- 1Programa de Pós-Graduação em Etnobiologia e Conservação da Natureza, Universidade Federal Rural de Pernambuco, Departamento de Biologia, Recife, Brazil
- 2Programa de Pós-Graduação em Ecologia, Universidade Federal de Santa Catarina, Departamento de Ecologia e Zoologia, Florianópolis, Brazil
- 3Laboratório de Síntese Ecológica e Conservação da Biodiversidade, Departamento de Biologia, Universidade Federal Rural de Pernambuco, Recife, Brazil
- 4Núcleo de Pesquisa em Arqueologia e História Natural, Universidade Católica de Pernambuco, Recife, Brazil
- 5Instituto Nacional de Pesquisas da Amazônia, Manaus, Brazil
- 6Universidade Federal de Juiz de Fora, Departamento de Botânica, Juiz de Fora, Brazil
The tropical South American savannas have been occupied and manipulated by humans since the late Pleistocene. Ecologists consider that soils, hydrology, and seasonal precipitation influence the structure and composition of plants and the fire-proneness of savannas. However, the human influence on these dynamics remains uncertain. This is because little is known about human activities and what influence they have on the diversity of ecosystems. Considering this, our study sought to synthesize the management practices used by small-scale societies of the South American savannas, compile the species that are the focus of direct management, and demonstrate the role of this management in maintaining the diverse ecosystems that make up the savannas. We also set out to test the hypotheses that forms of management differ depending on the ecosystem and cultural matrices. To do so, we conducted a systematic review, in which we collected 51 articles with information about the management carried out by small-scale societies. From this, we categorized 10 management practices directed to ecosystems: protection of the ecosystem, enrichment of species, topographic changes, increased soil fertility, cleaning, prevention of fire, resource promotion, driving of game, swidden-fallow, and maintenance of ecosystem structure. We identified 19 native plant species whose populations are managed in-situ. These management practices have proven capable of keeping savanna and grassland ecosystems open and increasing the occurrence of forest ecosystems in the mosaic, as well as favoring plants of human interest in general. We note that there is a relationship between management practices with ecosystems and cultures, which suggests that both factors influence the management of landscapes. We conclude that management practices of small-scale societies are responsible for domesticating South American tropical savannas and that these savannas are composed of a mosaic of culturally constructed niches. The small-scale societies that inhabit these environments have important traditional ecological knowledge and strategies that enable the use, conservation, and restoration of savannas, extremely threatened by agribusiness today.
Systematic Review Registration: [website], identifier [registration number].
Introduction
Humans across the planet evolved in different ecosystems and their activities influenced these habitats, as well as the evolution of populations in numerous taxonomic groups (Cooke, 1998; Boivin et al., 2016; Roberts, 2019). Within the diversity of environments in the Neotropical region, savannas were a key part of early occupations (Lombardo et al., 2020), either because of their diversity of ecosystems and resources, or because of the evolutionary preferences inherited from Pleistocene hominids (Ellenberg and Mueller-Dombois, 1967; Eiten, 1972; Orians, 1980; Harris and Hillman, 2014; Roberts, 2019). The prevailing ecological thinking considers that the ecosystems that make up tropical savannas are mainly influenced by soils, hydrology, and seasonal precipitation, as these factors determine the structure and composition of plants and their propensity to fire (Staver et al., 2011; Hoffmann et al., 2012; Silva et al., 2013). Some authors draw attention to the fact that humans can influence these dynamics, as they can modify floristic composition and edaphic conditions (Hirota et al., 2011; Pinho et al., 2011; Lombardo et al., 2020) and they are mostly responsible for the appearance of fire (Ramos-Neto and Pivello, 2000). However, the extent of human influence on savannas remains unclear, as it is not known which practices are used, in what combinations, how they influence different ecosystems, and which plant species are most affected in this process.
Human influences on environments occur from the moment humans settle in new territories (Lombardo et al., 2020), and tropical South American savannas have a diverse occupation history (Denevan, 1966; Morey, 1976; Bueno and Isnardis, 2018). Early records are sparse, but make clear that the savannas were inhabited in the late Pleistocene and that, in this period, human activities were characterized by a dynamic of high mobility and initial recognition of environments (Bocanegra and Mora, 2012; Bueno and Dias, 2015; Vialou et al., 2017). In the Holocene, there is a greater number of archaeological records, which suggests sedentarization associated with population growth and diversification of strategies to adapt to the temperature oscillations and rainfall variations common in the period (Erickson, 1995; Gassón, 2002; Mayle et al., 2004; Rostain, 2008; Bueno and Dias, 2015; Bueno and Isnardis, 2018; Lombardo et al., 2020; Stier et al., 2020).
At present, the tropical savannas of South America are the focus of urban expansion and intensive agriculture and ranching; these activities take advantage of open ecosystems and eliminate native vegetation, endangering biodiversity in general (Klink and Machado, 2005; Hernández-Hernández et al., 2011; Eufemia et al., 2019). On the other hand, parts of these regions continue to be occupied by small-scale societies, who base their lifestyles on an intimate relationship with nature (Ploeg, 2009). Smith (2012) points out that these people, in the past or the present, share the following behavioral patterns: have well-defined territories; maintain and update knowledge about local ecosystems, passing it on to future generations; create strategies to control wild resources; have the inherent capacity to modify ecosystems; and, through these modifications, increase the abundance and accessibility of resources of interest.
These modifications made by small-scale societies generally do not result in a decrease in plant diversity (Balée, 2006). However, these modifications reduce the impact of existing natural selection pressures, making it easier for humans to adapt (Albuquerque et al., 2015). These modifications also influence other species that interact with resources of human interest, directly or indirectly (Laland et al., 2016; Albuquerque et al., 2019). This process is the basis of Niche Construction Theory (NCT), which affirms that living beings can change environmental conditions at different scales, consequently becoming more adapted to the transformed environment (Laland and O’Brien, 2010; Odling-Smee et al., 2013; Albuquerque et al., 2019). When humans are the agents of landscape transformation, it is necessary to recognize that cultural aspects also influence their activities (Albuquerque et al., 2015; Coca et al., 2021). NCT helps explain and substantiate the process of plant domestication (Smith, 2012), which consists of humans selecting and managing phenotypes in wild populations, resulting in genetic, morphological, and demographic changes in the resulting populations (Clement, 1999).
This relationship between humans and plants is mutualistic and occurs through selection combined with management; it can occur in different locations and not only in food production systems, such as cultivated areas (Clement et al., 2021). By domesticating plant populations in their natural environments, even if at incipient levels, humans also domesticate landscapes (Casas et al., 1997; Clement, 1999; Allaby et al., 2021; Clement et al., 2021). Domesticated landscapes are created by the conscious and unconscious processes of manipulating ecosystems and the plants that compose them, resulting in more productive environments suitable for humans (Terrell et al., 2003; Casas et al., 2017; Hecht, 2017; Clement et al., 2021). This feedback between practice and result is one of the differentials of NCT (Matthews et al., 2014; Huebert and Allen, 2020; Davis and Douglass, 2021).
According to recent research, domesticated landscapes are formed and maintained as a result of a set of management practices that alter vegetation structure, floristic composition, and ecological processes (Smith, 2011; Levis et al., 2018). For tropical humid forests, theoretical models explain the mechanisms of management of species of human interest by indigenous and traditional communities that result in richer and more diverse forests (Clement, 1999; Levis et al., 2018). Similarly, Smith (2011), who focused his investigation on the rich diversity of environments in North America, presented another conceptual model on the set of strategies focused on making environments richer in food. In the case of savannas, there are reports of small-scale societies in northern Australia that have a set of organized and directed burning practices, aimed at maintaining grasslands and savannas (Russell-Smith et al., 1997; Yibarbuk et al., 2001; Fletcher et al., 2020). In the savannas of northern Tanzania, there are reports of non-fire management practices, where the Chagga constantly manage their forest yards employing practices such as the toleration of species of interest and the removal of species of no use to humans (Fernandes et al., 1985). In general, these management practices are responsible for lasting legacies in ecosystems (Arroyo-Kalin, 2016), another feature of niche construction (Albuquerque et al., 2015).
In these varied theoretical perspectives, the process of constructing niches and domesticating landscapes is the result of valuing and promoting species and environments (Smith, 2012; Harris and Hillman, 2014; Allaby et al., 2021). Once occupied by humans, ecosystems become dependent on complex interactions with human societies, which can be described by interactive matrices of species and management strategies over time (Terrell et al., 2003; Crumley, 2007; Albuquerque et al., 2019). Valuation procedures, also called management practices, are learned collectively and created through an intimate relationship with the landscape, common in small-scale societies (Abraão et al., 2010; Smith, 2011; Silva et al., 2016; Balée, 2018; Levis et al., 2018). For a long time, it has been debated which factors guide these practices; some authors argue that practices are developed in the social environment and consequently are influenced by different cultures (Smith, 2012; Albuquerque et al., 2015); others adopted deterministic thinking where the environment would be the main factor related to this set of practices and strategies (Meggers, 2001). Today, historical ecology proposes a middle ground by assuming that environments and cultures evolved together and that landscapes are the result of this relationship, and therefore it is impossible to separate their effects (Arroyo-Kalin, 2016; Balée, 2018).
Considering this context, our study uses a systematic review to: 1) synthesize the management practices carried out by small-scale societies in South American savannas; 2) highlight the role of this management in maintaining the diversity of ecosystems that make up the savannas; and 3) compile the focus species for human management. We also propose to test the hypotheses that management strategies vary with the type of vegetation formation (H1) and the cultural matrices that use them (H2). From this effort, we hope to contribute to the understanding of how humans domesticate landscapes and plants, with reference to savannas, which are characterized by specific ecological processes, limiting environmental factors and their own taxonomic composition.
Materials and Methods
Definition of Terms
Savannah formations expanded globally in the late Miocene, about eight million years ago, due to the decrease in atmospheric CO2 and the arid climate at the time, factors that favored the occurrence of fires and, consequently, savannas (Keeley and Rundel, 2005; Beerling and Osborne, 2006). Today, tropical savannas cover one-eighth of the earth’s terrestrial surface, with representation in the Americas, Africa, and Australia (Scholes and Archer, 1997). The present study is focused on South American tropical savannas, which include the following areas (Figure 1): 1) the Cerrado, the largest savanna in Brazilian territory 2) the Orinoco Llanos, with parts in the territories of Colombia and Venezuela; 3) the Magdalena Valley in Colombia; 4) the savannas of the Guianas, located in the Guiana shield; 5) the Beni Savanna, located in Bolivia, also known as Llanos de Mojos. Besides these larger areas, there are small fragments of savanna within the boundaries of Amazonia, called 6) Amazonian Savannas (Huber, 1987; Olson et al., 2001; Adeney et al., 2016; ONF, 2017).
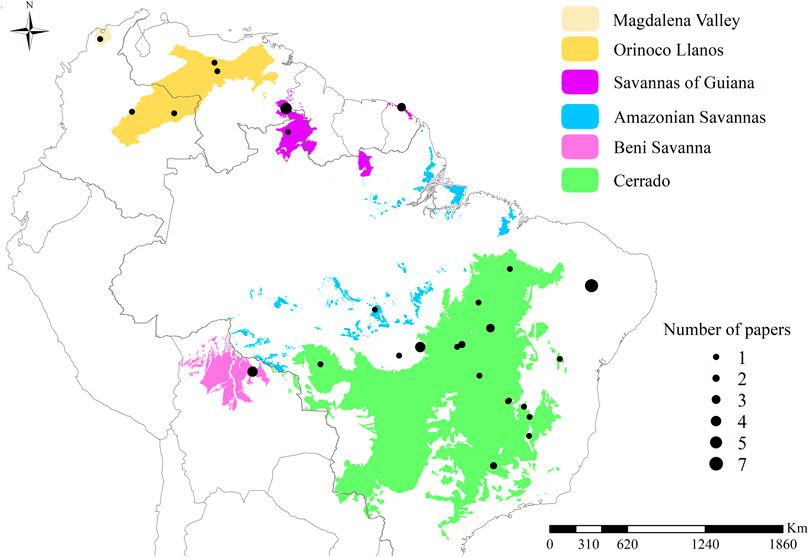
FIGURE 1. Map showing the location of tropical savannas in South America. To compose this map, we combined the following classifications: from Adeney et al. (2016) for Amazonian savannas; from Huber (1987) for the Magdalena valley; from ONF (2017) for the coastal savannas of the Guianas; and from Olson et al. (2001) for the remaining areas. The black dots represent the sampling sites, the size of the dots corresponds to the number of papers included in this review at each site. The point located in northeastern Brazil corresponds to seven studies carried out in a patch of savannah detached from the main Cerrado area; the point overlaps the patch and cannot be seen on the map, differing from the others.
Broadly, savannas can be defined as terrestrial domains or ecosystems whose herbaceous stratum is ecologically predominant and continuous, and woody individuals may or may not be present (Huber, 1987; FAO, 2000). The climate of these regions is classified as tropical savanna, Aw or As in the Köppen system, determined by having a well-marked dry season, in winter or summer (Kottek et al., 2006). The length of the dry season is one of the most important factors in the occurrence and current distribution of savannas (Walter et al., 2008). Soils are generally classified as dystrophic to acidic because they have base saturation of less than 50%, low to medium fertility, and high aluminum contents (Cole, 1986; Reatto et al., 2008). Other determinants of savanna distribution are fire and herbivory; both play strong roles in continental-scale tree cover (Staver et al., 2011).
More detailed definitions exist which propose that savannas are made up of forest, savanna and grassland ecosystems distributed as a mosaic across the landscape (Ellenberg and Mueller-Dombois, 1967; Eiten, 1972; Ribeiro and Walter, 2008). In these different ecosystems there are environmental variations in the general characteristics described above.
