
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Environ. Sci. , 07 September 2022
Sec. Freshwater Science
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.854835
Wetlands are among the most productive ecosystems globally characterized by dynamic interactions between terrestrial and aquatic habitats at different scales. These systems support valuable floodplain fisheries that are a major livelihood for riparian communities. Understanding the dynamics of these systems is important for developing adaptive fisheries management paradigms that will facilitate access and sustainability to this cheap but high-quality food and nutrition source. The Okavango Delta in Botswana is a large land-locked complex river-floodplain ecosystem, with a diverse biota, and high environmental heterogeneity due to periodic drying and flooding along a space and time gradient. It is characterized by a multi-species, multi-gear fishery adapted to the seasonal flood pulse. The Delta’s fish species assemblage undergoes seasonal changes driven by the flood regime. There is also a dynamic inter-annual variability in the fish species assemblage, particularly between “good” and “bad” flood years. During the wet season, high flows increase connectivity in three dimensions (longitudinal, lateral, and vertical) which facilitates dispersal of aquatic biota, nutrients, and other material among successive locations in the riverscape. However, the dry season results in alteration or reduction in aquatic habitats available for fish reproduction. Similarly, low floods may reduce inputs of nutrient resources from the terrestrial environment that support aquatic food webs and can lead to community disruption, even to the point of local extirpation of stranded fish in fragmented ephemeral pools in the floodplain. Consequently, the periodicity, magnitude and predictability of flows are the major drivers of the systems’ capacity to sustain persistent fisheries production and other ecosystem services affecting human welfare. We argue that identification of the processes that sustain production and biodiversity patterns is an essential step towards a better ecological understanding and natural resource management of river-floodplain systems. Based on this review, we debate that floodplain fisheries, like in the Okavango Delta, should be exploited using a diverse exploitation pattern to ensure a harvesting regime in balance with system productivity. Such balanced fishing pattern, based on traditional fishing practices, facilitates the provision of food and nutritional value of the fishery to marginalized communities.
Tropical inland fisheries, while producing at least 15–20% of the global fish production, are based on the tiny fraction (≈0.04%) that tropical aquatic freshwater systems contribute to the world’s freshwater resources (Kolding and van Zwieten, 2006). Most importantly, inland fisheries provide vital proteins, micro-nutrients, jobs and income for some of the most marginalized communities of the world (Allan et al., 2005; Welcomme, 2011; HLPE 2014; Béné et al., 2015), but a growing global population, with a consequent increase in food demand, will place increased pressure on the global water resources (e.g. http://www.waterforfood.org/). According to Molden and de Fraiture (2004), this situation is of particular concern in Africa, where pressure on water resources is expected to increase rapidly within the next two decades. In addition, climate change will likely increase water stress in southern Africa (Boko et al., 2007) because of reduced (Clark, 2006) or increased variability in precipitation across the continent (Tadross et al., 2005), which will affect fish productivity (Magadza, 2011; Gownaris et al., 2018) and increase food insecurity. An increased pressure on resources has raised concerns of overexploitation exacerbated by lack of knowledge on ecosystem response to changes in species, size, and trophic composition of fish assemblages (Allan et al., 2005). However, “where there is water there is fish” (Kolding et al., 2016) and since the hydrological regimes are key drivers of productivity and structure in freshwater ecosystems (Gownaris et al., 2018) there is compelling need to understand and appreciate the dynamics of floodplain fisheries better because of their prevalence, high productivity, and intrinsic value to riparian communities in Africa.
Floodplain fisheries are generally considered among the most productive in the tropics (Junk, et al., 1989; Welcomme, 2009), with an average potential fish production rate of 2.5–4 times that of tropical lakes and reservoirs on a water surface area basis (Bayley, 1991). The Okavango Delta (Figure 1) is one of the largest inland river deltas in the world (Allanson, et al., 1990) with a fishery which is predominantly artisanal and subsistence, combined with a small-scale commercial gillnet fishery (Mosepele, et al., 2003). In common with most African inland fisheries, it is characterized by a multi-species, multi-gear fishery harvesting the fish community across different trophic levels, species, and sizes (Mosepele, 2019). Approximately 65% of the 25,000 people (based on 1995 population estimates) who live within the periphery of the Delta depend on the fishery as a source of livelihood (Mosepele, 2001). Due to competing interests in the Delta’s fish resources, particularly between the flourishing tourist and angling industry and the local people, there has been a long history of stakeholder conflicts and repeated allegations of over-exploitation of the fish resource and deterioration of the environment (Mosepele et al., 2014). However, apart from a preliminary analysis (Mosepele and Kolding, 2003) there have been no informed assessment studies on the Okavango Delta fishery. Because of the complex and dynamic nature of the fishery (approximately 71 species and high seasonal variability, Mosepele, 2019), using conventional single-species fish stock assessment, based on steady state assumptions, is considered only partly adequate for a comprehensive and accommodating evaluation of the fishery. The Okavango Delta is subject to seasonal flooding which, like elsewhere, plays a key role in determining the potential and nature of its fishery (Mosepele et al., 2009). However, a comprehensive understanding of the relationship between the hydrological regime and the dynamics of the fishery, the productivity, and the trophic interrelationships remains limited and dispersed.
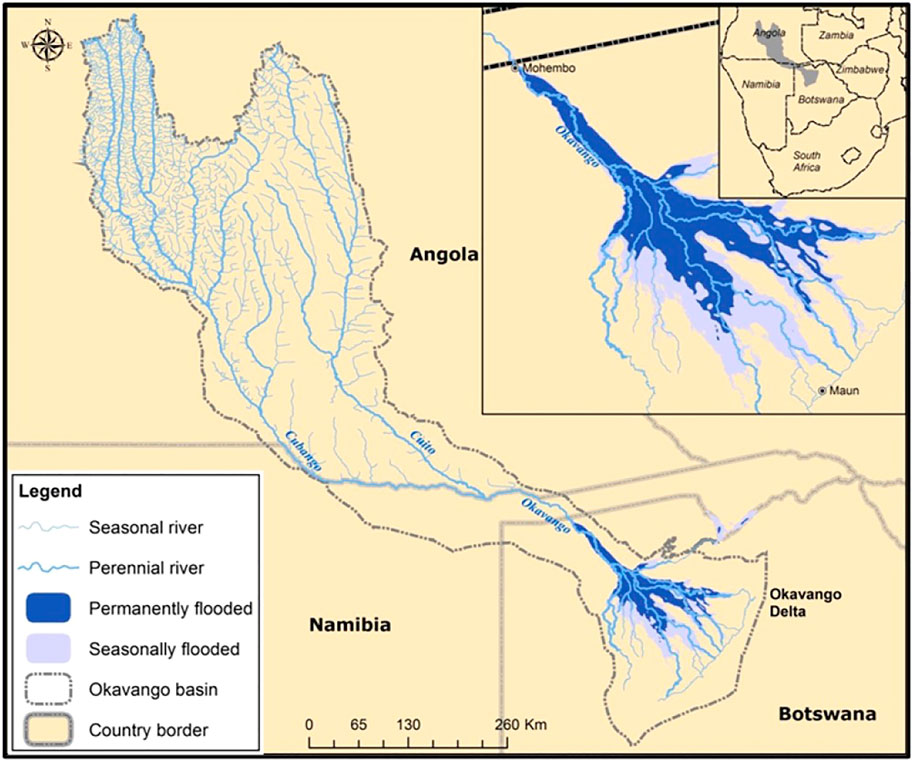
FIGURE 1. Map of the Okavango River basin in southern Africa, with the three countries sharing the drainage basin. The insert shows the Okavango Delta inside Botswana, which is the focus of this study (Source: The ORI GIS Laboratory).
This study is a systematic review of over two decades of research work in the Okavango Delta. Data used in Figure 3–5 are based on data that were collected over several years as part of a PhD work and are described comprehensively in Mosepele (2019). The aim of this review is to examine the relationship between fish dynamics and environmental variability which allows for assessment of an optimum fishing regime in a flood-pulsed floodplain fishery of the Okavango Delta. Establishing this relationship is important towards identifying the key drivers of change, the potential range of fluctuations, and resilience in floodplain fish communities. Understanding this relationship will aid in floodplain fisheries and water management, as a step beyond prevailing management regimes based on steady state theories and models (Mosepele, 2014).
Tropical and sub-tropical floodplains are dynamic pulsating systems, which are constantly changing at various spatio-temporal scales, but where the seasonal fluctuations are also essential for regeneration and maintenance of the ecosystem. Proper understanding of floodplains is essential towards their conservation aligned with the socio-economic development of riparian communities. The fundamental philosophy underpinning this review is that floodplains are dynamic, interconnected aquatic-terrestrial systems driven by seasonal flooding at variable intra and inter-annual scales and that management needs to be equally dynamic, flexible, and adaptive.
The Okavango River basin (Figure 1) is located in a semi-arid environment with one of most sparsely populated basins in southern Africa. It is a large endorheic (no outlet) system that spans three countries (Angola, Namibia, and Botswana) (Ashton and Neal, 2003; McCarthy et al., 2003).
The catchment of the Okavango River Basin is estimated to be approximately 530 000 km2 at its largest extent (Andersson et al., 2003). The basin is located in a water scarce region, and future planned water abstractions are projected to amount to about 3% of the mean annual daily runoff of the Okavango River when entering Botswana at Mohembo at the distant end of the so-called panhandle (Figure 1). According to Steudel et al. (2013) mean annual daily runoff (1974–1998) at Mohembo is 263m−3s−1. However, there is not enough knowledge to accurately predict the scale, significance and resilience of ecosystem responses within the Delta to the anticipated decreased flows (Ashton and Neal, 2003).
Currently, the delta is still relatively pristine (Milzow et al., 2009; Black et al., 2011), which nevertheless, does not discount threats to its ecological integrity. Anthropogenic threats to the delta do not only come from within the country driven by local population development pressures (Porter and Muzila, 1989), but also from transboundary threats which have increased with the advent of peace in Angola (Andersson et al., 2003; Milzow, et al., 2009; Milzow, et al., 2010). After a prolonged civil war, a repopulation of the headwaters of the Okavango has begun (Mendelsohn et al., 2010), where approximately one million people are expected to settle within the river basin (Andersson et al., 2003). Concomitant human activities like agriculture (including irrigation), water abstraction and hydropower development in both Angola and Namibia are expected to place an increased demand on the water resources of the basin (Andersson et al., 2003; Junk et al., 2006; Milzow et al., 2009) and may negatively affect water quantity and quality (Masamba and Mazvimavi, 2008).
The Okavango Delta is a vast mosaic of various habitats consisting of swamps, islands and river channels whose aquatic, semi-aquatic and terrestrial phases change constantly at different temporal scales, driven by the flood regime (McCarthy et al., 2003; Ramberg and Wolski, 2008). It is located in a dry sub-tropical area with a mean annual rainfall of 475 mm and experiences large annual variations in temperature where October is the hottest month while July is the coldest (Milzow et al., 2009). Rain normally falls in the period November—March while annual flooding from the Angolan highlands occurs in the period April - September (Ramberg and Wolski, 2008). Annual precipitation, which is out of phase with seasonal flooding (Porter and Muzila, 1989; Ramberg et al., 2006a), contributes approximately between 5% (Andersson et al., 2003) and 42% of the total water input into the delta, while the rest comes as discharge from the Angolan highlands (Ramberg and Wolski, 2008). Total water storage in the delta is about 10 Km3 (about a year’s inflow of water) which supports diverse vegetation (Porter and Muzila, 1989) “aquatic” and wildlife species (Ramberg, et al., 2006b). The delta’s hydrology is dynamic (i.e. changes in flow patterns from one part of the delta to the other), that varies in response to changes in seismic activity, vegetation dynamics, animal activity (such as hippos) and human intervention (Wilson, 1973; Porter and Muzila, 1989; Wolski and Murray-Hudson, 2006; Milzow et al., 2009), causing the flow in the anastomosis of channels to change at any given time due to variations in these factors.
Peak discharge in the delta’s panhandle occurs in March/April (Wolski, et al., 2005) and the flood pulse travels progressively down the delta, taking a maximum of 6 months to reach the distal ends of the system (Andersson et al., 2003). The sinusoidal flooding cycle (Figure 2) in the delta results in a period of minimum inundation (November - March) to a period of maximum inundation (May - September) (Andersson et al., 2003; McCarthy et al., 2003; Wolski et al., 2005). Water depth variations in the permanently flooded areas are usually very small, while normally in the order of 1–2 m in the seasonally inundated parts of the Delta (Ramberg et al. (2006b).
