- 1Key Laboratory of Estuarine Ecological Security and Environmental Health, Education Department of Fujian, Tan Kah Kee College, Xiamen University, Zhangzhou, China
- 2Department of Environmental Science and Engineering, Tan Kah Kee College, Xiamen University, Zhangzhou, China
Microplastics have been widely detected in the environment, while mangrove wetlands are considered barriers to land-based plastic transport to the ocean, requiring special attention. However, the current literature is distributed and broad besides limited information on the fate characteristics and pollution levels. This study uses a systematic literature review method to analyze the current research status and future trends. In this study, the literature is summarized and concluded that Characteristics including color, shape, size, polymer chemistry and surface microstructure are the basic information for microplastic research in mangrove wetlands. Size is the key to studying distribution and convergence without international standards. The shape is vital to study its sources and environmental processes. Color affects biological predation and is important information for studying ecological risk. The chemical composition of plastics is the key to studying microplastics’ fingerprint information, source, and sink. The surface microstructure is an important basis for studying adsorption behavior and aging processes. Mangrove microplastic studies in China are mainly on the southern and southeastern coasts, and microplastic pollution is more severe in Fujian, Guangdong, and Guangxi than in Hainan. In contrast, studies on mangrove microplastics abroad are mainly concentrated in Southeast Asia, the Middle East, and South America. Overall, microplastic contamination was detected in the major distribution areas of mangroves worldwide and was correlated with mangrove density and human activities.
Introduction
Plastics rapidly increase as man-made goods in the Anthropocene (Hardesty et al., 2017). The issues caused by the accumulation of improperly disposed plastic solid waste in marine and coastal zone environments due to their inability to degrade in the environment effectively is a major international concern (Lusher, 2015) (Plastic under natural environmental conditions takes decades to centuries to completely degrade and disappear (Andrady, 2011). However, before disappearing, physical, chemical, and biological processes reduce the structural integrity of plastics, leading to their fragmentation (Browne et al., 2008). Internationally, plastics smaller than 5 mm are microplastics (Thompson et al., 2004; Hidalgo-Ruz et al., 2012). Microplastics have been widely detected in water, sediments, and other environmental media and organisms (Browne et al., 2011; Naji et al., 2017; Zuo et al., 2020). The amount of plastic and microplastics floating in the ocean is about 5.25 trillion pieces, equivalent to 65,269,000 tons (Eriksen et al., 2014). These particles exist throughout the Earth’s ecosystems, including rivers, oceans, and mangrove wetlands. Because of their small size, microplastics can be ingested by various organisms, such as birds, mammals, and turtles, and fugacity has been detected from single cells in mammals (Stolte et al., 2015). More seriously, several problems have been reported in the metabolism of these organisms, such as microplastics blocking and scratching the digestive organs, leading to pseudo-satiety, reduced nutritional capacity, fertility and reproduction, induced organ wounds, rupture, and death (Maghsodian et al., 2021). Also, microplastics contain an average of 4% plastic additives, and such compounds may release toxic substances. In addition, microplastics in the environment can accumulate polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and heavy metals, which can be enriched at higher trophic levels through the food chain, leading to the death of various marine organisms (Barnes et al., 2009; Teuten et al., 2009; Rios et al., 2010; Gallagher et al., 2016), and may pose human health and potential socioeconomic threats (Devriese et al., 2015; Leslie et al., 2017) (Browne et al., 2007; Thompson et al., 2004; Vethaak and Leslie, 2016).
To study microplastic fugacity characteristics and pollution levels, scientists usually collect samples from oceans, mangrove wetlands, and rivers (Claessens et al., 2011). Mangrove wetlands, as special habitats and important buffers, are considered barriers to land-based plastic transport to the ocean and require special attention for research. As a critical interface for river-to-ocean transport, mangrove wetlands play a vital role in trapping suspended matter in the water column and sediments while providing food and habitat for various marine and terrestrial organisms (J. Deng et al., 2020; Kathiresan, 2003). Mangroves reduce coastal erosion, act as filters for pollutants trapped in their sediments, and stop dispersing to open water (MacFarlane et al., 2007). According to a study on the distribution of microplastics in mangrove sediments, mangroves attenuate waves through strut roots, thereby affecting microplastic accumulation in sediments (Duan et al., 2021). Therefore, it is important to summarize the current study of the mechanism and relationship between microplastics and mangrove wetlands.
As one of the most important marine ecosystems, Mangrove ecosystems have sensitive and outstanding ecological values. Mangrove conservation and restoration are necessary to stabilize coastlines and maintain healthy coastal environments. Protecting and maintaining mangrove ecosystems for human benefit has also become a “biodiversity hotspot” for various species. Studies have shown that most of the solid waste polluting mangroves is plastic, textiles, glass, and wood, with plastic accounting for about 70% of the total (van Bijsterveldt et al., 2021). The high fertility of mangrove wetlands leads to a different mechanism of microplastic decomposition than other habitats such as aquatic environments (Li et al., 2019). Mangroves prevent erosion by stabilizing sediments, maintaining water clarity, absorbing large amounts of CO2, filtering pollutants, and capturing sediments from land, as evidenced by the important contribution of organic carbon (Meynecke et al., 2007), especially the ecological function of mangrove carbon sinks to mitigate global climate change (Donato et al., 2011). Pollution from plastic waste has been reported to pose a serious threat to mangrove vegetation height and density (J. Deng et al., 2020). Over the last few decades, mangroves have been subjected to multiple threats and are disappearing worldwide at an alarming rate of 1–2% per year. (Richards and Friess, 2016). However, the current literature is distributed and broad besides limited information on the fate characteristics and pollution levels of microplastics in mangrove ecosystems. Literature reviews are necessary to summarize the research status and trends.
Literature reviews occupy a fundamental position in scientific research and serve as a bridge to thematic studies. This is because any study is based on the contributions of previous authors. A review of prior contributions in the field before conducting a study on a particular topic is not only a way to respect previous authors’ contributions but also a way to illustrate the innovation through comparison. Studies addressing microplastic fugacity characteristics and pollution levels in mangrove ecosystems are relatively limited (J. Deng et al., 2020). Based on the important ecological value of mangrove wetlands and the increasing threat of microplastics, further research related to mangrove microplastics needs to be sorted out and summarized to draw the attention of more scholars. In this paper, a systematic literature review was conducted to review the characteristics of microplastics in mangrove wetlands and their pollution levels at home and abroad. The objectives are: 1) To summarize the overall characteristics of microplastics, including color, shape, size, polymer chemistry, and surface microstructure. 2) To summarize the global and Chinese mangrove microplastic pollution levels and distribution information. 3) Suggest future research directions.
The next section 2 discusses the study background and research methodology while section 3 focuses on the study results. The section 4 presents manuscript discussion, conclusion and future study suggestions.
Materials and methods
Literature reviews are divided into “narrative literature reviews” and “systematic literature reviews” (Petticrew, 2001). The Systematic Review Methodology (SRM) is a standardized literature research method that utilizes different databases and multiple search and analysis techniques to comprehensively capture research progress on a topic and draw conclusions accurately. The systematic literature review method ensures clarity in all processes, from identifying literature selection criteria to the specific selection of literature, especially the distillation of findings from the scattered research literature. It is a transparent mechanism for literature review research (Borenstein et al., 2021). This paper systematically analyzed the related literature through the systematic literature review method with the following flowchart and determination criteria (Figure 1).
