- Agricultural and Ecological Research Unit, Indian Statistical Institute, Kolkata, India
Phytoremediation is gaining interest in recent years as it is a simple and effective strategy for heavy metal decontamination. The most straightforward strategy for successful heavy metal clean-up is searching for efficient hyperaccumulator species that grow naturally in contaminated sites. The present study, therefore, is the first detailed account of hyperaccumulator potentialities of a neglected and underutilized (NUS) species, Cleome rutidosperma DC. Hydroponic screening experiment against cadmium and lead revealed that even at 10 mg/kg concentration, it could accumulate 42.49 mg/kg of Cd and 27.79 mg/kg of Pb in shoots, while it could accumulate 134.71 mg/kg Cd and 491.35 mg/kg of Pb in its roots, and these values were significantly higher than those of the control plants. This plant could efficiently accumulate as high as 639.07 mg/kg of Cd, 8,726.03 mg/kg of Pb in its roots, while it could accumulate 752.83 mg/kg Cd and 3,732.64 mg/kg Pb in its shoots as evident from the pot experiments. In the case of Cd, there was no significant effect of toxicity on the phytophysiological parameters. But increasing concentrations of Pb did have toxic effects on the total chlorophyll content. This plant showed to have a BCF >1 in most of the tested concentrations. At the highest treatment concentration, however, both the BCF and TF were found to be greater than 1. This indicated that C. rutidosperma can accumulate and translocate the heavy metals to its aerial parts when the metal concentration is extremely high, proving itself to be an efficient hyperaccumulator. In order to decode the chemical signals, this plant may emit through the roots to cope with stress; root exudates were collected, purified, and analyzed through GCMS. This revealed the presence of five major compounds, namely, palmitic acid, linoleic acid, oleic acid, campesterol, and stigmasterol, which mainly are metabolic markers for detoxification mechanisms triggered by various stresses. Therefore, based on this study, C. rutidosperma can be termed a potent hyperaccumulator and can further be exploited for remediation of other classes of environmental pollutants.
1 Introduction
Pollution and heavy metal contamination of the environment is one of the most severe problems in the world (Liu et al., 2009; Mishra et al., 2019). Such contamination is posing serious threats to the health of all the living beings on this planet by degrading the quality of the main resources which allow life to thrive, that is, water, air, and soil. The main reasons behind such deterioration are anthropogenic activities like imprudent industrialization, exhaustive agricultural activities, and uncontrolled mining practices. These have led to a drastic increase in inorganic contaminants like cadmium (Cd), chromium (Cr), zinc (Zn), arsenic (As), and lead (Pb) in the soil (Zhang et al., 2010; Alengebawy et al., 2021). Cd and Pb are two of the major environmental pollutants, and are known to contaminate the food chain. Cd can enter the human body through food crops (Rigby and Smith 2020). Even if present at low concentrations in soil, it may cause toxic effects (Sharma et al., 2010). Cd has been listed seventh on the priority list of hazardous substances (Agency for Toxic Substances and Disease Registry, 2019).
Lead (Pb) is released into the environment from the burning of coal, vehicular exhausts, electric batteries, paints, explosives, sewage sludge, petroleum, and metallurgical activities (Sharma and Dubey 2005; Masindi, and Muedi 2018; Weldeslassie et al., 2018). Pb has been ranked second on the priority list of hazardous substances (Agency for Toxic Substances and Disease Registry, 2019). In plants, Cd and Pb have no physiological relevance, and are readily taken up by the plants to toxic concentrations, posing a serious threat to the consumers (Ramesar et al., 2014). The toxic effects include retardation of various metabolic processes like photosynthesis, respiration, water transport, nitrogen metabolism and nutrient uptake (Amari et al., 2017).
Recently, many conventional and non-conventional techniques have been suggested for the decontamination of polluted soils. The major goals of such strategies are as follows: 1) to decrease the heavy metal content of the contaminated soils and 2) to re-establish the chemical and biological qualities of the soil to maintain its fertility. The conventional clean-up techniques like soil excavation, solidification, vitrification, stabilization, incineration, soil flushing, electro-kinetic systems, and landfill have several drawbacks including cost, procedural complexity, regulatory burden, and lack of complete degradation (Agnello et al., 2016; Ghosal et al., 2016; Yan et al., 2020).
The use of plants to reduce contaminants and restore our natural resources like soil, water, and the air is gaining much attention from environmental perspectives (Yan et al., 2020). Phytoremediation has emerged as a promising, in situ, cost-effective, green, and cleaner technology that employs hyperaccumulator plant species for the treatment of contaminants (Datta and Sarkar 2005; de Souza Miranda et al., 2022). Hyperaccumulator plants have higher fitness to tolerate and grow on metal-rich soils (Cappa and Pilon-Smits 2014). Phytoextraction and phytostabilization are the two main strategies implemented by the plants, to decrease the metal content and/or immobilize those in the polluted soils (Anjum et al., 2014). A remediation system can be successful depending on the efficiency of the plant of interest to endure and grow luxuriantly under heavy metal stress during phytoextraction and phytostabilization (Ali et al., 2013). The most forthright strategy for heavy metal clean-up is searching for efficient hyperaccumulator species. A feasible approach for the same is the observation of naturally proliferating species at the contaminated sites, which can help in identifying potential plant species. It has been widely reported that plants that are native to contaminated sites like sewage disposal sites and household dumpyards have shown promise for phytoextraction (Merkl et al., 2005). Therefore, identification and exploration of novel species inhabiting such areas that yield high biomass and grow naturally were our foremost criteria for the successful phytoremediation of Cd- and Pb-contaminated soils. According to the earlier studies, most of the plants that were reported to be highly efficient in phytoextraction mostly belonged to family Brassicaceae. While screening the literature, we came across family Cleomaceae which is the sister family of Brassicaceae (Chase and Reveal 2009). This family has three species, namely, Cleome rutidosperma, C. viscosa, and C. gynandra that are ubiquitously present in India. Studies carried out by Abidemi et al., 2014 showed heavy metal accumulation abilities in Cleome viscosa. In our field studies, however, we observed that Cleome rutidosperma was growing profusely in local waste dumpyards throughout the year. Therefore, we were interested to study the capabilities of this species to tolerate and proliferate in such contaminated soil.
Cleome rutidosperma DC. is an annual herb that grows up to the height of 1 m. This plant was selected for our study due to its distinctive growth habitat. This species characteristically grows and proliferates naturally in disturbed, waste dump areas (Burkill 1995). The plant habit is erect, and has alternate trifoliate leaves and an extensive root system. Flowers are small, and pink or violet in color. The fruits are capsules with an average length of 5–7 cm producing seeds as small as 2 mm in diameter (Hooker 1872; Saxena and Saxena 2001). C. rutidosperma is a native species of West Africa but has naturalized in vast areas of tropical America as well as Southeast Asia (Burkill 1995). According to traditional use, the different parts like leaves, roots, and seeds of the plants of the Cleome genus are used as stimulants, antiscorbutic, diuretic, anthelmintic, rubefacient, vesicant, and carminative (Ghosh et al., 2019).
