- 1School of Environment, Harbin Institute of Technology, Harbin, China
- 2Shenzhen Yuchi Testing Technology Co LTD, Shenzhen, China
- 3State Key Laboratory of Urban Water Resource and Environment, School of Civil and Environmental Engineering, Harbin Institute of Technology (Shenzhen), Shenzhen, China
In order to clarify the pollution characteristics and human health risks of PFASs pollutants in typical drinking water sources in Zhejiang Province, this study relies on ultra -performance liquid chromatography-mass spectrometry (UPLC-MS/MS) technology to analyze the pollution of 26 PFASs in 7 reservoirs in Zhejiang Province. The detected concentrations of PFASs were evaluated to further assess the human health risks. Total PFASs concentrations in the seven reservoirs ranged from 1.30 ng L−1–24.90 ng L−1. Among the 26 PFASs pollutants analyzed, PFOA and PFBA were the main PFASs pollutants, the detected concentrations of PFOA and PFBS ranging from 0.50 ng L−1–13.70 ng L−1 and 0 ng L−1–1.70 ng L−1, respectively. Then we evaluated 15 PFASs and calculated the results of the HQ value of the reproductive toxicity and hepatotoxicity of the total PFASs in this study ranged from 2.30 × 10–8 to 1.16 × 10–4 and 9 × 10–8 to 5.24 × 10–4 respectively, which were both lower than 0.01, indicating that there is no significant risk to the human body.
Introduction
Per- and polyfluoroalkyl substances (PFASs) are widely used in industrial and consumer products, including food packaging, metal plating materials, aqueous film-forming foams (AFFFs), textile coatings, and nonstick coatings, due to their unique amphiphilic nature and high chemical stability (strong C-F bonding energy) (Knutsen et al., 2019; Li et al., 2020). Moreover, many PFASs are also bioaccumulative and toxic (Lau et al., 2007). Many researchers have detected their presence in the aqueous environment, sediment, soil, and atmosphere (Ahrens et al., 2010; Li et al., 2011; Yang et al., 2011; Wang et al., 2015; Hu et al., 2019; Park et al., 2020; Xu et al., 2021), and they have even been reported in human blood and serum (Poothong et al., 2020). Perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkane sulfonic acids (PFSAs), such as perfluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA), have been found to be persistent in the environment and in living organisms and are difficult to remove by conventional drinking water treatment techniques (e.g., coagulation, filtration, and oxidation) (Rahman et al., 2014). PFASs can enter the human body through drinking water, which is considered to be one of the main exposure pathways for PFASs in the general population (Jian et al., 2018). High contamination (>100 ng L−1) of PFASs in source water and drinking water has been found in many countries (Gebbink et al., 2017; Guelfo et al., 2018; Kaboré et al., 2018; Liu et al., 2021). It has been shown that different PFASs show different toxicological profiles, including hepatotoxicity, reproductive and developmental toxicity, immune system toxicity, thyroid toxicity, neurotoxicity, cardiovascular toxicity, and endocrine toxicity (DeWitt, 2015).
Drinking water has been identified as a substantial source of PFAS exposure for many populations, particularly those living near contaminated sites (Hu et al., 2016; Banzhaf et al., 2017). Next to contaminated sites, drinking water has been reported to account for up to 75% of total PFAS exposure (Vestergren and Cousins, 2009; Hoffman et al., 2011). In recent years, several health-protective guidelines have been proposed, such as the United States Environmental Protection Agency (U.S. EPA) proposed a lifetime health advisory level for PFOS + PFOA of 70 ng L−1 in drinking water in 2016 (U.S. EPA, 2016). In 2018, the Agency for Toxic Substances and Disease Registry (ATSDR) in the US further lowered the Minimum Risk Levels (MRLs) for PFOS and PFOA by approximately an order of magnitude compared to the reference dose (RfD) used by the U.S. EPA to develop the 2016 lifetime advisory (ATSDR, 2018). Despite industry has rapidly replaced PFOS and PFOA with shorter chain length PFASs and new chemicals due to these regulatory interventions, these chemicals are difficult to detect by using standard methods (Wang et al., 2017). Emerging evidence from animal experiments suggests some of these alternative PFASs can be equally hazardous (Gomis et al., 2018). Currently, most studies focus only on PFASs in rivers and water plants, and limited work has been done on PFASs in reservoirs. Given this, and the fact that reservoir water, as the front end of the drinking water supply chain, is an important part of ensuring water safety for the population, it is necessary to investigate the concentration levels and human health risks of PFASs contaminants in reservoir water.
In this study, seven reservoirs in a region of Zhejiang Province, China, were used as target areas, and 4–11 sampling sites were set up in each reservoir, for a total of 42 sampling sites, to investigate the distribution and concentrations of different PFASs contaminants in the region. An ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS) was used to detect and analyze 26 PFASs pollutants, and the hazard quotient (HQ) modeling system was used to assess the human health risk of the target PFASs pollutants in the region. This work is expected to provide basic data and theoretical support for the potential risk of PFASs pollutant levels in urban reservoirs.
Materials and Methods
Materials and Reagents
Analytical standards for 26 natural PFASs were purchased from Wellington Laboratories Inc. (Canada). The 26 natural PFASs include 18 PFCAs and eight PFSAs. All stock solutions were prepared in methanol and stored at 4 C in polypropylene (PP) tubes. Other reagents included methanol for UPLC analysis, ≥ 99.9% (Sigma-Aldrich, USA), ammonium hydroxide (25%, ACOS Organics, USA), and ammonium acetate (CNW, Germany). The solid phase extraction (SPE) column was purchased from Waters (6 ml, 150 mg, Oasis® WAX).
