- 1Department of Geobotany and Plant Ecology, Faculty of Biology and Environmental Protection, University of Lodz, Lodz, Poland
- 2Department of Biodiversity Research, Global Change Research Institute AS CR, Brno, Czech Republic
- 3Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdańsk, Gdańsk, Poland
Climate change affects populations of plants, animals, and fungi not only by direct modifications of their climatic niches but also by altering their ecological interactions. In this study, the future distribution of suitable habitats for the small-white orchid (Pseudorchis albida) was predicted using ecological niche modeling. In addition, the effect of global warming on the spatial distribution and availability of the pollen vectors of this species was evaluated. Due to the inconsistency in the taxonomic concepts of Pseudorchis albida, the differences in the climatic preferences of three proposed subspecies were investigated. Due to the overlap of both morphological and ecological characters of ssp. albida and ssp. tricuspis, they are considered to be synonyms, and the final analyses were carried out using ssp. albida s.l. and ssp. straminea. All of the models predict that with global warming, the number of suitable niches for these orchids will increase. This significant increase in preferred habitats is expected to occur in Greenland, but habitat loss in continental Europe will be severe. Within continental Europe, Pseudorchis albida ssp. albida will lose 44%–98% of its suitable niches and P. albida ssp. straminea will lose 46%–91% of its currently available habitats. An opposite effect of global warming was predicted for pollinators of P. albida s.l., and almost all insects studied will be subject to habitat loss. Still, within the predicted potential geographical ranges of the orchid studied, some pollen vectors are expected to occur, and these can support the long-term survival of the small-white orchid.
1 Introduction
The sole representative of the genus Pseudorchis Ség., the small-white orchid (Pseudorchis albida (L.) Á. Löve and D. Löve), is a tuberous perennial geophyte growing in most of Europe and northern Asia from Spain and Iceland to northwest Siberia (Jersáková et al., 2011). This species is variable within its geographical range, and the morphological differences prompted taxonomists to divide P. albida into three subspecies: ssp. albida, ssp. straminea (Fern.) Ä. Löve and D. Löve, and ssp. tricuspis (Beck) Klein (Figure 1; Reinhammar, 1998; Klein, 2000; Jersáková et al., 2011). However, the recognition of these taxa is still debated. Reinhammar (1995, 1998) recognized two species in the genus Pseudorchis, with moderate morphometric support. The studies on their allozymes also indicate that it is reasonable to accept the species status of the lowland to subalpine P. albida s.s., and alpine P. straminea (Reinhammar and Hedren, 1998). Klein (2000) accepted three subspecies of P. albida: ssp. tricuspis (calcicolous, with an alpine–boreal distribution), ssp. albida (acidophilous, with alpine–temperate–boreal distribution), and ssp. straminea (basiphilous, with west Arctic–north Atlantic distribution). Jersáková et al. (2011) considered that the taxa of Pseudorchis characterized by differences in distribution are not well-defined and accept the broad concept of P. albida s.l. On the other hand, Bateman et al. (2017) recognized Pseudorchis albida and P. straminea as separate species, pointing out morphological features and molecular divergence (ITS) sufficient for species-level distinction. The same authors rejected the separateness of P. tricuspis due to overlap in supposedly taxonomically useful characters with P. albida and P. straminea (Bateman et al., 2017). Considering the differences in the taxonomic approach, it was decided to accept all taxa as subspecies in this ecological study. The results of the analyses can be used in further taxonomic studies on Pseudorchis.

FIGURE 1. Photographs of the small-white orchid in its natural habitat. Pseudorchis albida ssp. albida in Rhön, Germany (A), and Zillertal Alps, Austria [(B); photographer: Marco Klüber/www.m-klueber.de], Pseudorchis albida ssp. straminea in Newfoundland, Canada [(C,D); photographer: James Fowler], and Pseudorchis albida ssp. tricuspis on Mt. Mangart, Julian Alps, Slovenia [(E,F); photographer: Amadej Trnkoczy].
Pseudorchis albida ssp. albida occurs in areas with a boreal–montane climate and is found from United Kingdom across Scandinavia to the northern Urals in the European part of Russia as well as in mountain ranges from Spain across the Alps to the Eastern Carpathians (Jersáková et al., 2011). Pseudorchis albida ssp. straminea is restricted to areas with a west Arctic–north Atlantic climate (Iceland, Faroes, Greenland, and Scandinavia; Jersáková et al., 2011), and Pseudorchis albida ssp. tricuspis is restricted to alpine–boreal areas (Swiss, Italian and Austrian Alps, Tatra Mountains, and Eastern Carpathian; Jersáková et al., 2011).
Populations of small-white orchid reproduce mainly sexually (Jersáková et al., 2011), and vegetative propagation by tubers contributes little to population growth (Summerhayes, 1951). As a species that provides a nectar reward, several species of Lepidoptera (Claessens and Kleynen, 2011) are reported pollinators of Pseudorchis albida. More recently, Jersáková et al. (2011) also reported species of Empis (Diptera) as diurnal pollen vectors.
In terms of anthropogenic threats, P. albida is endangered by agricultural development and afforestation (Reinhammar et al., 2002; Foley and Clarke, 2005; Forbes and Northridge, 2012). Reduction in traditional mowing and grazing has resulted in it being overgrown by more competitive species (Reinhammar et al., 2002; Holland et al., 2008). On the other hand, reduced seed set and recruitment can result from over-grazing (Duffy et al., 2009; Jersáková et al., 2011). The effect of global warming on this species is yet to be evaluated.
According to the IUCN Red List, Pseudorchis albida is assessed as a species of least concern because it is rather widespread (Rankou, 2011). However, due to a considerable decline in its distribution, it is currently considered to be critically endangered in Greece (small population found by Tsiftsis and Antonopoulos, 2011), vulnerable in Great Britain (Cheffings and Farrell, 2005) and Bulgaria (Petrova and Vladimirov, 2009), endangered in Ireland (Curtis and McGough, 1988), Czech Republic (Holub and Procházka, 2000), Germany (Ludwig and Schnittler, 1996), and Sweden (Gärdenfors, 2010), and near threatened in Norway (Artsdatabanken, 2010) and Poland (Kaźmierczakowa et al., 2016). It is also protected in many European countries (Reinhammar et al., 2002; Bilz et al., 2011), e.g., Poland (Kaźmierczakowa et al., 2016), Czech Republic (Danihelka et al., 2012), Denmark (Damgaard et al., 2020), Romania (Sârbu et al., 2020), Ukraine (Kricsfalusy et al., 1999, 2010), Slovakia (Turis et al., 2014), Norway (subordinate agency, 2022), Sweden (Naturvårdsverket, 2022), Austria (Zulka et al., 2001; Jersáková et al., 2011), Germany (Jersáková et al., 2011), Switzerland (Jersáková et al., 2011), and Italy (Jersáková et al., 2011).
This study aimed to estimate the effect of global warming on the distribution of climatic niches suitable for P. albida s.l. Since this orchid relies mainly on sexual reproduction, the effect of climate change was also evaluated for the pollinators of this orchid. To improve the estimates and because the taxonomic separateness of P. albida ssp. tricuspis is questioned by some authors (Bateman et al., 2017), the differences in the preferred climatic niches of the three-known subspecies of P. albida were evaluated in order to assess their ecological distinctiveness.
