- 1Defence Research Laboratory, DRDO, Tezpur, Assam, India
- 2Indian Institute of Science Education and Research Mohali, Mohali, Punjab, India
The non-ending needs of growing human population are being met by rapid industrialization and globalization, which have nowadays become an indispensable component of growth. Although these activities have led to phenomenal growth of the human civilization, at the same time, they have resulted in severe environmental pollution by discharge of highly toxic waste. This waste is severely detrimental not only for the environment but also for the health of the human population. Among different classes of pollutants, one being considered as one of the highly toxic ones is that of persistent organic pollutants (POPs). Advanced oxidation technologies (AOTs) play a major role in the degradation of pollutants by converting organic pollutants into CO2, H2O, and mineralized inorganic ions. AOTs include UV-based photocatalysis, ozonation, electrochemical oxidation, and Fenton and Fenton-like processes There are some difficulties and challenges associated with AOT, such as being highly capital intensive and high consumption of energy. To overcome these bottlenecks, photocatalytic degradation is a promising method that uses solar energy for the degradation of such pollutants. Photocatalysis is further classified into homogenous and heterogenous photocatalysis. As a part of heterogenous photocatalysis, semiconductor photocatalysts have received great attention; but because of their drawbacks such as the recombination of the electron/hole pair, low adsorption rate, and low surface area coverage, nanotechnology was considered for bringing a novel and enhanced remediation photocatalysis process. To this end, the designing of a more efficient photocatalyst by modifying morphology, composition, and structure and reducing toxicity is the need of the hour for the abatement of environmental pollutants. This review focuses on the degradation and removal of highly toxic persistent organic pollutants by using photocatalytic degradation with a detailed account of the various pollutants, their degradation mechanism, process shortcomings, remedial measures, and future prospects.
Introduction
Environmental pollutants are the major cause of adverse effects on the normal physical and biological environmental processes. The type of pollutants that needs global attention is persistent organic pollutants (POPs). POPs are toxic chemical compounds that remain very stable in the environment for a longer period, are geographically distributed all over the world, gets accumulated in the fatty tissue of the living organism, and are toxic to human health and wildlife. POPs are found around the globe, and some of them are listed and recognized by the United Nations Environment Programme (UNEP); Inter-governmental Negotiating Committee, Montreal, Canada; International POPs Elimination Network (IPEN); and Stockholm Convention (Fiedler et al., 2019). Initially, there were 12 POPs listed in the treaty. These 12 POPs are pesticides, industrial chemicals, and by-products. After that, 16 new POPs were included as of 2017 by 181 parties (Madaj et al., 2018). Anthropogenic sources of POPs play a major role in the harmful effects of pollutants.
There are different types of POPs in the environment, and their toxicity varies. POPs are generally of two types: intentional POPs and unintentional POPs (Gaur et al., 2018). Organochlorine insecticides and industrial chemicals such as polychlorinated biphenyls (PCBs), polychlorinated dibenzodioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), polycyclic aromatic hydrocarbons (PAH), and polybrominated diphenyl ethers (PBDEs) are included in the range of POPs, which are used in a variety of products. Persistent organic pollutants are the silent killers, and they are present everywhere in our environment including in the tissues of the human, plants, and animals (Fry and Power, 2017). Exposure to POPs has chronic and acute effects on the organisms such as diabetes, obesity, reproductive impairment, neurological disorder, cancer, and damage to the liver, kidney, lung, and nervous system (Cao et al., 2020). Organisms can be exposed to natural and synthetically manufactured chemicals through diet, by accident, or in the environment, and this exposure is very detrimental because it can cause genotoxicity, ecotoxicity, immunotoxicity, reproductive toxicity, and chronic toxicity.
Various techniques to remove persistent organic pollutants need to be established. One of the chemical approaches well known for the degradation of POPs is the advanced oxidation technologies. AOTs are promising techniques for the removal of POPs, which are an emerging concern for the environment (Gmurek et al., 2017). The advanced oxidation methods that use light as a driving catalyst to create hydroxyl radicals include photolysis, photo-Fenton process, and photocatalysis. Photocatalysis is widely studied and used for the remediation of persistent organic pollutants. Photocatalysis is a process that combines photochemistry and catalysis and uses a synthetic substance as a catalyst. Photocatalysts can be classified into a variety of categories depending on their compositions, sizes, diameters, electrical properties, and other characteristics. It includes typical semiconductors, molecular, plasmonic, 2D, quantum dots, metal organic frameworks, etc.(Jouyandeh et al., 2021). Some of the applications of photocatalysis include toxicity reduction of real wastewaters, abatement of air pollutants, disinfection, self-cleaning, green chemistry, degradation of natural organic matter, removal of inorganic compounds, medical applications, photodynamic therapy, hydrogen production, removal of contaminants in wastewater, and so on (Ibhadon and Fitzpatrick, 2013). The two types of photocatalysis processes that are well known are homogenous and heterogenous photocatalysis. The homogenous photocatalysis technique uses homogenous photocatalysts, in which the medium is in the same phase as the photocatalyst and the reaction. Heterogenous photocatalysis has become one of the most promising options for environmental remediation due to its capacity to produce highly reactive oxidizing species that can remove a wide spectrum of contaminants (Kar et al., 2021). In addition to several applications, there are challenges and disadvantages that are associated with the photocatalysis process. Advances in technology have opened new doors to overcome problems associated with photocatalytic degradation, but photocatalysis still faces challenges such as longer reaction times, lower efficiency, less recyclability of the photocatalyst for continuous use, high recombination rate, and less adsorption of the photocatalyst’s active surface (Tahir et al., 2020).
Considering the detrimental effects of POPs and the urgent need for sustainable mitigation of persistent organic pollutants, this review, therefore, aims to summarize recent methods for removal of POPs through advanced oxidation technologies and photocatalysis. In addition, this review also dispenses data about POPs, their types, sources, and their effects on human health. The review mostly emphasizes on the photocatalysis-mediated remediation of POPs and the photocatalytic degradation mechanism, its types, and challenges faced during this process. Heterogenous photocatalysis has been described in detail with the challenges, future aspects, advantages, and the photocatalytic mechanism. While reviewing the literature, photocatalytic degradation was found to be effective as compared to the other organic pollutant-degradation techniques.
Techniques for the degradation of persistent organic pollutants
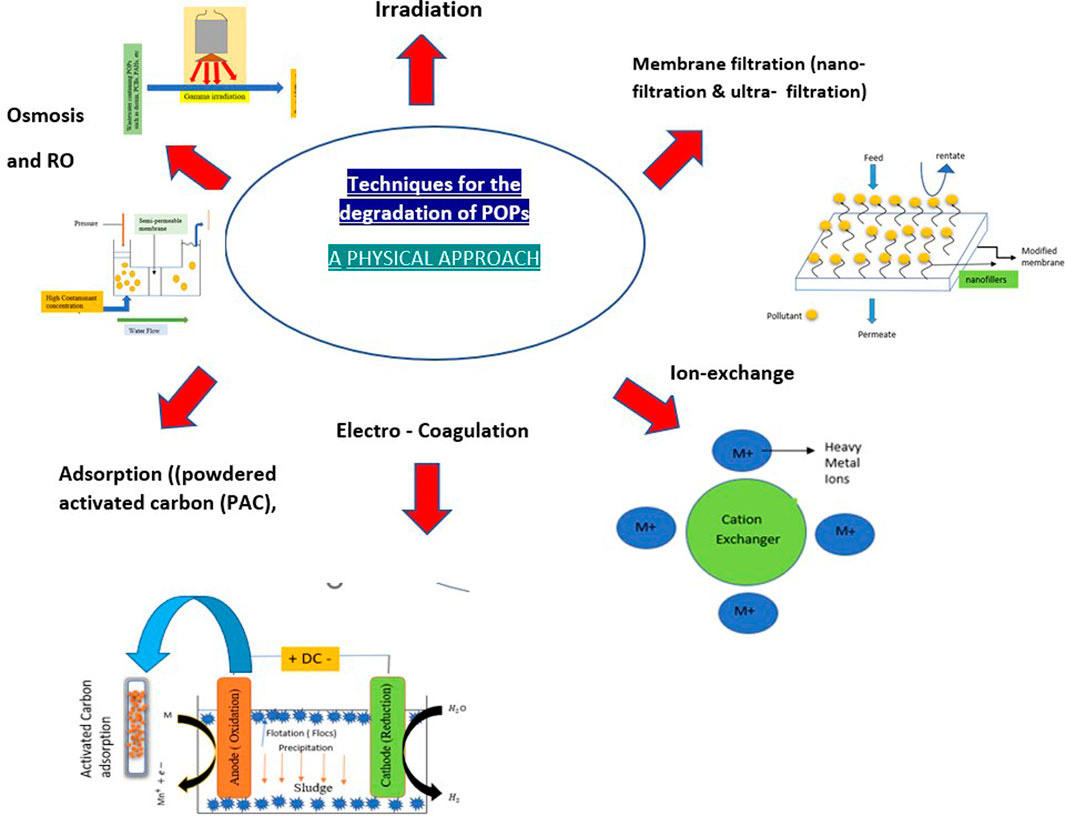
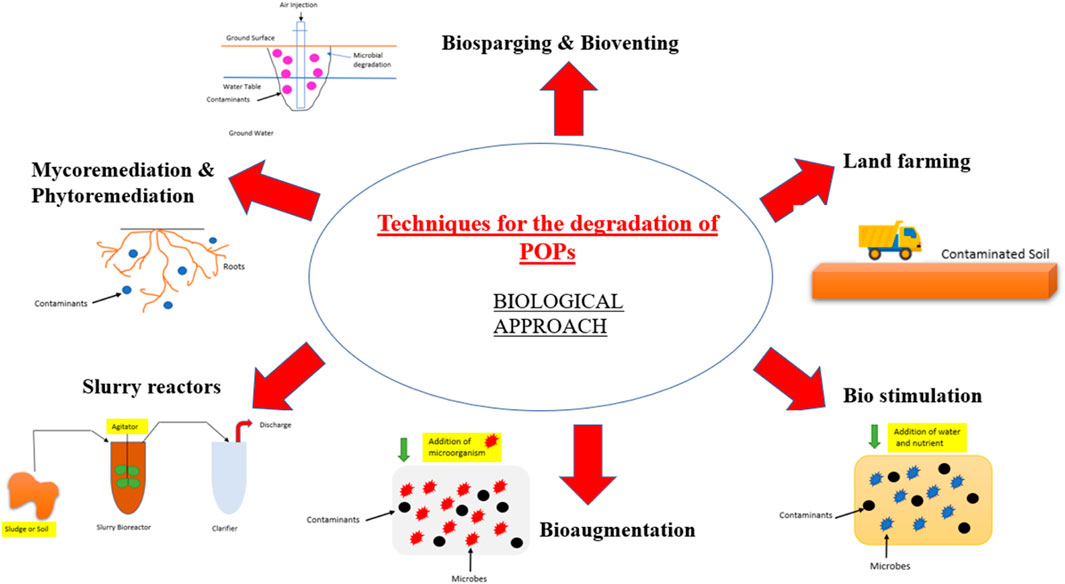
Persistent organic pollutants are environmental hazards that are resistant to degradation and may not be entirely eliminated by treatment techniques. Despite bans on some of them and their restricted use, we can still find their residues in the environment. POPs are known to have a toxic and deleterious effect on the health of the human population and environment, so it becomes very crucial to remove these substances from the environment. There are generally three types of approaches that are used to degrade organic pollutants: chemical approach, physical approach, and biological approach (Figures 1, 2, 3).
Advanced oxidation technologies are an important part of chemical approaches, which include electrochemical routes, sonolysis, photocatalysis, ozonation, photo-Fenton, and Fenton’s reaction (Nguyen et al., 2020). The biological approach makes use of selected microorganisms for the degradation of unwanted substances present in the environment such as pesticides, textile dyes, hydrocarbons, and organic pollutants (Guo et al., 2019b). Methods that are generally included in the biological approach are biosparging, bioventing, land farming, composting, slurry reactors, biostimulation, bioaugmentation, and mycoremediation (Akhtar and Mannan 2020; Taoufik et al., 2021). Physical approaches are in the developing phase and incorporate techniques such as membrane filtration (nano-filtration and ultrafiltration), coagulation, adsorption (powdered activated carbon (PAC), granular activated carbon (GAC), etc), ion exchange, irradiation, osmosis, and reverse osmosis.
Conventional techniques include adsorption, incineration, solvent extraction, landfill stabilization/curing, composting, coagulation–flocculation–sedimentation, membrane filtration, ozonation, biological methods, phytoremediation, catalytic membrane reactors, and advanced oxidation technologies (AOTs). Table 2 presents the typical findings observed in the degradation of organic pollutants by using various remediation methods, which also include various conventional techniques. Biological processes generally involve living organisms such as bacteria, fungi, algae, and plants for the remediation of pollutants. Bioremediation is a useful technique with high throughput, cost-effectiveness, and eco-friendly properties. It uses microorganisms for the removal of organic pollutants under either aerobic or anaerobic conditions (Natarajan et al., 2020). The presence of enzymes in the microbes make them useful for the remediation process of the pollutants because they attack the pollutant and convert them into less toxic substances (Mishra et al., 2020). Another technique used is phytoremediation, which is usually carried out by using the plants or through the involvement of bacteria in the system (Irga et al., 2018). This technique has been previously used in the removal of organochloride pesticides from the soil and water. Phytoremediation involves various processes, such as rhizoremediation, phytodegradation, phytovolatilization, phytoaccumulation, and phytoextraction. The biological methods are not convenient for the degradation of complex organic pollutants; in addition, physical and chemical methods are also not quite successful in the degradation because of the chemicals that remain untreated and the sludge treatment (Valizadeh et al., 2021). The physical methods have drawbacks while treating wastewater, such as the production of solid waste and secondary contamination. One of the techniques, coagulation–flocculation via chemicals, generates lots of sludge and chemicals, which are hazardous to the health of the environment and organisms. Adsorption is also costly because of the need for regular regeneration of the adsorbent as the contaminant gets adsorbed on its surface (Gusain et al., 2019). Incineration is also used for the reduction of textile dyeing sludge, but the main drawback of this technique is that it produces harmful gases during the process, which are generally released into the environment (de Titto and Savino, 2019). Landfills are used to discard the textile dyeing sludge, but it is very toxic for the environment because it contains harmful aromatic amines which are absorbed in the soils (Vaverková, 2019). When techniques such as membrane filtration, osmosis, and reverse osmosis are used for large-scale remediation of wastewater, these techniques do not remain cost-effective in addition to operational difficulties.
