
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 03 May 2022
Sec. Toxicology, Pollution and the Environment
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.869727
This article is part of the Research TopicChallenges in Characterizing Nano- to Macro-Plastics and Adhered Substances in the Aquatic EnvironmentView all 7 articles
A few studies assessed how natural products can protect fish from the neurotoxic effects of Microplastics (MPs). Therefore, the goal of this study was to look into the neurotoxicity of PE-MPs on the brain tissue of African catfish (C. gariepinus), and whether dietary feeding on Chlorella, citric acid, and lycopene could help alleviate their toxicity. Five groups of fish were used: The first group received a standard diet (control). The second group was fed 500 mg/kg PE-MP. The third group was fed PE-MP + lycopene (500 mg/kg diet). The fourth group was fed PE-MP + citric acid (30 g/kg diet). And the fifth group was fed PE-MP + Chlorella (50 g/kg diet) for 15 days. The activities of Acetylcholinesterase (Ach), Monoamine Oxidase (MAO), Aldehyde Oxidase (AO), and Nitric Oxide (NO), and the histological effect on brain tissues were then assessed. The activity of the four neurological biomarker enzymes investigated was altered significantly in fish subjected to PE-MP alone compared with the control group. For fish exposed to PE-MP with lycopene, citric acid, or Chlorella, the activities of these neurological enzymes significantly improved particularly with Chlorella compared with fish fed PE-MP individually. Histological investigations illustrated that being subjected to PE-MPs effected cellular alterations in the telencephalon, including diffuse distorted and degraded neurons, encephalomalacia, aggregated neuroglial cells (gliosis), as well as deformed and necrotic neurons, neuropil vacuolation (spongiosis), aggregated neuroglial cells (gliosis), pyknotic neurons, and shrunken Purkinje cells which were found in the cerebellum. Most histological alterations induced by exposure to PE-MP feeding were restored by dietary feeding on Chlorella, citric acid, and lycopene. Accordingly, this study recommends using citric acid, lycopene, and Chlorella as a natural remedy against MP neurotoxicity particularly with Chlorella.
In recent years, plastic debris pollution in freshwater and marine habitats garnered the attention of the scientific community and the general public (Andrady, 2011). Synthetic polymers are only a matter of time until they become a global ecological and environmental issue due to their high production combined with their physicochemical properties, such as buoyancy and slow (bio)degradation rate, and ineffective and irresponsible waste collection and recycling (Free et al., 2014). Plastic debris is physically fragmented into Microplastics (MPs) via photochemical degradation and mechanical abrasion (Thompson et al., 2004; Andrady, 2011). Therefore, “MPs” stands for all plastic items smaller than 5.0 mm in size (Thompson et al., 2004), classifiable into primary MPs—intentionally produced at a microscopic scale (Costa et al., 2009; Browne, 2015) and secondary MPs—resulting from the degradation of larger plastics into smaller pieces because of hydrolysis and biodegradation under environmental conditions weathering and photo-oxidation (Mathalon and Hill, 2014; Gewert et al., 2015). Fragmentation occurs over time due to the culmination of biological physical, and chemical processes that diminish plastic debris’ structural integrity (Browne et al., 2007).
Manufacturing of synthetic polymers has increased rapidly in recent decades, led by Polyethylene (PE), with aquatic habitats functioning as a global sink. PE is non-biodegradable; however, it does break down into MPs that are easily absorbed by biota in coastal areas (Beiras et al., 2019). PE, polypropylene plastic polymers, are more frequently generated and disposed of, resulting in the presence of significant quantities of them in marine habitats (Andrady, 2011; Abidli et al., 2018; Abidli et al., 2019; James et al., 2020). Yet knowledge on the occurrence and effects of MPs on the marine environment surpasses those on freshwater. Continental aquatic ecosystems can contain and collect many microparticles and plastic fibers (Li et al., 2018 and, 2020; Wagner and Lambert, 2018). This is exacerbated by urban areas near rivers and lakes (Imhof et al., 2013; Faure et al., 2015; Anderson et al., 2017).
MPs can be part of the aquatic food web through direct ingestion of MPs—which could happen by accident due to being mistaken for food—or ingestion of a prey species that already contains MPs (Wright et al., 2013). There are filter feeders, such as the mussel Mytilus edulis (Browne et al., 2008) and the copepod Centropages typicus (Cole et al., 2013), deposit-feeders in the lab, such as the sea cucumber Holothuria floridana and Holothuria grisea (Graham and Thompson, 2009), and scavenging invertebrates in the field, such as the decapod Nephro (Murray and Cowie, 2011). MPs have also been found in the digestive tracts of commercial fish species, according to research (Lusher et al., 2013; Neves et al., 2015; Romeo et al., 2015; Miranda and de Carvalho-Souza, 2016; Hamed et al., 2019; Sayed et al., 2021a). MPs pose risks because chemicals and pollutants become encrusted on the surface (Rochman et al., 2013).
Ingestion of MPs could have both physical and physiological consequences for marine organisms. Internal abrasions and gut obstructions could cause malnutrition as a result of the physical repercussions (Gall and Thompson, 2015). The toxicity of plastic monomers and additives causes carcinogenesis and endocrine disruption, resulting in physiological repercussions (Wright et al., 2013; Hamed et al., 2019; Hamed et al., 2020; Hamed et al., 2021). Several studies on the neurotoxicity of various MPs in fish have been reported (Barboza et al., 2018b; Ding et al., 2018; Miranda and de Carvalho-Souza, 2016; Oliveira et al., 2013). Additionally, MPs can increase cellular oxidative stress by altering antioxidant defense responses, resulting in Lipid Peroxidation (LPO) of cellular membranes (Alomar et al., 2017; Barboza et al., 2018a). These findings are concerning because enzymes, such as cholinesterase, some of which are necessary for cholinergic neurotransmission in neuromuscular junctions and cholinergic brain synapses (Massoulié et al., 1993), and LPO have been deemed as important molecular mechanisms related to oxidative damage to cell structures and the toxicity process that causes death (Massoulié et al., 1993; Repetto et al., 2012). Moreover, MPs in the stomachs of commercially important fish species are concerning due to the potential for these small plastic particles and/or related to pollutants to be transported to edible fish tissue, endangering human health (Fossi et al., 2018). As a result, it is imperative to begin investigating how to manage the ecotoxicity of MPs in fish and whether dietary feeding on natural products could help reduce their toxicity.
Carotenoids, particularly lycopene, act as antioxidants since they can interact with reactive oxygen species. As a result, consuming lycopene as an antioxidant in fish traps active oxygen species, reducing oxidative stress and the risk of oxidation of cellular components such as lipids, proteins, and DNA (Waliszewski and Blasco, 2010). As a result, many functional foods are now developed to give a high level of antioxidants while lowering the risk of diseases linked to oxidative stress (Roberfroid, 2002). Similarly, Chlorella vulgaris is a unicellular green alga found in freshwater and saltwater and is commonly utilized as a food supplement (Kay and Barton, 1991). It is a nutrient-dense superfood with 60% protein, 18 amino acids, and different vitamins and minerals. Calcium, iron, potassium, phosphorous, magnesium, pro-vitamin A, vitamins C, B1, B2, B5, B6, B12, E, and K, biotin, inositol, and folic acid are among the vitamins and minerals in Chlorella (Nick, 2003). Furthermore, citric acid is useful not only in terrestrial animal studies (Liu et al., 2014), but also in aquatic animals, such as fish, for improving calcium, phosphorus, and zinc intake (Sugiura et al., 1998). Citric acid has been shown to improve the availability of phosphorus in rainbow trout (Pandey and Satoh, 2008), red sea bream (Pagrus major) (Hossain et al., 2007), beluga (Huso huso) (Khajepour and Hosseini, 2012), rohu (Labeo rohita) (Baruah et al., 2007), and yellowtail (Seriola quinqueradiata) (Sarker et al., 2012). Recently, Sayed et al., 2021a and Sayed et al., 2022 stated that lycopene, citric acid, and chlorella can be recommended as a feed supplement to improve hemato-biochemical alterations and oxidative damage as well as reproductive impairment induced by MPs toxicity in the African catfish (C. gariepinus). To the best of the authors’ knowledge, no study has been conducted to investigate the moderating effects of Chlorella, citric acid, and lycopene on MP neurotoxicity in fish brain tissue (Sayed et al., 2021b). Therefore, this study used a controlled laboratory experimental design to analyze the neurotoxicity of PE-MPs on the brain tissue of African catfish (C. gariepinus), and whether dietary feeding on Chlorella, citric acid, and lycopene could help alleviate their toxicity. In catfish exposed to PE-MP alone or with Chlorella, citric acid, and lycopene, the activities of Acetylcholinesterase (Ach), Monoamine Oxidase (MAO), Aldehyde Oxidase (AO), and Nitric Oxide (NO), and the histological effect were assessed.
PE-MPs make up of unevenly shaped raw powder particles. More than 90% of PE-MPs were larger than 100 nm. Toxemerge Pty Ltd. provided PE-MPs for this study (Melbourne, Australia). Per the manufacturer’s instructions, a stock solution was made from the powder using purified water (Milli-Q) and stored at 4°C in the dark. Before each use, the stock solution (2.5 g MP/L) was sonicated. More dilutions were made from this stock right away. Sigma-Aldrich provided lycopene and citric acid (Cairo, Egypt). The National Research Center provided the C. vulgaris extract (Cairo, Egypt).
A total of 150 adult African catfish (C. gariepinus), males and females (weighting 250–300 g, 20–25 cm long), were obtained and delivered to the Fish Biology and Pollution Laboratory, Faculty of Science, Assuit University, from an aquaculture farm in Assuit Governorate, Egypt. For acclimation, the fish were housed in 100 L tanks with dechlorinated tap water and air pumps under laboratory conditions for 4 weeks. Conductivity was 260.8 mM cm-1, pH 7.4, dissolved oxygen 6.9 mg L−1, temperature 20.5°C, and photoperiod 12:12 h light/dark as the physicochemical parameters of the test water. Fish commercial feed was administered at a rate of approximately 3% body weight per day, divided into two portions, during the acclimation phase. The feed contained 30% protein and consisted of soybean meal, wheat bran, maize, crude protein, fats, crude fiber, fish meal, calcium, sodium chloride, vitamins, and mineral salt. The water was changed daily (40%), and redosing was done frequently to purify water from fish waste.
