- 1Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, China
- 2College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing, China
- 3Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters, Nanjing University of Information Science and Technology, Nanjing, China
- 4Department of Life Science, Faculty of Science and Engineering, Kindai University, Osaka, Japan
- 5LAGEO, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, China
- 6Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, China
- 7Earth System Physics, The Abdus Salam International Centre for Theoretical Physics, Trieste, Italy
- 8Department of Environmental Sciences, International Islamic University, Islamabad, Pakistan
Dust events moving at high altitudes by westerly wind can transport aerosols from Asian deserts to eastern Asia deposition areas such as China. Aerosols do not include only mineral particles but also microbial particles, which are called bioaerosols, and impact the ecosystem and air environment of the deposition area. For identifying the airborne microbial communities transported from the source area to the deposition area, bioaerosol samples were collected in the typical source region (Tsogt-Ovoo in Gobi deserts) and the deposition region (Beijing in Chinese industrial area) during dust events and non-dust days and the sampling sites were compared. The microscopic observation using DAPI fluorescent techniques revealed that the concentration of bioaerosols increased during the dust events in both the source and deposition regions. For the community structures of airborne bacteria at both sites, the dust-event occurrences changed the structure of the bacterial community and increased the diversity of bacterial communities during dust events. Some specific bacterial populations, such as members of Bacteroidetes, dominated during dust events. There is the possibility that specific bacteria can be maintained for a longer time in the atmosphere and might be transported from the source area to the deposition area.
Introduction
Bioaerosol means biological particles suspended in the atmosphere, and they are an important part of aerosols (Després et al., 2012). Bioaerosols include viruses, bacteria, fungi, spores, and pollen. Ariya and Amyot (2003), Iwasaka et al. (2009), and Smets et al. (2016) have reported on the negative influence of bioaerosols on humans (Wu et al., 2020) and animal health (Onishi et al., 2012; Ma et al., 2017), which can easily cause various diseases such as asthma (Walser et al., 2015; Fröhlich-Nowoisky et al., 2016; Li et al., 2018). Bioaerosols are distributed all over the atmosphere and stay in the atmosphere for a long time (Burrows et al., 2009). The small bacterial particles can suspend for a long time in the atmosphere and can sometimes reach the upper troposphere (DeLeon-Rodriguez et al., 2013) withstanding desiccation, ultraviolet (UV) radiation, extreme temperature, and oxygen limitation (Rothschild and Mancinelli, 2001), so the sandstorms can carry bioaerosols across thousands of kilometers to the deposition area (Okamoto et al., 2004). These microorganisms (i.e., bioaerosols) may impact the reflection and absorption of sunlight, and affect the formation of cloud condensation nuclei (CCN) and ice nuclear (IN) (Peter et al., 2011; Morris et al., 2011; Šantl-Temkiv et al., 2020). Du et al. (2018) found the seasonal variations of bioaerosol, which were identified by the high-throughput DNA sequencing, showing obvious seasonal variation in PM2.5 days. The abundance and diversity of bioaerosol communities are reported to be related to haze episodes (Xu et al., 2017). Kobayashi et al. (2015) found that bioaerosols are metabolically active and well adapted to harsh atmospheric stress conditions. Asian dust events transport the bioaerosols including bacteria as well as mineral particles from Asian desert regions and influence the airborne bacterial communities in downwind areas (Maki et al., 2017b). The dispersion of bioaerosols by dust events from desert areas may be related to the ecosystems of subsidence areas (Pointing and Belnap, 2014). Some studies have confirmed that bioaerosols play an important role in meteorological processes and chemical mechanisms of dust deposition areas (Pratt et al., 2009; Creamean et al., 2013). The major source areas of Asian dust events are known to be the Gobi Desert, Taklimakan desert, and Loess Plateau (Duce et al., 1980; Iwasaka et al., 1983; Kurosaki and Mikami, 2015). In particular, the Gobi Desert located in Mongolia is one of the major sand sources in the Asian continent. The release and transport of dust in this region could have a significant impact on the quality of air and the global geochemical cycle (Chen et al., 2017).
Several researchers have focused on the microbial community structures and diversities of bioaerosols influenced by Asian dust. Maki et al. (2015) found that the compositions of bacterial communities sampled in the air displayed differences among the three altitudes of 3000, 1,000, and 10 m. over the Noto Peninsula during a dust event period. The fungal and bacterial community structures in bioaerosols collected in autumn and winter on the Korean Peninsula had high biodiversity, with seasonal changes causing changes in diversity (Lee and Jo, 2006).
The tethered balloon sampling in Dunhuang’s study of bioaerosols indicated that the local-scale distribution of bioaerosols is caused by the air convective mixing and regional scale diffusion (Chen et al., 2011). Tang et al. (2018) studied Asian dust in 2016 and compared the diversity of airborne bacterial communities between dust samples and no-dust samples, showing that the bacterial relative abundances differ from those in the surface, sand, or soil. In general, only a few studies have focused on the changes of the bioaerosols traveling from the source area in different altitudes (Maki et al., 2017a; Maki et al., 2019), but the characteristics of bioaerosols in the deposition area, especially in large cities, have not yet been compared to those in the dust source regions.
This research focuses on the long-range transport of bioaerosols from the dust source region, Tsogt-Ovoo, to the dust deposition region, Beijing. To compare the composition and structural characteristics of bioaerosols between both sampling sites, the bioaerosol characteristics were analyzed using microscopic observation and high-throughput DNA sequencing. This study explores the influence of the horizontal transport of dust on bioaerosols and their impact on health and the deposition environment.
Materials and Methods
Sampling Site and Aerosol’s Collection
Aerosol samplings were performed at Tsogt-Ovoo, which is located in the middle of the Gobi Desert (44.2304°N and 105.17°E) in Mongolia. As a typical source area, the sampler was placed in the desert, about 5 km away from the downtown and about 2 m above the ground. The air samples were collected from 7 to 11 March 2015 and from 26 to 27 April 2015 and were named as 15To-1 to 15To-9 (Table 1). Other samples were collected on the office roof of the IAP (Institute of Atmospheric Physics, Chinese Academy of Science) building (39.98° N and 116.38° E) in Chaoyang District of Beijing, where it has typical urban underlying surface features (Wu et al., 2019) and is a typical dust deposition area. The air samples were collected on March 30, March 31, April 1, April 2, April 15, and April 16, which were named as 15Bj-1 to 15Bj-6, respectively (Table 1). The sampling site is about 42 m above the ground.
