- 1Department of Applied Sciences, Amity University, Raipur, India
- 2Department of Chemistry, Government Nagarjuna Post Graduate College of Science, Raipur, India
- 3Department of Chemistry, School of Applied Sciences, Kalinga Institute of Information Technology (KIIT), Bhubaneswar, Odisha, India
- 4Instituto de Investigación en Ciencias Ambientales de Aragón (IUCA), Universidad de Zaragoza. Escuela Politécnica Superior, Huesca, Spain
- 5Laboratory for inorganic environmental geochemistry and chemodynamics of nanoparticles, Division for Marine and Environmental Research, Ruđer Bošković Institute, Zagreb, Croatia
- 6KTH-International Groundwater Arsenic Research Group, Department of Sustainable Development, Environmental Science and Engineering, KTH Royal Institute of Technology, Teknikringen, Sweden
- 7Environmental Standards Research Group,Research Institute for Material and Chemical Measurement,National Metrology Institute of Japan (NMIJ), National Institute of Advanced Industrial Science and Technology (AIST), Ibaraki, Japan
Uranium, thorium, and rare earth elements (REEs) are important strategic elements in today’s world with a range of applications in high and green technology and power generation. The expected increase in demand for U, Th, and REEs in the coming decades also raises a number of questions about their supply risks and potential environmental impacts. This review provides an overview of the current literature on the distribution of these elements in different environmental compartments. For example, the processes of extraction, use, and disposal of U-, Th-, and REE-containing materials have been reported to result in elevated concentrations of these elements in air, in some places even exceeding permissible limits. In natural waters, the above processes resulted in concentrations as high as 69.2, 2.5, and 24.8 mg L−1 for U, Th, and REE, respectively, while in soils and sediments they sometimes reach 542, 75, and 56.5 g kg−1, respectively. While plants generally only take up small amounts of U, Th, and REE, some are known to be hyperaccumulators, containing up to 3.5 and 13.0 g kg−1 of U and REE, respectively. It appears that further research is needed to fully comprehend the fate and toxicological effects of U, Th, and REEs. Moreover, more emphasis should be placed on developing alternative methods and technologies for recovery of these elements from industrial and mining wastes.
1 Introduction
Uranium, thorium, and rare earth elements (REEs) are vital components of today’s modern world. They play a critical role in power generation and in many emerging technologies such as magnetic resonance imaging, satellites, batteries, light-emitting diodes (LED), and solar cells, as well as in the production of advanced materials such as catalysts, high-temperature ceramics and welding electrodes.
According to the 2020 Joint Report of the Nuclear Energy Agency (NEA) and the International Atomic Energy Agency (IAEA) (OECD, 2020), sufficient uranium resources are available to support the long-term, sustainable use of nuclear energy for low-carbon electricity generation, as well as for other uses such as industrial heat applications and hydrogen production. However, some recent events, such as the COVID pandemic and its impact on the industry, as well as recent declines in uranium production and exploration, could affect available supplies. On the other hand, the limiting factor in REEs production is the extraction process itself. Despite their name, REEs are relatively abundant (Suli et al., 2017; Balaram, 2019). However, the process of extraction and conversion into usable materials is expensive and environmentally harmful. Currently, REE resources are obtained from various geological settings, including carbonatites, ion-absorbing clay deposits, and monazite xenotime placer deposits (OECD, 2020; PMF IS, 2020), with China being the leading producer. The main production of REEs in China comes from carbonatite deposits, where the main mineral is bastnaesite. Outside China, minerals such as monazite, xenotime, and apatite are the main sources. It should be emphasized that monazite is also the most abundant thorium mineral, while thorium in uranium deposits is concentrated in thorite and thorianite. In addition, high Th contents have been found in carbonatites where it is associated with REEs in bastnaesite.
While primary U, Th, and REE resources currently account for the majority of industrial production and use, secondary resources are becoming increasingly important from an environmental and resource conservation perspective (Amaral et al., 2018). Since these elements often occur in the same mineral assemblages and source rocks (Rhodes, 2011; Jonathan, 2012; Goodenough et al., 2016; Ramos et al., 2016; Schulz et al., 2017), e.g., uraniferous phosphates processed by the fertilizer industry or monazite processed to extract REEs (Barthel and Tulsidas, 2014; Suli et al., 2017), the residues of the above processes are the most obvious target. Although some of the tailings have been extensively explored for the above purposes, they have generally been quickly abandoned for economic reasons. To date, such unconventional resources account for just over 11% of historical uranium production (OECD 2020), and even less for REEs and thorium.
A major advantage of secondary resources is that the main costs of mining and processing are incurred in the extraction of primary resources, and only the additional costs of processing them need to be considered. A second major advantage in terms of U and Th comes from radiation protection requirements. Primary sources are associated with higher levels of radioactive daughter products due to their higher uranium and thorium content (IAEA 2018), resulting in stringent radiological safety standards for mining and processing personnel, industry, and the environment. Secondary raw materials, on the other hand, require few additional safety measures for source materials.
As demand for U, Th, and REEs increases, the amount of tailings, byproducts, and wastes generated in the production and use of these elements continues to grow, inevitably leading to increasing environmental impacts. Due to industrial emissions, leaching of minerals, soils, and industrial wastes (e.g., coal ash, red mud, cement, and metal wastes, e-waste, used catalysts, etc.), the natural concentrations of these elements are increasing in various environmental compartments (e.g., Neves et al., 2009; Erickson, 2018; Arome et al., 2019; Wang et al., 2019b; Li Z et al., 2019; Piarulli et al., 2021; Pereirada et al., 2022). This increase highlights the need to understand not only their sources, but also their fate, mobility, and toxicity in the environment, especially in contaminated areas. Further research is also needed to understand the environmental impacts of industry from a life-cycle perspective in the secondary resource sector.
The objective of this review is to discuss the occurrence of U, Th, and REE in the environment, the composition of their resource materials, the potential of industrial wastes and end-use materials for recovery purposes, and the major environmental issues (contamination, sources, fate, mobility, and toxicity) associated with their increasing anthropogenic emissions.
2 Results and discussion
2.1 Literature search and selection criteria
Regarding the occurrence, use and distribution of REEs, U and Th, as well as the extraction, separation and purification techniques, a literature search on these elements was performed in four major academic databases, namely Web of Science, ScienceDirect, PubMed, and Google Scholar. After screening the results of the literature search considering the mobility and fate of these elements in different environmental compartments, 197 references were selected, including research articles (151), reviews (13), monographs, reports or books (27), and conference proceedings (6) from 1960 to 2022. In general, the publication of research articles on these critical elements has greatly increased since 2015 due to their wide use and industrial application.
Of the 197 references, 56% addressed the distribution of these elements in primary and secondary resource materials (P/SRM), air, water, soil, sediments, and biota. The remaining 44% of citations addressed their use, sources, fate, mobility, recycling, and toxicity.
Most studies (80%) were conducted in Asia and Europe, and only 20% involved other continents.
2.2 Occurrence of U, Th, and REEs
2.2.1 Uranium and thorium
Uranium gained importance with the development of the practical use of nuclear energy. In addition to its use in electricity generation, nuclear weapons, and fission reactors, it also found application in photography, lamps, X-ray equipment, etc. It is mainly extracted from minerals such as uraninite, pitchblende, carnotite, and brannerite (Lauf, 2016), and its occurrence is related to various geological conditions, including intrusive rocks, volcanic rocks, and polymetallic iron oxide breccia complexes. Economically mineable U mineral resources currently exist in many countries (PMF IS 2020), a large amount of uranium is also contained in seawater, given the relatively high U concentration of about 3 ng ml−1 (Dungan et al., 2017).
In geological matrices, uranium is found in a wide range of concentrations, e.g., from below 1–23 g kg−1 in igneous rocks, 2–5 mg kg−1 in metamorphic rocks, from 3 to 647 mg kg−1 in phosphatized limestones, from 46 to 85 mg kg−1 in phosphate ores, and around 71 and 11 mg kg−1 in slate and bauxites, respectively (Adams and Richardson, 1960; Godinez et al., 1997; Gupta et al., 2008; Tzifas et al., 2014; Wu et al., 2014; Al-Eshaikh et al., 2016; Santos-Francés et al., 2018; Asokan et al., 2020) (Table 1).
Thorium abundance is three times that of uranium in the Earth’s crust (Adams and Richardson, 1960; Korna et al., 2014; Asokan et al., 2020), it is usually mined from monazite, a phosphate mineral, although it also occurs in minerals such as thorite or thorianite (Barthel and Tulsidas 2014). Thorium concentrations in the lithosphere range from 8 to 12 μg g−1. It is a weakly radioactive metal, similar to uranium, and occurs naturally in various minerals, soils, and waters (Lauf, 2016). Thorium is not only critical for power generation, but also has applications in the production of alloys, arc welding, heat-resistant ceramics, ceramics, glass, etc.
2.2.2 Rare earth elements
The REEs are a group of 17 chemical elements in the periodic table, namely the 15 lanthanides (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) and the lighter scandium and yttrium. Scandium and yttrium are included among the REEs because they generally occur in the same ore deposits as the lanthanides and have similar physical and chemical properties. They represent a coherent group of elements typically found together in minerals, some of which are technologically critical elements: La, Ce, Gd, Nd, Pr, and Dy (Malhotra et al., 2020).
Precisely because of their exceptional physicochemical properties, REEs have found widespread application in industrial goods, which has led to a steady increase in global demand for these elements (Balaram, 2019). REEs are essential components of many technological devices, including electric, and hybrid vehicles, flat-screen displays, cell phones, and computer hard drives (Lucas et al., 2014), but they also have many other applications (Figure 1; LePan 2021).
For example, the pyrophoric alloy of Ce and Fe is used as “flint” in gas and cigarette lighters (Cotton, 1991); the mixed lanthanide alloys of Ce, La, Nd, and Pr are widely used in metallurgy (Sastri et al., 2003); many lanthanides are used as catalysts for petroleum refining (Kilbourn, 1986; Balaram, 2019) or in nuclear technology to absorb neutrons; Eu and Yb oxides are widely used in the color television industry; La2O3 is used to color ceramics and glasses; Ce2O3 is used to polish glass; Nd, Pr, and Dy have unusual magnetic properties; and other lanthanide compounds are used in lighting technology (Zhang et al., 2020). In addition, lanthanides have been used in agriculture for several decades, especially in China, as fertilizers to increase yields (Pang et al., 2002).
Despite their name, REEs are indeed relatively abundant in the Earth’s crust, with a mean total concentration (∑REEs) in the Earth’s crust of up to 240 mg kg−1 (Balaram, 2019), but are rarely found in concentrations that are economically mineable (Suli et al., 2017). Current resources are associated with several primary geologic settings: ion-absorption clay deposits, igneous systems including carbonatites which are the main source, and monazite-xenotime placer deposits. Ore production of REEs per country and projected mineral reserves are shown in Figure 2 (LePan, 2021).
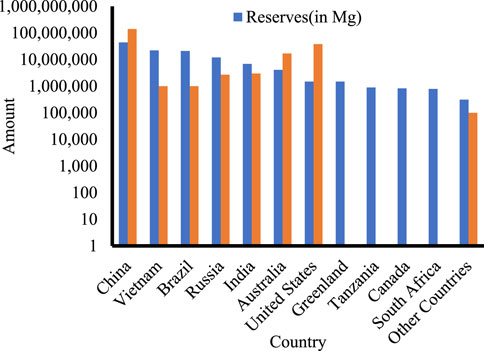
FIGURE 2. Current production and predicted reserves of REEs per country plotted based on the data by (LePan, 2021).
As shown in Table 1, bauxite, bituminous coal, and lignite contain REEs in the range of 250–675 mg kg−1 (Mayfield and Lewis 2013; Deady et al., 2016; Hower et al., 2016; Montross et al., 2020). Much higher REEs concentrations (172–3385 mg kg−1) have been found in granite, and phosphorus deposits (Hua et al., 2010; Emsbo et al., 2015; Asokan et al., 2020), while extremely high concentrations (17%–20%) have been recorded for carbonatites (Castor, 2008; RMREI, 2012; Fan et al., 2016).
