- 1Key Laboratory of Aquatic Biodiversity and Conservation of Chinese Academy of Sciences, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China
- 2School of Ecology and Environment, Anhui Normal University, Wuhu, China
Anthropogenic disturbances are one of the primary factors that drive biodiversity loss. Temporal changes in biodiversity respond to such disturbances. In the present study, we investigated the temporal changes in taxonomic and functional diversity of fish assemblages in different habitats (Three Gorges Reservoir, TGR; running water above the TGR, UTGR; and tributary Chishui River, CSR) in the upper Yangtze basin after impoundment of the TGR from 2008 to 2015. During the survey, the taxonomic and functional composition of fish assemblages varied among the habitats. Although taxonomic diversity increased and functional diversity decreased significantly in the entire upper Yangtze basin, no significant changes in the diversities were observed in each habitat. The habitats showed directional changes in the composition of fish species. The slope of the directional changes in the TGR was more than that in the UTGR and CSR. These results indicated that the distribution of fish species was associated with the habitat after the impoundment. The assembly of the fish assemblage was driven primarily by the neutral process in the entire survey area but was promoted by a combination of species sorting and neutral process in each habitat. The impoundment caused more effects on the TGR than on the UTGR, but it slightly affected the CSR. Regarding fishing closure, conservation measures for fish diversity should be established according to the different habitats. The study findings suggest the removal of small dams in tributaries and ecological regulation in the mainstream and continuous monitoring of fish biodiversity.
1 Introduction
Biodiversity loss is currently one of the most severe environmental issues (Ceballos et al., 2015). Anthropogenic disturbances such as agriculture, urbanization, overlogging, pollution, and damming are one of the most crucial drivers of biodiversity loss (van der Plas, 2019; Suárez-Castro et al., 2022). These disturbances eliminate the sensitive species by reducing the abundance of resources and degrading the habitats (Steudel et al., 2012; Gámez-Virués et al., 2015). From another perspective, anthropogenic disturbances are also considered to be the mechanism responsible for structuring biota communities by transforming the patterns of species coexistence (Suárez-Castro et al., 2022).
Freshwater biodiversity is more vulnerable to anthropogenic disturbances, such as overfishing, damming, and pollution, than terrestrial and marine realms (Winemiller, 2018; Dudgeon, 2019). More than 20% of the freshwater species have become extinct or nearly extinct (Wishart and Davies, 2003). Dam is one of the major disturbances that affect river ecosystems and aquatic biodiversity by obstructing the river continuum and changing the natural hydrological regime (García et al., 2011; Liermann et al., 2012), thereby leading to a decreased population density of migratory fish (Gao et al., 2009), delayed timing of fish breeding (Li et al., 2016), and altered taxonomic and functional composition (Dugan et al., 2010; Liu X. et al., 2019; Zhang C et al., 2020).
Temporal directional or nondirectional changes in biodiversity respond to the changes in both abiotic and biological factors (Martin, 2001; Hollister et al., 2015). The temporal changes can also reflect the underlying mechanisms that influence the community assembly processes under disturbance gradients, such as species sorting and neutral processes (Larson et al., 2021). Recent studies on the response of biodiversity to disturbance events have raised serious concerns, particularly regarding long-term changes under pressure and after pulse disturbances (Jentsch and White, 2019). These studies have provided insights into the protection of native species, habitat management and restoration, and prediction of ecosystem functions (Grman et al., 2010; Betts et al., 2019).
The Yangtze River is the third longest river in the world, with a length of 6,300 km and a mean annual runoff of 951.3 billion m3. The river flows through 11 provinces or cities from west to east and merges with the East China Sea in Shanghai city. The Yangtze River basin covers an area of 1.8 × 106 km2, accounting for 18.8% of China’s land area. More than 400 fish species or subspecies inhabit the Yangtze River basin, and it is, therefore, recognized as a biodiversity hotspot. The upper Yangtze River basin serves as a habitat for approximately 300 fish species, of which about 50% of the species are endemic to the upper Yangtze River (Institute of Hydrobiology, unpublished data). The Three Gorges Dam (TGD), one of the largest dams in the world, is located in the Yichang reach of the middle Yangtze River. Since 2010, the water of the Three Gorges Reservoir (TGR) fills up to 175 m above sea level (ASL). After the water level reaches the 175 m mark, the TGR inundates the riverine and reaches 660 km above the TGD, with a catchment area of 1.0 × 106 km2.
While the TGD offered great social and economic benefits, it has also caused a series of ecological and environmental problems (Chinese Academy of Engineering Three Gorges Project Construction Third Party Independent Evaluation Project Group, 2020). Previous studies have found that the TGD induced negative effects on the fish population in the Yangtze River. For example, after the impoundment, the fish species diversity in the Yangtze River basin significantly decreased, and the species composition verged to homogenization (Liu X. et al., 2019). A total of 44 endemic species in the upper Yangtze River have a higher extinction risk (Park et al., 2003); this is because a shift in the species composition in fish assemblages occurred in the river area above and below the TGD (Gao et al., 2019; Zhang C et al., 2020). After the impoundment, in terms of hydrological regime, the fish habitats in the upper Yangtze River are roughly classified into three categories: reservoir, running water of the main channel, and tributary. Few current studies have investigated the differences in temporal changes in fish diversity between the habitats after the impoundment.
In the present study, we analyzed the temporal changes in taxonomic and functional diversity and composition in the TGR, the main channel above the TGR, and a tributary after the impoundment to understand the effects of the TGD on fish diversity in different habitats. Based on a comprehensive analysis framework of temporal changes in taxonomic and functional diversity, we expect to understand to some extent the mechanisms driving the assembly processes of fish assemblage under the effects of the impoundment.
