- 1Dept of Environ. Eng. Yeungnam University, Gyungsan, Korea
- 2Groundwater Environment Research Center, Korea Institute of Geoscience and Mineral Resources (KIGAM), Daejeon, Korea
The bioluminescence activity and biokinetics of a recombinant Pseudomonas putida mt-2 were investigated in the presence of various inducers: three chlorotoluenes (CTs) and two nitrotoluenes (NTs). The effects of binary mixtures (40 combinations) of eleven inducers on the bioluminescence activity were also studied. Different responses and toxicities were observed depending on the type of inducers and concentrations. The intensity of the bioluminescent response at 1 mM of individual inducers was in the following order: o-CT > p-CT > m-CT > o-NT and p-NT. The biokinetics calculated based on the bioluminescence activity was in the range of 0.109–8.417 mM for the half-saturation constant (Ks) and 6.083–19.880 h−1 for the maximum SBR (µmax). In the case of binary mixtures, the observed bioluminescence was 4–810% (avg. 80.4%) of the expected bioluminescence intensity depending on the combinations (0.0001 < p < 0.5226). Among these binary mixture combinations, 27 out of 40 combinations indicated antagonistic effects (3–83% of expected activity; p < 0.0391) under the exposure of inducer mixtures. Overall, this study demonstrated that the proper biomonitoring for specific chemicals could be established by considering the characteristics of mixture pollutants for their possible usages as a preliminary rapid and field analytical bio-tool.
Introduction
Many chemicals in petroleum hydrocarbons and their products are one of the most common and important contaminants in soil and groundwater systems worldwide (Onwurah et al., 2007; Truskewycz et al., 2019). Among many technologies developed to remediate contaminated environments, biotechnologies, such as bioremediation and biomonitoring, based on various microbial mechanisms for adapting to and catabolizing contaminants, have been known to be effective (Tang et al., 2012; Bak et al., 2015). Microbial techniques are generally simple and cost effective compared to traditional chemicals and physical techniques. Several microbial processes based on the whole-cell activity, specific enzyme activity, and intact and recombinant gene expression can be adopted as rapid and straightforward on-site bioassessments for the prebioassays of contaminated sites (Choi and Gu, 2003; Velazquez et al., 2006).
Genetically engineered microorganisms (GEMs) have attracted interest for the biomonitoring and bioremediation of contaminated sites because of the understanding of genes and their regulatory systems, such as bioluminescent whole-cell-based biosensors (Ford et al., 1999; Burlage, 2002; Voon et al., 2022). For example, the recombination of light-producing lux-gene encoding luciferase found in Aliivibrio fischeri has attracted considerable interest (Mirjani et al., 2021). The lux genes of Aliivibrio fischeri (luxCDABE) with a promoter of the TOL plasmid are effective bioreporters for real-time and environmental monitoring assays based on the bacterial production of detectable bioluminescence (Inouye et al., 1987; Harayama and Rekik, 1990; Burlage, 2002). The TOL plasmid in bacteria can transform toluene analogs (gasoline components) into nontoxic metabolites and contains two promoters under the process of two regulatory genes (Ps and Pr), which produce transcriptional activator proteins on the upper and lower (meta) pathways operons. Under suitable conditions, a recombinant strain can produce luminescent light that can be correlated directly with the bioavailable specific chemical (Kong et al., 2004). This type of bio-tool can give important information about the bioavailability and biodegradation of chemicals in the environment as valuable alternatives to chemical analytical methods for in situ or on-site monitoring (Kong, 2006).
Biomonitoring studies for contaminated sites have mostly focused on individual chemicals under laboratory and controlled conditions (Pavlaki et al., 2011; Kong et al., 2021). On the other hand, the results of investigations on individual chemicals alone cannot provide complete data for biomonitoring because the contaminated sites are generally exposed to chemical mixtures. The interactive effects of mixtures rather than only on individual chemicals should reflect the actual conditions and characteristics of contaminated sites. Therefore, a study of the effects of mixtures on the bioluminescence activity using the bioluminescence-producing recombinant strain is strongly recommended for the biomonitoring of a specific inducer contaminant. The effects of inducer mixtures on the activity of bioluminescence production may be similar (additive effect) or different significantly (antagonistic or synergistic effects) to the theoretically expected effects calculated based on the exposure of individual inducer (Norwood et al., 2003). Researchers evaluated the interactive effects of chemicals using various models, such as independent action (response addition), concentration addition, a mathematical model based on the theory of probabilities (Kungolos et al., 2009), or by the toxic unit (TU) approach (Olmstead and LeBlanc, 2005; Horvat et al., 2007; Ferreira et al., 2008). Proper model adoption is based on the characteristics of chemical action on metabolic processes (An, 2004; Pavlaki et al., 2011).
This study adopted the previously constructed recombinant strain of Pseudomonas putida mt-2 (called KG1206), containing Pm-lux fused plasmid (the lux-gene was expressed under the control of promoter Pm of the TOL plasmid). The objectives of this study were as follows: 1) to evaluate the characteristics of the bioluminescence activity and biokinetics exposed to single inducers of three chlorotoluenes (CTs) and two nitrotoluenes (NTs); 2) to assess the effects of binary mixtures with eleven single inducers for the recombinant strain KG1206.
