationship between PM2.5 and lipids (Chuang et al., 2011; Jacobs et al., 2011) in human studies is relatively limited. In addition, previous studies have examined the relationship between PM2.5, abnormal lipid metabolism, and abnormal lipid metabolism (Poursafa et al., 2014; Shanley et al., 2016; Yitshak Sade et al., 2016; Wallwork et al., 2017; McGuinn et al., 2019). A study in the United States (US) reported that PM2.5 was associated with increased TG, TC, HDL-C, and LDL-C levels (McGuinn et al., 2019). However, Wallwork et al. demonstrated that PM2.5 does not correspond to an increased risk of hypoalphalipoproteinemia (Wallwork et al., 2017).
However, existing research has focused on the relationship between urban regions with relatively high pollutant levels of PM2.5 and blood lipids (Li et al., 2021). Little evidence has been reported on the relationship between PM2.5, blood lipid levels, and dyslipidaemia in a rural Chinese area exposed to relatively low levels of air pollution. PM2.5 is an urgent problem in rural regions of China, and the relationship between long-term PM2.5, blood lipids, and dyslipidaemia in rural populations with low PM2.5, is unclear.
In this study, we explored the relationships among long-term PM2.5, blood lipids, and dyslipidaemia in rural areas with low air pollution levels using baseline data from the China Northwest Cohort-Ningxia Project study.
In the present study, we included a baseline of participants in the China Northwest Natural Population Cohort: Ningxia Project conducted from 2018 to 2019 (Zhang et al., 2021). Briefly, individuals were randomly collected from rural residents living in two counties (Pingluo and Qingtongxia), including four townships in Ningxia (https://www.resdc.cn/data.aspx?DATAID=200), a landlocked province in northwestern China (Figure 1). A total of 15,802 participants, aged 35–74 years, were recruited. Written informed consent was obtained from all participants before the survey. The study was approved by the “Ningxia Medical University Ethics Committee” (approval code:2018-012).
Baseline data, such as demographic profiles and lifestyle (alcohol consumption, smoking, intake of vegetables and fruits, intake of meat and poultry, and physical activity), were collected using standardised questionnaires. According to Chinese dietary guidelines, intake of vegetables and fruit was defined as consumption of an average of 500 g of vegetables or fruit each day, and intake of meat and poultry was defined as a mean intake of 75 g of meat or poultry each day. Body fat was estimated using the body mass index (BMI).
Blood specimens were collected from participants over 8 h after their last meal. HDL-C and LDL-C levels were analysed using a direct determination method. TC was assessed using the cholesterol oxidase method and TG was assessed using an enzymatic process.
The concentration of PM2.5 was predicted according to previous studies (Van Donkelaar et al., 2019; Hammer et al., 2020). The adjusted coefficient of determination and slope for annual prediction was 0.82 and 0.90, respectively. The participants were recruited from four townships. Long-term exposure was aggregated into 3-years average air pollution exposure prior to the start of the investigation. 5-years average air pollution exposure was utilized in the sensitivity analysis.
According to the Guidelines of Chinese Adult Prevention and Treatment Dyslipidaemia (2019), participants were defined as having hypercholesterolaemia with TC greater or equal to 6.22 mmol/L, hypertriglyceridaemia with TG greater or equal to 2.26 mmol/L, hypoalphalipoproteinaemia with HDL-C less than 1.04 mmol/L, and hyperbetalipoproteinaemia with LDL-C greater or equal to 4.14 mmol/L.
R4.0.3 software was utilized in this metabolic syndrome (MS), and the location of sampling sites in the China Northwest Cohort-Ningxia Project Study was described using ArcGIS 10.6 software. Multiple linear regression analyses were performed to quantify the associations between exposure to PM2.5 and blood lipids (TC, TG, LDL-C, and HDL-C). Odds ratios (OR) with 95% confidence intervals (CI were evaluated using a binary logistic regression model to quantify associations between PM2.5 and dyslipidaemia. Stratified analyses were employed to assess the modifying influences of sex, age, BMI, and lifestyle characteristics. Statistical significance was set at p value < 0.05.
