- 1Institute of Environmental and Plant Protection, Chinese Academy of Tropical Agricultural Sciences, Haikou, China
- 2Danzhou Scientific Observing and Experimental Station of Agro-Environment, Ministry of Agriculture and Rural Affairs, National Agricultural Experimental Station for Agricultural Environment, Danzhou, China
- 3School of Marine Sciences, Guangxi University, Nanning, China
Root exudates play a pivotal role in the behaviors of polycyclic aromatic hydrocarbons (PAHs) in mangrove sediments, but the knowledge of how mangrove root exudates response to PAHs pollutants is limited. This study examined the root exudates of Bruguiera gymnorrhiza (L.) (B. gymnorrhiza) under exposure in phenanthrene, pyrene, and benzo[a]pyrene solution through a 45 days hydroponic cultivation. The results showed that the root exudates of B. gymnorrhiza were mainly hydrocarbon compounds. Tartaric acid was the dominant low molecular weight organic acids (LMWOAs) in root exudates. Under PAHs stress, the proportion of hydrocarbon compounds in root exudates decreased, while the proportion of amide compounds increased. At the first 15 days exposure, the amounts of dissolved organic carbon, soluble total sugars, total organic acids and LWMOAs all increased and reached the maximum values, subsequently, the amounts of root exudates had dropped. The degradation rates of PAHs followed the sequence of phenanthrene > pyrene > benzo [a] pyrene, and the presence of root exudates can significantly enhance the degradation of PAHs. The results illustrated that PAHs stress can significantly change the concentrations and species of root exudates. This study provides the scientific reference for understanding the ability of B. gymnorrhiza response to PAHs stress.
Introduction
Mangroves are important inter-tidal estuarine wetlands located along coastlines of tropical and subtropical regions. They serve important ecological functions because of high productivity, high return rate, high decomposition rate and high resistance to extreme weather events, and anthropogenic activities (Wang and Gu, 2021). They also have important environmental protection functions because they can easily accumulate varieties of pollutants mainly derived from rivers or tidal waters (Zhang et al., 2014). Polycyclic aromatic hydrocarbons (PAHs), as a class of organic pollutants, have been widely investigated for monitoring pollution level, and source identification and ecological risk assessment over the last several decades (Huang et al., 2021). Over the past few decades, mangrove wetlands have been exposed to multiple poisonous PAHs due to various kinds of human activities (Qiu et al., 2018; Balu et al., 2020; Verâne et al., 2020; Garcia and Martins, 2021). Phytoremediation and biodegradation by indigenous degrading microbes are important processes of PAHs in contaminated mangrove sediments, and rhizosphere has been proved to effectively promote the depletion of PAHs in contaminated sediments in both cases (Tam and Wong, 2008; Lu et al., 2011).
Plant roots secrete a wide range of organic compounds into the rhizosphere, known as root exudates, which play an important role in rhizodegradation (Gao et al., 2011; Lu et al., 2017; Turkovskaya and Muratova, 2019; Sivaram et al., 2020; Wang et al., 2021a). Root exudates are often divided into two groups: low and high-molecular weight exudates. Low-molecular-weight organic acids (LMWOAs) are chemically active. They can be easily involved in a series of rhizospheric processes, such as, microbial metabolism, and detoxify of harmful elements (Jia et al., 2018). Root exudates also have been implicated in many soil processes, and affect the behavior of PAHs. For example, the previous studies showed that root exudates could influence the sorption, desorption, and transport of PAHs, and subsequently influence their degradation and phytoremediation in mangrove sediments (Wang et al., 2014; Jia et al., 2016a; Jia et al., 2016b; Lu et al., 2017). Our former study has shown that the organic components in the mangrove root exudates included hydrocarbons, esters, phenols, and aromas (Liu et al., 2017). However, most of the current studies focused on LMWOAs, how the other components of mangrove root exudates response to PAHs pollutants is still limited.
This study aims to evaluate the properties of exudates from mangrove roots, and compare the root exudates under different PAH stress through a hydroponic culture experiment. We selected Bruguiera gymnorrhiza (L.) as the object of this study because it is a typical mangrove species and has exhibited clear promotion of the depletion of PAHs in mangrove sediments in previous research (Song et al., 2012; Naidoo and Naidoo, 2016; Qiu et al., 2018). Phenanthrene, pyrene, and benzo[a]pyrene were selected as representative PAHs because they are commonly found in mangrove sediments (Tam et al., 2002; Tian et al., 2008; Aziz et al., 2018). The results of this study will provide guidelines to remediation strategies at mangrove-contaminated sediments.
Materials and Methods
Hydroponic Culture Experiment
B. gymnorrhiza seedlings was sampled from a mangrove nature reserve at Dongzhai Harbor in Hainan, China within a B. gymnorrhiza community (E 110°34′51.7″, N 19°57′18.0″). A number of B. gymnorrhiza seedlings with similar shapes, leaf numbers, stem internodes, and maturities, and no disease or insect pests were sampled and taken to laboratory for study. The sampled B. gymnorrhiza seedlings were transplanted into 1 L beakers, and treated with distilled water for 2 days, and then put into the 600 ml culture solutions containing phenanthrene (Phe), pyrene (Pyr), and benzo [a] pyrene (Bap) for 45 days, respectively. The culture solutions was prepared according to a modification of Hoagland nutrient solution for mangrove growth (Jiang et al., 2017). The salinity was adjusted to 4‰, which is consistent with the sediment environment in our sampling location. The culture solutions without receiving exogenous PAH, and spiked with single PAH compound were set as the control and PAH stress treatments respectively. Methane (0.5%) was added to enhance dissolution of PAH, and the final concentrations of each PAH in the nutrient solutions were 0.1, 1.0, and 10 mg/L respectively. The culture solution was supplemented daily to maintain the liquid level. The beakers were wrapped in tin foil to avoid light degradation of PAHs and the treatments of without plants were also set for comparing the natural degradation of PAHs without plants. After 15, 30, and 45 days cultivation, the plants and culture solutions were sampled for analyzing the composition of root exudates and the content of residual PAHs.
