- 1Bangladesh Marine Fisheries Association, Dhaka, Bangladesh
- 2Institute of Marine Sciences, Faculty of Marine Sciences and Fisheries, University of Chittagong, Chittagong, Bangladesh
Microplastics (MPs) are regarded as a global issue due to their toxicity effects on fish and humans. Fish is a vital origin of human protein, which is necessary for body growth. Contamination of fish by MPs is a major hazard that requires special focus. After exposure to MPs alone or in combination with other pollutants, fish may experience a variety of health issues. MPs can cause tissue damage, oxidative stress, and changes in immune-related gene expression as well as antioxidant status in fish. After being exposed to MPs, fish suffer from neurotoxicity, growth retardation, and behavioral abnormalities. The consequences of MPs on human health are poorly understood. Due to the abundance of MPs in environment, exposure may occur via consumption, inhalation, and skin contact. Humans may experience oxidative stress, cytotoxicity, neurotoxicity, immune system disruption, and transfer of MPs to other tissues after being exposed to them. The toxic effects of MPs in both fish and human are still unknown. This detailed review has the potential to add to existing knowledge about the ecotoxicity effects of MPs in both fish and humans, which will be useful for the forthcoming study.
Introduction
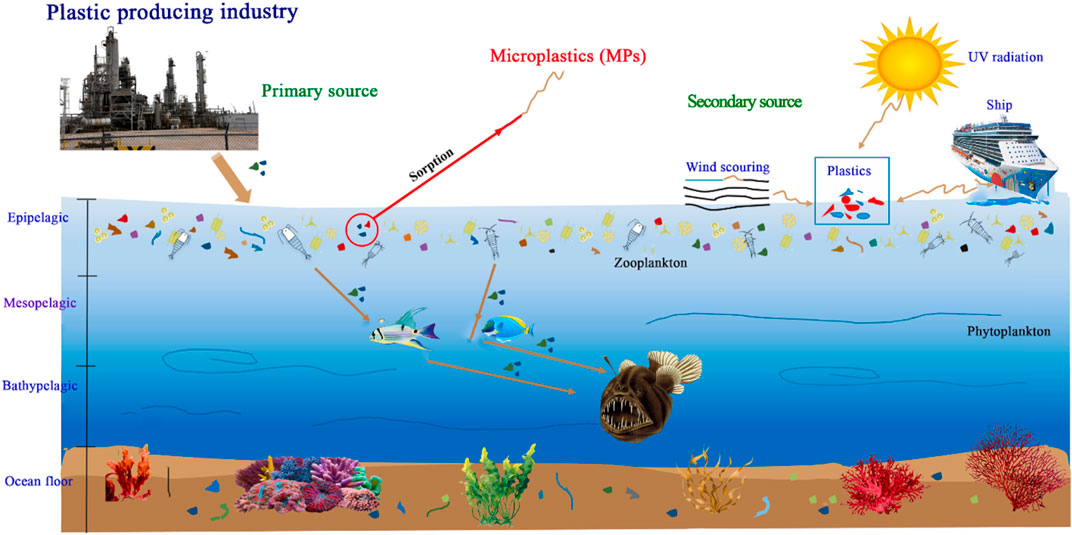
Microplastics (MPs) are a global issue because they are released all over the world (Yu et al., 2018; Alimba and Faggio, 2019). In 2018, global plastics output increased to over 359 million tonnes (Mt), up from 348 Mt in 2017 (PlasticsEurope, 2019). Since the 1950s, when plastic items were widely available, global plastic manufacturing has expanded substantially, from 0.5 Mt/year in 1960 to 348 Mt/year in 2017 (PlasticsEurope, 2018). China is the leading producer of plastics, with 107.7 Mt produced in 2018, accounting for 30% of worldwide plastic production (PlasticsEurope, 2019). Borrelle et al. (2020) projected plastic emissions to 2030 for 173 countries, estimating that plastic emissions to aquatic environment will range between 20 and 53 Mt/year by 2030. Different plastic goods have become commonplace in people’s daily lives. Plastics’ use has expanded 25-fold in the previous 40 years owing to minimal cost, durability, low weight, and elasticity (Sutherland et al., 2016). Worldwide, plastics are widely utilized in food packaging, building and construction, automobile items, electrical devices, domestic sports and recreational, farming, healthcare, and plastic furnishings (PlasticsEurope, 2019).
MPs are microscopic plastic grains that are said to be common in discarded plastic fragment goods (Thompson et al., 2004). Primary MPs are produced small plastic granules to be used in facial-cleansers and cosmetics, air blasting technology, and vectors for drugs in medicine, while secondary nano plastics are tiny plastic remnants deteriorated from MPs debris (Cole et al., 2011; González-Pleiter et al., 2019). The most prevalent waste materials are brought to the seas by rivers, floods, and winds that pollute the ocean and beaches ecosystem. Discarded fishing craft, plastic bags, food containers, and plastic drinks bottles (water and cold drinks) pollute the water ecosystem (Zhou et al., 2018). Mishandling of enormous anthropogenic activities could introduce many xenobiotic pollutants to water environments around the planet, either deliberately or accidentally (Alimba and Faggio, 2019). MPs are reported to be present at all levels of aquatic environments, posing threat to major biota (Ma et al., 2020; Aragaw, 2021).
MPs have been found in edible fish, according to various research, and as a result of biomagnifications, MPs penetrate human systems (Alfaro-Núñez et al., 2021; Goswami et al., 2020; James et al., 2020). MP-induced impairments in species ranged from minimal biological systems disturbance to substantial unfavorable consequences that resulted in mortality (Mallik et al., 2021). Physiological harm as a result of MPs accumulating within the digestive system; disruption of organisms’ energy flow as a result of MPs expelling as pseudofeces; and inner body tissue exposed to MPs after transfer within the body were all designated as harmful by Ma et al. (2020). They also serve as a pathway for organic contaminants and trace metals to reach aquatic habitats (Gholizadeh and Patimar, 2018). MPs can affect predatory behavior in fish and cause misunderstanding between MPs and genuine prey (de Sá et al., 2015), leading to malnutrition and MP storage in key organs such the gills, gut, and stomach (Lu K. et al., 2018; Güven et al., 2017; Greven et al., 2016). MPs were also found in fish muscle/meat, which is mainly consumed by humans (Thiele et al., 2021; Barboza et al., 2020a,b; Akhbarizadeh et al., 2018; Abbasi et al., 2018). Growth retardation, hormone disruption, metabolic perturbation, oxidative stress, immunological and neurotoxicity malfunction, and genotoxicity behavioural alterations are all caused by a buildup of MPs (Güven et al., 2017; Choi et al., 2018).
As fish is a major source of protein for humans, the prevalence and ecotoxicological effects of MPs in fish may influence aquatic food security (Wright and Kelly, 2017; Barboza et al., 2018a). The harmful effects of MPs in fish and humans have been studied in a small number of researches. The goals of this review are to: 1) assess the potentially toxic effects of MPs on fish; 2) determine the impact of MPs on human health, and 3) identify existing knowledge gaps and suggest potential solutions to minimize MPs contamination.
Materials and Methods for the Data Collection and Analyses
Online Study Sites
Data have been collected from many countries around the world. Results from the publications of various scientists and researchers are presented.
Data Collection
The first phase involved the identification of related studies. In order to conduct a systematic literature search, the following specifications were created for the database:
Searching Database
• Scopus, Web of Science, Google Scholar, PubMed, Dimension
Searching Conditions
• Journal articles written in English
• Impact of MPs on fish and human-related journals, book chapters, conference proceedings
• Accessible over the internet (No time limitation)
Searching Strings
The data were tagged with the keywords “Impacts of microplastics on fish” “Effects of microplastics on fish” “Toxicity of microplastics on fish” or “Adverse impacts of microplastics on fish” or “Biomagnification of microplastics on fish” or “Impacts of microplastics on human” or “Effects of microplastics on human” or “Toxicity of microplastics on human” or “Adverse impacts of microplastics on human”, etc. A search for “Impacts of microplastics on fish and human” in the Dimension database (https://app.dimensions.ai/discover/publication) revealed numerous research papers (Figures 1A,B). To gather information on the effects of microplastics in fish and humans, a total of 800 relevant papers were screened. Following the collection of data, two distinct tables were created from these studies.
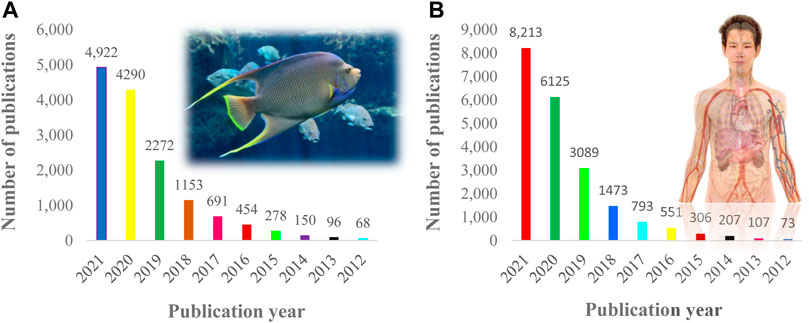
FIGURE 1. (A) Number of publications on MPs effects in fish (B) MPs effect related research trends on human.
Research Trends About Effects of MPs in Fish and on Human
MPs pollution is a new environmental concern that poses a risk to fish and human health (Garrido Gamarro et al., 2020; Kutralam-Muniasamy et al., 2020; Huang W. et al., 2021; Aragaw and Mekonnen, 2021). Fish is being contaminated with MPs worldwide and it finds its way to human body through food (Sequeira et al., 2020). Many scientists have recently focused on the effects of MPs in fish and on human health (Chae and An, 2018). Despite the fact that the number of publications is growing, the possible consequences are still largely unknown (Kutralam-Muniasamy et al., 2020). Figures 1A,B depicted research patterns, demonstrating how the effect of MPs studies is growing.
Bioavailability of MPs to Fish
MPs are found in practically most of aquatic settings which are the similar size as sediments as well as few planktonic species, subjected them accessible to a variety of aquatic creatures, particularly fish (Pazos et al., 2017; Kumar et al., 2018; Sequeira et al., 2020). The bioavailability of MPs to fish is influenced by a variety of circumstances. Filters and deposit-eating fishes are thought to be more vulnerable to MPs ingestion than predator fishes due to their non-selective feeding behavior (Wesch et al., 2016; Lusher et al., 2020). Mizraji et al. (2017) examined the relationships among tidal fish-eating patterns and the possibility of MPs ingestion and discovered that omnivore fish ingested more MPs than plant-eating and carnivore fish.
MPs particles that resemble natural prey objects seem to be more likely to be ingested by types of fish that are visual eaters. White MPs were ingested more selectively by Pomatoschistus microps than black and red particles (de Sa et al., 2015). A suitable form or size could improve the likelihood of MPs being consumed by fish (Auta et al., 2017). Boerger et al. (2010) discovered that the most prevalent size class of MPs swallowed by the Myctophidae in the North Pacific Central Gyre was 1–2.79 mm, which is close to the size category of plankton species, that are the principal food supply for all of these fishes.
MPs’ vertically positioning in the water column is mostly determined by their volume, which may affect the probability of fish encountering MPs in various fish regions (de Sá et al., 2018). Pelagic fishes, for example, seem to be more likely to come across low-density plastics, whereas demersal fish may be more prone to high MPs (Lusher et al., 2013). Moreover, there are many unknowns concerning the fundamental factors that regulate fish choice eating patterns for MPs. The interaction method involving MPs and fish still has to be studied extensively.
Biomagnifications and Trophic Transfer
Biomagnification has been observed in a variety of fish and other species higher up the food chain (Figure 2). MPs were found in planktivorous fish, according to Boerger et al. (2010), that biomagnified to bigger predatory feeding on the fish. Biomagnifications have been observed in bluefin tuna, albacore tuna, and swordfish in the Mediterranean Sea (Romeo et al., 2015). MPs were found to be transferred tropically from Scombrus scombrus to Halichoerus grypus, reported by Nelms et al. (2019). Low density MPs can be expelled as pseudofeces by fish though most of the MPs remain in gastrointestinal tract of fish (Capone et al., 2020; Zhang et al., 2021a; Prata et al., 2022). Biomagnifications from marine species to humans could occur in the same way. Although biomagnification research has been explored, there are few of them. The big facts of biomagnifications and trophic transmission cannot be fully appreciated with these limited investigations. As a result, more research in this area is required.
How Do Fish React to the Toxicity of Microplastics?
Many researchers have studied the negative consequences of microplastics on species, which can vary from interruption of biological functions to death. MPs poisoning is categorized as follows depending on the nature of MPs after intake:
1) build-up in the gastrointestinal tract, producing physical harm such as blockage and damage;
2) release as pseudofeces, disrupting organisms’ energy transfer;
3) transfer inside the body, exposing inner organs and tissues to MPs.
MPs-caused detrimental effects on species were outlined to provide a solid research foundation for sustainable MPs toxicological investigations and to evaluate the potential for huge ecological disruption.
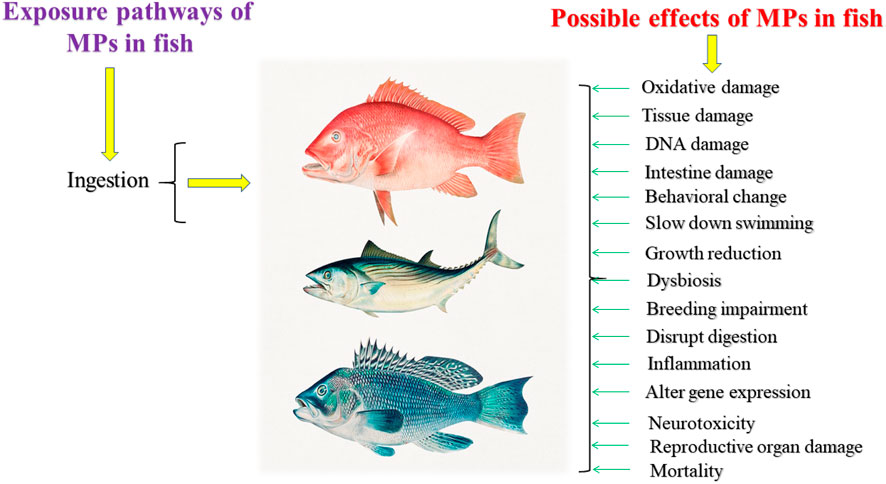
Impacts of MPs, in Fish
MPs exposure has been studied in respect of particular physical or biological reactions. So far, most investigations on the effects of MPs on fish have been undertaken in the laboratory (Supplementary Table S1). The fish used in the MPs exposure tests came from a variety of environments, with the bulk coming from the sea. MPs may build up in the gastrointestinal system of fish after consumption, producing obstructions across the digestive tract and limiting feeding owing to appetite (Lusher et al., 2013; Wright et al., 2013). Intake of MPs could also induce anatomical and functional changes in the digestive tracts, causing dietary and development issues in fish (Huang et al., 2022; Jabeen et al., 2018; Borrelle et al., 2017; Peda et al., 2016). Many pieces of research have been carried to demonstrate that MPs pose a threat to fish, with mortality occurring frequently before they reach maturity owing to MPs intake (Figure 3). Most of the studies were conducted on Danio rerio. Oxidative stress, decreased mobility, gene expression disruption and damage of reproductive organs are the most common effects of MPs in Danio rerio (Mu et al., 2021; Zhao et al., 2021; Zhang et al., 2022).