The data used in this paper were all obtained from the China National Knowledge Internet (CNKI) and Web of Science (WOS). In CNKI, the Chinese words “microplastics + mangroves” were used as the keyword for the subject search from 2006-01-01 to 2021-12-31. In WOS, the core collection database was searched with “microplastic mangrove” as the keyword for 2006-01-01 to 2021-12-31. In addition, to cover the research papers as much as possible, terms such as plastics deris, plastics pellets, sediment, and wetland were used to supplement the search, and the content ranking was verified manually.
Results and discussion
The systematic literature review method was used to summarize the characteristics of microplastic fugacity in mangrove ecosystems from the literature, including color, size, composition, surface microstructure, etc.; to summarize the level of microplastic pollution in mangrove wetlands at home and abroad; and then to speculate future research trends.
Fugacity characteristics as basic information for microplastic research in mangrove ecosystems
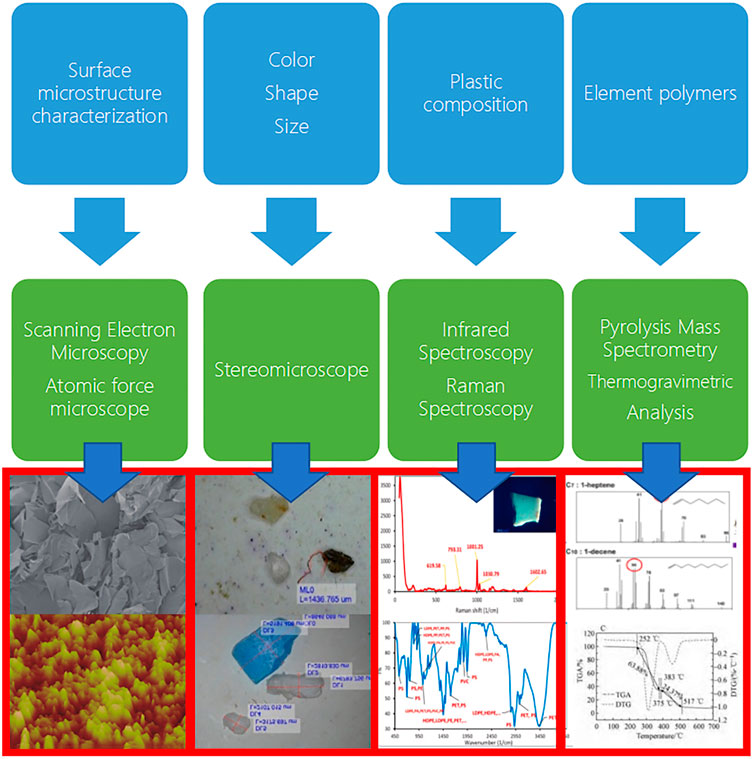
Microplastic abundance is the main feature to describe the pollution level of the mangrove ecosystem in terms of unit quantity. In addition, microplastic fate characteristics include their physical and chemical properties, and the latter has become important basic information for microplastic studies in mangrove ecosystems. Many scholars use the fugacity characteristics of microplastics to infer their sources, analyze environmental processes, and assess ecological risks (Peng et al., 2017). Characteristics, such as their size, color, shape, composition, surface microstructure, and other properties, have been explored in various reports (Figure 2). Usually, physical identification methods use microscopic identification, divided into three parameters: size, color, and shape (Frias et al., 2018). Furthermore, infrared spectroscopy (μ-FTIR), fourier transform reflectance infrared spectroscopy (ATR-FTIR), micro-Raman spectroscopy (μ-Raman), pyrolysis-gas chromatography-mass spectrometry (Py-GCMS) and thermogravimetric analysis (TGA) are used to identify different elements and polymers of microplastics. Some scholars also observed their microstructure by scanning electron microscopy (SEM) and atomic force microscopy (AFM) to study their aging process and environmental behaviors.
Microplastic particles’ abundance, distribution, and fate are influenced by their size (Duan et al., 2020). It has been reported that the smaller plastic particles are, the more frequent they occur in sediments (Batel et al., 2018). Studies have shown that smaller microplastics in mangrove sediments may pose a greater potential threat to biological communities because smaller microplastics are more likely to adsorb persistent organic pollutants (POPs) and are more readily transferred to organisms. Therefore, it is necessary to study the size of microplastics. However, there is no international standard for sizing microplastics in mangrove ecosystems. Even in the more mature marine plastic litter research field, there is still no internationally accepted classification standard for marine microplastics. However, marine litter can be classified according to size.
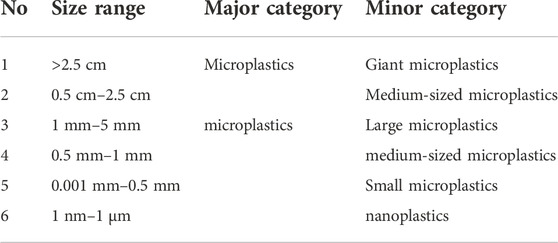
Based on a review of the relevant literature, the most widely accepted definition of “microplastics” is those with the longest diameter of less than 5 mm (Hidalgo-Ruz et al., 2012). Some scholars have simply divided them into large microplastic particles (1–5 mm) and small microplastic particles (0.1–1000 μm) (Imhof et al., 2012; Qiu et al., 2016). Some researchers have considered further subdivisions of plastic particles. For example, the Marine Litter Technical Group for the implementation of the Marine Strategy Framework Directive (MSFD) recommends further subdivision into large microplastics (1–5 mm), medium-sized microplastics (5–25 mm), and giant microplastics (>25 mm), while those that are not visible to the naked eye (<1000 μm) are called “small microplastics” (Galgani et al., 2013). Gigault et al. (2018) classify microplastics larger than 2.5 cm as giant microplastics. Particles with a size of 0.5–2.5 cm are called medium-sized microplastics, while particles with a size of 0.5–1 mm are called large microplastics, and particles with a size of 0.001–0.5 mm are called small microplastics. Nano plastics in this classification are the last group with dimensions of 1 nm–1 μm (Gigault et al., 2018). In another study on the microplastics collected from the Atlantic, sizes were classified into five groups: 0.25–0.5 mm, 0.5–0.75 mm, 0.75–1 mm, 1–2 mm, and 2–5 mm (La Daana et al., 2017). Maghsodian et al. (2021) classified plastic particles found in mangrove sediments into three groups based on size: microplastics >2.5 cm, medium-sized plastics of 0.5–2.5 cm, and microplastics of 0.5–3 mm (Maghsodian et al., 2021). Combining the above literature on microplastic and microplastic size classification, this paper summarizes them into six groups for reference.
There have been many studies on the size characteristics of microplastics from environmental media and organisms in mangrove ecosystems. In terms of environmental media, the main studies have been on mangrove sediments; for example, microplastics smaller than 20 μm have been found in Singapore mangrove sediments (Nor and Obbard, 2014). Naji et al. (2019) classified microplastics isolated from mangrove sediments of Ramsar (Iran) into two groups (10–300 μm and 300–1000 μm) based on size, with small particles accounting for 70–97% (Naji et al., 2019). Zhou et al. (2020) reported the highest percentage of particles below 2.5 mm in size in a study of microplastics in mangrove sediments along the southeast coast of China (Zhou et al., 2020). Microplastics, mainly smaller than 1 mm were detected in the mangrove sediments of the Maowei Sea (J. Li et al., 2018). The above studies indicate that smaller sizes mainly dominate microplastics in mangrove sediment.
