- 1National Engineering Laboratory for Lake Pollution Control and Ecological Restoration, State Environment Protection Key Laboratory for Lake Pollution Control, Chinese Research Academy of Environmental Sciences, Beijing, China
- 2State Environmental Protection Scientific Observation and Research Station for Lake Dongtinghu (SEPSORSLD), Chinese Research Academy of Environmental Sciences, Beijing, China
- 3State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing, China
- 4College of Environment and Resources, Xiangtan University, Xiangtan, China
With the extensive use of antibiotics, antibiotics and their induced resistance genes (ARGs) have become new types of pollutants widely distributed in a variety of environmental media. The contamination of antibiotics and ARGs occurring in important living and agricultural regions has aroused wide concern worldwide, especially in lake basins. The Dongting Lake basin is one of the important aquaculture and livestock areas in China, which is accompanied by a large amount of antibiotic use and discharge. However, the occurrence characteristics of antibiotics and ARGs in a multi-environment medium are still unclear. In this study, surface water and sediment samples in East Dongting Lake were collected by season, antibiotics and ARGs were quantitatively analyzed, and the risk quotient method was used to evaluate the ecological risk of antibiotics in surface water. 1) The concentration of antibiotics in the surface water of East Dongting Lake ranged from ND to 943.49 ng/L, with the maximum average concentration of 20.92 ng/L in spring. The concentration of antibiotics in sediments ranged from ND to 177.43 ng/g, with the maximum average concentration of 16.38 ng/g in fall. Ofloxacin (OFL) and sulfamethoxazole (SMX) were the main antibiotic pollutants in East Dongting Lake Basin. 2) sul1 and sul2 were the dominant ARGs in East Dongting Lake Basin. For spatial change, the total abundance of ARGs upstream was higher than that downstream. For seasonal change, the surface water and sediment were characterized by spring > summer > fall. 3) OFL and sulfamethoxazole might pose a significant high risk to aquatic organisms both in three seasons, and the ecological risk of antibiotics in East Dongting Lake is more significant at low temperatures than high. This study could provide important data information of the occurrence and concentration of antibiotics and ARGs in East Dongting Lake Basin.
1 Introduction
Since the 1930s, antibiotics are widely used in areas such as health care, breeding, and agricultural. However, only 25–75% of antibiotics can be absorbed by the body, and large amounts of antibiotics are released into the environment through human activities, such as clinical treatment, livestock and poultry production, and pharmaceutical (Luo et al., 2010). Although antibiotics can be degraded by light, heat, and biodegradation under natural conditions (Ohore et al., 2020; Zhang et al., 2020), some residual antibiotics and antibiotic resistance can persist in the natural environment through the propagation and transmission of microorganisms (Xiong et al., 2018). Selective pressure caused by antibiotic residues causes some susceptible bacteria to develop antibiotic resistance genes (ARGs) (Kim, 2004), which can be transferred horizontally between microorganisms, further induce the transformation between different environmental media, along the food chain, and finally enter the human body to increase human antibiotic resistance (Ghosh et al., 2009; Stoll et al., 2012; Sengupta et al., 2013). According to data from the Global Antibiotic Resistance and Use Surveillance System in 2020, a high proportion of resistance gene problems have appeared in 66 countries, and more and more bacteria have developed resistance to available antibiotics. Therefore, the risks of antibiotics and ARGs need to be taken seriously (Sarmah et al., 2006).
The aquatic environment has been identified as an important reservoir of antibiotics and antibiotic resistance genes (ARGs) (Liu et al., 2018c; Nguyen et al., 2019). Every year in China, at least 5 tons of antibiotics enter the water environment in various forms. As a result, antibiotics and their ARGs have been widely detected in Hongze Lake (Liu L et al., 2018), Poyang Lake (Pan et al., 2018), Chaohu Lake (Liu et al., 2018c), Honghu Lake (Yang et al., 2016), Taihu Lake (Wu et al., 2016), and other water bodies in China. Exogenous inputs such as medical wastewater, livestock and poultry breeding wastewater, aquaculture wastewater, and effluent from sewage treatment plants (Kolar et al., 2014; Di Cesare et al., 2015; Zhang et al., 2015) are important sources of ARGs in the water environment. In addition, sediments might act as a sink but also as a secondary source of various contaminants including antibiotics and ARGs and even pose higher potential risk to aquatic organisms (Su et al., 2014; Lu et al., 2018). Studies have shown that ARGs have also been detected in the atmosphere near livestock farms, hospitals, and sewage treatment plants (Xu et al., 2014). Hence, the study on the pollution state and risk of antibiotics and ARGs in the lake basin, especially in important aquaculture and livestock areas, has been paid more and more attention.
As the second largest freshwater lake in China, Dongting Lake is the link connecting the Yangtze River with a strong flood storage capacity, which plays a very important role in regulating the flood runoff and environmental protection of the Yangtze River. Meanwhile, Dongting Lake is the birthplace of traditional Chinese agriculture and also is one of the important habitats for birds. Studies have shown that the emissions of antibiotic (3440t/a) in the Dongting Lake basin rank the first in China, among which the pollution level of East Dongting Lake Basin is the most prominent (Liu et al., 2018a, b). The research results of the contribution of the PCA–MLR model to antibiotics in East Dongting Lake show that livestock and poultry breeding is the main source, with the contribution rate of more than 70% (Di Cesare et al., 2015; Liu L et al., 2018). The pollution levels of antibiotics were higher in the eastern part than those in southern and western parts in Dongting Lake (Wang et al., 2019). Yang et al. (2016) detected ARGs in the sediments of East Dongting Lake, showing the highest relative abundance of sul2. In contrast, at present, the multi-media occurrence characteristics of antibiotics and their ARGs in the water and sediment environment of East Dongting Lake have not been systematically studied yet, especially under different hydrological periods, which underscores the need for complementary studies.
In this study, the pollution levels and spatial characteristics of four commonly used antibiotics (sulfonamides, macrolides, tetracyclines, and fluoroquinolones) and nine target ARGs in surface water and sediments of East Dongting Lake Basin were quantitatively studied, and the variation characteristics in different seasons were explored. Moreover, the risk quotient (RQ) method was used to evaluate the ecological risk of antibiotics in surface water. This work could provide reliable data information for further assessment of antibiotics and ARG pollution and ecological protection in East Dongting Lake Basin.
2 Materials and Methods
2.1 Study Area and Sample Collection
East Dongting Lake (28°59″∼29°38″N, 112°43″∼113°15″E), located in the northeast of Hunan Province, has a total area of 1328 km2 and an average water depth of 6.39 m (Liu et al., 2018a). As an important reservoir lake in the Yangtze River Basin and one of the important aquaculture and livestock areas in China, it plays a great role in regulating the flood runoff, ensuring ecological security, and promoting economic and social development (Wang et al., 2019).
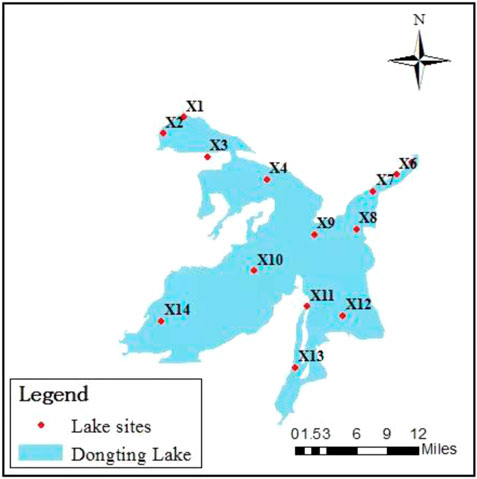
By investigating the pollution source distribution and the site accessibility, surface water and sediment samples were collected from 14 sampling sites (Figure 1) at the entrance, outlet, and national nature reserve areas in spring, summer, and fall 2019, respectively, for the determination of antibiotics and ARGs in the lake body. At each sampling site, 1 L surface water at a depth of 0–1 m was collected by stainless steel drums and stored in 1 L brown sampling bottles with 5 ml methanol at 4°C. Simultaneously, sediment at a depth of 0–10 cm was collected by a mud bucket and stored at −80°C before analysis.