The experimental design of current study was based on our previous studies (Sayed et al., 2021a; Sayed et al., 2022). Fish were classified into five groups (30 fish per each). Each treatment group was separated and placed into glass aquaria (100 cm × 70 cm × 50 cm) on a triplicate base (10 fish each) for the 15 days of the experiment. The first group was the control (fed on normal commercial feed which contained 30% protein. The second group was given PE-MP (500 mg/kg diet for 15 days in compliance with (Espinosa et al., 2019). The third group got PE-MP (500 mg/kg diet) + lycopene (500 mg/kg diet). The fourth group got PE-MP (500 mg/kg diet) + citric acid (30 g/kg diet). And the fifth group got PE-MP (500 mg/kg diet) + Chlorella (50 g/kg diet). Concentrations of lycopene, citric acid, and chlorella were employed as previously described (Abd El-Gawad et al., 2019; Mahmoud et al., 2019; Carneiro et al., 2020, respectively). Six fish from each group were randomly picked from each replica at the end of the experiment and sedated with ice to lessen stress for subsequent studies and sample collection (Wilson et al., 2009).
Blood samples were acquired from the caudal veins of every fish in each treatment group and placed in non-heparinized clean and dry tubes, which were then left to clot at room temperature before being centrifuged at 5,000 rpm for 20 min at 4°C. The neurotoxicological and antioxidant characteristics were measured through the separation of the sera. Burtis-method Ashwood’s for analyzing ACh was used (Burtis 1992). Reagent I (2 ml) was combined with the sample (0.1 ml) and incubated at 37°C for 5 minutes. Then, at 37°C, reagent II (0.5 ml) was added and vigorously mixed for 2 minutes. A spectrophotometer was used to measure the absorbance for 3 minutes at 340 nm. As per (Naseem and Parvez 2014), MAO was gauged via Holt et al.’s (1997) method, based on the oxidation of BAHC to benzaldehyde (Ashafaq et al., 2014). AO was measured according to (Johnson et al., 1984), and NO was measured according to (Tatsch et al., 2011).
Randomly, four fish from each group were dissected. The brains were rinsed in saline water to remove the blood and then washed in phosphate-buffered saline thrice, fixed in a Davidson fixative for 24 h, dehydrated through a tiered series of ethanol. Then, they were embedded in a paraffin wax block. Blocks were sectioned using a microtome at a thickness of 7 µm. To analyze standard histopathology, the sections were stained with Harris Hematoxylin and Eosin (H&E) stain (Bancroft and Stevens, 1982) and cresyl violet (Pilati et al., 2008). Finally, slide examination was done under a ×40 objective with a ×10 eyepiece using an OMAX microscope with a 14 MP USB digital camera (CS-M837ZFLR-C140U).
GraphPad Prism version 8.00 for Windows was used to analyze the data (www.graphpad.com). When required, and to better quantify the normality and homogeneity of the variance, the data were changed to log10. To compare differences in Ach, MO, AO, and NO activity between treatment groups, researchers employed a one-way analysis of variance followed by Tukey’s multiple comparison test. p < 0.05 was the value used to determine whether differences were significant.
The Research Ethical Committee of Assuit University’s Faculty of Science approved the experimental setup and fish handling (Assuit, Egypt).
During the 15-days exposure period, mortality was observed daily. Only three fish perished in the PE-MP group, while only one fish died in the PE-MP + citric acid group. All of the fish in the PE-MP + Chlorella, PE-MP + Lycopene, and control groups survived. The fish in the control group was swimming normally. Those under PE-MP exposure either individually or in combination with food supplements showed abnormal behaviors in the swimming pattern, which is illustrated as follows: PE-MP + Chlorella < PE-MP + lycopene < PE-MP + citric acid < PE-MP. Losses of movement coordination, as well as lateral and vertical swimming, were among the behavioral alterations noted. There was also an increase in respiration.
In fish chronically exposed to PE-MP for 15 days, the activities of Ach, NO, MAO, and AO significantly changed compared with the control group (Table 1). Ach and NO’s activities were significantly suppressed (p < 0.05), while MO and AO’s activities were significantly surged compared with the control group. In fish subjected to PE-MP with lycopene or Chlorella, the activities of these neurological enzymes were significantly improved or/and restored (p < 0.05) compared with fish fed PE-MP individually (Table 1). Dietary feeding citric acid significantly (p < 0.05) restored the activities of only Ach and AO in fish compared with those exposed to PE-MP alone (Table 1).
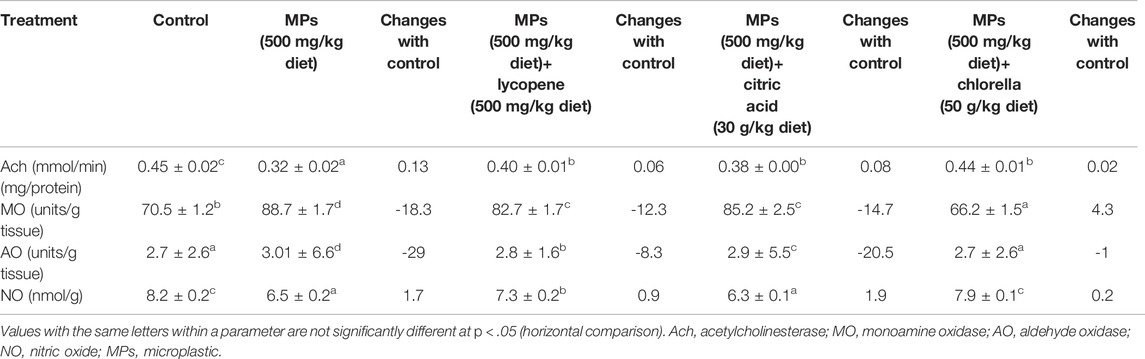
TABLE 1. Effect of microplastic on neurotoxiclogical biomarkers as mean ± SE and changes with control in African catfish (Clarias gariepinus) exposed to MPs (500 mg/kg diet), MPs (500 mg/kg diet)+ lycopene (500 mg/kg diet), MPs (500 mg/kg diet)+ citric acid (30 g/kg diet) and MPs (500 mg/kg diet)+ chlorella (50 g/kg diet) for 15 days.
Histological investigations showed normal architecture of telencephalon and no clear histological alterations in the control sections stained by H&E. Neurons with their dendrites contain basophilic homogenous cytoplasm-localized perinuclear and vesicular, round, and centrally located nuclei. Deeply stained neuroglia cells were dispersed in the homogenous ground substances of neuropil (Figure 1A). In the PE-MP group, there was severe degeneration in both neuropil and neurons. Neuropil contains patches of degenerated unstained ground substances (encephalomalacia). Most neurons lost their processes and became shrunken, containing eccentric vesicular nuclei and deeply basophilic cytoplasm localized perinuclei. Sever shrunken neurons contained aggregated, deeply stained basophilic cytoplasm surrounded by unstained space. Few pyknotic and degenerated neurons with karyolitic nuclei were observed. Hemorrhage and dilated blood vessels with leukocytic inflammatory cells were observed (Figures 1B,C). The PE-MP + lycopene group showed amelioration in neuronal morphology with vesicular nuclei and neuronal processes containing basophilic cytoplasm localized perinuclei. The beginning appearance of small patches of aggregated glial cells (gliosis) and an increase of randomly distributed glial cells were observed. The start of newly formed blood capillaries angiogenesis was noticed. There were congested blood vessels with leukocytic inflammatory cells and neuropil degeneration (Figures 1D,E). In the PE-MP + citric acid group, there was amelioration in both neuropil and neuron morphology, especially the appearance of their dendrites. There was a disappearance of shrunken neurons, even though there were still round neurons with eccentric nuclei and degenerated ones. The presence of neuroglial cells beside degenerated neurons and an increase of randomly distributed ones were observed (Figures 1F,G). In contrast, the PE-MP + Chlorella group showed the restoration of neuropil and neuron morphology, especially their sizes. There was a disappearance of shrunken neurons, where most appeared round or ovoid with centrally located nuclei and a few of them were with eccentric ones. Few degenerated cells were noticed. Random distribution of glial cells was noticed, but there were some aggregated ones besides degenerated neurons (gliosis Figures 1H,J).
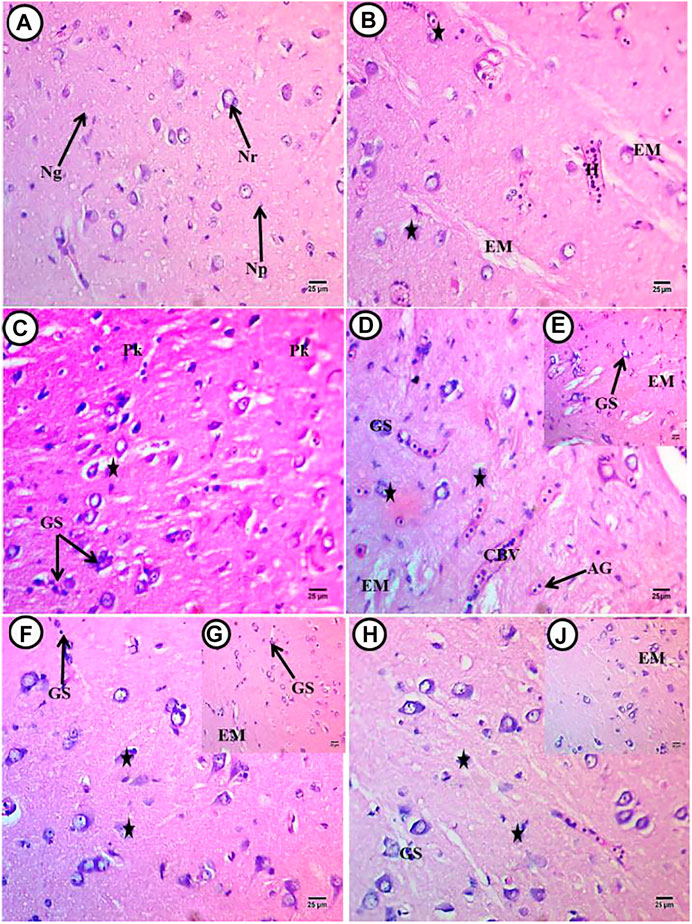
FIGURE 1. Transverse sections of telencephalon of African catfish (C. gariepinus) exposed to PE-MPs and PE-MPs with lycopene, citric acid and chlorella as antioxidants for 15 days. (A) Control fish showing normal histology of brain tissue (Telencephalon), Nr; neurons, Np; neuropil and Ng; neuroglial cells, (B,C) (PE-MPs 500 mg/kg diet), (D,E) PE-MPs + lycopene (500 mg/kg diet), (F,G) PE-MPs + citric acid (30 g/kg diet) and (H,J) PE-MPs + chlorella (50 g/kg diet). (star); diffuse deformed and degenerated neurons, EM; encephalomalacia, GS; aggregated neuroglial cells (gliosis) and edema, H; hemorrhage and inflammatory cells, AG; angiogenesis, CBV; congested blood vessels and Pk; pyknotic neurons. H&E. Scale bar 25 µm.