Materials and methods
Characteristics of the recombinant strain
The recombinant Pseudomonas putida mt-2 KG1206 contains two types of plasmid: the intact TOL and Pm-lux fusion plasmids. The recombinant plasmid is responsible for producing the bioluminescence indirectly and directly in the presence of toluene analogs and their metabolites, respectively (Harayama and Rekik, 1990; Burlage et al., 1994; Kong et al., 2004). The strain was maintained and stored at −70°C until needed. The cultures were grown in Luria–Bertanika (LB; with 50 mg/L of kanamycin) medium at 27°C with shaking (130 rpm). The culture was grown to an optical density (OD600) of approximately 0.6 and diluted equally with minimal salt medium for the bioluminescence test (Ko and Kong 2017). The inducers, o-, m-, and p-chlorotoluene (CT) and o- and m-nitrotoluene (NT) were used to determine the bioluminescence characteristics. In addition, six more inducers (toluene, xylene isomers, m-toluate, and benzoate) were adopted for the binary mixture investigation. All chemicals were obtained from Sigma-Aldrich Chemical (St. Louis, MO, United States), except o-NT (Alfa Aesar, Millstone, United States). During incubation, the bioluminescence intensity was measured using a Turner 20/20 luminometer (Turner, Sunnyvale, CA United States) and expressed in relative light units (RLUs). The maximum detection limit was 9999 RLU. Figure 1 presents a schematic diagram of these lux fusions, which were modified from previous reports (Kong et al., 2004; Kong, 2006).
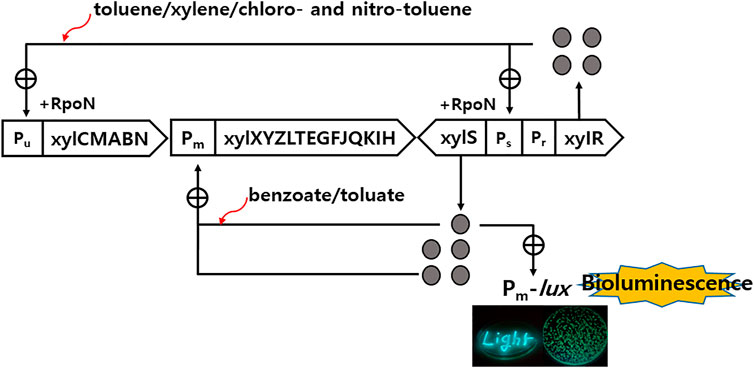
FIGURE 1. Schematic diagram of the regulatory systems of TOL catabolic and recombinant Pm-lux genes (⊕: positive control; ●: regulatory protein) (modified from Kong et al., 2004; Kong 2006; Kong et al., 2021).
Bioluminescence characteristics and biokinetics
A determined concentration of the inducer chemical in the range of 0.1–10 mM (0.1, 0.5, 1, 3, 5, 8, and 10 mM) was added to culture vials and sealed with a Teflon septum to prevent the loss of the volatile organic inducers. The culture was incubated at 27°C with shaking at 130 rpm. The biokinetics was calculated based on the bioluminescence production at the exponential phase during the incubation periods. The specific bioluminescence-producing rate (SBR; μ), which could be correlated with the growth rate, was calculated based on exposure to different concentration ranges of inducers and their corresponding bioluminescence activity. Based on the SBR of various concentration exposures, the maximum SBR (μmax) and the half-saturated constant (Ks) were calculated using a Lineweaver–Burk equation, which was derived from the Monod equation.
Effects of inducer mixtures on the activity of the recombinant strain
Based on the preliminary test, one concentration of the five inducers (1 mM of three CTs, 10 mM o-NT, and 3 mM p-NT) and six previously tested inducers (0.5 mM m-toluate, 1 mM of toluene, benzoate, and three xylene isomers) were equally mixed with 40 combinations of binary mixtures. The effects of binary mixtures were examined by measuring the bioluminescence intensity after transferring a bacterial culture (9.8 ml) into the serum vials with predetermined concentrations of two compounds (each 0.1 ml). During the 2.5 h incubation periods (130 rpm shaking at 27°C), bioluminescence production was measured every 30 min, and the total bioluminescence (sum of bioluminescence produced at 0.5, 1.0, 1.5, 2.0, and 2.5 h) was used to compare the effect of a binary mixture. All tests were performed in triplicate. The mixture effects were examined by comparing the P(O) (observed bioluminescence intensity) with the P(E) (theoretically expected bioluminescence intensity), which were determined by combining both individual test results. In this mixture test, the theoretically expected bioluminescence of the binary mixture was evaluated using a concentration addition model (Ferreira et al., 2008).
P(E) = P1 + P2, where P1 and P2 are the bioluminescence intensity by inducer 1 and 2, respectively.