One of the major drawbacks of physical methods is that it does not completely remove the pollutant from the environment. Aerobic and anaerobic biological treatments are also not suitable for the high concentration of the pollutant. The capacity of biodegradability becomes limited when this technology is used for the removal of pharmaceuticals and non-organic compounds from wastewater. Biodegradation is not effective for the removal of pollutants because some of the pollutants are non-biodegradable; in addition, the time required for the degradation is high along with use of sophisticated equipment (Akhtar and Mannan 2020). These conventional techniques have shortcomings, but by understanding them and making improvements, overcoming these shortcomings is possible.
Wastewater, which is difficult to treat using biological treatment, is treated with advanced oxidation technologies because of their effectiveness in treating refractory pollutants present in the wastewater. Advanced oxidation technologies are more expensive in comparison to biological remediation techniques because of the additional energy and expensive chemicals used. In recent years, sulfate radical (
AOTs are widely used for the remediation of toxic, complex, and bio-refractory contaminants because of several advantages, such as high efficiency, non-selectivity, great reproducibility, simplicity, and easy operation. In AOTs, many pollutants are oxidized by hydroxyl radicals and sulfate radicals to become less harmful compounds and later ultimately mineralized to the CO2 and H2O. Recent research studies show that the AOTs are effectively used for the degradation of common pollutants such as organic dyes, surfactants, hydrocarbons, phenols, pharmaceutical active ingredients, PAHs, and pesticides (Baruah et al., 2016; Singh et al., 2020).
Table 1 provides a brief overview of some of the strategies used to remove persistent organic pollutants, including those that have been used in the past and present and those that are currently being developed. The discussion in the list significantly points to one direction: the present and future of mitigation of persistent organic pollutants are greener technologies, that is, photocatalytic degradation, which is briefly discussed in the upcoming section of this study. The photocatalytic treatment technique is an alternative that will soon be commercialized. The photocatalytic technique effectively eliminates contaminants ranging from traditional to emergent organic pollutants, including pathogens, viruses, detergents, and pesticides (Nguyen et al., 2020). In order to broaden the range of applications, it is also necessary to investigate the potential for combining various techniques with other technologies to increase the quantum yield and decrease the toxicity of the by-products and secondary pollutants that are produced. Many technological constraints must be overcome in the near future, from catalyst development to reactor design and process optimization, in order to make photocatalytic treatment technology more practical and feasible. In order to use semiconductor metal oxides and other nanoparticles for photocatalytic treatment in the future, it will be necessary to examine and assess the toxicity impact, cost analysis research, regeneration potential, and reusability potential. There is an identified gap between the commercialization of remediation techniques from the pilot scale. For a better understanding of the application at full scale, more research and investigation must be carried out at a pilot scale for usefulness so that the identified gap can be minimized (Daramola and Adebayo, 2021).
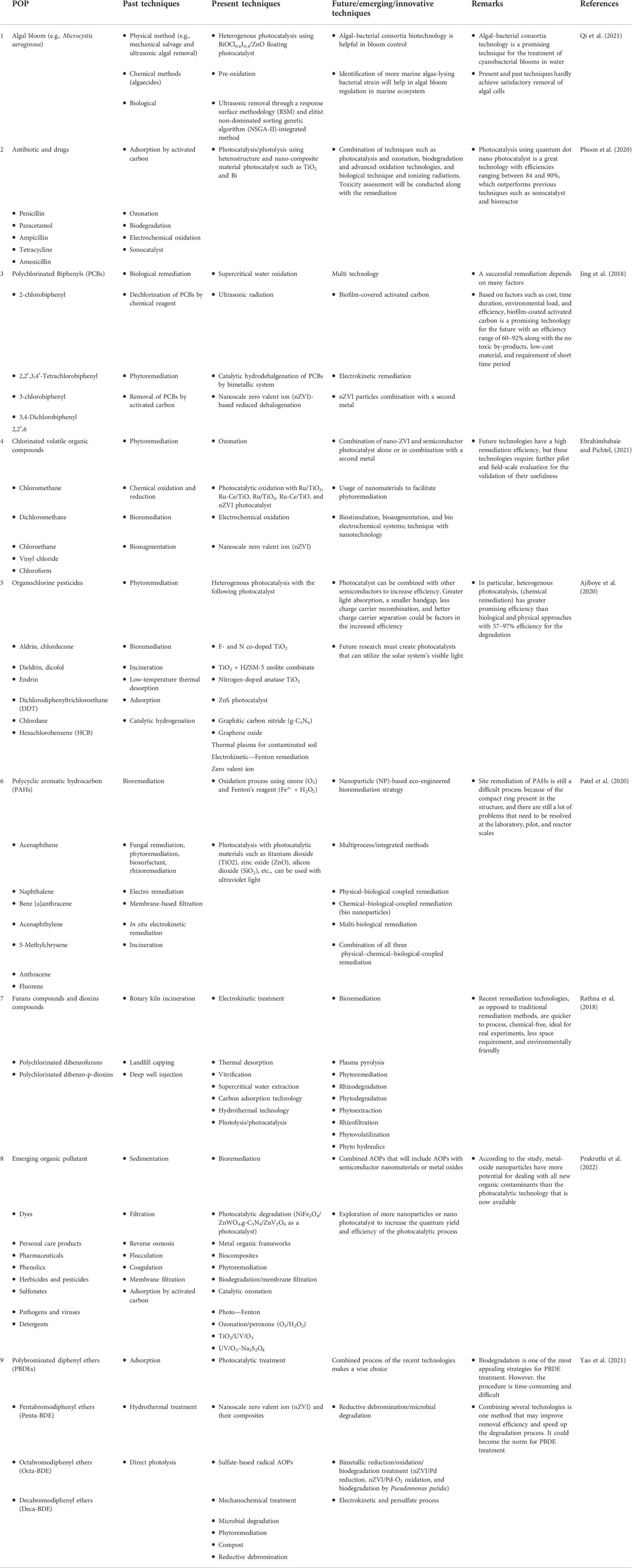
TABLE 1. List of strategies used for the remediation of persistent organic pollutants, covering past, present, and future/emerging/innovative techniques.
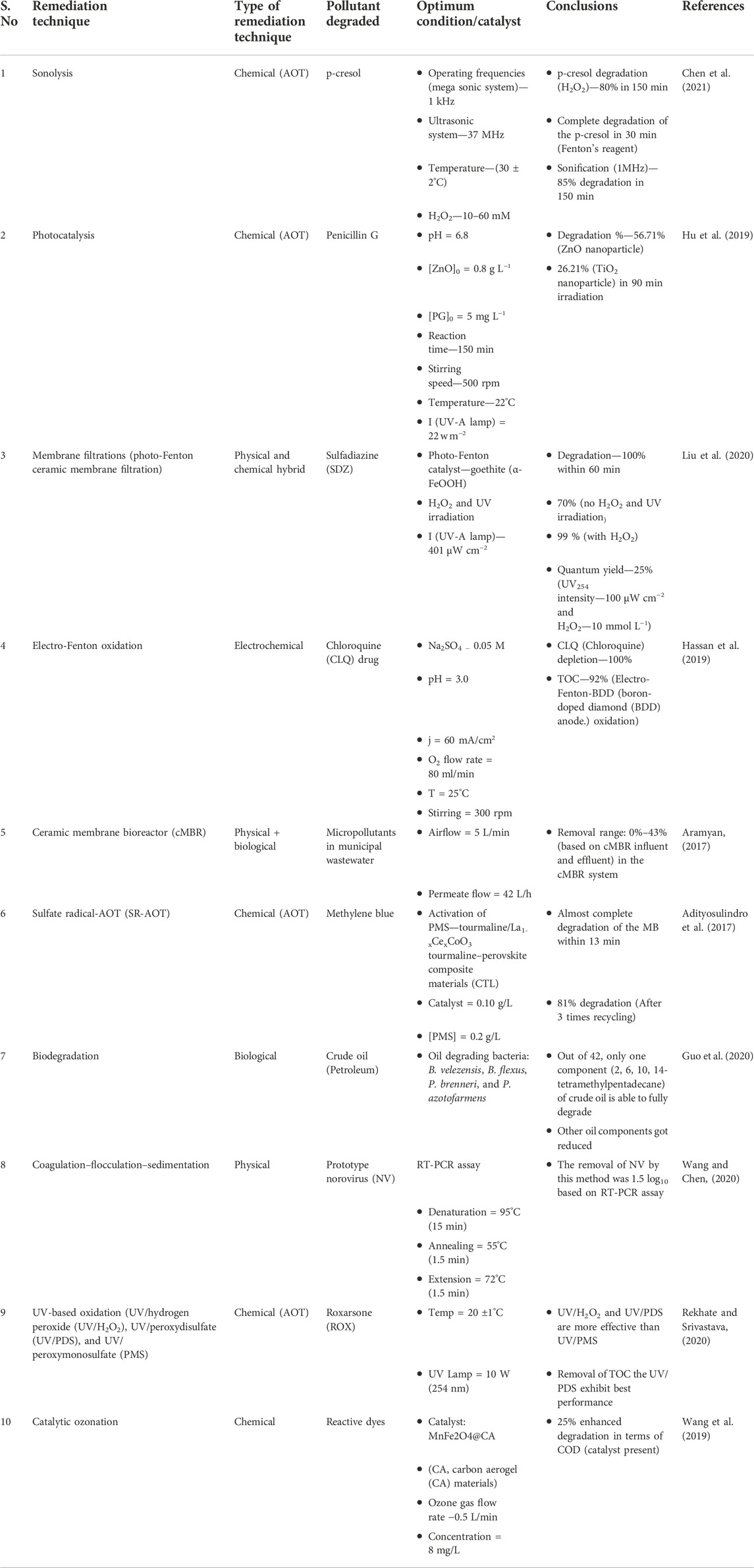
TABLE 2. Typical findings observed in the degradation of organic pollutants by using various remediation methods.
Techniques for the detection of pollutants
For effective remediation processes, there is a need for reliable, sensitive, and fast techniques for the screening and detection of pollutants (Moldovan et al., 2021). For detection of pollutants, electrochemical sensors, absorbance sensors, surface plasmon resonance (SPR), piezoelectric sensors, thermal sensors, enzyme/antibiotic sensors, MS (mass spectrometry), inductively coupled plasma mass spectrometry (ICPMS), OES (optical emission spectroscopy), Rayleigh scattering and post-sample fluorescence method, luminescence sensors, atomic absorption/emission spectroscopy, X-ray absorption spectroscopy, and surface-enhanced Raman scattering (SERS) are the techniques available (Singh et al., 2020). The available techniques require sophisticated ultra-modern instrumentation, which increases their cost price; therefore, these techniques are highly expensive and time-consuming. Sensors have received a great amount of attention for environmental pollutant detection in recent years. They are reliable, inexpensive, and accurate. Various functional materials are also used in environmental sensors, such as graphene, carbon nanotubes, graphitic carbon nitride, nano metals and their oxide materials, conductive polymer materials, nanoparticles, and mesoporous materials. The sensing method for the detection of environmental pollutants is still in the laboratory research stage, but they have good potential because of the attributes they have such as low cost, flexibility, portability, high selectivity, and high accuracy (Zhang et al., 2021).
Biosensors have been emerging over the past decade because they are easy, sensitive, fast, selective, and cost-effective. They can be classified into various types based on the type of analyte signal generated by the transducing element. Electrochemical and optical biosensors are the two types of biosensors that are used for pollutant detection (Yadav et al., 2021). There are still challenges to using the biosensor technique because of the low flexibility for the analyte and the low detection limit, but nanotechnology came as an alternative for increasing specificity and flexibility (Sposito et al., 2018). The introduction of nanomaterials with biosensors has reduced the disadvantages associated with them. The combination of nanomaterials with biosensors has been used for the detection of heavy metals, pathogens, pesticides, etc. The most recent emerging technique used for environmental pollutant detection is metal nanoclusters (MNCs). MNCs are made up of a few to hundreds of metal atoms with particle sizes that are close to the electron Fermi wavelength. Because of their low toxicity, intense fluorescence, and great biocompatibility, MNCs have great potential as fluorescent probes. MNCs are used for the detection of inorganic, organic, and microbial pollutants (MU et al., 2021). Another promising tool used for the monitoring of hazardous chemicals in the environment is nanosensors. These nanosensors detect pollutants by detecting pollutants’ surface markers or boosting the analytical signal. Compared to conventional approaches, nanosensors’ unique characteristics make them dependable for sensitive detection of extremely low pollutant concentrations. Silver, zinc oxide, and silicon oxide nanoparticles are used in these nanosensors (Potes-Lesoinne et al., 2022). To address the toxicity hazards of chemically produced nanomaterials, green nanomaterials have received increased attention. As a result, nanotechnology has become a platform with several applications for creating a sustainable environment for current and upcoming generations.