Sampling Campaign
The sampling activities in this study were conducted in June 2021 in the province of Zhejiang. A total of 42 water samples were collected in seven reservoirs from A to G. From four to eleven sampling sites were set up in each reservoir. Samples were collected from 0.5 to 1 m below the water surface using stainless steel buckets and stored in PP bottles (2 L). Samplers and collection containers were washed with methanol as well as the water column at the sampling sites, respectively, prior to sample collection. Water samples were transported to the laboratory at low temperature, pretreated within 2 days, and stored in cold storage at -20 C prior to pretreatment.
Sample Extraction
The protocol for the extraction of PFASs from water samples is based on ISO-25101. Briefly, the pH of unfiltered water samples (500 ml) is adjusted to 3 with acetic acid. The samples were then spiked with 5 ng of alternative standards using an Oasis® WAX SPE column. Extracted fractions were collected in PP medium tubes and then concentrated to 1 ml under a gentle nitrogen stream and prepared for injection into UPLC-MS/MS. Information on instrumental parameters is listed in Supplementary Table S1.
The main work of the extraction was divided into three parts: filtration, SPE, and nitrogen blowing concentration. 1) Measure 500 ml of water sample in a PP beaker and add 5 ng of internal standard. Using a circulating water vacuum pump and filtration device, the mixed solution was passed through a 0.45 μm filter membrane to separate the aqueous phase from the suspended particulate phase. In order to avoid the interference of the membrane and other organic components on the quantitative results, the membrane used in the experiment was preheated and dried to a constant weight in an electric blast dryer. 2) A WAX SPE column (6 ml, 150 mg) was used for the SPE. The solid-phase extraction process consisted of activation of the column, sample loading, solid-phase extraction, and elution of the target compounds. The SPE column was activated with 4 ml of 0.1% ammonia-methanol mixture, 4 ml of methanol solution and 4 ml of high purity water in turn. After activation of the column, the water sample was flowed through the column at a rate of six to eight ml min−1. After all the target mixture flowed through the column, the column was washed with 4 ml of 25 mM ammonium acetate solution to clean any impurities other than the target compounds. After that, the SPE device and the column were dried under vacuum for 10 min using a vacuum pump, and the target compounds were eluted with 5 ml of methanol solution and 5 ml of 0.1% ammonia-methanol solution in turn, and the eluate was collected in a 15 ml centrifuge tube. 3) The eluate was concentrated by 12-well nitrogen blowing apparatus with 99.7% purity of nitrogen, and the water bath temperature was set at 40 C during the nitrogen blowing process. The auto-sampling vial was stored under refrigeration and protected from light.
Quality Assurance and Quality Control
PP bottles and tubes, ultrapure water, methanol, and nitrogen were tested prior to sampling and no contamination was found in any of these blanks. Procedural blank samples and procedural spiked samples were performed for each set of extractions, and duplicate samples and archived blanks were performed for each sampling event. The limit of detection (LOD) was defined as the analyte peak required to give a signal-to-noise ratio (S/N) of 3:1, and the limit of quantification (LOQ) was defined as the analyte required to produce a S/N of 10:1, or as the lowest point of the calibration curve calculated to be within 30% of its actual value. Compound concentrations below the LOQ were reported as non-detected. Matrix spiked recoveries for water samples ranged from 75% to 117%. Proxy recoveries for water samples ranged from 81% to 115%. Internal calibration was used for quantification.
Risk Assessment
Since it is extremely difficult to remove PFASs by the current conventional water treatment process, this study assumes that PFASs in this reservoir can enter the drinking water network without loss and be absorbed into the body by humans. Based on this assumption, we selected the hazard quotient (HQ) model for risk assessment of 15 PFASs (data for other compounds of PFASs were not available). We adopted the assessment method of Borg (Borg et al., 2013) et al. for the hepatotoxicity as well as the reproductive toxicity of the target contaminants. The specific calculation equation is as follows:
In the above formula, Exp is exposure concentration of PFASs; RfD is reference dose; POD is point of departure; Afs are assessment factors; HQ is the health quotient. When HQ < 0.01, very low risk; 0.01 < HQ < 0.1, low risk; 0.1 < HQ < 1, medium risk; HQ > 1, high risk (Lemly, 1996). POD is the PFASs serum/plasma concentration at the respective no observed adverse effect level (NOAEL), lowest observed adverse effect level (LOAEL), or baseline dose (BMD). The data were collected from available studies. An Af of three was applied to calculate chronic toxic effects for hepatotoxicity and reproductive toxicity, respectively, according to the REACH guidelines for EU chemicals legislation (ECHA, 2010).