Oryzias melastigma was second most studied fish that subjected to physical impairment due to MPs ingestion (Xia et al., 2022). Growth inhibition, dysbiosis of fish gut, reduction of weight, disturbance of anti-oxidative condition of the liver, damaging reproductive organs and growth retardation are visible effects in Oryzias melastigma (Wang et al., 2022; Zhang X. et al., 2021; Feng et al., 2021; Li et al., 2021; Le Bihanic et al., 2020) (Supplementary Table S1). Sparus aurata is another important consumable fish that affected by MPs ingestion. This fish faced stress, oxidative damage, survival, Behavior changes and damage of immune system’s key functions (Espinosa et al., 2017; Pannetier et al., 2020; Jacob et al., 2021; Rios-Fuster et al., 2021; Solomando et al., 2021) (Supplementary Table S1).
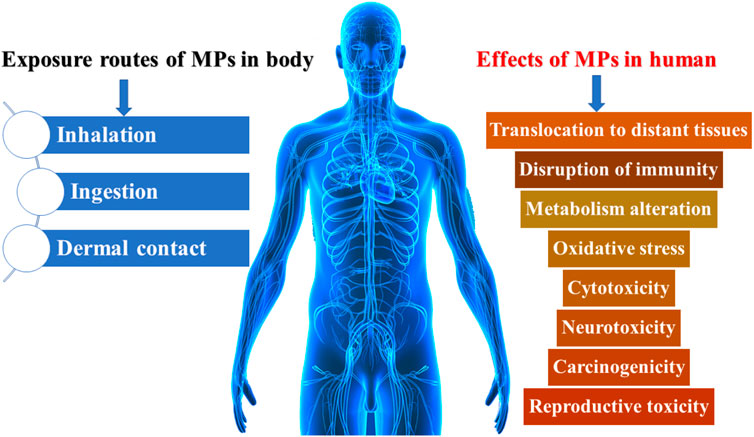
Microplastics in humans
The presence of MPs in seafood poses a major hazard to human health. Seafood is an essential part of the human diet. MPs contamination of the intestinal system poses a serious risk of spreading to other regions of the body. Endocytosis and persorption are two of the most common methods for MPs to enter the human body (Figure 4). Toxicological impacts may reduce fish performance, which is of considerable consideration of humans who eat fish as a major part of their meal, and may have severe impacts on catching fish (Naji et al., 2017; Kor et al., 2020; Kor and Mehdinia, 2020). More examination into these concerns is required, taking into account realistic MP and pollutant levels in the ecosystem (Neves et al., 2015).
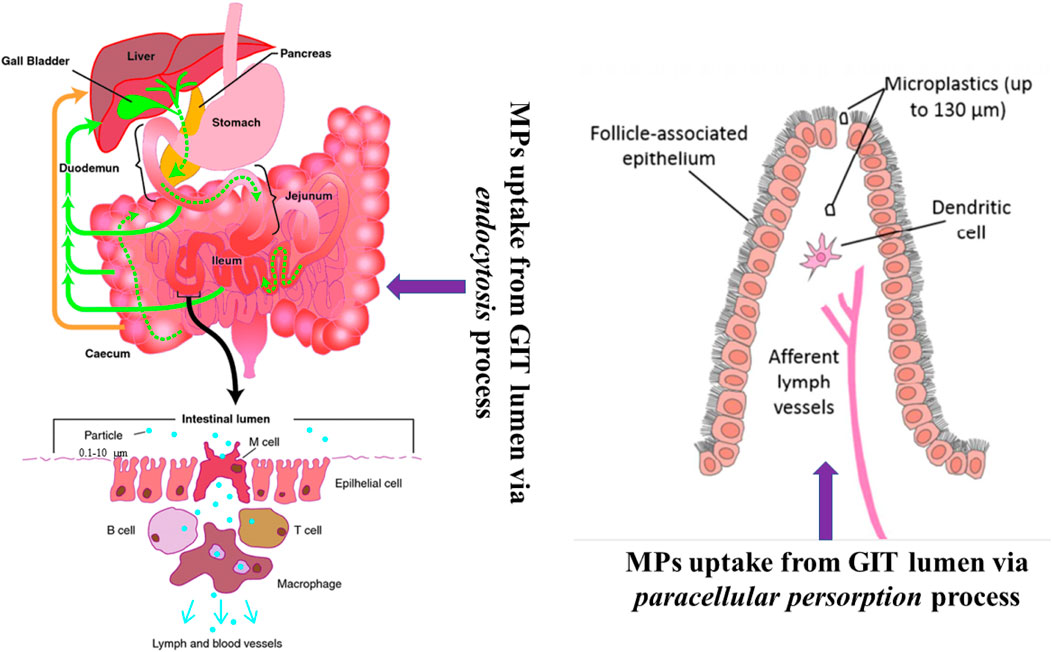
FIGURE 4. Different process of MPs uptake from gastrointestinal tract (GIT) after exposure (Modified from Galloway, 2015 and Wright and Kelly, 2017).
Toxicological Effects in Human Health
Swelling and blockage are caused due to the buildup of MPs and nano plastics in tissues (Wang et al., 2016; Wright et al., 2013). In vitro tests revealed that MPs concentrate in the gills, stomachs, and metabolic systems of crabs and cause unfavorable cellular alterations in fish (Karbalaei et al., 2018; Brennecke et al., 2015). Microorganisms and pollutants have also been shown to be transported by them (Manzoor et al., 2020; Wang et al., 2016). Such negative consequences were mostly determined by the individual’s level of exposure and sensitivity (Prata et al., 2020). Exposure to MPs has also been linked to oxidative stress, cytotoxicity, and transfer to other tissues (Figure 5) (Galloway, 2015; Schirinzi et al., 2017; Wright and Kelly, 2017; Anbumani and Kakkar, 2018).
MPs are long-lasting in the ecosystem and biological organisms. As a result, the animals are exposed to MPs for an extended period, potentially leading to chronic discomfort, swelling, cell growth, and death, as well as immune cell impairment (Smith et al., 2018). Inflammatory bowel disease was significantly higher in patients with MPs than the healthy people (Yan et al., 2021). PS MPs decreased the growth of Caco-2 cells over time (Wu et al., 2020). The mitochondrial membrane potential was disturbed by PS MPs, according to Wu et al. (2019). MPs may also serve as vectors for a variety of microbes (Kirstein et al., 2016). They can eject compounds from their matrixes or absorb substances from the surrounding (Crawford and Quinn, 2017).
Oxidative Stress and Cytotoxicity
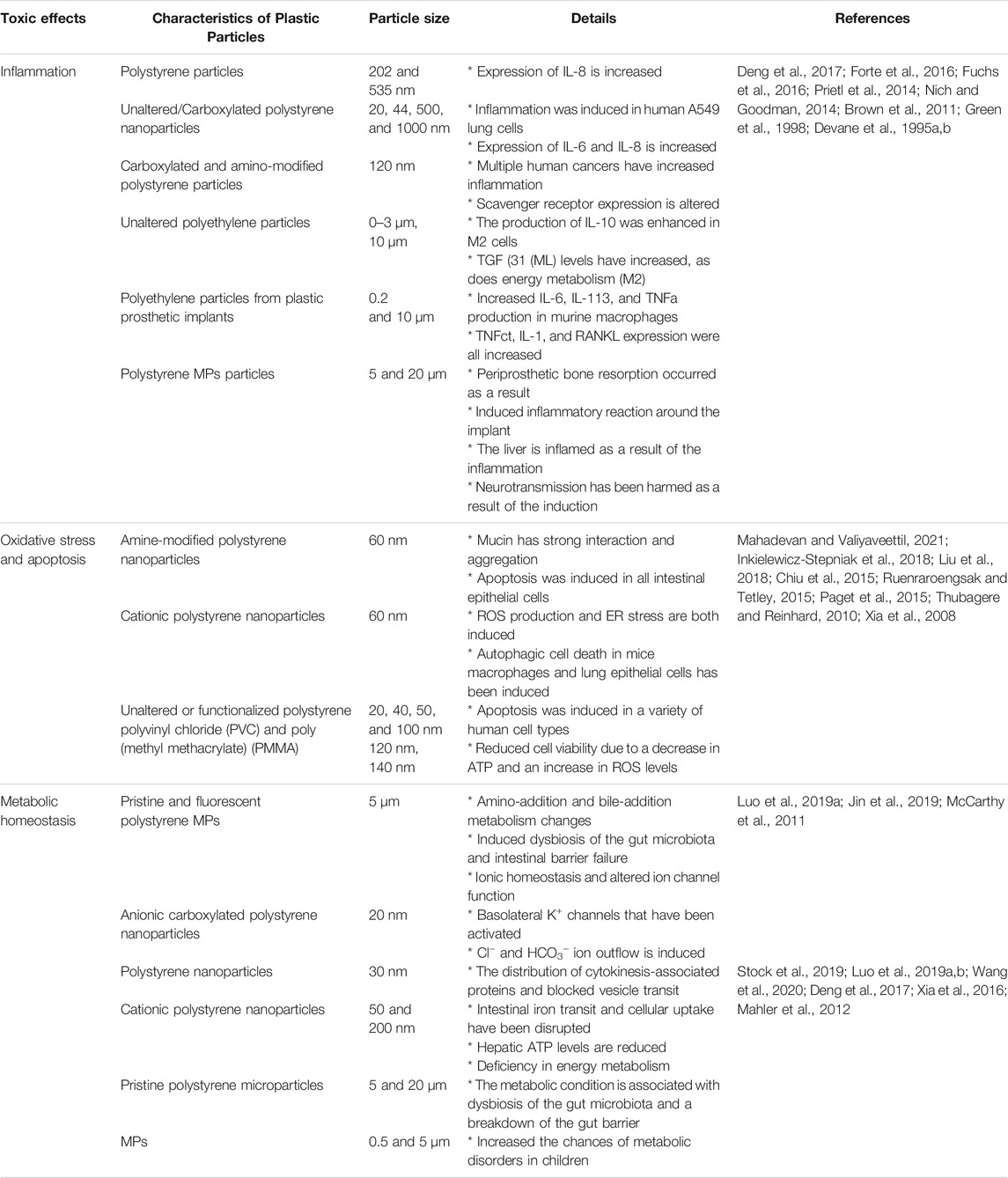
Oxidative stress, consequent inflammatory, and cytotoxic impacts were thought to be the main effects for MPs toxicity in inhalation exposure experiments (Table 1). MPs can induce oxidative stress by producing oxidizing substances adsorbing to their surface, as well as reactive oxygen radicals created by the host during the inflammation (Kelly and Fussel, 2012; Valavanidis et al., 2013). Forte et al. (2016) used nanoparticles to stimulate pro-inflammatory replies in A549 lung cells and human gastric cancer cells. Green et al. (1998) found that larger polyethylene particles (0.3–10 m) promote the development of cytokines such as IL-6, IL-1b, and TNF-a, some of which are inflammatory agents.
Due to the polymerization and processing, MPs include reactive oxygen species. Nevertheless, reactions with UV radiation or the existence of reactive metals may cause such free radicals to be greatly amplified. MPs aging also resulted in the formation of free radicals, which oxidized the target tissues (Gewert et al., 2015). Polystyrene nanoparticles link with the surface of the intestinal epithelial, according to Inkielewicz-Stepniak et al. (2018). Nanoplastics caused epithelial cells to oxidize, according to the research (Inkielewicz-Stepniak et al., 2018). Acute toxins and free radicals have been observed to be released by limbs and joint prostheses incorporating MPs in humans because of severe inflammatory reaction. Those oxidants were shown to cause hydrolysis, which led to polymer degradation, breaking, and leaking. This free radical manufacturing process may cause the prosthesis to be rejected by the human organs (Sternschuss et al., 2012).
MPs were found to be cytotoxic as an effect of oxidative damage and inflammation (Table 1). MPs can be absorbed by certain cells, such as macrophages, as revealed in animal research and in vitro tests (Geiser et al., 2005; Yacobi et al., 2008). MPs interacted easily with intracellular organelles since they were not membrane-bound, posing a risk of damage (Geiser et al., 2005). In vitro studies by Furukuma and Fujii, 2016) revealed that MPs particles are cytotoxic. Schirinzi et al. (2017) found that MPs at levels of 0.05–10 mg/L produced reactive oxygen species to high levels, contributing to cytotoxicity in the human brain and epithelial cells in in vitro research. Furthermore, in vitro contact of macrophages and lung epithelial cells to nano plastics enhanced reactive oxygen radicals, causing unfolded proteins particle agglomeration in the endoplasmic reticulum and cytolysis (Chiu et al., 2015). Thubagere and Reinhard (2010) found that polystyrene particles were cytotoxic to adenomatous cells of the tiny intestine. As a result, individuals exposed to MPs may cause cytotoxicity and oxidative damage. Moreover, no strong cytotoxic effects were found in multiple in vitro investigations even at high levels (Magri et al., 2018; Stock et al., 2019; Wu et al., 2019).
Changing the Body’s Metabolism and Energy Flow
MPs can either directly influence metabolism by altering metabolic enzymes or circuitously by upsetting the energy equilibrium. By boosting or lowering energy consumption, decreasing nutritional intake, and regulating metabolic enzymes, MPs exhibit metabolic impacts (Table 1). Humans, on the other hand, have higher energy requirements and more sophisticated metabolic processes than the creatures studied, which could affect the metabolic impacts (Table 1).
Immune System Dysfunction
MPs were reported to cause systemic or local immune responses after exposure, based on their dispersion and human reaction. Environmental exposure to MPs, on the other hand, was enough to impair immune systems in biologically vulnerable individuals, resulting in autoimmune disorders or immunosuppression (Table 1) (Prata, 2018; Prata et al., 2020). According to Farhat et al. (2011), chronic damage in cells, the production of immune modulators, and the incorrect stimulation of immune cells may all contribute to MP-induced autoimmune disorders. Antibodies against self-antigens would be produced as an outcome of this chain of events (Farhat et al., 2011). In addition, exposure to MPs has been linked to autoimmune rheumatic illness and systemic lupus erythematosus (Fernandes et al., 2015; Bernatsky et al., 2016). MPs have the potential to alter human immunological function, albeit this has yet to be proven. As a result, more research into the impacts on human immune systems is required.
Translocation of Cells to Other Tissues
MPs may transfer to distal tissues via the circulatory system after exposure. Internalization of MPs in the cardiovascular system instigated an inflammatory reaction, blood cell cytotoxicity, vascular swelling, obstructions, and respiratory high blood pressure (Table 1) (Wright and Kelly, 2017; Campanale et al., 2020). In vitro tests revealed that nanoparticles exposure can cause red blood cell coagulation and endothelium wall adherence (Barshtein et al., 2016). MPs accelerated hemolysis and contributed to the production of histamine, a pro-inflammatory molecule, according to Hwang et al. (2019). The most significant method of MP transfer is enhanced porosity of the epithelial membrane as a result of inflammation (Prata et al., 2020). Wick et al. (2010) revealed that 240 nm nanoparticles may easily pass the placental barrier in a perfusion model of the human placenta. Polystyrene particles were taken up by an ex vivo human placental perfusion model, which then penetrated the placental barrier without affecting the stability of the explant. Particles were transferred through intracellular and extracellular transporter proteins, as well as diffusion. The results from this study point to the possibility of nanoparticles being transported across the placenta (Wick et al., 2010). Grafmueller et al. (2015) used an ex vivo human placental perfusion model to show that polystyrene nanoparticles have a similar capability to breach the placenta barrier.