Collection and Determination of Root Exudates
After the set culture time, the seedlings were taken out and rinsed thoroughly in distilled water, and then rinsed again in distilled water containing an antimicrobial agent to prevent microbial degradation of the exudates during their collection. The roots were then immersed in a collection volume of 600 ml of distilled water for 4 h for the collection of root exudates (Lu et al., 2007). Then a portion of 200 ml collected solutions were removed for extraction with 50 ml ethyl acetate three times, and the extractions were evaporated to 5 ml under reduced pressure at 40°C, and filtered with a 0.22-μm organic filter membrane, then the filter liquors was analyzed for the organic compounds of root exudates by Gas Chromatography-Mass Spectrometer (GC-MS) determination. The other 400 ml collected solutions were evaporated to 20 ml under reduced pressure at 40°C and filtered with a 0.22-μm aqueous filter membrane, and the filter liquors were used for analyzing LMWOAs by High Performance Liquid Chromatography (HPLC) determination, and also the analysis of dissolved organic carbon (DOC), soluble total sugars (TS), and total organic acids (TOA).
The GC-MS determination was conducted by using an Agilent 7890A-5975C (Agilent, United States), with a chromatographic column HP-5MS (30 m × 250 μm × 0.25 µm). The instrument parameters were described by our former study (Liu et al., 2017), in which, the injection port temperature was 230°C; the gas flow rate was 1.0 ml/min; the column temperature started at 80°C, then underwent a 15°C/min ramp to 200°C, then an 8°C/min ramp to 250°C, which was held for 8 min. The ion-source temperature was 230°C and mass spectra were acquired between m/z 10 and 450. Compounds were identified by comparison of their mass spectra with those in the NIST11 library.
The LMWOAs of root exudates were analyzed using an HPLC method as described by our former study (Liu et al., 2017). The analysis was carried out on a Dionex 3000 (ThermoFisher, Germany) with an AtlantisTM C18 chromatographic column. The mobile phase was a mixture of 10 mmol/L KH2PO4 (pH 2.7) and CH3OH with v/v =95:5. The flow rate was 0.5 ml/min. The wavelength of the detector was 220 nm. Five types of organic acids including oxalic, tartaric, malic, maleic, and fumaric acid were determined. All samples and solutions of the mobile phase were filtered through a 0.22-μm membrane prior to being injected into the chromatographic column. The method recovery was above 83.6%.
The DOC concentrations were measured by carbon and nitrogen analyzer. The TOA and TS concentration was determined by colorimetric method. The unit of each type of LMWOA, DOC, TOA, and TS were expressed as mg C per kilogram of wet plant weight.
Determination of Polycyclic Aromatic Hydrocarbons
Phenanthrene, pyrene and benzo [a] pyrene in solutions were determined by HPLC using Waters2695 chromatograph, detector was Waters 2489 UV detector, absorption wavelength of 245nm, and chromatographic column was Athena c18-wp (5 μm, 4.6 × 150 mm). The mobile phase was 95% methanol and 5% water at a flow rate of 1 ml/min. The external standard method was used to determine PAHs, and the peak area method was used to calculate PAHs content.
Statistical Analyses
All of these experiments were performed in triplicate and the results presented are average values of the three replicates. Data were analyzed statistically using analysis of variance and the Duncan’s multiple range test was used to determine the significance of the differences between the parameters. Statistics were calculated using the SPSS statistical software package (Version 11.0) and the confidence limit was 95%.
Results
Changes of Organic Compounds in Root Exudates
The organic compounds in root exudates analyzed by GC-MS determination at different cultural time were shown in Table 1, 2, 3, in which the components with a similarity of more than 80% and relative content of more than 0.5% in root exudates were listed. Through the GC-MS analysis, it can be seen that the organic compounds in root exudates contained hydrocarbons, esters, phenols, and amides. Comparing the change of the detected organic compounds in root exudates under PAHs stress, it can be seen that after 15 days of PAH stress (Table 1), the proportion of hydrocarbons significantly decreased (p < 0.05), the proportion of phenols and esters slightly decreased, while the proportion of amides significantly increased (p < 0.05), and which were the main components of organic compounds in root exudates. For each PAH, the most species of compounds produced under the medium concentration (1 mg/L), and comparing the stress under different PAH with the same concentration, phenanthrene stress showed the most species of compounds. Under 30 days of PAH stress (Table 2), the proportion of hydrocarbon and ester compounds decreased continuously, while the proportion of phenolic compounds increased. The composition of root exudates was still dominated by amide compounds, and its proportion increased comparing with that of 15 days. Highest diversity of exudate was produced by roots under high PAHs concentration (10 mg/L). After 45 days of cultivation, as shown in Table 3, with PAHs stress, the proportion of hydrocarbon compounds continually decreased, and amide and phenolic compounds became the main compounds in root exudates, and the varieties of detected organic compounds reached the highest.
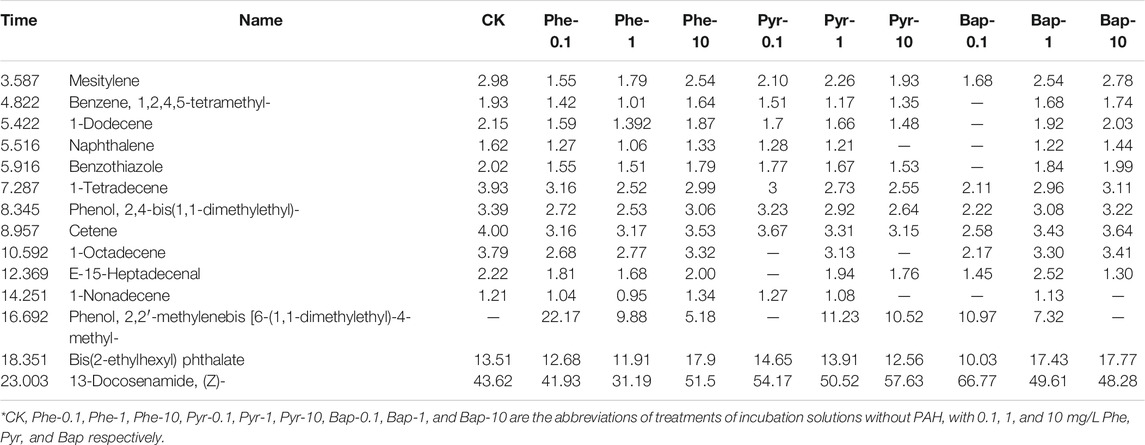
TABLE 1. GC-MS measurement results of composition and relative content of root exudates after15 days PAHs hydroponic stress.