Histological investigations showed normal architecture of the cerebellum and no clear histological alterations in the control sections stained by H&E. There was a normal architecture of neuropil, and Purkinje cells appeared normal as a flask shape and located at the boundary between the granular and molecular layers. These cells contained basophilic cytoplasm and small vesicular nuclei. Neuroglial cells were observed with deeply stained small nuclei (Figure 2A). In the PE-MP group, there was a deconstruction of cerebellum structures, and neuropil showed severe spongiosis with different size vacuoles that appeared as parenchymal morphology. There were shrunken Purkinje cells displaced toward the molecular layer and appeared deformed in shapes containing eccentric nuclei and aggregated basophilic cytoplasm mainly localized at the lateral side. Many cells have unstained areas, and pyknotic nuclei were also observed. Congested blood capillaries with inflammatory cells and an increase of randomly distributed glial cells were noticed (Figures 2B,C). In the PE-MP + lycopene, PE-MP + citric acid, and PE-MP + Chlorella groups, there was marked amelioration in cerebellum structures compared with the PE-MP group, with slight spongiosis in these three groups (Figures 2D–F). Shrinking and degeneration in Purkinje cells left a large unstained space, which was more observed in the PE-MP + citric acid group (Figure 2E) compared with the PE-MP + lycopene (Figure 2D) and PE-MP + Chlorella (Figure 2F) groups. There was an increase in basophilic and perinuclear cytoplasm in the PE-MP + lycopene and PE-MP + Chlorella groups, supporting the synthesis of basophilic materials (RNA). There was an increase in glial cells in the PE-MP + lycopene and PE-MP + Chlorella groups compared with the PE-MP + citric acid group but a total decrease compared with the PE-MP group. Displacement of Purkinje cells toward the molecular layer was found in the PE-MP + Chlorella group, followed by the PE-MP + lycopene group and finally the PE-MP + citric acid group.
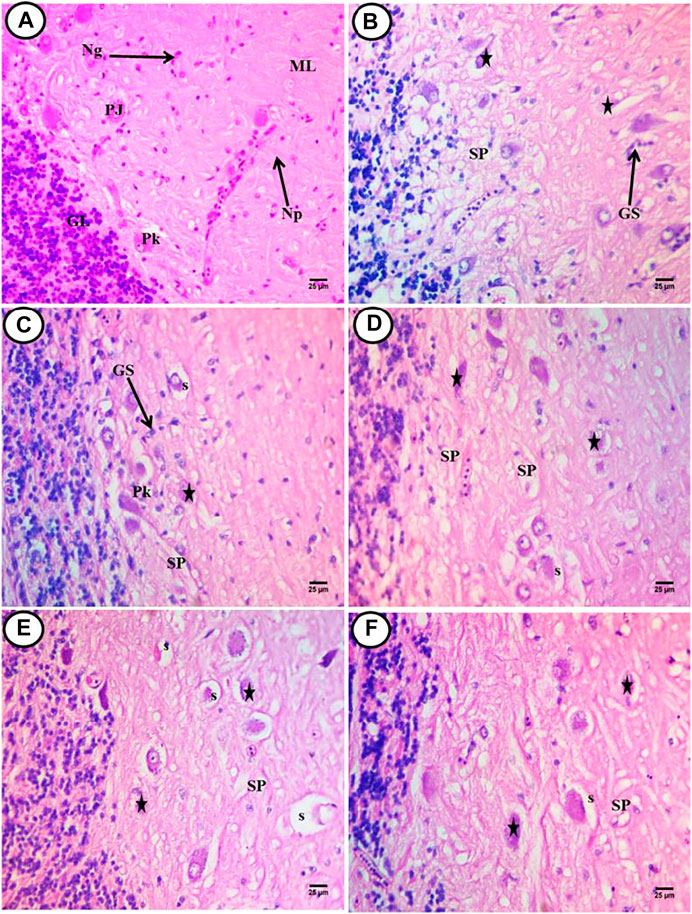
FIGURE 2. Transverse sections of cerebellum of African catfish (C. gariepinus) exposed to PE-MPs and PE-MPs with lycopene, citric acid and chlorella as antioxidants for 15 days. (A) control fish showing normal histology of brain tissue (cerebellum), PJ; purkinje cells, Np; neuropil, Ng; neuroglial cells, GL; granular layer and ML; molecular layer. (B,C) (PE-MPs 500 mg/kg diet) dose, (D) PE-MPs + lycopene (500 mg/kg diet) dose, (E) PE-MPs + citric acid (30 g/kg diet) dose and (F) PE-MPs + chlorella (50 g/kg diet). (Star); deformed and necrotic neurons, SP; vacuolization of the neuropil (spongiosis), GS; aggregated neuroglial cells (gliosis), Pk; pyknotic neurons and s; shrunken purkinje cells. H&E. Scale bar 25 µm.
Transverse telencephalon sections stained by cresyl violet showed a normal distribution of RNA substances that localized perinuclei in neurons and neuritis in control fish (Figure 3A). In the PE-MP group, there was an increase in RNA content distributed in shrunken neurons and appeared deeply stained (black arrow). Other neurons showed perinuclei RNA located at the eccentric side in a few cells, and a few glial cells were noticed (Figure 3B). In the PE-MP + lycopene, PE-MP + citric acid, and PE-MP + Chlorella groups, neuropil and neurons restored their normal appearance in these groups with antioxidants. There was an increase and homogenous distribution of RNA-localized perinuclei in the neurons and their neuritis (Figures 3C–E), with a remarkable increase in these contents in the PE-MP + citric acid group (Figure 3D). However, compared with the PE-MP group (Figure 3B), there was a decrease in RNA content in the PE-MP + lycopene and PE-MP + Chlorella groups (Figures 3C,E), whereas the PE-MP + citric acid group had the most increase (Figure 3D), and a positive reaction was seen in glial cells.
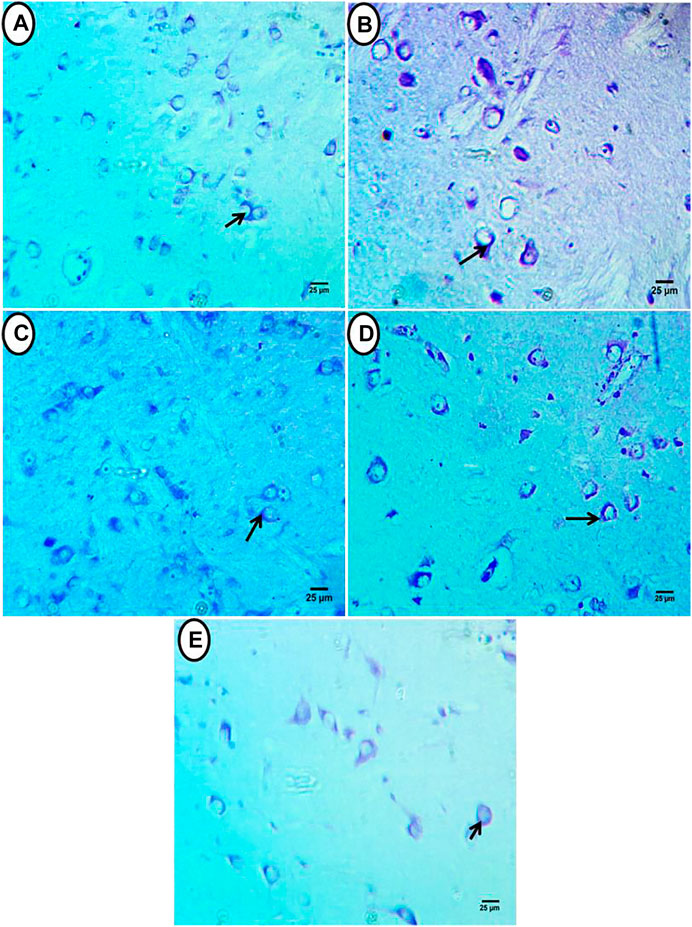
FIGURE 3. Transverse sections of telencephalon of African catfish (C. gariepinus) exposed to PE-MPs and PE-MPs with lycopene, citric acid and chlorella as antioxidants for 15 days. (A) Control group showed normal appearance of neurons and its contents of RNA (black arrow). (B) (PE-MPs 500 mg/kg diet) exposed group showed increase in RNA contents which distributed in shrunken neurons and appeared deeply stained. (C) PE-MPs + lycopene (500 mg/kg diet), (D) PE-MPs + citric acid (30 g/kg diet) and. (E) PE-MPs + chlorella (50 g/kg diet) exposed groups, showed increase in RNA contents located perinuclei in the neurons and their neuritis with remarkable increase in PE-MPs + citric acid (30 g/kg diet) exposed group (D), Cresyl violet, Scale bar 25 µm.
In the cerebellum of the control group, Purkinje cells showed a homogenous distribution of Nissle substance localized perinuclei in neurons and glial cells (Figure 4A). Fish treated with PE-MPs showed shrunken Purkinje cells with deeply stained Nissle granules; in other cells, RNA content was surrounded by unstained space. Glial cells showed deeply stained Nissle granules (Figure 4B). In the PE-MP + lycopene, PE-MP + citric acid, and PE-MP + Chlorella groups, there was a slight decrease in RNA content compared with the PE-MP group, with amelioration in the homogenous distribution of Nissle bodies localized perinuclei (Figures 4C–E), and a remarkable decrease was observed in the PE-MP + citric acid group (Figure 4D). In glial cells, there was a faint reaction in Nissle substances.

FIGURE 4. Transverse sections of cerebellum of African catfish (C. gariepinus) exposed to PE-MPs and PE-MPs with lycopene, citric acid, and chlorella as antioxidants for 15 days. (A) Control group showed Purkinje cells with normal distribution of Nissle substabnces perinuclei. (B) (PE-MPs 500 mg/kg diet) exposed group showed shrunken Purkinje cells containing deeply stained Nissle granules. (C) PE-MPs + lycopene (500 mg/kg diet), (D) PE-MPs + citric acid (30 g/kg diet) and (E) PE-MPs + chlorella (50 g/kg diet) exposed groups, showed a slight decrease in RNA contents. Remarkable decrease was observed in PE-MPs + citric acid (30 g/kg diet) exposed group, Cresyl violet, Scale bar 25 µm.