Results and Discussions
Concentration and Composition Profile of Exposure
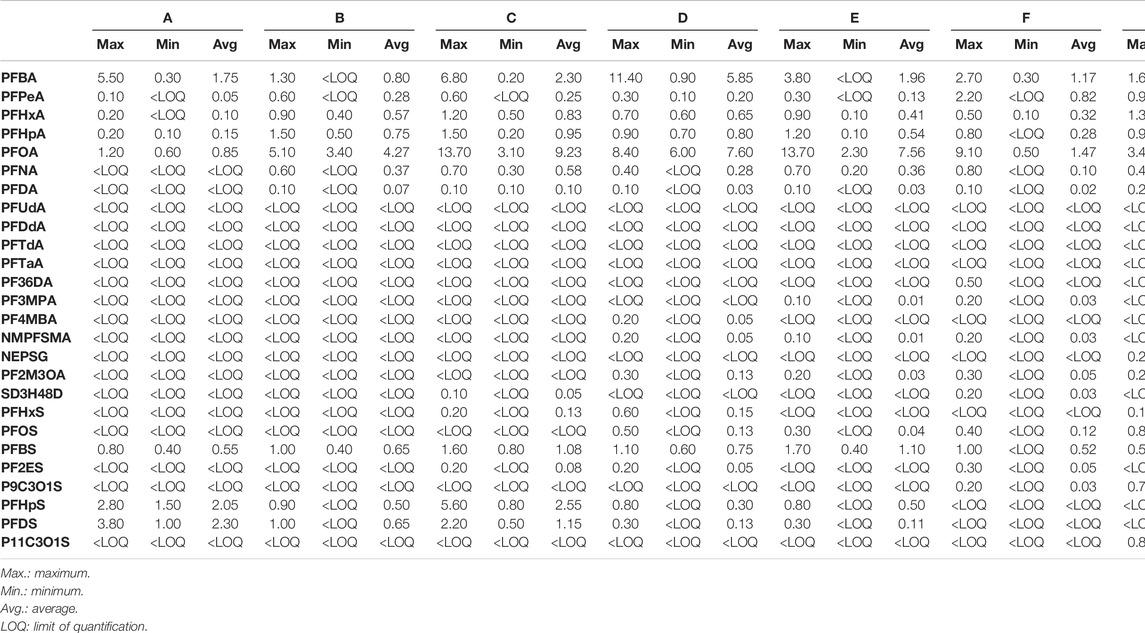
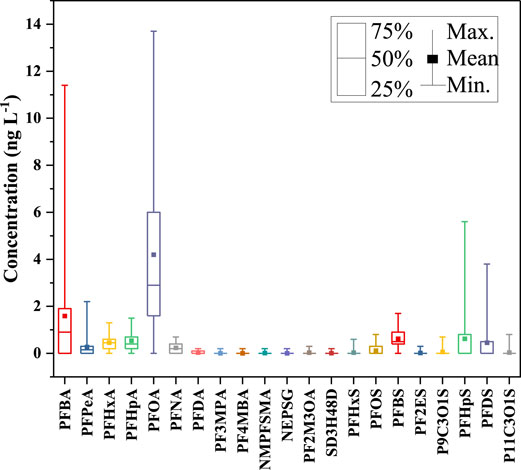
The maximum, minimum, and average concentrations of PFASs in a reservoir in a certain area of Zhejiang Province were shown in Table 1. In this study, total PFASs concentrations in the seven reservoirs ranged from 1.30 ng L−1–24.90 ng L−1. Some PFASs in the reservoir have become common, and the detection rates of 7 pollutants in the 26 PFASs were above 50%. Among them, the detection rates of perfluorohexanoic acid (PFHxA) with 6 (C6) carbons, PFOA and perfluorobutane sulfonate (PFBS) with 8 (C8) carbons were more than 90%. As can be seen from Table 1, the detected concentrations of PFOA and PFBS ranged from 0.50 ng L−1–13.70 ng L−1 and 0 ng L−1–1.70 ng L−1, respectively. In contrast, the detection concentrations of Perfluoro (3-methoxy)propionic acid (PF3MPA), Perfluoro (4-methoxy)butyric acid (PF4MBA), N-methyl perfluorooctane sulfonyl aminoacetic acid (NMPFSMA), N-Ethyl Perfluorooctane Sulfonyl Glycine (NEPSG), Sodium dodecafluoro-3H-4,8-dioxonanonate (SD3H48D) in PFCAs, and Potassium 11-chloroecosfluoro-3-oxaundecane-1-sulfonate (P11C3OLS) in PFSAs were lower, and the detection rates were in the range of 2%–8%, and the long-chain complete PFCAs (greater than or equal to 11 carbons) and Perfluoro-3,6-dioxaheptanoic acid (PF36DA) were not detected at all sampling points.
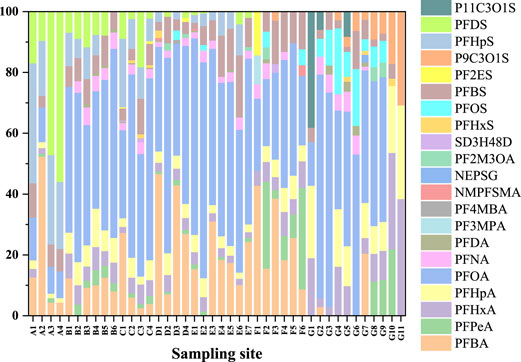
The composition and concentration of PFASs pollutants in various sampling points in this area of Zhejiang Province were shown in Figures 1, 2. As can be seen from Figure 1, among all the 26 PFASs pollutants detected, PFOA and Perfluorobutanoic acid (PFBA) were the main PFASs pollutants. The average contribution rate of PFOA with eight fluorinated carbon atoms to the overall PFASs was 39.42%. The composition and concentration of PFASs pollutants detected at each reservoir sampling point were shown in Supplementary Figure S1–S14. As shown in Supplementary Figures S5, S9, the highest PFOA concentrations was 13.7 ng L−1 sampled at C4 and E2, followed by C2 and E1, the concentrations of PFOA were 12.9 ng L−1and 12.6 ng L−1, respectively. In Supplementary Figures S1, S11, S13, lower concentrations of PFOA detected were 0.6 ng L−1, 0.6 ng L−1, 0.5 ng L−1, and 0.3 ng L−1 sampled at A3, A4, F1, and G1, even no PFOA were detected at sampling points G10 and G11. In particular, the average contribution rate of PFOA in reservoir E was 56.48%, the average contribution rate of PFOA in reservoir A was 10.79%.Followed by the average contribution rate of PFBA in the seven reservoirs was 13.87%. The sampling points where the highest concentration of PFBA was 11.4 ng L−1 detected was D1 (Supplementary Figure S7), no PFBA were detected at sampling points B2, E2, and reservoir G except G2, G7 (Supplementary Figure S3, S9, S13). This shows that the concentrations of PFOA and PFBA varies greatly at different sampling points. There are many reasons for this, such as a degree of temporal variation in PFASs concentrations in the target reservoir, the use of a sewage treatment plant sludge as a soil amendment in the area, or the presence of fluoropolymer manufacturing plants in the area impacted the drinking water with PFOA or PFBA, etc. A more comprehensive survey of the area is needed to understand the details that are currently unknown to the authors.