Forest ecosystems have a predominant arboreal stratum and a continuous canopy, with heights varying between 3 and 30 m (Oliveira-Filho, 2009). Included in this category are moist tropical forest enclaves that occur within the domain of savannas and forested savannas; both types do not have a dense and continuous herbaceous layer and have a sub-canopy layer that covers a large part of the terrain (FAO, 2000; IBGE, 2012). This type of ecosystem commonly occurs in mesotrophic soils, with moderate nutrient contents, and in places where water is available year-round; these forests are species-rich compared to other savanna ecosystems (Oliveira-Filho and Ratter, 2002). These characteristics mean that these forests do not burn easily and consequently their species are not adapted to fire, being considered fire-sensitive ecosystems (Pivello et al., 2021).
Savanna ecosystems, the predominant formation from which the domain’s name originates, occur in interfluves with well-drained and low-fertility soils (Oliveira-Filho and Ratter, 2002). These environments have two representative strata, herbaceous and arboreal-bushy, the latter reaching heights of up to 8 m (Kauffman et al., 1994). The flora of the savannas presents adaptations to fire and drought, such as the presence of underground organs for water storage, protection of the buds below ground or by a dense arrangement of the leaves, thickening of the bark and sclerophilia, resulting in a set of evergreen or semideciduous species (Oliveira-Filho and Ratter, 2002). These adaptations to fire demonstrate that the flora of these environments co-evolved with this disturbance and benefited from it, being considered fire-dependent (Pivello et al., 2021). There is, however, a subclass in savanna ecosystems considered to be fire-sensitive (Flores et al., 2021; Pivello et al., 2021), the swamp savannas also called veredas, a pioneer formation without canopy where the arboreal palm Mauritia flexuosa L. f. is dominant to the shrub-herbaceous stratum; these occur on hydromorphic and floodable soils (Ribeiro and Walter, 1998).
Grassland ecosystems are dominated by the herbaceous and subshrub strata, being poor in woody vegetation that reaches a maximum of 5 m in height. (Kauffman et al., 1994; Filgueiras, 2002). These ecosystems can occur on very well-drained, dystrophic sites or in areas that experience seasonal flooding (Oliveira-Filho and Ratter, 2002). Grasslands tend to have a high diversity of herbaceous species, and it is rare to find monodominant grasslands (Filgueiras, 2002). Like savannas, grasslands are adapted to and dependent on fire, and benefit from its action (Pivello et al., 2021).
Data Collection
We conducted a systematic literature review, using the practices recommended by PRISMA-EcoEvo (O’Dea et al., 2021) and Collaboration for Environmental Evidence (CEE, 2018). For this we used the online search engines Web of Science, Scielo and Scopus. The survey was conducted in June 2021, using the following search key in the fields 1) title, 2) abstract and 3) keywords: (traditional OR local OR indigen*) NEAR/4 (management OR manejo OR practi* OR pratic* OR colet* OR harvest* OR cosecha) (OR domestic*) AND (savanna OR savana OR sabana OR llanos OR cerrado), which returned 1,555 documents. We initially refined the results so that only papers about South America were displayed, leaving 597 articles. We excluded duplicate articles and were left with 393 unique articles (for details see Figure 1; Supplementary Material).
We first evaluated these documents by reading their titles and abstracts, selecting 1) peer-reviewed articles, 2) written in English, Portuguese or Spanish, 3) that addressed the management carried out by small-scale societies. We excluded from our review all documents developed 1) outside the scope of the study, and 2) outside our spatial frame. We selected 147 documents, which were read in their entirety and excluded those that: 1) did not detail how the management is done; 2) did not mention ways of managing ecosystems or native plants; 3) did not make explicit the ecosystem in which management occurred; 4) did not mention management with species in situ; and 5) did not make clear which human group performed a particular form of management (for list of documents evaluated and specific exclusion criteria see Supplementary Table S1). As a result of this process, 37 articles were included in our review.
To increase coverage, we included in our sample 14 articles previously known to contain relevant information on savanna management, but which did not appear in the search strategy described above. We evaluated these articles individually to understand why they did not appear in our systematic search and observed that five of them presented our key search terms but were published in non-indexed journals, and nine did not present our key search terms in the title, abstract, or keywords, but contained them in the body of the text (Supplementary Table S1). Thus, our final sample size was 51 articles.
The objectives of the papers were not always to investigate the management of ecosystems or plant populations, but all those included in our sample included this information to support or complete the main objective; thus, there were differences in the detailing of the information. Such differences may also be related to the different methodologies used by the researchers.
Data Extraction and Categorization of Information
We extracted from the selected articles: 1) the types of ecosystems managed; 2) the human groups investigated; 3) all forms of management cited; and 4) the species targeted for individual or population management. These data were categorized according to the following criteria:
Ecosystems: We categorized the ecosystems where the work was carried out into: 1) Forests: papers whose authors cited the terms gallery forests, riparian forests, forest islands and patches, secondary forests, inundated forests, cerradão or just forests; 2) Savannas: papers in which the authors used the terms savannas, cerrado-like vegetation, and swamp savannas (veredas); and 3) Grasslands: articles whose authors referred to grasslands, open savanna, treeless open savannas, seasonally flooded savannas or wet grasslands, and natural pastures. For the main structural and ecological differences between these ecosystems see Definition of Terms.
Cultural Matrices: Human communities were assigned to the following groups: 1) Indigenous peoples: human groups that self-identify as indigenous and that inhabited the country or a geographical region belonging to the country at the time of European colonization or the establishment of current state borders. Included in this group are pre-Columbian peoples and their descendants who, regardless of their legal status, retain their own social, economic, cultural, and political institutions, or parts of them (ILO, 1989; ISA, 2005; Brondízio et al., 2021). 2) Local communities: human groups acting collectively in a way that defines a territory and culture over time (Brondízio et al., 2021). In this group were included family farmers and local communities that do not necessarily agree on one concept of political self-determination. 3) Traditional communities: groups that maintain knowledge, identities, and territorialities linked to a historical, collective, and communal territory (Brasil, 1988; Colombia, 1997; Brandão, 2012; Eidt and Udry, 2019; Brondízio et al., 2021). In this group were included “geraizeiros,” “apanhadores de sempre-vivas,” groups formed from the historical mixture between indigenous, black and European people, and “quilombolas” groups that originated from enslaved black people who resisted slavery (Monteiro, 2011; Dayrell, 2012; CPI-SP, 2021). It is important to emphasize that all groups share the behavioral patterns of small-scale societies, such as direct dependence on biological resources from a rural territory (Ploeg, 2009; Smith, 2012), and yet they have unique traditions passed down through generations (Almeida, 2004).
Management citations: Based on the information from the selected articles, we grouped the various citations of ways to manage ecosystems into categories, called management practices. Citations were considered: observations of management practices made by the researcher responsible for the article, and practices reported by small-scale societies to the researchers. Our grouping was based on the following criteria: 1) similarity among the citations, 2) result of the management practice, and 3) use or not of fire as a tool (Supplementary Table S2).
Target species: We list the target species for some types of management in the different ecosystems of the savannas. In this list we include information about the species, such as popular names, scientific name, life form and uses; and on indicators of patterns and processes of plant domestication, based on categories that already exist in the literature (Casas et al., 1997, 2017; Clement, 1999; Levis et al., 2018; Clement et al., 2021). We follow this categorization: 1) Patterns: genetic or phenotypic differences, occurrence of intraspecific diversity or evidence of pre-Columbian use; and 2) Processes: evidence of protection or tolerance, techniques to reduce competition, evidence of propagule dispersal, selection, cultivation, soil preparation, evidence of fire management.
Quantitative Analyses
To test whether the different ecosystems and cultural matrices are associated with management practices we used a chi-square test of independence. For this, we used the number of records in each category organized in a contingency table. The correlation between the predictor variables was also evaluated using the chi-square test for independence. All analyses were performed in the R (R Team Core, 2021) and p values ≤0.05 were considered significant.
Results
Management Practices
We registered 147 citations of management practices distributed in the 10 categories summarized in Supplementary Table S2 and presented in detail below:
Protection of the ecosystem—This involves avoiding actions that damage systems that are more sensitive to fire, especially dry season fires (Mistry et al., 2005; Welch, 2015; Figueira et al., 2016; Eloy et al., 2018a). This protection is done essentially without fire, by establishing firebreaks (Bilbao et al., 2010; Falleiro, 2011; Batista et al., 2018), and in some cases secured by collective agreements (Sletto and Rodriguez, 2013). The fires near these protected sites are closely monitored; if the flames approach they are put out with natural smothering devices, such as branches of native trees (Posey, 1985). Fire-protected sites are also considered areas rich in resources, such as fruits and animals, with high potential for gathering and hunting (Mistry et al., 2005). The practice of protecting the system as a whole can also be designed to protect some species and resources, such as water (Eloy et al., 2018a). In addition to protecting the resources present, certain sites are also protected from burning because they are considered sacred (Sletto and Rodriguez, 2013).
Enrichment of species—This consists of planting seeds and transplanting seedlings of species of human interest. It can extend to the creation of home gardens and forest islands, in which arboreal individuals are intentionally planted to enrich the vegetation of an area (Rostain, 2010; Pinho et al., 2011; Dayrell, 2012; Iriarte et al., 2012; Lombardo et al., 2020). This practice can also be characterized by the accidental dumping of food waste, such as seeds and fruits, by humans or non-humans (Lúcio et al., 2014). This practice does not use fire and creates resource-rich supporting points for living, hiking, and camping (Posey, 1985). These places are used by humans and species with some utility are protected and tolerated, making the area even more favorable (Posey, 1985).
Topographic changes—This consists in the movement of earth with the intention of creating elevated environments that do not suffer from the action of floods or sunken areas to store water. In this practice earth mounds are raised, often following structural and geometric patterns (Erickson, 2000). Fire is not used (Iriarte et al., 2012). Mounds often occur beside ditches and have complementary purposes, for example growing food on the mound and raising fish in the ditch (Posey, 1985; Iriarte et al., 2010; Rostain, 2010; Carson et al., 2014; Leal et al., 2019). Large mounds may have human settlements and even forest islands (Lombardo et al., 2013, Lombardo et al., 2020). After these changes in topography are established, other practices can be carried out in the same locations, such as system protection and/or species enrichment. These patterns are common among the peoples who inhabited South America before the arrival of Europeans and were discovered mainly through archaeological research.
Increased soil fertility—This consists in improving the nutritional characteristics of the soil of a certain place in the ecosystem, thus favoring plant species of human interest (Pinho et al., 2011). It often occurs through the creation of piles of organic matter, prepared from sticks, branches, and leaves, remains of termite mounds, and anthills (Posey, 1985; Rostain, 2010; Lombardo et al., 2020). This practice also involves the conservation of fertility in cultivated areas by covering the soil to avoid its exposure (Hernández-Hernández et al., 2011). In this practice, fire is not used.
Cleaning—This practice uses fire in a controlled manner with the intention of making the place safe and comfortable. It consists of removing part of the herbaceous layer, especially removing excess dry materials, as these make walking and hunting difficult (Bilbao et al., 2010; Sletto and Rodriguez, 2013; Carson et al., 2014). Not cleaning the place makes it dangerous, because it increases the chance of finding venomous animals, such as snakes and scorpions (Posey, 1985; Mistry et al., 2005). Thus, it is common to maintain clean sites near habitations There are no reports on when this practice is carried out.
Prevention—This strategy literally fights fire with fire. It is carried out in fire-dependent ecosystems adjacent to fire-sensitive ones; for this reason, it is directly linked to the practice of protection of the ecosystem (Bilbao et al., 2010; Sletto and Rodriguez, 2013; Eloy et al., 2018a, Eloy et al., 2018b). It is intended to reduce combustible material loads, which are responsible for large, out-of-control fires that commonly occur in the dry season (Mistry et al., 2005; Rodríguez, 2007; Falleiro, 2011; Melo and Saito, 2011; Welch et al., 2013; Lúcio et al., 2014; Batista et al., 2018). Prevention is done through controlled burning in the transition from the rainy to the dry season when the savannas and grasslands still have ideal amounts of moisture (Rodríguez, 2007; Melo and Saito, 2011; Batista et al., 2018). The records of this practice make it clear that it is necessary to understand the climatic conditions, such as the seasonality of rainfall and wind direction, so that the fires do not get out of control and turn into megafires.
Resource promotion—This practice has the purpose of maintaining the herbaceous vegetation with new leaves, promoting and accelerating regrowth. This renewal is mainly aimed at stimulating animal feed for wild and domestic species, because new leaves are more palatable (Rodríguez, 2007; Falleiro, 2011; Hernández-Hernández et al., 2011; Sampaio et al., 2012; Sletto and Rodriguez, 2013; Welch et al., 2013; Eloy et al., 2018a; Batista et al., 2018). It is done using controlled burning and, like the previous practice, is carried out in the transition from the rainy to the dry season. This practice goes beyond promoting the regrowth of important herbaceous species for livestock; it also stimulates flowering and fruiting in the following year of species of economic importance that are adapted to fire (Posey, 1985; Mistry et al., 2005; Falleiro, 2011; Schmidt et al., 2011; Monteiro et al., 2012; Schmidt and Ticktin, 2012; Figueira et al., 2016; Assunção et al., 2017; Eloy et al., 2018b).