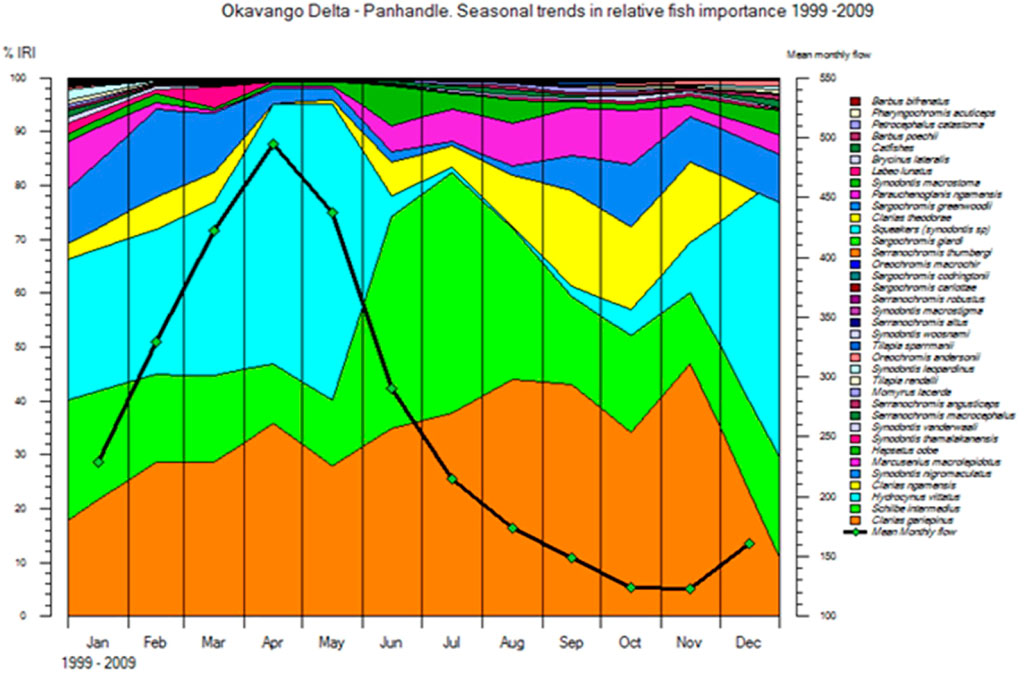
FIGURE 2. Intra annual variations in fish species biodiversity in the Okavango Delta where the black line shows seasonal variability in mean discharge (Source: Mosepele et al., 2017).
Also inter-annually the flows in the delta have a cyclical behaviour with a 17.5 year periodicity in the annual average and maximum flows (Mazvimavi and Wolski, 2006). However, there is high inter-annual variability in flooding patterns where good flood years may be followed by poor flood years and the extent of flooding in the previous year and local rainfall also affect the extent of flooding in any 1 year (Milzow et al., 2009; Mendelsohn et al., 2010). While inter-annual variations in rainfall cause variability (lows and highs) in its flooding regime (Wolski and Murray-Hudson, 2006), seismic Earth movements also cause different parts of the delta to periodically undergo drying episodes (Milzow et al., 2009). Thus, flooding dynamics in the delta are highly dynamic and critical towards a comprehensive understanding of ecological processes in the delta.
Seasonal flooding liberates nutrients from the inundated soils as new floodwaters enter the floodplains (Welcomme, 1988; Lindholm et al., 2007). The delta has a heterogeneous mosaic of micro-habitats (Siziba et al., 2011a) characterized by low nutrient concentrations (Krah et al., 2006) and oligotrophic waters (Cronberg et al., 1995; McKay et al., 2011). Despite its oligotrophic state, the delta is a productive system (Høberg et al., 2002) as evidenced by relatively high fish production/biomass in some lower delta lagoons (Fox, 1976; Mosepele et al., 2011) and fast vegetation growth (Ramberg et al., 2006a). Several key processes contribute to nutrient dynamics in the delta; (i) surface waters (Cronberg et al., 1995; Garstang et al., 1998; McKay et al., 2011) (ii) soil nutrients (Krah et al., 2006), (iii) dung from mammals in the seasonal floodplains (Mosepele et al., 2009), (iv) mineralization (from senescent plant material and peat) (Ramberg et al., 2006a), and (v) windblown dust/atmospheric deposition (Krah et al., 2006), the latter is a major nutrient source at receding water levels in the seasonal floodplains.
When the new floods arrive, they carry allotropic nutrients from upstream runoff, which facilitate the primary production processes in the delta (Krah et al., 2006). The new floods also dissolve embedded soil nutrients from the terrestrial dry phase, which increase nutrient concentration and availability (Tsheboeng et al., 2014). This is also coupled with an increase in Dissolved Organic Carbon (DOC) in the seasonal floodplains (Mladenov, et al., 2005), due to high organic matter loading (Mladenov, et al., 2007). Additionally, dung from the herds of large herbivores (elephants, buffaloes, antelopes) also contributes to the organic matter loading in the seasonal floodplains (Mosepele et al., 2012). Hippos also play a major role in nutrient cycling of aquatic ecosystems by converting terrestrial biomass (ingested grass) into aquatic nutrients in the delta’s waters where they defecate (Garstang et al., 1998). Ultimately, water borne and internal nutrient loading switches to atmospheric deposition when the floods have reached their maximum extent in the seasonal floodplains (Krah et al., 2006). The alternating wetting and drying processes in the delta facilitate optimum conditions for enhanced primary production in the system (Ramberg et al., 2006b). This is consistent with studies from elsewhere (Junk et al., 1989; Ward and Stanford, 1995) which observed that regular flooding and drying in floodplains is an essential recycling nutrient pump for biological production.
Average biomass of large mammals in the delta is approximately 12 t km−2, and is among the highest in wetlands around the world (Junk et al., 2006). The density of mammals in the Okavango Delta is 4–8 times higher than expected from its standing nutrient status, primarily because of its high efficiency in primary productivity from recycling nutrients (Ramberg et al., 2006b). This positive feedback loop in fertilization makes the delta highly efficient in transforming plant carbon into higher food-web levels through terrestrial mammals (Junk et al., 2006).
Regular flooding and drying episodes in the delta increase plant diversity (Tsheboeng et al., 2014), in accordance with Huston’s (1979) “intermediate disturbance hypothesis”. Other “disturbing factors” include erosion and sediment deposition, and actions by biological engineers like elephants, hippos and termites (Mosepele et al., 2009). Frequent disturbances in the Delta create small-scale habitat patches, which facilitate the co-existence of different successional stages of plant communities (Tsheboeng and Murray-Hudson, 2013). Generally, flood pulsed systems provide diverse food items to food webs, and act as dry season refuges for migrating mammals (Junk et al., 1989; Junk et al., 2006; Bartlaam-Brooks et al., 2011). Flooding dynamics in the delta, coupled with the “out-of-phase” rainfall season, ensure that fresh primary vegetation is available much longer in the Delta for herbivore mammals, which increases the land’s carrying capacity (Junk et al., 2006). All these interrelated dynamics enhance ecosystem productivity, and contribute to the high productivity in the Delta, despite its oligotrophic clear water.
In addition to a high average biological basis production, the aquatic processes in subtropical and tropical floodplains systems undergo “boom and bust” conditions driven by seasonal flooding (Lowe-McConnell, 1987; Junk et al., 1989; Bunn et al., 2006; Schongart and Junk, 2007; Kolding and van Zwieten 2012). The seasonal flooding in the Okavango Delta initiates a “boom” in the aquatic primary production when the new annual floods inundate the peripheral floodplains (Høberg et al., 2002). As the floodwaters submerge the floodplains, microbial decomposition begins to degrade the accumulated detritus, dung, perennial plants, and other organic matter. There is an initial build-up in nitrogen and phosphorous concentrations at the start of the flooding season, but these are gradually depleted over time through photolytic degradation and burning in the dry floodplains. There are spatio-temporal variations in dissolved oxygen (DO) (Høberg et al., 2002), conductivity and phosphorous concentrations (Siziba et al., 2011b). DO levels are initially low at the onset of the floods and increase gradually, before reducing again at decreasing flood levels (Høberg et al., 2002). There is also diurnal variability in DO levels where anoxic conditions are observed at sunrise while peak DO saturation levels occur at sunset (Høberg et al., 2002).
The initial flooding in the delta results in a “boom” in chlorophyll a and primary production processes, followed by a “bust” towards the end of the flooding cycle. During the first week of flooding, chlorophyll a concentration increases from 2.6 to 23.5 μg L−1 before receding to 10 μg L−1 by the end of the flooding season (Høberg et al., 2002). Similarly, primary production increases from 63 μg C L−1 day −1 at the onset to 264 μg C L−1 day −1 within a week of flooding, before settling to 82 μg C L−1 day −1 by the end of the first month of flooding. However, there is spatial variability in chlorophyll a concentration across the delta’s microhabitats (Siziba et al., 2011a). The seasonally inundated floodplains in the delta have higher concentrations of DOC, K, SiO2, Mg, HCO3, Na and NO3 than permanently flooded areas (Mackay et al., 2011). Like the mosaic pattern of the delta itself, there are spatial and temporal variations in water chemistry. This complex system is further exacerbated by a rolling time lag where new floods arrive at Mohembo (northern delta), while the previous year’s flood are still receding at Maun (southern delta) (Mackay et al., 2011).
The zooplankton biomass “boom” at the onset of the floods is inoculated from egg banks in the seasonal floodplains (Høberg et al., 2002; Siziba et al., 2012). Regular flooding is important in maintaining micro-crustacean propagules and the diversity of these micro-fauna in the Delta’s floodplains (Siziba et al., 2012). Cladocerans, copepods and ostracods are the three major groups whose emergence from floodplain sediments is initiated by inundation. These micro-crustacea, which are key fish food (Siziba et al., 2013), then inoculate new flood waters in the seasonal floodplains (Siziba et al., 2012). Riding on the wave of seasonal flooding are strong fluctuations in zooplankton biomass over the flooding season in the seasonal floodplains (Høberg et al., 2002). Zooplankton biomass peaks at about 10 mg DW L−1 during the first month of flooding, which gradually declines to 1 mg DW L−1 towards the end of the flooding season. Høberg et al., (2002) also observed a species succession in zooplankton species during the flooding season. Moina micrura is the dominant species during the onset of the flood, whose populations then decrease to the end of the first month of flooding. Zooplankton populations are then dominated by Daphnia laevis during the second month of flooding, while Chydorus spp. dominates the zooplankton community at the end of the flooding season.
Structure of the fishery: Based on two previous frame surveys in the delta, there are approximately 3000 fishers in the Okavango Delta fishery (Mosepele, 2001; Bokhuto et al., 2007). Generally, the number of fishers has gradually decreased over time (SADC, 2016). Approximately 7% of the fishers constitute a small-scale commercial fishery (Mosepele, 2001; Bokhuto et al., 2007) composed of modern gill nets and aluminium boats outfitted with outboard engines (Mosepele, 2001). Earliest records of fishing in the delta include extensive use of traditional fishing traps, weirs, traps, spear fishing, and traditional hook and line all used in different habitats and across hydroperiods (Kay, 1962; Maar, 1965) and these fishing gears are still the most common gears in the fishery (Mosepele et al., 2003).
These different fishing gears and methods are used across different habitats and hydroperiods in the delta (Mmopelwa et al., 2009). This suggests that generally, the Okavango Delta has retained its traditional/artisanal character (Cassidy et al., 2011). According to Mosepele et al. (2003), there are five different types of fishers in the Okavango Delta; basket fishers, traditional hook and line fishers, gill net fishers, trap fishers and spear fishers. Recreational tourist fishers are another key group in the delta’s fishery (Mosepele, 2000; SADC, 2016). Traditional hook and line and basket fishers are the major groups in the delta (Mosepele, 2001; Mosepele et al., 2003). NORFICO (1986) defined fishers as either occasional, seasonal or professional. Furthermore, Turpie et al. (2006) defined fishers as either “traditional” or “modern” where the latter own at least one gillnet.
Catch statistics are a major challenge in most fisheries around the world (FAO, 2020) particularly in inland fisheries (Welcomme, 2011) and Botswana is not an exception. Generally, data on fish catches are fragmented (Arntzen, 2005) and irregular. Therefore, there is a lack of accurate catch statistics from the Okavango Delta, and most of the initial records are best estimates (Mosepele, 2003). Subsequently, the period between 1970 and 1987 is characterized as a time of poor/uncertain data, while fish yield data from 1996 are relatively better (Mosepele, 2003). Table 1 summarizes fish production data from the Okavango Delta. Turpie et al. (2006) attributes these discrepancies to the presence of different actors in the delta’s fishery. However, inter-annual variability in flooding patterns can also cause discrepancies in fish production estimates. Fishing effort in the delta is driven by the seasonal flood regime (Mosepele et al., 2018), where most traditional/artisanal fishers are only active during good flood years (Mosepele, 2001). Furthermore, Mosepele (2000) and Mosepele (2001) highlights that catch data is only collected from gill net fishers, which would underestimate the total annual production from the fishery. The extent of flooding in the delta also opens up new fishing grounds which would increase the total annual fish production from the system. According to Mosepele (2019), fishery yield is driven by the delta’s flood regime at a 2-year time lag. This fishery-flood relationship is an illustration of Junk et al.’s (1989) flood pulse concept.
