
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 16 March 2022
Sec. Water and Wastewater Management
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.848245
This study is a novel approach toward the development of a chemical-free and sustainable textile dyeing process with minimum environmental risks. Cotton fabrics were cationized with (3-chloro-2-hydroxypropyl) trimethylammonium chloride in four concentrations and subsequently dyed with the black tea extract. Eco-friendly colorant extraction from raw black tea leaves was carried out in aqueous media avoiding the use of hazardous organic solvents. The major coloring components in the black tea extract are polyphenols like theaflavins and thearubigin. Cationized cotton fabrics were dyed in four different shade depths without employing auxiliary chemicals in the dyeing process. For comparison, un-cationized cotton was dyed with the same extract in the same shades. It was observed that un-cationized cotton samples exhibited very low color strength (K/S) values and excellent colorfastness rating. However, the cationized samples showed remarkable enhancement in their color strength with an increase in the concentration of the cationizing agent. Furthermore, colorfastness to washing, rubbing, and perspiration was excellent, but lightfastness was poor. Deep shades (K/S = 8.996) were obtained for cotton sample cationized (20 g/l) and dyed (6%) shades. Thus, the extraction of natural colorants without toxic solvents, economically viable surface modification of cotton, and chemical-free dyeing render the dyeing process cleaner, sustainable, and practicable at an industrial scale. The textile units could easily adopt this approach to regulate a pollution-free dyeing process without modifying their existing infrastructure.
The textile dyeing and finishing industry contributes more to water contamination and environmental pollution around the world (Hossain et al., 2018; Khan et al., 2018; Khan et al., 2020). The textile wastewater loaded with hazardous synthetic dyes, organic acids, inorganic salts, etc. becomes a pollution challenge threatening the living organisms and sustainability of the ecosystem (Dasgupta et al., 2015; Khan et al., 2019). In the recent past, the increasing demand for eco-safety has forced textile industries to implement cleaner production technologies, rather than the adoption of effluent treatment strategies. Generally, the effluent treatment approaches are expensive and inefficient to reduce pollutants completely (Ciardelli et al., 2001; Khatri et al., 2015). Thus, it is better to adopt cleaner production approaches to minimize the waste generation in textile dyeing and processing units. To this end, several advancements including the production of synthetic dyes with better fixing properties and improvements in dyeing processes have helped to reduce the use of auxiliary chemicals. But much progress is warranted to make the dyeing process green and sustainable with ideally zero emissions (von Sperling and Augusto de Lemos Chernicharo, 2002; Imtiazuddin et al., 2012). There has been an increasing demand to minimize the negative ecological impacts of synthetic dyes in textile dyeing and replace them with bio-based colorants (Vankar et al., 2007; Vankar and Shanker, 2008; Haji, 2017). Over the years, natural dyes have emerged as biocompatible, environment-friendly, and non-toxic substitutes, and their facile applications could validate the concept of pollution-free dyeing of textile materials (Ibrahim et al., 2010; Baaka et al., 2018).
The natural dyes are also known as mordant dyes as they do not adhere to cotton without the use of mordants (Ibrahim et al., 2010; Pisitsak et al., 2016). The mordants are generally inorganic salts of heavy metals (aluminum, iron, tin, copper, chrome, etc.) or other acidic or basic chemical agents which facilitate the fixing of natural dyes on fabric (cotton) through chemical reactions (Prabhu and Bhute, 2012). However, during the last few decades, the growing consciousness about eco-friendly textiles has compelled the textile industry to use natural dyes without employing toxic mordants in the dyeing process. To this end, a number of developments have been made during the past few years (Baaka et al., 2019; Manyim et al., 2021; Zhang et al., 2022a; Zhang et al., 2022b). The introduction of biodegradable organic salts and green chemical auxiliaries in textile processing has helped to reduce the generation of effluent wastes (Senthil Kumar and Gunasundari, 2018). Cationization is a novel approach to achieve better exhaustion rates, shade depth, and colorfastness on cotton (Wolela, 2019). Recently, ovalbumin was employed to modify the cotton surface to achieve better colorfastness and shade depth with a cochineal natural dye (Giacomini et al., 2020). In another study, chitosan was used to enhance the dyeability of cotton fibers before the application of reactive dyes (Rehman et al., 2020). In a recent study, chitosan was used for surface functionalization of cotton and wool that improved fastness properties and dyeability for reactive, acid and walnut dyes (Haji, 2020). The cotton surface was functionalized with N+(CH3)3 groups using poly[styrene-butyl acrylate-(P-vinylbenzyl trimethyl ammonium chloride)] nanospheres for dyeing with reactive dyes. The pad–steam dyeing of cationic cotton improved washing and rubbing fastness, significantly reducing steaming time and salt consumption (Fang et al., 2018). According to another report, a bifunctional cationic polymer was used for surface modification of cotton for low-temperature and salt-free dyeing with reactive dyes. The surface functionalization caused a significant improvement in leveling properties, colorfastness, and dyeability of modified cotton (Niu et al., 2020). A recent study describes the cationization of cotton with plasma-induced graft polymerization of diallyldimethylammonium chloride before salt-free dyeing with reactive dyes. The surface modification of cotton improved dye fixation, wash fastness, and colorfastness compared to conventional dyeing techniques (Meng et al., 2021).