2 Materials and methods
2.1 List of localities
The databases of localities of Pseudorchis albida s.l. in continental Europe as well as records of pollinators of this orchid were compiled based on information in public facilities accessed through the Global Biodiversity Information Facility (GBIF 2020; Supplementary Table S1). The information on pollen vectors was obtained from previous reports on pollination of P. albida by Claessens and Kleynen (2011) and Jersáková et al. (2011). There was an insufficient number of occurrences for Empis bistortae Meigen, 1822 for performing an analysis for this insect. From a total of 4518 localities for Pseudorchis albida (ssp. albida—316, ssp. straminea—4170, and tricuspis—32) and 69424 for insects (Chrysoteuchia culmella (Linnaeus, 1758)—46299, Crambus ericella (Hübner, 1813)—1098, Crambus pascuella L.—4032, Plutella xylostella (Linnaeus, 1758)—17643, and Udea uliginosalis (Stephens, 1834)—352) available in the repositories, only records that were georeferenced with a minimum of 1 km precision were selected. To reduce sampling bias, spatial thinning was carried out using SDMtoolbox 2.3 for ArcGIS (Kremen et al., 2008; Brown, 2014). The data were rarified by designating a minimal distance of 5 km for calculating climatic habitat heterogeneity. The final database included 28 localities for P. albida ssp. albida, 414 for P. albida ssp. straminea, 11 for P. albida ssp. tricuspis (Supplementary Data Sheet S1), and 3694 for its pollinators (Chrysoteuchia culmella—1472, Crambus ericella—249, Crambus pascuella—707, Plutella xylostella—1244, and Udea uliginosalis—22; Supplementary Data Sheet S2).
2.2 Principal component analysis
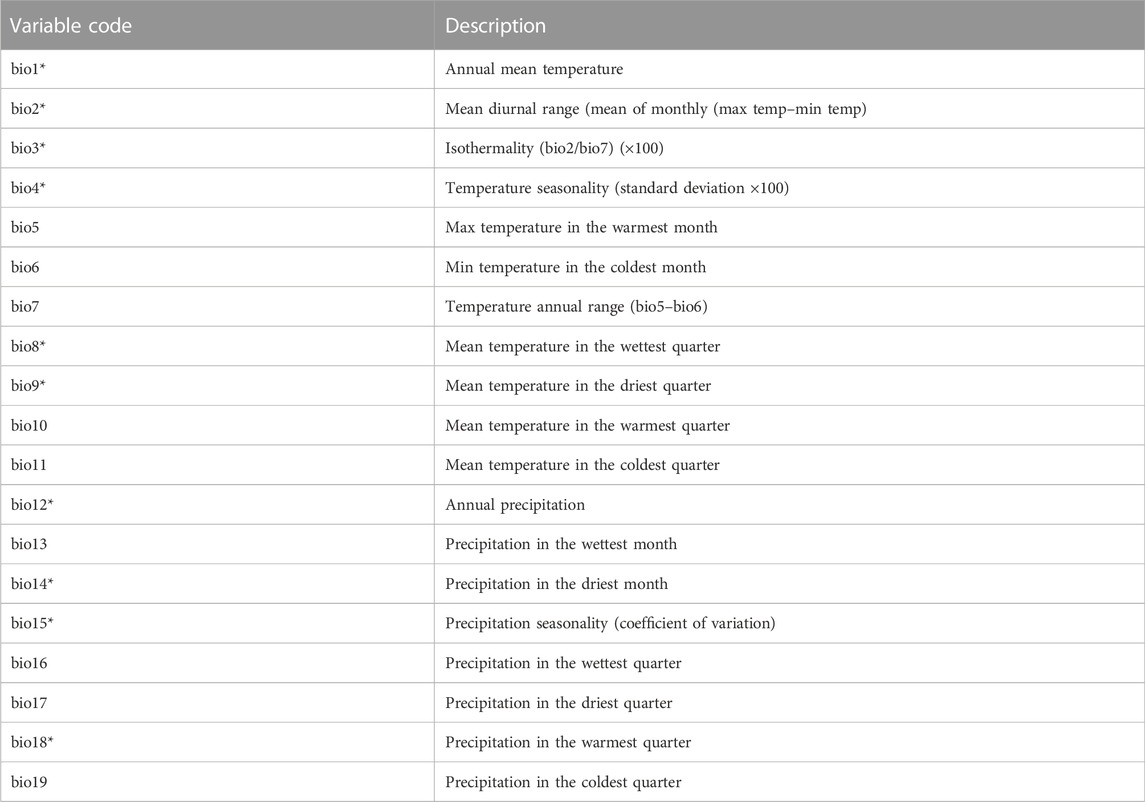
Principal components analysis (PCA) was used to evaluate the differences between populations of P. albida ssp. straminea, P. albida ssp. albida, and P. albida ssp. tricuspis based on 19 bioclimatic variables from WorldClim v. 2.1 (Table 1; Fick and Hijmans, 2017). Calculations were carried out using the software package Statistica PL. ver. 13.3 (StatSoft Inc. 2011). The data matrix was transformed (square root) before carrying out the ordination analysis.
2.3 Ecological niche modeling
The modeling of the current and future distribution of the species studied was carried out using the maximum entropy method implemented in MaxEnt version 3.3.2 (Phillips et al., 2004, 2006; Elith et al., 2011) based on presence-only observations. For the modeling, bioclimatic variables in 30 arc-seconds of the interpolated climate surface downloaded from WorldClim v. 2.1 were used (Fick and Hijmans, 2017). Nine of 19 variables were removed from the analyses due to their high correlation with other variables as indicated by Pearson’s correlation coefficient (Table 1; Supplementary Data Sheet S3) computed using SDMtoolbox 2.3 for ArcGIS (Kremen et al., 2008; Brown, 2014). Because some previous studies (Barve et al., 2011) suggest that modeling based on data for a restricted area is more reliable than calculating habitat suitability at a global scale, the area included in the analysis was restricted to 84.65–34.43˚N and 74.65˚W–45.43˚E. Since this study investigated the effect of climate change on the distribution of the species and soil characteristics have little effect on models of Australian terrestrial orchid, Leporella fimbriata (Kolanowska et al., 2021a), we did not use these variables in the analyses.
Predictions of the future extent of the climatic niches of P. albida and its pollinator in 2080–2100 were made using climate projections developed by the CNRM/CERFACS modeling group for the coupled model intercomparison project (CNRM–CM6-1) for four shared socio-economic pathways (SSPs; O’Neill et al., 2014): SSP1-2.6, SSP2-4.5, SSP3-7.0, and SSP5-8.5. The layers in 2.5 arc-minutes were re-scaled to fit bioclimatic variables. SSPs are trajectories adopted by the Intergovernmental Panel on Climate Change (IPCC), which provide a broader view of a “business as usual” world without a climate policy, with global warming in 2100 ranging from a low of 3.1°C to a high of 5.1°C above pre-industrial levels (O’Neill et al., 2014).
In all the analyses, the maximum number of iterations was set to 10000 and that of convergence threshold to 0.00001. The neutral (= 1) regularization multiplier value and auto features were used. All samples were added to the background. The “random seed” option, which provided a random test partition and background subset for each run, was applied, and 20% of the samples were used as test points. The run was performed as a bootstrap with 100 replicates. The output was set to logistic. In addition, the “fade by clamping” function in MaxEnt was enabled to preclude extrapolations outside the environmental range of the training data (Phillips et al., 2006). All analyses of GIS data were carried out on ArcGIS 10.6 (Esri, Redlands, CA, United States). The evaluation of the models was conducted using the area under the curve (AUC; Mason and Graham 2002; Evangelista et al., 2008) and True Skill Statistic (TSS; Allouche et al., 2006).