Among the reported microplastic characteristics of organisms in mangrove ecosystems, a study on the size of microplastics in crab and fish in mangroves in Beibu Bay, China, showed that 1–500 μm accounted for 85.84% (Zhang et al., 2021). Maghsodian et al. (2021) observed microplastics of size 0.5–3 mm in mudskippers (Maghsodian et al., 2021). Smaller microplastic sizes were reported in a study on the quantification and characterization of microplastics ingested by juvenile fish associated with mangroves in KwaZulu-Natal, South Africa, with an average microplastic size of 0.77 ± 0.89 μm (R. Naidoo, 2020).
Microplastic shape and environmental processes
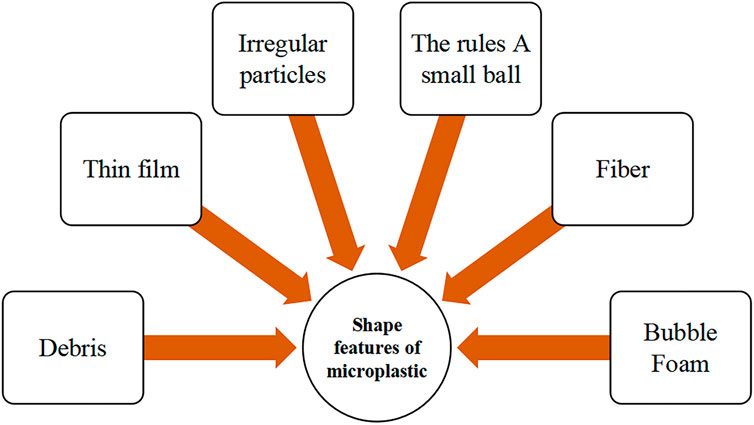
There is no official unified naming standard or specification for microplastic shapes (Gago et al., 2019). The detection of different shapes of microplastics may be due to different pollution sources or different environmental processes (e.g., erosion, solar radiation, biodegradation). Microplastics can take regular and irregular shapes. Regular shapes are usually from direct release (primary microplastics) and are generally beads or small balls. At the same time, irregular shapes are mainly due to the decomposition of larger plastic fragments (secondary microplastics), which contain fibers, debris, and other irregular shapes (Khatmullina and Isachenko, 2017). Based on various literature reports, microplastic shape characteristics were divided into six groups (Figure 3). The first group is irregularly shaped, coarse-edged, sharp microplastics called fragments, which are the most abundant. The second group is like movie film, transparent, thin, and flexible, called film-like. The third category is round, irregular particles and larger than other microplastics. The fourth category is also round particles, more regular and about 1 mm in size, but less diverse than the third category. The fifth category is elongated, called fibrous, and comes in various sizes, with a relatively high percentage as granular. The sixth category is foam-like, formed by the fragmentation of polystyrene foam (Pflieger et al., 2017).
The main shapes of microplastics observed in marine organisms are fibrous or granular, which may come from discarded fishing gear (Zhang et al., 2021). The shape of microplastics in the aquatic environment can be regular or irregular and indirectly affect their environmental processes. For example, when microplastics are in the form of fibers or films, they are more buoyant, difficult to settle in the water, and more likely to spread to different marine areas, so more organisms may mistakenly ingest them as food. On the other hand, when microplastics are present as spheres, they are relatively easier to settle or precipitate (Jung et al., 2021).
For the shape characteristics of microplastics in mangrove sediments, domestic and foreign scholars have also done a lot of research. Domestically, the highest percentage of microplastics in the Pearl River Estuary, China mangrove sediments was found in particles and fibers (Zuo et al., 2020). Most microplastics detected in Beibu Bay, China mangrove forests were also particles and fibers (Zhang et al., 2021). In another study on mangrove microplastics in Dongzhai Harbor, Hainan, four types of microplastics were detected: elastic fibers, soft films, foams, and fragments, among which elastic fibers (EF) were detected at a high level in all the study areas, accounting for 57.02%, followed by films accounting for 21.11%. Microplastics detected in mangroves of Qinzhou Bay, Guangxi were fragmented, fibrous, and spherical. It was found that the highest percentage of isolated microplastics in mangrove sediments and fish in southern Iran was fibers (Maghsodian et al., 2021). Particles and fibers were the most abundant microplastics detected in the north coast of the Persian Gulf (Naji et al., 2019). In the study of mangrove microplastics in the Colombian Caribbean Sea, particles, fibers, and film forms were the most common (Garcés-Ordóñez et al., 2019). Fibrous microplastics were mainly detected in mangrove sediments from Ramsar (Iran), where sewage discharge may be the main source (Naji et al., 2019). Synthetic resinous material was detected in all seven habitats of Singapore mangroves, and most microplastics were fibrous (Nor and Obbard, 2014). The fibrous shape was the most commonly isolated in mangrove microplastic studies. This may be because this morphology is more likely to be enriched in organic matter-rich mangrove sediments by adsorption (Zhou et al., 2020).
Microplastic color and biological predation
Color is one of the physical characteristics influencing microplastic feeding by organisms at different trophic levels (John et al., 2021). Microplastics vary in color and look similar to plankton. Some of the most important commercial fish and their larvae are visual predators that may ingest more prey-like microplastics, such as white, brown, and yellow plastics (Maghsodian et al., 2021). Another study showed that dark-colored microplastics (such as black and blue) were ingested more by organisms than light-colored microplastics due to their attractiveness or color similarity to prey (John et al., 2021).
In studies of mangroves in the Persian Gulf, the highest proportion of white microplastics was found in sediments, probably due to secondary microplastic degradation (Naji et al., 2019). The proportion of microplastics in particles isolated from seafood such as fish is highest in black (60%) and lowest in white (7%) (Maghsodian et al., 2021). The most common microplastic particles observed in a study of juvenile mangrove fish in KwaZulu-Natal, South Africa were blue (Naidoo et al., 2020). Blue, black and white microplastics are most common on the northern coast of the Persian Gulf (Naji et al., 2019). Transparent white, black and blue are the three main colors of microplastics observed in mangroves in southern China (Li et al., 2019). The microplastics detected in Qinzhou Bay in Guangxi have a variety of colors, such as white, transparent, yellow, green, red, and blue. Combined with the above reports, the most common colors identified in the mangrove include black, blue, white, transparent, red, green, etc. (Frias et al., 2018). However, the isolation and identification of microplastics cannot be based solely on color differences. Because color discrimination is subjective, the visual identification of microplastics is highly controversial. Some particles are white, which is very different from transparent particles. The difference between white and transparent particles is their turbidity. Transparent particles are completely colorless, high transparency. White microplastics are opaque, as opposed to transparent particles. Further evaluation is needed to determine whether it is the original color or discoloration caused by environmental processes such as UV from sunlight and whether it is primary or secondary plastic (Frias et al., 2018; Khan et al., 2021a; Khan et al., 2021b). The usefulness of the green approach has ensured sustainable economic and environmental performance (Naseem et al., 2021; Sarfraz et al., 2022a; Ivascu et al., 2022).