2 Materials and Methods
2.1 Study Area and Sampling
The filling of the TGR was completed in four stages. The first filling raised the water level to 135 m in 2003, followed by a raise to 156 and 172.5 m in 2006 and 2008, respectively. Finally, the water level was raised to 175 m in 2010. For flood control, electricity generation, and shipping, the operation schedule of the TGR involves raising the water level to 175 m in the dry season (from October to April) and lowering the water level to 145 m in the wet season (from May to September) (Gao et al., 2019). The present study was conducted from Yibin to Zigui in the mainstream of the upper Yangtze River and the mainstream of the Chishui River. The Chishui River is one of the largest tributaries with a length of 436.5 km and a drainage area of 20,440 km2 in the upper Yangtze River, and it is the only tributary without dams built in the mainstream (Figure 1). The survey area that has no dams and maintains riverine continuity covers approximately 1,400 km long reaches, and it includes about 1,000 km reaches of the Yangtze River, and about 400 km reaches of the Chishui River.
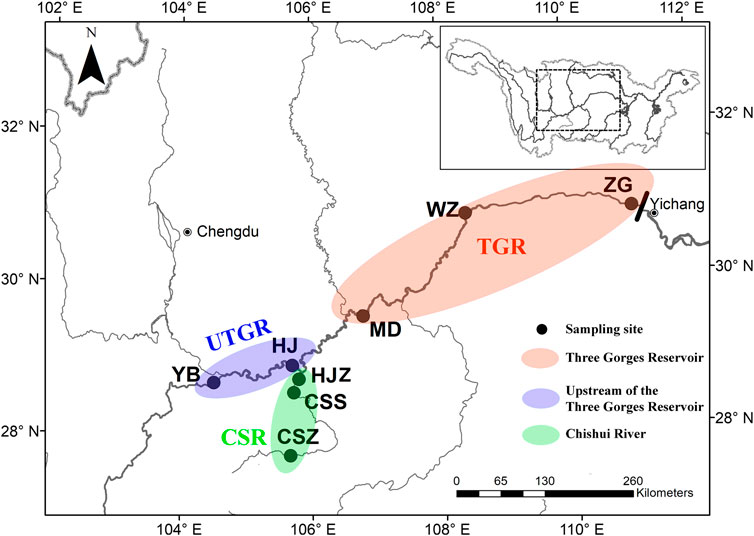
FIGURE 1. Study area and sampling sites upstream of the Three Gorges Dam. The red ellipse indicates the Three Gorges Reservoir area; the blue ellipse indicates the upper reaches of the Three Gorges Reservoir; and the green ellipse indicates the Chishui River.
Data regarding the presence/absence of fish species were collected at eight sites from 2008 to 2015. These sites were grouped into three types of habitats: reservoir (TGR), riverine reach (upstream of the TGR, UTGR), and tributary (Chishui River, CSR). The UTGR and CSR are the major components of the National Nature Reserve for Rare and Endemic Fishes in the upper Yangtze River, which approximately account for 65% of the total length of the reserve. Mudong (MD), Wanzhou (WZ), and Zigui (ZG) sites are located in the TGR, which is defined as the lacustrine zone. Yibin (YB) and Hejiang in the Yangtze (HJY) sites are located in the UTGR, which is the riverine zone. Hejiang in Chishui River (HJC), Chishui city (CSS), and Chishuizhen town (CSZ) sites are located in the CSR, which show a natural flow (Figure 1). A 10-year fishing closure has been implemented progressively in the different regions of the Yangtze River since 2017. For the present study, we used data only up to 2015, which were collected in a consistent environment to reduce analysis bias.
A fish survey was conducted using at least two local fishing boats in spring (May–June) and autumn (September–November) each year from 2008 to 2015 at each site. Each survey was performed for approximately 15–20 days by local fishermen, with a region of 10–30 km surveyed within each site. The fishermen captured fish in different habitats using multiple fishing methods depending on their experience. They preferred to capture fish in the area where they could obtain as many fish as possible at each site. In the mid-channel, the fishermen caught fish using drifting gillnets (height: 1–2.3 m; length: 50–180 m; and mesh size: 1–14 cm), multi-cod-end seines (height: 1.5 m; length: 150 m; mesh size: 1, 1.5, or 2 cm; cod ends: 500–800), and trawl nets (net opening: 4.5 m × 1.8 m; net depth: 8 m; mesh size: one or 2 cm) for approximately every 2 h during a 12-h period on each sampling day. In near-shore areas, stationary gillnets (height: 5 m; length: 35–100 m; mesh size: 1–11 cm), hoop nets (mesh size: 0.5, 1, 1.5 cm), and trotlines (200 to1900 hooks per line) baited with worms or an artificial bait were used. The fishermen set these fishing gears in the river water for 10–12 h at night (from 6 p.m. to 6–8 am on the next day). Moreover, we captured rare species in each survey using electrofishing, lift net, cast net, and trap net. The fish specimens were sorted and identified according to the guidelines of Ding (1994). Each fishing gear has its inherent biases. Therefore, we used multiple fishing gears that almost covered a variety of fish habitats and caught more fish species for obtaining a consistent representation of fish species composition at the same site. These consistent biases can eliminate the impact of sampling error on the analysis of diversity and structure of fish assemblages to a certain extent.
2.2 Data Analysis
In this study, the seasonal data were pooled as the annual data for each site. All statistical analyses were performed using the R software (R Core Team, 2017). Functional diversity can effectively reflect the long-term effects of changes in an aquatic system on fish communities (Villéger et al., 2010; Oliveira et al., 2018). In the present study, we selected eight characteristics based on fish habitat preferences, life history, and feeding mode, which included four continuous variables (maximum length, body length, tail shaft length, head length and diameter ratio, and body length and body height ratio) and four categorical variables (vertical position, water flow preference, feeding, and spawning type). All functional trait values or trait categories were obtained from the FishBase website (www.fishbase.org) and published studies or books (Institute of Hydrobiology, 1976; Ding, 1994). When trait values or categories were lacking for a specific species, the mean value within the same or a similar genus was used (Zhang C et al., 2020).
2.2.1 Differences in Taxonomic and Functional Composition Among the Different Habitats
To evaluate the differences in species composition between the different habitat types, we analyzed the multivariate homogeneity of groups’ dispersions (variances) of different habitats using the “betadisper” function of the vegan package (Oksanen et al., 2013). This method used principal coordinate analysis (PCoA) to reduce the dimensions of the multidimensional space composed of the matrix of the community at each section during the entire investigation and showed the difference between the communities of the different habitats in a two-dimensional space (Anderson, 2006). We then used permutational multivariate analysis of variance (PERMANOVA) to quantify the differences in the fish community composition of the different habitats.