The test results of binary mixtures were determined to be synergistic, antagonistic, or additive effects based on the differences between the observed and theoretically predicted bioluminescence activity. The significance of the difference between experimental groups was determined based on the calculated p-values using Student’s t-test (GraphPad. Available online: http://www.graphpad.com).
Results and discussion
The recombinant strain, KG1206, produces bioluminescence during growth at the exposure of toluene analogs and their catabolic intermediates. These inducers directly or indirectly activate the inactive regulatory protein of the XylS or XylR, which controls the Pm promoter positively, producing bioluminescence from the Pm-lux recombinant gene of the plasmid. In a previous study, several inducers (toluene, xylene isomers, methylbenzylalcohol, benzoate, and m-toluate) over a wide concentration range were tested to analyze the bioluminescence activity over several hours (Kong et al., 2004; Kong, 2014). In line with previous studies, the bioluminescence characteristics and biokinetics with inducers of the CTs and NTs were investigated. Patterns of the bioluminescence response of recombinant strain were also observed in the presence of binary inducer mixtures.
Response of the Pm-lux recombinant gene to CTs and NTs
The concentration dependence of the bioluminescence activity for three CTs and two NTs was tested over time using the recombinant strain KG1206 at concentration ranges of 0.1–10 mM. Overall, exposure to CTs showed greater bioluminescence activity than exposure to NTs. Different bioluminescence activities were also observed between each isomer. Moreover, the concentration for the maximum bioluminescence activity and inhibition differed according to the type of inducer. Among bioluminescence activities with various inducers tested, Figure 2 shows two representative exposure results with m-CT and p-NT. The maximum bioluminescence activity at each exposure concentration was mostly induced after 2 h incubation and lasted approximately for 4 h, similar to other inducers investigated previously (Kong et al., 2004; Kong, 2014), but this is dependent on the type of inducer and its initial concentration (Figure 2). For example, maximum bioluminescence activity appeared at 2 h of 8 mM m-CT exposure, showing a 960 ± 95 RLU activity. In the case of p-NT exposure, lower bioluminescence activity was observed compared to CT exposure, and the maximum activity appeared mostly after 2 h incubation with a prolonged increase in bioluminescence activity (Figure 2B). At the end of the 4 h incubation period, the bioluminescence activity for CTs was almost extinguished, and very low bioluminescence (less than 30 RLU) was observed compared to maximum activity. In the case of NTs, the bioluminescence activities gradually increased with the incubation time (Figure 2B). The concentration at which bioluminescence inhibition began differed for each isomer and inducers. In the presence of CTs, bioluminescence inhibition appeared over 3 mM, above 5 mM, and 5 mM for o-, m-, and p-CT exposure, respectively. The degree of bioluminescence activity was compared based on the maximum bioluminescence activity of each inducer (Figure 3). The response of o- and m-CT on the bioluminescence activity of KG1206 was weaker than that for p-CT. Among the three CT isomers, the maximum activity was observed at 3 mM p-CT, 1 mM o-CT, and 8 mM m-CT, showing >9999 RLU, 3617 ± 333 RLU, and 960 ± 95 RLU, respectively. The magnitude of maximum bioluminescence and concentration of the tested inducers appeared in the following order (Figure 3): 3 mM p-CT (>9999 RLU) > 1 mM o-CT (3617 RLU) > 8 mM m-CT (960 RLU) > 10 mM o-NT (165 RLU) and 5 mM p-NT (177 RLU).
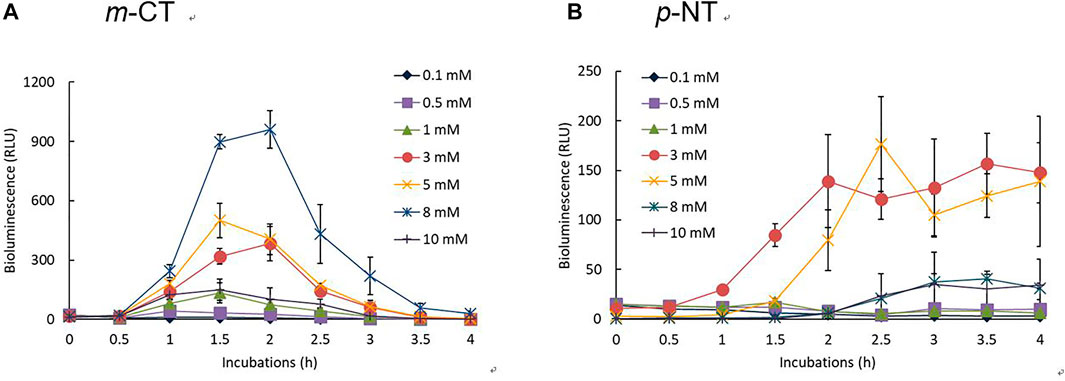
FIGURE 2. Two representative results of the profiles of the bioluminescence activities of KG1206 with different concentrations of m-CT (A) and p-NT (B) over time.