SDMtoolbox 2.3 for ArcGIS (Kremen et al., 2008; Brown, 2014) was used to visualize changes in the distribution of suitable niches of the orchid studied and its pollinator due to global warming. To compare the prediction of the model of the current distribution with future predictions, all SDMs were converted into binary rasters and projected using the Goode homolosine. The presence threshold was estimated based on the values for grids in which the species studied were predicted to occur using present-time data. Because about 70%–84% of known localities of P. albida and its pollinators were located in grids with values > 0.4, this threshold value was used to create binary rasters. To determine the availability of pollinators for the orchid, the overlap of the binary models of both organisms was calculated.
3 Results
3.1 Ecological differences between subspecies of Pseudorchis
The result of PCA analyses indicate that although the preferred niche of P. albida ssp. straminea differs from that of the two other taxa, P. albida ssp. albida and P. albida ssp. tricuspis occupy similar habitats. This is indicated by the second axis, which separated P. albida ssp. albida and P. albida ssp. tricuspis from most of the records of P. albida ssp. straminea. Our analyses indicate significant differences in the bioclimatic preferences of the subspecies. of P. albida. Along the gradient represented by the first axis, P. albida ssp. straminea is correlated especially with precipitation in the warmest quarter (bio18) and the mean temperature in the wettest quarter (bio8). The ordination diagrams of PCA explained 68.96% of the total variance. The first component accounted for 51.77% of the total variance and the second for 17.19% (Figure 2; Supplementary Table S2). Based on the morphological similarities of the two latter orchids, a broader concept of P. albida ssp. albida was used, which also includes P. albida ssp. tricuspis.
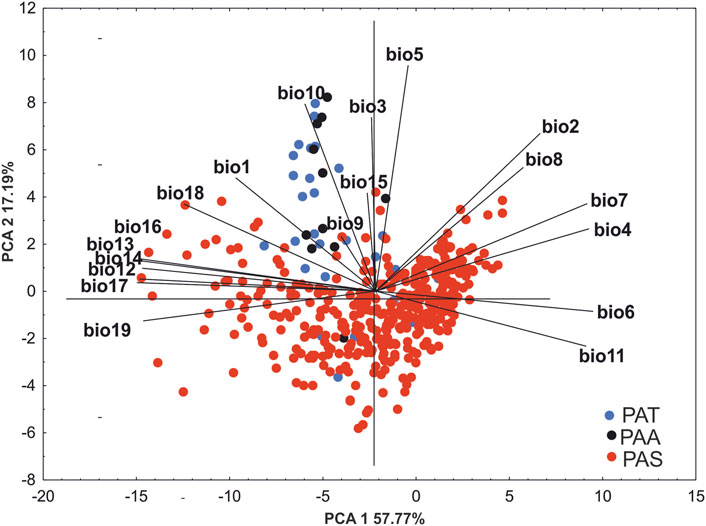
FIGURE 2. PCA ordination diagram (principal component analysis) of the distributions of populations of P. albida ssp. straminea (red dots), P. albida ssp. albida (black dots), and P. albida ssp. tricuspis (blue dots) based on 19 bioclimatic variables.
3.2 Model evaluation and limiting factors
The models had high AUC (0.871–0.998) and TSS (0.517–0.9924) scores, indicating their predictions are very reliable (Figure 3; Table 2). The most important variable limiting the distribution of P. albida ssp. albida was precipitation in the warmest quarter (bio18—47.5%). Much less significant for its occurrence were the annual precipitation (bio12—18.5%) and the annual mean temperature (bio1—14.9%). The latter factor was crucial (42.6%) for the distribution of P. albida ssp. straminea, followed by the mean temperature in the wettest quarter (bio8—29.8%) and precipitation in the warmest quarter (bio18—7.9%).
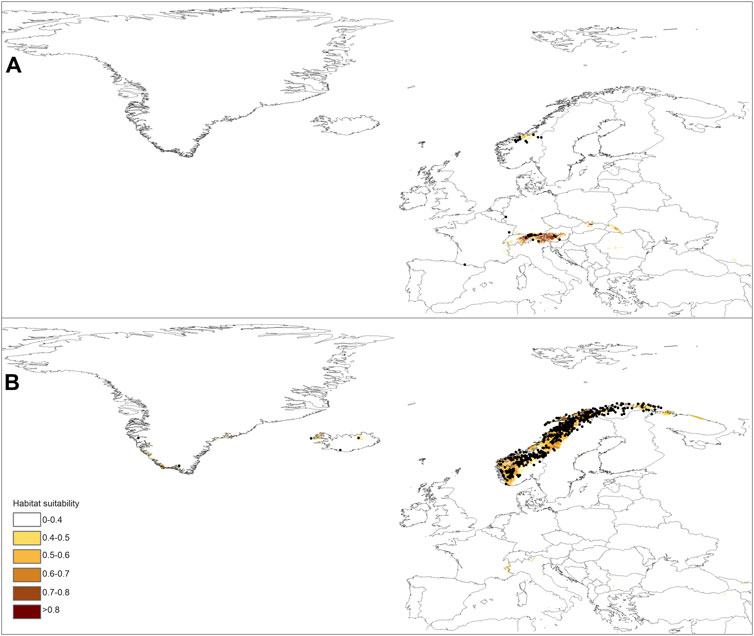
FIGURE 3. Current distribution of suitable niches for P. albida ssp. albida (A) and P. albida ssp. straminea (B) along with the localities included in the models (marked by black dots).
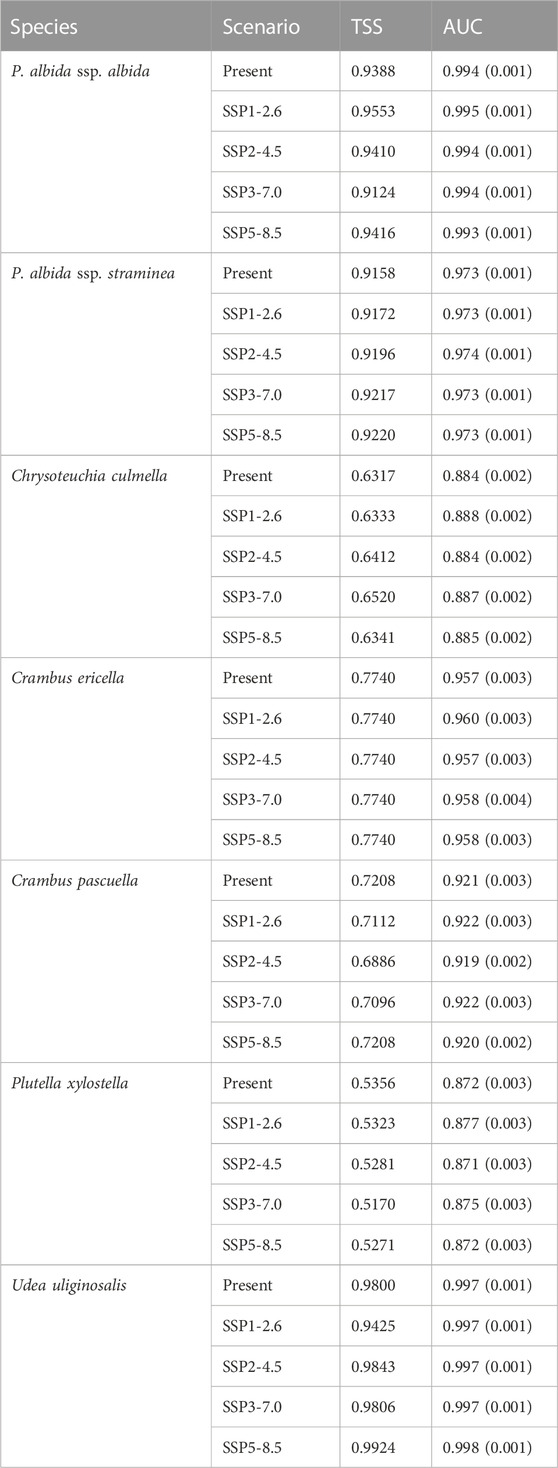
TABLE 2. TSS scores, average training AUC, and standard deviations (in brackets) for the replicate runs of the models.