In vitro tests revealed that when subjected to nano plastics (44 nm), human renal cortical epithelial cells ingested them without cleaning, even after 90 min (Monti et al., 2015). The buildup of particles, on the other hand, caused considerable impairments in renal active operation (Monti et al., 2015). MPs may cause persistent inflammation, impaired organ function, and a higher risk of neoplasia when transferred to distal tissues (Prata et al., 2020). MPs may promote bone loss by stimulating osteoclasts after they reach the bone (Liu et al., 2015; Ormsby et al., 2016). Inconsistencies with the data have been discovered by Braeuning (2019), such as mass disparity of MP tissue burden vs. actual ingestion and the failure to account for MPs’ existence in other bodily tissues. As a result, toxicokinetic features of MPs must be considered when evaluating their absorption, dispersion, and effects (Böhmert et al., 2019).
Neurotoxicity
In vivo neurotoxicity has been documented following persistent exposure to particulate matter, particularly MPs, perhaps due to immune cell activation and oxidative stress in the brain (Table 1). These events could be the result of direct interaction with teleported particles or the effects of circulating pro-inflammatory cytokines that result in long-term neuronal injury (MohanKumar et al., 2008). MPs have been shown to have an effect on neuronal function and behaviour in vivo. Exposure to MPs elevated AChE activity in the brain and altered serum neurotransmitters, according to Deng et al. (2017). Nanoplastics were capable of creating toxicity and impairing metabolic activity in brain cell types in an in vitro investigation due to the high level of bioactive chemicals.
Carcinogenicity
For generations, human contact with plastic items has been associated with malignancies. Nevertheless, no conclusive proof has been established until now. According to Prata (2018), prolonged inflammation and irritation caused by MPs consumption may induce cancer by causing DNA damage (Table 1). According to Chang (2010), oxidative stress and persistent irritation generated by nano plastics revealed evidence of pro-inflammatory agents, which stimulated vasculature, that led to the creation and development of cancers.
Combine Effects (MPs With Other Pollutants)
Since MPs inclusion in the manufacturing process, they were observed to include a variety of exogenous chemical additions and colors. MPs were frequently detected with persistent organic pollutants (POPs) and phthalates, bisphenol A (BPA), bisphenone, triclosan, organotin, and brominated flame retardants. During the production process of the items, these POPs are applied as additives to plastics (Galloway 2015; EFSA, 2016; Prata et al., 2020). Emollients such as phthalates, for example, are used to soften plastics by lowering the attraction of molecular chains inside the synthetic polymer matrix. BPA is a component monomer in polycarbonate that is used in catering packaging (Cole et al., 2011).
POPs, such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), as well as heavy metals like lead, nickel, cadmium, and zinc, were found in MPs retrieved from various environments (Crawford and Quinn, 2017; Wright and Kelly, 2017). Such chemicals are prone to leaching since no chemical linkages between them and the matrix of MPs (Wright and Kelly, 2017). Structural substances on the surface of MPs were said to travel along the concentration gradient at a consistent rate when they were broken. When MPs come into touch with bodily surfaces and are transported to the deeper tissues, contaminants like these may be produced (Browne et al., 2011). Those chemicals showed the capacity to interact with endogenous hormones even at low doses (Cole et al., 2011).
MPs worked as vectors, delivering germs to target tissues, protecting them from the immune structure, causing pro-inflammatory replies, and potentially facilitating infections (Kirsten et al., 2016). When MPs came into touch with bacteria and chemicals, their large surface area made them vulnerable to becoming vectors (Prata et al., 2020). When compared to the daily consumption of food and dust, MPs may have a minor impact on harmful chemical exposure (Bakir et al., 2014). Numerous MP exposures, on the other hand, could significantly increase their amounts. Monomers, additives, and degradation products carried by MPs, according to Rodrigues et al. (2019), could offer significant human health concerns if transmitted.
Vibrio sp., among the most aggressive bacteria, could populate the surface of MPs, according to Kirsten et al., 2016). MPs, on the other hand, were discovered to affect the variety and activity of the gut microbiome reported by Zhu et al. (2018). Once humans are exposed to a large number of MPs through consumption, such impacts could have serious consequences. According to West-Eberhard (2019), exposure to MPs can affect the gut microbiota, resulting in the rapid growth of opportunistic species, an upsurge in pro-inflammatory responses, and endotoxemia (Table 1). The harmful effects of chemicals or microbes adsorbed onto MPs, on the other hand, are greatly reliant on the types and concentrations of swallowed particle, vector particle transportation, release profile, and pollutant lethality in human body cells (Campanale et al., 2020; Prata et al., 2020).
BPA is utilized as an antioxidant and a stabilizer that has the potential to damage the endocrine system (Yamamoto et al., 2001; Halden, 2010). It can move out of polycarbonates and adhere to consumable food, allowing it to be consumed by humans (Calafat et al., 2008). BPA poisoning has been found in tuna fish, pork, and tap water, demonstrating how this toxin can enter highly eaten foods (Colin et al., 2014). BPA levels in the urine of 167 men were shown to be negatively proportionate to serum levels of inhibin B and the estradiol: testosterone ratio, indicating a detrimental effect on hormone levels, according to Meeker et al. (2010). BPA may potentially have a role in the development of overweight by disrupting alpha and beta receptors in fat tissues, changing fat tissue hormone levels, and interacting with the action of lipoprotein lipase, aromatase, and lipogenesis regulators (Vom Saal et al., 2012; Michalowicz, 2014). It has the potential to cause breast and prostate cancer in mammalian species, as well as cancer in humans (Michalowicz, 2014). Many chemical substances found in plastics or firmly attached to MPs, such as leftover low molecular weight styrenes, PAHs, PVC monomer, PCBs, PBDEs, OCPs, and pharmaceutical drugs, which include their metabolites, are toxic to humans, genotoxicity, and hormonal disruptors after being ingested, according to study.
Future Perspectives/Knowledge Gaps
→ The majority of research is done in laboratories, although wild fish must be used in some experiments. The conditions in the lab are very different from those in the real world. Experiment with wild fish to see if there is a difference in the results. A significant future scientific goal should be to increase the number of comparative laboratory-field experiments with parallel exposure in order to evaluate and strengthen our understanding of the real-world impact of MPs.
→ Non-destructive sampling should be maintained. Destructive sampling damages tissue and genetic components in the specimens collected. Destructive sampling makes species-level identification of fish nearly impossible in most circumstances, rendering this methodology inappropriate.
→ MPs’ harmful impact on aquatic organisms at various phases of development. Most of the research was carried out in the juvenile stage. Research should be conducted in other stages (e.g., larval, adult stage). MPs exposure must be compared throughout life stages and sexes in order to draw appropriate conclusions about a species’ sensitivity. At the moment, findings from a small number of researches imply that younger people and women have a higher inclination to consume more MPs and are more vulnerable to MPs pollution.
→ Proof of the negative effects of MP on individuals, groups, and ecosystems. There are very few negative consequences available. More research is needed to confirm MPs’ deleterious impacts. Journals, newspapers, magazines, and different websites are not interested to publish findings if the experiments have no effects on fish and humans. That’s why some researchers always try to show negative effects instead of actual findings.
→ Ingestion of MP, internalization and transport potential should be identified. Ingestion routes in the body and how they connect to the gut wall, as well as MPs trophic transfer.
→ MPs’ role as vectors for sorbed persistent organic pollutants and metals exposure and bioaccumulation. Many pathogens and contaminants are transported by MPs. It is necessary to define the role of MPs as carriers to identify the ecotoxicological effects of MPs in fish.
→ The influence of related additional compounds on the effects of MPs on aquatic creatures. MPs, in combination with other pollutants, have severe negative effects on organisms, which MPs alone cannot always achieve. It is necessary to determine how much the presence of MPs contributes to fish exposure to these hazardous compounds. Given the importance of fish as a significant source of protein for humans, ongoing research is essential to demonstrate the ecotoxicological effects of MPs on fish at all stages, from individual to community, to experimentally assess the general risks of these emerging pollutants to fish.
→ MPs’ specific forms of harmful activity and how they compare to ‘typical’ oceanic contaminants. Identify the severity of conventional oceanic pollutants and MPs. The toxicity level of MPs in fish is quite necessary to know the extent of MPs’ effects in humans.
→ Testing for MP should not be restricted to the gastrointestinal system, but should be done across different tissues and organs as well. The research of tissues of animals used for human sustenance (e.g., fish muscle) is one idea for examining the accumulation of MPs, given their possible role in human healthcare.
Conclusion
Plastic is a valuable, useful, and useful material that is used to make up the bulk of the items in daily life; however, in today’s world, mismanagement, improper handling, and abuse of plastics have resulted in MPs pollution in every edge of the aquatic environment, from the highest-ranked pelagic layer to seafloor sedimentary rocks. Because MPs are abundant in aquatic ecosystems, fish species have easy access to them. A growing body of research demonstrates that MPs are toxic to a wide range of fish. MPs can collect in the gastrointestinal system of fish after intake and then disperse to other body tissues. MPs can cause a variety of health concerns in fish. Toxic substances and dangerous germs may potentially be transmitted to fish through MPs. Humans eat plastic-tainted fish and are exposed to plastic particles. As a consequence, several chronic illness outbreaks occur, and people suffer the effects. As a result, reducing MPs contamination is critical. Implementing efficient waste management methods, enhancing the shelf life of plastic items, and increasing awareness can substantially limit the input of litter into environments, allowing the aquatic ecosystem to be recovered.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.827289/full#supplementary-material
References
Abarghouei, S., Hedayati, A., Raeisi, M., Hadavand, B. S., Rezaei, H., and Abed-Elmdoust, A. (2021). Size-dependent Effects of Microplastic on Uptake, Immune System, Related Gene Expression and Histopathology of Goldfish (Carassius auratus). Chemosphere 276, 129977. doi:10.1016/j.chemosphere.2021.129977
Abbasi, S., Soltani, N., Keshavarzi, B., Moore, F., Turner, A., and Hassanaghaei, M. (2018). Microplastics in Different Tissues of Fish and Prawn from the Musa Estuary, Persian Gulf. Chemosphere 205, 80–87. doi:10.1016/j.chemosphere.2018.04.076
Ahmadifar, E., Kalhor, N., Dawood, M. A. O., Ahmadifar, M., Moghadam, M. S., Abarghouei, S., et al. (2021). Effects of Polystyrene Microparticles on Inflammation, Antioxidant Enzyme Activities, and Related Gene Expression in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 28 (12), 14909–14916. doi:10.1007/s11356-020-11731-x
Ahrendt, C., Perez-Venegas, D. J., Urbina, M., Gonzalez, C., Echeveste, P., Aldana, M., et al. (2020). Microplastic Ingestion Cause Intestinal Lesions in the Intertidal Fish Girella Laevifrons. Mar. Pollut. Bull. 151, 110795. doi:10.1016/j.marpolbul.2019.110795
Akhbarizadeh, R., Moore, F., and Keshavarzi, B. (2018). Investigating a Probable Relationship between Microplastics and Potentially Toxic Elements in Fish Muscles from Northeast of Persian Gulf. Environ. Pollut. 232, 154–163. doi:10.1016/j.envpol.2017.09.028
Alfaro-Núñez, A., Astorga, D., Cáceres-Farías, L., Bastidas, L., Villegas, C. S., Macay, K., et al. (2021). Microplastic Pollution in Seawater and marine Organisms across the Tropical Eastern Pacific and Galápagos. Scientific Rep. 11 (1), 1–8.
Alimba, C. G., and Faggio, C. (2019). Microplastics in the marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 68, 61–74. doi:10.1016/j.etap.2019.03.001
Almeida, D. S. M. (2017). Histopathological Evaluation of Two Blennius Fishes Exposed to MPs Via Feeding. Doctoral dissertation.
Alomar, C., Sureda, A., Capó, X., Guijarro, B., Tejada, S., and Deudero, S. (2017). Microplastic Ingestion by Mullus Surmuletus Linnaeus, 1758 Fish and its Potential for Causing Oxidative Stress. Environ. Res. 159, 135–142. doi:10.1016/j.envres.2017.07.043
Anbumani, S., and Kakkar, P. (2018). Ecotoxicological Effects of Microplastics on Biota: a Review. Environ. Sci. Pollut. Res. 25 (15), 14373–14396. doi:10.1007/s11356-018-1999-x
Aragaw, T. A., and Mekonnen, B. A. (2021). Distribution and Impact of Microplastics in the Aquatic Systems: A Review of Ecotoxicological Effects on Biota. Microplastic Pollution. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer Singapore, 65–104. doi:10.1007/978-981-16-0297-9_3
Aragaw, T. A. (2021). Microplastic Pollution in African Countries’ Water Systems: a Review on Findings, Applied Methods, Characteristics, Impacts, and Managements. SN Appl. Sci. 3 (6), 1–30. doi:10.1007/s42452-021-04619-z
Ašmonaitė, G., Tivefälth, M., Westberg, E., Magnér, J., Backhaus, T., and Carney Almroth, B. (2020). MPs as a Vector for Exposure to Hydrophobic Organic Chemicals in Fish: A Comparison of Two Polymers and Silica Particles Spiked with Three Model Compounds. Front. Environ. Sci. 8, 87.
Assas, M., Qiu, X., Chen, K., Ogawa, H., Xu, H., Shimasaki, Y., et al. (2020). Bioaccumulation and Reproductive Effects of Fluorescent Microplastics in Medaka Fish. Mar. Pollut. Bull. 158, 111446. doi:10.1016/j.marpolbul.2020.111446
Auta, H. S., Emenike, C. U., and Fauziah, S. H. (2017). Distribution and Importance of Microplastics in the marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 102, 165–176. doi:10.1016/j.envint.2017.02.013
Bakir, A., Rowland, S. J., and Thompson, R. C. (2014). Enhanced Desorption of Persistent Organic Pollutants from Microplastics under Simulated Physiological Conditions. Environ. Pollut. 185, 16–23. doi:10.1016/j.envpol.2013.10.007
Banaee, M., Soltanian, S., Sureda, A., Gholamhosseini, A., Haghi, B. N., Akhlaghi, M., et al. (2019). Evaluation of Single and Combined Effects of Cadmium and Micro-plastic Particles on Biochemical and Immunological Parameters of Common Carp (Cyprinus carpio). Chemosphere 236, 124335. doi:10.1016/j.chemosphere.2019.07.066
Banihashemi, E. A., Soltanian, S., Gholamhosseini, A., and Banaee, M. (2021). Effect of MPs on Yersinia ruckeri Infection in Rainbow trout (Oncorhynchus mykiss). Environ. Sci. Pollut. Res., 1–12.