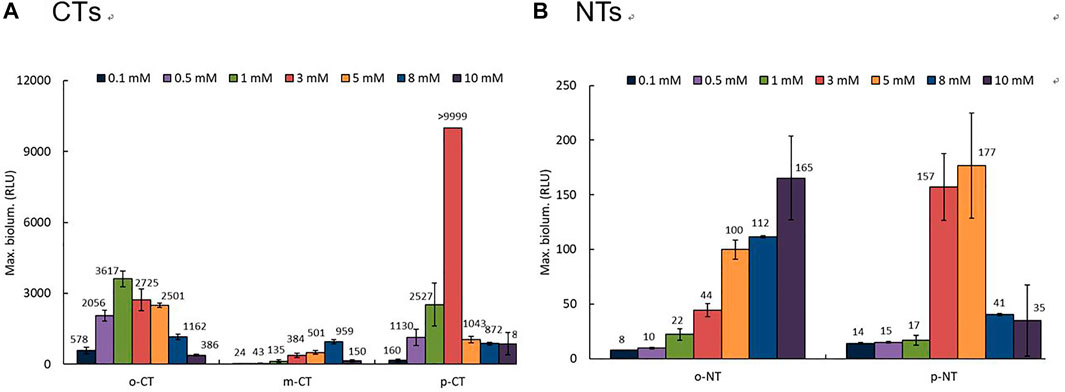
FIGURE 3. Profiles of maximum bioluminescence intensity of KG1206 at the exposure to different concentrations of inducer: (A) three CTs and (B) two NTs.
The tested inducers of CTs and NTs could activate the XylS protein indirectly. In addition to controlling the upper pathway promoter (Pu), a XylR regulatory gene also induces expression from the xylS regulatory promoter (Ps). When the concentration of XylS protein was overproduced through XylR induction in the presence of inducers, the amount of active proteins would increase and become sufficient to initiate the transcription of the Pm promoter in the absence of the activator of XylS protein (Inouye et al., 1987; Holtel et al., 1994). In this investigation, the strong expression from the Pm promoter was also revealed in the presence of indirect upper pathway inducers (CTs and NTs). The different responses of isomers and types of inducers (e.g., CT and NT) highlight the specificity and the different binding affinity on an inactive XylR regulatory protein.
Comparisons of the characteristics of biokinetics and bioluminescence activity
The bioluminescence characteristics of KG1206 on each inducer were examined by determining the growth rate characteristics based on the bioluminescence activity at the exponential phase in the presence of an individual inducer. Figure 4 shows two representative results of the KG1206 recombinant strain on changes in “μ” in terms of the concentration inducer (p-CT and o-NT). The strains exposed to CTs showed significantly higher “μ” than those exposed to the inducer NTs. For example, SBRs of 7.99 h−1 and 1.14 h−1 were observed at 1 mM of p-CT and o-NT exposure, respectively (Figure 4). Under the tested concentration range, the observed SBR of KG1206 was in the range of 2.995–8.514, 4.268–8.464, and 2.452–7.980 h−1 for o-, m-, and p-CT mM, respectively; very low bioluminescence activity (<500 RLU) was observed for NTs exposure and SBRs of 0–2.477 and 0.233–5.345 h−1 for o- and p-NT under tested conditions, respectively (Table 1). The Ks measures the affinity for each substrate, and a low Ks value indicates a high affinity for substrate compared to a high Ks value. Based on the “μ” values calculated from the bioluminescence activity at various initial inducer concentrations, the Ks and maximum μ (μmax) were calculated using the Lineweaver–Bulk equation (Table 1). Among the inducers tested, m-CT exposure showed the highest bioluminescence activity with the lowest Ks (equal to 0.1086 mM; corresponding to the highest affinity), whereas p-NT exposure showed the lowest bioluminescence activity with the highest Ks value (6.191 mM). The Ks values were in the following order: m-CT (0.1086 mM) > o-CT (0.2208 mM) > p-CT (0.2769 mM) > o-NT (5.461 mM) > p-NT (6.191 mM). Compared to the Ks values, no significant differences were observed for μmax, showing values in the range of 6.083–10.661 h−1 (Table 1).
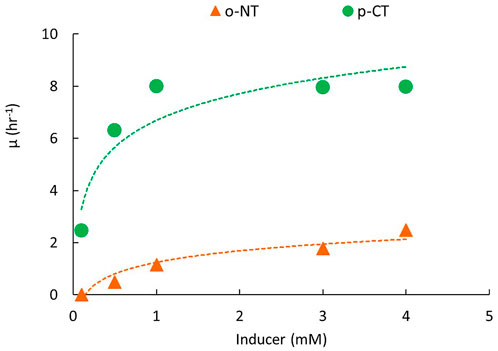
FIGURE 4. Representative results of changes in the specific bioluminescence activity of strain KG1206 associated with changes in the concentrations of p-CT and o-NT.

TABLE 1. Summary of the values of half-saturated constant (Ks) and maximum specific bioluminescence producing rate (SBR; µmax) for the KG1206 recombinant strain.