2.3 Secondary resources of U, Th and REEs
Table 2 summarizes the concentrations of U, Th, and REEs found in some industrial wastes. As shown in Table 2, all these elements can be found in elevated concentrations in red mud, coal ash, and magnetic scrap. During the Bayer process, most REEs pass almost completely into the bauxite residue. Depending on the initial REE concentration in the bauxite ore, the bauxite residue may have a total REE concentration of up to 2500 mg kg−1, as in the case of Jamaican red mud (Wagh and Pinnock, 1987). In addition to REEs, such residues may also be enriched in uranium and thorium. Gu et al. (2017) reported U, Th, and REEs ranging from 20 to 31, 72 to 159, and 468–1237 mg kg−1, respectively, in Chinese red mud, while the resulting red mud after alumina extraction from Greek bauxites (497–674 mg kg−1 REEs) had REEs up to 1777 mg kg−1 (Binnemans et al., 2013; Deady et al., 2016). Coal combustion processes also lead to enrichment of REEs in ash by a factor of six to ten compared to coal (e.g., Seredin and Dai, 2012; Franus et al., 2015; Fiket et al., 2018a; Okeme et al., 2020). For example, in southwestern China (in Guizhou), bottom and fly ashes from five coal-fired power plants were found to be enriched in REEs and yttrium, with average concentrations of 658 ± 296 mg kg−1 and some elements reaching levels as high as 1257 mg kg−1 (Li C et al., 2019). The contents of REEs and critical REEs (Y, Nd, Eu, Tb, Dy, and Er) in various coal ashes reported by Kolker et al. (2017) were even higher, ranging from 192 to 1668 mg kg−1 and from 28.3% to 44.5%, respectively.
Recently, Costis et al. (2021) presented a comprehensive review of the potential recovery of REE from abundant secondary sources such as mine drainage, phosphogypsum residues, and U mine waste. Such mining and industrial wastes contain a wide range of REEs, with concentrations reaching extremely high levels in certain locations. Moraes et al. (2017) reported values as high as 130 mg L−1 in the drainage of the Osamu-Utsumi mine in Brazil, while phosphogypsum extracted from sedimentary and especially igneous phosphate rock reaches even higher values. For example, 481 mg kg−1 of REE was measured in phosphogypsum derived from sedimentary rocks from Egypt (Gasser et al., 2019), while up to 26 g kg−1 REE was found for phosphogypsum derived from igneous rocks from Brazil (Brückner et al., 2020).
Nowadays, only some countries rely on recycling U, Th, and REEs from coal ash, red mud, pre-consumer scrap, industrial residues, mine waste, metallurgical slags, and e-waste (Kumar et al., 2014; Binnemans et al., 2015; IAEA, 2018). Among these secondary resources, some authors also include the so-called “unconventional sources” that have been exploited for some time, especially as uranium sources (e.g., uranium-bearing phosphate and black shale deposits). There are estimates that such unconventional uranium resources may contain up to 7.6 million tons of U (OECD, 2020). Although unconventional resources are currently uneconomic, recovery of uranium from these deposits could become profitable through improved extraction techniques or upgrading of byproducts.
Chemical and biological leaching methods, followed by solvent extraction or alkali smelting, are used to achieve substantial recovery of these metals from secondary sources (Maslov et al., 2010; Santos and Ladeira, 2011; Amaral et al., 2018; Dinal et al., 2019; Jyothi et al., 2020; Ahmed et al., 2021). The methods used for REE recycling of used electronic devices also include crushing and grinding the devices into powder form, from which the essential components are extracted by further separating the elements using pyrometallurgical methods (Jowitt et al., 2018).
Some of the conventional methods involve the use of various acid mixtures, e.g., uranium (54.3 mg kg−1) and REEs are often leached from coal ash with a mixture of HNO3 and HF, using an anion exchanger to extract uranium from solution, followed by further purification (Maslov et al., 2010; Mondal et al., 2019). On the other hand, effective extraction and recovery of REEs in contaminated soil and other waste materials has been achieved by using reusable biosurfactants (Gao et al., 2012; Li C et al., 2019). Chour et al. (2018) developed a method to recover REEs from the hyperaccumulator plant Dicranopteris dichotoma by an improved ion-exchange leaching process, which resulted in a REE purity of 81.4% and a recovery of 78%. Santos and Ladeira (2011) extracted uranium (0.25%) from waste materials composed of Ca, S, Mn, and Al by using Na2CO3 and NaHCO3 as sequestering agents, while other elements were crystallized as ettringite, gypsum, calcite, and bassanite. Recently, the macromolecule lanmodulin (binding protein) was proposed for the selective extraction of REEs from low-grade sources (Deblonde et al., 2020).
However, in order to optimize extraction processes, achieve much higher efficiency, and make them robust enough for different waste types, further efforts are needed, and not only at laboratory scale. This is especially true for the efficiency of extraction from technical waste. Most scrap magnets are based on REEs-Fe-B and generally consist of more than 30% neodymium, praseodymium and dysprosium. Since direct melting is not considered beneficial (Voßenkaul et al., 2013), a direct leaching step offers the possibility to recover REEs very easily and with lower energy consumption. Subsequent separation is also much easier compared to primary extraction, as only four of the seventeen REEs are in solution.
2.4 Environmental impact of U, Th, and REEs
Intensive REE mining and production have resulted in significant environmental and health impacts. Mining and extraction releases REE-containing dust, other toxic metals, and chemicals into the air and surrounding waters, which can affect not only humans but also soil, wildlife, and plants. In addition, landfills exposed to weathering have the potential to further pollute air, soil, and water, resulting in further environmental degradation and human health hazards (Wang and Liang, 2015; Balaram, 2019). Because REE minerals often contain significant amounts of uranium and thorium, mining residues and their weathering can contaminate air, water, soil, and groundwater with radioactive elements. In addition, the extensive use of REEs in modern technologies, as well as the burning of biomass and coal, fertilizers, livestock feed, medical facilities, electronic waste, recycling facilities, and petroleum refining, further increases their concentration in the environment (Ganguli and Cook, 2018; Gwenzi et al., 2018; Shao et al., 2020). In the following text, the levels of U, Th, and REE in different environmental compartments (Tables 3, 4, 5) are discussed to provide an overview of their levels in natural and contaminated environments.
2.4.1 Air
As mentioned earlier, uranium, thorium, and REEs can be released and mobilized from primary and secondary deposits and tailings piles through natural weathering and erosion, as well as through processes of coal combustion and biomass burning, and can be further transported to various environmental compartments (Erickson, 2018; Li C et al., 2019; Piarulli et al., 2021). Consequently, the processes of extraction, use, and disposal of U-, Th-, and REE-containing materials lead to an increase in their concentrations in the ambient air as aerosols or particulates above the permissible or prescribed levels.
Background concentrations of uranium in ambient air vary widely from 0.02 to 0.40 ng m−3, with an average value of about 0.1 ng m−3 or 1 μBq m−3 in radioactivity units (Bem and Bou-Rabee, 2004). In Canada, the recommended limit for U to protect human health is 30 ng m−3 (AAQCs 2012), while in India, the prescribed annual dose limit for Th (weighted sum of Ra, Th, and K activities) is 30 mSv for the population (Parthasarathy, 2018). The average maximum allowable concentrations (MAC) of REEs fluoride compounds (leading to pneumoconiosis) are 0.5 mg m−3 (Rim et al., 2013).
Concentrations exceeding the recommended limits set by AAQCs (2012) are often found near mining and industrial areas (Tracy and Meyerhof, 1967; Shaltout et al., 2013; Wang et al., 2014; Dai et al., 2016; Wang et al., 2016; Hussein et al., 2018; Fesenko and Emlutina 2021; Kolawole et al., 2021), Table 3. For example, U concentrations of 2–200 ng m−3 have been measured within 2 km of a refinery (Tracy and Meyerhof, 1967), which exceeds the recommended limits by a factor of 6. In addition, thorium and REE concentrations of up to 1 × 10−4 Bq·m−3 and 173–298 ng m−3, respectively, have been detected in atmospheric particles from Chinese cities (Wang et al., 2014; Wang et al., 2016; Fesenko and Emlutina 2021). The average total concentration of REEs in total PM, PM10 (coarse dust), and PM2.5 (fine dust) measured in the Yangtze River Delta region of China was 12.0, 9.4, and 2.2 ng m−3, respectively (Dai et al., 2016), with particle size distribution indicating significant fractionation in the coarse fraction (Dai et al., 2016; Hussein et al., 2018). REE concentrations in collected dust samples from Greater Cairo (Egypt) ranged from 1 to 60 μg g−1, with the highest concentrations found in dust samples outside the city, indicating a possible natural source of REE (Shaltout et al., 2013). Even higher REE levels were found in atmospheric dust from the city of Ibadan (Nigeria), ranging from 42.5 μg g−1 (residential) to 785 μg g−1 (industrial), with mean ΣREE levels in industrial, transportation, landfill, residential, remote areas, and local soil of 638, 283, 130, 163, 96.0, and 144 μg g−1. The average ΣREE content in dust in industrial and transportation areas was about 4.5 and 2.0 times higher than the average ΣREE content in soil, respectively (Kolawole et al., 2021). The main sources of airborne thorium are believed to be the sites of processing and extraction of thorium, uranium, and radium from ores and concentrates, but data on the fate and transport of airborne thorium are rather limited.
2.4.2 Water
Major anthropogenic sources of U, Th, and REE include wastes and effluents from medical facilities, mines, industrial mineral processing plants, nuclear power plants, the electronics industry, oil refineries, fertilizer and feed mills, etc. (e.g., Harmsen and Haan, 1980; Brindha and Elango, 2013; Keesari et al., 2014; Coyte et al., 2018; Gwenzi al, 2018; CGWB, 2020; etc.). Although the concentrations of U, Th, and REEs in natural waters are relatively low, about 3.3, 0.006, and 0.004–0.024 μg L−1, respectively (Deng et al., 2017; Degueldre and Joyce, 2020), numerous processes, such as mining, extraction, weathering, and disposal of these elements, can affect their natural levels (Table 4).
Maximum permissible levels (MPLs) for U and Th in water are set at 30 μg L−1 (0.75 ng m−3 or 20.3 pCi L−1) and 15 pCi L−1 (0.555 Bq·L−1), respectively (Sneller et al., 2000; ATSDR, 2009). MPLs for fresh and (marine) water for Eu, La, Ce, Pr, Nd. Sm, Gd, and Dy are 62 (0.72), 10 (1.0), 22 (0.15), 9 (0.92), 1.4 (0.85), 7.6 (0.42), 6.8 (0.52), and 9.1 (0.16) μg L−1, respectively (Sneller et al., 2000).
However, uranium concentrations in surface and groundwater exceeding 6 μg L−1 have been reported in several countries (notably China, India, UAE, Saudi Arabia, Korea, Nigeria, and Tanzania), with concentrations in the waters of many countries exceeding the ATSDR (2009) permissible limit of 30 μg L−1 (Aleissa et al., 2004; Ahmed et al., 2014; Flanagan et al., 2014; Wu et al., 2014; Cordeiro et al., 2016; Shin et al., 2016; Pant et al., 2017; Kaishwa et al., 2018; Bergmann and Graca, 2020; CGWB, 2020). In India, for example, U concentrations in groundwater vary from 0.6 μg L−1–1443 μg L−1 and often exceed the above limit of 30 μg L−1, especially in areas of uranium mineralization that have a natural anomaly of U (Babu et al., 2008; Brindha and Elango, 2013; Keesari et al., 2014; Raghavendra et al., 2014; Thivya et al., 2014; Saini and Bajwa, 2016; Pant et al., 2017; Rishi et al., 2017; Sharma et al., 2017; Coyte et al., 2018; CGWB, 2020; Sahu et al., 2020; Tanwer, et al., 2022) or near industries such as cement factories, fertilizer plants, chemical factories, and coal-fired thermal power plants (Saini and Bajwa, 2016; CGWB, 2020).