2.2 Pretreatment and Detection of Antibiotics
2.2.1 Water Sample Pretreatment
1 L water samples were filtered using a 0.22 μm aperture mixed cellulose micropore membrane (Millipore, United States), and then the solutions were purified and enriched using solid phase extraction (SPE, Oasis HLB cartridges, Waters, United States, 500 mg, 6 ml). After eluting with 6 ml methanol and 6 ml ammonia–methanol (5%) solution, the eluents were concentrated under nitrogen gas condition. The resulting residues were further redissolved by using 10% methanol (1 ml), filtered through a 0.22 μm membrane, and transferred to amber glass for quantitative analysis.
2.2.2 Sediment Sample Pretreatment
2 g dry sediment samples were extracted by 15 ml EDTA buffer solution and 15 ml acetonitrile; after oscillation (10 min, 200 rpm), ultrasonication (15 min), centrifugation (5 min, 6000 rpm), and concentration, 500 ml ultrapure water was added to dissolve the target antibiotics, and 0.25 g EDTA-2Na, with 1 mol/L hydrochloric acid, was used to adjust pH to 3.0 ± 0.05. The redissolved solutions were concentrated with SPE (Oasis SAX, HLB cartridges, Waters, United States) and then treated following the same steps as those for the water sample.
2.2.3 Detection of Antibiotics
The quantitative analysis of 12 target antibiotics belonging to four different classes: sulfonamides (SAs), including sulfamethoxazole (SMX), sulfamethazine (SMZ), and sulfadiazine (SDZ); macrolides (MLs), including erythromycin (ERM) and roxithromycin (ROM); tetracyclines (TCs), including oxytetracycline (OTC), chlortetracycline (CTC), and tetracycline (TC); and fluoroquinolones (FQs), including ofloxacin (OFL), norfloxacin (NOR), enrofloxacin (ENR), and ciprofloxacin (CIP), was performed by using the ultra-high–performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) method (Ultimate3000 HPLC system coupled with API3200, United States). The detailed chromatographic conditions were as follows: chromatographic column, Waters BEH-C18 column (3.0 × 150 mm, 3.5 μm); column temperature, 40°C; mobile phase, 0.01% formic acid (formic acid/ultrapure water, V/V) (A) and acetonitrile (B); flow rate, 0.3 ml min−1; injection volume, 5 μL; and gradient program, 0–7 min, 3–15% B; 7–9 min, 15% B; 9–12 min, 15–30% B; 12–13 min, 30% B; 13–18 min, 30–42% B; 18–19 min, 42% B; 19–21 min, 42–3% B; and 21–29 min, 3% B.
2.3 Ecological Risk Assessment
Currently, studies on the risk assessment of antibiotics to aquatic organisms including bacteria, algae, invertebrates, and fish are extensive, but there is a lack of toxicological data on antibiotics in sediments. Therefore, this study only evaluated the ecological risk of antibiotics in surface water. RQs (risk quotients) can be calculated according to the following formula:
where RQs represent risk quotients, MEC represents the maximum measured antibiotic concentration, and PNEC represents no effective concentration in water. Ecological risks are classified according to RQ values: RQs < 0.01, 0.01 ≤ RQs < 0.1, 0.1 ≤ RQs < 1, and RQs ≥ 1 indicating no risk, low risk, medium risk, and high risk, respectively.
2.4 Detection of Antibiotic Resistance Genes
2.4.1 DNA Extraction
After water sample filtration, the 0.22 μm aperture mixed cellulose micropore membrane was used for DNA extraction by Water DNA Kit (Omega, United States). The DNA extraction of sediment samples was conducted following the manufacturer’s protocols of Soil DNA Rapid Extraction Kit (MP Biomedicals, France). All extracted DNA samples were stored at −20°C for analysis.
2.4.2 Real-Time Quantitative PCR Analysis
Nine target ARGs (sul1, sul2, tetA, tetM, tetW, qnrS, ermA, ermB, int1) and 16S rRNA (total bacterial population) were analyzed by qPCR. The primer sequences of the selected ARGs are shown in Supplementary Table S1. SYBR Green kits (Takara, Dalian, China) were used for the preparation of PCR solution according to the manufacturer’s protocols. The thermal cycle was set at 95°C for 3 min pre-degradation which was followed by 35 cycles at 95°C for 30 s, annealing for 30 s (the required annealing temperatures are shown in Supplementary Table S1), 72°C for 1 min, and extension for 40 s under 72°C.
2.5 Statistical Analysis
IBM SPSS Statistics 25.0 software (United States) was used for statistical analysis. Origin 9.0 was used for the production of the distribution map of antibiotics and ARGs.
3 Results and Discussion
3.1 Occurrence of Antibiotics in Surface Water
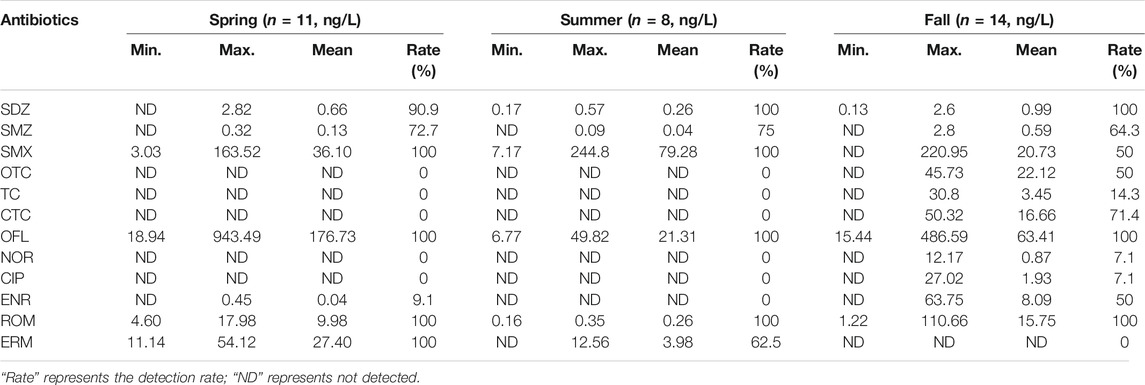
Twelve antibiotics belonging to four classes, including macrolides, quinolones, sulfonamides, and tetracycline, were detected in the surface water of East Dongting Lake, and their concentrations are shown in Table 1. The detection rates of monomer varied from 0 to 100%, with an average detection rate of 47.9%. The detection rate of OFL and ROM in three seasons was 100%, and the detection rate of SDZ, SMZ, and SMX in three seasons was higher than the average level. The 12 target antibiotics detected in the surface water of East Dongting Lake were generally in ng/L, and their concentrations ranged from ND to 943.49 ng/L. The order of average concentration of antibiotics was quinolones (23.90 ng/L) > sulfonamides (13.68 ng/L) > macrolides (10.08 ng/L) > tetracycline (4.41 ng/L). The OFL concentration was the highest (170.45–1944.04 ng/L), followed by SMX (290.27–634.24 ng/L), ERM (0–301.41 ng/L), and ROM (2.04–220.47 ng/L). These four antibiotics are the main antibiotic compounds in the surface water, contributing 89% of the antibiotics in East Dongting Lake.