A wide range of biokinetic values is generally reported depending on the types of organisms and chemicals. According to the researchers, Ks and μmax of toluene in the genus Pseudomonas were reported to be 0.003–0.823 mM and 0.035–0.86 h−1, respectively (Reardon et al., 2000; Maliyekkal et al., 2004; Kim et al., 2005). In this investigation, Ks showed similar or slightly higher values to the reported results, but μmax based on the bioluminescence activity was significantly higher than the reported values (Table 1). The high SBR value may be due to the bioluminescence process that responds quickly upon exposure to the inducer chemicals, compared to cell growth.
Effects of binary mixture inducers on the bioluminescence activity
The effects of binary mixtures on the bioluminescence activity were examined by determining one concentration of each inducer (direct and indirect) based on the previous results of the effects of various concentration ranges of single inducers (0.5–10 mM). Overall, 40 combinations of binary mixtures of eleven single inducers (1 mM toluene, benzoate, three xylenes, and three CT isomers; 0.5 mM m-toluate, 10 mM o-NT; 3 mM p-NT) were exposed for 2.5 h to examine the mixture effects on the bioluminescence activity. For example, among the 40 mixture combinations tested, Figure 5 shows the bioluminescence activities of the individual inducers and binary mixtures of m-toluate with CTs and NTs during 2.5 h incubation periods. Maximum bioluminescence activity mostly appeared at 1.5 h or 2 h incubation for both sets of single and binary mixtures. On the other hand, under a mixture of m-toluate and p-NT, the observable bioluminescence appeared at 1.5 h (925 RLU), which continued to 8986 RLU at 2.5 h incubation. At 1.5 h incubation, the bioluminescence activities of a single inducer were in the range of 1.3 RLU (p-NT) to 9223 RLU (m-toluate), whereas mixtures in the range of 925 RLU (m-toluate and p-NT) to >9999 RLU (m-toluate and o-NT). Inducer m-toluate and m-CT produced 9225 RLU and 1066 RLU at 1.5 h, respectively, while a binary mixture produced 6049 RLU.
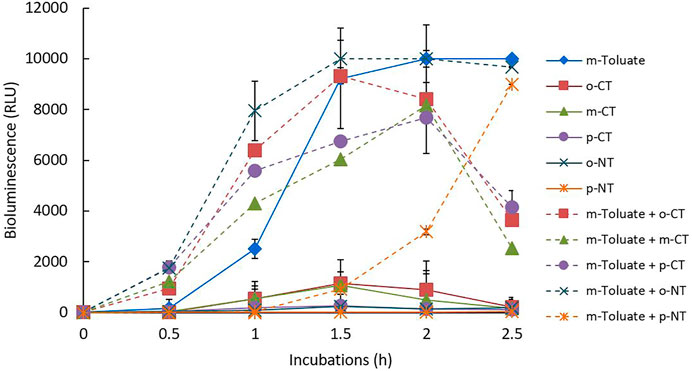
FIGURE 5. Representative results of the bioluminescence activities of individual inducers and binary mixtures of m-toluate, CTs, and NTs among the tested 40 mixture combinations during 2.5 h incubation periods; individual inducer (solid line) and mixtures (dotted line).
As shown in the example, all results of the tested binary mixture exhibited one of the three modes of effects, such as antagonistic (less than additive; weaker than those of single inducers), synergistic (greater than additive; stronger than those of single inducers), and additive effects (Figure 6A). The mode of the mixture effects was evaluated by comparing the observed and expected bioluminescence of each combination based on the total bioluminescence production during the 2.5 h incubation period (Figure 6A). The expected bioluminescence was determined using the concentration addition model, in which the inducer concentrations of the binary mixture were added to predict the theoretical effects because of similar bioluminescence-producing processes of each inducer (Norwood et al., 2003). The observed total bioluminescence ranged from 42 RLU (mixture of p-NT and o-xyl) to 43,375 RLU (mixture of m-CT and benzoate). In comparison, the expected total bioluminescence ranged from 1,166 RLU (mixture of p-NT and p-xylene) to 34,192 RLU (mixture of m-CT and m-toluate) (Figure 6A). Overall, the observed bioluminescence appeared in the range of 4–810% (average 80.4%) of the expected bioluminescence intensity depending on the combinations. Regarding Student’s t-test, statistically significant differences were observed between P(E) and P(O) in all the tested combinations (p-values ranging from 0.0001 to 0.5226). Among these binary mixture combinations, 27 out of 40 combinations indicated antagonistic activity, ranging from 3% to 83% of the expected activity (0.0001 < p < 0.0391). Among the 40 combinations tested, synergistic and additive activities were observed with seven and six combinations, showing in the range of 143–811% (avg. 300%) (0.0001 < p < 0.0037) and 38–146% (avg. 96%) (0.0643 < p < 0.5226) of expected activity, respectively. This suggests that the effects of binary mixtures of inducers on the bioluminescence activity may have antagonistic effects because of various characteristics on the environmental conditions and type of inducers. The regression coefficient (R2) of the correlation relationship between P(O) and P(E) was 0.7704 (Figure 6B). The P(O)-to-P(E) ratios were in the range of 0.034–8.109 (avg. 0.804; p = 0.0001–0.5226) under the tested concentration ranges. However, four out of five binary mixtures with the direct inducer benzoate showed synergistic activity, and the one remaining combination showed an additive activity. In the case of binary mixture with p-NT, nine out of ten combinations exhibited an antagonistic activity; the remaining combination showed an additive activity. Different test organisms showed different modes of toxic action in previous studies. Mainly synergistic (67%) and additive (67%) effects have been observed in bacterial bioluminescence and algal growth tests, respectively, whereas both additive (50%) and synergistic (47%) effects on seed germination have been observed (Ko et al., 2017, 2018). As shown in these results, the effects of inducer mixtures on the bioluminescence activities of recombinant strains can be affected by many factors, such as the conformational changes of the related proteins and different binding affinities of inducers. In general, the activity of bioluminescence production of Pm-lux recombinant strain can be affected by the binding affinities of the inducers to inactive proteins, as well as by other factors with the presence of toxic chemicals, cell growth status, and membrane-perturbing organic solvents (Bundy et al., 2003; Trogl et al., 2010). All these factors affecting the bioluminescence activity should be considered to evaluate the effects of the mixture properly, particularly based on quantifying the expressed protein levels due to the different levels of active processes.
