- South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences/ Guangdong Provincial Key Laboratory of Fishery Ecology and Environment, Guangzhou, China
Considering the unique characteristics of rare earth elements (REEs), the presence of REEs beyond specific limits will adversely affect the environment and it can be employed as a powerful probe for investigating hydrogeochemical processes. This requires sensitive determination of REEs in natural seawater. A matrix separation and pre-concentration technique using the mini-column packed with crab shell particles (CSPs) by inductively coupled plasma mass spectrometry (ICP-MS) as a means of determination has been developed. The aim of the proposed method was to simultaneously determine 16 REEs (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) at trace or ultra-trace concentrations in seawater. The biosorption capacity of CSPs was found to achieve 1.246–1.250 mg g−1 for all elements. In order to optimize performance of the method, the effects of analytical parameters concerning oscillation time, solution pH, salt concentration and eluent concentration were explored. Under the optimal conditions, the detection limits of REEs ranged 0.0006–0.0088 μg L−1, and relative standard deviations (n = 7) varied between 0.55 and 1.39%. The accuracy of developed method was evidenced by applying it to the analysis of REEs in seawater samples, with the overall recoveries at a level of 95.3 and 104.4%. Together, this work provides a promising and cost-effective CSPs-based pretreatment approach for REEs detection in sea environment.
Introduction
In recent years, rare earth elements (REEs: consisting of La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y, Sc, and Pm) are extensively used in several industries and high-tech devices, such as petrochemical, ceramic, metallurgy, laser and fiber optic industries (Balaram, 2019). With the ever-increasing industrialization, concerns on the accumulation and release of REEs, and their adverse effects on ecological environment have already been observed worldwide. On one hand, REEs in seawater originate from various anthropogenic and natural processes including river runoff, eolian dust, hydrothermal activity along with glacier discharge, which occur as a valuable tracer for investigating hydrological and geological acivities (Dubinin, 2004; Crocket et al., 2018). Furthermore, the data of REEs provide significant information about hydrogeochemical processes, covering ocean circulation, water physical mixing, external water transport and water-rock interface interactions (Hathorne et al., 2020). As a consequence, it is essential to propose an accurate, rapid and selective method for the sensitive measurement of REEs at trace or ultra-trace concentrations in natural environment with complicated matrix.
Among several analytical techniques employed for measuring multi-elements in various environmental samples, neutron activation analysis (NAA), inductively coupled plasma-optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS) have gained increasing attentions (Li et al., 2017; Zhu and Zheng, 2018; Montalvan-Olivares et al., 2021). It is worth noting that ICP-MS is a most powerful and sensitive technique with high precision, wide linear dynamic range and low detection limits for REEs determination, which only requires a small amount of samples (Zhu et al., 2009; Wysocka and Vassileva, 2017; Wysocka, 2020). Nevertheless, the direct determination of REEs by this technique suffers from the interferences of complicated matrix and the ultra-low concentration (e.g., ng L−1 or pg L−1) of target elements. Albeit the significant progress in the development of analytical technologies, direct determination of REEs in a highly saline water still remains a challenge. To circumvent these difficulties, a variety of approaches including co-precipitation (Zhu, 2020), ion-exchange (Barrat et al., 2020), liquid-liquid extraction (Guo et al., 2014) and solid phase extraction (SPE) (Pyrzynska et al., 2016; Chen et al., 2019) have been used for matrix separation and analyte pre-concentration, aiming to improve detection capability of REEs. In terms of these techniques, SPE has been preferred as a sample preparation approach owing to its flexibility of adsorbent, simple operation, low risk of contamination and high recovery (Tazoe et al., 2021). However, the certain limitation of SPE technique is attributed to the high cost of commercially available adsorbent. Therefore, the development of cost-effective SPE adsorbents has drawn special attention in the filed of analyte pretreatment.
Crab shell, a by-product from seafood processing, shows superior binding capacity for various heavy metal ions such as lead, cadmium, chromium, and cobalt, which has been identified as an effective SPE adsorbent (Boulaiche et al., 2019; Jeon, 2019; Richards et al., 2019). Attributed to its abundant contents of calcium carbonate, chitin along with proteins, crab shell was successfully applied to adsorb REEs in aqueous solution (Vijayaraghavan and Balasubramanian, 2010; Cadogan et al., 2014). Micro-precipitation of REE carbonate followed by adsorption to the chitin on the surface of crab shell was found to be the major mechanism responsible for REEs extraction by crab shell (Vijayaraghavan et al., 2009). Being a straight-chain polymer comprised of β-1,4-N-acetylglucosamin, chitin has available acetamido groups (Zhou et al., 2021). In particular, acetamido groups of chitin exhibit a high potential for non-specific metal chelation. Due to its good biosorption capacity, excellent mechanical stability, ease in desorption and ability of withstanding extreme conditions, crab shell is suited for column applications involving REEs extraction and pre-concentration prior to their detection (Su et al., 2019). Recycling crab shell is capable to potentially eliminate the disposal problem and convert useless waste into high value-added products. Furthermore, in the case of high biocompatibility, cost-effectiveness and great availability, crab shell can serve as a powerful REEs adsorbent. However, studies related to the adsorption and extraction of crab shell for rare earth elements are still scanty.