Several factors influence the neurotoxicity of MPs and nanoplastics, including material type, particle size, concentration, and exposure time (Liu et al., 2020). According to Bhagat et al. (2020), MPs can suppress the release of dopamine, melatonin, aminobutyric acid, vasopressin, oxytocin, serotonin, and kisspeptin, among other neurotransmitters. ACh is a prominent indication of neurotoxicity among multiple neurotransmitters since it offers information on possible neuromuscular cholinergic damage (Barboza et al., 2018a). Ach is required for optimal neuromuscular functioning and contributes to Acetylcholine (ACh) inactivation, which is required for cholinergic neurotransmission at neuromuscular junctions and cholinergic brain synapses. Exposure to PE-MPs caused neurotoxicity in fish by causing LPO and disrupting nerve-related enzymes (Kim et al., 2021). Here, the significant inhibition of brain Ach resulting from exposure to PE-MP alone indicates its severe neurotoxicity on catfish. This may be explained by higher amounts of MPs were observed in the gills, stomach, and feces of group 2 (MPs), group 3 (MPs + lycopene), groups 4 (MPs + CA), and groups 5 (MPs + chorella) compared to the control group, with the obvious presence of MPs in the gills, stomach and feces (Sayed et al., 2021b). Ach inhibition increases the levels of acetylcholine in the brain, inhibiting the nervous system’s functioning. Acetylcholine accumulation in the synaptic cleft effects excessive stimulation of receptors, impedes neurotransmission and paralysis, then eventually death (Chen et al., 2017). These findings are consistent with (Oliveira et al., 2013) who reported that ACh inhibition of Pomatoschistus microps after MP (PE) exposure, which was high enough to cause side effects in neurological function. Also, a significant inhibition of ACh activity at higher MPs concentration (0.69 mg.L−1) (Barboza et al., 2018b). Ding et al. (2018) reported that PS-MPs significantly reduced ACh activity in tilapia (Oreochromis niloticus). Wen et al. (2018) found that exposure to fluorescent PE-microspheres inhibits ACh activity in zebrafish, adversely affecting cholinergic neurotransmission, and leading to potential neurological and neuromuscular dysfunction. Acetylcholinesterase was significantly inhibited in Cu and Cu + MPs groups in early life stages of zebrafish, indicating neurotoxicity (Santos et al., 2020). Barboza et al. (2018a) reported that MPs cause neurotoxicity in Dicentrarchus labrax through ACh inhibition and LPO induction. Umamaheswari et al. (2021) observed the ACh activity in the brain and liver of zebrafish (Danio rerio) was notably inhibited in PS-MPs exposed groups compared to the control group. Usman et al. (2021) found a significant decrease in the activity of ACh between the MP-MED and MP-HIGH groups and between the same groups and the control in Javanese Medaka fish. The levels of acetylcholine in the groups exposed to micro-PS in mice were significantly decreased (Wang et al., 2022). In the other hand, Hoyo-Alvarez et al. (2022) showed no significant effects on ACh activity of seabream brains at low MPs concentration.
Monoamine oxidase (MAO) activity greatly affects neurotransmitters’ metabolism, such as norepinephrine, dopamine, serotonin, and epinephrine (Devi et al., 2005). It is crucial in regulating several monoamines, and its decrease may result in an imbalance of the monoaminergic system (Tabassum et al., 2015). In this study, the MAO level increased significantly in PE-MP-exposed fish compared with the control. Also, Borges and Drujan (1971) found an increase in the brain monoamine oxidase (MAO) activity in both fish and mice occurred on the third day after a single dose of 2 krads of γ-irradiation. In contrast, Basu et al. (2007) stated that a negative correlation was calculated between the concentrations of brain Hg (i.e., total Hg and MeHg) and the activities of MAO in the cerebral cortex of North American river otters. Also, Li et al. (2015) found that TBT decreased NO production in the brains of exposed juvenile common carp (Cyprinus carpio). Mukherjee et al. (2019) observed a statistically significant difference in the mean MAO level between all the different treatments of pH and type of carp. Li and Li (2021) found MAO activities were significantly decreased compared to control in brain tissues of zebrafish exposed to TBT concentrations at 100 and 300 ng/L. On the other hand, PS-microbead mixtures did not change the activity of MO in freshwater zebra mussel Dreissena polymorpha (Magni et al., 2018). These discrepancies might be attributed to several parameters, including material type, particle size, concentration, and the duration of exposure (Liu et al., 2020).
Nitric oxide (NO) plays a critical role in practically every biological system (Asl et al., 2008). Its biological importance in neurotransmission, anti-inflammation, and vascular dilatation justifies its measurement in clinical and experimental fields (Ricart-Jané et al., 2002). In this study, the significant decrease of brain NO resulting from exposure to PE-MP alone indicates its severe neurotoxicity on catfish. Inhibition of NO synthase can lead to decreased locomotor activity (Motahari et al., 2016). The NO system of fish responds to many environmental stressors, such as: tributyltin (Zhang et al., 2008; Li, et al., 2015), hyperammonia (Choudhury and Saha 2012b; Kumari et al., 2019), temperature elevation (Jørgensen et al., 2014), desiccation stress (Choudhury and Saha, 2012a; Garofalo et al., 2015), waterborne cadmium (Zheng et al., 2016), hyperosmotic stress (Gerber et al., 2018), and hypoxia/anoxia (Jensen et al., 2015; Hansen et al., 2016).
The roles of Aldehyde oxidase (AO) in fish have been examined in the metabolism of pollutants, and the use of AO as a biomarker in response to pollution has been examined in the metabolism of pollutants in fish (Tatsumi et al., 1992; Ueda et al., 2002). In addition, AO plays a very important role in the biotransformation of drugs and xenobiotics (Beedham, 1985). Lakshmanan et al. (1964) reported that AO is responsible for the metabolism of AO substrates, such as endogenous vitamins, in fish. In this study, the increase in brain AO activity in PE-MP-exposed fish was consistent with Ichipi-Ifukor et al. (2013) who observed increase in the activity of brain aldehyde oxidase in the African cat fish (Clarias gariepienus) after cadmium and arsenic exposure.
Histopathological investigations can introduce a clear picture of cytoarchitectural alterations resulting from intoxication with chemicals, although the idea of the animal pathological condition can be indicated by biochemical studies (Lakshmaiah, 2017). PE-MP exposure modifications were identified in the telencephalon and cerebellum of brain tissue in this study, with varying degrees of impact and alterations. Santos et al. (2020) observed that microplastics alone or co-exposed with copper in zebrafish embryos caused some signs of pathological changes, namely, epithelial detachment, edema, changes in midbrain-hindbrain boundary (MHB) and cell death. Also, inflammatory responses such as cytoplasmic vacuolation, inflammatory cell infiltration, the occurrence of degenerated, and necrotic neurons in the brain of zebrafish exposed to PS-MPs group (10 and 100 mg L_1) was noted at the end of 7th and 35th day (Umamaheswari et al., 2021). Hamed et al. (2021) observed degeneration and protruding of the outer meninges of the spinal cord in tilapia (Oreochromis niloticus) after exposure to MPs. The MP-LOW and MP-MED brain tissue slices showed no obvious abnormality while, 26 ± 6% of the 50 slices of MP-HIGH sections showed features of cerebral edema (Usman et al., 2021). Wang et al. (2021) found numerous vacuoles were visible in the brains of fish in the 3,000 μg/L PS-NPs exposure group. Wang et al. (2022) mentioned the cells of the hippocampal region of the mice exposed to micro-PS appeared irregular. The cells of the hippocampus of the 0.1 mg/d micro-PS group were not compact, while the cells of the 1 mg/d micro-PS group were even more loosely arranged. Moreover, Jeong et al. (2022) showed that the thickness of the neuronal layer in the CA3 region was clearly lower in the mice exposed to PSNP, whereas no change was observed in the CA1 or the dentate gyrus of the hippocampus. Besides the neuronal soma, the thickness of the corpus callosum was also clearly lower in both the medial and lateral hemispheric regions of PSNP-exposed mice.
Our previous studies have been reported the ability of different pollutants to cause brain neuropathological conditions in fish, such as; ultraviolet radiation-A (UVA) caused vacuoles, blood congestion, degeneration of neuropils, and pyknotic nuclei in neurons of brain in adult Japanese medaka (Sayed et al., 2019). Moreover, the brain showed severe gliosis, dark neurons, and vacuolation in fish exposed to tramadol (Soliman and Sayed, 2020). The brains of Nile tilapia treated with CuSO4 or CuO nanoparticles showed neuropil degeneration and pyknotic nuclei (Soliman et al., 2021). Recently, Eid et al. (2021) reported brain neuropathological abnormalities in juvenile C. gariepinus after exposure to 4-nonylphenol, including gliosis, encephalomalacia, and neuron degeneration. In this study, the brain of PE-MP-exposed fish showed increased aggregated neuroglial cells around deteriorated neurons. Polystyrene MP particles were detected in fish brain tissue for the first time, and plastic nanoparticles were found to be carried across the blood-brain barrier (Mattsson et al., 2017). Additionally, alterations observed in the brain might have been caused by specific interactions between the plastics and the brain tissue (Mattsson et al., 2017). As a result, more studies could help determine how MPs interact with brain tissue and whether this varies depending on the MP size, shape, and type. MP and nanoplastic particles can reach the brain when consumed (Prüst et al., 2020), although the quantity and potential neurotoxicity of the particles reaching the brain is still not determined. Furthermore, following 14 days of exposure, Ding et al. (2018) found PE-MP accumulation in the brain of O. niloticus, implying that MPs as small as 0.1 mm might reach the fish brain via blood circulation. For the first time (Mattsson et al., 2017), demonstrated that 0.1 g/L PE-MP of 0.18 mm size could pass the blood-brain barrier.
In our study, fish treated with PE-MPs showed an increase in RNA content distributed in shrunken neurons and appeared deeply stained. Other neurons showed perinuclei RNA located at the eccentric side in a few cells, and a few glial cells were noticed. In addition, shrunken Purkinje cells with deeply stained Nissle granules; in other cells, RNA content was surrounded by unstained space. Glial cells showed deeply stained Nissle granules. Also, Wang et al. (2022) mentioned the damage to the pyramidal cells is related to the exposure to micro-PS, and the number of Nissl bodies in the cells of the exposed mice was reduced. In the 1 mg/d micro-PS group, the Nissl bodies were significantly reduced, the pyramidal neurons were scattered, and the main dendrites were reduced or had even disappeared.
Dietary feeding lycopene, citric acid, and Chlorella improved the activities of neurological enzymes studied and restored most histological alterations induced by exposure to PE-MP feeding particularly Chlorella. Also, both lycopene and Chlorella supplements acted as potent antioxidants in detoxifying the reproductive damage induced by MPs, whereas citric acid was found to be an ineffective antioxidant in ameliorating the MPs-induced reproductive toxicity in male catfish (Sayed et al., 2022). Furthermore, Wang et al. (2022) found that the pathological changes were significantly reduced after treatment with Vit E. Vit E treatment attenuated the damage done by micro-PS exposure, the cell arrangement was more regular, the number of Nissl bodies was increased, and there was less damage to the pyramidal cells. In other pollutants and toxins, Prakash and Kumar (2014) found lycopene significantly progresses the cerebral functions and obstruct apoptosis, through preventing mitochondrial oxidative impairment, then reduction in inflammatory signs and protective properties against amyloid influenced neurotoxicity in rat cortical neurons. Abd Al Hassen, 2019 observed lycopene in co-treated groups enhanced the harmful effect of MSG on brain tissue probably because lycopene is a potent antioxidant. Farouk et al. (2021) observed that co-administration of lycopene markedly counteracted the histological alterations induced by acrylamide in brain tissues of rat. Lycopene’s neuroprotective benefits are mediated by mechanisms such as inhibition of oxidative stress and neuroinflammation and neuronal death and restoration of mitochondrial functions. The antioxidant activity of lycopene has been linked to the multiple conjugated doubled bonds and energy transfer between electrophilic singlet oxygen and their polyene backbone (Olasehinde et al., 2017). Other processes may be entailed in lycopene’s neuroprotective benefits, such as suppression of nuclear factor-B and c-Jun N-terminal kinase, activation of nuclear factor erythroid 2-related factor and brain-derived neurotrophic factor signaling, and the restoration of intracellular Ca2+ equilibrium (Chen et al., 2017).