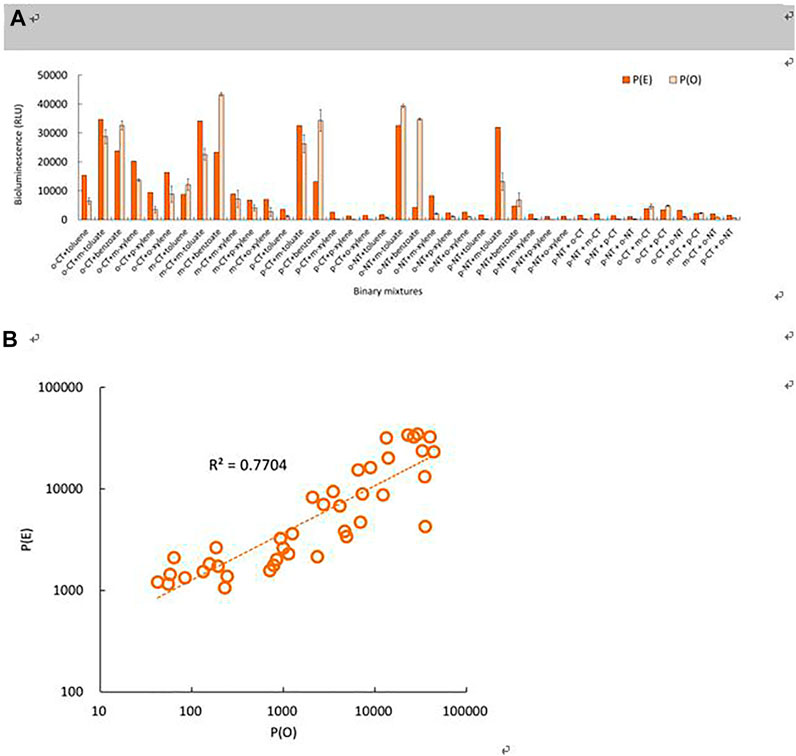
FIGURE 6. Comparisons of the P(E) and P(O) of the binary mixtures of inducers based on the total bioluminescence produced during 2.5 h incubation periods of KG1206: (A) labels show the combinations of the binary mixtures and (B) correlation between P(O) and P(E).
Conclusion
The bioluminescence characteristics of individual CT and NT inducers and the effects of binary mixtures of eleven inducers on the bioluminescence activity were studied using the Pm-lux recombinant strain. Depending on the inducer types (CTs and NTs) and initial concentrations, different bioluminescence activities were observed. The bioluminescence activity of KG1206 was particularly stimulated in the presence of CTs, rather than NTs. The high affinity (low value of half-saturation constant Ks) on bioluminescence activity was also observed with CTs. Exposure to binary inducer mixtures strongly affected the bioluminescence activity, showing 65% antagonistic effects of the tested combinations.