The chemical composition of plastics and fingerprint information
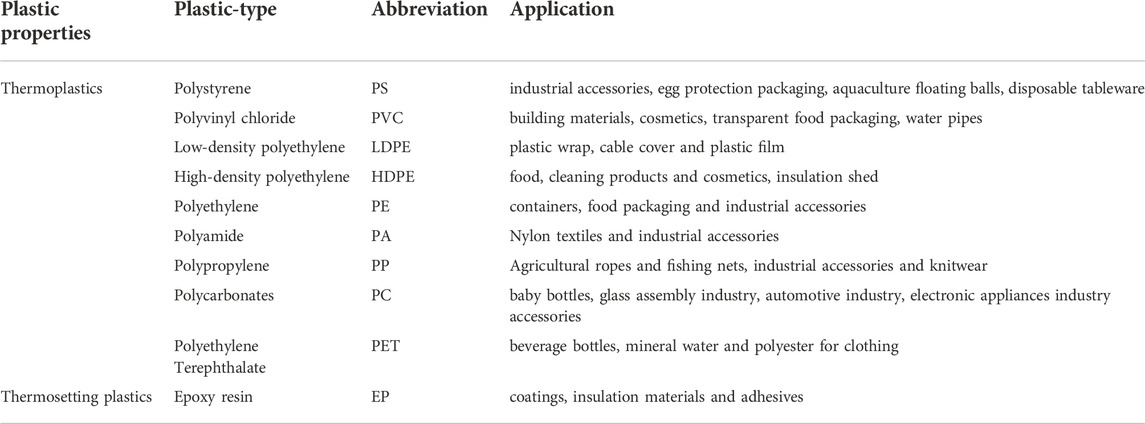
Synthetic resins are plastics’ major components and determine the microplastics' chemical structure or properties (John et al., 2021). Plastics are macromolecules synthesized by addition polymerization or condensation polymerization of monomers as raw materials, as shown in Table 1. Plastic is commonly divided into thermoplastic and thermosetting plastic; the distinction depends on whether to melt when heated. Epoxy and phenolic resins are thermosetting plastics, while polyethylene, polypropylene, polystyrene, and polyvinyl chloride are thermoplastic polymers. Most household plastics are thermoplastics. According to previous statistics, about 90% of global plastic production is thermoplastic polymers (Antony and Neal., 2009).
The physical properties of plastics and non-plastics make microplastics accurately identifiable. In addition to visual recognition, other, more precise methods are needed to identify microplastics. According to the literature, infrared spectroscopy and Raman spectroscopy are widely accepted methods to identify different polymer types of microplastics (Maghsodian et al., 2021; Pan and Chen, 2021; Khan et al., 2022). The analysis of microplastic polymer is the main basis for determining its origin. For example, when PP, PE, or PS are the main polymers, most of the detected microplastics are generated from the fragmentation of larger plastic products (Eerkes-Medrano et al., 2015; Sarfraz et al., 2022b; Mohsin et al., 2022).
PET was more abundant than other polymers in a study of microplastic fugacity characteristics in Brazilian mangroves. In a study of Indonesian mangrove sediments, 50% of the isolated microplastics were PS, and the other 50% were PE and PP (Cordova et al., 2021). PE and PP were detected in mangrove sediments in Singapore, with the highest levels of PA and PVC microplastics (Nor and Obbard, 2014). The most abundant polymers found in mangrove Vietnam was PE (Khuyen et al., 2021). PS, PE, PP, and PET are almost the most abundant microplastics in Iran’s Persian Gulf (Maghsodian et al., 2021). The abundance of PP, PA, and PS is higher than in other polymers in the Colombian Caribbean (Garcés-Ordóñez et al., 2019). The highest abundance of PE and PP was found in the port of Kingston (Rose & Webber, 2019). PE and PP were detected more frequently than other polymers in four estuaries on the east coast of South Africa (Govender et al., 2020). In the study of African mangrove fishes, the percentage of isolated microplastics were PP (70.4%), PET (10.4%), PA (5.2%), and PVC (3.0%) (Naidoo et al., 2020).
Three major microplastics, PS, PP, and PE, were detected in the Qinzhou Bay, Guangxi mangrove forests. In the survey of mangrove sediment in the Pearl River Estuary, PE and PP had the highest abundance among the polymers (Zuo et al., 2020). PE and PET were most abundant in the mangrove forests of Zhanjiang, Guangdong Province, China (Huang et al., 2020). Mangrove sediments in Beibu Gulf, China, had the highest abundance of PE and PET (Zhang et al., 2021). Microplastics in mangroves of Maowei Sea, South China Sea area were mainly PE, PP, and PS. Overall, thermoplastic microplastics were detected more frequently, and PE and PP were the most frequently detected microplastics in mangrove sediments along the Chinese coast (Zuo et al., 2020).
Microplastic surface microstructure, adsorption behavior, and aging process
High temperatures and strong solar UV light can accelerate plastic fragmentation (Lambert et al., 2013). Microplastic particles may bind and integrate into aggregated material with other organic fragments (Rillig, 2018). During cracking, the surface morphology of plastic particles may change significantly due to corrosion and biological effects (Imhof et al., 2012). Roughness and cracks may appear on the surface of the microplastic, while clay minerals and quartz particles can be embedded in the pores or cracks of the microplastic (Kowalski et al., 2016). Environmental erosion and biofouling can significantly alter the surface morphology of plastic particles, which may show roughness and cracks (Imhof et al., 2012).
Further, inorganic and organic substances may enrich and alter the overall morphological characteristics of microplastics (Kowalski et al., 2016). Naji et al. (2019) used scanning electron microscopy to isolate some non-plastic particles from suspected plastic-like particles in mangrove sediments (Naji et al., 2019). Electron microscopy scanning is a common method to identify microplastics indicating microstructure, and some scholars have also used atomic force microscopy to observe their surface roughness change process (Qian Zhou, 2021).
Pollution levels and distribution of microplastic in mangrove wetlands
The spatial distribution of microplastics can provide important clues to the location of plastic release (Heo et al., 2013; Lee et al., 2013). In recent years, microplastic pollution in mangrove wetlands has been rising, and their pollution levels are significantly different from other coastal environments due to their unique ecological characteristics. Investigations of mangrove microplastics may significantly change our estimates of plastic stocks in the marine environment (Nor and Obbard, 2014). However, systematic investigation of the microplastic distribution and pollution assessment in mangrove wetlands are limited (Li et al., 2019). Based on the existing literature reports, the following summarizes the microplastic pollution levels in mangrove wetlands from domestic and international dimensions.