Each air sample was collected by applying four sterilized polycarbonate filters (0.22 μm pore size, Whatman, Japan) with an air sampling system. The sampling system used the 13-mm Swinnex filter holder sampler (Merck, Germany) connected to an air pump (AS ONE, MAS-1, Japan). Each filter was sampled with a flow rate of approximately 0.3L min−1, the sampling time is about 10–18 h based on the air quality conditions, and the filters were changed after each sampling period. Detailed information on the samples is provided in Table 1.
After the sampling, two of them were used to determine the concentration of bioaerosols using a fluorescence microscope after DAPI staining, and the other two samples were stored in a sterile environment at −80°C, before the use for DNA high-throughput sequencing analysis.
Aerosol Counts Using the Fluorescent Microscopic Observation
The number of concentrations of microbial particles was determined using the direct counting method by the DAPI fluorescence staining. Aerosol particles were fixed with a 4% concentration of paraformaldehyde for 1 hour and washed with pure water; then samples were stained with 0.5
DNA High-Throughput Sequencing Analysis
The other two filters were used for DNA high-throughput sequencing analysis. After the aerosol was suspended on the filters in 500 µL of sterile 0.6% NaCl solution, the particles were pelleted by centrifugation at 15,000 × g for 10 min. The gDNA was then extracted using SDS (10% w/w), proteinase K, and lysozyme at 50 ̊C for 30 min and purified by phenol–chloroform extraction (c.f., Maki et al., 2008). Before sequencing, PCR was used to amplify the fragments of 16 S rDNA which was extracted from gDNA by the universal primers 515F (5′-TGTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) (c.f., Caporaso et al., 2011). The two-step PCR method was performed according to the method of Maki et al. (2017a), and detailed steps were described by Maki et al. (2017a). The gene sequence of bacteria was performed using a Illumina MiSeq Genome Sequencer (Illumina, USA), which is widely used in multiplexed sequencing of 16S rDNA (c.f., Maki et al., 2017b). Finally, an average read length of 250 bp was obtained.
Before the analysis of microbial community structures, the bioinformatics of sequences was performed with QIIME 2-2020.2 (c.f., Bolyen et al., 2019). Raw sequence data were demultiplexed and quality-filtered using the q2-demux plugin followed by denoising with DADA2 (c.f., Callahan et al., 2016), and the related species of the isolates were searched using BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST/) and Silva132 database (Quast et al., 2013). The R software package (version 4.0.2) was employed to analyze the experimental data. The alpha-rarefaction and PCoA plots were computed using R software with the Amplicon package (Liu et al., 2021). The Interactive Tree of Life (ITOL) was used to display the phylogenetic trees. As an online tool (https://itol.embl.de), it can annotate trees easily (Letunic and Bork, 2007).
The meteorological parameters of Tsogt-Ovoo were observed during the sampling period by measuring the concentration of PM10 to evaluate the occurrences of dust events using an aerosol mass monitor (DustTrakTM DRX 8533, TSI Inc., Shoreview, MN, USA). Other meteorological observations of Tsogt-Ovoo were monitored using the observation systems described by Ishizuka et al. (2012). The meteorological parameters of Beijing (temperature, relative humidity, wind velocity, and PM2.5) that were obtained from the China Meteorological Data Network (http://data.cma.cn/) are shown in Supplementary Table S1.
Results and Discussion
Meteorological Condition and Backward Trajectory
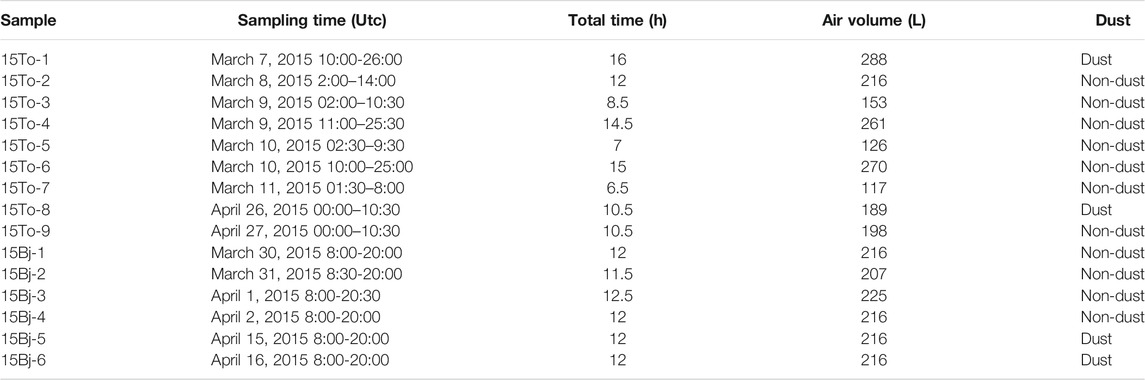
The concentrations of PM10 increased in the 15To-1 and 15To-8 samples, suggesting these samples of Tsogt-Ovoo were collected during dust events. During March and April in Beijing, strong sandstorms occurred. So, the air mass backward trajectories after 48 h in March and April 2015 were conducted using Meteoinfo (Wang, 2014; 2019). Figure 1 shows that there are six back trajectories of air masses in Beijing from March to April 2015 where four of them originated from northwest China. The air masses that came from the Gobi Desert accounted for 25.78% as the largest proportion of all sources during sampling. In conclusion, Beijing is in a downwind area of the Gobi Desert and the characteristic of bioaerosols in Beijing may be influenced by the transported dust.