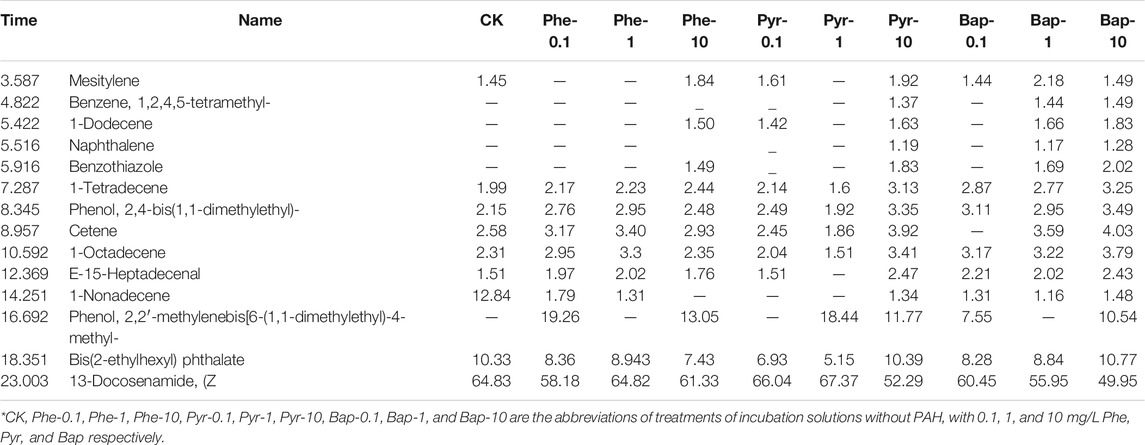
TABLE 2. GC-MS measurement results of composition and relative content of root exudates after 30 days PAHs hydroponic stress.
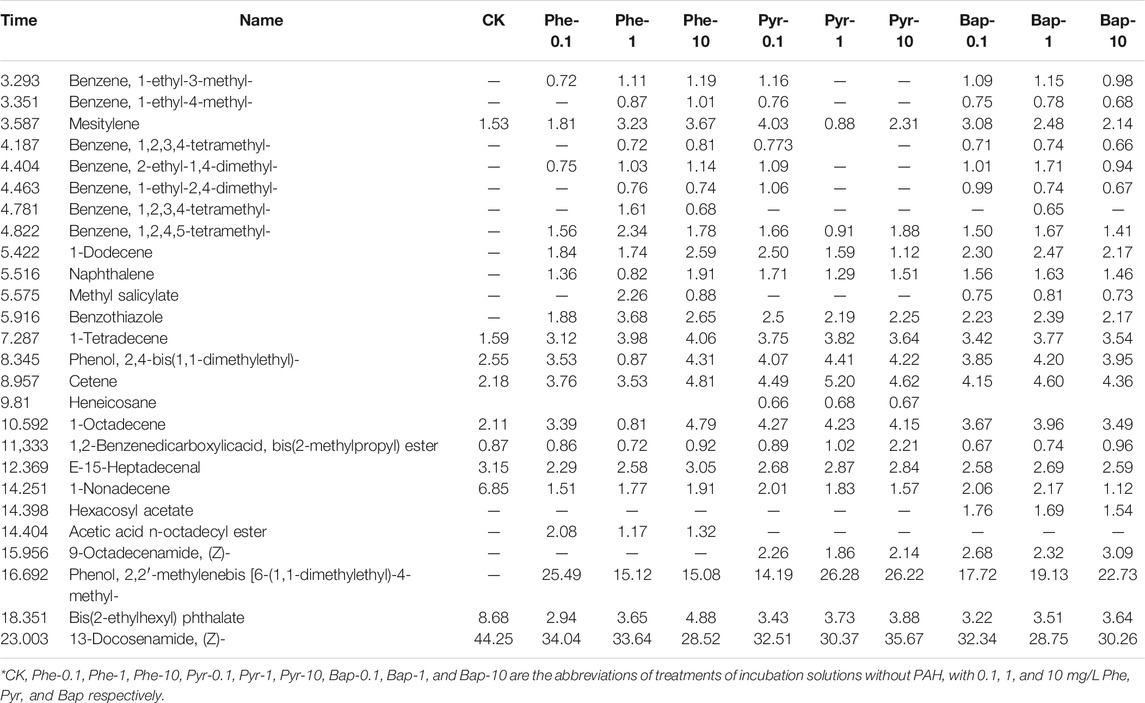
TABLE 3. GC-MS measurement results of composition and relative content of root exudates after 45 days PAHs hydroponic stress.
From the above results, it can be seen that the varieties and portions of organic compounds were both influenced by PAHs stress, with the concentration of PAH, and the time of exposure were the main influence factors. Generally, the proportion of hydrocarbon compounds in root exudates decreased, while the varieties of detected organic compounds increased with the increase of PAH concentration. Also, the proportion of amide and phenolic compounds increased with the increase of PAH exposure time.
