
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 02 December 2022
Sec. Toxicology, Pollution and the Environment
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.1058408
 Acharee Kaewlaoyoong1†
Acharee Kaewlaoyoong1† Shih-Ting Huang1,2†
Shih-Ting Huang1,2† Shu-Li Wang1,3†
Shu-Li Wang1,3† Chien-Wen Sun3
Chien-Wen Sun3 Jia-Jen Chen1
Jia-Jen Chen1 Chao-Hung Kuo4,5
Chao-Hung Kuo4,5 Chih-Hsing Hung6,7,8
Chih-Hsing Hung6,7,8 Szu-Chia Chen2,4,9
Szu-Chia Chen2,4,9 Ching-Chao Liang10
Ching-Chao Liang10 Hsiao-Wen Tsai11
Hsiao-Wen Tsai11 Chia-Fang Wu2,12†
Chia-Fang Wu2,12† Wen-Yi Lin2,13†
Wen-Yi Lin2,13† Ming-Tsang Wu1,8,14,15*†
Ming-Tsang Wu1,8,14,15*†The public in southwestern Taiwan’s Kaohsiung City have expressed concern over risk of arsenic (As) to people living in six villages of that city nearby a coastal heavy-industrial area. To investigate, we first analyzed urinary total As (TAs) levels in 328 adult subjects from the Nutrition and Health Survey in Taiwan in 2005-2008 (NAHSIT 2005-8). We found the top three highest median urinary TAs levels in residents from the Penghu islands (150.90 µg/L, n = 21) and the upper northern region (78.04 µg/L, n = 56) and the southern region (75.21 µg/L, n = 33) of Taiwan. Then, urinary TAs levels in 1,801 and 1,695 voluntary adult residents of the above-mentioned six villages in 2016 and 2018 respectively were compared with those from the top three highest TAs levels of NAHSIT 2005-8. Median urinary As levels were 84.60 µg/L in 2016 and 73.40 µg/L in 2018, similar to those in the southern region of Taiwan, but far below those in the Penghu islands (p < 0.05). Finally, in 2020, we interviewed 116 healthy adult residents from the same six villages and analyzed one-spot urine samples of total inorganic-related As (TiAs), a summation of As3+, As5+, monomethylarsonic acid, and dimethylarsinic acid. Subjects consuming seafood 2 days before urine sampling (n = 15) were significantly higher TiAs levels than those not (n = 101, p = 0.028). These findings suggest that seafood consumption is probably the main source of urinary TAs and TiAs in people residing close to that coastal heavy-industrial area.
Arsenic, one of the most abundant elements in the Earth’s crust, is naturally present in the environment. However, the burning of fossil fuel, metal smelting, the manufacturing of insecticides/herbicides and other man-made sources also release significant amounts of As into the environment (Chung et al., 2014; WHO, 2019). People can be potentially exposed to As via drinking water, dietary ingestion, inhalation of polluted air including tobacco smoke, and dermal absorption (National Research Council, 1999; Chung et al., 2014; Molin et al., 2015; WHO, 2019).
As is biomethylated in the human liver via enzymatic conversion catalyzed by a methyltransferase (AS3MT) (Khairul et al., 2017). This bioconversion reduces inorganic As such as arsenite (As3+) and arsenate (As5+), to lower toxic methylated metabolites such as dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA), and nontoxic forms of organic As (e.g., arsenobetaine (Asb) and arsenocholine (Asc)) (Khairul et al., 2017; González-Martínez et al., 2020). Inorganic As is considered a Group 1 human carcinogen by the International Agency for Research on Cancer (IARC), while MMA and DMA are classified as possible human carcinogens (IARC Group 2B) (WHO, 2019). The half-life of inorganic As3+ and As5+ is short in humans, with 35% excreted through urine within 2 days (Fowler et al., 2015). The sum concentration of As3+, As5+, DMA, and MMA in urine, named as urinary total inorganic-related As (TiAs) levels, is commonly used to assess the harmful health exposure to humans (Chang et al., 2019).
Kaohsiung, a major city located in southern Taiwan, has a population of 2.77 million people. It is one of Taiwan’s most industrialized cities and home to three large heavy-industrial areas in southern Kaohsiung, including Da-Fa, Lin-Yuan, and Lin-Hai industrial park areas (Figure 1). Lin-Hai industrial area is the largest heavy industrial zone in Taiwan (Yuan et al., 2022). It was established and completed during 1960-1972, and the area covers 15.69 km2 (Maynard et al., 2020). The major industrial activities in the Lin-Hai are metals and the related processing (33.7%), followed by machinery and equipment (16.8%), nonmetallic mineral products (9.3%), and others. Table 1 shows the summary of the top 80% of manufacturing types and their possible pollutants released in the Lin-Hai industrial area and the other two major industrial areas of southern Kaohsiung city. People residing near one coast heavy-industrial area (Lin-Hai industrial area) concerned they were exposed to As and blamed the source as being the Lin-Hai industrial area; thus, the Kaohsiung city government provided free health check-ups for the inhabitants living close to the Lin-Hai industrial area at three different periods and assigned us (an independent party) to determine their possible source of As exposure. To do this, we analyzed these three waves of existing health check-up data (2016, 2018, and 2020) and compared the data from the Nutrition and Health Survey in Taiwan (NAHSIT) 2005-2008 to elucidate possible As exposure source in people residing close to the Lin-Hai industrial area.
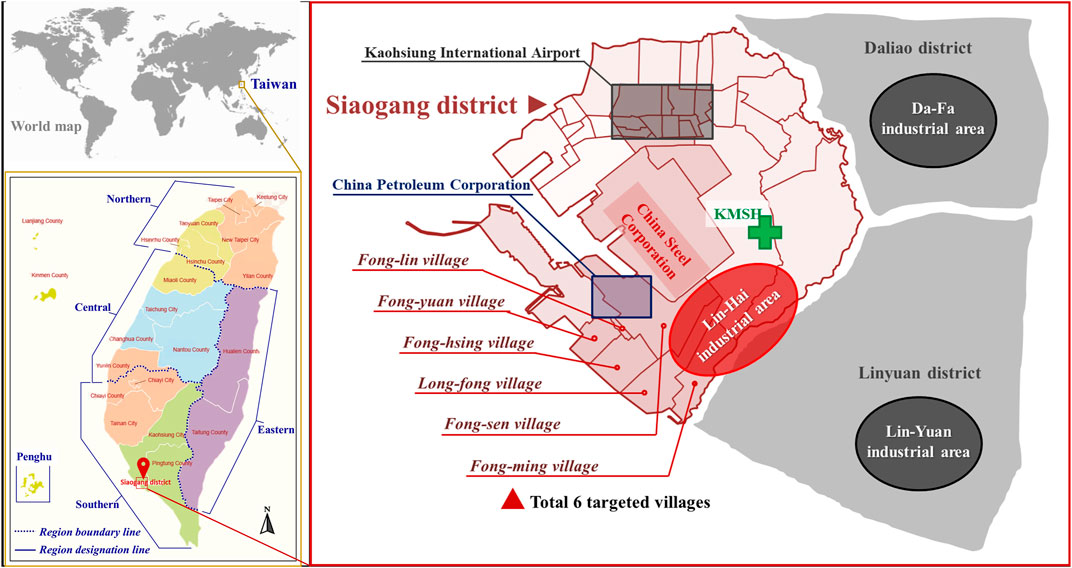
FIGURE 1. Maps of six villages close to one coastal heavy-industrial area (Lin-Hai industrial area) in southwestern Kaohsiung, Taiwan. KMSH: Kaohsiung Municipal Siaogang Hospital.