Our present work is the first report of Cleome rutidosperma as a potent phytoremediator plant. Therefore, we have encompassed a meticulous study of the phytoremediation potential of Cleome rutidosperma against two of the most notorious heavy metals cadmium and lead through hydroponic as well as pot culture experiments. It accounts for the uptake and immobilization of both the tested heavy metals by the plant body and takes into consideration the toxic effects of the high concentrations of metals mainly depicted as the biomass content and total chlorophyll content. The plants interact with the environment through signaling compounds that code for their unique language. The plants mainly communicate through the root with the help of chemical signals. Therefore, we were also interested in analyzing the bioactive compounds present in the root exudates of this plant with the help of GCMS in order to decrypt the distinctive vocabulary this species uses that enables it to survive in the highly contaminated areas.
2 Materials and Methods
2.1 Plant Material and Soil Collection
Mature plants with flowers and fruits were collected from the local waste disposal areas around Kolkata (22.6494° N, 88.3805° E), West Bengal, India, in August 2019, and identified with the type specimen at the Botanical Survey of India, Shibpur, Howrah, West Bengal, India. A voucher specimen (Cr02a) is preserved in our departmental herbarium for future reference. Seeds of the identified plants were collected after proper ripening of fruits. Those were surface-sterilized with 0.01% of HgCl2 and washed properly with distilled water repeatedly (Sidhu et al., 2017). Then the seeds were placed on moist filter papers and were allowed to germinate for 10 days. The healthy seedlings of 3–4 cm were selected for further experiments.
The soil required for the pot experiments was collected from the institute garden. The garden was mainly a region of natural vegetation and was free from any external disturbance. The topsoil (up to 20 cm depth) was taken manually. The soil was then air-dried inside the laboratory storehouse for a week. The air-dried soil was then crushed manually and sieved through a sieve of <4 mm mesh size to remove small stones or gravels or any unwanted debris. The dimension of the pots were 12 cm × 8 cm (dia x height). The pots filled with soil were kept for 7 days for maturing, and the planting was done afterward (Sidhu et al., 2018).
2.2 Hydroponic Screening Experiment
Plants were grown in a hydroponic environment to study their ability to accumulate and tolerate heavy metal stress of Cd and Pb (Niu et al., 2007). Seedlings were placed through perforations in a plastic platform in a 1000-ml glass jar containing 500 ml of Hoagland’s solution (Hoagland and Arnon, 1938) so that the roots were immersed in a liquid medium and the shoots remained above the platform. The heavy metal salts (reagent grade) used in this study included CdCl2·2.5H2O and Pb(NO3)2·H2O. The salts were separately dissolved in deionized water and added into hydroponic plant culture, respectively. Treatments were prepared at 10 mg/kg concentrations for both metals. A control that contained a nutrient medium devoid of the metal salts was maintained. All solutions were adjusted to pH 7.0–7.2. The experimental setup was maintained for 60 days. After the incubation in greenhouse conditions, the plants were harvested and phytophysiological parameters were recorded.
2.3 Pot Experiments
Pot experiments were performed following a completely randomized design under greenhouse conditions (Sidhu et al., 2018). The natural soil was spiked with heavy metals salts, namely, CdCl2·2.5H2O and Pb(NO3)2·H2O. Three replicates were setup for each of the 10 different concentration levels of the metal treatments including control. Altogether, 30 individual pots were maintained in this study. There were nine different Pb and Cd concentrations, equivalent to 10, 20, 30, 40, 50, 75, 100, 150, and 200 mg/kg soil, respectively. The spiked soils (1 kg each) were filled in polythene bags and placed in plastic pots to avoid metal leaching from soils. After spiking prior to the seedling plantation, the soils were equilibrated for 7 days. The plants were maintained for 60 days under the same conditions before harvesting. Triplicates of the control plants were maintained in soil devoid of metal salts. The moisture content was maintained at 60% throughout the experimental period using distilled water.
2.4 Analysis of Heavy Metals
The heavy metal concentration in the plant tissue as well as in the soil was analyzed using the AAS. Plants were harvested after 60 days and separated into root and shoot. The plant parts were washed repeatedly with distilled water and 0.01% EDTA solution to get rid of any heavy metal residue in them. The plant tissues were then oven-dried at 80°C for 72 h. The dried plant parts were then weighed, grounded, and digested using the tri-acid mixture (Hseu 2004).
2.5 Soil Properties
Physicochemical analyses of the soil that is used in the experiment including pH, organic carbon, and available NPK (Jackson 1958) were carried out along with Cd and Pb contents for the same. The physicochemical characteristics revealed pH of 7.45 ± 0.05, organic carbon of 0.83 ± 0.17 (%), phosphorous of 11.3 ± 0.54 mg/kg, potassium of 93.2 ± 1.21 mg/kg, available nitrogen of 112 ± 1.93 mg/kg, Cd content of 3.875 ± 0.125 mg/kg, and Pb content of 3.75 ± 0.5 mg/kg. The residual Cd and Pb contents in the soil (in the control and different treatments) and removal percentages for both the metals, after 60 days of growth period, are given in Supplementary Table S1.
2.6 Determination of Phytophysiological Effects of Toxicity
The phytophysiological effects of the heavy metal stress were determined using two parameters, namely, change in dry biomass and total chlorophyll content. The total chlorophyll content was measured using the following equation (Arnon 1949).
where A = absorbance of specific wavelength, V = final volume of chlorophyll extract in 80% acetone, and W = fresh weight of the tissue extract.
2.7 Determination of Bioconcentration Factor and Translocation Factor
In order to determine the type of remediation a plant performs, that is, whether it has phytoextraction abilities or it immobilizes the heavy metals by phytostabilization, two main indices, namely, bioconcentration factor (BCF) and translocation factor (TF), are calculated based on the heavy metal contents of the plant parts. Plants can be considered good phytoextractors if the BCF >1 and TF > 1 which indicate that those plants can translocate the heavy metals into their shoots. On the other hand, if a plant exhibits the BCF >1 but TF < 1, then those species can be categorized as phytostabilizer which have the ability to immobilize the heavy metal in its rhizosphere (Ali et al., 2013).
BCF signifies the ability of the plant to accumulate and immobilize the heavy metals in its tissues. It is expressed as the ratio of metal accumulated in the below-ground tissues (roots) of the plant to that present in the soil (Zhuang, et al., 2007; Bonanno and Vymazal 2017).
TF is the efficiency of plants to translocate the metal accumulated to its above-ground tissues (stem and leaves). It is expressed as the ratio of the metal content in the shoots to that of the roots of a plant (Zhuang et al., 2007; Bonanno and Vymazal 2017).
2.8 Collection and Purification of Root Exudates of C. rutidosperma
Cleome rutidosperma plants were grown in special root exudate trapping systems (Figure 1) which consist of a Buchner funnel (dia = 110 mm) and conical flasks of 500 ml capacity (Jana and Biswas 2011). The sieve inside the Buchner funnel was removed. The funnel was filled with soil after placing a piece of cotton cloth at the mouth of the funnel to hold the soil. The conical flasks were painted black to avoid the growth of fungus or algae. The germinated seeds (6–10) of C. rutidosperma were sown in each funnel. An average of 5–6 plants depending on the growth, size, and number of leaves were allowed to grow in each set till maturity. Plant roots penetrated the soil in the funnel and extended into the flasks after 20–25 days. The flasks contained distilled water. The plants release compounds into the water, which is further referred to as the root exudates. Root exudates were collected every 7 days, and the flasks were filled with fresh distilled water. This procedure was performed for a period of 4 months. The collected exudates were dried in a vacuum evaporator, extracted, and purified using the solvent extraction method followed by TLC and column chromatography. The purified root exudates were then sent for identification using GCMS analysis.