Table 1 shows the basic characteristics of all individuals. In total, 6,334 men and 9,468 women were included in the study. The average age of individuals was 56.97 years, and the mean BMI was 24.97 kg/m2. The mean levels of TG, TC, HDL-C, and LDL-C were 1.70, 4.86, 2.84, and 1.35 mmol/L, respectively. The prevalence of dyslipidaemia, hypercholesterolaemia, hypertriglyceridaemia, hypoalphalipoproteinemia, and hyperbetalipoproteinemia in this rural population was 31.99, 8.45, 18.66, 14.47, and 5.52%, respectively. Long-term PM2.5 in our study was 35.36 ± 4.21 μg/m3, ranging from 28.73 to 39.37 μg/m3.
Table 2 summarises the relationships between PM2.5 and blood lipids. In the baseline model, higher PM2.5 was related to an increase in TC and decreased TG, HDL-C, and LDL-C levels. In the adjusted model, higher PM2.5 was related to elevated TG levels and reduced TC, HDL-C, and LDL-C levels.
We tested the interactions among age, sex, BMI, and lifestyle characteristics (Figure 2; Supplementary Table S1). The effects of PM2.5 were more robust in men than in women for LDL-C (p < 0.05). Interactions with age were associated with PM2.5, TC, HDL-C, and LDL-C levels. The relationship between PM2.5 and TC in individuals ≥65 years of age was significantly lower than in those aged <45 years. Furthermore, a modifying influence of BMI on the effects of PM2.5 was present for TG, HDL-C, and LDL-C. Our study also showed that a high-fat diet could alleviate the associations between PM2.5, TG, TC, and LDL-C. The intake of adequate vegetables and fruits adjusted the relationships between PM2.5, TG, TC, and LDL-C.
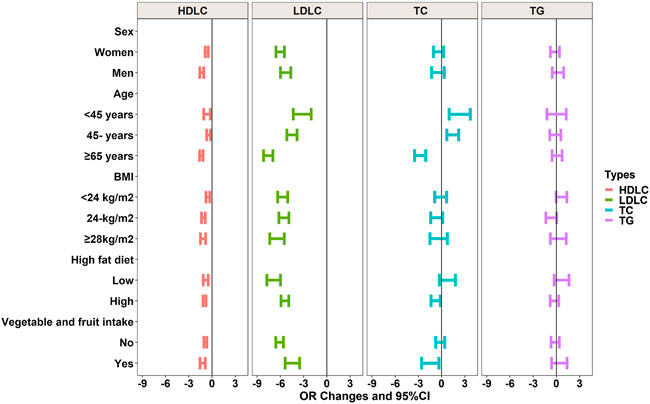
FIGURE 2. Interactions of age, sex, BMI, and lifestyle on relationships between PM2.5 and blood lipids.
Covariates are the same as in Table 2 above.
Figure 3 shows the relationship between PM2.5 and dyslipidaemia. In the baseline and adjusted models, elevated PM2.5 was related to an increased risk of dyslipidaemia and hypoalphalipoproteinemia and a reduced risk of hyperbetalipoproteinemia (see Figure 3; Supplementary Table S2). In the adjusted model, the OR (95% CI) for dyslipidaemia, hypoalphalipoproteinemia, and hyperbetalipoproteinemia were 1.040 (1.031–1.050), 1.179 (1.161–1.197), and 0.885 (0.869–0.900), respectively.
Table 3 shows the results of the interaction analyses between PM2.5, dyslipidaemia, age, sex, BMI, and lifestyle factors. Men are more vulnerable to PM2.5. For instance, the impact of PM2.5 on dyslipidaemia in women (OR:1.02, 95% CI:1.01–1.04) was stronger than that in men (OR:1.07, 95% CI:1.06–1.09). The influence of PM2.5 on dyslipidaemia, hypercholesterolaemia, hypoalphalipoproteinemia, and hyperbetalipoproteinemia was significant according to age. In addition, the intake of vegetables and fruit adjusted the relationships of PM2.5, dyslipidaemia, hypertriglyceridaemia, hypoalphalipoproteinemia, and hyperbetalipoproteinemia.