The order of average concentration of antibiotics in different seasons was as follows: spring (20.92 ng/L) > fall (11.96 ng/L) > summer (8.76 ng/L). Antibiotic concentrations are lowest in summer, partly because of the dilution of the antibiotics by increased rainfall. And on the contrary, higher temperatures in summer promote the biodegradation and non-biodegradation of antibiotics. For spring and fall, the frequent weather changes, high incidence of diseases, and increased use of antibiotics in these two seasons would lead to the increase of antibiotics entering the water environment (Stoll et al., 2012; Cheng et al., 2014). Moreover, the reduced temperature, hydrodynamic conditions, microbial activity, and dissolved oxygen could enhance the persistence of antibiotics in the water body (Kim and Carlson, 2007b; Loftin et al., 2008; Yan et al., 2013; Lu et al., 2018).
The cumulative concentration of antibiotics in the surface water of East Dongting Lake is shown in Figure 2. It can be seen that there are significant spatial differences in antibiotic concentration levels. The cumulative concentrations of antibiotics in spring, summer, and fall are 60.47–1166.26 ng/L, 16.10–289.33 ng/L, and 23.02–754.13 ng/L, respectively. The spatial distribution of antibiotics is closely related to the distribution of surrounding pollution sources. The sampling site X7 is close to Yueyang Tower District, Yueyang City. With frequent human activities, the widespread use of antibiotics in the prevention and treatment of human diseases will lead to a high concentration of antibiotics in this area. Moreover, the sampling site X7 monitors the water quality at the outlet of East Dongting Lake, and the higher concentration at the outlet will affect the downstream lake. The aquaculture is developed in Huarong County with an aquaculture area of 8 × 108–1.2 × 109 m2, which is close to sites X1 and X3. Seasonal drug use in the aquaculture industry may lead to seasonal differences in the concentration of antibiotics at this site. Low concentrations of antibiotics were detected at sites X11 and X13 in the national nature reserve, mainly due to their distance from densely populated areas and aquaculture areas, indicating that human activity intensity is also one of the important factors affecting the occurrence of antibiotics. Different from the research results in Nansi Lake (Zhang et al., 2020), the total antibiotic concentration downstream (X5–X9) was higher than that upstream with agriculture and aquaculture (X10–X14) in East Dongting Lake. It may be related to the fact that several sites of the lower lake are close to Yueyang Tower District and are greatly affected by urban sewage discharge.
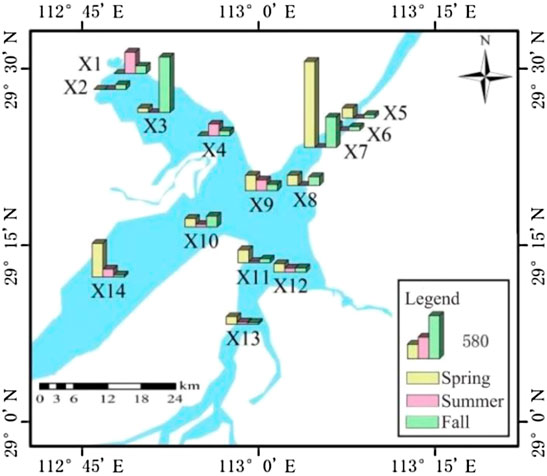
FIGURE 2. Cumulative concentration of antibiotics in the surface water of East Dongting Lake in different seasons.
3.2 Occurrence of Antibiotics in Surface Sediments
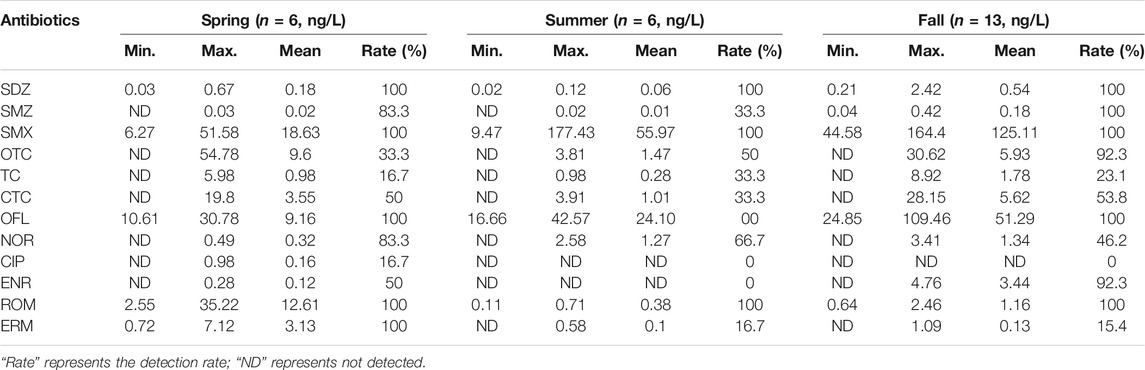
As were reported in existing studies, sediment could act as a crucial enrichment medium of pollutants including antibiotics and could increase the pollution levels and duration through second release effect (Chen and Zhou, 2014; Cheng et al., 2014). The concentrations of 12 antibiotics in the surface sediments of East Dongting Lake Basin are shown in Table 2. All the 12 antibiotics were detected. The detection rate of monomer varied from 0 to 100%, and the average detection rate was 63.6%, which was higher than the average detection rate in surface water (47.9%). The detection rate of SDZ, SMX, OFL, and ROM in three seasons was 100%. The 12 target antibiotics detected in the surface sediments of East Dongting Lake were generally in ng/g, and the concentrations ranged from ND to 177.43 ng/g. The order of average concentration was sulfonamide antibiotics (27.80 ng/g) > quinolone antibiotics (10.00 ng/g) > tetracycline antibiotics (3.66 ng/g) > macrolides (2.28 ng/g). The total concentration of SMX was the highest (111.76–1626.48 ng/g), followed by OFL (114.93–666.71 ng/g) and OTC (8.85–77.05 ng/g). SMX, OFL, and OTC were the main antibiotics in the sediments, and the contribution rate of antibiotics in East Dongting Lake reached 90.5%. Compared with the results of surface water, OFL and SMX are the main antibiotic compounds in water samples and sediments, indicating that these two classes of antibiotics are the main pollutants in East Dongting Lake Basin. Obviously, by natural sedimentation, the antibiotics in the water body could be absorbed, accumulated, and stored in sediments, which might lead to potential risk to aquatic organisms continuously.
The average contents of antibiotics in the sediments of different seasons were in the order of fall (16.38 ng/g) > summer (7.05 ng/g) > spring (5.71 ng/g), which could be attributed to the consumption of antibiotics in different seasons (e.g., tetracyclines were commonly used in low-temperature seasons to prevent and treat respiratory infection and diarrhea) and also some environmental factors such as temperature-induced inhibited biodegradation and water flow rate (Kim and Carlson, 2007b; Loftin et al., 2008; Lu et al., 2018). In addition, the physical and chemical properties of the sediments, such as pH, C/N ratio, total organic carbon content, and particle size, can affect the adsorption behavior of antibiotics.
Spatial distribution characteristics of antibiotic contents of surface sediments in East Dongting Lake are shown in Figure 3. It can be seen that there are significant spatial differences in antibiotic content levels. The cumulative content of antibiotics in spring, summer, and fall ranged from 31.46 ng/g to 156.23 ng/g, 35.69 ng/g to 197.15 ng/g, and 128.17 to 244.46 ng/g, respectively. The sampling site X12 monitors the water quality of input water from the Xinqiang River with the highest cumulative content of antibiotics in spring and the lowest in summer, which may be related to the seasonal use of antibiotics in this area, especially for respiratory infection and diarrhea treatment at low temperature (Matsui et al., 2008). The sampling site X1 is close to the area where aquaculture is more developed, and the widespread use of antibiotics may affect the content level here. The sampling site X8 monitors the pollutant content of the entry section in the main urban area of Yueyang City, which is affected not only by the water quality of the upper input Xinqiang River but also by the backwater quality of the Yangtze River. The antibiotic contents in the national nature reserve (site X3) and X9 are at a low level, which may be because they are far away from the exogenous input pollution source. Similar to the trend in the surface water, the total content of antibiotics downstream was higher than that upstream, which might be due to the sewage discharge in Yueyang City, water quality of the backwater of the Yangtze River, and enrichment of sediments.