This was similar to the study by Ding (Ding et al., 2018) et al. on PFASs in the seawater in the coastal waters of Dalian Bay, where PFOA is one of the main pollutants. Li (Li et al., 2016) et al. researched on PFASs occurrence in groundwater in rural areas in Liaoning peninsula located in the northeast of China, where PFOA and PFBA were the most frequently detected compounds. Wan (Wan et al., 2017) et al. found that the concentration range of total PFASs in seawater in the coastal waters of Shandong Peninsula was 23.69 ng L−1–148.48 ng L−1, and PFOA, PFOS and PFHxA were the main PFASs in seawater in this area. In addition, Shao (Shao et al., 2016) et al. studied PFASs in the surface layer and low seawater of Shuangtaizi Estuary in Liaodong Bay and found that the total PFASs concentration was between 66.2 ng L−1–185 ng L−1 and 44.8–209 ng L−1, respectively, and PFBA was the main short-chain pollutants. However, in previous studies, PFBS was the most abundant PFASs in the rivers of the Pearl River Delta (Liu et al., 2015), Wuhan, China (Zhou et al., 2017), and five major river basins in Italy (Valsecchi et al., 2015). 6:2 fluorotelomer alcohol (6:2 FTOH) and Perfluoroheptanoic acid (PFHpA) were detected as the most important PFASs in river water and sediment samples collected from a tributary of the Liu Xi River, a part of the Pearl River near Guangzhou (Si et al., 2021). The differences may be due to the different PFASs that were mainly used in different areas.
As shown in Figure 2, among all the measured PFASs, PFCAs were the most important class of PFASs, with a total concentration ranged from 0.90 ng L−1–22.30 ng L−1 and the proportion ranged from 69.23% to 89.56%. PFOA and PFBA were the main PFASs pollutants with the average concentrations of 4.24 ng L−1 and 1.64 ng L−1, respectively. Due to the limitation of long-chain PFASs, short-chain PFASs account for a relatively high proportion of total PFASs in aquatic environments, especially PFBA and PFBS are widely used as substitutes worldwide (Li et al., 2020). However, their adsorption to sediments and soil is limited (Zhao et al., 2016). In addition, the removal efficiency of short-chain PFASs is relatively limited even if advanced treatment processes such as granular activated carbon, ion exchange, membrane treatment are used in drinking water treatment (Zhao et al., 2016). Similarly, the results of Chen (Chen et al., 2017) et al. researched on PFASs in seawater in the Bohai Sea showed that the average concentration of PFOA was as high as 4.97 ng L−1, which was the main PFASs pollutant. Liu (Liu et al., 2021) et al. found a survey of different enterprises in China in 2018 that extremely high concentrations of PFOA were mainly concentrated in polytetrafluoroethylene (PTFE)-producing provinces, such as Sichuan (3,165 ng L−1), Zhejiang (115.4 ng L−1), Shanghai (78 ng L−1), Jiangsu (61.4 ng L−1), and Guangdong (53.4 ng L−1). The patterns and concentrations of PFASs in drinking water can be affected due to the emission sources, especially fluorination plants, major local industrial users of PFASs, use of PFAS-containing firefighting foams (Hu et al., 2016; Mumtaz et al., 2019b; Li et al., 2019), the quality of drinking water treatment processes, and their precursor conversions. In this research, compared with PFCAs, PFSAs accounted for a smaller proportion of total PFASs, and among the eight detected PFSAs, the average concentrations of PFBS and Perfluoroheptane sulfonic acid (PFHpS) were higher, the concentration levels were 0 ng L−1–1.6 ng L−1 and 0 ng L−1–5.6 ng L−1, respectively.