In fact, U concentrations in water were found to be many times above the permissible limit in 14 Indian states: Panjab, Haryana, Telangana, Delhi, Rajasthan, Andhra Pradesh, Uttar Pradesh, Karnataka, Madhya Pradesh, Tamilnadu, Jharkhand, Chhattisgarh, Gujrat, Himachal Pradesh, Maharashtra, Odisha, West Bengal, and Bihar (CGWB, 2020). Even unexploited uranium ores have been reported to contribute to uranium levels in local waters (Cordeiro et al., 2016). For example, uranium contamination of groundwater by naturally occurring uranium was observed in the Horta da Vilariça region of Portugal, with U concentrations in groundwater as high as 3.5 mg L−1 (Cordeiro et al., 2016).
Ahmed et al. (2014) reported thorium concentrations in groundwater ranging from 0.2–2.5 μg L−1. As for REEs, concentrations in surface water, groundwater, and wastewater were found to range from 1.4 to 2.9, 0.1 to 229, and 4.5–118 g L−1, respectively, in different countries (Smedley, 1991; Li et al., 2013; Medas et al., 2013; Tang et al., 2020; Tian et al., 2020). Extremely high REE water contents are usually associated with mining sites or associated mine drainages, as in the Wiśniówka waters in Poland, where 8.5 and 17.9 mg L−1 total REE associated with As-rich pyrite mineralization were detected in ponds and drainage ditches of the Wiśniówka mining area (Migaszewski et al., 2019). Moreover, the increasing use of REEs, especially Gd, for medicinal purposes in recent decades has led to an increase in their concentrations not only in wastewater but also in streams (e.g., Bau and Dulski, 1996; Bau et al., 2006; Kulaksız and Bau, 2013; Klaver et al., 2014), up to two orders of magnitude higher than natural concentrations.
Regardless of their origin, aquatic environments play a dual role with respect to these elements: either as temporary or long-term storage (Gwenzi et al., 2018; Gwenzi et al., 2021). Because REEs readily bind to colloidal particles and suspended solids or are complexed at the surface with inorganic and/or organic ligands (Gwenzi et al., 2018), they are eventually removed from the water column into the sediment. Their co-precipitation with solids and removal in sediments is generally dominated by Fe and Mn oxyhydroxides, which are characterized by a large surface area and high adsorption capacity, while their return to solution depends on changing environmental conditions. Whether they are in sediment, sediment pore water, or water itself, elevated concentrations of U, Th, and REEs can cause a wide range of toxicities in aquatic organisms (ATSDR, 2009; Briner 2010; Tai et al., 2010; Pagano et al., 2015; Gwenzi et al., 2018; Malhotra et al., 2020).
2.4.3 Soil and sediments
Because the elements U, Th, and REE are ubiquitous in the Earth’s crust (Kasar et al., 2019), their main sources in surface soils and sediments are predominantly geogenic; however, inputs from industrial and mining processes and atmospheric deposition should not be ignored when interpreting their distribution in the environment (Duplay et al., 2014; Kritsananuwat et al., 2014; Gu et al., 2020). While the mobility of U in the ecosystem depends on redox conditions (IRNS, 2012) and complexation by organic matter (Bone et al., 2020), with the most stable and mobile form being U(VI), the mobility of Th in soil is very limited due to strong sorption to soil particles (Torstenfelt, 1986). In contrast, the content of REEs in soil is related to redox potential and pH as well as to the presence of clay, carbonate, organic matter, and Al, Fe, and Mn oxides and oxyhydroxides (Aide and Aide, 2012; Felipe-Sotelo et al., 2017; Mihajlovic and Rinklebe, 2018; Aide, 2019; Tang et al., 2020; Ogawa et al., 2021). Their content generally decreases with the decrease of pH, redox potentials, and Al-Mn-Fe contents (Aide and Aide, 2012; Mihajlovic and Rinklebe, 2018; Aide, 2019). At the same time, their residence time in sediments increases under less oxidative and alkaline conditions in an aquatic reservoir (Felipe-Sotelo et al., 2017; Ogawa et al., 2021).
The average concentrations of U and Th in soils reported by Harmsen and Hann (1980) are 1–2 and 6 mg kg−1, respectively, while Sneller et al. (2000) reported MPLs at least 10-fold higher for Y, La, Ce, Pr, Nd, Sm, Gd, and Dy in freshwater sediments than in marine sediments: 1.4(0.18), 4.7(0.51), 18.8(0.22), 5.8(0.61), 7.5(0.48), 2.5(0.15), 1.8(0.14), and 2.2 (0.89) g·kg−1 (dry weight).
However, uranium, thorium, and REE levels of 630, 75, and 56500 mg kg−1, respectively, have been detected in various surface soils, with the highest levels measured in some areas of the United States of America, Bulgaria, and China (Neves et al., 2009; Caldwell et al., 2012; Mihaylova et al., 2013; Sadeghi et al., 2013; Wang and Liang, 2015; Fiket et al., 2016; Ramos et al., 2016; Han et al., 2017; Brito et al., 2018; Kaishwa et al., 2018; Santos-Francés et al., 2018; Arome et al., 2019; Wang et al., 2019a; Freitas Rodrigo et al., 2021; Wang et al., 2022; Table 5). In sediments, U, Th, and REE values of up to 126, 12, and 4000 mg kg−1, respectively, were detected, with the highest values measured in some areas of Malaysia, Thailand, and Japan (Zhu et al., 1997; Munksgaard et al., 2003; Borrego et al., 2005; Marmolejo-Rodríguez et al., 2007; Prego et al., 2009; Tranchida et al., 2011; Deepulal et al., 2012; Antonina et al., 2013; Kritsananuwat et al., 2014; Cordeiro et al., 2016; Sahu et al., 2016; Fiket et al., 2018b; Brito et al., 2018; El-Taher et al., 2018; El-Taher et al., 2019; Elias et al., 2019; Nascimento et al., 2019; Sivasamandy et al., 2019; Bergmann and Graça, 2020; Tanaka et al., 2020; Wu et al., 2020; Freitas Rodrigo et al., 2021; Kim et al., 2022; Ndjama et al., 2022).
Although some sources are associated with nearby ore deposits (e.g., Neves et al., 2009; Mihaylova et al., 2013; Kaishwa et al., 2018; Wang et al., 2019a) or secondary sources of these elements, such as the remains of the phosphorus fertilizer industry (Brito et al., 2018) or coal ash deposits (Fiket et al., 2018a), elevated U, Th, or REE concentrations in soils and sediments can also be attributed to other influences, such as the impact of steel mills (Arome et al., 2019), different agricultural practices (Han et al., 2017), or mining areas for other commodities (Pereirada et al., 2022). For example, soils in the gold mining region of the Amazon were found to be contaminated with REEs, with Y being most abundant in urban, agricultural, and mining areas and enriched in soil by a factor of 18.2, 39.0, and 44.4, respectively (Pereirada et al., 2022).
2.4.4 Biota accumulation
Due to their widespread distribution in the environment, U, Th, and REE are also found in certain concentrations in biota. Current literature indicates their occurrence in macroalgae, benthic invertebrates, including bivalves, echinoderms and crustaceans, fish and plants (Hegazy and Emam, 2011; Kovaříková et al., 2019; Piarulli et al., 2021), and some species can be considered hyperaccumulators. The transfer of these elements from soil to plant is a complex process that depends on many factors: pH, redox potential, organic matter in plants, and presence of certain ions (Kasar et al., 2019; Kovaříková et al., 2019). In general, element content decreases from leaves to stems to fruits and seeds (Tyler 2004). It is related to the nutrient content of the soil (Tyler, 2004; Rodrı́guez et al., 2005; Oufni et al., 2011) and the content of clay and organic matter in the soil (Tyler, 2004; Hegazy and Emam, 2011). A selection of data on uranium and REEs in plants, including mosses, ferns, algae, macrophytes, and freshwater organisms, is presented in Table 6. However, the table does not include data for Th because such data are very limited or non-existent.
Plants and aquatic organisms have been reported to accumulate U from soils and water in the range of 0.08–3500 mg kg−1 (Huang et al., 1998; Dienemann et al., 2002; Chang et al., 2005; Sakamoto et al., 2008; Viehweger and Geipel, 2010; Caldwell et al., 2012; Cordeiro et al., 2016; Boryło et al., 2017; Li C et al., 2019; Bergmann and Graca, 2020). Hyperaccumulation of U from contaminated sites has been reported for several plant species and aquatic organisms (Dienemann et al., 2002; Chang et al., 2005; Viehweger and Geipel, 2010; Caldwell et al., 2012; Li C et al., 2019; Bergmann and Graca, 2020). Following the above mentioned general rule, U is accumulated more in plant leaves (up to 100 μg kg−1) than in stems, fruits, and seeds (Anke et al., 2009). For marine biota, U concentrations of up to 178 ± 45 mg kg−1 have been reported (Abdullah et al., 2015; Bergmann and Graça, 2020; Matara-aho, 2020).
Plants, mosses, fungi, tea trees, algae, and trees accumulate REEs at trace to low levels, ranging from 0.2 to 109 g kg−1 (Markert 1987; Zhang et al., 2007; Li et al., 2012; Mashitah et al., 2012; Brioschi et al., 2013; Mahmood et al., 2015; Pratas et al., 2017; Zhuang et al., 2017; Mędyk and Falandysz, 2022; Shi et al., 2022), while Phytolacca icosandra has been found to hyperaccumulate REEs with concentrations up to 13 g kg−1 (Yuan et al., 2018; Grosjean et al., 2019). In general, light REEs are more readily taken up by plants than heavy REEs due to their higher mobility in soil. For example, citrus plants have been reported to act as bioaccumulators for light REEs (Ramos et al., 2016).
As part of research efforts aimed at recycling these elements in a stepwise and cost-effective manner (Sinha et al., 2016), studies on the use of plants (Sytar et al., 2016) or microorganisms (Jalali and Lebeau, 2021) for REE recycling are receiving increasing attention due to their ability to accumulate these elements in significant concentrations.
The positive effects of REEs on quality and growth have also been studied in crops and livestock (Vítová et al., 2018). In the former, positive effects on photosynthesis, biomass production, cytoplasmic membranes, nutrient metabolism, enzymes, water use efficiency, germination and growth, amount of sugars and other metabolites, and number of vitamins, among others, have been found (Kovaříková et al., 2019). In animals, REEs are used as food additives because they can improve the production of milk, eggs, and meat. However, some studies show just the opposite effect, with toxic effects associated with substitution of Mg, Ca, Fe, and other elements by REEs that disrupt bone integrity, cellular signaling, fluid peroxidation, and phosphate deficiency in a variety of animals and plants (Sneller et al., 2000). Such positive or negative effects depend on the REE dose. Therefore, further research on this topic is needed to accurately determine whether REEs are “essential” or merely support growth and development (Ramos et al., 2016).
Human activities have caused concentrations of U, Th, and REEs to increase above natural levels in many environmental compartments, making them accessible to humans through various pathways. According to the Agency for Toxic Substances and Disease Registry (ATSDR, 1990), the health effects of natural and depleted uranium are due to chemical effects rather than radiation. To primarily prevent kidney damage, emission limits for human body exposure to uranium have been established for ingestion of contaminated food (0.6–1.0 pCi d−1, 1 µg = 0.72 pCi), drinking water (0.6–1.0 pCi d−1), and inhalation of contaminated air (0.0007–0.007 pCi d−1), respectively (USEPA, 1983; ATSDR, 1990). On the other hand, inhalation of thorium dust has been reported to increase the risk of lung and pancreatic cancer (USEPA, 2015). The health effects of REEs have been found to be associated with modulation of oxidative stress on Nuclear Factor Erythroid-2-Related Factor-2 (transcription factor) protein and endocrine effects on the hypothalamic-pituitary-thyroid axis (Pagano et al., 2015; Guo et al., 2020). The standard sanitary limit regulated by national standard of China for REEs in vegetables and fruits is 0.70 mg kg−1 (Jin et al., 2015).