Advanced oxidation technologies
Advanced oxidation technologies (AOTs) are emergent, simple, cost-effective, and eco-friendly methods without secondary pollutant generation that effectively degrade recalcitrant organic pollutants such as aromatic amines and polycyclic aromatic hydrocarbons (PAHs). AOTs work on the in situ production of strong oxidizing agents including reactive oxygen species, such as singlet oxygen (1O2), hydroxyl radical (OH·), superoxide radicals (O2−), sulfate radicals (
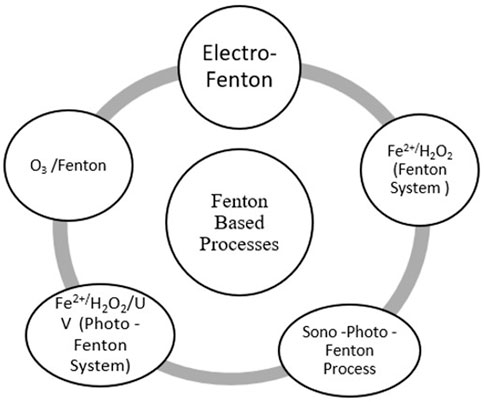
On the basis of photo-driven reactions, AOTs can be classified into two processes: 1) photochemical process and 2) non-photochemical process, as mentioned in Figure 4.
Fenton and Fenton system: The Fenton system includes Fenton, electro-Fenton, photo-Fenton, sono-photo-Fenton, sono-electro-Fenton, and photo-electro-Fenton, which are typical hydroxyl radical (OH) AOTs, as mentioned in Figure 5 (Rueda-Márquez et al., 2020). Fenton reagents are used when the activation of H2O2 is carried out by iron salts with the help of the oxidation process. Eq. 4 is a Fenton process that takes place at ambient temperature and pressure. It is a simple way to generate hydroxyl radicals that will destroy the organic compound by reacting with them. To produce the maximum amount of hydroxyl radicals, the process requires an acidic condition (pH 2–3). This method has wide applications because it requires easily available hydrogen peroxide and hydrogen salts.
Easy availability of the iron salts and H2O2 makes this AOT cost-effective and practically viable. It shows high efficiency in the mineralization of organic pollutants into simpler compounds such as non-toxic carbon dioxide. Another attribute of this system is that it requires a shorter reaction time for the generation of hydroxyl radicals by the rapid reaction between the H2O2 and iron salts. Complicated reactor facilities are not required, and the system can work in ambient temperature and pressure. The Fenton process can be easily integrated with the pre-existing organic pollutant remediation processes such as coagulation and filtration because of the uncomplicated, flexible, and straightforward nature of the process. The Fenton system is popularly used for the remediation of wastewater and soil (Thakur and Chauhan, 2016). There are some drawbacks that have been identified such as a rigid pH range, excessive solid ferric sludge formation, ineffective utilization of hydrogen peroxide, and radical scavenging of H2O2, which lead to the wastage of oxidants. The rate of degradation of pollutants can be increased by the combination of H2O2 and UV radiation with Fe+2 or Fe+3 oxalate ions. This photo-Fenton (PF) process increases the hydroxyl radical formation (Eq. 7).
Ozonation: Ozonation is one of the efficient methods of AOTs. In ozonation reactions, organic pollutants react directly or indirectly with the O3 molecule. O3 molecule decomposition leads to the generation of hydroxyl radicals. Ozone is also the strongest oxidant (Eq. 8) with high reactivity. There are two main mechanisms of ozonation: indirect and direct. In the direct mechanism, the molecular ozone directly does the electrophilic attack, and the indirect method involves an indirect attack by the hydroxyl radicals generated through the decomposition of ozone. The main ozonation reaction takes place in the alkaline medium because the disintegration of the ozone molecule with the water molecule is improved (de Titto and Savino, 2019). Ozone alone is not sufficient to cause complete degradation of the refractory organic pollutant that is why it is combined with H2O2, UV light, catalyst, ultrasound, etc. to enhance the generation of hydroxyl radicals by the ozonation process. An appropriate catalyst or UV light irradiation can enhance the efficiency of the ozonation reaction (Eqs 10–12). The ozonation process can also be sped up with the help of homogenous and heterogenous catalysts (Vaverková, 2019). Transition metal ions such as Co(II), Mn(II), Ni(II), Fe(II), Mn(IV), Cu(II), Cr(II), Cd(II), Ag(II), and Zn(II) are involved in the homogenous catalytic ozonation, whereas in heterogenous catalytic ozonation, metal oxides such as MnO2, TiO2, or metals on metal oxide are present. Ozonation AOT includes O3/UV, O3/Fe (II), O3/metal oxide catalyst, O3/activated carbon, O3/ultrasound, O3/Fenton, photocatalytic ozonation, and O3/H2O2 (Eq. 9); additionally, a simple ozone molecule is insufficient because ozone alone does not facilitate complete oxidation due to a low reaction rate for the degradation of pollutant. pH, ozone dosage, ozone flow rate, catalyst loading, temperature, and reaction time are the influencing factors in the catalytic ozonation reaction.
Ozonation is the most commonly used AOT for the degradation and oxidation of organic, inorganic, and micropollutants. Water disinfection or wastewater released from the petroleum refineries, textile industries, pharmaceutical industries, food industry, pulp, etc. is another area where ozonation-based AOT is most commonly employed (Singh and Borthakur, 2018). Ozonation is efficient in wastewater reclamation because of the reduced sludge formation during the process and the effective removal of the recalcitrant organic contaminants from the wastewater released from the various industries (Balachandran et al., 2016)
Sulfate radical–based advanced oxidation technologies (SR-AOTs): SR-AOTs have recently emerged as a promising alternative for the degradation of the organic contaminants and removal of the recalcitrant organic pollutant present in the air, soil, and water (Berkani et al., 2022). The reactive oxygen species generated in this type of AOT is sulfate radicals (
(peroxymonosulfate)
where PDS is peroxydisulfate.
In comparison to the hydroxyl radical, the sulfate radical selectively reacts with many refractory contaminants such as endocrine disruptor, pharmaceuticals, perfluorinated compounds, perfluorooctanoic acid (PFOA), and personal care products (Sun B. et al., 2020; Sun S. et al., 2020). The treatment of landfill leachate (LL) is mostly carried out by SR-AOTs. SR-AOTs have shown good performance across a wide pH range (3–9), especially in the alkaline and neutral media. SR-AOTs have been used for the treatment of petroleum wastewater, pharmaceutical wastewater, pulp and paper wastewater, textile wastewater, winery wastewater, coking wastewater, etc (Midassi et al., 2020). There are some disadvantages to using SR-AOTs: the presence of residual cation ions and sulfate ions in the effluent, costly expenses, higher amount of PMS/PDS is required for the elimination of contaminates, and the occasional formation of toxic by-products in the presence of Cl− and Br−.
Photocatalytic degradation
Photocatalysis can be described as the absorption of photons on the photocatalyst (solid material), which induces the chemical reaction that results in the decomposition of the adsorbed molecules on the photocatalyst (Hassan et al., 2019). The process of photocatalysis started with the transfer of the pollutant to the photocatalyst surface, and this leads to the adsorption of the pollutants on the surface. When the photocatalytic material of the photocatalyst is exposed to the UV or visible light with equal or greater energy than that of the photocatalyst’s bandgap, it results in the generation of strong reducing and oxidizing agents (electron–hole pair) that will eventually disassociate into electrons (e−) and holes (h+) in the conduction band and valence band, respectively. This photonic activation (e− and h+) causes the reduction and the oxidation of the adsorbed molecule present on the surface of the photocatalytic material. This is followed by desorption of the products that are obtained after the reaction and their removal from the photocatalyst surface. Oxidation and reduction reactions occur simultaneously in the photocatalytic reaction (equation 22–33). The crystal structure of the catalyst and the energy of the photons coming from the UV or visible light determine the reaction rate of the reaction. The specific mechanism of photocatalysis using TiO2 photocatalyst is the reaction between the organic pollutants and the strong oxidizing and reducing agents (electron–hole pair) under irradiation of the UV or visible light (Aramyan 2017)(Eqs 17–21). Figure 6 illustrates the mechanism of photocatalysis degrading organic pollutants using the
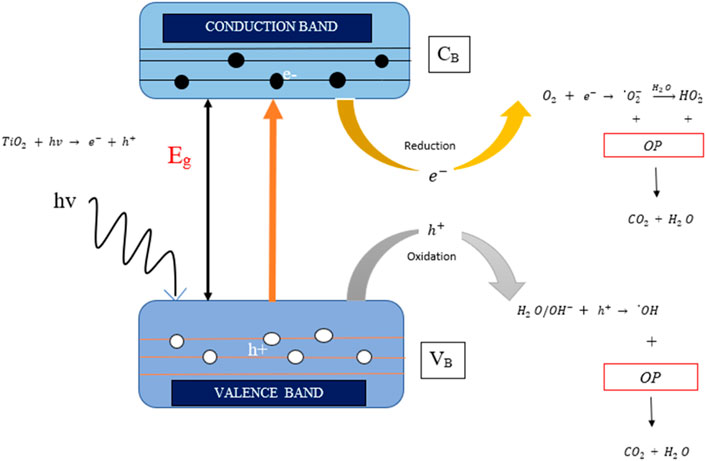
FIGURE 6. Mechanism of photocatalysis degrading organic pollutant using
The detailed mechanism reaction of the fundamental process during photocatalysis is as follows:
There are various advantages of the photocatalysis process which makes it a promising advanced oxidation technologies (AOT) to decompose and mineralize organic pollutants into harmless products, CO2 and H2O, while only utilizing the atmospheric oxygen under ambient operating temperature and pressure. Another reason that this AOT is environmentally friendly is that it does not cause secondary pollution. This technique saves a lot of energy because it utilizes solar energy for the degradation process (Adityosulindro et al., 2017).
Photocatalysts are the central element of the photocatalysis process that converts solar energy into a chemical process to degrade the organic pollutant (Guo et al., 2020). For the photocatalysis process, the semiconductor photocatalyst is widely used because semiconductors have a moderate bandgap and oxidation and reduction can simultaneously occur on the surface of the photocatalyst (Wang and Chen 2020). Metal oxides are best suitable as photocatalysts because of their favorable light absorption, electronic structure, and bandgap, which is within the UV-visible range (Rekhate and Srivastava 2020). Among all of them, TiO2 (titanium dioxide) gained the most attention because of its efficient and excellent performance under UV irradiation (Wang et al., 2019). Other semiconductor catalysts are used; some of them are ZnO (n-type semiconductor), WOx (transition metal oxide and n-type semiconductor), SnO2 (n-type semiconductor), CdS, graphene, graphite carbon nitride, etc. (Hou et al., 2021; Ma et al., 2021). Many photocatalysts were synthesized and used for the degradation of the organic pollutant, but some of the drawbacks of the semiconductor photocatalyst remain, such as rapid recombination of the electron/hole resulting from the low quantum yield of the process, low adsorption, and low surface coverage of the photocatalyst which decreases the efficiency of the process. Therefore, a novel photocatalyst gained attention for improving the photocatalysis process (Table 4).

TABLE 4. Some of the reaction mechanisms for the degradation of persistent organic pollutants using advanced oxidation technologies, such as photocatalysis.
The photocatalysis process has been used for the removal of toxic compounds from aqueous solutions such as dyes, phenolic compounds, and petroleum hydrocarbons (Yang et al., 2019). Microbes and toxic chemicals are also eliminated by the photocatalysis process (Mohammed and Ali 2019). Photocatalysis is also used for the production of hydrogen gas (Ismail and Bahnemann 2014). Wastewater treatment, air treatment, and disinfection are other applications of the photocatalysis process (Teodosiu et al., 2018; Escobedo and Lasa 2020; Kouchakpour et al., 2021). Depending on the catalyst phase, the AO mechanism can be divided into two categories: homogenous and heterogenous photocatalysis. In homogenous photocatalysis, the reactant and the photocatalyst are present in the same phase and include ozone and Fenton’s reagent, which is a mixture of hydrogen peroxide and Fe+2 salts, to produce reactive species hydroxyl radicals under UV irradiation. In heterogenous photocatalysis, the catalyst and the reactant are not in the same phase and semiconductor oxides are most commonly used as a photocatalyst. Photocatalytic ozonation includes the presence of photo-catalyst, UV-vis radiation, and ozone. In this process, the ozone molecule is adsorbed on the surface of the photocatalyst, which leads to the generation of active oxygen radicals and is followed by the reaction of water with active oxygen radicals to form hydroxyl radicals (Eq. 34).
Operational factors affect photocatalysis efficiency in addition to the intrinsic properties of the photocatalyst. Factors that affect photocatalytic activity are bandgap energy, surface area/structure of the catalyst, light intensity of the irradiated light, temperature, pH, recombination rate, and electron–hole pair separation (Shivaraju et al., 2016). Haque and Muneer (2007) briefly discuss the significance of pH. The pH value affects the surface charge properties of the photocatalyst, size of the photocatalytic aggregate, and even the sites of conductance, making it one of the significant operational elements in heterogenous photocatalysis. Gaya and Abdullah (2008) explained the directly proportional relationship between rate of photocatalysis reaction and photocatalyst dosage. However, the photocatalyst dosage should be used in the optimal level. Because of the light screening effect brought on by the extra photocatalytic particles, the response rate reduces when the dose exceeds the ideal amount. When there are too many photocatalytic particles in the system, less light illuminates the surface, which changes the photocatalytic efficacy. Temperature is another crucial parameter that directly affects the photocatalytic efficacy. Herrmann (2010) estimated that the ideal temperature is between 20°C and 80°C. The quantum yield, radiant flux, and light intensity are also operational factors that affect the photocatalytic efficacy. To achieve a high photocatalytic degradation rate in wastewater treatment, strong light intensity is required to provide photocatalytic particles with enough photon energy. Saquib and Muneer (2003) also clarified that pollutant loading also causes variations in the photocatalytic efficiency. It has been demonstrated that significant concentrations of organic contaminants can saturate the photocatalytic surface, decreasing photonic efficiency and deactivating the photocatalyst in use.