Barboza, L. G. A., Vieira, L. R., Branco, V., Carvalho, C., and Guilhermino, L. (2018c). Microplastics Increase Mercury Bioconcentration in Gills and Bioaccumulation in the Liver, and Cause Oxidative Stress and Damage in Dicentrarchus labrax Juveniles. Sci. Rep. 8 (1), 15655–15659. doi:10.1038/s41598-018-34125-z
Barboza, L. G. A., Cunha, S. C., Monteiro, C., Fernandes, J. O., and Guilhermino, L. (2020b). Bisphenol A and its Analogs in Muscle and Liver of Fish from the North East Atlantic Ocean in Relation to Microplastic Contamination. Exposure and Risk to Human Consumers. J. Hazard. Mater. 393, 122419. doi:10.1016/j.jhazmat.2020.122419
Barboza, L. G. A., Dick Vethaak, A., Lavorante, B. R. B. O., Lundebye, A.-K., and Guilhermino, L. (2018a). Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 133, 336–348. doi:10.1016/j.marpolbul.2018.05.047
Barboza, L. G. A., Lopes, C., Oliveira, P., Bessa, F., Otero, V., Henriques, B., et al. (2020a). Microplastics in Wild Fish from North East Atlantic Ocean and its Potential for Causing Neurotoxic Effects, Lipid Oxidative Damage, and Human Health Risks Associated with Ingestion Exposure. Sci. total Environ. 717, 134625. doi:10.1016/j.scitotenv.2019.134625
Barboza, L. G. A., Vieira, L. R., Branco, V., Figueiredo, N., Carvalho, F., Carvalho, C., et al. (2018d). Microplastics Cause Neurotoxicity, Oxidative Damage and Energy-Related Changes and Interact with the Bioaccumulation of Mercury in the European Seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 195, 49–57. doi:10.1016/j.aquatox.2017.12.008
Barboza, L. G. A., Vieira, L. R., and Guilhermino, L. (2018b). Single and Combined Effects of Microplastics and Mercury on Juveniles of the European Seabass (Dicentrarchus labrax): Changes in Behavioural Responses and Reduction of Swimming Velocity and Resistance Time. Environ. Pollut. 236, 1014–1019. doi:10.1016/j.envpol.2017.12.082
Barshtein, G., Livshits, L., Shvartsman, L. D., Shlomai, N. O., Yedgar, S., and Arbell, D. (2016). Polystyrene Nanoparticles Activate Erythrocyte Aggregation and Adhesion to Endothelial Cells. Cell Biochem Biophys 74 (1), 19–27. doi:10.1007/s12013-015-0705-6
Bernatsky, S., Smargiassi, A., Barnabe, C., Svenson, L. W., Brand, A., Martin, R. V., et al. (2016). Fine Particulate Air Pollution and Systemic Autoimmune Rheumatic Disease in Two Canadian Provinces. Environ. Res. 146, 85–91. doi:10.1016/j.envres.2015.12.021
Boerger, C. M., Lattin, G. L., Moore, S. L., and Moore, C. J. (2010). Plastic Ingestion by Planktivorous Fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 60 (12), 2275–2278. doi:10.1016/j.marpolbul.2010.08.007
Böhmert, L., Stock, V., and Braeuning, A. (2019). Plausibility of microplastic uptake in a paper by Deng et al. Scientific reports 7. Arch. Toxicol. Archiv für Toxikologie 93 (1), 46687217–46687218.
Bonfanti, P., Colombo, A., Saibene, M., Motta, G., Saliu, F., Catelani, T., et al. (2021). Microplastics from Miscellaneous Plastic Wastes: Physico-Chemical Characterization and Impact on Fish and Amphibian Development. Ecotoxicology Environ. Saf. 225, 112775. doi:10.1016/j.ecoenv.2021.112775
Borrelle, S. B., Ringma, J., Law, K. L., Monnahan, C. C., Lebreton, L., McGivern, A., ., , Murphy, E., Jambeck, J., Leonard, G. H., Hilleary, M. A., Eriksen, M., Possingham, H. P., De Frond, H., Gerber, L. R., Polidoro, B., Tahir, A., Bernard, M., Mallos, N., Barnes, M., and Rochman, C. M. (2020). Predicted Growth in Plastic Waste Exceeds Efforts to Mitigate Plastic Pollution. Science 369 (6510), 1515–1518. doi:10.1126/science.aba3656
Borrelle, S. B., Rochman, C. M., Liboiron, M., Bond, A. L., Lusher, A., Bradshaw, H., et al. (2017). Opinion: Why We Need an International Agreement on marine Plastic Pollution. Proc. Natl. Acad. Sci. USA 114 (38), 9994–9997. doi:10.1073/pnas.1714450114
Bour, A., Sturve, J., Höjesjö, J., and Carney Almroth, B. (2020). Microplastic Vector Effects: Are Fish at Risk when Exposed via the Trophic Chain? Front. Environ. Sci. 8, 90. doi:10.3389/fenvs.2020.00090
Braeuning, A. (2019). Uptake of microplastics and related health effects: a critical discussion of Deng et al., Scientific reports 7:46687, 2017. Arch. Toxicolarchives Toxicology 793 (1), 46687219–46687220. doi:10.1007/s00204-018-2367-9
Brennecke, D., Ferreira, E. C., Costa, T. M., Appel, D., da Gama, B. A., and Lenz, M. (2015). Ingested Microplastics (>100 μm) Are Translocated to Organs of the Tropical Fiddler Crab Uca Rapax. Mar. Pollut. Bull. 96 (1-2), 491–495. doi:10.1016/j.marpolbul.2015.05.001
Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., et al. (2011). Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 45 (21), 9175–9179. doi:10.1021/es201811s
Bucci, K., Bikker, J., Stevack, K., Watson‐Leung, T., and Rochman, C. (2021). Impacts to Larval Fathead Minnows Vary between Preconsumer and Environmental Microplastics. Environ. Toxicol. Chem. 20. doi:10.1002/etc.5036
Calafat, A. M., Ye, X., Wong, L.-Y., Reidy, J. A., and Needham, L. L. (2008). Exposure of the U.S. Population to Bisphenol A and 4- Tertiary -Octylphenol: 2003-2004. Environ. Health Perspect. 116 (1), 39–44. doi:10.1289/ehp.10753
Campanale, C., Massarelli, C., Savino, I., Locaputo, V., and Uricchio, V. F. (2020). A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Ijerph 17 (4), 1212. doi:10.3390/ijerph17041212
Campos, D., Rodrigues, A. C. M., Rocha, R. J. M., Martins, R., Candeias-Mendes, A., Castanho, S., et al. (2021). Are Microplastics Impairing Marine Fish Larviculture?-Preliminary Results with Argyrosomus Regius. Water 13 (1), 104. doi:10.3390/w13010104
Capó, X., Company, J. J., Alomar, C., Compa, M., Sureda, A., Grau, A., et al. (2021). Long-term Exposure to virgin and Seawater Exposed Microplastic Enriched-Diet Causes Liver Oxidative Stress and Inflammation in Gilthead Seabream Sparus aurata, Linnaeus 1758. Sci. Total Environ. 767, 144976.
Capone, A., Petrillo, M., and Misic, C. (2020). Ingestion and Elimination of Anthropogenic Fibres and Microplastic Fragments by the European Anchovy (Engraulis Encrasicolus) of the NW Mediterranean Sea. Mar. Biol. 167 (11), 1–15. doi:10.1007/s00227-020-03779-7
Chae, Y., and An, Y.-J. (2018). Current Research Trends on Plastic Pollution and Ecological Impacts on the Soil Ecosystem: A Review. Environ. Pollut. 240, 387–395. doi:10.1016/j.envpol.2018.05.008
Chang, C. (2010). The Immune Effects of Naturally Occurring and Synthetic Nanoparticles. J. Autoimmun. 34 (3), J234–J246. doi:10.1016/j.jaut.2009.11.009
Chen, J.-C., Chen, M.-Y., Fang, C., Zheng, R.-H., Jiang, Y.-L., Zhang, Y.-S., et al. (2020c). Microplastics Negatively Impact Embryogenesis and Modulate the Immune Response of the marine Medaka Oryzias Melastigma. Mar. Pollut. Bull. 158, 111349. doi:10.1016/j.marpolbul.2020.111349
Chen, Q., Lackmann, C., Wang, W., Seiler, T.-B., Hollert, H., and Shi, H. (2020a). Microplastics Lead to Hyperactive Swimming Behaviour in Adult Zebrafish. Aquat. Toxicol. 224, 105521. doi:10.1016/j.aquatox.2020.105521
Chen, Q., Lv, W., Jiao, Y., Liu, Z., Li, Y., Cai, M., et al. (2020b). Effects of Exposure to Waterborne Polystyrene Microspheres on Lipid Metabolism in the Hepatopancreas of Juvenile Redclaw Crayfish, Cherax Quadricarinatus. Aquat. Toxicol. 224, 105497. doi:10.1016/j.aquatox.2020.105497
Chiu, H.-W., Xia, T., Lee, Y.-H., Chen, C.-W., Tsai, J.-C., and Wang, Y.-J. (2015). Cationic Polystyrene Nanospheres Induce Autophagic Cell Death through the Induction of Endoplasmic Reticulum Stress. Nanoscale 7 (2), 736–746. doi:10.1039/c4nr05509h
Choi, J. S., Jung, Y.-J., Hong, N.-H., Hong, S. H., and Park, J.-W. (2018). Toxicological Effects of Irregularly Shaped and Spherical Microplastics in a marine Teleost, the Sheepshead Minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 129 (1), 231–240. doi:10.1016/j.marpolbul.2018.02.039
Cole, M., Lindeque, P., Halsband, C., and Galloway, T. S. (2011). Microplastics as Contaminants in the marine Environment: A Review. Mar. Pollut. Bull. 62 (12), 2588–2597. doi:10.1016/j.marpolbul.2011.09.025
Colin, A., Bach, C., Rosin, C., Munoz, J.-F., and Dauchy, X. (2014). Is Drinking Water a Major Route of Human Exposure to Alkylphenol and Bisphenol Contaminants in France? Arch. Environ. Contam. Toxicol. 66 (1), 86–99. doi:10.1007/s00244-013-9942-0
Cong, Y., Jin, F., Tian, M., Wang, J., Shi, H., Wang, Y., et al. (2019). Ingestion, Egestion and post-exposure Effects of Polystyrene Microspheres on marine Medaka (Oryzias Melastigma). Chemosphere 228, 93–100. doi:10.1016/j.chemosphere.2019.04.098
Cormier, B., Le Bihanic, F., Cabar, M., Crebassa, J.-C., Blanc, M., Larsson, M., et al. (2021). Chronic Feeding Exposure to virgin and Spiked Microplastics Disrupts Essential Biological Functions in Teleost Fish. J. Hazard. Mater. 415, 125626. doi:10.1016/j.jhazmat.2021.125626
Crawford, C. B., and Quinn, B. (2017). The Interactions of Microplastics and Chemical Pollutants. Microplastic pollutants 1, 131–157. doi:10.1016/b978-0-12-809406-8.00006-2
Critchell, K., and Hoogenboom, M. O. (2018). Effects of Microplastic Exposure on the Body Condition and Behaviour of Planktivorous Reef Fish (Acanthochromis polyacanthus). PloS one 13 (3), e0193308. doi:10.1371/journal.pone.0193308
Cruz, C. A. B. (2019). Single and Combined Effects of MPs and Cadmium on Innate Immunity and Antioxidant Defence in European Seabass (Dicentrarchus labrax).
de Sá, L. C., Oliveira, M., Ribeiro, F., Rocha, T. L., and Futter, M. N. (2018). Studies of the Effects of Microplastics on Aquatic Organisms: What Do We Know and where Should We Focus Our Efforts in the Future? Sci. Total Environ. 645, 1029–1039. doi:10.1016/j.scitotenv.2018.07.207
de Sá, L. C., Luís, L. G., and Guilhermino, L. (2015). Effects of MPs on Juveniles of the Common Goby (Pomatoschistus Microps): Confusion with Prey, Reduction of the Predatory Performance and Efficiency, and Possible Influence of Developmental Conditions. Environ. Pollut. 196, 359–362.
Deng, Y., Zhang, Y., Lemos, B., and Ren, H. (2017). Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 7 (1), 46687–46710. doi:10.1038/srep46687
Devane, P. A., Bourne, R. B., Rorabeck, C. H., Hardie, R. M., and Home, J. G. (1995a). Measurement of Polyethylene Wear in Metal-Backed Acetabular Cups. Clin. orthopaedics Relat. Res. NA, 303–316. doi:10.1097/00003086-199510000-00033
Devane, P. A., Bourne, R. B., Rorabeck, C. H., MacDonald, S., and Robinson, E. J. (1995b). Measurement of Polyethylene Wear in Metal-Backed Acetabular Cups. Clin. orthopaedics Relat. Res. NA (319), 317–326. doi:10.1097/00003086-199510000-00034
Dimitriadi, A., Papaefthimiou, C., Genizegkini, E., Sampsonidis, I., Kalogiannis, S., Feidantsis, K., et al. (2021). Adverse Effects Polystyrene Microplastics Exert on Zebrafish Heart - Molecular to Individual Level. J. Hazard. Mater. 416, 125969. doi:10.1016/j.jhazmat.2021.125969
Dinani, F. S. H., Baradaran, A., and Ebrahimpour, K. (2021). Acute Toxic Effects of Polyurethane MPs on Adult Zebra Fish (Danio rerio). Int. J. Environ. Health Eng. 10 (1), 9.
Ding, J., Huang, Y., Liu, S., Zhang, S., Zou, H., Wang, Z., et al. (2020). Toxicological Effects of Nano- and Micro-polystyrene Plastics on Red tilapia: Are Larger Plastic Particles More Harmless? J. Hazard. Mater. 396, 122693. doi:10.1016/j.jhazmat.2020.122693
Ding, J., Zhang, S., Razanajatovo, R. M., Zou, H., and Zhu, W. (2018). Accumulation, Tissue Distribution, and Biochemical Effects of Polystyrene Microplastics in the Freshwater Fish Red tilapia (Oreochromis niloticus). Environ. Pollut. 238, 1–9. doi:10.1016/j.envpol.2018.03.001
Duan, Z., Duan, X., Zhao, S., Wang, X., Wang, J., Liu, Y., et al. (2020). Barrier Function of Zebrafish Embryonic Chorions against Microplastics and Nanoplastics and its Impact on Embryo Development. J. Hazard. Mater. 395, 122621. doi:10.1016/j.jhazmat.2020.122621
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2016). Presence of MPs and Nanoplastics in Food, with Particular Focus on Seafood. Efsa J. 14 (6), e04501.
Espinosa, C., Cuesta, A., and Esteban, M. Á. (2017). Effects of Dietary Polyvinylchloride Microparticles on General Health, Immune Status and Expression of Several Genes Related to Stress in Gilthead Seabream (Sparus aurata L.). Fish Shellfish Immunol. 68, 251–259. doi:10.1016/j.fsi.2017.07.006
Espinosa, C., Esteban, M. Á., and Cuesta, A. (2019). Dietary Administration of PVC and PE Microplastics Produces Histological Damage, Oxidative Stress and Immunoregulation in European Sea Bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 95, 574–583. doi:10.1016/j.fsi.2019.10.072
Espinosa, C., García Beltrán, J. M., Esteban, M. A., and Cuesta, A. (2018). In Vitro effects of virgin Microplastics on Fish Head-Kidney Leucocyte Activities. Environ. Pollut. 235, 30–38. doi:10.1016/j.envpol.2017.12.054
Farhat, S. C. L., Silva, C. A., Orione, M. A. M., Campos, L. M. A., Sallum, A. M. E., and Braga, A. L. F. (2011). Air Pollution in Autoimmune Rheumatic Diseases: a Review. Autoimmun. Rev. 11 (1), 14–21. doi:10.1016/j.autrev.2011.06.008
Feng, S., Zeng, Y., Cai, Z., Wu, J., Chan, L. L., Zhu, J., et al. (2021). Polystyrene Microplastics Alter the Intestinal Microbiota Function and the Hepatic Metabolism Status in marine Medaka (Oryzias Melastigma). Sci. Total Environ. 759, 143558. doi:10.1016/j.scitotenv.2020.143558
Fernandes, E. C., Silva, C. A., Braga, A. L. F., Sallum, A. M. E., Campos, L. M. A., and Farhat, S. C. L. (2015). Exposure to Air Pollutants and Disease Activity in Juvenile-Onset Systemic Lupus Erythematosus Patients. Arthritis Care Res. 67 (11), 1609–1614. doi:10.1002/acr.22603
Fonte, E., Ferreira, P., and Guilhermino, L. (2016). Temperature Rise and Microplastics Interact with the Toxicity of the Antibiotic Cefalexin to Juveniles of the Common Goby ( Pomatoschistus Microps ): Post-exposure Predatory Behaviour, Acetylcholinesterase Activity and Lipid Peroxidation. Aquat. Toxicol. 180, 173–185. doi:10.1016/j.aquatox.2016.09.015
Forte, M., Iachetta, G., Tussellino, M., Carotenuto, R., Prisco, M., De Falco, M., et al. (2016). Polystyrene Nanoparticles Internalization in Human Gastric Adenocarcinoma Cells. Toxicol. Vitro 31, 126–136. doi:10.1016/j.tiv.2015.11.006
Fuchs, A.-K., Syrovets, T., Haas, K. A., Loos, C., Musyanovych, A., Mailänder, V., et al. (2016). Carboxyl- and Amino-Functionalized Polystyrene Nanoparticles Differentially Affect the Polarization Profile of M1 and M2 Macrophage Subsets. Biomaterials 85, 78–87. doi:10.1016/j.biomaterials.2016.01.064
Furukuma, S., and Fujii, N. (2016). In Vitro cytotoxicity Evaluation of Plastic marine Debris by colony-forming Assay. Jpn. J. Environ. Toxicol. 19 (2), 71–81.