Accordingly, the objective of the present work is to develop a simple and cost-effective method for the extraction and pre-concentration of 16 REEs in seawater samples using a mini-column packed with crab shell particles (CSPs) prior to their measurement with ICP-MS. To explore the applicability of this approach, the effects of oscillation time, pH, salt concentration and eluent concentration were investigated by batch experiments. The analytical performances including limits of detection (LOD) and relative standard deviations (RSD) were also studied. Additionally, validation of the analytical procedure has been carried out, which was applied to the determination of REEs in spiked and unspiked natural seawater samples. To the best of our knowledge, crap shell particles have rarely been used as an adsorbent for the separation and pre-concentration of REEs at ultra-low level in seawater samples.
Experimental
Instruments
Mutielemental determination of REEs was carried out utilizing the quadrupole ICP-MS (Agilent 7,700, Agilent, America) equipped with a concentric nebulizer, a quartz injector (a sample tubing of 0.3 mm i.d. along with 1.6 mm o.d.), and a quartz cyclonic spray chamber including a third-generation octopole reaction system (ORS3) using helium gas. The resolution adjustement and mass calibration were weekly performed with the AutoTune function to stabilize the sensitivity of ICP-MS instrument. To correct instrument drift and signal fluctuation, the internal standard solution including indium (In) and rhodium (Rh) was mixed on-line with the samples. The optimized instrumental parameters of ICP-MS measurements are provided in Table 1.
Reagents and Solutions
All chemicals used in the experiment were of ultrapure grade. Ultra-pure water supplied by Guangzhou Chemical Reagent Factor was used throughout the present work. HNO3 and NaOH solutions were used for pH adjustment. China Series Standard Seawater D191 (R15 = 0.9995) was employed to evaluate the effect of salt concentration on this method. The working standard solutions of REEs were prepared by gradual dilution of multi-element calibration standard (10 mg L−1, Agilent Technologies, United States) with the matrix of 5% HNO3. The glassware employed in this work was immersed in 30% (v/v) HNO3 overnight and rinsed with ultra-pure water before use.
Synthesis of the Column Packed With Crab Shell Particles
Waste shells of Portunus sanguinolentus were obtained from the seafood market located in Zhoushan, Zhejiang. The crab shells were firstly rinsed adequately with distilled water to remove flesh and other impurities on the surface, then sun-dried for several days, crushed to particles, and sieved with 100 mesh. The crab shell particles were placed in a tube furnace for activation by calcining at 700°C for 2 h under nitrogen. The other chemical pretreament was not performed and CSPs were kept in the desiccators. A polyethylene mini-column (10 × 0.46 cm) was packed with CSPs, which was used in an off-line mode. A suitable glass wool was placed onto the bottom of column, and then 0.30 g of CSPs were placed upper part with the bed length of 5 mm. Another glass wool was fitted closely above the layer of CSPs.
Procedure
Biosorption Studies
The biosorption capacity of the CSPs was conducted by means of a batch procedure. The CSPs with a dosage of 0.4 g L−1 were contacted with 20 ml of 500 μg L−1 REEs standard solution at pH 6.0 in a 50 ml centrifuge tube, which was kept on a rotary shaker at 300 rpm and at room temperature (24 ± 2 °C) for a period of 2 h, and then CSPs were separated from aqueous solution by filtration. The experiment was performed in triplicate.
The amount of REEs sorbed by CSPs was calculated using the following equation:
Where Q is the REEs uptake (mg g−1); Co and Cf are the initial and equilibrium concentrations of REEs in solution (mg L−1), respectively; V is the volume of solution (L) and M is the mass of CSPs (g).
Off-Line Preconcentration and Elution Procedure
The sample solution was passed through the CSPs mini-column at a flow rate of 1.0 ml min−1 with a peristaltic pump. After the loading period, ultra-pure water was pumped through the column at a flow rate of 1.5 ml min−1 for 20 s to remove any un-retained matrix ions. The REEs retained on the sorbent were eluted utilizing HNO3 at a flow rate of 1.0 ml min−1. The eluent solution was collected for analyte concentrations measurement by ICP-MS.
Applications to Natural Seawater Samples
In order to assess accuracy of the proposed method, it was applied to the analysis of natural seawater samples spiked with standard reference REE solutions. The natural seawater samples were collected from Xisha sea area (113°E, 13°N). Before performing the extraction steps, 200 ml of the seawater sample was firstly filtered through a cellulose membrane with a pore size of 0.45 μm and then adjusted the pH to the optimum value if necessary. The recovery tests were carried out using 2 ml 4 M HNO3 for eluting analyte, and then pre-concentration by a factor of 100 was therefore acheieved. To obtain reliable high-quality data, the eluent solution was diluted (corresponding to a dilution factor of 20) before the analysis. Basically, due to its non-existence in natural environment except uranium minerals (Wysocka, 2020), radioactive promethium (Pm) is not determined in natural waters.