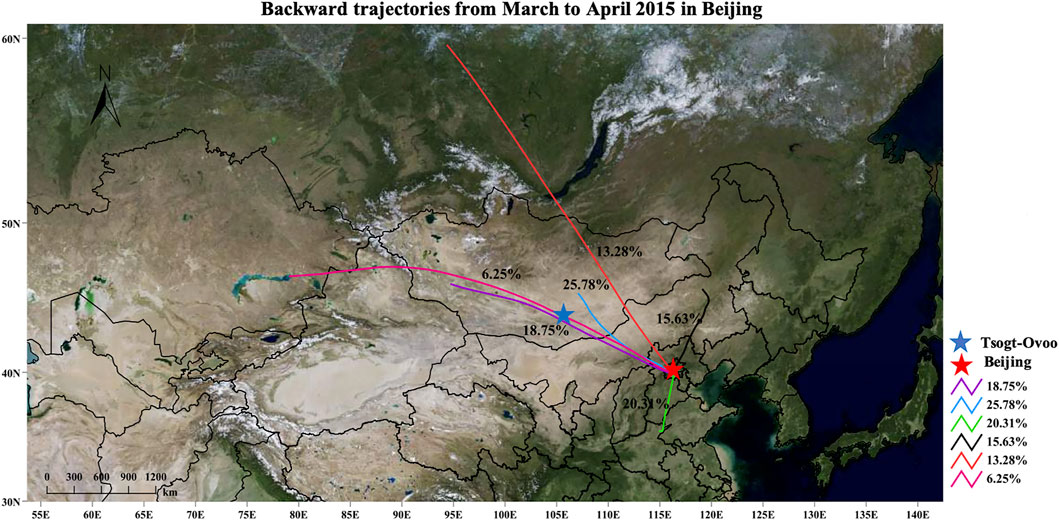
FIGURE 1. Back trajectories of air masses in Beijing were calculated using the MeteoInfo from March 2015 to April 2015.
Variations in the Concentrations of Bioaerosols Sampled in Beijing and Tsogt-Ovoo
During non-dust events in Tsogt-Ovoo, the bacterial and organic matter particles showed low concentrations ranging from 104 to 105 particulars/m3, and they increased to the higher concentrations ranging from 106 to 107 particulars/m3 (Figure 2A). The concentration of 15To-8 was as high as 108 particulars/m3. At the same time, the changes in bacterial and organic particle concentration appeared in similar patterns with dust. During the observation period in Beijing, the particle concentrations of bacteria and organic matter exhibited a high peak at 15Bj-5 (more than 105 particles/m3) and a low peak at 15Bj-3 (104–105 particles/m3) (Figure 2B). The dust events may transport abundant dust and microbes which can increase the concentrations of organic particles, dust, and bacteria. In addition, the concentrations of three particles in the dust source area are at least one order higher than those collected in the deposition area. The observation concentration ranges are consistent with those measured by Fang et al. (2008) in Beijing and observed by Maki et al. (2017b) in Mongolian spring.
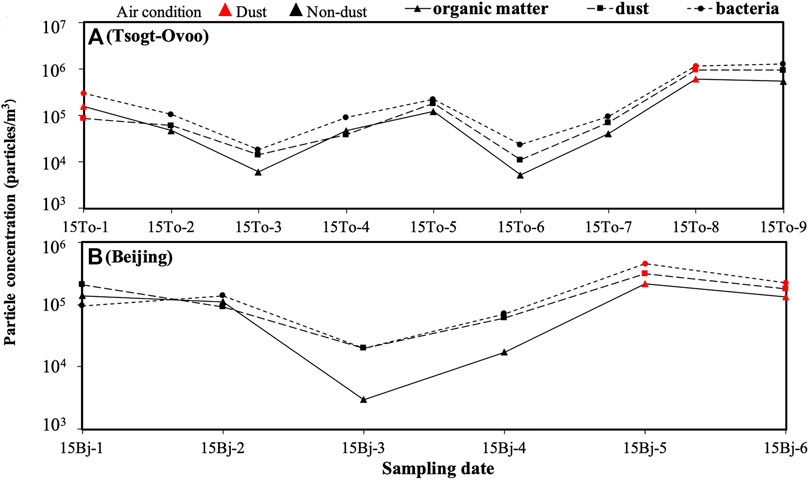
FIGURE 2. Changes in particle concentrations at the sampling site collected during dust events and non-dust events: (A) Tsogt-Ovoo and (B) Beijing.
Analysis of the Microbial Community Structure Characteristics
Diversity Analysis of Sequencing Samples
After DNA sequencing, the chimeric sequences were removed and the effective sequences were obtained, that is, a total of 623,992 and 88,729 respectively. The percentage of the input non-chimeric value is used to represent the sequencing coverage. All of the percentages have passed 50%, which indicated that the processed sequences have good coverage and can be used for subsequent analysis. The amplicon sequence variants (ASVs) of sequences had shown a significant increase following the dust event in two sampling sites, suggesting that the occurrence of dust events increased the abundance and diversity of microorganisms in the atmosphere.
The Shannon–Wiener alpha-rarefaction curves of all samples were classified into four patterns, which indicated that when the sequence reads reached about 500, the curve tends to be flat, indicating that the sequencing amount is sufficient (Figure 3). The microbial information contained in the samples is fully expressed, and the sequencing data can be analyzed for community diversity (Wang et al., 2012). The Shannon index is used to draw the ordinate, reflecting the higher microbial diversity by the higher index. The diversity of the microbial community in the dust samples was higher than that collected in non-dust samples (Figure 3); meanwhile, the microbial diversity of Beijing was lower than that of samples in Tsogt-Ovoo under the same air condition (Figure 3). The result is in accordance with another study (Cha et al., 2016), which illustrates that dust events can enrichen microbial diversity.
FIGURE 3. Alpha-rarefaction curves using the Shannon index showing the bacterial diversity observed in the air samples.
To analyze the community similarity of each sample, principal coordinate analysis (PCoA) with weighted UniFrac distances was performed. The composition of the bacterial community differed significantly between the samples between Beijing and Tsogt-Ovoo (Figure 4). The local bacterial populations are thought to be regulated by the bacterial compositions in the atmosphere of each sampling site. The dust sample of 15Bj-6 showed a high degree of similarity with the dust sample 15To-8 in Tsogt-Ovoo, indicating the possibility that some bacterial populations were transported from Tsogt-Ovoo to Beijing.