The bioluminescence activity may display large variability under various field conditions because of its reactivity with environmental constituents. Therefore, additional interdisciplinary and more systematic studies have to be performed to assess the real-time effects of inducer chemicals in environmental systems. Although this study provides information on the potential environmental impact of exposed inducer chemicals, future studies at the molecular level of the bioluminescent processes will be needed to provide more clear additional information about the responses.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
KK and KH partly prepared the manuscript, designed the experiment, and analyzed the data. SL prepared and partly performed the experiment. IK performed the research and mainly contributed to writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Environment Industry and Technology Institute (KEITI) through the Demand Responsive Water Supply Service Program, funded by the Korean Ministry of Environment (MOE). This researh was also supported by the National Research Council of Science and Technology (NST) grant by the Korea Government (MSIP) (No. CAP-17–05-KIGAM).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, Y-J. (2004). Soil ecotoxicity assessment using cadmium sensitive plants. Environ. Pollut. 127, 21–26. doi:10.1016/S0269-7491(03)00263-X
Bak, F., Bonnichsen, L., Jorgensen, N. O. G., Nicolaisen, M. H., and Nybroe, O. (2015). The biosurfactant viscosin transiently stimulates n-hexadecane mineralization by a bacterial consortium. Appl. Microbiol. Biotechnol. 99, 1475–1483. doi:10.1007/s00253-014-6054-3
Bundy, J. G., Maciel, H., Cronin, M. T. D., and Paton, G. I. (2003). Limitations of a cosolvent for ecotoxicity testing of hydrophobic compounds. Bull. Environ. Contam. Toxicol. 70, 1–8. doi:10.1007/s00128-002-0148-9
Burlage, R. S. (2002). “Emerging technologies: Bioreporters, biosensors, and microprobes,” in Manual of environmental microbiology. 2nd edition (Washington, D.C. ASM Press), 147–157.
Burlage, R. S., Palumbo, A. V., Heitzer, A., and Sayler, G. S. (1994). Bioluminescent reporter bacteria detect contaminants in soil samples. Appl. Biochem. Biotechnol. 45/46, 731–740. doi:10.1007/bf02941845
Choi, S. H., and Gu, M. B. (2003). Toxicity biomonitoring of degradation byproducts using freeze-dried recombinant bioluminescent bacteria. Anal. Chim. Acta X. 481, 229–238. doi:10.1016/s0003-2670(03)00091-6
Ferreira, A. L. G., Loureiro, S., and Soares, A. M. V. M. (2008). Toxicity prediction of binary combinations of cadmium, carbendazim and low dissolved oxygen on Daphnia magna. Aquat. Toxicol. 89, 28–39. doi:10.1016/j.aquatox.2008.05.012
Ford, C. Z., Sayler, G. S., and Burlage, R. S. (1999). Containment of a genetically engineered microorganism during a field bioremediation application. Appl. Microbiol. Biotechnol. 51, 397–400. doi:10.1007/s002530051409
Harayama, S., and Rekik, M. (1990). The meta cleavage operon of TOL degradative plasmid pWWO comprises 13 genes. Molec. Gen. Genet. 221, 113–120. doi:10.1007/BF00280375
Holtel, A., Marques, S., Mohler, I., Jakubzik, U., and Timmis, K. N. (1994). Carbon source-dependent inhibition of xyl operon expression of the Pseudomonas putida TOL plasmid. J. Bacteriol. 176, 1773–1776. doi:10.1128/jb.176.6.1773-1776.1994
Horvat, T., Vidakovic-Cifrek, Z., Orescnin, V., Tkalec, M., and Pevalek-Kozlina, B. (2007). Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci. Total Environ. 384, 229–238. doi:10.1016/j.scitotenv.2007.06.007
Inouye, S., Nakazawa, A., and Nakazawa, T. (1987). Overproduction of the xylS gene product and activation of the xylDLEGF operon on the TOL plasmid. J. Bacteriol. 169, 3587–3592. doi:10.1128/jb.169.8.3587-3592.1987
Kim, D. J., Choi, J. W., Cho, N. C., Mahendran, B., and Lee, C. E. (2005). Modeling of growth kinetics for Pseudomonas spp. during benzene degradation. Appl. Microbiol. Biotechnol. 69 (4), 456–462. doi:10.1007/s00253-005-1997-z
Ko, K.-S., Koh, D. C., and Kong, I. C. (2017). Evaluation of the effects of nanoparticle mixtures on Brassica seed germination and bacterial bioluminescence activity based on the theory of probability. Nanomaterials 7, 344. doi:10.3390/nano7100344
Ko, K.-S., Koh, D. C., and Kong, I. C. (2018). Toxicity evaluation of individual and mixtures of nanoparticles based on algal chlorophyll content and cell count. Materials 11, 121. doi:10.3390/ma11010121
Ko, K-S., and Kong, I. C. (2017). Application of the freeze-dried bioluminescent bioreporter Pseudomonas putida mt-2 KG1206 to the biomonitoring of groundwater samples from monitoring wells near gasoline leakage sites. Appl. Microbiol. Biotechnol. 101, 1709–1716. doi:10.1007/s00253-016-7974-x