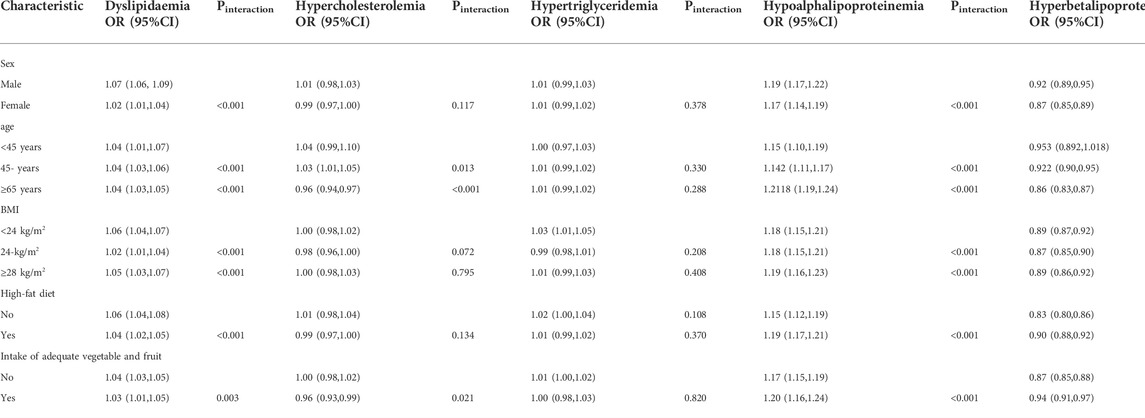
TABLE 3. Interactions of age, sex, and BMI on relationships between a 1-μg/m3 increase of PM2.5 and dyslipidaemia.
Covariates are the same as in Table 2 above.
Compared with the results of previous studies, the relationships of PM2.5, blood lipids, and dyslipidaemia were consistent in sensitivity analyses (see Supplementary Table S3, 4), and 1-year and 5-years PM2.5 exposure was utilized.
This study in a rural population of China provides new evidence regarding the harmful effects of long-term PM2.5 exposure on blood lipids and dyslipidaemia, which has implications for preventing CVD. Greater long-term PM2.5 was related to increased TC and decreased HDL-C and LDL-C levels. Greater PM2.5 was also related to an elevated risk of dyslipidaemia and hypoalphalipoproteinemia, as well as a reduced risk of hyperbetalipoproteinemia. Our study implies that males, the elderly, and overweight individuals may be more susceptible to the harmful effects of PM2.5.
PM2.5 showed adverse effects on blood lipids and dyslipidaemia. The positive effects of PM2.5 on TC level were similar. Our results are comparable to those reported by Zhang (Zhang, 2021). In an urban population of North China, the authors found that per 1-μg/m3 increase in PM2.5 corresponding to a 0.065% (95% CI:0.003–0.128%], 0.056% (95% CI:0.033–0.079%), and 0.063% (95% CI:0.035–0.091%) increase in TG, TC, and LDL-C, respectively, and a 0.091% (95% CI:0.068–0.113%) decrease in HDL-C. Our study findings showed that every 1-μg/m3 increment of PM2.5 was related to an increase in TG and non-significant decreases in TC, LDL-C, and HDL-C. This change implies that urban populations may be less susceptible to PM2.5 than rural populations.
