
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Environ. Sci. , 14 December 2021
Sec. Soil Processes
Volume 9 - 2021 | https://doi.org/10.3389/fenvs.2021.756378
The organic matter of living plants is the precursor material of the organic matter stored in terrestrial soil ecosystems. Although a great deal of knowledge exists on the carbon turnover processes of plant material, some of the processes of soil organic matter (SOM) formation, in particular from microbial necromass, are still not fully understood. Recent research showed that a larger part of the original plant matter is converted into microbial biomass, while the remaining part in the soil is modified by extracellular enzymes of microbes. At the end of its life, microbial biomass contributes to the microbial molecular imprint of SOM as necromass with specific properties. Next to appropriate environmental conditions, heterotrophic microorganisms require energy-containing substrates with C, H, O, N, S, P, and many other elements for growth, which are provided by the plant material and the nutrients contained in SOM. As easily degradable substrates are often scarce resources in soil, we can hypothesize that microbes optimize their carbon and energy use. Presumably, microorganisms are able to mobilize biomass building blocks (mono and oligomers of fatty acids, amino acids, amino sugars, nucleotides) with the appropriate stoichiometry from microbial necromass in SOM. This is in contrast to mobilizing only nutrients and consuming energy for new synthesis from primary metabolites of the tricarboxylic acid cycle after complete degradation of the substrates. Microbial necromass is thus an important resource in SOM, and microbial mining of building blocks could be a life strategy contributing to priming effects and providing the resources for new microbial growth cycles. Due to the energy needs of microorganisms, we can conclude that the formation of SOM through microbial biomass depends on energy flux. However, specific details and the variability of microbial growth, carbon use and decay cycles in the soil are not yet fully understood and linked to other fields of soil science. Here, we summarize the current knowledge on microbial energy gain, carbon use, growth, decay, and necromass formation for relevant soil processes, e. g. the microbial carbon pump, C storage, and stabilization. We highlight the factors controlling microbial necromass contribution to SOM and the implications for soil carbon use efficiency (CUE) and we identify research needs for process-based SOM turnover modelling and for understanding the variability of these processes in various soil types under different climates.
A large amount of organic C in terrestrial ecosystems is stored in soil organic matter (SOM), and was estimated as 1,500–2,500 Pg C with significant losses within the last 200 years, for review see (Scharlemann et al., 2014) and the references therein. Therefore, knowledge-based management of SOM is needed. The amount of C stored in SOM is a steady state between CO2 fixed in annual plant primary production and the release from SOM by soil microbial degradation and mineralization. Reduced C in the form of plant biomass enters the soil and provides energy and C for the growth of heterotrophic microbial decomposers. These microorganisms drive C and nutrient cycling and are thus relevant for many of the ecosystem services that soils provide, e.g., C sequestration, nutrient retention, provision of food, fibers and fuel, habitat for organisms, water retention and purification, etc. (Baveye et al., 2016). They transform many C sources to microbial biomass C that is subsequently distributed to various trophic levels of the soil fauna. For decades, microorganisms were thought to contribute to SOM mainly through their activity, i.e. degrading and modifying plant organic matter, but this view has changed over the last decade.
Research on soil C transformation and sequestration has shifted towards studying the processes of SOM formation (Schimel and Weintraub, 2003; Schmidt et al., 2011; Cotrufo et al., 2013; Lehmann and Kleber, 2015a; Barre et al., 2018; Sokol et al., 2018; Sokol and Bradford, 2019). This has also been accompanied by a shift in paradigms: SOM is increasingly seen as being comprised of molecules that are the result of microbial metabolism, including microbial biomass components and microbial-processed plant compounds (Lehmann et al., 2008; Miltner et al., 2012; Wang et al., 2021b). Microbe-mediated turnover of plant materials into stabilized SOM has long been conceptualized (Guggenberger et al., 1999; Kögel-Knabner, 2002; Kögel-Knabner, 2017) but it is only recently that evidence for a large contribution of microbial necromass to SOM formation has accumulated (Craig et al., 2018; Kästner and Miltner, 2018; Liang et al., 2019; Liang et al., 2020). This evidence is based on the NMR spectra of microbial biomolecules in SOM (Simpson et al., 2007), on specific analyses of cell envelope amino sugars of bacteria and fungi (Guggenberger et al., 1999; Amelung, 2001; Appuhn and Joergensen, 2006; Fan and Liang, 2015; Liang et al., 2017), on turnover studies of microbial proteins and cell envelope structures (Rillig et al., 2007; Miltner et al., 2009; Miltner et al., 2012; Schweigert et al., 2015), on elemental stoichiometry and the C:N ratio (del Giorgio and Cole, 1998), or on SOM development from farmyard manure or defined substrate materials in artificial soils (Pronk et al., 2013; Kallenbach et al., 2016; Pronk et al., 2017). Contributions of microbial biomass components to SOM were recently summarized (Starke et al., 2017; Liang et al., 2019; Angst et al., 2021; Wang et al., 2021a; Wang et al., 2021b) but also depend on microbial taxa (Dong et al., 2021). Microbial contributions to SOM play a much greater role in C sequestration into soils than traditionally believed, particularly because a significant portion of those inputs were found to be sometimes stabilized more than plant inputs (Ma et al., 2018). However, the specific roles of microbial C utilization, transformation, as well as necromass stabilization processes in SOM and their interactions with ecosystem conditions, remain largely elusive. There seems to be a discrepancy between the low amounts of living biomass and the relatively high amounts of residues of dead cells, showing not only a high formation and turnover of microbial biomass but also effective and preferential stabilization of microbial residues.
Due to macromolecular aggregations, e.g., in cell envelopes, microbial biomolecules are partially stabilized by the complex composition of these materials. They can be additionally stabilized either by aggregation with themselves or by physical interaction, sequestration, or trapping within the soil matrix and aggregates (Miltner et al., 2012; Liang et al., 2017; Kästner and Miltner, 2018). A significant part of these materials is normally present in particulate matter of nm size: colloidal proteins, ribosomes, or cell envelope fragments. Based on its size, this material is by definition part of the dissolved organic C fraction (<0.45 μm, DOC) (Bridgewater, 2012). Recent calculations based on microbial cell envelope residues inferred by amino sugar analysis of 122 soil site samples showed an average contribution of microbial necromass to SOM varying between 33% in forest soils and up to 62% in grasslands (Liang et al., 2019). The concept of microbial biomass formation combined with mineral matrix stabilization (Cotrufo et al., 2013) has been conceptualized to a soil “microbial C pump,” MCP (as an analogy to the marine “carbon pump” (Jiao et al., 2010)) in which microbes degrade plant-derived C to produce own biomass, which is stabilized later as necromass by various processes of mineral interaction and in soil aggregates (Liang et al., 2017; Liang et al., 2020). In terrestrial systems, this “pump” transforms microbe-synthesized compounds into SOM where they are stabilized in a kind of “entombing” effect (Liang et al., 2017); however, detailed understanding of these processes is still a scientific challenge (Liang, 2020; Zhu et al., 2020). In order to step forward from empirical understanding towards mechanistic process control, we need to relate the MCP idea to the details of the microbial growth and decay cycle processes, as well as post mortem modifications of biomolecules combined with matrix stabilization including the effects of redox cycling. Therefore, we need to recall the principles and factors of microbial energy and C use, and this knowledge needs to be thoroughly and consistently used in soil science.
In order to understand C transformation and sequestration in soils, an approach that focuses only on C pools and C storage without considering energy and matter fluxes is too limited (Waring et al., 2020; Manzoni et al., 2021). Soil fertility and many other soil functions depend on the activity of various soil microbial communities and thus on continuous energy and C fluxes through the soil system (Janzen, 2015; Waring et al., 2020; Manzoni et al., 2021). For maintaining microbial diversity and ecosystem functions in soil including C storage, both fluxes and stoichiometry issues need to be considered. More information on the link between element cycling and energy fluxes is thus necessary in order to understand C turnover and sequestration in terrestrial ecosystems. Here we provide a conceptual synopsis about microorganisms as drivers for SOM formation, with the potential for an improved understanding of C sequestration processes in soils. Our goal is to relate microbial growth behavior and energy consumption to bio- and necromass formation in SOM. In addition, we aim to establish the relationship with the factors controlling necromass stabilization in SOM, together with their implications on C use efficiency (CUE) and nutrient cycling. Finally, we identify resulting open questions and research needs in SOM turnover research, including suggestions for the improvement of SOM contents.
Most of the solar energy retained in C by plant primary production enters soil (>90%) (Gessner et al., 2010), nurturing ecosystems and biodiversity. Megatons of organic matter per hectare with hundreds of gigajoule of Gibbs energy are intermediately stored in the soil on its way through food webs until there is a final mineralization and release of this energy as heat and entropy (von Stockar, 2010). Microbial bio- and necromass thus represent intermediate stages of C and energy retained in SOM.
All living organisms need energy, C sources, and nutrients to accommodate their requirements for maintenance metabolism, growth, and activity. In soils, some microorganisms are photolithoautotrophs (green algae, cyanobacteria) or chemolithoautotrophs that use inorganic molecules, e.g. NH4+ or Fe2+ as energy sources and electron donors as well as CO2 as C source; for more details see (Lengler et al., 1999; Schink, 2006). However, most of the microorganisms in soil are chemoorganoheterotrophs and organic molecules are their C and energy sources. The C of these organic substrates can be shared between catabolism (= degradation) and anabolism with biomolecule synthesis and eventually growth. Thereby the catabolism provides the energy for the anabolism (Figure 1). Under starvation or unbalanced elemental supply conditions in soil, anabolic C turnover can be decoupled from catabolic processes, resulting in variable C use from different substrates. However, no anabolism is possible without energy gain from catabolism (Russel and Cook, 1995; Lengler et al., 1999). Degradation processes in catabolism apply irrespective of the origin of the substrates for both plant materials and microbial necromass. The turnover of microbial bio- and necromass enables element cycling of N and P through microbial food webs (Kästner and Miltner, 2018). Productive degradation of a substrate, combined with cycling of biomass building blocks, can increase the overall amount of biomass produced.
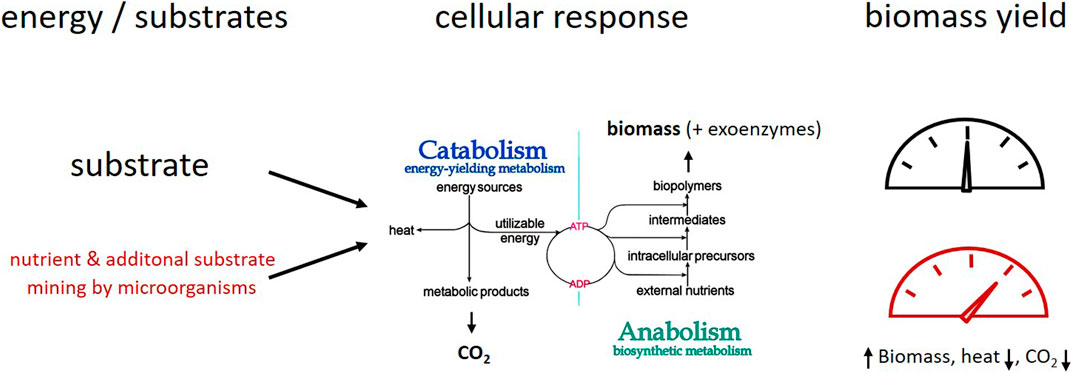
FIGURE 1. Simplified sketch of microbial metabolism under growth conditions: C and energy from substrate degradation feeds anabolism with biomass formation. Microbial mining of nutrients and additional substrates–a concept to explain variation of carbon use efficiency (CUE) small amounts of SOM or biomass “building” blocks’ result in higher biomass yield from main substrates; more C and energy from the substrates can be allocated towards biomass formation resulting in higher CUE and C retention because of lower energy requirements for biomass synthesis.
Energy is the driver of all biotic processes in nature and energy metabolism depends on redox reactions that couple two proceeding half-reactions (oxidation and reduction). The total energy gain (ΔG, Gibbs energy) is the energy balance of the half-reactions in heterotrophic organisms: 1) the oxidation of C substrates, often to CO2, and 2) the transfer of released electrons to terminal electron acceptors in respiration processes, e.g. the reduction of O2 to H2O (Russel and Cook, 1995; Schink, 2006). The energy gain from the oxidation of substrates varies with different electron acceptors; O2 as electron acceptor sustains the highest turnover rates of organic matter (Schink, 2006). Microbial activity generally reduces the redox potential and O2 content in the vicinity of the cells. Alternative electron acceptors such as NO3−, Fe3+, Mn4+/3+, SO42- can also be used, but with decreasing energy gains due to lower redox potentials. Anoxic electron acceptors result in various degradation efficiencies of molecule classes, which results in selective stabilization (Keiluweit et al., 2017). Thermodynamics can thus provide mechanistic explanations for SOM formation and turnover and for understanding soil processes in general.
Microbial growth and biomass formation by anabolism in soils depends on many factors such as 1) soil properties, texture, and environmental conditions (pH, clay and SOM content as well as humidity, aeration, temperature), 2) the presence and general activity of potential litter degraders in microbial communities, and 3) the availability of nutrients, electron acceptors, and substrates (Gavrilescu, 2005; Waring et al., 2020). Bioavailability is controlled by the characteristics of the substrate (water solubility, molecular size, Koc/Kow values) as well as their interaction with other components of the soil matrix, e.g., dissolution, sorption, aggregation, and entrapment (Bollag and Liu, 1990; Sims et al., 1990; Gavrilescu, 2005).