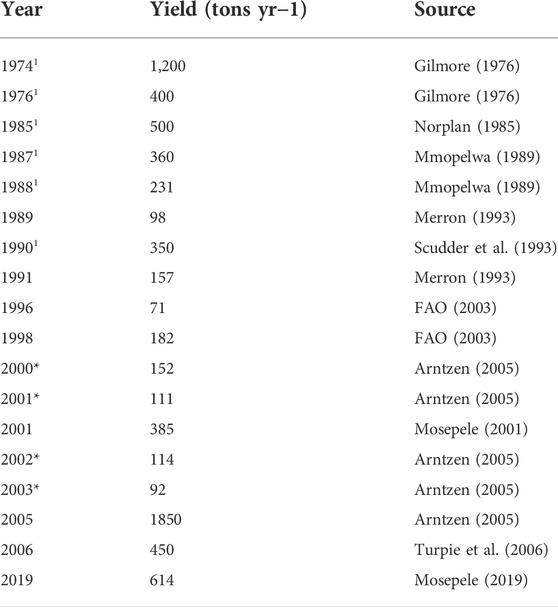
TABLE 1. Total annual fish yield/estimates from the Okavango Delta fishery based on various sources where data with an * are estimates from the Fisheries Division while data with1 are from various sources.
Turpie et al. (2006) estimated the total annual private use value of the delta’s fishery at approximately US$ 490,000 (based on 2005 exchange rates), which was approximately 9% of the total value from the delta’s wetland resources. Despite this relatively low use value, the delta’s fishery is a source of livelihoods and food security for subsistence fishers (Mosepele et al., 2006) who increase fish catches as the first and second coping strategy during periods of food shortage (Mosepele et al., 2006; Ngwenya and Mosepele, 2008; Mmopelwa et al., 2009). Subsistence fishers either sell their surplus fish for cash or barter them for grain (Ministry of Agriculture, 1997; Mosepele, 2001; Mmopelwa et al., 2009). Therefore, subsistence fishing is a source of income for 40% of subsistence fisher households in the delta, where fishing income is used on food, toiletry, and clothing (Ngwenya and Mosepele, 2008). This is in accordance with Mmopelwa et al. (2009) who observed that subsistence fishing has socio-cultural, socio-economic and food security value to the delta’s subsistence fishers.
Furthermore, the presence of a small-scale and yet profitable commercial fishery (Mmopelwa et al., 2005) makes it an integral part of the delta’s rural livelihoods strategy (Mosepele and Ngwenya, 2010). The small-scale commercial fishery is not only a source of rural employment (Mosepele, 2001; Mmopelwa et al., 2005; Mosepele and Ngwenya, 2010), but fishery revenue is also invested into either agriculture (Mendelson et al., 2010; Mosepele and Ngwenya, 2010) or other economic activities (Mosepele, 2001). Kgathi et al. (2018) observed that the small-scale commercial fishery also contributes to fish regional trade, which may contribute to a reduction of Botswana’s food import bill. Generally, most of the fish catch goes towards livelihood support (Arntzen, 2005) which makes fish not only a valuable safety-net (Mendelson et al., 2010) but also a resource (Mosepele, 2001). Traditional (artisanal) fishing is also identified as a natural safety net against HIV/AIDS comorbidities in the delta (Ngwenya and Mosepele, 2008). Based on income generated, fishing was the second most important economic activity in the region after cattle farming in the 1990s (Mosepele, 2003).
Newly inundated floodplains are an important nursery habitat for fish recruitment (Junk et al., 1989; de Oliveira et al., 2020). In the Okavango Delta the inundated areas are dominated by juvenile cichlids (e.g. Oreochromis andersonii, Tilapia sparrmanii and Coptodon rendalli), catfish (Clarias gariepinus), and cyprinids (e.g. Barbus bifrenatus and B. barnardi) during the first month of flooding. Fish fry and juveniles were observed at increasing frequency starting from the second month of flooding (Høberg et al., 2002). The boom of primary producers and zooplankton initiated by the seasonal flooding, serves as abundant food sources for the juvenile fish and small fishes (Siziba et al., 2013) and also some adult fish (Mosepele et al., 2012). The subsequent decrease in zooplankton biomass corresponding with an increased frequency of juvenile fish over the flooding season is largely due to predation and decreased primary productivity (Høberg et al., 2002; Siziba et al., 2013). This suggests that failed or poor floods cause a bottle neck in fish production due to failed zooplankton production (Siziba et al., 2012).
Juvenile fish growth on the inundated floodplains is rapid within the first year of life (Dudley, 1974). Rapid growth ensures that juvenile fish are large enough to (i) avoid being stranded in the floodplains at receding floods, and (ii) avoid heavy predation when migrating into the permanent channels at draw-down (Booth and Merron, 1996). Foraging by juvenile fish in the inundated areas is an adaptation for taking advantage of high zooplankton biomass (Lindholm et al., 2007), and these shallow areas also act as a predator refuge due to highly fluctuating DO concentrations (Kolding 1993; Mosepele et al., 2017). Less frequently flooded areas (those only flooded occasionally at very high flows) show exceptional “booms” in zooplankton biomass and juvenile fish (Siziba et al., 2011b), especially after a low flood year (Siziba et al., 2013). During poor flood (i.e. low flood) years, the zooplankton biomass is less exposed to fish grazing, while predation appears to be a strong regulator of zooplankton biomass during good flood years (Lindholm et al., 2007). Large flood years result in extensive flooded areas which appear to particularly facilitate fish breeding, growth and survival and ultimately increased fish production (Lowe-McConnell, 1987; de Graff, 2003). Thus, the flood volume in the Okavango Delta is a major driver of fish production, where relative fish biomass during a high flood year can be double that of a low flood year (Lindholm et al., 2007).
Alternating wetting and drying processes are necessary in floodplains to increase nutrient turnover, maintain primary production dynamics (Junk et al., 1989) and hence fish production. However, the pattern of rise and fall of the hydrograph is influencing floodplain fish production. According to King et al. (2003), a “relatively slow rate of rise and fall” of the seasonal hydrograph creates optimum conditions for fish species to utilize the floodplain for recruitment. Conversely, a rapid rise and fall in the hydrograph may offset the balanced time lag between primary production and fish production (Tockner et al., 2000), which may result in less successful fish production. However, short lived hardy species in floodplain systems can adjust quickly to extreme hydrological events (Junk et al., 1989; Junk, 2002).
Floodplain fish communities are structured along a hydrology-water chemistry gradient at both seasonal and annual scales (Zeug et al., 2005; Zeug and Winemiller, 2007; Mosepele et al., 2017). However, due to inter-annual differences in flooding regimes, fish communities among years are stochastically different driven by the seasonal dilution and expansion dynamics of the hydrological cycle (Mosepele et al., 2009; Mosepele et al., 2017).
Studies from other areas have shown that poor flood years are dominated by opportunistic fish species (Laë, 1995; Petry et al., 2003), which have fast growth rates and high fecundities. Other studies show that good flood years are dominated by iliophagous (mud-eaters) species, which are preceded by piscivores in poor flood years (Agostinho et al., 2001). Similar kinds of species dynamics driven by flooding at an annual scale have also been observed in the Okavango delta. Seasonally, the Delta’s fish community, as judged by experimental catch rates, is dominated by C. gariepinus at maximum flooded area, while tiger fish (H. vittatus) dominates the fish community in the channels at minimum flooded area (Mosepele et al., 2017). Furthermore, poor flood years are dominated by hardy, multiple spawning species (i.e. C gariepinus) while good/high flood years are dominated by opportunistic, highly fecund, total spawning species (i.e. Schilbe intermedius) (Mosepele et al., 2017). There are, however, spatial differences in fish community structure among lagoons across the delta (Mosepele et al., 2011). Generally, upper delta lagoons have higher fish species richness than lower Delta lagoons. One factor that may contribute to these community differences is relative hydrological stability in the upper delta vs increased hydrological variability in the lower delta.
While spawning for some floodplain fish species is cued by rising water levels (Dudley, 1974; van der Waal, 1985; Welcomme, 1985; Godinho et al., 2010; Montcho et al., 2011), others spawn at low water levels (Vasquez et al., 2009). In the Okavango Delta, peak spawning for some fish species occurs at low flood levels in the main channel at high water temperatures, while other species spawn during high water levels in the floodplains at low water temperatures (Merron et al., 1990; Mosepele et al., 2017). Van der Waal (1985) observed that spawning for some cichlids was apparently not associated with hydrology, while other studies (Dudley, 1974; Mosepele et al., 2017), found that spawning for the majority of cichlids is associated with a hydrological gradient, However, for some cichlids (e.g. Serranochromis macrocephalus and C. rendalli) spawning was mostly associated with water temperature, which agrees with van der Waal’s (1985) observations.
Floodplain fish growth is fastest during increasing water levels (Power, 1984; Bayley, 1988; Bokhutlo et al., 2015) and peaks at maximum flooded area to take advantage of the available abundant food in the floodplains (Booth and Merron, 1996; Bokhutlo et al., 2015). During the low flood season, intra-specific competition for food (Mosepele et al., 2012) decreases growth rates (Dudley, 1974; Martin et al., 2011). At inter-annual scale, growth of floodplain fish in Kafue, Zambia, differed significantly among years according to flooding and temperature (Dudley, 1974). In the Okavango, there are significant phenotypic differences in maximum size between upper and lower delta Clarias gariepinus populations (Mosepele et al., 2011) and some cichlid species (Mosepele and Mosepele, 2005). The phenotypic differences in size for C. gariepinus are attributed to hydrological differences between the upper and lower delta (Bokhutlo et al., 2016). Similarly, Merron and Bruton (1988) observed that differences in hydrology between upper and lower delta habitats account for the phenotypic differences in cichlids between these habitats.
Like most other features, the diet and feeding ecology of floodplain fish species is flood-pulse driven (Lowe-McConnell, 1987; Mosepele et al., 2012). After the feeding and growth of the juveniles on the floodplains during high water, a dominant feature is increased piscivory at receding water levels by fish predators when all the young fish are forced back into the main channels (Bayley, 1988; Mosepele et al., 2012). This “concentration effect” at receding water levels facilitates predation by piscivorous fish, as well as fishers. These dynamic processes illustrate the variability of floodplain fish dynamics and the need for adaptive approaches in both exploitation and conservation.
The preceding overview has highlighted the dynamic interactions and processes between floodplain fish communities and the highly dynamic environment in the delta. Floodplains are unstable, seasonally fluctuating ecosystems characterized by strong intra and inter annual variability, where the flood pulse is a key driver of practically all processes (Junk et al., 1989; Schongart and Junk, 2007). Inland fisheries in Africa are generally small-scale and labour intensive (Welcomme, 2011). They are characterized by multi-species assemblages, of different sizes exploited by diverse fishing gears and methods (van Zwieten et al., 2003; Welcomme, 2011; Kolding and van Zwieten 2014; Mosepele, 2019). In the Okavango Delta, the hydrological regime is a major driver of change in the biology and ecology of the fish community (Lindholm et al., 2007; Mosepele et al., 2009; Linhoss et al., 2012; Mosepele et al., 2012; Bokhutlo et al., 2015; Mosepele et al., 2017). Like the habitat, the fisheries are dynamic, fluctuating, and constantly changing and are never in stable equilibrium and the environmental drivers are in general much more important in regulating productivity than the fishing effort (Jul-Larsen et al., 2003, Kolding and van Zwieten 2006; Kolding and van Zwieten 2012). This makes conventional management approaches based on steady state assumptions inconsistent and difficult (Staples et al., 2004; Mosepele, 2008; Welcomme et al., 2010; Mosepele, 2014).
Except for a few highly commercialized fisheries in freshwater systems like the Amazon and Mekong (Welcomme et al., 2014), most tropical floodplain fisheries are a major source of localized food and nutrition and mostly serving as subsistence for riparian households (Junk, 2002; Mosepele et al., 2006; Welcomme, 2011). Their primary value to local communities is their contribution towards household income and food security (Mosepele et al., 2006), though some African inland fisheries are slowly morphing towards commercial or recreational fishing as well (Kolding and van Zwieten, 2014). Fishers in floodplain fisheries systems use various traditional techniques (Cerdeira et al., 2000; Kolding et al., 2003; van Zwieten et al., 2003) to adapt and optimize utilization of the ever changing fish assemblages, and the same is observed in the Okavango Delta (Mosepele et al., 2007; Mmopelwa et al., 2009; Mosepele, 2019). Floodplain fisheries are thus also a major source of traditional ecological knowledge (Mosepele, 2008) and cultural heritage (Junk, 2002). Therefore, floodplain fisheries management plans should incorporate these characteristics (i.e. cultural values and traditional knowledge) into their management objectives.
Gear restrictions and mesh regulations are fixed constant attributes and remain some of the easiest and cheapest regulations to implement in fisheries management regimes (Misund et al., 2002), and these have been widely implemented in floodplain fisheries. The fundamental question in fisheries management is how to regulate the fishing mortality, which is a combination of how to catch the fish (regulated by gear and mesh restrictions) and how much fish to catch (which is based on effort regulation). The key approach to regulate the ‘how’ question is to control gear selectivity (see next section), while effort on the other hand is sometimes regulated to maintain the aggregate fishing effort to obtain a “maximum sustainable yield” (MSY). An efficient economic exploitation of the fishery is assumed to save fish stocks from over-exploitation/collapse (Bene et al., 2010; Kolding and van Zwieten, 2014). Arguments such as these are attractive to policy makers and introduce policies aimed at effort reduction. The classical argument is that fishing effort is the main factor influencing fish stock dynamics, which is otherwise assumed in “steady state” and since catch is a function of effort, it needs to be managed. The alternative assumption would be that effort is controlled by the current production (Kolding and van Zwieten 2011; Kolding and van Zwieten 2014), and therefore largely self-regulated as in natural predator-prey relationships. According to Mosepele and Kolawole (2017), law enforcement in fisheries is prioritised over rural people’s livelihoods. This is a consequence of implementing classical management approaches in fisheries management. Subsequently, anecdotal evidence indicates that people’s livelihoods were curtailed through the implementation of these management approaches in the delta (Daily Maverick, 2017).