The functional composition of fish communities in the different habitats was analyzed based on the functional space comprising multidimensional volume (Villéger et al., 2011). In this method, PCoA was performed on the fish functional traits, and the multidimensional volume was calculated for each habitat type (Villéger et al., 2011). Based on the functional space constituted by the first three axes of PCoA, the functional dissimilarity between the fish communities of different habitats is calculated and decomposed into functional nestedness and functional turnover (Villéger et al., 2013). The functional dissimilarity is the difference in the distribution and occupied space of the different communities in the functional space (Villéger et al., 2011). Functional space and functional dissimilarity were calculated based on the “multi-dimFbetaD” function provided by Villéger (http://villeger.sebastien.free.fr/Rscripts.html).
2.2.2 Temporal Changes in Taxonomic and Functional Composition
To investigate the temporal variation of fish communities in each section, species diversity and species evenness were measured using Shannon–Wiener diversity (H) and Pielou’s evenness (J), respectively (Hill, 1973). Functional richness (FRic), functional divergence (FDiv), functional dispersion (FDis), and Rao’s quadratic entropy index (RaoQ) were used to calculate the functional characteristics of fish communities, following the multifaceted framework proposed by Villéger et al. (2008).
Inter-annual variation trends of species and functional diversity indices in the different habitats were analyzed based on the Mann–Kendall trend multivariate analysis (Maire et al., 2019). The Mann–Kendall trend test is widely used to study whether the change in variables over time has a general monotonous upward or downward trend, and it can identify the nonlinear change trends (Hamed and Rao, 1998). Before multivariate analysis, based on the methods of Hamed and Rao (1998), the variation in statistical data was corrected for temporal autocorrelation. In the present study, a random-effects model based on the Gaussian correlation structure was used to analyze the spatial autocorrelation of the different habitat communities in the upper reaches of the Yangtze River (Cressie, 2015). A 4 × 4 distance matrix was developed based on the data of the three habitats (TGR, UTGR, and CSR) and the total data (the dataset created by pooling the data of the UTGR, TGR, and CSR together and abbreviated as all habitat (AHT)). The distance between the sampling units was calculated as river channel distance (km). Among them, AHT was obtained by summarizing the fish species data of the other three sampling units; thus, the distance from this unit to the other three units was defined as zero. The random-effects model was constructed using the “rma.mv” function of the metafor package (Viechtbauer, 2010). Multivariate analysis of the trend of community diversity was performed using the R codes provided by Maire et al. (2019).
Directional change trend and variation degree of the fish community structure of the different habitat types in the upper reaches of the Yangtze River were studied using the method of Collins et al. (2000). This method is based on the Euclidean distance of species composition in the entire time series and calculates the differences in the species composition between paired communities in different time intervals. The Euclidean distance value is subjected to regression analysis according to the time interval. The slope of the regression line indicates the rate of community composition change and direction (Collins et al., 2000). The analysis was completed using the “rate_change_interval” function in the codyn package in R software (Hallett et al., 2016).
3 Results
3.1 Difference in Taxonomic and Functional Composition Among the Different Habitats
During the survey period, 171 fish species belonging to 30 families were collected. Cyprinidae and Bagridae species accounted for 56.1 and 7.6% of the collected species, respectively. In the UTGR, 131 fish species belonging to 26 families were captured, where Cyprinidae and Bagridae species accounted for 52.7 and 8.4%, respectively; in the TGR, 143 fish species belonging to 29 families were captured, where Cyprinidae and Bagridae species accounted for 53.8 and 7.0%, respectively; and in the CSR, 141 fish species belonging to 22 families were captured, where Cyprinidae and Bagridae species accounted for 58.2 and 7.8%, respectively.
The results of PERMANOVA showed significant differences in the species compositions among the TGR, UTGR, and CSR (p < 0.05). PCoA showed that the species composition in the CSR completely varied from those in the TGR and UTGR (Figure 2). However, there was some overlap in the species composition between the TGR and UTGR (Figure 2).
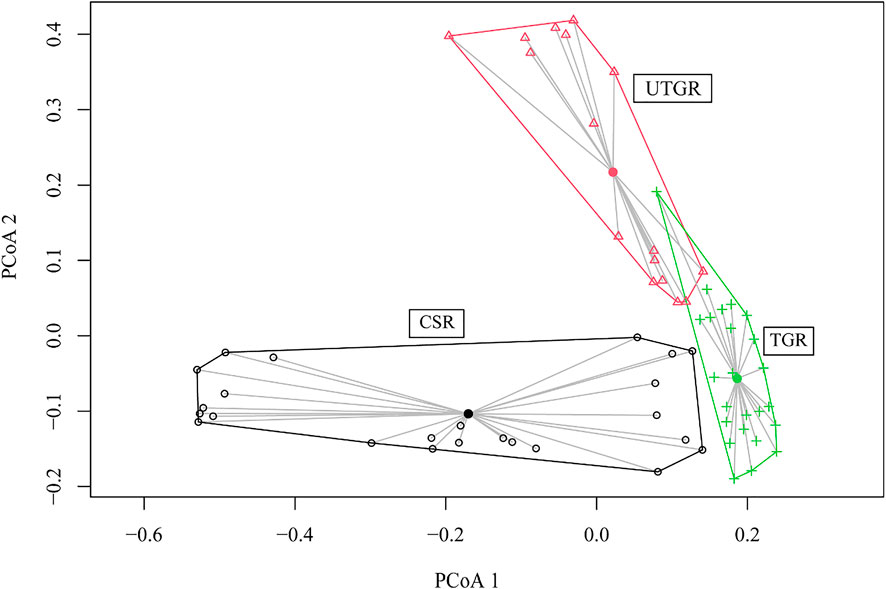
FIGURE 2. Principal coordinate analysis of fish communities in the different habitat types in the upper reaches of the Yangtze River.