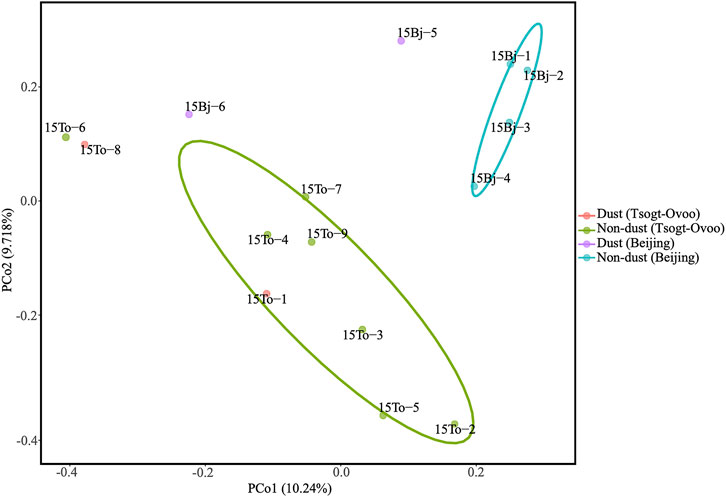
FIGURE 4. Principal coordinate analysis of bacteria by 16 S rRNA sequencing data indicating phylogenetic clustering from 15 samples (PCo: principal coordinate).
Analysis of Microbial Community Composition in Tsogt-Ovoo and Beijing
The microbial community compositions of two sample sites are shown in Figure 5 by the histograms. The majority (>90%) of abundance were represented by 8 bacterial phyla and 14 classes (Figure 5).
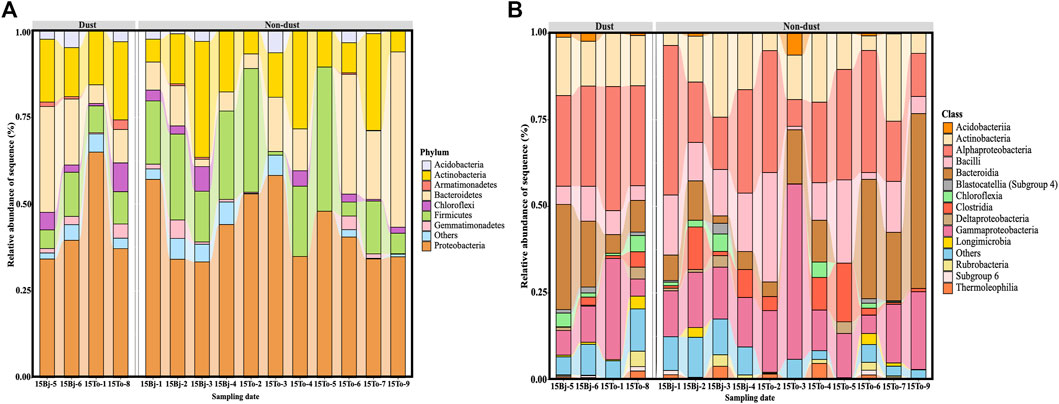
FIGURE 5. Variations of bacterial community compositions at (A) phylum levels (B) and class levels by the alluvial plot.
Comparative analysis revealed that the main phylotypes include members of the phyla Acidobacteria, Actinobacteria, Armatimonadia, Bacteroidetes, Chloroflexi, Firmicutes, Gemmatimonadetes, and especially Proteobacteria in the two sample sites (Figure 5A). The proportion of them had passed 90%, while the phylum Proteobacteria accounts for more than 40%. These members are typically phyla found in the atmospheric and terrestrial environments of dust source areas including the Gobi Desert, Taklamakan Desert, and Sahara Desert (Griffin et al., 2007; Zhang et al., 2008; An et al., 2013), and dust deposition areas like Beijing (Hu et al., 2017; Yan et al., 2017). The phyla of Chloroflexi and Gemmatimonadetes appeared in all sample days but had lower relative abundances except dust samples. The members of the phylum Chloroflexi were often detected from grassland and alpine soils (Costello and Schmidt, 2006). Will et al. (2010) found the members of Gemmatimonadetes were adapted to the arid environment and are found in prairie and pasture soil. Accordingly, these particles would be transported from desert areas by dust. The phylum Bacteroidetes showed higher relative abundances in dust days of 15Bj-5, 15Bj-6, and 15To-9 compared with other samples, and the abundance increased may be impacted by dust events. Maki et al. (2017b) pointed out that Bacteroidetes which dominate in the desert atmosphere can form endospores and attach with coarse particles such as organic particles to sustain in the air for a longer time (Maki et al., 2017b); thus, other studies also found their relative abundance increased during Asia events (Newton et al., 2011; Thomas et al., 2011). It is worth mentioning that the relative abundances of phylum Firmicutes decreased in the dust samples compared with non-dust samples. Tang (et al., 2018) mentioned that due to the existence of environmental stressors, only a small fraction of Firmicutes remain during the dust events.
At the class level, the relative abundances of Actinobacteria, Alpha-proteobacteria, Bacilli, Delta-proteobacteria, and Gamma-proteobacteria account for more than 80%. The relative abundances of Alpha-proteobacteria and Gamma-proteobacteria showed a significant climb compared with other samples (Figure 5B). The phyla of Proteobacteria that mainly belong to the classes of Alpha-proteobacteria and Gamma-proteobacteria have strong adaptability and survivability (Ma et al., 2016) which help them survive in poor conditions such as high-altitude ice clouds and mineral particles (Sheridan et al., 2003; Hara et al., 2012) that are widely distributed (Nicholson et al., 2000; Puspitasari et al., 2015). In addition, the class member of Actinobacteria presented a high abundance collected during the dust events in two sample sites. Cao et al. (2014) indicated that the Actinobacteria group was primarily detected from anthropogenic particles collected in Beijing. The abundances of Bacilli in non-dust samples are higher than that of samples on dust days. The phylum Firmicutes mainly belongs to the classes Bacilli (Maki et al., 2017b) and are often collected from the Chinese desert (Jeon et al., 2011). The result shows a different pattern would be that all samples collected from the air are different from those on the surface of the sand.
The analysis of the differences in community composition between samples is shown in Figure 6. The four dust samples showed a higher similarity and could be clustered as one group, while other non-dust samples are similar. The cluster tree is consistent with the principal coordinate analysis which indicates the occurrence of dust could transport bacterial particles from the dust source area and change the microbial community composition of the dust deposition area.