3.3 Effect of climate change on P. albida and its pollinators
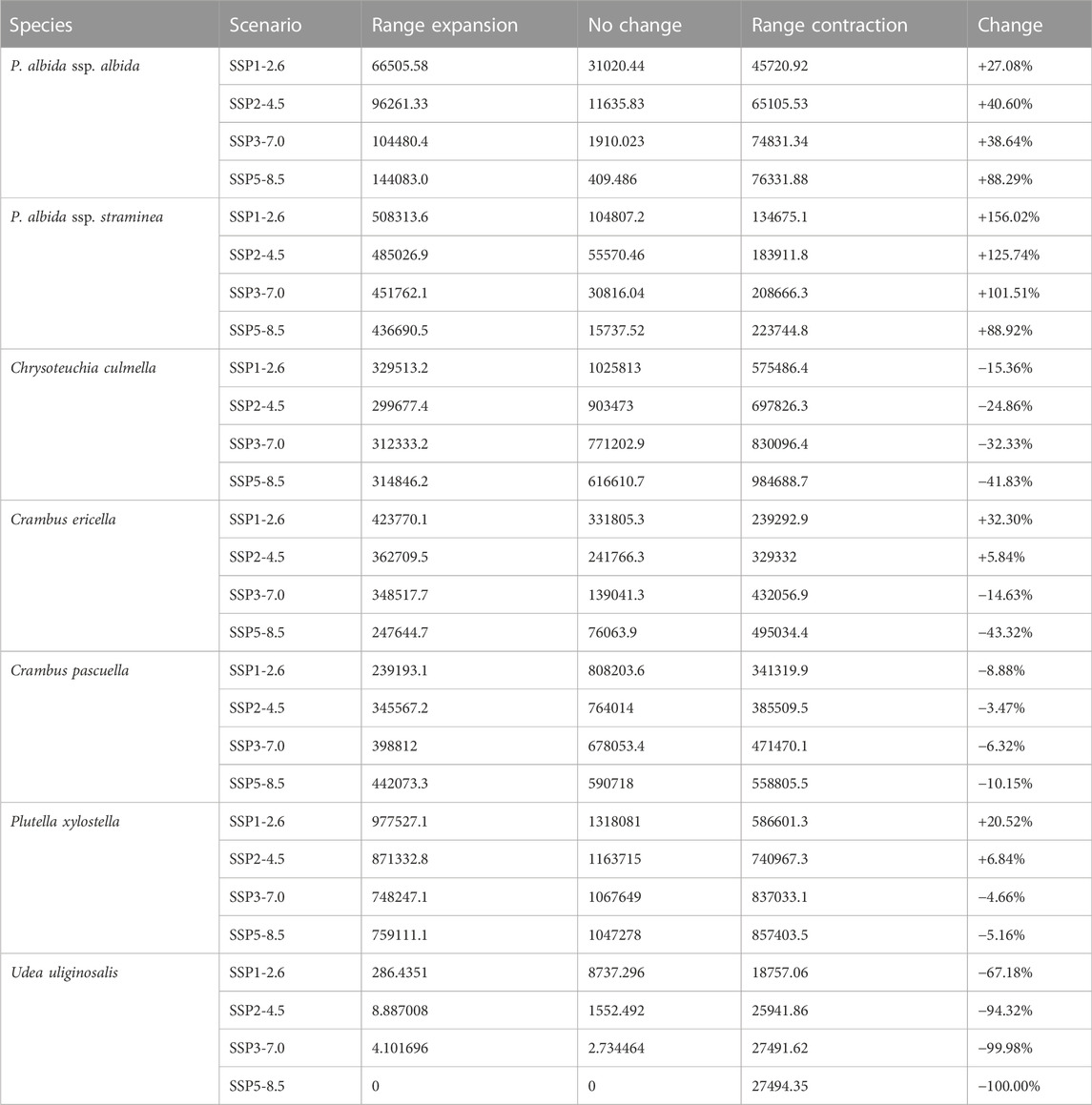
The predictions of the present-time models are congruent with the known geographical ranges of P. albida ssp. albida and P. albida ssp. straminea (Figure 3). Our analyses indicate the critical changes in the distribution of small-white orchid (Figures 4–7). All models predict that the availability of suitable niches for the orchids studied will increase as a result of global warming (Table 3), but the significant increase in suitable niches is expected to occur in Greenland, whereas habitat loss in continental Europe will be severe. Overall, the potential range of P. albida ssp. albida will be 27%–88% greater than at present, whereas that of P. albida ssp. straminea will be 88%–156% greater. The unexpected result is that while SSP1-2.6 is expected to be the most advantageous climate change scenario for the latter taxon, the same scenario is the least optimistic for P. albida ssp. albida, which will mostly benefit from SSP5-8.5.
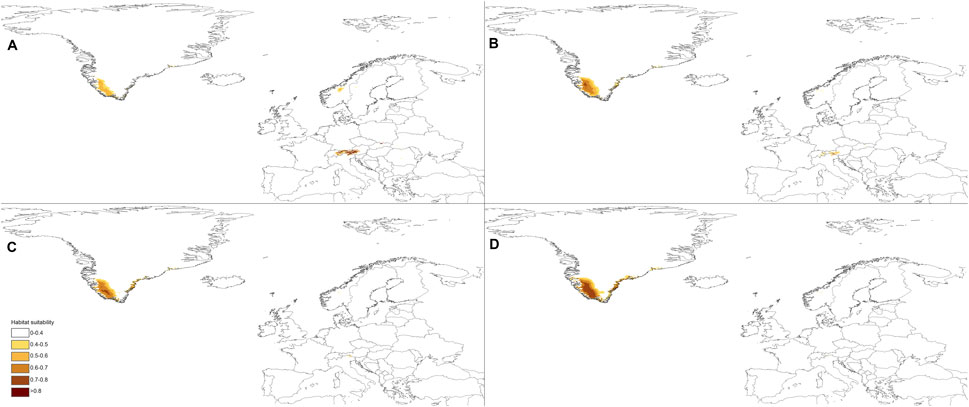
FIGURE 4. Future distribution of suitable niches for P. albida ssp. albida predicted under SSP1-2.6 (A), SSP2-4.5 (B), SSP3-7.0 (C), and SSP5-8.5 (D) climates.

FIGURE 5. Future distribution of suitable niches for P. albida ssp. straminea predicted under SSP1-2.6 (A), SSP2-4.5 (B), SSP3-7.0 (C), and SSP5-8.5 (D) climates.