A comparison of the functional composition revealed some differences in the functional composition of fish assemblage among the three habitat types (Figure 3). The functional space area of fish assemblage in the TGR was 93.9% of the total functional space area, which was larger than that of other habitats. The functional space area in the UTGR and CSR was 87 and 81%, respectively (Figure 3). The fish assemblage in the TGR showed the most specific traits. For example, some species, including Polyodon spathula, Anguilla japonica, and Monopterus albus were located at the margins of the functional space.
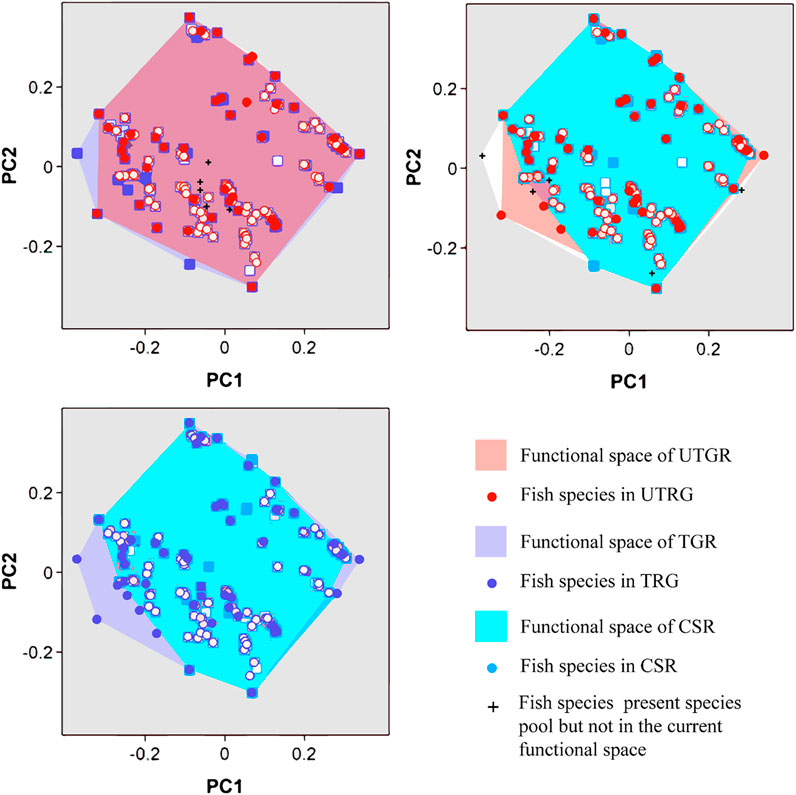
FIGURE 3. Functional space of fish assemblages in the different habitat types in the upper reaches of the Yangtze River.
The functional dissimilarity between the fish assemblages in the UTGR and TGR was 0.157, of which the functional nestedness value was 0.064, and the functional turnover value was 0.093 (Figure 3A). The functional dissimilarity between the fish assemblages in the UTGR and CSR was 0.166, comprising functional nestedness of 0.058 and functional turnover of 0.108 (Figure 3B). The functional dissimilarity between the fish assemblages in the TGR and CSR was 0.21, comprising functional nestedness of 0.12 and functional turnover of 0.09 (Figure 3C).
3.2 Temporal Changes in Taxonomic and Functional Diversity and Composition
The multivariate analysis based on the Mann–Kendall trend test showed that the temporal trends of the taxonomic and functional diversity indices among the different habitat types were inconsistent from 2008 to 2015 (Figures 4, 5). The species richness and Shannon–Wiener diversity of AHT showed a significant increasing trend (Figures 4A, B, p < 0.05); however, Pielou’s evenness index showed a significant decreasing trend (Figure 4C, p < 0.05). No significant trend was observed in species richness and taxonomic diversity indices in each type of habitat (Figure 4). The functional diversity indices in the different habitat types also showed varying trends (Figure 5). All the functional diversity indices of AHT showed no significant trend, except for the RaoQ index, which showed a significant decreasing trend (Figure 5D, p < 0.05). The TGR showed a significant decreasing trend in functional divergence (Figure 5C, p < 0.05), while the CSR showed a significant increasing trend in functional dispersion (Figure 5D, p < 0.05).

FIGURE 4. Temporal changes in the species diversity of fish communities in the different habitat types in the upper reaches of the Yangtze River. (A) Species richness; (B) Shannon-Wiener diversity; (C) Pielou’s evenness. Temporal changes in the species diversity of fish communities in the different habitat types in the upper reaches of the Yangtze River.