Several studies have evaluated the effects of PM2.5. For instance, a study in Europe showed that PM2.5 was related to increased TC and LDL-C (Sorensen et al., 2015). However, our findings regarding TC and TG levels are inconsistent with those reported in previous studies. Some studies have shown no relationship between PM2.5, HDL-C, and the prevalence of hypoalphalipoproteinemia (Wallwork, 2017). However, other studies have shown that higher PM2.5 exposure is associated with increased TC and LDL-C levels and reduced TG and HDL-C, with a higher risk of hypercholesterolaemia, hyperbetalipoproteinemia, and hypoalphalipoproteinemia (Mao, 2020). Laura et al. reported consistently elevated associations between long-term PM2.5 and HDL-C (McGuinn et al., 2019). However, our study demonstrated that an increase in PM2.5 was related to a decrease in LDL-C and HDL-C levels. Recent research has shown that statins, one of the most widely used lipid-lowering drugs, significantly reduce levels of LDL-C than TG (Awad et al., 2017). PM2.5 and LDL-C could be associated with lipid-lowering drugs, even though we did not collect the exact drugs. Rats exposed to PM2.5 develop atherosclerosis, which is correlated with cholesterol levels, oxidative stress, and inflammation. Atorvastatin significantly reduced the levels of LDL-C induced by PM2.5 in rats (Yao and Lv, 2017). A previous study also found that serum HDL-C was reduced in the ApoE−/− mice after exposure to diesel exhaust particles (Qu et al., 2022).
There are several possible explanations for these discrepancies. First, different levels, compositions, and sources of air pollution may have diverse effects (Valavanidis et al., 2008). For example, large disparities in air pollution concentrations exist in diverse areas of China. Second, differences in the distribution of risk factors among populations may also account for these differences, including the way of life and health status (Cao et al., 2011). The participants of this study were from rural areas. The intake of meat, eggs, dairy, fish, and shellfish in rural populations is lower than that in urban populations (Guo et al., 2017). Additionally, lipid-lowering medications may influence the blood lipid levels (Awad et al., 2017).
The mechanism of lipid metabolism and the potential adverse effects of air pollution exposure remain unclear. Several potential pathways may be supported by existing evidence. Previous studies have suggested that long-term exposure to air pollution could induce oxidative stress and inflammation, interfere with lipid metabolism, and alter dyslipidaemia (Araujo and Nel, 2009; Xu et al., 2011; Shanley et al., 2016). Some evidence has demonstrated that air pollution can induce changes in DNA methylation of genes associated with lipid metabolism (Mendez et al., 2013; Bind et al., 2014; Chen et al., 2016). Differences in hazard factor distributions among participants may also illustrate these differences, including health status (Cao et al., 2011). As noted above, the dietary intake of dairy, meat, and other foods is lower in rural residents than in their urban counterparts (Guo et al., 2017).
Sex and age may adjust for the toxic effects of PM2.5 on blood lipid. This result is consistent with the findings (Shanley et al., 2016). Men have robust relationships between exposure to PM10 and TG and TC. Additionally, women living in urban areas have been found to be more susceptible to PM2.5 exposure than men (Yang et al., 2018). However, our study findings implied that sex did not affect the relationships between PM2.5, TC, and TG, which is consistent with the results of a previous study (Sorensen et al., 2015). Differences in the biological and lifestyle characteristics between men and women may be responsible for this difference. First, smoking and drinking may influence the effects of PM2.5. In China, the rates of smoking and drinking are lower in women than in men, which can lead to a higher risk of dyslipidaemia. Moreover, most individuals in the present study were peasants. Male peasants in rural regions spend considerable time outdoors and are more heavily exposed to PM2.5. Some studies have also suggested that long-term air pollution can activate oestrogen disruptors and play a vital role in the production of oxidative stress (Chen et al., 2013; Bell et al., 2017).
A previous study reported a greater susceptibility to PM2.5 among older people (Yang et al., 2018). However, several studies have shown no modifying effects of age on the relationship between PM2.5 and blood lipid (Sorensen et al., 2015; Shanley et al., 2016). Changes in body components associated with aging can induce numerous metabolic complications, resulting in pro-inflammatory conditions and interference with lipid metabolism (Liu and Li, 2015). This difference may be attributed to the utilisation of health services. Older people have low local health service utilisation, which may result in a greater toxic impact of air pollutants (Zhang X. et al., 2018).