Galloway, T. S. (2015). “Micro- and Nano-Plastics and Human Health,” in Marine Anthropogenic Litter (Cham: Springer), 343–366. doi:10.1007/978-3-319-16510-3_13
Garrido Gamarro, E., Ryder, J., Elvevoll, E. O., and Olsen, R. L. (2020). Microplastics in Fish and Shellfish - A Threat to Seafood Safety? J. Aquat. Food Product. Technology 29 (4), 417–425. doi:10.1080/10498850.2020.1739793
Geiser, M., Rothen-Rutishauser, B., Kapp, N., Schürch, S., Kreyling, W., Schulz, H., et al. (2005). Ultrafine Particles Cross Cellular Membranes by Nonphagocytic Mechanisms in Lungs and in Cultured Cells. Environ. Health Perspect. 113 (11), 1555–1560. doi:10.1289/ehp.8006
Gewert, B., Plassmann, M. M., and MacLeod, M. (2015). Pathways for Degradation of Plastic Polymers Floating in the marine Environment. Environ. Sci. Process. Impacts 17 (9), 1513–1521. doi:10.1039/c5em00207a
Gholizadeh, M., and Patimar, R. (2018). Ecological Risk Assessment of Heavy Metals in Surface Sediments from the Gorgan Bay, Caspian Sea. Mar. Pollut. Bull. 137, 662–667. doi:10.1016/j.marpolbul.2018.11.009
González-Pleiter, M., Tamayo-Belda, M., Pulido-Reyes, G., Amariei, G., Leganés, F., Rosal, R., et al. (2019). Secondary Nanoplastics Released from a Biodegradable Microplastic Severely Impact Freshwater Environments. Environ. Sci. Nano 6 (5), 1382–1392.
Goswami, P., Vinithkumar, N. V., and Dharani, G. (2020). First Evidence of Microplastics Bioaccumulation by marine Organisms in the Port Blair Bay, Andaman Islands. Mar. Pollut. Bull. 155, 111163. doi:10.1016/j.marpolbul.2020.111163
Grafmueller, S., Manser, P., Diener, L., Diener, P.-A., Maeder-Althaus, X., Maurizi, L., et al. (2015). Bidirectional Transfer Study of Polystyrene Nanoparticles across the Placental Barrier in an Ex Vivo Human Placental Perfusion Model. Environ. Health Perspect. 123 (12), 1280–1286. doi:10.1289/ehp.1409271
Granby, K., Rainieri, S., Rasmussen, R. R., Kotterman, M. J. J., Sloth, J. J., Cederberg, T. L., et al. (2018). The Influence of Microplastics and Halogenated Contaminants in Feed on Toxicokinetics and Gene Expression in European Seabass (Dicentrarchus labrax). Environ. Res. 164, 430–443. doi:10.1016/j.envres.2018.02.035
Green, T., Fisher, J., Stone, M., Wroblewski, B. M., and Ingham, E. (1998). Polyethylene Particles of a 'critical Size' Are Necessary for the Induction of Cytokines by Macrophages In Vitro. Biomaterials 19 (24), 2297–2302. doi:10.1016/s0142-9612(98)00140-9
Greven, A.-C., Merk, T., Karagöz, F., Mohr, K., Klapper, M., Jovanović, B., et al. (2016). Polycarbonate and Polystyrene Nanoplastic Particles Act as Stressors to the Innate Immune System of Fathead Minnow (Pimephales promelas). Environ. Toxicol. Chem. 35 (12), 3093–3100. doi:10.1002/etc.3501
Gu, L., Tian, L., Gao, G., Peng, S., Zhang, J., Wu, D., ., , Huang, J., Hua, Q., Lu, T., Zhong, L., Fu, Z., Pan, X., Qian, H., and Sun, L. (2020). Inhibitory Effects of Polystyrene Microplastics on Caudal Fin Regeneration in Zebrafish Larvae. Environ. Pollut. 266, 114664. doi:10.1016/j.envpol.2020.114664
Guimarães, A. T. B., Charlie-Silva, I., and Malafaia, G. (2021). Toxic Effects of Naturally-Aged MPs on Zebrafish Juveniles: A More Realistic Approach to Plastic Pollution in Freshwater Ecosystems. J. Hazard. Mater. 407, 124833.
Guven, O., Bach, L., Munk, P., Dinh, K. V., Mariani, P., and Nielsen, T. G. (2018). Microplastic Does Not Magnify the Acute Effect of PAH Pyrene on Predatory Performance of a Tropical Fish ( Lates calcarifer ). Aquat. Toxicol. 198, 287–293. doi:10.1016/j.aquatox.2018.03.011
Güven, O., Gökdağ, K., Jovanović, B., and Kıdeyş, A. E. (2017). Microplastic Litter Composition of the Turkish Territorial Waters of the Mediterranean Sea, and its Occurrence in the Gastrointestinal Tract of Fish. Environ. Pollut. 223, 286–294.
Halden, R. U. (2010). Plastics and Health Risks. Annu. Rev. Public Health 31, 179–194. doi:10.1146/annurev.publhealth.012809.103714
Hamed, M., Soliman, H. A. M., Badrey, A. E. A., and Osman, A. G. M. (2021). Microplastics Induced Histopathological Lesions in Some Tissues of tilapia (Oreochromis niloticus) Early Juveniles. Tissue and Cell 71, 101512. doi:10.1016/j.tice.2021.101512
Hamed, M., Soliman, H. A. M., Osman, A. G. M., and Sayed, A. E.-D. H. (2019). Assessment the Effect of Exposure to Microplastics in Nile Tilapia (Oreochromis niloticus) Early Juvenile: I. Blood Biomarkers. Chemosphere 228, 345–350. doi:10.1016/j.chemosphere.2019.04.153
Hamed, M., Soliman, H. A., Osman, A. G., and Sayed, A. E. D. H. (2020). Antioxidants and Molecular Damage in Nile Tilapia (Oreochromis niloticus) after Exposure to MPs. Environmental Science and Pollution Research 27, 14581–14588.
Hanachi, P., Karbalaei, S., and Yu, S. (2021). Combined Polystyrene MPs and Chlorpyrifos Decrease Levels of Nutritional Parameters in Muscle of Rainbow trout (Oncorhynchus mykiss). Environ. Sci. Pollut. Res., 1–13.
Huang, J.-N., Wen, B., Meng, L.-J., Li, X.-X., Wang, M.-H., Gao, J.-Z., et al. (2020a). Integrated Response of Growth, Antioxidant Defense and Isotopic Composition to Microplastics in Juvenile Guppy (Poecilia reticulata). J. Hazard. Mater. 399, 123044. doi:10.1016/j.jhazmat.2020.123044
Huang, J.-N., Wen, B., Xu, L., Ma, H.-C., Li, X.-X., Gao, J.-Z., et al. (2022). Micro/nano-plastics Cause Neurobehavioral Toxicity in Discus Fish (Symphysodon aequifasciatus): Insight from Brain-Gut-Microbiota axis. J. Hazard. Mater. 421, 126830. doi:10.1016/j.jhazmat.2021.126830
Huang, J.-N., Wen, B., Zhu, J.-G., Zhang, Y.-S., Gao, J.-Z., and Chen, Z.-Z. (2020b). Exposure to Microplastics Impairs Digestive Performance, Stimulates Immune Response and Induces Microbiota Dysbiosis in the Gut of Juvenile Guppy (Poecilia reticulata). Sci. Total Environ. 733, 138929. doi:10.1016/j.scitotenv.2020.138929
Huang, J. N., Zhang, Y., Xu, L., He, K. X., Wen, B., Yang, P. W., et al. (2021b). MPs: A Tissue-specific Threat to Microbial Community and Biomarkers of Discus Fish (Symphysodon aequifasciatus). J. Hazard. Mater., 127751. doi:10.1016/j.jhazmat.2021.127751
Huang, W., Song, B., Liang, J., Niu, Q., Zeng, G., Shen, M., et al. (2021a). Microplastics and Associated Contaminants in the Aquatic Environment: A Review on Their Ecotoxicological Effects, Trophic Transfer, and Potential Impacts to Human Health. J. Hazard. Mater. 405, 124187. doi:10.1016/j.jhazmat.2020.124187
Hwang, J., Choi, D., Han, S., Choi, J., and Hong, J. (2019). An Assessment of the Toxicity of Polypropylene MPs in Human Derived Cells. Sci. Total Environ. 684, 657–669. doi:10.1016/j.scitotenv.2019.05.071
Iheanacho, S. C., Igberi, C., Amadi-Eke, A., Chinonyerem, D., Iheanacho, A., and Avwemoya, F. (2020). Biomarkers of Neurotoxicity, Oxidative Stress, Hepatotoxicity and Lipid Peroxidation in Clarias gariepinus Exposed to Melamine and Polyvinyl Chloride. Biomarkers 25 (7), 603–610. doi:10.1080/1354750x.2020.1821777
Iheanacho, S. C., and Odo, G. E. (2020a). Dietary Exposure to Polyvinyl Chloride Microparticles Induced Oxidative Stress and Hepatic Damage in Clarias gariepinus (Burchell, 1822). Environ. Sci. Pollut. Res. 27 (17), 21159–21173. doi:10.1007/s11356-020-08611-9
Iheanacho, S. C., and Odo, G. E. (2020b). Neurotoxicity, Oxidative Stress Biomarkers and Haematological Responses in African Catfish (Clarias gariepinus) Exposed to Polyvinyl Chloride Microparticles. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 232, 108741. doi:10.1016/j.cbpc.2020.108741
Inkielewicz-Stepniak, I., Tajber, L., Behan, G., Zhang, H., Radomski, M. W., Medina, C., et al. (2018). The Role of Mucin in the Toxicological Impact of Polystyrene Nanoparticles. Materials 11 (5), 724. doi:10.3390/ma11050724
Ismail, R. F., Saleh, N. E., and Sayed, A. E. D. H. (2021). Impacts of MPs on Reproductive Performance of Male tilapia (Oreochromis niloticus) Pre-fed on Amphora Coffeaeformis. Environ. Sci. Pollut. Res., 1–13.
Jabeen, K., Li, B., Chen, Q., Su, L., Wu, C., Hollert, H., et al. (2018). Effects of virgin MPs on Goldfish (Carassius auratus). Chemosphere 213, 323–332. doi:10.1016/j.chemosphere.2018.09.031
Jacob, H., Besson, M., Oberhaensli, F., Taylor, A., Gillet, B., Hughes, S., et al. (2021). A Multifaceted Assessment of the Effects of Polyethylene MPs on Juvenile Gilthead Seabreams (Sparus aurata). Aquat. Toxicol., 106004.
Jakubowska, M., Białowąs, M., Stankevičiūtė, M., Chomiczewska, A., Pažusienė, J., Jonko-Sobuś, K., et al. (2020). Effects of Chronic Exposure to MPs of Different Polymer Types on Early Life Stages of Sea trout Salmo trutta. Sci. Total Environ. 740, 139922. doi:10.1016/j.scitotenv.2020.139922
James, K., Vasant, K., Padua, S., Gopinath, V., Abilash, K. S., Jeyabaskaran, R., et al. (2020). An Assessment of Microplastics in the Ecosystem and Selected Commercially Important Fishes off Kochi, South Eastern Arabian Sea, India. Mar. Pollut. Bull. 154, 111027. doi:10.1016/j.marpolbul.2020.111027
Jin, Y., Lu, L., Tu, W., Luo, T., and Fu, Z. (2019). Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 649, 308–317. doi:10.1016/j.scitotenv.2018.08.353
Jin, Y., Xia, J., Pan, Z., Yang, J., Wang, W., and Fu, Z. (2018). Polystyrene MPs Induce Microbiota Dysbiosis and Inflammation in the Gut of Adult Zebrafish. Environ. Pollut. 235, 322–329. doi:10.1016/j.envpol.2017.12.088
Jovanović, B., Gökdağ, K., Güven, O., Emre, Y., Whitley, E. M., and Kideys, A. E. (2018). Virgin MPs Are Not Causing Imminent Harm to Fish after Dietary Exposure. Mar. Pollut. Bull. 130, 123–131. doi:10.1016/j.marpolbul.2018.03.016
Kaloyianni, M., Bobori, D. C., Xanthopoulou, D., Malioufa, G., Sampsonidis, I., Kalogiannis, S., et al. (2021). Toxicity and Functional Tissue Responses of Two Freshwater Fish after Exposure to Polystyrene MPs. Toxics 9 (11), 289. doi:10.3390/toxics9110289
Kang, H. M., Byeon, E., Jeong, H., Kim, M. S., Chen, Q., and Lee, J. S. (2021). Different Effects of Nano-And MPs on Oxidative Status and Gut Microbiota in the marine Medaka Oryzias Melastigma. J. Hazard. Mater. 405, 124207. doi:10.1016/j.jhazmat.2020.124207
Karami, A., Romano, N., Galloway, T., and Hamzah, H. (2016). Virgin MPs Cause Toxicity and Modulate the Impacts of Phenanthrene on Biomarker Responses in African Catfish (Clarias gariepinus). Environ. Res. 151, 58–70. doi:10.1016/j.envres.2016.07.024
Karbalaei, S., Hanachi, P., Rafiee, G., and Seifori, P. (2021). Toxicity of Polystyrene MPs on Juvenile Oncorhynchus mykiss (Rainbow trout) after Individual and Combined Exposure with Chlorpyrifos. J. Hazard. Mater., 403, 123980.doi:10.1016/j.jhazmat.2020.123980
Karbalaei, S., Hanachi, P., Walker, T. R., and Cole, M. (2018). Occurrence, Sources, Human Health Impacts and Mitigation of Microplastic Pollution. Environ. Sci. Pollut. Res. 25 (36), 36046–36063. doi:10.1007/s11356-018-3508-7
Kelly, F. J., and Fussell, J. C. (2012). Size, Source and Chemical Composition as Determinants of Toxicity Attributable to Ambient Particulate Matter. Atmos. Environ. 60, 504–526. doi:10.1016/j.atmosenv.2012.06.039
Kirstein, I. V., Kirmizi, S., Wichels, A., Garin-Fernandez, A., Erler, R., Löder, M., et al. (2016). Dangerous Hitchhikers? Evidence for Potentially Pathogenic Vibrio Spp. On Microplastic Particles. Mar. Environ. Res. 120, 1–8. doi:10.1016/j.marenvres.2016.07.004
Kor, K., Ghazilou, A., and Ershadifar, H. (2020). Microplastic Pollution in the Littoral Sediments of the Northern Part of the Oman Sea. Mar. Pollut. Bull. 155, 111166. doi:10.1016/j.marpolbul.2020.111166
Kor, K., and Mehdinia, A. (2020). Neustonic Microplastic Pollution in the Persian Gulf. Mar. Pollut. Bull. 150, 110665. doi:10.1016/j.marpolbul.2019.110665
Kumar, V. E., Ravikumar, G., and Jeyasanta, K. I. (2018). Occurrence of MPs in Fishes from Two landing Sites in Tuticorin, South East Coast of India. Mar. Pollut. Bull. 135, 889–894. doi:10.1016/j.marpolbul.2018.08.023
Kumkar, P., Gosavi, S. M., Verma, C. R., Pise, M., and Kalous, L. (2021). Big Eyes Can't See MPs: Feeding Selectivity and Eco-Morphological Adaptations in Oral Cavity Affect Microplastic Uptake in Mud-Dwelling Amphibious Mudskipper Fish. Sci. Total Environ. 786, 147445. doi:10.1016/j.scitotenv.2021.147445
Kutralam-Muniasamy, G., Pérez-Guevara, F., Elizalde-Martínez, I., and Shruti, V. C. (2020). Review of Current Trends, Advances and Analytical Challenges for Microplastics Contamination in Latin America. Environ. Pollut. 267, 115463.doi:10.1016/j.envpol.2020.115463
Le Bihanic, F., Clérandeau, C., Cormier, B., Crebassa, J. C., Keiter, S. H., Beiras, R., et al. (2020). Organic Contaminants Sorbed to MPs Affect marine Medaka Fish Early Life Stages Development. Mar. Pollut. Bull. 154, 111059. doi:10.1016/j.marpolbul.2020.111059
LeMoine, C. M., Kelleher, B. M., Lagarde, R., Northam, C., Elebute, O. O., and Cassone, B. J. (2018). Transcriptional Effects of Polyethylene MPs Ingestion in Developing Zebrafish (Danio rerio). Environ. Pollut. 243, 591–600. doi:10.1016/j.envpol.2018.08.084
Li, Y., Wang, J., Yang, G., Lu, L., Zheng, Y., Zhang, Q., et al. (2020). Low Level of Polystyrene MPs Decreases Early Developmental Toxicity of Phenanthrene on marine Medaka (Oryzias Melastigma). J. Hazard. Mater. 385, 121586. doi:10.1016/j.jhazmat.2019.121586
Li, Y., Yang, G., Wang, J., Lu, L., Li, X., Zheng, Y., et al. (2021). MPs Increase the Accumulation of Phenanthrene in the Ovaries of marine Medaka (Oryzias Melastigma) and its Transgenerational Toxicity. J. Hazard. Mater., 127754. doi:10.1016/j.jhazmat.2021.127754
Li'ang Li, R. X., Jiang, L., Xu, E. G., Wang, M., Wang, J., Li, B., et al. (2021). Effects of MPs on Immune Responses of the Yellow Catfish Pelteobagrus fulvidraco under Hypoxia. Front. Physiol. 12. doi:10.3389/fphys.2021.753999
Limonta, G., Mancia, A., Abelli, L., Fossi, M. C., Caliani, I., and Panti, C. (2021). Effects of MPs on Head Kidney Gene Expression and Enzymatic Biomarkers in Adult Zebrafish. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 245, 109037. doi:10.1016/j.cbpc.2021.109037
Limonta, G., Mancia, A., Benkhalqui, A., Bertolucci, C., Abelli, L., Fossi, M. C., et al. (2019). MPs Induce Transcriptional Changes, Immune Response and Behavioral Alterations in Adult Zebrafish. Scientific Rep. 9 (1), 1–11. doi:10.1038/s41598-019-52292-5
Ling, H. E., ZhanG, Y., Yuanjiao, W. A. N. G., Yijiao, W. U., Lixiao, C. H. E. N., Ruilei, F. U., et al. (2018). Toxic Effects of Micro-plastics on Zebrafish Embryos. Agric. Biotechnol. 7 2164–49935.