Under optimal conditions with ideal water contents, electron acceptor availability, nutrient and substrate supply, the growth rates of microorganisms depend on available C and energy substrate concentrations. For growth, each cell needs to divert a minimum substrate flux in order to maintain metabolism, cell integrity, and survival (the maintenance threshold (van Uden, 1967; van Bodegom, 2007)). Thus, minimum substrate and energy fluxes significantly different from zero are needed, even if cells do not grow. Low concentrations of substrates or low turnover rates due to limited supply conditions may not provide sufficient energy for this maintenance metabolism, resulting in dormancy or spore formation and eventually in cell death (Kovarova and Egli, 1998). For a long time it was thought that the maintenance rates of various bacteria as well as death rates and growth yields were nearly constant over wide substrate ranges, but this view has changed (van Bodegom, 2007). More detailed examinations with a model bacterium showed that fast-growing bacteria die faster upon substrate deprivation and have lower biomass yields due to increased maintenance requirements (Biselli et al., 2020). This means that growth rates are positively correlated to maintenance needs and death rates; however, they are negatively correlated with biomass yields and increasing growth rates can thus result in decreasing biomass yields relative to substrate uptake. Higher growth rates thus can lower C retention in biomass resulting in highly flexible microbial C use in soil. Slow-growing cells on the other hand survive longer due to lower maintenance requirements (Russel and Cook, 1995) and have an evolutionary advantage in environments where substrate supply varies over time (Kovarova and Egli, 1998; van Bodegom, 2007; Biselli et al., 2020). This advantage is based on lower maintenance needs and thus the ability to survive longer (Kovarova and Egli, 1998; van Bodegom, 2007; Biselli et al., 2020). Not surprisingly, the maintenance requirements and survival of organisms also depend on the recycling yield from dead cells in microbial cultures. Organisms usually feed on their deceased neighbors and the recycling yield is exponentially related to the growth rate (Biselli et al., 2020). Under optimal growth conditions a recycling yield of 12% can be obtained. In other words, only eight dead cells are needed to produce one new cell, which supports the hypothesis that microbes are capable of generally mining microbial necromass. The requirements are much lower under more limited conditions. This mining can explain why microbes in deeper soil layers can survive by merely feeding on the necromass seeping downwards; the availability of these resources directly regulates the C use (Ludwig et al., 2015; Ni et al., 2020).
With decreasing concentrations of degradable SOM compounds, the overall growth in soil decreases (Kästner and Miltner, 2018; Zheng et al., 2019). However, the activity of microorganisms is determined by the energy content of the substrates, the availability of nutrients, terminal electron acceptors as well as soil water contents, and typically by the most limiting factor (Russel and Cook, 1995; Lengler et al., 1999; Waring et al., 2020). For optimum growth, all factors required by the soil microorganisms have to match their needs. This does not only include C but also other nutrients in the required stoichiometric ratios. The growth of soil microorganisms is often limited by one of these factors, which then controls the growth yield and energy use efficiency (EUE = energy retained in the system, here in biomass or SOM) (Harris et al., 2012). The ratio of C used for biomass production (= growth) in anabolism over total substrate C use (= C used for growth + respired C) has been defined as yield coefficients (YX/S) in microbiology (Schink, 2006) which is similar to C use efficiency (CUE) (del Giorgio and Cole, 1998).
The organic matter of the photoauthotrophic primary producers enters the soil as litter and root exudates (Schmidt et al., 2011; Lehmann and Kleber, 2015b; Janzen, 2015; Paul, 2016). Litter mostly consists of macroscopically visible aggregated plant-derived biopolymers (such as starch, cellulose, and lignin); for details see (Kögel-Knabner, 2017) and the references therein. Due to their large molecular size, these compounds cannot be taken up by microorganisms (with cell sizes of a just a few μm), because the transport across the cell envelope is considered to be limited to smaller molecules (<600–1,000 Da) (Ekschmitt et al., 2008; Lehmann and Kleber, 2015a). Similarly, microbial biomass and their aggregates of proteins, nucleic acids, cell envelope fragments, and extracellular polymeric substances are also macromolecules, but of a much smaller size in the order of nm. All of these macromolecules need to be depolymerized (often hydrolyzed) to oligomers outside of microbial cells by extracellular enzymes before they can be taken up as substrates (Schimel and Weintraub, 2003; Kästner and Miltner, 2018). Microorganisms producing extracellular enzymes were shown to have a lower growth yield than non-producers (Malik et al., 2019). The accumulation of microbial necromass alters the relative C and N limitation during litter decomposition and accelerates N cycling, ultimately resulting in increased SOM content. Such effects partly decouple C and N use and the spatial heterogeneity may explain different C and N turnover dynamics and stabilization in SOM. The resulting monomers and oligomers can then be taken up by microorganisms and used for cell maintenance and growth.
Due to their rapid consumption, such low-molecular-weight compounds are only present at very low steady-state concentrations in the soil, although the flux into the cells may be considerable (Schimel and Schaeffer, 2012). Sorption reactions often take place very quickly and can reduce the availability of substrates for microorganisms, resulting in a competition between uptake with degradation by microorganisms and external reactions. As bioavailability of C substrates and nutrients is often limited in soil, starving microorganisms are eager to take up whatever they are able to access and able to degrade (Schimel and Schaeffer, 2012; Waring et al., 2020). Described transport mechanisms for the uptake of organic molecules into microbes are (Lengler et al., 1999; Madigan et al., 2014): 1) diffusion, or 2) porin-mediated transporter systems, which are typically transmembrane tunnel proteins in the cell membrane that can be associated to electron transfer, acceptor molecules, or a phosphorylation process. The abundance and properties of such porines can be modulated by the microorganisms and depend on environmental and growth conditions as well as exposition to substrates and chemicals (Denyer and Maillard, 2002). In addition, facilitated transport of molecules into degrading microbial cells by unknown mechanisms related to carrier molecules, e.g. by the presence of humic acids or particularly after compost addition, has also been described (Li et al., 2021; Adam et al.). Even sorbed compounds can be degraded (Feng and Boyd, 2008) and for Gram-positive bacteria the uptake of N-containing compounds of much higher molecular size, e.g. proteins even from sites of strong sorption, has been observed (Enggrob et al., 2020).
Temperature, water and O2 contents of a soil are all important regulators of plant primary production, soil microbial activity, and energy use, controlling the input and compound turnover in the soil system. Access to water is vital for bacterial and fungal biodegradation and growth (Moyano et al., 2013; Rath et al., 2017) and a lack of bio-accessible water leads to limited depolymerization and degradation in general (Masoom et al., 2016). In addition, O2 limitations may be the least understood regulators of SOM turnover (Keiluweit et al., 2017). This is also valid for microbial growth and necromass stabilization related to redox cycling. The importance of temporary anaerobic conditions for SOM accumulation is underlined by results from wet rice-cropping in paddy soils, which show a profound enrichment of SOM over 2000 years (Kalbitz et al., 2013).
Moreover, self-organization and interaction dynamics in decomposer communities are generally assumed in soils and can result in increased biomass amounts as SOM is thought to be utilized by functionally redundant soil-specific microbial communities (Rillig et al., 2015; Fierer, 2017). Based on the modelling approach of Allison (2005), Kaiser et al. (2014); Kaiser et al. (2015) showed that SOM development depends not only on the stoichiometric response of microbes to a high C:N ratio substrate but also on the community dynamics. They showed that the activity of microbes producing extracellular enzymes (“decomposers”) and microbes exploiting the catalytic activities of others (“cheaters”) lead to the regulation of SOM turnover. The presence of “cheaters” increased N retention by down-regulation of the ratio of extracellular enzymes to total microbial biomass, resulting in increased bio- and necromass formation. This resulted in increased N-rich necromass accumulation, increased C retention, decreased turnover rates, and an increase in spatial heterogeneity of microorganisms and necromass distribution.
In spite of the importance of energy fluxes through the soil systems, thermodynamics have only partly been considered for soil systems (Bosatta and Agren, 1999; Lueders et al., 2004; Harris et al., 2012; Di Lonardo et al., 2017; Manzoni et al., 2021), but have been applied predominantly to technological, chemical, and biochemical processes, such as the production of proteins, and in order to determine the energy use, heat production, and nutrient requirements (Westerhoff et al., 1982; McCarty, 2007; Xiao and VanBriesen, 2008; Kleerebezem and Van Loosdrecht, 2010). The determination of thermodynamic state variables has been applied to soil or natural organic matter processes only in a handful of cases (Barros and Feijóo, 2003; Barros et al., 2007; Herrmann et al., 2014; Barros et al., 2016; Boye et al., 2018). Currie (2003) identified the link between energy and C content in the soil by combining the principle of energy balance with a biogeochemical process model and using calorimetric analysis of different litter materials and soil samples for parameterization of the turnover processes. However, the main obstacle for considering energy fluxes was the difficulty in finding suitable experimental approaches that link energy to matter fluxes in soil systems. For soil they have to be related to complex and diverse energy-consuming processes and including the formation of microbial biomass, necromass, and subsequently SOM (Chakrawal et al., 2020; Barros, 2021).
Thermodynamic properties of low-molecular-weight organic compounds have been related to their elemental composition and the nominal oxidation state of C (NOSC) in organic compounds (including SOM) and were suggested as a proxy for the potential release of Gibbs energy during the oxidative degradation of these compounds (LaRowe and van Cappellen, 2011). The NOSC was then used to explain qualitatively the linkage between organic C transformation, soil microbial communities and land use/management (Keiluweit et al., 2017). However, NOSC was not found to explain the preferential substrate use of various organic compounds in pure culture experiments (Cyle et al., 2020). Emphasizing the thermodynamic potential factors as a driving force for microbial metabolism (Ji and Bethke, 2007), thermodynamic constraints were shown to lead to a limited degradation of highly reduced compounds (e.g., lipids) under anoxic conditions (Keiluweit et al., 2017), resulting in the relative enrichment of such compounds in anoxic microsites of soil aggregates. Thermodynamic aspects thus play a much more important role in SOM protection and stabilization than was previously assumed.
Quantitative approaches using thermodynamics in the soil are currently limited to defined organic compounds. The potential growth yield of microbes feeding on a certain compound has been modelled in order to predict their turnover to biomass in soils (Brock et al., 2017; Trapp et al., 2018), including stabilization in SOM. The Microbial Turnover to Biomass (MTB) model is based on the estimation of the energy available for biomass formation (growth) obtained from the catabolic reactions of a substrate. The model calculates the electron transfers that can be exploited by microorganisms from the substrate to the respective electron acceptors for a specific turnover equation (Brock et al., 2017; Trapp et al., 2018); it predicts the resulting potential microbial yields and enables the formation of biomass and necromass to be related to the CO2 released, hence it enables a direct link to potential C use in soil. The approach can be applied for many organic compounds in order to predict their potential contribution to microbial biomass formation and ultimately the stabilization of organic compounds in the soil.
Considering the thermodynamic issues, we can conclude that energy is needed for SOM formation through microbial growth and the decay cycle in which some energy is retained within the necromass. A comparison of the total Gibbs energy released from an oxidation reaction with the potential energy available for microbial growth provides an estimate of the actual energy retention within microbial biomass and later necromass in a soil in relation to the release as heat and may provide a sound basis for further research approaches for improving SOM contents.
There are still huge gaps in our understanding of C conversion and microbial CUE in soils, which is defined as the C stored in soil per substrate consumed (del Giorgio and Cole, 1998; Mooshammer et al., 2014; Nunan et al., 2015; Spohn, 2016; Spohn et al., 2016; Manzoni et al., 2018; Geyer et al., 2019; Manzoni et al., 2021). CUE will always be lower than 1 and the empirically determined upper limit is 0.6 (Roels, 1980); it is not a constant value and depends on the microbial physiology and the environmental conditions (temperature, water content, electron acceptor availability). CUE was often expected to be constant; however, the related CUE in complex environmental systems must be calculated based upon the sum of all consumed substrates and thus it may be variable (Manzoni et al., 2021). CUE is a key parameter that is closely linked with the biotic energy use principles on all trophic levels and that varies with the input of C and energy of the substrates and the specific environmental conditions. Reports consider various influencing factors such as depolymerization of plant matter, substrate elemental composition (stoichiometry), substrate molecular structure, and nutrient availability (Liu et al., 2016; Spohn et al., 2016; Takriti et al., 2018). However, microbial necromass as a substrate and nutrient resource also plays a significant role. Due to the variation and environmental conditions of soils, the CUE was observed in the range of 0.3–0.55 (Sinsabaugh et al., 2016; Soares and Rousk, 2019). Substantial differences in CUE were determined by various methods targeting different aspects of microbial metabolism, e.g. general growth, biomarker formation, energy gain by ATP formation, etc. (Geyer et al., 2019). CUE based on the assimilation of radiolabeled thymine and acetate often provide relatively low values (0.03–0.3) (Soares and Rousk, 2019). These differences complicate comparisons of CUE reported in different studies.
CUE in soils shows an expected high sensitivity and negative correlation to temperature (Manzoni et al., 2012). The response of CUE to short-term changes in temperature and moisture as well as O2 content was found to depend mainly on microbial growth response and not on respiration responses in various soils and land use types (Zheng et al., 2019). The C:N ratios of plant inputs are negatively correlated to CUE; the highest CUE in terrestrial ecosystems are found with plant input material C:N ratios around 10, which are in the range of microbial biomass C/N (Manzoni et al., 2012). Such materials meet the stoichiometric requirements for microbial biomass production and thus favor microbial growth. CUE declines as resource C:N moves further away from biomass stoichiometry but also depends on the availability of inorganic and organic N (Manzoni et al., 2008; Manzoni et al., 2021). Under N-limited conditions, CUE is reduced and nitrogen use efficiency (NUE) is maximized, whereas under non-limited conditions NUE decreases (Mooshammer et al., 2014). Conversely, C:N:P stoichiometry regulates SOM mineralization; N and P provision increases biomass formation and lowers CO2 emission (Wei et al., 2020). In addition, soil warming increases growth, decreases CUE and increases microbial turnover to CO2, finally affecting SOM dynamics, see Figure 2 (Li et al., 2019b). With the focus on necromass, high CUE in soils occurred by necromass retention in relation to the substrate consumption for biomass production, and is thus a matter of microbial physiology as well as of matter and energy fluxes. Therefore, we can hypothesize that rapid degradation processes of a pulsed substrate input and the related microbial growth result in peak energy and C fluxes through the soil, whereas slow processes result in much broader flux behavior with higher overall C and energy retention (Figure 2). This results in higher cumulative microbial biomass formation as the organisms are able to meet their maintenance requirements over longer periods of time, translating into more necromass and SOM formation.