Citric acid may have clinical benefits in neurodegenerative illnesses because both elevated brain oxidative stress and chronic inflammation have been associated with the development of such diseases. Citric acid might find utility in treating toxic and inflammatory conditions of the brain and liver tissues. This can take the form of supplementation as nutraceutical citric acid (Abdel-Salam et al., 2014). However, citric acid was found to be an ineffective antioxidant in ameliorating the MPs-induced neurotoxicity in catfish. Also, the citric acid was not effective in mitigation the MP-induced reproductive stress (Sayed et al., 2022).
Chlorella vulgaris has been found to have high vitamin K content (phytomenadione), although vitamins B12 (cobalamin) and B3 (niacin) were detected only in trace amounts as well as carotenoids and chlorophylls (Alagawany et al., 2021). Safafar et al. (2015) reported that coumaric, gallic and caffeic acids contributed to the antioxidant activities of Chlorella sp. Additionally, C. vulgaris in fish was found to enhance dietary lipid utilization, productivity, and muscle pigmentation, all identified as product quality improvement in fish (Gouveia et al., 1998). It is also used for medical treatment (Justo et al., 2001; Morris et al., 2009) due to its immunomodulating and anticancer properties and protection against hematopoiesis and age-related diseases (Safi et al., 2014). Yun et al. (2011) showed that the administration of Chlorella vulgaris is capable of reducing free radical damage by directly acting as a free radical scavenger and by indirectly stimulating antioxidant enzyme activities when animals were given a subchronic low-level exposure to lead. The protective effects of Chlorella vulgaris against lead-induced toxicity may be due to various bioactive ingredients in Chlorella vulgaris, which react with various ROS as well as inhibits oxidation processes in lipids and in the cellular compartment. Nicula et al. (2018) observed the efficiency of chlorella to alleviate the lead impact on homeostasis of trace elements from brain in Carassius gibelio Bloch. Yanuhar et al. (2020) noticed the administration of Chlorella vulgaris extract has the potential to be used as a natural bioactive of antivirus in VNN-infected brain of Grouper fish.
The variations of alleviation between these materials may be due to their different composition. The protective role of chlorella could be attributed to its natural antioxidant contents, such as chlorophyll, polyphenol, vitamins, and sulfur-containing compounds that have the capacity to scavenge free radicals (Abdelhamid et al., 2020). Lycopene is an antioxidant carotenoid compound and composed entirely of hydrogen and carbon (Stahl and Sies, 2003; Hussein et al., 2019). Citric Acid is a tricarboxylic acid found in citrus fruits. Citric acid is used as an excipient in pharmaceutical preparations due to its antioxidant properties (Nangare et al., 2021).
The present study demonstrated that MPs ingestion induced alterations in both enzymes and histology in the brain of catfish (Clarias gariepinus). In addition, dietary feeding lycopene, citric acid, and Chlorella improved the activities of neurological enzymes and restored most histological alterations. Chlorella (50 g/kg diet) was the most optimal then lycopene (500 mg/kg diet) whereas citric acid (30 g/kg diet) was found to be an ineffective antioxidant in ameliorating the MPs-induced neurotoxicity in catfish. Further studies must consider a wider range of citric acid concentrations on diet, as well as on exposure time could also be considered.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Assiut university committe.
MH, YA, ZE, HS and AS: conceptualization, AS, MH, and ZE: methodology, AS, MH, and ZE: visualization, investigation, MH, YA, ZE, HS, and AS: data curation, writing- original draft preparation. All authors: final draft writing- reviewing and editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge the Science and Technology Development Fund, Egypt, for financially supporting this study (project ID 38327).
Abd Al Hassen, M. N. (2019). Effect of Lycopene In Vivo and In Vitro on Some Physiological and Histological Changes Induced by Monosodium Glutamate in Rats. Doctoral dissertation. Iraq: Council of The College of Veterinary Medicine, University of Basrah.
Abd El-Gawad, E. A., Wang, H.-P., Yao, H., and Yao, H. (2019). Diet Supplemented with Synthetic Carotenoids: Effects on Growth Performance and Biochemical and Immunological Parameters of Yellow Perch (Perca flavescens). Front. Physiol., 10, 1056. doi:10.3389/fphys.2019.01056
Abdel-Salam, O. M. E., Youness, E. R., Mohammed, N. A., Morsy, S. M. Y., Omara, E. A., and Sleem, A. A. (2014). Citric Acid Effects on Brain and Liver Oxidative Stress in Lipopolysaccharide-Treated Mice. J. Med. Food 17, 588–598. doi:10.1089/jmf.2013.0065
Abdelhamid, F. M., Elshopakey, G. E., and Aziza, A. E. (2020). Ameliorative Effects of Dietary Chlorella Vulgaris and β-glucan against Diazinon-Induced Toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 96, 213–222. doi:10.1016/j.fsi.2019.12.009
Abidli, S., Antunes, J. C., Ferreira, J. L., Lahbib, Y., Sobral, P., and Trigui El Menif, N. (2018). Microplastics in Sediments from the Littoral Zone of the North Tunisian Coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 205, 1–9. doi:10.1016/j.ecss.2018.03.006
Abidli, S., Lahbib, Y., and Trigui El Menif, N. (2019). Microplastics in Commercial Molluscs from the Lagoon of Bizerte (Northern Tunisia). Mar. Pollut. Bull. 142, 243–252. doi:10.1016/j.marpolbul.2019.03.048
Alagawany, M., Taha, A. E., Noreldin, A., El-Tarabily, K. A., and Abd El-Hack, M. E. (2021). Nutritional Applications of Species of Spirulina and Chlorella in Farmed Fish: A Review. Aquaculture, 542, 736841. doi:10.1016/j.aquaculture.2021.736841
Alomar, C., Sureda, A., Capó, X., Guijarro, B., Tejada, S., and Deudero, S. (2017). Microplastic Ingestion by Mullus Surmuletus Linnaeus, 1758 Fish and its Potential for Causing Oxidative Stress. Environ. Res. 159, 135–142. doi:10.1016/j.envres.2017.07.043
Anderson, P. J., Warrack, S., Langen, V., Challis, J. K., Hanson, M. L., and Rennie, M. D. (2017). Microplastic Contamination in Lake Winnipeg, Canada. Environ. Pollut. 225, 223–231. doi:10.1016/j.envpol.2017.02.072
Andrady, A. L. (2011). Microplastics in the Marine Environment. Mar. Pollut. Bull. 62, 1596–1605. doi:10.1016/j.marpolbul.2011.05.030
Ashafaq, M., Varshney, L., Khan, M. H., Salman, M., Naseem, M., Wajid, S., et al. (2014). Neuromodulatory Effects of Hesperidin in Mitigating Oxidative Stress in Streptozotocin Induced Diabetes. Biomed. Res. Int. 2014, 249031. doi:10.1155/2014/249031
Asl, S. Z., Ghasemi, A., and Azizi, F. (2008). Serum Nitric Oxide Metabolites in Subjects with Metabolic Syndrome. Clin. Biochem. 41 (16–17), 1342–1347. doi:10.1016/j.clinbiochem.2008.08.076
Bancroft, D., and Stevens, A. (1982). Theory and Practice of Histological Techniques Churchill Livingstone. Edinburgh, London, Melaborne: Elsevier.
Barboza, L. G. A., Vieira, L. R., Branco, V., Carvalho, C., and Guilhermino, L. (2018a). Microplastics Increase Mercury Bioconcentration in Gills and Bioaccumulation in the Liver, and Cause Oxidative Stress and Damage in Dicentrarchus labrax Juveniles. Sci. Rep. 8, 15655. doi:10.1038/s41598-018-34125-z
Barboza, L. G. A., Vieira, L. R., Branco, V., Figueiredo, N., Carvalho, F., Carvalho, C., et al. (2018b). Microplastics Cause Neurotoxicity, Oxidative Damage and Energy-Related Changes and Interact with the Bioaccumulation of Mercury in the European Seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 195, 49–57. doi:10.1016/j.aquatox.2017.12.008
Baruah, K., Sahu, N. P., Pal, A. K., Jain, K. K., Debnath, D., and Mukherjee, S. C. (2007). Dietary Microbial Phytase and Citric Acid Synergistically Enhances Nutrient Digestibility and Growth Performance of Labeo Rohita (Hamilton) Juveniles at Sub‐optimal Protein Level. Aquac. Res. 38, 109–120. doi:10.1111/j.1749-7345.2007.00092.x
Basu, N., Scheuhammer, A. M., Evans, R. D., O'Brien, M., and Chan, H. M. (2007). Cholinesterase and Monoamine Oxidase Activity in Relation to Mercury Levels in the Cerebral Cortex of Wild River Otters. Hum. Exp. Toxicol. 26 (3), 213–220. doi:10.1177/0960327107070570
Beedham, C. (1985). Molybdenum Hydroxylases as Drug-Metabolizing Enzymes. Drug Metab. Rev. 16, 119–156. doi:10.3109/03602538508991432
Beiras, R., Muniategui-Lorenzo, S., Rodil, R., Tato, T., Montes, R., López-Ibáñez, S., et al. (2019). Polyethylene Microplastics Do Not Increase Bioaccumulation or Toxicity of Nonylphenol and 4-MBC to Marine Zooplankton. Sci. Total Environ. 692, 1–9. doi:10.1016/j.scitotenv.2019.07.106
Bhagat, J., Zang, L., Nishimura, N., and Shimada, Y. (2020). Zebrafish: An Emerging Model to Study Microplastic and Nanoplastic Toxicity. Sci. Total Environ. 728, 138707. doi:10.1016/j.scitotenv.2020.138707
Borges, J. M. D., and Drujan, B. D. (1971). The Effects of γ-Irradiation upon Monoamine Oxidase Activity. Radiat. Res. 45 (3), 589–597.
Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M., and Thompson, R. C. (2008). Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environ. Sci. Technol. 42, 5026–5031. doi:10.1021/es800249a
Browne, M. A., Galloway, T., and Thompson, R. (2007). Microplastic-an Emerging Contaminant of Potential Concern? Integr. Environ. Assess. Manag. 3, 559–561. doi:10.1002/ieam.5630030412
Browne, M. A. (2015). Sources and Pathways of Microplastics to Habitats. Mar. Anthropog. litter, 229–244. doi:10.1007/978-3-319-16510-3_9
Burtis, W. J. (1992). Parathyroid Hormone-Related Protein: Structure, Function, and Measurement. Clin. Chem. 38, 2171–2183. doi:10.1093/clinchem/38.11.2171
Carneiro, W. F., Castro, T. F. D., Orlando, T. M., Meurer, F., Paula, D. A. d. J., Virote, B. d. C. R., et al. (2020). Replacing Fish Meal by Chlorella Sp. Meal: Effects on Zebrafish Growth, Reproductive Performance, Biochemical Parameters and Digestive Enzymes. Aquaculture 528, 735612. doi:10.1016/j.aquaculture.2020.735612
Chen, Q., Yin, D., Jia, Y., Schiwy, S., Legradi, J., Yang, S., et al. (2017). Enhanced Uptake of BPA in the Presence of Nanoplastics Can Lead to Neurotoxic Effects in Adult Zebrafish. Sci. Total Environ. 609, 1312–1321. doi:10.1016/j.scitotenv.2017.07.144
Choudhury, M. G., and Saha, N. (2012a). Expression of Inducible Nitric Oxide Synthase and Nitric Oxide Production in the Mud-Dwelled Air-Breathing Singhi Catfish, Heteropneustes Fossilis under Condition of Water Shortage. Nitric Oxide 27, 219–227. doi:10.1016/j.niox.2012.07.006
Choudhury, M. G., and Saha, N. (2012b). Influence of Environmental Ammonia on the Production of Nitric Oxide and Expression of Inducible Nitric Oxide Synthase in the Freshwater Air-Breathing Catfish (Heteropneustes Fossilis). Aquat. Toxicol. 116-117, 43–53. doi:10.1016/j.aquatox.2012.03.006
Cole, M., Lindeque, P., Fileman, E., Halsband, C., Goodhead, R., Moger, J., et al. (2013). Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 47, 6646–6655. doi:10.1021/es400663f
Costa, M. F., Ivar do Sul, J. A., Silva-Cavalcanti, J. S., Araújo, M. C. B., Spengler, Â., and Tourinho, P. S. (2009). On the Importance of Size of Plastic Fragments and Pellets on the Strandline: A Snapshot of a Brazilian Beach. Environ. Monit. Assess. 168, 299–304. doi:10.1007/s10661-009-1113-4
Devi, C. B., Reddy, G. H., Prasanthi, R. P. J., Chetty, C. S., and Reddy, G. R. (2005). Developmental Lead Exposure Alters Mitochondrial Monoamine Oxidase and Synaptosomal Catecholamine Levels in Rat Brain. Int. J. Dev. Neurosci. 23, 375–381. doi:10.1016/j.ijdevneu.2004.11.003
Ding, J., Zhang, S., Razanajatovo, R. M., Zou, H., and Zhu, W. (2018). Accumulation, Tissue Distribution, and Biochemical Effects of Polystyrene Microplastics in the Freshwater Fish Red tilapia (Oreochromis niloticus). Environ. Pollut. 238, 1–9. doi:10.1016/j.envpol.2018.03.001
Eid, Z., Mahmoud, U. M., Mekkawy, I. A., Abdel-Tawab, H. S., and Sayed, A. E.-D. H. (2021). 4-Nonylphenol Induced Brain Damage in Juvenile African Catfish (Clarias Garepinus). Toxicol. Environ. Health Sci., 1–14. doi:10.1007/s13530-021-00080-y
Espinosa, C., Esteban, M. Á., and Cuesta, A. (2019). Dietary Administration of PVC and PE Microplastics Produces Histological Damage, Oxidative Stress and Immunoregulation in European Sea Bass (Dicentrarchus labrax L.). Fish shellfish Immunol. 95, 574–583. doi:10.1016/j.fsi.2019.10.072
Farouk, S. M., Gad, F. A., Almeer, R., Abdel-Daim, M. M., and Emam, M. A. (2021). Exploring the Possible Neuroprotective and Antioxidant Potency of Lycopene against Acrylamide-Induced Neurotoxicity in Rats' Brain. Biomed. Pharmacother. 138, 111458. doi:10.1016/j.biopha.2021.111458
Faure, F., Demars, C., Wieser, O., Kunz, M., and De Alencastro, L. F. (2015). Plastic Pollution in Swiss Surface Waters: Nature and Concentrations, Interaction with Pollutants. Environ. Chem. 12, 582–591. doi:10.1071/en14218
Fossi, M. C., Pedà, C., Compa, M., Tsangaris, C., Alomar, C., Claro, F., et al. (2018). Bioindicators for Monitoring Marine Litter Ingestion and its Impacts on Mediterranean Biodiversity. Environ. Pollut. 237, 1023–1040. doi:10.1016/j.envpol.2017.11.019
Free, C. M., Jensen, O. P., Mason, S. A., Eriksen, M., Williamson, N. J., and Boldgiv, B. (2014). High-levels of Microplastic Pollution in a Large, Remote, Mountain Lake. Mar. Pollut. Bull. 85, 156–163. doi:10.1016/j.marpolbul.2014.06.001
Gall, S. C., and Thompson, R. C. (2015). The Impact of Debris on Marine Life. Mar. Pollut. Bull. 92, 170–179. doi:10.1016/j.marpolbul.2014.12.041
Garofalo, F., Amelio, D., Icardo, J. M., Chew, S. F., Tota, B., Cerra, M. C., et al. (2015). Signal Molecule Changes in the Gills and Lungs of the African Lungfish Protopterus annectens, during the Maintenance and Arousal Phases of Aestivation. Nitric Oxide 44, 71–80. doi:10.1016/j.niox.2014.11.017
Gerber, L., Jensen, F. B., and Madsen, S. S. (2018). Dynamic Changes in Nitric Oxide Synthase Expression Are Involved in Seawater Acclimation of Rainbow Trout Oncorhynchus mykiss. Am. J. Physiology-Regulatory, Integr. Comp. Physiology 314, R552–R562. doi:10.1152/ajpregu.00519.2016
Gewert, B., Plassmann, M. M., and MacLeod, M. (2015). Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 17, 1513–1521. doi:10.1039/c5em00207a
Gouveia, L., Choubert, G., Gomes, E., Rema, P., and Empis, J. (1998). Use of Chlorella Vulgaris as a Carotenoid Source for Rainbow Trout: Effect of Dietary Lipid Content on Pigmentation, Digestibility and Retention in the Muscle Tissue. Aquac. Int. 6, 269–279. doi:10.1023/a:1009251714573
Graham, E. R., and Thompson, J. T. (2009). Deposit- and Suspension-Feeding Sea Cucumbers (Echinodermata) Ingest Plastic Fragments. J. Exp. Mar. Biol. Ecol. 368, 22–29. doi:10.1016/j.jembe.2008.09.007
Hamed, M., Soliman, H. A. M., Badrey, A. E. A., and Osman, A. G. M. (2021). Microplastics Induced Histopathological Lesions in Some Tissues of tilapia (Oreochromis niloticus) Early Juveniles. Tissue Cell. 71, 101512. doi:10.1016/j.tice.2021.101512
Hamed, M., Soliman, H. A. M., Osman, A. G. M., and Sayed, A. E.-D. H. (2020). Antioxidants and Molecular Damage in Nile Tilapia (Oreochromis niloticus) after Exposure to Microplastics. Environ. Sci. Pollut. Res., 27(13), 14581–14588. doi:10.1007/s11356-020-07898-y
Hamed, M., Soliman, H. A. M., Osman, A. G. M., and Sayed, A. E.-D. H. (2019). Assessment the Effect of Exposure to Microplastics in Nile Tilapia (Oreochromis niloticus) Early Juvenile: I. Blood Biomarkers. Chemosphere 228, 345–350. doi:10.1016/j.chemosphere.2019.04.153
Hansen, M. N., Gerber, L., and Jensen, F. B. (2016). Nitric Oxide Availability in Deeply Hypoxic Crucian Carp: Acute and Chronic Changes and Utilization of Ambient Nitrite Reservoirs. Am. J. Physiology-Regulatory, Integr. Comp. Physiology 310, R532–R540. doi:10.1152/ajpregu.00515.2015
Holt, A., Sharman, D. F., Baker, G. B., and Palcic, M. M. (1997). A Continuous Spectrophotometric Assay for Monoamine Oxidase and Related Enzymes in Tissue Homogenates. Analyt. Biochem. 244 (2), 384–392.
Hossain, M. A., Pandey, A., and Satoh, S. (2007). Effects of Organic Acids on Growth and Phosphorus Utilization in Red Sea Bream Pagrus major. Fish. Sci. 73, 1309–1317. doi:10.1111/j.1444-2906.2007.01469.x
Hoyo-Alvarez, E., Arechavala-Lopez, P., Jiménez-García, M., Solomando, A., Alomar, C., Sureda, A., et al. 2022). Effects of Pollutants and Microplastics Ingestion on Oxidative Stress and Monoaminergic Activity of Seabream Brains. Aquat. Toxicol., 242, 106048. doi:10.1016/j.aquatox.2021.106048
Hussein, M. M. A., Elsadaawy, H. A., El-Murr, A., Ahmed, M. M., Bedawy, A. M., Tukur, H. A., et al. (2019). Endosulfan Toxicity in Nile tilapia (Oreochromis niloticus) and the Use of Lycopene as an Ameliorative Agent. Comp. Biochem. Physiology Part C Toxicol. Pharmacol. 224, 108573. doi:10.1016/j.cbpc.2019.108573
Ichipi-Ifukor, P. C., Evwhre, O. L., and Eferobohwo, A. (2013). A Comparative Study of the Effect of Cadmium and Arsenic on the Activities of Brain Sulphite Oxidase and Aldehyde Oxidase in the African Cat Fish (Clarias Gariepienus). J. Res. Biosci. 9 (1), 68–71.