The aqueous extracts of black tea are known for antioxidant and antimicrobial properties; thus, the dyed cotton samples exhibited antibacterial, antifungal, and antioxidant activities, eliminating the use of any additional finishing agent. Black tea mainly contains polyphenols (20–35%) in the form of catechins including epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate (Balentine et al., 1997; Liang et al., 2003; Bydoon, 2017). These polyphenolic compounds undergo enzymatic oxidation to produce theaflavins, which further get converted into thearubigin in black tea. In fact, thearubigin constitutes the largest mass of extractable matter of black tea. The polyphenols carry several negatively charged components and could not be applied directly on cotton. Therefore, natural dyes generally show low adsorption efficiency. However, the dyeability of the fabric is increased by treatment with mordants in the dyeing process. Here, we report the successful dyeing of cationized cotton with aqueous extracts of tea without the involvement of any additive chemical during the whole progression of the extraction and dyeing process. This pollution-free dyeing with simultaneous bio-functionalization of cotton represents a premier example of cleaner production with ideally zero emissions into the environment.
Black tea was purchased from Khan Tea Dealers, Jhang Bazaar (Faisalabad, Pakistan). Methanol was used for the extraction of colorants from raw black tea. The cotton fabric with 120 g/m2 was used and provided by the Textile Processing Department for the project. The fabric was cationized using (3-chloro-2-hydroxypropyl) trimethylammonium chloride (65% w/w) and NaOH (50% (w/v) in the presence of the commercial wetting agent. The cationizing agent was purchased from Tokyo Chemical Industries (TCI), Japan. A non-ionic detergent was used for washing dyed samples. Sodium perborate (1 g/l) and ECE phosphate (4 g/l) were applied in washing fastness measurements. All laboratory-grade chemicals were purchased from Sigma Aldrich (Faisalabad, Pakistan). Double-distilled water was used throughout the research.
Raw black tea (25 g) was poured into a 500-ml beaker, and 100 ml water was added to it. The beaker containing the mixture was covered with aluminum foil and placed on a stirring plate for continuous stirring at room temperature for 24 h. Then, the beaker containing the mixture was removed from the stirring plate and filtered using a Whatman filter paper. The filtrate was transferred to another beaker and allowed to stand open at room temperature for 3 days. The remaining water was evaporated using a hot plate at 40 C. After complete evaporation of water, the dried black tea extract (5.5 g) was obtained, which was used for the dyeing purpose.
The FTIR spectrum of the tea extract was found to be in agreement with that of previous studies (Figure 1). The presence of polyphenolic compounds was identified due to the characteristic peaks of C-O-C, C=C, C=O, C-H, and –OH (Hamdan and Haider, 2018; Brza et al., 2020).
(3-chloro-2-hydroxypropyl) trimethylammonium chloride in four different concentrations (5, 10, 15, and 20 g/l) was added to separate beakers containing 100 ml distilled water. Then wetting agent (10 g/l) and NaOH (20 g/l) were added to each beaker to achieve a uniform solution. Finally, the total solution was made up to 200 ml by adding distilled water and again stirred for some time. The solution was then ready for padding. Cotton was padded using this pad liquor with a pick-up of 100% using a padder (TSUJI, Japan). After padding, the fabric was batched for 18 h by covering it with a polythene film to make it airtight (Iqbal et al., 2020). After batching overnight, the fabric was properly washed to make sure that there was no chemical left inside the fabric and subsequently dried (Acharya et al., 2014).
Four dyeing solutions (1%, 2%, 4%, and 6%) were made using black tea extract and distilled water. Cationized cotton samples (5 g) were dyed using these dyeing solutions at 100 C for 60 min using High Temperature-machine with a liquor ratio of 50:1. Then, the dyed cotton samples were washed with a non-ionic detergent for 15 min at 70 C and subsequently dried at room temperature. The dyed cotton samples were investigated for color strength and colorfastness measurements. For comparison purposes, bleached un-cationized cotton samples were dyed under the same dyeing conditions.
The color strength of dyed cotton samples was measured from the reflectance (%) values recorded by using a Datacolor 650™ spectrophotometer. The wavelength of maximum absorption (λmax) used for the calculation of color strength (K/S) of treated samples was 618 nm. Kubelka–Munk’s theory was used to get the color strength values from recorded reflectance (%) of the samples (Khan et al., 2018).
where R is the reflectance, K is the absorption coefficient, and S is the scattering.
The ISO 105-A05 standard method was followed for the assessment of colorfastness to washing of dyed samples, and the rating was determined using the gray scale. The samples specimen (04 cm × 10 cm) was conditioned for 4 h and sewed with a multifiber strip at one corner. The solution of ECE phosphate (4 g/l) and sodium perborate (1 g/l) was prepared, and the specimen with a multifiber fabric was placed into the solution. The experiment was performed on the Rotawash machine at a temperature of 40 C, time 30 min, and steel ball 25 pieces. After completion, the samples were rinsed and dried at room temperature. The multifiber strip and dyed samples were evaluated with the gray scale (rating range 1–5).
