- 1Institute of Marine Sciences, National Research Council (CNR-ISMAR), Bologna, Italy
- 2Dipartimento Dei Beni Culturali, Università di Bologna, Ravenna, Italy
- 3Consorzio Nazionale Interuniversitario per le Scienze Del Mare (CoNISMa), Roma, Italy
- 4Institute of Polar Sciences, National Research Council (CNR-ISP), Bologna, Italy
- 5Lamont Doherty Earth Observatory of Columbia University, Palisades, NY, United States
- 6Stazione Zoologica Anton Dohrn, Naples, Italy
Mesophotic ecosystems in the Mediterranean Sea are biodiversity hotspots distributed from ca 30 m down to 180 m, depending upon the depth of the light compensation point. Overall, the taxonomic composition of Mediterranean mesophotic ecosystems is dominated by corals and sponges, with subordinate bryozoans, mollusks, ascidians, and shade-adapted algae. As for most marine ecosystems, the mesophotic habitats are increasingly exposed to natural and anthropogenic threats, including seawater-temperature rise, more intense and frequent heat waves, progressive ocean acidification, fishing activities, and littering. The establishment of effective governance guidelines is, therefore, the necessary rationale to guarantee the good environmental status of such widespread, highly diverse, service-provider natural resources. However, an in-depth quantification of the extent to which Mediterranean mesophotic habitats and taxa are included in conservation measures is lacking. In this article, we review the available literature information on mesophotic habitats in the Mediterranean Sea to evaluate the efficiency of the current legislative framework in providing instruments to protect this natural heritage. Our analysis allows identifying gaps in the current conservation network, ultimately suggesting functional integrative actions for effective conservation measures and the long-term survival of the Mediterranean mesophotic ecosystems.
1 Introduction
Global biodiversity loss is the largest ecological crisis our society is facing together with climate change. Invaluable genetic resources are being lost and ecosystem processes destroyed due to anthropogenic activities (Lande, 1998; Brooks et al., 2006; Danovaro et al., 2021). Current rates of extinction are 1,000 times higher than those of pre-human levels, and future rates might be 100 times higher than those of today (Pimm et al., 1995). The Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services assessment highlighted that almost two-thirds of marine environments have been “severely altered” by human activity causing massive marine biodiversity loss in the last 40 years (Díaz et al., 2019) with a substantial erosion of the environmental services and goods on which we depend (Worm et al., 2006).
The international community delineated the path to strengthen marine protection by 2020 and strike the Aichi Target 11 of the Convention on Biological Diversity, which called for 10% of coastal and marine areas to be “conserved through effectively and equitably managed, ecologically representative, and well-connected systems of protected areas and other effective area-based conservation measures.” Several European member states claimed the achievement of the target, but nearly 90% of the European Marine Protected Areas (MPAs) are not managed effectively (WWF, 2019) and the network of MPAs is not ecologically coherent yet (i.e., representing all natural communities within an area, maintaining ecological and evolutionary processes, and ensuring resilience to large-scale disturbances and to long-term changes), according to the European Environment Agency assessment (EEA, 2015).
Protecting the marine species and resources in their totality is arguably utopistic (Brooks et al., 2006), and we need to identify priorities for conservation (habitats and species) and guide government agencies and environmental organizations toward the best compromise. Many “shortcuts” have been adopted for monitoring management plans, defining “keystone,” “indicator,” “flagship,” “umbrella,” and “charismatic” species (Vane-Wright et al., 1991; Roberge and Angelstam, 2004; Mace et al., 2006).
The information on the species identified as priorities is, however, dramatically scarce. Despite various studies estimating that between 1.4 and 1.6 million species live in the oceans (Bouchet, 2006), currently, less than 15% of the about 240,000 known marine species are considered by the IUCN Red List, the most comprehensive indicator of the health of the world’s biodiversity (https://www.iucnredlist.org/about/barometer-of-life). Summing up, if the status of marine species and habitats is still practically unknown, how can we effectively define what is of priority?
Providing an answer is tremendously and worryingly hard. Geographical gaps exist in implementing conservation measures, with an unbalance in the coverage of protected areas across regions (with Mediterranean and Macaronesian areas as the tail light, EEA, 2015), and between coastal and deep habitats, which are operationally more difficult to reach and remain strongly underrepresented in the conservation and monitoring plans (MedPAN and SPA/RAC, 2017).
Currently, protected areas between 50 m and 200 m depth cover 13.18% of the European designations (MedPAN and SPA/RAC, 2017). This depth range largely overlaps with the mesophotic domain (from 30 m depth down to the photosynthetic compensation point) that might cover a consistent portion of the entire Mediterranean Sea (Castellan et al., 2022). Ecologically relevant habitats occur within this depth layer, whose composition largely varies depending on the geographic area (Pyle and Copus, 2019). Coralligenous formations (Ballesteros, 2006), rhodoliths s.l. (Foster et al., 2013; Basso et al., 2017), sponge grounds (Idan et al., 2018; Goren et al., 2021), structures built by stony corals and mollusks (Taviani et al., 2012; Corriero et al., 2019; Angeletti and Taviani, 2020; Angeletti et al., 2020; Cardone et al., 2020), and cnidarian forests (Bo et al., 2011; Cau et al., 2015; Boavida et al., 2016; Chimienti et al., 2020 among many others) dominate the mesophotic zone of the Mediterranean Sea. It is well established that mesophotic habitats provide various ecosystem services, for example, acting as hotspots of biodiversity, potential sources of commercial species, and carbon sinks (Rossi et al., 2017). Despite their recognized importance, mesophotic habitats do not directly receive protection from marine conservation networks (Rocha et al., 2018; Soares et al., 2020). The lack of a clear definition of the mesophotic zone (Castellan et al., 2022) together with the complex patterns of genetic connectivity of mesophotic assemblages, characterized by critical areas of discontinuities (Costantini et al., 2018), surely did not facilitate the delineation of conservation measures specifically targeting mesophotic habitats. Given their heterogeneous nature in terms of the main structuring taxa, mesophotic habitats are characterized as the perfect ground to test if the current conservation network in the Mediterranean Sea is good enough to favor their long-term preservation or whether we need ad hoc measures.
