
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 24 March 2022
Sec. Water and Wastewater Management
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.845448
This article is part of the Research TopicMicropollutants in the Aquatic EnvironmentView all 6 articles
 Fras Baasher1
Fras Baasher1 Tian-Nyu Wang2
Tian-Nyu Wang2 Muhammad Zulhelmi Bin Yusnan3
Muhammad Zulhelmi Bin Yusnan3 Mohsen Alkahtani4
Mohsen Alkahtani4 Yasir M. Bashawri5
Yasir M. Bashawri5 Hamed Al Qarni5
Hamed Al Qarni5 Pei-Ying Hong1,2,6*
Pei-Ying Hong1,2,6*This study characterizes a total of 21 wastewater samples collected from Al Amal hospital, and aims to determine if untreated hospital wastewater may impose a potentially detrimental impact on the downstream municipal biological wastewater treatment process. By means of solid phase extraction and liquid chromatography with tandem mass spectrometry (LC-MS/MS), chemical contaminants in these wastewater samples were determined in a non-targeted manner. In-silico characterization for the mutagenicity and reactive oxygen species (ROS) producing capabilities was performed by checking against database and literature. However, majority of the chemical contaminants have no prior information available and remain uncharacterized for both traits. Instead, in-vitro mutagenicity tests by means of Ames test showed that majority of the samples were non-mutagenic except for 5 samples that imposed mutagenic effect at high concentrations of >×10. In-vitro tests to determine for intracellular ROS production further showed that one of the mutagenic samples collected on Jun-22 positively induce ROS production and subsequently increased horizontal gene transfer via natural transformation. The findings in this study suggest that a specialty hospital like Al Amal does not frequently contribute mutagenic compounds and ROS to the wastewater streams, and in instances where it contributed positively, would require a high concentration to do so. Hence in general, wastewater streams from a specialty hospital like Al Amal may be unlikely to significantly perturb the downstream environment.
As demand for water increases due to population growth, wastewater treatment and its subsequent reuse are increasingly becoming an important aspect of integral water resources management to enhance water supply reliability (Jiménez and Asano, 2008). Water reuse is practiced for municipal, industrial, agricultural or ecological purposes. Depending on the reuse purposes, wastewater is cleaned to varying degrees of final quality that would be fit for that specific purpose. Among the various reuse purposes, reclaiming water for potable drinking purposes would require demonstration of a wastewater treatment facility that is able to achieve reliable pathogen control and attenuation of chemicals that are present in the wastewater (Trussell et al., 2018). For example, the state of California adopts the “12/10/10 rule”, meaning viruses, Cryptosporidium and Giardia should be reduced by 12-logs, 10-logs and 10-logs, respectively, from the untreated wastewater (Crook et al., 2013; Nappier et al., 2018). In addition, source control programs are put in place to control toxic chemicals from entering the wastewater collection system as toxic chemicals may interfere with or pass through the wastewater treatment system to pollute the environment and affect aquatic ecosystems (Neemann et al., 2020).
Municipal wastewater, which is most commonly used as the source to obtain reclaimed potable waters, contains discharges from homes, industries, hospitals and public and private institutions. Among these sources contributing to the municipal wastewaters, hospital wastewaters are particularly of interest as high consumptions of pharmaceutical drugs and disinfectants happen within these facilities. Pharmaceuticals such as paracetamol and diclofenac were detected at 7.4–65 μg/L, and others like ofloxacin (an antibiotic) were detected at up to 200 μg/L in hospital wastewater sampled in Turkey (Yilmaz et al., 2017). Another study found concentrations as high as 212 μg/L of paracetamol and 141 μg/L of ibuprofen in hospital wastewater in Cameroon (Mayoudom et al., 2018). Compared to municipal wastewater from the same urban area, pharmaceuticals in hospital wastewater were more concentrated and can contribute up to 67% of the pharmaceutical load into the municipal wastewater treatment plant (WWTP) (Verlicchi et al., 2012).
There are four potential scenarios by which hospital wastewaters are handled. The first is a direct discharge to the environment without any prior treatment on-site of the hospital. The second is a direct discharge to the municipal sewer without any prior treatment on-site of the hospital but rely on the downstream municipal wastewater treatment to remove chemical contaminants. The third is to have an on-site hospital wastewater treatment plant that typically involve an activated sludge processes before discharge. The fourth is to have both on-site and subsequent municipal wastewater treatment for hospital wastewaters. There are currently no data on the occurrence of each of these four different scenarios in most countries (Pauwels and Verstraete, 2006) but a recent review on the global hospital wastewater treatment scenario stated that most developing countries often drain the hospital wastewaters into municipal wastewater systems or discharge them into water bodies directly without any prior treatment (Kumari et al., 2020). Regardless of which scenario is prevalent, chemical contaminants from the untreated hospital wastewater, with their chemical contaminants, may potentially interfere with either the natural ecosystems receiving these wastewaters or the biological processes in the wastewater treatment plants.
Potential impacts may include detrimental effect on the functionality and performance of the biological treatment process or by contributing towards new gene traits among microorganisms. For example, recent studies have shown an increase in horizontal gene transfer rates caused by non-antibiotic pharmaceuticals such as ibuprofen (Wang et al., 2020). The pharmaceutical compounds were further shown to increase horizontal gene transfer rates for competent bacterial cell hosts by enhancing the stress levels that resulted in reactive oxygen species (ROS) production. In separate studies, it was also shown that chemicals or external stressors that are mutagenic can increase DNA repair rates, in turn triggering natural transformation (i.e., uptake of extracellular DNA) among competent Acinetobacter baylyi ADP1 (Augsburger et al., 2019; Mantilla-Calderon et al., 2019). This is a major concern especially in hospital wastewater due to the higher presence of antibiotics and antibiotic resistance genes (ARGs). Different studies have reported that hospital wastewater have concentration of ARGs between 0.4 log to 1.8-fold more than communal wastewater (Paulus et al., 2019; Wang et al., 2019). The presence of ARGs along with a mutagen can potentially facilitate the increase in antibiotic resistance in the microbial community through natural transformation events, which poses a great health hazard. Other impacts may include harmful effects on the aquatic life where the treated wastewater is discharged. A study has shown that the treated wastewater generated by a psychiatric hospital damaged the reproduction and biological activities of macroinvertebrate communities (Mazzitelli et al., 2018).
Hence, understanding the level of toxicity imposed by chemicals that are present in the hospital wastewater would facilitate subsequent assessment on potential detrimental impact on the recipient environment. To study the toxicity of the wastewater produced by hospitals, it is important to first have an understanding of its composition and properties. Several studies were conducted on wastewater collected from hospitals in which they target specific compounds and evaluate their toxicity and environmental impact. In one study, single grab samples from three hospitals in Turkey were collected and analyzed for the presence of 55 compounds that include pharmaceuticals, corrosion inhibitors and pesticides (Yilmaz et al., 2017). The hazard quotients were estimated for each characterized compound and the cytotoxicity and mutagenicity assessment was done to evaluate the environmental risk imposed by the hospital wastewater. However, the study provides only a snapshot view of the hospital wastewater as it did not sample the wastewater over a temporal scale (Yilmaz et al., 2017). A similar approach was done by Mendoza et al. in which 25 pharmaceuticals were detected, quantified for their concentration and determined for their hazard quotients (Mendoza et al., 2015). However, those studies targeted general hospital. A study by Mazzitelli el al. targeted and quantified 10 compounds used in psychiatric hospitals. Some of those compounds, which include cyamemazine and diazepam were not found in the mentioned studies (Mazzitelli et al., 2018). This is due to the fact that psychiatric hospitals use different medications for their patients compared to the general hospital, hence the difference in the chemical composition of their wastewater. Earlier studies focused on targeting a selected group of pharmaceuticals and quantifying the concentrations of specific compounds to estimate hazard quotients (Escher et al., 2011; Verlicchi et al., 2012; Mendoza et al., 2015; Yilmaz et al., 2017). However, such approach does not provide a holistic profile of the suite of chemical contaminants that may be present in the complex hospital wastewater matrix over a certain temporal duration.