A key theoretical argument for regulating the gear selectivity is to protect the young fish and target the big fish in order to prevent so-called growth overfishing (Kolding and van Zwieten, 2011). Most fishing gears are selective regarding species, sizes and habitats fished (Kolding and van Zwieten, 2014, Figure 3 and Table 2) but regulating selectivity on certain sizes or species will invariably change the natural composition of the various components in the ecosystem (Garcia et al., 2012). For example, males of O. andersonii, O. macrochir and C. rendalli (these are the three most important commercial fish species in the Okavango Delta), grow larger than females (Dudley, 1974). Hence, selective harvesting with large mesh sizes would tend to select the males from the populations of these three species resulting in unbalanced sex ratios. Such scenario can alter the breeding sex ratio of an exploited population and ultimately reduce its reproductive potential (Fenberg and Roy 2008). Focusing exploitation exclusively on the mature part of the population will also alter the demographic composition and potential recruitment. It therefore makes ecological sense to also target younger and more productive age classes than only old big fish, the so-called (BOFFFs, Big Old Fat Fecund Females, Hixon et al., 2014), which are the engines of new recruitment by being more fecund and having better egg quality than smaller/younger fish (Trippel, 1995; Walsh et al., 2006; Kolding et al., 2015a). Smaller/younger fish are also relatively more productive than bigger/older fish (Law et al., 2012). Therefore, in order to maintain the natural structure and composition of fish communities it has been suggested to exploit populations in proportion to their natural productivity, the so-called ‘Balanced harvest’ concept (Garcia et al., 2012; Law et al., 2012; Zhou et al., 2019).
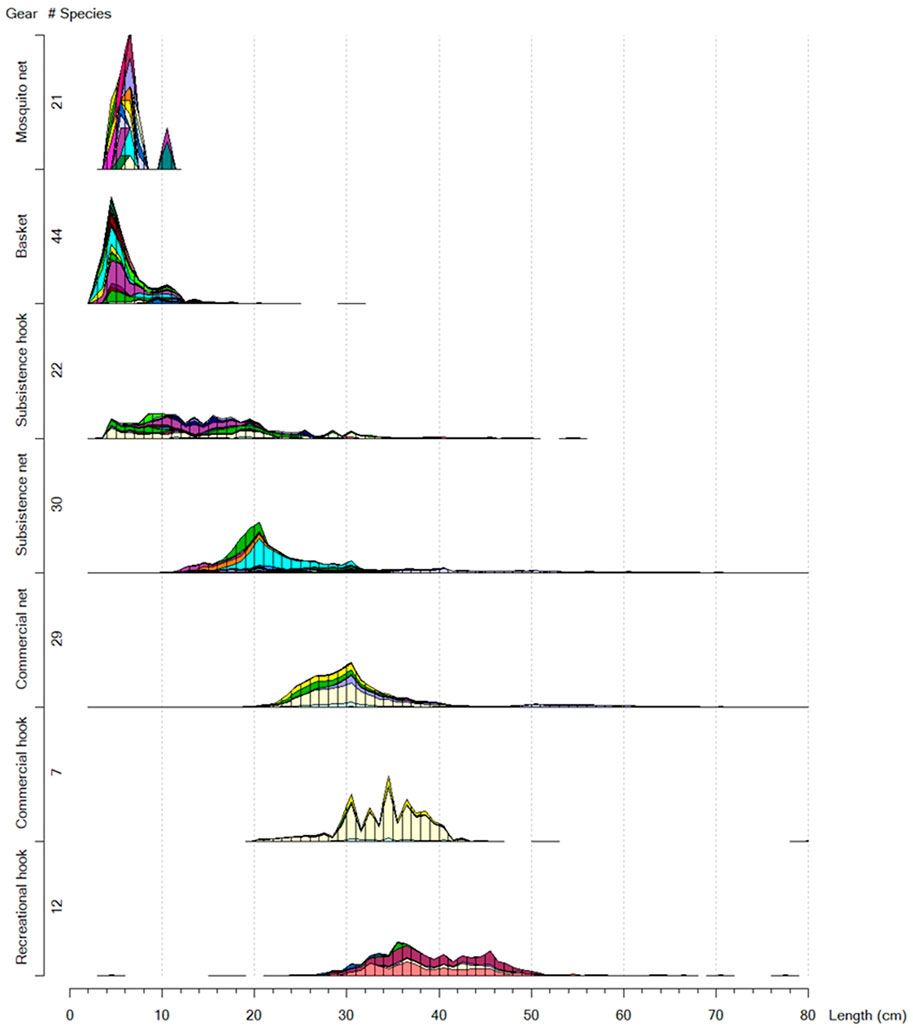
FIGURE 3. Gear signatures in terms of species and size selectivity of the seven most frequently encountered fishing methods in the Okavango Delta. The order of the species is according to the maximum lengths encountered in the catch. Each gear has its own specific signature and there is a clear overall difference in sizes and partly targeted species between the commercial/recreational fishery, targeting larger species and sizes, and the subsistence fishery, targeting smaller species and sizes (Source: Mosepele, 2019)
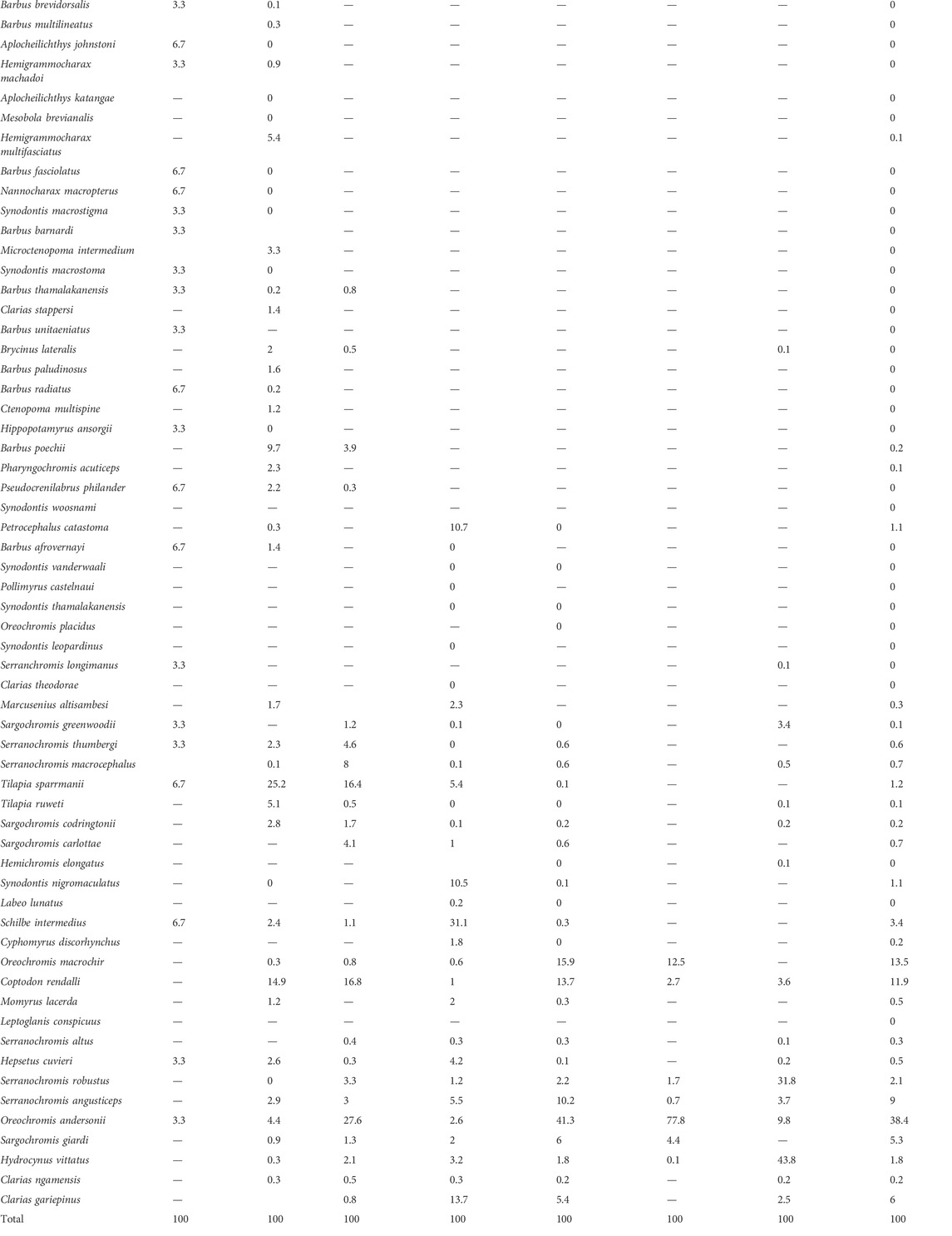
TABLE 2. List of 63 fish species caught in the Okavango fishery and relative abundance (%) in each of the common gear types. Sorted by ascending maximum size as shown in Figure 3.
Similar species from different habitats in the delta display different phenotypic life history strategies (Merron and Bruton, 1988; Mosepele, 2000; Mosepele and Mosepele 2005; Bokhutlo et al., 2015) where lower delta species are generally smaller, upper delta species are generally larger (Merron and Bruton, 1988; Mosepele et al., 2011). While O. andersonii from the lower delta has slower growth than those from upper Delta, O. macrochir and C. rendalli from the lower Delta grow faster than their upper delta conspecifics (Mosepele, 2000). Moreover, lower delta populations of these three cichlids were found to mature earlier than those from the upper delta (Mosepele and Mosepele 2005). A similar observation was made for C. gariepinus (Bokhutlo et al., 2015). Wild tilapias are frequently observed to mature early and breed prolifically in small shallow water bodies, but not in larger, deeper environments (Lowe-McConnell 1982; Kolding 1993), and this ‘stunting’ is apparently driven by the fluctuating oxygen conditions found in shallow environments (Kolding et al., 2008).
From a multispecies point of view, the smallest fish species (Total Length) in the delta is approximately 3 cm while the largest species is over 1 m with a graduation of sizes in between them (Mosepele, 2019, Figures 3, 4). Implementing conventional mesh (or gear) regulations will skew fishing mortality towards larger sizes of the community size spectrum (Figure 4), causing a structural and demographic change of the fish community, and possibly also effecting functional changes. Selective fishing, can in the long run also cause evolutionary change in exploited populations (Rochet, 1998; Law, 2000). As a consequence, exploited stocks undergo changes in growth and maturation (Rochet, 1998; Law, 2000), and selective fishing essentially causes ecosystem imbalances (Schindler et al., 1998; Law, 2000; Kolding and van Zwieten, 2011). According to the Convention on Biological Diversity (CBD), a major component of the Ecosystem Approach to Fisheries (EAF) is to maintain the structure and function of the natural communities as close as possible to the natural stages.
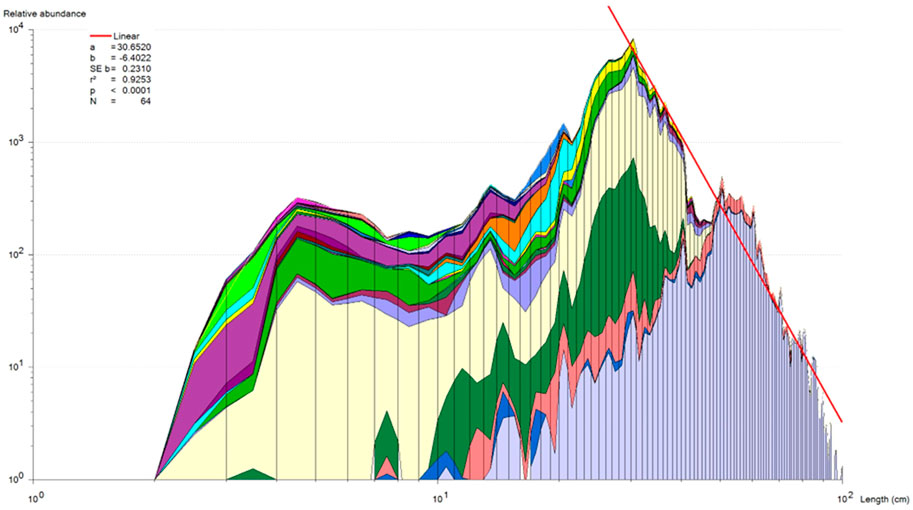
FIGURE 4. Relative biomass-size distribution of the Okavango Delta fishery represented by cumulated standardized (number caught per gear sample) log-log converted catch-curve in the seven most frequently encountered fishing methods (see Figure 3). The slope of the catch curve from 28 cm TL to 100 cm TL is almost identical (p = 0.073) to the theoretical undisturbed Sheldon spectrum slope of −6, which indicate that the fishery is pretty balanced in this size range, consisting of mainly catfishes, tigerfish and larger cichlids. Species and sizes below 28 cm TL are relatively underutilized in terms of a fully balanced fishery (Source: Mosepele, 2019)
Classical single-species assessment models are incompatible with multi-species, multi-gear fisheries (Mosepele, 2008; Welcomme, et al., 2010; Welcomme, 2011). A more balanced exploitation pattern, harvesting species of all sizes and all trophic levels in proportion to their individual productivity, is likely the best management approach for floodplain fisheries in terms of both yield and maintaining the fish community structure (Kolding et al., 2003; Mosepele 2014; Kolding et al., 2015b; Mosepele 2019). There are at least nine different fishing gears/methods observed in the Okavango Delta, with specific catch signatures (Figure 3), but which collectively harvest the fish community across different age classes, species and trophic levels (Figures 4, 5) (Mosepele et al., 2003; Mmopelwa et al., 2009). A cumulated log-converted multispecies and multi-gear catch curve (Figure 4; Table 3) shows that the fishery is approximately “balanced” on all sizes above 28 cm TL (being not significantly different from a theoretical slope of −6), but smaller sizes and species are still under exploited compared to larger sizes. Such multi-species harvesting pattern, by the diversified gear assemblage (Figure 3) is a common attribute of floodplain fisheries (Kolding et al., 2003; Kolding and van Zwieten, 2014). The only fish stock assessment of the delta so far (Mosepele and Kolding, 2003), showed that i) the fish stocks were generally under-exploited and ii) that the fish community was being rationally exploited by using several different fishing gears and methods to harvest the delta’s diverse species assemblage (Mosepele, 2019, Figure 5).