Here, we analyze the available information on mesophotic-benthic habitats and their taxonomic composition in the Mediterranean Sea to evaluate the efficiency of the current legislative framework in providing instruments to protect this natural heritage. Our contribution not only aims at identifying persisting biases and gaps but also provides a first assessment of the extent to which the conservation network addresses mesophotic habitats across the basin, suggesting potential integrative actions for their long-term survival.
2 Materials and methods
2.1 Literature review
A systematic analysis of the literature was conducted up to 31 May 2022. To identify documents regarding mesophotic habitats in the Mediterranean Sea, the query “Mediterranean” was used in the mesophotic.org database (http://www.mesophotic.org/), while “twilight AND Mediterranean” and “mesophotic AND Mediterranean” were used in Elsevier’s Scopus database (scopus.com). A cross-check between the results from these two databases was performed to exclude duplicates. The records were then screened to remove non-benthic studies (e.g., fish fauna). The typology of habitat, according to the definitions provided in the literature records, and taxonomic lists, whenever present, were extracted (Supplementary Tables S1, S2).
2.2 Data repositories
Literature records were integrated with information from the open-access Ocean Biogeographic Information System (OBIS), held by the UNESCO/IOC project office for IODE in Oostende (Belgium), which provides taxonomically and geographically resolved data for over 47 million observations of marine species. Taxonomic occurrences for the Mediterranean Basin were sorted using the depth range of 30–190 m as a constraint (according to the estimation in Castellan et al., 2022). Duplicated taxa were removed to obtain a list of single taxa observed in the mesophotic depth range. Finally, records were filtered to isolate only benthic taxa (Supplementary Table S2).
2.3 Conservation status
International binding and not-binding instruments in the field of conservation of marine environments were extensively analyzed to extract lists of habitats and taxa currently identified as protected or used to define areas that might deserve management and/or conservation measures. Mesophotic benthic habitat typologies and taxa from the literature and data stored in repositories were compared to those listed in conservation instruments, reporting information on the conservation rank, whenever specified. The number of habitats and taxa currently listed in policy instruments was calculated as percentages.
3 Results
3.1 Conservation status of Mediterranean mesophotic habitats
The screening of literature records on mesophotic benthic habitats in the Mediterranean Sea resulted in 93 scientific documents, including peer-reviewed articles and technical reports (Figure 1; Supplementary Table S1). Most of the literature is represented by single-taxon studies, whilst community composition assessments and area-based censuses are scarce. About 80% of the records defined or reported a description of the target habitats, while the remaining 20% lacked this information since they were represented by large-scale studies encompassing various and unspecified situations or because they were simply not provided (Figure 2). We identified eight categories of habitats, as listed in Table 1.
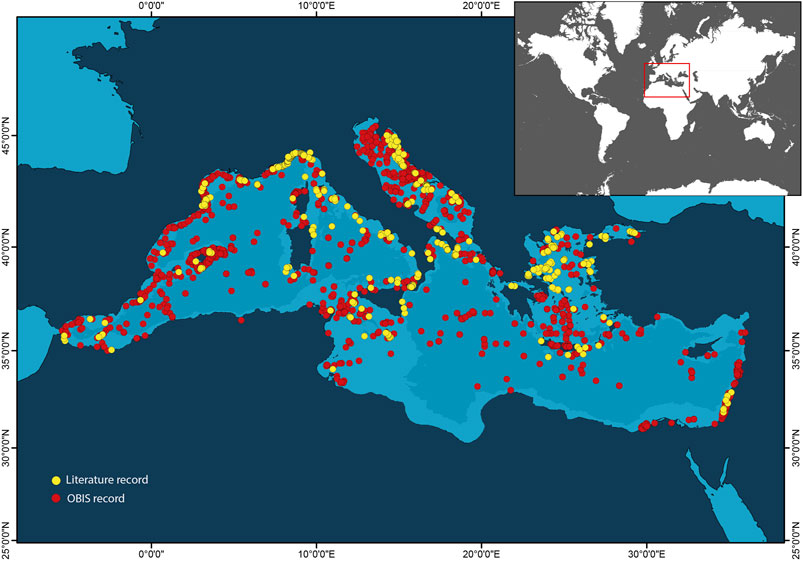
FIGURE 1. Map showing the distribution of available information on mesophotic habitats and taxa in the Mediterranean Sea. Yellow and red dots refer to the literature records (scientific articles and reports) and species occurrences from OBIS repository (obis.org), respectively.
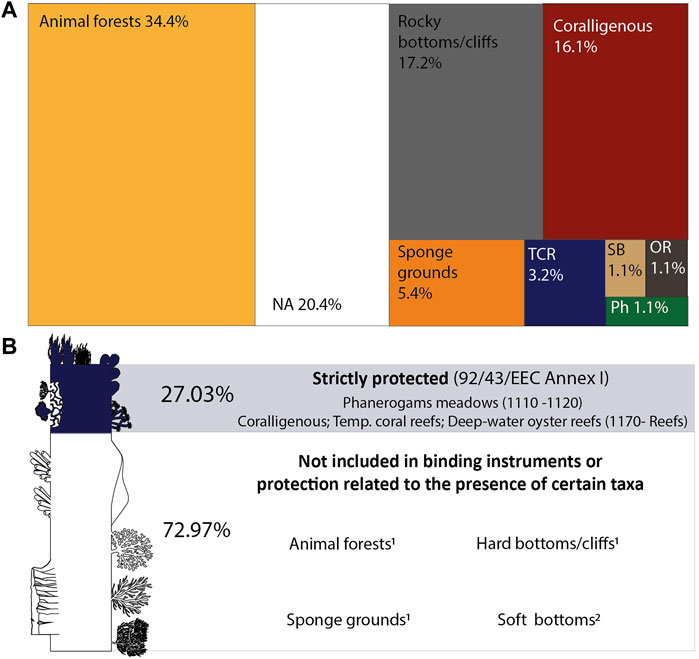
FIGURE 2. Proportion of mesophotic habitats studied in the Mediterranean Sea (A) and the proportion of records targeting habitats included in the current International Policy Framework (B). 1Habitats defined as VME in FAO, 2009. 2Only soft-bottom habitats (1110-Sandbanks) shallower than 20 m depth are listed under the Habitats Directive (Romão, 1996). TCR: temperate coral reefs; SB: soft bottom; OR: deep-water oyster reef; Ph: Phanerogam meadows; NA: not available.