Ecological Risk Assessment
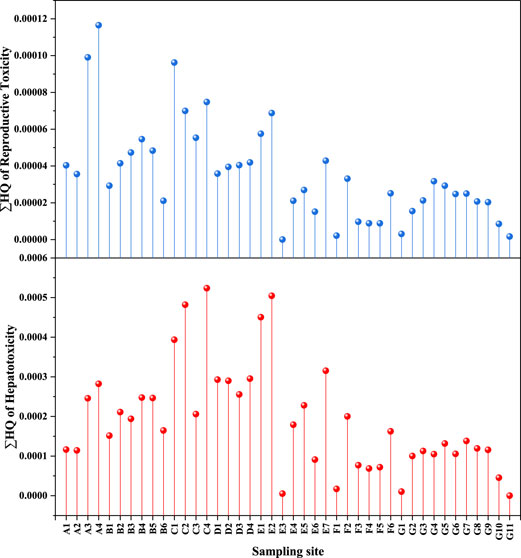
This study adopts the Hazard Quotient (HQ) method to assess the level of potential health risk. HQ value is widely used to assess the potential health risk of different chemicals, including PFASs (Ludwicki et al., 2015; Habibullah-Al-Mamun et al., 2016; Mumtaz et al., 2019a). Due to the lack of toxicological data for some mixtures of PFAS in this assessment, toxicity data may not be completely accurate for all mixtures. However, some studies have shown that the toxicological endpoints evaluated were hepatotoxicity (hepatocellular hypertrophy, hepatocellular vacuolation, increased liver weight and liver-to-body ratio) and reproductive toxicity (reduced fetal/perinatal/neonatal viability, reduced body weight/body weight gain and litter) loss in the dams (Si et al., 2021). Reproductive toxicity and hepatotoxicity were considered to be the more common methods for assessing accumulation of PFASs. We evaluated 15 PFASs pollutants and the calculated results of the HQ value in this study were shown in Figure 3. It can be seen from Figure 3 that the HQ values of the reproductive toxicity and hepatotoxicity of the total PFASs at different sampling points ranged from 2.30 × 10–8 to 1.16 × 10–4 and 9 × 10–8 to 5.24 × 10–4 respectively, which were low than 0.01, indicating that the potential health risk was very low. It was worth noting that the HQ values of them were much lower than 0.01, although PFOA and PFBA were the main PFASs pollutants. The average HQ values of reproductive toxicity and hepatotoxicity of PFOA were 6.02 × 10–4 and 5.75 × 10–3, respectively, and the average HQ values of reproductive toxicity and hepatotoxicity of PFBA were 6.68 × 10–7 and 1.34 × 10–6, respectively, indicating that PFOA and PFBA didn’t pose the health risk to the target area. Some researchers believed that the detection of PFOS and PFOA in soil and groundwater near a fluorine chemical plant in China poses a much greater health risk than other fluorinated compounds (Wei et al., 2018; Xie et al., 2021). PFOS can suppress sheep red blood cell-specific immunoglobulin M production on mice, and cause hepatocellular hypertrophy in livers of rats (Peden-Adams et al., 2008; Butenhoff et al., 2012), even caused the death of newborn mice (Yahia et al., 2010). PFOA can derive stunted mammary epithelial growth on offspring of mice, and increased peroxisome proliferation and relative liver weights on rats (Perkins et al., 2004; Macon et al., 2011). The diversity of PFAS classes complicates the ecotoxicological study of PFAS. Although bioaccumulation of some persistent organic pollutants (POPs) is usually associated with lipid partition coefficients, PFAS are not exclusively lipid-related (De Silva et al., 2021). Bioaccumulation models suggest that both protein interactions and lipid partitioning are important parameters for accurate PFAS assessment (Ng and Hungerbühler, 2014; Dassuncao et al., 2018). Differences for specific physicochemical properties, such as chain length, can lead to different distribution of PFAS in biological tissues (Chen et al., 2021). In this study, although the assessed risks of all sampling points were low, long-term ecological effects and risks cannot be ignored due to the strong bioaccumulation of PFASs and their difficulty in degrading. In early 2020, China’s Ministry of Ecology and Environment stated that more POPs would be included in the national monitoring system. PFASs, particularly PFOS and PFOA, should be added to the candidate list (Zhang et al., 2021).
Conclusion
In this study, water samples from 7 reservoirs in a certain area of Zhejiang Province were detected, and 26 PFASs pollutants were selected for comprehensive evaluation. At the same time, the potential health risk assessment of the reservoir was carried out using the HQ method. Among the 26 PFASs pollutants, the detection rates of 7 pollutants ranged above 50%, and the detection rates of PFHxA, PFOA, and PFBS were all above 90%. The concentrations and detection rates of the mixtures tested show that PFOA and PFBA were the main PFASs pollutants, and the average contribution rates of PFOA and PFBA to the overall PFASs were 39.42% and 13.87%, respectively.
According to the potential health risk analysis, the HQ values of the reproductive toxicity and hepatotoxicity of the total PFASs at different sampling points were low than 0.01, indicating that there was no immediate risk in this area. When conducting water potential health risk assessment in the future, it is necessary to develop a comprehensive evaluation method for these compounds to comprehensively investigate the impacts and risks of PFASs pollutants on the water environment. In addition, PFASs pollutants have strong bioaccumulation and are difficult to degrade. Long-term healthy impacts and risks cannot be ignored. Strengthen the management of pollution sources and continue to pay attention to the situation of PFASs pollutants in the region.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
SH: Analysis and writing NR: Revision.
Funding
This work was financially supported by Shenzhen Science and Technology Program (Grant Nos. KQTD20190929172630447).
Conflict of Interest
Author SH was employed Shenzhen Yuchi Testing Technology Co LTD.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.913997/full#supplementary-material
References
Ahrens, L., Taniyasu, S., Yeung, L. W. Y., Yamashita, N., Lam, P. K. S., and Ebinghaus, R. (2010). Distribution of Polyfluoroalkyl Compounds in Water, Suspended Particulate Matter and Sediment from Tokyo Bay, Japan. Chemosphere 79 (3), 266–272. doi:10.1016/j.chemosphere.2010.01.045
ATSDR (2018). Toxicological Profile for Perfluoroalkyls: Draft for Public Comment, June 2018. Atlanta, GA: Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services.