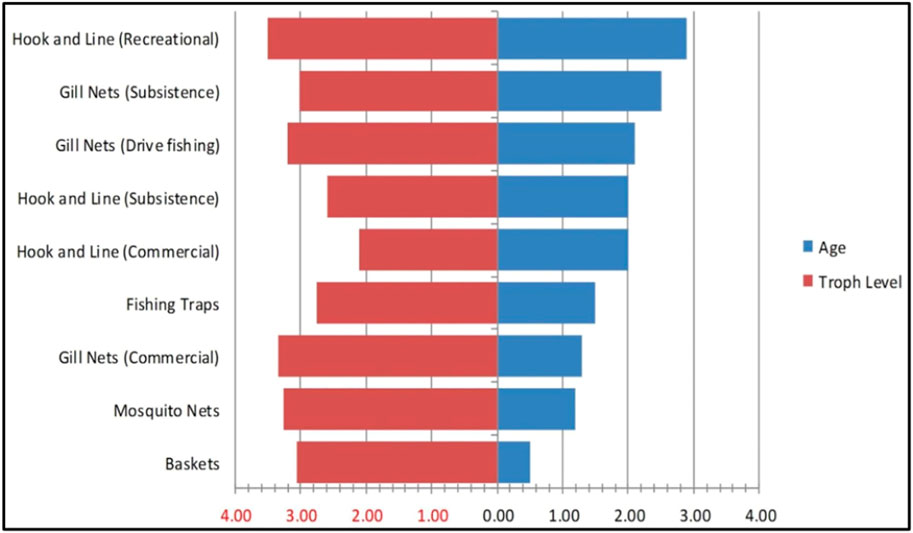
FIGURE 5. Effect of various fishing gears and methods on the Okavango Delta’s fish community where the red scale on the x-axis represents the mean trophic level of each species calculated from Mosepele et al., 2012, while the black scale represents the mean age of each fish species calculated from Froese and Binohlan (2000) (Source: Mosepele, 2019)
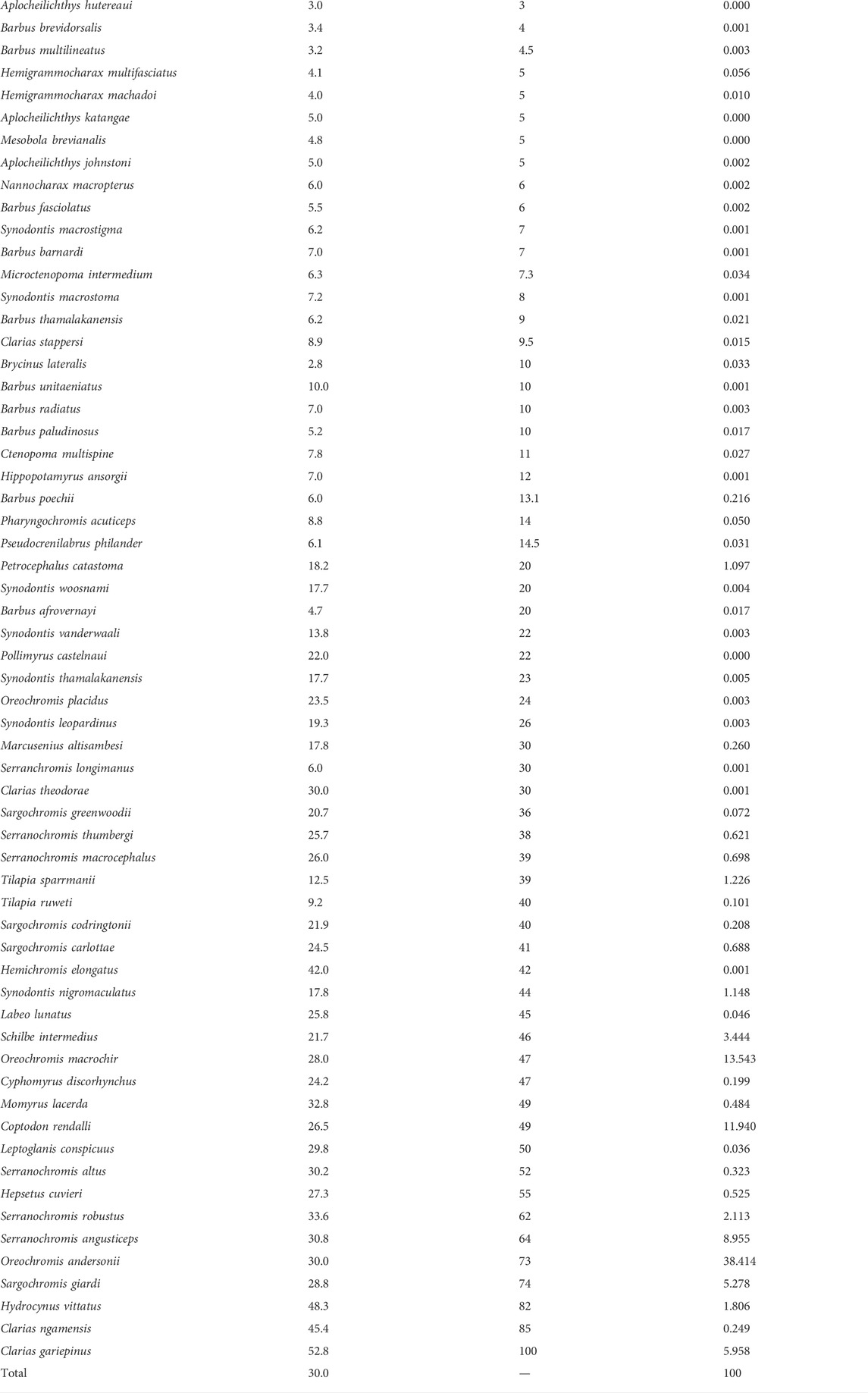
TABLE 3. List of 63 fish species caught in the Okavango fishery with mean size, maximum size, and relative abundance in the catch (%). Sorted by ascending maximum size as shown in Figure 4.
Currently, some of these gears (e.g. mosquito nets) and fishing methods (e.g. drive fishing) are prohibited in the delta (Botswana Government, 2008; Mosepele, 2008; Mosepele, 2014). However, there is no empirical evidence to justify these regulations. About 70% of the species exploited by mosquito nets are generally very small species (e.g. Barbus radiatus, Aplocheilichthys johnstoni, etc.), which are not caught by other methods (Mosepele et al., 2003; Mosepele, 2019, Figure 3). Restricting this gear will result in decreased catches of these small sized underutilized species, which are primarily harvested by women for subsistence household consumption. Moreover, small fish are usually eaten whole, with heads, bones, skin and viscera, and represent a concentrated source of multiple essential nutrients compared to only eating the flesh of larger species (Longley et al., 2014). Drive fishing is a traditional and efficient method for exploiting O. andersonii, O. macrochir and C. rendalli (Mosepele et al., 2007; Mosepele, 2019). However, prohibiting drive fishing (which would only legalise stationary gill net setting) will skew gill net fishing mortality towards O. andersonii (Mosepele, 2000; Mosepele, 2019), while Coptodon rendalli, well known for escaping stationary gillnets (Mosepele, 2000; Kolding et al., 2003; Mosepele et al., 2007), will remain relatively unexploited. In addition, prohibiting drive fishing will result in reduced revenue for the delta’s commercial fishers which are primarily targeting cichlids. A blanket prohibition of some fishing methods and gears, without informed justification may not only cause ecosystem imbalances, but may also reduce the food security aspect and socio-economic value of the fishery to riparian communities. The principle of Balanced Harvest (BH) has been strongly criticized by Froese et al. (2015), because they argue it does not conform to ‘basic population dynamics’ as developed by Beverton and Holt (1957). However, the basic population dynamics were single-species, steady state models where fish grow without eating (Tilley et al., 2020), whereas BH is a multi-species concept with a concrete proposal for implementing the Ecosystem Approach to Fisheries (EAF) (Kolding et al., 2015b), which does not only make ecological and biological sense in floodplain fisheries (Kolding et al., 2003; Mosepele, 2014), but it is also sensitive to the traditional fishing patterns and cultural value of floodplain fisheries (Mosepele, 2008).
Diversified fishing techniques, as traditionally practiced in the Okavango Delta and many other African inland fisheries (Kolding and van Zwieten, 2014), ensure that most species across various sizes and habitats-in the fish community are exploited. It also allows impoverished households (especially those headed by women), to have access to high quality protein and nutrients, which again ensures that young children from these fishing households have a relatively good nutritional status (Nnyepi et al., 2007; Longley et al., 2014; Kolding et al., 2019). BH was intended to reduce adverse ecological impacts of fishing while also supporting sustainable fisheries (Garcia et al., 2012). Fisheries management should also preserve cultural and heritage practices of fishing communities when these are not proven destructive, because, “culture is a fundamental human right” (Junk, 2002). Therefore, we advocate for balanced fishing that allows the utilisation of diverse fishing gears and methods to exploit the delta’s fish community.
The seasonal flood pulse in the Okavango Delta, driving the dry and wet floodplain phases, is the main contributor towards enhanced ecosystem production in an otherwise oligotrophic and semi-arid environment. Seasonal flooding not only changes the physical landscape of the delta, by re-connecting isolated lagoons and creating a multitude of diverse micro-habitats, it also enhances nutrient dynamics in both the terrestrial and aquatic system. These alternating micro-habitats ensure continuous succession in plant communities and enhanced plant biomass production (much of which is grazed by large herbivores), thereby contributing to nutrient recycling in the system. Much of this shifting terrestrial and aquatic based food web is eventually transformed into fish biomass.
Fish production is dynamic and fluctuating both seasonally and interannually and comprises many species of various sizes, trophic levels, and life histories, which can only be exploited by deploying a wide range of seasonally adapted fishing methods. Single-species management, based primarily on regulating selectivity towards larger species will distort the fish community structure, will lower the overall yields, and does not comply with an ecosystem approach towards maintaining the structure and function of the natural community composition. It also prevents marginalised groups from using traditional fishing methods for essential and nutritious subsistence household consumption. For these communities, the fish resource is a key source of household food and nutrition security. Leveraging these resources for local communities will contribute significantly to socio-economic development of these communities. Management interventions in floodplain fisheries should be adaptive, practical, realistic, and implementable, which in practice means acceptable to the stakeholders. Most developing countries have limited resources (particularly financial and human resources), and these should be spent on achievable, agreeable, and practical activities. Informed management also necessitates continuous long-term monitoring of exploited fisheries to follow changes and to gradually improve our understanding of fishing patterns and their impact on the fish communities. This involves the collection of fisheries related data across a broad spectrum of activities (e.g. fish consumption, employment creation, various kinds of biological data on species exploited, gear use and efficiencies, etc.) and associated factors/variables (e.g. environmental factors, various land-use activities, etc.). Once these have been documented and understood, they can be integrated into a flexible management system, which will allow for more adaptive management of these resources. Such integration is currently lacking in the Okavango Delta in line with many other floodplain fisheries.
This review has shown that periodic drying and flooding episodes in the delta are necessary for primary production. Therefore, any management efforts implemented in this system should ensure that these natural cycles are maintained. This is consistent with Junk et al.’s (1989) flood pulse concept which highlights the importance of the seasonal flood pulse in maintaining ecosystem productivity in floodplain systems. Mosepele (2009), Mosepele (2019) also highlighted the key role of seasonality in maintaining productivity of the delta’s fish resources.
KM: Conceptualization of the paper and did all the major work on the paper. JK: Assisted with the conceptualization and drafting of the paper TB: Assisted with the drafting of the paper. BM and MM: Assisted with the discussion section of the paper, particularly on fisheries management in the Okavango Delta. All the authors also reviewed the final iterations of this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agostinho, A. A., Gomes, L. C., and Zalewski, M. (2001). The importance of floodplains for the dynamics of fish communities of the upper river Parana. Ecohydrol. Hydrobiology 1 (1-2), 209–217.