Liu, A., Richards, L., Bladen, C. L., Ingham, E., Fisher, J., and Tipper, J. L. (2015). The Biological Response to Nanometre-Sized Polymer Particles. Acta Biomater. 23, 38–51. doi:10.1016/j.actbio.2015.05.016
Liu, X., Tian, X., Xu, X., and Lu, J. (2018). Design of a Phosphinate‐based Bioluminescent Probe for Superoxide Radical Anion Imaging in Living Cells. Luminescence 33 (6), 1101–1106. doi:10.1002/bio.3515
Liu, Y., Jia, X., Zhu, H., Zhang, Q., He, Y., Shen, Y., et al. (2022). The Effects of Exposure to MPs on Grass Carp (Ctenopharyngodon Idella) at the Physiological, Biochemical, and Transcriptomic Levels. Chemosphere 286, 131831. doi:10.1016/j.chemosphere.2021.131831
Lu, K., Qiao, R., An, H., and Zhang, Y. (2018a). Influence of MPs on the Accumulation and Chronic Toxic Effects of Cadmium in Zebrafish (Danio rerio). Chemosphere 202, 514–520. doi:10.1016/j.chemosphere.2018.03.145
Lu, L., Wan, Z., Luo, T., Fu, Z., and Jin, Y. (2018b). Polystyrene MPs Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci. Total Environ. 631, 449–458. doi:10.1016/j.scitotenv.2018.03.051
Lu, Y., Zhang, Y., Deng, Y., Jiang, W., Zhao, Y., Geng, J., et al. (2016). Uptake and Accumulation of Polystyrene MPs in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 50 (7), 4054–4060. doi:10.1021/acs.est.6b00183
Luís, L. G., Ferreira, P., Fonte, E., Oliveira, M., and Guilhermino, L. (2015). Does the Presence of MPs Influence the Acute Toxicity of Chromium (VI) to Early Juveniles of the Common Goby (Pomatoschistus Microps)? A Study with Juveniles from Two Wild Estuarine Populations. Aquat. Toxicol. 164, 163–174. doi:10.1016/j.aquatox.2015.04.018
Luo, T., Wang, C., Pan, Z., Jin, C., Fu, Z., and Jin, Y. (2019b). Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 53 (18), 10978–10992. doi:10.1021/acs.est.9b03191
Luo, T., Zhang, Y., Wang, C., Wang, X., Zhou, J., Shen, M., et al. (2019a). Maternal Exposure to Different Sizes of Polystyrene MPs during Gestation Causes Metabolic Disorders in Their Offspring. Environ. Pollut. 255, 113122. doi:10.1016/j.envpol.2019.113122
Lusher, A. L., Mchugh, M., and Thompson, R. C. (2013). Occurrence of MPs in the Gastrointestinal Tract of Pelagic and Demersal Fish from the English Channel. Mar. Pollut. Bull. 67 (1-2), 94–99. doi:10.1016/j.marpolbul.2012.11.028
Lusher, A. L., Welden, N. A., Sobral, P., and Cole, M. (2020). Sampling, Isolating and Identifying Microplastics Ingested by Fish and Invertebrates. Anal. Nanoplastics Microplastics Food, 119–148. doi:10.1201/9780429469596-8
Ma, H., Pu, S., Liu, S., Bai, Y., Mandal, S., and Xing, B. (2020). MPs in Aquatic Environments: Toxicity to Trigger Ecological Consequences. Environ. Pollut. 261, 114089. doi:10.1016/j.envpol.2020.114089
Magrì, D., Sánchez-Moreno, P., Caputo, G., Gatto, F., Veronesi, M., Bardi, G., et al. (2018). Laser Ablation as a Versatile Tool to Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS nano 12 (8), 7690–7700. doi:10.1021/acsnano.8b01331
Mahadevan, G., and Valiyaveettil, S. (2021). Understanding the Interactions of Poly (Methyl Methacrylate) and Poly (Vinyl Chloride) Nanoparticles with BHK-21 Cell Line. Scientific Rep. 11 (1), 1–15. doi:10.1038/s41598-020-80708-0
Mahler, G. J., Esch, M. B., Tako, E., Southard, T. L., Archer, S. D., Glahn, R. P., et al. (2012). Oral Exposure to Polystyrene Nanoparticles Affects Iron Absorption. Nat. nanotechnology 7 (4), 264–271. doi:10.1038/nnano.2012.3
Mak, C. W., Yeung, K. C. F., and Chan, K. M. (2019). Acute Toxic Effects of Polyethylene Microplastic on Adult Zebrafish. Ecotoxicology Environ. Saf. 182, 109442. doi:10.1016/j.ecoenv.2019.109442
Mallik, A., Xavier, K. M., Naidu, B. C., and Nayak, B. B. (2021). Ecotoxicological and Physiological Risks of Microplastics on Fish and Their Possible Mitigation Measures. Sci. Total Environ. 779, 146433. doi:10.1016/j.scitotenv.2021.146433
Manzoor, J., Sharma, M., Sofi, I. R., and Dar, A. A. (2020). “Plastic Waste Environmental and Human Health Impacts,” in Handbook of Research on Environmental and Human Health Impacts of Plastic Pollution (Hershey, USA: IGI Global), 29–37. doi:10.4018/978-1-5225-9452-9.ch002
Mazurais, D., Ernande, B., Quazuguel, P., Severe, A., Huelvan, C., Madec, L., et al. (2015). Evaluation of the Impact of Polyethylene Microbeads Ingestion in European Sea Bass (Dicentrarchus labrax) Larvae. Mar. Environ. Res. 112, 78–85. doi:10.1016/j.marenvres.2015.09.009
McCarthy, J., Gong, X., Nahirney, D., Duszyk, M., and Radomski, M. W. (2011). Polystyrene Nanoparticles Activate Ion Transport in Human Airway Epithelial Cells. Int. J. nanomedicine 6, 1343. doi:10.2147/ijn.s21145
McCormick, M. I., Chivers, D. P., Ferrari, M. C., Blandford, M. I., Nanninga, G. B., Richardson, C., et al. (2020). Microplastic Exposure Interacts with Habitat Degradation to Affect Behaviour and Survival of Juvenile Fish in the Field. Proc. R. Soc. B 287, 20201947. doi:10.1098/rspb.2020.1947
Meeker, J. D., Ehrlich, S., Toth, T. L., Wright, D. L., Calafat, A. M., Trisini, A. T., et al. (2010). Semen Quality and Sperm DNA Damage in Relation to Urinary Bisphenol A Among Men from an Infertility Clinic. Reprod. Toxicol. 30 (4), 532–539. doi:10.1016/j.reprotox.2010.07.005
Michałowicz, J. (2014). Bisphenol A–Sources, Toxicity and Biotransformation. Environ. Toxicol. Pharmacol. 37 (2), 738–758. doi:10.1016/j.etap.2014.02.003
Miranda, T., Vieira, L. R., and Guilhermino, L. (2019). Neurotoxicity, Behavior, and Lethal Effects of Cadmium, MPs, and Their Mixtures on Pomatoschistus Microps Juveniles from Two Wild Populations Exposed under Laboratory Conditions―implications to Environmental and Human Risk Assessment. Int. J. Environ. Res. Public Health 16 (16), 2857. doi:10.3390/ijerph16162857
Mizraji, R., Ahrendt, C., Perez-Venegas, D., Vargas, J., Pulgar, J., Aldana, M., et al. (2017). Is the Feeding Type Related with the Content of MPs in Intertidal Fish Gut? Mar. Pollut. Bull. 116 (1-2), 498–500. doi:10.1016/j.marpolbul.2017.01.008
MohanKumar, S. M., Campbell, A., Block, M., and Veronesi, B. (2008). Particulate Matter, Oxidative Stress and Neurotoxicity. Neurotoxicology 29 (3), 479–488. doi:10.1016/j.neuro.2007.12.004
Montero, D., Rimoldi, S., Torrecillas, S., Rapp, J., Moroni, F., Herrera, A., et al. (2022). Impact of Polypropylene MPs and Chemical Pollutants on European Sea Bass (Dicentrarchus labrax) Gut Microbiota and Health. Sci. Total Environ. 805, 150402. doi:10.1016/j.scitotenv.2021.150402
Monti, D. M., Guarnieri, D., Napolitano, G., Piccoli, R., Netti, P., Fusco, S., et al. (2015). Biocompatibility, Uptake and Endocytosis Pathways of Polystyrene Nanoparticles in Primary Human Renal Epithelial Cells. J. Biotechnol. 193, 3–10. doi:10.1016/j.jbiotec.2014.11.004
Mu, X., Qi, S., Liu, J., Yuan, L., Huang, Y., Xue, J., et al. (2021). Toxicity and Behavioral Response of Zebrafish Exposed to Combined Microplastic and Bisphenol Analogues. Environ. Chem. Lett., 1–8. doi:10.1007/s10311-021-01320-w
Müller, C., Erzini, K., Teodósio, M. A., Pousão-Ferreira, P., Baptista, V., and Ekau, W. (2020). Assessing Microplastic Uptake and Impact on Omnivorous Juvenile white Seabream Diplodus sargus (Linnaeus, 1758) under Laboratory Conditions. Mar. Pollut. Bull. 157, 111162.
Naidoo, T., and Glassom, D. (2019). Decreased Growth and Survival in Small Juvenile Fish, after Chronic Exposure to Environmentally Relevant Concentrations of Microplastic. Mar. Pollut. Bull. 145, 254–259. doi:10.1016/j.marpolbul.2019.02.037
Naji, A., Esmaili, Z., Mason, S. A., and Vethaak, A. D. (2017). The Occurrence of Microplastic Contamination in Littoral Sediments of the Persian Gulf, Iran. Environ. Sci. Pollut. Res. 24 (25), 20459–20468. doi:10.1007/s11356-017-9587-z
Nelms, S. E., Barnett, J., Brownlow, A., Davison, N. J., Deaville, R., Galloway, T. S., et al. (2019). MPs in marine Mammals Stranded Around the British Coast: Ubiquitous but Transitory? Scientific Rep. 9 (1), 1–8. doi:10.1038/s41598-018-37428-3
Nematdoost Haghi, B., and Banaee, M. (2017). Effects of Micro-plastic Particles on Paraquat Toxicity to Common Carp (Cyprinus carpio): Biochemical Changes. Int. J. Environ. Sci. Technol. 14 (3), 521–530. doi:10.1007/s13762-016-1171-4
Neves, D., Sobral, P., Ferreira, J. L., and Pereira, T. (2015). Ingestion of MPs by Commercial Fish off the Portuguese Coast. Mar. Pollut. Bull. 101 (1), 119–126. doi:10.1016/j.marpolbul.2015.11.008
Nich, C., and Goodman, S. B. (2014). Role of Macrophages in the Biological Reaction to Wear Debris from Joint Replacements. J. long-term effects Med. Implants 24 (4). doi:10.1615/jlongtermeffmedimplants.2014010562
Oliveira, M., Ribeiro, A., Hylland, K., and Guilhermino, L. (2013). Single and Combined Effects of MPs and Pyrene on Juveniles (0+ Group) of the Common Goby Pomatoschistus Microps (Teleostei, Gobiidae). Ecol. indicators 34, 641–647. doi:10.1016/j.ecolind.2013.06.019
Ormsby, R. T., Cantley, M., Kogawa, M., Solomon, L. B., Haynes, D. R., Findlay, D. M., et al. (2016). Evidence that Osteocyte Perilacunar Remodelling Contributes to Polyethylene Wear Particle Induced Osteolysis. Acta Biomater. 33, 242–251. doi:10.1016/j.actbio.2016.01.016
Ouyang, M. Y., Feng, X. S., Li, X. X., Wen, B., Liu, J. H., Huang, J. N., et al. (2021). MPs Intake and Excretion: Resilience of the Intestinal Microbiota but Residual Growth Inhibition in Common Carp. Chemosphere 276, 130144. doi:10.1016/j.chemosphere.2021.130144
Paget, V., Dekali, S., Kortulewski, T., Grall, R., Gamez, C., Blazy, K., et al. (2015). Specific Uptake and Genotoxicity Induced by Polystyrene Nanobeads with Distinct Surface Chemistry on Human Lung Epithelial Cells and Macrophages. PloS one 10 (4), e0123297. doi:10.1371/journal.pone.0123297
Pannetier, P., Cachot, J., Clérandeau, C., Faure, F., Van Arkel, K., de Alencastro, L. F., et al. (2019). Toxicity Assessment of Pollutants Sorbed on Environmental Sample MPs Collected on Beaches: Part I-Adverse Effects on Fish Cell Line. Environ. Pollut. 248, 1088–1097. doi:10.1016/j.envpol.2018.12.091
Pannetier, P., Morin, B., Le Bihanic, F., Dubreil, L., Clérandeau, C., Chouvellon, F., et al. (2020). Environmental Samples of MPs Induce Significant Toxic Effects in Fish Larvae. Environ. Int. 134, 105047. doi:10.1016/j.envint.2019.105047
Pazos, R. S., Maiztegui, T., Colautti, D. C., Paracampo, A. H., and Gómez, N. (2017). MPs in gut contents of coastal freshwater fish from Río de la Plata estuary. Mar. Pollut. Bull. 122 (1-2), 85–90. doi:10.1016/j.marpolbul.2017.06.007
Peda, C., Caccamo, L., Fossi, M. C., Gai, F., Andaloro, F., Genovese, L., et al. (2016). Intestinal Alterations in European Sea Bass Dicentrarchus labrax (Linnaeus, 1758) Exposed to MPs: Preliminary Results. Environ. Pollut. 212, 251–256. doi:10.1016/j.envpol.2016.01.083
PlasticsEurope, (2018). Annual Review 2017–2018. Available at: https://www.plasticseurope.org/download_file/force/1830/181.