FIGURE 1. Collection of root exudates from Cleome rutidosperma DC. (A) Total experimental setup, (B) root exudates trapping system, and (C) roots extend downward into the conical flasks containing distilled water.
2.9 GC-MS Analysis of Purified Root Exudates of C. rutidosperma
Purified root exudates of C. rutidosperma were subjected to GC-MS analysis (Model No. Agilent Technologies, GC-6860N Network GC System with 5973 inert Mass Selective Detector) for detecting bioactive compounds. The GC-MS analysis was done at the National Test House, Salt Lake, Sector V, Kolkata, WB, India. HP-1MS column (25 m × 0.33 mm, i. d. 0.25 μm) was used; 0.1 μL of root exudates of C. rutidosperma (dissolved in chloroform) was injected into GC set in the split mode for analysis at an injector temperature of 280°C. Helium gas was used as the carrier gas with a flow rate of 1 ml/min. The oven temperature was programmed as follows: 45°C (1 min hold), 45–220°C at 7°C/min, and 220–300°C at 6°C/min, 200°C (2 min). The electron ionization mode with ionization energy of 70 eV was employed by the MS. A full scan mode was used with an ion source temperature of 280°C and an acquisition rate of 0.2 s. The mass range was adjusted to 50–350 Da.
The compound identification was performed by comparing the mass spectra with the spectral data of the NBS75K library provided by the GC/MS control and data processing software.
2.10 Statistical Analysis
The data were tested for normality using the Kruskal–Wallis test. T-test and one-way ANOVA were performed to analyze the differences in the parameters of the treated plants with respect to the experimental control. The correlation between the parameters and the tested concentrations was also studied. The statistical analysis was performed using SPSS 21 (IBM Corp. Released, 2012) software.
3 Results and Discussion
3.1 Hydroponic Screening Experiment
The hydroponic screening experiment revealed that the biomass of root for the control was 0.56 ± 0.04 g, while the biomass for the Cd and Pb treated plants were 0.65 ± 0.06 g and 0.58 ± 0.03 g, respectively (Figure 2). The total chlorophyll content was recorded to be 1.576 ± 0.17 mg/g in the control, while it was 1.39 ± 0.35 mg/g and 1.35 ± 0.09 mg/g in Cd- and Pb-treated plants, respectively. Statistically, no significant differences were observed in the total biomass and total chlorophyll content compared to the control, which indicate that the plant can tolerate heavy metal stress without showing any significant signs of toxicity. Our results were in accordance with hydroponic studies done on Brassica juncea, which showed no significant difference along the Cd treatments ranging from 1 to 50 µM Cd (Ying et al., 2021). But the AAS analysis revealed that even at the low concentration of 10 mg/kg, C. rutidosperma could accumulate 42.49 mg/kg of Cd in shoots and 134.71 mg/kg of Cd in roots. In the case of Pb, the plant could store 27.79 mg/kg in shoots and 491.35 mg/kg in its roots. These values were significantly higher than those of control plants (Figure 3).

FIGURE 2. Phytophysiological effects of heavy metal stress on Cleome rutidosperma based on hydroponic experiments: (A) and (B) biomass of shoot and root, respectively, and (C) total chlorophyll content. The results did not differ significantly w.r.t control (F values and “degrees of freedom (df)” are given in the respective graphs).
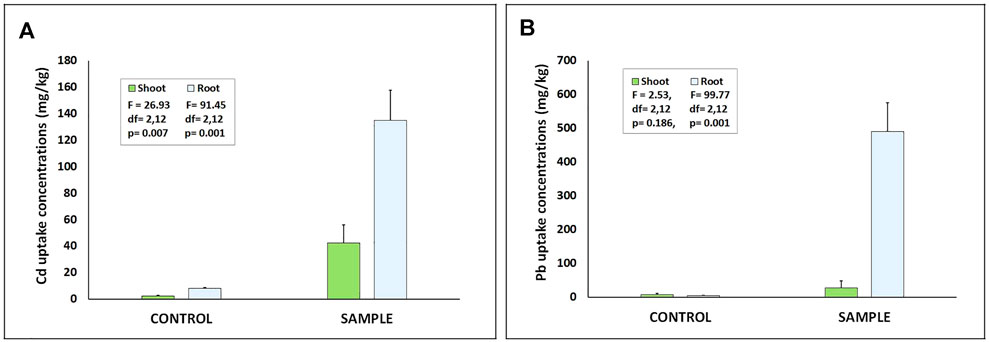
FIGURE 3. Heavy metal uptake by roots and shoots of Cleome rutidosperma recorded in hydroponic experiments at 10 mg/kg tested concentration of (A) cadmium and (B) lead. There was a significant difference in the metal concentrations in the sample w.r.t control as well as the roots w.r.t to the shoots (F values and “df” are given in the respective graphs).
3.2 Pot Experiments
C. rutidosperma could efficiently accumulate as high as 639.07 mg/kg of Cd, 8,726.03 mg/kg of Pb in its roots, while could accumulate 752.83 mg/kg of Cd and 3,732.64 mg/kg of Pb in its shoots at the highest treatment concentrations of 200 mg/kg of Cd and 150 mg/kg of Pb, respectively (Figure 4). The Cd content significantly increased from 256.5 mg/kg to 639.07 mg/kg in roots and from 90.93 mg/kg to 752.83 mg/kg in shoots. There was a linear increase in the uptake of both the metals in their shoots and roots with increasing heavy metal exposure (10–200 mg/kg). The R2 values for Cd uptake in shoots and roots were 0.991 and 0.738, respectively.