Allan, J. D., Abell, R., Hogan, Z., Revenga, C., Taylor, B. W., Welcomme, R. L., et al. (2005). Overfishing of inland waters. Bioscience 55 (12), 1041–1051. doi:10.1641/0006-3568(2005)055[1041:ooiw]2.0.co;2
Allanson, B. R., Hart, R. C., O’Keeffe, J. H., and Robarts, R. D. (1990). Inland waters of southern Africa; an ecological perspective. Netherlands: Kluiwer Academic Publishers.
Andersson, L. G., Thomas, H., Denis, K. D., Ringrose, S., Savenije, H. T., Martin, W. J., et al. (2003). Water flow dynamics in the Okavango River basin and delta––a prerequisite for the ecosystems of the delta. Phys. Chem. Earth 28, 1165–1172. doi:10.1016/j.pce.2003.09.002
Arntzen, J. (2005). Livelihoods and biodiversity in the Okavango delta, Botswana. Gaborone, Botswana: Centre for Applied Research.
Ashton, P., and Neal, M. (2003). “An overview of key strategic issues in the Okavango basin,” in Transboundary rivers, sovereignty and development: Hydropolitical drivers in the Okavango River basin. Editors A. R. Turton, P. J. Ashton, and T. E. Cloete (Geneva: Green Cross International), 31–63.
Bartlam-Brooks, H. L. A., Bonyongo, M. C., and Harris, S. (2011). Will reconnecting ecosystems allow long-distance mammal migrations to resume? A case study of a zebra Equus burchelli migration in Botswana. Oryx 45 (2), 210–216. Fauna and Flora International. doi:10.1017/S0030605310000414
Bayley, P. B. (1988). Factors affecting growth rates of young tropical floodplain fishes: Seasonality and density-dependence. Environ. Biol. Fishes 21 (2), 127–142. doi:10.1007/bf00004848
Bayley, P. B. (1991). The flood pulse advantage and the restoration of river-floodplain systems. Regul. Rivers Res. Mgmt. 6, 75–86. doi:10.1002/rrr.3450060203
Béné, C., Barange, M., Subasinghe, R., Pinstrup-Andersen, P., Merino, G., Hemre, G., et al. (2015). Feeding 9 billion by 2050 – putting fish back on the menu. Food Secur. 7, 261–274. doi:10.1007/s12571-015-0427-z
Bene, C., Hersoug, B., and Allison, E. H. (2010). Not by rent alone: Analysing the pro-poor functions of small-scale fisheries in developing countries. Dev. Policy Rev. 28 (3), 325–358. doi:10.1111/j.1467-7679.2010.00486.x
Beverton, R. J. H., and Holt, S. J. (1957). On the dynamics of exploited fish populations. Fish. Investig. 19, 1–533.
Black, F. J., Bokhutlo, T., Somoxa, A., Maethamako, M., Modisaemang, O., Kemosedile, T., et al. (2011). The tropical African mercury anomaly: Lower than expected mercury concentrations in fish and human hair. Sci. Total Environ. 409 (10), 1967–1975. doi:10.1016/j.scitotenv.2010.11.027
Bokhutlo, T., Weyl, O., Mosepele, K., and Wilson, G. (2015). Age and growth of sharp-tooth catfish, Clarias gariepinus (burchell 1822) (clariidae), in the lower Okavango delta, Botswana. Mar. Freshw. Res. 66, 420–429. doi:10.1071/mf13322
Bokhutlo, T., Weyl, O., Mosepele, K., and Wilson, G. (2016). Influence of the annual flood-pulse on catch per unit effort, condition and reproduction of Clarias gariepinus (Burchell 1882) in the upper Okavango Delta, Botswana. Afr. J. Aquatic Sci. 41 (2), 235–239. doi:10.2989/16085914.2016.1138279
Bokhuto, T., Kootsositse, M. V., and Mosepele, K. (2007). Okavango Delta fishery frame survey. Gaborone, Botswana: Department of Wildlife and National Parks.
Boko, M., Niang, I., Nyong, A., Vogel, C., Githeko, A., Medany, M., et al. (2007). in Africa climate change 2007: Impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Editors M. L. Parry, O. F. Canziani, J. P. Palutikof, P. J. van der Linden, and C. E. Hanson (Cambridge UK: Cambridge University Press), 433–467.
Booth, A. J., and Merron, G. S. (1996). The age and growth of the greenhead tilapia Oreochromis macrochir (pisces: Cichlidae) from the Okavango delta, Botswana. Hydrobiologia 321 (2), 29–34. doi:10.1007/bf00018674
Botswana Government (2008). Fish protection regulations. Botswana: Botswana Government Printing and Publishing Services Gaborone.
Bunn, S. E., Thoms, M. C., Hamilton, S. K., and Capon, S. J. (2006). Flow variability in dry land rivers: Boom, bust and the bits in between. River Res. Appl. 22, 179–186. doi:10.1002/rra.904
Cassidy, L., Wilk, J., Kgathi, D. L., Bendsen, H., Ngwenya, B. N., and Mosepele, K. (2011). “Indigenous knowledge, livelihoods and government policy,” in Rural livelihoods, risk and political economy of access to natural resources in the Okavango delta, Botswana”. Editors D. L. Kgathi, B. N. Ngwenya, and M. B. K. Darkoh (USA: Nova Science Publishers), 75–98.
Cerdeira, R. G. P., and Ruffino, M. L. (2000). Fish catches among riverside communities around Lago Grande de Monte Alegre, Lower Amazon. Fisheries Management and Ecology 7, 355–374. doi:10.1046/j.1365-2400.2000.007004355.x
Clark, B. M. (2006). Climate change: A looming challenge for fisheries management in southern Africa. Mar. Policy 30, 84–95. doi:10.1016/j.marpol.2005.06.006
Cronberg, G., Gieske, A., Martins, E., Nengu, J., and Stenstrom, I-M. (1995). Hydrobiological studies of the Okavango delta and the kwando/linyanti/chobe river, Botswana. I. Surface water quality analysis. Botsw. Notes Rec. 27, 151–226.
Daily Maverick (2017). Botswana: Government fishing ban cuts lifeline to impoverished villagers. Available at: https://www.dailymaverick.co.za/article/2017-10-06-botswana-government-fishing-ban-cuts-lifeline-to-impoverished-villagers/(Accessed May 6, 2022).
de Graff, G. (2003). The flood pulse and growth of floodplain fish in Bangladesh. Fish. Manag. Ecol. 10, 241–247. doi:10.1046/j.1365-2400.2003.00341.x
de Oliveira, A. G., Lopes, T. M., Angulo-Valencia, M. A., Dias, R. M., Suzuki, H. I., Costa, I. C. B., et al. (2020). Relationship of freshwater fish recruitment with distinct reproductive strategies and flood attributes: A long-term view in the upper paraná river floodplain. Front. Environ. Sci. 8, 577181. doi:10.3389/fenvs.2020.577181
Dudley, R. G. (1974). Growth of Tilapia of the Kafue floodplain, Zambia: Predicted effects, of the Kafue gorge dam. Trans. Am. Fish. Soc. 2, 281–291. doi:10.1577/1548-8659(1974)103<281:gototk>2.0.co;2
FAO (2003). Information on fisheries management in the Republic of Botswana. Available at: https://www.fao.org/fi/oldsite/FCP/en/bwa/BODY.HTM (Accessed February 28, 2022).
FAO (2020). “The state of world fisheries and aquaculture 2020,” in Sustainability in action (Rome: FAO). doi:10.4060/ca9229en
Fox, P. J. (1976). “Preliminary observations on fish communities of the Okavango Delta,” in Proceedings of the symposium on the Okavango Delta and its future utilization. (Gaborone, Botswana: National Museum), 125–130.
Fenberg, P. B., and Roy, K. (2008). Ecological and evolutionary consequences of size-selective harvesting: How much do we know? Mol. Ecol. 17, 209–220. doi:10.1111/j.1365-294x.2007.03522.x
Froese, R., and Binohlan, C. (2000). Empirical relationships to estimate asymptotic length, length at first maturity, and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 56, 758–773. doi:10.1111/j.1095-8649.2000.tb00870.x
Froese, R., Walters, C., Pauly, D., Winker, H., Weyl, O. L. F., Demirel, N., et al. (2015). A critique of the balanced harvesting approach to fishing. ICES J. Mar. Sci. 73, 1640–1650. doi:10.1093/icesjms/fsv122
Garcia, S. M., Kolding, J., Rice, J., Rochet, M.-J., Zhou, S., Arimoto, T., et al. (2012). Reconsidering the consequences of selective fisheries. Science 335 (6072), 1045–1047. doi:10.1126/science.1214594
Garstang, M., Ellery, W. N., McCarthy, T. S., Scholes, M. C., Swap, R. J., and Tyson, P. O . (1998). The contribution of aerosol and water borne nutrients to the functioning of the Okavango Delta ecosystem, Botswana. S. Afr. J. Sci. 94 (5), 223–229.
Gilmore, K. S. (1976). Development potential and constraints of a fishing industry in the Okavango Delta,” in Proceedings of the symposium on the Okavango Delta and its future utilization. (Gaborone, Botswana: National Museum), 223–229.
Godinho, A. L., Lamas, I. R., and Godinho, H. P. (2010). Reproductive ecology of Brazilian freshwater fishes. Environ. Biol. Fishes 87, 143–162. doi:10.1007/s10641-009-9574-4
Gownaris, N. J., Rountos, K. J., Kaufman, L., Kolding, J., Lwiza, K. M. M., and Pikitch, E. K. (2018). Water level fluctuations and the ecosystem functioning of lakes. J. Gt. Lakes. Res. 44, 1154–1163. doi:10.1016/j.jglr.2018.08.005
Hixon, M. A., Johnson, D. W., and Sogard, S. M. (2014). BOFFFFs: On the importance of conserving old-growth age structure in fishery populations. ICES J. Mar. Sci. 71 (8), 2171–2185. doi:10.1093/icesjms/fst200
HLPE (2014). Food losses and waste in the context of sustainable food systems in A report by the high level panel of experts on food security and nutrition of the committee on world food security (Rome: FAO).
Høberg, P., Lindholm, M., Ramberg, L., and Hessen, D. O. (2002). Aquatic food web dynamics on a floodplain in the Okavango Delta, Botswana. Hydrobiologia 470, 23–30. doi:10.1023/a:1015693520169
Huston, M. (1979). A general hypothesis of species diversity. Am. Nat. 113 (1), 81–101. doi:10.1086/283366
Junk, W. J., Bayley, P. B., and Sparks, R. E. (1989). “The flood pulse concept in river-floodplain systems, p 110-127,” in Proceedings of the international large river symposium. Editor D. P. Dodge (Honey Harbour, Ontario, Canada: Canadian Journal of Fisheries and Aquatic Sciences), 106. Canadian Special Publications in Fisheries and Aquatic Sciences.
Junk, W. J., Brown, M., Campbell, I. C., Finlayson, M., Gopal, B., Ramberg, L., et al. (2006). The comparative biodiversity of seven globally important wetlands: A synthesis. Aquat. Sci. 68, 400–414. doi:10.1007/s00027-006-0856-z
Jul-Larsen, E., Kolding, J., Overå, R., Raakjær Nielsen, J., and Zwieten, P. A. M. van (Editors) (2006). Management, co-management or no management? Major dilemmas in southern African freshwater fisheries. 2. Case studies. Rome, FAO: FAO Fisheries Technical Paper.
Junk, W. (2002). Long-term environmental trends and the future of tropical wetlands. Environ. Conserv. 29 (4), 414–435. doi:10.1017/s0376892902000310
Kgathi, D. L., Matlhola, D. M., Mosepele, K., Kolawole, O. D., and Motho, M., (2018). Emerging regional fish trade and food security in the lower Okavango delta, Botswana: Results of a pilot survey, p 219–245. In D. L. Kgathi, E. N. Mosimanyana, J. E. Mbaiwa, and W. R. L. Masamba (Eds) Ecosystem services and human well-being at lake ngami, Botswana: Implications for sustainability (New York: Nova Science Publishers, Inc).
King, A. J., Humphries, P., and Lake, P. S. (2003). Fish recruitment on floodplains: the roles of patterns of flooding and life history characteristics Canadian J. Fisheries Aquatic Sci. 60 (7), 773–786.
Kolding, J., and van Zwieten, P. A. M. (2011). The tragedy of our legacy: How do global management discourses affect small scale fisheries in the south? Forum Dev. Stud. 38 (3), 267–297. doi:10.1080/08039410.2011.577798
Kolding, J., and van Zwieten, P. A. M. (2006). Improving productivity in tropical lakes and reservoirs. Challenge program on water and food - aquatic ecosystems and fisheries review series 1. Cairo: WorldFish Center. Theme 3 of CPWF.