Long-term dietary habits are strongly associated with air pollution, blood lipid levels, and dyslipidaemia. Unhealthy eating habits, including the consumption of more added sugar and fats, increase the prevalence of dyslipidaemia. In this study, we analysed the interactions of nutritional habits in the relationship between PM2.5, blood lipids, and dyslipidaemia. Surprisingly, a high-fat diet was found to alleviate the relationship between PM2.5, TC, HDL-C, and LDL-C in the current study. A similar study was conducted in a previous report (Lin et al., 2017), in which a high-fat diet was inversely related to high TC. The intake of vegetables and fruits was also shown to have a modifying effect. Studies have shown that consuming fruits and vegetables could mitigate the toxic health effects of outdoor air pollution owing to the high levels of carotenoids, vitamin C, and flavonoids, given the role of antioxidants in response to oxidative stress (Bowler and Crapo, 2002). Differences in food supplies, food choices, and dietary customs among different populations influence the intake of vegetables and fruits, which is linked to inadequate intake of antioxidants in rural areas (Jia et al., 2018).
Our study had several limitations. First, the major limitation is the cross-sectional design, which hinders us from drawing firm conclusions regarding causality. The results should be validated using a longitudinal method with repeated measurements. Second, the effects of other air pollutants should be considered, some of which may be highly correlated with our measures of interest (Chen et al., 2012). Third, although permanent rural residents were collected as individuals and we excluded those who had migrated to the study area within the 3 years prior to the investigation, the exact locations of some participants during the past 3 years were unknown. Additionally, we lacked information on individual levels that might affect exposure to PM2.5, such as indoor exposure to pollutants, which might have induced exposure misclassification errors and underestimation of the associations.
In conclusion, this study demonstrated that long-term exposure to PM2.5 was related to altered lipid levels and a higher risk of hypoalphalipoproteinemia and lower risk of hyperbetalipoproteinemia in rural regions of northwestern China. In particular, men and the elderly may be more susceptible to the toxic effects of air pollutants. More well-designed studies in the future are needed to confirm our findings because there are several limitations to this study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the study was Ethics Review Board of Ningxia Medical University (Ethics ID 2018-012). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
KW conceptualized the study and contributed to the acquisition of data, analysis, interpretation of data, and manuscript drafting. YZ analyzes, interprets data, and provides critical feedback on the manuscript. QW contributed to the interpretation of data. YZ contributed to the interpretation of data and provided critical feedback on the manuscript. YuZ contributed to manuscript and supervised the project.
This research was funded by the National Natural Science Foundation of China, grant number 81860603; the National Key R and D Program of China, grant number 2017YFC0907204; and the Natural Science Foundation of Ningxia Hui Autonomous Region, grant number 2021AAC03167.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.955279/full#supplementary-material
SD, standard deviation.
Araujo, J. A., and Nel, A. E. (2009). Particulate matter and atherosclerosis: Role of particle size, composition and oxidative stress. Part. Fibre Toxicol. 6, 24. doi:10.1186/1743-8977-6-24