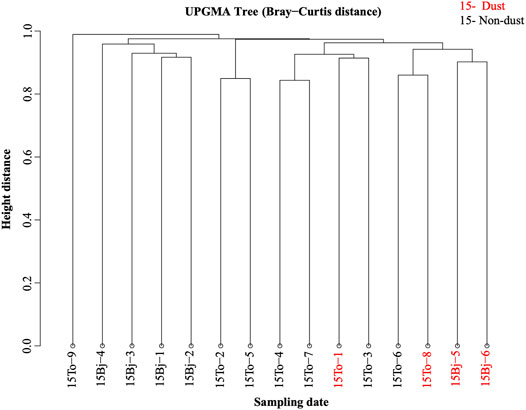
FIGURE 6. UPGMA cluster tree of samples. The degree of dissimilarity was calculated and the dissimilarity value was used to deal with hierarchical clustering analysis.
Comparison of the Phylogenetic Tree Between Dust Source Area and Dust Deposition Area
The phylogenetic tree can represent the genetic relationship among species or genes by using the tree branch graph. The mathematical statistics algorithm was used to calculate the evolutionary relationship, and the result was visualized. On the phylogenetic trees in Tsogt-Ovoo and Beijing, the main phyla and classes of bioaerosols collected from the two sites are similar (Supplementary Figure S1). This result also indicated that the dust can drive the long-distance transport process of bioaerosols, and there are slight differences between the dust source area and the deposition area.
Relationship Between Microbial Community Structure and Environmental Factors
The correlation between the bacterial community structures and environmental factors was compared using the Mantel test (Figure 7). There was no correlation between environmental factors and community structure in Tsogt-Ovoo (Figure 7A). In the samples of Beijing, the Mantel test’s p-value of Actinobacteria and temperature and the value between Proteobacteria and PH were all in the range of 0.01–0.05, and their R-values were between 0.25 and 0.5. The members of Actinobacteria and Proteobacteria change in correspondence to temperature and RH, relatively. Rui et al. (2015) also found that Actinobacteria is sensitive to temperature, and the relative abundance of Actinobacteria increases as temperature increases. The difference in the relationship with environmental factors indicated some differences between the community structures of the two sampling areas.
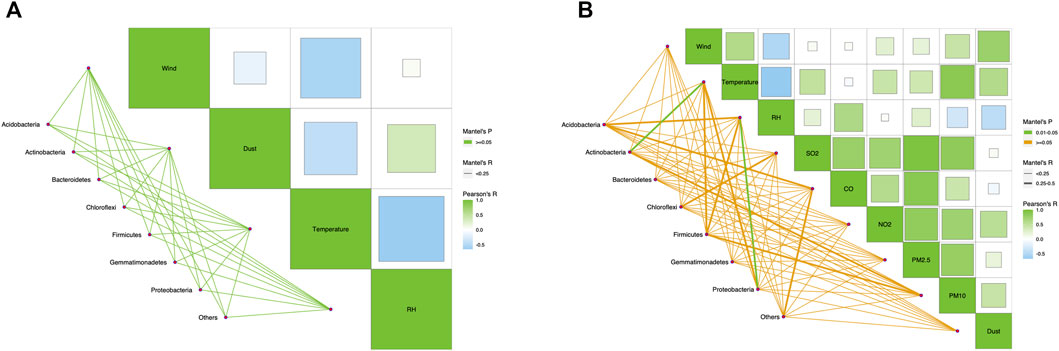
FIGURE 7. The Mantel test between microbial community and environmental factors: (A) Tsogt-Ovoo and (B) Beijing. The relativities were analyzed according to the calculated R-value and significance level p-value based on the R package of ggcor. The color of the left line represents the p-value, and the thickness represents the R-value. On the right part, Pearson correlation analysis was performed for each environmental factor, and the color and size of the squares represent the positive and negative of correlation and the value of correlation, respectively.
Conclusion
In this study, we conducted quantitative investigations of bioaerosols collected at the dust source region, Tsogt-Ovoo, and the dust depositions region, Beijing. Bioaerosol particle sampling was taken 15 times using a self-made biological particle sampler during dust seasons. Under microscopic observation, we found that the concentrations of bacteria and organic matter increased after dust events at both sites, and the particle concentrations in Tsogt-Ovoo were higher than those in Beijing. The rarefaction curves and PCoA plots indicated that there are significant differences between Tsogt-Ovoo and Beijing. The high degree of similarity between some samples in dust events of Beijing and Tsogt-Ovoo suggests that some bacterial populations such as members of Bacteroidetes were transported from the source area in Tsogt-Ovoo to the deposition area in Beijing. These results suggest that the long-range transport of dust in the atmosphere may play a significant role in the dispersal procedure for bioaerosols on local, regional, and even global scales. Our findings serve as a vital reference for the further cooperation of countries to reduce dust impact and improve environmental quality.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
XD: data analyses and manuscript writing. BC: field investigations, collection of microbial data, and suggestion and support for data analyses and manuscript writing. TM: field investigations, collection of microbial data, and suggestion and support for data analyses and manuscript writing. GS: supports for summarizing manuscript writing. MD: supports for summarizing manuscript writing. BK: supports for summarizing manuscript writing.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 42175150, 42030107) and International Partnership Program of the Chinese Academy of Sciences (Grant No. 134111KYSB20180021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.795489/full#supplementary-material
References
An, S., Couteau, C., Luo, F., Neveu, J., and DuBow, M. S. (2013). Bacterial Diversity of Surface Sand Samples from the Gobi and Taklamaken Deserts. Microb. Ecol. 66, 850–860. doi:10.1007/s00248-013-0276-2