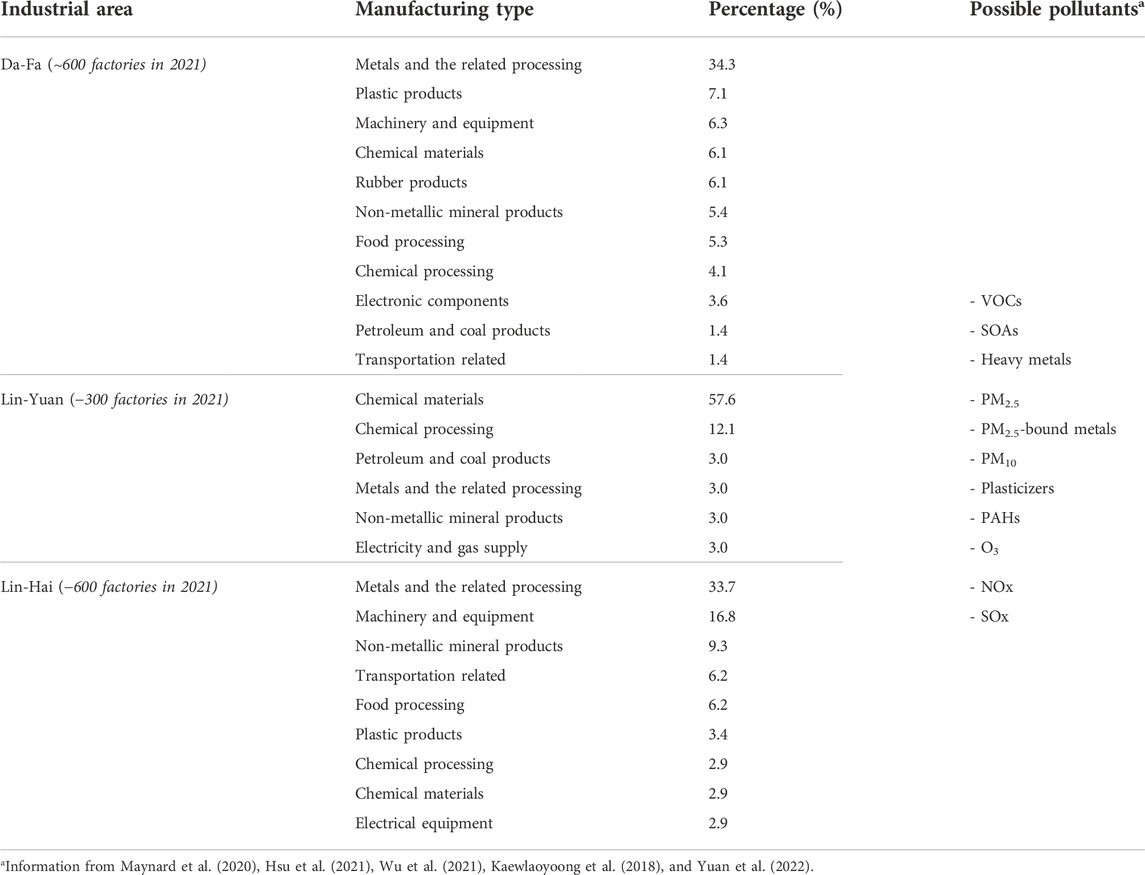
TABLE 1. Summary of the top 80% of manufacturing types in three major industrial areas of southern Kaohsiung city and some possible pollutants.
The Nutrition and Health Survey in Taiwan (NAHSIT) is the nationwide representative monitoring of Taiwanese nutritional and health status. The detailed sampling methods of this cross-sectional survey has been described previously (Tu et al., 2011; Liao et al., 2019). During the NAHSIT survey period of 2005 and 2008 (NAHSIT 2005-8), one-spot urine samples were also collected by the researchers of Taiwan National Health Research Institutes (NHRI) for the measurement of metal elements, including cadmium (Cd), lead (Pb), chromium (Cr), nickel (Ni), copper (Cu), cobalt (Co), indium (In), thallium (Tl), mercury (Hg), and total arsenic (TAs). The study subjects in the NAHSIT 2005-8 were mainly from eight geographical areas of Taiwan (Figure 1), including the upper northern region (Taipei, New Taipei, and Keelung), lower northern region (Yilan County, Taoyuan County, and Hsinchu County and City), central region (Miaoli County, Taichung City, Changhua County, Nantou County, and Yunlin County), southern region (Chiayi County, Chiayi City, Tainan city, Kaohsiung City, and Pingtung County), eastern region (Hualien County and Taitung County), Hakka (many tribes spread Taiwan islandwide), mountainous region (central parts of New Taipei City, Hsinchu County, Yilan County, Miaoli County, Taichung City, Nantou County, Hualien County, Taitung County, Chiayi City, Kaohsiung City, or Pingtung County), and the Penghu islands (Penghu County).
Data of urinary Cd, Pb, Cu, and Co levels, but not urinary TAs, have been published previously (Liao et al., 2019). In this study, we only presented the subjects whose ages ≥18 years and who had the data of TAs levels in urine, measured by Agilent 7700 Series Inductively coupled plasma-mass spectrometry (ICP-MS, Agilent Technologies, Inc., Santa Clara, CA, United States) in the central laboratory of NHRI. This laboratory was accredited by the German External Quality Assessment Scheme (G-EQUAS) for the international laboratory competence test for biological samples of environmental pollutants (Figure 2).