Araujo, J. A. (2011). Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual. Atmos. Health 4 (1), 79–93. doi:10.1007/s11869-010-0101-8
Awad, K., Mikhailidis, D. P., Toth, P. P., Jones, S. R., Moriarty, P., Lip, G. Y. H., et al. (2017). Efficacy and safety of alternate-day versus daily dosing of statins: A systematic review and meta-analysis. Cardiovasc. Drugs Ther. 31 (4), 419–431. doi:10.1007/s10557-017-6743-0
Bell, G., Mora, S., Greenland, P., Tsai, M., Gill, E., and Kaufman, J. D. (2017). Association of air pollution exposures with high-density lipoprotein cholesterol and particle number: The multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 37 (5), 976–982. doi:10.1161/ATVBAHA.116.308193
Bind, M. A., Lepeule, J., Zanobetti, A., Gasparrini, A., Baccarelli, A., Coull, B. A., et al. (2014). Air pollution and gene-specific methylation in the normative aging study: Association, effect modification, and mediation analysis. Epigenetics 9 (3), 448–458. doi:10.4161/epi.27584
Bowler, R. P., and Crapo, J. D. (2002). Oxidative stress in airways: Is there a role for extracellular superoxide dismutase? Am. J. Respir. Crit. Care Med. 166 (12), S38–S43. doi:10.1164/rccm.2206014
Cao, J., Yang, C., Li, J., Chen, R., Chen, B., Gu, D., et al. (2011). Association between long-term exposure to outdoor air pollution and mortality in China: A cohort study. J. Hazard. Mat. 186 (2-3), 1594–1600. doi:10.1016/j.jhazmat.2010.12.036
Chen, R., Kan, H., Chen, B., Huang, W., Bai, Z., Song, G., et al. (2012). Association of particulate air pollution with daily mortality: The China air pollution and health effects study. Am. J. Epidemiol. 175 (11), 1173–1181. doi:10.1093/aje/kwr425
Chen, R., Meng, X., Zhao, A., Wang, C., Yang, C., Li, H., et al. (2016). DNA hypomethylation and its mediation in the effects of fine particulate air pollution on cardiovascular biomarkers: A randomized crossover trial. Environ. Int. 94, 614–619. doi:10.1016/j.envint.2016.06.026
Chen, S. T., Lin, C. C., Liu, Y. S., Lin, C., Hung, P. T., Jao, C. W., et al. (2013). Airborne particulate collected from central Taiwan induces DNA strand breaks, Poly(ADP-ribose) polymerase-1 activation, and estrogen-disrupting activity in human breast carcinoma cell lines. J. Environ. Sci. Health Part A 48 (2), 173–181. doi:10.1080/10934529.2012.717809
Chuang, K-J., Yan, Y-H., and Cheng, T-J. (2010). Effect of air pollution on blood pressure, blood lipids, and blood sugar: A population-based approach. J. Occup. Environ. Med. 52, 258–262. doi:10.1097/jom.0b013e3181ceff7a
Chuang, K-J., Yan, Y-H., Chiu, S-Y., and Cheng, T-J. (2011). Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup. Environ. Med. 68 (1), 64–68. doi:10.1136/oem.2009.052704
Cohen, A. J., Brauer, M., Burnett, R., Anderson, H. R., Frostad, J., Estep, K., et al. (2017). Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the. Glob. Burd. Dis. Study 389 (10082), 1907–1918.
Feigin, V., Roth, G., Naghavi, M., Parmar, P., Krishnamurthi, R., Chugh, S., et al. (2016). Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet Neurol. 15 (9), 913–924. doi:10.1016/s1474-4422(16)30073-4
Franssen, R., Monajemi, H., Stroes, E. S., and Kastelein, J. J. (2011). Obesity and dyslipidemia. Med. Clin. North Am. 95 (5), 893–902. doi:10.1016/j.mcna.2011.06.003
Guo, Q. Y., Zhao, L. Y., He, Y. N., Fang, Y. H., Fang, H. Y., Xu, X. L., et al. (2017). Survey on dietary nutrients intake of Chinese residents between 2010 and 2012. Zhonghua Yu Fang. Yi Xue Za Zhi 51 (6), 519–522. doi:10.3760/cma.j.issn.0253-9624.2017.06.012
Hammer, M. S., Van Donkelaar, A., Li, C., Lyapustin, A., Sayer, A. M., Hsu, N. C., et al. (2020). Global estimates and long-term trends of fine particulate matter concentrations (1998–2018). Environ. Sci. Technol. 54 (13), 7879–7890. doi:10.1021/acs.est.0c01764