Banzhaf, S., Filipovic, M., Lewis, J., Sparrenbom, C. J., and Barthel, R. (2017). A Review of Contamination of Surface-, Ground-, and Drinking Water in Sweden by Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs). Ambio 46 (3), 335–346. doi:10.1007/s13280-016-0848-8
Borg, D., Lund, B.-O., Lindquist, N.-G., and Håkansson, H. (2013). Cumulative Health Risk Assessment of 17 Perfluoroalkylated and Polyfluoroalkylated Substances (PFASs) in the Swedish Population. Environ. Int. 59, 112–123. doi:10.1016/j.envint.2013.05.009
Butenhoff, J. L., Chang, S. C., Olsen, G. W., and Thomford, P. J. (2012). Chronic Dietary Toxicity and Carcinogenicity Study with Potassium Perfluorooctanesulfonate in Sprague Dawley Rats. Toxicology 293 (1-3), 1–15. doi:10.1016/j.tox.2012.01.003
Chen, H., Wang, X., Zhang, C., Sun, R., Han, J., Han, G., et al. (2017). Occurrence and Inputs of Perfluoroalkyl Substances (PFASs) from Rivers and Drain Outlets to the Bohai Sea, China. Environ. Pollut. 221, 234–243. doi:10.1016/j.envpol.2016.11.070
Chen, Y., Fu, J., Ye, T., Li, X., Gao, K., Xue, Q., et al. (2021). Occurrence, Profiles, and Ecotoxicity of Poly- and Perfluoroalkyl Substances and Their Alternatives in Global Apex Predators: A Critical Review. J. Environ. Sci. 109, 219–236. doi:10.1016/j.jes.2021.03.036
Dassuncao, C., Hu, X. C., Nielsen, F., Weihe, P., Grandjean, P., and Sunderland, E. M. (2018). Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Environ. Sci. Technol. 52 (6), 3738–3747. doi:10.1021/acs.est.7b06044
De Silva, A. O., Armitage, J. M., Bruton, T. A., Dassuncao, C., Heiger‐Bernays, W., Hu, X. C., et al. (2021). PFAS Exposure Pathways for Humans and Wildlife: a Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 40 (3), 631–657. doi:10.1002/etc.4935
DeWitt, J. C. (2015). Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances, Editor J. C. DeWitt. Cham: Humana Press. doi:10.1007/978-3-319-15518-0
Ding, G., Xue, H., Yao, Z., Wang, Y., Ge, L., Zhang, J., et al. (2018). Occurrence and Distribution of Perfluoroalkyl Substances (PFASs) in the Water Dissolved Phase and Suspended Particulate Matter of the Dalian Bay, China. Chemosphere 200, 116–123. doi:10.1016/j.chemosphere.2018.02.093
ECHA (2010). Guidance on Information Requirements and Chemical Safety Assessment Chapter R.8: Characterisation of Dose [Concentration]-Response for Human Health. Available at: http://echa.europa.eu/de/guidance-documents/guidance-on-information-requirements-and-chemicalsafety-assessment.
Gebbink, W. A., Van Asseldonk, L., and Van Leeuwen, S. P. J. (2017). Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water Near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol. 51 (19), 11057–11065. doi:10.1021/acs.est.7b02488
Gomis, M. I., Vestergren, R., Borg, D., and Cousins, I. T. (2018). Comparing the Toxic Potency In Vivo of Long-Chain Perfluoroalkyl Acids and Fluorinated Alternatives. Environ. Int. 113, 1–9. doi:10.1016/j.envint.2018.01.011
Guelfo, J. L., Marlow, T., Klein, D. M., Savitz, D. A., Frickel, S., Crimi, M., et al. (2018). Evaluation and Management Strategies for Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water Aquifers: Perspectives from Impacted U.S. Northeast Communities. Environ. Health Perspect. 126 (6), 065001. doi:10.1289/ehp2727
Habibullah-Al-Mamun, M., Ahmed, M. K., Raknuzzaman, M., Islam, M. S., Negishi, J., Nakamichi, S., et al. (2016). Occurrence and Distribution of Perfluoroalkyl Acids (PFAAs) in Surface Water and Sediment of a Tropical Coastal Area (Bay of Bengal Coast, Bangladesh). Sci. total Environ. 571, 1089–1104. doi:10.1016/j.scitotenv.2016.07.104
Hoffman, K., Webster, T. F., Bartell, S. M., Weisskopf, M. G., Fletcher, T., and Vieira, V. M. (2011). Private Drinking Water Wells as a Source of Exposure to Perfluorooctanoic Acid (PFOA) in Communities Surrounding a Fluoropolymer Production Facility. Environ. health Perspect. 119 (1), 92–97. doi:10.1289/ehp.1002503
Hu, X. C., Andrews, D. Q., Lindstrom, A. B., Bruton, T. A., Schaider, L. A., Grandjean, P., et al. (2016). Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 3 (10), 344–350. doi:10.1021/acs.estlett.6b00260
Hu, X. C., Tokranov, A. K., Liddie, J., Zhang, X., Grandjean, P., Hart, J. E., et al. (2019). Tap Water Contributions to Plasma Concentrations of Poly- and Perfluoroalkyl Substances (PFAS) in a Nationwide Prospective Cohort of U.S. Women. Environ. Health Perspect. 127 (6), 067006. doi:10.1289/ehp4093
Jian, J.-M., Chen, D., Han, F.-J., Guo, Y., Zeng, L., Lu, X., et al. (2018). A Short Review on Human Exposure to and Tissue Distribution of Per- and Polyfluoroalkyl Substances (PFASs). Sci. total Environ. 636, 1058–1069. doi:10.1016/j.scitotenv.2018.04.380
Kaboré, H. A., Duy, S. V., Munoz, G., Méité, L., Desrosiers, M., Liu, J., et al. (2018). Worldwide Drinking Water Occurrence and Levels of Newly-Identified Perfluoroalkyl and Polyfluoroalkyl Substances. Sci. total Environ. 616, 1089–1100. doi:10.1016/j.scitotenv.2017.10.210
Knutsen, H., Mæhlum, T., Haarstad, K., Slinde, G. A., and Arp, H. P. H. (2019). Leachate Emissions of Short- and Long-Chain Per- and Polyfluoralkyl Substances (PFASs) from Various Norwegian Landfills. Environ. Sci. Process. Impacts 21 (11), 1970–1979. doi:10.1039/c9em00170k