In this study, we performed a non-targeted qualitative assessment of the chemical composition of wastewater samples collected from a hospital in Jeddah, Saudi Arabia. Mutagenicity and ROS production capacity of each individual chemical identified after solid phase extraction followed by liquid chromatography-mass spectrometry was further assessed by referencing against literature and databases (i.e., in-silico). Mutagenicity and ROS production were also characterized in-vitro for each wastewater sample to complement the in-silico observations. As untreated hospital wastewater is in contact with either the natural environment or the biological wastewater treatment processes, it is essential to have an understanding on how each constituent chemicals can impose stress on the microbial community. Hence, natural transformation test was performed with hospital wastewater samples that exhibit both mutagenicity and ROS induction. By understanding the chemical composition of the wastewater and demonstrating the extent at which hospital wastewater can impose stress conditions, such findings will allow us to better understand the extent of detrimental impact that may arise from untreated hospital wastewaters.
Al Amal Hospital is a psychiatric medical facility that specializes in the treatment of patients with mental illnesses as well as addiction rehabilitation. This hospital has a tertiary wastewater treatment system that consists of equalization tank, primary clarifier, aeration tank, secondary clarifier, sand filter and chlorination. The hospital generates approximately 200 m3/day of wastewater, while its designed capacity is 400 m3. This hospital has 210 beds with an average of 75 ± 6 patients/day during the sampling period. Grab samples were collected from the equalization tank in the morning on each sampling date and immediately transported to KAUST. A total of 21 untreated wastewater samples were collected from Al Amal Hospital in Jeddah from Apr-12 to Jul-8, 2020, during the first wave of the COVID-19 pandemic in Saudi Arabia. The sample collection dates were spaced out unevenly due to COVID-19 restrictions imposed within the city during the initial phase of the pandemic, and sampling can only be carried out when permission was granted on an ad-hoc basis. The sampling dates are listed in Supplementary Figure S1. Sample collected on Jun-22 was collected into two different sterile collection bottles and individually processed by solid phase extraction (SPE) and subsequent analytical characterization independently. This is to serve as an internal control to determine technical reproducibility of protocols and analytical instruments. Samples were stored at 4°C until further analysis.
200 ml of each sample was individually filtered through Whatman® glass microfiber filters (GF/F grade) and collected for concentration and purification through SPE. SPE was done using Dionex AutoTrace 280 through 500 mg Oasis HLB cartridges. The cartridges were pre-conditioned with 5 ml HPLC-grade methanol followed by 5 ml of HPLC water (pH 2.5). The sample was loaded into the cartridge with a flow rate of 3 ml/min, then washed with 10 ml of HPLC-grade water. The sample-loaded cartridge was then dried with nitrogen gas for 60 min, then elution of bound sample was done using 10 ml HPLC-grade methanol. Concentrated samples were collected in sterile tube and lyophilized in a 40°C water bath under a nitrogen gas blower. The lyophilized sample was then dissolved in 1 ml of HPLC-grade methanol and stored at 4°C prior to analysis on the liquid chromatography with tandem mass spectrometry. The recovery efficiency of the SPE method was evaluated by assessing the recovery of two pharmaceuticals, namely carbamazepine and atenolol. After running wastewater samples spiked with known concentrations of carbamazepine and atenolol, the concentrate derived from SPE was ran through the LC-MS/MS and the recovered concentration of each pharmaceutical was measured. The measured concentration is subtracted from a negative control, which is the same wastewater without spiking the pharmaceuticals. Observed recovery efficiency for carbamazepine and atenolol are 90.9% and 91.5%, respectively. Standard curve at different spiked concentrations was determined for the R2 value to determine the linearity range for concentration detection (Supplementary Figure S2).
LC-MS/MS was used to provide a non-targeted approach to characterize the chemical constituents that are present in each of the untreated hospital wastewater sample. The system consists of Gemini-NX C18, 4 × 2.0 mm (Phenomenex Inc.) column, Agilent 1260 infinity, and ABSCIEX QTRAP 5500. The system was using two mobile phases: Mobile phase A with 4 mM of ammonium formate and 0.1% formic acid in HPLC-grade methanol and mobile phase B with 4 mM of ammonium formate and 0.1% formic acid in HPLC-grade water. The LC-MS-MS ran on multiple reaction monitoring (MRM) at flow rate of 200 uL/min for 18 min (Zaouri et al., 2021). The scan range was limited between 150 and 1000 m/z to improve signal intensity since a preliminary run showed only noise before 150 and after 1000 m/z. The peaks were recorded for data analysis and were compared to the online database mzCloud to find matches. A positive match is defined based on having at least 80% match similarity with a specific compound listed in the database. For each identified chemical, the occurrence frequency was noted and subsequently categorized into five major classes, namely 1) medication (i.e., chemicals such as antibiotics, non-steroidal anti-inflammatory drugs NSAIDs, allergy medication etc, needed for medical treatment), 2) illicit drugs (i.e., chemicals that were used as illicit or recreational drugs), 3) pesticides (contaminants that are used in agricultural activities), 4) natural bioactives (chemicals that are secreted naturally by the microorganisms in the wastewater such as toxins), and 5) withdrawn chemicals that have been discontinued from the market. With the exception of the final class, the remaining four classes were further organized into three subclasses to form a total of 13 categories. The first subclass was “Parent Compounds” which was defined as chemicals that retained their original chemical structure and did not undergo any reaction. The second subclass was “Analogs” which was defined as chemicals that have similar chemical structure and function to the parent compounds but may differ in their pharmacokinetic properties, such as their absorption and toxicity to the human body, amongst others. The third subclass was “Metabolites” which was defined as parent compounds that have undergone a reaction which resulted in an entirely different chemical compound altogether.
For each chemical, in-silico characterization on its mutagenicity and ROS production data was done based on search conducted on databases collated by US Food and Drug Administration (US FDA), European Chemicals Agency (ECHA). Studies and articles that are within the accessible public domain, written in English and were published within the duration of 1991–2021 were included as information database to search for the mutagenicity and ROS production capacity of each individual chemical. To search, the name of the chemical and the primary search terms used were “mutagen”, “Ames assay”, “Ames test”, “Salmonella” and “reactive oxygen species”. These databases were also used to determine if the Ames assay results of each contaminant were available. Apart from the results, the details of such studies, such as the strains of the model organism used and concentration of contaminants tested amongst others, were also noted to construct a comprehensive catalog. In addition, a computational model, admetSAR accessed through DrugBank (https://go.drugbank.com) was also used to computationally predict the Ames mutagenicity of the contaminant using a probability score, so as to further enhance the comprehensiveness of the in-silico characterization. For chemicals that have no literature data, they were indicated as “inconclusive”. Similarly, these databases were also used to determine if each contaminant had been tested in vitro for their capability to induce, suppress or to have no effect on ROS production. Contaminants that do not have such data related to ROS available in literature or databases will be denoted as “Inconclusive”. After analyzing each individual chemical for its mutagenicity and ROS production capacity, the relative percentage of mutagenicity and ROS production capacity for each wastewater sample was further tabulated.