The colorfastness to light was tested by AATCC TM16 in a Q-SUN XE-2 Xenon test chamber for 72 h and evaluated against blue wool standards (BS -1006- B01-1978, rating range 1–8). The colorfastness to rubbing of the samples (wet and dry) was measured by using the ISO 105-X12:2001(E) standard test protocol using the gray scale (rating range 1–5). Furthermore, the colorfastness to perspiration was measured by using the ISO 105 E04:2013 standard test method.
Liang et al. (2003) reported the chemical composition of the black tea extract in detail. Thus, in our study, the extract was applied directly after filtration for cotton dyeing. Color strength (K/S) values of the dyed samples were measured. It was observed that cotton cationized in the concentration (5 g/l) revealed better color strength (K/S) values when dyed with black tea extract in various shade depths (Figure 2). The modification of cotton was used useful in getting dark shades on cotton. The trend line with regression (R2) value 0.9866 exhibits best fitting to the polynomial equation (y = 0.1802x2 + 0.0381x + 2.1132) for cationized dyed cotton samples with an increase in shade depth (%). In contrast, bleached cotton dyed samples showed no significant enhancement in K/S values with an increase in shade depth (%).
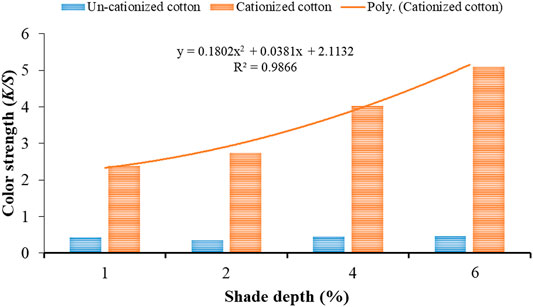
FIGURE 2. Color strength (K/S) values of cationized (5 g/l) and un-cationized samples dyed with black tea extract.
Figure 3 reveals the K/S values obtained for dyed cotton samples cationized (10 g/l cationizing agent). The trend line with regression (R2) value 0.9972 shows best fitting to the polynomial equation (y = −0.1265x2 + 1.8107x + 1.4245) for cationized dyed cotton samples with an increase in shade depth (%). Thus, it is also confirmed that increasing the concentration of cationizing agent and shade depth (%) simultaneously could yield higher K/S values.
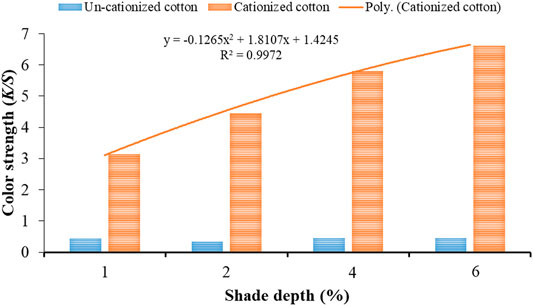
FIGURE 3. Color strength (K/S) values of cationized (10 g/l) and un-cationized samples dyed with black tea extract.
The K/S values obtained for dyed samples cationized with 15 and 20 g/l cationizing agent are shown in Figures 4, 5, respectively. The trend line with regression (R2) value 0.9934 (Figure 1) exhibits best fitting to polynomial equation (y = −0.312x2 + 2.8952x + 0.615) for cationized dyed cotton (75 g/l) samples with an increase in shade depth (%). Also, in case of cationizing concentration (100 g/l), the trend line with regression (R2) value 0.9969 confirmed best fitting with the respective polynomial equation (y = 0.1278x2 + 1.2215x + 2.0138). Thus, at these cationizing concentrations (Figures 4, 5), it is confirmed that increasing the concentration of cationizing agent and shade depth (%) simultaneously could yield higher K/S values.
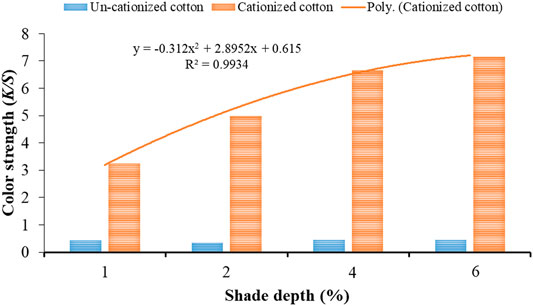
FIGURE 4. Color strength (K/S) values of cationized (15 g/l) and un-cationized samples dyed with black tea extract.
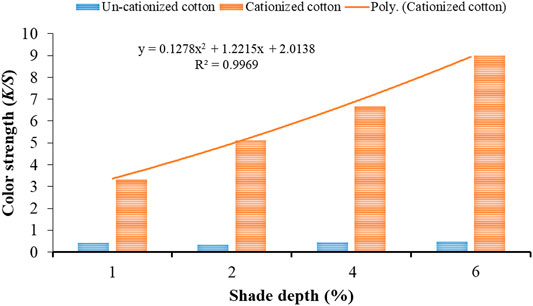
FIGURE 5. Color strength (K/S) values of cationized (20 g/l) and un-cationized samples dyed with black tea extract.