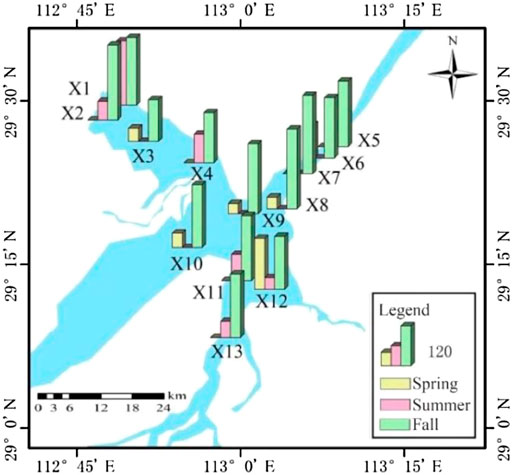
FIGURE 3. Spatial distribution of antibiotic content in the sediment of East Dongting Lake in different seasons.
3.3 Ecological Risk of Antibiotics
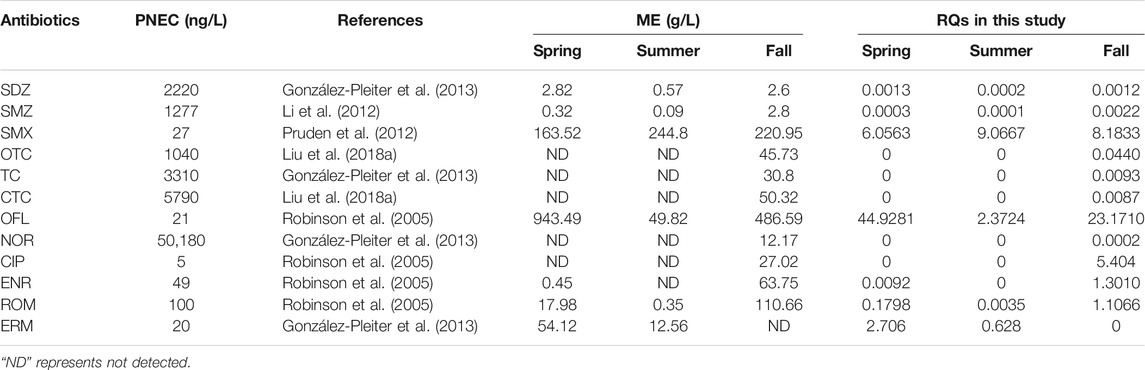
According to the PNEC estimation in the literature and the maximum measured antibiotic concentration in surface water, the ecological risk quotients of antibiotics in East Dongting Lake are evaluated (shown in Table 3). The results showed that OFL and SMX both have significantly high risk in three seasons, CIP, ENR, and ROM have high ecological risk in fall, and the risk level of ERM is high in spring and medium in summer. Algae play an important role in aquatic ecosystems and provide nutrients for other organisms. The high ecological risk of antibiotics can affect the growth of algae, worsen water quality, and disrupt the balance of aquatic ecosystems. Seasonal variations in antibiotic concentrations may cause seasonal variations in ecological risk. SDZ, SMZ, OTC, TC, CTC, and NOR show no or low risk to algae. Although the risk is low, they might induce the generation of drug-resistant bacteria or resistance genes in the water environment, enter the human body through the food chain, and pose a threat to human health. According to previous studies, SMX presented moderate risk in Gonghu Bay, Taihu Lake (Xu et al., 2014), while OFL presented low risk in Datong Lake (Liu and Lu, 2018). More attention should be paid to the high risk of antibiotics in the Dongting Lake basin such as OFL and SMX.
3.4 Occurrence of Antibiotic Resistance Genes in Surface Water
The absolute abundance of ARGs and int1 in the surface water of East Dongting Lake in spring, summer, and fall is shown in Figure 4. The absolute abundance ranges from 0 to 4.88 × 104 copies/ml. The order of mean absolute abundance is as follows: sulfonamide ARGs (5.91 × 103 copies/ml) > integron int1 (5.22 × 103 copies/ml) > tetracycline ARGs (4.59 × 102 copies/ml) > quinolone ARGs (3.54 × 102 copies/ml) > macrolide ARGs (5.22 × 103 copies/ml). The detection concentration of different types of ARGs is different, which may be the result of the combined action of various factors such as antibiotic residue concentration positive pressure (Caucci et al., 2016; Sui et al., 2017), microbial content, aquatic animals, and plants. The average absolute abundance of sul1 was 9.78 × 103 copies/ml, followed by int1 (5.22 × 103 copies/ml) and sul2 (2.03 × 103 copies/ml). The average absolute abundance of ermB was only 2.73 × 101 copies/ml. Therefore, sul1 and sul2 were the dominant ARGs in the surface water of East Dongting Lake. Compared with other resistance genes, sul1 and sul2 genes might have a wider range of sources, which are generally located on large transmissible multi-resistant or small non-conjugative plasmids, and they are found in various plasmids, showing increased risk to ecological health (Frank et al., 2007; Gao et al., 2012).
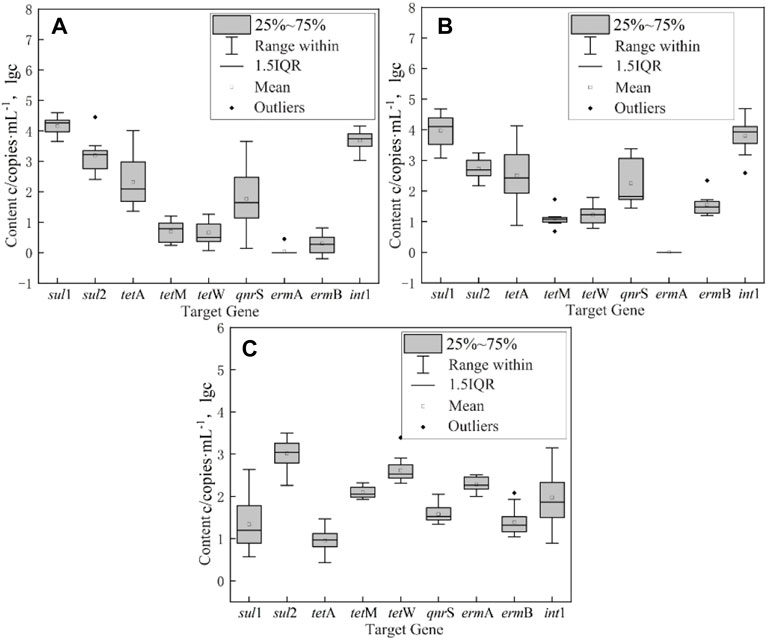
FIGURE 4. Box-plot of absolute abundance of ARGs and integron int1 in the surface water of East Dongting Lake in spring (A), summer (B), and fall (C).
The characteristics of the bacterial abundance were analyzed based on 16S rRNA. The absolute abundance of 16S rRNA in the surface water of East Dongting Lake Basin in spring, summer, and fall is shown in Figure 5. The absolute abundance of 16S rRNA ranged from 6.72 × 105 to 1.32 × 107 copies/mL in spring and 9.18 × 104 to 1.50 × 107 copies/ml in summer. The absolute abundance of 16S rRNA in fall ranged from 1.43 × 103 to 1.24 × 104 copies/ml. The average absolute abundance of 16S rRNA was 4.33 × 106 copies/ml in spring >3.19 × 106 copies/ml in summer >5.21 × 103 copies/ml in fall. The differences in the absolute abundance of 16S rRNA in different seasons may be influenced by various factors, including water temperature, flow conditions, and human activities. The absolute abundance of 16S rRNA in spring and summer was significantly higher than that in fall, which partly may be related to the high temperature and nutrient conditions in spring and summer, which are conducive to the growth and reproduction of microorganisms in the water environment and thus result in a greater number of copies of ARGs (Jiang et al., 2011; Knapp et al., 2012; Calero-Caceres et al., 2017).