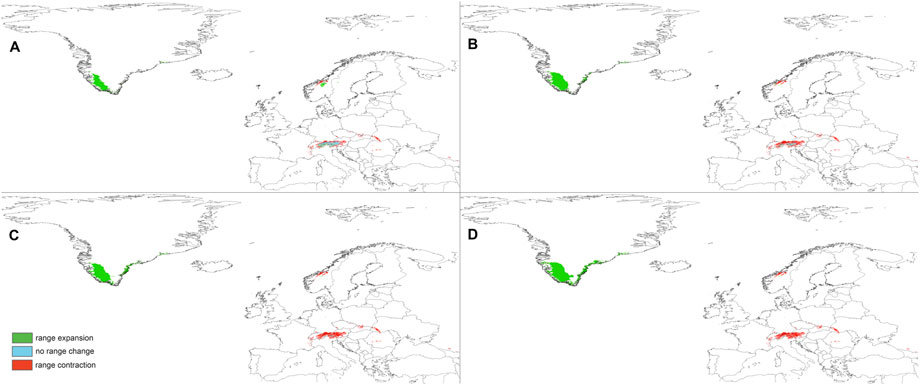
FIGURE 6. Changes in the distribution of suitable niches for P. albida ssp. albida predicted under SSP1-2.6 (A), SSP2-4.5 (B), SSP3-7.0 (C), and SSP5-8.5 (D) climates.
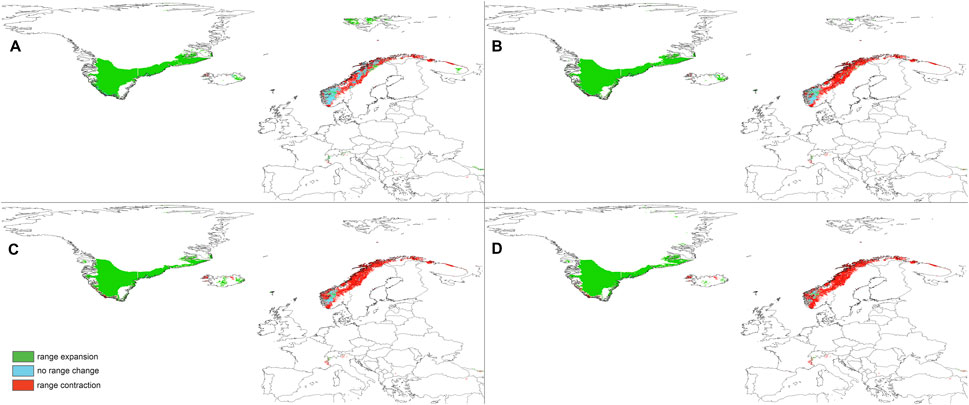
FIGURE 7. Changes in the distribution of suitable niches for P. albida ssp. straminea predicted under SSP1-2.6 (A), SSP2-4.5 (B), SSP3-7.0 (C), and SSP5-8.5 (D) climates.
Pseudorchis albida ssp. albida is currently known to occur only in continental Europe, but apparently its suitable habitats will be located mainly in Greenland in the future and will become extinct in continental Europe based on SSP5-8.5. P. albida ssp. straminea will also face significant loss of habitats in this part of its range; however, it could potentially extend its range to Svalbard (only in the less severe scenarios, such as SSP1-2.6 and SSP2-4.5, is its occurrence in Iceland not completely threatened). Within continental Europe, Pseudorchis albida ssp. albida will lose 44% (SSP1-2.6)–99% (SSP5-8.5) of its suitable niches, and P. albida ssp. straminea will lose 46% (SSP1-2.6)–91% (SSP5-8.5) of its current habitat.
While in the future P. albida is predicted to occupy different areas, the situation is completely different for the pollinators of this species (Table 3). All models predict a significant loss of habitat for them, which in the case of Udea uliginosalis could result in its extinction (Table 3).
3.4 Availability of pollinators
Based on the analyses, Udea uliginosalis is currently present in ca. 10% of the potential range of P. albida ssp. straminea, but will not be present there by 2100 (Supplementary Data Sheet S4; Table 4).
Plutella xylostella is predicted to be the most important pollinator of P. albida, with a range overlap of 75% (SSP5-8.5)–88% (SSP3-7.0) with P. albida ssp. albida and 70% (SSP2-4.5)–100% (SSP1-2.6) with P. albida ssp. straminea. Chrysoteuchia culmella is currently present in 74% of the range of P. albida ssp. albida and 52% of that of P. albida ssp. straminea. The predicted future distribution of this insect will overlap partially with both subspecies of the small-white orchid, overlapping 66% (SSP1-2.6)–91% (SSP3-7.0) of that of P. albida ssp. albida and 38% (SSP2-4.5)–56% (SSP3-7.0) of that of P. albida ssp. straminea. The statistics for Crambus ericella and C. pascuella are similar (Table 4).
4 Discussion
4.1 Implication for taxonomy
The recognition of three taxa within the Pseudorchis albida group remains a topic of taxonomic discussion and concern in terms of both their distinction and rank. This study indicates that ssp. tricuspis occupies niches similar to those occupied by ssp. albida, even if ssp. tricuspis is considered to be an alpine taxon and ssp. albida associated with lowland to subalpine regions (Reinhammar et al., 2002; Jersáková et al., 2011). On the other hand, Klein (2000) argues that ssp. tricuspis should be considered to be a separate subspecies, and this concept is also accepted by other scientists (Moore, 1980; Reinhammar, 1998; Bournérias and Prat, 2005; Perazza, 2016). Reinhammar (1995), Reinhammar (1998) based on the results of a multivariate morphometric study considering plants of ssp. tricuspis as conspecific with P. straminea. The position of “tricuspis” as a variety is proposed by Kreutz (2004), Delforge (2006), and Jersáková et al. (2011). Landwehr (1977) believes that this taxon is just a form of P. albida. Unfortunately, no molecular studies have included ssp. tricuspis. The results presented indicate that their morphological characteristics are very similar, which supports merging them under ssp. albida.
Unlike Pseudorchis albida ssp. tricuspis, ssp. straminea is more distinct. Only the rank of this taxon is debated. Analyses presented in this paper reveal differences in the climatic requirements of ssp. albida and ssp. straminea, which could be a potential argument and area for research on whether to elevate the latter taxon to a separate species. This is proposed based on its morphology (Reinhammar 1995; Reinhammar 1998) and differences in allozymes (Reinhammar and Hedren, 1998). According to Duffy et al. (2011) the AFLP markers for P. albida are very polymorphic, and there are significant differences both within and among populations, and population genetic isolation increases with distance but did not find any differences in plastid microsatellites between Irish populations of ssp. albida and Swedish ssp. straminea. Based on molecular studies, Bateman et al. (2003); Bateman et al. (2017) show that the differences in DNA sequences (nrITS, rbcL, and trnL-F) of the two taxa are near the lowest level of acceptance for their being separate species. Bateman et al. (2017) also reported at least 14 morphometric characters that can be used to identify these taxa. Based on previous studies and the results presented, it is proposed that “straminea” is a subspecies.
4.2 Effect of global warming on occurrence of P. albida s.l. and its conservation
The effect of predicted climate change will adversely affect populations of P. albida in continental Europe. In the best-case scenario (SSP1-2.6) both subspecies, ssp. albida and ssp. straminea, will lose almost half of their current suitable niches (44% and 46%, respectively). In the most damaging SSP5-8.5, only 1%–9% of the currently available habitats will still be suitable for small-white orchids. Global warming is one of the most important causes of changes in habitat (Opdam and Wascher, 2004; Troia et al., 2019). This is particularly so for alpine species, the available habitat for which is likely to significantly decrease (Freeman et al., 2018; Lamprecht et al., 2018) and other species with very specific ecological requirements (Tsiftsis et al., 2019). Geppert et al. (2020) indicated that ranges of some alpine orchids are or will decrease, especially since they are also threatened by other factors, i.e., habitat modification and loss of specific ecological relationships. Similar results are reported in a study on another orchid with a Scandinavian-alpine distribution in Europe, Nigritella nigra s.l. (Kolanowska et al., 2021b). However, global warming will result in the transformation of currently unsuitable habitats in Greenland. Shifts in the ranges of species may enable them to access and colonize these areas (Kelly and Goulden, 2008; Cannone and Pignatti, 2014; Geppert et al., 2020). However, as the populations of P. albida are usually very small (Jeřábková, 2006; Pearman et al., 2008; Jersáková et al., 2011), it is unlikely that ssp. albida will be able to colonize and adapt to new habitats in Greenland in the next few decades. These should be accessible for ssp. Straminea, which is more likely to be able to colonize this area. That distributions of orchids can change as a result of global warming is unlikely, but is suggested in some previous studies (van der Meer et al., 2016; Kolanowska et al., 2017).