The potential simultaneous effect of increment in the concentration of cationizing agent and shade depth (%) can be witnessed from Figure 6. Based upon these color strength (K/S) investigations, it can be confidently claimed that cationization of cotton with (3-chloro-2-hydroxypropyl) trimethylammonium chloride can be applied as an alternative to heavy metal mordants used in various industries. Also, the increase in cationization agent concentration helped achieve higher color strength values on cotton dyed with black tea extract (Figure 6).
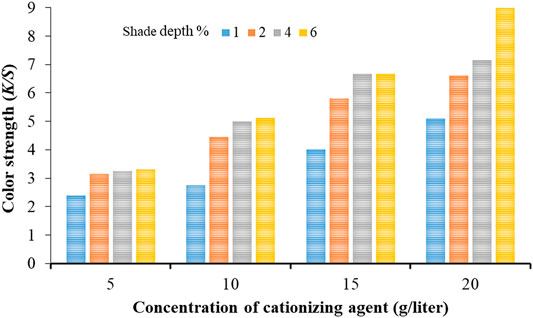
FIGURE 6. Comparing the color strength (K/S) values obtained by increment in the concentration of cationizing agent and shade depth (%) simultaneously.
In the first step of colorfastness measurements, the un-cationized cotton dyed samples were investigated, and the results are listed in Table 1. The colorfastness to washing, rubbing, and perspiration was excellent, while lightfastness was good. The better colorfastness rating observed may be due to very low color strength (K/S) values of un-cationized samples (Supplementary Figure S1).
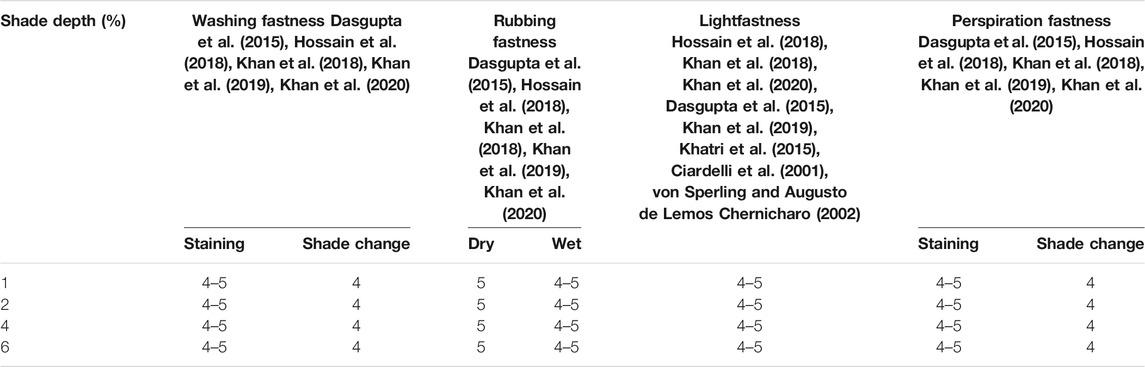
TABLE 1. Colorfastness results of un-cationized cotton dyed samples recorded according to standard test protocols.
The standard test protocols were followed to measure colorfastness to washing of cationized cotton dyed samples. The colorfastness to washing was evaluated by investigating the staining on the multifiber strip and shade change of dyed samples. Supplementary Figure S2 shows the dyed samples along with the multifiber strip obtained after the washing fastness test. From the washing fastness results presented in Table 2, it is obvious that colorfastness to staining falls in the excellent category according to gray scale ratings. However, the shade change results are fair to good. The increase in the concentration of cationization agent rendered no remarkable improvement in colorfastness to both staining and shade change. It can be assumed on the basis of the excellent colorfastness to staining and fair to good shade change results that dyeing of cationized cotton with black tea extract is a step toward the development of sustainable textiles.
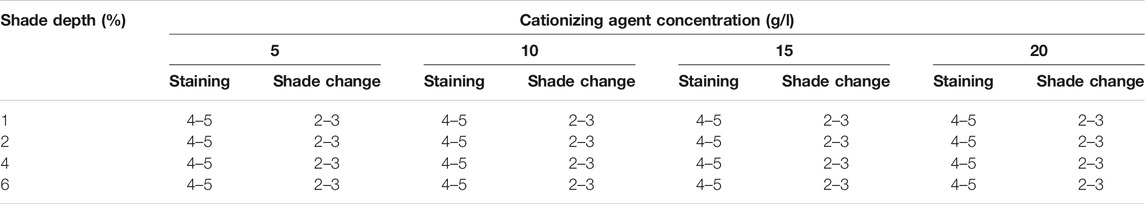
TABLE 2. Colorfastness to washing results of cationized cotton dyed samples recorded by following the ISO 105-A05 standard protocol.
The lightfastness of the samples recorded according to the blue wool scale is given in Table 3, and it is observed that black tea extract delivered good fastness on cationized cotton samples. It can be attributed to the relatively stable chromophore of the black tea extract because lightfastness mainly depends on the chromophore of coloring species. The difference between the cationized cotton samples (20 g/l) dyed before and after the lightfastness test is illustrated in Supplementary Figure S3.