Kolding, J., Bundy, A., van Zwieten, P. A. M., and Plank, M. J. (2015a). Fisheries, the inverted food pyramid. ICES J. Mar. Sci. 73, 1697–1713. doi:10.1093/icesjms/fsv225
Kolding, J., Haug, L., and Stefansson, S. (2008). Effect of oxygen on growth and reproduction in Nile tilapia (Oreochromis niloticus). Can. J. Fish. Aquat. Sci. 65 (7), 1413–1424. doi:10.1139/F08-059
Kolding, J., Jacobsen, N. S., Andersen, K. H., and van Zwieten, P. A. M. (2015b). Maximizing fisheries yields while maintaining community structure. Can. J. Fish. Aquat. Sci. 73 (4), 644–655. doi:10.1139/cjfas-2015-0098
Kolding, J., Musando, B., and Songore, N. (2003). “Inshore fisheries and fish population changes in Lake Kariba,” in Management, Co-management, or No management? Major dilemmas in southern african freshwater fisheries, 2: Case studies. Editors E. Jul-Larsen, J. Kolding, R. Overa, J. R. Nielsen, and P. A. M. Zwieten (Rome: FAO), 67–99. FAO Fisheries Technical Paper 426/2.
Kolding, J. (1993). Population dynamics and life history styles of nile tilapia (Oreochromis niloticus) in fergusons gulf, lake turkana, Kenya. Environ. Biol. Fishes 37, 25–46. doi:10.1007/bf00000710
Kolding, J., van Zwieten, P. A. M., and Mosepele, K. (2016). “Where there is water there is fish” – small-scale inland fisheries in Africa: Dynamics and importance,” in A history of water, series 3, volume 3. Water and food: From hunter-gatherers to global production in Africa. Editors T. Tvedt, and T. Oestigaard (London: I.B. Tauris), 440–460.
Kolding, J., and van Zwieten, P. A. M. (2012). Relative lake level fluctuations and their influence on productivity and resilience in tropical lakes and reservoirs. Fish. Res. 115-116, 99–109. doi:10.1016/j.fishres.2011.11.008
Kolding, J., and van Zwieten, P. A. M. (2014). Sustainable fishing of inland waters. J. Limnol. 73 (1), 132–148. doi:10.4081/jlimnol.2014.818
Kolding, J., van Zwieten, P. A. M., Marttin, F., Funge-Smith, S., and Poulain, F. (2019). Freshwater small pelagic fish and fisheries in major African lakes and reservoirs in relation to food security and nutrition. FAO Fisheries and Aquaculture Technical Paper No. 642 Rome, FAO
Krah, M., McCarthy, T. S., Mapila, P. H., Wolski, P., Annegarn, H., and Sethebe, K. (2006). Nutrient budget in the seasonal wetland of the Okavango Delta, Botswana. Wetl. Ecol. Manag. 14, 253–267. doi:10.1007/s11273-005-1115-0
Laë, R. (1995). Climatic and anthropogenic effects on fish diversity and fish yields in the Central Delta of the Niger River. Aquat. Living Resour. 8 (1), 43–58. doi:10.1051/alr:1995004
Law, R. (2000). Fishing, selection, and phenotypic evolution. ICES J. Mar. Sci. 57, 659–668. doi:10.1006/jmsc.2000.0731
Law, R., Plank, M. J., and Kolding, J. (2012). On balanced exploitation of marine ecosystems: Results from dynamic size spectra. ICES J. Mar. Sci. 69, 602–614. doi:10.1093/icesjms/fss031
Lindholm, M., Hessen, D. O., Mosepele, K., and Wolski, P. (2007). Food webs and energy fluxes on a seasonal floodplain: The influence of flood size. Wetlands 27 (4), 775–784. doi:10.1672/0277-5212(2007)27[775:fwaefo]2.0.co;2
Linhoss, A. C., Muñoz-Carpena, R., Allen, M., Kiker, G., and Mosepele, K. (2012). A flood pulse driven fish population model for the Okavango Delta, Botswana. Ecol. Model. 228, 27–38. doi:10.1016/j.ecolmodel.2011.12.022
Longley, C., Thilsted, S. H., Beveridge, M., Cole, S., Nyirenda, D. B., Heck, S., et al. (2014). The Role of fish in the first 1,000 days in Zambia. Brighton, UK: Institute of Development Studies. IDS Special Collection.
Lowe-McConnell, R. H. (1987). Ecological studies in tropical fish communities. Cambridge: Cambridge University Press, 400.
Lowe-McConnell, R. H. (1982). “Tilapias in fish communities,” in The biology and culture of tilapias, ICLARM conference proceedings 7. Editors R. S. V. Pullin, and R. H. Lowe-McConnell (Manila: International Center for Living Aquatic Resources Management), 83–113.
Mackay, A. W., Davidson, T., Wolski, P., Mazebedi, R., Masamba, W. R. L., Huntsman-Mapila, P., et al. (2011). Spatial and seasonal variability in surface water chemistry in the Okavango delta, Botswana: A multivariate approach. Wetlands 31 (5), 815–829. doi:10.1007/s13157-011-0196-1
Magadza, C. H. D. (2011). Indications of the effects of climate change on the pelagic fishery of Lake Kariba, Zambia–Zimbabwe. Lakes and Reservoirs 16 (1), 15–22. doi:10.1111/j.1440-1770.2011.00462.x
Martin, S. M., Lorenzen, K., Arthur, R. I., Kaisone, P., and Souvannalangsy, K. (2011). Impacts of fishing by dewatering on fish assemblages of tropical floodplain wetlands: A matter of frequency and context. Biol. Conserv. 144 (1), 633–640. doi:10.1016/j.biocon.2010.11.005
Masamba, W. R., and Mazvimavi, D. (2008). Impact on water quality of land uses along Thamalakane-Boteti River: An outlet of the Okavango Delta. Phys. Chem. Earth Parts A/B/C 33 (8-13), 687–694. doi:10.1016/j.pce.2008.06.035
Mazvimavi, D., and Wolski, P. (2006). Long-term variations of annual flows of the Okavango and zambezi rivers. Phys. Chem. Earth 31, 944–951. doi:10.1016/j.pce.2006.08.016
McCarthy, J. M., Gumbricht, T., McCarthy, T., Frost, P., Wessels, K., and Seidel, F. (2003). Flooding patterns of the Okavango wetland in Botswana between 1972 and 2000. AMBIO A J. Hum. Environ. 32 (7), 453–457. doi:10.1579/0044-7447-32.7.453
Mendelsohn, J. M., van der Post, C., Ramberg, L., Murray-Hudson, M., Wolski, P., and Mosepele, K. (2010). Okavango delta: Floods of life. Windhoek, Namibia): RAISON.
Merron, G. S., and Bruton, M. N. (1988). The ecology and management of the fishes of the Okavango delta, Botswana, with special reference to the role of the seasonal floods. J. L. B. Smith Institute of Ichthyology, Grahamstown, South Africa. Investigational Report No. 29. 291
Merron, G. S., Holden, K. K., and Bruton, M. N. (1990). The reproductive biology and early development of the African pike, Hepsetus odoe, in the Okavango Delta, Botswana. Environ. Biol. Fishes 28, 215–235. doi:10.1007/bf00751036
Merron, G. S. (1993). A synopsis of presentations and discussions on the fish and fishery in the Okavango Delta, Botswana. Botswana Notes Rec. 25, 133–140.
Milzow, C., Kgotlhang, L., Gottwein, P. B., Meier, P., and Kinzelbach, W. (2009). Regional review: The hydrology of the Okavango delta, Botswana—processes, data and modelling. Hydrogeol. J. 17, 1297–1328. doi:10.1007/s10040-009-0436-0
Milzow, C., Burg, V., and Kinzelbach, W. (2010). Estimating future ecoregion distributions within the Okavango Delta Wetlands based on hydrological simulations and future climate and development scenarios. J. Hydrol. 381, 89–100. doi:10.1016/j.jhydrol.2009.11.028
Ministry of Agriculture (1997). 1996/97 Fisheries annual report. Gaborone: Technical Report of the Ministry of AgricultureBotswana.
Misund, O. A., Kolding, J., and Fréon, P. (2002). “Fish capture devices in industrial and artisanal fisheries and their influence on management,” in Handbook of fish biology and fisheries. Editors P. J. B. Hart, and J. D. Reynolds (London: Blackwell Science), Vol. II, 13–36.
Mladenov, N., Mcknight, D. M., Wolski, P., and Murray-Hudson, M. (2007). Simulation of DOM fluxes in a seasonal floodplain of the Okavango Delta, Botswana. Ecol. Model. 205, 181–195. doi:10.1016/j.ecolmodel.2007.02.015
Mladenov, N., McKnight, D. M., Wolski, P., and Ramberg, L. (2005). Effects of annual flooding on dissolved organic carbon dynamics within a pristine wetland, the Okavango Delta, Botswana. Wetlands 25 (3), 622–638. doi:10.1672/0277-5212(2005)025[0622:eoafod]2.0.co;2
Mmopelwa, G., Mosepele, K., Mosepele, B., Moleele, N., and Ngwenya, B. (2009). Environmental variability and the fishery dynamics of the Okavango delta, Botswana: The case of subsistence fishing. Afr. J. Ecol. 47 (1), 119–127. doi:10.1111/j.1365-2028.2008.01058.x
Mmopelwa, G., Raletsatsi, S., and Mosepele, K. (2005). Cost Benefit Analysis of Commercial Fishing in Shakawe, Ngamiland. Botswana Notes Rec. 37 (1), 11–21.
Molden, D., and de Fraiture, C. (2004). Investing in water for food, ecosystems and livelihoods. Blue Paper Stockholm Comprehensive Assessment of Water Management in Agriculture. (Colombo, Sri Lanka: CGIAR).
Montcho, S. A., Chikou, A., Lalèyè, P. A., and Linsenmair, K. E. (2011). Population structure and reproductive biology of Schilbe intermedius (teleostei: Schilbeidae) in the pendjari river, Benin. Afr. J. Aquatic Sci. 36 (2), 139–145. doi:10.2989/16085914.2011.589111
Mosepele, B., Mosepele, K., Mogotsi, S., and Thamage, D. (2014). “Fisheries co-management in the Okavango Delta’s panhandle: The Okavango fisheries management committee (OFMC) case study,” in Governance for justice and environmental sustainability: Lessons across natural resource sectors in sub-saharan Africa. Editors M. Sowman, and R. Wynberg (UK: Routledge Publishers), 180–199.
Mosepele, K., Mmopelwa, T. G., and Mosepele, B. (2003). “Characterization and monitoring of the Okavango delta artisanal fishery,” in Environmental monitoring of tropical and subtropical wetlands. Editors T. Bernard, K. Mosepele, and L. Ramberg (Gainesville, FLA: University of Botswana, Maun and University of Florida), 391–413.
Mosepele, K., and Mosepele, B. (2005). Spatial and temporal variability in fishery and fish community structure in the Okavango delta, Botswana: Implications towards fisheries management. Botsw. Notes Rec. 37, 280–291. doi:10.2307/40980420
Mosepele, K., and Kolawole, O. (2017). Fisheries governance, management, and marginalization in developing countries: Insights from Botswana. Cogent Food and Agriculture 3 (1), 1338637. doi:10.1080/23311932.2017.1338637
Mosepele, K., and Ngwenya, B. (2010). Socio-economic survey of commercial fishing in the Okavango Delta, Botswana. Gaborone, Botswana): Bay Publishing.
Mosepele, K. (2014). Classical fisheries theory and inland (floodplain) fisheries management; is there need for a paradigm shift? Lessons from the Okavango delta, Botswana. Fish. Aquac. J. 5, 101. doi:10.4172/2150-3508.1000101
Mosepele, K. (2001). Description of the Okavango delta fishery. Technical report to the fisheries section. Gaborone, Botswana: Ministry of Agriculture.
Mosepele, K. (2019). Dynamics of the seasonal floodplain fishery of the Okavango delta, Botswana. Norway: University of Bergen. PhD Thesis.
Mosepele, K. (2008). “Flood pulse in a subtropical floodplain fishery and the consequences for steady state management,” in Water Resource Management; Science and technology innovation for sustainable development. Editor O. Totolo (Canada: Acta Press), 56–62.
Mosepele, K., Kolding, J., and Bokhutlo, T. (2017). Fish community dynamics in an inland floodplain system of the Okavango Delta, Botswana. Ecohydrol. Hydrobiology 17, 89–102. doi:10.1016/j.ecohyd.2017.01.005
Mosepele, K., and Kolding, J. (2003). “Fish Stock Assessment in the Okavango delta, Botswana – preliminary results from a length-based analysis, pp 363–390,” in Environmental monitoring of tropical and subtropical wetlands. Okavango report series No. 1. Editors T. Bernard, K. Mosepele, and L. Ramberg (Gainesville, FLA: University of Botswana, Maun and University of Florida).
Mosepele, K. (2000). Length based fish stock assessment of the main exploited fish stocks of the Okavango delta, Botswana. Bergen, Norway: MPhil Thesis with the University of Bergen.
Mosepele, K., Makati, K., Mosie, I., and Murray-Hudson, M., (2018). Fish stock assessment of a shallow tropical sump lake; Lake Ngami, Botswana, p 321 – 353. In D. L. Kgathi, E. N. Mosimanyana, J. E. Mbaiwa, and W. R. L. Masamba (Eds) Ecosystem services and human well-being at lake ngami, Botswana: Implications for sustainability (New York: Nova Science Publishers, Inc).