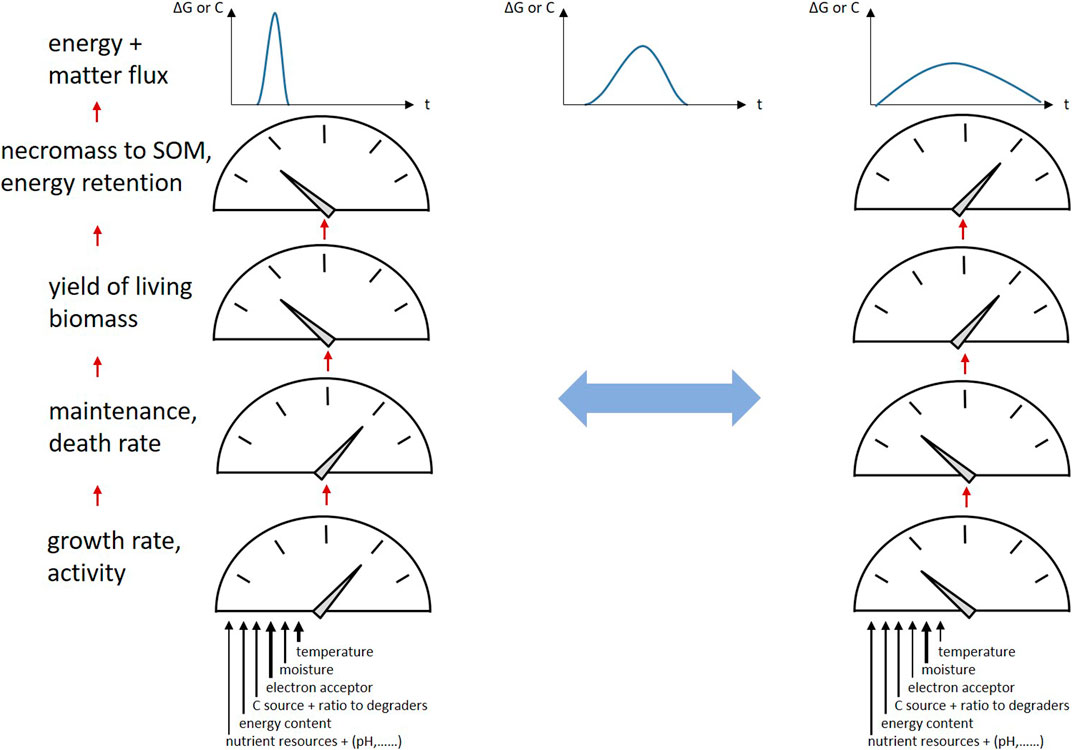
FIGURE 2. Energy and C fluxes through SOM triggered by environmental conditions: transitions of “peak flow” to much broader flux behavior and vice versa; factors causing variation of microbial growth, maintenance and death rates, biomass yield, and finally necromass retention in SOM resulting in variation of CUE and EUE.
In addition to C and energy, microorganisms need other elements, such as H, O, N, etc. at appropriate stoichiometric ratios for homoeostatic growth. The elemental composition of microbial biomass may vary from C4H7O1,5 N (Lengler et al., 1999) to C5H7O2N (Christensen and McCarty, 1975) and C5H8O0,8 N (Rittmann and McCarty, 2001), with C:N ratios of about 4-5 in microbial cultures. In addition, small amounts of P and S as well as trace elements (e.g. Fe, K, Mg, Ca) must be available (Lengler et al., 1999). Soil microbial biomass C:N ratios were reported to be higher, ranging from 4.5 to 12.5, with 7 as a generally accepted average (Xu et al., 2013; Mooshammer et al., 2014). Soil microorganisms usually grow on plant litter material, which has higher C:N ratios than microbial biomass. The microorganisms have several options to meet their stoichiometric requirements, e.g. they could preferentially degrade nutrient-rich compounds from plant matter, mine SOM for specific elements, or utilize other substrates with the excess C released into the environment. In addition, living microorganisms can also recycle cell internal materials (Spohn et al., 2016; Capek et al., 2021). The elemental needs are the key element of the theory of ecological stoichiometry and are one of the potential mechanisms for a variation in CUE and priming effects in soils (Nunan et al., 2015; Spohn et al., 2016). From turnover analyses of organic compounds in the soil we know that multiple C sources are often used by microbes in order to obtain the optimum stoichiometry by “mixing” substrate molecules (Kovarova and Egli, 1998; Brock et al., 2019; Cyle et al., 2020).
CUE in soil cannot be related to the transformation of a single substrate, because soil microbial communities use different C sources at the same time, which is also related to the stoichiometry needs. CUE is thus notoriously difficult to analyze related to both method and system properties (Geyer et al., 2016; Geyer et al., 2019; Li et al., 2019b). This, together with the pronounced effect of environmental boundary conditions, results in the observed variability of CUE, reflecting the plasticity of microbes reacting to their overall growth conditions (Anthony et al., 2020). The CUE concept relates C turnover to microbial growth and provides much deeper insight into the relevant processes of C dynamics when considering biomass and the resulting necromass formation as substrate and nutrient resources in soil. However, over the last decade the role of energy fluxes has hardly been given any consideration, with the main focus having been on the turnover of C, N, and P. Combined mass turnover and energy balances enable evaluation of the amount of energy derived from C sources degradation as well as the amount of energy retained in soil by microbial biomass (Barros and Feijóo, 2003; Barros, 2021).
With every microbial compound oxidation, a part of the energy is dissipated as heat, whereas the remainder may be used for driving microbial anabolism and growth. The additional use of necromass as a source of building blocks for microbial biomass (here defined as amino acids and peptides, amino sugars and peptidoglycan oligomers, fatty acids and lipids, nucleotides and nucleic acids of microbial origin) may improve growth yield, CUE and EUE considerably. For microorganisms, it is economic not to degrade all substrate molecules to central metabolites of the tricarboxylic acid cycle and then synthesize the complex biomolecules from these metabolites (see Figure 3 and also Figure 1). Recycling of building blocks from microbial necromass thus saves considerable energy, which would otherwise be needed to re-synthesize such compounds.
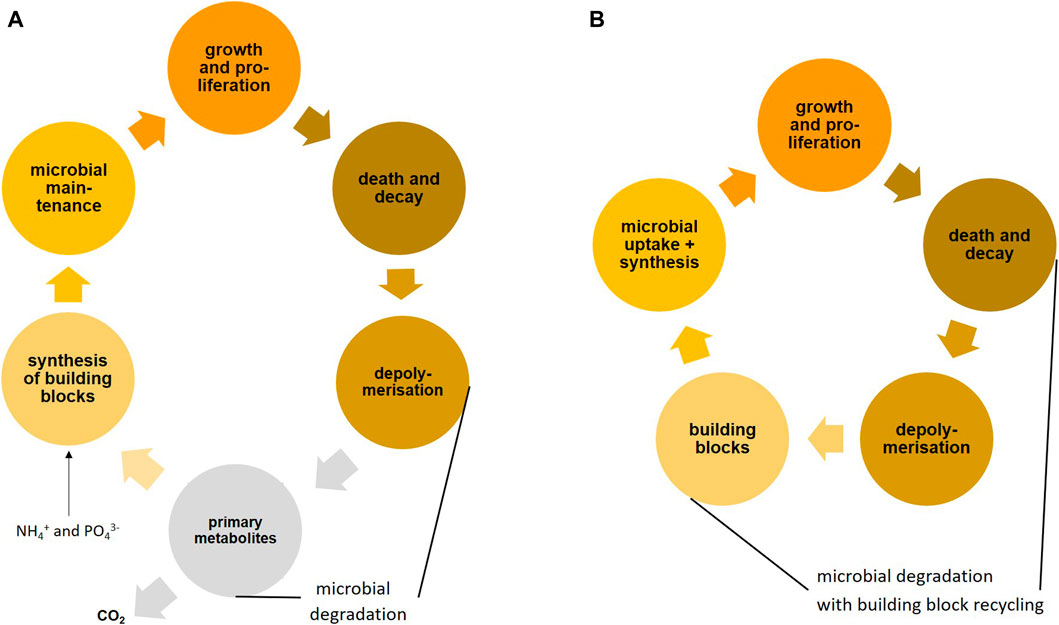
FIGURE 3. Microbial turnover fed by plant primary production with mining of nutrients in soil: (A) full degradation cycle resulting in mineralization with new synthesis of microbial biomass from primary metabolites of the tricarboxylic acid cycle, typically considered in microbiology, (B) microbial resource mining (building block mining from microbial necromass with appropriate stoichiometry) in addition to full degradation, which is much more effective, because a lower part of the substrate is mineralized with lower energy demand for anabolism and growth (The viral shunt in soil may provide similar resources during the lytic cycle in bacteria).
Based on these energy considerations, we suggest an extended mining concept of SOM decomposition, which does not only focus on the stoichiometry of elements (Mooshammer et al., 2014; Zechmeister-Boltenstern et al., 2015) but on mining of biomass building blocks that can be mobilized by living microbes. The use of biomolecules from microbial necromass improves the energy budgets and fluxes of the cells, as they do not need to consume energy for their synthesis. Microbial necromass is a good mining substrate, as the elemental and chemical composition is similar to microbial biomass and the enzymes for the depolymerization and re-polymerization are generally present in the cells. Using building blocks enables better microbial growth and biomass yields. In comparison, plant detritus has a much higher C:N ratio, is more difficult to degrade and requires depolymerization by exoenzymes, whereas plant exudates in the vicinity of roots may also directly provide building blocks.
Accordingly, a direct incorporation of labelled fatty acids from PLFA into Actinobacteria has been observed (Apostel et al., 2018). The observed higher priming effects after the addition of a single amino acid to soils compared to glucose and inorganic N (Mason-Jones et al., 2018) also support the “microbial resource mining” hypothesis, because amino acids and peptides provided as building blocks stimulate growth and additional mining. Position-specific 13C label showed a much higher mean residence time of ribose compared to glucose in soils, indicating the reuse or preservation of intact ribose-derived cell components in SOM (Bore et al., 2019). The addition of biomass building blocks (yeast extract) compared to glucose alone actually increased the amount of active enzymes and microbial yield in a forest soil, but did not increase the averaged growth rates or mineralization of the soil (Loeppmann et al., 2020). In addition, fungi were shown to mobilize resources from Gram-negative necromass and distribute it within the microbial food web (Zheng et al., 2021). In a rhizosphere soil an active Saccharibacterium was identified, which lacks genes for nucleotide synthesis (Starr et al., 2018) and thus depends on the uptake of complete nucleotides for DNA and RNA synthesis. Growing evidence shows that N mining can be considered to be directly targeting N-containing building blocks from microbial necromass, since a large part of N in SOM is derived from microbial products (Mooshammer et al., 2014).
However, the stabilization of microbial necromass in soil systems limits the availability for mining and is thus a competing process. Depending on the availability of nutrients, other substrates, and the biochemical effort for mining building blocks, soil microbes optimize their resource utilization in order to achieve maximum growth but with minimum energy loss (Westerhoff et al., 1982; Kovarova and Egli, 1998; Roller and Schmidt, 2015). This allows optimum growth and maintenance (= trade-off between rate and efficiency) under the given environmental conditions, and suggests that the Maximum Power Principle (Odum and Pinkerton, 1955) is relevant for the formation of SOM through microbial necromass.
Reports are inconsistent about the role of influencing factors, but the existence of optimized microbial regulation of CUE has been demonstrated along resource stoichiometry gradients (Craine et al., 2007; Manzoni et al., 2017; Manzoni et al., 2018). Increased availability of nutrients (either in organic or inorganic form) allows microorganisms to use more C for microbial growth, whereas nutrient deficiency results in less C allocation to growth and more to mineralization. The supplementation of soils with easily degradable organic compounds leads to priming effects resulting in an immediate response of the SOM turnover (Kuzyakov, 2010). Although the processes underlying priming effects are still elusive, they are presumably linked to microbial resource mining.
Predation by other organisms (Richter et al., 2019) and in particular phage lysis play important roles in determining microbial survival and death in the soil and in releasing microbial cell material (Kuzyakov and Mason-Jones, 2018). Viruses are extremely abundant in the soil (up to 1010 viral particles g−1 soil (Williamson et al., 2017); and thus infection and lysis of the cells are major factors for microbial life and death in soils. After death, microbial cells start to decay and the integrity of the cell membrane is no longer given (Lengler et al., 1999). Microbial necromass can be considered as a continuum from active biomass to necromass in all stages of decay or stabilization (Kästner and Miltner, 2018). Necromass, including cell envelope fragments, is material for degradation reactions in soil, since it is intrinsically biodegradable and will be metabolized according to the same rules and principles as described for organic substrates and plant materials in general. However, due to the much smaller size of microbial biomass materials and cell envelope fragments, the depolymerization and degradation may be facilitated in comparison to plant detritus material. In addition, mining of bioavailable necromass supports the formation of new biomass, which partly ends up as necromass again within the microbial food web of necromass turnover (Lueders et al., 2006), especially in deeper soil layers (Ludwig et al., 2015; Angst et al., 2018; Ni et al., 2020). The necromass can be decomposed by enzymes in the soil matrix that are either present as exoenzymes or released by cell autolysis (Miltner et al., 2012; Schimel and Schaeffer, 2012). In addition, mechanical disruption of the residual cell envelopes by physical processes, e.g., shrinking and swelling of SOM as well as soil mixing due to bioturbation by macrofauna may also determine the fate (Bohlen et al., 2004; Basile-Doelsch et al., 2020). The ultimate fate of the necromass then depends on many factors: microbial decay, clay content, Fe or Al oxides, water content and pore system of the soil aggregates (Schimel et al., 2007).
Microbial necromass (including cell envelope fragments) can be stabilized within the soil matrix through its small size, spatial conformation, and by interaction with itself, e.g., by the formation of stacks of cell-wall fragments that may hamper degradation due to limited accessibility of the interior regions (Miltner et al., 2012; Buckeridge et al., 2020). Necromass may easily be occluded in soil aggregates and therefore not be bioavailable for degradation. Interactions of biomolecules with other organic and mineral matter can occur by ionic or hydrogen bonds or hydrophobic interactions (Galicia-Andres et al., 2021). However, various types of biomolecules are partly stabilized in SOM to different extents: proteins > bulk biomass C > lipids (e.g. PLFA) (Kindler et al., 2006; Kindler et al., 2009; Miltner et al., 2009; Spence et al., 2011; Miltner et al., 2012). An overarching stabilization process of SOM and necromass can also be caused by the formation of hydrophobic domains with a decreased chemical activity of water (Masoom et al., 2016) by drying processes, or by loss of pore water connectivity. The authors impressively provided evidence that around 75% of the necromass-derived SOM material is actually not in contact with water.
In addition, post mortem modifications of biomass components can be hypothesized, in particular for proteins, peptidoglycans and chitins, which can alter these materials and cause their stabilization, and may mask them for analysis. This could well be one reason why the high contribution of stabilized biomolecules in SOM has been overlooked for decades (Simpson et al., 2007). Necromass–necromass interactions through ionic or hydrogen bonds or hydrophobic interactions have recently been demonstrated (Buckeridge et al., 2020; Galicia-Andres et al., 2021). However, if we consider post-mortem modifications for both plant- and microbe-derived molecules, there must be some molecules resulting from these modifications. These modifications may be derived from bridging by metal cations or hydrogen bonds (Galicia-Andres et al., 2021), multiple cross-linking of proteins, peptidoglycan, lipopolysaccharides and nucleic acids, which can no longer be directly identified as the original molecule classes. For example, amorphous Fe precipitation may coat ribosomes of cellular origin. Such processes may be of high interest and particularly valid for proteins, since some authors found up to 100% of N in SOM may be present in amide bonds (Knicker, 2011).