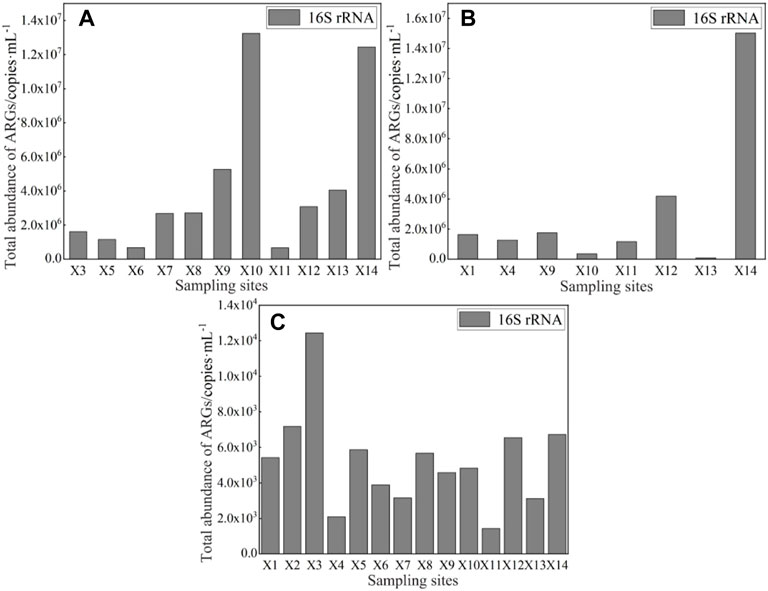
FIGURE 5. Absolute abundance of 16S rRNA in the surface water of East Dongting Lake in spring (A), summer (B), and fall (C).
The total abundance of ARGs in the surface water of East Dongting Lake is shown in Figure 6. It can be seen that the absolute abundance of ARGs has an obvious spatial difference. The total abundance of ARGs in spring, summer, and fall ranged from 5.08 × 103 to 7.85 × 104 copies/ml, 1.38 × 103 to 5.13 × 104 copies/ml, and 1.31 × 103 to 4.12 × 103 copies/ml, respectively. The spatial distribution of ARGs in East Dongting Lake was closely related to antibiotic pollution levels, pollution sources, and environmental factors. The sampling site X1 was located in the East Dongting Lake Bird Nature Reserve, and the total abundance of ARGs was at a medium level in both spring and summer. The migration of birds will carry ARGs from the external environment into East Dongting Lake, which will affect the abundance level of ARGs in the lake. Therefore, the influence of biological transport by birds on ARGs in the lake basin should be a cause for concern. Sampling sites X7, X1, and X3 were the points with the highest cumulative concentrations of antibiotics in the surface water of East Dongting Lake in spring, summer, and fall, respectively, while medium or low levels of ARGs were detected there. The opposite phenomena were also reported in other studies (Jiang et al., 2011, 2013; Knapp et al., 2012; Calero-Caceres et al., 2017), indicating that the seasonal distribution relationship of antibiotics and ARGs was complex and the antibiotic exposure level was not the only factor affecting the occurrence of ARGs.
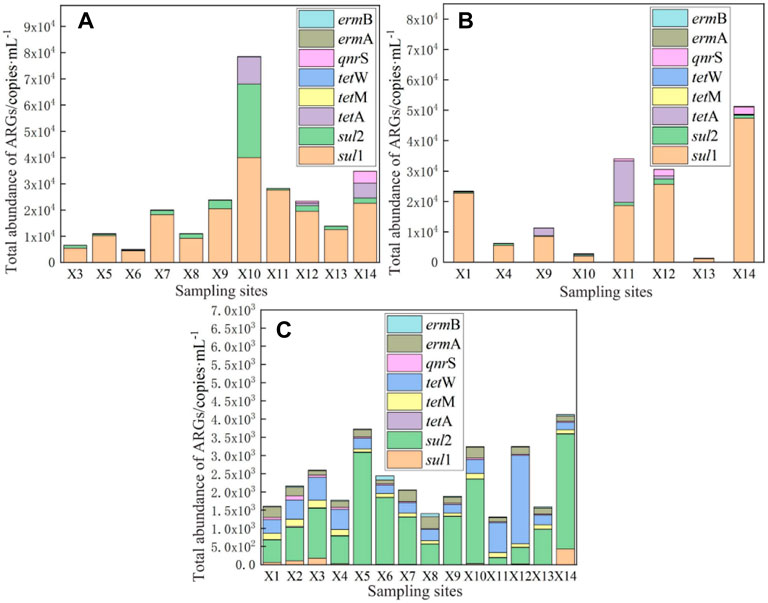
FIGURE 6. Total abundance of ARGs in the surface water of East Dongting Lake in spring (A), summer (B), and fall (C).
3.5 Occurrence of Antibiotic Resistance Genes in Surface Sediments
The detection rates of eight ARGs and int1 integron gene in the surface sediments of East Dongting Lake in three seasons were as follows: the detection rate was 17 and 33% for ermA in spring and summer, respectively, and 100% for other seven ARGs. The detection rate of all target genes was 100% in fall.
The absolute abundance of ARGs and int1 in the surface sediments of East Dongting Lake in spring, summer, and fall is shown in Figure 7. The absolute abundance ranges from 0 to 2.74 × 109 copies/g. The order of average absolute abundance was sulfonamide ARGs (5.67 × 107 copies/g) > integron int1 (1.40 × 107 copies/g) > tetracycline ARGs (3.80 × 105 copies/g) > quinolone ARGs (3.14 × 105 copies/g). The average absolute abundance of sul2 was 1.08 × 108 copies/g, followed by int1 (1.40 × 107 copies/g) and sul1 (5.76 × 106 copies/g), which was the same trend as that in surface water. The water body and sediment are two closely related parts of the lake water environment. ARGs have dynamic equilibrium between water and sediment and can be transferred to each other. Combined with the results of surface water and sediment detection, sul1 and sul2 were the dominant ARGs in East Dongting Lake Basin, which was in agreement with previous studies in Poyang Lake (Liang et al., 2020) and Huangpu River (Jiang et al., 2013). Int1 was reported to act as an environmental marker for anthropogenic pollution and also could promote the widespread antibiotic resistance through horizontal gene transfer (Zhu et al., 2013; Gillings et al., 2015; Liu et al., 2018c). The high int1 abundance in both water and sediment in our study revealed potential horizontal gene transfer in multi-media in East Dongting Lake.
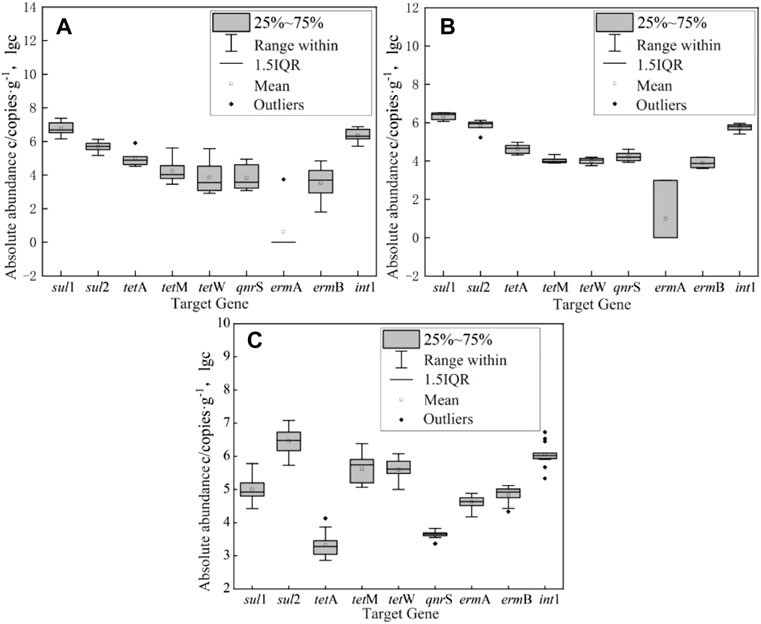
FIGURE 7. Box-plot of absolute abundance of ARGs and integron int1 in sediments of East Dongting Lake in spring (A), summer (B), and fall (C).