Due to the distinct physical characteristics of materials at the nanoscale, nanotechnology has attracted a lot of attention in recent years. Nanotechnology also contributes to environmental remediation by the synthesis of the nanoparticles, nanowires, and nano-thin films, which are used in photocatalysis as nanocatalysts (Tang et al., 2020). Given that the nanoparticles have a high surface area-to-volume ratio and generally exhibit higher reactivity due to this, nanotechnology-based materials are especially suitable for environmental remediation operations due to their improved characteristics and efficacy. One of the many distinct types of materials that may be used effectively for a number of environmental remediation applications is inorganic, bio-fabricated, carbonaceous, and polymeric nanoparticles. Metal (Ag NPs/Ag ions, TiO2 NPs, metal-doped TiO2, and titanate nanotubes), metal oxide, and silica nanomaterials (amine-modified xerogels, amine-modified aluminosilicates and porous silica, and thiol-functionalized mesoporous silica) are included in the inorganic nanomaterials. Fullerene C60, fullerene C540, single-walled nanotubes, multi-walled nanotubes, and graphene are included in carbon-based nanomaterials because of their unique chemical, physical, and electronic properties of the carbonaceous material (Guerra et al., 2018). ZnO and CuO nanoparticles (metal nanoparticles), polymeric nanoparticles (organic nanoparticles with the shape of nanosphere or nano capsular), and carbon-based nanoparticles (graphene nanotubes, carbon nanotubes, and carbon fullerenes) are used as the photocatalyst for the solar-driven photodegradation process of persistent organic pollutants (Tang and Wang 2018; Kråkström et al., 2020).
The creation of diversely sized and shaped nanoparticles by a general synthesis involves the use of a variety of physical, chemical, and biological methods. A variety of techniques are included in the nanoparticle synthesis with the use of top-down method and the bottom-up approaches, employing the physical procedures such as crushing and grinding with many methods, including laser and sputtering obliteration and chemical reductions. Bottom-up strategies include laser pyrolysis, chemical vapor deposition (CVD), and modeling such as spinning and green synthesis (Bhavya et al., 2021). Nanotechnology increases the efficiency of the photocatalytic reaction (Shu et al., 2016). However, the occurrence of aggregation, non-specificity, and low stability might restrict the usage of these nanotechnologies due to the lack of functionality, even if the large surface area-to-volume ratio of nanomaterials leads to increased reactivity with accompanying better performance. The application of solar photocatalysis for the degradation of organic pollutants at the industry-level scale is very costly. The implementation at a large scale is very high, but the nanotechnology ensures a cost-effective successful photothermal process for the wastewater treatment (Pandey et al., 2021). To fully utilize the potential of nanomaterials for environmental applications, these difficulties must be solved. However, there are additional strategies that can be used to mitigate environmental pollutants because of the nanotechnology.
Homogenous photocatalysis
Homogenous photocatalysts are used for the homogenous photocatalysis process (Guo et al., 2017). In this photocatalyst and the reaction, the medium is in the same phase. For example, the degradation of aqueous organic dye is photo-assisted using water-soluble carbon dots. In homogenous photocatalysis, the separation of used catalysts is a tedious task in comparison to heterogenous photocatalysis, as we can easily separate the catalyst material after the work. Homogenous photocatalyst is generally formed through coordination chemistry that contains well-defined monoatomic metal centers in the molecular complexes. Homogenous photocatalyst has high photocatalytic activity and selectivity because of the easy changes that can be implemented in the coordination of the central metal atoms with various organic ligands. Homogeneous photocatalysis is more expensive than heterogeneous photocatalysis because the homogeneous photocatalyst is more costlier and more difficult to separate for product purification and reuse for a longer period of time (Cheng et al., 2022).
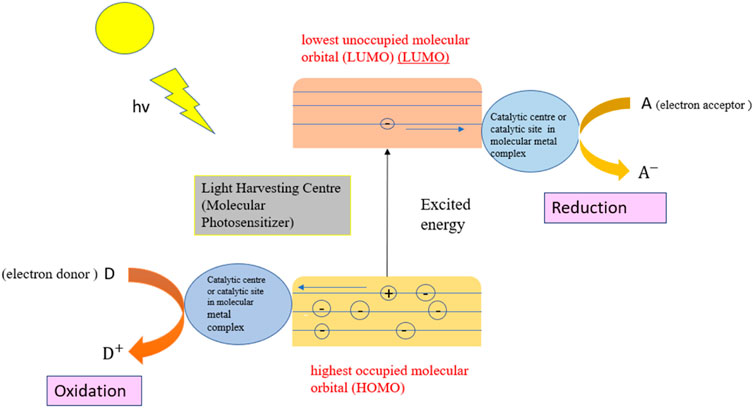
Light-harvesting centers and catalytically active sites are the two main components of the photocatalytic system. The light-harvesting center for homogenous photocatalysis is the light-absorbing system which is also called a molecular photosensitizer. The photosensitizer and catalytic units can also be homogenous molecules dissolved in an aqueous solution or any other medium. Under light irradiation, the electron gets excited from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) energy levels of the molecular photosensitizer. Figure 7 illustrates the photocatalytic process in a typical homogenous photocatalytic system. The large separation between HOMO and LUMO results in a very low percentage of absorption of the solar energy, so from the broad spectrum of solar energy, the photocatalyst absorbs very narrowly. The molecular photosensitizer is inherently very unstable by nature, which results in limited photocatalytic activity and stability of the homogenous photocatalyst.
Over the years, great effort and time have been devoted to obtaining the homogenous photocatalyst that can absorb a broad spectrum of solar energy and remain stable. Re(CO)3 (bpy)+ and [Ru (bpy)3]2+ are the two examples of homogenous photocatalyst (Nippatlapalli et al., 2022; Song et al., 2022). The light-harvesting centers and catalytic active sites are ideal in homogenous photocatalysts in comparison to heterogenous photocatalysts. The coordination chemistry has been utilized to unite the merits of homogenous and heterogenous photocatalysts to upgrade the photocatalysis process (Zeng et al., 2018). The homogeneous photocatalyst frequently uses the ozone and photo-Fenton processes (Capodaglio 2020). Fenton process is one of the ways to generate the hydroxyl radical. In an acidic medium, the hydroxyl radicals are generated from the mixture of hydrogen peroxide and Fe+2 (Eq. 35). The acidic medium prevents iron precipitation. Due to the consumption of Fe+2 during the reaction, Fe+3 is generated which decreases the rate of reaction because of the HO2 (E° = 1.65 V) generated after the reaction which is a weaker oxidant than the hydroxyl radical (E° = 2.80 V) (Eq. 36).
To accelerate the photoreduction of Fe+3 to Fe+2, the photo-Fenton process is used in which the process is enhanced by UV-visible radiation. This photo-Fenton results in the extra generation of the hydroxyl radical (Eqs 37, 38).
The photo-Fenton process is used for the remediation of the organic contaminants from the wastewater (Prete et al., 2021). Iron sludge formation after the reaction creates another task, which needs to be removed later. The homogenous Fenton process requires a high concentration of the ferrous catalyst, whereas in photo-Fenton, the amount of sludge produced and the concentration of the ferrous catalyst required is optimal. The concentration of ions dissolved in the wastewater significantly affects the photo-Fenton process. The low toxicity of Fe and its high abundance in the environment makes the photo-Fenton process highly environment friendly.
Solar-powered photocatalysis treatment of wastewater is a promising sustainable method for resolving the worldwide water crisis. For wastewater treatment, heterogenous and homogenous photocatalysis approaches were used. Homogenous photocatalysis was used by (Dükkanci et al., 2014) for the effective Orange II dye degradation. After 2 h, 80.8 percent decolorization was achieved using the homogenous Fenton device and a combination of ultrasound and ultraviolet light, indicating that UV promotes Orange II degradation. The use of ferric (Fe(III)) ions in homogenous photocatalysis appears to be an efficient and cost-effective approach of producing •OH radicals for dye degradation. Using ferric ions (Fe(III)) and visible light (VL) irradiation, the homogenous photocatalytic oxidation (PCO) of Reactive Black 5 (RB5) dye was investigated (SL) by Sadhu et al. (2020). Under artificial VL, more over 80% of the original 20 mg/L RB5 was decolorized in 60 min in the presence of 5 mM ferric ions at pH 2.6. The combination of both homogenous and heterogenous photocatalysis can be used for H2 production. One such example is dye-sensitized photocatalysis. It is a relatively recent method for converting sunlight into a fuel such as H2. The self-assembly of a molecular dye and an electrocatalyst on a semiconductor nanoparticle is the basis of dye-sensitized photocatalysis (DSP) using molecular catalysts (Willkomm et al., 2016). Tributyltin (TBT) is one of the hazardous organotin compounds that have been introduced into the marine environment by mankind. Brosillon et al. (2016) showed that the homogenous photocatalysis (Photo-Fenton) is a viable solution for the decontaminating marine debris problem in comparison to the heterogenous photocatalysis (TiO2 + UV).After 1 h of the photo-Fenton reaction, degradation yields for MBT (monobutyltin), DBT (dibutyltin), and TBT (tributyltin) varied from 52% to 76%, 27 %–73%, and 51%–79%, respectively. In homogenous photocatalytic processes, homogenous cocatalysts proved to be very effective electron-capturing species.
Using molecular cocatalysts in composite photocatalytic systems to capture more photogenerated electrons from photosensitizers might be a promising strategy. Irfan et al. (2021) showed that the homogenous cocatalysts (aminopyridine derived Co-complex) have led the way for increased electron capturing from photoexcited composite photocatalysts (CdS/Ni3C), and they offer a lot of potential for improving photocatalytic performance for low-cost H2 evolution under visible light. Nanoparticles have become popular as homogenous photocatalysts due to their enhanced features, such as a large surface-to-volume ratio, regulated uniform particle size and composition which enhances the degradation rate (Padmanaban et al., 2016). 3d complexes have been extensively researched as potential catalysts for solar fuel generation via water splitting and CO2 reduction. One of the examples of 3d complexes used for CO2 reduction is the CuII quarterpyridine complex [Cu(qpy)]2+. [Cu(qpy)]2+ was shown to be a highly efficient and selective catalyst for visible light-driven CO2 reduction in CH3CN, employing [Ru (bpy)3]2+(bpy:bipyridine) as a photosensitizer and BIH/TEOA (1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo [d]imidazole/triethanolamine) as the sacrificial reductant The inclusion of H2O (1–4% v/v) considerably enhances the photocatalytic process, and a turnover number of >12,400 for CO generation can be attained with 97 percent selectivity, making it one of the most efficient molecular 3d CO2 reduction catalysts (Guo et al., 2017). With atomically dispersed catalytic sites and tunable light absorption, homogenous photocatalysis is gaining a lot of attention because it offers higher activity and selectivity. As a result, there is a growing trend to combine the advantages of both homogenous and heterogenous photocatalysts, with coordination chemistry serving as the connecting link (Gao et al., 2017)
Heterogenous photocatalysis
In heterogenous photocatalytic reactions, two or more phases are used in which the semiconductor photocatalyst such as TiO2 gets excited by the UV or visible radiation coming from the solar spectrum in the presence of oxygen. For water and air treatment, heterogenous photocatalysis is a promising and rapidly advancing technology. The basic principle of heterogenous photocatalysis relies on the oxidative and reductive reaction that takes place on the surface of the semiconductor photocatalyst when the photocatalyst material gets exposed to the photon with energy equal to or greater than the semiconductor bandgap results in the generation of electron–hole pair. The electronic structure of the semiconductor consists of the valence band and the conduction band which plays a pivotal role in the photocatalytic process. Before excitation, the electrons and holes are present in the valence band, but when the photocatalyst is exposed to the photons with energies greater than the bandgap energy, this can result in the excitation of electrons in the valence band to the conduction band and the holes are there in the valence band. pH, photocatalyst concentration, substrate concentration, light intensity, wavelength, and the oxidizing agent are the operational factors influencing the photocatalytic reaction.
The most studied semiconductor photocatalyst is titanium dioxide. TiO2 is a naturally occurring mineral with excellent pigmentary properties, high ultraviolet (UV) absorption, and high stability which make it suitable for application in photocatalysis. TiO2 is an effective and notable catalyst that is used for the photocatalytic degradation of chemicals, organic pollutants such as herbicides, dyes, pesticides, phenolic compounds, and inorganic materials and also helped in the inactivation of microorganisms such as bacteria, molds, and yeasts present in the air or water (Bui et al., 2016.) One of the noteworthy points of TiO2 is that it operates only in the UV region. One of the advantages of TiO2 is that it is inexpensive because its continuous re-use is possible as it can be supported on various substrates such as glass, fibers, stainless steel, and sand. TiO2 photocatalyst has a lower quantum yield because of the fast recombination of the electron–hole pair which is generated during the reaction (Peiris et al., 2021). The visible light absorption is also low because of the larger bandgap which limits its utility, and absorption only takes place from the UV irradiation which makes the cost higher. To enhance the photoresponse activity of TiO2, the doping, formation of nanocomposites, surface modification, dye sensitization, noble metal, and non-noble metal deposition are tried out (Byrne et al., 2018; Cheng and Xu 2019; Vilar et al., 2019).
ZnO is an n-type semiconductor that has a bandgap similar (3.37 eV) to the TiO2 photocatalyst. The degradation mechanism and the drawbacks are also the same as the TiO2 photocatalyst, but the absorption efficiency of the ZnO is greater than that of the TiO2. ZnO is mostly suggested as the alternative photocatalyst to TiO2. The mechanism of the photodegradation of the organic pollutant in the presence of solar radiation using ZnO photocatalyst is as follows (equation 39–43):
1) Transport of the electrons from the valence band to the conduction band when ZnO is photo-induced by the solar light with (photonic energy) hv ≥ Eg (excitation energy).