An important aspect of the occurrence of Pseudorchis in Greenland is that currently most of the island is covered by ice (GrIS). Studies indicate that by 2100, the thickness of the GrIS will decrease significantly, but the area occupied will not differ much (Muntjewerf et al., 2020; Greve and Chambers 2022; Yang et al., 2022). This means that many areas predicted suitable by the models will still be inaccessible to Pseudorchis, and its occurrence will be limited to the island’s coastal zone. Of course, this has implications for the future, when the area of the GrIS is expected to decrease significantly and thus there will be new areas for colonization by plants (Chambers et al., 2022; Greve and Chambers 2022; Yang et al., 2022).
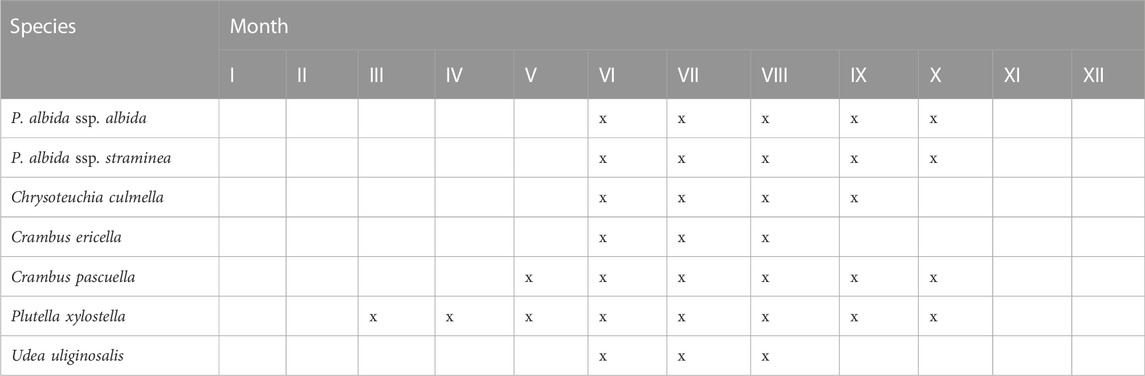
While a similar decline in the availability of a pollinator previously predicted for the Australian orchid Leporella fimbriata (Kolanowska et al., 2021a) is unlikely to affect P. albida, changes in climate will probably not limit the long-term survival of this species. According to data available in GBIF (Table 5), at the beginning of the flowering season (June–August) of both subspecies of Pseudorchis, their pollinators are active and can transfer pollen. For September and October, there are no reports of Crambus ericella and Udea uliginosalis, so late-flowering populations are unlikely to reproduce. The effect of climate change on the flowering time of orchids and activity of their pollinators is poorly known; however, previous studies indicate that global warming can lead to desynchronization and decline in the fruiting process of plants (Robbirt et al., 2014; Hutchings et al., 2018). Similar findings are reported by Tsiftsis and Djordjević (2020) for two deceptive species of the genus Ophrys, and they highlight a disruption of plant–pollinator interactions due to climate change, resulting in serious conservation consequences for these species. On the other hand, Molnár et al. (2012) reported that the phenology of nectar-rewarding orchids or short-lived species with non-Mediterranean distributions is less affected by global warming than that of autogamous or deceptive, long-lived species with mainly Mediterranean distributions. Pseudorchis albida belongs to the first group of species.
As the predicted changes in the ranges of the taxa studied differ, their future need of conservation is also likely to differ. Pseudorchis albida ssp. straminea is not threatened in the near future by changes in climate, whereas populations of P. albida ssp. albida are, especially in Central and Eastern Europe. Nevertheless, Pfeifer et al. (2010) indicated that relict areas are likely to occur in which this taxon can survive much longer than in new areas, which could be affected by various non-climate related factors. It is, therefore, best to maintain current populations in the best possible condition. Reinhammar et al. (2002) studied the population dynamics of P. albida over 6 years in two permanent plots (3 × 3 m), one mown and the other left to succession revealed that in the mown plot, the number of new individuals appearing annually was large and stable, whereas in the unmanaged plot, there was little or no recruitment. It is, therefore, important to maintain the stability of semi-natural habitats inhabited by P. albida.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
MK designed the research and collected data. AR performed statistical analyses. MK, AR, and SN defined the methodology and conducted the research, prepared figures, and wrote and reviewed the manuscript.
Acknowledgments
We are grateful to Marco Klüber, Walter Ezell on behalf of James Fowler and Amadej Trnkoczy for giving us permission to use their photographs in this paper. We are very thankful to the prof. Anthony Dixon for all his suggestions and language corrections. We thank the reviewers for all their valuable comments on the first version of the text.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.912428/full#supplementary-material
Supplementary Data Sheet S1 | Localities of P. albida used in analyses.
Supplementary Data Sheet S2 | Localities of P. albida pollinators used in analyses.
Supplementary Data Sheet S3 | Correlations between bioclimatic variables calculated using Pearson’s correlation coefficient.
Supplementary Data Sheet S4 | Overlap of suitable niches for the orchids studied and their pollinators currently and in various climate change scenarios.
Supplementary Table S1 | List of GBIF Occurrence Download used in the study.
Supplementary Table S2 | Factor coordinates of the cases based on correlation.
References
Allouche, O., Tsoar, A., and Kadmon, R. (2006). Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 43 (6), 1223–1232. doi:10.1111/j.1365-2664.2006.01214.x
Artsdatabanken (2010). Red List Database (Informasjon om rødlistede arter er nå i Artsportalen). Trondheim: Artsdatabanken.
Barve, N., Barve, V., Jimenez-Valverde, A., Lira-Noriega, A., Maher, S. P., Peterson, A. T., et al. (2011). The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Modell. 222, 1810–1819. doi:10.1016/j.ecolmodel.2011.02.011
Bateman, R. M., Hollingsworth, P. M., Preston, J., Yi-Bo, L. U. O., Pridgeon, A. M., and Chase, M. W. (2003). Molecular phylogenetics and evolution of orchidinae and selected habenariinae (orchidaceae). Bot. J. Linn. Soc. 142 (1), 1–40. doi:10.1046/j.1095-8339.2003.00157.x
Bateman, R. M., Rudall, P. J., and Denholm, I. (2017). Morphometric comparison of British Pseudorchis albida with Icelandic P. straminea (orchidaceae: Orchidinae). New J. Bot. 7 (2-3), 78–93. doi:10.1080/20423489.2017.1408191
Bilz, M., Kell, S. P., Maxted, N., and Lansdown, R. V. (2011). European red list of vascular plants. Luxembourg: Publications Office of the European Union.
Bournérias, M., and Prat, D. (Editors) (2005). Les orchidées de France, Belgique et Luxembourg. 2nd ed. (Biotope: Mezé).