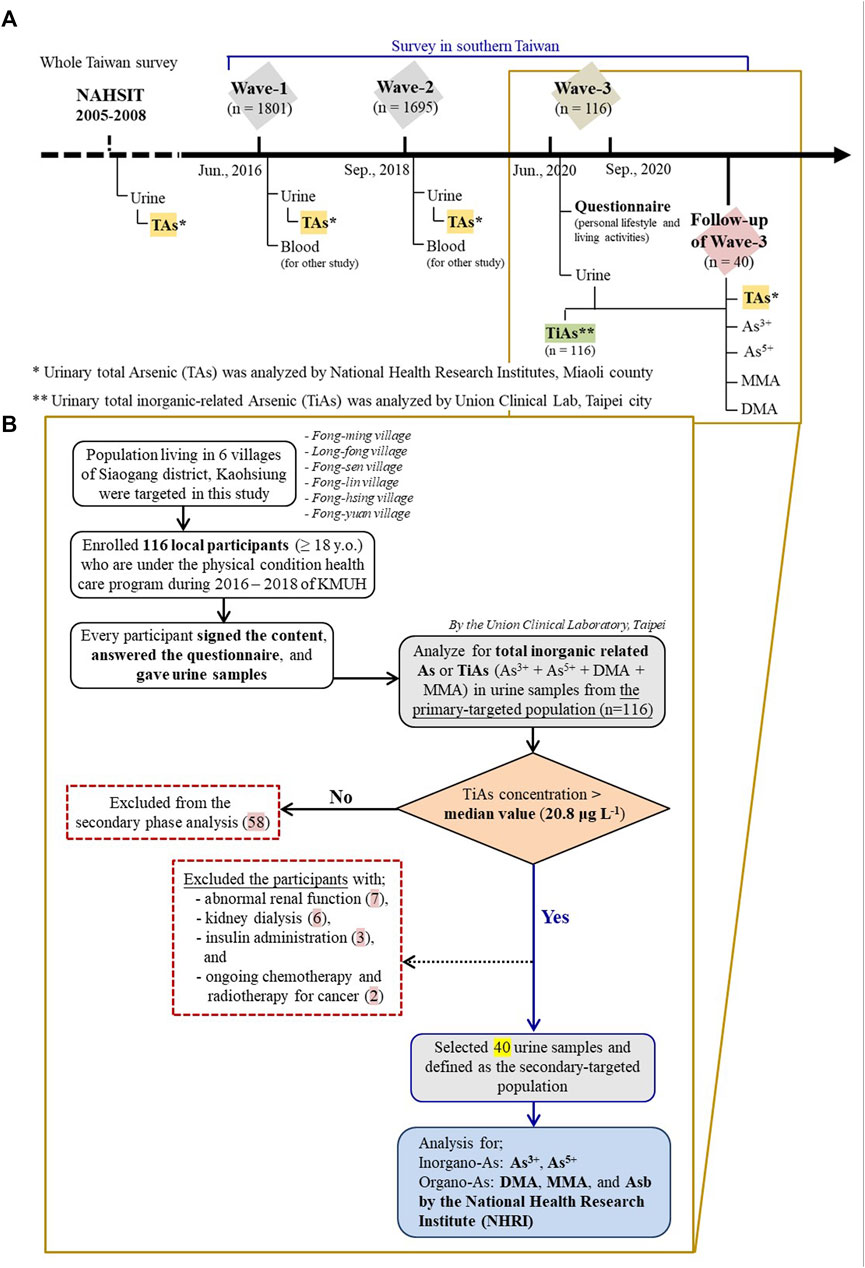
FIGURE 2. Timeline of measurement of arsenic (As) in urine in different cohorts (≥18 years old); (A) Taiwan’s Nutrition and Health Survey in 2005-2008 (NAHSIT 2005-8) and three survey waves (2016, 2018, and 2020) of six villages close to one coastal heavy industry in Kaohsiung city; (B) Study flowchart of those six villages in the latest survey wave of 2020.
About 2,000 inhabitants live in the six villages (Fong-ming, Long-fong, Fong-sen, Fong-lin, Fong-hsing, and Fong-yuan) close to the Lin-Hai industrial area (Figure 1). Under the support of Kaohsiung city government, residents in those six villages were invited voluntarily to participate the health check-up in Kaohsiung Municipal Siaogang Hospital (KMSH) in three different survey periods (2016, 2018, and 2020) (Figure 2). During the health examination, one-spot urine sample was also collected. The total of 1,801 subjects in 2016 (Wave-1) and 1,927 subjects in 2018 (Wave-2) had the data of urinary TAs, analyzed by one central laboratory (the Union Clinical Laboratory (UCL)) in Taipei, Taiwan officially accredited by Taiwan Accreditation Foundation, with its certification effective between 16 March 2015 and 15 March 2018 (Certificate No. L1447-150325). The detailed study design of the Wave-1 and Wave-2 has been published previously (Tsai H. et al., 2021; Liu et al., 2021). Because the majority of voluntary subjects in the three-wave surveys were adults, only subjects whose ages ≥18 years (n = 1,801 for Wave-1; n = 1,695 for Wave-2; n = 116 for Wave-3) were included in this study.
Due to lack of data of lifestyle information and urinary inorganic As levels in Wave-1 and Wave-2, another 116 voluntary adult residents, joining any of Wave-1 or Wave-2 previously, from the same six villages were interviewed by one standardized questionnaire and one-spot midstream urine samples in 10 ml polypropylene centrifuge tubes were also collected in August and September, 2020 (Wave-3) for the subsequent analysis of total inorganic-related As (TiAs), a summation of As3+, As5+, monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA), in the same central laboratory in Taipei (Figure 2). The samples were preserved at −20°C until the day before analysis. Urinary TiAs levels were analyzed using a high-performance liquid chromatography (HPLC) coupled with the inductively coupled plasma mass spectrometry (ICP-MS, NexION 300 Series, Perkin Elmer). Details regarding the operation of the instrument and analysis have been reported by the CAP (College of American Pathologists) accredited Union Clinical Laboratory (UCL) Taipei, Taiwan (CAP Number: 6979606).
The questionnaire included information about sociodemographic characteristics, self-reported medical history, lifestyle and living activities. This study protocol was approved by the Ethics Committee of Taiwan’s National Health Research Institutes (NHRI) and Kaohsiung Medical University Hospital (KMUH) (KMUHIRB-F(I)-20190128). All study subjects signed informed consents.
Among the 116 subjects recruited in Wave-3, we chose 40 who were healthy and had urinary TiAs levels higher than median value (20.8 μg/L) of urinary TiAs in 116 subjects to further analyze TAs and four speciations of TiAs in the urines (Supplementary Figure S1). In brief, each sample (1 ml) was diluted 10-times using 1% Nitric acid (1:9, v/v) in a 15 ml polypropylene tube, then it was analyzed using HPLC coupled with the ICP-MS Agilent 7800 in NHRI central laboratory for another external validation and reassurance (Supplementary Table S1). Urinary creatinine levels in these 40 subjects were also measured.
The calibration curve was established as a 7-point calibration model of standard solutions (0–100 μg/L) of urinary TAs. The calibration curve for sample quantification was characterized by a high correlation coefficient (R2 > 0.995), and the relative standard deviation (RSD) was within ±20%. The method detection limit of TiAs reported by UCL was 0.3 μg/L (Supplementary Table S1). With regard to the speciation analysis of As, the recovery percentages of As3+, As5+, DMA, MMA, and TAs ranged from 80%–120% while detection limits are shown in Supplementary Table S1. In addition, routine internal and external quality control tests (duplicate sample, blank, and spike recovery) were performed daily.