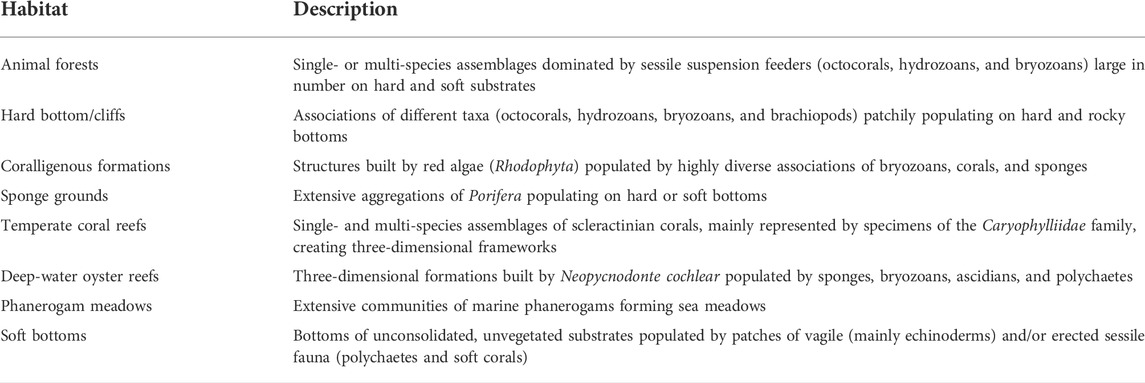
TABLE 1. Description of the target mesophotic habitats from the literature analysis. Definitions reported here summarize those provided in the bibliographic records. See Supplementary Table S1 for further information.
Most of the studies providing information on habitats focused on animal forests (34.4%), with those formed by octocorals and antipatharians as the preferred targets (Figure 2). Although not forming animal forests, cnidarians also represented a frequent focus in the mesophotic literature on hard bottoms/cliffs, accounting for 17.2% of the whole record. Coralligenous formations were the third most frequently studied habitat (16%), followed by sponge grounds (5.4%), temperate coral reefs (3.2%) and deep-water oyster reefs, phanerogam meadows, and soft bottoms (about 1.1% each).
By analyzing the policy framework currently in force, the Habitats Directive resulted as the main instrument for the establishment of binding measures on marine habitats in the Mediterranean area, with four out of the eight habitats identified in the literature listed in its annexes. Phanerogam meadows are listed as “Posidonia beds” (code 1120) and “Mediterranean Cymodocea and Zostera beds” under “sandbanks which are slightly covered by seawater all the time” (code 1110). Coral and oyster reefs and coralligenous are listed as biogenic or geogenic concretions under “reefs” (code 1170).
Although habitats related to soft bottoms may fall under Habitat 1110, the interpretation manual (Romão, 1996) that specifies this category mainly refers to situations shallower than 20 m depth, thus not encompassing mesophotic situations. Animal forests, sponge grounds, and hard and soft bottoms are, instead, not directly included in the Habitats Directive and conservation or management actions are strictly related to the presence of taxa that are listed under Annex IV or other binding instruments (Figure 2).
3.2 Conservation status of Mediterranean mesophotic taxa
The taxonomic lists included in the literature documented the occurrence of 507 benthic taxa within the mesophotic depth range and 3,146 taxa were further obtained from the OBIS repository, resulting in 3,653 different mesophotic-benthic taxa for the Mediterranean Sea (Supplementary Table S1). The final dataset was highly diverse, encompassing 21 Phyla, 53 Orders, and more than 800 Families. Arthropods, mollusks, and annelids accounted for ca. 68% of the entire dataset (∼ 26%, ∼ 21%, and ∼ 20%, respectively), followed by sponges (∼10%), cnidarians (∼8%), and bryozoans (∼5%).
About 69.5% of the identified taxa are currently not included in the legal framework, whilst ca. 30.5% resulted as listed under policy instruments, comprising both those are binding and not-binding (Figure 3). Most of these were represented by sponges, annelids, and bryozoans, followed, by arthropods and cnidarians. The proportions of the listed taxa varied significantly when considering only binding instruments, with the annelids completely disappearing and cnidarians covering about 53% of all the species included in the policy framework, followed by sponges, arthropods, and mollusks (Figure 3).
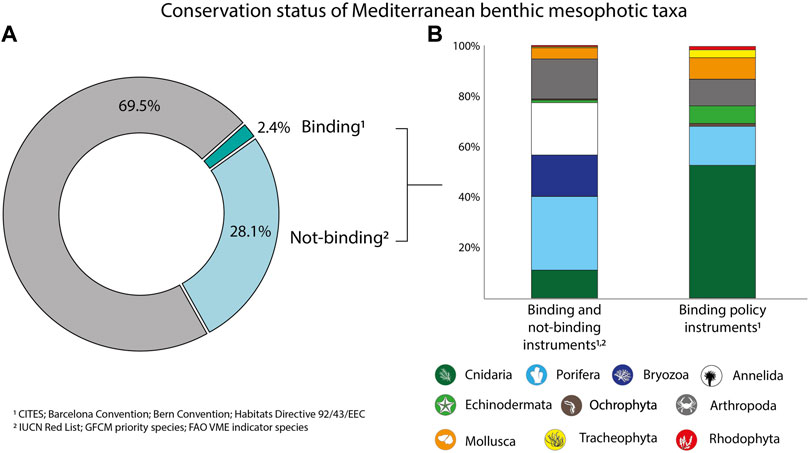
FIGURE 3. Portion of benthic mesophotic taxa included in the International Legal Framework, considering both binding and not-binding instruments (A), and percentage contribution of the identified Phyla to taxa listed under legal instruments (B).