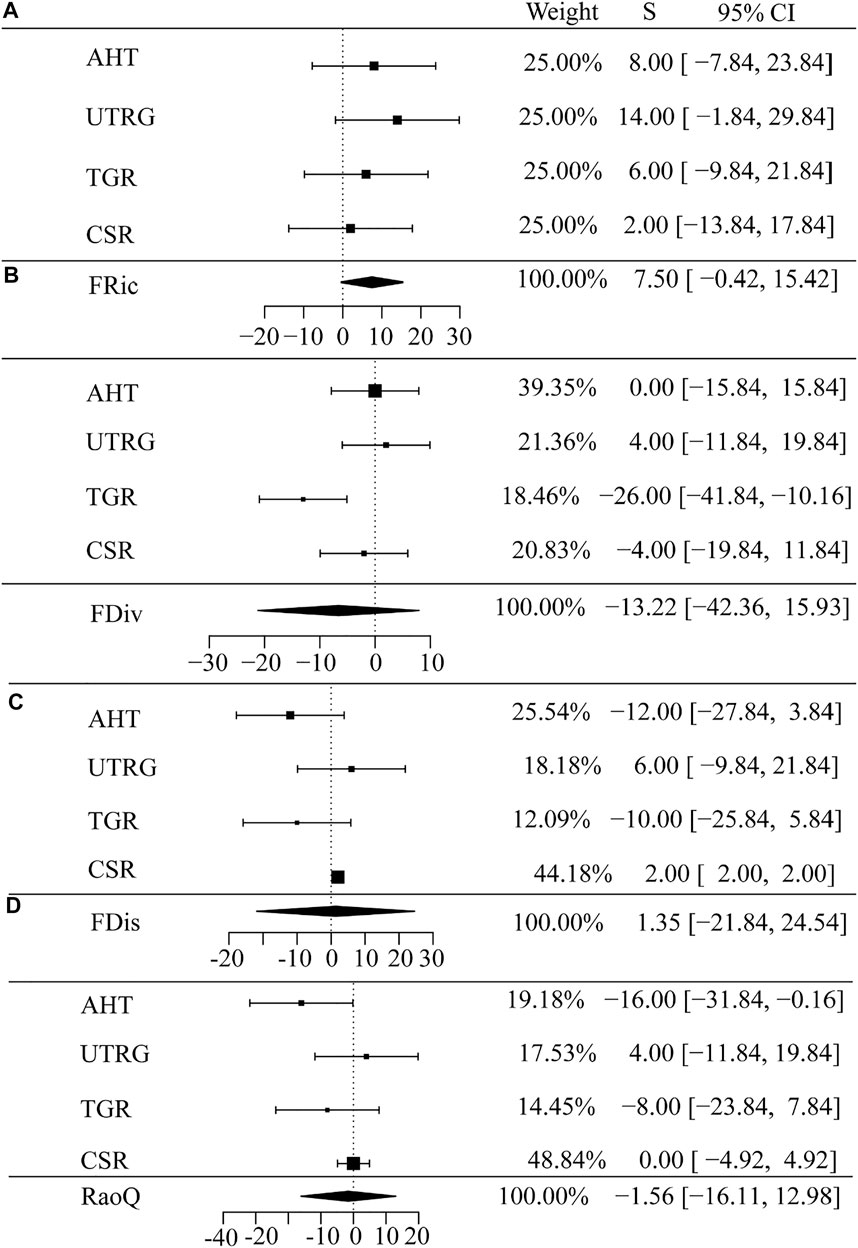
FIGURE 5. Temporal changes in functional diversity of fish communities in different habitat types in the upper reaches of the Yangtze River. (A) Functional richness; (B) Functional divergence; (C) Functional dispersion; (D) Rao’s quadratic entropy index.
The species composition in the TGR, UTGR, and CSR had directional trends from 2008 to 2015 (p < 0.05) (Figure 6). The variation value of the temporal trend in the TGR was 0.17, which was more than that in the CSR (0.12) and UTGR (0.07).
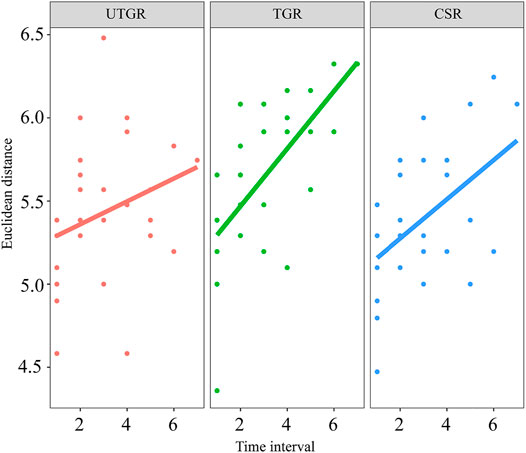
FIGURE 6. Directional changes in the composition of fish communities in different habitat types in the upper reaches of the Yangtze River.
4 Discussion
The distribution of fish species in the upper Yangtze River basin was associated with the habitat after the impoundment of the TGR. Fish species composition is determined by the habitat-trait relationship (Chittaro, 2004; Villéger et al., 2010). The lentic fish and generalist dominated in the TGR, while the lotic fish were still the dominant groups in the UTGR. In particular, endemic fish such as Coreius guichenoti, Rhinogobio ventralis, and Rhinogobio cylindricus preferred inhabiting the UTGR, rather than the TGR (Supplementary Table S1). Moreover, in this study, more non-native species such as Piaractus brachypomus and Micropterus salmoides occurred in the TGR. Twenty-three non-native fish species were recorded in the TGR (Ba and Chen, 2012). The fish assemblage composition of the tributary was different from that in the main channel (Gorman, 1986). Shi et al. (2020) reported that the fish assemblage structures in the tributaries varied from that in the mainstream within the Ganjiang River basin, China. Our results also indicated the significant differences in the fish species composition between the tributary and mainstream (Figure 2). Fish with small body sizes and omnivorous feeding patterns preferred the CSR as their habitat (Liu F. et al., 2019).
The functional diversity offers complementary information for taxonomic diversity when ecologists study the mechanisms driving assemblage assembly (Larson et al., 2021). Our results revealed increased species richness and intact functional richness in the entire survey area after the impoundment. This implied that the species with equivalent functionality were benefited, and the functional redundancy was increasingly high, which indicated that the neutral process was primarily responsible for fish assemblage assembly under the disturbance (Larson et al., 2021). Fish assemblage assembly in each habitat was, however, dominated by a combination of species sorting and neutral process due to the unchanged species and functional richness. All local communities do not respond similarly to the regional assembly processes (Legendre and De Cáceres, 2013). Because of the differences in environmental factors, species composition, and functional traits among local communities, the assembly mechanisms of some local communities and the global metacommunities may vary (Leibold et al., 2022).
The present results indicated that the directional change of species composition was more apparent in the TGR than in the UTGR and CSR (Figure 6). Directional change in community structure is a response to a long-term persistent disturbance (Collins et al., 2000). For example, biodiversity and species composition in a biological community will change significantly in response to the changes in the habitat environment caused by land usage or climate change (Dornelas et al., 2014; Hoover et al., 2014; Frishkoff et al., 2016). Disturbances with high frequency and intensity make communities more prone to directional changes (Collins et al., 2000). The effects of disturbances are related to the distance from a dam (Gao et al., 2019). The CSR also showed a directional change in species composition. Liu et al. (2021a) found significant temporal changes in the structures of the local fish communities. However, these changes were primarily caused by human activities on a local scale, such as the construction of small dams in the branches, overfishing, pollution, and navigation (Liu et al., 2021b).