Imhof, H. K., Ivleva, N. P., Schmid, J., Niessner, R., and Laforsch, C. (2013). Contamination of Beach Sediments of a Subalpine Lake with Microplastic Particles. Curr. Biol. 23, R867–R868. doi:10.1016/j.cub.2013.09.001
James, K., Vasant, K., Padua, S., Gopinath, V., K S, A., R, J., et al. (2020). An Assessment of Microplastics in the Ecosystem and Selected Commercially Important Fishes off Kochi, South Eastern Arabian Sea, India. Mar. Pollut. Bull. 154, 111027. doi:10.1016/j.marpolbul.2020.111027
Jensen, F. B., Gerber, L., Hansen, M. N., and Madsen, S. S. (2015). Metabolic Fates and Effects of Nitrite in Brown Trout under Normoxic and Hypoxic Conditions: Blood and Tissue Nitrite Metabolism and Interactions with Branchial NOS, Na+/K+-ATPase and Hsp70 Expression. J. Exp. Biol. 218, 2015–2022. doi:10.1242/jeb.120394
Jeong, B., Baek, J. Y., Koo, J., Park, S., Ryu, Y.-K., Kim, K.-S., et al. 2022). Maternal Exposure to Polystyrene Nanoplastics Causes Brain Abnormalities in Progeny. J. Hazard. Mater., 426, 127815. doi:10.1016/j.jhazmat.2021.127815
Johnson, C., Stubley-Beedham, C., and Stell, J. G. P. (1984). Elevation of Molybdenum Hydroxylase Levels in Rabbit Liver after Ingestion of Phthalazine or its Hydroxylated Metabolite. Biochem. Pharmacol. 33, 3699–3705. doi:10.1016/0006-2952(84)90159-x
Jørgensen, S. M., Castro, V., Krasnov, A., Torgersen, J., Timmerhaus, G., Hevrøy, E. M., et al. (2014). Cardiac Responses to Elevated Seawater Temperature in Atlantic Salmon. BMC Physiol. 14, 2–11. doi:10.1186/1472-6793-14-2
Justo, G. Z., Silva, M. R., and Queiroz, M. L. S. (2001). Effects of the Green Algaechlorella Vulgarison the Response of the Host Hematopoietic System to Intraperitoneal Ehrlich Ascites Tumor Transplantation in Mice. Immunopharmacol. Immunotoxicol. 23, 119–132. doi:10.1081/iph-100102573
Kay, R. A., and Barton, L. L. (1991). Microalgae as Food and Supplement. Crit. Rev. Food Sci. Nutr. 30, 555–573. doi:10.1080/10408399109527556
Khajepour, F., and Hosseini, S. A. (2012). Citric Acid Improves Growth Performance and Phosphorus Digestibility in Beluga (Huso huso) Fed Diets where Soybean Meal Partly Replaced Fish Meal. Animal Feed Sci. Technol. 171, 68–73. doi:10.1016/j.anifeedsci.2011.10.001
Kim, J.-H., Yu, Y.-B., and Choi, J.-H. (2021). Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Neurotoxicity in Fish Exposed to Microplastics: A Review. J. Hazard. Mater. 413, 125423. doi:10.1016/j.jhazmat.2021.125423
Kumari, S., Choudhury, M. G., and Saha, N. (2019). Hyper-ammonia Stress Causes Induction of Inducible Nitric Oxide Synthase Gene and More Production of Nitric Oxide in Air-Breathing Magur Catfish, Clarias Magur (Hamilton). Fish. Physiol. Biochem. 45 (3), 907–920. doi:10.1007/s10695-018-0593-y
Lakshmaiah, G. (2017). Brain Histopathology of the Fish Cyprinus carpio Exposed to Lethal Concentrations of an Organophosphate Insecticide Phorate. Int. J. Adv. Res. Develop. 2, 668–672.
Lakshmanan, M., Vaidyanathan, C., and Cama, H. (1964). Oxidation of Vitamin A1 Aldehyde and Vitamin A2 Aldehyde to the Corresponding Acids by Aldehyde Oxidase from Different Species. Biochem. J. 90, 569–573. doi:10.1042/bj0900569
Li, C., Busquets, R., and Campos, L. C. (2020). Assessment of Microplastics in Freshwater Systems: A Review. Sci. Total Environ. 707, 135578. doi:10.1016/j.scitotenv.2019.135578
Li, J., Liu, H., and Paul Chen, J. (2018). Microplastics in Freshwater Systems: A Review on Occurrence, Environmental Effects, and Methods for Microplastics Detection. Water Res. 137, 362–374. doi:10.1016/j.watres.2017.12.056
Li, P., and Li, Z. H. (2021). Neurotoxicity and Physiological Stress in Brain of Zebrafish Chronically Exposed to Tributyltin. J. Toxicol. Environ. Health 84 (1), 20–30.
Li, Z.-H., Li, P., and Shi, Z.-C. (2015). Chronic Exposure to Tributyltin Induces Brain Functional Damage in Juvenile Common Carp (Cyprinus carpio). PloS one 10, e0123091. doi:10.1371/journal.pone.0123091
Liu, Q., Chen, C., Li, M., Ke, J., Huang, Y., Bian, Y., et al. (2020). Neurodevelopmental Toxicity of Polystyrene Nanoplastics in Caenorhabditis elegans and the Regulating Effect of Presenilin. ACS omega 5, 33170–33177. doi:10.1021/acsomega.0c04830
Liu, S. T., Hou, W. X., Cheng, S. Y., Shi, B. M., and Shan, A. S. (2014). Effects of Dietary Citric Acid on Performance, Digestibility of Calcium and Phosphorus, Milk Composition and Immunoglobulin in Sows during Late Gestation and Lactation. Animal Feed Sci. Technol. 191, 67–75. doi:10.1016/j.anifeedsci.2014.01.017
Lusher, A. L., McHugh, M., and Thompson, R. C. (2013). Occurrence of Microplastics in the Gastrointestinal Tract of Pelagic and Demersal Fish from the English Channel. Mar. Pollut. Bull. 67, 94–99. doi:10.1016/j.marpolbul.2012.11.028
Magni, S., Gagné, F., André, C., Della Torre, C., Auclair, J., Hanana, H., et al. (2018). Evaluation of Uptake and Chronic Toxicity of Virgin Polystyrene Microbeads in Freshwater Zebra Mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ. 631-632, 778–788. doi:10.1016/j.scitotenv.2018.03.075
Mahmoud, N., Eid, A., Wahdan, A. A., Enany, M. E., and Abd El-Nab, A. S. (2019). Effect of Phytase and Citric Acid on Growth Performance, Feed Utilization and its Antibacterial Activity against Fish Pathogens of Nile tilapia Fingerlings. Egypt. J. Aquac. 9, 35–53. doi:10.21608/EJA.2019.47194
Massoulié, J., Pezzementi, L., Bon, S., Krejci, E., and Vallette, F. M. (1993). Molecular and Cellular Biology of Cholinesterases. Prog. Neurobiol. 41, 31–91. doi:10.1016/0301-0082(93)90040-y
Mathalon, A., and Hill, P. (2014). Microplastic Fibers in the Intertidal Ecosystem Surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 81, 69–79. doi:10.1016/j.marpolbul.2014.02.018
Mattsson, K., Johnson, E. V., Malmendal, A., Linse, S., Hansson, L.-A., and Cedervall, T. (2017). Brain Damage and Behavioural Disorders in Fish Induced by Plastic Nanoparticles Delivered through the Food Chain. Sci. Rep. 7, 11452. doi:10.1038/s41598-017-10813-0
Miranda, D. d. A., and de Carvalho-Souza, G. F. (2016). Are We Eating Plastic-Ingesting Fish? Mar. Pollut. Bull. 103, 109–114. doi:10.1016/j.marpolbul.2015.12.035
Morris, H. J., Carrillo, O. V., Almarales, Á., Bermúdez, R. C., Alonso, M. E., Borges, L., et al. (2009). Protein Hydrolysates from the Alga Chlorella Vulgaris 87/1 with Potentialities in Immunonutrition. Biotecnol. Apl. 26, 162–165.
Motahari, A. A., Sahraei, H., and Meftahi, G. H. (2016). Role of Nitric Oxide on Dopamine Release and Morphine-Dependency. Basic Clin. Neurosci. 7, 283–290. doi:10.15412/J.BCN.03070401
Mukherjee, A., Bhowmick, A. R., Mukherjee, J., and Moniruzzaman, M. (2019). Physiological Response of Fish under Variable Acidic Conditions: a Molecular Approach through the Assessment of an Eco-Physiological Marker in the Brain. Environ. Sci. Pollut. Res., 26(23), 23442–23452. doi:10.1007/s11356-019-05602-3
Murray, F., and Cowie, P. R. (2011). Plastic Contamination in the Decapod Crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 62, 1207–1217. doi:10.1016/j.marpolbul.2011.03.032
Nangare, S., Vispute, Y., Tade, R., Dugam, S., and Patil, P. (2021). Pharmaceutical Applications of Citric Acid. Future J. Pharm. Sci., 7(1), 1–23. doi:10.1186/s43094-021-00203-9
Naseem, M., and Parvez, S. (2014). Hesperidin Restores Experimentally Induced Neurotoxicity in Wistar Rats. Toxicol. Mech. Methods 24, 512–519. doi:10.3109/15376516.2014.945108
Neves, D., Sobral, P., Ferreira, J. L., and Pereira, T. (2015). Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Mar. Pollut. Bull. 101, 119–126. doi:10.1016/j.marpolbul.2015.11.008
Nick, G. L. (2003). Addressing Human Exposure to Environmental Toxins with Chlorella pyrenoidosa. (Medicinal Properties in Whole Foods). Townsend Lett. Dr. Patients 237, 28–33.
Nicula, M., Pacala, N., Stef, L., Pet, I., Dronca, D., Ahmadi, M., et al. (2018). Garlic and Chlorella Biomodulate Lead Toxicity on Manganese Homeostasis in Carassius gibelio Bloch. Rev. Chim., 69, 986–989. doi:10.37358/rc.18.4.6242
Olasehinde, T., Olaniran, A., and Okoh, A. (2017). Therapeutic Potentials of Microalgae in the Treatment of Alzheimer's Disease. Molecules, 22(3), 480. doi:10.3390/molecules22030480
Oliveira, M., Ribeiro, A., Hylland, K., and Guilhermino, L. (2013). Single and Combined Effects of Microplastics and Pyrene on Juveniles (0+ Group) of the Common Goby Pomatoschistus Microps (Teleostei, Gobiidae). Ecol. Indic. 34, 641–647. doi:10.1016/j.ecolind.2013.06.019
Pandey, A., and Satoh, S. (2008). Effects of Organic Acids on Growth and Phosphorus Utilization in Rainbow troutOncorhynchus Mykiss. Fish. Sci. 74, 867–874. doi:10.1111/j.1444-2906.2008.01601.x
Pilati, N., Barker, M., Panteleimonitis, S., Donga, R., and Hamann, M. (2008). A Rapid Method Combining Golgi and Nissl Staining to Study Neuronal Morphology and Cytoarchitecture. J. Histochem Cytochem. 56, 539–550. doi:10.1369/jhc.2008.950246
Prakash, A., and Kumar, A. (2014). Implicating the Role of Lycopene in Restoration of Mitochondrial Enzymes and BDNF Levels in β-Amyloid Induced Alzheimer’s Disease. Eur. J. Pharmacol. 741, 104–111.