Distribution of microplastic pollution in mangrove wetlands of China
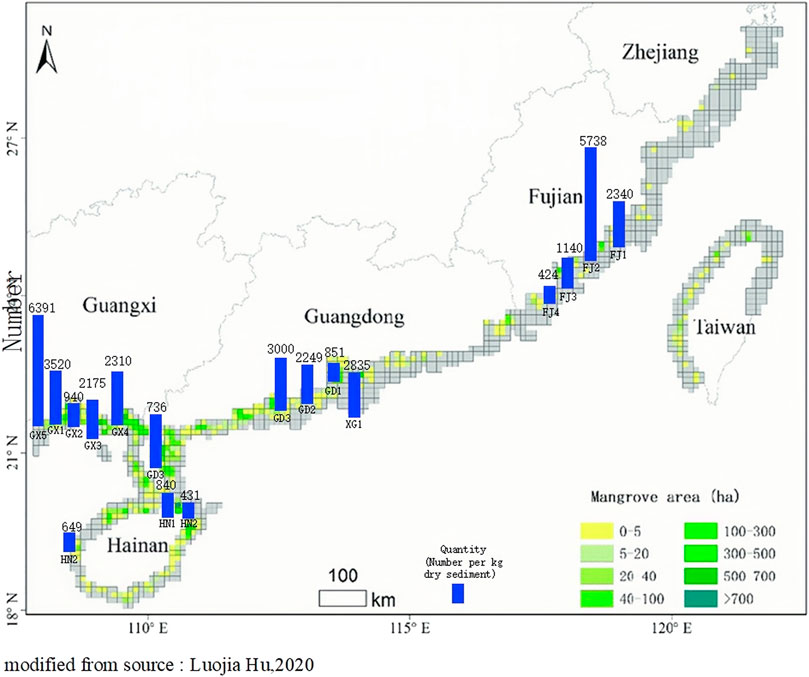
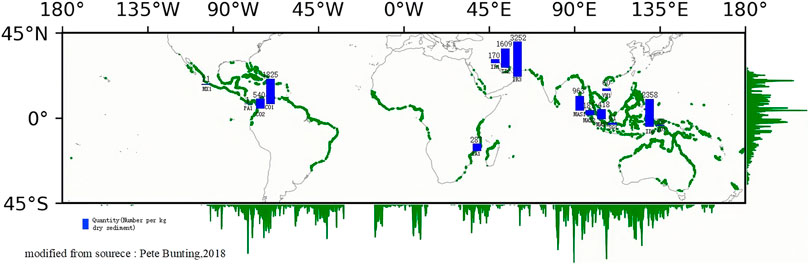
From the analysis of microplastic pollution levels in the Chinese mangrove distribution map (Hu L et al., 2020), it can be summarized that current studies mainly focus on microplastic pollution in mangrove sediments, and the study areas are mainly concentrated in South China and Southeast coast (Figure 4). Deng et al. (2020) studied mangrove forests in the Jinjiang estuary, Fujian Province. They found that the overall abundance of microplastics in sediments was high, ranging from 980 ± 255 to 2340 ± 188 n/kg (H. Deng et al., 2021). Zuo et al. (2020) investigated microplastic contamination in sediments from three mangrove areas in the Pearl River Estuary, Guangdong Province. They found that microplastic abundances ranged from 100–7900 n/kg with a mean value of 851 ± 177 n/kg (Zuo et al., 2020). The microplastics' mass concentrations in Dongzhai Harbor, Hainan mangrove sediments ranged from 1.39 to 13.65 mg/kg. The concentration of microplastics in mangrove forest sediments outside Qinzhou Bay, Guangxi, ranged from 306–6168 n/kg, with an average concentration of 2174.5 ± 2206.8 n/kg. In contrast, the abundance of microplastics in the sediments inside the mangrove forest was much lower, with an average concentration of 42.9 ± 26.8 n/kg. This may be related to the transport hindrance of plastic waste by the dense aerial root system of mangroves and tides (Li et al., 2018). In a study of mangrove microplastics in coastal mangroves in southeast China, an average of 5738.3 ± 8.3 n/kg microplastic particles were detected in mangrove sediments (Zhou et al., 2020). An average of 15 ± 12.85 n/kg of microplastics in sediment was found in the sandy mangrove coast (Li et al., 2018). Current studies and trends show that investigating microplastic pollution in intertidal mangrove wetlands is receiving more and more attention from scholars. Li et al. (2019) suspected that microplastic mangrove pollution in the semi-enclosed sea is significantly different from other coastal types due to its unique geographical characteristics and found that the abundance of microplastics in mangroves of the Maowei Sea estuary was much lower than that in the ocean entry zone, with abundances ranging from 520 ± 8 to 940 ± 17 n/kg (Li et al., 2019). However, data on microplastic distribution and fugacity characteristics in mangrove sediments from semi-enclosed seas are still very limited. On the whole, microplastic pollution in Fujian, Guangdong, and Guangxi provinces’ mangroves is somewhat more serious than that in Hainan. The average number of plastic particles isolated from mangrove sediments in southern China was 851 ± 177 n/kg (Zuo et al., 2020).
Pollution levels of microplastics in mangrove wetlands abroad
From the map in Figure 5, it can be seen that global mangrove forests are mainly distributed in tropical and subtropical regions. Since the data of the global mangrove microplastic system study is not comprehensive, some of the research data are listed in this paper. Figure 5 shows that the investigated regions are mainly concentrated in Southeast Asia, the Middle East, and South America. Barasarathi et al. (2014) conducted a study in Kapar, a remote area in Malaysia far from human industrial and commercial activities. They found that this region’s abundance of microplastics in the surface sample of mangrove sediment (50 cm × 50 cm, depth 5 cm) was 418 n/kg. The microplastics were mainly distributed on the surface 1–2 cm depth (Barasarathi et al., 2014). Smith (2012) Smith (2012) found an abundance of 3349 n/kg of microplastics in mangrove sediments from Motupore Island, Papua New Guinea. Nor and Obbard (2014) investigated microplastic abundance in mangrove wetlands in Singapore and found an overall abundance range of 12.0–62.7 n/kg (dry weight) (Nor & Obbard, 2014). The average microplastics in mangrove sediments from Ramsar (Iran) ranged from 19.5 to 34.5 n/kg of dry sediment, respectively (Naji et al., 2019). However, the total number of microplastic particles found in mangrove sediments in southern Iran was 2657n (Maghsodian et al., 2021). Microplastic contamination was detected in both seawater and sediments of mangroves in South Africa (Govender et al., 2020). In another study, the average amount of microplastics isolated from mangrove sediments in the northern Persian Gulf was 34.5 ± 19.5 n/kg (Naji et al., 2019). Areas with lower microplastic contamination indicate lower anthropogenic activity, and areas with higher anthropogenic activity may generate more plastic solid waste, resulting in higher abundance levels of microplastics (Nor and Obbard, 2014).
Overall, no matter whether in China or abroad, microplastic contamination was detected in all major mangrove distribution areas and correlated with mangrove density and human activities. As an important coastal zone special habitat, mangrove wetlands are an important interface for sea-land transport and an important barrier to blocking land-based inputs of plastic pollution. Mangrove wetlands provide a habitat for a variety of organisms and also contain significant carbon sinks. Microplastic pollution poses a significant ecological risk and potential threat to human health and social and economic development (Browne et al., 2007; Devriese et al., 2015; Thompson et al., 2004; Vethaak and Leslie, 2016). Microplastic pollution and distribution in mangrove wetlands is attracting increasing scholarly attention (Zhang et al., 2021).
Future studies should focus on the microplastic transport process, ecological risk assessments, the effects on the carbon cycle, and policies and management plans for microplastic pollution.
Discussion and conclusion
In this paper, we systematically reviewed the published articles on mangrove-related microplastics. Microplastic fugacity characteristics are the basis for studying mangrove ecosystems, which include color, shape, size, and polymer, based on physical sensory identification, chemical composition analysis, and electron microscopic micrographs. The size of microplastics is the key to studying the distribution and convergence, but there is no internationally accepted classification standard, and this paper summarizes them into six groups (Table 2). The microplastics in mangrove sediments are relatively small in size, and those detected in organisms are also mainly small in size. The shape of microplastics is the key to studying their origin and environmental processes. Generally, the shape of primary microplastics is relatively regular, and the shape of secondary microplastics is influenced by environmental processes and plastic raw material types. Most scholars agree to describe the shape characteristics of microplastics by six categories (Figure 3), such as fragments, films, and fibers. The fibrous shape is the most commonly isolated in mangrove microplastic studies (Zhou et al., 2020). Microplastic color affects biological predation and is important information for studying ecological risk, which may be related to biological feeding behavior and microplastic attachment. However, environmental influences may alter microplastic color. Further assessment is needed to determine whether it is the original color or environmental processes that bring about the discoloration and whether it is primary or secondary plastic (Frias et al., 2018).