The species richness in the upper Yangtze River basin increased after the impoundment. However, even though species richness can increase on a local scale, the species number could decrease on regional and global scales (Sax and Gaines, 2003). The yield of captured fish in the entire Yangtze River did not significantly change from 2008 to 2016 (Zhang H et al., 2020). Assuming a positive relationship between fish diversity and catch (McIntyre et al., 2016), we roughly inferred that the fish species richness did not significantly change in the entire Yangtze River after the impoundment. The fish species richness in the Yangtze River is currently possibly decreased to a low level because the yield has decreased to only 25% of a historical peak in the late 1950s (Zhang H. et al., 2020).
Since 2021, the 10-year fishing closure has been imposed completely in the Yangtze River, which is predicted to recover the fish resources effectively (Zhang H. et al., 2020; Mei et al., 2020). However, other disturbances such as dams and pollution are still influencing fish diversity and population density. Our results revealed that the fish species distribution and temporal changes in fish species composition varied among the different habitats. For protecting the fish biodiversity, in the context of fishing closure, we suggested conservation measures related to the different habitats. For example, the removal of small dams should be initiated to restore the continuum of the tributaries. Ecological regulation should be implemented in the mainstream. Moreover, the monitoring of fish biodiversity should be conducted continuously to gain long-term data, which could provide an important basis for explaining the impact of multiple disturbances on the river ecosystems (Daufresne et al., 2015; Counihan et al., 2018).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee for Animal Experiments of the Institute of Hydrobiology, Chinese Academy of Sciences.
Author Contributions
HL and XG conceived the ideas and designed methodology. FL and PL performed field work. CZ analyzed the data. CZ, CW, and XG wrote the manuscript.
Funding
This work was funded by the National Key R and D Program of China (2018YFD0900804), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31040000), the Comprehensive Safety Monitoring System of Three Gorges Project (Reservoir Operation and Management Fund: 2136703, 12620200600020J003), and the Sino BON–Inland Water Fish Diversity Observation Network.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.875789/full#supplementary-material
References
Anderson, M. J. (2006). Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 62 (1), 245–253. doi:10.1111/j.1541-0420.2005.00440.x
Ba, J. W., and Chen, D. Q. (2012). Invasive Fishes in Three Gorges Reservoir Area and Preliminary Study on Effects of Fish Invasion Owing to Impoundment. J. Lake Sci. 24, 185–189. doi:10.18307/2012.0203
Betts, M. G., Wolf, C., Pfeifer, M., Banks-Leite, C., Arroyo-Rodríguez, V., Ribeiro, D. B., et al. (2019). Extinction Filters Mediate the Global Effects of Habitat Fragmentation on Animals. Science 366 (6470), 1236–1239. doi:10.1126/science.aax9387
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., García, A., Pringle, R. M., and Palmer, T. M. (2015). Accelerated Modern Human-Induced Species Losses: Entering the Sixth Mass Extinction. Sci. Adv. 1, e1400253. doi:10.1126/sciadv.1400253
Chinese Academy of Engineering Three Gorges Project Construction Third Party Independent Evaluation Project Group (2020). Comprehensive Report of the Third Party Independent Evaluation of the Construction of the Three Gorges Project. Beijing, China: China WaterPower Press. [In Chinese].
Chittaro, P. M. (2004). Fish-habitat Associations across Multiple Spatial Scales. Coral Reefs 23 (2), 235–244. doi:10.1007/s00338-004-0376-z
Collins, S. L., Micheli, F., and Hartt, L. (2000). A Method to Determine Rates and Patterns of Variability in Ecological Communities. Oikos 91 (2), 285–293. doi:10.1034/j.1600-0706.2000.910209.x
Counihan, T. D., Waite, I. R., Casper, A. F., Ward, D. L., Sauer, J. S., Irwin, E. R., et al. (2018). Can Data from Disparate Long-Term Fish Monitoring Programs Be Used to Increase Our Understanding of Regional and continental Trends in Large River Assemblages? PloS one 13 (1), e0191472. doi:10.1371/journal.pone.0191472
Daufresne, M., Veslot, J., Capra, H., Carrel, G., Poirel, A., Olivier, J.-M., et al. (2015). Fish Community Dynamics (1985-2010) in Multiple Reaches of a Large River Subjected to Flow Restoration and Other Environmental Changes. Freshw. Biol. 60 (6), 1176–1191. doi:10.1111/fwb.12546
Ding, R. H. (1994). Fishes of Sichuan. Chengdu, China: Sichuan Science and Technology Press. (In Chinese).