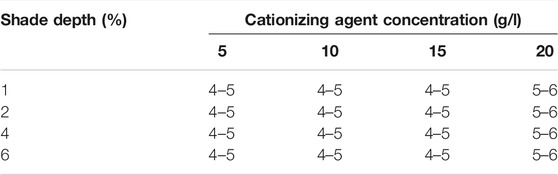
TABLE 3. Colorfastness to light results of cationized cotton dyed samples recorded according to the AATCC TM16 in Q-SUN XE-2 Xenon test chamber for 72 h.
The colorfastness to perspiration was tested according to the ISO 105 E04:2013 standard test protocol, and the results are given in Table 4. The colorfastness to shade change and staining was categorized as excellent (Dasgupta et al., 2015; Khan et al., 2019) and medium (Khan et al., 2018; Khan et al., 2020). No significant changes were observed with increment in shade depth and cationization concentration simultaneously. The cationized dyed cotton samples (20 g/l) obtained after the perspiration fastness test along with the test fabric (Supplementary Figure S4) also confirmed that the black tea extract rendered good fastness to perspiration.
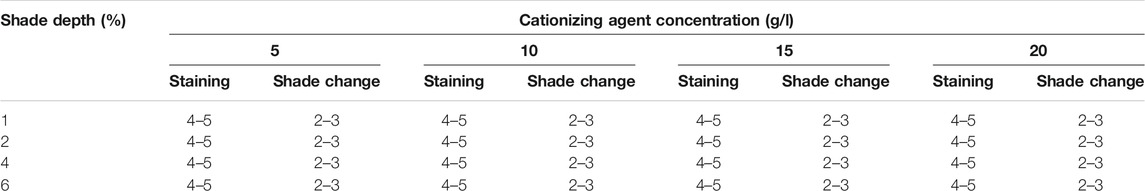
TABLE 4. Colorfastness to perspiration results recorded according to the ISO 105 E04:2013 standard protocol for cationized cotton dyed samples.
The colorfastness to wet and dry rubbing of cationized (20 g/l) is given in Supplementary Figure S5. It was observed that colorfastness to dry and wet rubbing was outstanding and very good, respectively. Table 5 represents the results of all cationized cotton samples dyed with different shade depths. A significant resistance to wet and dry rubbing was displayed by cationized dyed cotton samples.
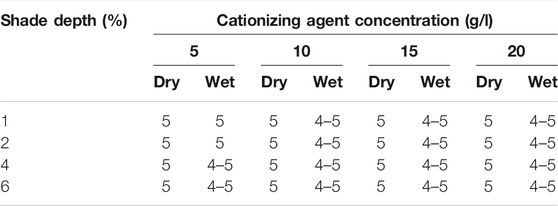
TABLE 5. Colorfastness to rubbing (dry and wet) results recorded according to the ISO 105-X12:2001(E) standard protocol for cationized cotton dyed samples.
The FTIR analysis revealed the enrichment of carboxylic acid, hydroxyl, and phenolic groups in tea extract. The presence of polyphenols provides negatively charged sites, which are critical for the formation of complex with heavy metals. However, the presence of surface hydroxyl groups on cotton fabric hinders the direct application of such polyphenolic dyes. Therefore, generally, the adsorption efficiency of natural dyes including polyphenols of tea extract is low, and dyeability is improved by employing salts of heavy metal as mordants. Accordingly, Deo et al. exploited the negatively charged sites of polyphenols of tea extract and applied it on jute and cotton. They employed alum, copper sulfate, and ferrous sulfate in pre-, meta-, and post-mordanting in the dyeing process. The mordant applications enhanced the lightfastness and wash fastness properties, providing deeper shades with less dulling and darkening than the control (Deo and Desai, 1999). Syamili et al. (2012)used tea extracts to develop complexes with copper for application as antibacterial finishes on cotton. This was an attempt to develop an eco-friendly textile finish using natural polyphenols; however, the involvement of heavy metals rendered the process unsustainable. Eman A. Bydoon also employed tea extracts for the dyeing of cotton fabric using alum, copper sulfate, and ferrous sulfate as mordants (Bydoon, 2016). The samples dyed using ferrous sulfate showed better K/S values, and different chemicals and mordants were suggested to achieve a variety of colors and shades. Although the tea extract is non-toxic and eco-friendly, the applications of metallic salts have made the dyeing course unsustainable.
In recent years, some alternative strategies have been exploited to minimize the usage of toxic chemicals in natural dyeing. Several natural mordants have been tested as eco-friendly options to replace the conventional type of metal-based mordants. Bonet-Aracil et al. (2016) used chitosan as bio-mordant to pretreat cotton which was subsequently dyed with different tea extracts. The dyed samples were used for color strength, total antioxidant capacity (TAC), and UV protection studies. Samples dyed with green tea extracts showed highest TAC, while highest UV protection was exhibited by both samples dyed with black and green tea extracts (Bonet-Aracil et al., 2016). Over the last decade, researchers have tried to exclude mordants from natural dyeing, and there has been a great surge in the development of alternative strategies. Therefore, attempts have been made to replace metal-based mordants with eco-friendly natural mordants (İşmal and Yıldırım, 2019). The natural dyes were successfully applied on textile materials using chitosan as bio-mordant in dyeing (Mehrparvar et al., 2016). However, the film-forming properties of this biopolymer create problems with the feel of the dyed fabric and challenge its large-scale applications. Some other phytophenols have also been exploited as non-toxic bio-mordants. However, technical and cost-effective issues have posed several challenges to their industrial scale applications in natural dyeing (Samanta, 2020). Therefore, the usage of natural dyes in textile dyeing has raised several ecological issues due to the lack of pollution-free dyeing methods.