Game driving—This practice uses controlled fire in order to drive hunted animals in a specific direction, thus facilitating slaughter in a desired location (Mistry et al., 2005; Bilbao et al., 2010; Melo and Saito, 2011; Welch et al., 2013; Figueira et al., 2016; Leal et al., 2019). This type of hunting is done collectively and is associated with indigenous ceremonies at the beginning of the dry season (Welch, 2015; Welch and Coimbra Jr, 2019).
Swidden-fallow—This practice involves the felling of vegetation and subsequent burning of the area (Rosa et al., 2018). The intention is to open areas for horticulture, known as slash-and-burn cultivation (Bilbao et al., 2010; Iriarte et al., 2012; Miller, 2016). It is part of a rotational system of shifting cultivation, in which prescribed burning occurs in small cleared areas, followed by a period of cultivation and harvest (Mistry et al., 2005; Rodríguez, 2007; Schmidt et al., 2011, 2018; Sampaio et al., 2012; Leal et al., 2019). After harvest, the fields go through a regeneration period (fallow) to recover soil nutrients and the pre-existing ecosystem (Kingsbury, 2003; Dayrell, 2012).
Maintenance of ecosystem structure—This consists of setting fire to fire-dependent ecosystems at the beginning of the dry season or the beginning of the rainy season in order to keep them open (Falleiro, 2011), preventing the proliferation of tree species (Rodríguez, 2007).
Ecosystem Use and Management
The management practices presented above are related to the types of ecosystems in which they are carried out (x2 = 71.5; p < 0.001) (Figure 2). We observed 32 citations of management of forest ecosystems; there were no reports of prevention with fire, nor of fire for maintenance of ecosystem structure. Savanna ecosystems had 44 management citations, with no reports of increased soil fertility. The grassland ecosystems, on the other hand, had 71 management citations, with the only practice not reported being swidden-fallow.
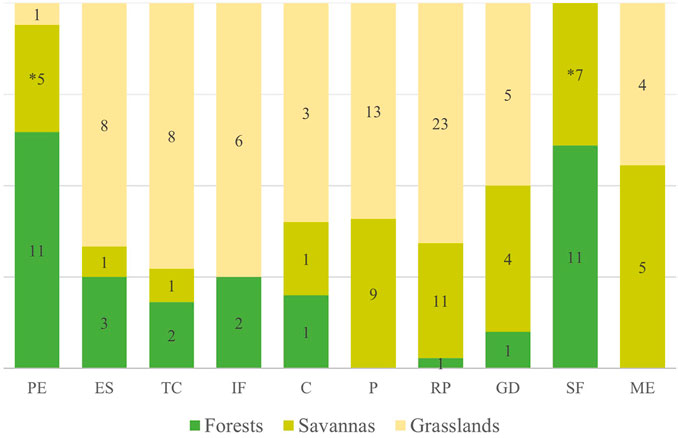
FIGURE 2. Proportions and variation of management categories according to the ecosystem in which it occurs. The acronyms represent the different categories: protection of the ecosystem (PE), enrichment of species (ES), topographic changes (TC), increased soil fertility (IF), cleaning (C), prevention (P), resource promotion (RP), game driving (GD), swidden-fallow (SF), maintenance of ecosystem structure (ME). The values within the bars represent the number of citations in each ecosystem; and the asterisk represents that the practice is carried out in a subclass of savannas, swampy areas (veredas), with only one citation for typical savannas.
The two most cited management practices for forests are complementary, since the first one prioritizes their protection to conserve resources (32.2%) and the second consists in using fire to open areas for swidden-fallow cultivation (32.2%). In addition to these practices, forests undergo management that favors characteristics considered important to humans, such as increasing the abundance of managed species (9.4%) and increasing soil fertility and changing topography (6.3% each). The other practices with fire were rarely mentioned in this type of ecosystem.
Forests had 25 cited uses, with transformation into food cultivation areas (56%) being the most significant. These are also used for gathering (28%) and non-fire hunting (12%). This type of ecosystem is used as a resting or security point, serving as a shelter in times of conflict, or even as a barrier to protect housing areas that are in more open formations (4%).
The savannah ecosystems had management citations focused mainly on management with fire to promote resources of both human and animal interest (25%), and with prescribed fire for wildfire prevention (20.5%). Fire in savannas is also used for hunting (9%) and to keep the physiognomies open (11.4%). The practices of swidden-fallow (15.9%) and protection against fire (11.4%) were cited mainly in one sub-class of savanna ecosystems, the swampy areas (veredas). The other management practices in the savannas were not mentioned or were rare.
The savannas had 56 reports of use. These areas are mainly used for gathering and hunting (32.1 and 23.2%, respectively). Savannah ecosystems are also used for cattle raising (16.1%), due to their abundant herbaceous stratum, and sporadically for housing construction (3.6%). The swamps are much sought after for cultivating food (16.1%) and provide drinking water for humans and domestic and wild animals, which makes it common to use them as water reservoirs (8.9%).
The most cited practice for managing grassland areas is promoting resources with fire (32.4%), followed by wildfire prevention (18.3%). Besides these, the other practices with fire were also expressive, cleaning (4.2%), game driving (7%) and maintaining the ecosystem (5.6%). The grasslands are also managed without burning, through species enrichment, topographic changes (11.3% each) and changes in soil fertility (8.5%). Other forms of management are rare or absent.
We recorded 73 citations of uses for the grasslands. These are mainly used for gathering and cattle raising (24.7% each). As open areas, they are used for hunting (16.4%) and are preferred sites for establishing camps and dwellings (11%). In addition to these uses, grasslands, especially humid ones, were used by pre-Columbian indigenous peoples for cultivation on raised fields without the use of fire (11%). Grasslands with rocky outcrops, a subclass of the category, are considered by some peoples as ritualistic sites (5.5%).
We recorded in our survey that these ecosystem management practices are carried out by 26 human groups, including 16 indigenous peoples, six local communities and four traditional communities (see Supplementary Table S2). We observed no correlation between the ecosystems and the cultural matrices recorded (p = 0.24), that is, all cultures manage forests, savannas and grasslands in some way.
These practices are also related to the cultural matrices that practice them (x2 = 36.2; p = 0.007) (Figure 3). Five practices were recorded in all recorded human groups: protection of the ecosystem, enrichment of species, prevention, resource promotion, and swidden-fallow. The practices of topographic changes, increased soil fertility, and game driving with fire were cited only by indigenous peoples and local communities. In addition, two practices were recorded exclusively among indigenous peoples, using fire for cleaning and for maintaining open ecosystems.
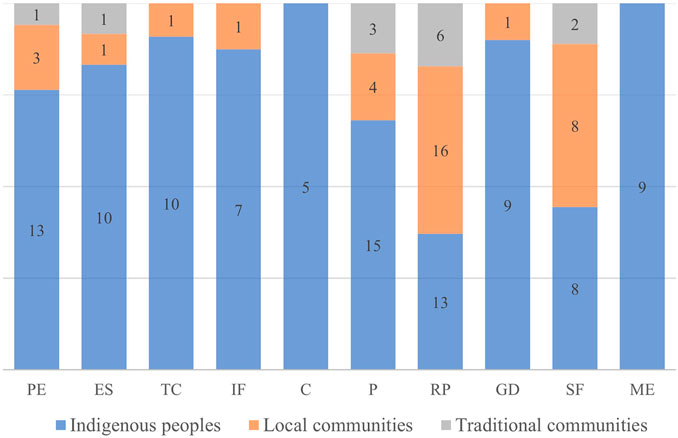
FIGURE 3. Proportions and variation of management categories according to the culture that practices it. The acronyms represent the different categories: protection of the ecosystem (PE), enrichment of species (ES), topographic changes (TC), increased soil fertility (IF), cleaning (C), prevention (P), resource promotion (RP), game driving (GD), swidden-fallow (SF), maintenance of ecosystem structure (ME). The values within the bars represent the number of citations in each cultural matrix.
Use, Management and Domestication of Plant Species
In addition to the ecosystem management practices, 81 citations were registered for management practices directed to 19 species of human interest. Eight species occur only in forest ecosystems and five in savannas; two occur in both environments. In grasslands only four species are the focus of some type of management, two of them are herbs from the most representative stratum of this ecosystem. Most of them are food resources (12), of which four were registered only for food and eight have complementary uses, three are used exclusively for folk medicine, three are used only for making handicrafts for self-consumption and for sale, and 1 is exploited as fuel and for ritualistic purposes (Table 1).
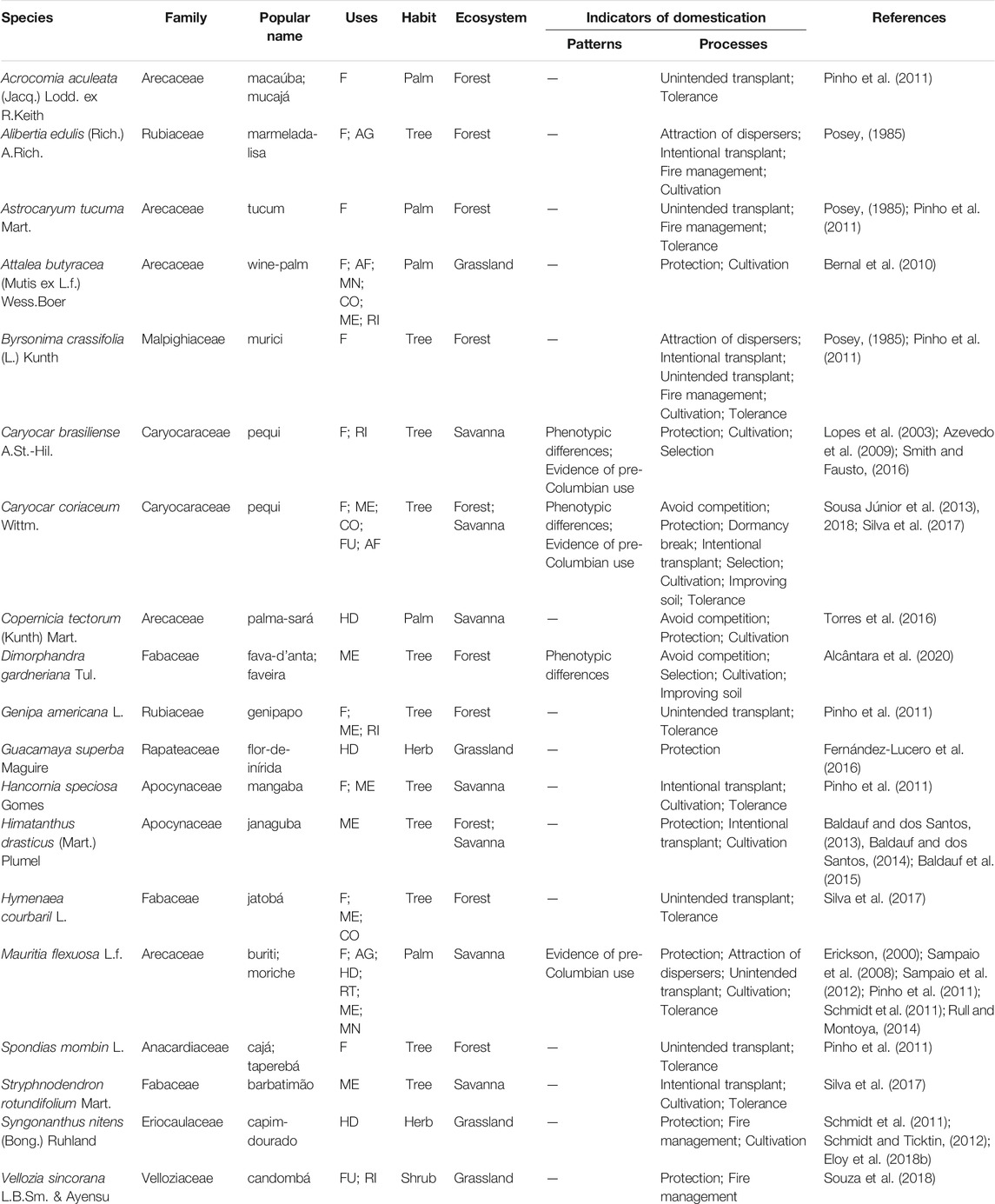
TABLE 1. List of target species for management in South American savannas, including: scientific name and botanical family, accepted by Plants of the World Online; popular names; uses, the codes represent categories (F, food; AG, attracts game animals; AF, animal food; MN, manufacturing, tool making; CO, construction; ME, medicinal; RI, ritualistic; FU, fuel; HD, handicraft; RT, Roofing thatch); habit; ecosystem of occurrence; domestication patterns; management that indicates the domestication process; and references. Indicators of domestication follow the categories proposed by Casas et al. (1997, 2017), Clement (1999), and Levis et al. (2018).
We observed that the different management practices that demonstrate the process of domestication of plant populations of human interest were cited in different proportions. The practice of protection by keeping individuals of different ages represented 24.7% of the citations and was reported for 9 species. Cultivation, which consists of planting seeds and seedlings, represented 19.8% of the reports and is carried out with 12 species, two of which reported the additional practice of improving soil structure and fertility. The tolerance strategy, which occurs when a species is not removed from the environment, accounted for 13.6% and is directed at 10 species. Transplanting seedlings or intentional seedling exchange accounted for 8.6% and targeted six species. This process can also occur unintentionally (8.6%) and occurs with seven species. Management using fire to encourage a certain resource, such as fruits or flowers, represented 8.6% of the citations and is aimed at five species. The practice of avoiding competition, which consists of removing useless plants to benefit species of human interest, represented 4.9% of the citations and is aimed at only three species. Selection of specific phenotypes of plants of interest by promoting morphological and/or genetic divergence from wild populations represented 3.7% of the citations and was reported for three species. The strategy of attracting dispersers of plants of human interest was also recorded (3.7%) for three species. The practice of breaking seed dormancy to accelerate the germination process represented 1.2% of the citations and was recorded for only one specie.