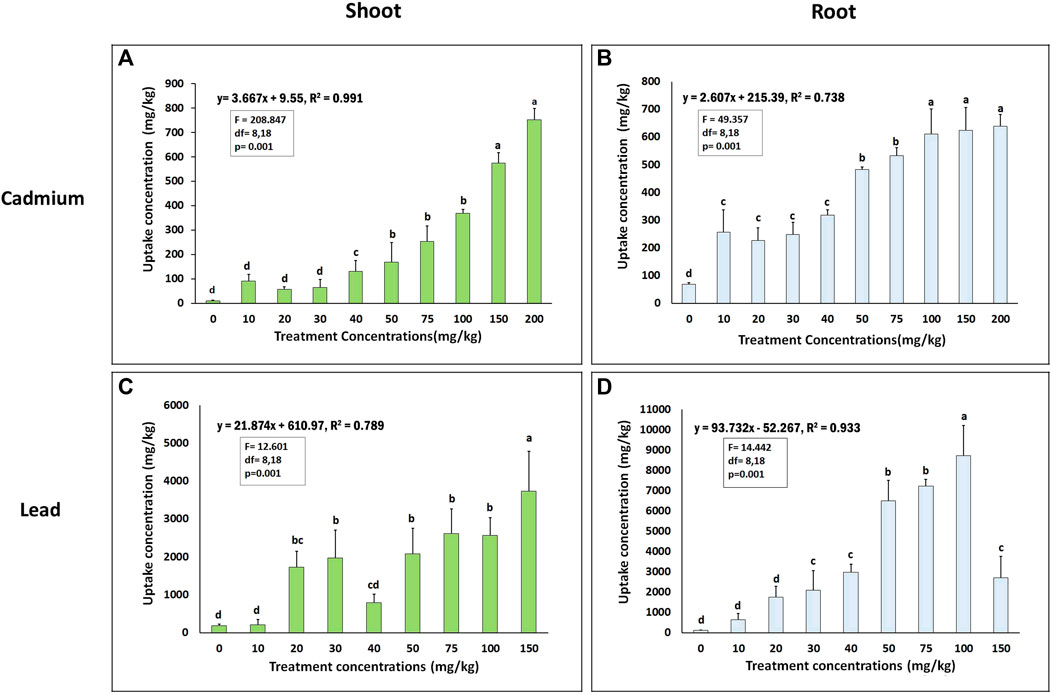
FIGURE 4. Heavy metal uptake by roots and shoots of Cleome rutidosperma recorded in pot experiments under different treatment concentrations of (A) and (B) cadmium, (C) and (D) lead in shoots and roots, respectively. The bars marked with different alphabets (a,b,c,d) are significantly different from each other (F values and “df” are given in the respective graphs). R2 represents the correlation between the content of metal in root–shoot tissues versus the soil at p ≤ 0.05.
In the case of Pb, C. rutidosperma could efficiently accumulate about 80 times more Pb in its roots as compared to the amount of Pb in the soil. In several studies, Pb has been reported to be preferentially stored in roots (Wang et al., 2014). The plant could accumulate a maximal amount of Pb, that is, 8,726.03 mg/kg, in its roots at the 100 mg/kg concentration, while the plant accumulated 3,732 mg/kg of Pb in its shoots at 150 mg/kg treatment. There was a linear correlation in the Pb uptake for both the shoot and roots with an R2 value of 0.789 and 0.933, respectively.
In agreement with other findings, we observed that the roots of the plants that were primarily exposed to heavy metal stress retained a significant amount of Cd as well as Pb (Gavrilescu 2022). As reported in Cd hyperaccumulator species, namely, Arabis paniculata, Brassica napus, and Calendula officinalis, the main organic compounds are metallothioneins and phytochelatins that help to sequester and accumulate Cd within the root cells (Liu et al., 2008; Zeng et al., 2009; Ehsan et al., 2014; Awa and Hadibarata 2020). Celosia cristata pyramidalis, an ornamental plant, has been reported to accumulate up to three times more Pb in its roots than shoots (Cui et al., 2013). As explained by the earlier studies, such accumulation of metals in roots could be attributed to the subcellular compartmentalization of metals in vacuoles by the plant that helps it to cope with the possible toxicity imposed by increased heavy metal uptake (Riyazuddin et al., 2021). Another reason could be the formation of insoluble metal phosphates, carbonates, and bicarbonates that precipitate in the intercellular root spaces (Brennan and Shelley 1999), which in turn reduces the translocation of the same from roots to the shoots (Cunningham and Berti 2000).
C. rutidosperma may employ such strategies to restrict the excess translocation of Cd and Pb, therefore, protecting itself from metal-induced toxicity. However, C. rutidosperma also showed the tendency to translocate significant amounts of Cd and Pb in its aerial parts, which is a key criterion for a plant to have phytoextraction efficiency. This plant showed significant translocation at the higher treatment concentrations, which was also the trend observed in species of Brassica (Mourato et al., 2015). The plant accumulated 752.8 mg/kg of Cd and 3,732.63 mg/kg of Pb in its shoots at 200 mg/kg and 150 mg/kg treatment concentration, respectively. Such translocation of metals may have occurred due to the increase in the internal transport of aqueous free Cd and Pb ions, a process mediated by xylem loading while being regulated by xylem flux and endodermis (Uraguchi et al., 2009). The uptake of these metals may take place along with the essential metal nutrients like Zn, Cu, and Fe via the membrane transporters (Zheng et al., 2011) and also by the production of phytochelatins followed by formation of Pb–phytochelatin complexes within the vascular tissues (Andra et al., 2009).
In summary, the heavy metal content in roots and shoots of C. rutidosperma plants at all the treatments was well above the threshold level for Cd hyperaccumulators (˃100 mg/kg) and for Pd hyperaccumulators (>1,000 mg/kg) (Pollard et al., 2002; Sidhu et al., 2017). Moreover, unpalatability, high biomass yield, and shorter life span provide added advantages to make C. rutidosperma, a novel plant species to be exploited for Cd and Pb extraction from the polluted soils. These findings strongly support the potential of C. rutidosperma for both phytostabilization and phytoextraction of Cd and Pb from the polluted soils.
3.3 Phytophysiological Effects of Heavy Metal Toxicity on C. rutidosperma
Plant biomass and growth were significantly impacted by increasing heavy metal stress (Figure 5). In the case of Cd, the dry wt of both root and shoot increased significantly at Cd treatment concentrations of 20–40 mg/kg, respectively. The exact reason for such promotion in growth cannot be explained. However, such a response may be attributed to the phenomenon called hormesis in which a stimulatory effect in growth is noticed under the physiological toxic doses of heavy metal ions (Tang et al., 2009; Poschenrieder et al., 2013).
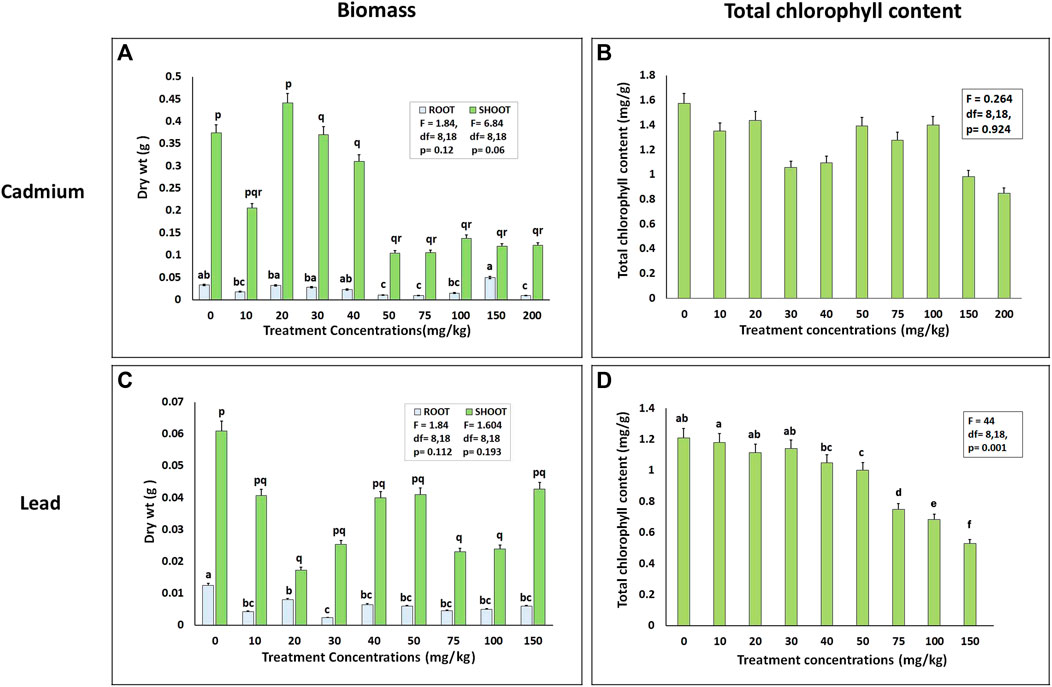
FIGURE 5. Phytophysiological effects of heavy metal stress on Cleome rutidosperma at different tested concentrations of (A) and (B) cadmium, (C), and (D) lead in terms of biomass and total chlorophyll content, respectively. The bars marked with different alphabets (a,b,c,d and p,q,r,s) are significantly different from each other (F values and “df” are given in the respective graphs).