Dissolved Organic Carbon, Soluble Total Sugars, and Total Organic Acid in Root Exudates
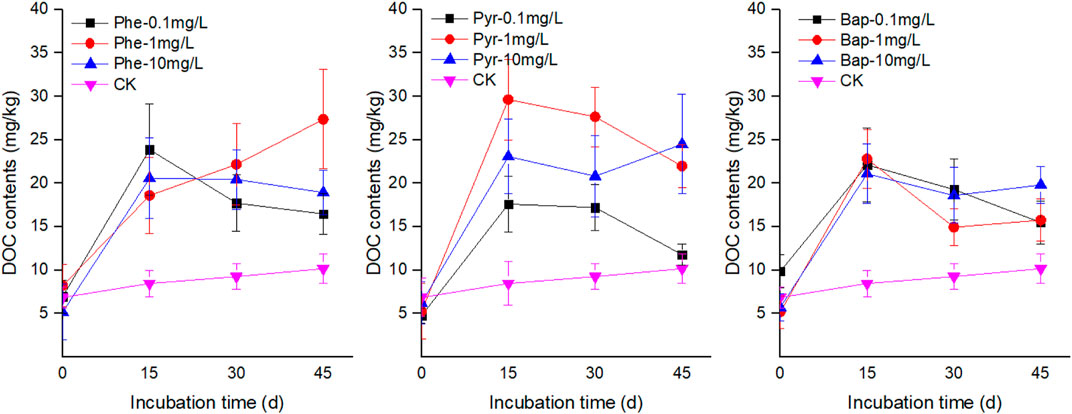
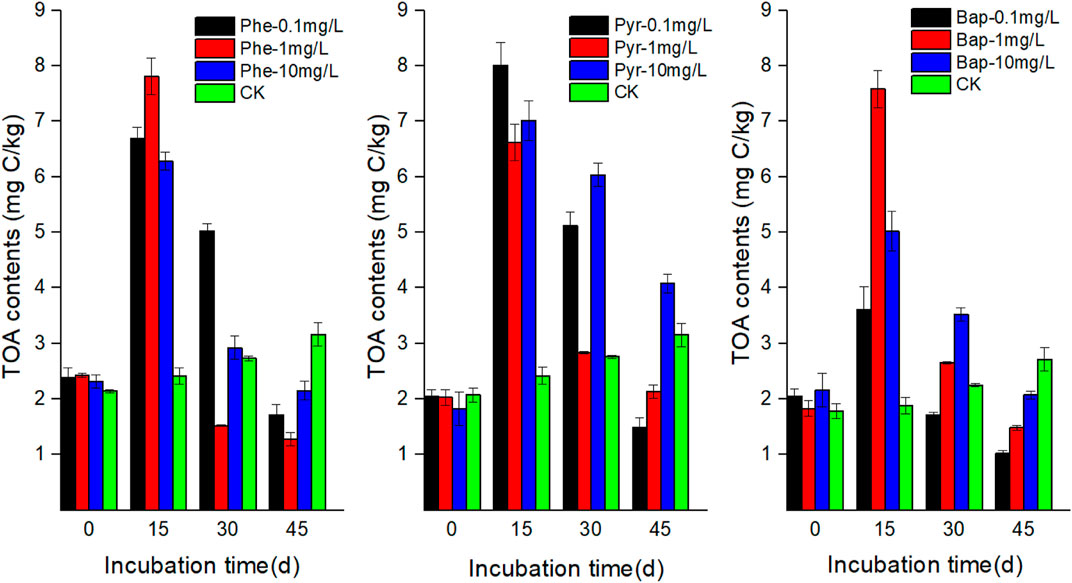
The concentrations of DOC in root exudates with PAHs stress were higher than that of control during the incubation time (Figure 1). At the first 15 days cultivation time, the concentrations of DOC in root exudates increased significantly under PAHs stress (p < 0.05), and then maintained at a higher level than the control with a slight fluctuation. The concentrations of TS and TOA in root exudates increased firstly and then decreased with PAHs stress, and the maximum values existed at 15 days (Figures 2, 3). However, at the end of cultivation time, the concentrations of TS and TOA in root exudates under PAH stress had no significant difference with those of control (p < 0.05).
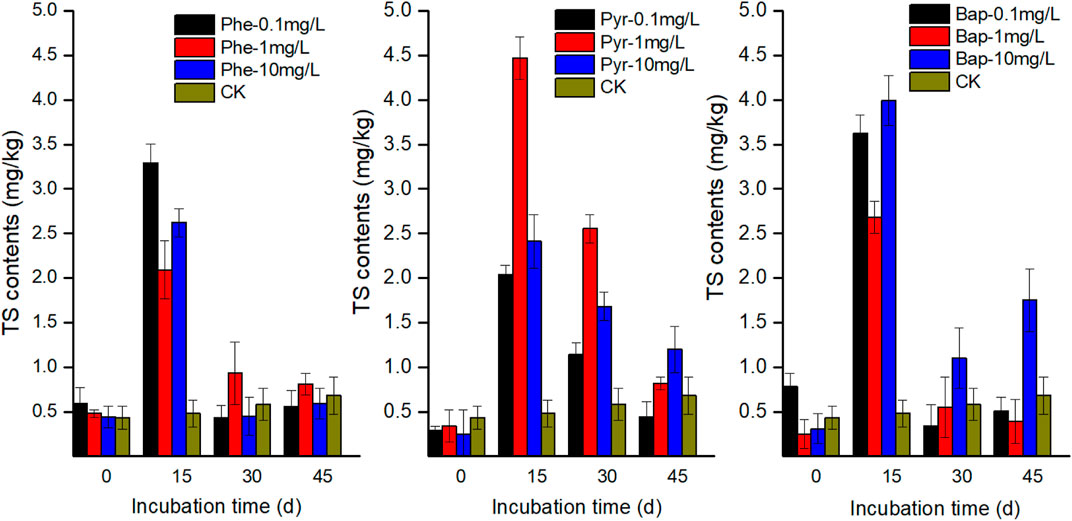
FIGURE 2. Effect of PAH stress on the amount of total soluble sugar (TS) excreted by B. gymnorrhiza.