Lau, C., Anitole, K., Hodes, C., Lai, D., Pfahles-Hutchens, A., and Seed, J. (2007). Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci. 99 (2), 366–394. doi:10.1093/toxsci/kfm128
Lemly, A. D. (1996). Evaluation of the Hazard Quotient Method for Risk Assessment of Selenium. Ecotoxicol. Environ. Saf. 35 (2), 156–162. doi:10.1006/eesa.1996.0095
Li, J., Del Vento, S., Schuster, J., Zhang, G., Chakraborty, P., Kobara, Y., et al. (2011). Perfluorinated Compounds in the Asian Atmosphere. Environ. Sci. Technol. 45 (17), 7241–7248. doi:10.1021/es201739t
Li, X., Shang, X., Luo Sun, T., Du, X., Wang, Y., Xie, Q., et al. (2016). Screening and Health Risk of Organic Micropollutants in Rural Groundwater of Liaodong Peninsula, China. Environ. Pollut. 218, 739–748. doi:10.1016/j.envpol.2016.07.070
Li, Y., Li, J., Zhang, L., Huang, Z., Liu, Y., Wu, N., et al. (2019). Perfluoroalkyl Acids in Drinking Water of China in 2017: Distribution Characteristics, Influencing Factors and Potential Risks. Environ. Int. 123, 87–95. doi:10.1016/j.envint.2018.11.036
Li, F., Duan, J., Tian, S., Ji, H., Zhu, Y., Wei, Z., et al. (2020). Short-chain Per- and Polyfluoroalkyl Substances in Aquatic Systems: Occurrence, Impacts and Treatment. Chem. Eng. J. 380, 122506. doi:10.1016/j.cej.2019.122506
Liu, B., Zhang, H., Xie, L., Li, J., Wang, X., Zhao, L., et al. (2015). Spatial Distribution and Partition of Perfluoroalkyl Acids (PFAAs) in Rivers of the Pearl River Delta, Southern China. Sci. total Environ. 524-525, 1–7. doi:10.1016/j.scitotenv.2015.04.004
Liu, L., Qu, Y., Huang, J., and Weber, R. (2021). Per-and Polyfluoroalkyl Substances (PFASs) in Chinese Drinking Water: Risk Assessment and Geographical Distribution. Environ. Sci. Eur. 33 (1), 1–12. doi:10.1186/s12302-020-00425-3
Ludwicki, J. K., Góralczyk, K., Struciński, P., Wojtyniak, B., Rabczenko, D., Toft, G., et al. (2015). Hazard Quotient Profiles Used as a Risk Assessment Tool for PFOS and PFOA Serum Levels in Three Distinctive European Populations. Environ. Int. 74, 112–118. doi:10.1016/j.envint.2014.10.001
Macon, M. B., Villanueva, L. R., Tatum-Gibbs, K., Zehr, R. D., Strynar, M. J., Stanko, J. P., et al. (2011). Prenatal Perfluorooctanoic Acid Exposure in CD-1 Mice: Low-Dose Developmental Effects and Internal Dosimetry. Toxicol. Sci. 122 (1), 134–145. doi:10.1093/toxsci/kfr076
Mumtaz, M., Bao, Y., Li, W., Kong, L., Huang, J., and Yu, G. (2019a). Screening of Textile Finishing Agents Available on the Chinese Market: An Important Source of Per-And Polyfluoroalkyl Substances to the Environment. Front. Environ. Sci. Eng. 13 (5), 1–10. doi:10.1007/s11783-019-1145-0
Mumtaz, M., Bao, Y., Liu, L., Huang, J., Cagnetta, G., and Yu, G. (2019b). Per- and Polyfluoroalkyl Substances in Representative Fluorocarbon Surfactants Used in Chinese Film-Forming Foams: Levels, Profile Shift, and Environmental Implications. Environ. Sci. Technol. Lett. 6 (5), 259–264. doi:10.1021/acs.estlett.9b00154
Ng, C. A., and Hungerbühler, K. (2014). Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environ. Sci. Technol. 48 (9), 4637–4648. doi:10.1021/es404008g
Park, M., Wu, S., Lopez, I. J., Chang, J. Y., Karanfil, T., and Snyder, S. A. (2020). Adsorption of Perfluoroalkyl Substances (PFAS) in Groundwater by Granular Activated Carbons: Roles of Hydrophobicity of PFAS and Carbon Characteristics. Water Res. 170, 115364. doi:10.1016/j.watres.2019.115364
Peden-Adams, M. M., Keller, J. M., Eudaly, J. G., Berger, J., Gilkeson, G. S., and Keil, D. E. (2008). Suppression of Humoral Immunity in Mice Following Exposure to Perfluorooctane Sulfonate. Toxicol. Sci. 104 (1), 144–154. doi:10.1093/toxsci/kfn059
Perkins, R. G., Butenhoff, J. L., Kennedy, G. L., and Palazzolo, M. J. (2004). 13‐Week Dietary Toxicity Study of Ammonium Perfluorooctanoate (APFO) in Male Rats. Drug Chem. Toxicol. 27 (4), 361–378. doi:10.1081/dct-200039773
Poothong, S., Papadopoulou, E., Padilla-Sánchez, J. A., Thomsen, C., and Haug, L. S. (2020). Multiple Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs): From External Exposure to Human Blood. Environ. Int. 134, 105244. doi:10.1016/j.envint.2019.105244
Rahman, M. F., Peldszus, S., and Anderson, W. B. (2014). Behaviour and Fate of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Drinking Water Treatment: A Review. Water Res. 50, 318–340. doi:10.1016/j.watres.2013.10.045
Shao, M., Ding, G., Zhang, J., Wei, L., Xue, H., Zhang, N., et al. (2016). Occurrence and Distribution of Perfluoroalkyl Substances (PFASs) in Surface Water and Bottom Water of the Shuangtaizi Estuary, China. Environ. Pollut. 216, 675–681. doi:10.1016/j.envpol.2016.06.031
Si, Y., Huang, J., Liang, Z., Liu, G., Chen, D., Guo, Y., et al. (2021). Occurrence and Ecological Risk Assessment of Perfluoroalkyl Substances (PFASs) in Water and Sediment from an Urban River in South China. Arch. Environ. Contam. Toxicol. 81 (1), 133–141. doi:10.1007/s00244-021-00855-x
U.S. EPA (2016). Lifetime Health Advisories and Health Effects Support Documents for Perfluorooctanoic Acid and Perfluorooctane Sulfonate Washington, DC: Environmental Protection Agency 81 (101), 33250–33251.