Brown, J. L. (2014). SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic, and species distribution model analyses. Methods Ecol. Evol. 5 (7), 694–700. doi:10.1111/2041-210x.12200
Cannone, N., and Pignatti, S. (2014). Ecological responses of plant species and communities to climate warming: Upward shift or range filling processes? Clim. Change 123 (2), 201–214. doi:10.1007/s10584-014-1065-8
Chambers, C., Greve, R., Obase, T., Saito, F., and Abe-Ouchi, A. (2022). Mass loss of the Antarctic ice sheet until the year 3000 under a sustained late-21st-century climate. J. Glaciol. 68 (269), 605–617. doi:10.1017/jog.2021.124
Cheffings, C., and Farrell, L. (2005). The vascular plant red data list for Great Britain. Species Status 7. Peterborough: Joint Nature Conservation Committee.
Claessens, J., and Kleynen, J. (2011). The flower of the European orchid. Form and function. Netherlands: Jean Claessens and Jacques Kleynen Publishers.
Curtis, T. G. F., and McGough, H. N. (1988). The Irish red data book. 1. Vascular plants. Dublin: The Stationery Office.
Damgaard, C., Moeslund, J. E., and Wind, P. (2020). Changes in the abundance of Danish orchids over the past 30 years. Diversity 12 (6), 244. doi:10.3390/d12060244
Danihelka, J., Chrtek, J., and Kaplan, Z. (2012). Checklist of vascular plants of the Czech Republic. Preslia 84, 647–811.
Duffy, K. J., Fay, M. F., Smith, R. J., and Stout, J. C. (2011). Population genetics and conservation of the small white orchid, Pseudorchis albida, in Ireland. Biol. Environ. 111B (2), 73–81. doi:10.1353/bae.2011.0004
Duffy, K. J., Kingston, N. E., Sayers, B. A., Roberts, D. L., and Stout, J. C. (2009). Inferring national and regional declines of rare orchid species with probabilistic models. Conserv. Biol. 23 (1), 184–195. doi:10.1111/j.1523-1739.2008.01064.x
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., and Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57. doi:10.1111/j.1472-4642.2010.00725.x
Evangelista, P. H., Kumar, S., Stohlgren, T. J., Jarnevich, C. S., Crall, A. W., Norman, J. B., et al. (2008). Modelling invasion for a habitat generalist and a specialist plant species. Divers. Distrib. 14, 808–817. doi:10.1111/j.1472–4642.2008.00486.x
Fick, S. E., and Hijmans, R. J. (2017). WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37 (12), 4302–4315. doi:10.1002/joc.5086
Forbes, R. S., and Northridge, R. H. (2012). The flora of county fermanagh. Holywood: National Museums Northern Ireland.
Freeman, B. G., Lee-Yaw, J. A., Sunday, J. M., and Hargreaves, A. L. (2018). Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Glob. Ecol. Biogeogr. 27 (11), 1268–1276. doi:10.1111/geb.12774
Gärdenfors, U. (2010). Rödlistade arter i sverige - the 2010 red list of Swedish species. Uppsala: ArtDatabanken, SLU.
Geppert, C., Perazza, G., Wilson, R. J., Bertolli, A., Prosser, F., Melchiori, G., et al. (2020). Consistent population declines but idiosyncratic range shifts in Alpine orchids under global change. Nat. Commun. 11 (1), 5835. doi:10.1038/s41467-020-19680-2
Greve, R., and Chambers, C. (2022). Mass loss of the Greenland ice sheet until the year 3000 under a sustained late-21st-century climate. J. Glaciol. 68 (269), 618–624. doi:10.1017/jog.2022.9
Holland, J. P., Pollock, M. L., and Waterhouse, A. (2008). From over-grazing to under-grazing: Are we going from one extreme to another? Aspects Appl. Biol. 85, 25–30.
Holub, J., and Procházka, F. (2000). Red List of the Flora of the Czech Republic (state in the year 2000. Červený seznam květeny České repub. 72, 187–230.
Hutchings, M. J., Robbirt, K. M., Roberts, D. L., and Davy, A. J. (2018). Vulnerability of a specialized pollination mechanism to climate change revealed by a 356-year analysis. Botanical J. Linn. Soc. 186 (4), 498–509. doi:10.1093/botlinnean/box086
Jeřábková, K. (2006). Ecological demands and optimal management of Pseudorchis albida. Master thesis in Czech. Prague: Czech University of Life Sciences.
Jersáková, J., Malinová, T., Jeřábková, K., and Dötterl, S. (2011). Biological flora of the British isles: Pseudorchis albida (L.) Á. & D. Löve. J.f Ecol. 99 (5), 1282–1298. doi:10.1111/j.1365-2745.2011.01868.x
Kaźmierczakowa, R., Bloch-Orłowska, J., Celka, Z., Cwener, A., Dajdok, Z., Michalska-Hejduk, D., et al. (2016). Polska czerwona lista paprotników i roślin kwiatowych. Polish red list of pteridophytes and flowering plants. Kraków: Instytut Ochrony Przyrody Polskiej Akademii Nauk.
Kelly, A. E., and Goulden, M. L. (2008). Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. U. S. A. 105, 11823–11826. doi:10.1073/pnas.0802891105
Klein, E. (2000). Pseudorchis albida subsp. tricuspis (Beck) Klein stat. nov., eine weitgehend übersehene, calcicole, alpisch-boreale Sippe (Orchidaceae – orchideae). Phyton 40, 141–159.
Kolanowska, M., Kras, M., Lipińska, M., Mystkowska, K., Szlachetko, D. L., and Naczk, A. M. (2017). Global warming not so harmful for all plants-response of holomycotrophic orchid species for the future climate change. Sci. Rep. 7 (1), 12704–12713. doi:10.1038/s41598-017-13088-7
Kolanowska, M., Michalska, E., and Konowalik, K. (2021a). The impact of global warming on the niches and pollinator availability of sexually deceptive orchid with a single pollen vector. Sci. Total Environ. 795, 148850. doi:10.1016/j.scitotenv.2021.148850
Kolanowska, M., Rewicz, A., and Nowak, S. (2021b). Significant habitat loss of the black vanilla orchid (Nigritella nigra sl, Orchidaceae) and shifts in its pollinators availability as results of global warming. Glob. Ecol. Conservation 27, e01560. doi:10.1016/j.gecco.2021.e01560
Kremen, C. A., Cameron, A., Moilanen, S. J., Phillips, C. D., Thomas, H., Beentje, J., et al. (2008). Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 320, 222–226. doi:10.1126/science.1155193
Kricsfalusy, V., Budnikov, G., and Lesio, I. (2010). Rare and protected plant species of the uzhansky national nature park (transcarpathia, Ukraine), taiszia -. J. Bot. Košice 20, 115–125.
Kricsfalusy, V., Budnikov, G., and Mihaly, A. (1999). Red list of transcarpathia: Threatened plant species and plant communities. Uzhgorod: Zakarpattya Publishing.
Lamprecht, A., Semenchuk, P. R., Steinbauer, K., Winkler, M., and Pauli, H. (2018). Climate change leads to accelerated transformation of high-elevation vegetation in the central Alps. New Phytol. 220, 447–459. doi:10.1111/nph.15290
Ludwig, G., and Schnittler, M. (1996). Red list of threatened plants in Germany (rote liste gefährdeter pflanzen deutschlands). Bonn: Bundesamt für Naturschutz.
Mason, S. J., and Graham, N. E. (2002). Areas beneath the relative operating characteristics (ROC) and relative operating levels (ROL) curves statistical significance and interpretation. Q. J. R. Meteorol. Soc. 128, 2145–2166. doi:10.1256/003590002320603584
Molnár, V. A., Tökölyi, J., Végvári, Z., Sramkó, G., Sulyok, J., and Barta, Z. (2012). Pollination mode predicts phenological response to climate change in terrestrial orchids: A case study from central Europe. J. Ecol. 100, 1141–1152. doi:10.1111/j.1365-2745.2012.02003.x
Moore, D. M. (1980). in Pseudorchis. Flora europaea. Editors T. G. Tutin, V. H. Heywood, N. A. Burges, D. M. Moore, D. H. Valentine, S. M. Walterset al. (Cambridge, U.K.: Cambridge University Press), 332.