2). e−/h+ pair is generated, and they will migrate to the ZnO surface and be involved in the redox reaction.
3). In the redox reaction, hydroxyl radicals and superoxide radical anions are generated. The hydroxyl radicals will degrade the pollutants adsorbed on the surface of the ZnO. Intermediate compounds are rapidly produced during the reaction, which will be converted into carbon dioxide and water.
ZnO has a higher efficiency because of the higher absorption of the light from the solar spectrum, which is UV/visible light. The photodegradation reaction of the ZnO is also perturbed by the rapid recombination of the electron/hole pair and its optical absorption because of its larger bandgap energy. To improve the ZnO efficiency, various attempts are done to reduce the recombination rate of the photogenerated electron–hole pair and decrease the energy bandgap. Transition metal oxide, SnO2, CdS, graphene, graphene carbon nitride (g-C3N4), and Zn2SnO4 are the various semiconductor photocatalyst that is used (Védrine 2017; Zhu and Zhou 2019; Chen X. et al., 2020). To enhance the semiconductor photocatalysis, the metal doping of the semiconductor and photodegradation of the pollutant by the hybrid nanomaterial photocatalyst is attempted to increase the mobility of electron/hole, improve the separation between the electron/hole pair, and enhance the visible light adsorption. Figure 8 illustrates the photodegradation mechanism of the pollutant by hybrid metal oxide photocatalyst nanomaterial. Non-metal doping and mix doping enhance photocatalytic activity by narrowing the bandgap and by the formation of the intra-bandgap energy states (Kuo et al., 2021). Coupling between the semiconductors with desirable matching electronic bandgap hinders the recombination of the photogenerated electron–hole pair. Ag-based semiconductor photocatalysis is an emerging method of photocatalysis. Nanomaterials (nanoparticles, nano-catalyst, nanofilms, nanofibers, nanomembranes, colloidal semiconductor nanocrystals, “quantum dots” (QDs), nanosheets, nanorods, etc.) are also used in the heterogenous photocatalysis as they possess the increased surface area-to-volume ratios and high surface reactivity compared to the other photocatalyst (Adhikari et al., 2018).
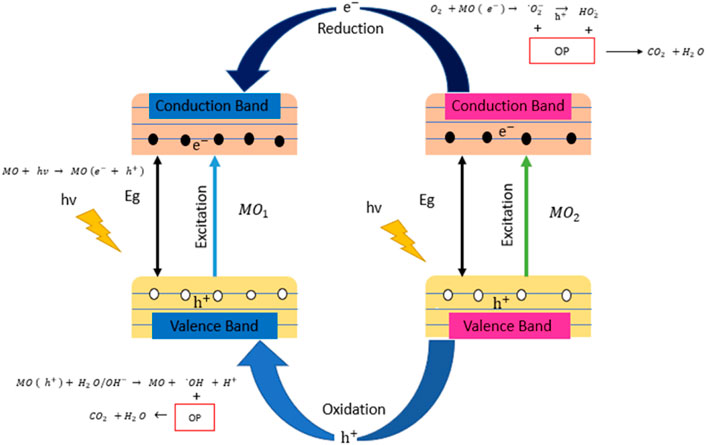
FIGURE 8. Illustration of the photodegradation of the pollutant by hybrid metal oxide photocatalyst nanomaterial. MO, metal oxide photocatalyst.
The ability to completely mineralize the organic pollutant in the environment makes the heterogenous photocatalysis the effective AOT for the wastewater treatment, which cannot be done effectively using secondary water treatment (Parul et al., 2020). No consumable chemicals are required during the heterogenous photocatalysis process, which makes this AOT inexpensive. The heterogenous photocatalysis process is relatively safe in comparison to other processes because reactions mostly proceed in ordinary temperature and pressure, which are mild conditions (Rueda-Marquez et al., 2020). One of the drawbacks of heterogenous photocatalysis is that its large-scale synthesis is not suitable because of the problem of the electron–hole recombination and lower absorption because of the smaller surface areas (Pawar and Lee 2015). The large-scale utilization of the heterogenous photocatalysis was successful only for the wastewater treatment with lower concentration of contaminants present in the wastewater.
The scientific community is interested in photocatalysis for POP degradation because it can generate hydroxyl radicals (•OH) for effective chemical oxidation of organic pollutants, including organic micropollutants (OMPs) (Bertagna Silva et al., 2021).UV-LED TiO2 photocatalysis is used for the degradation of ibuprofen with 97% degradation after 25 min (Ding and Hu, 2020). Photocatalysts based on semiconductors are also used for the detoxification of emerging pharmaceutical contaminants in aquatic systems. In the presence of Zr, Belver et al. observed almost complete (90 percent) antipyrine degradation within 6 h using Zr-doped TiO2 (Belver et al., 2017). Under UV illumination, acetaminophen photodegraded well when TiO2 was supported on zeolite. Under optimum TiO2 loading (40 wt% and 1.0 g/L conc) on the surface of zeolite, 96.6% of the degradation was ascribed to enhanced charge separation (Chang et al., 2015). Bisphenol A and 2–4 dichlorophenol organic pollutants were effectively degraded by the catalytic ozonation and photocatalysis synergy system using a double-functional MgO/g-C3N4 catalyst. Within 2 min, the degradation efficiency was approximately 100%, which was 18 and 1.5 times greater than that of the individual photocatalytic and catalytic ozonation activity, respectively (An et al., 2020). Hu et al., (2020) used the FeOOH/Bi2MoO6-OVs (oxygen vacancies) photocatalyst for effective degradation of phenol. The 10 % FeOOH/Bi2MoO6-OVs showed the best degrading activity when exposed to visible light. The elimination efficiency of phenol was 100% within 3 h, which was 1.54 times and 1.33 times faster than photocatalysis and Fenton alone, respectively.
Sonophotocatalytic processes are advanced integrated AOPs that combine sonolysis and photocatalysis to degrade organic pollutants effectively. To eliminate harmful wastewater impurities, these ecologically friendly technologies rely on ultrasound and the appropriate catalysts (Theerthagiri et al., 2021). Ghalamchi and Aber (2020) looked into the sonophotocatalytic activity of aminated silver phosphate/GCN (NH2-Ag3PO4/GCN) in the degradation of methylene blue (MB) dye molecules, achieving an 82% removal efficiency. For the degradation of 2,4-dichlorophenol (2,4-DCP) and rhodamine B, Neena et al. produced ZnO nanorods with nitrogen-doped reduced graphene oxide (N-rGO). They evaluated the photocatalytic performance of ZnO, GO, N-rGO, ZnO/rGO, and ZnO/N-rGO and discovered that the ZnO/N-rGO composite had the highest photocatalytic activity in all of the model processes (Neena et al., 2019).
Challenges of the photocatalysis
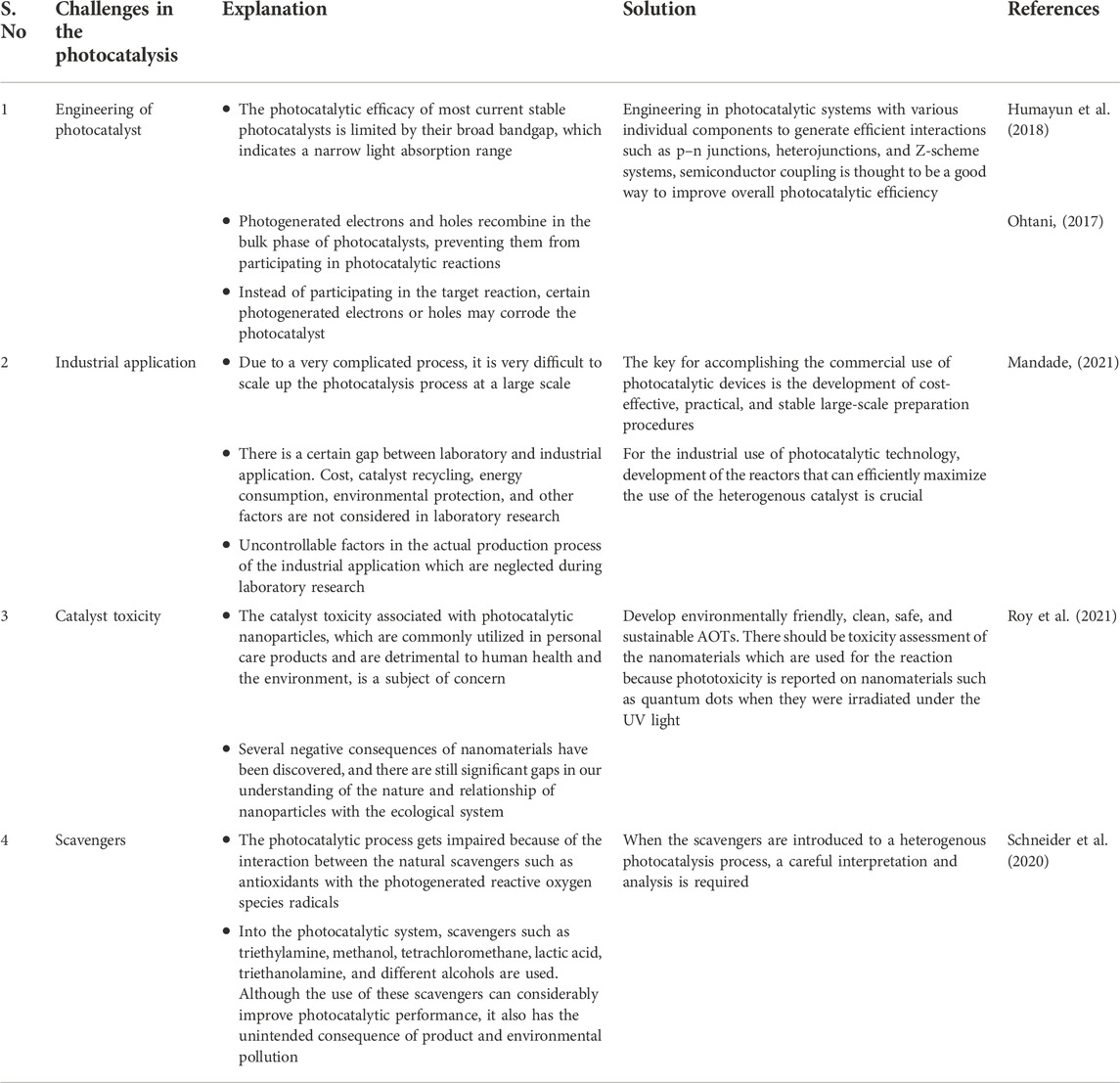
One of the major research challenges is to overcome the limitations of the photocatalysis process. There are various challenges that arise while engineering the photocatalyst. In general, the efficiency and stability of today’s heterogenous photocatalysts are still far from satisfactory because of the difficulties in controlling and balancing multiple competing processes, such as carrier generation, charge separation, and transportation, especially under the highly corrosive conditions of photochemical reactions (Guo et al., 2019a). When one component is introduced into photocatalysts to address one weakness, it frequently creates a new set of problems. Some of the examples of challenges in the photocatalysis are as follows: for the nanocomposite catalytic material, the attachment of the nanostructure to the substrate is a challenge; under solar irradiation, quantum efficiency reduces due to larger bandgap energy in the electronic structure of the semiconductor photocatalyst and photocatalytic activity and reactive oxygen species production reduce due to the electron/hole pair recombination on the active surface of the photocatalyst (Xu, 2021). Interaction with the intermediate products that are formed during the reaction results in the fast active surface site deactivation or poising, which will finally reduce the kinetic reaction rate. Due to a very complicated process, it is very difficult to scale up the photocatalysis process at a large scale. Integrating very dissimilar (e.g., metal and semiconductor) materials together at the nanoscale with well-defined size, shape, and interface, which are required for effective charge cascading in photocatalytic processes, continues to be a major issue (Saravanan et al., 2017). The research on heterogenous photocatalysts is still in its early stages, and more systematic research studies are certainly needed. Some of these challenges are briefly discussed in the Table 5. One of the most urgent challenges is to develop environmentally friendly, clean, safe, and sustainable AOTs. The solution to these issues is to investigate model systems and gain a better understanding of the basic properties of various photocatalysts used in the photocatalysis process (Younis and Kim, 2020).
Conclusion
Conventional remediation methods such as biological, chemical, and physical methods are inadequate and insufficient to completely eradicate and degrade the persistent organic pollutants. Advanced oxidation processes have been widely used to completely mineralize refractory organic pollutants because it involves the generation of hydroxyl radical species in sufficient concentration under ambient temperature and pressure. Among all of the AOTs, the photocatalysis has proven to be very effective in the treatment of wastewater, air purification, and disinfection because of the ambient operating conditions, complete mineralization of the substrate into inorganic carbon dioxide and water, utilization of solar energy, and cost-effectiveness. Photocatalysis is a low-cost, ecologically friendly technique that may be used everywhere since it uses sunlight or UV rays. This technique has also been used to successfully destroy pathogens and algae blooms in fresh water sources. Photo disinfection sensitized by TiO2 has been used to degrade green algae, treat humic substances that act as bacterial growth substrates, and inhibit bacterial degradation of impurities in natural water. Photocatalysis is suitable for the abatement of most air pollutants, including organic compounds, such as alkanes, alkenes, alkynes, aromatics, aldehydes, ketones, and inorganic molecules. Nanomaterials have gained significant attention for photocatalytic degradation. Advances in nanotechnology have opened new doors to overcome the problems related to the heterogenous photocatalytic degradation, but still, heterogenous photocatalysis faces challenges such as higher reaction time, lower efficiency, less recyclability of the photocatalyst for continuous use, high recombination rate, and less adsorption of the active surface of the photocatalyst. Therefore, efforts have been made to enhance the efficiency of the process by reactor design, putting efforts for more novel photocatalyst, modifications of the photocatalysis by doping and heterojunction, hybridizing the semiconductors, etc. To increase the efficiency, the photocatalysis process can be coupled with the other advanced oxidation process, which can be highly effective for both of them. For the detection of the intermediates qualitatively and quantitatively, various techniques can be used such as mass spectrometry with chromatography. The focus should be more on the detection of the intermediate products formed during the reaction. The synthesis of the nanomaterial should be in an eco-friendly and sustainable way by collaborating with different researchers. But still, there are challenges ahead to enhance the advanced oxidation process for the better degradation of the environmental pollutants of wide variety at large scale. In the end, the main focus should be on the elimination and degradation of POPs from the environment in an eco-friendly way.