The cadmium stress, however, did not show any significant effect on the total chlorophyll content of C. rutidosperma. The difference was not significant with respect to the control sets. On the contrary, the total chlorophyll content of C. rutidosperma exposed to lead stress did decrease significantly compared to the control in a dose-dependent manner (y = −0.005x + 1.2256; R2 = 0.957). The plants treated with 200 mg/kg Pb, in our studies, failed to survive. Therefore, we have the data of plants treated with 10–150 mg/kg of Pb. Similar observations were also reported in Coronopus didymus (Sidhu et al., 2017), Brassica napus (Shakoor et al., 2014), and Eichornia crassipes (Malar et al., 2014) under Pb stress where a decrease in the total chlorophyll content was detected.
3.4 Analysis of Bioconcentration Factor and Translocation Factor for C. rutidosperma
BCF was observed as greater than 1 for all the treatment concentrations in both the targeted heavy metals, that is, Cd and Pb. In the hydroponic experiments, with a treatment concentration of 10 mg/kg, the plant showed the highest BCF of 16.12 for Cd and 57.82 for Pb (Table 1). However, the TF values were lesser than 1 for both the metals tested. Pot culture experiments showed a similar trend. BCF values were all greater than 1 for both the metals at all the tested concentrations. The highest BCF recorded for Cd was 27, while that for Pb was 847.23 (Table 1). Here, an interesting fact observed was that the TF > 1 at the highest treatment concentration. The TF was 1.18 at 200 mg/kg concentration of Cd and 1.38 at 150 mg/kg concentration of Pb. An explanation to this may be that the higher heavy metal content in the rhizospheric soil had catalyzed the rapid uptake of heavy metal in the roots and from roots to its aerial parts (Islam et al., 2020). If we look closely, the metal uptake of Pb reached maximum at the preceding concentration of 100 mg/kg in the roots. Interestingly, however, the uptake drastically decreased at 150 mg/kg concentration. This may be due to the toxic effects of heavy metals induced at this threshold concentration (Ying et al., 2021). Additionally, the TF > 1 at that concentration, which indicates that the plant was translocating the Pb taken up by the roots efficiently (Wang et al., 2014). According to the studies done on heavy metal sequestration and detoxification, it was reported that plants tend to cope with the heavy metal stress by translocating and sequestering them in the aerial tissues (Singh et al., 2016). But when exposed to even higher stress levels, that is, 200 mg/kg, the plant failed to survive (Dubey et al., 2018).
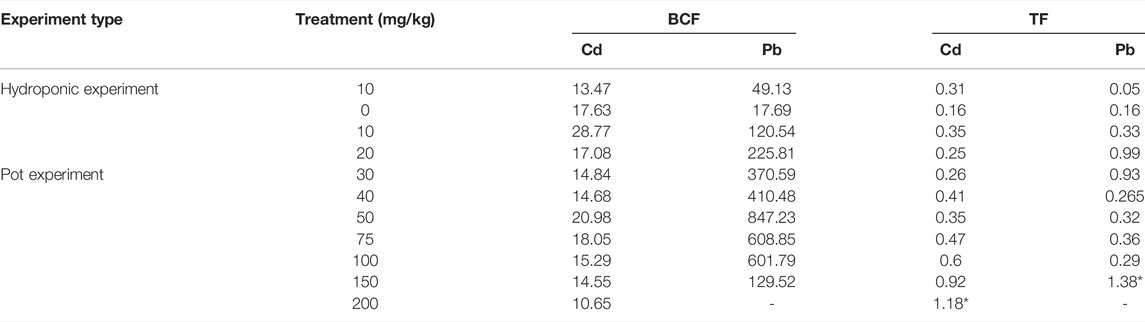
TABLE 1. Bioconcentration factor (BCF) and translocation factor (TF) of Cleome rutidosperma for both the hydroponic and pot experiments against Cd and Pb. The values marked with (*) are greater than 1, showing positive translocation of heavy metal to the aerial parts of the plant.
3.5 Chemical Profiling of the Purified Root Exudates Using GCMS
GCMS spectra of purified root exudates of C. rutidosperma revealed the presence of five major peaks along some minor peaks based on the percentage area of the peak (Figure 6). The major compounds detected were palmitic acid (retention time = 22.847), linoleic acid (retention time = 25.801), oleic acid (retention time = 25.89), campesterol (retention time = 39.099), and stigmasterol (retention time = 39.427). The minor compounds include stearic acid, ethyl lineolate, ethyl oleate, behenic acid, tricosanoic acid, and lignoceric acid.
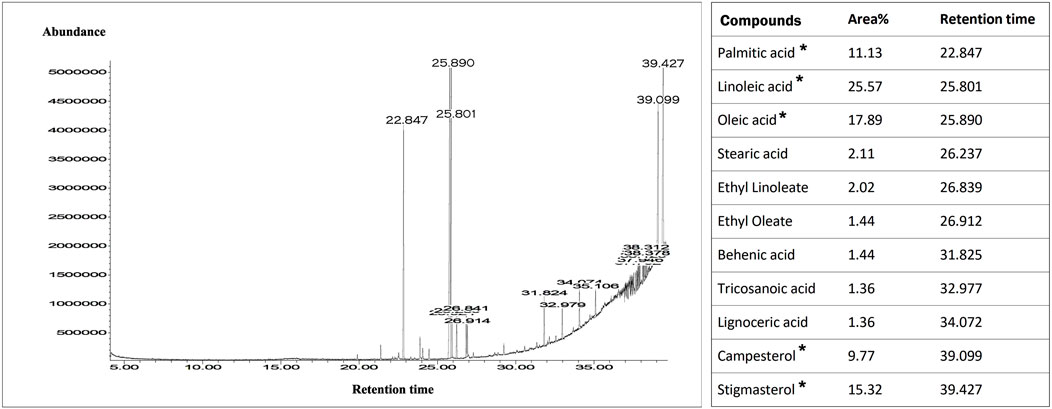
FIGURE 6. GCMS spectra of the purified root exudates of Cleome rutidosperma. The list of main compounds with the respective retention times is given in the table provided. The area % provides information about the abundance of the respective compounds. The compounds marked with (*) are the main compounds based on the abundance and sharp peaks.