Our review found, through nine citations, that only four species showed the expected patterns in plant populations with some degree of domestication: Mauritia flexuosa, Caryocar brasiliense, Caryocar coriaceum and Dimorphandra gardneriana. For the first three species, evidence of pre-Columbian use was recorded, and phenotypic differences only for the latter three.
Discussion
Our review identified a diverse set of management practices carried out by small-scale societies that are co-responsible for creating and maintaining the diversity of ecosystems of South American savannas; these practices vary with both environments and cultural matrices, corroborating our hypotheses. In addition, there is a set of native species that are being favored by human management. Thus, it is possible to affirm that these small-scale societies play a fundamental role not only in maintaining the savannas, but also in transforming them into more favorable environments for humans themselves.
Influence of Management on the Diversity of Ecosystems
Management practices were distributed heterogeneously, forming subsets by ecosystems, and these ecosystems tend to be influenced by these interventions in different ways (Figure 4). Protection of the ecosystem is a practice aimed at forest ecosystems and savanna swamps (veredas). This practice is also common among the Akan people of the Ghana savannas, where forests are protected because they are sacred and provide food, medicine, wood and other resources important for subsistence (Sarfo-Mensah and Oduro, 2007). Besides not allowing burning, another practice, the non-extraction of plants of human interest, is carried out to protect these ecosystems (Sarfo-Mensah and Oduro, 2007); in South American savannas this practice was also cited for six forest species, but we categorized it as protection and tolerance (Table 1). The protection of the ecosystem favors the structure and floristic composition, because fire significantly affects both in these environments (Pivello et al., 2021). Furthermore, when uncontrolled, fire in these ecosystems causes chemical and water stress in the soil, which drastically alters the amount of biomass, making these ecosystems more susceptible to megafires, which—when recurrent—convert forests into more open vegetation ecosystems (Dezzeo and Chacón, 2005). With this, we can affirm that the practice of protection maintains these fire-sensitive ecosystems, also known as “Fire refugia” (Meddens et al., 2018), and consequently conserves important ecological functions for the entire biome, because these sites help maintain trophic chains, act as a refuge for fauna, and function as a seed bank to repopulate burned areas (Meddens et al., 2018; Flores et al., 2021).
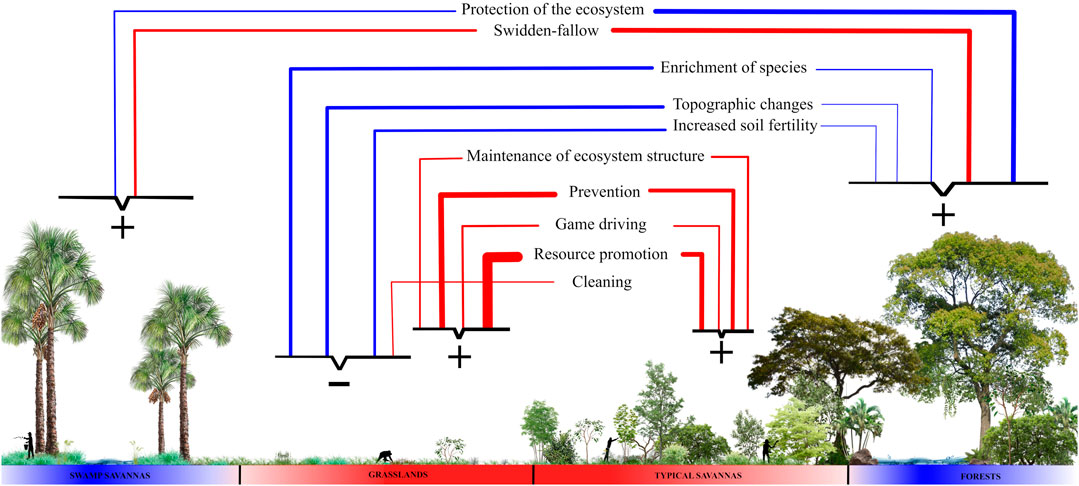
FIGURE 4. Summary diagram of how the most expressive management practices are distributed and influence the following ecosystems: swamp savannas (veredas), grasslands, typical savannas, and forests. The direction of the lines indicates which ecosystem is the focus of each management practice, and the thickness of the lines the number of citations; those considered rare were excluded for having only one citation (see Figure 2). The colors of the lines indicate whether the practice uses fire (red) or not (blue). On the basis of the diagram, we categorize ecosystems according to the classification by Pivello et al. (2021), in blue the fire-sensitive and in red the fire-dependent. Each ecosystem is targeted by a subset of practices grouped by keys and the signs represent the influence that each subset exerts on the ecosystem: maintenance due to management (positive) or ecosystems altered by management (negative).
Complementary to protection, prevention is done in savanna and grassland ecosystems. It can reduce the accumulated fine fuel load, composed of leaves and thinner branches, alive or dead, of the herbaceous layer and from the lower part of the arboreal-shrub layer (Miranda, 2010). Fuel accumulation, together with extreme droughts and hot weather events, are responsible for the megafires (Fidelis et al., 2018), which are those with a large extension of burning, that are difficult to control, and that cause a negative impact on biodiversity (Fidelis et al., 2018). This practice has the consequence of maintaining structure and floristic composition because excluding fire permanently from the ecosystems dependent on it generates negative social and ecological consequences (Aslan et al., 2018; Pivello et al., 2021), which explains why protection management is rarely applied to them. Studies in Australian savannas show that this practice is common among small-scale tropical savanna societies and that, in addition to intentionally preventing large fires, it protects food resources of unburned ecosystems (Russell-Smith et al., 1997; Yibarbuk et al., 2001; Hill and Baird, 2003; Fletcher et al., 2020). The negative ecological consequences of fire exclusion are related to the breaking of the circular dynamics present in grasslands and savannas, which regulate the establishment of woody plants (Kauffman et al., 1994). This relationship occurs as follows: grassland ecosystems that contain fewer woody plants tend to have intense fires, which result in higher mortality of living tissue and consequently less chance for woody plants to establish themselves and remain structurally open; in savanna ecosystems that are woodier, fires tend to be mild, because they do not consume shrubs and trees completely, which consequently facilitates the persistence of these individuals, remaining structurally denser (Kauffman et al., 1994).
Fire-sensitive ecosystems are also sought after and preferred by savanna inhabitants for swidden-fallow; this practice was not reported in articles on grasslands and rarely in typical savannas. This can be explained by the fact that forests and swamp savannas (veredas) have wetter soils and higher levels of organic matter, important attributes for the soil’s capacity to retain and exchange cations and keep the microbiota in balance, which consequently facilitates the establishment of plants of human interest (Reatto et al., 2008). Fire in these ecosystems is considered destructive (Pivello et al., 2021), but in this practice, it is used in a controlled manner on a small scale to remove the original vegetation before planting and to further improve soil characteristics through the ash that provides an initial nutritional increase of K and Ca (Kauffman et al., 1994). Another important point is that this practice involves the rotation of areas and long fallow periods, which makes it less aggressive to the soil, since it leads to the eventual reestablishment of the vegetation, creating a mosaic of heterogeneous areas in different regenerative stages, increasing diversity as a whole (Kingsbury, 2003; Adamou et al., 2007). This food production system is common throughout the Neotropics and in tropical savannas, and allows the soil to regain its fertility and native species to re-establish themselves (Sarfo-Mensah and Oduro, 2007; Clement et al., 2021); From then on, these recovering forests are protected again. We can affirm that this practice is favorable for the ecosystems in which it is carried out because even if it partially alters ecosystems in the short term, in the long term it allows for their recovery and favors species of human interest.
Species enrichment is mainly done in grasslands, occasionally mentioned in forests, and rarely in savannas. This practice was considered to alter the structure and floristic composition of the grasslands, because the establishment of woody species in these ecosystems can alter nutrient cycling, soil structure, biomass levels, and organic matter concentration, resulting in changes to the entire local microclimate (Scholes and Archer, 1997; Ayalew and Mulualem, 2018). Giles et al. (2021) evaluated the process of invasion of woody plants into grassland ecosystems used for grazing and proposed that a management approach to remove woody species would be ideal to conserve the characteristics of these ecosystems. We observe that the practice of enrichment with woody species is done in or near inhabited grasslands, that is, the intention here is not to maintain the grassland structure but to bring the environment closer to the forest structure rich in human interest resources and to create a milder microclimate around the dwellings. Similarly, there are records in the savannas of West Africa of non-forested areas being enriched with tree species to become forest patches because this type of ecosystem is considered the most important to the people who inhabit the region (Sarfo-Mensah and Oduro, 2007).
Two other practices done near dwellings are cleaning and increasing soil fertility, also recorded for the grassland ecosystems, the preferred areas to establish human habitation. Both change the structure of the grasslands, as the cleaning reduces competition between trees and herbs, favoring the trees, as it allows them to have more access to nutrients and water, and their roots reach deeper into the soil. (Scholes and Archer, 1997). The increase in fertility also favors the establishment of trees, because it reduces nutritional stress and allows their growth by providing greater nutritional support (Scholes and Archer, 1997). In the savannas of Western Sudan, small-scale farmers have developed similar practices aimed at increasing soil fertility, such as creating mounds of organic matter and fertilizing with home-grown organic residues, which allow for the support of arboreal individuals (Adamou et al., 2007).
The wet grasslands close to human dwellings also had more citations of alteration of topography, mainly by indigenous peoples before European colonization. These people significantly changed the shape and ecology of these grasslands, which made human population growth possible throughout the Holocene (Erickson, 2000; Rostain, 2010), but did not change their structure to the point of becoming forest or savanna ecosystems. Paleoenvironmental research has shown that the Holocene climate favored the expansion of forests, but human care meant that the grassland structure was maintained (Carson et al., 2014). Hence this practice was considered to maintain the structure of the grasslands. In addition, it was common in tropical savannas, as it occurred locally in the humid grasslands of the Amazonian savannas, the Beni Savannah, the Guianas savannas (Erickson, 2000; Iriarte et al., 2010, 2012; Rostain, 2010; Pinho et al., 2011; Carson et al., 2014; Leal et al., 2019; Rodrigues et al., 2020).
In our review, we observed that the most cited activities for grassland and savannah ecosystems are cattle raising and gathering, and the peoples of the South American savannas have developed management practices that differ from those proposed by Giles et al. (2021). Four practices are geared towards these ecosystems and make use of prescribed fire: prevention, resource promotion, game drives, and ecosystem maintenance. Fire, the central element of these practices, consumes the fine, flammable tissues of the grasses quickly enough to not kill them and the heat remains at the surface, which allows seeds and perennial parts to survive (Pivello et al., 2021). The mature shrub layer present in savannas is also able to recover because the accumulation of bark prevents the trunks from dying (Hoffmann et al., 2012). After the fire event, the herbaceous stratum, the focus of human interest in this case, quickly regrows and recovers its flammability, becoming susceptible to another fire event (Miranda, 2010). These practices with fire are commonly used recurrently (Schmidt et al., 2011; Schmidt et al., 2018; Monteiro et al., 2012; Sletto and Rodriguez, 2013), which ends up favoring that the dominance of the herbaceous layer over the arboreal, as the fire-sensitive woody trees, which could change the structure of these ecosystems, have no chance of establishing themselves, as they cannot thicken their bark before the next fire (Hoffmann et al., 2012).
Moreover, these fire practices are done in the transitions between the rainy and dry seasons. The time when a fire event occurs influences its intensity and is directly linked to the chances of it getting out of human control and reaching fire-sensitive ecosystems (Schmidt and Eloy, 2020). Fire at the end of the dry season, a period of low humidity, tends to be more aggressive and cause mortality of woody individuals more than fires at the beginning of the dry season, when management is done, because at this time the environments still contain moisture and occasional rainfall occurs, which makes the fire milder (Miranda, 2010). According to Ramos-Neto and Pivello (2000), fires in the rainy season are mainly caused by lightning and not by human action; this causes only small areas to be burned, as the rain that proceeds or follows the lightning does not allow the fire to spread over large areas. Furthermore, it is known that management practices that use fire in an organized and controlled manner are essential for open formations to continue to occur in tropical savannas (Russell-Smith et al., 1997; Hill and Baird, 2003; Roberts et al., 2021). Considering these factors, we can say that in general these four practices, prevention, resource promotion, game driving, and ecosystem maintenance, tend to maintain the grassland and savanna ecosystems used for cattle grazing and gathering.
The influence of the practices presented above allowed us to observe feedbacks between practice and result, an important dynamic for confirming the niche construction process (Baedke et al., 2021). In simplified form, we can affirm that fire-dependent ecosystems are managed mainly with fire and fire-sensitive ones are managed without it. Over time, this influenced the evolution of the ecosystems and consequently the evolution of the practices used by the small-scale societies of the South American savannas, making explicit the evolutionary feedback present in this process (Spengler, 2021).