The conversion of macroscopic macromolecular plant matter to microbial biomass causes a flux of diverse plant polymers towards small-size microbial polymers of lower diversity. Macromolecular plant matter often comprises homopolymers (e.g., starch, cellulose) as well as heteropolymers (e.g., lignin, lignocelluloses, proteins) with very high molecular weights and a size range of µm to cm (Bresinski et al., 2008). Through microbial degradation, this material is converted to microbial heteropolymers (proteins, peptidoglycan and chitines) with comparatively low molecular weights and a much smaller size (10–100 fold). The conversion of plant polymers to microbial polymers results in the “entombing effect” (Liang et al., 2017) by increasing small-scale heterogeneity, and is presumably also accompanied by an increase in entropy (von Stockar, 2010). Soil C stabilization by molecular complexity was recently conceptualized as a driving factor for the persistent behavior of generally easily degradable organic molecules (Lehmann et al., 2020), which causes the spatial separation and temporal variability of SOM. The general conversion from plant litter towards microbial OM increases small-scale molecular complexity and finally decreases the likelihood of the co-location of a substrate molecule next to the decomposer organisms, and thus the turnover rates, resulting in higher turnover times.
Experiments on the turnover of common farmyard manure in artificial soils with various mineral composition revealed relatively similar CO2 emissions in all samples (Pronk et al., 2013). However, the highly different compositions of the developed microbial communities (Babin et al., 2013) indicated that microbial activity and utilization of complex substrates are adapted to reach the optimal CUE and are not primarily related to microbial community compositions. Although minerals and substrates strongly affect the composition of microbial communities, they obviously do not have a pronounced effect on microbial activity and overall biomass formation. The stabilization of the biomass and necromass formed, however, depends on the mineralogy and the redox dynamics of the soil. Minerals, in particular clay minerals, were shown to be the most correlating factors of necromass and SOM storage in various forest soils (Angst et al., 2018; Angst et al., 2021; Kleber et al., 2021).
The stabilization of non-living 13C-labelled microbial biomass by the soil matrix was tested in soils of two contrasting forest ecosystems (temperate forest in California; tropical forest in Puerto Rico (Throckmorton et al., 2015)). That particular study traced microbial biomass from fungi, actinobacteria, as well as Gram-positive and Gram-negative bacteria into soil density fractions. As expected, for both ecosystems the highest amount of soil C was found in the heaviest mineral-associated fraction. After prolonged incubation, the highest percentage of the microbial necromass-derived 13C was recovered in the mineral-associated fraction, irrespective of the type of microbial cell material. Mineral associations are thus more important for stabilizing microbial necromass in soil than the cellular origin. However, the exact fate of the necromass was under the strong control of site-specific edaphic factors. Fungal hyphae appear to have a relatively fast turnover, with a reported life span of only 7–10 days (Godbold et al., 2006). This would imply higher turnover times for fungal necromass compared to bacterial necromass, and a potential shift in the fungal/bacterial ratio when going from biomass to necromass (Liang et al., 2019). Other results showed a lower mineralization of ectomycorrhizal fungi (Schweigert et al., 2015).
Microbial necromass is stabilized like all other types of organic matter mostly by aggregation and bonding to the mineral matrix of the soil (Cotrufo et al., 2013; Angst et al., 2018; Angst et al., 2021; Kleber et al., 2021). It is generally considered that SOM is dominantly stabilized at the mineral phase as organo-mineral associated organic matter (MOM) (Kleber et al., 2015; Angst et al., 2018; Gao et al., 2019; Haddix et al., 2020; Angst et al., 2021; Gerrit et al., 2021). However, mineral association comprises a multitude of interactions dominantly considered as molecular interaction (Kleber et al., 2021). As microbes frequently live attached to particle surfaces, microbial necromass already is in direct contact to minerals when it is formed. In addition, due to the small size, microbial necromass can be associated with small-scale minerals, resulting in a considerable stabilization; however, these associations can also be metabolized by living microbes (Omoike and Chorover, 2006; Mikutta et al., 2007; Mikutta et al., 2011; Throckmorton et al., 2015; Gao et al., 2019; Kleber et al., 2021).
The mineral phase controls the long-term (up to millennia) C storage (Mikutta et al., 2007; Mikutta et al., 2009; Doetterl et al., 2015) as well as N storage in soil (Jilling et al., 2018). Biogeochemistry studies of MOM across long-term mineralogical soil gradients and chronosequences on the Hawaiian Islands (Mikutta et al., 2009) showed that SOM contents increased significantly with increasing surface area of poorly crystalline Fe and Al minerals. This correlation got lost, however, when formation of secondary minerals commenced. A significant impact of the plant cover of topsoils was detected, whereas mineral composition was more important in deeper layers. Studies in a glacier forefield chronosequence along a 120 ky ecosystem gradient (Mikutta et al., 2019) confirmed that MOM was formed through association with minerals of both microbial necromass and modified plant-derived compounds. Litter quality was less important for the development of MOM, and plant-derived C that had not been microbially processed was present in all soil depths.
The association with and occlusion in minerals and aggregates was considered to be the most important stabilization mechanism for microbial necromass in soil (Guggenberger et al., 1999) and Fe and Al oxides play a dominant role in these stabilization processes (Eusterhues et al., 2003; von Lützow et al., 2006; Schneider et al., 2010). Association of SOM to minerals is typically considered as interaction of separate molecules with mineral surfaces (Kleber et al., 2021) but often such materials are aggregations of biomolecules or cell envelope fragments of colloidal size in the nm range, which is not yet in the scientific focus. The association of microbial necromass with amorphous minerals or freshly precipitated metal oxides in soils has been demonstrated using various methods, suggesting important relations to the redox cycles of Fe (Ludwig et al., 2015; Kunhi Mouvenchery et al., 2016; Woche et al., 2017). The biogenic oxidation of reduced Fe, accompanied by precipitation, may result in incrustation of the cells and necromass materials, thus leading to strong preservation of cell organic matter on timescales up to 100,000 years under extreme conditions (Posth et al., 2014) as long as the Fe oxides are not reduced again. Microbially enhanced incrustation of SOM by Fe, Al, Mn, or Si oxides additionally slows down degradation (Kappler et al., 2005; Posth et al., 2014). Apart from sorption, the specific molecular architecture of the necromass fragments, as well as the self-aggregation of this material and the embedding in EPS of biofilm materials, contribute to physical separation of the materials, making them inaccessible for degrading enzymes. In addition to complexation with other organic compounds, microbial components and necromass are protected through occlusion within aggregates, creating physical barriers against enzymatic attack (Balesdent et al., 2000; Christensen, 2001; Rasmussen et al., 2005; Plaza et al., 2013). In addition, bacteria and necromass were found to be predominantly associated with clay particles forming clay “hutches” as a kind of shelter (Lünsdorf et al., 2000; Plaza et al., 2013).
Even if sorbed to minerals, microbial necromass is not protected from recycling by other microbes (Creamer et al., 2019), which is an important pathway for microbial resource mining. The authors identified two mechanisms of OM enrichment on two different minerals (feldspar and Al(OH)3): the molecular enrichment of non-living OM and the enrichment of living or dead cells on surfaces. They also proved that microbial necromass is strongly sorbed to Al(OH)3, making it poorly bioavailable. Feldspar in contrast has a lower accumulation potential for organic compounds in general. However, necromass retention on Al(OH)3 was decreased in the presence of living bacteria compared to the control. This indicates that living cells attached to the minerals can recycle mineral-sorbed necromass C. Therefore, both mechanisms, stabilization by mineral-sorption as well as destabilization by mobilization, have to be considered. Mobilization of sorbed materials was particularly found for mineral-associated N-containing compounds (Turner et al., 2017; Creamer et al., 2019; Enggrob et al., 2020), supporting the microbial resource mining hypothesis.
Redox cycling strongly controls both C and P storage, e.g. in the rhizosphere of paddy soils (Huang et al., 2018; Wei et al., 2020; Kleber et al., 2021). The majority of stored P and C is associated with freshly deposited ferrihydrite, often formed by Fe-oxidizing bacteria in oxic microhabitats (Kappler et al., 2005; Posth et al., 2014). The current state of knowledge is that Fe-metabolizing bacteria are intimately linked to the Fe cycle in all environments and provide the key preservation of OM in soils and sediments (Posth et al., 2014). In addition, physical protection in concretions and aggregates is also correlated to changes in the moisture regime (Cates et al., 2019). Conversely, the release of C and P depends on Fe-reducing bacteria utilizing electron donors from root exudates as C and the energy source in anoxic habitats (Kappler et al., 2005; Achtenhagen, 2015). The dynamics of both processes depend on drying and rewetting cycles; there is also a strong link to N cycling. Excess of ammonium in the rhizosphere was shown to result in significant Fe-reduction-related ammonia oxidation (Feammox) accompanied by the release of soluble Fe2+ (Li et al., 2019a).
After exposure to redox fluctuations mimicking wet rice production with and without addition of rice straw, SOM and microbial community structures have been traced in soils with different mineralogy (Winkler et al., 2019). In comparison to continuously oxic conditions, lower overall mineralization was shown for the samples from redox cycling with a tendency to store more C from straw addition. Under cycling, more straw-derived C was allocated to C-retaining MOM, irrespective of the soil type (Winkler et al., 2019). Dissolution and precipitation of Fe oxides is essential for MOM formation and thus SOM protection, as more MOM was retained in soil with higher amounts of redox reactive minerals and cycling. This, however, requires a sufficient input of fresh OM as electron donor and C-source in order to induce sufficient microbial activity to induce Fe oxide dissolution (Posth et al., 2014). Similar effects of dissolution of amorphous Al(OH)3 with subsequent co-precipitation with OM due to shifts in pH can also protect OM from decomposition (Mikutta et al., 2011), although the role of microbial activities for this process are not clear yet. Overall, mineralogy had no or only little effect on the turnover of straw-derived C. However, the capacity of the redox-active minerals is responsible for whether newly formed MOM replaces older MOM or is retained in addition to older MOM sorbed by the minerals (Winkler et al., 2019). We can hypothesize that these processes will apply in a similar way to necromass.
Redox cycling and the related dissolution and precipitation of Fe oxides are thus important key factors for SOM, in particular for necromass stabilization with continuous seasonal or annual cycling. In the wet period of wet-dry cycles, the redox potential of soil can drop dramatically, with SOM released from Fe oxides upon microbial reductive dissolution. With recurring oxidative conditions, fresh Fe oxides are formed that have low crystallinity and small particle sizes in the nm range. Furthermore, they cover existing surfaces, and thus offer large surface areas for sorption, protecting SOM and causing encrustation of larger biomaterials. Co-precipitation of oxidized Fe with necromass fragments (cell envelopes) was shown to result in strong stabilization through spatial protection (Achtenhagen, 2015). Such dynamics provide a close link between SOM stabilization and release of C, P and organic N, including microbial biomass building blocks.
Overall, we can conclude that the processes of the microbial growth and decay cycle combined with the matrix stabilization are the mechanistic principles of the MCP concept (Liang et al., 2017). It is based on the following interwoven factors: microbial growth and decay cycle, post mortem modifications of the biomolecules combined with matrix and mineral associated stabilization of these compounds as well as increasing molecular complexity (Figure 4). They are consistent with the observed similarity in the chemical composition of SOM in various soil ecosystems.
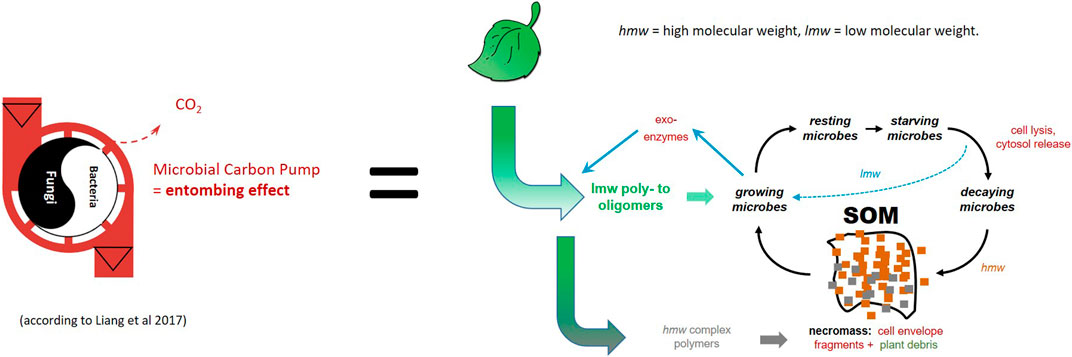
FIGURE 4. Intriguing conceptual models need to be filled with real process understanding: the microbial growth and decay cycle (macro-polymer conversion to micro-polymers) combined with matrix stabilization of necromass are the driving forces of the microbial C pump (MCP).
There appears to be a discrepancy between the low amounts of living biomass and the relatively high amounts of residues of dead cells, showing not only a high formation and turnover of microbial biomass but also effective and preferential stabilization of microbial residues. The CUE concept relates C turnover to microbial growth and provides much deeper insight into the relevant processes of C dynamics when considering biomass and the resulting necromass formation as substrate and nutrient resource in soil. Combined mass turnover and energy balances enable evaluation of the amount of energy derived from C sources degradation as well as the amount of energy retained in soil microbial biomass (Barros and Feijóo, 2003; Barros, 2021). Energy is needed for microbial biomass and necromass formation, but it is also stored in the soil by necromass retention. A comparison of the total Gibbs energy released from an oxidation reaction with the potential energy available for microbial growth provides an estimate of the actual energy retention within microbial biomass in a soil in relation to the release as heat, and may provide a sound basis for further research approaches for improving SOM contents.
A missing contact to water, the complexity of biomolecule aggregations and cell envelope fragments, the increased small-scale molecular complexity of SOM after turnover of plant materials (Lehmann et al., 2020) as well as post-mortem modifications of microbial biomass, including mineral incrustation of biomaterials, can explain not only the discrepancy between the amounts of necromass and living biomass in soil but also why the highly contributive necromass may have been overlooked for decades. In addition, researchers should take into account that organo-mineral associations are often not only single-molecule interactions, but also interactions of biomolecule aggregations, which additionally interact with themselves. Therefore, future research should focus on assessing the impacts of each of these processes to the storage and stabilization of necromass C in SOM. Moreover, it should always be kept in mind that C turnover in soil is a matter not only of C stocks but also of fluxes (Janzen, 2015; Waring et al., 2020), which link aboveground to belowground ecosystems by organic-matter-based energy fluxes through soils from plants via microbes and their necromass to CO2.