Heavy metal stress induces the plant cells to generate highly reactive oxygen species (ROS) which can oxidize and degenerate cellular macromolecules such as DNA, pigments, proteins, lipids, and other essential molecules irreversibly (Berni et al., 2019; Castro et al., 2021). In order to prevent that, plants enable their defense strategies mainly by producing some alternate bioactive molecules which act as reaction centers for the generated ROS (Singh et al., 2016). Unsaturated fatty acids and esters like linoleic acid, ethyl linoleate, oleic acid, and ethyl oleate are compounds acting as molecular targets that scavenge ROS. The presence of these compounds in the root exudates clearly indicates their increased production to cope with the heavy metal stress (De Bigault Du Granrut and Cacas 2016). Peroxidation of reactive target molecules like linoleic acid and its methyl ester derivatives through radicals generated by the heavy metal stress protects the cells from extensive injury to cellular DNA (Guerzoni et al., 2001; Berni et al., 2019). Plants communicate with the environment using these chemical signals. In a study done by Yi and Crowley (2007), it has been reported that fatty acids act as a metabolic marker which stimulates poly-aromatic-hydrocarbon (PAH) degradation through roots. Therefore, this plant may also have a tendency to remediate such PAHs, which provides a future scope that requires thorough investigation. Apart from fatty acids, the precursor of steroidal compounds mainly brassinosteroids, that is, campesterol and stigmasterol, were also identified in the root exudates. There have been reports about the roles of these compounds in detoxification mechanisms in heavy metal stress conditions (Kapoor et al., 2022). The studies done on Cd-treated plants of Arabidopsis thaliana support these statements as concentrations of these compounds were observed to be considerably increased in the stress exposed plants as compared to the untreated control (Sun et al., 2010).
4 Conclusion
Our observations, therefore, provide enough information about a novel candidate plant species that can further be used for phytoremediation implications. It was also observed that there is a threshold value of Cd and Pb concentrations after which C. rutidosperma translocates the heavy metal to its aerial parts. Thus, it can be hypothesized that Cleome rutidosperma DC. may act as a phytostabilizer in low metal-contaminated areas, but may also act as an efficient hyperaccumulator in highly contaminated areas. The plant also possessed the characteristics of being able to remediate poly-aromatic-hydrocarbons either with help of its root exudates or by using microbes as mediators. Hence, to conclude, we proposed this plant as a potential candidate to be incorporated into successful phytoremediation strategies based on the results of our study supported by its natural habit and habitat.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
EB performed the experiments, analyzed the data, and prepared the manuscript. SMB has designed the experiments, provided the resources, and edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.830087/full#supplementary-material
References
Abidemi, I. O., Bosede, O. M., and Oladele, O. (2014). Lead and Cadmium Phytoremediation Potentials of Plants from Four lead Smelting Slags Contaminated Sites. Nat. Environ. 2 (3), 33–38. doi:10.12966/ne.11.01.2014
Agency for Toxic Substances and Disease Registry (ATSDR) (2019). CERCLA Priority List of Hazardous Substances. Available at: https://www.atsdr.cdc.gov/spl/index.html. Accessed on 20th Oct 2021.
Agnello, A. C., Bagard, M., van Hullebusch, E. D., Esposito, G., and Huguenot, D. (2016). Comparative Bioremediation of Heavy Metals and Petroleum Hydrocarbons Co-contaminated Soil by Natural Attenuation, Phytoremediation, Bioaugmentation and Bioaugmentation-Assisted Phytoremediation. Sci. Total Environ. 563-564, 693–703. doi:10.1016/j.scitotenv.2015.10.061
Alengebawy, A., Abdelkhalek, S. T., Qureshi, S. R., and Wang, M.-Q. (2021). Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 9 (3), 42. doi:10.3390/toxics9030042
Ali, H., Khan, E., and Sajad, M. A. (2013). Phytoremediation of Heavy Metals-Concepts and Applications. Chemosphere 91, 869–881. doi:10.1016/j.chemosphere.2013.01.075
Amari, T., Ghnaya, T., and Abdelly, C. (2017). Nickel, Cadmium and lead Phytotoxicity and Potential of Halophytic Plants in Heavy Metal Extraction. South Afr. J. Bot. 111, 99–110. doi:10.1016/j.sajb.2017.03.011
Andra, S. S., Datta, R., Sarkar, D., Makris, K. C., Mullens, C. P., and Sahi, S. V. (2009). Induction of lead-binding Phytochelatins in Vetiver Grass [Vetiveria Zizanioides (L.)]. J. Environ. Qual. 38, 868–877. doi:10.2134/jeq2008.0316
Anjum, N. A., Umar, S., and Iqbal, M. (2014). Assessment of Cadmium Accumulation, Toxicity, and Tolerance in Brassicaceae and Fabaceae Plants—Implications for Phytoremediation. Environ. Sci. Pollut. Res. 21, 10286–10293. doi:10.1007/s11356-014-2889-5
Arnon, D. I. (1949). Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 24, 1–15. doi:10.1104/pp.24.1.1
Awa, S. H., and Hadibarata, T. (2020). Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: a Review. Wat. Air Soil Poll. 231 (2), 1–15. doi:10.1007/s11270-020-4426-0
Berni, R., Luyckx, M., Xu, X., Legay, S., Sergeant, K., Hausman, J. F., et al. (2019). Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell wall and Secondary Metabolism. Environ. Exp. Bot. 161, 98–106. doi:10.1016/j.envexpbot.2018.10.017
Bonanno, G., and Vymazal, J. (2017). Compartmentalization of Potentially Hazardous Elements in Macrophytes: Insights into Capacity and Efficiency of Accumulation. J. Geochem. Explor. 181, 22–30. doi:10.1016/j.gexplo.2017.06.018
Brennan, M. A., and Shelley, M. L. (1999). A Model of the Uptake, Translocation, and Accumulation of lead (Pb) by maize for the Purpose of Phytoextraction. Ecol. Eng. 12, 271–297. doi:10.1016/S0925-8574(98)00073-1
Burkill, H. M. (1995). The Useful Plants of West Tropical Africa. 2. ed., 1-3. Richmond, UK: Royal Botanic Gardens, Kew, 976.
Cappa, J. J., and Pilon-Smits, E. A. (2014). Evolutionary Aspects of Elemental Hyperaccumulation. Planta 239, 267–275. doi:10.1007/s00425-013-1983-0
Castro, B., Citterico, M., Kimura, S., Stevens, D. M., Wrzaczek, M., and Coaker, G. (2021). Stress-induced Reactive Oxygen Species Compartmentalization, Perception and Signalling. Nat. Plants 7 (4), 403–412. doi:10.1038/s41477-021-00887-0
Chase, M. W., and Reveal, J. L. (2009). A Phylogenetic Classification of the Land Plants to Accompany APG III. Bot. J. Linn. Soc. 161 (2), 122–127. doi:10.1111/j.1095-8339.2009.01002.x
Cui, S., Zhang, T., Zhao, S., Li, P., Zhou, Q., Zhang, Q., et al. (2013). Evaluation of Three Ornamental Plants for Phytoremediation of Pb-Contamined Soil. Int. J. Phytorem 15, 299–306. doi:10.1080/15226514.2012.694502
Cunningham, S. D., and Berti, W. R. (2000). “Phytoextraction and Phytostabilization: Technical, Economic and Regulatory Considerations of the Soil-lead Issue,” in Phytoremediation of Contaminated Soil and Water. Editors N. Terry, and G. S. Banuelos (Boca Raton, FL: CRC Press), 359–376.