Cultural Influence: Construction of Niches
In addition to management practices varying between ecosystems, our results also showed that the different cultural matrices of South America share only a few ways of managing these ecosystems (Figure 3). Two of these widely used practices are aimed at increasing the abundance of edible species: species enrichment and swidden-fallow. This can be explained by the fact that, in general, humans tend to optimize environments by increasing food resources, transforming them into domesticated landscapes (Clement, 1999; Hill and Baird, 2003; Terrell et al., 2003; Clement et al., 2021). Furthermore, of the 19 species managed in-situ, 12 are used for food; of these, 12 are cultivated or transplanted intentionally or not, and two are protected in their original ecosystems (Table 1). All these forms of management aimed at food acquisition corroborate the analysis of Smith (2011), who says that niche constructing humans create strategies to increase the abundance of food resources. While in the tropical savannas of South America we have found that enriching areas with plants of interest is common, this is not the case in the savannas of Nigeria where rural communities claim that the food trees of the forests are only planted by God, but should always be preserved (Olorunfemi et al., 2016). Swidden-fallow in turn is a system widely used for food production by small-scale societies in the Neotropics, but it is not the only one (Terrell et al., 2003; Clement et al., 2021). For example, in the Bolivian savannas, food production was done by indigenous people on raised fields without the use of fire (Iriarte et al., 2012). This system of topographic changes for cultivation without fire has also been recorded today in the savannas of Congo (Rodrigues et al., 2020).
Two other practices performed by all cultural matrices are resource promotion and prevention. Our review found no records of savanna or grassland patches created by humans, however, the practice of burning open environments is described by Laland and O’Brien (2010) as one of the most efficient ways of constructing niches by humans. Clark (1980) reported that in the early Holocene African savannas and grasslands were burned regularly and seasonally by hunters and gatherers, a practice that encouraged the growth of herbaceous plants and consequently maintained the structure of these ecosystem types. We, therefore, defend that these environments in South America occur naturally due to environmental factors and are perpetuated by anthropic action.
Complementarily, the practice of protecting forest ecosystems is also common to all the cultures sampled in our study, because these environments are rich in important resources for human survival. In parallel, we have observed that some forest patches in our survey were constructed by indigenous people through a combination of the following practices: increased soil fertility, topographic changes, followed by species enrichment. Research suggests that the extent of savannas is not entirely the result of human action, but the location and extent of ecotones, forests and grasslands, may have been determined by human actions in the past (Hammond, 1980). In the same sense, Casas et al. (1997) observed that forms of management aimed at wild plants in situ influence regeneration processes and may be responsible for the creation of anthropogenic forests. As observed by these authors, our review showed that ten species are managed in different ways in South American savanna forests (Table 1), which suggests that—even if they were not intentionally constructe—forests can be considered legacies of human management (Arroyo-Kalin, 2016), and are therefore protected due to their importance.
These common practices are widely spread across the continent and this can be explained by the fact that Neotropical savannas were connected in the past (Silva and Bates, 2002) and people were able to exchange information. The distribution of these practices among different human cultures is maintained today, and this may be related to the fact that humans, when successful niche constructors, inherit from their ancestors information about how to manage these environments, thus favoring more than one generation (Smith, 2011; Albuquerque et al., 2015; Coca et al., 2021). This makes explicit one of the principles of NCT, relocation, which affirms that practices can migrate with constructing organisms and be adopted by other populations when they meet in time and space (Davis and Douglass, 2021). Moreover, these common practices refute one of the current criticisms of NCT, because they are not merely singular human behaviors but behaviors that are regularly repeated at the population level (Spengler, 2021).
Together with these management practices aimed at ecosystems, we recorded the occurrence of practices aimed at 19 species important for humans (Table 1). Management practices aimed at plant populations may be responsible for altering landscapes as a whole, transforming them into domesticated landscapes (Casas et al., 1997; Clement, 1999; Terrell et al., 2003; Levis et al., 2018). This is because management practices favor these species in the environment, which can make them more abundant, and consequently the environments more favorable to humans (Smith, 2012). In conjunction with changes in the environment, population management can generate phenotypic and genetic changes, indicating the occurrence of a domestication process (Casas et al., 1997; Clement, 1999; Allaby et al., 2021; Clement et al., 2021). We observed that only four species present some component of the domestication syndrome (Meyer et al., 2012), but the other 16 managed species may be under-going domestication, even if in early stages (Terrell et al., 2003; Clement et al., 2021). All these plant populations are domesticated as components of the landscape and not separately (i.e., not necessarily in cultivation), because the domestication process is the result of interactions between cultural matrices and selection over time; this results in increased gene flow even with incipient changes in phenotypic traits (Rindos, 2013; Allaby et al., 2021). Clement et al. (2021) showed that 2,384 plant species are used in the Brazilian Cerrado and other Neotropical savannas and some may be undergoing domestication, but our review found a much smaller number. This may be related to our search methodology, as we included in our review only articles that detailed the forms of management and not those that only cited plants used by small-scale societies; we also did not search for information in databases like the cited authors. However, this restricted number indicates which species are in fact managed in the natural ecosystems of the South American savannas and which may be at different stages of the domestication process, thus being able to guide further studies on this theme.
Traditional Ecological Knowledge: a Tool for Conservation and Restoration
The extent and durability of human manipulations, and the diversity and specificity of management practices demonstrate extensive traditional knowledge about the ecological processes of the South American savannas. Small-scale societies have this knowledge due to the intimate relationship they have with their respective territories, a relationship marked by long periods of observation and use (Abraão et al., 2010; Smith, 2011; Silva et al., 2016).
The practice of fire management to keep these ecosystems open demonstrates traditional knowledge about the ecological processes of savanna succession. These peoples have a clear understanding that savanna and grassland areas left without human manipulation for even a short period can change due to the establishment of arboreal individuals, becoming denser (Pinheiro and Durigan, 2009; Santos et al., 2017). Another factor that seems well understood by small-scale societies is tree-herb interaction in the savannas, very relevant knowledge for restoration of degraded environments. According to Hoffmann et al. (2012), for forest seedlings to establish naturally in savanna ecosystems, long periods without fire are required and for saplings typical of savannas to establish themselves in forest ecosystems, prolonged droughts and intense fires are required. However, these authors point out that humans can induce changes in this process. Our survey confirmed this argument, as it showed that humans are actively transplanting species of interest between environments and subsequently caring for them to reach adulthood. Furthermore, we observed that there is protection of already established trees in both environments (Table 1), which proves that humans do indeed know how to influence the distribution dynamics of woody individuals.
Of the 10 management practices aimed at ecosystems, six of them have fire as a central element. Traditional knowledge of fire is extremely complex and involves a detailed understanding of several elements: seasons, effect of fire on fauna, rainfall seasonality, current legislation, moisture of combustible material, fire intensity, heat production, necessary intervals between fires, consequences of not burning, how to control fires, and plant phenology of each ecosystem (Huffman, 2013). This set of details makes it possible to say that fire management is reliable, but it is also dependent on extensive knowledge acquired over many generations.
Drastic changes in the planet’s climate are a real problem to be mitigated (IPCC, 2021) and several studies have shown that high temperatures, extreme droughts and accumulation of combustible material are the main causes of the megafires that have been occurring more frequently in tropical savannas and that prescribed burning may be a feasible solution for this problem (Eloy et al., 2018a; Fidelis et al., 2018; Mistry et al., 2018; Schmidt et al., 2018; Moura et al., 2019; Schmidt and Eloy, 2020; Pivello et al., 2021; Roberts et al., 2021). Flores et al. (2021) stated that integrated management plans require strategies that consider forests as a vulnerable element of the system. The practices proposed by scientists are part of the strategies carried out by small-scale societies throughout the history of occupation of the South American savannas, but our results show that prevention and ecosystem protection do not maintain ecosystems on their own (Figure 4). This suggests to us that other practices need to be further analyzed and possibly included in these conservation and restoration strategies. When incorporating these niche-construction practices into conservation and restoration plans, it is important to keep in mind that they go beyond the momentary and have long-term ecological consequences, including evolutionary consequences (Albuquerque et al., 2019).
Concluding Remarks
Our review demonstrated that small-scale societies of South American savannas have a diverse set of management practices that contribute, along with environmental factors, to keeping savanna and grassland ecosystems open and to increase the occurrence of forest ecosystems in the mosaic of ecosystems, favoring human sustenance. These practices vary with the ecosystems in which they are used and with the different cultures that use them. They have also proven to be a very useful sources of information for restoration, conservation, and integrated management programs for these endangered ecosystems.
We found that the small-scale societies of the South American savannas are remarkable niche constructors, changing the selective pressures of the ecosystems and leaving important legacies for following generations. These ranged from very expressive and persistent constructions, such as anthropogenic forests or raised fields, to less visible footprints, such as 19 native species being domesticated in situ by diverse cultures, consequently making the landscapes more favorable for humans. We conclude, therefore, that South American savannas are domesticated landscapes because they are composed of a mosaic not only of natural ecosystems, but also of culturally constructed niches.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MF, GS, CC, and CL designed the study; MF and LC designed and executed the methodological part; MF collected and synthesized the data; MF and LC analyzed the data; MF wrote the manuscript and designed the graphics; CC, GS, and CL supervised the study; and all authors contributed to previous versions and approved the final one.
Funding
The authors thank the Postgraduate Program in Ethnobiology and Nature Conservation, Universidade Federal Rural de Pernambuco, for the academic and financial support to the first author’s PhD. MF thanks the Coordenação de Aperfeicoamento de Pessoal de Nível Superior (CAPES) for a scholarship (88882.436667/2019-01) and CL thanks CAPES for a postdoctoral fellowship (88887.474568/2020). CC thanks the Brazilian National Research Council (CNPq) for a research fellowship (303477/2018-0). GS thanks the Brazilian National Research Council (CNPq) (425908/2016-0) and the Research Support Foundation of the State of Minas Gerais (Fapemig) (CRA APQ 03937/16) for research support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the researchers who conducted the studies included in our review for the rich information they provided. We are also grateful to Nivaldo Peroni, Cristina Baldauf, and Taline C. da Silva for useful comments on a previous version of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.809404/full#supplementary-material
References
Abraão, M. B., Shepard, G. H., Nelson, B. W., Baniwa, J. C., Andrello, G., and Yu, D. W. (2010). “Baniwa Vegetation Classification in the white-sand Campinarana Habitat of the Northwest Amazon, Brazil,” in Landscape Ethnoecology: Concepts Of Biotic And Physical Space. Editors L. M. Johnson, and E. S. Hunn (New York, NY: Berghahn Books), 83–115.
Adamou, I., Pierre, N. J., Pogenet, P., Tchimbi, B., and Gonlaina, G. (2007). Soil Degradation in the Sudano-guinea Savannas of Mbe, Cameroon: Farmers’ Perception, Indicators and Soil Fertility Management Strategies. Res. J. Agric. Biol. Sci. 3, 907–916.
Adeney, J. M., Christensen, N. L., Vicentini, A., and Cohn‐Haft, M. (2016). White‐sand Ecosystems in Amazonia. Biotropica 48, 7–23. doi:10.1111/btp.12293
Albuquerque, U. P., do Nascimento, A. L. B., da Silva Chaves, L., Feitosa, I. S., de Moura, J. M. B., Gonçalves, P. H. S., et al. (2019). A Brief Introduction to Niche Construction Theory for Ecologists and Conservationists. Biol. Conservation 237, 50–56. doi:10.1016/j.biocon.2019.06.018
Albuquerque, U. P., Júnior, W. S. F., Santoro, F. R., Torres-Avilez, W. M., and Júnior, J. R. S. (2015). “Niche Construction Theory and Ethnobiology,” in Evolutionary Ethnobiology. Editors U. P. Albuquerque, P. M. De Medeiros, and A. Casas (Cham: Springer International Publishing), 73–87. doi:10.1007/978-3-319-19917-7_6
Allaby, R. G., Stevens, C. J., Kistler, L., and Fuller, D. Q. (2021). “Genetic Revelations of a New Paradigm of Plant Domestication as a Landscape Level Process,” in Plant Breeding Reviews. Editor I. Goldman (Oxford, UK: John Wiley & Sons), 321–343. doi:10.1002/9781119828235.ch8
Almeida, A. W. B. d. (2004). Terras tradicionalmente ocupadas: processos de territorialização e movimentos sociais. Rbeur 6, 9–32. doi:10.22296/2317-1529.2004v6n1p9
Arroyo-Kalin, M. (2016). “Landscaping, Landscape Legacies, and Landesque Capital in Pre-columbian Amazonia,” in The Oxford Handbook of Historical Ecology and Applied Archaeology. Editors C. Isendahl, and D. Stump (Oxford University Press), 90–109. doi:10.1093/oxfordhb/9780199672691.013.16
Aslan, C. E., Samberg, L., Dickson, B. G., and Gray, M. E. (2018). Management Thresholds Stemming from Altered Fire Dynamics in Present-Day Arid and Semi-arid Environments. J. Environ. Manage. 227, 87–94. doi:10.1016/j.jenvman.2018.08.079
Assunção, R. de., Tetto, A. F., and Batista, A. C. (2017). O Uso Tradicional Do Fogo No Assentamento Vale Verde, Em Gurupi/TO. Espacios 38, 19–32.
Ayalew, S., and Mulualem, G. (2018). A Review on Bush Encroachment Effect on Cattle Rearing in Rangelands. J. Rangel. Sci. 8, 403–415.