The following questions still remain unanswered: what predominantly determines SOM and necromass stabilization processes in soils: the matter and energy fluxes, the water contents, the microbial communities with their biochemical traits, the mineral matrix, or their redox dynamics combined with the microbial growth and decay cycles including molecular complexity? The energy and matter fluxes feeding microbial communities in soils are currently the research focus of a joint research initiative launched by the German Research Foundation (DFG; SPP2322, https://www.www.SoilSystems.uni-trier.de).
Understanding and, in particular, controlling these processes will be imperative for a sustainable knowledge-based management of SOM. Necromass stabilization can explain why SOM content is higher in arable soils fertilized with organic material compared to mineral fertilizers. The addition of farmyard manure obviously helps to increase active and fertile SOM because manure is comprised of almost pure necromass from the cattle gut digestion processes (Chen et al., 2020; Liu et al., 2020). Necromass-based SOM formation also explains why perennial plants, in particular grasses with their dense root systems, improve microbial necromass and SOM contents by feeding microbes with their exudates (Fester et al., 2014). The conversion of grasslands and forests to arable land will release part of the stored C by mineralization (Guo and Gifford, 2002). Conversely, more plants and biodiversity are needed in order to produce more microbial biomass and ultimately SOM in soils (van der Heijden et al., 2008).
Therefore, the management of SOM in soil ecosystems and arable land equates to the management of energy, C and N fluxes. Maintaining biodiversity, fertility and many soil functions will depend on active microorganisms, and thus on the decomposition of SOM and the energy use derived from this turnover. The management of C and energy fluxes should therefore become a strategy as opposed to simply increasing C stocks, and the management of agroecosystems should thus also focus on optimizing CUE with the aim of increasing SOM content in soils (Kallenbach et al., 2019).
The addition of clay minerals e.g. to sandy soils will contribute to higher C sequestration (Churchman et al., 2020). As was found for wet rice cropping, redox cycling may be included in a clever strategy increasing the turnover of the microbial growth and decay cycle, and the addition of clay minerals may boost storage capacities in the soil (Kalbitz et al., 2013). Such treatment and supplementation concepts may be applied in order to increase SOM contents in general and to mitigate excess CO2 in the atmosphere. Research on channeling of C and energy through plant residues and the microbial growth and decay cycle as dominating driver provides the unique opportunity to gain a biota-controlled mechanistic understanding of SOM formation and turnover.
A great deal of research has investigated microbial activity and community structures, with very little research focusing on microbial necromass stabilization and its implications. The extension of an established model (MIMICS-CN) considers not only litter quality but also more detailed microbial processes of N mobilization from necromass and SOM, including the spilling of excess C or N (Kyker-Snowman et al., 2020). It shows prognostic value but is still not able to represent the dynamics of CUE and NUE based on climate factors, e.g., temperature and moisture, which are the controlling determinants of microbial activity and growth. The inclusion of microbial interactions in SOM models can explain persistence as a feedback of substrate availability, mineral protection, and microbial population size (Woolf and Lehmann, 2019) and was able to reproduce the C dynamics of 22 long-term agricultural experiments without the need for assuming an inherently stable C pool in SOM. However, the high amounts of necromass in SOM are currently not really considered as a resource of substrates and building blocks, although recent model developments (Manzoni et al., 2021) do at least consider recycling of C and N within the microbial food web.
For a thorough understanding and predictive modelling of SOM formation and turnover processes, the energy fluxes and the microbial growth and decay cycle need to be applied in modelling soil C storage processes. Models reflecting the microbial growth, decay, and necromass contribution to SOM as well as redox cycling may show promise for improving our knowledge and ability to predict effects of global changes on SOM in soils and may finally enable us to assess the determinants of the C storage capacities of different soils.
MK: corresponding author, writing and conceptualisation of the manuscript AM: writing and conceptualization of the manuscript STB: writing of the manuscript CL: writing of the manuscript.
We acknowledge financial support from the Helmholtz-Centre for Environmental Research UFZ, from the University of Trier, the German Research Foundation (SPP2322), and the National Natural Science Foundation of China (No. 31930070) as well as the Humboldt Fellowship to CL by the Alexander von Humboldt Foundation of Germany.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achtenhagen, J. (2015). Dynamic (Redox) Interfaces in Soil - Carbon Turnover of Microbial Residues and Their Impact on Soil Properties. PhD. Thesis. Munich: Technical University of Munich.
Allison, S. D. (2005). Cheaters, Diffusion and Nutrients Constrain Decomposition by Microbial Enzymes in Spatially Structured Environments. Ecol. Lett. 8, 626–635. doi:10.1111/j.1461-0248.2005.00756.x
Amelung, W. (2001). “Methods Using Amino Sugars as Markers for Microbial Residues in Soil,” in Assessment Methods for Soil Carbon. Editors R. Lal, J. M. Kimble, R. F. Follett, and B. A. Stewart (Boca Raton, FL: CRC/Lewis Publishers), 233–270.
Angst, G., Messinger, J., Greiner, M., Häusler, W., Hertel, D., Kirfel, K., et al. (2018). Soil Organic Carbon Stocks in Topsoil and Subsoil Controlled by Parent Material, Carbon Input in the Rhizosphere, and Microbial-Derived Compounds. Soil Biol. Biochem. 122, 19–30. doi:10.1016/j.soilbio.2018.03.026
Angst, G., Mueller, K. E., Nierop, K. G. J., and Simpson, M. J. (2021). Plant- or Microbial-Derived? A Review on the Molecular Composition of Stabilized Soil Organic Matter. Soil Biol. Biochem. 156, 108189. doi:10.1016/j.soilbio.2021.108189
Anthony, M. A., Crowther, T. W., Maynard, D. S., van den Hoogen, J., and Averill, C. (2020). Distinct Assembly Processes and Microbial Communities Constrain Soil Organic Carbon Formation. One Earth 2, 349–360. doi:10.1016/j.oneear.2020.03.006
Apostel, C., Herschbach, J., Bore, E. K., Spielvogel, S., Kuzyakov, Y., and Dippold, M. A. (2018). Food for Microorganisms: Position-specific 13 C Labeling and 13 C-PLFA Analysis Reveals Preferences for Sorbed or Necromass C. Geoderma 312, 86–94. doi:10.1016/j.geoderma.2017.09.042
Appuhn, A., and Joergensen, R. (2006). Microbial Colonisation of Roots as a Function of Plant Species. Soil Biol. Biochem. 38 (5), 1040–1051. doi:10.1016/j.soilbio.2005.09.002
Babin, D., Ding, G.-C., Pronk, G. J., Heister, K., Kögel-Knabner, I., and Smalla, K. (2013). Metal Oxides, clay Minerals and Charcoal Determine the Composition of Microbial Communities in Matured Artificial Soils and Their Response to Phenanthrene. FEMS Microbiol. Ecol. 86, 3–14. doi:10.1111/1574-6941.12058
Balesdent, J., Chenu, C., and Balabane, M. (2000). Relationship of Soil Organic Matter Dynamics to Physical protection and Tillage. Soil Tillage Res. 53, 215–230. doi:10.1016/s0167-1987(99)00107-5
Barré, P., Quénéa, K., Vidal, A., Cécillon, L., Christensen, B. T., Kätterer, T., et al. (2018). Microbial and Plant-Derived Compounds Both Contribute to Persistent Soil Organic Carbon in Temperate Soils. Biogeochemistry 140, 81–92. doi:10.1007/s10533-018-0475-5
Barros, N., and Feijóo, S. (2003). A Combined Mass and Energy Balance to Provide Bioindicators of Soil Microbiological Quality. Biophysical Chem. 104 (3), 561–572. doi:10.1016/S0301-4622(03)00059-0
Barros, N., Hansen, L. D., Piñeiro, V., Pérez-Cruzado, C., Villanueva, M., Proupín, J., et al. (2016). Factors Influencing the Calorespirometric Ratios of Soil Microbial Metabolism. Soil Biol. Biochem. 92, 221–229. doi:10.1016/j.soilbio.2015.10.007
Barros, N., Salgado, J., and Feijóo, S. (2007). Calorimetry and Soil. Thermochim. Acta 458, 11–17. doi:10.1016/j.tca.2007.01.010
Barros, N. (2021). Thermodynamics of Soil Microbial Metabolism: Applications and Functions. Appl. Sci. 11, 4962. doi:10.3390/app11114962
Basile-Doelsch, I., Balesdent, J., and Pellerin, S. (2020). Reviews and Syntheses: The Mechanisms Underlying Carbon Storage in Soil. Biogeosciences 17, 5223–5242. doi:10.5194/bg-2020-4910.5194/bg-17-5223-2020
Baveye, P. C., Baveye, J., and Gowdy, J. (2016). Soil “Ecosystem” Services and Natural Capital: Critical Appraisal of Research on Uncertain Ground. Front. Environ. Sci. 4, 41. doi:10.3389/fenvs.2016.00041
Biselli, E., Schink, S. J., and Gerland, U. (2020). Slower Growth of Escherichia coli Leads to Longer Survival in Carbon Starvation Due to a Decrease in the Maintenance Rate. Mol. Syst. Biol. 16, e9478. doi:10.15252/msb.20209478
Bohlen, P. J., Pelletier, D. M., Groffman, P. M., Fahey, T. J., and Fisk, M. C. (2004). Influence of Earthworm Invasion on Redistribution and Retention of Soil Carbon and Nitrogen in Northern Temperate Forests. Ecosystems 7, 13–27. doi:10.1007/s10021-003-0127-y
Bollag, J. M., and Liu, S. Y. (1990). “Biological Transformation Processes of Pesticides,” in Pesticides in the Soil Environment: Processes, Impacts and Modeling. Editor H. H. Cheng (Madison, WI, USA: Soil Science Society of America), 169–211.
Bore, E. K., Kuzyakov, Y., and Dippold, M. A. (2019). Glucose and Ribose Stabilization in Soil: Convergence and Divergence of Carbon Pathways Assessed by Position-specific Labeling. Soil Biol. Biochem. 131, 54–61. doi:10.1016/j.soilbio.2018.12.027
Bosatta, E., and Ågren, G. I. (1999). Soil Organic Matter Quality Interpreted Thermodynamically. Soil Biol. Biochem. 31, 1889–1891. doi:10.1016/s0038-0717(99)00105-4
Boye, K., Herrmann, A. M., Schaefer, M. V., Tfaily, M. M., and Fendorf, S. (2018). Discerning Microbially Mediated Processes during Redox Transitions in Flooded Soils Using Carbon and Energy Balances. Front. Environ. Sci. 6, e15. doi:10.3389/fenvs.2018.00015
Bresinski, A., Körner, C., Kadereit, J. W., Neuhaus, G., and Sonnewald, U. (2008). Strasburger - Lehrbuch der Botanik. Springer Spektrum.
Bridgewater, L. (2012). Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association.
Brock, A. L., Kästner, M., and Trapp, S. (2017). Microbial Growth Yield Estimates from Thermodynamics and its Importance for Degradation of Pesticides and Formation of Biogenic Non-extractable Residues. SAR QSAR Environ. Res. 28 (8), 629–650. doi:10.1080/1062936X.2017.1365762
Brock, A. L., Rein, A., Polesel, F., Nowak, K. M., Kästner, M., and Trapp, S. (2019). Microbial Turnover of Glyphosate to Biomass: Utilization as Nutrient Source and Formation of AMPA and Biogenic NER in an OECD 308 Test. Environ. Sci. Technol. 53, 5838–5847. doi:10.1021/acs.est.9b01259
Buckeridge, K. M., La Rosa, A. F., Mason, K. E., Whitaker, J., McNamara, N. P., Grant, H. K., et al. (2020). Sticky Dead Microbes: Rapid Abiotic Retention of Microbial Necromass in Soil. Soil Biol. Biochem. 149, 107929–107933. doi:10.1016/j.soilbio.2020.107929
Capek, P., Choma, M., Tahovská, K., Kaňa, J., Kopáček, J., and Šantrůčková, H. (2021). Coupling the Resource Stoichiometry and Microbial Biomass Turnover to Predict Nutrient Mineralization and Immobilization in Soil. Geoderma 385, 114884. doi:10.1016/j.geoderma.2020.114884
Cates, A. M., Braus, M. J., Whitman, T. L., and Jackson, R. D. (2019). Separate Drivers for Microbial Carbon Mineralization and Physical protection of Carbon. Soil Biol. Biochem. 133, 72–82. doi:10.1016/j.soilbio.2019.02.014
Chakrawal, A., Herrmann, A. M., Šantrůčková, H., and Manzoni, S. (2020). Quantifying Microbial Metabolism in Soils Using Calorespirometry - A Bioenergetics Perspective. Soil Biol. Biochem. 148, 107945–107966. doi:10.1016/j.soilbio.2020.107945
Chen, X., Xi, Y., Rui, Y., Ning, Z., Hu, Y., Tang, H., et al. (2020). Microbial Carbon Use Efficiency, Biomass Turnover, and Necromass Accumulation in Paddy Soil Depending on Fertilization. Agri. Eco. Environ. 292, 106816. doi:10.1016/j.agee.2020.106816
Christensen, B. T. (2001). Physical Fractionation of Soil and Structural and Functional Complexity in Organic Matter Turnover. Eur. J. Soil Sci. 52, 345–353. doi:10.1046/j.1365-2389.2001.00417.x
Christensen, D. R., and McCarthy, P. L. (1975). Multi-Process Biological Treatment Model. J. Water Pollut. Control. Fed. 47, 2652–2664.