Results and Discussion
Biosorption Capacity of Crab Shell Particles
To estimate the biosorption capacity of CSPs towards REEs, the proposed method was conducted under the condition as described in Biosorption studies. According to Equation 1, it can be calculated that the sorption capacity of CSPs for all elements were in the range 1.246 mg g−1–1.250 mg g−1 (Supplementary Table S1), denoting that CSPs possessed high affinity towards each REE. It should be mentioned that the present approach could extract 1.246–1.250 μg REE in 1 ml aqueous solution when the SPE column was packed with 1 g CSPs. Undoubtedly, the biosorption capacity of CSPs was sufficient to extract REEs from aqueous solution in following experiments.
Optimization of Analytical Parameters
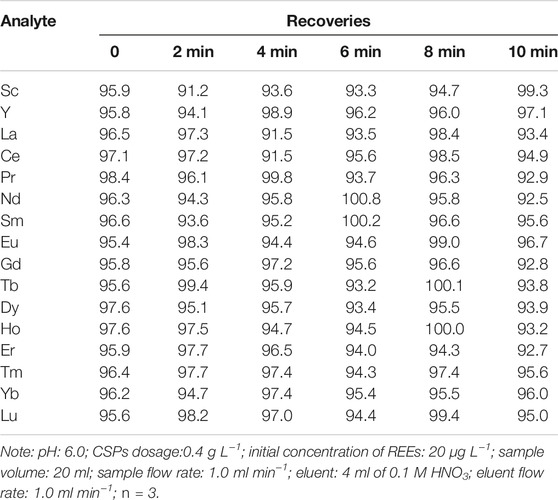
Effect of Oscillation Time
Biosorption of REEs onto CSPs was possibly connected with oscillation time. The effect of oscillation time on the recoveries of REEs was investigated by performing equilibrium sorption experiments at varying time intervals (0, 2, 4, 6, 8, and 10 min). As shown in Table 2, it should be noted that direct passing through the column packed with CSPs possessed high recoveries (95.6–98.4%) of REEs, which almost unchanged with oscillation time. Obviously, biosorption of REEs from aqueous solutions onto CSPs was observed to be a rapid process. Therefore, sample solutions could be directly pumped through the column packed with CSPs without the oscillation procedure, improving the simplicity and efficiency of the present work.
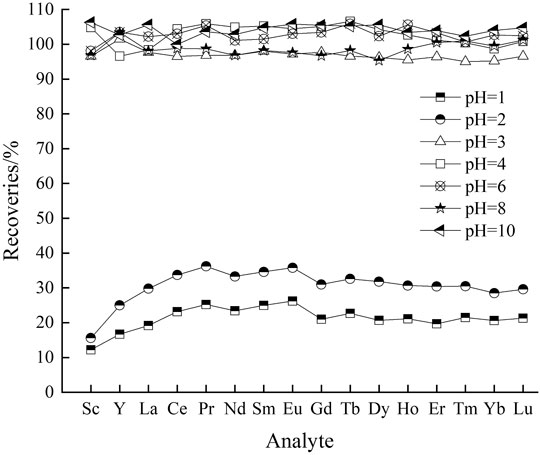
Effect of Solution pH
As the main constituent of crab shells, calcium carbonate favors micro-precipitation of REEs by dissociating to Ca2+ and CO32-, which is related to the pH of solution. In this respect, one of the most significant variables on preconcentration procedure is the pH level of samples. The effect of pH on the extraction of REEs with CSPs was evaluated within the range from 1.0 to 10.0. Beyond pH 10, noticeable precipation of REEs was found, thus the range of pH was not explored further. Unfortunately, the results (Figure 1) indicated that the overall recoveries of REEs were in the range 12.2–26.2% and 15.6–36.2% at the pH of 1 and 2 respecetively, which could be attributed to the low level uptake of REE ions by CSPs. On one hand, the lowering of solution pH can cause the surface charge on CSPs to become positive, which will inhibit the attraction of positively charged REE ions by means of electrostatic repulsion (Cadogan et al., 2014). On the other hand, it is worth noting that the competition among the REE ions enhances due to the limited quantity of available adsorption sites when the pH decreases (Ramasamy et al., 2017). As solution pH increased, it was clearly observed that the quantitative recoveries of REEs were close to 95.0–106.3% at the pH ranges of 3.0–10.0. Namely, the proposed method had a high applicability over a wide pH range. All further experiments were carried out at pH 6.