Azevedo, A. I. d., Martins, H. T., and Drummond, J. A. L. (2009). A dinâmica institucional de uso comunitário dos produtos nativos Do cerrado no município de japonvar (Minas Gerais). Soc. Estado. 24, 193–228. doi:10.1590/s0102-69922009000100009
Baedke, J., Fábregas-Tejeda, A., and Prieto, G. I. (2021). Unknotting Reciprocal Causation Between Organism and Environment. Biol. Philos. 36, 1–29. doi:10.1007/s10539-021-09815-0
Baldauf, C., Corrêa, C. E., Ferreira, R. C., and dos Santos, F. A. M. (2015). Assessing the Effects of Natural and Anthropogenic Drivers on the Demography of Himatanthus Drasticus (Apocynaceae): Implications for Sustainable Management. For. Ecol. Manag. 354, 177–184. doi:10.1016/j.foreco.2015.06.022
Baldauf, C., and Dos Santos, F. A. M. (2014). The Effect of Management Systems and Ecosystem Types on Bark Regeneration in Himatanthus Drasticus (Apocynaceae): Recommendations for Sustainable Harvesting. Environ. Monit. Assess. 186, 349–359. doi:10.1007/s10661-013-3378-x
Baldauf, C., and Maës dos Santos, F. A. (2013). Ethnobotany, Traditional Knowledge, and Diachronic Changes in Non-timber Forest Products Management: A Case Study of Himatanthus Drasticus (Apocynaceae) in the Brazilian Savanna1. Econ. Bot. 67, 110–120. doi:10.1007/s12231-013-9228-5
Balée, W. (2018). Brief Review of Historical Ecology. Les Nouv. L’archéologie 152, 1–11. doi:10.4000/nda.4150
Balée, W. (2006). The Research Program of Historical Ecology. Annu. Rev. Anthropol. 35, 75–98. doi:10.1146/annurev.anthro.35.081705.123231
Batista, E. K. L., Russell-Smith, J., França, H., and Figueira, J. E. C. (2018). An Evaluation of Contemporary savanna Fire Regimes in the Canastra National Park, Brazil: Outcomes of Fire Suppression Policies. J. Environ. Manage. 205, 40–49. doi:10.1016/j.jenvman.2017.09.053
Beerling, D. J., and Osborne, C. P. (2006). The Origin of the Savanna Biome. Glob. Chang. Biol. 12, 2023–2031. doi:10.1111/j.1365-2486.2006.01239.x
Bernal, R., Galeano, G., García, N., Olivares, I. L., and Cocomá, C. (2010). Uses and Commercial Prospects for the Wine Palm, Attalea Butyracea, in Colombia. Ethnobot. Res. App. 8, 255–268. doi:10.17348/era.8.0.255-268
Bilbao, B. A., Leal, A. V., and Méndez, C. L. (2010). Indigenous Use of Fire and Forest Loss in Canaima National Park, Venezuela. Assessment of and Tools for Alternative Strategies of Fire Management in Pemón Indigenous Lands. Hum. Ecol. 38, 663–673. doi:10.1007/s10745-010-9344-0
Bocanegra, F. J. A., and Mora, S. R. (2012). Del paleoindio al formativo: 10.000 años para la historia de la tecnología lítica en Colombia. Boletín Antropol 26, 124–156.
Boivin, N. L., Zeder, M. A., Fuller, D. Q., Crowther, A., Larson, G., Erlandson, J. M., et al. (2016). Ecological Consequences of Human Niche Construction: Examining Long-Term Anthropogenic Shaping of Global Species Distributions. Proc. Natl. Acad. Sci. USA 113, 6388–6396. doi:10.1073/pnas.1525200113
Brandão, C. R. (2012). “A Comunidade Tradicional,” in Cerrado, Gerais, Sertão: Comunidades Tradicionais Nos Sertões Roseanos. Editors J. B. de. A. Costa, and C. L. de Oliveira (São Paulo: Editora Intermeios), 367–380.
Brasil (1988). Constituição da República Federativa do Brasil: texto constitucional promulgado em 5 de outubro de 1988, com as alterações determinadas pelas Emendas Constitucionais de Revisão nos 1 a 6/94, pelas Emendas Constitucionais nos 1/92 a 91/2016 e pelo Decreto. Brasília, DF: Senado Federal: Centro Gráfico, 496.
Brondízio, E. S., Aumeeruddy-Thomas, Y., Bates, P., Carino, J., Fernández-Llamazares, Á., Ferrari, M. F., et al. (2021). Locally Based, Regionally Manifested, and Globally Relevant: Indigenous and Local Knowledge, Values, and Practices for Nature. Annu. Rev. Environ. Resour. 46, 481–509. doi:10.1146/annurev-environ-012220-012127
Bueno, L., and Dias, A. (2015). Povoamento inicial da América Do Sul: contribuições Do contexto brasileiro. Estud. Av. 29, 119–147. doi:10.1590/S0103-4014201500010000710.1590/s0103-40142015000100009
Bueno, L., and Isnardis, A. (2018). Peopling central Brazilian Plateau at the Onset of the Holocene: Building Territorial Histories. Quat. Int. 473, 144–160. doi:10.1016/j.quaint.2018.01.006
Cardoso Da Silva, J. M., and Bates, J. M. (2002). Biogeographic Patterns and Conservation in the South American Cerrado: A Tropical Savanna Hotspot. Bioscience 52, 225–233. doi:10.1641/0006-3568(2002)052[0225:bpacit]2.0.co;2
Carson, J. F., Whitney, B. S., Mayle, F. E., Iriarte, J., Prumers, H., Soto, J. D., et al. (2014). Environmental Impact of Geometric Earthwork Construction in Pre-columbian Amazonia. Proc. Natl. Acad. Sci. 111, 10497–10502. doi:10.1073/pnas.1321770111
Casas, A., Caballero, J., Mapes, C., and Zárate, S. (1997). Manejo de la vegetación, domesticación de plantas y origen de la agricultura en Mesoamérica. Bot. Sci. 61, 31–47. doi:10.17129/botsci.1537
Casas, A., Torres-Guevara, J., and Parra-Rondinel-Rondinel, F. (2017). in Domesticación en el continente americano. Editors A. Casas, J. Torres-Guevara, and F. Parra-. Rondinel-Rondinel.
CEE (2018). Guidelines and Standards for Evidence Synthesis in Environmental Management. Version 5.0. AS Pullin GK Fram. B Livoreil G Petrokofsky. Available at: www.environmentalevidence.org/information-for-authors. Acessed in: 03/16/2021.
Clark, J. D. (1980). “Early Human Occupation of African savanna Enviroment,” in Human Ecology in savanna Enviroments. Editor D. R. Harris (London: Academic Press), 41–71.
Clement, C. R. (1999). 1492 and the Loss of Amazonian Crop Genetic Resources. I. The Relation Between Domestication and Human Population Decline. Econ. Bot. 53, 188–202. doi:10.1007/BF02866498
Clement, C. R., Casas, A., Parra-Rondinel, F. A., Levis, C., Peroni, N., Hanazaki, N., et al. (2021). Disentangling Domestication from Food Production Systems in the Neotropics. Quaternary 4, 4–35. doi:10.3390/quat4010004
Coca, J. R., Soto, A., Mesquita, C., Lopes, R. P., and Cordero-Rivera, A. (2021). Biosociological Ethodiversity in the Social System. BioSystems 210, 104552. doi:10.1016/j.biosystems.2021.104552
Cole, M. M. (1986). in The Savannas: Biogeography and Geobotany. Editor M. M. Cole (London: Academic Press). Available at: https://www.jstor.org/stable/635398?origin=crossref.
Cooke, R. (1998). Human Settlement of Central America and Northernmost South America (14,000-8000Bp). Quat. Int. 49-50, 177–190. doi:10.1016/s1040-6182(97)00062-1
CPI-SP (2021). Direitos quilombolas. Comissȧo prɃ-ȷndio Sȧo Paulo. Available at: https://cpisp.org.br/direitosquilombolas/. Acessed: September 06, 2021.
Crumley, C. L. (2007). “Historical Ecology: Integrated Thinking at Multiple Temporal and Spatial Scales,” in The World System and the Earth System: Global Socioenvironmental Change and Sustainability since the Neolithic. Editors A. Hornborg, and C. L. Crumley (Abingdon: The Routledge), 1–13. doi:10.1017/CBO9781107415324.004
da Silva, T. C., Medeiros, M. F. T., Peroni, N., and Paulino Albuquerque, U. (2016). Folk Classification as Evidence of Transformed Landscapes and Adaptative Strategies: a Case Study in the Semiarid Region of Northeastern Brazil. Landscape Res. 42, 521–532. doi:10.1080/01426397.2016.1258047
Davis, D. S., and Douglass, K. (2021). Remote Sensing Reveals Lasting Legacies of Land-Use by Small-Scale Foraging Communities in the Southwestern Indian Ocean. Front. Ecol. Evol. 9, 689399. doi:10.3389/fevo.2021.689399
de Alcântara, M. S., Duarte, A. E., Boligon, A. A., de Campos, M. M. A., de Lucena, R. F. P., Pinheiro, M. A., et al. (2020). Effects of Different Levels of Exploration on the Ecological Processes of Dimorphandra Gardneriana, a Tropical savanna Tree. Environ. Monit. Assess. 192, 1–15. doi:10.1007/s10661-020-08344-9
Dezzeo, N., and Chacón, N. (2005). Carbon and Nutrients Loos in Aboveground Biomass along a Fire Induced forest-savanna Gradient in the Gran Sabana, Southern Venezuela. For. Ecol. Manag. 209, 343–352. doi:10.1016/j.foreco.2005.02.008
Eidt, J. S., and Udry, C. (2019). Sistemas Agrícolas Tradicionais No Brasil. Editors J. S. Eidt, and C. Udry Brasília. 1st ed. (Embrapa: DF).
Ellenberg, H., and Mueller-Dombois, D. (1967). Tentative Physiognomic-Ecological Classification of Plant Formations of the Earth. Berichte Des Geobot. Institutes der Eidg. Techn. 37, 21–55. doi:10.5169/seals-377650
Eloy, L., Bilbao, A. B., Schmidt, I. B., and Schmidt, I. B. (2018a). From Fire Suppression to Fire Management: Advances and Resistances to Changes in Fire Policy in the Savannas of Brazil and Venezuela. Geogr. J. 185, 10–22. doi:10.1111/geoj.12245
Eloy, L., Schmidt, I. B., Borges, S. L., Ferreira, M. C., and dos Santos, T. A. (2018b). Seasonal Fire Management by Traditional Cattle Ranchers Prevents the Spread of Wildfire in the Brazilian Cerrado. Ambio 48, 890–899. doi:10.1007/s13280-018-1118-8
Erickson, C. L. (2000). An Artificial Landscape-Scale Fishery in the Bolivian Amazon. Nature 408, 190–193. doi:10.1038/35041555
Erickson, C. L. (1995). “Archaeological Methods for the Study of Ancient Landscapes of the Llanos de Mojos in the Bolivian Amazon,” in Archaeology In the Lowland American Tropics: Current Analytical Methods And Applications. Editor P. Stahl (Cambridge University Press), 66–95. doi:10.1017/cbo9780511521188.004
Eufemia, L., Morales, H., Bonatti, M., Graser, M., Lana, M., and Sieber, S. (2019). Collective Perception of Anthropic and Extractive Interventions in the Colombian Llanos. Soc. Sci. 8, 259–315. doi:10.3390/socsci8090259
Falleiro, R. D. M. (2011). Resgate Do manejo tradicional Do Cerrado com fogo para proteção das terras indígenas Do oeste Do Mato Grosso: um estudo de caso. Biodiversidade Bras 1, 86–96.
Fernandes, E. C. M., Oktingati, A., and Maghembe, J. (1984). The Chagga Homegardens: a Multistoried Agroforestry Cropping System on Mt. Kilimanjaro (Northern Tanzania). Agroforest Syst. 2, 73–86. doi:10.1007/BF00131267
Fernández-Lucero, M., Madriñán, S., and Campbell, L. M. (2016). Morphology and Anatomy ofGuacamaya superba(Rapateaceae) and Schoenocephalieae with Notes on the Natural History of theFlor de inírida. Harv. Pap. Bot. 21, 105–123. doi:10.3100/hpib.v21iss1.2016.n9
Fidelis, A., Alvarado, S., Barradas, A., and Pivello, V. (2018). The Year 2017: Megafires and Management in the Cerrado. Fire 1, 49–11. doi:10.3390/fire1030049
Figueira, J. E. C., Ribeiro, K. T., Ribeiro, M. C., Jacobi, C. M., França, H., de Oliveira Neves, A. C., et al. (2016). “Fire in Rupestrian Grasslands: Plant Response and Management,” in Ecology and Conservation of Mountaintop Grasslands in Brazil. Editor G. W. Fernandes (Cham: Springer International Publishing), 415–448. doi:10.1007/978-3-319-29808-5_18
Filgueiras, T. S. (2002). “Herbaceous Plant Communities,” in The Cerrados of Brazil: Ecology and Natural History of a Neotropical savanna. Editors P. S. Oliveira, and R. J. Marquis (Columbia University Press), 121–139.
Fletcher, M.-S., Hall, T., and Alexandra, A. N. (2020). The Loss of an Indigenous Constructed Landscape Following British Invasion of Australia: An Insight into the Deep Human Imprint on the Australian Landscape. Ambio 50, 138–149. doi:10.1007/s13280-020-01339-3
Flores, B. M., Sá Dechoum, M., Schmidt, I. B., Hirota, M., Abrahão, A., Verona, L., et al. (2021). Tropical Riparian Forests in Danger from Large savanna Wildfires. J. Appl. Ecol. 58, 419–430. doi:10.1111/1365-2664.13794
Gassón, R. A. (2002). Orinoquia: The Archaeology of the Orinoco. J. World Prehistory 16, 237–311. doi:10.1023/a:1020978518142
Giles, A. L., Flores, B. M., Rezende, A. A., Weiser, V. d. L., and Cavassan, O. (2021). Thirty Years of clear-cutting Maintain Diversity and Functional Composition of Woody-Encroached Neotropical Savannas. For. Ecol. Manag. 494, 119356. doi:10.1016/j.foreco.2021.119356
Hammond, N. (1980). “Prehistoric Human Utilization of the savanna Enviroments of Middle and South America,” in Human Ecology in savanna Enviroments. Editor D. R. Harris (London: Academic Press), 73–105.