Churchman, G. J., Singh, M., Schapel, A., Sarkar, B., and Bolan, N. (2020). Clay Minerals as the Key to the Sequestration of Carbon in Soils. Clays Clay Miner. 68, 135–143. doi:10.1007/s42860-020-00071-z
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K., and Paul, E. (2013). The Microbial Efficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter?. Glob. Change Biol. 19 (4), 988–995. doi:10.1111/gcb.12113
Craig, M. E., Turner, B. L., Liang, C., Clay, K., Johnson, D. J., and Phillips, R. P. (2018). Tree Mycorrhizal Type Predicts Within‐site Variability in the Storage and Distribution of Soil Organic Matter. Glob. Change Biol. 24, 3317–3330. doi:10.1111/gcb.14132
Craine, J. M., Morrow, C., and Fierer, N. (2007). Microbial Nitrogen Limitation Increases Decomposition. Ecology 88, 2105–2113. doi:10.1890/06-1847.1
Creamer, C. A., Foster, A. L., Lawrence, C., McFarland, J., Schulz, M., and Waldrop, M. P. (2019). Mineralogy Dictates the Initial Mechanism of Microbial Necromass Association. Geochimica et Cosmochimica Acta 260, 161–176. doi:10.1016/j.gca.2019.06.028
Currie, W. S. (2003). Relationships between Carbon Turnover and Bioavailable Energy Fluxes in Two Temperate forest Soils. Glob. Change Biol. 9 (6), 919–929. doi:10.1046/j.1365-2486.2003.00637.x
Cyle, K. T., Klein, A. R., Aristilde, L., and Martínez, C. E. (2020). Ecophysiological Study of Paraburkholderia Sp. Strain 1N under Soil Solution Conditions: Dynamic Substrate Preferences and Characterization of Carbon Use Efficiency. Appl. Environ. Microbiol. 86, e01851. doi:10.1128/AEM.01851-20
del Giorgio, P. A., and Cole, J. J. (1998). Bacterial Growth Efficiency in Natural Aquatic Systems. Annu. Rev. Ecol. Syst. 29, 503–541. doi:10.1146/annurev.ecolsys.29.1.503
Di Lonardo, D. P., De Boer, W., Klein Gunnewiek, P. J. A., Hannula, S. E., and Van der Wal, A. (2017). Priming of Soil Organic Matter: Chemical Structure of Added Compounds Is More Important Than the Energy Content. Soil Biol. Biochem. 108, 41–54. doi:10.1016/j.soilbio.2017.01.017
Doetterl, S., Stevens, A., Six, J., Merckx, R., Van Oost, K., Casanova Pinto, M., et al. (2015). Soil Carbon Storage Controlled by Interactions between Geochemistry and Climate. Nat. Geosci 8 (10), 780–783. doi:10.1038/ngeo2516
Dong, W., Song, A., Yin, H., Liu, X., Li, J., and Fan, F. (2021). Decomposition of Microbial Necromass Is Divergent at the Individual Taxonomic Level in Soil. Front. Microbiol. 12, 679793. doi:10.3389/fmicb.2021.679793
Ekschmitt, K., Kandeler, E., Poll, C., Brune, A., Buscot, F., Friedrich, M., et al. (2008). Soil-carbon Preservation through Habitat Constraints and Biological Limitations on Decomposer Activity. J. Plant Nutr. Soil Sci. 171, 27–35. doi:10.1002/jpln.200700051
Enggrob, K. L., Larsen, T., Peixoto, L., and Rasmussen, J. (2020). Gram-positive Bacteria Control the Rapid Anabolism of Protein-Sized Soil Organic Nitrogen Compounds Questioning the Present Paradigm. Sci. Rep. 10, 15840. doi:10.1038/s41598-020-72696-y
Eusterhues, K., Rumpel, C., Kleber, M., and Kögel-Knabner, I. (2003). Stabilisation of Soil Organic Matter by Interactions with Minerals as Revealed by mineral Dissolution and Oxidative Degradation. Org. Geochem. 34, 1591–1600. doi:10.1016/j.orggeochem.2003.08.007
Fan, Z., and Liang, C. (2015). Significance of Microbial Asynchronous Anabolism to Soil Carbon Dynamics Driven by Litter Inputs. Sci. Rep. 5, 9575. doi:10.1038/srep09575
Feng, Y., and Boyd, S. A. (2008). “Bioavailability of Soil-Sorbed Pesticides and Organic Contaminants,” in Soil Mineral Microbe-Organic Interactions: Theories and Applications.. Editors P. M. H. Qiaoyun Huang, and A Violante (Springer), 259–279.
Fester, T., Giebler, J., Wick, L. Y., Schlosser, D., and Kästner, M. (2014). Plant-microbe Interactions as Drivers of Ecosystem Functions Relevant for the Biodegradation of Organic Contaminants. Curr. Opin. Biotechnol. 27, 168–175. doi:10.1016/j.copbio.2014.01.017
Fierer, N. (2017). Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 15 (10), 579–590. doi:10.1038/nrmicro.2017.87
Galicia-Andres, E., Escalona, Y., Oostenbrink, C., Tunega, D., and Gerzabek, M. H. (2021). Soil Organic Matter Stabilization at Molecular Scale: The Role of Metal Cations and Hydrogen Bonds. Geoderma 401, 12. doi:10.1016/j.geoderma.2021.115237
Gao, J., Mikutta, R., Jansen, B., Guggenberger, G., Vogel, C., and Kalbitz, K. (2019). The Multilayer Model of Soil mineral-organic Interfaces-A Review. J. Plant Nutr. Soil Sci. 183, 27–41. doi:10.1002/jpln.201900530
Gavrilescu, M. (2005). Fate of Pesticides in the Environment and its Bioremediation. Eng. Life Sci. 5 (6), 497–526. doi:10.1002/elsc.200520098
Gerrit, A., Mueller, K. E., Nierop, K. G. J., and Simpson, M. J. (2021). Plant- or Microbial-Derived? A Review on the Molecular Composition of Stabilized Soil Organic Matter. Soil Biol. Biochem. 156, 108189–108201. doi:10.1016/j.soilbio.2021.108189
Gessner, M. O., Swan, C. M., Dang, C. K., McKie, B. G., Bardgett, R. D., Wall, D. H., et al. (2010). Diversity Meets Decomposition. Trends Ecol. Evol. 25, 372–380. doi:10.1016/j.tree.2010.01.010
Geyer, K. M., Dijkstra, P., Sinsabaugh, R., and Frey, S. D. (2019). Clarifying the Interpretation of Carbon Use Efficiency in Soil through Methods Comparison. Soil Biol. Biochem. 128, 79–88. doi:10.1016/j.soilbio.2018.09.036
Geyer, K. M., ,, K., Grandy, A. S., Frey, S. D., and Frey, S. D. (2016). Microbial Carbon Use Efficiency: Accounting for Population, Community, and Ecosystem-Scale Controls over the Fate of Metabolized Organic Matter. Biogeochemistry 127, 173–188. doi:10.1007/s10533-016-0191-y
Godbold, D. L., Hoosbeek, M. R., Lukac, M., Cotrufo, M. F., Janssens, I. A., Ceulemans, R., et al. (2006). Mycorrhizal Hyphal Turnover as a Dominant Process for Carbon Input into Soil Organic Matter. Plant Soil 281, 15–24. doi:10.1007/s11104-005-3701-6
Guggenberger, G., Frey, S. D., Six, J., Paustian, K., and Elliott, E. T. (1999). Bacterial and Fungal Cell‐Wall Residues in Conventional and No‐Tillage Agroecosystems. Soil Sci. Soc. Am. J. 63 (5), 1188–1198. doi:10.2136/sssaj1999.6351188x
Guo, L. B., and Gifford, R. M. (2002). Soil Carbon Stocks and Land Use Change: a Meta Analysis. Glob. Change Biol. 8, 345–360. doi:10.1046/j.1354-1013.2002.00486.x
Haddix, M. L., Gregorich, E. G., Helgason, B. L., Janzen, H., Ellert, B. H., and Francesca Cotrufo, M. (2020). Climate, Carbon Content, and Soil Texture Control the Independent Formation and Persistence of Particulate and mineral-associated Organic Matter in Soil. Geoderma 363, 114160. doi:10.1016/j.geoderma.2019.114160
Harris, J. A., Ritz, K., Coucheney, E., Grice, S. M., Lerch, T. Z., Pawlett, M., et al. (2012). The Thermodynamic Efficiency of Soil Microbial Communities Subject to Long-Term Stress Is Lower Than Those under Conventional Input Regimes. Soil Biol. Biochem. 47, 149–157. doi:10.1016/j.soilbio.2011.12.017
Herrmann, A. M., Coucheney, E., and Nunan, N. (2014). Isothermal Microcalorimetry Provides New Insight into Terrestrial Carbon Cycling. Environ. Sci. Technol. 48 (8), 4344–4352. doi:10.1021/es403941h
Huang, X., Tang, H., Kang, W., Yu, G., Ran, W., Hong, J., et al. (2018). Redox Interface-Associated Organo-mineral Interactions: A Mechanism for C Sequestration under a rice-wheat Cropping System. Soil Biol. Biochem. 120, 12–23. doi:10.1016/j.soilbio.2018.01.031
Janzen, H. H. (2015). Beyond Carbon Sequestration: Soil as Conduit of Solar Energy. Eur. J. Soil Sci. 66 (1), 19–32. doi:10.1111/ejss.12194
Jiao, N., Herndl, G. J., Hansell, D. A., Benner, R., Kattner, G., Wilhelm, S. W., et al. (2010). Microbial Production of Recalcitrant Dissolved Organic Matter: Long-Term Carbon Storage in the Global Ocean. Nat. Rev. Microbiol. 8, 593–599. doi:10.1038/nrmicro2386
Jilling, A., Keiluweit, M., Contosta, A. R., Frey, S., Schimel, J., Schnecker, J., et al. (2018). Minerals in the Rhizosphere: Overlooked Mediators of Soil Nitrogen Availability to Plants and Microbes. Biogeochemistry 139, 103–122. doi:10.1007/s10533-018-0459-5
Jin, Q., and Bethke, C. M. (2007). The Thermodynamics and Kinetics of Microbial Metabolism. Am. J. Sci. 307, 643–677. doi:10.2475/04.2007.01
Kaiser, C., Franklin, O., Dieckmann, U., and Richter, A. (2014). Microbial Community Dynamics Alleviate Stoichiometric Constraints during Litter Decay. Ecol. Lett. 17, 680–690. doi:10.1111/ele.12269
Kaiser, C., Franklin, O., Richter, A., and Dieckmann, U. (2015). Social Dynamics within Decomposer Communities lead to Nitrogen Retention and Organic Matter Build-Up in Soils. Nat. Commun. 6, 8960. doi:10.1038/ncomms9960
Kalbitz, K., Kaiser, K., Fiedler, S., Kölbl, A., Amelung, W., Bräuer, T., et al. (2013). The Carbon Count of 2000 Years of rice Cultivation. Glob. Change Biol. 19, 1107–1113. doi:10.1111/gcb.12080
Kallenbach, C. M., Frey, S. D., and Grandy, A. S. (2016). Direct Evidence for Microbial-Derived Soil Organic Matter Formation and its Ecophysiological Controls. Nat. Commun. 7, 13630. doi:10.1038/ncomms13630
Kallenbach, C. M., Wallenstein, M. D., Schipanksi, M. E., and Grandy, A. S. (2019). Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and a Path Forward. Front. Microbiol. 10, 1146. doi:10.3389/fmicb.2019.01146
Kappler, A., Schink, B., and Newman, D. K. (2005). Fe(III) mineral Formation and Cell Encrustation by the Nitrate-dependent Fe(II)-oxidizer Strain BoFeN1. Geobiology 3 (4), 235–245. doi:10.1111/j.1472-4669.2006.00056.x
Kästner, M., and Miltner, A. (2018). “SOM and Microbes-What Is Left from Microbial Life,” in The Future of Soil Carbon – its Conservation and Formation. Editors C. Garcia, P. Nannipieri, and T. Hernandez (Elsevier Academic Press.), 125–163. doi:10.1016/b978-0-12-811687-6.00005-5
Keiluweit, M., Wanzek, T., Kleber, M., Nico, P., and Fendorf, S. (2017). Anaerobic Microsites Have an Unaccounted Role in Soil Carbon Stabilization. Nat. Commun. 8, 1771. doi:10.1038/s41467-017-01406-6
Kindler, R., Miltner, A., Richnow, H., and Kästner, M. (2006). Fate of Gram-Negative Bacterial Biomass in Soil-Mineralization and Contribution to SOM. Soil Biol. Biochem. 38 (9), 2860–2870. doi:10.1016/j.soilbio.2006.04.047
Kindler, R., Miltner, A., Thullner, M., Richnow, H.-H., and Kästner, M. (2009). Fate of Bacterial Biomass Derived Fatty Acids in Soil and Their Contribution to Soil Organic Matter. Org. Geochem. 40, 29–37. doi:10.1016/j.orggeochem.2008.09.005
Kleber, M., Bourg, I. C., Coward, E. K., Hansel, C. M., Myneni, S. C. B., and Nunan, N. (2021). Dynamic Interactions at the mineral-organic Matter Interface. Nat. Rev. Earth Environ. 2, 402–421. doi:10.1038/s43017-021-00162-y
Kleber, M., Eusterhues, K., Keiluweit, M., Mikutta, C., Mikutta, R., and Nico, P. S. (2015). “Mineral-organic Associations: Formation, Properties, and Relevance in Soil Environments,” in Advances in Agronomy, 1–140. doi:10.1016/bs.agron.2014.10.005
Kleerebezem, R., and Van Loosdrecht, M. C. M. (2010). A Generalized Method for Thermodynamic State Analysis of Environmental Systems. Crit. Rev. Environ. Sci. Techn. 40 (1), 1–54. doi:10.1080/10643380802000974
Knicker, H. (2011). Soil Organic N - an Under-rated Player for C Sequestration in Soils? Soil Biol. Biochem. 43, 1118–1129. doi:10.1016/soilbio.2011.02.020
Kögel-Knabner, I. (2002). The Macromolecular Organic Composition of Plant and Microbial Residues as Inputs to Soil Organic Matter. Soil Biol. Biochem. 34, 139–162. doi:10.1016/s0038-0717(01)00158-4
Kögel-Knabner, I. (2017). The Macromolecular Organic Composition of Plant and Microbial Residues as Inputs to Soil Organic Matter: Fourteen Years on. Soil Biol. Biochem. 105, A3–A8. doi:10.1016/j.soilbio.2016.08.011
Kovárová-Kovar, K., and Egli, T. (1998). Growth Kinetics of Suspended Microbial Cells: From Single-Substrate-Controlled Growth to Mixed-Substrate Kinetics. Microbiol. Mol. Biol. Rev. 62, 646–666. doi:10.1128/MMBR.62.3.646-666.1998
Kuzyakov, Y., and Mason-Jones, K. (2018). Viruses in Soil: Nano-Scale Undead Drivers of Microbial Life, Biogeochemical Turnover and Ecosystem Functions. Soil Biol. Biochem. 127, 305–317. doi:10.1016/j.soilbio.2018.09.032
Kuzyakov, Y. (2010). Priming Effects: Interactions between Living and Dead Organic Matter. Soil Biol. Biochem. 42, 1363–1371. doi:10.1016/j.soilbio.2010.04.003
Kyker-Snowman, E., Wieder, W. R., Frey, S. D., and Grandy, A. S. (2020). Stoichiometrically Coupled Carbon and Nitrogen Cycling in the MIcrobial-MIneral Carbon Stabilization Model Version 1.0 (MIMICS-CN v1.0). Geosci. Model. Dev. 13, 4413–4434. doi:10.5194/gmd-13-4413-2020
LaRowe, D. E., and van Cappellen, P. (2011). Degradation of Natural Organic Matter: A Thermodynamic Analysis. Geochimica et Cosmochimica Acta 75, 2030–2042. doi:10.1016/j.gca.2011.01.020
Lehmann, J., Hansel, C. M., Kaiser, C., Kleber, M., Maher, K., Manzoni, S., et al. (2020). Persistence of Soil Organic Carbon Caused by Functional Complexity. Nat. Geosci. 13, 529–534. doi:10.1038/s41561-020-0612-3
Lehmann, J., and Kleber, M. (2015a). The Contentious Nature of Soil Organic Matter. Nature 528 (7580), 60–68. doi:10.1038/nature16069
Lehmann, J., and Kleber, M. (2015b). The Contentious Nature of Soil Organic Matter. Nature 528, 60–68. doi:10.1038/nature16069
Lehmann, J., Solomon, D., Kinyangi, J., Dathe, L., Wirick, S., and Jacobsen, C. (2008). Spatial Complexity of Soil Organic Matter Forms at Nanometre Scales. Nat. Geosci 1 (4), 238–242. doi:10.1038/ngeo155
Lengler, J. W., Drews, G., and Schlegel, H. G. (1999). Biology of the Prokaryotes. Blackwell Science: Thieme Verlag.