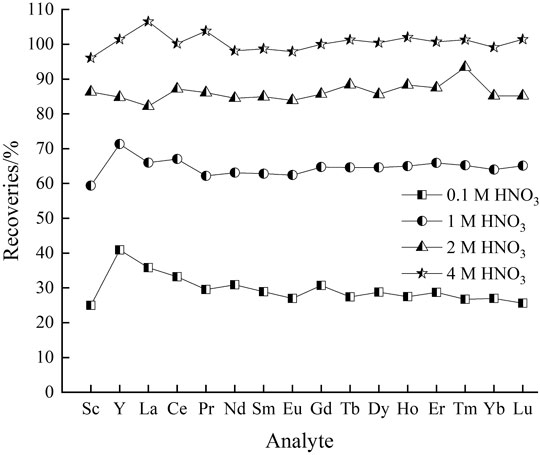
Effect of Eluent Concentration
Desorption procedure is of utmost importance to recover the retained REEs in concentrated form and recycle the biomass. Generally, appropriate selection of eluent solutions is required for a successful desorption process. In order to enhance sequential stripping of REEs retained by CSPs, the effect of HNO3 solution at different strengths (0.1, 1, 2 and 4 M) was explored using series standard seawater containing 20 μg L−1 REEs ions with the salt concentration of 5‰. Results concerning the influence of salt concentration are depicted in Figure 2. The obtained results revealed that 4 M HNO3 exhibited effective in desorption with the elution efficiencies of 96.1–106.5%. By contrast, the overall recoveries of REEs were in the range 25–40.9%, 59.4–71.3% and 82.2–93.4%, while uziling 0.1, 1 and 2 M HNO3 solution as eluent, respectively. It could be speculated that REEs in seawater were intensely bound onto the surface of sorbent and thus resistant to eluting with mild acid solution. Hence, 4 M HNO3 solution was selected as the optimum eluent for further experiments.
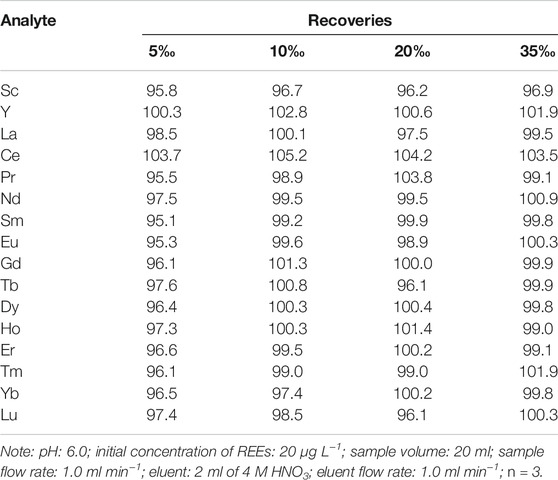
Effect of Salt Concentration
The salt content in seawater can pose the problems of clogging the inlet of cones to ICP-MS instrument, resulting in signal drifting and memory effect. The effect of salt concentrations on biosorption and elution procedure of REEs was studied using series standard seawater containing 20 μg L−1 REEs ions with the salt concentration in the range of 5–35‰. Results concerning the influence of salt concentration are provided in Table 3. The obtained results indicated that the overall recoveries were between 95.1 and 105.2% (95.1–103.7%, 96.7–105.2%, 96.1–104.2% and 96.9–103.5% at salt concentration of 5, 10, 20 and 35‰, respectively). From the high recovery of all REEs in varying salt concentrations, it could be concluded that the separation process effectively eliminated severe matrix interferences from seawater and the proposed method could successfully extract REEs from highly saline seawater samples.
Analytical Performance of the Proposed Method
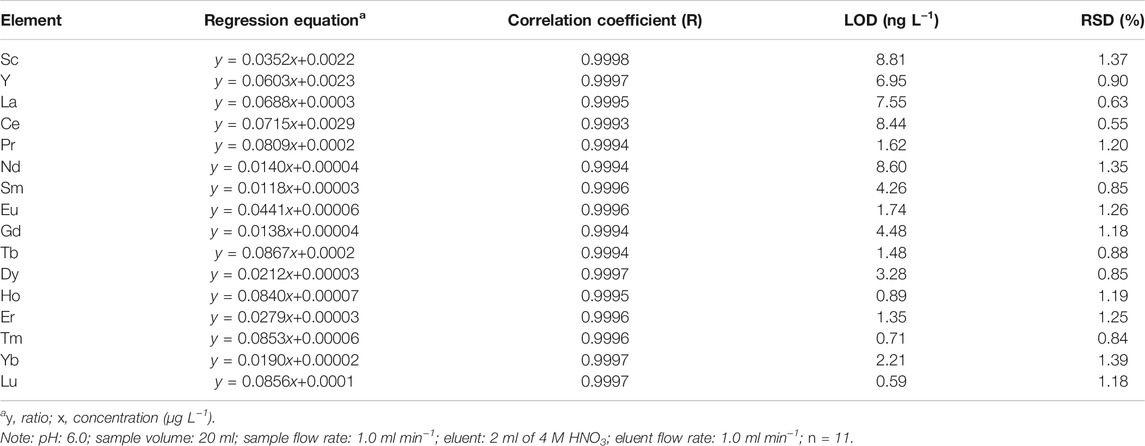
The detection limit of individual REE was affected by a variety of factors including atomic mass, natural abundance of isotopes, sample preparation steps, potential contamination and performances of ICP-MS system. The blank test was prepared using 20 ml ultra-pure water, and water was passed through the CSP column followed by elution with 2 ml 4 M HNO3. Based on the results of 11-set of replicated blank test, the detection limits of REEs were calculated as the concentration corresponding to three-fold standard deviation of the blank signal. The standard curves of 16 REEs are shown in Supplementary Figure S1, and the results of regression equation, correlation coefficient and LOD are given in Table 4. Under the optimum conditions, the detection limits of the method ranged from 0.59 ng L−1 (Lu) to 8.81 ng L−1 (Sc), which were low enough for the measurement of REEs in seawater samples. It should be noted that monoisotopic elements such as Pr, Tb, Ho, and Tm possessed lower detection limits than most polyisotopic ones (La, Ce, Nd, Sm, Eu, Gd, Dy, and Yb), which was in accordance with the results of previous studies (Hathorne et al., 2013; Rousseau et al., 2013; Zheng et al., 2014; Wysocka and Vassileva, 2017). The detection limits of REEs achieved in this work lowered than those reported values for REEs (e.g., 2.0–10.3 ng L−1; 2.0–60.0 ng L−1) in seawater determined by ICP-MS (Karada et al., 2011; Zhu et al., 2021). A relatively lower value for LOD might be attributed to lower background signal intensity, higher sensitivity, accurate blank correction, optimization of pretreating steps or a combination of some of these parameters.