The mutagenic effects of the wastewater were evaluated using Salmonella enterica serovar Typhimurium TA98 and TA100 strains. TA98 strain evaluates for on-point mutation while TA100 tests for base pair substitution mutation (Maron and Ames, 1983). The positive control was 100 µg of sodium azide/plate for TA100 and 5 µL dichloromethane/plate for TA98, respectively (Jongen et al., 1978). ×1 potassium phosphate buffer (PPB) was used as the negative control. Both strains were prepared by growing them overnight in LB broth with ampicillin at 25 mg/L. The mutagenic effect was evaluated by the Ames test as previously described (Maron and Ames, 1983). The SPE-extracted wastewater samples which were stored in methanol-dissolved form were prepared by lyophilizing them again and dissolving them in sterile Milli-Q water prior to mutagenicity experiments. The Salmonella cells were standardized to 2 × 109 cells/mL and incubated with the SPE-extracted samples (which has an original concentration of ×200) at a final concentration of either ×1, ×10 or ×20 for 1 h and 37°C. Samples of each concentration were done in three technical replicates and plated onto minimal glucose plates supplemented with Vogel−Bonner (VB) salt solution (recipe listed in earlier paper by (Vijay et al., 2018). After 48 h of incubation at 37°C, the number of histidine revertant colonies was counted and normalized against the number of colonies derived from the negative control. Moreover, the standardized cells were grown in a separate plate along with sterile disk that absorbed crystal violet to ensure that the cells contain rfa mutation, which allows higher permeability for larger molecules (Ames et al., 1973).
A single colony of Acinetobacter baylyi ADP1 (Rizzi et al., 2008) was cultured in LB Lenox medium at 37°C in a shaker incubator set at 200 rpm. Once at exponential growth phase, the culture was centrifuged at 5,000 g, removed of its supernatant and the pellet washed once with ×1 PBS buffer prior to resuspending it to a density of approximately 2 × 107 cfu/ml. The cells were stained with 5 μM of CM-H2DCFDA (Thermo Fisher Scientific, Waltham, MA) at 37°C for 30 min. Afterwards, 100 µL of the stained cells were mixed with 100 µL of PBS buffer (as control) or with SPE-concentrates (with original concentration of ×200) derived from hospital wastewater samples to become a final concentration of either ×1, ×10 and ×20. The SPE-extracted wastewater samples which were stored in methanol-dissolved form were prepared by lyophilizing them again and dissolving them in sterile Milli-Q water prior to ROS determination experiments. To facilitate high throughput measurements, the samples were placed individually within a well on a 96-well plate, and the plate was sealed with a plastic film and wrapped with aluminum foil to avoid light and cultured at 37°C for 30 min and 1 h. Thereafter, the samples were put on ice and diluted 10-fold in ×1 PBS buffer before analysis using BD LSRFortessa Cell analyzer (Becton Dickinson, Franklin Lakes, NJ) outfitted with a microplate autosampler. A total of 50,000 events was analyzed at a speed of 2 μL/s for each sample for fluorescence determination. The detection parameter was FSC 500, SSC 250 and FL1-A 500, with a threshold on FSC set at a value of 500. Culture lacking fluorescent dye was included as a control for background auto fluorescence. The bacteria treated with 10 μM, 100 μM and 1 mM hydrogen peroxide were used as positive control.
The tests were performed in 3 biological replicates. Each sample was recorded in technical replicates. The ROS data recorded by flow cytometry was presented in two ways. First, mean fluorescence intensity for total cells indicate the level of intracellular ROS produced by the cells upon exposure to hospital wastewater. The second way of indicating intracellular ROS was by showing the percentage of stressed cells with an increased production of ROS. This is indicated by first determining the gating region of a flow cytogram that would indicate the percentage of non-stressed cells in the presence of negative control, and the gating region on the right would indicate the percentage of cells that are stressed (Ameziane-El-Hassani and Dupuy, 2013; Zhang et al., 2021). The same gating region is then used for all subsequent flow cytograms derived from the test treatment (Supplementary Figure S3) to tabulate percentage of stressed cells.
Statistical significance was calculated for each set of technical triplicate measurements using one-way ANOVA, making comparison to the unstained auto fluorescence control included in each replicate. Measurements were regarded as statistically significant when the p value were ≤0.05.
For those hospital wastewater samples that exhibit both mutagenicity and ROS production, the change in the transformation frequency was measured using the transformation assay as described previously (Rizzi et al., 2008; Augsburger et al., 2019; Mantilla-Calderon et al., 2019). A. baylyi ADP1 was inoculated in 50 ml LB broth along with rifampicin (50 μg/ml) and kanamycin (50 μg/ml) and incubated overnight at 37°C at 200 rpm. The cells were adjusted to OD of 0.05 at 600 nm. For each reaction, 2 µg of donor DNA was incubated along with 100 μL of the adjusted cells and ×20 concentrate of the SPE-extracted wastewater samples that was resuspended in Milli-Q. ×20 concentrate was chosen for this experiment because earlier studies performed by our group indicated that the Acinetobacter baylyi ADP1 system along with the plate counting technique require high concentration to induce positive observations (i.e., detection sensitivity issues). Hence, false-negative issues may arise when using low concentration of tested factor. Cells that undergo successful natural transformation will integrate this donor DNA into their DNA, which will give them resistance to spectinomycin. The reactions were incubated at 37°C at 200 rpm for 18 h. The number of transformants was determined by plating 100 μL of 10−1 diluted cells were plated in LB media agar plate supplemented with 50 μg/ml rifampicin, 50 μg/ml kanamycin and 100 μg/ml spectinomycin. The total number of cells was determined by plating 100 μL of 10−6 diluted cells in LB media agar plate supplemented with 50 μg/ml rifampicin and 50 μg/ml kanamycin. Those plates were incubated at 37°C for 18 h and the colonies were counted. The transformation frequency was calculated by dividing the number of transformed cells by the total cells. The frequency is used to calculate the fold change by dividing the transformation frequency of the sample by the transformation frequency of the control (×1 potassium phosphate buffer, PPB). Transformation experiments for each sample were done in triplicate.
A total of 824 chemical contaminants was identified from all wastewater samples collected from Al Amal hospital (Supplementary Table S1). 32% of the identified chemicals were classified into medications in their parent compound form and include medications such as antibiotics, antidepressants, and antipsychotics (Figure 1) The percentage of medications that are in its metabolites form account for only 6.3% of the total detected chemicals. In contrast, illicit drugs (19%) and their metabolites (13%) account for the next two major categories of chemicals detected in the hospital wastewater (Figure 1). Illicit drugs are drugs that were discharged by patients of the hospital in their pure form, while metabolites are the final products after they are metabolized in the patient’s body. Those illicit drugs include multiple types of synthetic cannabinoids, heroin, Lysergic Acid Diethylamide (LSD), and others.