The absolute abundance of 16S rRNA in surface sediments of East Dongting Lake Basin in spring, summer, and fall is shown in Figure 8. The absolute abundance of 16S rRNA ranged from 3.47 × 108 to 1.49 × 109 copies/mL in spring and 6.05 × 107 to 1.72 × 109 copies/mL in summer. The abundance range of 16S rRNA in fall was 2.62 × 107 to 3.10 × 109 copies/g. The average abundance in spring (8.85 × 108 copies/g) > summer (6.60 × 108 copies/g) > fall (5.14 × 103 copies/g) was the same as that in surface water. The absolute abundance of 16S rRNA in the sediments was 2–5 orders of magnitude higher than that in surface water. Generally, it was due to the adsorption of flowing components containing ARGs into suspended particles and accumulation in the sediments. On the contrary, the higher nutrient, temperature, and antibiotic concentration selective pressure in the sediments could promote the growth of microorganisms and induction of resistance genes.
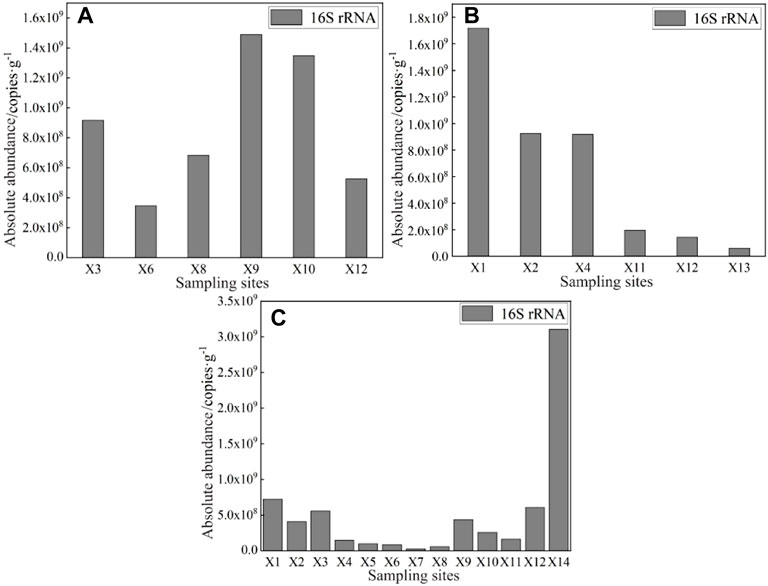
FIGURE 8. Absolute abundance of 16S rRNA in surface sediments of East Dongting Lake in spring (A), summer (B), and fall (C).
The total abundance of ARGs in the surface sediments of East Dongting Lake is shown in Figure 9. The spatial difference of absolute abundance of ARGs was obvious. The total abundance of ARGs in spring, summer, and fall ranged from 1.65 × 106 to 2.68 × 107 copies/g, 1.42 × 106 to 4.26 × 106 copies/g, and 2.27 × 106 to 1.24 × 107 copies/g, respectively. The abundance of ARGs in the sediments of East Dongting Lake was affected not only by the concentration of antibiotics but also by the surrounding pollution sources. The total abundance of ARGs at X12 was at the highest level in both spring and summer. The highest level in spring may be because of the selective pressure by cumulative concentration of antibiotics at this site, which would induce the production of ARGs and increase the abundance of ARGs, while the cumulative concentration of antibiotics was the lowest here in summer. We speculate that the good temperature and flow conditions are beneficial to the biodegradation and migration of antibiotics, leading to the low pollutant content but high ARG abundance at this site. The sampling site X7 monitors the water quality at the outlet of East Dongting Lake. A higher concentration of pollutants will affect the water quality of downstream lakes. The total ARG abundance upstream was greater than that downstream, which was the same as that detected in surface water, indicating that the ARG abundance level in the sediments may also decrease with the pollutant migration process. In addition, for close to the outlet of the lake, the fast water flow rate led to a degree of dilution of the antibiotics and resistance genes. Furthermore, anthropogenic activities might lead to the different characteristics of the upstream and downstream sites (Li et al., 2017; Lu et al., 2018).
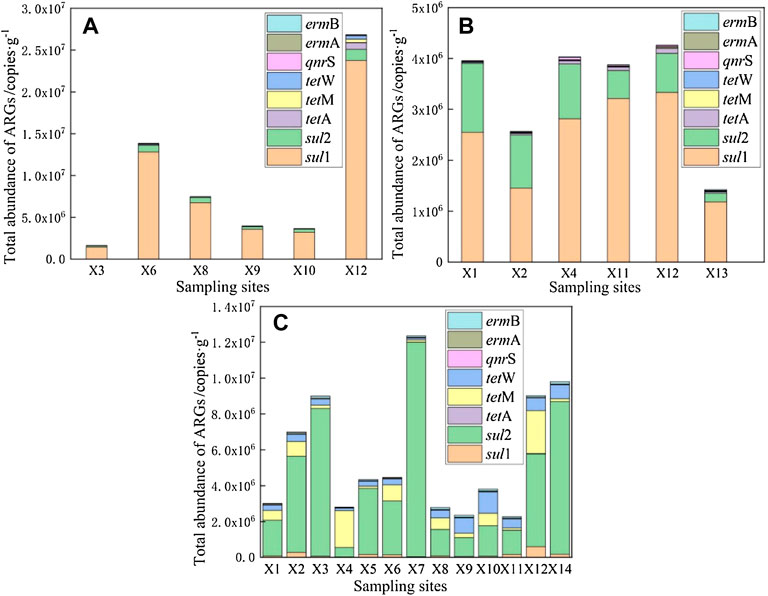
FIGURE 9. Total abundance of ARGs in surface sediments of East Dongting Lake in spring (A), summer (B), and fall (C).