4 Discussion
Conservation efforts in Europe, similarly to other regions around the globe, against biodiversity loss and the impact on ecosystem functions and services has been focused on setting the instruments and priorities for the management of habitat degradation and species protection, their sustainable exploitation, and their monitoring (Figure 4). Considerable advances in the conservation of biodiversity have been documented in the last decades, with 18.5% of the European land area and almost 10% of the total EU marine area currently covered by conservation and/or management measures (EEA, 2020). This substantial effort, however, proves insufficient to reduce biodiversity loss (European Commission, 2020), with only 15% of the habitats and 27% of the species listed in the Habitats Directive have been saved from the risk of extinction to date (European Commission, 2020). Although this insufficient advance is surely related to the multiple impact the biodiversity is facing, lessons from the past provide evidence that conservation goals need to be coupled with adequate planning and prompt integration of scientific information into governance in order to be effective (Guidetti et al., 2008; Yates et al., 2019).

FIGURE 4. International Legislative Framework, comprising binding (bold font) and not-binding instruments, which also includes Mediterranean mesophotic habitats and taxa. The establishment years and depositary organisms are reported.
4.1 Policy framework on the conservation and management of marine natural resources in the Mediterranean Sea
4.1.1 International Union for Conservation of Nature, Red List
The IUCN Red List (iucnredlist.org) was established in 1964 and is the world’s most comprehensive inventory on the extinction risk for flora and fauna that aims at catalyzing action for biodiversity conservation and promoting the protection of species. It collects information on geographic distribution range, population size trends, habitat and ecology, and the extinction risk of more than 142,500 species by classifying them into nine threatening levels based on reports performed by experts. Despite being largely used as a reference to integrate conservation directives by government agencies, wildlife departments, and conservation-related organizations, the list does not have legislative implications and cannot establish binding restrictions or measures. The list mainly includes land species, whilst marine species represent a small amount of the species assessed (less than 15% https://www.iucnredlist.org/about/barometer-of-life).
4.1.2 Convention on International Trade in Endangered Species of wild fauna and flora
CITES (cites.org) was signed in 1973 and entered into force in 1975 to control international trade in wild species of flora and fauna and their by-products for conservation purposes and avoid them becoming threatened through international commerce. CITES does not directly address issues of habitat destruction and biodiversity loss, but it was intended to supplement the management and/or protection of wildlife. It represents a functional mechanism to control the commercial exploitation and alleviate pressure on wild populations (Vincent et al., 2014). The relevance of the convention is that it is legally binding for the states that joined CITES and remains one of the most world’s powerful tools for wildlife conservation. The species covered by CITES are listed in three appendices, according to the degree of restriction in trade. Appendix I includes species for which trade is forbidden. Appendix II refers to species for which trade is restricted and has to be authorized through an international licensing system supported by national managing and scientific authorities. Appendix III refers to species that are protected at least in one country, which can impose controls on trade. Appendices I and II are amended and updated every two/3 years at the Conference of the Parties, participated by 184 states. Currently, the lists contain roughly 1,000 marine species (https://cites.org/eng/app/appendices.php).
4.1.3 The Convention for the Protection of the Mediterranean Sea against Pollution—Barcelona Convention
The Barcelona Convention was adopted in 1976 in Barcelona and entered into force in 1978 in the European Union, while its amendments came into force in 2004 (unep.org/unepmap/who-we-are/contracting-parties/barcelona-convention-and-amendments). The convention comprises a protocol promoting the creation of protected areas and the conservation and regulation of threatened or endangered species of flora and fauna. Annex I of the convention delineates the criteria for the selection of the marine areas to be protected. Annexes II and III provide lists of threatened or endangered species and those whose exploitation requires regulation, including about 130 marine species (https://rac-spa.org/annexes).
4.1.4 The Council of Europe’s Convention on the Conservation of European Wildlife and Natural Habitats—Bern Convention
The Bern Convention (82/72/EEC) came into force in 1982, and it was among the first international agreements aimed at conserving habitats and wild species. The convention establishes general guidelines to develop conservation measures and includes a list of specific species to be protected. Despite its adoption occurred when the information on marine environments was in its infancy, its lists are constantly updated with biannual reports (coe.int/en/web/bern-convention/biennial-reports). The Bern Convention’s lists of species to be protected include ca. 200 marine species, encompassing mammals, invertebrates, fishes, and algae (https://eunis.eea.europa.eu/references/2443).
4.1.5 Council Directive 92/43/EEC—Habitats Directive
Building on the Bern Convention, the European Habitats Directive was first adopted in 1992 by the European Union (92/43/EEC). Contrary to the Bern Convention, this directive is a European law and is mandatorily transposed to the national laws of EU countries. The Habitats Directive is, together with the Birds Directive, the main legislation regarding Europe’s nature conservation policy as its annexes list the protected habitats and species in the EU. It went through a number of updates and corrections, mainly to the annexes, the last in 2007. Annexes II and IV form the basis for the protected species lists in many European countries, delineating the types of habitats and the animal and plant species whose conservation requires the designation of special areas of conservation and animal and plant species of community interest in need of strict protection (ec.europa.eu/environment/nature/legislation/habitatsdirective/index_en.htm). Together with the Birds Directive (2009/147/EC), species and habitats listed under the Habitats Directive represent the backbone of Natura 2000, the largest network of Sites of Community Importance (SCIs) and conservation areas in the world aiming at ensuring the long-term survival of species and habitats of community interest (ec.europa.eu/environment/nature/natura2000/index_en.htm). Unlike the Bern Convention, the Directive’s annexes are, however, not periodically updated and have remained practically unchanged from its establishment, including only five marine habitats and 18 marine species (rac-spa.org/annexes).