The relative standard deviation tests were carried out by seven preconcentration and determination cycles using the solutions with the concentration of 1 μg L−1 for REEs. The analytical reproducibility was among 0.55–1.39% (RSD). The precisions were higher than most of reported values in the literatures (e.g., 2.8–11%; 1.2–18%) (Freslon et al., 2011; Arslan et al., 2018). These results demonstrated that the low detection limits and high precision of the developed method were sufficient to quantify ultra-trace REEs in seawater.
Overall, in terms of the detection limits and precision, the proposed approach provided data that were comparable with those of other methods. Compared with other techniques of SPE in the reported literatures (e.g., optimum pH range of 5–8) (Karada et al., 2011; Kumar et al., 2011), the process in this work was no need for careful pH adjustment (optimum pH values ranging 3–10). Indeed, the proposed method did not require any hazardous or harmful organic solvents which were indispensable in solvent extraction. Further, in comparison with the method of co-precipitation (Hsieh et al., 2011; Arslan et al., 2018), one of its demerits was that the relatively high concentration of co-precipitate carrier might interfer with the determination of REEs, while the elemental impurities in CSPs were negligible for REEs measurement. The present method apparently improved the capability for the determination of REEs in sea environment, and exhibited the advantages of relatively simple pretreatment procedure, high sensitivity, cost-effectiveness along with eco-friendliness.
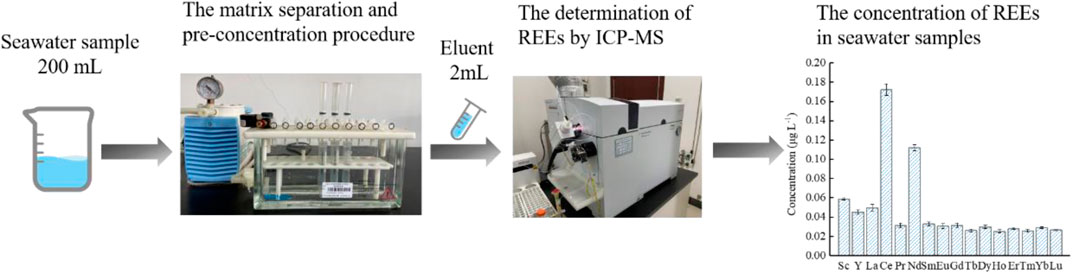
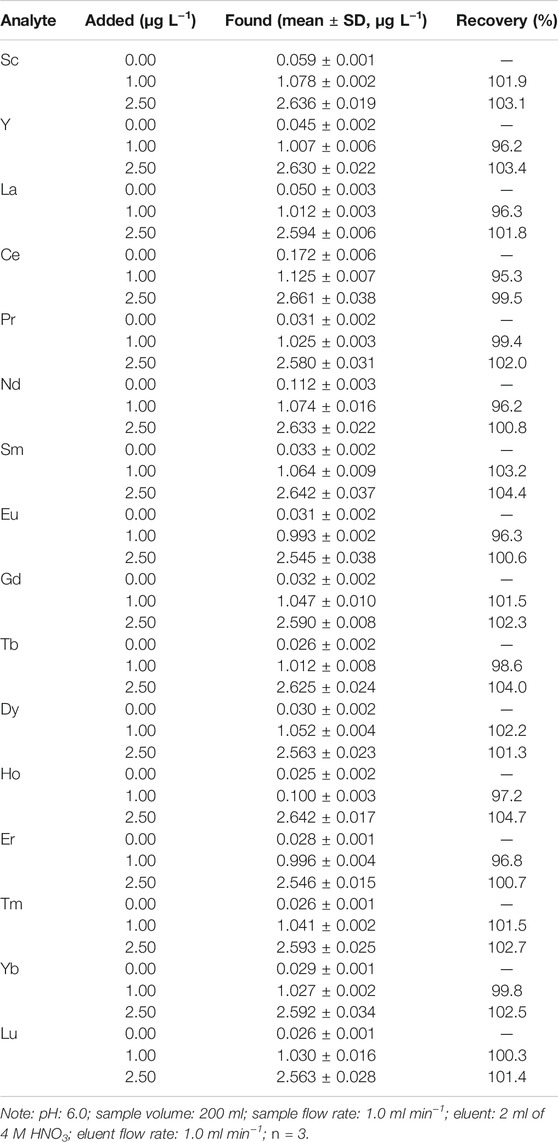
Application of the Proposed Method to Natural Seawater Samples
In order to verify the applicability and accuracy of the developed method, this method was applied to the measurement of REEs in spiked and unspiked seawater samples under the optimum condition described above. The analytical results of REEs concentrations in seawater samples collected from Xisha sea area are presented in Table 5. The total REEs concentrations in seawater samples ranged from 0.025 μg L−1 to 0.172 μg L−1. It could be noted that the mean concentrations of light REEs (ranging from La to Nd) were between 0.031 μg L−1 and 0.172 μg L−1, while the medium REEs (ranging from Sm to Ho) concentrations were between 0.025 μg L−1 and 0.033 μg L−1, and the level of heavy REEs (ranging from Er to Lu) were among 0.026–0.029 μg L−1. Generally, cerium (0.172 μg L−1) was the most abundant element, followed by neodymium (0.112 μg L−1). The obtained results were compared with those obtained using other SPE method reported previously (e.g., 0.007–0.138 μg L−1) (Karada et al., 2011), which were well within one order of magnitude. The recoveries of the varying spiked samples were between 95.3 and 104.4%, indicating that there was no significant loss of REEs during the extraction and elution process with the present method. The results clearly demonstrated the successful application of proposed method in the determination of ultra-low REEs in natural seawater samples.
Conclusion
After optimization of the separation and pre-concentration of REEs with crap shell particles as a unique sorbent, the results of accuracy and sensitivity for 16 REEs were sufficient for their precise determination in seawater samples. Based on the obtained results, it can be summarized that: 1) Biosorption experiments indicated that CSPs exhibited a high biosorption capacity towards REEs (1.246–1.250 mg g−1), which was capable to meet the demand of subsequent experiments. 