Kong, I. C. (2006). An optimization of a bioassay for toluene analogs using bioluminescence reporter strain KG1206. Soil Sediment Contam. An Int. J. 15, 231–239. doi:10.1080/15320380600646241
Kong, I. C. (2014). Effects of binary mixtures of inducers (toluene analogs) and of metals on bioluminescence induction of a recombinant bioreporter strain. Sensors 14, 18993–19006. doi:10.3390/s141018993
Kong, I. C., Ko, S-K., Lee, S., Koh, D-C., and Burlage, R. (2021). Exposure of metal oxide nanoparticles on the bioluminescence process of Pu- and Pm-lux recombinant P. putida mt-2 strains. Nanomaterials 11, 2822. doi:10.3390/nano11112822
Kong, I. C., Suh, H., Yang, Z., and Burlage, R. S. (2004). A bioluminescent reporter strain utilizing the lower pathway promoter (Pm) of the xyl operon of Pseudomonas: Optimization of a bioassay for m-toluate. Adv. Environ. Res. 8 (3-4), 647–654. doi:10.1016/S1093-0191(03)00037-6
Kungolos, A., Emmanouil, C., Tsiridis, V., and Tsiropoulos, N. (2009). Evaluation of toxic and interactive toxic effects of three agrochemicals and copper using a battery of microbiotests. Sci. Total Environ. 407, 4610–4615. doi:10.1016/j.scitotenv.2009.04.038
Maliyekkal, S. M., Rene, E. R., Philip, L., and Swaminathan, T. (2004). Performance of BTX degraders under substrate versatility conditions. J. Hazard. Mat. 109 (1-3), 201–211. doi:10.1016/j.jhazmat.2004.04.001
Mirjani, M., Soleimani, M., and Salari, V. (2021). Toxicity assessment of total petroleum hydrocarbon in aquatic environments using the bioluminescent bacterium Aliivibrio fischeri. Ecotoxicol. Environ. Saf. 207, 111554. doi:10.1016/j.ecoenv.2020.111554
Norwood, W. P., Borgmann, U., Doxon, D. G., and Wallace, A. (2003). Effects of metal mixtures on aquatic biota: A review of observations and methods. Hum. Ecol. Risk Assess. An Int. J. 9, 795–811. doi:10.1080/713610010
Olmstead, A. W., and LeBlanc, G. A. (2005). Toxicity assessment of environmentally relevant pollutant mixtures using a heuristic model. Integr. Environ. Assess. Manag. 1, 114–122. doi:10.1897/IEAM_2004-005R.1
Onwurah, I. N. E., Ogugua, V. N., Onyike, N. B., Ochonogor, A. E., and Otitoju, O. F. (2007). Crude oil spills in the environment, effects and some innovative clean-up biotechnologies. Int. J. Environ. Res. 1, 307–320. doi:10.22059/IJER.2010.142
Pavlaki, M. D., Pereira, R., Loureiro, S., and Soares, A. M. V. M. (2011). Effects of binary mixtures on the life traits of Daphnia magna. Ecotoxicol. Environ. Saf. 74, 99–110. doi:10.1016/j.ecoenv.2010.07.010
Reardon, K. F., Mosteller, D. C., and Rogers, J. B. (2000). Biodegradation kinetics of benzene, toluene, and phenol as single and mixed substrates for Pseudomonas putida F1. Biotechnol. Bioeng. 69 (4), 384–400. doi:10.1002/1097-0290(20000820)69:4<385::AID-BIT5>3.0.CO;2-Q
Tang, J., Lu, X., Sun, Q., and Zhu, W. (2012). Aging effect of petroleum hydrocarbons in soil under different attenuation conditions. Agric. Ecosyst. Environ. 149, 109–117. doi:10.1016/j.agee.2011.12.020
Trogl, J., Kuncova, G., and Kuran, P. (2010). Bioluminescence of Pseudomonas fluorescens HK44 in the course of encapsulation into silica gel. Effect of methanol. Folia Microbiol. (Praha). 55, 569–575. doi:10.1007/s12223-010-0091-9
Truskewycz, A., Gundry, T. D., Khudur, L. S., Kolobaric, A., Taha, M., Aburto-Medina, A., et al. (2019). Petroleum hydrocarbon contamination in terrestrial ecosystems-fate and microbial responses. Molecules 24, 3400. doi:10.3390/molecules24183400
Velazquez, F., De Lorenzo, V., and Valls, M. (2006). The m-xylene biodegradation capacity of Pseudomonas putida mt-2 is submitted to adaptation to abiotic stresses: Evidence from expression profiling of xyl genes. Environ. Microbiol. 8, 591–602. doi:10.1111/j.1462-2920.2005.00936.x
Keywords: biokinetics, bioluminescence, Pm-lux recombinant strain, chloro-toluene, nitro-toluene
Citation: Kong IC, Lee S, Ha K and Ko K-S (2022) Evaluation of the bioluminescence activity, biokinetics, and the effects of binary mixtures of inducers on the Pm-lux recombinant strain. Front. Environ. Sci. 10:910346. doi: 10.3389/fenvs.2022.910346
Received: 01 April 2022; Accepted: 30 August 2022;
Published: 20 September 2022.
Edited by:
Manosij Ghosh, KU Leuven, BelgiumReviewed by:
Naga Raju Maddela, Technical University of Manabi, EcuadorPankaj Bhatt, Purdue University, United States
Elena Sezenna, Politecnico di Milano, Italy
Copyright © 2022 Kong, Lee, Ha and Ko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyung-Seok Ko, a3l1bmdzb2tAa2lnYW0ucmUua3I=
 In Chul Kong
In Chul Kong Sohyeon Lee1
Sohyeon Lee1 Kyung-Seok Ko
Kyung-Seok Ko