Valsecchi, S., Rusconi, M., Mazzoni, M., Viviano, G., Pagnotta, R., Zaghi, C., et al. (2015). Occurrence and Sources of Perfluoroalkyl Acids in Italian River Basins. Chemosphere 129, 126–134. doi:10.1016/j.chemosphere.2014.07.044
Vestergren, R., and Cousins, I. T. (2009). Tracking the Pathways of Human Exposure to Perfluorocarboxylates. Environ. Sci. Technol. 43 (15), 5565–5575. doi:10.1021/es900228k
Wan, Y., Wang, S., Cao, X., Cao, Y., Zhang, L., Wang, H., et al. (2017). Perfluoroalkyl Acids (PFAAs) in Water and Sediment from the Coastal Regions of Shandong Peninsula, China. Environ. Monit. Assess. 189 (3), 100–114. doi:10.1007/s10661-017-5807-8
Wang, T., Wang, P., Meng, J., Liu, S., Lu, Y., Khim, J. S., et al. (2015). A Review of Sources, Multimedia Distribution and Health Risks of Perfluoroalkyl Acids (PFAAs) in China. Chemosphere 129, 87–99. doi:10.1016/j.chemosphere.2014.09.021
Wang, Z., Dewitt, J. C., Higgins, C. P., and Cousins, I. T. (2017). A Never-Ending Story of Per-And Polyfluoroalkyl Substances (PFASs)?. Environ. Sci. Technol. 51 (5), 2508–2518. doi:10.1021/acs.est.6b04806
Wei, C., Wang, Q., Song, X., Chen, X., Fan, R., Ding, D., et al. (2018). Distribution, Source Identification and Health Risk Assessment of PFASs and Two PFOS Alternatives in Groundwater from Non-industrial Areas. Ecotoxicol. Environ. Saf. 152, 141–150. doi:10.1016/j.ecoenv.2018.01.039
Xie, L.-N., Wang, X.-C., Dong, X.-J., Su, L.-Q., Zhu, H.-J., Wang, C., et al. (2021). Concentration, Spatial Distribution, and Health Risk Assessment of PFASs in Serum of Teenagers, Tap Water and Soil Near a Chinese Fluorochemical Industrial Plant. Environ. Int. 146, 106166. doi:10.1016/j.envint.2020.106166
Xu, B., Liu, S., Zhou, J. L., Zheng, C., Weifeng, J., Chen, B., et al. (2021). PFAS and Their Substitutes in Groundwater: Occurrence, Transformation and Remediation. J. Hazard. Mater. 412, 125159. doi:10.1016/j.jhazmat.2021.125159
Yahia, D., El-Nasser, M. A., Abedel-Latif, M., Tsukuba, C., Yoshida, M., Sato, I., et al. (2010). Effects of Perfluorooctanoic Acid (PFOA) Exposure to Pregnant Mice on Reproduction. J. Toxicol. Sci. 35 (4), 527–533. doi:10.2131/jts.35.527
Yang, L., Zhu, L., and Liu, Z. (2011). Occurrence and Partition of Perfluorinated Compounds in Water and Sediment from Liao River and Taihu Lake, China. Chemosphere 83 (6), 806–814. doi:10.1016/j.chemosphere.2011.02.075
Zhang, Y., Zhou, Y., Zhang, A., Li, J., Yu, J., Dou, Y., et al. (2021). Perfluoroalkyl Substances in Drinking Water Sources along the Yangtze River in Jiangsu Province, China: Human Health and Ecological Risk Assessment. Ecotoxicol. Environ. Saf. 218, 112289. doi:10.1016/j.ecoenv.2021.112289
Zhao, P., Xia, X., Dong, J., Xia, N., Jiang, X., Li, Y., et al. (2016). Short- and Long-Chain Perfluoroalkyl Substances in the Water, Suspended Particulate Matter, and Surface Sediment of a Turbid River. Sci. total Environ. 568, 57–65. doi:10.1016/j.scitotenv.2016.05.221
Keywords: detection, hazard quotient, Pafs, risk assessment, reservoir
Citation: He S and Ren N (2022) Occurrence and Risk Assessment of per- and Polyfluoroalkyl Substances in Water Source Protection Area of Southeastern China. Front. Environ. Sci. 10:913997. doi: 10.3389/fenvs.2022.913997
Received: 06 April 2022; Accepted: 30 May 2022;
Published: 24 June 2022.
Edited by:
Wojciech Mrozik, Newcastle University, United KingdomCopyright © 2022 He and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanqi Ren, cm5xQGhpdC5lZHUuY24=
 Shu He
Shu He Nanqi Ren
Nanqi Ren