Infrared and Raman spectroscopy are recognized methods for detecting different polymer types of microplastics and are key to studying microplastic fingerprint information and source sinks (Maghsodian et al., 2021). Overall, thermoplastic microplastics were detected more frequently, and PS, PP, and PE had the highest frequency of microplastic detection in Chinese coastal mangrove sediments (Zhou et al., 2020). The surface microstructure of microplastics is an important basis for studying adsorption behavior and aging processes and is generally observed using scanning electron microscopy. Microplastic studies in China are mainly on southern and southeastern mangrove coasts, and pollution is more serious in Fujian, Guangdong, and Guangxi than in Hainan. Studies on mangrove microplastics abroad are mainly concentrated in Southeast Asia, the Middle East, and South America. Overall, microplastic contamination was detected in the major distribution areas of mangroves worldwide and was correlated with mangrove density and human activities. Although this study may not be rigorous enough due to the limited data, microplastic research in mangrove wetlands has attracted increasing attention from scholars (Zhang et al., 2021).
Study recommendations
Based on the above summary of the microplastic fugacity characteristics and pollution distribution in mangrove wetlands, this paper then proposes several suggestions for future research trends: 1) To study the microplastic transport process from land-based sources to the ocean from the perspective of microplastic environmental behavior, and the mechanism of microplastic blockage by mangrove wetlands. 2) Strengthen the census of mangrove microplastics and pollution source analysis, and systematically study the spatial and temporal distribution characteristics, fingerprint information, and influencing factors of global microplastics. 3) Study the effects of microplastic pollution on different mangrove forest species, tree ages, growth and development, and mortality, as well as the effects on various organisms and communities inhabiting the forest, and then comprehensively assess the ecological risks induced by them from macroscopic and microscopic perspectives. 4) To study the effects of microplastics on the role of various microorganisms and algae and the carbon cycle in mangrove wetlands from the perspective of carbon neutrality and the ecological value of abundant mangroves. 5) Finally, policies for microplastic pollution in mangrove wetlands and integrated management of plastic throughout its life cycle are proposed based on the above studies.
Author contributions
The author BC confirms being the sole contributor of this work and has approved it for publication.
Funding
This research was supported by Natural Science Foundation of Zhangzhou City of China (ZZ2021J34), and Education Research Project for Youth and Middle-aged Teacher of Fujian Province (JAT210632).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrady, A. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull. 62 (8), 1596–1605. doi:10.1016/j.marpolbul.2011.05.030
Anthony, A. L., and Neal, M. A. (2009). Applications and societal benefits of plastics. Phil. Trans. R. Soc. B 364 (1526), 1977–1984. doi:10.1098/rstb.2008.0304
Barasarathi, J., Agamuthu, P., Emenike, C. U., and Fauziah, S. H. (2014). Microplastic abundance in selected mangrove forest in Malaysia. Proceeding ASEAN Conf. Sci. Technol. 5.
Barnes, D. K. A., Galgani, F., Thompson, R. C., and Barlaz, M. (2009). Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364 (1526), 1985–1998. doi:10.1098/rstb.2008.0205
Batel, A., Borchert, F., Reinwald, H., Erdinger, L., and Braunbeck, T. (2018). Microplastic accumulation patterns and transfer of benzo [a] pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 235, 918–930. doi:10.1016/j.envpol.2018.01.028
Borenstein, M., Hedges, L. V., Higgins, J. P. T., and Rothstein, H. R. (2021). Introduction to meta-analysis. Amsterdam: John Wiley & Sons.
Browne, Mark A., Dissanayake, A., Galloway, T. S., Lowe, D. M., and Thompson, R. C. (2008). Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 42 (13), 5026–5031. doi:10.1021/es800249a
Browne, Mark A., Galloway, T., and Thompson, R. (2007). Microplastic--an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 3 (4), 1–561. doi:10.1897/ieam_2007-048
Browne, Mark Anthony, Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., et al. (2011). Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 45 (21), 9175–9179. doi:10.1021/es201811s
Claessens, M., De Meester, S., Van Landuyt, L., De Clerck, K., and Janssen, C. R. (2011). Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 62 (10), 2199–2204. doi:10.1016/j.marpolbul.2011.06.030
Cordova, M. R., Ulumuddin, Y. I., Purbonegoro, T., and Shiomoto, A. (2021). Characterization of microplastics in mangrove sediment of muara angke wildlife reserve, Indonesia. Mar. Pollut. Bull. 163, 112012. doi:10.1016/j.marpolbul.2021.112012
Deng, H., He, J., Feng, D., Zhao, Y., Sun, W., Yu, H., et al. (2021). Microplastics pollution in mangrove ecosystems: A critical review of current knowledge and future directions. Sci. Total Environ. 753, 142041. doi:10.1016/j.scitotenv.2020.142041
Deng, J., Guo, P., Zhang, X., Su, H., Zhang, Y., Wu, Y., et al. (2020). Microplastics and accumulated heavy metals in restored mangrove wetland surface sediments at Jinjiang Estuary (Fujian, China). Mar. Pollut. Bull. 159, 111482. doi:10.1016/j.marpolbul.2020.111482
Devriese, L. I., Van der Meulen, M. D., Maes, T., Bekaert, K., Paul-Pont, I., Frère, L., et al. (2015). Microplastic contamination in Brown shrimp (Crangon crangon, linnaeus 1758) from coastal waters of the southern north sea and channel area. Mar. Pollut. Bull. 98 (1–2), 179–187. doi:10.1016/j.marpolbul.2015.06.051
Donato, D. C., Kauffman, J. B., Murdiyarso, D., Kurnianto, S., Stidham, M., and Kanninen, M. (2011). Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 4 (5), 293–297. doi:10.1038/ngeo1123
Duan, J., Han, J., Cheung, S. G., Chong, R. K. Y., Lo, C.-M., Lee, F. W.-F., et al. (2021). How mangrove plants affect microplastic distribution in sediments of coastal wetlands: Case study in Shenzhen Bay, South China. Sci. Total Environ. 767, 144695. doi:10.1016/j.scitotenv.2020.144695
Duan, J., Han, J., Zhou, H., Lau, Y. L., An, W., Wei, P., et al. (2020). Development of a digestion method for determining microplastic pollution in vegetal-rich clayey mangrove sediments. Sci. Total Environ. 707, 136030. doi:10.1016/j.scitotenv.2019.136030
Eerkes-Medrano, D., Thompson, R. C., and Aldridge, D. C. (2015). Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 75, 63–82. doi:10.1016/j.watres.2015.02.012
Eriksen, M., Lebreton, L. C. M., Carson, H. S., Thiel, M., Moore, C. J., Borerro, J. C., et al. (2014). Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250, 000 tons afloat at sea. PloS One 9 (12), e111913. doi:10.1371/journal.pone.0111913
Frias, J., Pagter, E., Nash, R., O’Connor, I., Carretero, O., Filgueiras, A., et al. (2018). Standardised protocol for monitoring microplastics in sediments. Deliverable 4.2.