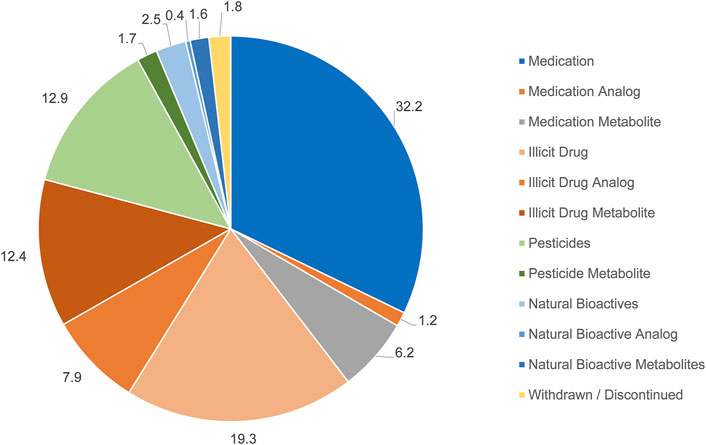
FIGURE 1. An overall depiction of the chemicals identified in the 21 wastewater samples and their respective categories. Numbers represent percentage of identified chemicals that are assigned to that respective category.
Pesticides were the fourth most common chemicals identified in the hospital wastewater, accounting for 12% of the total identified chemicals. This category includes pesticides, herbicides, and fungicides. Both chemicals classified as bioactives or withdrawn chemicals account for only a small percentage of the identified chemicals. Bioactives include chemicals that are secreted naturally by the microorganisms in the wastewater, while withdrawn chemicals include those that were discontinued from the target consumer markets but may be accessed through illegal routes.
The number of chemicals identified within each sample and classified to either mutagenic, non-mutagenic or inconclusive based on in-silico prediction is shown in Supplementary Table S2. For all samples, less than an average of 4% of the chemicals were in-silico predicted to be mutagenic (Figure 2). An average of 34% is predicted to be non-mutagenic. However, an average of 62% of the chemicals did not have data available about their mutagenicity, primarily because they are illicit drugs and their metabolites and analogs (i.e., inconclusive). The sample with a comparatively higher percentage of chemical contaminants classified as mutagens was Apr-25 (Figure 2). Moreover, one-way ANOVA was performed on the number of characterized mutagenic, non-mutagenic, and inconclusive chemicals to compare samples collected in April, May, and June 2020. July was excluded due to the low number of samples. While there was no statistically significant difference in the mutagenic category, there was a statistical difference among the different months for the percentage of inconclusive chemicals (p < 0.05). From Tukey’s Honest Significant Difference, the different months were April and May for the percentage of inconclusive chemicals. Sample collected on Jun-22 was analyzed twice in an independent manner from the SPE to the LC-MS/MS step, and both independently processed samples showed similar in-silico profiles, suggesting that the earlier processing protocol is reproducible.
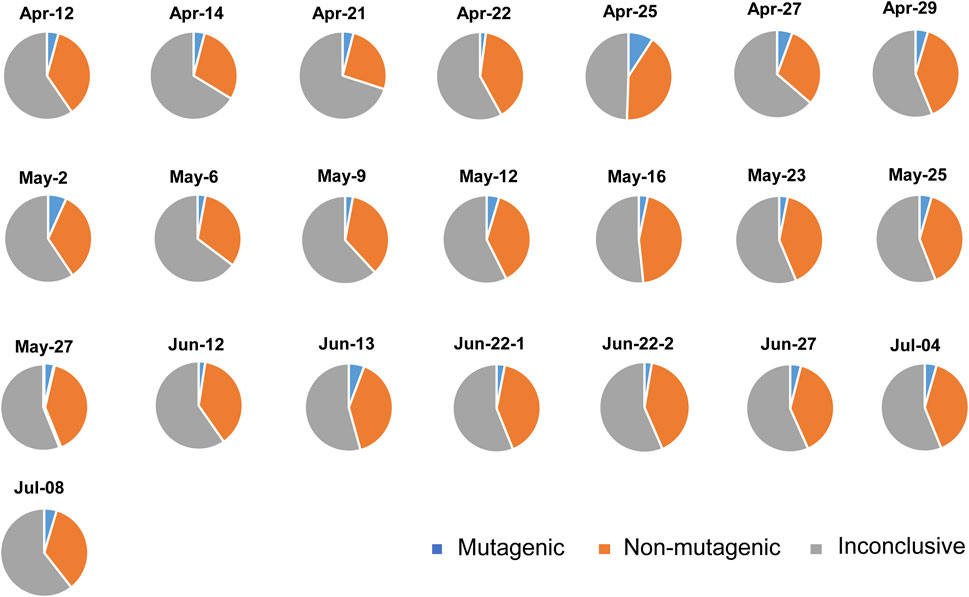
FIGURE 2. Percentage of compounds classified in-silico as mutagenic and non-mutagenic compounds, or do not have available literature and hence classified as inconclusive compounds.
Of the 21 samples, most of the samples did not show a mutagenic effect for either TA98 or TA100. The only sample that showed mutagenic response on TA98 was collected on Jun-27 at a ×20 concentration (Figure 3A), in which the number of revertants normalized against the negative control (NC) was 1.4-fold higher. Samples that showed mutagenic response on TA100 include those collected on Apr-27, Apr-29 and on May-27 (Figures 3B–D). Mutagenic response on TA100 was only observed when Apr-27 sample was of 20x concentrate, and that the fold increase in the number of revertants was 1.7 compared to NC. For Apr-29 sample, mutagenic response on TA100 was observed for both ×1 and ×20 concentrate but not for ×10, and that the fold increase in the number of revertants was 1.26 and 1.24, respectively, compared to NC. In contrast, May-27 sample exhibited a mutagenic response on TA100 at ×10 concentration. In addition, both independently processed samples collected on Jun-22 exhibited a sub-lethal response on TA100 when tested at ×10 and ×20 concentrations (Figures 3E,F). The number of revertants counted at both concentrations was 0.5 to 0.7-fold of that in NC. One of the Jun-22 repeats also showed a significant decrease in number of revertants at ×1 concentration compared to that of control (Figure 3F).