3 Conclusion
The rapid development of today’s society, accompanied by increasing demand for energy and environmentally friendly high technologies, requires an ever-increasing access to U, Th, and REEs. As the demand for these elements increases, so does the amount of waste, byproducts, and residues generated during the various steps of manufacturing and using products containing U, Th, and REEs. All this leads to an ever-increasing pollution of the environment and all its components. As a result, elevated concentrations of U, Th, and REE have been detected in air, water, and biota. However, there is still a lack of information on their fate in the environment, their toxicological effects on organisms, and the consequences for the ecosystem, prompting research on these issues.
In this context, secondary resources are gaining importance not only from the point of view of environmental friendliness and resource conservation, but also as a means of securing supply under conditions of an uncertain and unstable market. Recycling of U, Th, and REE from industrial wastes such as coal ash, red mud, magnets, catalysts, etc. Has been studied and applied for years, but not on a large scale. At the same time, alternative methods such as the use of hyperaccumulator plants, extraction from unconventional sources, or extraction with macromolecules or microorganisms are becoming increasingly important.
Author contributions
KP: Conceptualization, data curation, investigation, methodology, validation, visualization, writing—original draft. SS: Data curation, visualization, graphics drafting, review. JM: Investigation, validation, editing. PM-R: Data curation, graphic drafting, editing original draft. ŽF: Software, editing—original draft. PB: Review and editing. YZ, Discussion, review and editing.
Acknowledgments
The University Grants Commission (UGC), New Delhi is gratefully acknowledged for providing financial support through BSR grant no. F.18-1/2011(BSR)2016.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aaqcs, (2012). Ambient air quality criteria (AAQCs), ontario ministry of the environment. Canada. Available at: www.airqualityontario.com/downloads/AmbientAirQualityCriteria.pdf.
Abdullah, A., Hamzah, Z., Saat, A., Wood, A., and Alias, M. (2015). Accumulation of radionuclides in selected marine biota from Manjung coastal area. AIP Conf. Proceed. 1659, 050009. doi:10.1063/1.4916879
Adams, J. A. S., and Richardson, K. A. (1960). Thorium, uranium, and zirconium concentrations in bauxite. Econom. Geol. 55, 1653–1675. doi:10.2113/gsecongeo.55.8.1653
Ahmed, M., Dalal, A., Ala, A., and Xiaolin, H. (2014). Distribution of uranium and thorium in groundwater of arid climate region. Vienna, Austria: EGU General Assembly. 2014EGUGA.1613737M.
Ahmed, M. R., Mohammed, H. S., El-Feky, M. G., and Abdel-Monem, Y. K. (2021). Studies on uranium extraction from leach liquor of the mineralized shear zone at El-Missikat area using tri-n-octylamine. J. Radioanal. Nucl. Chem. 327, 731–743. doi:10.1007/s10967-020-07545-3
Aide, M. (2019). Lanthanide soil chemistry and its importance in understanding soil pathways: Mobility, plant uptake, and soil health. London, United Kingdom: Lanthanides. In book. doi:10.5772/intechopen.79238
Aide, M., and Aide, C. (2012). Rare earth elements: Their importance in understanding soil genesis. ISRN Soil Sci. 2012, 11. ID 783876. doi:10.5402/2012/783876
Al-Eshaikh, M. A., Kadachi, A. N., and Sarfraz, M. (2016). Determination of uranium content in phosphate ores using different measurement techniques. J. King Saud. Univer.–Engin. Sci. 28, 41–46. doi:10.1016/j.jksues.2013.09.007
Aleissa, A., Aleissa, K., Shabana, E., and Almasoud, F. I. (2004). Accumulation of uranium by filamentous green algae under natural environmental conditions. J. Radioanal. Nucl. Chem. 260, 683–687. doi:10.1023/B:JRNC.0000028232.52884.61
Amaral, J. C. B. S., Sá, M. L. C. G., and Morais, C. A. (2018). Recovery of uranium, thorium and rare Earth from industrial residues. Hydrometallurgy 181, 148–155. doi:10.1016/j.hydromet.2018.09.009
Anke, M., Seeber, O., Müller, R., Schäfer, U., and Zerull, J. (2009). Uranium transfer in the food chain from soil to plants, animals and man. Geochem 69 (2), 75–90. doi:10.1016/j.chemer.2007.12.001
Antonina, A. N., Shazili, N. A. M., Kamaruzzaman, B. Y., Ong, M. C., Rosnan, Y., and Sharifah, F. N. (2013). Geochemistry of the rare earth elements (ree) distribution in terengganu coastal waters: A study case from redang island marine sediment. Open J. Mar. Sci. 03 (03), 154–159. doi:10.4236/ojms.2013.33017
Arome, A., Gyuk, P. M., Ogwo, A. M., and Suleiman, I. (2019). Determination of the uranium content in some soil samples from ajaokuta, kogi state: Nigeria. Int. J. Res.–Granthaalayah. 7, 252–258. doi:10.29121/granthaalayah.v7.i9.2019.608
Asokan, A. D., Elangovan, R., Vishwakarma, N., Hari, H. R., and Mohan, M. R. (2020). Petrogenesis of the kanker granites from the bastar craton: Implications for crustal growth and evolution during the archean–proterozoic transition. Front. Earth Sci. 8. doi:10.3389/feart.2020.00212
ATSDR (Agency for Toxic Substances and Disease Registry) (2009). Environmental health and medicine education, uranium toxicity. Atlanta, GA: Public Health Service, U.S. Department of Health and Human Services.
ATSDR (Agency for Toxic Substances and Disease Registry) (1990). Toxicological profile for radium. Atlanta, GA: Public Health Service, U.S. Department of Health and Human Services.
Aytekin, H., and Baldık, R. (2012). Radioactivity of coals and ashes from Catalagzi coal-fired power plant in Turkey. Radiat. Prot. Dosim. 149 (2), 211–215. doi:10.1093/rpd/ncr225
Babu, M. N. S., Somashekar, R. K., Kumar, S. A., Shivanna, K., Krishnamurthy, V., and Eappen, K. P. (2008). Concentration of uranium levels in groundwater. Int. J. Environ. Sci. Technol. 5 (2), 263–266. doi:10.1007/bf03326020
Balaram, V. (2019). Rare Earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 10 (4), 1285–1303. doi:10.1016/j.gsf.2018.12.005
Barthel, F. H., and Tulsidas, H. (2014). Occurrences, geological deposits and resources. IAEA Int. Symposium Uranium Raw Material Nucl. Energy, 27. Vienna, Austria, 2014.
Bau, M., and Dulski, P. (1996). Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sci. Lett. 143, 245–255. doi:10.1016/0012-821X(96)00127-6
Bau, M., Knappe, A., and Dulski, P. (2006). Anthropogenic gadolinium as a micropollutant in river waters in Pennsylvania and in Lake Erie, northeastern United States. Chem. Erde 66 (2), 143–152. doi:10.1016/j.chemer.2006.01.002
Bem, H., and Bou-Rabee, F. (2004). Environmental and health consequences of depleted uranium use in the 1991 Gulf war. Environ. Int. 30 (1), 123–134. doi:10.1016/S0160–4120(03)00151–X
Bergmann, M., and Graça, M. A. S. (2020). Bioaccumulation and dispersion of uranium by freshwater organisms. Arch. Environ. Contam. Toxicol. 78, 254–266. doi:10.1007/s00244–019–00677–y
Binnemans, K., Jones, P. T., Blanpain, B., Gerven, T. V., and Pontikes, Y. (2015). Towards zero-waste valorisation of rare-Earth-containing industrial process residues: A critical review. J. Clean. Prod. 99, 17–38. doi:10.1016/j.jclepro.2015.02.089
Binnemans, K., Pontikes, Y., Jones, P. T., Gerven, T. V., and Blanpain, B. (2013). Recovery of rare earths from industrial waste residues: A concise review, 191–205.Proceeding of 3rd International Conference, Slag vaporization Symposium, Lauren, Belgium.
Bone, S. E., Cliff, J., Weaver, K., Takacs, C. J., Roycroft, S., Fendorf, S., et al. (2020). Complexation by organic matter controls uranium mobility in anoxic sediments. Environ. Sci. Techno. 54, 1493–1502. doi:10.1021/acs.est.9b04741
Borrego, J., López-González, N., Carro, B., and Lozano-Soria, O. (2005). Geochemistry of rare-Earth elements in holocene sediments of an acidic estuary: Environmental markers (tinto river estuary, south-western Spain). J. Geochem. Explor. 86 (3), 119–129. doi:10.1016/j.gexplo.2005.05.002
Boryło, A., Romańczyk, G., and Skwarzec, B. (2017). Lichens and mosses as polonium and uranium biomonitors on Sobieszewo Island. J. Radioanal. Nucl. Chem. 311 (1), 859–869. doi:10.1007/s10967–016–5079–8
Brindha, K., and Elango, L. (2013). Occurrence of uranium in groundwater of a shallow granitic aquifer and its suitability for domestic use in southern India. Radioanal. Nucl. Chem. 295, 357–367. doi:10.1007/s10967–012–2090–6
Briner, W. (2010). The toxicity of depleted uranium. Int. J. Environ. Res. Public Health 7 (1), 303–313. doi:10.3390/ijerph7010303
Brioschi, L., Steinmann, M., Lucot, E., Pierret, M. C., Stille, P., Prunier, J., et al. (2013). Transfer of rare Earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE. Plant Soil 366, 143–163. doi:10.1007/s11104-012-1407-0
Brito, P., Prego, R., Mil-Homens, M., Caçador, I., and Caetano, M. (2018). Sources and distribution of yttrium and rare Earth elements in surface sediments from Tagus estuary, Portugal. Port. Sci. Total Environ. 621, 317–325. doi:10.1016/j.scitotenv.2017.11.245
Brückner, L., Elwert, T., and Schirmer, T. (2020). Extraction of rare Earth elements from phosphogypsum: Concentrate digestion, leaching, and purification. Metals 10 (1), 13121. doi:10.3390/met10010131
Caldwell, E., Duff, M., Hicks, T., Coughlin, D., Hicks, R., and Dixon, E. (2012). Bio-monitoring for uranium using stream-side terrestrial plants and macrophytes. J. Environ. Monit. 14 (3), 968. doi:10.1039/c2em10738d
Castor, S. B. (2008). The Mountain Pass rare-Earth carbonatite and associated ultrapotassic rocks, California. Can. Mineralogist 46 (4), 779–806. doi:10.3749/canmin.46.4.779
CGWB (2020). Central groundwater board (CGWB), uranium occurrence in shallow aquifer in India. Central Groundwater Board. Uranium_Report_2019–20.pdf (cgwb.gov.in).
Chang, P., Kim, K., Yoshida, S., and Kim, S. (2005). Uranium Accumulation of crop plants enhanced by citric acid. Environ. Geochem. Health. 27, 529–538. doi:10.1007/s10653–005–8013–5
Chour, Z., Laubie, B., Morel, J. L., Tang, Y., Qiu, R., Simonnot, M., et al. (2018). Recovery of rare Earth elements from Dicranopteris dichotoma by an enhanced ion exchange leaching process. Chem. Engin. Proces. – Process Intensif. 130, 208–213. doi:10.1016/j.cep.2018.06.007
Cordeiro, C., Favas, P. J. C., Pratas, J., Sarkar, S. K., and Venkatachalam, P. (2016). Uranium accumulation in aquatic macrophytes in an uraniferous region: Relevance to natural attenuation. Chemosphere 156, 76–87. doi:10.1016/j.chemosphere.2016.04.105
Costis, S., Mueller, K. K., Coudert, L., Neculita, C. M., Reynier, N., and Blais, J.-F. (2021). Recovery potential of rare Earth elements from mining and industrial residues: A review and cases studies. J. Geochem. Explor. 221, 106699. doi:10.1016/j.gexplo.2020.106699
Cotton, S. (1991). Lanthanides and actinides. New York: Oxford University Press. ISBN-13: 978-0470010068.