PlasticsEurope, (2019). Plastics e the Facts 2019. Available at: https://www.plasticseurope.org/en/resources/publications/1804-plastics-facts-2019.
Prata, J. C. (2018). Airborne MPs: Consequences to Human Health? Environ. Pollut. 234, 115–126. doi:10.1016/j.envpol.2017.11.043
Prata, J. C., da Costa, J. P., Duarte, A. C., and Rocha-Santos, T. (2022). Suspected Microplastics in Atlantic Horse Mackerel Fish (Trachurus trachurus) Captured in Portugal. Mar. Pollut. Bull. 174, 113249. doi:10.1016/j.marpolbul.2021.113249
Prata, J. C., da Costa, J. P., Lopes, I., Duarte, A. C., and Rocha-Santos, T. (2020). Environmental Exposure to MPs: An Overview on Possible Human Health Effects. Sci. Total Environ. 702, 134455. doi:10.1016/j.scitotenv.2019.134455
Prietl, B., Meindl, C., Roblegg, E., Pieber, T. R., Lanzer, G., and Fröhlich, E. (2014). Nano-sized and Micro-sized Polystyrene Particles Affect Phagocyte Function. Cel Biol. Toxicol. 30 (1), 1–16. doi:10.1007/s10565-013-9265-y
Qiang, L., and Cheng, J. (2019). Exposure to MPs Decreases Swimming Competence in Larval Zebrafish (Danio rerio). Ecotoxicology Environ. Saf. 176, 226–233. doi:10.1016/j.ecoenv.2019.03.088
Qiang, L., and Cheng, J. (2021). Exposure to Polystyrene MPs Impairs Gonads of Zebrafish (Danio rerio). Chemosphere 263, 128161. doi:10.1016/j.chemosphere.2020.128161
Qiang, L., Lo, L. S. H., Gao, Y., and Cheng, J. (2020). Parental Exposure to Polystyrene MPs at Environmentally Relevant Concentrations Has Negligible Transgenerational Effects on Zebrafish (Danio rerio). Ecotoxicology Environ. Saf. 206, 111382. doi:10.1016/j.ecoenv.2020.111382
Qiao, R., Lu, K., Deng, Y., Ren, H., and Zhang, Y. (2019b). Combined Effects of Polystyrene MPs and Natural Organic Matter on the Accumulation and Toxicity of Copper in Zebrafish. Sci. Total Environ. 682, 128–137. doi:10.1016/j.scitotenv.2019.05.163
Qiao, R., Sheng, C., Lu, Y., Zhang, Y., Ren, H., and Lemos, B. (2019a). MPs Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 662, 246–253. doi:10.1016/j.scitotenv.2019.01.245
Qiu, X., Saovany, S., Takai, Y., Akasaka, A., Inoue, Y., Yakata, N., et al. (2020). Quantifying the Vector Effects of Polyethylene MPs on the Accumulation of Anthracene to Japanese Medaka (Oryzias latipes). Aquat. Toxicol. 228, 105643. doi:10.1016/j.aquatox.2020.105643
Rainieri, S., Conlledo, N., Larsen, B. K., Granby, K., and Barranco, A. (2018). Combined Effects of MPs and Chemical Contaminants on the Organ Toxicity of Zebrafish (Danio rerio). Environ. Res. 162, 135–143. doi:10.1016/j.envres.2017.12.019
Rangasamy, B., Malafaia, G., and Maheswaran, R. (2021). Evaluation of Antioxidant Response and Na+-K+-ATPase Activity in Zebrafish Exposed to Polyethylene MPs: Shedding Light on a Physiological Adaptation. J. Hazard. Mater., 127789.
Rios-Fuster, B., Arechavala-Lopez, P., García-Marcos, K., Alomar, C., Compa, M., Álvarez, E., et al. (2021). Experimental Evidence of Physiological and Behavioral Effects of Microplastic Ingestion in Sparus aurata. Aquat. Toxicol. 231, 105737. doi:10.1016/j.aquatox.2020.105737
Rochman, C. M., Hoh, E., Kurobe, T., and Teh, S. J. (2013). Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Scientific Rep. 3 (1), 1–7. doi:10.1038/srep03263
Rochman, C. M., Kurobe, T., Flores, I., and Teh, S. J. (2014). Early Warning Signs of Endocrine Disruption in Adult Fish from the Ingestion of Polyethylene with and without Sorbed Chemical Pollutants from the marine Environment. Sci. total Environ. 493, 656–661. doi:10.1016/j.scitotenv.2014.06.051
Roda, J. F. B., Lauer, M. M., Risso, W. E., and dos Reis Martinez, C. B. (2020). MPs and Copper Effects on the Neotropical Teleost Prochilodus lineatus: Is There Any Interaction? Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 242, 110659. doi:10.1016/j.cbpa.2020.110659
Rodrigues, M. O., Abrantes, N., Gonçalves, F. J. M., Nogueira, H., Marques, J. C., and Gonçalves, A. M. M. (2019). Impacts of Plastic Products Used in Daily Life on the Environment and Human Health: What Is Known? Environ. Toxicol. Pharmacol. 72, 103239. doi:10.1016/j.etap.2019.103239
Romeo, T., Pietro, B., Pedà, C., Consoli, P., Andaloro, F., and Fossi, M. C. (2015). First Evidence of Presence of Plastic Debris in Stomach of Large Pelagic Fish in the Mediterranean Sea. Mar. Pollut. Bull. 95 (1), 358–361. doi:10.1016/j.marpolbul.2015.04.048
Ruenraroengsak, P., and Tetley, T. D. (2015). Differential Bioreactivity of Neutral, Cationic and Anionic Polystyrene Nanoparticles with Cells from the Human Alveolar Compartment: Robust Response of Alveolar Type 1 Epithelial Cells. Part. fibre Toxicol. 12 (1), 1–20. doi:10.1186/s12989-015-0091-7
Santana, L. M., Rodrigues, A. C., Campos, D., Kaczerewska, O., Figueiredo, J., Silva, S., et al. (2022). Can the Toxicity of Polyethylene MPs and Engineered Nanoclays on Flatfish (Solea Senegalensis) Be Influenced by the Presence of Each Other? Sci. Total Environ. 804, 150188. doi:10.1016/j.scitotenv.2021.150188
Santana, M. F., Dawson, A. L., Motti, C. A., van Herwerden, L., Lefevre, C., and Kroon, F. J. (2021). Ingestion and Depuration of MPs by a Planktivorous Coral Reef Fish, Pomacentrus amboinensis. Front. Environ. Sci. 9, 79. doi:10.3389/fenvs.2021.641135
Santos, D., Félix, L., Luzio, A., Parra, S., Bellas, J., and Monteiro, S. M. (2021b). Single and Combined Acute and Subchronic Toxic Effects of MPs and Copper in Zebrafish (Danio rerio) Early Life Stages. Chemosphere 277, 130262. doi:10.1016/j.chemosphere.2021.130262
Santos, D., Félix, L., Luzio, A., Parra, S., Cabecinha, E., Bellas, J., et al. (2020). Toxicological Effects Induced on Early Life Stages of Zebrafish (Danio rerio) after an Acute Exposure to MPs Alone or Co-exposed with Copper. Chemosphere 261, 127748. doi:10.1016/j.chemosphere.2020.127748
Santos, D., Luzio, A., Matos, C., Bellas, J., Monteiro, S. M., and Félix, L. (2021a). MPs Alone or Co-exposed with Copper Induce Neurotoxicity and Behavioral Alterations on Zebrafish Larvae after a Subchronic Exposure. Aquat. Toxicol. 235, 105814. doi:10.1016/j.aquatox.2021.105814
Schirinzi, G. F., Pérez-Pomeda, I., Sanchís, J., Rossini, C., Farré, M., and Barceló, D. (2017). Cytotoxic Effects of Commonly Used Nanomaterials and MPs on Cerebral and Epithelial Human Cells. Environ. Res. 159, 579–587. doi:10.1016/j.envres.2017.08.043
Schmieg, H., Burmester, J. K., Krais, S., Ruhl, A. S., Tisler, S., Zwiener, C., et al. (2020). Interacting Effects of Polystyrene MPs and the Antidepressant Amitriptyline on Early Life Stages of Brown trout (Salmo trutta F. Fario). Water 12 (9), 2361. doi:10.3390/w12092361
Sequeira, I. F., Prata, J. C., da Costa, J. P., Duarte, A. C., and Rocha-Santos, T. (2020). Worldwide Contamination of Fish with Microplastics: A Brief Global Overview. Mar. Pollut. Bull. 160, 111681. doi:10.1016/j.marpolbul.2020.111681
Sheng, C., Zhang, S., and Zhang, Y. (2021). The Influence of Different Polymer Types of MPs on Adsorption, Accumulation, and Toxicity of Triclosan in Zebrafish. J. Hazard. Mater. 402, 123733. doi:10.1016/j.jhazmat.2020.123733
Shi, W., Sun, S., Han, Y., Tang, Y., Zhou, W., Du, X., et al. (2021). MPs Impair Olfactory-Mediated Behaviors of Goldfish Carassius auratus. J. Hazard. Mater. 409, 125016. doi:10.1016/j.jhazmat.2020.125016
Smith, M., Love, D. C., Rochman, C. M., and Neff, R. A. (2018). MPs in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 5 (3), 375–386. doi:10.1007/s40572-018-0206-z
Solomando, A., Capó, X., Alomar, C., Álvarez, E., Compa, M., Valencia, J. M., et al. (2020). Long-term Exposure to MPs Induces Oxidative Stress and a Pro-inflammatory Response in the Gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 266, 115295. doi:10.1016/j.envpol.2020.115295
Solomando, A., Capó, X., Alomar, C., Compa, M., Valencia, J. M., Sureda, A., et al. (2021). Assessment of the Effect of Long-Term Exposure to MPs and Depuration Period in Sparus aurata Linnaeus, 1758: Liver and Blood Biomarkers. Sci. Total Environ. 786, 147479. doi:10.1016/j.scitotenv.2021.147479
Sternschuss, G., Ostergard, D. R., and Patel, H. (2012). Post-implantation Alterations of Polypropylene in the Human. J. Urol. 188 (1), 27–32. doi:10.1016/j.juro.2012.02.2559
Stienbarger, C. D., Joseph, J., Athey, S. N., Monteleone, B., Andrady, A. L., Watanabe, W. O., et al. (2021). Direct Ingestion, Trophic Transfer, and Physiological Effects of MPs in the Early Life Stages of Centropristis striata, a Commercially and Recreationally Valuable Fishery Species. Environ. Pollut. 285, 117653. doi:10.1016/j.envpol.2021.117653
Stock, V., Böhmert, L., Lisicki, E., Block, R., Cara-Carmona, J., Pack, L. K., et al. (2019). Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles In Vitro and In Vivo. Arch. Toxicol. 93 (7), 1817–1833. doi:10.1007/s00204-019-02478-7
Sutherland, W. J., Broad, S., Caine, J., Clout, M., Dicks, L. V., Doran, H., et al. (2016). A Horizon Scan of Global Conservation Issues for 2016. Trends Ecology Evolution 31 (1), 44–53. doi:10.1016/j.tree.2015.11.007
Thiele, C. J., Hudson, M. D., Russell, A. E., Saluveer, M., and Sidaoui-Haddad, G. (2021). Microplastics in Fish and Fishmeal: an Emerging Environmental challenge? Scientific Rep. 11 (1), 1–12. doi:10.1038/s41598-021-81499-8
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W., et al. (2004). Lost at Sea: where Is All the Plastic? Science 304 (5672), 838. doi:10.1126/science.1094559
Thubagere, A., and Reinhard, B. M. (2010). Nanoparticle-induced Apoptosis Propagates through Hydrogen-Peroxide-Mediated Bystander Killing: Insights from a Human Intestinal Epithelium In Vitro Model. ACS nano 4 (7), 3611–3622. doi:10.1021/nn100389a
Tongo, I., and Erhunmwunse, N. O. (2022). Effects of Ingestion of Polyethylene MPs on Survival Rate, Opercular Respiration Rate and Swimming Performance of African Catfish (Clarias gariepinus). J. Hazard. Mater. 423, 127237. doi:10.1016/j.jhazmat.2021.127237
Tosetto, L., Williamson, J. E., and Brown, C. (2017). Trophic Transfer of MPs Does Not Affect Fish Personality. Anim. Behav. 123, 159–167. doi:10.1016/j.anbehav.2016.10.035
Umamaheswari, S., Priyadarshinee, S., Bhattacharjee, M., Kadirvelu, K., and Ramesh, M. (2021). Exposure to Polystyrene MPs Induced Gene Modulated Biological Responses in Zebrafish (Danio rerio). Chemosphere 281, 128592. doi:10.1016/j.chemosphere.2020.128592
Usman, S., Abdull Razis, A. F., Shaari, K., Amal, M. N. A., Saad, M. Z., Mat Isa, N., et al. (2021). Polystyrene MPs Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854). Int. J. Environ. Res. Public Health 18 (18), 9449. doi:10.3390/ijerph18189449
Uy, C., and Johnson, D. (2021). Effects of MPs on the Feeding Rates of Larvae of a Coastal Fish: Direct Consumption, Trophic Transfer, and Effects on Growth and Survival. Mar. Biol. 169, 27. doi:10.1007/s00227-021-04010-x
Valavanidis, A., Vlachogianni, T., Fiotakis, K., and Loridas, S. (2013). Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health 10 (9), 3886–3907. doi:10.3390/ijerph10093886
Varó, I., Osorio, K., Estensoro, I., Naya-Catala, F., Sitja-Bobadilla, A., Navarro, J. C., et al. (2021). Effect of virgin Low Density Polyethylene Microplastic Ingestion on Intestinal Histopathology and Microbiota of Gilthead Sea Bream. Aquaculture 545, 737245.