2) The preconcentration of REEs by CSPs was found to be pH dependent, with optimal recoveries occurring at pH 3.0–10.0. Furthermore, desorption process revealed that REEs retained on CSPs could be eluted with 2 ml 4 M HNO3 solution. The developed method was successfully applied to the analysis of high salinity samples (35‰), since severe matrix interference could be effectively eliminated. 3) The detection limits of REEs achieved 0.0006–0.0088 μg L−1 and the precision of REEs values was in the range of 0.55–1.39% (RSD). 4) The proposed method has been validated by analysis of natural seawater samples to confirm its sensitivity and reliability, with the recoveries ranging from 95.3 to 104.4%. Overall, rapid sorption process, high biosorption capacity, and performance of simplicity, accuracy coupled with cost-effectiveness make crab shell particles-based pretreatment procedure a powerful and attractive approach for the determination of ultra-low REEs in seawater.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
DL designed and performed the experiments, analyzed the data and completed the articles. XW and KH provided the guidance on the conception and design of this study. ZW provided the guidance on data analysis and structure. XW, KH, and ZW contributed to the revision and editing of manuscript. All authors conducted the experiments and approved the submitted version.
Funding
This work was supported by Key Laboratory of Fishery Ecology and Environment of Guangdong Province (FEEL-2017-14), Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2021SD20), and Guangdong Provincial Special Fund For Modern Agriculture Industry Technology Innovation Teams (2021KJ151).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors express great appreciation to South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences and Guangdong Provincial Key Laboratory of Fishery Ecology and Environment for their support and aid during the experimental works.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2021.781996/full#supplementary-material
References
Arslan, Z., Oymak, T., and White, J. (2018). Triethylamine-assisted Mg(OH)2 Coprecipitation/preconcentration for Determination of Trace Metals and Rare Earth Elements in Seawater by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Analytica Chim. Acta 1008, 18–28. doi:10.1016/j.aca.2018.01.017
Balaram, V. (2019). Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 10 (4), 1285–1303. doi:10.1016/j.gsf.2018.12.005
Barrat, J.-A., Bayon, G., Wang, X., Le Goff, S., Rouget, M.-L., Gueguen, B., et al. (2020). A New Chemical Separation Procedure for the Determination of Rare Earth Elements and Yttrium Abundances in Carbonates by ICP-MS. Talanta 219, 121244. doi:10.1016/j.talanta.2020.121244
Boulaiche, W., Hamdi, B., and Trari, M. (2019). Removal of Heavy Metals by Chitin: Equilibrium, Kinetic and Thermodynamic Studies. Appl. Water Sci. 9 (2), 39. doi:10.1007/s13201-019-0926-8
Cadogan, E. I., Lee, C.-H., Popuri, S. R., and Lin, H.-Y. (2014). Efficiencies of Chitosan Nanoparticles and Crab Shell Particles in Europium Uptake from Aqueous Solutions through Biosorption: Synthesis and Characterization. Int. Biodeterioration Biodegradation 95, 232–240. doi:10.1016/j.ibiod.2014.06.003
Chen, S., Yan, J., Li, J., and Lu, D. (2019). Magnetic ZnFe2O4 Nanotubes for Dispersive Micro Solid-phase Extraction of Trace Rare Earth Elements Prior to Their Determination by ICP-MS. Microchim. Acta 186 (4), 228. doi:10.1007/s00604-019-3342-8
Crocket, K. C., Hill, E., Abell, R. E., Johnson, C., Gary, S. F., Brand, T., et al. (2018). Rare Earth Element Distribution in the NE Atlantic: Evidence for Benthic Sources, Longevity of the Seawater Signal, and Biogeochemical Cycling. Front. Mar. Sci. 5, 147. doi:10.3389/fmars.2018.00147