Coyte, R. M., Jain, R. C., Srivastava, S. K., Sharma, K. C., Khalil, A., Ma, L., et al. (2018). Large–scale uranium contamination of groundwater resources in India. Environ. Sci. Technol. Lett. 5-6, 341–347. doi:10.1021/acs.estlett.8b00215
Dai, Q., Li, L., Li, T., Bi, X., Zhang, Y., Wu, J., et al. (2016). Atmospheric signature and potential sources of rare Earth elements in size–resolved particulate matter in a megacity of China. Aerosol Air Qual. Res. 16, 2085–2095. doi:10.4209/aaqr.2016.03.0108
Deady, É., Mouchos, E., Goodenough, K., Williamson, B., and Wall, F. (2016). A review of the potential for rare-Earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Mineral. Mag. 80 (1), 43–61. doi:10.1180/minmag.2016.080.052
Deblonde, G. J.-P., Mattocks, J. A., Park, D. M., Reed, D. W., Cotruvo, J. A., and Jiao, Y. (2020). Selective and efficient biomacromolecular extraction of rare-earth elements using lanmodulin. Inorg. Chem. 59 (17), 11855–11867. doi:10.1021/acs.inorgchem.0c01303
Deepulal, P. M., Gireesh Kumar, T. R., and Sujatha, C. H. (2012). Behaviour of REEs in a tropical estuary and adjacent continental shelf of southwest coast of India: Evidence from anomalies. J. Earth Syst. Sci. 121 (5), 1215–1227. doi:10.1007/s12040-012-0223-5
Degueldre, C., and Joyce, M. J. (2020). Evidence and uncertainty for uranium and thorium abundance: A review. Prog. Nucl. Energy. 124, 103299. doi:10.1016/j.pnucene.2020.103299
Deng, Y., Ren, J., Guo, Q., Cao, J., Wang, H., and Liu, C. (2017). Rare Earth element geochemistry characteristics of seawater and porewater from deep sea in Western Pacific. Sci. Rep. 7, 16539. doi:10.1038/s41598-017-16379-1
Dienemann, C., Dudel, G. E., Dienemann, H., and Stolz, L. (2002). “Retention of radionuclides and arsenic by algae downstream of u mining tailings,” in Uranium in the aquatic environment. Editors B. J. Merkel, B. Planer–Friedrich, and C. Wolkersdorfer (Berlin, Heidelberg: Springer).
Dinal, G. S., Ramos, S. J., Carvalho, T. S. de, Carvalho, G. S., Oliveira, C. de, Siqueira J. O., Guilherme, L. R. G., et al. (2019). Dissolution techniques for determination of rare Earth elements in phosphate products: Acid digestion or alkaline fusion? J. Geochem. Explor. 197, 114–121. doi:10.1016/j.gexplo.2018.11.016
Dungan, K., Butler, G., Livens, F. R., and Warren, L. M. (2017). Uranium from seawater–Infinite resource or improbable aspiration? Prog. Nucl. Ener. 99, 81–85. doi:10.1016/j.pnucene.2017.04.016
Duplay, J., Semhi, K., Mey, M., Messina, A., Quaranta, G., Huber, F., et al. (2014). Geogenic versus anthropogenic geochemical influence on trace elements contents in soils from the Milazzo Peninsula. Geochem 74 (4), 691–704. doi:10.1016/j.chemer.2014.04.006
El-Taher, A., Badawy, W. M., Khater, A. E. M., and Madkour, H. A. (2019). Distribution patterns of natural radionuclides and rare Earth elements in marine sediments from the Red Sea, Egypt. Appl. Rad. Isot. 151, 171–181. doi:10.1016/j.apradiso.2019.06.001
El-Taher, A., Zakaly, H. M. H., and Elsaman, R. (2018). Environmental implications and spatial distribution of natural radionuclides and heavy metals in sediments from four Harbours in the Egyptian Red Sea coast. Appl. Radiat. Isot. 131, 13–22. doi:10.1016/j.apradiso.2017.09.024
Elias, M. S., Ibrahim, S., Samuding, K., Kantasamy, N., Rahman, S. A., and Hashim, A. (2019). Rare Earth elements (REEs) as pollution indicator in sediment of Linggi river, Malaysia. Appl. Radiat. Isot. 151, 116–123. doi:10.1016/j.apradiso.2019.05.038
Emsbo, P., McLaughlin, P. I., Breit, G. N., Bray, E. A. du, and Koenig, A. E. (2015). Rare Earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 27 (2), 776–785. doi:10.1016/j.gr.2014.10.008
Fan, H.-R., Yang, K.-F., Hu, F.-F., Liu, S., and Wang, K.-Y. (2016). The giant Bayan Obo REE-Nb-Fe deposit, China: Controversy and ore Genesis. Geosci. Front. 7 (3), 335–344. doi:10.1016/j.gsf.2015.11.005
Felipe-Sotelo, M., Hinchliff, J., Field, L. P., Milodowski, A. E., Preedy, O., and Read, D. (2017). Retardation of uranium and thorium by a cementitious backfill developed for radioactive waste disposalfill developed for radioactive waste disposal. Chemosphere 179, 127–138. doi:10.1016/j.chemosphere.2017.03.109
Fesenko, S. V., and Emlutina, E. S. (2021). Thorium concentrations in the environment: A review of the global data. Biol. Bul.l Russ. Acad. Sci. 48, 2086–2097. doi:10.1134/S1062359021110030
Fiket, Ž., Medunić, G., and Kniewald, G. (2016). Rare Earth elements distribution in soil nearby thermal power plant. Environ. Earth Sci. 75, 598. doi:10.1007/s12665-016-5410-2
Fiket, Ž., Medunić, G., Furdek Turk, M., and Kniewald, G. (2018a). Rare Earth elements in superhigh-organic-sulfur Raša coal ash (Croatia). Int. J. Coal Geol. 194, 1–10. doi:10.1016/j.coal.2018.05.002
Fiket, Ž., Mlakar, M., and Kniewald, G. (2018b). Distribution of rare Earth elements in sediments of the marine lake Mir (Dugi Otok, Croatia). Geosci 8 (8), 301. doi:10.3390/geosciences8080301
Flanagan, S. M., Belaval, M., and Ayotte, J. D. (2014). Arsenic, iron, lead, manganese, and uranium concentrations in private bedrock wells in southeastern New Hampshire 2012–2013. U.S. geo. Surv. Fact. Sheet 3042, 6. doi:10.3133/fs20143042
Franus, W., Wiatros-Motyka, M. M., and Wdowin, M. (2015). Coal fly ash as a resource for rare Earth elements. Environ. Sci. Pollut. Res. Int. 22 (12), 9464–9474. doi:10.1007/s11356–015–4111–9
Freitas Rodrigo, T. O. P., de Pedreira, M. A., and Hatje, V. (2021). Distribution and fractionation of rare Earth elements in sediments and mangrove soil profiles across an estuarine gradient. Chemosphere 264, 128431. doi:10.1016/j.chemosphere.2020.128431
Galhardi, J., and Bonotto, D. (2017). Radionuclides (222Rn, 226Ra, 234U, and 238U) release in natural waters affected by coal mining activities in Southern Brazil. Water Air Soil Pollut. 228 (6), 207. doi:10.1007/s11270–017–3381–x
Ganguli, R., and Cook, D. R. (2018). Rare earths: A review of the landscape. MRS Energy Sustain 5, 6. doi:10.1557/mre.2018.7
Gao, L., Kano, N., Sato, Y., Li, C., Zhang, S., and Imaizumi, H. (2012). Behavior and distribution of heavy metals including rare Earth elements, thorium, and uranium in sludge from industry water treatment plant and recovery method of metals by biosurfactants application. Bioinorg. Chem. Appl. ID 2012, 1–11. doi:10.1155/2012/173819
Gasser, M. S., Ismail, Z. H., Abu Elgoud, A., Abdel Hai, F., Ali, O. I., and Aly, H. F. (2019). Process for lanthanides-Y leaching from phosphogypsum fertilizers using weak acids. J. Hazard. Mat. 378, 120762. doi:10.1016/j.jhazmat.2019.120762
Godinez, M., lturbe, J., Ordonez, E., and Solache–Rios, M. (1997). Determination of radium–226 in phosphate fertilisers and gypsum by gamma–ray spectrometry. Int. J. Environ. Pollut. 8 (1), 195–200. doi:10.1504/IJEP.1997.028167
Goodenough, K. M., Schilling, J., Jonsson, E., Kalvig, P., Charles, N., Tuduri, J., et al. (2016). Europe's rare Earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 72 (1), 838–856. doi:10.1016/j.oregeorev.2015.09.019
Grosjean, N., Le Jean, M., Berthelot, C., Chalot, M., Gross, E. M., and Blaudez, D. (2019). Accumulation and fractionation of rare Earth elements are conserved traits in the Phytolacca genus. Sci. Rep. 9, 18458. doi:10.1038/s41598–019–54238–3
Gu, F., Wu, G., Zhang, C., Yan, N., and Huang, J. (2020). Geochemistry of surface soil in the Eastern Pamirs and its implications for Muztagata ice core dust provenance. Appl. Geochem. 121, 104724. doi:10.1016/j.apgeochem.2020.104724
Gu, H., Wang, N., Yang, Y., Zhao, C., and Cui, S. (2017). Features of distribution of uranium and thorium in red mud. Physicochem. Probl. Min. Process. 53 (1), 110–121. doi:10.5277/ppmp170109
Guo, C., Wei, Y., Yan, L., Li, Z., Qian, Y., Liu, H., et al. (2020). Rare Earth elements exposure and the alteration of the hormones in the hypothalamic-pituitary-thyroid (hpt) axis of the residents in an e-waste site: A cross-sectional study. Chemosphere 252, 126488. doi:10.1016/j.chemosphere.2020.126488
Gupta, P. K., Ranjan, R., Mukundan, A. R., Deshpande, M. S. M., Shrivastava, V. K., and Yadava, R. S. (2008). Uranium mineralization along the northeastern margin of proterozoic Chhattisgarh basin around chitakhol, central India: A petromineralogical study. Explor. Res. At. Miner. 18, 33–53.
Gwenzi, W., Mangori, L., Danha, C., Chaukura, N., Dunjana, N., and Sanganyado, E. (2018). Sources, behaviour, and environmental and human health risks of high-technology rare Earth elements as emerging contaminants. Sci. Total Environ. 636, 299–313. doi:10.1016/j.scitotenv.2018.04.235
Gwenzi, W., Mupatsi, N. M., Mtisi, M., and Mungazi, A. A. (2021). “Sources and health risks of rare Earth elements in waters,” in Water pollution and remediation: Heavy metals. Environmental chemistry for a sustainable world, 53. Editors Inamuddin, M. I. Ahamed, and E. Lichtfouse (Cham: Springer).