Gago, J., Filgueiras, A., Pedrotti, M. L., Caetano, M., and Frias, J. (2019). Stand. Protoc. Monit. microplastics seawater. Deliv. 4, 1.
Galgani, F., Hanke, G., Werner, S., and De Vrees, L. (2013). Marine litter within the European marine strategy framework directive. ICES J. Mar. Sci. 70 (6), 1055–1064. doi:10.1093/icesjms/fst122
Gallagher, A., Rees, A., Rowe, R., Stevens, J., and Wright, P. (2016). Microplastics in the solent estuarine complex, UK: An initial assessment. Mar. Pollut. Bull. 102 (2), 243–249. doi:10.1016/j.marpolbul.2015.04.002
Garcés-Ordóñez, O., Castillo-Olaya, V. A., Granados-Briceño, A. F., García, L. M. B., and Díaz, L. F. E. (2019). Marine litter and microplastic pollution on mangrove soils of the Ciénaga Grande de Santa Marta, Colombian Caribbean. Mar. Pollut. Bull. 145, 455–462. doi:10.1016/j.marpolbul.2019.06.058
Gigault, J., Ter Halle, A., Baudrimont, M., Pascal, P.-Y., Gauffre, F., Phi, T.-L., et al. (2018). Current opinion: What is a nanoplastic? Environ. Pollut. 235, 1030–1034. doi:10.1016/j.envpol.2018.01.024
Govender, J., Naidoo, T., Rajkaran, A., Cebekhulu, S., Bhugeloo, A., and Sershen, S. (2020). Towards characterising microplastic abundance, typology and retention in mangrove-dominated estuaries. Water 12 (10), 2802. doi:10.3390/w12102802
Hardesty, B. D., Harari, J., Isobe, A., Lebreton, L., Maximenko, N., Potemra, J., et al. (2017). Using numerical model simulations to improve the understanding of micro-plastic distribution and pathways in the marine environment. Front. Mar. Sci. 4, 30. doi:10.3389/fmars.2017.00030
Heo, N. W., Hong, S. H., Han, G. M., Hong, S., Lee, J., Song, Y. K., et al. (2013). Distribution of small plastic debris in cross-section and high strandline on Heungnam beach, South Korea. Ocean. Sci. J. 48 (2), 225–233. doi:10.1007/s12601-013-0019-9
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C., and Thiel, M. (2012). Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 46 (6), 3060–3075. doi:10.1021/es2031505
Huang, J.-S., Koongolla, J. B., Li, H.-X., Lin, L., Pan, Y.-F., Liu, S., et al. (2020). Microplastic accumulation in fish from Zhanjiang mangrove wetland, South China. Sci. Total Environ. 708, 134839. doi:10.1016/j.scitotenv.2019.134839
Imhof, H. K., Schmid, J., Niessner, R., Ivleva, N. P., and Laforsch, C. (2012). A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 10 (7), 524–537. doi:10.4319/lom.2012.10.524
Ivascu, L., Domil, A., Sarfraz, M., Bogdan, O., Burca, V., and Pavel, C. (2022). New insights into corporate sustainability, environmental management and corporate financial performance in European union: An application of VAR and granger causality approach. Environ. Sci. Pollut. Res. Int. 20, 1–17. doi:10.1007/s11356-022-21642-8
John, J., Nandhini, A. R., Velayudhaperumal Chellam, P., and Sillanpää, M. (2021). Microplastics in mangroves and coral reef ecosystems: A review. Environ. Chem. Lett., 397–416. doi:10.1007/s10311-021-01326-4
Jung, J.-W., Park, J.-W., Eo, S., Choi, J., Song, Y. K., Cho, Y., et al. (2021). Ecological risk assessment of microplastics in coastal, shelf, and deep sea waters with a consideration of environmentally relevant size and shape. Environ. Pollut. 270, 116217. doi:10.1016/j.envpol.2020.116217
Khan, S. A. R., Ponce, P., Yu, Z., and Ponce, K. (2022). Investigating economic growth and natural resource dependence: An asymmetric approach in developed and developing economies. Resour. Policy 77, 102672. doi:10.1016/j.resourpol.2022.102672
Khan, S. A. R., Ponce, P., and Yu, Z. (2021a). Technological innovation and environmental taxes toward a carbon-free economy: An empirical study in the context of COP-21. J. Environ. Manag. 298, 113418. doi:10.1016/j.jenvman.2021.113418
Khan, S. A. R., Yu, Z., and Sharif, A. (2021b). No silver bullet for de-carbonization: Preparing for tomorrow, today. Resour. Policy 71, 101942. doi:10.1016/j.resourpol.2020.101942
Khatmullina, L., and Isachenko, I. (2017). Settling velocity of microplastic particles of regular shapes. Mar. Pollut. Bull. 114 (2), 871–880. doi:10.1016/j.marpolbul.2016.11.024
Khuyen, V. T. K., Le, D. V., Fischer, A. R., and Dornack, C. (2021). Comparison of microplastic pollution in beach sediment and seawater at UNESCO can gio mangrove biosphere reserve. Glob. Challenges 5 (11), 2100044. doi:10.1002/gch2.202100044
Kowalski, N., Reichardt, A. M., and Waniek, J. J. (2016). Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 109 (1), 310–319. doi:10.1016/j.marpolbul.2016.05.064
La Daana, K. K., Officer, R., Lyashevska, O., Thompson, R. C., and O’Connor, I. (2017). Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 115 (1–2), 307–314. doi:10.1016/j.marpolbul.2016.12.025
Lambert, S., Sinclair, C. J., Bradley, E. L., and Boxall, A. B. A. (2013). Effects of environmental conditions on latex degradation in aquatic systems. Sci. Total Environ. 447, 225–234. doi:10.1016/j.scitotenv.2012.12.067
Lee, J., Hong, S., Song, Y. K., Hong, S. H., Jang, Y. C., Jang, M., et al. (2013). Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pollut. Bull. 77 (1–2), 349–354. doi:10.1016/j.marpolbul.2013.08.013
Leslie, H. A., Brandsma, S. H., Van Velzen, M. J. M., and Vethaak, A. D. (2017). Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 101, 133–142. doi:10.1016/j.envint.2017.01.018
Li, J., Zhang, H., Zhang, K., Yang, R., Li, R., and Li, Y. (2018). Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Mar. Pollut. Bull. 136, 401–406. doi:10.1016/j.marpolbul.2018.09.025
Li, R., Zhang, L., Xue, B., and Wang, Y. (2019). Abundance and characteristics of microplastics in the mangrove sediment of the semi-enclosed Maowei Sea of the south China sea: New implications for location, rhizosphere, and sediment compositions. Environ. Pollut. 244, 685–692. doi:10.1016/j.envpol.2018.10.089
Lusher, A. (2015). “Microplastics in the marine environment: Distribution, interactions and effects,” in Marine anthropogenic litter (Cham: Springer), 245–307.