For the data of NAHSIT 2005-8, the Kruskal-Wallis ANOVA was first used to examine the overall geographic distributions of urinary TAs levels across eight regions in Taiwan. If the overall test urinary TAs levels reached significance, Dunn’s statistical method of pairwise multiple comparison procedures was used to examine the differences of urinary TAs levels in each of two different geographic regions of Taiwan. Subsequently, urinary TAs levels in voluntary inhabitants in those six villages from three-wave health check-ups were compared individually with those people residing in the top three highest TAs levels of NAHSIT 2005-8 using the Mann-Whitney rank-sum test. To account for the above multiple comparisons (n = 9 comparisons), we used the Benjamini-Hochberg method to correct p-values. Finally, for the data of 116 study subjects in Wave-3 with urinary TiAs levels, univariable and multiple linear regression were both used to examine the influential factors of dietary habits and lifestyle activities on urinary TiAs levels before and after adjusting for other covariates. Urinary TiAs levels were log-transformed to express normality before the analysis. All statistical operations were performed using Sigma Plot 14.0 for Windows (Systat Software Inc., Chicago, IL, United States), with significance set as a two-sided p < 0.05.
Among the 539 subjects with data of urinary TAs levels in NAHSIT, there were 328 of them, aged ≥18 years. Among the 328 subjects with data of urinary TAs levels, distributions of urinary TAs levels were significantly different across eight regions of NAHSIT 2005-8 (p < 0.001). The top three highest median urinary TAs levels in these eight regions were from the Penghu islands (150.90 µg/L, n = 21), the upper northern region of Taiwan (78.04 µg/L, n = 56), and the southern region of Taiwan (75.21 µg/L, n = 33) (Figure 3A; Supplementary Table S2). Pairwise multiple comparison statistics by Dunn’s Method found that median urinary TAs levels in the Penghu islands were significantly higher than those in the remaining seven regions each, but in contrast, the remaining comparisons in other regions were not significantly different (Figure 3A).
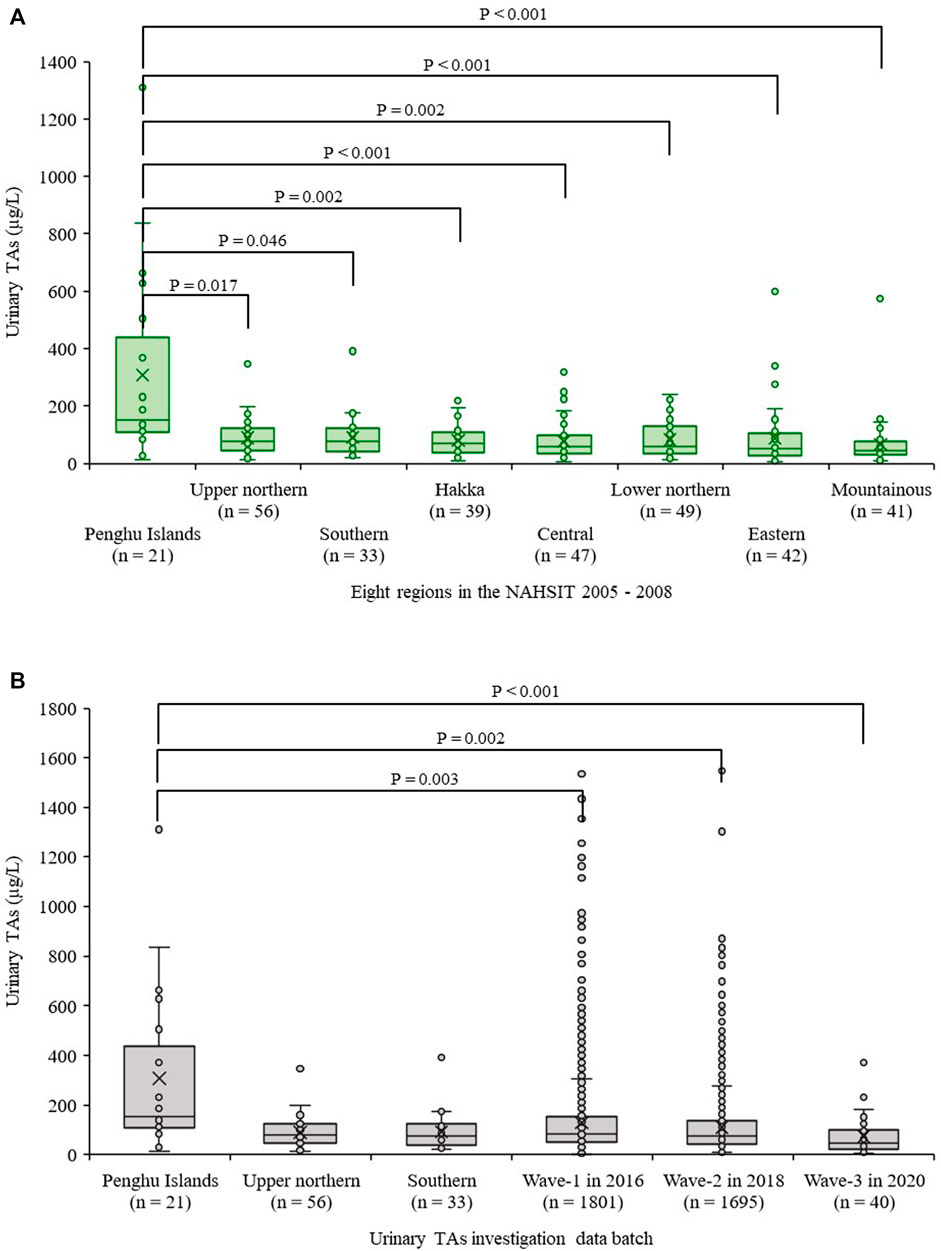
FIGURE 3. Comparisons of urinary total arsenic (TAs) levels in people from different regions of Taiwan; (A) Data solely from the Nutrition and Health Survey in Taiwan during the survey of 2005-2008 (NAHSIT 2005-8); (B) Distributions of urinary TAs levels from six townships in 2016, 2018, and 2020 vs. the top three highest TAs levels of NAHSIT 2005-8.
We compared median urinary TAs levels from top three highest regions of NAHSIT 2005-8, including Penghu islands and the upper northern region and the southern region of Taiwan, with Wave-1 (n = 1,801), Wave-2 (n = 1,695), and Wave-3 (n = 40 out of 116), whose age ≥18 years from those six villages (Supplementary Table S3). We found that median urinary TAs levels in Penghu islands were significantly higher than those from all three surveys (Wave-1, Wave-2, and Wave-3) (Figure 3B). By contrast, median urinary TAs levels in the southern region of Taiwan from NAHSIT 2005-8 were not significantly different from Wave-1, Wave-2, and Wave-3 (p = 0.218, 0.823, and 0.059 respectively). In addition, by observation from Wave-1 to Wave-3, urinary As concentrations tended to decrease.