Dornelas, M., Gotelli, N. J., McGill, B., Shimadzu, H., Moyes, F., Sievers, C., et al. (2014). Assemblage Time Series Reveal Biodiversity Change but Not Systematic Loss. Science 344 (6181), 296–299. doi:10.1126/science.1248484
Dudgeon, D. (2019). Multiple Threats Imperil Freshwater Biodiversity in the Anthropocene. Curr. Biol. 29 (19), R960–R967. doi:10.1016/j.cub.2019.08.002
Dugan, P. J., Barlow, C., Agostinho, A. A., Baran, E., Cada, G. F., Chen, D., et al. (2010). Fish Migration, Dams, and Loss of Ecosystem Services in the Mekong basin. Ambio 39 (4), 344–348. doi:10.1007/s13280-010-0036-1
Frishkoff, L. O., Karp, D. S., Flanders, J. R., Zook, J., Hadly, E. A., Daily, G. C., et al. (2016). Climate Change and Habitat Conversion Favour the Same Species. Ecol. Lett. 19 (9), 1081–1090. doi:10.1111/ele.12645
Gámez-Virués, S., Perović, D. J., Gossner, M. M., Börschig, C., Blüthgen, N., De Jong, H., et al. (2015). Landscape Simplification Filters Species Traits and Drives Biotic Homogenization. Nat. Commun. 6 (1), 8568–8. doi:10.1038/ncomms9568
Gao, X., Brosse, S., Chen, Y., Lek, S., and Chang, J. (2009). Effects of Damming on Population Sustainability of Chinese sturgeon, Acipenser Sinensis: Evaluation of Optimal Conservation Measures. Environ. Biol. Fish. 86 (2), 325–336. doi:10.1007/s10641-009-9521-4
Gao, X., Fujiwara, M., Winemiller, K. O., Lin, P., Li, M., and Liu, H. (2019). Regime Shift in Fish Assemblage Structure in the Yangtze River Following Construction of the Three Gorges Dam. Sci. Rep. 9 (1), 4212–4311. doi:10.1038/s41598-019-38993-x
García, A., Jorde, K., Habit, E., Caamaño, D., and Parra, O. (2011). Downstream Environmental Effects of Dam Operations: Changes in Habitat Quality for Native Fish Species. River Res. Applic. 27 (3), 312–327. doi:10.1002/rra.1358
Gorman, O. T. (1986). Assemblage Organization of Stream Fishes: the Effect of Rivers on Adventitious Streams. Am. Natural. 128 (4), 611–616. doi:10.1086/284592
Grman, E., Lau, J. A., Schoolmaster, D. R., and Gross, K. L. (2010). Mechanisms Contributing to Stability in Ecosystem Function Depend on the Environmental Context. Ecol. Lett. 13 (11), 1400–1410. doi:10.1111/j.1461-0248.2010.01533.x
Hallett, L. M., Jones, S. K., MacDonald, A. A. M., Jones, M. B., Flynn, D. F. B., Ripplinger, J., et al. (2016). Codyn: An R Package of Community Dynamics Metrics. Methods Ecol. Evol. 7 (10), 1146–1151. doi:10.1111/2041-210x.12569
Hamed, K. H., and Rao, A. R. (1998). A Modified Mann–Kendall Trend Test for Autocorrelated Data. J. Hydrol. 204 (1–4), 182–196. doi:10.1016/s0022-1694(97)00125-x
Hill, M. O. (1973). Diversity and Evenness: a Unifying Notation and its Consequences. Ecology 54 (2), 427–432. doi:10.2307/1934352
Hollister, R. D., May, J. L., Kremers, K. S., Tweedie, C. E., Oberbauer, S. F., Liebig, J. A., et al. (2015). Warming Experiments Elucidate the Drivers of Observed Directional Changes in Tundra Vegetation. Ecol. Evol. 5 (9), 1881–1895. doi:10.1002/ece3.1499
Hoover, D. L., Knapp, A. K., and Smith, M. D. (2014). Resistance and Resilience of a Grassland Ecosystem to Climate Extremes. Ecology 95 (9), 2646–2656. doi:10.1890/13-2186.1
Institute of Hydrobiology (1976). Fishes of the Yangtze River. Beijing, China: Science press. (In Chinese).
Jentsch, A., and White, P. (2019). A Theory of Pulse Dynamics and Disturbance in Ecology. Ecology 100 (7), e02734. doi:10.1002/ecy.2734
Larson, E. I., Poff, N. L., Funk, W. C., Harrington, R. A., Kondratieff, B. C., Morton, S. G., et al. (2021). A Unifying Framework for Analyzing Temporal Changes in Functional and Taxonomic Diversity along Disturbance Gradients. Ecology 102 (11), e03503. doi:10.1002/ecy.3503
Legendre, P., and De Cáceres, M. (2013). Beta Diversity as the Variance of Community Data: Dissimilarity Coefficients and Partitioning. Ecol. Lett. 16 (8), 951–963. doi:10.1111/ele.12141
Leibold, M. A., Rudolph, F. J., Blanchet, F. G., De Meester, L., Gravel, D., Hartig, F., et al. (2022). The Internal Structure of Metacommunities. Oikos 2022 (1), e08618. doi:10.1111/oik.08618
Li, M., Duan, Z., Gao, X., Cao, W., and Liu, H. (2016). Impact of the Three Gorges Dam on Reproduction of Four Major Chinese Carps Species in the Middle Reaches of the Changjiang River. Chin. J. Ocean. Limnol. 34 (5), 885–893. doi:10.1007/s00343-016-4303-2
Liermann, C. R., Nilsson, C., Robertson, J., and Ng, R. Y. (2012). Implications of Dam Obstruction for Global Freshwater Fish Diversity. BioScience 62 (6), 539–548. doi:10.1525/bio.2012.62.6.5
Liu, F., Wang, J., Zhang, F. B., Liu, H. Z., and Wang, J. W. (2021a). Spatial Organisation of Fish Assemblages in the Chishui River, the Last Free‐flowing Tributary of the Upper Yangtze River, China. Ecol. Freshw. Fish. 30 (1), 48–60. doi:10.1111/eff.12562
Liu, F., Yu, F., Xia, Z., Qin, Q., Xu, C., Wang, J., et al. (2021b). Changes in Fish Assemblages Following the Implementation of a Complete Fishing Closure in the Chishui River. Fish. Res. 243, 106099. doi:10.1016/j.fishres.2021.106099
Liu F, F., Wang, X., Wang, M., Liu, H., and Wang, J. (2019). Diet Partitioning and Trophic Guild Structure of Fish Assemblages in Chishui River, the Last Undammed Tributary of the Upper Yangtze River, China. River Res. Applic. 35 (9), 1530–1539. doi:10.1002/rra.3519
Liu X, X., Qin, J., Xu, Y., Ouyang, S., and Wu, X. (2019). Biodiversity Decline of Fish Assemblages after the Impoundment of the Three Gorges Dam in the Yangtze River Basin, China. Rev. Fish. Biol. Fish. 29 (1), 177–195. doi:10.1007/s11160-019-09548-0
Maire, A., Thierry, E., Viechtbauer, W., and Daufresne, M. (2019). Poleward Shift in Large‐river Fish Communities Detected with a Novel Meta‐analysis Framework. Freshw. Biol. 64 (6), 1143–1156. doi:10.1111/fwb.13291
Martin, T. E. (2001). Abiotic vs. Biotic Influences on Habitat Selection of Coexisting Species: Climate Change Impacts? Ecology 82 (1), 175–188. doi:10.1890/0012-9658(2001)082[0175:avbioh]2.0.co;2
McIntyre, P. B., Reidy Liermann, C. A., and Revenga, C. (2016). Linking Freshwater Fishery Management to Global Food Security and Biodiversity Conservation. Proc. Natl. Acad. Sci. U.S.A. 113 (45), 12880–12885. doi:10.1073/pnas.1521540113
Mei, Z., Cheng, P., Wang, K., Wei, Q., Barlow, J., and Wang, D. (2020). A First Step for the Yangtze. Science 367 (6484), 1314. doi:10.1126/science.abb5537
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’hara, R. B., et al. (2013). Package ‘vegan’. Community Ecology Package, Version 2, 1–295. Available at: https://cran.ism.ac.jp/web/packages/vegan/vegan.pdf.