Author contributions
NG: final draft preparation. DD: framework of manuscript. AS: first draft preparation. RD: editing of manuscript. DVK: overall guidance and final editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Salam, M. O., and Yoon, T. (2022). Cobalt-ferrite/Ag-fMWCNT hybrid nanocomposite catalyst for efficient degradation of synthetic organic dyes via peroxymonosulfate activation. Environ. Res. 205, 112424. doi:10.1016/j.envres.2021.112424
Adhikari, S., Sarath Chandra, K., Kim, D. H., Madras, G., and Sarkar, D. (2018). Understanding the morphological effects of WO3 photocatalysts for the degradation of organic pollutants. Adv. Powder Technol. 29, 1591–1600. doi:10.1016/j.apt.2018.03.024
Adityosulindro, S., Barthe, L., González-Labrada, K., Jauregui Haza, U. J., Delmas, H., and Julcour, C. (2017). Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochemistry 39, 889–896. doi:10.1016/j.ultsonch.2017.06.008
Ajiboye, T. O., Kuvarega, A. T., and Onwudiwe, D. C. (2020). Recent strategies for environmental remediation of organochlorine pesticides. Appl. Sci. Switz. 10, 6286. doi:10.3390/APP10186286
Akhtar, N., and Mannan, M. A. U. (2020). Mycoremediation: Expunging environmental pollutants. Biotechnol. Rep. 26, e00452. doi:10.1016/j.btre.2020.e00452
Alani, O. A., Ari, H. A., Alani, S. O., Offiong, N.-A. O., and Feng, W. (2022). Polyhedral magnetite nanoparticles modified with porous bio-templated copper oxide as catalyst for visible-light-driven photodegradation of methylene blue. Int. J. Environ. Sci. Technol. (Tehran). doi:10.1007/s13762-022-04249-x
An, W., Tian, L., Hu, J., Liu, L., Cui, W., and Liang, Y. (2020). Efficient degradation of organic pollutants by catalytic ozonation and photocatalysis synergy system using double-functional MgO/g-C3N4 catalyst. Appl. Surf. Sci. 534, 147518. doi:10.1016/j.apsusc.2020.147518
Aramyan, S. M. (2017). Advances in fenton and fenton based oxidation processes for industrial effluent contaminants control-A review. Int. J. Environ. Sci. Nat. Resour. 2. doi:10.19080/ijesnr.2017.02.555594
Ari, H. A., Alani, O. A., Zeng, Q. rui, Ugya, Y. A., Offiong, N. A. O., and Feng, W. (2022). Enhanced UV-assisted Fenton performance of nanostructured biomimetic α-Fe2O3 on degradation of tetracycline. J. Nanostructure Chem. 12, 45–58. doi:10.1007/s40097-021-00400-1
Balachandran, R., Patterson, Z., Deymier, P., Snyder, S. A., and Keswani, M. (2016). Understanding acoustic cavitation for sonolytic degradation of p-cresol as a model contaminant. Chemosphere 147, 52–59. doi:10.1016/j.chemosphere.2015.12.066
Balbayeva, G., Yerkinova, A., Inglezakis, V. J., and Poulopoulos, S. G. (2018). Photochemical degradation of organic pollutants in wastewaters. IOP Conf. Ser. Mater. Sci. Eng. 301, 012099. doi:10.1088/1757-899X/301/1/012099
Baruah, S., Najam Khan, M., and Dutta, J. (2016). Perspectives and applications of nanotechnology in water treatment. Environ. Chem. Lett. 14, 1–14. doi:10.1007/s10311-015-0542-2
Belver, C., Bedia, J., and Rodriguez, J. J. (2017). Zr-doped TiO 2 supported on delaminated clay materials for solar photocatalytic treatment of emerging pollutants. J. Hazard. Mater. 322, 233–242. doi:10.1016/j.jhazmat.2016.02.028
Benssassi, M. E., Mammeri, L., Sehili, T., and Canle, M. (2021). First evidence of a photochemical process including an iron-aspartate complex and its use for paracetamol elimination from aqueous solution. J. Photochem. Photobiol. A Chem. 409, 113132. doi:10.1016/j.jphotochem.2021.113132
Berkani, M., Smaali, A., Kadmi, Y., Almomani, F., Vasseghian, Y., Lakhdari, N., et al. (2022). Photocatalytic degradation of Penicillin G in aqueous solutions: Kinetic, degradation pathway, and microbioassays assessment. J. Hazard. Mater. 421, 126719. doi:10.1016/j.jhazmat.2021.126719
Bertagna Silva, D., Buttiglieri, G., and Babić, S. (2021). State-of-the-art and current challenges for TiO2/UV-LED photocatalytic degradation of emerging organic micropollutants. Environ. Sci. Pollut. Res. 28, 103–120. doi:10.1007/s11356-020-11125-z
Bhavya, G., Belorkar, S. A., Mythili, R., Geetha, N., Shetty, H. S., Udikeri, S. S., et al. (2021). Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 275, 129975. doi:10.1016/j.chemosphere.2021.129975
Boukhatem, H., Khalaf, H., Djouadi, L., Marin, Z., Navarro, R. M., Santaballa, J. A., et al. (2017). Diclofenac degradation using mont-La (6%)-Cu0.6Cd0.4S as photocatalyst under NUV-Vis irradiation. Operational parameters, kinetics and mechanism. J. Environ. Chem. Eng. 5, 5636–5644. doi:10.1016/j.jece.2017.10.054
Brosillon, S., Bancon-Montigny, C., and Mendret, J. (2016). Photo-oxidation of tributyltin, dibutyltin and monobutyltin in water and marine sediments. Int. J. Chem. React. Eng. 14, 719–726. doi:10.1515/ijcre-2014-0127
Bui, X. T., Vo, T. P. T., Ngo, H. H., Guo, W., and Nguyen, T. (2016). Multicriteria assessment of advanced treatment technologies for micropollutants removal at large-scale applications. Sci. Total Environ. 563-564, 5631050–5641067. doi:10.1016/J.SCITOTENV.2016.04.191
Byrne, C., Subramanian, G., and Pillai, S. C. (2018). Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 6, 3531–3555. doi:10.1016/j.jece.2017.07.080
Cao, Y., Chen, M., Dong, D., Xie, S., and Liu, M. (2020). Environmental pollutants damage airway epithelial cell cilia: Implications for the prevention of obstructive lung diseases. Thorac. Cancer 11, 505–510. doi:10.1111/1759-7714.13323
Capodaglio, A. G. (2020). Critical perspective on advanced treatment processes for water and wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 10, 4549. doi:10.3390/app10134549
Chang, C. T., Wang, J. J., Ouyang, T., Zhang, Q., and Jing, Y. H. (2015). Photocatalytic degradation of acetaminophen in aqueous solutions by TiO2/ZSM-5 zeolite with low energy irradiation. Mater. Sci. Eng. B 196, 53–60. doi:10.1016/j.mseb.2014.12.025
Chen D., D., Cheng, Y., Zhou, N., Chen, P., Wang, Y., Li, K., et al. (2020). Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 268, 121725. doi:10.1016/J.JCLEPRO.2020.121725
Chen, L., Li, H., and Qian, J. (2021). Degradation of roxarsone in UV-based advanced oxidation processes: A comparative study. J. Hazard. Mater. 410, 124558. doi:10.1016/j.jhazmat.2020.124558
Chen, X., Xu, X., Cui, J., Chen, C., Zhu, X., Sun, D., et al. (2020). Visible-light driven degradation of tetracycline hydrochloride and 2, 4-dichlorophenol by film-like N-carbon@N-ZnO catalyst with three-dimensional interconnected nanofibrous structure. J. Hazard. Mat. 392, 122331. doi:10.1016/j.jhazmat.2020.122331
Cheng, H., and Xu, W. (2019). Recent advances in modified TiO2 for photo-induced organic synthesis. Org. Biomol. Chem. 17, 9977–9989. doi:10.1039/c9ob01739a
Cheng, J., Xie, Y., Wei, Y., Xie, D., Sun, W., Zhang, Y., et al. (2022). Degradation of tetracycline hydrochloride in aqueous via combined dielectric barrier discharge plasma and Fe–Mn doped AC. Chemosphere 286, 131841. doi:10.1016/j.chemosphere.2021.131841
Daramola, I. O., and Adebayo, M. A. (2021). “Recent developments in the application of advanced oxidative processes for remediation of persistent organic pollutants from water,” in Persistent organic pollutants (POPs) - monitoring, impact and treatment (London, UK: IntechOpen). doi:10.5772/intechopen.101304
de Medeiros Lima, S. V., Padoin, N., and Soares, C. (2021). CFD analysis of a H2O2/UVC water treatment process in the annular FluHelik reactor. Environ. Sci. Pollut. Res. 28, 41224–41232. doi:10.1007/s11356-021-13566-6
de Titto, E., and Savino, A. (2019). Environmental and health risks related to waste incineration. Waste Manag. Res. 37, 976–986. doi:10.1177/0734242X19859700
Ding, H., and Hu, J. (2020). Degradation of ibuprofen by UVA-LED/TiO2/persulfate process: Kinetics, mechanism, water matrix effects, intermediates and energy consumption. Chem. Eng. J. 397, 125462. doi:10.1016/j.cej.2020.125462
Dükkanci, M., Vinatoru, M., and Mason, T. J. (2014). The sonochemical decolourisation of textile azo dye Orange II: Effects of Fenton type reagents and UV light. Ultrason. Sonochemistry 21, 846–853. doi:10.1016/j.ultsonch.2013.08.020
Ebrahimbabaie, P., and Pichtel, J. (2021). Biotechnology and nanotechnology for remediation of chlorinated volatile organic compounds: Current perspectives. Environ. Sci. Pollut. Res. 28, 7710–7741. doi:10.1007/s11356-020-11598-y
Escobedo, S., and de Lasa, H. (2020). Photocatalysis for air treatment processes: Current technologies and future applications for the removal of organic pollutants and viruses. Catalysts 10, 966. doi:10.3390/catal10090966
Fiedler, H., Kallenborn, R., de Boer, J., and Sydnes, L. K. (2019). The Stockholm convention: A tool for the global regulation of persistent organic pollutants. Chem. Int. 41, 4–11. doi:10.1515/ci-2019-0202
Fry, K., and Power, M. C. (2017). Persistent organic pollutants and mortality in the United States, NHANES 1999-2011. Environ. Health A Glob. Access Sci. Source 16. doi:10.1186/s12940-017-0313-6
Gao, C., Wang, J., Xu, H., and Xiong, Y. (2017). Coordination chemistry in the design of heterogeneous photocatalysts. Chem. Soc. Rev. 46, 2799–2823. doi:10.1039/c6cs00727a
Gaur, N., Narasimhulu, K., and PydiSetty, Y. (2018). Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 198, 1602–1631. doi:10.1016/j.jclepro.2018.07.076
Gaya, U. I., and Abdullah, A. H. (2008). Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 9, 1–12. doi:10.1016/j.jphotochemrev.2007.12.003
Ghalamchi, L., and Aber, S. (2020). An aminated silver orthophosphate/graphitic carbon nitride nanocomposite: An efficient visible light sonophotocatalyst. Mater. Chem. Phys. 256, 123649. doi:10.1016/j.matchemphys.2020.123649
Gmurek, M., Olak-Kucharczyk, M., and Ledakowicz, S. (2017). Photochemical decomposition of endocrine disrupting compounds – a review. Chem. Eng. J. 310, 437–456. doi:10.1016/j.cej.2016.05.014
Guateque-Londoño, J. F., Serna-Galvis, E. A., Silva-Agredo, J., Avila-Torres, Y., and Torres-Palma, R. A. (2020). Dataset on the degradation of losartan by TiO2-photocatalysis and UVC/persulfate processes. Data Brief 31, 105692. doi:10.1016/j.dib.2020.105692
Guerra, F. D., Attia, M. F., Whitehead, D. C., and Alexis, F. (2018). Nanotechnology for environmental remediation: Materials and applications. Molecules 23, 1760. doi:10.3390/molecules23071760
Guo, X., Xie, C., Wang, L., Li, Q., and Wang, Y. (2019b). Biodegradation of persistent environmental pollutants by Arthrobacter sp. Environ. Sci. Pollut. Res. 26, 8429–8443. doi:10.1007/s11356-019-04358-0
Guo, Y., Zhao, E., Wang, J., Zhang, X., Huang, H., Yu, G., et al. (2020). Comparison of emerging contaminant abatement by conventional ozonation, catalytic ozonation, O3/H2O2 and electro-peroxone processes. J. Hazard. Mater. 389, 121829. doi:10.1016/j.jhazmat.2019.121829
Guo, Z., Yu, F., Yang, Y., Leung, C. F., Ng, S. M., Ko, C. C., et al. (2017). Photocatalytic conversion of CO2 to CO by a copper(II) quaterpyridine complex. ChemSusChem 10, 4009–4013. doi:10.1002/cssc.201701354
Gusain, R., Gupta, K., Joshi, P., and Khatri, O. P. (2019). Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 272, 102009. doi:10.1016/j.cis.2019.102009
Haque, M. M., and Muneer, M. (2007). Photodegradation of norfloxacin in aqueous suspensions of titanium dioxide. J. Hazard. Mater. 145, 51–57. doi:10.1016/j.jhazmat.2006.10.086
Hassan, M., Olvera-Vargas, H., Zhu, X., Zhang, B., and He, Y. (2019). Microbial electro-Fenton: An emerging and energy-efficient platform for environmental remediation. J. Power Sources 424, 220–244. doi:10.1016/J.JPOWSOUR.2019.03.112