Demographic characteristics and personal lifestyle of 116 study subjects in Wave-3 are shown in Tables 2; Supplementary Table S4. Their mean age was 60.9 years and 60.3% were females. Median urinary TiAs, a summation of As3+, As5+, MMA, and DMA, in 15 study subjects consuming seafood 2 days before urine sampling was 24.0 µg/L, significantly higher than another 101 study subjects who did not consume seafood at this time (median = 20.5 µg/L, p = 0.029) (Table 2). The results remained significant after adjusting for other covariates (p = 0.028) (Table 3). Other covariates, including industry-related occupations, did not show any significance in a multiple linear regression model (Tables 2, 3; Supplementary Table S4).
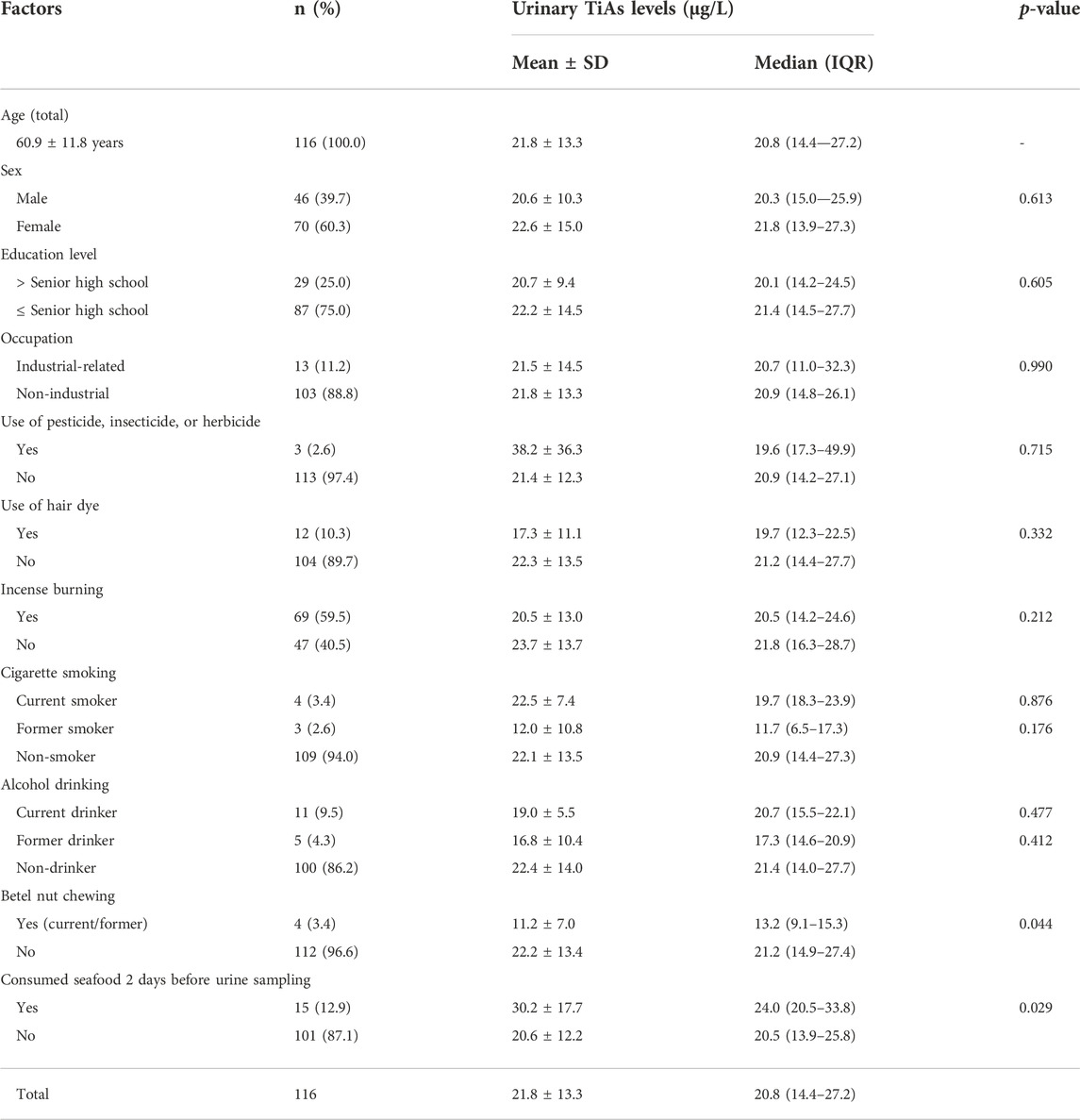
TABLE 2. Distribution of urinary total inorganic-related arsenic (TiAs) levels by different factors in 116 study subjects from six villages close to one coastal heavy-industrial area in Kaohsiung city in 2020 (Wave-3).
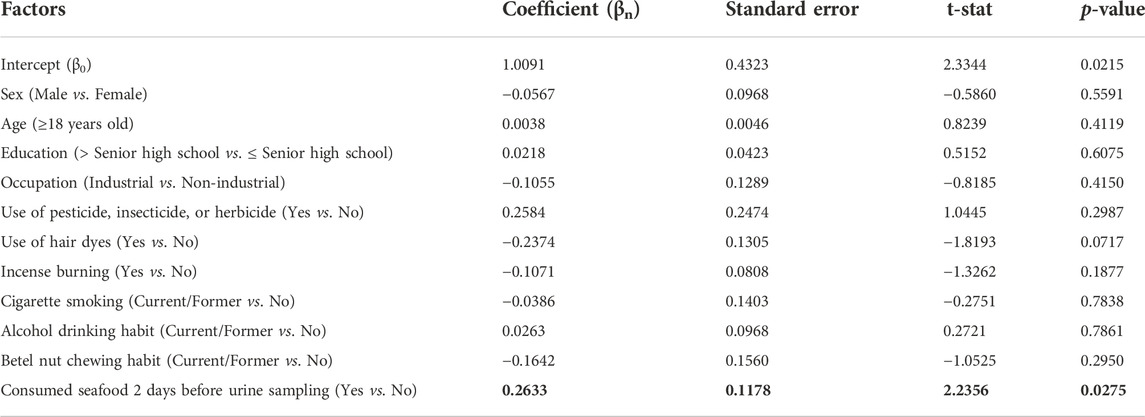
TABLE 3. Factors associated with urinary total inorganic-related arsenic (TiAs) levels in 116 study subjects in a multiple linear regression model.
In the cohort of NAHSIT 2005-8, subjects from the Penghu islands had the highest median urinary TAs levels among the eight geographic regions of Taiwan. These islands, an archipelago, consists of a couple of dozen islands and islets with a combined coastline of more than 300 km and is located approximately midway between Taiwan and Mainland China. Because the islands are a destination for nature tourism and fishery businesses and the location is far away from heavy industrial areas, it is unlikely that the highest levels of urinary As found in residents there are attributable to industrial activity. Since seafood has been recognized and reported as one of the important sources of As exposure in humans worldwide (Maulvault et al., 2015; Molin et al., 2015), it is reasonable to suspect the highest urinary TAs concentrations found in these residents of the Penghu islands are probably due to regular seafood consumption.