Han, G., Song, X., and Tang, Y. (2017). Geochemistry of rare Earth elements in soils under different land uses in a typical karst area, Guizhou Province, Southwest China. Can. J. Soil Sci. 97 (4). doi:10.1139/cjss–2017–0043
Harmsen, K., and Haan, De F. A. M. (1980). Occurance and behaviour of uranium and thorium in soil and water. Neth. J. Agric. Sci. 28, 40–62. doi:10.18174/njas.v28i1.17043
Hegazy, A. K., and Emam, M. H. (2011). Accumulation and soil-to-plant transfer of radionuclides in the Nile Delta coastal black sand habitats. Int. J. Phytoremed. 13 (2), 140–155. doi:10.1080/15226511003753961
Hower, J. C., Granite, E. J., Mayfield, D. B., Lewis, A. S., and Finkelman, R. B. (2016). Notes on contributions to the science of rare Earth element enrichment in coal and coal combustion byproducts. Minerals 6, 32–39. doi:10.3390/min6020032
Hua, R., Gao, J., Zhao, K., Long, G., Lu, H., Yao, J., et al. (2010). Rare Earth element and trace element features of gold-bearing pyrite in the Jinshan gold deposit, Jiangxi Province. J. Geol. Soc. China 84 (3), 614–623. doi:10.1111/j.1755-6724.2010.00077.x
Huang, J. W., Blaylock, M. J., Kapulnik, Y., and Ensley, B. D. (1998). Phytoremediation of uranium–contaminated soils: Role of organic acids in triggering uranium hyperaccumulation in plants. Environ. Sci. Technol. 32 (13), 2004–2008. doi:10.1021/es971027u
Hussein, T., Juwhari, H., Al Kuisi, M., Alkattan, H., and Lahlouh Bal-Hunaiti, A. (2018). Accumulation and coarse mode aerosol concentrations and carbonaceous contents in the urban background atmosphere in Amman, Jordan. Arab. J. Geosci. 11, 617. doi:10.1007/s12517–018–3970–z
IAEA (International Atomic Energy Agency) (2018). Status and trends in spent fuel and radioactive waste management. Vienna, Austria: International Atomic Energy Agency. Nuclear Energy Series No. NW-T-1.14.
IRNS (Institute for Radiological Protection and Nuclear Safety) (2012). Natural uranium, and the environment. Fontenay-aux-Roses: Institute for Radiological Protection and Nuclear Safety.
Jalali, J., and Lebeau, T. (2021). The role of microorganisms in mobilization and phytoextraction of rare earth elements: A review. Front. Environ. Sci. 9. doi:10.3389/fenvs.2021.688430
Jin, S. L., Huang, Y. Z., Wang, F., Xu, F., Wang, X. L., Gao, Z., et al. (2015). Rare Earth elements content in farmland soils and crops of the surrounding copper mining and smelting plant in Jiangxi province and evaluation of its ecological risk. Huan Jing Ke Xue 36 (3), 1060–1068. Chinese. PMID: 25929077.
Jonathan, O'C. (2012). Rare Earth minerals and the geochemistry and implications of their respective uranium and thorium contents. Available at: https://www.researchgate.net/publication/264647626.
Jowitt, S. M., Werner, T. T., Weng, Z., and Mudd, G. M. (2018). Recycling of the rare Earth elements. Curr. Opin. Green Susta. Chem. 13, 1–7. doi:10.1016/j.cogsc.2018.02.008
Jyothi, R. K., Thenepalli, T., Ahn, J. W., Parhi, P. K., Chung, K. W., and Lee, J.-Y. (2020). Review of rare Earth elements recovery from secondary resources for clean energy technologies: Grand opportunities to create wealth from waste. J. Clean. Product. 267, 122048. doi:10.1016/j.jclepro.2020.122048
Kaishwa, S. J., Marwa, E. M., Msaky, J. J., and Mwakalasya, W. N. (2018). Uranium natural levels in soil, rock and water: Assessment of the quality of drinking water in singida urban district, Tanzania. J. Water Health 16 (4), 542–548. doi:10.2166/wh.2018.254
Kasar, S., Sahoo, S. K., Área, H., Mishra, S., Tokonami, S., and Aono, T. (2019). Uranium, thorium, and rare Earth elements distribution in Fukushima soil samples. Radia. Prot. Dosim. 184 (3-4), 363–367. doi:10.1093/rpd/ncz075
Keesari, T., Mohokar, H. V., Sahoo, B. K., and Mallesh, G. (2014). Assessment of environmental radioactive elements in groundwater in parts of Nalgonda district, Andhra Pradesh, South India using scintillation detection methods. J. Radioanal. Nucl. Chem. 302, 1391–1398. doi:10.1007/s10967–014–3566–3
Kilbourn, B. T. (1986). The role of the lanthanides in applied catalysis. J. Less Common Met. 126, 101–106. doi:10.1016/0022–5088(86)90254–7
Kim, M. G., Hyeong, K., and Yoo, C. M. (2022). Distribution of rare Earth elements and yttrium in sediments from the Clarion-Clipperton fracture zone, northeastern Pacific Ocean. Geochem. Geophys. Geosyst. 23 (7). doi:10.1029/2022GC010454
Klaver, G., Verheul, M., Bakker, I., Petelet-Giraud, E., and Négrel, P. (2014). Anthropogenic rare Earth element in rivers: Gadolinium and lanthanum. Partitioning between the dissolved and particulate phases in the rhine river and spatial propagation through the rhine-meuse Delta (The Netherlands). Appl. Geochem. 47 (0), 186–197. doi:10.1016/j.apgeochem.2014.05.020
Kolawole, T. O., Olatunji, O. S., Ajibade, O. M., and Oyelami, C. A. (2021). Sources and level of rare Earth element contamination of atmospheric dust in Nigeria. J. Health Pollut. 11 (30), 210611. doi:10.5696/2156-9614-11.30.210611
Kolker, A., Scott, C., Hower, J. C., Vazquez, J. A., Lopano, C. L., and Dai, S. (2017). Distribution of rare Earth elements in coal combustion fly ash, determined by SHRIMP–RG ion microprobe. Int. J. Coal Geol. 184, 1–10. doi:10.1016/j.coal.2017.10.002
Korna, A. H., Fares, S. S., and El-Rahman, M. A. (2014). Natural radioactivity levels and radiation hazards for gypsum materials used in Egypt. Nat. Sci. 6 (1), 5–13. doi:10.4236/ns.2014.61002
Kovaříková, M., Tomášková, I., and Soudek, P. (2019). Rare Earth elements in plants. Biol. Plant. 63, 20–32. doi:10.32615/bp.2019.003
Kritsananuwat, R., Sahoo, S. R., Fukushi, M., and Chanyotha, S. (2014). Distribution of rare Earth elements, thorium, and uranium in Gulf of Thailand’s sediments. Environ. Earth Sci. 73 (7), 3361–3374. doi:10.1007/s12665–014–3624–8
Kulaksız, S., and Bau, M. (2013). Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare Earth element distribution in rivers. Earth Planet. Sci. Lett. 362, 43–50. doi:10.1016/j.epsl.2012.11.033
Kumar, V., Jha, M. K., Kumari, A., Panda, R., Kumar, J. R., and Lee, J. Y. (2014). Recovery of rare earth metals (REMs) from primary and secondary resources: A review. Rare Met. Tech., 81–88. doi:10.1002/9781118888551.ch16
LePan, N. (2021). Visual Capitalist, Rare earth elements: Where in the World are they. Canada: visualcapitalist.
Li, C., Wang, M., Luo, X., Liang, L., Han, X., and Lin, X. (2019). Accumulation and effects of uranium on aquatic macrophyte Nymphaea tetragona Georgi: Potential application to phytoremediation and environmental monitoring. J. Environ. Radioact. 198, 43–49. doi:10.1016/j.jenvrad.2018.12.018
Li, X., Chen, Z., Chen, Z., and Zhang, Y. (2013). A human health risk assessment of rare Earth elements in soil and vegetables from a mining area in Fujian province, southeast China. Chemosphere 93, 1240–1246. doi:10.1016/j.chemosphere.2013.06.085
Li, Y., Yang, J. L., and Jiang, Y. (2012). Trace rare Earth element detection in food and agricultural products based on flow injection walnut shell packed microcolumn preconcentration coupled with inductively coupled plasma mass spectrometry. J. Agric. Food Chem. 60, 3033–3041. doi:10.1021/jf2049646
Li, Z., Li, X., Zhang, L., Li, S., Chen, J., Feng, X., et al. (2019). Partitioning of rare Earth elements and yttrium (REY) in five coal–fired power plants in Guizhou, Southwest China. J. Rare Earths. 38 (11), 1257–1264. doi:10.1016/j.jre.2019.12.013
Lucas, J., Lucas, P., Le Mercier, T., Rollat, A., and Davenport, W. (2014). Rare earths: Science, technology, production and use. 1st Edit. Amsterdam: Elsevier.
Mahmood, Q., Mirza, N., and Shaheen, S. (2015). “Phytoremediation using algae and macrophytes,” in Phytoremediation. Editors A. Ansari, S. Gill, R. Gill, G. Lanza, and L. Newman (Germany: Springer).
Malhotra, N., Hua-Shu, H., Sung-Tzu, L., Marri, J. M. R., Jiann-Shing, L., Tzong-Rong, G., et al. (2020). An updated review of toxicity effect of the rare Earth elements (REEs) on aquatic organisms. Animal 10 (9), 1663. doi:10.3390/ani10091663
Markert, B. (1987). The pattern of distribution of lanthanide elements in soils and plants. Phytochem 26, 3167–3170. doi:10.1016/S0031–9422(00)82463–2
Marmolejo-Rodríguez, A. J., Prego, R., Meyer-Willerer, A., Shumilin, E., and Sapozhnikov, D. (2007). Rare Earth elements in iron oxy−hydroxide rich sediments from the Marabasco River-Estuary System (Pacific coast of Mexico). REE affinity with iron and aluminium. J. Geochem. Explor. 94, 43–51. doi:10.1016/j.gexplo.2007.05.003
Mashitah, S. M., Shazili, N. A. M., and Rashid, M. K. A. (2012). Elemental concentrations in Brown seaweed, padina sp. along the East Coast of peninsular Malaysia. Aquat. Ecosyst. Health 15, 267–278. doi:10.1080/14634988.2012.705774
Maslov, O. D., Tserenpil, S., Norov, N., Gustovaa, M. V., Filippova, M. F., Belova, A. G., et al. (2010). Uranium recovery from coal ash dumps of Mongolia. Solid Fuel Chem. 44, 433–438. doi:10.3103/S0361521910060133
Matara-aho, M. (2020). Speciation of uranium and thorium in seawater and bioaccumulation of uranium in sea urchins. Helsinki, Finland: Helsingin yliopisto. Available at: http://urn.fi/URN:NBN:fi:hulib-202012104890.
Mayfield, D. B., and Lewis, A. S. (2013). “Environmental review of coal ash as a resource for rare Earth and strategic elements,” in Proceedings of the 2013 World Ash Conference, Lexington, KY, 2013.
Medas, D., Cidu, R., Giudici de, G., and Podda, F. (2013). Geochemistry of rare Earth elements in water and solid materials at abandoned mines in SW Sardinia (Italy). J. Geochem. Explor. 133, 149–159. doi:10.1016/j.gexplo.2013.05.005
Mędyk, M., and Falandysz, J. (2022). Occurrence, bio-concentration and distribution of rare Earth elements in wild mushrooms. Sci. Total Environ. 158159, 158159. doi:10.1016/j.scitotenv.2022.158159
Migaszewski, Z. M., Gałuszka, A., and Dołęgowska, S. (2019). Extreme enrichment of arsenic and rare Earth elements in acid mine drainage: Case study of Wiśniówka mining area (south–central Poland). Environ. Pollut. 244, 898–906. doi:10.1016/j.envpol.2018.10.106
Mihajlovic, J., and Rinklebe, J. (2018). Rare Earth elements in German soils - a review. Chemosphere 205, 514–523. doi:10.1016/j.chemosphere.2018.04.059
Mihaylova, V., Todorov, B., and Djingova, R. (2013). Determination of uranium and thorium in soils and plants by ICP–MS. Case study of Buhovo region. Compt. Rend. Acad. Bulg. Sci. 66 (4), 513–518. doi:10.7546/CR-2013-66-4-13101331-6
Mihucz, V. G., Varga, Z., Tatár, E., Virág, I., Grieken, R., Koleszár, Z., et al. (2008). Redistribution of uranium and thorium by soil/plant interaction in a recultivated mining area. Microchem. J. 90, 44–49. doi:10.1016/j.microc.2008.03.004
Mondal, S., Ghar, A., Satpati, A. K., Sinharoy, P., Singh, D. K., Sharma, J. N., et al. (2019). Recovery of rare Earth elements from coal fly ash using TEHDGA impregnated resin. Hydrometallurgy 185, 93–101. doi:10.1016/j.hydromet.2019.02.005
Montross, S. N., Yang, J., Britton, J., McKoy, M., and Verba, C. (2020). Leaching of rare Earth elements from central Appalachian coal seam under clays. Mineral 10, 577. doi:10.3390/min10060577
Moraes, M., Marinho Saraiva, A., and Ladeira, A. (2017). “Recovery of rare earth elements by Co-precipitation with iron, aluminum and manganese (Hydr)oxides from acid mine drainage,” in Sustainable industrial processing summit SIPS 2017 volume 1. Barrios intl. Symp./non-ferrous smelting hydro/electrochemical processing. Editors F. Kongoli, M. Palacios, T. Buenger, J. H. Meza, E. Delgado, M. C. Joudrieet al. (Montreal, Canada: FLOGEN Star Outreach), 208–220.