Ariya, P. A., and Amyot, M. (2003). New Directions: The Role of Bioaerosols in Atmospheric Chemistry and Physics. Atmos. Environ. 38, 1231–1232. doi:10.1016/j.atmosenv.2003.12.006
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 37, 852–857. doi:10.1038/s41587-019-0209-9
Burrows, S. M., Elbert, W., Lawrence, M. G., and Pöschl, U. (2009). Bacteria in the Global Atmosphere - Part 1: Review and Synthesis of Literature Data for Different Ecosystems. Atmos. Chem. Phys. 9, 9263–9280. doi:10.5194/acp-9-9263-2009
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 13, 581–583. doi:10.1038/nmeth.3869
Cao, C., Jiang, W., Wang, B., Fang, J., Lang, J., Tian, G., et al. (2014). Inhalable Microorganisms in Beijing's PM2.5 and PM10 Pollutants during a Severe Smog Event. Environ. Sci. Technol. 48, 1499–1507. doi:10.1021/es4048472
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., et al. (2011). Global Patterns of 16S rRNA Diversity at a Depth of Millions of Sequences Per Sample. Proc. Natl. Acad. Sci. 108, 4516–4522. doi:10.1073/pnas.1000080107
Cha, S., Lee, D., Jang, J. H., Lim, S., Yang, D., Seo, T., et al. (2016). Alterations in the Airborne Bacterial Community During Asian Dust Events Occurring Between February and March 2015 in South Korea. Sci. Rep. 6, 1–9. doi:10.1038/srep37271
Chen, B., Kobayashi, F., Yamada, M., Kim, Y.-H., Iwasaka, Y., and Shi, G.-Y. (2011). Identification of Culturable Bioaerosols Collected over Dryland in Northwest China: Observation Using a Tethered Balloon. Asian J. Atmos. Environ. 5 (3), 172–180. doi:10.5572/ajae.2011.5.3.172
Chen, S., Huang, J., Li, J., Jia, R., Jiang, N., Kang, L., et al. (2017). Comparison of Dust Emissions, Transport, and Deposition between the Taklimakan Desert and Gobi Desert from 2007 to 2011. Sci. China Earth Sci. 60, 1338–1355. doi:10.1007/s114300169051010.1007/s11430-016-9051-0
Costello, E. K., and Schmidt, S. K. (2006). Microbial Diversity in alpine Tundra Wet Meadow Soil: Novel Chloroflexi from a Cold, Water-Saturated Environment. Environ. Microbiol. 8, 1471–1486. doi:10.1111/j.1462-2920.2006.01041.x
Creamean, J. M., Suski, K. J., Rosenfeld, D., Cazorla, A., DeMott, P. J., Sullivan, R. C., et al. (2013). Dust and Biological Aerosols from the Sahara and Asia Influence Precipitation in the Western U.S. Science 339, 1572–1578. doi:10.1126/science.1227279
Després, V. R., Huffman, J. A., and Burrows, S. M. (2012). Primary Biological Aerosol Particles in the Atmosphere: A Review. Tellus B: Chem. Phys. Meteorol. 64, 1–58.
DeLeon-Rodriguez, N., Lathem, T. L., Rodriguez-R, L. M., Barazesh, J. M., Anderson, B. E., Beyersdorf, A. J., et al. (2013). Microbiome of the Upper Troposphere: Species Composition and Prevalence, Effects of Tropical Storms, and Atmospheric Implications. Proc. Natl. Acad. Sci. 110, 2575–2580. doi:10.1073/pnas.1212089110
Du, P., Du, R., Ren, W., Lu, Z., and Fu, P. (2018). Seasonal Variation Characteristic of Inhalable Microbial Communities in PM2.5 in Beijing City, China. Sci. Total Environ. 610-611, 308–315. doi:10.1016/j.scitotenv.2017.07.097
Duce, R. A., Unni, C. K., Ray, B. J., Prospero, J. M., and Merrill, J. T. (1980). Long-Range Atmospheric Transport of Soil Dust from Asia to the Tropical North Pacific: Temporal Variability. Science 209, 1522–1524. doi:10.1126/science.209.4464.1522
Fang, Z., Ouyang, Z., Zheng, H., and Wang, X. (2008). Concentration and Size Distribution of Culturable Airborne Microorganisms in Outdoor Environments in Beijing, China. Aerosol Sci. Technol. 42 (5), 325–334. doi:10.1080/02786820802068657
Fröhlich-Nowoisky, J., Kampf, C. J., Weber, B., Huffman, J. A., Pöhlker, C., Andreae, M. O., et al. (2016). Bioaerosols in the Earth System: Climate, Health, and Ecosystem Interactions. Atmos. Res. 182, 346–376. doi:10.1016/j.atmosres.2016.07.018
Griffin, D. W., Kubilay, N., Koçak, M., Gray, M. A., Borden, T. C., and Shinn, E. A. (2007). Airborne Desert Dust and Aeromicrobiology over the Turkish Mediterranean Coastline. Atmos. Environ. 41, 4050–4062. doi:10.1016/j.atmosenv.2007.01.023
Hara, K., and Zhang, D. (2012). Bacterial Abundance and Viability in Long-Range Transported Dust. Atmos. Environ. 47, 20–25. doi:10.1016/j.atmosenv.2011.11.050
Hu, Y. D., Ma, A. Z., Lü, P. Y., Zhang, Y., and Zhuang, G. Q. (2017). Community Characteristics of Cultivable Bacteria in Fine Particles(PM2.5) of Beijing and Baoding. Huan Jing Ke Xue 38 (04), 1327–1339. doi:10.13227/j.hjkx.201603224
Ishizuka, M., Mikami, M., Yamada, Y., Kimura, R., Kurosaki, Y., Jugder, D., et al. (2012). Does Ground Surface Soil Aggregation Affect Transition of the Wind Speed Threshold for Saltation and Dust Emission? SOLA 8, 129–132. doi:10.2151/sola.2012-032