4.1.6 General Fisheries Commission for the Mediterranean—Priority and vulnerable species
The General Fisheries Commission for the Mediterranean (FAO-GFCM) is a regional fishery management organization under the Food and Agriculture Organization of the United Nations whose main objective is to ensure the conservation of living marine resources, including aquaculture systems and their sustainable use in the Mediterranean and Black seas (fao.org/gfcm/en/). FAO-GFCM was established in 1949 and counts 22 contracting partners (19 Mediterranean states, 3 Black Sea states, and the European Union). It has authority to deliberate binding recommendations for fishery monitoring and management. During the Ninth Session that took place in 2006, the Scientific Advisory Committee (SAC) on Fisheries identified a list of priority species for the Mediterranean and the Black seas (https://www.fao.org/3/a0889b/a0889b00.htm). Mainly having authority on fishing and aquaculture activities, the overwhelming majority of the roughly 100 marine species included in the lists are represented by cetaceans, sharks, and rays, whilst benthic species are only five (four decapod species and the cnidarian Corallium rubrum, https://www.fao.org/gfcm/activities/fisheries/stock-assessment/priority-species/en/).
4.1.7 FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas
The concept of vulnerable marine ecosystems (VMEs) was formally defined after the United Nations General Assembly (UNGA) in 2004 (A/RES/61/105, 2007). VMEs are groups of species, communities, or habitats that may be vulnerable to the impact from fishing activities. The FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas (FAO, 2009) were built on the UNGA Resolution 61/105 and provide details on the VME, criteria to identify them, and examples of species groups, communities, and potentially vulnerable habitats. Despite the presence of VMEs leading to the establishment of management measures, that, however, only act on restricting fishing activities (Fishery Restricted Areas, FRA, fao.org/gfcm/data/maps/fras/en/), the guidelines have no binding force (fao.org/in-action/vulnerable-marine-ecosystems/background/international-framework/en/). The list of taxa that may form VMEs has been also integrated into Annex 1.c of Monitoring the incidental catch of vulnerable species in the Mediterranean and Black Sea fisheries: methodology for data collection to promote the collection of data on VME-forming species (FAO. 2019).
To date, the European Union’s most important instrument for the constitution of conservation areas in the marine environment is represented by the Natura 2000 sites network whose designation is based upon the Habitats Directive (92/43/EEC). As established in Article 19 of the Directive, the list of habitats should be subjected to updates and amendments each time new countries join the European Union (Cardoso, 2012). However, the growth rate of technical and scientific progresses is arguably different from that of new member state inclusion, and no considerable modifications have been registered solely as a consequence of new knowledge so far (Fois et al., 2021). Consequently, the lists of habitats and species currently included in the Habitats Directive annexes rely upon outdated information, whilst some habitats of community interest are still not considered (Evans, 2006).
4.2 Conservation network addressing Mediterranean mesophotic habitats and species
Information on mesophotic habitats of the Mediterranean Sea has been largely collected in the early 2000s (e.g., Cerrano et al., 2019), providing evidence on the paramount ecological importance of habitats populating this depth range that serve as areas for spawning, breeding, feeding, and growth to maturity (e.g., Lesser et al., 2009; Bramanti et al., 2017; Capdevila et al., 2018; Santín et al., 2019). The analysis of the literature identified eight categories of mesophotic habitats in the Mediterranean Sea from reefs to soft bottoms patchily populated by erect megafauna. Four of these are included in binding instruments (i.e., Habitats Directive), corresponding to biogenic structures and phanerogam meadows. These habitats, however, covered a small portion of the literature, whilst about 73% of records focused on habitats currently listed in not-binding instruments or whose protection is related to the presence of certain taxa. As a case in point, animal forests resulted as the most studied habitat, accounting for ca. 34% of the literature records. These are known to represent hotspots of biodiversity and ecological services (Gori et al., 2017), but their protection is strictly related to the taxonomic composition: forests formed by Callogorgia verticillata, for instance, are considered of priority for protection and for the establishment of conservation measures since the species are listed in Barcelona Convention Annex II. On the contrary, Paramuricea clavata, gorgonid largely studied in the Mediterranean Sea (e.g., Linares et al., 2008 amongst many others), is currently not listed in any binding directives. So, identifying and collecting scientific information on situations hosting P. clavata forests may not be enough to lead to conservation actions.
Likewise, sponge grounds represented ca. 5% of the literature records, but this habitat is not included in the Habitats Directive and the chance to be subjected to conservation measures relies upon the presence of species listed under binding legal instruments. Despite 359 taxa of Porifera that were identified through the literature analysis, only 13 species, however, resulted as included in binding instruments to date. Similar arguments can be made for hard- and soft-bottom habitats, whose protection emerged as completely dependent on the occurrence of taxa listed in binding instruments.
If establishing conservation measures relying upon certain taxa which might surely represent a successful strategy to contrast biodiversity loss, it endows lists of species included in legally binding instruments a critical role. Of the more than 3,600 benthic mesophotic taxa identified from our analysis, 2.4% are currently listed under binding instruments, encompassing eight Phyla out of the 22 documented in the literature and open-access databases. Not only do the taxa need to be listed in binding instruments to be considered for protection, but also the different annexes or appendices within the same instrument have different reasoning. Annex II of the Habitats Directive, for instance, lists species for which members have to be designated protected areas, whilst Annex IV comprises strictly protected species but for which no legal obligation to protect the habitat exists. Listing species in Annex II is, therefore, more legally binding, but no marine benthic species are included yet. In natural systems, discerning species protection from habitat conservation might be tricky since the first concur in forming habitats, while habitats support the presence of species.
There is no doubt that finding solutions to contrast the jeopardization of benthic habitats is incredibly hard. A successful path toward the effective conservation of mesophotic-benthic habitats might be the improvement of legal conservation instruments to be more adaptive and promptly incorporate the available scientific knowledge (Manea et al., 2020).
However, the spatial distribution of information on mesophotic habitats and taxa in the Mediterranean Sea is heterogeneous. Most of the information comes from the northwestern sector of the basin, whilst in the easternmost Levantine Sea and the African coasts and margins, the number of available records is limited (Figure 1). The sole analysis of the literature records would lead to a notable underestimation of the diversity of mesophotic habitats and related taxa in the southeastern Mediterranean Sea. If the scientific knowledge that should fuel improvements in conservation measures to include mesophotic habitats and species occurring in the easternmost Mediterranean Sea is missing, evidence of the collapse of native mesophotic biodiversity by non-indigenous species is largely documented in the literature (Albano et al., 2021).