Datta, R., and Sarkar, D. (2005). Phytoextraction of Zinc and Cadmium from Soils Using Hyperaccumulator Plants. Water Encyclopedia 5, 369–374. doi:10.1002/047147844X.gw856
De Bigault Du Granrut, A., and Cacas, J. L. (2016). How Very-Long-Chain Fatty Acids Could Signal Stressful Conditions in Plants? Front. Plant Sci. 7, 1490. doi:10.3389/fpls.2016.01490
de Souza Miranda, R., Boechat, C. L., Bomfim, M. R., Santos, J. A. G., Coelho, D. G., Assunção, S. J. R., et al. (2022). “Phytoremediation: A Sustainable green Approach for Environmental Cleanup,” in Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water (Netherlands: Elsevier), 49–75. doi:10.1016/B978-0-323-85763-5.00017-9
Dubey, S., Shri, M., Gupta, A., Rani, V., and Chakrabarty, D. (2018). Toxicity and Detoxification of Heavy Metals during Plant Growth and Metabolism. Environ. Chem. Lett. 16 (4), 1169–1192. doi:10.1007/s10311-018-0741-8
Ehsan, S., Ali, S., Noureen, S., Mahmood, K., Farid, M., Ishaque, W., et al. (2014). Citric Acid Assisted Phytoremediation of Cadmium by Brassica Napus L. Ecotox. Environ. Safe 106, 164–172. doi:10.1016/j.ecoenv.2014.03.007
Gavrilescu, M. (2022). Enhancing Phytoremediation of Soils Polluted with Heavy Metals. Curr. Opin. Biotechnol. 74, 21–31. doi:10.1016/j.copbio.2021.10.024
Ghosal, D., Ghosh, S., Dutta, T. K., and Ahn, Y. (2016). Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): a Review. Front. Microbiol. 7, 1369. doi:10.3389/fmicb.2016.01369
Ghosh, P., Chatterjee, S., Das, P., Karmakar, S., and Mahapatra, S. (2019). Natural Habitat, Phytochemistry and Pharmacological Properties of a Medicinal weed-Cleome Rutidosperma DC. (Cleomaceae): A Comprehensive Review. Int. J. Pharm. Sci. Res. 10 (4), 1605–1612. doi:10.13040/IJPSR.0975-8232.10(4).1605-12
Guerzoni, M. E., Lanciotti, R., and Cocconcelli, P. S. (2001). Alteration in Cellular Fatty Acid Composition as a Response to Salt, Acid, Oxidative and thermal Stresses in Lactobacillus Helveticus. Microbiology 147 (8), 2255–2264. doi:10.1099/00221287-147-8-2255
Hoagland, D. R., and Arnon, D. I. (1938). The Water-Culture Method for Growing Plants without Soil[J]. Calif. Agric. Exp. Stn. Bull. 347, 36–39.
Hooker, J. D. (1872). Flora of British India, Dehra Dun: Reprint by Bishen Singh Mahendra Pal Singh, Publishers, 1. 169.
Hseu, Z. Y. (2004). Evaluating Heavy Metal Contents in Nine Composts Using Four Digestion Methods. Bioresour. Technol. 95 (1), 53–59. doi:10.1016/j.biortech.2004.02.008
Islam, M. D., Hasan, M. M., Rahaman, A., Haque, P., and Rahman, M. M. (2020). Translocation and Bioaccumulation of Trace Metals from Industrial Effluent to Locally Grown Vegetables and Assessment of Human Health Risk in Bangladesh. SN Appli. Sci. 2 (8), 1–11. doi:10.1007/s42452-020-3123-3
Jackson, M. L. (1958). Soil Chemical Analysis. New Delhi: Prentice Hall of India, 38–134. doi:10.2307/477540
Jana, A., and Biswas, S. M. (2011). Lactam Nonanic Acid, a New Substance from Cleome viscosa with Allelopathic and Antimicrobial Properties. J. Biosci. 36 (1), 27–35. doi:10.1007/s12038-011-9001-9
Kapoor, D., Bhardwaj, S., Gautam, S., Rattan, A., Bhardwaj, R., and Sharma, A. (2022). “Brassinosteroids in Plant Nutrition and Heavy Metal Tolerance,” in Brassinosteroids in Plant Developmental Biology and Stress Tolerance (London: Academic Press), 217–235. doi:10.1016/B978-0-12-813227-2.00008-4
Liu, J. N., Zhou, Q. X., Sun, T., Ma, L. Q., and Wang, S. (2008). Growth Responses of Three Ornamental Plants to Cd and Cd–Pb Stress and Their Metal Accumulation Characteristics. J. Hazard. Mater. 151, 261–267. doi:10.1016/j.jhazmat.2007.08.016
Liu, Z., He, X., Chen, W., Yuan, F., Yan, K., and Tao, D. (2009). Accumulation and Tolerance Characteristics of Cadmium in a Potential Hyperaccumulator—Lonicera japonica Thunb. J. Hazard. Mater. 169, 170–175. doi:10.1016/j.jhazmat.2009.03.090
Malar, S., Vikram, S. S., Favas, P. J., and Perumal, V. (2014). Lead Heavy Metal Toxicity Induced Changes on Growth and Antioxidative Enzymes Level in Water Hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 55, 1–11. doi:10.1186/s40529-014-0054-6
Masindi, V., and Muedi, K. L. (2018). “Environmental Contamination by Heavy Metals,” in Heavy Metals. London: IntechOpen, 10, 115–132. doi:10.5772/intechopen.76082
Merkl, N., Schultze-Kraft, R., and Infante, C. (2005). Assessment of Tropical Grasses and Legumes for Phytoremediation of Petroleum-Contaminated Soils. Water Air Soil Pollut. 165 (1), 195–209. doi:10.1007/s11270-005-4979-y
Mishra, S., Bharagava, R. N., More, N., Yadav, A., Zainith, S., Mani, S., et al. (2019). “Heavy Metal Contamination: an Alarming Threat to Environment and Human Health,” in Environmental Biotechnology: For Sustainable Future (Singapore: Springer), 103–125. doi:10.1007/978-981-10-7284-0_5
Mourato, M. P., Moreira, I. N., Leitão, I., Pinto, F. R., Sales, J. R., and Martins, L. L. (2015). Effect of Heavy Metals in Plants of the Genus Brassica. Int. J. Mol. Sci. 16 (8), 17975–17998. doi:10.3390/ijms160817975
Niu, Z. X., Sun, L. N., Sun, T. H., Li, Y. S., and Hong, W. A. N. G. (2007). Evaluation of Phytoextracting Cadmium and lead by sunflower, Ricinus, Alfalfa and Mustard in Hydroponic Culture. J. Environ. Sci. 19 (8), 961–967. doi:10.1016/S1001-0742(07)60158-2
Pollard, A. J., Powell, K. D., Harper, F. A., and Smith, J. A. C. (2002). The Genetic Basis of Metal Hyperaccumulation in Plants. Crit. Rev. Plant Sci. 21, 539–566. doi:10.1080/0735-260291044359
Poschenrieder, C., Cabot, C., Martos, S., Gallego, B., and Barceló, J. (2013). Do toxic Ions Induce Hormesis in Plants? Plant Sci. 212, 15–25. doi:10.1016/j.plantsci.2013.07.012
Ramesar, N. S., Tavarez, M., Ebbs, S. D., and Sankaran, R. P. (2014). Transport and Partitioning of Lead in Indian Mustard (Brassica Juncea) and Wheat (Triticum aestivum). Biorem. J. 18, 345–355. doi:10.1080/10889868.2014.933170
Riyazuddin, R., Nisha, N., Ejaz, B., Khan, M. I. R., Kumar, M., Ramteke, P. W., et al. (2021). A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 12 (1), 43. doi:10.3390/biom12010043
Saxena, N. B., and Saxena, S. (2001). Plant Taxonomy. Meerut, Uttar Pradesh: Reprint by Pragati Prakashan, 224–228.