Iwasaka, Y., Minoura, H., and Nagaya, K. (1983). The Transport and Spacial Scale of Asian Dust-Storm Clouds: a Case Study of the Dust-Storm Event of April 1979. Tellus 35B, 189–196. doi:10.1111/j.1600-0889.1983.tb00023.x
Iwasaka, Y., Shi, G. Y., Yamada, M., and Kobayashi, F. (2009). Mixture of Kosa (Asian Dust) and Bioaerosols Detected in the Atmosphere over the Kosa Particles Source Regions with Balloon-Borne Measurements: Possibility of Long-Range Transport. Air Qual. Atmosphere Health 2, 2938. doi:10.1007/s11869-009-0031-5
Jeon, E. M., Kim, H. J., Jung, K., Kim, J. H., Kim, M. Y., Kim, Y. P., et al. (2011). Impact of Asian Dust Events on Airborne Bacterial Community Assessed by Molecular Analyses. Atmos. Environ. 45, 4313–4321. doi:10.1016/j.atmosenv.2010.11.054
Kobayashi, F., Maki, T., Kakikawa, M., Yamada, M., Puspitasari, F., and Iwasaka, Y. (2015). Bioprocess of Kosa Bioaerosols: Effect of Ultraviolet Radiation on Airborne Bacteria within Kosa (Asian Dust). J. Biosci. Bioeng. 119 (5), 570–579. doi:10.1016/j.jbiosc.2014.10.015
Kurosaki, Y., and Mikami, M. (2015). Regional Difference in the Characteristic of Dust Event in East Asia: Relationship Among Dust Outbreak, Surface Wind, and Land Surface Condition. J. Meteorol. Soc. Jpn. Ser. 83A, 1–18. doi:10.1029/2003JD003913
Lee, J.-H., and Jo, W.-K. (2006). Characteristics of Indoor and Outdoor Bioaerosols at Korean High-Rise Apartment Buildings. Environ. Res. 101 (1), 11–17. doi:10.1016/j.envres.2005.08.009
Letunic, I., and Bork, P. (2007). Interactive Tree of Life (iTOL): An Online Tool for Phylogenetic Tree Display and Annotation. Bioinformatics 23 (1), 127–128. doi:10.1093/bioinformatics/btl529
Li, Y. P., Liu, P. X., and Xie, Z. S. (2018). Recent Research Progress and Perspective of Characteristics of Ambient Bioaerosols during Hazy Pollution in China (In Chinese). Chin. Sci. Bull. 63, 940–953. doi:10.1360/N97201701214
Liu, Y.-X., Qin, Y., Chen, T., Lu, M., Qian, X., Guo, X., et al. (2021). A Practical Guide to Amplicon and Metagenomic Analysis of Microbiome Data. Protein Cell 12, 315–330. doi:10.1007/s13238-020-00724-8
Ma, J., Ibekwe, A. M., Yang, C.-H., and Crowley, D. E. (2016). Bacterial Diversity and Composition in Major Fresh Produce Growing Soils Affected by Physiochemical Properties and Geographic Locations. Sci. Total Environ. 563-564, 199–209. doi:10.1016/j.scitotenv.2016.04.122
Ma, Y., Zhou, J., Yang, S., Zhao, Y., and Zheng, X. (2017). Assessment for the Impact of Dust Events on Measles Incidence in Western China. Atmos. Environ. 157, 1–9. doi:10.1016/j.atmosenv.2017.03.010
Maki, T., Susuki, S., Kobayashi, F., Kakikawa, M., Yamada, M., Higashi, T., et al. (2008). Phylogenetic Diversity and Vertical Distribution of a Halobacterial Community in the Atmosphere of an Asian Dust (KOSA) Source Region, Dunhuang City. Air Qual. Atmos. Health 1, 81–89. doi:10.1007/s11869-008-0016-9
Maki, T., Hara, K., Kobayashi, F., Kurosaki, Y., Kakikawa, M., Matsuki, A., et al. (2015). Vertical Distribution of Airborne Bacterial Communities in an Asian-Dust Downwind Area, Noto Peninsula. Atmos. Environ. 119, 282–293. doi:10.1016/j.atmosenv.2015.08.052
Maki, T., Hara, K., Iwata, A., Lee, K. C., Kawai, K., Kai, K., et al. (2017a). Variations in Airborne Bacterial Communities at High Altitudes over the Noto Peninsula (Japan) in Response to Asian Dust Events. Atmos. Chem. Phys. 17, 11877–11897. doi:10.5194/acp-17-11877-2017
Maki, T., Kurosaki, Y., Onishi, K., Lee, K. C., Pointing, S. B., Jugder, D., et al. (2017b). Variations in the Structure of Airborne Bacterial Communities in Tsogt-Ovoo of Gobi Desert Area during Dust Events. Air Qual. Atmos. Health 10 (3), 249–260. doi:10.1007/s11869-016-0430-3
Maki, T., Bin, C., Kai, K., Kawai, K., Fujita, K., Ohara, K., et al. (2019). Vertical Distributions of Airborne Microorganisms over Asian Dust Source Region of Taklimakan and Gobi Desert. Atmos. Environ. 214, 116848. doi:10.1016/j.atmosenv.2019.116848
Morris, C. E., Sands, D. C., Bardin, M., Jaenicke, R., Vogel, B., Leyronas, C., et al. (2011). Microbiology and Atmospheric Processes: Research Challenges Concerning the Impact of Airborne Micro-organisms on the Atmosphere and Climate. Biogeosciences 8 (80), 17–25. doi:10.5194/bg-8-17-2011
Newton, R. J., Jones, S. E., Eiler, A., McMahon, K. D., and Bertilsson, S. (2011). A Guide to the Natural History of Freshwater lake Bacteria. Microbiol. Mol. Biol. Rev. 75, 14–49. doi:10.1128/MMBR.00028-10
Nicholson, W. L., Munakata, N., Horneck, G., Melosh, H. J., and Setlow, P. (2000). Resistance of Bacillus Endospores to Extreme Terrestrial and Extraterrestrial Environments. Microbiology and Molecular Biology Reviews 64, 548–572.