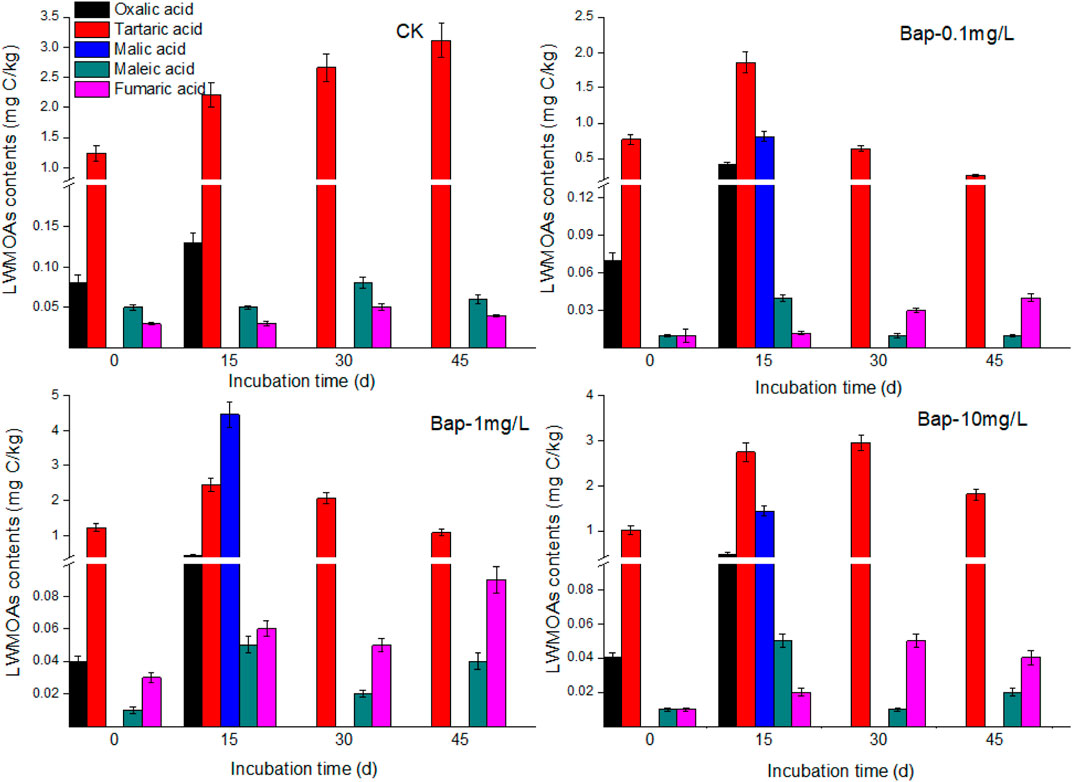
LMWOAs in Root Exudates
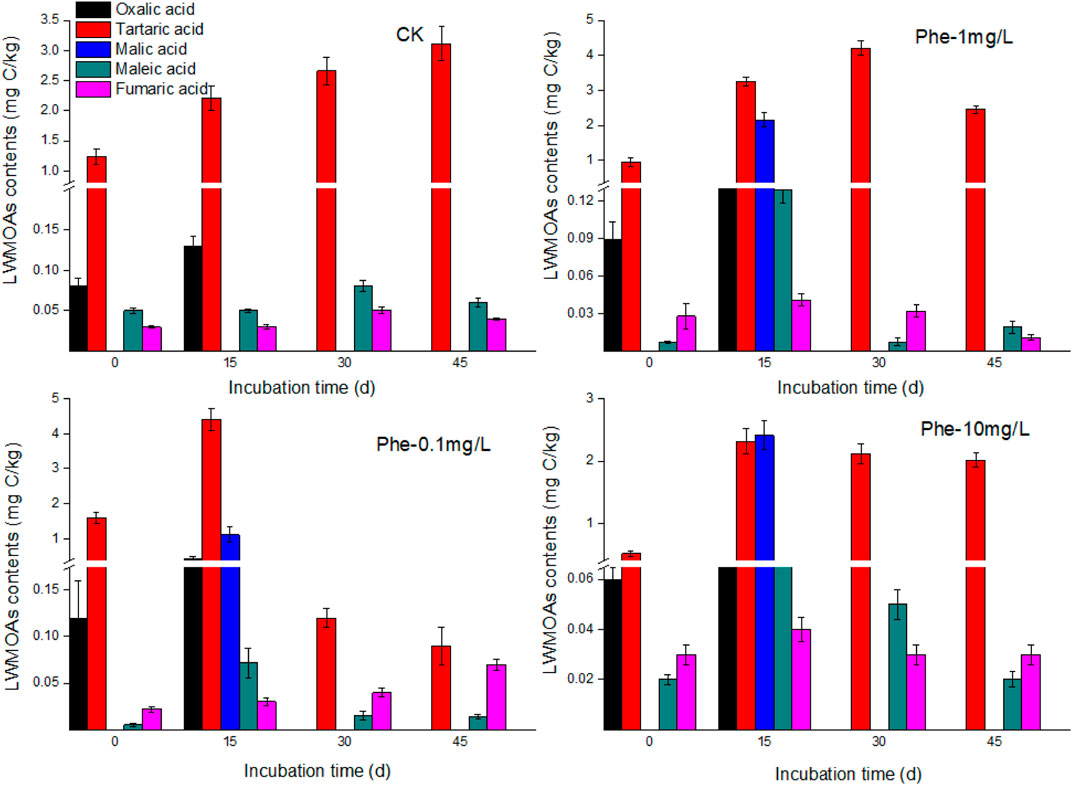
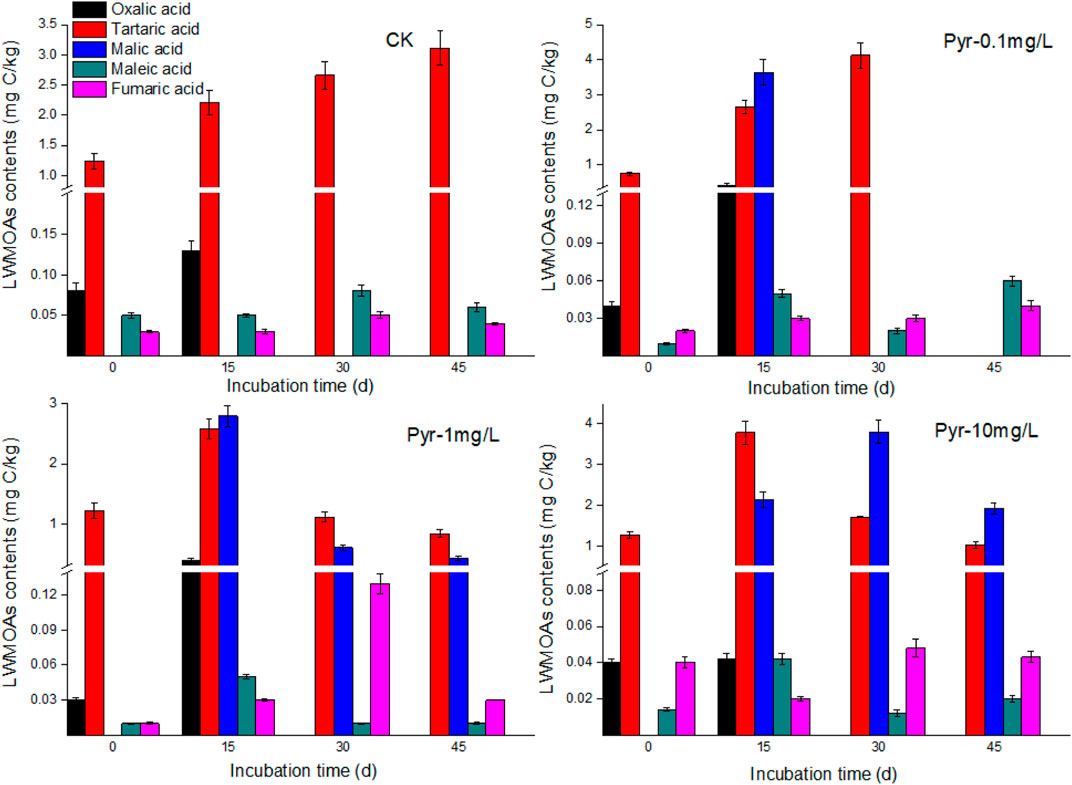
From the results of different LMWOAs in root exudates, as shown in Figures 4, 5, 6, tartaric acid, maleic acid, and fumaric acid were the commonly detected acids during the cultivation time, and tartaric acid was the predominate LMWOAs in root exudates of B. gymnorrhiza. The amounts of the three acids were significantly influenced by PAH stress. For tartaric acid, under PAH stress, it increased at first, and reached the maximum value at 15 days, subsequently decreased to a lower amount comparing with that of control. In contrast, there was no significant difference between the concentration of maleic acid and fumaric acid with and without PAH stress (p > 0.05). Specially, oxalic acid and malic acid were detected at different cultivation time in different treatments. For example, oxalic acid was only detected at first 15 days cultivation, and with PAH stress, the amounts of oxalic acid decreased with the increasing PAH concentration. Malic acid only detected after 15 days of PAH exposure. In total, the exudation of the detected LMWOAs had decreased in PAHs contaminated groups compared to the control, indicating that PAHs negatively impacted root metabolism, and activities due to their toxicity.