Black tea carries theaflavins and thearubigin as the main polyphenolic constituents (Anesini et al., 2008; Tanaka and Matsuo, 2020). In mordant-mediated dyeing, the carboxyl and hydroxyl functionalities on dye molecules develop complexes with electropositive metals, while in this study, the hydroxyl and carboxyl groups of polyphenols develop bonding with the cationized surface of cotton. The functionalization of the cotton surface with cationizing agents enhanced dye fixation and colorfastness properties without the involvement of toxic metals (Figure 7).
The cationized cotton fabrics were dyed in four different shade depths. The cationized samples exhibited significant enhancement in their color strength values with an increase in the concentration of cationization agent. The colorfastness to washing, rubbing, and perspiration was excellent, but the lightfastness was good. Deep shades (K/S = 8.996) were obtained for cotton sample cationized (20 g/l) and dyed (6%) shades. However, the un-cationized cotton fabrics showed no significant affinity to black tea extracts and revealed very low color strength (K/S) values. Thus, the economically viable surface modification of cotton with cationizing agents enhanced the fixation of natural dyes, rendering the dyeing process eco-friendly and sustainable.
This research reported a novel approach toward cationization of cotton with (3-chloro-2-hydroxypropyl) trimethylammonium chloride and their subsequent dyeing with an aqueous extract of black tea in a chemical-free dyeing process. The cationized samples displayed substantial enhancement in their color strength with the increase in the concentration of the cationizing agent. The colorfastness to washing, rubbing, and perspiration was excellent, but the lightfastness was good. The cationized cotton fabrics dyed in four different shade depths and deep shades (K/S = 8.996) were obtained for cotton sample cationized (20 g/l) and dyed (6%) shades. As expected, the un-cationized cotton fabrics showed no significant affinity to black tea extracts and revealed very low color strength (K/S) values. Thus, the eco-friendly extraction of natural colorants and economically viable surface modification of cotton renders the auxiliary-free dyeing process sustainable and practicable at an industrial scale. This risk-free dyeing approach could easily be adopted by the textile dyeing units without the modification of existing infrastructure and could help regulate the pollution-free textile production.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
AR supervised the dyeing process. MI carried out the extraction and dyeing process. AH interpreted of results. MS contributed to insightful discussion of results. MQ proofread/improved the quality of the manuscript. TF conceptualized the idea and supervised the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.848245/full#supplementary-material
A. Hamdan, and F. Haider (Editors) (2018). Study on tea Leaves Extract as green Corrosion Inhibitor of Mild Steel in Hydrochloric Acid Solution (Materials Science and Engineering Conference Series).
A. Khan, Z. Valicsek, and O. Horváth (Editors) (2019). Synthesis and Photochemical Reactions of Iron (II) Doped Copper Ferrites as Heterogeneous Catalysts (World Congress on Civil, Structural, and Environmental Engineering).
Acharya, S., Abidi, N., Rajbhandari, R., and Meulewaeter, F. (2014). Chemical Cationization of Cotton Fabric for Improved Dye Uptake. Cellulose 21 (6), 4693–4706. doi:10.1007/s10570-014-0457-2
Anesini, C., Ferraro, G. E., and Filip, R. (2008). Total Polyphenol Content and Antioxidant Capacity of Commercially Available tea (Camellia Sinensis) in Argentina. J. Agric. Food Chem. 56 (19), 9225–9229. doi:10.1021/jf8022782
Baaka, N., Ben Ticha, M., Haddar, W., Amorim, M. T. P., and Mhenni, M. F. (2018). Upgrading of UV protection Properties of Several Textile Fabrics by Their Dyeing with Grape Pomace Colorants. Fibers Polym. 19 (2), 307–312. doi:10.1007/s12221-018-7327-0
Baaka, N., Haddar, W., Ben Ticha, M., and Mhenni, M. F. (2019). Eco-friendly Dyeing of Modified Cotton Fabrics with Grape Pomace Colorant: Optimization Using Full Factorial Design Approach. J. Nat. Fibers 16 (5), 652–661. doi:10.1080/15440478.2018.1431997
Balentine, D. A., Wiseman, S. A., and Bouwens, L. C. M. (1997). The Chemistry of tea Flavonoids. Crit. Rev. Food Sci. Nutr. 37 (8), 693–704. doi:10.1080/10408399709527797
Bonet-Aracil, M. Á., Díaz-García, P., Bou-Belda, E., Sebastiá, N., Montoro, A., and Rodrigo, R. (2016). UV protection from Cotton Fabrics Dyed with Different tea Extracts. Dyes Pigm. 134, 448–452. doi:10.1016/j.dyepig.2016.07.045
Brza, M. A., Aziz, S. B., Anuar, H., Ali, F., Dannoun, E. M. A., Mohammed, S. J., et al. (2020). Tea from the Drinking to the Synthesis of Metal Complexes and Fabrication of PVA Based Polymer Composites with Controlled Optical Band gap. Sci. Rep. 10 (1), 18108–18117. doi:10.1038/s41598-020-75138-x
Bydoon, E. (2016). Extraction of Natural Dye from Tea Leaves and its Application on Giza 86 Egyptian Cotton Fabric. Int. J. Adv. Struct. Eng. 3, 455.