Mosepele, K., Mmopelwa, G., Mosepele, B., and Kgathi, D. L. (2007). “Indigenous knowledge and fish utilization in the Okavango Delta, Botswana: Implications for food security,” in Managing knowledge, technology and development in the era of information revolution. Editor A. Ahmed (Australia: Griffith University), 292–302.
Mosepele, K., Mosepele, B., Bokhutlo, T., and Amutenya, K. (2011). Spatial variability in fish species assemblage and community structure in four subtropical lagoons of the Okavango Delta, Botswana. Phys. Chem. Earth Parts A/B/C 36 (14-15), 910–917. doi:10.1016/j.pce.2011.07.060
Mosepele, K., Mosepele, B., Wolski, P., and Kolding, J. (2012). Dynamics of the feeding ecology of selected fish species from the Okavango River delta, Botswana. Acta Ichthyol. Piscat. 42 (4), 271–289. doi:10.3750/aip2012.42.4.01
Mosepele, K., Moyle, P. B., Merron, G. S., Purkey, D., and Mosepele, B. (2009). Fish, floods, and ecosystem engineers: Aquatic conservation in the Okavango delta, Botswana. Bioscience 59 (1), 53–64. doi:10.1525/bio.2009.59.1.9
Mosepele, K., Ngwenya, B. N., and Bernard, T. (2006). “Artisanal fishing and food security in the Okavango Delta, Botswana,” in Global and local resources in achieving sustainable development. Editor A. Ahmed (Geneva, Switzerland: Interdescience Publishers), 159–168.
Mosepele, K. (2009). Okavango River basin technical diagnostic analysis: Environmental flow module specialist report country: Botswana: Discipline: Fish. Biophysical series. Maun, Botswana: EPSMO-OKACOM.
Mosepele, K. (2003). Trends in fisheries development and fish utilization in the Okavango delta. Maun, Botswana: Okavango Research Institute, University of Botswana.
Ngwenya, B. N., and Mosepele, K. (2008). Socio-economic survey of subsistence fishing in the Okavango delta, Botswana. Gaborone: Bay Publishing.
Nnyepi, M., Ngwenya, B., and Mosepele, K. (2007). “Food (in) security and child nutrition in Ngamiland,” in Managing knowledge, technology and development in the era of information revolution. Editor A. Ahmed (Australia: Griffith University), 281–291.
NORFICO (1986). Botswana Fisheries: Fishing Gear and technology – a report from a consultancy in gear technology in Botswana in the period August – October 1986. Gaborone: Norwegian Agency for International Development. Report to the Republic of Botswana, Ministry of Agriculture.
Norplan, (1985). Botswana Fisheries: Status and development strategies. Gaborone, Botswana: Norwegian Agency for International Development.
Petry, A. C., Agostinho, A. A., and Gomes, L. C. (2003). Fish assemblages of tropical floodplain lagoons: Exploring the role of connectivity in a dry year. Neotrop. Ichthyol. 1 (2), 111–119. doi:10.1590/s1679-62252003000200005
Porter, J. W., and Muzila, I. L. (1989). Aspects of swamp hydrology in the Okavango. Botsw. Notes Rec. 21, 73–91.
Power, M. (1984). Depth distributions of armoured catfish Predator-induced resource avoidance? Ecology 65 (2), 523–528. doi:10.2307/1941414
Ramberg, L., Hancock, P., Lindholm, M., Meyer, T., Ringrose, S., Sliva, J., et al. (2006b). Species diversity of the Okavango delta, Botswana. Aquat. Sci. 68, 310–337. doi:10.1007/s00027-006-0857-y
Ramberg, L., and Wolski, P. (2008). Growing islands and sinking solutes: Processes maintaining the endorheic Okavango delta as a freshwater system. Plant Ecol. 196 (2), 215–231. doi:10.1007/s11258-007-9346-1
Ramberg, L., Wolski, P., and Krah, M. (2006a). Water balance and infiltration in a seasonal floodplain in the Okavango Delta, Botswana. Wetlands 26 (3), 677–690. doi:10.1672/0277-5212(2006)26[677:wbaiia]2.0.co;2
Rochet, M.-J. (1998). Short-term effects of fishing on life history traits of fishes. ICES J. Mar. Sci. 55 (3), 371–391. doi:10.1006/jmsc.1997.0324
SADC (2016). “Focus on the Botswana fisheries and aquaculture,” in SADC fisheries fact sheet (Gaborone, Botswana: SADC Secretariat), 1.4
Schindler, D. E., Kitchell, J. F., and Ogutu-Ohwayo, R. (1998). Ecological consequences of alternative gill net fisheries for nile perch in lake victoria. Conserv. Biol. 12 (1), 56–64. doi:10.1111/j.1523-1739.1998.96160.x
Schongart, J., and Junk, W. J. (2007). Forecasting the flood-pulse in central amazonia by ENSO-indices. J. Hydrology 335, 124–132. doi:10.1016/j.jhydrol.2006.11.005
Scudder, T., Manley, R. E., Coley, R. W., Davis, R. K., Green, J., Howard, G. W., et al. (1993). The IUCN review of the Southern Okavango Integrated Water Development Project. Gland, Switzerland: IUCN.
Siziba, N., Chimbari, M. J., Masundire, H., and Mosepele, K. (2011b). Spatial and temporal variations of microinvertebrates across temporary floodplains of the lower Okavango Delta, Botswana. Phys. Chem. Earth Parts A/B/C 36 (14-15), 939–948. doi:10.1016/j.pce.2011.07.055
Siziba, N., Chimbari, M. J., Masundire, H., Mosepele, K., and Ramberg, L. (2013). Variation in assemblages of small fishes and micro-crustaceans after inundation of rarely flooded wetlands of the lower Okavango delta, Botswana. Environ. Manag. 52, 1386–1399. doi:10.1007/s00267-013-0183-9
Siziba, N., Chimbari, M. J., Masundire, H., and Mosepele, K. (2012). Spatial variations of microinvertebrates across different microhabitats of temporary floodplains of lower Okavango Delta, Botswana. Afr. J. Ecol. 50 (1), 43–52. doi:10.1111/j.1365-2028.2011.01289.x
Siziba, N., Chimbari, M. J., Mosepele, K., and Masundire, H. (2011a). Spatial and temporal variations in densities of small fishes across different temporary floodplain types of the lower Okavango Delta, Botswana. Afr. J. Aquatic Sci. 36 (3), 309–320. doi:10.2989/16085914.2011.636912
Staples, D., Satia, B., and Gardiner, P. R. (2004). A research agenda for small-scale fisheries. Bangkok, Thailand: FAO Regional Office for Asia and the Pacific. RAP PUBLICATION No. 2004/21 and FIPL/C 10009 (En).
Steudel, T., Göhmann, H., Mosimanyana, E., Quintino, M., Flügel, W-A., and Helmschrot, J. (2013). “Okavango basi – hydrology,” in Environmental assessments in the Okavango region – biodiversity and ecology. Editors J. Oldeland, C. Erb, M. Finckh, and N. Jürgens (Hamburg, Germany: University of Hamburg), 5, 19–22. doi:10.7809/b-e.00238
Tadross, M., Jack, C., and Hewitson, B. (2005). On RCM based projections of change in southern African summer climate. Geophys. Res. Lett. 32, L23713. doi:10.1029/2005gl024460
Tilley, A., Mills, D., Short, R., and Kolding, J. (2020). Valuing small fish from mosquito nets: A comment on jones & unsworth. Ambio 49, 1257–1267. doi:10.1007/s13280-019-01280-0
Tockner, K., Malard, F., and Ward, J. V. (2000). An extension of the flood pulse concept. Hydrol. Process. 14, 2861–2883. doi:10.1002/1099-1085(200011/12)14:16/17<2861::aid-hyp124>3.0.co;2-f
Trippel, E. A. (1995). Age at maturity as a stress indicator in fisheries. BioScience 45 (11), 759–771. doi:10.2307/1312628
Tsheboeng, G., and Murray-Hudson, M. (2013). “Spatial variation of population size structure of selected riparian tree species in the Okavango Delta, Botswana,” in Environmental assessments in the Okavango region. Editors J. Oldeland, C. Erb, M. Finckh, and N. Jürgens, 5, 341–350. (Hamburg, Germany: University of Hamburg). Biodiversity & Ecology. doi:10.7809/b-e.00287
Tsheboeng, G., Bonyongo, M., and Murray-Hudson, M. (2014). Flood variation and soil nutrient content in floodplain vegetation communities in the Okavango Delta. S. Afr. J. Sci. 110 (3/4), 1–5. doi:10.1590/sajs.2014/20130168
Turpie, J., Barnes, J., Arntzen, J., Nherera, B., Lange, G-M., and Buzwani, B. (2006). Economic value of the Okavango Delta, Botswana, and implications for management. Gaborone, Botswana: IUCN.
van der Waal, B. C. W. (1985). Aspects of the biology of larger fish species of lake liambezi, caprivi, southwest Africa. Madouqua 14 (2), 101–1441.
van Zwieten, P. A. M., Goudswaard, P. C., and Kapasa, C. K. (2003). “Mweru-Luapula is an open exit fishery where a highly dynamic population of fishermen makes use of a resilient resource base,” in Management, Co-management or No management? Major dilemmas in southern african freshwater fisheries, 2: Case studies. Editors E. Jul-Larsen, J. Kolding, R. Overa, J. R. Nielsen, and P. A. M. Zwieten (Rome: FAO Fisheries), 1–33. Technical Paper 426/2.
Vasquez, A. G., Alonso, J.-C., Carvajal, F., Moreau, J., Nunez, J., Renno, J.-F., et al. (2009). Life-history characteristics of the large Amazonian migratory catfish Brachyplatystoma rousseauxii in the Iquitos region, Peru. J. Fish Biol. 75, 2527–2551. doi:10.1111/j.1095-8649.2009.02444.x
Walsh, M. R., Munch, S. B., Chiba, S., and Conover, D. O. (2006). Maladaptive changes in multiple traits caused by fishing: Impediments to population recovery. Ecol. Lett. 9, 142–148. doi:10.1111/j.1461-0248.2005.00858.x
Ward, J. V., and Stanford, J. A. (1995). The serial discontinuity concept: Extending the model to floodplain rivers. Regul. Rivers Res. Mgmt. 10, 159–168. doi:10.1002/rrr.3450100211
Welcomme, R. L. (2011). An overview of global catch statistics for inland fish. ICES J. Mar. Sci. 68, 1751–1756. doi:10.1093/icesjms/fsr035
Welcomme, R. L. (1988). “Concluding remarks I: On the nature of large tropical rivers, floodplains, and future research directions. J. North Am. Benthol. Soc., 7 (4), 525–526.
Welcomme, R. L., Cowx, I. G., Coates, D., Béné, C., Funge-Smith, S., Halls, A., et al. (2010). Inland capture fisheries. Phil. Trans. R. Soc. B 365 (1554), 2881–2896. doi:10.1098/rstb.2010.0168
Welcomme, R. L., Valbo-Jorgenson, J., and Halls, A. (2014). Inland fisheries evolution and management: Case studies from four continents. Rome: FAO.
Welcomme, R. L. (2009). World prospects for floodplain fisheries. Ecohydrol. Hydrobiology 8 (2-4), 169–182. doi:10.2478/v10104-009-0013-0
Wilson, B. H. (1973). Some natural and man-made changes in the channels of the Okavango delta. Botsw. Notes Rec. 5, 132–153.
Wolski, P., and Murray-Hudson, M. (2006). Flooding dynamics in a large low-gradient alluvial fan, the Okavango Delta, Botswana, from analysis and interpretation of a 30-year hydrometric record. Hydrol. Earth Syst. Sci. 10, 127–137. doi:10.5194/hess-10-127-2006
Wolski, P., Masaka, T., Raditsebe, L., and Murray-Hudson, M. (2005). Aspects of seasonal dynamics of flooding in the Okavango delta. Botsw. Notes Rec. 37, 179–195. doi:10.2307/40980412
Zeug, S. C., and Winemiller, K. O. (2007). Ecological correlates of fish reproductive activity in floodplain rivers: A life-history-based approach. Can. J. Fish. Aquat. Sci. 64 (10), 1291–1301. doi:10.1139/f07-094
Zeug, S. C., Winemiller, K. O., and Tarim, S. (2005). Response of Brazos River oxbow fish assemblages to patterns of hydrologic connectivity and environmental variability. Trans. Am. Fish. Soc. 134 (5), 1389–1399. doi:10.1577/t04-148.1
Keywords: Okavango Delta, floodplain fisheries management, fluctuating, balanced fishing, flood pulse
Citation: Mosepele K, Kolding J, Bokhutlo T, Mosepele BQ and Molefe M (2022) The Okavango Delta: Fisheries in a fluctuating floodplain system. Front. Environ. Sci. 10:854835. doi: 10.3389/fenvs.2022.854835
Received: 14 January 2022; Accepted: 28 July 2022;
Published: 07 September 2022.
Edited by:
Ana Pia Rabuffetti, National Institute of Limnology (INALI), ArgentinaReviewed by:
Matthew McCartney, International Water Management Institute, Sri LankaCopyright © 2022 Mosepele, Kolding, Bokhutlo, Mosepele and Molefe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeppe Kolding, SmVwcGUuS29sZGluZ0B1aWIubm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.