Munksgaard, N. C., Lim, K., and Parry, D. L. (2003). Rare Earth elements as provenance indicators in North Australian estuarine and coastal marine sediments. Estuar. Coast. Shelf Sci. 57, 399–409. doi:10.1016/S0272-7714(02)00368-2
Nascimento, R. C., da Silva, Y. J. A. B., do Nascimento, C. W. A., da Silva, Y. J. A. B., da Silva, R. J. A. B., and Collins, A. L. (2019). Thorium content in soil, water and sediment samples and fluvial sediment-associated transport in a catchment system with a semiarid-coastal interface, Brazil. Environ. Sci. Pollut. Res. 26, 33532–33540. doi:10.1007/s11356-019-06499-8
Ndjama, J., Mafany, G., Nkoue, R. G. N., Belmond, B. E., and Bessa, A. Z. E. (2022). Rare Earth elements in surface waters and sediments of the Mgoua watershed, south Western Cameroon. Arab. J. Geosci. 15, 1001. doi:10.1007/s12517-022-10278-0
Neves, O., Abreu, M. M., and Matias, M. J. (2009). Uranium distribution in the solid phases of soils from Cunha Baixa mining site (Portugal). Rev. Ciênc. Agrár. Port. 32 (1), 195–204.
OECD (2020). Uranium 2020: Resources, Production and Demand, 2020. A joint report by the nuclear energy agency and the international atomic energy agency. Paris, France: Nuclear Energy Agency, Organisation For Economic Co-operation and Development. Available at: https://www.oecd-nea.org/jcms/pl_52718/uranium-2020-resources-production-and-demand?details=true.
Ogawa, Y., Ishiyama, D., Đorđievski, S., Petrović, J., Milivojević, M., Saini-Eidukat, B., et al. (2021). Geochemical mobility of rare Earth elements (REEs) and actinides (U and Th) originating from Kusatsu acid thermal waters during neutralization and river transport: Effect of aqueous speciation on sorption onto suspended materials and fractionation among REEs and actinides. Chem. Geol. 586, 120559. doi:10.1016/j.chemgeo.2021.120559
Okeme, I. C., Martin, P. G., Jones, C., Crane, R. A., Ojonimi, T. I., Ignatyev, K., et al. (2020). An advanced analytical assessment of rare Earth element concentration, distribution, speciation, crystallography and solid-state chemistry in fly ash. Acta Part B At. Spectros 177, 105950. doi:10.1016/j.sab.2020.105950
Oufni, L., Taj, S., Manaut, B., and Eddouks, M. (2011). Transfer of uranium and thorium from soil to different parts of medicinal plants using SSNTD. J. Radioanal. Nucl. Chem. 287, 403–410. doi:10.1007/s10967-010-0888-7
Pagano, G., Guida, M., Tommasi, F., and Oral, R. (2015). Health effects and toxicity mechanisms of rare Earth elements—knowledge gaps and research prospects. Ecotoxico. Environ. Saf. 115, 40–48. doi:10.1016/j.ecoenv.2015.01.030
Pang, X., Li, D., and Peng, A. (2002). Application of rare-Earth elements in the agriculture of China and its environmental behavior in soil. Environ. Sci. Pollut. Res. 9 (2), 143–148. doi:10.1007/bf02987462
Pant, D., Keesari, T., Sharma, D., Rishi, M. S., Singh, G., Jaryal, A., et al. (2017). Study on uranium contamination in groundwater of Faridkot and Muktsar districts of Punjab using stable isotopes of water. J. Radioanal. Nucl. Chem. 313, 635–639. doi:10.1007/s10967–017–5284–0
Parthasarathy, K. S. (2018). Radiation doses, their limits: Related issues of public perception – analysis. Eurasia Rev.
Pereira, W. V., da, S., Ramos, S. J., Melo, L. C. A., Braz, A. M. de S., Dias, Y. N., et al. (2022). Levels and environmental risks of rare Earth elements in a gold mining area in the Amazon. Environ. Res. 211, 113090. doi:10.1016/j.envres.2022.113090
Piarulli, S., Hansen, B., Ciesielski, T., Zocher, A., Malzahn, A., Olsvik, P., et al. (2021). Sources, distribution and effects of rare Earth elements in the marine environment: Current knowledge and research gaps. Environ. Pollut. 291, 118230. doi:10.1016/j.envpol.2021.118230
Pmf, I. S. (2020). Uranium & thorium distribution across India & world. Available at: www.pmfias.com/uranium-thorium-distribution-advantages-uran.
Pratas, J., Favas, P. J. C., Varun, M., D’Souza, R., and Paul, M. S. (2017). Distribution of rare Earth elements, thorium and uranium in streams and aquatic mosses of Central Portugal. Environ. Earth. Sci. 76, 156. doi:10.1007/s12665-017-6459-2
Prego, R., Caetano, M., Vale, C., and Marmolejo-Rodríguez, J. (2009). Rare Earth elements in sediments of the vigo ria, NW iberian peninsula. Cont. Shelf Res. 29, 896–902. doi:10.1016/j.csr.2009.01.009
Raghavendra, T., Srilatha, K., Mahender, C., Elander, M., Vijayalakshmi, T., Himabindu, V., et al. (2013). Distribution of uranium concentration in groundwater samples from the Peddagattu/Nambapur and Seripally regions using laser fluorimetry. Radiat. Prot. Dosim. 158 (3), 325–330. doi:10.1093/rpd/nct228
Ramos, S. J., Dinali, G. S., Oliveira, C., Martins, C. G., Moreira, G. C., Siquiera, J. O., et al. (2016). Rare Earth elements in the soil environment. Curr. Pollut. Rep. 2, 28–50. doi:10.1007/s40726–016–0026–4
Rhodes, C. (2011). Rare earth elements and thorium power. Forbes. Availableat: https://www.forbes.com/sites/energysource/2011/03/28/rare-earth-elements-and-thorium-power/?sh=3d60a6952d76.
Rim, K. R., Koo, K. H., and Park, J. S. (2013). Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work. 4 (1), 12–26. doi:10.5491/SHAW.2013.4.1.12
Rishi, M. S., Keesari, T., Sharma, D. A., Pant, D., and Sinha, U. K. (2017). Spatial trends in uranium distribution in groundwaters of Southwest Punjab, India–A hydrochemical perspective. J. Radioanal. Nucl. Chem. 311, 1937–1945. doi:10.1007/s10967–017–5178–1
RMREI (2012). Rare metal and rare earth investigation (RMREI). Hyderabad, Telangana: Department of Atomic Energy, Atomic Minerals Directorate for Exploration and Research, Government of India. Available at: https://amd.gov.in/app16/content.aspx?link=40.
Rodríguez, P. B., Tomé, F. V., and Lozano, J. C. (2005). About the assumption of linearity in soil-to-plant transfer factors for uranium and thorium isotopes and 226Ra. Sci. Total Environ. 284 (1-3), 167–175. doi:10.1016/S0048-9697(01)00877-4
Sadeghi, M., Morris, G. A., Carranza, E. J. M., Ladenberger, A., and Andersson, M. (2013). Rare Earth element distribution and mineralization in Sweden: An application of principal component analysis to FOREGS soil geochemistry. J. Geochem. Explor. 133, 160–175. doi:10.1016/j.gexplo.2012.10.015
Sahoo, S., Hosoda, M., Kamagata, S., Sorimachi, A., Ishikawa, T., Tokonami, S., et al. (2011). Thorium, uranium and rare Earth elements concentration in weathered Japanese soil samples. Prog. Nucl. Sci. Techn. 1, 416–419. doi:10.15669/pnst.1.416
Sahu, B. L., Rajhans, K. P., Ramteke, S., Patel, K. S., Wysocka, I., and Jaron, I. (2016). Geochemistry of rare Earth elements in sediment of Central India. J. Environ. Prot. 7, 705–714. doi:10.4236/jep.2016.75063
Sahu, M., Sar, S. K., Baghe, l. T., and Dewangan, R. (2020). Seasonal and geochemical variation of uranium and major ions in groundwater at Kanker district of Chhattisgarh, central India. Groundw. Sustain. Dev. 10, 100330. doi:10.1016/j.gsd.2020.100330
Saini, K., and Bajwa, B. S. (2016). Uranium distribution study in the drinking water samples of SW Punjab, India. Adv. Appl. Sci. Res. 7 (2), 103–108.
Sakamoto, N., Kano, N., and Imaizumi, H. (2008). Biosorption of uranium and rare Earth elements using biomass of algae. Bioinorg. Chem. Appl. 2008, 1–8. doi:10.1155/2008/706240
Santos, E. A., and Ladeira, A. C. Q. (2011). Recovery of uranium from mine waste by leaching with carbonate–based reagents. Environ. Sci. Technol. 45 (8), 3591–3597. doi:10.1021/es2002056
Santos-Francés, F., Pacheco, E. G., Martínez-Graña, A., Rojo, P. A., Zarza, C. A., and Sánchez, A. G. (2018). Concentration of uranium in the soils of the west of Spain. Environ. Pollu. 236, 1–11. doi:10.1016/j.envpol.2018.01.038
Sastri, V. S., Perumareddi, J. R., Ramachandra Rao, V., Rayudu, G. V. S., and Bünzli, J.-C. (2003). Modern aspects of rare earths and their complexes. Amsterdam: Elsevier. ISBN: 9780444510105.