Dubinin, A. V. (2004). Geochemistry of Rare Earth Elements in the Ocean. Lithology Mineral. Resour. 39 (4), 289–307. doi:10.1023/B:LIMI.0000033816.14825.a2
Freslon, N., Bayon, G., Birot, D., Bollinger, C., and Barrat, J. A. (2011). Determination of Rare Earth Elements and Other Trace Elements (Y, Mn, Co, Cr) in Seawater Using Tm Addition and Mg(OH)2 Co-precipitation. Talanta 85 (1), 582–587. doi:10.1016/j.talanta.2011.04.023
Guo, X. Q., Tang, X. T., He, M., Chen, B. B., Nan, K., Zhang, Q. Y., et al. (2014). Dual Dispersive Extraction Combined with Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry for Determination of Trace REEs in Water and Sediment Samples. RSC Adv. 4 (38), 19960. doi:10.1039/c4ra01576b
Hathorne, E. C., Frank, M., and Mohan, P. M. (2020). Rare Earth Elements in Andaman Island Surface Seawater: Geochemical Tracers for the Monsoon? Front. Mar. Sci. 6, 767. doi:10.3389/fmars.2019.00767
Hathorne, E. C., Haley, B., Stichel, T., Grasse, P., Zieringer, M., and Frank, M. (2012). Online Preconcentration ICP-MS Analysis of Rare Earth Elements in Seawater. Geochem. Geophys. Geosyst. 13 (1), a–n. doi:10.1029/2011GC003907
Hsieh, H.-F., Chen, Y.-H., and Wang, C.-F. (2011). A Magnesium Hydroxide Preconcentration/matrix Reduction Method for the Analysis of Rare Earth Elements in Water Samples Using Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Talanta 85 (2), 983–990. doi:10.1016/j.talanta.2011.05.011
Jeon, C. (2019). Removal of Cr(VI) from Aqueous Solution Using Amine-Impregnated Crab Shells in the Batch Process. J. Ind. Eng. Chem. 77, 111–117. doi:10.1016/j.jiec.2019.04.025
Karadaş, C., Kara, D., and Fisher, A. (2011). Determination of Rare Earth Elements in Seawater by Inductively Coupled Plasma Mass Spectrometry with Off-Line Column Preconcentration Using 2,6-diacetylpyridine Functionalized Amberlite XAD-4. Analytica Chim. Acta 689 (2), 184–189. doi:10.1016/j.aca.2011.01.049
Kumar, S. A., Pandey, S. P., Shenoy, N. S., and Kumar, S. D. (2011). Matrix Separation and Preconcentration of Rare Earth Elements from Seawater by Poly Hydroxamic Acid Cartridge Followed by Determination Using ICP-MS. Desalination 281, 49–54. doi:10.1016/j.desal.2011.07.039
Li, F., Gong, A., Qiu, L., Zhang, W., Li, J., Liu, Y., et al. (2017). Simultaneous Determination of Trace Rare-Earth Elements in Simulated Water Samples Using ICP-OES with TODGA Extraction/back-Extraction. Plos One 12 (9), e0185302. doi:10.1371/journal.pone.0185302
Montalván-Olivares, D. M., Santana, C. S., Velasco, F. G., Luzardo, F. H. M., Andrade, S. F. R., Ticianelli, R. B., et al. (2021). Multi-element Contamination in Soils from Major Mining Areas in Northeastern of Brazil. Environ. Geochem. Health 43, 4553–4576. doi:10.1007/s10653-021-00934-x
Pyrzynska, K., Kubiak, A., and Wysocka, I. (2016). Application of Solid Phase Extraction Procedures for Rare Earth Elements Determination in Environmental Samples. Talanta 154, 15–22. doi:10.1016/j.talanta.2016.03.022
Ramasamy, D. L., Khan, S., Repo, E., and Sillanpää, M. (2017). Synthesis of Mesoporous and Microporous Amine and Non-amine Functionalized Silica Gels for the Application of Rare Earth Elements (REE) Recovery from the Waste Water-Understanding the Role of pH, Temperature, Calcination and Mechanism in Light REE and Heavy REE Separation. Chem. Eng. J. 322, 56–65. doi:10.1016/j.cej.2017.03.152
Richards, S., Dawson, J., and Stutter, M. (2019). The Potential Use of Natural vs Commercial Biosorbent Material to Remediate Stream Waters by Removing Heavy Metal Contaminants. J. Environ. Manage. 231, 275–281. doi:10.1016/j.jenvman.2018.10.019
Rousseau, T. C. C., Sonke, J. E., Chmeleff, J., Candaudap, F., Lacan, F., Boaventura, G., et al. (2013). Rare Earth Element Analysis in Natural Waters by Multiple Isotope Dilution - Sector Field ICP-MS. J. Anal. Spectrom. 28 (4), 573–584. doi:10.1039/c3ja30332b