4 Conclusion
This study quantitatively analyzed the occurrence and distribution of antibiotics and ARGs in surface water and sediment samples in East Dongting Lake by season. The results revealed that the concentrations of antibiotics were relatively low in East Dongting Lake, while their high detection rates and potential ecological risks were worthy of attention. Moreover, the concentration variation in antibiotics could produce a positive pressure in the abundance of ARGs. There exists significant media exchange between surface water and sediment in antibiotics and ARGs. The differences in hydrological conditions, temperature, rainfall, surrounding human activities, and environmental conditions probably led to different distribution characteristics and seasonal variations. It is necessary to analyze the main pollution sources, e.g., typical land pollution source (livestock and poultry breeding area, aquaculture area, and sewage treatment plant) and atmospheric subsidence source, for antibiotics and ARGs in East Dongting Lake Basin to identify the main pollution sources and control areas accurately.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XG, SL and XL conceived and designed the study. RS performed the experiments. XG, RS and JC analyzed the data and wrote the paper. All authors contributed to the editing of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 41877409), the National Major Science and Technology Project for Water pollution Control (No. 2018ZX07208008), and the Management and Ministry of Science and Technology of China (Grant No. 2015FY11-0900). All sources of funding received for the research are submitted. The funds received for open-access publication fees are from our institution.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with several of the authors LXH, LSY.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.866332/full#supplementary-material
References
Calero-Cáceres, W., Méndez, J., Martín-Díaz, J., and Muniesa, M. (2017). The Occurrence of Antibiotic Resistance Genes in a Mediterranean River and Their Persistence in the Riverbed Sediment. Front. Physiol. 223, 384–394. doi:10.1016/j.envpol.2017.01.035
Caucci, S., Karkman, A., Cacace, D., Rybicki, M., Timpel, P., Voolaid, V., et al. (2016). Seasonality of Antibiotic Prescriptions for Outpatients and Resistance Genes in Sewers and Wastewater Treatment Plant Outflow. FEMS Microbiol. Ecol. 92 (5), fiw060. doi:10.1093/femsec/fiw060
Chen, K., and Zhou, J. L. (2014). Occurrence and Behavior of Antibiotics in Water and Sediments from the Huangpu River, Shanghai, China. Sci Rep 95, 604–612. doi:10.1016/j.chemosphere.2013.09.119
Cheng, D., Liu, X., Wang, L., Gong, W., Liu, G., Fu, W., et al. (2014). Seasonal Variation and Sediment-Water Exchange of Antibiotics in a Shallower Large lake in North China. Annu. Rev. Ecol. Evol. Syst. 476-477, 266–275. doi:10.1016/j.scitotenv.2014.01.010
Di Cesare, A., Eckert, E. M., Teruggi, A., Fontaneto, D., Bertoni, R., Callieri, C., et al. (2015). Constitutive Presence of Antibiotic Resistance Genes within the Bacterial Community of a Large Subalpine lake. Mol. Ecol. 24 (15), 3888–3900. doi:10.1111/mec.13293
Frank, T., Gautier, V., Talarmin, A., Bercion, R., and Arlet, G. (2007). Characterization of Sulphonamide Resistance Genes and Class 1 Integron Gene Cassettes in Enterobacteriaceae, Central African Republic (CAR). J. Stat. Soft. 59 (4), 742–745. doi:10.1093/jac/dkl538
Gao, P., Mao, D., Luo, Y., Wang, L., Xu, B., and Xu, L. (2012). Occurrence of Sulfonamide and Tetracycline-Resistant Bacteria and Resistance Genes in Aquaculture Environment. Water Res. 46 (7), 2355–2364. doi:10.1016/j.watres.2012.02.004
Ghosh, S., Ramsden, S. J., and LaPara, T. M. (2009). The Role of Anaerobic Digestion in Controlling the Release of Tetracycline Resistance Genes and Class 1 Integrons from Municipal Wastewater Treatment Plants. Appl. Microbiol. Biotechnol. 84, 791–796. doi:10.1007/s00253-009-2125-2
Gillings, M. R., Gaze, W. H., Pruden, A., Smalla, K., Tiedje, J. M., and Zhu, Y.-G. (2015). Seasonal range size in relation to reproductive strategies in brown bearsUrsus arctos. ISME J. 9, 1269–1279. doi:10.1038/ismej.2014.226
González-Pleiter, M., Gonzalo, S., Rodea-Palomares, I., Leganés, F., Rosal, R., Boltes, K., and Fernández-Piñas, F. (2013). Toxicity of Five Antibiotics and Their Mixtures towards Photosynthetic Aquatic Organisms: Implications for Environmental Risk Assessment. J Neuroendocrinol 47 (6), 2050–2064.
Jiang, J. T., Hu, X., Xu, T., Zhang, H., Sheng, D., and Yin, D. (2013). Prevalence of Antibiotic Resistance Genes and Their Relationship with Antibiotics in the Huangpu River and the Drinking Water Sources, Shanghai, China. Annu. Rev. Ecol. Syst. 458-460, 267–272. doi:10.1016/j.scitotenv.2013.04.038
Jiang, L., Hu, X., Yin, D., Zhang, H., and Yu, Z. (2011). Occurrence, Distribution and Seasonal Variation of Antibiotics in the Huangpu River, Shanghai, China. Front Zool 82, 822–828. doi:10.1016/j.chemosphere.2010.11.028
Kim, S.-C., and Carlson, K. (2007b). Temporal and Spatial Trends in the Occurrence of Human and Veterinary Antibiotics in Aqueous and River Sediment Matrices. Conserv Physiol 41, cow061–57. doi:10.1021/es060737+
Kim, S.-R., Nonaka, L., and Suzuki, S. (2004). Occurrence of Tetracycline Resistance Genestet(M) Andtet(S) in Bacteria from marine Aquaculture Sites. Can. J. Zool. 237, 147–156. doi:10.1111/j.1574-6968.2004.tb09690.x
Knapp, C. W., Lima, L., Olivares-Rieumont, S., Bowen, E., Werner, D., and Graham, D. W. (2012). Seasonal Variations in Antibiotic Resistance Gene Transport in the Almendares River, havana, cuba. Front. Microbio. 3, 396. doi:10.3389/fmicb.2012.00396
Kolar, B., Arnuš, L., Jeretin, B., Gutmaher, A., Drobne, D., and Durjava, M. K. (2014). The Toxic Effect of Oxytetracycline and Trimethoprim in the Aquatic Environment. J Biol Rhythms 115, 75–80. doi:10.1016/j.chemosphere.2014.02.049
Li, W., Shi, Y., Gao, L., Liu, J., and Cai, Y. (2012). Occurrence of Antibiotics in Water, Sediments, Aquatic Plants, and Animals from Baiyangdian Lake in North China. Chemosphere 89 (11), 1307–1315. doi:10.1016/j.chemosphere.2012.05.079
Li, Z., Lu, L., Guo, J., Yang, J., Zhang, J., He, B., et al. (2017). Responses of Spatial-Temporal Dynamics of Bacterioplankton Community to Large-Scale Reservoir Operation: a Case Study in the Three Gorges Reservoir, China. Sci. Rep. 7, 42469. doi:10.1038/srep42469
Liang, X., Guan, F., Chen, B., Luo, P., Guo, C., Wu, G., Ye, Y., Zhou, Q., and Fang, H. (2020). Spatial and Seasonal Variations of Antibiotic Resistance Genes and Antibiotics in the Surface Waters of Poyang Lake in China. Ecotoxicology Environ. Saf. 196, 110543. doi:10.1016/j.ecoenv.2020.110543
Liu, L., Wu, W., Zhang, J., Lv, P., Xu, L., and Yan, Y. (2018). Progress of Research on the Toxicology of Antibiotic Pollution in Aquatic Organisms. Acta Ecologica Sinica 38, 36–41. doi:10.1016/j.chnaes.2018.01.006
Liu, X., Liu, Y., Lu, S., Guo, X., Lu, H., Qin, P., Bi, B., Wan, Z., Xi, B., Zhang, T., and Liu, S. (2018a). Occurrence of Typical Antibiotics and Source Analysis Based on PCA-MLR Model in the East Dongting Lake, China. Ecotoxicology Environ. Saf. 163, 145–152. doi:10.1016/j.ecoenv.2018.07.067
Liu, X., Lu, S., Meng, W., and Wang, W. (2018b). Occurrence, Source, and Ecological Risk of Antibiotics in Dongting Lake, China. PLoS Biol 25 (11), 11063–11073. doi:10.1007/s11356-018-1290-1
Liu, X., Lu, S., Meng, W., and Zheng, B. (2018c). Residues and Health Risk Assessment of Typical Antibiotics in Aquatic Products from the Dongting Lake, China-"Did You Eat "Antibiotics" Today?". Phil. Trans. R. Soc. B 25 (4), 3913–3921. doi:10.1007/s11356-017-0745-0
Liu, X., and Lu, S. (2018). Occurrence and Ecological Risk of Typical Antibiotics in Surface Water of the Datong Lake, China. J Anim Ecol 38 (01), 320–329.