Veneman, W. J., Spaink, H. P., Brun, N. R., Bosker, T., and Vijver, M. G. (2017). Pathway Analysis of Systemic Transcriptome Responses to Injected Polystyrene Particles in Zebrafish Larvae. Aquat. Toxicol. 190, 112–120. doi:10.1016/j.aquatox.2017.06.014
Vom Saal, F. S., Nagel, S. C., Coe, B. L., Angle, B. M., and Taylor, J. A. (2012). The Estrogenic Endocrine Disrupting Chemical Bisphenol A (BPA) and Obesity. Mol. Cell. Endocrinol. 354 (1-2), 74–84. doi:10.1016/j.mce.2012.01.001
Wan, Z., Wang, C., Zhou, J., Shen, M., Wang, X., Fu, Z., et al. (2019). Effects of Polystyrene MPs on the Composition of the Microbiome and Metabolism in Larval Zebrafish. Chemosphere 217, 646–658. doi:10.1016/j.chemosphere.2018.11.070
Wang, J., Li, X., Gao, M., Li, X., Zhao, L., and Ru, S. (2022). Polystyrene MPs Increase Estrogenic Effects of 17α-Ethynylestradiol on Male marine Medaka (Oryzias Melastigma). Chemosphere 287, 132312. doi:10.1016/j.chemosphere.2021.132312
Wang, J., Li, Y., Lu, L., Zheng, M., Zhang, X., Tian, H., et al. (2019). Polystyrene MPs Cause Tissue Damages, Sex-specific Reproductive Disruption and Transgenerational Effects in marine Medaka (Oryzias Melastigma). Environ. Pollut. 254, 113024. doi:10.1016/j.envpol.2019.113024
Wang, J., Tan, Z., Peng, J., Qiu, Q., and Li, M. (2016). The Behaviors of MPs in the marine Environment. Mar. Environ. Res. 113, 7–17. doi:10.1016/j.marenvres.2015.10.014
Wang, J., Zheng, M., Lu, L., Li, X., Zhang, Z., and Ru, S. (2021a). Adaptation of Life-History Traits and Trade-Offs in marine Medaka (Oryzias Melastigma) after Whole Life-Cycle Exposure to Polystyrene MPs. J. Hazard. Mater. 414, 125537. doi:10.1016/j.jhazmat.2021.125537
Wang, S., Xie, S., Wang, Z., Zhang, C., Pan, Z., Sun, D., et al. (2021b). Single and Combined Effects of MPs and Cadmium on the Cadmium Accumulation and Biochemical and Immunity of Channa argus. Biol. Trace Elem. Res., 1–11.
Wang, W., Ge, J., and Yu, X. (2020). Bioavailability and Toxicity of Microplastics to Fish Species: A Review. Ecotoxicology Environ. Saf. 189, 109913. doi:10.1016/j.ecoenv.2019.109913
Wen, B., Jin, S. R., Chen, Z. Z., Gao, J. Z., Liu, Y. N., Liu, J. H., et al. (2018). Single and Combined Effects of MPs and Cadmium on the Cadmium Accumulation, Antioxidant Defence and Innate Immunity of the Discus Fish (Symphysodon aequifasciatus). Environ. Pollut. 243, 462–471. doi:10.1016/j.envpol.2018.09.029
Wesch, C., Bredimus, K., Paulus, M., and Klein, R. (2016). Towards the Suitable Monitoring of Ingestion of MPs by marine Biota: A Review. Environ. Pollut. 218, 1200–1208. doi:10.1016/j.envpol.2016.08.076
West-Eberhard, M. J. (2019). Nutrition, the Visceral Immune System, and the Evolutionary Origins of Pathogenic Obesity. Proc. Natl. Acad. Sci. 116 (3), 723–731. doi:10.1073/pnas.1809046116
Wick, P., Malek, A., Manser, P., Meili, D., Maeder-Althaus, X., Diener, L., et al. (2010). Barrier Capacity of Human Placenta for Nanosized Materials. Environ. Health Perspect. 118 (3), 432–436. doi:10.1289/ehp.0901200
World Population Review (2021). Plastic Pollution by Country 2021. Availableat: https://worldpopulationreview.com/country-rankings/plastic-pollution-by-country.
Wright, S. L., and Kelly, F. J. (2017). Plastic and Human Health: a Micro Issue? Environ. Sci. Technol. 51 (12), 6634–6647. doi:10.1021/acs.est.7b00423
Wright, S. L., Thompson, R. C., and Galloway, T. S. (2013). The Physical Impacts of MPs on marine Organisms: a Review. Environ. Pollut. 178, 483–492. doi:10.1016/j.envpol.2013.02.031
Wu, B., Wu, X., Liu, S., Wang, Z., and Chen, L. (2019). Size-dependent Effects of Polystyrene MPs on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 Cells. Chemosphere 221, 333–341. doi:10.1016/j.chemosphere.2019.01.056
Wu, S., Wu, M., Tian, D., Qiu, L., and Li, T. (2020). Effects of Polystyrene Microbeads on Cytotoxicity and Transcriptomic Profiles in Human Caco‐2 Cells. Environ. Toxicol. 35 (4), 495–506. doi:10.1002/tox.22885
Xia, B., Sui, Q., Du, Y., Wang, L., Jing, J., Zhu, L., et al. (2022). Secondary PVC MPs Are More Toxic Than Primary PVC MPs to Oryzias Melastigma Embryos. J. Hazard. Mater. 424, 127421. doi:10.1016/j.jhazmat.2021.127421
Xia, L., Gu, W., Zhang, M., Chang, Y. N., Chen, K., Bai, X., et al. (2016). Endocytosed Nanoparticles Hold Endosomes and Stimulate Binucleated Cells Formation. Part. fibre Toxicol. 13 (1), 1–12. doi:10.1186/s12989-016-0173-1
Xia, T., Kovochich, M., Liong, M., Zink, J. I., and Nel, A. E. (2008). Cationic Polystyrene Nanosphere Toxicity Depends on Cell-specific Endocytic and Mitochondrial Injury Pathways. ACS nano 2 (1), 85–96. doi:10.1021/nn700256c
Xia, X., Sun, M., Zhou, M., Chang, Z., and Li, L. (2020). Polyvinyl Chloride MPs Induce Growth Inhibition and Oxidative Stress in Cyprinus carpio Var. Larvae. Sci. Total Environ. 716, 136479. doi:10.1016/j.scitotenv.2019.136479
Xie, M., Lin, L., Xu, P., Zhou, W., Zhao, C., Ding, D., et al. (2021). Effects of Microplastic Fibers on Lates calcarifer Juveniles: Accumulation, Oxidative Stress, Intestine Microbiome Dysbiosis and Histological Damage. Ecol. Indicators 133, 108370. doi:10.1016/j.ecolind.2021.108370
Xu, K., Zhang, Y., Huang, Y., and Wang, J. (2021). Toxicological Effects of MPs and Phenanthrene to Zebrafish (Danio rerio). Sci. Total Environ. 757, 143730. doi:10.1016/j.scitotenv.2020.143730
Xue, Y. H., Sun, Z. X., Xu, Z. Y., Feng, L. S., Zhao, F. Y., Wen, X. L., et al. (2021). Effects of Polyethylene MPs Exposure on Intestinal Flora of Zebrafish. Polish J. Environ. Stud. 30, 1–14. doi:10.1007/s10646-021-02469-4
Yacobi, N. R., DeMaio, L., Xie, J., Hamm-Alvarez, S. F., Borok, Z., Kim, K. J., et al. (2008). Polystyrene Nanoparticle Trafficking across Alveolar Epithelium. Nanomedicine: Nanotechnology, Biol. Med. 4 (2), 139–145. doi:10.1016/j.nano.2008.02.002
Yamamoto, Y., Kobayashi, Y., and Matsumoto, H. (2001). Lipid Peroxidation Is an Early Symptom Triggered by Aluminum, but Not the Primary Cause of Elongation Inhibition in Pea Roots. Plant Physiol. 125 (1), 199–208. doi:10.1104/pp.125.1.199
Yan, W., Hamid, N., Deng, S., Jia, P. P., and Pei, D. S. (2020). Individual and Combined Toxicogenetic Effects of MPs and Heavy Metals (Cd, Pb, and Zn) Perturb Gut Microbiota Homeostasis and Gonadal Development in marine Medaka (Oryzias Melastigma). J. Hazard. Mater. 397, 122795. doi:10.1016/j.jhazmat.2020.122795
Yan, Z., Liu, Y., Zhang, T., Zhang, F., Ren, H., and Zhang, Y. (2021). Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. doi:10.1021/acs.est.1c03924
Yang, H., Xiong, H., Mi, K., Xue, W., Wei, W., and Zhang, Y. (2020). Toxicity Comparison of Nano-Sized and Micron-Sized MPs to Goldfish Carassius auratus Larvae. J. Hazard. Mater. 388, 122058. doi:10.1016/j.jhazmat.2020.122058
Yin, L., Chen, B., Xia, B., Shi, X., and Qu, K. (2018). Polystyrene MPs Alter the Behavior, Energy reserve and Nutritional Composition of marine Jacopever (Sebastes Schlegelii). J. Hazard. Mater. 360, 97–105. doi:10.1016/j.jhazmat.2018.07.110
Yin, L., Liu, H., Cui, H., Chen, B., Li, L., and Wu, F. (2019). Impacts of Polystyrene MPs on the Behavior and Metabolism in a marine Demersal Teleost, Black Rockfish (Sebastes Schlegelii). J. Hazard. Mater. 380, 120861. doi:10.1016/j.jhazmat.2019.120861
Yu, X., Ladewig, S., Bao, S., Toline, C. A., Whitmire, S., and Chow, A. T. (2018). Occurrence and Distribution of MPs at Selected Coastal Sites along the southeastern United States. Sci. Total Environ. 613, 298–305. doi:10.1016/j.scitotenv.2017.09.100
Zhang, C., Wang, J., Pan, Z., Wang, S., Zhang, L., Wang, Q., et al. (2021c). A Dosage-Effect Assessment of Acute Toxicology Tests of Microplastic Exposure in Filter-Feeding Fish. Fish Shellfish Immunol. 113, 154–161. doi:10.1016/j.fsi.2021.04.010
Zhang, C., Wang, J., Zhou, A., Ye, Q., Feng, Y., Wang, Z., and Zou, J. (2021a). Species-specific Effect of Microplastics on Fish Embryos and Observation of Toxicity Kinetics in Larvae. J. Hazard. Mater. 403, 123948. doi:10.1016/j.jhazmat.2020.123948
Zhang, J., Meng, H., Kong, X., Cheng, X., Ma, T., He, H., and Zhang, L. (2021d). Combined Effects of Polyethylene and Organic Contaminant on Zebrafish (Danio rerio): Accumulation of 9-Nitroanthracene, Biomarkers and Intestinal Microbiota. Environ. Pollut. 277, 116767. doi:10.1016/j.envpol.2021.116767
Zhang, R., Wang, M., Chen, X., Yang, C., and Wu, L. (2020). Combined Toxicity of MPs and Cadmium on the Zebrafish Embryos (Danio rerio). Sci. Total Environ. 743, 140638. doi:10.1016/j.scitotenv.2020.140638
Zhang, S., Ding, J., Razanajatovo, R. M., Jiang, H., Zou, H., and Zhu, W. (2019). Interactive Effects of Polystyrene MPs and Roxithromycin on Bioaccumulation and Biochemical Status in the Freshwater Fish Red tilapia (Oreochromis niloticus). Sci. total Environ. 648, 1431–1439. doi:10.1016/j.scitotenv.2018.08.266
Zhang, X., Wen, K., Ding, D., Liu, J., Lei, Z., Chen, X., et al. (2021b). Size-dependent Adverse Effects of MPs on Intestinal Microbiota and Metabolic Homeostasis in the marine Medaka (Oryzias Melastigma). Environ. Int. 151, 106452. doi:10.1016/j.envint.2021.106452
Zhang, X., Xia, M., Zhao, J., Cao, Z., Zou, W., and Zhou, Q. (2022). Photoaging Enhanced the Adverse Effects of Polyamide MPs on the Growth, Intestinal Health, and Lipid Absorption in Developing Zebrafish. Environ. Int. 158, 106922. doi:10.1016/j.envint.2021.106922
Zhao, Y., Bao, Z., Wan, Z., Fu, Z., and Jin, Y. (2020). Polystyrene Microplastic Exposure Disturbs Hepatic Glycolipid Metabolism at the Physiological, Biochemical, and Transcriptomic Levels in Adult Zebrafish. Sci. Total Environ. 710, 136279. doi:10.1016/j.scitotenv.2019.136279
Zhao, Y., Qin, Z., Huang, Z., Bao, Z., Luo, T., and Jin, Y. (2021). Effects of Polyethylene MPs on the Microbiome and Metabolism in Larval Zebrafish. Environ. Pollut. 282, 117039. doi:10.1016/j.envpol.2021.117039
Zheng, J. L., Chen, X., Peng, L. B., Wang, D., Zhu, Q. L., Li, J., et al. (2021). Particles rather Than Released Zn2+ from ZnO Nanoparticles Aggravate MPs Toxicity in Early Stages of Exposed Zebrafish and Their Unexposed Offspring. J. Hazard. Mater., 127589. doi:10.1016/j.jhazmat.2021.127589
Zhou, Q., Zhang, H., Fu, C., Zhou, Y., Dai, Z., Li, Y., et al. (2018). The Distribution and Morphology of MPs in Coastal Soils Adjacent to the Bohai Sea and the Yellow Sea. Geoderma 322, 201–208. doi:10.1016/j.geoderma.2018.02.015
Zhu, D., Chen, Q. L., An, X. L., Yang, X. R., Christie, P., Ke, X., et al. (2018). Exposure of Soil Collembolans to MPs Perturbs Their Gut Microbiota and Alters Their Isotopic Composition. Soil Biol. Biochem. 116, 302–310. doi:10.1016/j.soilbio.2017.10.027
Zhu, M., Chernick, M., Rittschof, D., and Hinton, D. E. (2020). Chronic Dietary Exposure to Polystyrene MPs in Maturing Japanese Medaka (Oryzias latipes). Aquat. Toxicol. 220, 105396. doi:10.1016/j.aquatox.2019.105396
Zitouni, N., Bousserrhine, N., Missawi, O., Boughattas, I., Chèvre, N., Santos, R., et al. (2021). Uptake, Tissue Distribution and Toxicological Effects of Environmental MPs in Early Juvenile Fish Dicentrarchus labrax. J. Hazard. Mater. 403, 124055. doi:10.1016/j.jhazmat.2020.124055
Zou, W., Xia, M., Jiang, K., Cao, Z., Zhang, X., and Hu, X. (2020). Photo-oxidative Degradation Mitigated the Developmental Toxicity of Polyamide MPs to Zebrafish Larvae by Modulating Macrophage-Triggered Proinflammatory Responses and Apoptosis. Environ. Sci. Technology 54 (21), 13888–13898. doi:10.1021/acs.est.0c05399
Keywords: microplastics, toxic effects, fish, contamination, human health risks
Citation: Bhuyan MS (2022) Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 10:827289. doi: 10.3389/fenvs.2022.827289
Received: 01 December 2021; Accepted: 28 February 2022;
Published: 16 March 2022.
Edited by:
Veerasingam S., Qatar University, QatarReviewed by:
Tadele Assefa Aragaw, Bahir Dar University, EthiopiaJamila Patterson, Suganthi Devadason Marine Research Institute (SDMRI), India
Beth Polidoro, Arizona State University, United States
Copyright © 2022 Bhuyan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Simul Bhuyan, c2ltdWxiaHV5YW5AZ21haWwuY29t
 Md. Simul Bhuyan
Md. Simul Bhuyan