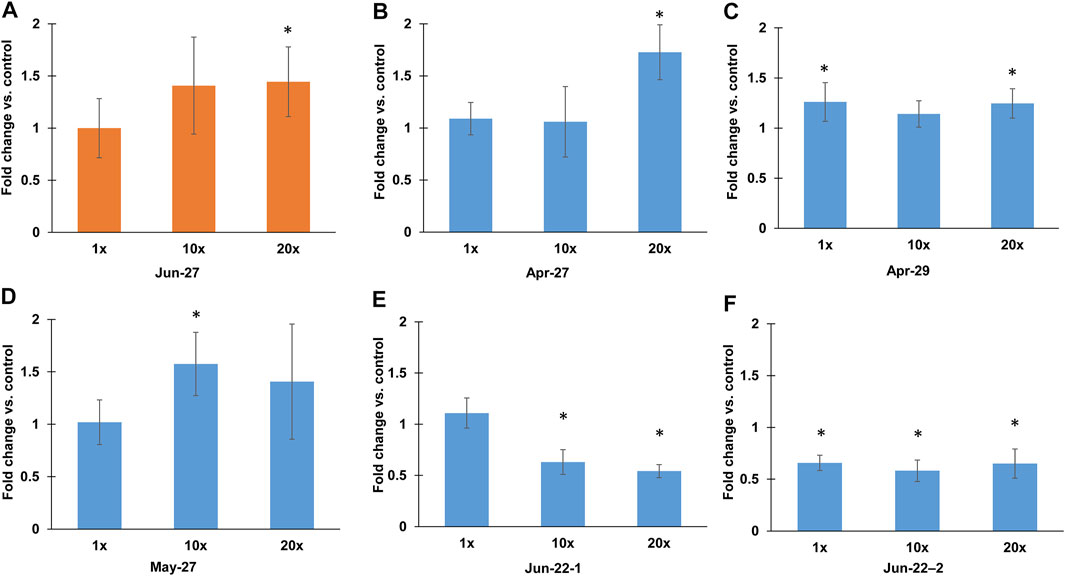
FIGURE 3. Fold changes of number of Salmonella Typhimurium revertants compared to the negative control. (A) Fold changes of S. Typhimurium TA98 at ×1, ×10 and ×20 concentration of 27 June sample (B–F) Fold changes of S. Typhimurium TA100 at ×1, ×10 and ×20 concentration of (B) 27 April sample, (C) 29 April sample, (D) 27 May sample, (E) 22 June sample replicate 1, (F) 22 June sample replicate 2. Asterisks denote significant difference compared to negative control.
The number of chemicals identified within each sample and classified to ROS inducers, ROS non-inducers, ROS-suppressants or inconclusive based on in-silico prediction is shown in Supplementary Table S3. Similar to the in-silico prediction of mutagenicity, majority of chemical contaminants (average 75.3%) were classified as inconclusive and without data available on its ROS production capabilities. For the remaining chemical contaminants, an average of 15% were predicted to induce ROS while only an average 2.6% of chemical contaminants showed no effect. The remaining average of 7.1% of chemical contaminants may potentially counteract ROS production. Samples collected on Apr-21, Apr-25, Apr-27, as well as that on May-16 and Jun-22 had a comparatively higher percentage of chemical contaminants that induce ROS than other samples (Figure 4). There was no statistically significant difference in the percentage of chemicals classified within the ROS production category among the different months.
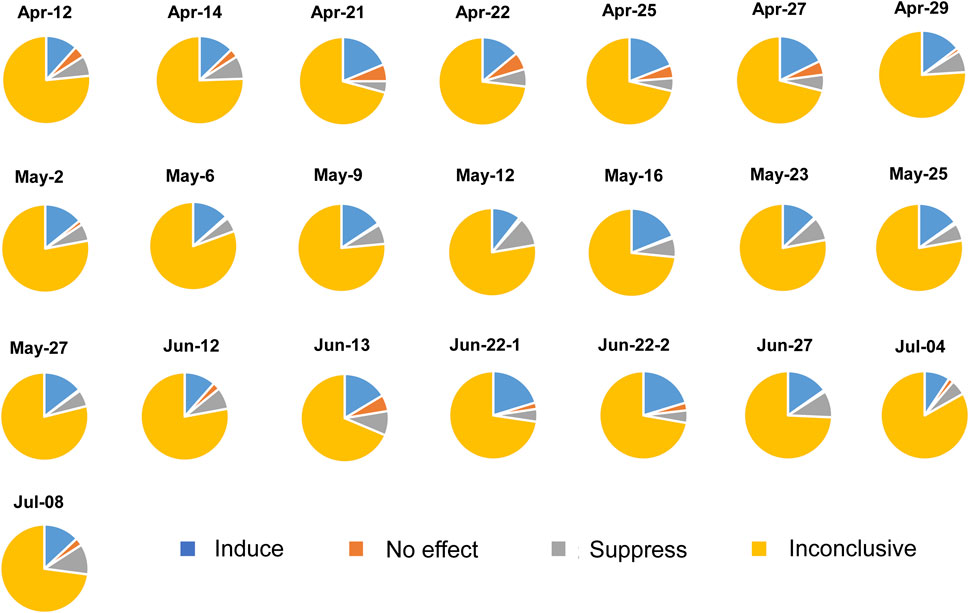
FIGURE 4. Percentage of chemical contaminants that induce, has no effect or suppress reactive oxygen species (ROS). Chemical contaminants that do not have available literature on ROS production are classified as inconclusive compounds.
The ROS data recorded by flow cytometry was presented in two ways. First, mean fluorescence intensity for total cells indicate the level of intracellular ROS produced by the cells upon exposure to hospital wastewater. Most of the 21 samples did not induce a significant generation of intracellular ROS compared with the positive control, H2O2 after both 30 min and 1 h incubation (Figures 5A,B) (p > 0.05), However, 2 hospital samples collected on Jun-13 and Jun-22 generated intracellular ROS by A. baylyi ADP1 after both 30 min and 1 h incubation. Specifically, there was 1.1-, 10.7- and 19.1-fold (p < 0.05) increases of ROS production observed for the 107 cfu/mL A. baylyi ADP1 in response to Jun-13 samples at ×1, ×10 and ×20 concentrations, respectively (Figure 5D). Similarly, both Jun-22 samples resulted in intracellular ROS increases within A. baylyi ADP1 at x10 (1.2- and 1.03-) and ×20 (1.37- and 1.21-) concentrations after 30 min reaction (Figure 5C).
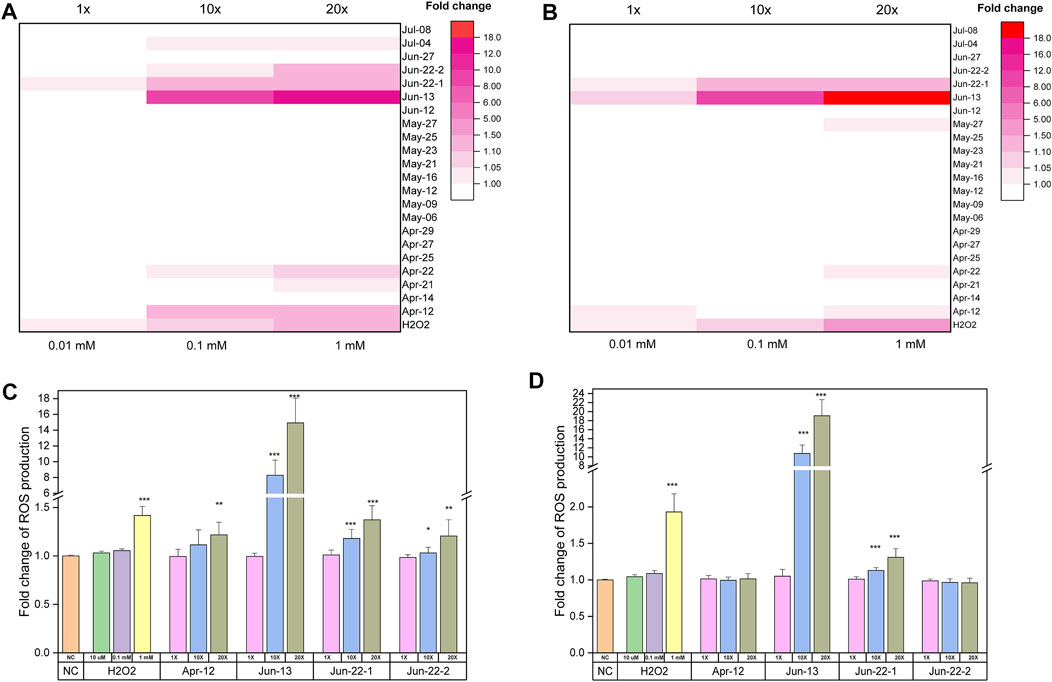
FIGURE 5. Flow cytometry detection results of intracellular ROS generation when exposing 107 cfu/ml of A. baylyi ADP1 to hospital samples. (A) and (C) Fold change of ROS production of A. baylyi ADP1 cells after 30 min treatment by hospital samples at ×1, ×10 and ×20 concentration. (B) and (D) Fold change of ROS production of A. baylyi ADP1 cells after 1 h treatment by hospital samples at ×1, ×10 and ×20 concentration, *p < 0.05, **p < 0.01,***p < 0.001.