A starting point might be to update lists of species included in binding instruments by integrating information included in not legally binding conservation tools. These already provide data on population trends (IUCN Red List) and/or scientific evidence that some benthic taxa occurring within the mesophotic depths may form ecologically relevant habitats crucial for human supplies (GFCM priority species and VME indicator species). For instance, considering the taxa listed in binding and not-binding (at any “concerning” level) instruments, the portion of mesophotic benthic taxa in the Mediterranean Sea considered by the conservation network would increase from 2.4% to about 30% of those identified from our analysis. Including these taxa and habitats into legally binding instruments does not necessarily lead to their strict protection but might fuel the monitoring of their conservation status through programs already in force. Despite presenting strong legal (Fraschetti et al., 2018) and conceptual limitations (Fanelli et al., 2021), the Marine Strategy Framework Directive (MSFD 2008/56/EC) set the path for monitoring the Good Environmental Status (GES) of marine biodiversity in the EU, channeling scientific information into conservation instruments (Danovaro et al., 2020).
Ensuring routine monitoring of mesophotic and deep-sea habitats is, however, much more demanding in terms of funds, time, and on-field effort with respect to coastal situations (Danovaro et al., 2020). Stable funding for the onset of innovative cabled ocean observatories, infrastructure that provides real-time data on benthic-mesophotic habitats (Levin et al., 2019), represents a new frontier that might not only lead to novel scientific insights but also provide long-term data to improve the efficiency of the current marine conservation framework.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
Conceived the study: GC; methods: GC and LA; writing original draft: GC; discussion writing, review, and editing: GC, MA, LA, VG, PM, and MT; data interpretation: GC, LA, FF, and VG.
Funding
This work was supported by the H2020 Project Reliance (grant agreement no.: 101017501), the DG Environment programme IDEM (grant agreement no.: 11.0661/2017/750680/SUB/EN V.C2), and MIUR-PRIN 2017 GLIDE 2017FREXZY. This contribution is an overgrowth of a chapter of GC dissertation, co-financed by the Ph.D. program in Cultural and Natural Heritage of the University of Bologna.
Acknowledgments
The authors thank the guest editors Elisabetta Manea and Caterina Bergami for accepting the kind invitation to contribute to the special issue. The authors also thank the Department of Cultural and Natural Heritage of the University of Bologna for supporting the activities performed during the Ph.D. program. This is an ISMAR-CNR Bologna scientific contribution n. 2069.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.1009033/full#supplementary-material
References
Albano, P. G., Steger, J., Bošnjak, M., Dunne, B., Guifarro, Z., Turapova, E., et al. (2021). Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc. B 288, 20202469. doi:10.1098/rspb.2020.2469
Angeletti, L., Canese, S., Cardone, F., Castellan, G., Foglini, F., and Taviani, M. (2020). A brachiopod biotope associated with rocky bottoms at the shelf break in the central Mediterranean Sea: Geobiological traits and conservation aspects. Aquat. Conserv. 30, 402–411. doi:10.1002/aqc.3255
Angeletti, L., and Taviani, M. (2020). Offshore Neopycnodonte oyster reefs in the Mediterranean Sea. Diversity 12, 92. doi:10.3390/d12030092
Ballesteros, E. (2006). Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biology:An Annu. Rev. 44, 123–195. doi:10.1201/9781420006391-7
Basso, D., Babbini, L., Ramos-Esplá, A. A., and Salomidi, M. (2017). “Mediterranean rhodolith beds,” in Rhodolith/maërl beds: A global perspective coastal research library. Editors R. Riosmena-Rodríguez, W. Nelson, and J. Aguirre (Cham: Springer International Publishing), 281–298. Available at:Accessed March 27, 2019]. doi:10.1007/978-3-319-29315-8_11
Bo, M., Bertolino, M., Borghini, M., Castellano, M., Harriague, A. C., Camillo, C. G. D., et al. (2011). Characteristics of the mesophotic megabenthic assemblages of the vercelli seamount (north tyrrhenian sea). PLOS ONE 6, e16357. doi:10.1371/journal.pone.0016357
Boavida, J., Assis, J., Silva, I., and Serrão, E. A. (2016). Overlooked habitat of a vulnerable gorgonian revealed in the Mediterranean and Eastern Atlantic by ecological niche modelling. Sci. Rep. 6, 36460. doi:10.1038/srep36460
Bouchet, P. (2006). “The magnitude of marine biodiversity,” in The exploration of marine biodiversity: Scientific and technological challenges. Editor C. M. Duarte (Madrid: Fundación BBVA), 31–62.