Schulz, K. J., DeYoung, J. H., Bradley, D. C., and Seal, R. R. (2017). “Critical mineral resources of the United States— an introduction,” in Critical mineral resources of the United States—economic and environmental geology and prospects for future supply. Editors A. chap, K. J. Schulz, J. H. DeYoung, R. R. Seal, and D. C. Bradley (Reston, VA: U.S. Geological Survey Professional Paper 1802), A1–A14. doi:10.3133/pp1802A
Seredin, V. V., and Dai, S. (2012). Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 94, 67–93. doi:10.1016/j.coal.2011.11.001
Shaltout, A. A., Khoder, M. I., El-Abssawy, A. A., Hassan, S. K., and Borges, D. L. G. (2013). Determination of rare Earth elements in dust deposited on tree leaves from Greater Cairo using inductively coupled plasma mass spectrometry. Environ. Pollut. 178, 197–201. doi:10.1016/j.envpol.2013.03.044
Shao, L., Chang, L., Finkelman, R. B., Wang, W., Liu, J., Li, J., et al. (2020). Distribution of rare Earth elements in PM10 emitted from burning coals and soil–mixed coal briquettes. J. Environ. Sci. 97, 96–101. doi:10.1016/j.jes.2020.05.010
Sharma, D. A., Rishi, M. S., Keesari, T., Pant, D., Singh, R., Thakur, N., et al. (2017). Distribution of uranium in groundwaters of bathinda and mansa districts of Punjab, India: Inferences from an isotope hydrochemical study. J. Radioanal. Nucl. Chem. 313 (4), 625–633. doi:10.1007/s10967–017–5288–9
Shi, Z., Yong, L., Liu, Z., Wang, Y., Sui, H., Mao, W., et al. (2022). Risk assessment of rare Earth elements in fruits and vegetables from mining areas in China. Environ. Sci. Pollut. Res. Int. 29 (32), 48694–48703. doi:10.1007/s11356-022-19080-7
Shin, W., Oh, J., Choung, S., Cho, B.-W., Lee, K.-S., Yun, U., et al. (2016). Distribution and potential health risk of groundwater uranium in Korea. Chemosphere 163, 108–115. doi:10.1016/j.chemosphere.2016.08.021
Sinha, S., AbhilashMeshram, P., and Pandey, B. D. (2016). Metallurgical processes for the recovery and recycling of lanthanum from various resources—a review. Hydrometallurgy 160, 47–59. doi:10.1016/j.hydromet.2015.12.004
Sivasamandy, R., Satyanarayanan, M., and Ramesh, R. (2019). Distribution of rare Earth elements in core sediments of Kolakkudi lake, Southern India. Acta Geodyn. Geomater. 16 (4), 473–486. doi:10.13168/AGG.2019.0040
Smedley, P. L. (1991). The geochemistry of rare Earth elements in groundwater from the Carnmenellis area, southwest England. Geochim. Cosmochim. Acta. 55 (10), 2767–2779. doi:10.1016/0016–7037(91)90443–9
Sneller, F. E. C., Kalf, D. F., Lennart, W., and Annemarie, W. (2000). Maximum Permissible concentrations and negligible concentrations for rare Earth elements (REEs). RIVM (Rijksinstituut voor Volksgezondheid en Milieu). report 601501011. Available at: http://hdl.handle.net/10029/9551.
Suli, L. M., Ibrahim, W. H. B. W., Badhrulhisham, A. A., Deraman, M. R., and Ismail, N. (2017). A Review of rare Earth mineral processing technology. Chem. Engin. Res. Bull. 19, 20. doi:10.3329/cerb.v19i0.33773
Sytar, O., Brestic, M., Taran, N., and Zivcak, M. (2016). Plants used for biomonitoring and phytoremediation of trace elements in soil and water. Emerg. Remed. Tech., 361–384. doi:10.1016/B978–0–12–803158–2.00014–X
Tai, P., Zhao, Q., Su, D., Li, P., and Stagnitti, F. (2010). Biological toxicity of lanthanide elements on algae. Chemosphere 80 (9), 1031–1035. doi:10.1016/j.chemosphere.2010.05.030
Tanaka, E., Nakamura, K., Yasukawa, K., Mimura, K., Fujinaga, K., Iijima, K., et al. (2020). Chemostratigraphy of deep-sea sediments in the Western North Pacific Ocean: Implications for Genesis of mud highly enriched in rare-Earth elements and yttrium. Ore Geol. Rev. 119, 103392. doi:10.1016/j.oregeorev.2020.103392
Tang, S., Zheng, C., Chen, M., Du, W., and Xu, X. (2020). Geobiochemistry characteristics of rare Earth elements in soil and ground water: A case study in baotou, China. China. Sci. Rep. 10, 11740. doi:10.1038/s41598–020–68661–4
Tanwer, N., Khyalia, P., Deswal, M., Laura, J., and Khosla, B. (2022). Spatial distribution of uranium in groundwater and its health risk assessment in Haryana, India. Ras. J. Chem. 15, 343–349. doi:10.31788/RJC.2022.1516608
Thivya, C., Chidambaram, S., Keesari, T., Prasanna, M. V., Thilagavathi, R., and Nepolian, M. (2014). Occurrence of the radionuclides in groundwater of crystalline hard rock regions of central Tamil Nadu, India. J. Radioanal. Nucl. Chem. 302, 1349–1355. doi:10.1007/s10967–014–3630–z
Tian, L., Chang, H., Tang, P., Li, T., Zhang, X., Liu, S., et al. (2020). Rare Earth elements occurrence and economical recovery strategy from shale gas wastewater in the Sichuan Basin. China. ACS Sustain. Chem. Engin. 8 (32), 11914–11920. doi:10.1021/acssuschemeng.0c04971
Torstenfelt, B. (1986). Migration of the actinides thorium, protactinium, uranium, neptunium, plutonium and americium in clay. Radiochim. Acta. 39, 105–112. doi:10.1524/ract.1986.39.2.105
Tracy, B. L., and Meyerhof, D. P. (1967). Uranium concentrations in air near a Canadian uranium refinery. Atmos. Environ. 21 (1), 165–172. doi:10.1016/0004–6981(87)90281–2
Tranchida, G., Oliveri, E., Angelone, M., Bellanca, A., Censi, P., D'Elia, M., et al. (2011). Distribution of rare Earth elements in marine sediments from the strait of sicily (Western mediterranean sea): Evidence of phosphogypsum waste contamination. Mar. Pollut. Bul. 62 (1), 182–191. doi:10.1016/j.marpolbul.2010.11.003
Tyler, G. (2004). Rare Earth elements in soil and plant systems - a review. Plant Soil 267, 191–206. doi:10.1007/s11104-005-4888-2
Tzifas, I. T., Godelitsas, A., Magganas, A., Androulakaki, E., Eleftheriou, G., Mertzimekis, T. J., et al. (2014). Uranium–bearing phosphatized limestones of NW Greece. J. Geochem. Explor. 143, 62–73. doi:10.1016/j.gexplo.2014.03.0090
USEPA (U.S. Environmental Protection Agency) (1983). Final environmental impact statement for standards for the control of byproduct materials from uranium ore processing. Washington, D.C.: U.S. Environmental Protection Agency, D–1213.
USEPA (U.S. Environmental Protection Agency) (2015). Radiation protection: Radionuclide basics: Thorium. Washington, DC: U.S. Environmental Protection Agency.
Viehweger, K., and Geipel, G. (2010). Uranium accumulation and tolerance in Arabidopsis Halleri under native versus hydroponic conditions. Environ. Experim. Bot. 69 (10), 39–46. doi:10.1016/j.envexpbot.2010.03.001
Virolainen, S., Repo, E., and Sainio, T. (2019). Recovering rare Earth elements from phosphogypsum using a resin-in-leach process: Selection of resin, leaching agent, and eluent. Hydrometallurgy 189, 105125. doi:10.1016/j.hydromet.2019.105125
Vítová, M., Čížková, M., and Zachleder, V. (2018). Lanthanides and algae. IntechOpen. doi:10.5772/intechopen.80260
Voßenkaul, D., Kruse, S., and Friedrich, B. (2013). “Recovery of rare Earth elements from small scale consumer scrap magnets,” in Proceedings of EMC 2013 conference: Rare Earth Elements and Compounds Conference (REEC), Münster/Germany. doi:10.13140/RG.2.2.23197.82400
Wagh, A. S., and Pinnock, W. R. (1987). Occurrence of scandium and rare Earth elements in Jamaican bauxite waste. Econ. Geol. 82, 757–761. doi:10.2113/gsecongeo.82.3.757
Wang, L., and Liang, T. (2015). Geochemical fractions of rare Earth elements in soil around a mine tailing in Baotou, China. Sci. Rep. 5, 12483. doi:10.1038/srep12483
Wang, L., Liang, T., Zhang, Q., and Li, K. (2014). Rare Earth element components in atmospheric particulates in the Bayan Obo mine region. Environ. Res. 131, 64–70. doi:10.1016/j.envres.2014.02.006
Wang, L., Zhong, B., Liang, T., Xing, B., and Zhu, Y. (2016). Atmospheric thorium pollution and inhalation exposure in the largest rare Earth mining and smelting area in China. Sci. Total Environ. 572, 1–8. doi:10.1016/j.scitotenv.2016.07.192
Wang, Z., Dai, S., Zou, J., French, D., and Graham, I. T. (2019a). Rare Earth elements and yttrium in coal ash from the Luzhou power plant in Sichuan, Southwest China: Concentration, characterization and optimized extraction. Int. J. Coal Geol. 203, 1–14. doi:10.1016/j.coal.2019.01.001
Wang, Z., Qin, H., and Wang, J. (2019b). Accumulation of uranium and heavy metals in the soil–plant system in Xiazhuang uranium ore field, Guangdong Province, China. Environ. Geochem. Health 41, 2413–2423. doi:10.1007/s10653–019–00286–7
Wang, Z. W., Liu, Y. M., Wang, Z. L., and Miao, Y. T. (2022). Distribution and environmental significance of rare Earth elements in typical protected vegetable soil, Northern China. Huan Jing Ke Xue 43 (4), 2071–2080. Chinese. doi:10.13227/j.hjkx.202108030
Wu, K., Liu, S., Shi, X., Lou, Z., Kandasamy, S., Wu, B., et al. (2020). Distribution of rare Earth elements in surface sediments of the Western Sunda Shelf: Constraints from sedimentology and mineralogy. Contin. Shelf Res. 206, 104198. doi:10.1016/j.csr.2020.104198
Wu, Y., Wang, Y., and Xie, X. (2014). Occurrence, behavior and distribution of high levels of uranium in shallow groundwater at Datong basin, northern China. Sci. Total Environ. 472, 809–817. doi:10.1016/j.scitotenv.2013.11.109
Yuan, M., Liu, C., Liu, W.-S., Guo, M.-N., Morel, J. L., Huot, H., et al. (2018). Accumulation and fractionation of rare Earth elements (REEs) in the naturally grown Phytolacca americana L. in southern China. Int. J. Phytoremediation. 20 (5), 415–423. doi:10.1080/15226514.2017.1365336
Zhang, N., Huang, C. H., and Hu, B. (2007). ICP–AES determination of trace rare Earth elements in environmental and food samples by on–line separation and preconcentration with acetylacetonemodifi ed silica gel using microcolumn. Anal. Sci. 23, 997–1002. doi:10.2116/analsci.23.997
Zhang, Y., Gu, F., Su, Z., Liu, S., Anderson, C., and Jiang, T. (2020). Hydrometallurgical recovery of rare earth elements from NdFeB permanent magnet scrap: A review. Metals 10, 841. doi:10.3390/met10060841
Zhu, W., Kennedy, M., Leer, E. W. B., Zhou, H., and Alaerts, G. J. F. R. (1997). Distribution and modelling of rare Earth elements in Chinese river sediments. Sci. Total Environ. 3 (1), 233–243. doi:10.1016/S0048–9697(97)00172–1
Keywords: rare earth elements, environment, secondary resource, uranium, thorium
Citation: Patel KS, Sharma S, Maity JP, Martín-Ramos P, Fiket Ž, Bhattacharya P and Zhu Y (2023) Occurrence of uranium, thorium and rare earth elements in the environment: A review. Front. Environ. Sci. 10:1058053. doi: 10.3389/fenvs.2022.1058053
Received: 30 September 2022; Accepted: 14 December 2022;
Published: 04 January 2023.
Edited by:
Chenyang Shuai, Chongqing University, ChinaReviewed by:
Piero Lattanzi, National Research Council (CNR), ItalyYu Zhang, Chongqing Jiaotong University, China
Copyright © 2023 Patel, Sharma, Maity, Martín-Ramos, Fiket, Bhattacharya and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khageshwar Singh Patel, a3NwYXRlbEBycHIuYW1pdHkuZWR1; Željka Fiket, emVsamthLmZpa2V0QGlyYi5ocg==; Yanbei Zhu, eWItemh1QGFpc3QuZ28uanA=
 Khageshwar Singh Patel
Khageshwar Singh Patel Saroj Sharma
Saroj Sharma Jyoti Prakash Maity
Jyoti Prakash Maity Pablo Martín-Ramos
Pablo Martín-Ramos Željka Fiket
Željka Fiket Prosun Bhattacharya
Prosun Bhattacharya Yanbei Zhu
Yanbei Zhu