Harris, D. R., and Hillman, G. C. (2014). Foraging and Farming - the Evolution of Plant Exploitation. Editors D. R. . Harris, and G. C. Hillman (London: Routledge).
Hecht, S. B. (2017). “Domestication, Domesticated Landscapes, and Tropical Nature,” in The Routledge Companion to the Environmental Humanities. Editors U. K. Heise, J. Christensen, and M. Niemann (Abingdon: The Routledge), 23–36.
Hernández-Hernández, R. M., Morros, M. E., Medina, C. A. B., Pérez, Z. L., Díaz, P. E. H., Hernández, A. O., et al. (2011). The Integration of Local and Scientific Knowledge in the Sustainable Management of Soils in savanna Agroecosystems. Interciencia 36, 104–112.
Hill, R., and Baird, A. (2003). Kuku-Yalanji Rainforest Aboriginal People and Carbohydrate Resource Management in the Wet Tropics of Queensland, Australia. Hum. Ecol. 31, 27–52. doi:10.1023/A:1022830123349
Hirota, M., Holmgren, M., Van Nes, E. H., and Scheffer, M. (2011). Global Resilience of Tropical forest and savanna to Critical Transitions. Science 334, 232–235. doi:10.1126/science.1210657
Hoffmann, W. A., Geiger, E. L., Gotsch, S. G., Rossatto, D. R., Silva, L. C. R., Lau, O. L., et al. (2012). Ecological Thresholds at the savanna-forest Boundary: How Plant Traits, Resources and Fire Govern the Distribution of Tropical Biomes. Ecol. Lett. 15, 759–768. doi:10.1111/j.1461-0248.2012.01789.x
Huber, O. (1987). Neotropical Savannas: Their flora and Vegetation. Trends Ecol. Evol. 2, 67–71. doi:10.1016/0169-5347(87)90151-0
Huebert, J. M., and Allen, M. S. (2020). Anthropogenic Forests, Arboriculture, and Niche Construction in the Marquesas Islands (Polynesia). J. Anthropological Archaeology 57, 101122. doi:10.1016/j.jaa.2019.101122
Huffman, M. R. (2013). The many Elements of Traditional Fire Knowledge: Synthesis, Classification, and Aids to Cross-Cultural Problem Solving in Fire-dependent Systems Around the World. E&S 18, 3. doi:10.5751/ES-05843-180403
IBGE (2012). Manual técnico da vegetação brasileira. second. , ed. I. B. de G, and e. E. IBGE Rio de Janeiro.
ILO (1989). Indigenous and Tribal Peoples Convention. The Foundations Mod. Int. L. Indigenous Tribal Peoples 1989 (No. 169), 12. doi:10.1163/9789004289062_010
Iriarte, J., Glaser, B., Watling, J., Wainwright, A., Birk, J. J., Renard, D., et al. (2010). Late Holocene Neotropical Agricultural Landscapes: Phytolith and Stable Carbon Isotope Analysis of Raised fields from French Guianan Coastal Savannahs. J. Archaeological Sci. 37, 2984–2994. doi:10.1016/j.jas.2010.06.016
Iriarte, J., Power, M. J., Rostain, S., Mayle, F. E., Jones, H., Watling, J., et al. (2012). Fire-free Land Use in Pre-1492 Amazonian Savannas. Proc. Natl. Acad. Sci. 109, 6473–6478. doi:10.1073/pnas.1201461109
ISA (2005). Povos Indigenas No Brasil: Quem São? Available at: https://pib.socioambiental.org/pt/Quem_são Acessed in: 06/09/2021.
Kauffman, J. B., Cummings, D. L., and Ward, D. E. (1994). Relationships of Fire, Biomass and Nutrient Dynamics along a Vegetation Gradient in the Brazilian Cerrado. J. Ecol. 82, 519–531. doi:10.2307/2261261
Keeley, J. E., and Rundel, P. W. (2005). Fire and the Miocene Expansion of C4 Grasslands. Ecol. Lett. 8, 683–690. doi:10.1111/j.1461-0248.2005.00767.x
Kingsbury, N. D. (2003). Same Forest, Different Countries. J. Sust. For. 17, 171–188. doi:10.1300/J091v17n01_10
Klink, C. a., and Machado, R. B. (2005). A Conservação Do Cerrado Brasileiro. Megadiversidade 1, 147–155. doi:10.1590/S0100-69912009000400001
Kottek, M., Grieser, J., Beck, C., Rudolf, B., and Rubel, F. (2006). World Map of the Köppen-Geiger Climate Classification Updated. metz 15, 259–263. doi:10.1127/0941-2948/2006/0130
Laland, K., Matthews, B., and Feldman, M. W. (2016). An Introduction to Niche Construction Theory. Evol. Ecol. 30, 191–202. doi:10.1007/s10682-016-9821-z
Laland, K. N., and O’Brien, M. J. (2010). Niche Construction Theory and Archaeology. J. Archaeol. Method Theor. 17, 303–322. doi:10.1007/s10816-010-9096-6
Leal, A., Gassón, R., Behling, H., and Sánchez, F. (2019). Human-made Fires and forest Clearance as Evidence for Late Holocene Landscape Domestication in the Orinoco Llanos (Venezuela). Veget Hist. Archaeobot 28, 545–557. doi:10.1007/s00334-019-00713-w
Levis, C., Flores, B. M., Moreira, P. A., Luize, B. G., Alves, R. P., Franco-Moraes, J., et al. (2018). How People Domesticated Amazonian Forests. Front. Ecol. Evol. 5, 1–21. doi:10.3389/fevo.2017.00171
Lombardo, U., Denier, S., May, J.-H., Rodrigues, L., and Veit, H. (2013). Human-environment Interactions in pre-Columbian Amazonia: The Case of the Llanos de Moxos, Bolivia. Quat. Int. 312, 109–119. doi:10.1016/j.quaint.2013.01.007
Lombardo, U., Iriarte, J., Hilbert, L., Ruiz-Pérez, J., Capriles, J. M., and Veit, H. (2020). Early Holocene Crop Cultivation and Landscape Modification in Amazonia. Nature 581, 190–193. doi:10.1038/s41586-020-2162-7
Lopes, P. S. N., Souza, J. C. d., de, Reis, P. R., Reis, J. M., and Rocha, I. D. F. (2003). Caracterização Do ataque da broca dos frutos Do pequizeiro. Rev. Bras. Frutic. 25, 540–543. doi:10.1590/s0100-29452003000300046
Lúcio, S. L. B., Pereira, L. E. C., and Ludewigs, T. (2014). O Gado que circulava : Desafios da gestão participativa e impactos da proibição Do uso Do fogo aos criadores de gado de solta da Reserva de Desenvolvimento Sustentável Veredas Do Acari. Biodiversidade Bras 4, 130–155.
Matthews, B., De Meester, L., Jones, C. G., Ibelings, B. W., Bouma, T. J., Nuutinen, V., et al. (2014). Under Niche Construction: An Operational Bridge between Ecology, Evolution, and Ecosystem Science. Ecol. Monogr. 84, 245–263. doi:10.1890/13-0953.1
Mayle, F. E., Beerling, D. J., Gosling, W. D., and Bush, M. B. (2004). Responses of Amazonian Ecosystems to Climatic and Atmospheric Carbon Dioxide Changes since the Last Glacial Maximum. Phil. Trans. R. Soc. Lond. B 359, 499–514. doi:10.1098/rstb.2003.1434
Meddens, A. J. H., Kolden, C. A., Lutz, J. A., Smith, A. M. S., Cansler, C. A., Abatzoglou, J. T., et al. (2018). Fire Refugia: What Are They, and Why Do They Matter for Global Change? Bioscience 68, 944–954. doi:10.1093/biosci/biy103
Meggers, B. J. (2001). The Continuing Quest for El Dorado: Round Two. Latin Am. Antiq. 12, 304–325. doi:10.2307/971635
Melo, M. M. De., and Saito, C. H. (2011). Regime de queima das caçadas com uso Do fogo realizadas pelos Xavante no Cerrado. Biodiversidade Bras 1, 97–109.
Meyer, R. S., Duval, A. E., and Jensen, H. R. (2012). Patterns and Processes in Crop Domestication: An Historical Review and Quantitative Analysis of 203 Global Food Crops. New Phytol. 196, 29–48. doi:10.1111/j.1469-8137.2012.04253.x
Miller, T. L. (2016). Living Lists: How the Indigenous Canela Come to Know Plants through Ethnobotanical Classification. J. Ethnobiol. 36, 105–124. doi:10.2993/0278-0771-36.1.105
Miranda, H. S. (2010). “Efeitos Do regime Do fogo sobre a estrutura de comunidades de cerrado: Resultados Do Projeto Fogo,” in DF: Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. Editor H. S. Miranda Brasília.
Mistry, J., Berardi, A., Andrade, V., Krahô, T., Krahô, P., and Leonardos, O. (2005). Indigenous Fire Management in the Cerrado of Brazil: The Case of the Krahô of Tocantíns. Hum. Ecol. 33, 365–386. doi:10.1007/s10745-005-4143-8
Mistry, J., Schmidt, I. B., Eloy, L., and Bilbao, B. (2018). New Perspectives in Fire Management in South American Savannas: The Importance of Intercultural Governance. Ambio 48, 172–179. doi:10.1007/s13280-018-1054-7
Monteiro, F. T. (2011). Os (as) apanhadores(as) de flores e o parque nacional das sempre-vivas (MG): Travessias e contradições ambientais, 241.
Monteiro, F. T., Pereira, D. B., and Del Gaudio, R. S. (2012). Os(as) apanhadores(as) de flores e o Parque Nacional das Sempre-Vivas: entre ideologias e territorialidades. Soc. Nat. 24, 419–433. doi:10.1590/s1982-45132012000300004
Morey, N. C. (1976). Ethnohistorical Evidence for Cultural Complexity in the Western Llanos of Venezuela and the Eastern Llanos of Colombia. Antropologica 45, 41–69.
Moura, L. C., Scariot, A. O., Schmidt, I. B., Beatty, R., and Russell-Smith, J. (2019). The Legacy of Colonial Fire Management Policies on Traditional Livelihoods and Ecological Sustainability in Savannas: Impacts, Consequences, New Directions. J. Environ. Manage. 232, 600–606. doi:10.1016/j.jenvman.2018.11.057
O'Dea, R. E., Lagisz, M., Jennions, M. D., Koricheva, J., Noble, D. W. A., Parker, T. H., et al. (2021). Preferred Reporting Items for Systematic Reviews and Meta‐analyses in Ecology and Evolutionary Biology: a PRISMA Extension. Biol. Rev. 96, 1695–1722. doi:10.1111/brv.12721
Odling-Smee, J., Erwin, D. H., Palkovacs, E. P., Feldman, M. W., and Laland, K. N. (2013). Niche Construction Theory: A Practical Guide for Ecologists. Q. Rev. Biol. 88, 3–28. doi:10.1086/669266
Oliveira-Filho, A. T. (2009). Classificação das fitofisionomias da América Do Sul cisandina tropical e subtropical: proposta de um novo sistema - prático e flexível - ou uma injeção a mais de caos? Rodriguésia 60, 237–258. doi:10.1590/2175-7860200960201
Oliveira-Filho, A. T., and Ratter, J. A. (2002). “Vegetation Physiognomies and Woody flora of the Cerrado Biome,” in The Cerrados of Brazil: Ecology and Natural History of a Neotropical savanna. Editors P. S. Oliveira, and R. J. Marquis (Columbia University Press New), 91–120.
Olorunfemi, F., Fasona, M., Oloukoi, G., Elias, P., and Adedayo, V. (2016). Traditional Knowledge in the Use and Management of forest Ecosystem for Livelihoods and Food Security in Nigerian Savanna. J. Hum. Ecol. 53, 167–175. doi:10.1080/09709274.2016.11906969
Olson, D. M., Dinerstein, E., Wikramanayake, E. D., Burgess, N. D., Powell, G. V. N., Underwood, E. C., et al. (2001). Terrestrial Ecoregions of the World: A New Map of Life on Earth. Bioscience 51, 933–938. doi:10.1641/0006-3568(2001)051[0933:teotwa]2.0.co;2
ONF (2017). Occupation du sol en 2015 sur la bande littorale de la Guyane et son évolution entre 2005 et 2015, 92. Paris: Ministère de L'agriculture et de l'alimentation.
Orians, G. H. (1980). “Habitat Selection: General Theory and Applications to Human Behavior,” in The Evolution of Human Social Behavior. Editor J. S. Lockard (Elsevier/North Holland), 49–66.
Pinheiro, E. D. S., and Durigan, G. (2009). Dinâmica espaço-temporal (1962-2006) das fitofisionomias em unidade de conservação Do Cerrado no sudeste Do Brasil. Rev. Bras. Bot. 32, 441–454. doi:10.1590/s0100-84042009000300005
Pinho, R. C., Alfaia, S. S., Miller, R. P., Uguen, K., Magalhães, L. D., Ayres, M., et al. (2011). Islands of Fertility: Soil Improvement Under Indigenous Homegardens in the Savannas of Roraima, Brazil. Agroforest Syst. 81, 235–247. doi:10.1007/s10457-010-9336-5
Pivello, V. R., Vieira, I., Christianini, A. V., Ribeiro, D. B., da Silva Menezes, L., Berlinck, C. N., et al. (2021). Understanding Brazil's Catastrophic Fires: Causes, Consequences and Policy Needed to Prevent Future Tragedies. Perspect. Ecol. Conservation 19, 233–255. doi:10.1016/j.pecon.2021.06.005
Ploeg, J. (2009). “Sete teses sobre a agricultura camponesa,” in Agricultura Familiar Camponesa Na Construção Do Futuro. Editor P. Petersen (Rio de Janeiro: AS-PTA), 17–32.