Bramanti, L., Benedetti, M. C., Cupido, R., Cocito, S., Priori, C., Erra, F., et al. (2017). “Demography of animal forests: The example of mediterranean gorgonians,” in Marine animal forests: The ecology of benthic biodiversity hotspots. Editors S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer International Publishing), 529. Available at:. doi:10.1007/978-3-319-21012-4_13
Brooks, T. M., Mittermeier, R. A., da Fonseca, G. A. B., Gerlach, J., Hoffmann, M., Lamoreux, J. F., et al. (2006). Global biodiversity conservation priorities. Science 313, 58–61. doi:10.1126/science.1127609
Capdevila, P., Linares, C., Aspillaga, E., Riera, J. L., and Hereu, B. (2018). Effective dispersal and density-dependence in mesophotic macroalgal forests: Insights from the Mediterranean species Cystoseira zosteroides. PLOS ONE 13, e0191346. doi:10.1371/journal.pone.0191346
Cardone, F., Corriero, G., Longo, C., Mercurio, M., Onen Tarantini, S., Gravina, M. F., et al. (2020). Massive bioconstructions built by Neopycnodonte cochlear (Mollusca, Bivalvia) in a mesophotic environment in the central Mediterranean Sea. Sci. Rep. 10, 6337. doi:10.1038/s41598-020-63241-y
Cardoso, P. (2012). Habitats directive species lists: Urgent need of revision. Insect Conserv. divers. 5, 169–174. doi:10.1111/j.1752-4598.2011.00140.x
Castellan, G., Angeletti, L., Montagna, P., and Taviani, M. (2022). Drawing the borders of the mesophotic zone of the Mediterranean Sea using satellite data. Sci. Rep. 12, 5585. doi:10.1038/s41598-022-09413-4
Cau, A., Follesa, M. C., Moccia, D., Alvito, A., Bo, M., Angiolillo, M., et al. (2015). Deepwater corals biodiversity along roche du large ecosystems with different habitat complexity along the south Sardinia continental margin (CW Mediterranean Sea). Mar. Biol. 162, 1865–1878. doi:10.1007/s00227-015-2718-5
Cerrano, C., Bastari, A., Calcinai, B., Camillo, C. D., Pica, D., Puce, S., et al. (2019). Temperate mesophotic ecosystems: Gaps and perspectives of an emerging conservation challenge for the Mediterranean Sea. Eur. Zoological J. 86, 370–388. doi:10.1080/24750263.2019.1677790
Chimienti, G., De Padova, D., Mossa, M., and Mastrototaro, F. (2020). A mesophotic black coral forest in the Adriatic Sea. Sci. Rep. 10, 8504. doi:10.1038/s41598-020-65266-9
Corriero, G., Pierri, C., Mercurio, M., Marzano, C. N., Tarantini, S. O., Gravina, M. F., et al. (2019). A Mediterranean mesophotic coral reef built by non-symbiotic scleractinians. Sci. Rep. 9, 3601. doi:10.1038/s41598-019-40284-4
Costantini, F., Ferrario, F., and Abbiati, M. (2018). Chasing genetic structure in coralligenous reef invertebrates: Patterns, criticalities and conservation issues. Sci. Rep. 8, 5844. doi:10.1038/s41598-018-24247-9
Danovaro, R., Aronson, J., Cimino, R., Gambi, C., Snelgrove, P. V. R., and Van Dover, C. (2021). Marine ecosystem restoration in a changing ocean. Restor. Ecol. 29, e13432. doi:10.1111/rec.13432
Danovaro, R., Fanelli, E., Canals, M., Ciuffardi, T., Fabri, M.-C., Taviani, M., et al. (2020). Towards a marine strategy for the deep Mediterranean Sea: Analysis of current ecological status. Mar. Policy 112, 103781. doi:10.1016/j.marpol.2019.103781
Díaz, S., Settele, J., Brondízio, E. S., Ngo, H. T., Agard, J., Arneth, A., et al. (2019). Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366 (6471), Eaax3100. doi:10.1126/science.aax3100
EEA (2020). Management effectiveness in the EU’s Natura 2000 network of protected areas. Prepared for the EEA by The Institute for European Environment Policy (IEEP), UNEP-WCMC and Trinomics. Copenhagen, Denmark, 87.
EEA (2015). Marine protected areas in Europe's seas -An overview and perspectives for the future. EEA Report, no 3/2015.
European Commission (2020). The state of nature in the European Union. Report on the status and trends in 2013 - 2018 of species and habitat types protected by the Birds and Habitats Directives. Brussels: European Commission. 15.10.2020 COM 635 final.
Evans, D. (2006). The habitats of the European Union habitats directive. Biol. Environ. 106B, 167–173. doi:10.1353/bae.2006.0032
Fanelli, E., Bianchelli, S., Foglini, F., Canals, M., Castellan, G., Güell-Bujons, Q., et al. (2021). Identifying priorities for the protection of deep Mediterranean Sea ecosystems through an integrated approach. Front. Mar. Sci. 8, 698890. doi:10.3389/fmars.2021.698890
Food and Agriculture Organization (FAO) (2009). International guidelines for the management of deep-sea fisheries in the High seas. Rome, Italy: FAO, 73.
Food and Agriculture Organization (FAO) (2019). Monitoring the incidental catch of vulnerable species in Mediterranean and Black Sea fisheries: Methodology for data collection. Rome: FAO. FAO Fisheries and Aquaculture Technical Paper No. 640.
Foster, M. S., Amado-Filho, G. M., Kamenos, N. A., Riosmena-Rodriguez, R., and Steller, D. L. (2013). in Rhodoliths and rhodolith beds” in research and discoveries: The revolution of science through SCUBA. Editors M. A. Lang, R. L. Marinelli, S. J. Roberts, and P. R. Taylor (Washington, DC: Smithsonian Contributions to the Marine Sciences), 143–155.