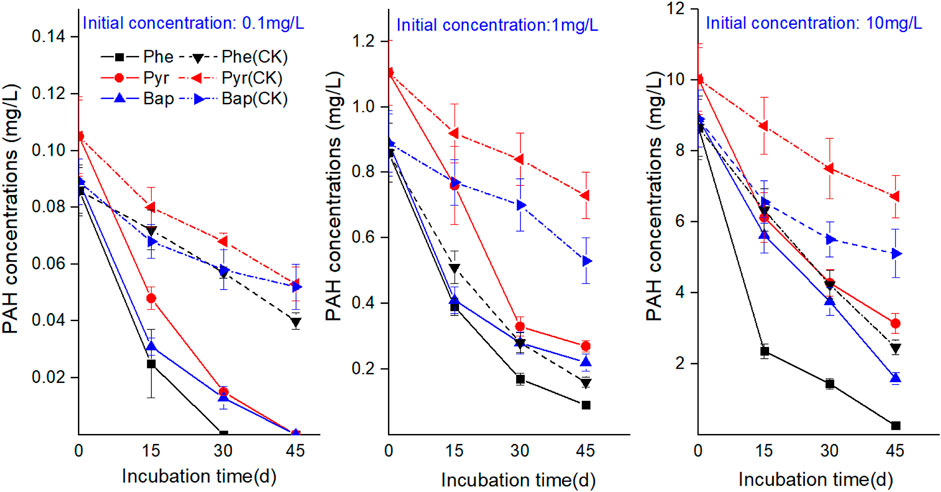
Polycyclic Aromatic Hydrocarbons Concentrations in Solutions
Figure 7 reflects the change of residual PAH in hydroponic solution during the cultivation time. The three PAHs at different concentrations showed a decreasing trend with the increase of cultivation time. At the same sampling time, the PAHs concentrations detected in hydroponic solution of the treatments with plants were significantly lower than those of the control treatments without plants (p < 0.05), indicating a depletion of PAHs in water by B. gymnorrhiza. The concentrations of PAHs rapidly decreased at the first 15 days incubation with the sequence of phenanthrene > pyrene > benzo [a] pyrene. The correlation analysis between residual PAHs in hydroponic solution and root exudates (Table 4) showed that there was no significant correlation between three PAHs in hydroponic solution and DOC, TS, and LWMOAs in root exudates, indicating that the change of components in root exudates under PAH stress did not influence the increased depletion of PAHs in water by B. gymnorrhiza.
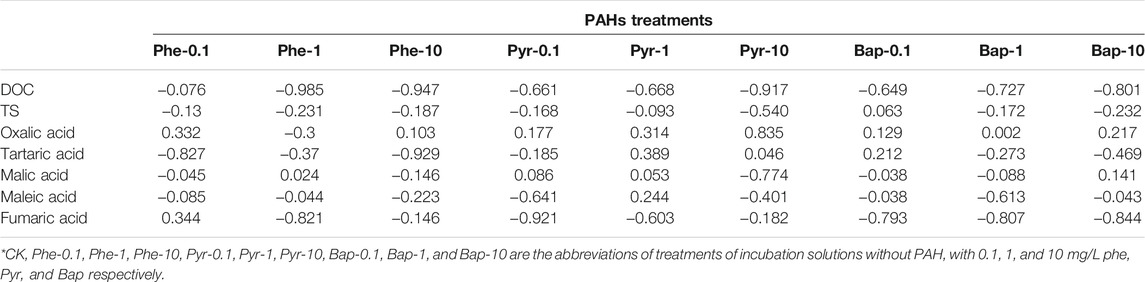
TABLE 4. The correlation between the residual amounts of PAHs and components of root exudates in hydroponic solution.
Discussion
Root exudates such as carboxylic acids, alcohols, and proteins can serve as nutrients for the growth of microbes. LMWOAs such as citric, lactic, oxalic, and glutaric acid, are active components in root exudates. Recently, more evidence is emerging that LMWOAs can remarkably influence the biodegradation of PAHs (Wang et al., 2021a; Zhang et al., 2021). However, the root exudates could also be influenced by PAHs stress. It made a difficult for evaluating the degradation of PAHs in plant rhizosphere. The variations of root exudates composition and its content are the concentrated expression of stimuli-responsive of the plant to the environment. Most of the researchers attributed this enhancing of root exudation by adjustment their metabolic process against stress. The changes observed in the quantities, relative proportions, and chemical composition. According to the former studies, the response of exudation of root exudates under PAHs stress varied with different plant species (Meier et al., 2012; Wang et al., 2021b).
For mangrove plants, there is little knowledge about how root exudates response to PAH stress except LMWOAs. In this study, it was found that the varieties and portions of organic compounds were both influenced by PAHs stress. For example, the proportions of hydrocarbon compounds in root exudates decreased, while the proportions of amide and phenolic compounds increased with the increasing PAH exposure time. With the increase of PAH concentration, the varieties of detected organic compounds increased. The previous studies also found that the largest proportions of phenols were detected under phenanthrene stress in root exudates of trees, and it was hypothesized that phenanthrene stress activated the phenol metabolism (Wang et al., 2021b). It is known that phenolics play a key role in plant-microbe interactions, which is related to the degradation of organic pollutants.