Posey, D. A. (1985). Indigenous Management of Tropical forest Ecosystems: the Case of the Kayapó Indians of the Brazilian Amazon. Agroforest Syst. 3, 139–158. doi:10.1007/BF00122640
R Team Core (2021). R: A Language and Environment for Statistical Computing. Available at: https://www.r-project.org/.
Ramos-Neto, M. B., and Pivello, V. R. (2000). Lightning Fires in a Brazilian savanna national park: Rethinking Management Strategies. Environ. Manage. 26, 675–684. doi:10.1007/s002670010124
Reatto, A., Correia, J. R., Spera, S. T., and Martins, É. de. S. (2008). “Solos Do Bioma Cerrado: Aspectos Pedólogicos,” in Cerrado: Ecologia e flora. Editor S. M. Sano (Brasília, DF: Embrapa Informação Tecnológica), 107–150.
Ribeiro, J. F., and Walter, B. M. T. (2008). “As Principais Fitofisionomias Do Bioma Cerrado,” in ” in Cerrado: Ecologia e flora. Editors S. M. Sano, S. P. de Almeida, and J. F. Ribeiro, 152–212.
Ribeiro, J. F., and Walter, B. M. T. (1998). “Fitofisionomias Do Bioma Cerrado,” in Cerrado: Ambiente e flora. Editors S. M. Sano, and S. P. de Almeida (Brasília, DF: Embrapa CPAC), 89–166.
Rindos, D. (2013). The Origins of Agriculture: An Evolutionary Perspective. San Diego: Academic Press.
Roberts, P., Buhrich, A., Caetano-Andrade, V., Cosgrove, R., Fairbairn, A., Florin, S. A., et al. (2021). Reimagining the Relationship between Gondwanan Forests and Aboriginal Land Management in Australia's "Wet Tropics". iScience 24, 102190. doi:10.1016/j.isci.2021.102190
Roberts, P. (2019). Tropical Forests in Prehistory, History, and Modernity. Oxford, UK: Oxford University Press.
Rodrigues, L., Sprafke, T., Bokatola Moyikola, C., Barthès, B. G., Bertrand, I., Comptour, M., et al. (2020). A Congo Basin Ethnographic Analogue of Pre-columbian Amazonian Raised fields Shows the Ephemeral Legacy of Organic Matter Management. Sci. Rep. 10, 10851. doi:10.1038/s41598-020-67467-8
Rodríguez, I. (2007). Pemon Perspectives of Fire Management in Canaima National Park, Southeastern Venezuela. Hum. Ecol. 35, 331–343. doi:10.1007/s10745-006-9064-7
Rosa, R. dos. S., Almeida, S. A. de., Araujo, A. C. de., and Moura, A. A. (2018). A agricultura tradicional na comunidade Kalunga Vão de Almas: Um estudo de caso. Facit Bus. Technol. J. 5, 121–141.
Rostain, S. (2008). “Agricultural Earthworks on the French Guiana Coast,” in “Agricultural Earthworks on the French Guiana Coast,” in Handbook Of South American Archaeology. Editors H. Silverman, and W. Isbell (New York, NY: Springer), 217–233. doi:10.1007/978-0-387-74907-5_13
Rostain, S. (2010). Pre-Columbian Earthworks in Coastal Amazonia. Diversity 2, 331–352. doi:10.3390/d2030331
Rull, V., and Montoya, E. (2014). Mauritia Flexuosa palm Swamp Communities: Natural or Human-Made? A Palynological Study of the Gran Sabana Region (Northern South America) within a Neotropical Context. Quat. Sci. Rev. 99, 17–33. doi:10.1016/j.quascirev.2014.06.007
Russell-Smith, J., Lucas, D., Gapindi, M., Gunbunuka, B., Kapirigi, N., Namingum, G., et al. (1997). Aboriginal Resource Utilization and Fire Management Practice in Western Arnhem Land, Monsoonal Northern Australia: Notes for Prehistory, Lessons for the Future. Hum. Ecol. 25, 159–195. doi:10.1023/A:1021970021670
IPCC (2021). in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Editors B. Z. Masson-Delmotte, C. Péan, S. Berger, P. Zhai, A. Pirani, S. L. Connorset al. (Cambridge University Press). In Press. doi:10.1080/03736245.2010.480842
Sampaio, M. B., Schmidt, I. B., and Figueiredo, I. B. (2008). Harvesting Effects and Population Ecology of the Buriti Palm (Mauritia Flexuosa L. f., Arecaceae) in the Jalapão Region, Central Brazil1. Econ. Bot. 62, 171–181. doi:10.1007/s12231-008-9017-8
Sampaio, M. B., Ticktin, T., Seixas, C. S., and dos Santos, F. A. M. (2012). Effects of Socioeconomic Conditions on Multiple Uses of Swamp Forests in central Brazil. Hum. Ecol. 40, 821–831. doi:10.1007/s10745-012-9519-y
Santos, G. L. d., Pereira, M. G., Delgado, R. C., and Torres, J. L. R. (2017). Natural Regeneration in Anthropogenic Environments Due to Agricultural Use in the Cerrado, Uberaba, MG, Brazil. Biosci. J. 33, 169–176. doi:10.14393/BJ-v33n1a2017-35036
Sarfo-Mensah, P., and Oduro, W. (20072007). Traditional Natural Resources Management Practices and Biodiversity Conservation in Ghana: A Review of Local Concepts and Issues on Change and Sustainability. SSRN J. 90, 1–19. doi:10.2139/ssrn.1017238
Schmidt, I. B., and Eloy, L. (2020). Fire Regime in the Brazilian Savanna: Recent Changes, Policy and Management. Flora 268, 151613. doi:10.1016/j.flora.2020.151613
Schmidt, I. B., Moura, L. C., Ferreira, M. C., Eloy, L., Sampaio, A. B., Dias, P. A., et al. (2018). Fire Management in the Brazilian Savanna: First Steps and the Way Forward. J. Appl. Ecol. 55, 2094–2101. doi:10.1111/1365-2664.13118
Schmidt, I. B., Sampaio, M. B., Figueiredo, I. B., and Ticktin, T. (2011). Fogo e artesanato de Capim-dourado no Jalapão – Usos tradicionais e consequências ecológicas. Biodiversidade Bras 1, 67–85.
Schmidt, I. B., and Ticktin, T. (2012). When Lessons from Population Models and Local Ecological Knowledge Coincide - Effects of Flower Stalk Harvesting in the Brazilian savanna. Biol. Conservation 152, 187–195. doi:10.1016/j.biocon.2012.03.018
Scholes, R. J., and Archer, S. R. (1997). Tree-grass Interactions in Savannas. Annu. Rev. Ecol. Syst. 28, 517–544. doi:10.1146/annurev.ecolsys.28.1.517
Silva, L. C. R., Hoffmann, W. A., Rossatto, D. R., Haridasan, M., Franco, A. C., and Horwath, W. R. (2013). Can Savannas Become Forests? A Coupled Analysis of Nutrient Stocks and Fire Thresholds in central Brazil. Plant Soil 373, 829–842. doi:10.1007/s11104-013-1822-x
Silva, T. C. d., Campos, L. Z. d. O., Balée, W., Medeiros, M. F. T., Peroni, N., and Albuquerque, U. P. (2017). Human Impact on the Abundance of Useful Species in a Protected Area of the Brazilian Cerrado by People Perception and Biological Data. Landscape Res. 44, 75–87. doi:10.1080/01426397.2017.1396304
Sletto, B., and Rodriguez, I. (2013). Burning, Fire Prevention and Landscape Productions Among the Pemon, Gran Sabana, Venezuela: Toward an Intercultural Approach to Wildland Fire Management in Neotropical Savannas. J. Environ. Manage. 115, 155–166. doi:10.1016/j.jenvman.2012.10.041
Smith, B. D. (2012). A Cultural Niche Construction Theory of Initial Domestication. Biol. Theor. 6, 260–271. doi:10.1007/s13752-012-0028-4
Smith, B. D. (2011). General Patterns of Niche Construction and the Management of 'wild' Plant and Animal Resources by Small-Scale Pre-industrial Societies. Phil. Trans. R. Soc. B 366, 836–848. doi:10.1098/rstb.2010.0253
Smith, M., and Fausto, C. (2016). Socialidade e diversidade de pequis (Caryocar brasiliense, Caryocaraceae) entre os Kuikuro Do alto rio Xingu (Brasil). Bol. Mus. Para. Emílio Goeldi. Ciênc. Hum. 11, 87–113. doi:10.1590/1981.81222016000100006
Sousa Júnior, J. R., Albuquerque, U. P., and Peroni, N. (2013). Traditional Knowledge and Management of Caryocar Coriaceum Wittm. (Pequi) in the Brazilian Savanna, Northeastern Brazil1. Econ. Bot. 67, 225–233. doi:10.1007/s12231-013-9241-8
Sousa Júnior, J. R., Collevatti, R. G., Lins Neto, E. M. F., Peroni, N., and Albuquerque, U. P. (2018). Traditional Management Affects the Phenotypic Diversity of Fruits with Economic and Cultural Importance in the Brazilian Savanna. Agroforest Syst. 92, 11–21. doi:10.1007/s10457-016-0005-1
Souza, J. M., Schmidt, I. B., and Conceição, A. A. (2018). How Do Fire and Harvesting Affect the Population Dynamics of a Dominant Endemic Velloziaceae Species in campo Rupestre? Flora 238, 225–233. doi:10.1016/j.flora.2017.02.007
Spengler, R. N. (2021). Niche Construction Theory in Archaeology: A Critical Review. J. Archaeol. Method Theor. 28, 925–955. doi:10.1007/s10816-021-09528-4
Staver, A. C., Archibald, S., and Levin, S. A. (2011). The Global Extent and Determinants of savanna and forest as Alternative Biome States. Science 334, 230–232. doi:10.1126/science.1210465
Stier, A., de Carvalho, W. D., Rostain, S., Catzeflis, F., Claessens, O., Dewynter, M., et al. (2020). The Amazonian Savannas of French Guiana: Cultural and Social Importance, Biodiversity, and Conservation Challenges. Trop. Conservation Sci. 13, 194008291990047–21. doi:10.1177/1940082919900471
Terrell, J. E., Hart, J. P., Barut, S., Cellinese, N., Curet, A., Denham, T., et al. (2003). Domesticated Landscapes: The Subsistence Ecology of Plant and Animal Domestication. J. Archaeological Method Theor. 10, 323–368. doi:10.1023/b:jarm.0000005510.54214.57
Torres Romero, M. C., Galeano Garces, G. A., and Bernal, R. (2016). Cosecha y manejo de Copernicia tectorum (Kunth) Mart. Para uso artesanal en el caribe colombiano. Colomb. For. 19, 5. doi:10.14483/udistrital.jour.colomb.for.2016.1.a01
Vialou, D., Benabdelhadi, M., Feathers, J., Fontugne, M., and Vialou, A. V. (2017). Peopling South America's centre: the Late Pleistocene Site of Santa Elina. Antiquity 91, 865–884. doi:10.15184/aqy.2017.101
Walter, B. M. T., Carvalho, A. M. de., and Ribeiro, J. F. (2008). “O conceito de savana e de seu componente Cerrado,” in Cerrado: Ecologia e flora. Editors S. M. Sano, S. P. de Almeida, and J. F. Ribeiro (Brasília, DF: Embrapa Informação Tecnológica), 19–45.
Welch, J. R., Brondízio, E. S., Hetrick, S. S., and Coimbra, C. E. A. (2013). Indigenous Burning as Conservation Practice: Neotropical Savanna Recovery amid Agribusiness Deforestation in Central Brazil. PLoS One 8, e81226. doi:10.1371/journal.pone.0081226
Welch, J. R., and Coimbra Jr., C. E. A. (2021). Indigenous Fire Ecologies, Restoration, and Territorial Sovereignty in the Brazilian Cerrado: The Case of Two Xavante Reserves. Land use policy 104, 104055. doi:10.1016/j.landusepol.2019.104055
Welch, J. R. (2015). Learning to hunt by Tending the Fire: Xavante Youth, Ethnoecology, and Ceremony in central Brazil. J. Ethnobiol. 35, 183–208. doi:10.2993/0278-0771-35.1.183
Keywords: landscape domestication, plant domestication, cultural niche construction, savannas, local management, indigenous management, traditional management, small scale societies
Citation: Ferreira MJ, Levis C, Chaves L, Clement CR and Soldati GT (2022) Indigenous and Traditional Management Creates and Maintains the Diversity of Ecosystems of South American Tropical Savannas. Front. Environ. Sci. 10:809404. doi: 10.3389/fenvs.2022.809404
Received: 05 November 2021; Accepted: 05 January 2022;
Published: 26 January 2022.
Edited by:
Ulysses Paulino Albuquerque, Federal University of Pernambuco, BrazilReviewed by:
Henrique Fernandes Magalhães, Federal University of Pernambuco, BrazilAlejandro Casas, Universidad Nacional Autónoma de México, Mexico
Ernani Lins Neto, Universidade Federal do Vale do São Francisco, Brazil
Copyright © 2022 Ferreira, Levis, Chaves, Clement and Soldati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Julia Ferreira, ZmVycmVpcmEuanVsaWEyMjA4QGdtYWlsLmNvbQ==
 Maria Julia Ferreira
Maria Julia Ferreira Carolina Levis
Carolina Levis Leonardo Chaves
Leonardo Chaves Charles Roland Clement
Charles Roland Clement Gustavo Taboada Soldati
Gustavo Taboada Soldati