Oliveira, A. G., Baumgartner, M. T., Gomes, L. C., Dias, R. M., and Agostinho, A. A. (2018). Long-term Effects of Flow Regulation by Dams Simplify Fish Functional Diversity. Freshw. Biol. 63 (3), 293–305. doi:10.1111/fwb.13064
Park, Y.-S., Chang, J., Lek, S., Cao, W., and Brosse, S. (2003). Conservation Strategies for Endemic Fish Species Threatened by the Three Gorges Dam. Conserv. Biol. 17 (6), 1748–1758. doi:10.1111/j.1523-1739.2003.00430.x
R Core Team (2017). R: A Language and Environment for Statistical Computing. Available at: https://www.r-project.org/.
Sax, D. F., and Gaines, S. D. (2003). Species Diversity: from Global Decreases to Local Increases. Trends Ecol. Evol. 18 (11), 561–566. doi:10.1016/s0169-5347(03)00224-6
Shi, Z., Zhang, J., Wu, H., Yang, J., and Hu, M. (2020). Taxonomic Diversity Pattern and Composition of Fish Species in the Upper Reaches of Ganjiang River, Jiangxi, China. PloS one 15 (11), e0241762. doi:10.1371/journal.pone.0241762
Steudel, B., Hector, A., Friedl, T., Löfke, C., Lorenz, M., Wesche, M., et al. (2012). Biodiversity Effects on Ecosystem Functioning Change along Environmental Stress Gradients. Ecol. Lett. 15 (12), 1397–1405. doi:10.1111/j.1461-0248.2012.01863.x
Suárez‐Castro, A. F., Raymundo, M., Bimler, M., and Mayfield, M. M. (2022). Using Multi‐scale Spatially Explicit Frameworks to Understand the Relationship between Functional Diversity and Species Richness. Ecography. e05844. doi:10.1111/ecog.05844
van der Plas, F. (2019). Biodiversity and Ecosystem Functioning in Naturally Assembled Communities. Biol. Rev. Camb Philos. Soc. 94 (4), 1220–1245. doi:10.1111/brv.12499
Viechtbauer, W. (2010). Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 36 (3), 1–48. doi:10.18637/jss.v036.i03
Villéger, S., Mason, N. W. H., and Mouillot, D. (2008). New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 89 (8), 2290–2301. doi:10.1890/07-1206.1
Villéger, S., Miranda, J. R., Hernández, D. F., and Mouillot, D. (2010). Contrasting Changes in Taxonomic vs. Functional Diversity of Tropical Fish Communities after Habitat Degradation. Ecol. Appl. 20 (6), 1512–1522. doi:10.1890/09-1310.1
Villéger, S., Novack-Gottshall, P. M., and Mouillot, D. (2011). The Multidimensionality of the Niche Reveals Functional Diversity Changes in Benthic marine Biotas across Geological Time. Ecol. Lett. 14 (6), 561–568. doi:10.1111/j.1461-0248.2011.01618.x
Villéger, S., Grenouillet, G., and Brosse, S. (2013). Decomposing Functional β-diversity Reveals that Low Functional β-diversity Is Driven by Low Functional Turnover in European Fish Assemblages. Glob. Ecol. Biogeogr. 22 (6), 671–681. doi:10.1111/geb.12021
Winemiller, K. O. (2018). “Trends in Biodiversity: Freshwater,” in The Encyclopedia of the Anthropocene. Editors D. A. Dellasala, and M. I. Goldstein (Amsterdam, Netherlands: Elsevier), 3, 151–161. doi:10.1016/b978-0-12-809665-9.09820-7
Wishart, M. J., and Davies, B. R. (2003). Beyond Catchment Considerations in the Conservation of Lotic Biodiversity. Aquat. Conserv: Mar. Freshw. Ecosyst. 13 (5), 429–437. doi:10.1002/aqc.600
Zhang C, C., Fujiwara, M., Pawluk, M., Liu, H., Cao, W., and Gao, X. (2020). Changes in Taxonomic and Functional Diversity of Fish Communities after Catastrophic Habitat Alteration Caused by Construction of Three Gorges Dam. Ecol. Evol. 10 (12), 5829–5839. doi:10.1002/ece3.6320
Keywords: community assembly, habitat changes, fish biodiversity, species traits, conservation
Citation: Zhang C, Liu F, Liu H, Wang C, Lin P and Gao X (2022) Temporal Changes in Taxonomic and Functional Diversity of Fish Assemblages in the Upper Yangtze River After Impoundment of the Three Gorges Reservoir, China. Front. Environ. Sci. 10:875789. doi: 10.3389/fenvs.2022.875789
Received: 16 February 2022; Accepted: 28 March 2022;
Published: 16 May 2022.
Edited by:
Min Wang, Los Alamos National Laboratory (DOE), United StatesReviewed by:
Binsong Jin, Hangzhou Normal University, ChinaZeng Yu, China West Normal University, China
Copyright © 2022 Zhang, Liu, Liu, Wang, Lin and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengcheng Lin, bGlucGNAaWhiLmFjLmNu; Xin Gao, Z2FveGluQGloYi5hYy5jbg==
 Chen Zhang
Chen Zhang Fei Liu1
Fei Liu1 Chunling Wang
Chunling Wang Pengcheng Lin
Pengcheng Lin Xin Gao
Xin Gao