Herrmann, J. M. (2010). Environmental photocatalysis: Perspectives for China. Sci. China Chem. 53, 1831. doi:10.1007/s11426-010-4076-y
Hou, J., He, X., Zhang, S., Yu, J., Feng, M., and Li, X. (2021). Recent advances in cobalt-activated sulfate radical-based advanced oxidation processes for water remediation: A review. Sci. Total Environ. 770, 145311. doi:10.1016/J.SCITOTENV.2021.145311
Hu, E., Shang, S., and Chiu, A. K. L. (2019). Removal of reactive dyes in textile effluents by catalytic ozonation pursuing on-site effluent recycling. Mol. (Basel, Switz. 24, 2755. doi:10.3390/molecules24152755
Hu, J., Li, J., Cui, J., An, W., Liu, L., Liang, Y., et al. (2020). Surface oxygen vacancies enriched FeOOH/Bi2MoO6 photocatalysis- fenton synergy degradation of organic pollutants. J. Hazard. Mater. 384, 121399. doi:10.1016/j.jhazmat.2019.121399
Humayun, M., Raziq, F., Khan, A., and Luo, W. (2018). Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 11, 86–102. doi:10.1080/17518253.2018.1440324
Ibhadon, A. O., and Fitzpatrick, P. (2013). Heterogeneous photocatalysis: Recent advances and applications. Catalysts 3, 189–218. doi:10.3390/catal3010189
Irfan, R. M., Khan, S. A., Tahir, M. H., Ahmad, T., Ali, L., Afzal, M., et al. (2021). Integration of an aminopyridine derived cobalt based homogenous cocatalyst with a composite photocatalyst to promote H2evolution from water. New J. Chem. 45, 5561–5567. doi:10.1039/d1nj00086a
Irga, P. J., Pettit, T. J., and Torpy, F. R. (2018). The phytoremediation of indoor air pollution: A review on the technology development from the potted plant through to functional green wall biofilters. Rev. Environ. Sci. Biotechnol. 17, 395–415. doi:10.1007/s11157-018-9465-2
Ismail, A. A., and Bahnemann, D. W. (2014). Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 128, 85–101. doi:10.1016/j.solmat.2014.04.037
Jing, R., Fusi, S., and Kjellerup, B. v. (2018). Remediation of Polychlorinated Biphenyls (PCBs) in contaminated soils and sediment: State of knowledge and perspectives. Front. Environ. Sci. 6. doi:10.3389/fenvs.2018.00079
Jouyandeh, M., Mousavi Khadem, S. S., Habibzadeh, S., Esmaeili, A., Abida, O., Vatanpour, V., et al. (2021). Quantum dots for photocatalysis: Synthesis and environmental applications. Green Chem. 23, 4931–4954. doi:10.1039/d1gc00639h
Kar, P., Shukla, K., Jain, P., Sathiyan, G., and Gupta, R. K. (2021). Semiconductor based photocatalysts for detoxification of emerging pharmaceutical pollutants from aquatic systems: A critical review. Nano Mater. Sci. 3, 25–46. doi:10.1016/j.nanoms.2020.11.001
Kouchakpour, F., Chaibakhsh, N., and Naeemi, A. S. (2021). Efficient removal of cytotoxic drugs from wastewater by single-stage combined photocatalysis–algae treatment process. Environ. Technol. 42, 3178–3190. doi:10.1080/09593330.2020.1725139
Kråkström, M., Saeid, S., Tolvanen, P., Salmi, T., Eklund, P., and Kronberg, L. (2020). Catalytic ozonation of the antibiotic sulfadiazine: Reaction kinetics and transformation mechanisms. Chemosphere 247, 125853. doi:10.1016/J.CHEMOSPHERE.2020.125853
Kuo, C. Y., Jheng, H. K., and Syu, S. E. (2021). Effect of non-metal doping on the photocatalytic activity of titanium dioxide on the photodegradation of aqueous bisphenol A. Environ. Technol. (United Kingdom) 42, 1603–1611. doi:10.1080/09593330.2019.1674930
Kwon, D., and Kim, J. (2020). Silver-doped ZnO for photocatalytic degradation of methylene blue. Korean J. Chem. Eng. 37, 1226–1232. doi:10.1007/s11814-020-0520-7
Lin, S., Du, W., Tong, L., Ji, T., and Jiao, X. (2019). Photocatalytic degradation of 4-chlorophenol by Gd-doped β-Bi 2 O 3 under visible light irradiation. Chem. Res. Chin. Univ. 35, 120–124. doi:10.1007/s40242-018-8170-6
Liu, C., Zhang, A. Y., Si, Y., Pei, D. N., and Yu, H. Q. (2017a). Photochemical anti-fouling approach for electrochemical pollutant degradation on facet-tailored TiO2 single crystals. Environ. Sci. Technol. 51, 11326–11335. doi:10.1021/acs.est.7b04105
Liu, G., You, S., Tan, Y., and Ren, N. (2017b). In situ photochemical activation of sulfate for enhanced degradation of organic pollutants in water. Environ. Sci. Technol. 51, 2339–2346. doi:10.1021/acs.est.6b05090
Liu, X., Wu, D., Jiang, H., and Song, N. (2020). The production process of reactive oxygen radicals in the degradation process of grass-source dissolved organic matter. J. Lake Sci. 32, 440–449. doi:10.18307/2020.0213
Liu, Y., Zhou, B., Bai, J., Li, J., Zhang, J., Zheng, Q., et al. (2009). Efficient photochemical water splitting and organic pollutant degradation by highly ordered TiO2 nanopore arrays. Appl. Catal. B Environ. 89, 142–148. doi:10.1016/j.apcatb.2008.11.034
Luo, Z., Min, Y., Qu, L., Song, Y., and Hong, Y. (2021). Remediation of phenanthrene contaminated soil by ferrous oxalate and its phytotoxicity evaluation. Chemosphere 265, 129070. doi:10.1016/j.chemosphere.2020.129070
Ma, J., Ding, Y., Gu, C., Zhai, G., Liu, Y., Wen, J., et al. (2021). Degradation of benzothiazole pollutant by sulfate radical-based advanced oxidation process. Environ. Technol. (United Kingdom) 43, 2834–2843. doi:10.1080/09593330.2021.1906326
Madaj, R., Sobiecka, E., and Kalinowska, H. (2018). Lindane, kepone and pentachlorobenzene: Chloropesticides banned by Stockholm convention. Int. J. Environ. Sci. Technol. (Tehran). 15, 471–480. doi:10.1007/s13762-017-1417-9
Mahasti, N. N. N., Shih, Y. J., and Huang, Y. H. (2020). Recovery of magnetite from fluidized-bed homogeneous crystallization of iron-containing solution as photocatalyst for Fenton-like degradation of RB5 azo dye under UVA irradiation. Sep. Purif. Technol. 247, 116975. doi:10.1016/j.seppur.2020.116975
Mandade, P. (2021). Introduction, basic principles, mechanism, and challenges of photocatalysis. Handb. Nanomater. Wastewater Treat. 2021, 137. doi:10.1016/b978-0-12-821496-1.00016-7
Masoud, G., Ali, R., and Khorshid, M. (2021). Photochemical degradation of an environmental pollutant by pure zno and mgo doped zno nanocatalysts. Iran. J. Chem. Chem. Eng. 40. doi:10.30492/ijcce.2019.36825
Matmin, J., Jalani, M. A., Osman, H., Omar, Q., Ab’lah, N., Elong, K., et al. (2019). Photochemical synthesis of nanosheet tin di/sulfide with sunlight response on water pollutant degradation. Nanomaterials 9, 264. doi:10.3390/nano9020264
Midassi, S., Bedoui, A., and Bensalah, N. (2020). Efficient degradation of chloroquine drug by electro-Fenton oxidation: Effects of operating conditions and degradation mechanism. Chemosphere 260, 127558. doi:10.1016/j.chemosphere.2020.127558
Mishra, B., Varjani, S., Kumar, G., Awasthi, M., Awasthi, S. K., Sindhu, R., et al. (2020). Microbial approaches for remediation of pollutants: Innovations, future outlook, and challenges. Energy Environ. 32. doi:10.1177/0958305X19896781
Mohammed, S. A., and Ali, A. H. (2019). Textile wastewater treatment by application of advance oxidation (AOPS) process and recycling for industrial uses. Indian J. Public Health Res. Dev. 10, 4946. doi:10.5958/0976-5506.2019.03933.0
Moldovan, R., Iacob, B. C., Farcău, C., Bodoki, E., and Oprean, R. (2021). Strategies for SERS detection of organochlorine pesticides. Nanomaterials 11, 304. doi:10.3390/nano11020304
Mu, J., Yang, J. L., Zhang, D. W., and Jia, Q. (2021). Progress in preparation of metal nanoclusters and their application in detection of environmental pollutants. Chin. J. Anal. Chem. 49, 319–329. doi:10.1016/S1872-2040(21)60082-8
Muthukumaran, M., Dhinagaran, G., Venkatachalam, K., Sagadevan, S., Gunasekaran, S., Podder, J., et al. (2020). Green synthesis of cuprous oxide nanoparticles for environmental remediation and enhanced visible-light photocatalytic activity. Optik 214, 164849. doi:10.1016/j.ijleo.2020.164849
Natarajan, V., Karunanidhi, M., and Raja, B. (2020). A critical review on radioactive waste management through biological techniques. Environ. Sci. Pollut. Res. 27, 29812–29823. doi:10.1007/s11356-020-08404-0
Neena, D., Kondamareddy, K. K., Humayun, M., Mohan, V. B., Lu, D., Fu, D., et al. (2019). Fabrication of ZnO/N-rGO composite as highly efficient visible-light photocatalyst for 2, 4-DCP degradation and H2 evolution. Appl. Surf. Sci. 488, 611–619. doi:10.1016/j.apsusc.2019.05.302
Nguyen, V. H., Smith, S. M., Wantala, K., and Kajitvichyanukul, P. (2020). Photocatalytic remediation of persistent organic pollutants (POPs): A review. Arabian J. Chem. 13, 8309–8337. doi:10.1016/J.ARABJC.2020.04.028
Nippatlapalli, N., Ramakrishnan, K., and Philip, L. (2022). Enhanced degradation of complex organic compounds in wastewater using different novel continuous flow non – thermal pulsed corona plasma discharge reactors. Environ. Res. 203, 111807. doi:10.1016/j.envres.2021.111807
Ohtani, B. (2017). Great challenges in catalysis and photocatalysis. Front. Chem. 5, 79. doi:10.3389/fchem.2017.00079
Padervand, M., Lammel, G., Bargahi, A., and Mohammad-Shiri, H. (2019). Photochemical degradation of the environmental pollutants over the worm-like Nd2CuO4-Nd2O3 nanostructures. Nano-Structures Nano-Objects 18, 100258. doi:10.1016/j.nanoso.2019.100258
Padmanaban, V. C., Giri Nandagopal, M. S., Madhangi Priyadharshini, G., Maheswari, N., Janani Sree, G., and Selvaraju, N. (2016). Advanced approach for degradation of recalcitrant by nanophotocatalysis using nanocomposites and their future perspectives. Int. J. Environ. Sci. Technol. (Tehran). 13, 1591–1606. doi:10.1007/s13762-016-1000-9
Pandey, A. K., Reji Kumar, R., Laghari, I. A., Samykano, M., Kothari, R., Abusorrah, A. M., et al. (2021). Utilization of solar energy for wastewater treatment: Challenges and progressive research trends. J. Environ. Manage. 297, 113300. doi:10.1016/j.jenvman.2021.113300
Parul, K. K., Badru, R., Singh, P. P., and Kaushal, S. (2020). Photodegradation of organic pollutants using heterojunctions: A review. J. Environ. Chem. Eng. 8, 103666. doi:10.1016/J.JECE.2020.103666
Patel, A. B., Shaikh, S., Jain, K. R., Desai, C., and Madamwar, D. (2020). Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 11, 562813. doi:10.3389/fmicb.2020.562813
Patidar, R., and Srivastava, V. C. (2021). Evaluation of the sono-assisted photolysis method for the mineralization of toxic pollutants. Sep. Purif. Technol. 258, 117903. doi:10.1016/j.seppur.2020.117903
Pawar, R. C., and Lee, C. S. (2015). “Basics of photocatalysis,” in Heterogeneous nanocomposite-photocatalysis for water purification (Amsterdam, Netherlands: Elsevier). doi:10.1016/B978-0-323-39310-2.00001-1
Peiris, S., de Silva, H. B., Ranasinghe, K. N., Bandara, S. V., and Perera, I. R. (2021). Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 68, 738–769. doi:10.1002/jccs.202000465
Phoon, B. L., Ong, C. C., Mohamed Saheed, M. S., Show, P. L., Chang, J. S., Ling, T. C., et al. (2020). Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 400, 122961. doi:10.1016/j.jhazmat.2020.122961
Potes-Lesoinne, H. A., Ramirez-Alvarez, F., Perez-Gonzalez, V. H., Martinez-Chapa, S. O., and Gallo-Villanueva, R. C. (2022). Nanomaterials for electrochemical detection of pollutants in water: A review. Electrophoresis 43, 249–262. doi:10.1002/elps.202100204
Prakruthi, K., Ujwal, M. P., Yashas, S. R., Mahesh, B., Kumara Swamy, N., and Shivaraju, H. P. (2022). Recent advances in photocatalytic remediation of emerging organic pollutants using semiconducting metal oxides: An overview. Environ. Sci. Pollut. Res. 29, 4930–4957. doi:10.1007/s11356-021-17361-1