Prüst, M., Meijer, J., and Westerink, R. H. (2020). The Plastic Brain: Neurotoxicity of Micro-and Nanoplastics. Part. fibre Toxicol. 17, 1–16.
Repetto, M., Semprine, J., and Boveris, A. (2012). “Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination,” in Lipid Peroxidation. Editor A. Catala, Vol. 1, 3–30.
Ricart-Jané, D., Llobera, M., and López-Tejero, M. D. (2002). Anticoagulants and Other Preanalytical Factors Interfere in Plasma Nitrate/nitrite Quantification by the Griess Method. Nitric Oxide 6, 178–185.
Roberfroid, M. B. (2002). Global View on Functional Foods: European Perspectives. Br. J. Nutr. 88, S133–S138. doi:10.1079/bjn2002677
Rochman, C. M., Hoh, E., Kurobe, T., and Teh, S. J. (2013). Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Sci. Rep. 3, 3263. doi:10.1038/srep03263
Romeo, T., Pietro, B., Pedà, C., Consoli, P., Andaloro, F., and Fossi, M. C. (2015). First Evidence of Presence of Plastic Debris in Stomach of Large Pelagic Fish in the Mediterranean Sea. Mar. Pollut. Bull. 95, 358–361. doi:10.1016/j.marpolbul.2015.04.048
Safafar, H., Wagenen, J., Møller, P., and Jacobsen, C. (2015). Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 13, 7339–7356. doi:10.3390/md13127069
Safi, C., Zebib, B., Merah, O., Pontalier, P.-Y., and Vaca-Garcia, C. (2014). Morphology, Composition, Production, Processing and Applications of Chlorella Vulgaris: A Review. Renew. Sustain. Energy Rev. 35, 265–278. doi:10.1016/j.rser.2014.04.007
Santos, D., Félix, L., Luzio, A., Parra, S., Cabecinha, E., Bellas, J., et al. (2020). Toxicological Effects Induced on Early Life Stages of Zebrafish (Danio rerio) after an Acute Exposure to Microplastics Alone or Co-exposed with Copper. Chemosphere, 261, 127748. doi:10.1016/j.chemosphere.2020.127748
Sarker, M. S. A., Satoh, S., Kamata, K., Haga, Y., and Yamamoto, Y. Supplementation effect(s) of organic acids and/or lipid to plant protein-based diets on juvenile yellowtail, Seriola quinqueradiata Temminck et Schlegel 1845, growth and, nitrogen and phosphorus excretion. Aquac. Res. 2012;43:538–545. doi:10.1111/j.1365-2109.2011.02859.x
Sayed, A. E.-D. H., Hamed, M., Badrey, A. E. A., Ismail, R. F., Osman, Y. A. A., Osman, A. G. M., et al. (2021b). Microplastic Distribution, Abundance, and Composition in the Sediments, Water, and Fishes of the Red and Mediterranean Seas, Egypt. Mar. Pollut. Bull., 173, 112966. doi:10.1016/j.marpolbul.2021.112966
Sayed, A. E.-D. H., Hamed, M., Badrey, A. E. A., and Soliman, H. A. M. (2021a). Bioremediation of Hemotoxic and Oxidative Stress Induced by Polyethylene Microplastic in Clarias gariepinus Using Lycopene, Citric Acid, and Chlorella. Comp. Biochem. Physiology Part C Toxicol. Pharmacol. 250, 109189. doi:10.1016/j.cbpc.2021.109189
Sayed, A. E.-D. H., Soliman, H. A. M., and Mitani, H. (2019). UVA-induced Neurotoxicity in Japanese Medaka (Oryzias latipes). Photochem. Photobiol. Sci., 18(1), 71–79. doi:10.1039/c8pp00169c
Sayed, A. E. D. H., Hamed, M., and Ismail, R. F. 2022. Natural Antioxidants Can Improve Microplastics-Induced Male Reproductive Impairment in the African Catfish (Clarias gariepinus). Front. Environ. Sci., 756.doi:10.3389/fenvs.2021.811466
Soliman, H. A. M., Hamed, M., and Sayed, A. E. H. (2021). Investigating the Effects of Copper Sulfate and Copper Oxide Nanoparticles in Nile tilapia (Oreochromis niloticus) Using Multiple Biomarkers: the Prophylactic Role of Spirulina. Environ. Sci. Pollut. Res. Int. 28, 30046–30057. doi:10.1007/s11356-021-12859-0
Soliman, H. A. M., and Sayed, A. E.-D. H. (2020). Poikilocytosis and Tissue Damage as Negative Impacts of Tramadol on Juvenile of Tilapia (Oreochromis niloticus). Environ. Toxicol. Pharmacol. 78, 103383. doi:10.1016/j.etap.2020.103383
Stahl, W., and Sies, H. (2003). Antioxidant Activity of Carotenoids. Mol. aspects Med., 24(6), 345–351. doi:10.1016/s0098-2997(03)00030-x
Sugiura, S. H., Dong, F. M., Rathbone, C. K., and Hardy, R. W. (1998). Apparent Protein Digestibility and Mineral Availabilities in Various Feed Ingredients for Salmonid Feeds. Aquaculture 159, 177–202. doi:10.1016/s0044-8486(97)00177-4
Tabassum, H., Afjal, M. A., Khan, J., Raisuddin, S., and Parvez, S. (2015). Neurotoxicological Assessment of Pendimethalin in Freshwater Fish Channa punctata Bloch. Ecol. Indic. 58, 411–417. doi:10.1016/j.ecolind.2015.06.008
Tatsch, E., Bochi, G. V., Pereira, R. d. S., Kober, H., Agertt, V. A., Anraku de Campos, M. M., et al. (2011). A Simple and Inexpensive Automated Technique for Measurement of Serum Nitrite/nitrate. Clin. Biochem. 44, 348–350. doi:10.1016/j.clinbiochem.2010.12.011
Tatsumi, K., Kitamura, S., Kato, M., and Hiraoka, K. (1992). Metabolism of Sodium Nifurstyrenate, a Veterinary Antimicrobial Nitrofuran, in Animals and Fish. Drug Metab. Dispos. 20, 226–233.
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W., et al. (2004). Lost at Sea: where Is All the Plastic? Science 304, 838. doi:10.1126/science.1094559
Ueda, O., Kitamura, S., and Ohta, S. (2002). Metabolism of 2-nitrofluorene, an Environmental Pollutant, by Liver Preparations of Sea Bream, Pagrus major. Xenobiotica 32, 667–682. doi:10.1080/00498250210144839
Umamaheswari, S., Priyadarshinee, S., Bhattacharjee, M., Kadirvelu, K., and Ramesh, M. (2021). Exposure to Polystyrene Microplastics Induced Gene Modulated Biological Responses in Zebrafish (Danio rerio). Chemosphere, 281, 128592. doi:10.1016/j.chemosphere.2020.128592
Usman, S., Abdull Razis, A. F., Shaari, K., Amal, M. N. A., Saad, M. Z., Mat Isa, N., et al. (2021). Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854). Ijerph, 18(18), 9449.doi:10.3390/ijerph18189449
Wagner, L., and Lambert, S. (2018). Freshwater Microplastics: Emerging Environmental Contaminants? Springer Nature, 303.
Waliszewski, K. N., and Blasco, G. (2010). Propiedades nutraceúticas del licopeno. Salud pública Méx 52, 254–265. doi:10.1590/s0036-36342010000300010
Wang, Q., Duan, X., Huang, F., Cheng, H., Zhang, C., Li, L., et al. (2021). Polystyrene Nanoplastics Alter Virus Replication in Orange-Spotted Grouper (Epinephelus coioides) Spleen and Brain Tissues and Spleen Cells. J. Hazard. Mater., 416, 125918. doi:10.1016/j.jhazmat.2021.125918
Wang, S., Han, Q., Wei, Z., Wang, Y., Xie, J., and Chen, M. (2022). Polystyrene Microplastics Affect Learning and Memory in Mice by Inducing Oxidative Stress and Decreasing the Level of Acetylcholine. Food Chem. Toxicol. 162, 112904. doi:10.1016/j.fct.2022.112904
Wen, B., Jin, S.-R., Chen, Z.-Z., Gao, J.-Z., Liu, Y.-N., Liu, J.-H., et al. (2018). Single and Combined Effects of Microplastics and Cadmium on the Cadmium Accumulation, Antioxidant Defence and Innate Immunity of the Discus Fish (Symphysodon aequifasciatus). Environ. Pollut. 243, 462–471. doi:10.1016/j.envpol.2018.09.029
Wilson, J. M., Bunte, R. M., and Carty, A. J. (2009). Evaluation of Rapid Cooling and Tricaine Methanesulfonate (MS222) as Methods of Euthanasia in Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 48, 785–789.
Wright, S. L., Thompson, R. C., and Galloway, T. S. (2013). The Physical Impacts of Microplastics on Marine Organisms: a Review. Environ. Pollut. 178, 483–492. doi:10.1016/j.envpol.2013.02.031
Yanuhar, U., Arfiati, D., Musa, M., Junirahma, N. S., and Caesar, N. R. (2020). The Status of VNN (Viral Nervous Necrosis)-Lnfected Grouper Fish Tissue with Chlorella Vulgaris Extract as Anti-virus Candidate. J. Phys. Conf. Ser. 1665 (No. 1), 012036. doi:10.1088/1742-6596/1665/1/012036
Yun, H., Kim, I., Kwon, S. H., Kang, J. S., and Om, A. S. (2011). Protective Effect of Chlorella Vulgaris against Lead-Induced Oxidative Stress in Rat Brains. J. Health Sci. 57 (3), 245–254.
Zhang, J., Zuo, Z., Chen, R., Chen, Y., and Wang, C. (2008). Tributyltin Exposure Causes Brain Damage in Sebastiscus marmoratus. Chemosphere 73 (3), 337–343.
Keywords: microplastics, lycopene, citric acid, chlorella, natural remedy, aquatic toxicology
Citation: Hamed M, Soliman HAM, Eid Z, Al Naggar Y and Sayed AE-DH (2022) Dietary Feeding Lycopene, Citric Acid, and Chlorella Alleviated the Neurotoxicity of Polyethylene Microplastics in African Catfish (Clarias gariepinus). Front. Environ. Sci. 10:869727. doi: 10.3389/fenvs.2022.869727
Received: 04 February 2022; Accepted: 13 April 2022;
Published: 03 May 2022.
Edited by:
Veerasingam S., Qatar University, QatarReviewed by:
Gurusamy Kutralam-Muniasamy, Instituto Politécnico Nacional de México (CINVESTAV), MexicoCopyright © 2022 Hamed, Soliman, Eid, Al Naggar and Sayed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alaa El-Din H. Sayed, YWxhYXNheWVkQGF1bi5lZHUuZWc=, YWxhYV9oMjU0QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.