Bydoon, E. A. (2017). Extraction of Natural Dye from Tea Leaves and its Application on Giza 86 Egyptian Cotton Fabric. Int. J. Adv. Sci. Eng. 3 (4), 455–462.
Ciardelli, G., Corsi, L., and Marcucci, M. (2001). Membrane Separation for Wastewater Reuse in the Textile Industry. Resour. conservation recycling 31 (2), 189–197. doi:10.1016/s0921-3449(00)00079-3
Dasgupta, J., Sikder, J., Chakraborty, S., Curcio, S., and Drioli, E. (2015). Remediation of Textile Effluents by Membrane Based Treatment Techniques: a State of the Art Review. J. Environ. Manag. 147, 55–72. doi:10.1016/j.jenvman.2014.08.008
Deo, H., and Desai, B. (1999). Dyeing of Cotton and Jute with tea as a Natural Dye. Coloration Tech. 115 (7‐8), 224–227. doi:10.1111/j.1478-4408.1999.tb00360.x
Fang, K., Shu, D., Liu, X., Cai, Y., An, F., and Zhang, X. (2018). Reactive Pad-Steam Dyeing of Cotton Fabric Modified with Cationic P(St-BA-VBT) Nanospheres. Polymers 10 (6), 564. doi:10.3390/polym10060564
Giacomini, F., de Souza, A. A. U., and de Barros, M. A. S. D. (2020). Cationization of Cotton with Ovalbumin to Improve Dyeing of Modified Cotton with Cochineal Natural Dye. Textile Res. J., 0040517519899652. doi:10.1177/0040517519899652
Haji, A. (2017). Improved Natural Dyeing of Cotton by Plasma Treatment and Chitosan Coating; Optimization by Response Surface Methodology. Cellulose Chem. Tech. 51 (9-10), 975–982.
Haji, A. (2020). Plasma Activation and Chitosan Attachment on Cotton and Wool for Improvement of Dyeability and Fastness Properties. Pigment & Resin Technology.
Hossain, L., Sarker, S. K., and Khan, M. S. (2018). Evaluation of Present and Future Wastewater Impacts of Textile Dyeing Industries in Bangladesh. Environ. Dev. 26, 23–33. doi:10.1016/j.envdev.2018.03.005
Ibrahim, N. A., El-Gamal, A. R., Gouda, M., and Mahrous, F. (2010). A New Approach for Natural Dyeing and Functional Finishing of Cotton Cellulose. Carbohydr. Polym. 82 (4), 1205–1211. doi:10.1016/j.carbpol.2010.06.054
Imtiazuddin, S., Mumtaz, M., and Mallick, K. A. (2012). Pollutants of Wastewater Characteristics in Textile Industries. Science 3 (3), 143–153. doi:10.6000/1927-5129.2012.08.02.47
Iqbal, K., Javid, A., Rehman, A., Rehman, A., Ashraf, M., and Abid, H. A. (2020). Single bath Dyeing of Modified Nylon/cotton Blended Fabrics Using Direct/acid Dyes. Pigment & Resin Technology.
İşmal, Ö. E., and Yıldırım, L. (2019). Metal Mordants and Biomordants. The Impact and Prospects of green Chemistry for Textile Technology. Elsevier, 57–82.