Prete, P., Fiorentino, A., Rizzo, L., Proto, A., and Cucciniello, R. (2021). Review of aminopolycarboxylic acids–based metal complexes application to water and wastewater treatment by (photo-)Fenton process at neutral pH. Curr. Opin. Green Sustain. Chem. 28, 100451. doi:10.1016/j.cogsc.2021.100451
Qi, J., Ma, B., Miao, S., Liu, R., Hu, C., and Qu, J. (2021). Pre-oxidation enhanced cyanobacteria removal in drinking water treatment: A review. J. Environ. Sci. (China) 110, 160–168. doi:10.1016/j.jes.2021.03.040
Quiton, K. G. N., Lu, M. C., and Huang, Y. H. (2021). Synthesis and catalytic utilization of bimetallic systems for wastewater remediation: A review. Chemosphere 262, 128371. doi:10.1016/j.chemosphere.2020.128371
Rajendrachari, S., Taslimi, P., Karaoglanli, A. C., Uzun, O., Alp, E., and Jayaprakash, G. K. (2021). Photocatalytic degradation of Rhodamine B (RhB) dye in waste water and enzymatic inhibition study using cauliflower shaped ZnO nanoparticles synthesized by a novel One-pot green synthesis method. Arabian J. Chem. 14, 103180. doi:10.1016/j.arabjc.2021.103180
Rathna, R., Varjani, S., and Nakkeeran, E. (2018). Recent developments and prospects of dioxins and furans remediation. J. Environ. Manag. 223, 797–806. doi:10.1016/j.jenvman.2018.06.095
Rekhate, C. v., and Srivastava, J. K. (2020). Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem. Eng. J. Adv. 3, 100031. doi:10.1016/j.ceja.2020.100031
Roy, S. D., Das, K. C., and Dhar, S. S. (2021). Conventional to green synthesis of magnetic iron oxide nanoparticles; its application as catalyst, photocatalyst and toxicity: A short review. Inorg. Chem. Commun. 134, 109050. doi:10.1016/j.inoche.2021.109050
Rueda-Marquez, J. J., Levchuk, I., Fernández Ibañez, P., and Sillanpää, M. (2020). A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 258, 120694. doi:10.1016/j.jclepro.2020.120694
Rueda-Márquez, J. J., Levchuk, I., Manzano, M., and Sillanpää, M. (2020). Toxicity reduction of industrial and municipal wastewater by advanced oxidation processes (Photo-fenton, UVC/H2O2, electro-fenton and galvanic fenton): A review. Catalysts 10, 612. doi:10.3390/catal10060612
Sadhu, S. P., Ruparelia, J. P., and Patel, U. D. (2020). Homogeneous photocatalytic degradationof azo dye Reactive Black 5 using Fe(III) ions under visible light. Environ. Technol. (United Kingdom) 43, 199–206. doi:10.1080/09593330.2020.1782995
Saquib, M., and Muneer, M. (2003). TiO2/mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dyes Pigments 56, 37–49. doi:10.1016/S0143-7208(02)00101-8
Saravanan, R., Gracia, F., and Stephen, A. (2017). “Basic principles, mechanism, and challenges of photocatalysis,” in Nanocomposites for visible light-induced photocatalysis, 19–40. doi:10.1007/978-3-319-62446-4_2
Schneider, J. T., Firak, D. S., Ribeiro, R. R., and Peralta-Zamora, P. (2020). Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 22, 15723–15733. doi:10.1039/d0cp02411b
Shivaraju, H. P., Muzakkira, N., and Shahmoradi, B. (2016). Photocatalytic treatment of oil and grease spills in wastewater using coated N-doped TiO2 polyscales under sunlight as an alternative driving energy. Int. J. Environ. Sci. Technol. (Tehran). 13, 2293–2302. doi:10.1007/s13762-016-1038-8
Shu, H. Y., Huang, S. W., and Tsai, M. K. (2016). Comparative study of acid blue 113 wastewater degradation and mineralization by UV/persulfate and UV/Oxone processes. Desalination Water Treat. 57, 29517–29530. doi:10.1080/19443994.2016.1172031
Singh, P., and Borthakur, A. (2018). A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean. Prod. 196, 1669–1680. doi:10.1016/J.JCLEPRO.2018.05.289
Singh, S., Halder, A., Sinha, O., Sarkar, P. K., Singh, P., Banerjee, A., et al. (2020). Nanoparticle-based “turn-on” scattering and post-sample fluorescence for ultrasensitive detection of water pollution in wider window. PLoS ONE 15, e0227584. doi:10.1371/journal.pone.0227584
Song, G., Du, X., Zheng, Y., Su, P., Tang, Y., and Zhou, M. (2022). A novel electro-Fenton process coupled with sulfite: Enhanced Fe3+ reduction and TOC removal. J. Hazard. Mater. 422, 126888. doi:10.1016/j.jhazmat.2021.126888
Sposito, A. J., Kurdekar, A., Zhao, J., and Hewlett, I. (2018). Application of nanotechnology in biosensors for enhancing pathogen detection. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 10, e1512. doi:10.1002/wnan.1512
Suliman, M. A., Gondal, M. A., Dastageer, M. A., Chuah, G., and Basheer, C. (2019). Method for visible light-induced photocatalytic degradation of methylparaben in water using nanostructured Ag/AgBr@m-WO3. Photochem. Photobiol. 95, 1485–1494. doi:10.1111/php.13118
Sun, B., Li, Q., Zheng, M., Su, G., Lin, S., Wu, M., et al. (2020a). Recent advances in the removal of persistent organic pollutants (POPs) using multifunctional materials:a review. Environ. Pollut. 265, 114908. doi:10.1016/j.envpol.2020.114908
Sun, S., Yao, H., Fu, W., Xue, S., and Zhang, W. (2020b). Enhanced degradation of antibiotics by photo-fenton reactive membrane filtration. J. Hazard. Mater. 386, 121955. doi:10.1016/j.jhazmat.2019.121955
Tahir, M. B., Sohaib, M., Sagir, M., and Rafique, M. (2020). Role of nanotechnology in photocatalysis. Reference Module Mater. Sci. Mater. Eng. 2020. doi:10.1016/b978-0-12-815732-9.00006-1
Tang, J., and Wang, J. (2018). Metal organic framework with coordinatively unsaturated sites as efficient fenton-like catalyst for enhanced degradation of sulfamethazine. Environ. Sci. Technol. 52, 5367–5377. doi:10.1021/acs.est.8b00092
Tang, Y., He, D., Guo, Y., Qu, W., Shang, J., Zhou, L., et al. (2020). Electrochemical oxidative degradation of X-6G dye by boron-doped diamond anodes: Effect of operating parameters. Chemosphere 258, 127368. doi:10.1016/j.chemosphere.2020.127368
Taoufik, N., Boumya, W., Achak, M., Sillanpaa, M., and Barka, N. (2021). Comparative overview of advanced oxidation processes and biological approaches for the removal pharmaceuticals. J. Environ. Manag. 288, 112404. doi:10.1016/J.JENVMAN.2021.112404
Tarkwa, J. B., Oturan, N., Acayanka, E., Laminsi, S., and Oturan, M. A. (2019). Photo-Fenton oxidation of Orange G azo dye: Process optimization and mineralization mechanism. Environ. Chem. Lett. 17, 473–479. doi:10.1007/s10311-018-0773-0
Teodosiu, C., Gilca, A. F., Barjoveanu, G., and Fiore, S. (2018). Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 197, 1210–1221. doi:10.1016/j.jclepro.2018.06.247
Thakur, S., and Chauhan, M. S. (2016). Electro-coagulation integrated with advance oxidation processes: Technical review on treatment of industrial wastewater. Int. J. Innovative Res. Sci. Eng. Technol. (An ISO Certified Organization) 5. doi:10.15680/IJIRSET.2015.0506159
Theerthagiri, J., Lee, S. J., Karuppasamy, K., Arulmani, S., Veeralakshmi, S., Ashokkumar, M., et al. (2021). Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. Journal of Hazardous Materials 412, 125245. doi:10.1016/j.jhazmat.2021.125245
Trubetskoi, O. A., Patsaeva, S. v., and Trubetskaya, O. E. (2019). Photochemical degradation of organic pollutants in solutions of soil humic acids. Eurasian Soil Sc. 52, 1075–1080. doi:10.1134/S1064229319090102
Valizadeh, S., Lee, S. S., Baek, K., Choi, Y. J., Jeon, B. H., Rhee, G. H., et al. (2021). Bioremediation strategies with biochar for polychlorinated biphenyls (PCBs)-contaminated soils: A review. Environmental Research 200, 111757. doi:10.1016/j.envres.2021.111757
Vaverková, M. D. (2019). Landfill impacts on the environment— Review. Geosciences (Switzerland) 9, 431. doi:10.3390/geosciences9100431
Védrine, J. (2017). Heterogeneous catalysis on metal oxides. Catalysts 7, 341. doi:10.3390/catal7110341
Vilar, V. J. P., Dionysiou, D. D., Torres-Palma, R., Malato, S., and Puma, G. L. (2019). Future trends in photocatalysis for environmental applications. Journal of Hazardous Materials 372. doi:10.1016/j.jhazmat.2019.03.073
Wang, B., Zhang, H., Wang, F., Xiong, X., Tian, K., Sun, Y., et al. (2019). Application of heterogeneous catalytic ozonation for refractory organics in wastewater. Catalysts 9, 241. doi:10.3390/catal9030241
Wang, H., Zhang, C., Kong, L., Wang, Y., Zhang, S., Zhang, X., et al. (2022). Solar light photocatalytic transformation of heptachlorobiphenyl (PCB 180) using g-C3N4 based magnetic porous photocatalyst. J. Hazard. Mat. 427, 128105. doi:10.1016/j.jhazmat.2021.128105
Wang, J., and Chen, H. (2020). Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Science of the Total Environment 704, 135249. doi:10.1016/j.scitotenv.2019.135249
Wang, S., Yang, Q., Chen, F., Sun, J., Luo, K., Yao, F., et al. (2017). Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review. Chemical Engineering Journal 328, 927–942. doi:10.1016/j.cej.2017.07.076
Wang, Y., Wang, C., Hu, Q., Liu, C., and Ding, L. (2022b). 0d/3d nico2o4/defected uio-66 catalysts for enhanced degradation of tetracycline in peroxymonosulfate/simulated sunlight systems: Degradation mechanisms and pathways. SSRN Journal. doi:10.2139/ssrn.4056739
Willkomm, J., Orchard, K. L., Reynal, A., Pastor, E., Durrant, J. R., and Reisner, E. (2016). Dye-sensitised semiconductors modified with molecular catalysts for light-driven H2 production. Chem. Soc. Rev. 45, 9–23. doi:10.1039/c5cs00733j
Xu, Y. J. (2021). Promises and challenges in photocatalysis. Front. Catal. 1, 708319. doi:10.3389/fctls.2021.708319
Yadav, N., Garg, V. K., Chhillar, A. K., and Rana, J. S. (2021). Detection and remediation of pollutants to maintain ecosustainability employing nanotechnology: A review. Chemosphere 280, 130792. doi:10.1016/j.chemosphere.2021.130792
Yang, Q., Ma, Y., Chen, F., Yao, F., Sun, J., Wang, S., et al. (2019). Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chemical Engineering Journal 378, 122149. doi:10.1016/j.cej.2019.122149
Yao, B., Luo, Z., Zhi, D., Hou, D., Luo, L., Du, S., et al. (2021). Current progress in degradation and removal methods of polybrominated diphenyl ethers from water and soil: A review. Journal of Hazardous Materials 403, 123674. doi:10.1016/j.jhazmat.2020.123674
Younis, S. A., and Kim, K. H. (2020). Heterogeneous photocatalysis scalability for environmental remediation: Opportunities and challenges. Catalysts 10, 1109. doi:10.3390/catal10101109
Zeng, X., Liu, J., and Zhao, J. (2018). Highly efficient degradation of pharmaceutical sludge by catalytic wet oxidation using CuO-CeO2/γ-Al2O3 as a catalyst. PLoS ONE 13, e0199520. doi:10.1371/journal.pone.0199520
Zhang, Y., Zhu, Y., Zeng, Z., Zeng, G., Xiao, R., Wang, Y., et al. (2021). Sensors for the environmental pollutant detection: Are we already there? Coordination Chemistry Reviews 431, 213681. doi:10.1016/j.ccr.2020.213681
Keywords: advanced oxidation technologies(AOTs), persistent organic pollutants, remediation, heterogeneous photocatalysis, photocatalytic degradation
Citation: Gaur N, Dutta D, Singh A, Dubey R and Kamboj DV (2022) Recent advances in the elimination of persistent organic pollutants by photocatalysis. Front. Environ. Sci. 10:872514. doi: 10.3389/fenvs.2022.872514
Received: 09 February 2022; Accepted: 10 October 2022;
Published: 30 November 2022.
Edited by:
Nsikak U. Benson, Covenant University, NigeriaReviewed by:
Zaneta Polkowska, Gdansk University of Technology, PolandNnanake-Abasi Offiong, Topfaith University, Nigeria
Copyright © 2022 Gaur, Dutta, Singh, Dubey and Kamboj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rama Dubey, cl9kdWJleTE3MkByZWRpZmZtYWlsLmNvbQ==
 Nisha Gaur
Nisha Gaur Dhiraj Dutta1
Dhiraj Dutta1 Ayushi Singh
Ayushi Singh Rama Dubey
Rama Dubey