It has been reported that the contents of soluble organic acids, oxalic acid, and total soluble sugar in the root exudations of ryegrass were higher than that of non-contaminated control in PAHs stress, which increased with the increase of phenanthrene, anthracene, and naphthalene concentration in the culture solution (Mleczek et al., 2018). In response to hazardous elements, oxalic and malic acid were recognized as part of the response mechanism (Montiel-Rozas et al., 2016). In this study, the amounts of TOA, TS, and LMWOAs in root exudates increased firstly, but decreased to the corresponding level of control at the end of cultivation, indicating that B. gymnorrhiza had the ability to gradually adapt to PAHs stress.
Conclusion
This study showed that the components in root exudates of B. gymnorrhiza varied significantly under the stress of Phe, Pyr, and Bap. The proportions of hydrocarbon compounds in root exudates decreased, but the proportion of amide compounds increased with PAHs stress. The amounts of DOC, TS, TOA, and LWMOAs of root exudates increased and reached the maximum values after 15 days PAHs exposure, and then dropped after 45 days of exposure. The exist of B. gymnorrhiza promoted the depletion of PAHs in solutions. These results indicated that B. gymnorrhiza had the ability to adapt to PAHs stress and promote the degradation of PAHs in water.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
BL, BL, and RL contributed to conception and design of the study. BL wrote the first draft of the article. LW and PP conducted the experiments and organized the database. All authors contributed to article revision, read, and approved the submitted version.
Funding
This work was funded by the Hainan Provincial Key Research and Development Projects (ZDYF2021SHFZ066, ZDYF2019221), and the National Natural Science Foundation of China (41301555).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aziz, A., Agamuthu, P., Alaribe, F. O., and Fauziah, S. H. (2018). Biodegradation of Benzo[a]pyrene by Bacterial Consortium Isolated from Mangrove Sediment. Environ. Tech. 39 (4), 527–535. doi:10.1080/09593330.2017.1305455
Balu, S., Bhunia, S., Gachhui, R., and Mukherjee, J. (2020). Assessment of Polycyclic Aromatic Hydrocarbon Contamination in the Sundarbans, the World's Largest Tidal Mangrove forest and Indigenous Microbial Mixed Biofilm-Based Removal of the Contaminants. Environ. Pollut. 266, 115270. doi:10.1016/j.envpol.2020.115270
Gao, Y., Yang, Y., Ling, W., Kong, H., and Zhu, X. (2011). Gradient Distribution of Root Exudates and Polycyclic Aromatic Hydrocarbons in Rhizosphere Soil. Soil Sci. Soc. America J. 75 (5), 1694–1703. doi:10.2136/sssaj2010.0244
Garcia, M. R., and Martins, C. C. (2021). A Systematic Evaluation of Polycyclic Aromatic Hydrocarbons in South Atlantic Subtropical Mangrove Wetlands under a Coastal Zone Development Scenario. J. Environ. Manage. 277, 111421. doi:10.1016/j.jenvman.2020.111421
Haoliang, L., Chongling, Y., and Jingchun, L. (2007). Low-molecular-weight Organic Acids Exuded by Mangrove (Kandelia candel (L.) Druce) Roots and Their Effect on Cadmium Species Change in the Rhizosphere. Environ. Exp. Bot. 61 (2), 159–166. doi:10.1016/j.envexpbot.2007.05.007
Huang, Q., Zhu, Y., Wu, F., and Zhang, Y. (2021). Parent and Alkylated Polycyclic Aromatic Hydrocarbons in Surface Sediments of Mangrove Wetlands across Taiwan Strait, China: Characteristics, Sources and Ecological Risk Assessment. Chemosphere 265, 129168. doi:10.1016/j.chemosphere.2020.129168
Jia, H., Hou, D., Dai, M., Lu, H., and Yan, C. (2018). Effects of Root Exudates on the Mobility of Pyrene in Mangrove Sediment-Water System. CATENA 162, 396–401. doi:10.1016/j.catena.2017.10.022
Jia, H., Lu, H., Dai, M., Hong, H., Liu, J., and Yan, C. (2016a). Effect of Root Exudates on Sorption, Desorption, and Transport of Phenanthrene in Mangrove Sediments. Mar. Pollut. Bull. 109 (1), 171–177. doi:10.1016/j.marpolbul.2016.06.004
Jia, H., Lu, H., Liu, J., Li, J., Dai, M., and Yan, C. (2016b). Effects of Root Exudates on the Leachability, Distribution, and Bioavailability of Phenanthrene and Pyrene from Mangrove Sediments. Environ. Sci. Pollut. Res. 23 (6), 5566–5576. doi:10.1007/s11356-015-5772-0
Jiang, S., Xie, F., Lu, H., Liu, J., and Yan, C. (2017). Response of Low-Molecular-Weight Organic Acids in Mangrove Root Exudates to Exposure of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Pollut. Res. 24 (13), 12484–12493. doi:10.1007/s11356-017-8845-4
Liu, B., Liu, X., Huo, S., Chen, X., Wu, L., Chen, M., et al. (2017). Properties of Root Exudates and Rhizosphere Sediment of Bruguiera Gymnorrhiza (L.). J. Soils Sediments 17 (1), 266–276. doi:10.1007/s11368-016-1541-z
Lu, H., Sun, J., and Zhu, L. (2017). The Role of Artificial Root Exudate Components in Facilitating the Degradation of Pyrene in Soil. Sci. Rep. 7 (1), 7130. doi:10.1038/s41598-017-07413-3
Lu, H., Zhang, Y., Liu, B., Liu, J., Ye, J., and Yan, C. (2011). Rhizodegradation Gradients of Phenanthrene and Pyrene in Sediment of Mangrove (Kandelia candel (L.) Druce). J. Hazard. Mater. 196, 263–269. doi:10.1016/j.jhazmat.2011.09.031
Meier, S., Alvear, M., Borie, F., Aguilera, P., Ginocchio, R., and Cornejo, P. (2012). Influence of Copper on Root Exudate Patterns in Some Metallophytes and Agricultural Plants. Ecotoxicology Environ. Saf. 75, 8–15. doi:10.1016/j.ecoenv.2011.08.029
Mleczek, M., Gsecka, M., Kaniuczak, J., Goliński, P., Szostek, M., Magdziak, Z., et al. (2018). Dendroremediation: The Role of Trees in Phytoextraction of Trace Elements.