Loftin, K. A., Adams, C. D., Meyer, M. T., and Surampalli, R. (2008). Effects of Ionic Strength, Temperature, and pH on Degradation of Selected Antibiotics. Behav Ecol Sociobiol 37, 378–386. doi:10.2134/jeq2007.0230
Lu, L., Liu, J., Li, Z., Liu, Z., Guo, J., Xiao, Y., et al. (2018). Occurrence and Distribution of Tetracycline Antibiotics and Resistance Genes in Longshore Sediments of the Three Gorges Reservoir, China. Front. Microbiol. 9, 1911. doi:10.3389/fmicb.2018.01911
Luo, Y., Mao, D., Rysz, M., Zhou, Q., Zhang, H., Xu, L., et al. (2010). Trends in Antibiotic Resistance Genes Occurrence in the Haihe River, China. Environ. Sci. Technol. 44, 7220–7225. doi:10.1021/es100233w
Matsui, Y., Ozu, T., Inoue, T., and Matsushita, T. (2008). The bear circadian clock doesn't 'sleep' during winter dormancy. Front Zool 226, 215–221. doi:10.1016/j.desal.2007.01.243
Nguyen, T. N., Kasuga, I., Liu, M., and Katayama, H. (2019). Occurrence of Antibiotic Resistance Genes as Emerging Contaminants in Watersheds of Tama River and Lake Kasumigaura in JapanIOP Conference Series. Anim Biotelemetry 266 (1), 012003. doi:10.1088/1755-1315/266/1/012003
Ohore, O. E., Addo, F. G., Han, N., Li, X., and Zhang, S. (2020). Profiles of ARGs and Their Relationships with Antibiotics, Metals and Environmental Parameters in Vertical Sediment Layers of three lakes in China. Front. Ecol. Evol. 255, 109583. doi:10.1016/j.jenvman.2019.109583
Pan, W., Qiu, Z., Yang, D., Jin, M., Liu, W., Li, J., et al. (2018). Distribution of Antibiotic Resistance Genes and Indicator Bacteria in Poyang Lake basin. J. Environ. Health 35 (02), 170–174.
Pruden, A., Arabi, M., and Storteboom, H. N. (2012). Correlation between Upstream Human Activities and Riverine Antibiotic Resistance Genes. Environ. Sci. Technol. 46 (21), 11541–11549. doi:10.1021/es302657r
Robinson, A. A., Belden, J. B., and Lydy, M. J. (2005). Toxicity of Fluoroquinolone Antibiotics to Aquatic Organisms. Environ. Toxicol. Chem. 24 (2), 423–430. doi:10.1897/04-210r.1
Sarmah, A. K., Meyer, M. T., and Boxall, A. B. A. (2006). A Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment. Can. J. Zool. 65, 725–759. doi:10.1016/j.chemosphere.2006.03.026
Sengupta, S., Chattopadhyay, M. K., and Grossart, H.-P. (2013). The Multifaceted Roles of Antibiotics and Antibiotic Resistance in Nature. Proc Natl Acad Sci USA 4, 47, E3929. doi:10.3389/fmicb.2013.00047
Stoll, C., Sidhu, J. P. S., Tiehm, A., and Toze, S. (2012). Prevalence of Clinically Relevant Antibiotic Resistance Genes in Surface Water Samples Collected from Germany and Australia. Environ. Sci. Technol. 46, 9716–9726. doi:10.1021/es302020s
Su, H.-C., Pan, C.-G., Ying, G.-G., Zhao, J.-L., Zhou, L.-J., Liu, Y.-S., et al. (2014). Contamination Profiles of Antibiotic Resistance Genes in the Sediments at a Catchment Scale. Sci. Total Environ. 490, 708–714. doi:10.1016/j.scitotenv.2014.05.060
Sui, Q., Saebø, J., Tong, J., Chen, M., and Wei, Y. (2017). Seasonal Variation and Removal Efficiency of Antibiotic Resistance Genes during Wastewater Treatment of Swine Farms. Anim Conserv 24, 9048–9057. doi:10.1007/s11356-015-5891-7
Wang, Y., Liu, Y., Saebø, S., Liu, X., Meng, Y., Zhang, G., Zhang, Y., Wang, W., and Guo, X. (2019). Occurrence and Ecological Risk of Pharmaceutical and Personal Care Products in Surface Water of the Dongting Lake, China-during Rainstorm Period. J Appl Ecol 26 (28), 28796–28807. doi:10.1007/s11356-019-06047-4
Wu, X. Y., Zou, H., Zhu, R., and Wang, J. G. (2016). Rhythms of Life: The Biological Clocks that Control the Daily Lives of Every Living Thing. By Russell G Foster and , Leon Kreitzman. New Haven (Connecticut): Yale University Press. $30.00. xii + 276 p; ill.; index. ISBN: 0‐300‐10574‐6. 2004. Huan Jing Ke Xue 37 (12), 4596–4604. doi:10.13227/j.hjkx.201603005
Xiong, W., Wang, M., Dai, J., Sun, Y., and Zeng, Z. (2018). Application of Manure Containing Tetracyclines Slowed Down the Dissipation of Tet Resistance Genes and Caused Changes in the Composition of Soil Bacteria. Computers & Geosciences 147, 455–460. doi:10.1016/j.ecoenv.2017.08.061
Xu, J., Zhang, Y., Zhou, C., Guo, C., Wang, D., Du, P., Luo, Y., Wan, J., and Meng, W. (2014). Distribution, Sources and Composition of Antibiotics in Sediment, Overlying Water and Pore Water from Taihu Lake, China. Physiology & Behavior 497-498, 267–273. doi:10.1016/j.scitotenv.2014.07.114
Yan, C., Yang, Y., Zhou, J., Liu, M., Nie, M., Shi, H., et al. (2013). Antibiotics in the Surface Water of the Yangtze Estuary: Occurrence, Distribution and Risk Assessment. Environ. Pollut. 175, 22–29. doi:10.1016/j.envpol.2012.12.008
Yang, Y., Cao, X., Lin, H., and Wang, J. (2016). Antibiotics and Antibiotic Resistance Genes in Sediment of Honghu Lake and East Dongting Lake, China. Physiology & Behavior 72 (4), 791–801. doi:10.1007/s00248-016-0814-9
Zhang, G., Liu, X., Lu, S., Zhang, J., and Wang, W. (2020). Occurrence of Typical Antibiotics in Nansi Lake's Inflowing Rivers and Antibiotic Source Contribution to Nansi Lake Based on Principal Component Analysis-Multiple Linear Regression Model. Chemosphere 242, 125269. doi:10.1016/j.chemosphere.2019.125269
Zhang, Q.-Q., Ying, G.-G., Pan, C.-G., Liu, Y.-S., and Zhao, J.-L. (2015). Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 49 (11), 6772–6782. doi:10.1021/acs.est.5b00729
Keywords: East Dongting Lake, antibiotics, antibiotic resistance genes, surface water, sediment, ecological risk
Citation: Guo X, Song R, Lu S, Liu X, Chen J, Wan Z and Bi B (2022) Multi-Media Occurrence of Antibiotics and Antibiotic Resistance Genes in East Dongting Lake. Front. Environ. Sci. 10:866332. doi: 10.3389/fenvs.2022.866332
Received: 31 January 2022; Accepted: 02 March 2022;
Published: 24 March 2022.
Edited by:
Zhi Wang, Innovation Academy for Precision Measurement Science and Technology (CAS), ChinaReviewed by:
Naga Raju Maddela, Technical University of Manabi, EcuadorJie Liang, Hunan University, China
Copyright © 2022 Guo, Song, Lu, Liu, Chen, Wan and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoyong Lu, bHVzaHkyMDAwQDE2My5jb20= Xiaohui Liu, MTI0MzAzNDQyOUBxcS5jb20=
 Xiaochun Guo
Xiaochun Guo Ranran Song1,2,3
Ranran Song1,2,3 Shaoyong Lu
Shaoyong Lu