The second way of indicating intracellular ROS was by showing the percentage of stressed cells with an increased production of ROS. Supplementary Figures S4A,B indicate the percentage of bacterial cells with an increase production of ROS after exposure to different hospital samples for 30 min and 60 min. Most of the cells showed increase in ROS level after exposure to hospital samples. The stress effect of hospitals sample on A. baylyi ADP1 increases with the sample concentrations and treatment duration. Among all samples, Jun-13 sample induces the highest stress on the bacterial cells, with 18.8% and 33.2% of cells showing an increase in ROS level after exposure to Jun-13 sample at ×10 and ×20 concentrations, respectively, for 30 min (Supplementary Figure S4A).
Considering that the 2 samples collected independently on Jun-22 exhibited both sub-lethality and ROS production, both samples were further tested to determine if they would induce horizontal gene transfer via natural transformation. ×20 concentrate of Jun-22-1 and Jun-22-2 samples were observed to significantly increase natural transformation by an average 1.2-fold compared to the control (p < 0.05), with an average transformation frequency of 1.9 × 10−5.
Although Al Amal hospital is a psychiatric medical facility, non-targeted characterization of the wastewaters from this hospital showed that the most abundant (32%) chemical constituents are medications including antibiotics, antidepressants and antipsychotics. Meza et al. detected the presence of 52 organic contaminants in Hershey Medical Center wastewater and observed 83% of the identified chemicals to be pharmaceuticals, while the rest were pharmaceutical metabolites or antifungals (Meza et al., 2020). Another study also characterized the wastewater collected from 4 general hospitals and detected 31 out of 55 (56%) identified chemicals to be pharmaceuticals. The remaining components were pharmaceutical metabolites, corrosion inhibitors and pesticides (Yilmaz et al., 2017). Those profiles are not entirely similar in terms of chemical composition to our study due to a higher percentage of chemicals belonging to medications in the earlier studies. There was also a lack of illicit drugs present in their wastewater streams. These differences in chemical profiles could be explained due to the nature of Al Amal hospital as a psychiatric medical facility (i.e., a specialty hospital), and hence the use of medications to treat ailments may be of lower frequency than in general hospitals. Escher et al. also observed differences in the predicted environmental concentrations of the top 100 pharmaceuticals present in the general hospital and psychiatric hospital wastewaters (Escher et al., 2011), reiterating that the type of chemical contaminants found in the waste streams is dependent on the hospital management and operation practices.
Using an in-silico approach, majority of the chemicals identified to be present in Al Amal hospital cannot be characterized for its mutagenicity/non-mutagenicity (Figure 2) nor for their ROS production capacities (Figure 4). In terms of mutagenicity, the highest number of identified inconclusive chemicals was from the sample collected on Apr-21. The inconclusive results were due to a lack of information for the mutagenic properties for different pharmaceuticals, for example, quinupramine, finasteride and robenidine that were identified in Apr-21 sample. In addition, Al Amal hospital is a specialty hospital that uses medications to alleviate the side effects of drug addictions. Patients can discharge illicit drugs and metabolites of the drugs they ingested or injected to the waste stream. Therefore, unless databases improve by expanding characterization effort for both mutagenicity and ROS production to a wider suite of chemical contaminants, particularly that of illicit drugs and drug metabolites, it remains difficult to ascertain the overall chemical profile of the hospital wastewater in an in-silico manner. This is especially if we were to consider the extent of complexity in such wastewater streams (in this instance, > 800 different types of chemical constituents are present).
Hence, reliance on in-vitro characterization of both mutagenicity and ROS production capacities are still needed to understand the chemical constituents in hospital wastewaters. It was observed that almost all, except for 5 samples, are non-mutagenic, suggesting that mutagenicity of such wastewaters occur on an intermittent basis. This observation conflicted with studies that reported that hospital wastewater is mutagenic in majority of their samples. Sharma et al. showed that wastewater from four hospitals in India is mutagenic but proper treatment of the hospital wastewater can lower the extent of mutagenicity (Sharma et al., 2015). Similarly, Yilmaz also showed that wastewater samples collected from hospitals in Turkey showed strong mutagenic activity (Yilmaz et al., 2017). The difference in observation may be due to the earlier studies sampling wastewater from general hospitals that provide treatment for a wider suite of ailments and diseases. In addition, the studies only presented data on a small sample size of grab samples, and hence do not provide information on the occurrence frequency of mutagenic compounds in the wastewater over a temporal scale.
For those five Al Amal wastewater samples that demonstrate mutagenicity or toxicity, there were 2 samples that induced a positive effect at ×1 concentration (e.g., Apr-29 and Jun-22-2) and with the other 4 samples (Apr-27, May-27, Jun-22-1 and Jun-27) requiring ≥×10 concentration to induce a positive effect (Figure 3). In earlier studies, it was determined that the contribution of untreated hospital wastewater into municipal wastewater can range from 0.2% v/v (Łuczkiewicz et al., 2010) to approximately 1% v/v (Galvin et al., 2010). Hence, accounting for dilution effect, the contribution of mutagenicity effect on bulk water would be comparable with the observed results noted from the x1 concentration (Figure 3), and that the frequency of contributing mutagenicity effect to the bulk water would be particularly low or possibly on an intermittent basis. However, there may be a need to monitor for potential detrimental impact on the activated sludge (AS) processes in the downstream biological wastewater treatment plants where bioaccumulation occurs. AS systems that are operated with long sludge retention time, SRT, (e.g., 20–40 days) may bioaccumulate the chemical contaminants to a high enough level that induce mutagenicity within the sludge blanket. In addition, although only one sample collected independently twice (e.g., Jun-22-1 and Jun-22-2) was determined to induce toxic effect at both ×10 and ×20 concentration, bacterial cells like A. baylyi ADP1 were stressed when exposed to all hospital wastewater samples (Supplementary Figure S4). Hence, there may be a potential, albeit low possibility, to detrimentally affect COD, nutrient and organic micropollutants removal via biodegradation by the AS with longer SRT (Majewsky et al., 2011; Falås et al., 2016).