Next, when comparing median urinary TAs levels in the southern region of Taiwan from NAHSIT 2005-8 with those from three-waves of health checkup in six villages close to the Lin-Hai industrial area (Wave-1, Wave-2, and Wave-3), we did not find any significant differences. Because subjects in the southern region of Taiwan of NAHSIT 2005-8 were recruited from any city/county of Chiayi county, Chiayi city, Tainan city, Kaohsiung city, or Pingtung county, the results of non-significant differences between NAHSIT 2005-8 and any of Wave-1, Wave-2, or Wave-3 suggest the Lin-Hai industrial area might not be the contributing factor of the increased urinary TAs levels in the inhabitants nearby this area.
One recent environmental study characterized the distributions of PM2.5-bound multiple metals, collected from the urban, suburban, rural, and industrial regions of Taiwan between 2016 and 2018 in identifying the potential sources of metal pollutants (Hsu et al., 2021). They found the constituents of metals from emissions of heavy industries in Kaohsiung city were mostly Fe, Zn, Cu, and Mn. Besides PM2.5-bound particular metals (e.g., Fe, Zn, Cu, and Mn) are the main concerns in Kaohsiung city, other harmful industry-related pollutants such as PAHs, PM10, O3, NOx, SOx, and VOCs can also be found in the Kaohsiung area (Hsu et al., 2021; Wu et al., 2021). By contrast, the studies did not report as a major metal pollutant released from the industrial processes in this heavy-industrial city (Hsu et al., 2021), consistent with our findings that, among the 116 study subjects from Wave-3, the variable of consuming seafood 2 days before urine collection was the most important contributing factor of urinary TiAs levels.
While ground water as a common source of As pollution could have been another concern in Taiwan (Liang et al., 2017; Tsai T.-L. et al., 2021), it can be ruled out because residents in the sampled six villages did not consume it. In addition, well water is nowadays rarely consumed and tap water is often filtered in Taiwan, which might explain why the urinary As levels we found in this study are lower than those found by other studies focusing on contaminated groundwater consumptions (Liao et al., 2012; Tsai T.-L. et al., 2021).
In general, methylation of As proceeds by two steps to metabolize inorganic As (As+3 and As+5) into the organic As: 1) As+3 and As+5 are methylated to MMA; and then 2) MMA is methylated to DMA (Khairul et al., 2017; González-Martínez et al., 2020), catalyzed by As (+3 oxidation state) methyltransferase (AS3MT) and S-adenosylmethionine (SAM) as methyl-ion donor (Khairul et al., 2017). MMA and DMA are less toxic than As+3 and As+5 and more readily excreted via urine. TAs levels in human urine consist of 10-30% As+3 and As+5, 10%–20% MMA, and 60%–80% DMA (Jansen et al., 2016); thus, to have information about the speciation of TAs in human biomonitoring becomes important to evaluate toxic health effects.
Unfortunately, we did not have the speciation data of urinary TAs in the cohorts of NAHSIT 2005-8 and the surveys of Wave-1 (2016) and Wave-2 (2018), although we did have the data of urinary TiAs in 116 adult subjects of Wave-3 and, among them, 40 subjects had the data of urinary TAs speciation corrected by urinary creatinine. Thus, we did a literature review to compare our results of urinary As3+, As5+, TiAs, and TAs concentrations with those of previous studies conducted in Taiwan within the past 10 years (2011-2021) (Table 4; Supplementary Table S5). Median (mean) urinary As3+, As5+, and TiAs levels corrected by urinary creatinine (µg/g creatinine) were 0.66 (0.77), 0.45 (0.93), and 32.06 (35.57), which were lower than other areas and much lower than subjects living nearby Lanyang basin with contaminated artesian water (Table 4). The comparisons further demonstrated not too much concerning high urinary toxic As (As+3 and As+5) levels in adults living in those six villages.
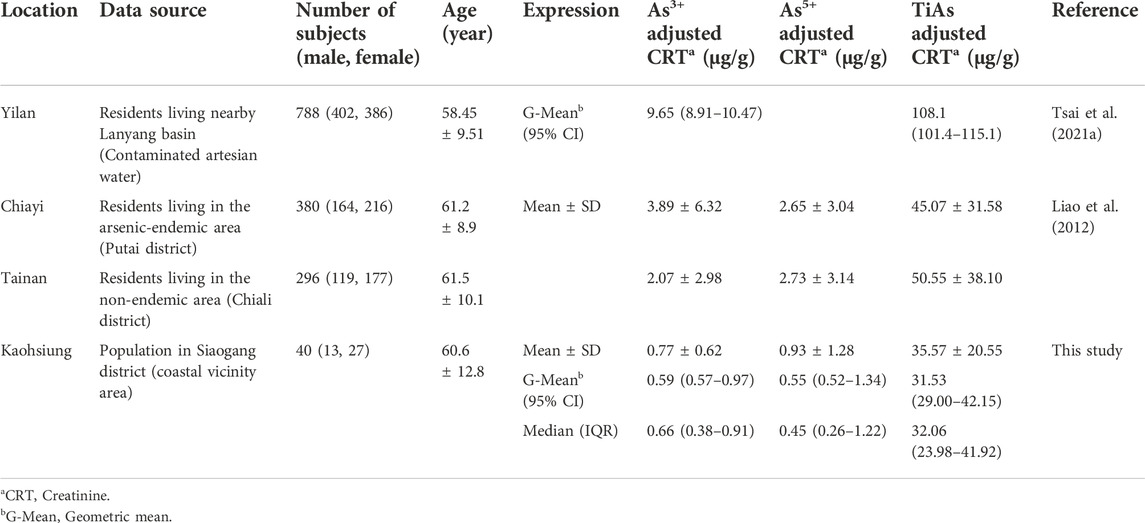
TABLE 4. Comparison of resident urinary arsenite (As3+), arsenate (As5+), and total inorganic-related arsenic (TiAs) levels in the previous As-endemic and nearby areas in Taiwan with this study.
We also investigated the results of As in other countries within the past 5 years (2016-2021) (Supplementary Table S6). We found the urinary inorganic As (As+3 + As+5) and TiAs concentrations in the present study to be much lower than those reported for residents living near the Yellow River, China (Wei et al., 2017), an area with the highest reported TiAs probably due to excessive exposure to contaminated drinking water. Mean urinary TAs in residents of Wuhan where there is a concern of As exposure source from inhalation of outdoor air PM2.5 from heavy industries, were relatively low, suggesting dietary exposure was more responsible for urinary TAs than inhalation from heavy industries (Mao et al., 2020). In northern Vietnam, the residents exposed to contaminated groundwater had also higher urinary TAs levels (125 ± 115 µg/L) than ours (Pham et al., 2017). Similarly, in Korea, higher urinary As3+ and As5+ concentrations have been found in people living near an abandoned metal mine (Chang et al., 2019). In eastern Croatia, residents living in rural areas have been found to have slightly higher urinary TAs than we found in our study (Ćurković et al., 2016). One intervention study conducted in Norway found a significant association between seafood consumption and urinary inorganic As, TiAs, and TAs concentrations (Molin et al., 2014). The urinary TAs concentrations after seafood consumption for 14 days found in the Norway study were much higher than those in this study, while inorganic As concentrations were similar.