Li, H., Su, J.-Q., Yang, X.-R., Zhou, G.-W., Lassen, S. B., and Zhu, Y.-G. (2019a). RNA Stable Isotope Probing of Potential Feammox Population in Paddy Soil. Environ. Sci. Technol. 53, 4841–4849. doi:10.1021/acs.est.8b05016
Li, J., Wang, G., Mayes, M. A., Allison, S. D., Frey, S. D., Shi, Z., et al. (2019b). Reduced Carbon Use Efficiency and Increased Microbial Turnover with Soil Warming. Glob. Change Biol. 25, 900–910. doi:10.1111/gcb.14517
Liang, C., Amelung, W., Lehmann, J., and Kästner, M. (2019). Quantitative Assessment of Microbial Necromass Contribution to Soil Organic Matter. Glob. Change Biol. 25, 3578–3590. doi:10.1111/gcb.14781
Liang, C., Kästner, M., and Joergensen, R. G. (2020). Microbial Necromass on the Rise: The Growing Focus on its Role in Soil Organic Matter Development. Soil Biol. Biochem. 150, 108000. doi:10.1016/j.soilbio.2020.108000
Liang, C., Schimel, J. P., and Jastrow, J. D. (2017). The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2, 17105. doi:10.1038/nmicrobiol.2017.105
Liang, C. (2020). Soil Microbial Carbon Pump: Mechanism and Appraisal. Soil Ecol. Lett. 2, 241–254. doi:10.1007/s42832-020-0052-4
Liu, S., Wang, J., Pu, S., Blagodatskaya, E., Kuzyakov, Y., and Razavi, B. S. (2020). Impact of Manure on Soil Biochemical Properties: A Global Synthesis. Sci. Total Environ. 745, 141003. doi:10.1016/j.scitotenv.2020.141003
Liu, X., Hu, G., He, H., Liang, C., Zhang, W., Bai, Z., et al. (2016). Linking Microbial Immobilization of Fertilizer Nitrogen to In Situ Turnover of Soil Microbial Residues in an Agro-Ecosystem. Agric. Ecosyst. Environ. 229, 40–47. doi:10.1016/j.agee.2016.05.019
Loeppmann, S., Breidenbach, A., Spielvogel, S., Dippold, M. A., and Blagodatskaya, E. (2020). Organic Nutrients Induced Coupled C- and P-Cycling Enzyme Activities during Microbial Growth in Forest Soils. Front. For. Glob. Change 3, 100. doi:10.3389/ffgc.2020.00100
Ludwig, M., Achtenhagen, J., Miltner, A., Eckhardt, K.-U., Leinweber, P., Emmerling, C., et al. (2015). Microbial Contribution to SOM Quantity and Quality in Density Fractions of Temperate Arable Soils. Soil Biol. Biochem. 81, 311–322. doi:10.1016/j.soilbio.2014.12.002
Lueders, T., Kindler, R., Miltner, A., Friedrich, M. W., and Kaestner, M. (2006). Identification of Bacterial Micropredators Distinctively Active in a Soil Microbial Food Web. Appl. Environ. Microbiol. 72, 5342–5348. doi:10.1128/aem.00400-06
Lueders, T., Pommerenke, B., and Friedrich, M. W. (2004). Stable-Isotope Probing of Microorganisms Thriving at Thermodynamic Limits: Syntrophic Propionate Oxidation in Flooded Soil. Appl. Environ. Microbiol. 70, 5778–5786. doi:10.1128/aem.70.10.5778-5786.2004
Lünsdorf, H., Erb, R. W., Abraham, W.-R., and Timmis, K. N. (2000). ‘Clay Hutches’: a Novel Interaction between Bacteria and clay Minerals. Environ. Microbiol. 2 (2), 161–168. doi:10.1046/j.1462-2920.2000.00086.x
Lützow, M. v., Kögel-Knabner, I., Ekschmitt, K., Matzner, E., Guggenberger, G., Marschner, B., et al. (2006). Stabilization of Organic Matter in Temperate Soils: Mechanisms and Their Relevance under Different Soil Conditions - a Review. Eur. J. Soil Sci. 57, 426–445. doi:10.1111/j.1365-2389.2006.00809.x
Ma, T., Zhu, S., Wang, Z., Chen, D., Dai, G., Feng, B., et al. (2018). Divergent Accumulation of Microbial Necromass and Plant Lignin Components in Grassland Soils. Nat. Commun. 9, 3480. doi:10.1038/s41467-018-05891-1
Madigan, M. T., Martinko, J. M., Bender, K. S., Buckley, D. H., and Stahl, D. A. (2014). Brock Biology of Microorganisms. 14th Edn Boston, USA: Pearson Inc.
Malik, A. A., Puissant, J., Goodall, T., Allison, S. D., and Griffiths, R. I. (2019). Soil Microbial Communities with Greater Investment in Resource Acquisition Have Lower Growth Yield. Soil Biol. Biochem. 132, 36–39. doi:10.1016/j.soilbio.2019.01.025
Manzoni, S., Čapek, P., Mooshammer, M., Lindahl, B. D., Richter, A., and Šantrůčková, H. (2017). Optimal Metabolic Regulation along Resource Stoichiometry Gradients. Ecol. Lett. 20, 1182–1191. doi:10.1111/ele.12815
Manzoni, S., Čapek, P., Porada, P., Thurner, M., Winterdahl, M., Beer, C., et al. (2018). Reviews and Syntheses: Carbon Use Efficiency from Organisms to Ecosystems - Definitions, Theories, and Empirical Evidence. Biogeosciences 15 (19), 5929–5949. doi:10.5194/bg-15-5929-2018
Manzoni, S., Chakrawal, A., Spohn, M., and Lindahl, B. D. (2021). Modeling Microbial Adaptations to Nutrient Limitation during Litter Decomposition. Front. For. Glob. Change 4, 686945. doi:10.3389/ffgc.2021.686945
Manzoni, S., Jackson, R. B., Trofymow, J. A., and Porporato, A. (2008). The Global Stoichiometry of Litter Nitrogen Mineralization. Science 321, 684–686. doi:10.1126/science.1159792
Manzoni, S., Taylor, P., Richter, A., Porporato, A., and Ågren, G. I. (2012). Environmental and Stoichiometric Controls on Microbial Carbon‐use Efficiency in Soils. New Phytol. 196, 79–91. doi:10.1111/j.1469-8137.2012.04225.x
Mason-Jones, K., Schmücker, N., and Kuzyakov, Y. (2018). Contrasting Effects of Organic and mineral Nitrogen challenge the N-Mining Hypothesis for Soil Organic Matter Priming. Soil Biol. Biochem. 124, 38–46. doi:10.1016/j.soilbio.2018.05.024
Masoom, H., Courtier-Murias, D., Farooq, H., Soong, R., Kelleher, B. P., Zhang, C., et al. (2016). Soil Organic Matter in its Native State: Unravelling the Most Complex Biomaterial on Earth. Environ. Sci. Technol. 50, 1670–1680. doi:10.1021/acs.est.5b03410
McCarty, P. L. (2007). Thermodynamic Electron Equivalents Model for Bacterial Yield Prediction: Modifications and Comparative Evaluations. Biotechnol. Bioeng. 97, 377–388. doi:10.1002/bit.21250
Mikutta, R., Mikutta, C., Kalbitz, K., Scheel, T., Kaiser, K., and Jahn, R. (2007). Biodegradation of forest Floor Organic Matter Bound to Minerals via Different Binding Mechanisms. Geochimica et Cosmochimica Acta 71 (10), 2569–2590. doi:10.1016/j.gca.2007.03.002
Mikutta, R., Schaumann, G. E., Gildemeister, D., Bonneville, S., Kramer, M. G., Chorover, J., et al. (2009). Biogeochemistry of mineral-organic Associations across a Long-Term Mineralogical Soil Gradient (0.3-4100kyr), Hawaiian Islands. Geochimica et Cosmochimica Acta 73, 2034–2060. doi:10.1016/j.gca.2008.12.028
Mikutta, R., Turner, S., Schippers, A., Gentsch, N., Meyer-Stüve, S., Condron, L. M., et al. (2019). Microbial and Abiotic Controls on mineral-associated Organic Matter in Soil Profiles along an Ecosystem Gradient. Sci. Rep. 9, 10294. doi:10.1038/s41598-019-46501-4
Mikutta, R., Zang, U., Chorover, J., Haumaier, L., and Kalbitz, K. (2011). Stabilization of Extracellular Polymeric Substances (Bacillus Subtilis) by Adsorption to and Coprecipitation with Al Forms. Geochimica et Cosmochimica Acta 75, 3135–3154. doi:10.1016/j.gca.2011.03.006
Miltner, A., Bombach, P., Schmidt-Brücken, B., and Kästner, M. (2012). SOM Genesis: Microbial Biomass as a Significant Source. Biogeochemistry 111, 41–55. doi:10.1007/s10533-011-9658-z
Miltner, A., Kindler, R., Knicker, H., Richnow, H.-H., and Kästner, M. (2009). Fate of Microbial Biomass-Derived Amino Acids in Soil and Their Contribution to Soil Organic Matter. Org. Geochem. 40 (9), 978–985. doi:10.1016/j.orggeochem.2009.06.008
Mooshammer, M., Wanek, W., Zechmeister-Boltenstern, S., and Richter, A. (2014). Stoichiometric Imbalances between Terrestrial Decomposer Communities and Their Resources: Mechanisms and Implications of Microbial Adaptations to Their Resources. Front. Microbiol. 5, 22–10. doi:10.3389/fmicb.2014.00022
Mouvenchery, Y. K., Miltner, A., Schurig, C., Kästner, M., and Schaumann, G. E. (2016). Linking Atomic Force Microscopy with Nanothermal Analysis to Assess Microspatial Distribution of Material Characteristics in Young Soils. J. Plant Nutr. Soil Sci. 179, 48–59. doi:10.1002/jpln.201500082
Moyano, F. E., Manzoni, S., and Chenu, C. (2013). Responses of Soil Heterotrophic Respiration to Moisture Availability: An Exploration of Processes and Models. Soil Biol. Biochem. 59, 72–85. doi:10.1016/j.soilbio.2013.01.002
Ni, X., Liao, S., Tan, S., Peng, Y., Wang, D., Yue, K., et al. (2020). The Vertical Distribution and Control of Microbial Necromass Carbon in forest Soils. Glob. Ecol. Biogeogr. 00, 1–11. doi:10.1111/geb.13159
Nunan, N., Lerch, T. Z., Pouteau, V., Mora, P., Changey, F., Kätterer, T., et al. (2015). Metabolising Old Soil Carbon: Simply a Matter of Simple Organic Matter? Soil Biol. Biochem. 88, 128–136. doi:10.1016/j.soilbio.2015.05.018
Odum, H. T., and Pinkerton, R. C. (1955). Time's Speed Regulator: The Optimum Efficiency for Maximum Power Output in Physical and Biological Systems. Am. Scientist 43 (2), 331–343.
Omoike, A., and Chorover, J. (2006). Adsorption to Goethite of Extracellular Polymeric Substances from Bacillus Subtilis. Geochimica et Cosmochimica Acta 70, 827–838. doi:10.1016/j.gca.2005.10.012
Paul, E. A. (2016). The Nature and Dynamics of Soil Organic Matter: Plant Inputs, Microbial Transformations, and Organic Matter Stabilization. Soil Biol. Biochem. 98, 109–126. doi:10.1016/j.soilbio.2016.04.001
Plaza, C., Courtier-Murias, D., Fernández, J. M., Polo, A., and Simpson, A. J. (2013). Physical, Chemical, and Biochemical Mechanisms of Soil Organic Matter Stabilization under Conservation Tillage Systems: A central Role for Microbes and Microbial By-Products in C Sequestration. Soil Biol. Biochem. 57, 124–134. doi:10.1016/j.soilbio.2012.07.026
Posth, N. R., Canfield, D. E., and Kappler, A. (2014). Biogenic Fe(III) Minerals: From Formation to Diagenesis and Preservation in the Rock Record. Earth-Science Rev. 135, 103–121. doi:10.1016/j.earscirev.2014.03.012
Pronk, G. J., Heister, K., and Kögel-Knabner, I. (2013). Is Turnover and Development of Organic Matter Controlled by mineral Composition?. Soil Biol. Biochem. 67, 235–244. doi:10.1016/j.soilbio.2013.09.006
Pronk, G. J., Heister, K., Vogel, C., Babin, D., Bachmann, J., Ding, G.-C., et al. (2017). Interaction of Minerals, Organic Matter, and Microorganisms during Biogeochemical Interface Formation as Shown by a Series of Artificial Soil Experiments. Biol. Fertil. Soils 53 (1), 9–22. doi:10.1007/s00374-016-1161-1
Rasmussen, C., Torn, M. S., and Southard, R. J. (2005). Mineral Assemblage and Aggregates Control Carbon Dynamics in a California conifer forest. Soil Sci. Soc. Am. J. 69, 1711–1721. doi:10.2136/sssaj2005.0040
Rath, K. M., Maheshwari, A., and Rousk, J. (2017). The Impact of Salinity on the Microbial Response to Drying and Rewetting in Soil. Soil Biol. Biochem. 108, 17–26. doi:10.1016/j.soilbio.2017.01.018
Richter, A., Kern, T., Wolf, S., Struck, U., and Ruess, L. (2019). Trophic and Non-trophic Interactions in Binary Links Affect Carbon Flow in the Soil Micro-food Web. Soil Biol. Biochem. 135, 239–247. doi:10.1016/j.soilbio.2019.04.010
Rillig, M. C., Antonovics, J., Caruso, T., Lehmann, A., Powell, J. R., Veresoglou, S. D., et al. (2015). Interchange of Entire Communities: Microbial Community Coalescence. Trends Ecol. Evol. 30, 470–476. doi:10.1016/j.tree.2015.06.004
Rillig, M. C., Caldwell, B. A., Wösten, H. A. B., and Sollins, P. (2007). Role of Proteins in Soil Carbon and Nitrogen Storage: Controls on Persistence. Biogeochemistry 85, 25–44. doi:10.1007/s10533-007-9102-6
Rittmann, B. E., and McCarty, P. L. (2001). Environmental Biotechnology: Principles and Applications. New York: McGraw-Hill.