Shakoor, M. B., Ali, S., Hameed, A., Farid, M., Hussain, S., Yasmeen, T., et al. (2014). Citric Acid Improves lead (Pb) Phytoextraction in Brassica Napus L. By Mitigating Pb-Induced Morphological and Biochemical Damages. Ecotox. Environ. Safe. 109, 38–47. doi:10.1016/j.ecoenv.2014.07.033
Sharma, P., and Dubey, R. S. (2005). Lead Toxicity in Plants. Braz. J. Plant Physiol. 17, 35–52. doi:10.1590/S1677-04202005000100004
Sharma, R. K., Agrawal, M., and Agrawal, S. B. (2010). Physiological and Biochemical Responses Resulting from Cadmium and Zinc Accumulation in Carrot Plants. J. Plant Nutr. 33, 1066–1079. doi:10.1080/01904161003729774
Sidhu, G. P. S., Bali, A. S., Singh, H. P., Batish, D. R., and Kohli, R. K. (2018). Phytoremediation of lead by a Wild, Non-edible Pb Accumulator Coronopus Didymus (L.) Brassicaceae. Int. J. Phytoremediation 20 (5), 483–489. doi:10.1080/15226514.2017.1374331
Sidhu, G. P. S., Singh, H. P., Batish, D. R., and Kohli, R. K. (2017). Alterations in Photosynthetic Pigments, Protein, and Carbohydrate Metabolism in a Wild Plant Coronopus Didymus L. (Brassicaceae) under lead Stress. Acta Physiol. Plant 39, 176. doi:10.1007/s11738-017-2476-8
Singh, S., Parihar, P., Singh, R., Singh, V. P., and Prasad, S. M. (2016). Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 6, 1143. doi:10.3389/fpls.2015.01143
Sun, X., Zhang, J., Zhang, H., Ni, Y., Zhang, Q., and Chen, J. (2010). The Responses of Arabidopsis thaliana to Cadmium Exposure Explored via Metabolite Profiling. Chemosphere 78 (7), 840–845. doi:10.1016/j.chemosphere.2009.11.045
Tang, Y. T., Qiu, R. L., Zeng, X. W., Ying, R. R., Yu, F. M., and Zhou, X. Y. (2009). Lead, Zinc, Cadmium Hyperaccumulation and Growth Stimulation in Arabis Paniculata Franch. Environ. Exp. Bot. 66 (1), 126–134. doi:10.1016/j.envexpbot.2008.12.016
Rigby, H., and Smith, S. R. (2020). The Significance of Cadmium Entering the Human Food Chain via Livestock Ingestion from the Agricultural use of Biosolids, with Special Reference to the UK. Environ. Int. 143, 105844. doi:10.1016/j.envint.2020.105844
Uraguchi, S., Mori, S., Kuramata, M., Kawasaki, A., Arao, T., and Ishikawa, S. (2009). Root-to-shoot Cd Translocation via the Xylem Is the Major Process Determining Shoot and Grain Cadmium Accumulation in rice. J. Exp. Bot. 60, 2677–2688. doi:10.1093/jxb/erp119
Wang, S., Shi, X., Sun, H., Chen, Y., Pan, H., Yang, X., et al. (2014). Variations in Metal Tolerance and Accumulation in Three Hydroponically Cultivated Varieties of Salix Integra Treated with lead. PloS one 9 (9), e108568. doi:10.1371/journal.pone.0108568
Weldeslassie, T., Naz, H., Singh, B., and Oves, M. (2018). “Chemical Contaminants for Soil, Air and Aquatic Ecosystem,” in Modern Age Environmental Problems and Their Remediation (Cham: Springer), 1–22. doi:10.1007/978-3-319-64501-8_1
Yan, A., Wang, Y., Tan, S. N., Mohd Yusof, M. L., Ghosh, S., and Chen, Z. (2020). Phytoremediation: a Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plan. Sci. 11, 359. doi:10.3389/fpls.2020.00359
Yi, H., and Crowley, D. E. (2007). Biostimulation of PAH Degradation with Plants Containing High Concentrations of Linoleic Acid. Environ. Sci. Technol. 41 (12), 4382–4388. doi:10.1021/es062397y
Ying, R., Xia, B., Zeng, X., Qiu, R., Tang, Y., and Hu, Z. (2021). Adsorption of Cadmium by Brassica Juncea (L.) Czern. And Brassica Pekinensis (Lour.) Rupr in Pot Experiment. Sustainability 14 (1), 429. doi:10.3390/su14010429
Zeng, X., Ma, L. Q., Qiu, R., and Tang, Y. (2009). Responses of Non-protein Thiols to Cd Exposure in Cd Hyperaccumulator Arabis Paniculata Franch. Environ. Exp. Bot. 66, 242–248. doi:10.1016/j.envexpbot.2009.03.003
Zhang, S., Chen, M., Li, T., Xu, X., and Deng, L. (2010). A Newly Found Cadmium Accumulator—Malva Sinensis Cavan. J. Hazard. Mater. 173, 705–709. doi:10.1016/j.jhazmat.2009.08.142
Zheng, R., Li, H., Jiang, R., Römheld, V., Zhang, F., and Zhao, F. (2011). The Role of Root Hairs in Cadmium Acquisition by Barley. Environ. Pollut. 159, 408–415. doi:10.1016/j.envpol.2010.10.034
Keywords: phytoremediation, phytoextraction, chemical signaling, heavy metal, campesterol
Citation: Bhattacharya E and Mandal Biswas S (2022) First Report of the Hyperaccumulating Potential of Cadmium and Lead by Cleome rutidosperma DC. With a Brief Insight Into the Chemical Vocabulary of its Roots. Front. Environ. Sci. 10:830087. doi: 10.3389/fenvs.2022.830087
Received: 06 December 2021; Accepted: 12 April 2022;
Published: 20 May 2022.
Edited by:
Ravi Naidu, University of Newcastle, AustraliaReviewed by:
Safdar Bashir, Ghazi University, Pakistan, PakistanAnjan Hazra, University of Calcutta, India
Nirjhar Dasgupta, Guru Nanak Institute of Pharmaceutical Science and Technology, India
Copyright © 2022 Bhattacharya and Mandal Biswas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ekta Bhattacharya, ZWt0YWJoYXR0YWNoYXJ5YTE5OTBAZ21haWwuY29t; Suparna Mandal Biswas, bW9uZGFsc3VwYUBnbWFpbC5jb20=
 Ekta Bhattacharya
Ekta Bhattacharya Suparna Mandal Biswas
Suparna Mandal Biswas