Several limitations are present in this study. One-spot urine samples instead of 24-h urine samples were collected to measure As levels, which might introduce random misclassification of exposure. Collection of urinary As speciation in most study subjects in this study (NAHSIT 2005-8 cohort and the surveys of Wave-1 and Wave-2) was not possible, which hampered the assessment of toxic As health effect. The study subjects in all three waves (Wave-1, Wave-2, and Wave-3) voluntarily participated in this study, so selection bias is likely. This was an observational study rather than a seafood-restriction interventional study; thus, we cannot completely rule out the possibility of residents living in those six villages with high As levels in urine not being due to the nearby Lin-Hai industrial area. In addition, this study did not have the data of the arsenic content of the effluents and/or waste from the Lin-Hai industrial area to completely rule out the possibility of the source of arsenic exposure from this heavy industry. Finally, the information from our questionnaire was subjective, information bias was likely. Although we only found the variable of consuming seafood 2 days before urine sampling were significantly higher TiAs levels than those not (Tables 2, 3), we cannot rule out the probability of seafood contamination with As in the local fish ponds through groundwater use, where were the common source of As pollution in the southwestern Taiwan area (Liang et al., 2017).
In conclusion, this study suggests that the consumption of seafood could be the main culprit of the elevation of urinary TAs and/or TiAs levels in residents living close to one coastal heavy-industrial area (Lin-Hai industrial area) in Kaohsiung city. Future diet-interventional studies are required for these residents with high urinary TAs and/or TiAs levels to re-confirm our findings.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by National Health Research Institutes and Kaohsiung Medical University Hospital, Taiwan. The patients/participants provided their written informed consent to participate in this study.
AK: Formal analysis, Data Curation, Writing—Original Draft, Writing—Review and Editing, and Visualization, S-TH: Methodology, Investigation, Data Curation, and Writing—Original Draft, S-LW: Methodology, Validation, Investigation, Resource, and Data Curation, C-WS: Investigation, Resources, and Data Curation, J-JC: Investigation and Data Curation, C-HK: Investigation and Resources, C-HH: Investigation, Resources, and Data Curation, S-CC: Conceptualization, Investigation, and Data Curation, C-CL: Validation and Data Curation, H-WT: Validation and Data Curation, C-FW: Investigation, Methodology, and Data Curation, W-YL: Conceptualization, Investigation, and Data Curation, M-TW: Conceptualization, Methodology, Validation, Investigation, Writing—Review and Editing, Supervision, and Funding acquisition. Each author had participated sufficiently in the work to take public responsibility for appropriate portions of the content.
This work was financially supported by grants from Kaohsiung Medical University Hospital (KMUH110-0R76); from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE), Kaohsiung Medical University Research Center Grant (KMU-TC109A01; KMUTC111IFSP01); from Kaohsiung Municipal Ta-Tung Hospital (KMTTH-106-02; KMTTH-109-49); from Ministry of Science and Technology (MOST 110-2314-B-037-047-MY3; 110-2314-B-239-001-MY3; 110-2314-B-037-133-; 110-2314-B-037-056-; 111-2314-B-037-004-); and from National Health Research Institutes (NHRI-EX110-10703PI), all in Taiwan. None of these institutions played any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.1058408/full#supplementary-material
KMSH, Kaohsiung Municipal Siaogang Hospital; As, Arsenic; TiAs, Total inorganic-related Arsenic; TAs, Total Arsenic; As3+, Arsenite; As5+, Arsenate; DMA, Dimethylarsinic acid; MMA, Monomethylarsonic acid; Asb, Arsenobetaine; Asc, Arsenocholine; NAHSIT 2005-8, The Nutrition and Health Survey in Taiwan 2005-2008; Wave-1, The health check-up in six villages close to Lin-Hai industrial area in 2016; Wave-2, The health check-up in six villages close to Lin-Hai industrial area in 2018; Wave-3, The health check-up in six villages close to Lin-Hai industrial area in 2020; CRT, Creatinine; G-Mean, Geometric mean; G-SD, Geometric standard deviation; M:F (%), Male per female (%); N/A, Not available; MDL, Method detection limit; LOQ, Limit of quantification; UCL, Union Clinical Laboratory (Taipei, Taiwan); NHRI, National Health Research Institutes (Miaoli, Taiwan).
Chang, J. Y., Ahn, S. C., Lee, J. S., Kim, J. Y., Jung, A. R., Park, J., et al. (2019). Exposure assessment for the abandoned metal mine area contaminated by arsenic. Environ. Geochem. Health 41, 2443–2458. doi:10.1007/s10653-019-00296-5
Chung, J.-Y., Yu, S.-D., and andHong, Y.-S. (2014). Environmental source of arsenic exposure. J. Prev. Med. Public Health 47, 253–257. doi:10.3961/jpmph.14.036
Ćurković, M., Sipos, L., Puntarić, D., Dodig-Ćurković, K., Pivac, N., and andKralik, K. (2016). Arsenic, copper, molybdenum, and selenium exposure through drinking water in rural eastern Croatia. Pol. J. Environ. Stud. 25, 981–992. doi:10.15244/pjoes/61777
Fowler, B. A., Selene, C. H., Chou, J. R., Jones, L. D., and Sullivan, W. (2015). “Chapter 28 - arsenic,” in Fourth E.nordberg (San Diego: Academic Press), 581–624. doi:10.1016/B978-0-444-59453-2.00028-7
González-Martínez, F., Sánchez-Rodas, D., Varela, N. M., Sandoval, C. A., Quiñones, L. A., and andJohnson-Restrepo, B. (2020). As3MT and GST polymorphisms influencing arsenic metabolism in human exposure to drinking groundwater. Int. J. Mol. Sci. 21, 4832. doi:10.3390/ijms21144832
Hsu, C.-Y., Chi, K.-H., Wu, C.-D., Lin, S.-L., Hsu, W.-C., Tseng, C.-C., et al. (2021). Integrated analysis of source-specific risks for PM2.5-bound metals in urban, suburban, rural, and industrial areas. Environ. Pollut. 275, 116652. doi:10.1016/j.envpol.2021.116652