Unlike mutagenicity that use Salmonella Typhimurium as a golden standard method, detecting intracellular ROS production is challenging due to limitations associated with the CM-H2DCFDA stain method. When establishing the ROS detection protocol, it was observed that the response of bacteria to stress factors varied with the ratio of bacterial cell density to test factor (e.g., H2O2), with a higher density of the cells being less affected by the test factor and hence not generating detectable fluorescence intensity to denote intracellular ROS production (i.e., lower detection sensitivity) (Supplementary Figure S5). Considering these limitations, this study used a cell density of A. baylyi that would be more responsive to treatment factors, and used flow cytometry to determine fluorescently-stained cells at 2 time points. In-vitro characterization of the hospital wastewater samples for ROS production suggests only 2 hospital wastewater samples collected on Jun-13 and Jun-22 induced intracellular ROS production in a time- and concentration-dependent manner (Figure 5).
Production of intracellular ROS may be concerning as an earlier study demonstrated an increase in the rate of conjugation in E. coli LE392 by up to 8-fold due to an increase in ROS generation caused by carbamazepine at 50 mg/L. When an ROS scavenger thiourea was added, the rate of conjugation was reversed, reiterating the role of ROS in horizontal gene transfer by means of conjugation (Wang et al., 2019). In a separate study, ROS production, along with enhanced stress levels and increased cell membrane permeability, also contributed to an increase in natural transformation (Wang et al., 2020). As such, we focused on determining if Jun-22 samples, which exhibited stress on the bacteria and ROS production, would contribute towards natural transformation. The untreated hospital wastewater samples at x20 concentration resulted in a positive increase in natural transformation, suggesting that potential accumulation of mutagenic and ROS producing chemicals that are discharged with the hospital wastewater may disseminate the acquisition of new functional gene traits in the downstream wastewater treatment processes. This is especially considering that activated sludge systems with longer SRT also have higher mixed liquor suspended solids concentration (i.e., biomass and extracellular DNA) that can favor horizontal gene transfer frequency.
This study demonstrated that a specialty hospital like Al Amal does not frequently contribute mutagenic compounds and ROS to the wastewater streams. Only a small number of samples (2 out of 6) contributed positively to mutagenicity at ×1 concentrations, with the remaining inducing positive response when present in high concentrations (≥×10). Only 2 samples produced ROS at both tested 30 min and 1 h exposure time, but both would require ≥×10 concentrate to induce ROS. In such specialty hospital wastewater, majority of the chemical components was medications, followed by illicit drugs, as was anticipated for a specialty hospital that provides drug addiction and psychological treatment to patients. The study utilized databases and literature to in-silico assess the overall mutagenicity and ROS production profile of the wastewater. In general, in-silico characterization resulted in a large number of inconclusive data, suggesting that further studies are needed to understand the nature of the chemical contaminants, particularly as it relates to their concentrations and stoichiometry, as well as their biochemical mechanisms. Meanwhile, in-vitro experiments to determine mutagenicity and ROS production denote that majority of the samples did not induce mutagenicity and ROS. Mutagenic effect was only observed in 4 samples when they are concentrated by at least ×10, and can be arising from the presence of the chemical constituents profiled within the hospital wastewater samples or due to other potentially mutagenic compounds (e.g., heavy metals and disinfectant residues used in the hospital) that may also be present but not elucidated in detail in this study. However, as is observed for the Jun-22 samples, mutagenicity and ROS production can result in an increase in natural transformation among competent bacterium like A. baylyi.
Overall, the results of this study demonstrated that there may still be a need to consider ad-hoc contributions of mutagenic and/or toxic wastewater streams from the hospitals that can in turn trigger horizontal gene transfer events. In those instances, there may be a likelihood of detrimentally impacting the downstream recipient environment, including biological wastewater treatment processes. Hence an on-site treatment at the point of generating hospital wastewaters may be an option to minimize such concerns.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
FB: Conceptualization, Methodology, Data acquisition, formal analysis, writing—original draft. T-NW: Methodology, Data acquisition, formal analysis. MZ: Methodology, formal analysis. MA: Sampling. YB: Sampling. HA: Sampling. P-YH: Conceptualization, resources, supervision, writing—review and editing.
This study was funded by KAUST baseline grant BAS/1/1033-01-01 and by Smart Health Initiative for KAUST Rapid Research Response Team awarded to P-YH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to acknowledge Daniele Daffonchio for gifting the Acinetobacter baylyi ADP1 used in ROS and natural transformation experiments. The authors would also like to thank Dr Shuo Zhang for his technical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.845448/full#supplementary-material
Ames, B. N., Lee, F. D., and Durston, W. E. (1973). An Improved Bacterial Test System for the Detection and Classification of Mutagens and Carcinogens. Proc. Natl. Acad. Sci. 70, 782–786. doi:10.1073/pnas.70.3.782
Ameziane-El-Hassani, R., and Dupuy, C. (2013). Detection of Intracellular Reactive Oxygen Species (CM-H2DCFDA). Bio-protocol 3, e313. doi:10.21769/bioprotoc.313
Augsburger, N., Mantilla-Calderon, D., Daffonchio, D., and Hong, P.-Y. (2019). Acquisition of Extracellular DNA by Acinetobacter Baylyi ADP1 in Response to Solar and UV-C254nm Disinfection. Environ. Sci. Technol. 53, 10312–10319. doi:10.1021/acs.est.9b01206
Castillo Meza, L., Piotrowski, P., Farnan, J., Tasker, T. L., Xiong, B., Weggler, B., et al. (2020). Detection and Removal of Biologically Active Organic Micropollutants from Hospital Wastewater. Sci. Total Environ. 700, 134469. doi:10.1016/j.scitotenv.2019.134469
Crook, J., Bull, R., Collins, H., Cotruvo, J., and Jakubowski, W. (2013). Examining the Criteria for Direct Potable Reuse: Recommendations of an NWRI Independent Advisory Panel. Fountain ValleyCalif: National Water Research Institute.
Escher, B. I., Baumgartner, R., Koller, M., Treyer, K., Lienert, J., and Mcardell, C. S. (2011). Environmental Toxicology and Risk Assessment of Pharmaceuticals from Hospital Wastewater. Water Res. 45, 75–92. doi:10.1016/j.watres.2010.08.019
Falås, P., Wick, A., Castronovo, S., Habermacher, J., Ternes, T. A., and Joss, A. (2016). Tracing the Limits of Organic Micropollutant Removal in Biological Wastewater Treatment. Water Res. 95, 240–249. doi:10.1016/j.watres.2016.03.009
Galvin, S., Boyle, F., Hickey, P., Vellinga, A., Morris, D., and Cormican, M. (2010). Enumeration and Characterization of Antimicrobial-Resistant Escherichia coli Bacteria in Effluent from Municipal, Hospital, and Secondary Treatment Facility Sources. Appl. Environ. Microbiol. 76, 4772–4779. doi:10.1128/aem.02898-09
Jiménez, B., and Asano, T. (2008). Water Reuse: An International Survey of Current Practice, Issues and Needs. IWA London.