Su, W., Yu, S., Wu, D., Xia, M., Wen, Z., Yao, Z., et al. (2019). A Critical Review of Cast-Off Crab Shell Recycling from the Perspective of Functional and Versatile Biomaterials. Environ. Sci. Pollut. Res. 26, 31581–31591. doi:10.1007/s11356-019-06318-0
Tazoe, H., Amakawa, H., Suzuki, K., Nishioka, J., Hara, T., and Obata, H. (2021). Determination of Nd Isotopic Composition in Seawater Using Newly Developed Solid Phase Extraction and MC-ICP-MS. Talanta 232, 122435. doi:10.1016/j.talanta.2021.122435
Vijayaraghavan, K., and Balasubramanian, R. (2010). Single and Binary Biosorption of Cerium and Europium onto Crab Shell Particles. Chem. Eng. J. 163, 337–343. doi:10.1016/j.cej.2010.08.012
Vijayaraghavan, K., Mahadevan, A., Joshi, U. M., and Balasubramanian, R. (2009). An Examination of the Uptake of Lanthanum from Aqueous Solution by Crab Shell Particles. Chem. Eng. J. 152, 116–121. doi:10.1016/j.cej.2009.03.040
Wysocka, I. (2021). Determination of Rare Earth Elements Concentrations in Natural Waters - A Review of ICP-MS Measurement Approaches. Talanta 221, 121636. doi:10.1016/j.talanta.2020.121636
Wysocka, I., and Vassileva, E. (2017). Method Validation for High Resolution Sector Field Inductively Coupled Plasma Mass Spectrometry Determination of the Emerging Contaminants in the Open Ocean: Rare Earth Elements as a Case Study. Spectrochimica Acta B: At. Spectrosc. 128, 1–10. doi:10.1016/j.sab.2016.12.004
Zheng, X.-Y., Yang, J., and Henderson, G. M. (2014). A Robust Procedure for High-Precision Determination of Rare Earth Element Concentrations in Seawater. Geostand. Geoanal. Res. 39 (3), 277–292. doi:10.1111/j.1751-908X.2014.00307.x
Zhou, D., Wang, H., and Guo, S. (2021). Preparation of Cellulose/Chitin Blend Materials and Influence of Their Properties on Sorption of Heavy Metals. Sustainability 13 (11), 6460–6511. doi:10.3390/su13116460
Zhu, Y. (2020). Determination of Rare Earth Elements in Seawater Samples by Inductively Coupled Plasma Tandem Quadrupole Mass Spectrometry after Coprecipitation with Magnesium Hydroxide. Talanta 209, 120536. doi:10.1016/j.talanta.2019.120536
Zhu, Y., Nakano, K., Shikamori, Y., and Itoh, A. (2021). Direct Determination of Rare Earth Elements in Natural Water Samples by Inductively Coupled Plasma Tandem Quadrupole Mass Spectrometry with Oxygen as the Reaction Gas for Separating Spectral Interferences. Spectrochimica Acta Part B: At. Spectrosc. 179, 106100. doi:10.1016/j.sab.2021.106100
Zhu, Y., Umemura, T., Haraguchi, H., Inagaki, K., and Chiba, K. (2009). Determination of REEs in Seawater by ICP-MS after On-Line Preconcentration Using a Syringe-Driven Chelating Column. Talanta 78 (3), 891–895. doi:10.1016/j.talanta.2008.12.072
Keywords: rare earth elements, seawater, inductively coupled plasma mass spectrometry, separation, preconcentration, crab shell particles
Citation: Li D, Wang X, Huang K and Wang Z (2021) Multielemental Determination of Rare Earth Elements in Seawater by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) After Matrix Separation and Pre-concentration With Crab Shell Particles. Front. Environ. Sci. 9:781996. doi: 10.3389/fenvs.2021.781996
Received: 23 September 2021; Accepted: 20 October 2021;
Published: 22 November 2021.
Edited by:
Chao Song, Chinese Academy of Fishery Sciences, ChinaReviewed by:
Ronghua Xu, Sun Yat-sen University, ChinaFuqiang Fan, Beijing Normal University, Zhuhai, China
Copyright © 2021 Li, Wang, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xunuo Wang, U2FucWlhbmxpLTE5ODNAMTYzLmNvbQ==; Ke Huang, eGlhbWlrZUAxNjMuY29t
 Danyi Li
Danyi Li Xunuo Wang*
Xunuo Wang*