Jansen, R. J., Argos, M., Tong, L., Li, J., Rakibuz-Zaman, M., Islam, M. T., et al. (2016). Determinants and consequences of arsenic metabolism efficiency among 4, 794 individuals: Demographics, lifestyle, genetics, and toxicity. Cancer Epidemiol. biomarkers Prev. a Publ. Am. Assoc. Cancer Res. cosponsored by Am. Soc. Prev. Oncol. 25, 381–390. doi:10.1158/1055-9965.EPI-15-0718
Kaewlaoyoong, A., Vu, C. T., Lin, C., and LiaoSenandChen, C. J.-R. (2018). Occurrence of phthalate esters around the major plastic industrial area in southern Taiwan. Environ. Earth Sci. 77, 457. doi:10.1007/s12665-018-7655-4
Khairul, I., Wang, Q. Q., Jiang, Y. H., Wang, C., and andNaranmandura, H. (2017). Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 8, 23905–23926. doi:10.18632/oncotarget.14733
Liang, C.-P., Chien, Y.-C., Jang, C.-S., Chen, C.-F., and andChen, J.-S. (2017). Spatial analysis of human health risk due to arsenic exposure through drinking groundwater in Taiwan’s Pingtung plain. Int. J. Environ. Res. Public Health 14, 81. doi:10.3390/ijerph14010081
Liao, K. W., Pan, W. H., Liou, S. H., Sun, C. W., Huang, P. C., and andWang, S. L. (2019). Levels and temporal variations of urinary lead, cadmium, cobalt, and copper exposure in the general population of Taiwan. Environ. Sci. Pollut. Res. 26, 6048–6064. doi:10.1007/s11356-018-3911-0
Liao, Y.-T., Chen, C.-J., Li, W.-F., Hsu, L.-I., Tsai, L.-Y., Huang, Y.-L., et al. (2012). Elevated lactate dehydrogenase activity and increased cardiovascular mortality in the arsenic-endemic areas of southwestern Taiwan. Toxicol. Appl. Pharmacol. 262, 232–237. doi:10.1016/j.taap.2012.04.028
Liu, Y.-H., Wang, C.-W., Wu, D.-W., Lee, W.-H., Chen, Y.-C., Li, C.-H., et al. (2021). Association of heavy metals with overall mortality in a Taiwanese population. Nutrients 13, 2070. doi:10.3390/nu13062070
Mao, X., Hu, X., Wang, Y., Xia, W., Zhao, S., and andWan, Y. (2020). Temporal trend of arsenic in outdoor air PM2.5 in wuhan, China, in 2015–2017 and the personal inhalation of PM-bound arsenic: Implications for human exposure. Environ. Sci. Pollut. Res. 27, 21654–21665. doi:10.1007/s11356-020-08626-2
Maulvault, A. L., Anacleto, P., Barbosa, V., Sloth, J. J., Rasmussen, R. R., Tediosi, A., et al. (2015). Toxic elements and speciation in seafood samples from different contaminated sites in Europe. Environ. Res. 143, 72–81. doi:10.1016/j.envres.2015.09.016
Maynard, N. J., Raj Kanagaraj Subramanian, V., Hua, C.-Y., and andLo, S.-F. (2020). Industrial symbiosis in taiwan: Case study on linhai industrial park. Sustainability 12, 4564. doi:10.3390/su12114564
Molin, M., Ulven, S. M., Dahl, L., Goessler, W., Fliegel, D., Holck, M., et al. (2014). Urinary excretion of arsenicals following daily intake of various seafoods during a two weeks intervention. Food Chem. Toxicol. 66, 76–88. doi:10.1016/j.fct.2014.01.030
Molin, M., Ulven, S. M., Meltzer, H. M., and andAlexander, J. (2015). Arsenic in the human food chain, biotransformation and toxicology – review focusing on seafood arsenic. J. Trace Elem. Med. Biol. 31, 249–259. doi:10.1016/j.jtemb.2015.01.010
National Research Council (1999). Arsenic in drinking water. Washington, DC: The National Academies Press. doi:10.17226/6444
Pham, L. H., Nguyen, H. T., VanTran, C., Nguyen, H. M., Nguyen, T. H., and andTu, M. B. (2017). Arsenic and other trace elements in groundwater and human urine in ha nam province, the northern Vietnam: Contamination characteristics and risk assessment. Environ. Geochem. Health 39, 517–529. doi:10.1007/s10653-016-9831-3
Tsai, H. J., Hung, C. H., Wang, C. W., Tu, H. P., Li, C. H., Tsai, C. C., et al. (2021a). Associations among heavy metals and proteinuria and chronic kidney disease. Diagnostics 11, 282. doi:10.3390/diagnostics11020282
Tsai, T.-L., Kuo, C.-C., Hsu, L.-I., Tsai, S.-F., Chiou, H.-Y., Chen, C.-J., et al. (2021b). Association between arsenic exposure, dna damage, and urological cancers incidence: A long-term follow-up study of residents in an arseniasis endemic area of northeastern taiwan. Chemosphere 266, 129094. doi:10.1016/j.chemosphere.2020.129094
Tu, S.-H., Chen, C., Hsieh, Y.-T., Chang, H.-Y., Yeh, C.-J., Lin, Y.-C., et al. (2011). Design and sample characteristics of the 2005-2008 nutrition and health survey in taiwan. Asia Pac. J. Clin. Nutr. 20, 225–237.
Wei, B., Yu, J., Wang, J., Yang, L., Li, H., Kong, C., et al. (2017). The relationships between arsenic methylation and both skin lesions and hypertension caused by chronic exposure to arsenic in drinking water. Environ. Toxicol. Pharmacol. 53, 89–94. doi:10.1016/j.etap.2017.05.009
WHO (2019). Preventing disease through healthy environments: Exposure to arsenic: A major public health concern. Geneva PP - Geneva: World Health Organization. Available at: https://apps.who.int/iris/handle/10665/329482.
Wu, D. W., Chen, S. C., Tu, H. P., Wang, C. W., Hung, C. H., Chen, H. C., et al. (2021). The impact of the synergistic effect of temperature and air pollutants on chronic lung diseases in subtropical taiwan. J. Pers. Med. 11, 819. doi:10.3390/jpm11080819
Keywords: arsenic, coastal heavy industry, seafood diet, urine, exposure assessment, southern Taiwan
Citation: Kaewlaoyoong A, Huang S-T, Wang S-L, Sun C-W, Chen J-J, Kuo C-H, Hung C-H, Chen S-C, Liang C-C, Tsai H-W, Wu C-F, Lin W-Y and Wu M-T (2022) Influential factors of urinary arsenic levels in the population residing close to one heavy-industrial area in Taiwan - A case study. Front. Environ. Sci. 10:1058408. doi: 10.3389/fenvs.2022.1058408
Received: 30 September 2022; Accepted: 21 November 2022;
Published: 02 December 2022.
Edited by:
Sudhir Kumar Pandey, Guru Ghasidas Vishwavidyalaya, IndiaReviewed by:
Md. Shiblur Rahaman, Noakhali Science and Technology University, BangladeshCopyright © 2022 Kaewlaoyoong, Huang, Wang, Sun, Chen, Kuo, Hung, Chen, Liang, Tsai, Wu, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Tsang Wu, OTYwMDIxQG1haWwua211aC5vcmcudHc=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.