Jongen, W. M. F., Alink, G. M., and Koeman, J. H. (1978). Mutagenic Effect of Dichloromethane on Salmonella typhimurium. Mutat. Research/Fundamental Mol. Mech. Mutagenesis 56, 245–248. doi:10.1016/0027-5107(78)90191-4
Kumari, A., Maurya, N. S., and Tiwari, B. (2020). Hospital Wastewater Treatment Scenario Around the globe. Curr. Dev. Biotechnol. Bioeng., 549–570. doi:10.1016/b978-0-12-819722-6.00015-8
Łuczkiewicz, A., Jankowska, K., Fudala-Książek, S., and Olańczuk-Neyman, K. (2010). Antimicrobial Resistance of Fecal Indicators in Municipal Wastewater Treatment Plant. Water Res. 44, 5089–5097. doi:10.1016/j.watres.2010.08.007
Majewsky, M., Gallé, T., Yargeau, V., and Fischer, K. (2011). Active Heterotrophic Biomass and Sludge Retention Time (SRT) as Determining Factors for Biodegradation Kinetics of Pharmaceuticals in Activated Sludge. Bioresour. Technol. 102, 7415–7421. doi:10.1016/j.biortech.2011.05.032
Mantilla-Calderon, D., Plewa, M. J., Michoud, G., Fodelianakis, S., Daffonchio, D., and Hong, P.-Y. (2019). Water Disinfection Byproducts Increase Natural Transformation Rates of Environmental DNA in Acinetobacter Baylyi ADP1. Environ. Sci. Technol. 53, 6520–6528. doi:10.1021/acs.est.9b00692
Maron, D. M., and Ames, B. N. (1983). Revised Methods for the Salmonella Mutagenicity Test. Mutat. Research/Environmental Mutagenesis Relat. Subjects 113, 173–215. doi:10.1016/0165-1161(83)90010-9
Mayoudom, E. V. T., Nguidjoe, E., Mballa, R. N., Tankoua, O. F., Fokunang, C., Anyakora, C., et al. (2018). Identification and Quantification of 19 Pharmaceutical Active Compounds and Metabolites in Hospital Wastewater in Cameroon Using LC/QQQ and LC/Q-TOF. Environ. Monit. Assess. 190, 723. doi:10.1007/s10661-018-7097-1
Mazzitelli, J.-Y., Budzinski, H., Cachot, J., Geffard, O., Marty, P., Chiffre, A., et al. (2018). Evaluation of Psychiatric Hospital Wastewater Toxicity: what Is its Impact on Aquatic Organisms? Environ. Sci. Pollut. Res. 25 (26), 26090–26102. doi:10.1007/s11356-018-2501-5
Mendoza, A., Aceña, J., Pérez, S., López de Alda, M., Barceló, D., Gil, A., et al. (2015). Pharmaceuticals and Iodinated Contrast media in a Hospital Wastewater: a Case Study to Analyse Their Presence and Characterise Their Environmental Risk and hazard. Environ. Res. 140, 225–241. doi:10.1016/j.envres.2015.04.003
Nappier, S. P., Soller, J. A., and Eftim, S. E. (2018). Potable Water Reuse: What Are the Microbiological Risks? Curr. Envir Health Rpt 5, 283–292. doi:10.1007/s40572-018-0195-y
Neemann, J., Colston, J., Krasner, S., Law, I., and Whitson, A. (2020). Enchanced Source Control Recommendations for Direct Potable Reuse in California.
Paulus, G. K., Hornstra, L. M., Alygizakis, N., Slobodnik, J., Thomaidis, N., and Medema, G. (2019). The Impact of On-Site Hospital Wastewater Treatment on the Downstream Communal Wastewater System in Terms of Antibiotics and Antibiotic Resistance Genes. Int. J. Hyg. Environ. Health 222 (4), 635–644. doi:10.1016/j.ijheh.2019.01.004
Pauwels, B., and Verstraete, W. (2006). The Treatment of Hospital Wastewater: an Appraisal. J. Water Health 4, 405–416. doi:10.2166/wh.2006.0024
Rizzi, A., Pontiroli, A., Brusetti, L., Borin, S., Sorlini, C., Abruzzese, A., et al. (2008). Strategy for In Situ Detection of Natural Transformation-Based Horizontal Gene Transfer Events. Appl. Environ. Microbiol. 74, 1250–1254. doi:10.1128/aem.02185-07
Sharma, P., Mathur, N., Singh, A., Sogani, M., Bhatnagar, P., Atri, R., et al. (2015). Monitoring Hospital Wastewaters for Their Probable Genotoxicity and Mutagenicity. Environ. Monit. Assess. 187, 4180–4189. doi:10.1007/s10661-014-4180-0
Trussell, R. S., Pecson, B. M., Pisarenko, A. N., Idica, E. Y., Howe, E. W., and Trussell, R. R. (2018). Demonstrating Redundancy and Monitoring to Achieve Reliable Potable Reuse.
Verlicchi, P., Al Aukidy, M., Galletti, A., Petrovic, M., and Barceló, D. (2012). Hospital Effluent: Investigation of the Concentrations and Distribution of Pharmaceuticals and Environmental Risk Assessment. Sci. Total Environ. 430, 109–118. doi:10.1016/j.scitotenv.2012.04.055
Vijay, U., Gupta, S., Mathur, P., Suravajhala, P., and Bhatnagar, P. (2018). Microbial Mutagenicity Assay: Ames Test. Bio Protoc. 8, e2763. doi:10.21769/BioProtoc.2763
Wang, Y., Lu, J., Mao, L., Li, J., Yuan, Z., Bond, P. L., et al. (2019). Antiepileptic Drug Carbamazepine Promotes Horizontal Transfer of Plasmid-Borne Multi-Antibiotic Resistance Genes within and across Bacterial Genera. Isme J. 13, 509–522. doi:10.1038/s41396-018-0275-x
Wang, Y., Lu, J., Zhang, S., Li, J., Mao, L., Yuan, Z., et al. (2020). Non-antibiotic Pharmaceuticals Promote the Transmission of Multidrug Resistance Plasmids through Intra-and Intergenera Conjugation. ISME J., 1–16.
Yilmaz, G., Kaya, Y., Vergili, I., Beril Gönder, Z., Özhan, G., Ozbek Celik, B., et al. (2017). Characterization and Toxicity of Hospital Wastewaters in Turkey. Environ. Monit. Assess. 189, 55. doi:10.1007/s10661-016-5732-2
Zaouri, N., Cheng, H., Khairunnisa, F., Alahmed, A., Blilou, I., and Hong, P.-Y. (2021). A Type Dependent Effect of Treated Wastewater Matrix on Seed Germination and Food Production. Sci. Total Environ. 769, 144573. doi:10.1016/j.scitotenv.2020.144573
Keywords: hospital wastewater, chemical contaminants, Ames Test, mutagenicity, intracellular ROS, natural transformation
Citation: Baasher F, Wang T-N, Zulhelmi Bin Yusnan M, Alkahtani M, Bashawri YM, Al Qarni H and Hong P-Y (2022) Characterizing the Chemical Contaminants Diversity and Toxic Potential of Untreated Wastewater From a Drug Rehabilitation Hospital: Understanding Impact on Downstream Environment. Front. Environ. Sci. 10:845448. doi: 10.3389/fenvs.2022.845448
Received: 29 December 2021; Accepted: 03 March 2022;
Published: 24 March 2022.
Edited by:
Parinda Thayanukul, Mahidol University, ThailandReviewed by:
Suthida Theepharaksapan, Srinakharinwirot University, ThailandCopyright © 2022 Baasher, Wang, Zulhelmi Bin Yusnan, Alkahtani, Bashawri, Al Qarni and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei-Ying Hong, cGVpeWluZy5ob25nQGthdXN0LmVkdS5zYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.