Muntjewerf, L., Petrini, M., Vizcaino, M., Ernani da Silva, C., Sellevold, R., Scherrenberg, M. D. W., et al. (2020). Greenland Ice Sheet contribution to 21st century sea level rise as simulated by the coupled CESM2.1-CISM2.1. Geophys. Res. Lett. 47, e2019GL086836. doi:10.1029/2019GL086836
Naturvårdsverket, (2022). Fridlysta orkidéer. Available at https://www.naturvardsverket.se/en/topics/species-protection/protected-species/(Access March 02, 2022).
O’Neill, B. C., Kriegler, E., Riahi, K., Ebi, K. L., Hallegatte, S., Carter, T. R., et al. (2014). A new scenario framework for climate change research: The concept of shared socioeconomic pathways. Clim. change 122 (3), 387–400. doi:10.1007/s10584-013-0905-2
Opdam, P., and Wascher, D. (2004). Climate change meets habitat fragmentation: Linking landscape and biogeographical scale levels in research and conservation. Biol. Conserv. 117, 285–297. doi:10.1016/j.biocon.2003.12.008
Pearman, D. A., Preston, C. D., Rothero, G. P., and Walker, K. J. (2008). The flora of rum: An atlantic island reserve. Cornwall, UK: Truro.
Perazza, G. (2016). in Orchidee d’ Italia. Editors P. Grünanger, and B. Barsella 2nd ed. (Milan: GIROS/Castello), 80–81.
Petrova, A., and Vladimirov, V. (2009). Red list of Bulgarian vascular plants. Phytol. Balc. 15 (1), 63–94.
Pfeifer, M., Passalacqua, N. G., Bartram, S., Schatz, B., Croce, A., Carey, P. D., et al. (2010). Conservation priorities differ at opposing species borders of a European orchid. Biol. Conserv. 143 (9), 2207–2220. doi:10.1016/j.biocon.2010.06.005
Phillips, S. J., Anderson, R., and Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259. doi:10.1016/j.ecolmodel.2005.03.026
Phillips, S. J., Dudík, M., and Schapire, R. E. (2004). “A maximum entropy approach to species distribution modeling,” in ICML '04. Proceedings of the twenty-first international conference on Machine learning (New York: ACM).
Rankou, H. (2011). Pseudorchis albida. The IUCN Red List of Threatened Species 2011: e.T176007A7169757. Accessed on 02 March 2022.
Reinhammar, L. G. (1995). Evidence for two distinctive species of Pseudorchis (orchidaceae) in Scandinavia. Nord. J. Bot. 15 (5), 469–481. doi:10.1111/j.1756-1051.1995.tb00180.x
Reinhammar, L. G., and Hedren, M. (1998). Allozyme differentiation between lowland and alpine populations of Pseudorchis albida s. lat.(Orchidaceae) in Sweden. Nordic J. Bot. 18 (1), 7–14. doi:10.1111/j.1756-1051.1998.tb01090.x
Reinhammar, L. G., Olsson, E. G., and Sormerland, E. (2002). Conservation biology of an endangered grassland plant species, Pseudorchis albida, with some references to the closely related alpine P. straminea (Orchideceae). Botanical J. Linn. Soc. 139, 47–66. doi:10.1046/j.1095-8339.2002.00041.x
Reinhammar, L. G. (1998). Systematics of Pseudorchis albida s.1. (Orchidaceae) in Europe and north America. Botanical J. Linn. Soc. 126, 363–382. doi:10.1111/j.1095-8339.1998.tb01388.x
Robbirt, K. M., Roberts, D. L., Hutchings, M. J., and Davy, A. J. (2014). Potential disruption of pollination in a sexually deceptive orchid by climatic change. Curr. Biol. 24 (23), 2845–2849. doi:10.1016/j.cub.2014.10.033
Sârbu, A., Janauer, G. A., Exler, N., Sarbu, I., and Anastasiu, P. (2020). The potential sensitivity to climate change of selected endangered and important Natura 2000 Habitats and plants from Bucegi Natural Park, Romania. Not. Bot. Horti Agrobot. Cluj. Napoca. 48 (1), 456–479. doi:10.15835/nbha48111756
StatSoft Inc (2011). STATISTICA (data analysis software system), version 13.1. Available at www.statsoft.com.
subordinate agency (2022). Norwegian biodiversity information centre. https://www.biodiversity.no/Pages/166308/Publications (Access March, 2022).02.
Troia, M. J., Kaz, A. L., Niemeyer, J. C., and Giam, X. (2019). Species traits and reduced habitat suitability limit efficacy of climate change refugia in streams. Nat. Ecol. Evol. 3, 1321–1330. doi:10.1038/s41559-019-0970-7
Tsiftsis, S., and Antonopoulos, Z. (2011). Pseudorchis albida: An enigmatic orchid for the Greek flora. J. Eur. Orch. 43 (4), 795–806.
Tsiftsis, S., and Djordjević, V. (2020). Modelling sexually deceptive orchid species distributions under future climates: The importance of plant-pollinator interactions. Sci. Rep. 10, 10623. doi:10.1038/s41598-020-67491-8
Tsiftsis, S., Djordjević, V., and Tsiripidis, I. (2019). Neottia cordata (orchidaceae) at its southernmost distribution border in Europe: Threat status and effectiveness of natura 2000 network for its conservation. J. Nat. Conserv. 48, 27–35. doi:10.1016/j.jnc.2019.01.006
Turis, P., Kliment, J., Feráková, V., Dítě, D., Eliáš, P., Hrivnák, R., et al. (2014). Red List of vascular plants of the Carpathian part of Slovakia. Thaiszia J. Bot. 24 (1), 35–87.
van der Meer, S., Jacquemyn, H., Carey, P. D., and Jongejans, E. (2016). Recent range expansion of a terrestrial orchid corresponds with climate-driven variation in its population dynamics. Oecologia 181, 435–448. doi:10.1007/s00442-016-3592-7
Yang, H., Krebs-Kanzow, U., Kleiner, T., Sidorenko, D., Rodehacke, C. B., Shi, X., et al. (2022). Impact of paleoclimate on present and future evolution of the Greenland Ice Sheet. PLoS ONE 17 (1), e0259816. doi:10.1371/journal.pone.0259816
Keywords: bioclimatic preferences, habitat loss, global warming, pollinators, Pseudorchis albida ssp. tricuspis, Pseudorchis albida ssp. straminea
Citation: Kolanowska M, Nowak S and Rewicz A (2022) Will Greenland be the last refuge for the continental European small-white orchid?Niche modeling of future distribution of Pseudorchis albida. Front. Environ. Sci. 10:912428. doi: 10.3389/fenvs.2022.912428
Received: 04 April 2022; Accepted: 07 December 2022;
Published: 28 December 2022.
Edited by:
Dennis Whigham, Smithsonian Institution, United StatesReviewed by:
Vladan Djordjević, University of Belgrade, SerbiaOle Bennike, Geological Survey of Denmark and Greenland, Denmark
Jasmina Šinžar-Sekulić, University of Belgrade, Serbia
Copyright © 2022 Kolanowska, Nowak and Rewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sławomir Nowak, slawomir.nowak@ug.edu.pl
 Marta Kolanowska
Marta Kolanowska