Khan, A., Hussain, M. T., and Jiang, H. (2018). Dyeing of Silk Fabric with Natural Dye from Camphor (Cinnamomum Camphora ) Plant Leaf Extract. Coloration Technol. 134 (4), 266–270. doi:10.1111/cote.12338
Khan, A., Hussain, M. T., Jiang, H., and Gul, S. (2020). Development of Functional Wool Fabric by Treatment with Aqueous and Alkaline Extracts of Cinnamomum Camphora Plant Leaves. J. Nat. Fibers 17 (4), 472–481. doi:10.1080/15440478.2018.1500339
Khatri, A., Peerzada, M. H., Mohsin, M., and White, M. (2015). A Review on Developments in Dyeing Cotton Fabrics with Reactive Dyes for Reducing Effluent Pollution. J. Clean. Prod. 87, 50–57. doi:10.1016/j.jclepro.2014.09.017
Liang, Y., Lu, J., Zhang, L., Wu, S., and Wu, Y. (2003). Estimation of Black tea Quality by Analysis of Chemical Composition and Colour Difference of tea Infusions. Food Chem. 80 (2), 283–290. doi:10.1016/s0308-8146(02)00415-6
Manyim, S., Kiprop, A. K., Mwasiagi, J. I., Achisa, C. M., and Odero, M. P. (2021). Dyeing of Cotton Fabric with Euclea Divinorum Extract Using Response Surface Optimization Method. Res. J. Textile Apparel. doi:10.1108/rjta-10-2020-0115
Mehrparvar, L., Safapour, S., Sadeghi-Kiakhani, M., and Gharanjig, K. (2016). Chitosan-polypropylene Imine Dendrimer Hybrid: a New Ecological Biomordant for Cochineal Dyeing of Wool. Environ. Chem. Lett. 14 (4), 533–539. doi:10.1007/s10311-016-0559-1
Meng, X., Wang, X., Wang, P., Miao, D., and Ning, X. (2021). Enhanced Dyeability and Wash Fastness through a Salt-free Plasma-Induced Grafting of Cationic Monomers on Cotton Fabrics. Fibers Polym. 22 (12), 3378–3384. doi:10.1007/s12221-021-1105-8
Niu, T., Wang, X., Wu, C., Sun, D., Zhang, X., Chen, Z., et al. (2020). Chemical Modification of Cotton Fabrics by a Bifunctional Cationic Polymer for Salt-free Reactive Dyeing. ACS Omega 5 (25), 15409–15416. doi:10.1021/acsomega.0c01530
Pisitsak, P., Hutakamol, J., Jeenapak, S., Wanmanee, P., Nuammaiphum, J., and Thongcharoen, R. (2016). Natural Dyeing of Cotton with Xylocarpus Granatum Bark Extract: Dyeing, Fastness, and Ultraviolet protection Properties. Fibers Polym. 17 (4), 560–568. doi:10.1007/s12221-016-5702-x
Prabhu, K., and Bhute, A. (2012). Plant Based Natural Dyes and Mordnats: a Review. J. Nat. Product. Plant Resour. 2 (6), 649–664.
Rehman, A., Iqbal, K., Azam, F., Safdar, F., Ashraf, M., Maqsood, H. S., et al. (2020). To Enhance the Dyeability of Cotton Fiber with the Application of Reactive Dyes by Using Chitosan. The Journal of The Textile Institute, 1–5.
Samanta, A. K. (2020). Bio-Dyes, Bio-Mordants and Bio-Finishes: Scientific Analysis for Their Application on Textiles. Chemistry and Technology of Natural and Synthetic Dyes and Pigments. IntechOpen.
Senthil Kumar, P., and Gunasundari, E. (2018). Green Chemistry in Textiles. Sustainable Innovations in Textile Chemistry and Dyes. Springer, 53–73. doi:10.1007/978-981-10-8600-7_3
Syamili, E., Elayarajah, B., Rajendran, R., Venkatrajah, B., and Kumar, P. A. (2012). Antibacterial Cotton Finish Using green tea Leaf Extracts Interacted with Copper. Asian J. Textile 2 (1), 6.
Tanaka, T., and Matsuo, Y. (2020). Production Mechanisms of Black Tea Polyphenols. Chem. Pharm. Bull. 68 (12), 1131–1142. doi:10.1248/cpb.c20-00295
Vankar, P. S., and Shanker, R. (2008). Ecofriendly Ultrasonic Natural Dyeing of Cotton Fabric with Enzyme Pretreatments. Desalination 230 (1-3), 62–69. doi:10.1016/j.desal.2007.11.016
Vankar, P. S., Shanker, R., and Verma, A. (2007). Enzymatic Natural Dyeing of Cotton and Silk Fabrics without Metal Mordants. J. Clean. Prod. 15 (15), 1441–1450. doi:10.1016/j.jclepro.2006.05.004
von Sperling, M., and Augusto de Lemos Chernicharo, C. (2002). Urban Wastewater Treatment Technologies and the Implementation of Discharge Standards in Developing Countries. Urban water 4 (1), 105–114. doi:10.1016/s1462-0758(01)00066-8
Wolela, A. D. (2019). An Overview on Surface Modification of Cotton Using Cationic Reagents for Salt-free or Low Salt Dyeing. Curr. Trends Fashion Tech. Textile Eng. 5 (1), 37–46.
Zhang, Y., Shahid-ul-Islam, S-u., Rather, L. J., and Li, Q. (2022). Recent Advances in the Surface Modification Strategies to Improve Functional Finishing of Cotton with Natural Colourants - A Review. J. Clean. Prod. 335, 130313. doi:10.1016/j.jclepro.2021.130313
Keywords: eco-friendly textiles, pollution-free dyeing, sustainable dyeing, cleaner production, chemical-free dyeing
Citation: Rehman A, Irfan M, Hameed A, Saif MJ, Qayyum MA and Farooq T (2022) Chemical-Free Dyeing of Cotton With Functional Natural Dye: A Pollution-Free and Cleaner Production Approach. Front. Environ. Sci. 10:848245. doi: 10.3389/fenvs.2022.848245
Received: 04 January 2022; Accepted: 01 February 2022;
Published: 16 March 2022.
Edited by:
Ahmed El Nemr, National Institute of Oceanography and Fisheries (NIOF), EgyptCopyright © 2022 Rehman, Irfan, Hameed, Saif, Qayyum and Farooq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tahir Farooq, dGFoaXJmYXJvb3Fmc2RAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.