MacFarlane, G. R., Koller, C. E., and Blomberg, S. P. (2007). Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere 69 (9), 1454–1464. doi:10.1016/j.chemosphere.2007.04.059
Maghsodian, Z., Sanati, A. M., Ramavandi, B., Ghasemi, A., and Sorial, G. A. (2021). Microplastics accumulation in sediments and Periophthalmus waltoni fish, mangrove forests in southern Iran. Chemosphere 264, 128543. doi:10.1016/j.chemosphere.2020.128543
Meynecke, J.-O., Lee, S. Y., Duke, N. C., and Warnken, J. (2007). Relationships between estuarine habitats and coastal fisheries in Queensland, Australia. Bull. Mar. Sci. 80 (3), 773
Mohsin, M., Naseem, S., Sarfraz, M., and Azam, T. (2022). Assessing the effects of fuel energy consumption, foreign direct investment and GDP on CO2 emission: New data science evidence from Europe & Central Asia. Fuel 314, 123098. doi:10.1016/j.fuel.2021.123098
Naidoo, R. (2020). A multi-level influence model of COVID-19 themed cybercrime. Eur. J. Inf. Syst. 29 (3), 306–321. doi:10.1080/0960085x.2020.1771222
Naidoo, T., Thompson, R. C., and Rajkaran, A. (2020). Quantification and characterisation of microplastics ingested by selected juvenile fish species associated with mangroves in KwaZulu-Natal, South Africa. Environ. Pollut. 257, 113635. doi:10.1016/j.envpol.2019.113635
Naji, A., Esmaili, Z., Mason, S. A., and Dick Vethaak, A. (2017). The occurrence of microplastic contamination in littoral sediments of the Persian Gulf, Iran. Environ. Sci. Pollut. Res. 24 (25), 20459–20468. doi:10.1007/s11356-017-9587-z
Naji, A., Nuri, M., Amiri, P., and Niyogi, S. (2019). Small microplastic particles (S-MPPs) in sediments of mangrove ecosystem on the northern coast of the Persian Gulf. Mar. Pollut. Bull. 146, 305–311. doi:10.1016/j.marpolbul.2019.06.033
Naseem, S., Mohsin, M., Zia-Ur-Rehman, M., Baig, S. A., and Sarfraz, M. (2021). The influence of energy consumption and economic growth on environmental degradation in BRICS countries: An application of the ARDL model and decoupling index. Environ. Sci. Pollut. Res. 29, 13042–13055. doi:10.1007/s11356-021-16533-3
Nor, N. H. M., and Obbard, J. P. (2014). Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 79 (1–2), 278–283. doi:10.1016/j.marpolbul.2013.11.025
Pan, D., and Chen, H. (2021). Border pollution reduction in China: The role of livestock environmental regulations. China Econ. Rev. 69, 101681. doi:10.1016/j.chieco.2021.101681
Peng, G., Zhu, B., Yang, D., Su, L., Shi, H., and Li, D. (2017). Microplastics in sediments of the changjiang estuary, China. Environ. Pollut. 225, 283–290. doi:10.1016/j.envpol.2016.12.064
Petticrew, M. (2001). Systematic reviews from astronomy to zoology: Myths and misconceptions. Bmj 322 (7278), 98–101. doi:10.1136/bmj.322.7278.98
Pflieger, M., Makorič, P., Viršek, M. K., and Koren, Š. (2017). Extraction of organochlorine pesticides from plastic pellets and plastic type analysis. J. Vis. Exp. 125, e55531. doi:10.3791/55531
Qiu, Q., Tan, Z., Wang, J., Peng, J., Li, M., and Zhan, Z. (2016). Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuar. Coast. Shelf Sci. 176, 102–109. doi:10.1016/j.ecss.2016.04.012
Richards, D. R., and Friess, D. A. (2016). Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl. Acad. Sci. U. S. A. 113 (2), 344–349. doi:10.1073/pnas.1510272113
Rios, L. M., Jones, P. R., Moore, C., and Narayan, U. V. (2010). Quantitation of persistent organic pollutants adsorbed on plastic debris from the Northern Pacific Gyre’s “eastern garbage patch. J. Environ. Monit. 12 (12), 2226–2236. doi:10.1039/c0em00239a
Rose, D., and Webber, M. (2019). Characterization of microplastics in the surface waters of Kingston Harbour. Sci. Total Environ. 664, 753–760. doi:10.1016/j.scitotenv.2019.01.319
Sarfraz, M., Mohsin, M., and Naseem, S. (2022a). A blessing in disguise: New insights on the effect of COVID-19 on the carbon emission, climate change, and sustainable environment. Environ. Sci. Pollut. Res. 29, 29651–29662. doi:10.1007/s11356-021-17507-1
Sarfraz, M., Naseem, S., Mohsin, M., Bhutta, M. S., and Jaffri, Z. u. A. (2022b). Recent analytical tools to mitigate carbon-based pollution: New insights by using wavelet coherence for a sustainable environment. Environ. Res. 212, 113074. doi:10.1016/j.envres.2022.113074
Smith, S. D. A. (2012). Marine debris: A proximate threat to marine sustainability in bootless Bay, Papua New Guinea. Mar. Pollut. Bull. 64 (9), 1880–1883. doi:10.1016/j.marpolbul.2012.06.013
Stolte, A., Forster, S., Gerdts, G., and Schubert, H. (2015). Microplastic concentrations in beach sediments along the German Baltic coast. Mar. Pollut. Bull. 99 (1–2), 216–229. doi:10.1016/j.marpolbul.2015.07.022
Teuten, E. L., Saquing, J. M., Knappe, D. R. U., Barlaz, M. A., Jonsson, S., Björn, A., et al. (2009). Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 364 (1526), 2027–2045. doi:10.1098/rstb.2008.0284
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., et al. (2004). Lost at sea: Where is all the plastic? Science 304 (5672), 838. doi:10.1126/science.1094559
van Bijsterveldt, C. E. J., van Wesenbeeck, B. K., Ramadhani, S., Raven, O. V., van Gool, F. E., Pribadi, R., et al. (2021). Does plastic waste kill mangroves? A field experiment to assess the impact of macro plastics on mangrove growth, stress response and survival. Sci. Total Environ. 756, 143826. doi:10.1016/j.scitotenv.2020.143826
Zhang, S., Sun, Y., Liu, B., and Li, R. (2021). Full size microplastics in crab and fish collected from the mangrove wetland of Beibu Gulf: Evidences from Raman Tweezers (1–20 μm) and spectroscopy (20–5000 μm). Sci. Total Environ. 759, 143504. doi:10.1016/j.scitotenv.2020.143504
Zhou, Q., Tu, C., Fu, C., Li, Y., Zhang, H., Xiong, K., et al. (2020). Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Sci. Total Environ. 703, 134807. doi:10.1016/j.scitotenv.2019.134807
Keywords: mangrove wetlands, research reviews, pollution levels, trends, environment, microplastic
Citation: Chen B (2022) Current status and trends of research on microplastic fugacity characteristics and pollution levels in mangrove wetlands. Front. Environ. Sci. 10:1021274. doi: 10.3389/fenvs.2022.1021274
Received: 18 August 2022; Accepted: 27 September 2022;
Published: 11 October 2022.
Edited by:
Syed Abdul Rehman Khan, Xuzhou University of Technology, ChinaReviewed by:
Kashif Iqbal, Shanghai Dianji University, ChinaMuhammad Ibrahim Abdullah, COMSATS University Islamabad, Lahore Campus, Pakistan
Copyright © 2022 Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Chen, Y2hlbmJpbkB4dWpjLmNvbQ==
 Bin Chen
Bin Chen