Montiel-Rozas, M. M., Madejón, E., and Madejón, P. (2016). Effect of Heavy Metals and Organic Matter on Root Exudates (Low Molecular Weight Organic Acids) of Herbaceous Species: An Assessment in Sand and Soil Conditions under Different Levels of Contamination. Environ. Pollut. 216, 273–281. doi:10.1016/j.envpol.2016.05.080
Naidoo, G., and Naidoo, K. (2016). Uptake of Polycyclic Aromatic Hydrocarbons and Their Cellular Effects in the Mangrove Bruguiera Gymnorrhiza. Mar. Pollut. Bull. 113 (1), 193–199. doi:10.1016/j.marpolbul.2016.09.012
Qiu, Y.-W., Qiu, H.-L., Li, J., and Zhang, G. (2018). Bioaccumulation and Cycling of Polycyclic Aromatic Hydrocarbons (PAHs) in Typical Mangrove Wetlands of Hainan Island, South China. Arch. Environ. Contam. Toxicol. 75 (3), 464–475. doi:10.1007/s00244-018-0548-4
Sivaram, A. K., Logeshwaran, P., Lockington, R., Naidu, R., and Megharaj, M. (2020). The Impact of Low Molecular Weight Organic Acids from Plants with C3 and C4 Photosystems on the Rhizoremediation of Polycyclic Aromatic Hydrocarbons Contaminated Soil. Environ. Tech. Innovation 19, 100957. doi:10.1016/j.eti.2020.100957
Song, H., Wang, Y.-S., Sun, C.-C., Wang, Y.-T., Peng, Y.-L., and Cheng, H. (2012). Effects of Pyrene on Antioxidant Systems and Lipid Peroxidation Level in Mangrove Plants, Bruguiera Gymnorrhiza. Ecotoxicology 21 (6), 1625–1632. doi:10.1007/s10646-012-0945-9
Tam, N. F., Guo, C. L., Yau, W. Y., and Wong, Y. S. (2002). Preliminary Study on Biodegradation of Phenanthrene by Bacteria Isolated from Mangrove Sediments in Hong Kong. Mar. Pollut. Bull. 45 (1), 316–324. doi:10.1016/S0025-326X(02)00108-X
Tam, N. F. Y., and Wong, Y. S. (2008). Effectiveness of Bacterial Inoculum and Mangrove Plants on Remediation of Sediment Contaminated with Polycyclic Aromatic Hydrocarbons. Mar. Pollut. Bull. 57 (6), 716–726. doi:10.1016/j.marpolbul.2008.02.029
Tian, Y., Luo, Y.-r., Zheng, T.-l., Cai, L.-z., Cao, X.-x., and Yan, C.-l. (2008). Contamination and Potential Biodegradation of Polycyclic Aromatic Hydrocarbons in Mangrove Sediments of Xiamen, China. Mar. Pollut. Bull. 56 (6), 1184–1191. doi:10.1016/j.marpolbul.2008.02.014
Turkovskaya, O., and Muratova, A. (2019). Plant-Bacterial Degradation of Polyaromatic Hydrocarbons in the Rhizosphere. Trends Biotechnol. 37 (9), 926–930. doi:10.1016/j.tibtech.2019.04.010
Verâne, J., dos Santos, N. C. P., da Silva, V. L., de Almeida, M., de Oliveira, O. M. C., and Moreira, Í. T. A. (2020). Phytoremediation of Polycyclic Aromatic Hydrocarbons (PAHs) in Mangrove Sediments Using Rhizophora Mangle. Mar. Pollut. Bull. 160, 111687. doi:10.1016/j.marpolbul.2020.111687
Wang, J., Chen, X., Yan, W., Ning, C., and Gsell, T. (2021a). Both artificial Root Exudates and Natural Koelreuteria Paniculata Exudates Modify Bacterial Community Structure and Enhance Phenanthrene Biodegradation in Contaminated Soils. Chemosphere 263, 128041. doi:10.1016/j.chemosphere.2020.128041
Wang, J., Farooq, T. H., Aslam, A., Shakoor, A., Chen, X., and Yan, W. (2021b). Non-targeted Metabolomics Reveal the Impact of Phenanthrene Stress on Root Exudates of Ten Urban Greening Tree Species. Environ. Res. 196, 110370. doi:10.1016/j.envres.2020.110370
Wang, Y.-S., and Gu, J.-D. (2021). Ecological Responses, Adaptation and Mechanisms of Mangrove Wetland Ecosystem to Global Climate Change and Anthropogenic Activities. Int. Biodeterioration Biodegradation 162, 105248. doi:10.1016/j.ibiod.2021.105248
Wang, Y., Fang, L., Lin, L., Luan, T., and Tam, N. F. Y. (2014). Effects of Low Molecular-Weight Organic Acids and Dehydrogenase Activity in Rhizosphere Sediments of Mangrove Plants on Phytoremediation of Polycyclic Aromatic Hydrocarbons. Chemosphere 99, 152–159. doi:10.1016/j.chemosphere.2013.10.054
Zhang, L., Li, X., Zuo, W., Li, S., Sun, G., Wang, W., et al. (2021). Root Exuded Low-Molecular-Weight Organic Acids Affected the Phenanthrene Degrader Differently: A Multi-Omics Study. J. Hazard. Mater. 414, 125367. doi:10.1016/j.jhazmat.2021.125367
Keywords: root exudate, mangrove, PAH, degradation, LMWOA
Citation: Liu B, Wu L, Pan P, Li R and Lin B (2022) Response of Root Exudates of Bruguiera gymnorrhiza (L.) to Exposure of Polycyclic Aromatic Hydrocarbons. Front. Environ. Sci. 9:787002. doi: 10.3389/fenvs.2021.787002
Received: 30 September 2021; Accepted: 13 December 2021;
Published: 13 January 2022.
Edited by:
Shuwen Yan, Fudan University, ChinaCopyright © 2022 Liu, Wu, Pan, Li and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruilong Li, bGlydWlsb25nQGd4dS5lZHUuY24=; Bigui Lin, bGluYmlndWlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Beibei Liu
Beibei Liu Lin Wu
Lin Wu Pan Pan
Pan Pan Ruilong Li
Ruilong Li Bigui Lin
Bigui Lin