Fraschetti, S., Pipitone, C., Mazaris, A. D., Rilov, G., Badalamenti, F., Bevilacqua, S., et al. (2018). Light and shade in marine conservation across European and contiguous seas. Front. Mar. Sci. 5. doi:10.3389/fmars.2018.00420
Goren, L., Idan, T., Shefer, S., Ilan, M., Hernandez, D., Yates, K. K., et al. (2021). Acidification in the U.S. Southeast: Causes, potential consequences and the role of the Southeast Ocean and coastal acidification network. Front. Mar. Sci. 7, 1–548. doi:10.3389/fmars.2020.00548
Gori, A., Bavestrello, G., Grinyó, J., Dominguez-Carrió, C., Ambroso, S., and Bo, M. (2017). “Animal forests in deep coastal bottoms and continental shelf of the Mediterranean Sea,” in Marine animal forests: The ecology of benthic biodiversity hotspots. Editors S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer International Publishing), 1–28. doi:10.1007/978-3-319-17001-5_5-2
Grassle, J. F., and Maciolek, N. J. (1992). Deep-sea species richness: Regional and local diversity estimates from quantitative bottom samples. Am. Nat. 139, 313–341. doi:10.1086/285329
Guidetti, P., Milazzo, M., Bussotti, S., Molinari, A., Murenu, M., Pais, A., et al. (2008). Italian marine reserve effectiveness: Does enforcement matter? Biol. Conserv. 141, 699–709. doi:10.1016/j.biocon.2007.12.013
Idan, T., Shefer, S., Feldstein, T., Yahel, R., Huchon, D., and Ilan, M. (2018). Shedding light on an East-Mediterranean mesophotic sponge ground community and the regional sponge fauna. Mediterr. Mar. Sci. 19, 84–106. doi:10.12681/mms.13853
Lande, R. (1998). Anthropogenic, ecological and genetic factors in extinction and conservation. Popul. Ecol. 40, 259–269. doi:10.1007/BF02763457
Lesser, M. P., Slattery, M., and Leichter, J. J. (2009). Ecology of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 375, 1–8. doi:10.1016/j.jembe.2009.05.009
Levin, L. A., Bett, B. J., Gates, A. R., Heimbach, P., Howe, B. M., Janssen, F., et al. (2019). Global observing needs in the deep ocean. Front. Mar. Sci. 6. doi:10.3389/fmars.2019.00241
Linares, C., Coma, R., Garrabou, J., Díaz, D., and Zabala, M. (2008). Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. J. Appl. Ecol. 45, 688–699. doi:10.1111/j.1365-2664.2007.01419.x
Manea, E., Bianchelli, S., Fanelli, E., Danovaro, R., and Gissi, E. (2020). Towards an ecosystem-based marine spatial planning in the deep Mediterranean Sea. Sci. Total Environ. 715, 136884. doi:10.1016/j.scitotenv.2020.136884
MedPAN and SPA/RAC (2017). The 2016 status of marine protected areas in the mediterranean. Editors B. Meola, and C. Webster. Tunis: SPA/RAC & MedPAN.
Pimm, S. L., Russell, G. J., Gittleman, J. L., and Brooks, T. M. (1995). The future of biodiversity. Science 269, 347–350. doi:10.1126/science.269.5222.347
Pyle, R. L., and Copus, J. M. (2019). “Mesophotic coral ecosystems: Introduction and overview, ” in Mesophotic coral ecosystems. Editors Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer International Publishing), 3–27. doi:10.1007/978-3-319-92735-0_1
Roberge, J. -M., and Angelstam, P. (2004). Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 18, 76–85. doi:10.1111/j.1523-1739.2004.00450.x
Rocha, L. A., Pinheiro, H. T., Shepherd, B., Papastamatiou, Y. P., Luiz, O. J., Pyle, R. L., et al. (2018). Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361, 281–284. doi:10.1126/science.aaq1614
Romão, C. (1996). Interpretation manual of European union habitats. Version EUR 15. Directorate general XI ‘environment, nuclear safety and civil protection’ of the European commission. Bruxelles: European Commission.
Rossi, S., Bramanti, L., Gori, A., and Orejas, C. (2017). “Animal forests of the world: An overview,”In. Marine animal forests: The ecology of benthic biodiversity hotspots. Editors S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer International Publishing), 1–28. Available at:. doi:10.1007/978-3-319-21012-4_1
Santín, A., Grinyó, J., Ambroso, S., Uriz, M. J., Dominguez-Carrió, C., and Gili, J. M. (2019). Distribution patterns and demographic trends of demosponges at the Menorca Channel (northwestern Mediterranean Sea). Prog. Oceanogr. 173, 9–25. doi:10.1016/j.pocean.2019.02.002
Soares, M. D. O., Araújo, J. T. D., Ferreira, S. M. C., Santos, B. A., Ruela Heimbürger Boavida, J., Costantini, F., et al. (2020). Why do mesophotic coral ecosystems have to be protected? Sci. Total Environ. 726, 138456. doi:10.1016/j.scitotenv.2020.138456
Taviani, M., Angeletti, L., Campiani, E., Ceregato, A., Foglini, F., Maselli, V., et al. (2012). Drowned karst landscape offshore the Apulian margin (southern Adriatic Sea, Italy). J. Caves. Karst Stud. 74/2, 197–212. doi:10.4311/2011JCKS0204
Vane-Wright, R. I., Humphries, C. J., and Williams, P. H. (1991). What to protect?—Systematics and the agony of choice. Biol. Conserv. 55, 235–254. doi:10.1016/0006-3207(91)90030-D
Vincent, A. C. J., Sadovy de Mitcheson, Y. J., Fowler, S. L., and Lieberman, S. (2014). The role of CITES in the conservation of marine fishes subject to international trade. Fish. Fish. (Oxf). 15, 563–592. doi:10.1111/faf.12035
Worm, B., Barbier, E. B., Beaumont, N., Duffy, J. E., Folke, C., Halpern, B. S., et al. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790. doi:10.1126/science.1132294
Keywords: marine conservation, biodiversity, policy, mesophotic ecosystems, monitoring, Mediterranean Sea
Citation: Castellan G, Abbiati M, Angeletti L, Foglini F, Grande V, Montagna P and Taviani M (2022) What are we protecting? An analysis of the current conservation framework addressing Mediterranean mesophotic habitats. Front. Environ. Sci. 10:1009033. doi: 10.3389/fenvs.2022.1009033
Received: 01 August 2022; Accepted: 18 October 2022;
Published: 04 November 2022.
Edited by:
Elisabetta Manea, National Research Council (CNR), ItalyReviewed by:
Soha Shabaka, National Institute of Oceanography and Fisheries (NIOF), EgyptLorenzo Bramanti, UMR8222 Laboratoire d'Ecogéochimie des Environnements Benthiques (LECOB), France
Copyright © 2022 Castellan, Abbiati, Angeletti, Foglini, Grande, Montagna and Taviani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: G. Castellan, Z2lvcmdpby5jYXN0ZWxsYW5AYm8uaXNtYXIuY25yLml0
†Present Address: L. Angeletti, National Research Council, Institute of Marine Biological Resources and Biotechnologies (CNR-IRBIM)
 G. Castellan
G. Castellan M. Abbiati
M. Abbiati L. Angeletti
L. Angeletti F. Foglini
F. Foglini V. Grande1
V. Grande1 P. Montagna
P. Montagna M. Taviani
M. Taviani