Okamoto, T., Maruyama, A., Imura, S., Takeyama, H., and Naganuma, T. (2004). Comparative Phylogenetic Analyses of Halomonas Variabilis and Related Organisms Based on 16S rRNA, gyrB and ectBC Gene Sequences. Syst. Appl. Microbiol. 27, 323–333. doi:10.1078/0723-2020-00271
Onishi, K., Kurosaki, Y., Otani, S., Yoshida, A., Sugimoto, N., and Kurozawa, Y. (2012). Atmospheric Transport Route Determines Components of Asian Dust and Health Effects in Japan. Atmos. Environ. 49, 94–102. doi:10.1016/j.atmosenv.2011.12.018
Peter, S. K., Kimberly, A. H., Paul, B. S., and Allan, K. (2011). Atmospheric Cloud Water Contains a Diverse Bacterial Community. Atmos. Environ. 45, 5399–5405. doi:10.1016/j.atmosenv.2011.06.041
Pointing, S. B., and Belnap, J. (2014). Disturbance to Desert Soil Ecosystems Contributes to Dust-Mediated Impacts at Regional Scales. Biodivers Conserv 23, 1659–1667. doi:10.1007/s10531-014-0690-x
Pratt, K. A., DeMott, P. J., French, J. R., Wang, Z., Westphal, D. L., Heymsfield, A. J., et al. (2009). In Situ detection of Biological Particles in Cloud Ice-Crystals. Nat. Geosci 2, 398–401. doi:10.1038/ngeo521
Puspitasari, F., Maki, T., Shi, G., Bin, C., Kobayashi, F., Hasegawa, H., et al. (2015). Phylogenetic Analysis of Bacterial Species Compositions in Sand Dunes and Dust Aerosol in an Asian Dust Source Area, the Taklimakan Desert. Air Qual. Atmos. Health 9, 631–644. doi:10.1007/s11869-015-0367-y
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 41, D590–D596. doi:10.1093/nar/gks1219
Rothschild, L. J., and Mancinelli, R. L. (2001). Life in Extreme Environments. Nature 409, 1092–1101. doi:10.1038/35059215
Rui, J., Li, J., Wang, S., An, J., Liu, W.-T., Lin, Q., et al. (2015). Responses of Bacterial Communities to Simulated Climate Changes in alpine Meadow Soil of the Qinghai-Tibet Plateau. Appl. Environ. Microbiol. 81 (17), 6070–6077. doi:10.1128/AEM.00557-15
Šantl-Temkiv, T., Sikoparija, B., Maki, T., Carotenuto, F., Amato, P., Yao, M., et al. (2020). Bioaerosol Field Measurements: Challenges and Perspectives in Outdoor Studies. Aerosol Sci. Technol. 54 (5), 520–546. doi:10.1080/02786826.2019.1676395
Sheridan, P. P., Miteva, V. I., and Brenchley, J. E. (2003). Phylogenetic Analysis of Anaerobic Psychrophilic Enrichment Cultures Obtained from a Greenland Glacier Ice Core. Appl. Environ. Microbiol. 69, 2153–2160. doi:10.1128/AEM.69.4.2153-2160.2003
Smets, W., Moretti, S., Denys, S., and Lebeer, S. (2016). Airborne Bacteria in the Atmosphere: Presence, Purpose, and Potential. Atmos. Environ. 139, 214–221. doi:10.1016/j.atmosenv.2016.05.038
Tang, K., Huang, Z., Huang, J., Maki, T., Zhang, S., Shimizu, A., et al. (2018). Characterization of Atmospheric Bioaerosols along the Transport Pathway of Asian Dust during the Dust-Bioaerosol 2016 Campaign. Atmos. Chem. Phys. 18, 7131–7148. doi:10.5194/acp187131201810.5194/acp-18-7131-2018
Thomas, F., Hehemann, J.-H., Rebuffet, E., Czjzek, M., and Michel, G. (2011). Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbio. 2, 93–111. doi:10.3389/FMICB.2011.00093
Walser, S. M., Gerstner, D. G., Brenner, B., Bünger, J., Eikmann, T., Janssen, B., et al. (2015). Evaluation of Exposure-Response Relationships for Health Effects of Microbial Bioaerosols - A Systematic Review. Int. J. Hyg. Environ. Health 218, 577–589. doi:10.1016/j.ijheh.2015.07.004
Wang, Y., Sheng, H.-F., He, Y., Wu, J.-Y., Jiang, Y.-X., Tam, N. F.-Y., et al. (2012). Comparison of the Levels of Bacterial Diversity in Freshwater, Intertidal Wetland, and Marine Sediments by Using Millions of Illumina Tags. Appl. Environ. Microbiol. 78 (23), 8264–8271. doi:10.1128/AEM.01821-12
Wang, Y. Q. (2014). MeteoInfo: GIS Software for Meteorological Data Visualization and Analysis. Met. Apps 21, 360–368. doi:10.1002/met.1345
Wang, Y. Q. (2019). An Open Source Software Suite for Multi-Dimensional Meteorological Data Computation and Visualisation. J. Open Res. Softw. 7 (1), 21. doi:10.5334/jors.267
Will, C., Thürmer, A., Wollherr, A., Nacke, H., Herold, N., Schrumpf, M., et al. (2010). Horizon-Specific Bacterial Community Composition of German Grassland Soils, as Revealed by Pyrosequencing-Based Analysis of 16S rRNA Genes. Appl. Environ. Microbiol. 76, 6751–6759. doi:10.1128/AEM.01063-10
Wu, X. C., Chen, B., Wen, T. X., Shahid, I., Habib, A., and Shi, G-Y. (2019). Comparison of Concentrations and Chemical Compositions of PM10 during Hazy and Non-Hazy Days in Beijing and Islamabad. J. Shandong Univ. (Natural Science) 54 (07), 42–49. doi:10.6040/j.issn.1671-9352.0.2019.039
Wu, X., Chen, B., Wen, T., Habib, A., and Shi, G. (2020). Concentrations and Chemical Compositions of PM10 during Hazy and Non-hazy Days in Beijing. J. Environ. Sci. 87, 1–9. doi:10.1016/j.jes.2019.03.021
Xu, C., Wei, M., Chen, J., Wang, X., Zhu, C., Li, J., et al. (2017). Bacterial Characterization in Ambient Submicron Particles during Severe Haze Episodes at Ji'nan, China. Sci. Total Environ. 580, 188–196. doi:10.1016/j.scitotenv.2016.11.145
Yan, W. Z., Wang, B. Y., Oscar, F. M., Jiang, J. K., and Hao, J. M. (2017). Impact of Gusty Northwesterly Winds on Biological Particles in Winter in Beijing. Huan Jing Ke Xue 38 (09), 3561–3568. doi:10.13227/j.hjkx.201701110
Keywords: bioaerosols, dust event, DAPI, high-throughput sequencing, community diversity
Citation: Dong X, Chen B, Maki T, Shi G, Duan M and Khalid B (2022) Characteristic Changes of Bioaerosols in Beijing and Tsogt-Ovoo During Dust Events. Front. Environ. Sci. 10:795489. doi: 10.3389/fenvs.2022.795489
Received: 15 October 2021; Accepted: 04 February 2022;
Published: 14 March 2022.
Edited by:
Sanat Kumar Das, Bose Institute, IndiaReviewed by:
Irma Rosas, National Autonomous University of Mexico, MexicoMagesh Peter Dhasssiah, National Institute of Ocean Technology, India
Copyright © 2022 Dong, Chen, Maki, Shi, Duan and Khalid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Chen, Y2hlbl9iaW5AbWFpbC5pYXAuYWMuY24=
 Xiaofei Dong
Xiaofei Dong Bin Chen
Bin Chen Teruya Maki
Teruya Maki Guangyu Shi1
Guangyu Shi1