Jacobs, L., Emmerechts, J., Hoylaerts, M. F., Mathieu, C., Hoet, P. H., Nemery, B., et al. (2011). Traffic air pollution and oxidized LDL. PloS one 6 (1), e16200. doi:10.1371/journal.pone.0016200
Jia, X., Wang, Z., Zhang, B., Su, C., Du, W., Zhang, J., et al. (2018). Food sources and potential determinants of dietary vitamin C intake in Chinese adults: A cross-sectional study. Nutrients 10 (3), 320. doi:10.3390/nu10030320
Lee, M. H., Kim, H. C., Ahn, S. V., Hur, N. W., Choi, D. P., Park, C. G., et al. (2012). Prevalence of dyslipidemia among Korean adults: Korea national health and nutrition survey 1998-2005. Diabetes Metab. J. 36 (1), 43–55. doi:10.4093/dmj.2012.36.1.43
Li, J., Yao, Y., Xie, W., Wang, B., Guan, T., Han, Y., et al. (2021). Association of long-term exposure to PM2. 5 with blood lipids in the Chinese population: Findings from a longitudinal quasi-experiment. Environ. Int. 151, 106454. doi:10.1016/j.envint.2021.106454
Li, R., Navab, M., Pakbin, P., Ning, Z., Navab, K., Hough, G., et al. (2013). Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J. lipid Res. 54 (6), 1608–1615. doi:10.1194/jlr.m035014
Lin, H., Guo, Y., Zheng, Y., Di, Q., Liu, T., Xiao, J., et al. (2017). Long-term effects of ambient PM2.5 on hypertension and blood pressure and attributable risk among older Chinese adults. Hypertension 69 (5), 806–812. doi:10.1161/HYPERTENSIONAHA.116.08839
Liu, H. H., and Li, J. J. (2015). Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 19, 43–52. doi:10.1016/j.arr.2014.12.001
Mao, S., Li, S., Wang, C., Liu, Y., Li, N., Liu, F., et al. (2020). Is long-term PM1 exposure associated with blood lipids and dyslipidemias in a Chinese rural population? Environ. Int. 138, 105637. doi:10.1016/j.envint.2020.105637
Mcguinn, L. A., Schneider, A., Mcgarrah, R. W., Ward-Caviness, C., Neas, L. M., Di, Q., et al. (2019). Association of long-term PM2.5 exposure with traditional and novel lipid measures related to cardiovascular disease risk. Environ. Int. 122, 193–200. doi:10.1016/j.envint.2018.11.001
Mendez, R., Zheng, Z., Fan, Z., Rajagopalan, S., Sun, Q., and Zhang, K. (2013). Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am. J. Transl. Res. 5 (2), 224–234.
Miller, M. R., Shaw, C. A., and Langrish, J. P. (2012). From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 8 (4), 577–602. doi:10.2217/fca.12.43
Poursafa, P., Mansourian, M., Motlagh, M. E., Ardalan, G., and Kelishadi, R. (2014). Is air quality index associated with cardiometabolic risk factors in adolescents? The CASPIAN-III study. Environ. Res. 134, 105–109. doi:10.1016/j.envres.2014.07.010
Qu, S., Deng, S., Yang, T., Yang, Y., Zhang, Y., Zheng, Z., et al. (2022). Shengmai Yin alleviated plaque vulnerability and ischemic myocardial damage in diesel exhaust particle-aggravated atherosclerosis with myocardial ischemia. Ecotoxicol. Environ. Saf. 234, 113379. doi:10.1016/j.ecoenv.2022.113379
Shanley, R. P., Hayes, R. B., Cromar, K. R., Ito, K., Gordon, T., and Ahn, J. (2016). Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology 27 (2), 291–298. doi:10.1097/EDE.0000000000000426
Sorensen, M., Hjortebjerg, D., Eriksen, K. T., Ketzel, M., Tjonneland, A., Overvad, K., et al. (2015). Exposure to long-term air pollution and road traffic noise in relation to cholesterol: A cross-sectional study. Environ. Int. 85, 238–243. doi:10.1016/j.envint.2015.09.021
Toth, P. P. (2008). Subclinical atherosclerosis: What it is, what it means and what we can do about it. Int. J. Clin. Pract. 62 (8), 1246–1254. doi:10.1111/j.1742-1241.2008.01804.x
Valavanidis, A., Fiotakis, K., and Vlachogianni, T. (2008). Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health Part C 26 (4), 339–362. doi:10.1080/10590500802494538