Roller, B. R., and Schmidt, T. M. (2015). The Physiology and Ecological Implications of Efficient Growth. Isme J. 9, 1481–1487. doi:10.1038/ismej.2014.235
Russell, J. B., and Cook, G. M. (1995). Energetics of Bacterial Growth: Balance of Anabolic and Catabolic Reactions. Microbiol. Rev. 59, 48–62. doi:10.1128/mr.59.1.48-62.1995
Scharlemann, J. P., Tanner, E. V., Hiederer, R., and Kapos, V. (2014). Global Soil Carbon: Understanding and Managing the Largest Terrestrial Carbon Pool. Carbon Manag. 5, 81–91. doi:10.4155/cmt.13.77
Schimel, J., Balser, T. C., and Wallenstein, M. (2007). Microbial Stress-Response Physiology and its Implications for Ecosystem Function. Ecology 88, 1386–1394. doi:10.1890/06-0219
Schimel, J. P., and Schaeffer, S. M. (2012). Microbial Control over Carbon Cycling in Soil. Front. Microbio. 3, 1–11. doi:10.3389/fmicb.2012.00348
Schimel, J., and Weintraub, M. N. (2003). The Implications of Exoenzyme Activity on Microbial Carbon and Nitrogen Limitation in Soil: a Theoretical Model. Soil Biol. Biochem. 35, 549–563. doi:10.1016/s0038-0717(03)00015-4
Schink, B. (2006). Microbially Driven Redox Reactions in Anoxic Environments: Pathways, Energetics, and Biochemical Consequences. Eng. Life Sci. 6, 228–233. doi:10.1002/elsc.200620130
Schmidt, M. W. I., Torn, M. S., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I. A., et al. (2011). Persistence of Soil Organic Matter as an Ecosystem Property. Nature 478 (7367), 49–56. doi:10.1038/nature10386
Schneider, M. P. W., Scheel, T., Mikutta, R., van Hees, P., Kaiser, K., and Kalbitz, K. (2010). Sorptive Stabilization of Organic Matter by Amorphous Al Hydroxide. Geochimica et Cosmochimica Acta 74, 1606–1619. doi:10.1016/j.gca.2009.12.017
Schurig, C., Smittenberg, R. H., Berger, J., Kraft, F., Woche, S. K., Goebel, M.-O., et al. (2013). Microbial Cell-Envelope Fragments and the Formation of Soil Organic Matter: a Case Study from a Glacier Forefield. Biogeochemistry 113, 595–612. doi:10.1007/s10533-012-9791-3
Schweigert, M., Herrmann, S., Miltner, A., Fester, T., and Kästner, M. (2015). Fate of Ectomycorrhizal Fungal Biomass in a Soil Bioreactor System and its Contribution to Soil Organic Matter Formation. Soil Biol. Biochem. 88, 120–127. doi:10.1016/j.soilbio.2015.05.012
Simpson, A. J., Simpson, M. J., Smith, E., and Kelleher, B. P. (2007). Microbially Derived Inputs to Soil Organic Matter: Are Current Estimates Too Low? Environ. Sci. Technol. 41 (23), 8070–8076. doi:10.1021/es071217x
Sims, J. L., Sims, R. C., and Matthews, J. E. (1990). Approach to Bioremediation of Contaminated Soil. Hazard. Waste Hazard. Mater. 7(2), 117–149. doi:10.1089/hwm.1990.7.117
Sinsabaugh, R. L., Turner, B. L., Talbot, J. M., Waring, B. G., Powers, J. S., Kuske, C. R., et al. (2016). Stoichiometry of Microbial Carbon Use Efficiency in Soils. Ecol. Monogr. 86 (2), 172–189. doi:10.1890/15-2110.1
Soares, M., and Rousk, J. (2019). Microbial Growth and Carbon Use Efficiency in Soil: Links to Fungal-Bacterial Dominance, SOC-Quality and Stoichiometry. Soil Biol. Biochem. 131, 195–205. doi:10.1016/j.soilbio.2019.01.010
Sokol, N. W., and Bradford, M. A. (2019). Microbial Formation of Stable Soil Carbon Is More Efficient from Belowground Than Aboveground Input. Nat. Geosci 12, 46–53. doi:10.1038/s41561-018-0258-6
Sokol, N. W., Sanderman, J., and Bradford, M. A. (2018). Pathways of mineral‐associated Soil Organic Matter Formation: Integrating the Role of Plant Carbon Source, Chemistry, and point of Entry. Glob. Change Biol. 25, 12–24. doi:10.1111/gcb.14482
Spence, A., Simpson, A. J., McNally, D. J., Moran, B. W., McCaul, M. V., Hart, K., et al. (2011). The Degradation Characteristics of Microbial Biomass in Soil. Geochimica Et Cosmochimica Acta 75, 2571–2581. doi:10.1016/j.gca.2011.03.012
Spohn, M. (2016). Element Cycling as Driven by Stoichiometric Homeostasis of Soil Microorganisms. Basic Appl. Ecol. 17, 471–478. doi:10.1016/j.baae.2016.05.003
Spohn, M., Pötsch, E. M., Eichorst, S. A., Woebken, D., Wanek, W., and Richter, A. (2016). Soil Microbial Carbon Use Efficiency and Biomass Turnover in a Long-Term Fertilization experiment in a Temperate Grassland. Soil Biol. Biochem. 97, 168–175. doi:10.1016/j.soilbio.2016.03.008
Starke, R., Bastida, F., Abadía, J., García, C., Nicolás, E., and Jehmlich, N. (2017). Ecological and Functional Adaptations to Water Management in a Semiarid Agroecosystem: a Soil Metaproteomics Approach. Sci. Rep. 7, 10221. doi:10.1038/s41598-017-09973-w
Starr, E. P., Shi, S., Blazewicz, S. J., Probst, A. J., Herman, D. J., Firestone, M. K., et al. (2018). Stable Isotope Informed Genome-Resolved Metagenomics Reveals that Saccharibacteria Utilize Microbially-Processed Plant-Derived Carbon. Microbiome 6, 122. doi:10.1186/s40168-018-0499-z
Takriti, M., Wild, B., Schnecker, J., Mooshammer, M., Knoltsch, A., Lashchinskiy, N., et al. (2018). Soil Organic Matter Quality Exerts a Stronger Control Than Stoichiometry on Microbial Substrate Use Efficiency along a Latitudinal Transect. Soil Biol. Biochem. 121, 212–220. doi:10.1016/j.soilbio.2018.02.022
Throckmorton, H. M., Bird, J. A., Monte, N., Doane, T., Firestone, M. K., and Horwath, W. R. (2015). The Soil Matrix Increases Microbial C Stabilization in Temperate and Tropical forest Soils. Biogeochemistry 122, 35–45. doi:10.1007/s10533-014-0027-6
Trapp, S., Brock, A. L., Nowak, K., and Kästner, M. (2018). Prediction of the Formation of Biogenic Nonextractable Residues during Degradation of Environmental Chemicals from Biomass Yields. Environ. Sci. Technol. 52 (2), 663–672. doi:10.1021/acs.est.7b04275
Turner, S., Meyer-Stüve, S., Schippers, A., Guggenberger, G., Schaarschmidt, F., Wild, B., et al. (2017). Microbial Utilization of mineral-associated Nitrogen in Soils. Soil Biol. Biochem. 104, 185–196. doi:10.1016/j.soilbio.2016.10.010
Uden, N. (1967). Transport-limited Growth in the Chemostat and its Competitive Inhibition; a Theoretical Treatment. Archiv. Mikrobiol. 58, 145–154. doi:10.1007/bf00406675
van Bodegom, P. (2007). Microbial Maintenance: A Critical Review on its Quantification. Microb. Ecol. 53, 513–523. doi:10.1007/s00248-006-9049-5
van der Heijden, M. G. A., Bardgett, R. D., and van Straalen, N. M. (2008). The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 11, 296–310. doi:10.1111/j.1461-0248.2007.01139.x
von Stockar, U. (2010). Biothermodynamics of Live Cells: a Tool for Biotechnology and Biochemical Engineering. J. Non-Equilibrium Thermodynamics 35, 415–475. doi:10.1515/jnetdy.2010.024
Wang, B., An, S., Liang, C., Liu, Y., and Kuzyakov, Y. (2021a). Microbial Necromass as the Source of Soil Organic Carbon in Global Ecosystems. Soil Biol. Biochem. 162, 108422. doi:10.1016/j.soilbio.2021.108422
Wang, C., Qu, L., Yang, L., LiuLiu, D., Morrissey, E., Miao, R., et al. (2021b). Large‐scale Importance of Microbial Carbon Use Efficiency and Necromass to Soil Organic Carbon. Glob. Change Biol. 27, 2039–2048. doi:10.1111/gcb.15550
Waring, B. G., Sulman, B. N., Reed, S., Smith, A. P., A., Creamer, C. A., et al. (2020). From Pools to Flow: The PROMISE Framework for New Insights on Soil Carbon Cycling in a Changing World. Glob. Change Biol. 26, 6631–6643. doi:10.1111/gcb.15365
Wei, X., Zhu, Z., Liu, Y., Luo, Y., Deng, Y., Xu, X., et al. (2020). C:N:P Stoichiometry Regulates Soil Organic Carbon Mineralization and Concomitant Shifts in Microbial Community Composition in Paddy Soil. Biol. Fertil. Soils 56, 1093–1107. doi:10.1007/s00374-020-01468-7
Westerhoff, H. V., Lolkema, J. S., Otto, R., and Hellingwerf, K. J. (1982). Thermodynamics of Growth. Non-equilibrium Thermodynamics of Bacterial Growth. The Phenomenological and the Mosaic Approach. Biochim. Biophys. Acta 683, 181–220. doi:10.1016/0304-4173(82)90001-5
Williamson, K. E., Fuhrmann, J. J., Wommack, K. E., and Radosevich, M. (2017). Viruses in Soil Ecosystems: An Unknown Quantity within an Unexplored Territory. Annu. Rev. Virol. 4, 201–219. doi:10.1146/annurev-virology-101416-041639
Winkler, P., Kaiser, K., Jahn, R., Mikutta, R., Fiedler, S., Cerli, C., et al. (2019). Tracing Organic Carbon and Microbial Community Structure in Mineralogically Different Soils Exposed to Redox Fluctuations. Biogeochemistry 143, 31–54. doi:10.1007/s10533-019-00548-7
Woche, S. K., Goebel, M.-O., Mikutta, R., Schurig, C., Kaestner, M., Guggenberger, G., et al. (2017). Soil Wettability Can Be Explained by the Chemical Composition of Particle Interfaces - an XPS Study. Sci. Rep. 7, 42877. doi:10.1038/srep42877
Woolf, D., and Lehmann, J. (2019). Microbial Models with Minimal mineral protection Can Explain Long-Term Soil Organic Carbon Persistence. Sci. Rep. 9, 6522. doi:10.1038/s41598-019-43026-8
Xiao, J., and VanBriesen, J. M. (2008). Expanded Thermodynamic True Yield Prediction Model: Adjustments and Limitations. Biodegradation 19 (1), 99–127. doi:10.1007/s10532-007-9119-5
Xu, X., Thornton, P. E., and Post, W. M. (2013). A Global Analysis of Soil Microbial Biomass Carbon, Nitrogen and Phosphorus in Terrestrial Ecosystems. Glob. Ecol. Biogeogr. 22, 737–749. doi:10.1111/geb.12029
Zechmeister-Boltenstern, S., Keiblinger, K. M., Mooshammer, M., Peñuelas, J., Richter, A., Sardans, J., et al. (2015). The Application of Ecological Stoichiometry to Plant-Microbial-Soil Organic Matter Transformations. Ecol. Monogr. 85 (2), 133–155. doi:10.1890/14-0777.1
Zheng, Q., Hu, Y., Zhang, S., Noll, L., Böckle, T., Richter, A., et al. (2019). Growth Explains Microbial Carbon Use Efficiency across Soils Differing in Land Use and Geology. Soil Biol. Biochem. 128, 45–55. doi:10.1016/j.soilbio.2018.10.006
Zheng, T., Miltner, A., Liang, C., Nowak, K. M., and Kästner, M. (2021). Turnover of Gram-Negative Bacterial Biomass-Derived Carbon through the Microbial Food Web of an Agricultural Soil. Soil Biol. Biochem. 152, 108070. doi:10.1016/j.soilbio.2020.108070
Keywords: microbial growth, energy, necromass, elemental stoichiometry, carbon use efficiency, energy use efficiency, nutrient mining, mineral interactions
Citation: Kästner M, Miltner A, Thiele-Bruhn S and Liang C (2021) Microbial Necromass in Soils—Linking Microbes to Soil Processes and Carbon Turnover. Front. Environ. Sci. 9:756378. doi: 10.3389/fenvs.2021.756378
Received: 10 August 2021; Accepted: 16 November 2021;
Published: 14 December 2021.
Edited by:
Rosa Francaviglia, Council for Agricultural and Economics Research (CREA), ItalyReviewed by:
Claire Chenu, AgroParisTech Institut des Sciences et Industries du Vivant et de L’environnement, FranceCopyright © 2021 Kästner, Miltner, Thiele-Bruhn and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Kästner, bWF0dGhpYXMua2Flc3RuZXJAdWZ6LmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.