Van Donkelaar, A., Martin, R. V., Li, C., and Burnett, R. T. (2019). Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ. Sci. Technol. 53 (5), 2595–2611. doi:10.1021/acs.est.8b06392
Wallwork, R. S., Colicino, E., Zhong, J., Kloog, I., Coull, B. A., Vokonas, P., et al. (2017). Ambient fine particulate matter, outdoor temperature, and risk of metabolic syndrome. Am. J. Epidemiol. 185 (1), 30–39. doi:10.1093/aje/kww157
Wallwork, R. S., Colicino, E., Zhong, J., Kloog, I., Coull, B. A., Vokonas, P., et al. (2017). Ambient fine particulate matter, outdoor temperature, and risk of metabolic syndrome. Am. J. Epidemiol. 185, 30–39. doi:10.1093/aje/kww157
Xu, M. X., Qin, Y. T., Ge, C. X., Gu, T. T., Lou, D. S., Li, Q., et al. (2018). Activated iRhom2 drives prolonged PM2.5 exposure-triggered renal injury in Nrf2-defective mice. Nanotoxicology 12 (9), 1045–1067. doi:10.1080/17435390.2018.1513093
Xu, M. X., Zhu, Y. F., Chang, H. F., and Liang, Y. (2016). Nanoceria restrains PM2.5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-κB pathway in Nrf2 deficient mice. Free Radic. Biol. Med. 99, 259–272. doi:10.1016/j.freeradbiomed.2016.08.021
Xu, Z., Xu, X., Zhong, M., Hotchkiss, I. P., Lewandowski, R. P., Wagner, J. G., et al. (2011). Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in Brown and white adipose tissues. Part. Fibre Toxicol. 8, 20. doi:10.1186/1743-8977-8-20
Yang, B. Y., Bloom, M. S., Markevych, I., Qian, Z. M., Vaughn, M. G., Cummings-Vaughn, L. A., et al. (2018). Exposure to ambient air pollution and blood lipids in adults: The 33 Communities Chinese Health Study. Environ. Int. 119, 485–492. doi:10.1016/j.envint.2018.07.016
Yao, H., and Lv, J. (2017). Statin attenuated myocardial inflammation induced by PM2.5 in rats. Acta Cardiol. Sin. 33 (6), 637–645. doi:10.6515/acs20170518a
Yitshak Sade, M., Kloog, I., Liberty, I. F., Schwartz, J., and Novack, V. (2016). The association between air pollution exposure and glucose and lipids levels. J. Clin. Endocrinol. Metab. 101 (6), 2460–2467. doi:10.1210/jc.2016-1378
Zhang, J. X., Li, J., Chen, C., Yin, T., Wang, Q. A., Li, X. X., et al. (2021). Reference values of skeletal muscle mass, fat mass and fat-to-muscle ratio for rural middle age and older adults in Western China. Arch. Gerontol. Geriatr. 95, 104389. doi:10.1016/j.archger.2021.104389
Zhang, K., Wang, H., He, W., Chen, G., Lu, P., Xu, R., et al. (2021). The association between ambient air pollution and blood lipids: A longitudinal study in shijiazhuang, China. Sci. Total Environ. 752, 141648. doi:10.1016/j.scitotenv.2020.141648
Zhang, M., Deng, Q., Wang, L., Huang, Z., Zhou, M., Li, Y., et al. (2018a). Prevalence of dyslipidemia and achievement of low-density lipoprotein cholesterol targets in Chinese adults: A nationally representative survey of 163, 641 adults. Int. J. Cardiol. 260, 196–203. doi:10.1016/j.ijcard.2017.12.069
Keywords: PM2.5, blood lipid level, dyslipidaemia, rural areas, cholesterol, north-western china
Citation: Wang K, Zhao Y, Wang Q, Zhang Y and Zhang Y (2022) Association of PM2.5 With blood lipids and dyslipidaemia in a rural population of north-western china. Front. Environ. Sci. 10:955279. doi: 10.3389/fenvs.2022.955279
Received: 28 May 2022; Accepted: 22 September 2022;
Published: 06 October 2022.
Edited by:
Oladele Ogunseitan, University of California, Irvine, United StatesReviewed by:
Xiaobo Liu, National Institute for Communicable Disease Control and Prevention (China CDC), ChinaCopyright © 2022 Wang, Zhao, Wang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajuan Zhang, emh5ajgzMDUxNUAxMjYuY29t; Yuhong Zhang, Email, emhhYm91ckAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.