
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 02 August 2021
Sec. Toxicology, Pollution and the Environment
Volume 9 - 2021 | https://doi.org/10.3389/fenvs.2021.727645
This article is part of the Research Topic Persistent Organic Pollutants in Soil and Sediments View all 10 articles
We developed a sensitive method for monitoring six natural (aldosterone) and synthetic mineralocorticoids (canrenone, spironolactone, 7β-spironolactone, 7α-thio spironolactone, and 7α-thiomethyl spironolactone) in sediment and water using ultra-performance liquid chromatography–electrospray tandem mass spectrometry, and then 30 water and 30 sediment samples were analyzed to reveal their occurrence and distributions in Taihu Lake. All target six mineralocorticoids were detected in sediment and water samples with the detection frequencies as high as 96–100%. The median concentrations of mineralocorticoids ranged from 0.04 ng/L (7α-thiomethyl spironolactone) to 14 ng/L (aldosterone) in water and 0.01 ng/g (7β-spironolactone and canrenone) to 1.44 ng/g (aldosterone) in sediment in dry weight. Natural aldosterone was the predominant mineralocorticoid detected in both water and sediment samples, indicating the mineralocorticoid pollution in Taihu Lake was mainly derived from human and/or animal excrement rather than pharmaceutical industry and usage. Two metabolites 7β-spironolactone and 7α-thio spironolactone were first found in this study. Low ratios of metabolites to spironolactone were observed in sediment (0.05–0.75) in contrast to water (0.12–2.26), indicating that spironolactone was prone to degradation in water phase compared to sediment environment.
Over the past 2 decades, steroid hormones in aquatic environments have attracted widespread attention due to their potential endocrine disrupting effects (Parks et al., 2001; Kolodziej et al., 2003; Runnalls et al., 2013; Shen et al., 2020). Steroid hormones include estrogens, androgens, progestogens, glucocorticoids, and mineralocorticoids, and all of them can be released into environment and present a risk to aquatic organisms (Larsson et al., 2000; Orlando et al., 2004; Sorensen et al., 2005; Zucchi et al., 2012; Zucchi et al., 2013 and; Zucchi et al., 2014; Liang et al., 2015; Fent, 2015). Studies have revealed the environmental relevance of mineralocorticoid spironolactone and its metabolite canrenone with the abnormal fishes downstream from pharmaceutical manufacture discharge (Sanchez et al., 2011). In addition, androgenic effects were observed on fish which were exposed to mineralocorticoid spironolactone (Natasha, 2011; Raut et al., 2011; LaLone et al., 2013; LaLone et al., 2013; Davis et al., 2017). However, in contrast to other classes, there are few reports about the occurrence and fate of mineralocorticoids in environment which is vital to assess their potential health and ecological risks.
Natural mineralocorticoid, such as aldosterone, are excreted by the adrenal cortex. They regulate water and salt metabolism and blood pressure, and play an important role in maintaining the constant environment in the body (Lifton et al., 2001; Kuhnle et al., 2004). Such properties have led to the usage of synthetic mineralocorticoids against a number of human diseases such as inflammation, allergies, adrenal insufficiency, diabetes and other related diseases (USEPA, 1997). Thus, a quantity of mineralocorticoids can be released into the aquatic environment via wastewater effluents of sewage treatment plants (STPs), hospitals and pharmaceutical factories. Recently, Weizel et al. (2018) reported the concentrations of spironolactone metabolites (canrenone and 7α-thiomethyl spironolactone) up to 19 ng/L in STP effluents. Ammann et al. (2014) found that the concentration of spironolactone in hospital wastewater was as high as 130 ng/L. Creusot et al. (2014) revealed the concentrations of spironolactone and canrenone up to 2.9 μg/L downstream of a French pharmaceutical factory. It was reported that spironolactone could be rapidly metabolized into canrenone, 7α-thiomethyl spironolactone and other metabolites in the body (Sadee et al., 1973; Vlase et al., 2011) and could fast degrade in contact with activated sludge and in aqueous solutions (Sulaiman et al., 2015).
Thus, spironolactone metabolites may originate from the biological metabolism in organisms, the degradation of spironolactone in water treatment processes and/or the natural environment, necessitating a systematic investigation of metabolites together with spironolactone. To our knowledge, only two studies have included metabolites canrenone and 7α-thiomethyl spironolactone in the investigations (Creusot et al., 2014; Weizel et al., 2018). Because spironolactone metabolites may still have endocrine disrupting effects on organisms (Sanchez et al., 2011; LaLone et al., 2013), it is essential to analyze as many as natural, synthetic mineralocorticoids and metabolites to obtain a more complete picture of the environmental contamination and to further assess the environmental risks.
In this study, we developed a method that allows the simultaneous analysis of six mineralocorticoids, including aldosterone, canrenone, 7α-thio spironolactone, 7α-thiomethyl spironolactone, spironolactone and 7β-spironolactone (Figure 1). We then applied this method to investigate the occurrence of each compound in sediment and water samples from Taihu Lake, which is one of the key lakes of water pollution control in China (Qin et al., 2007; Li et al., 2009).
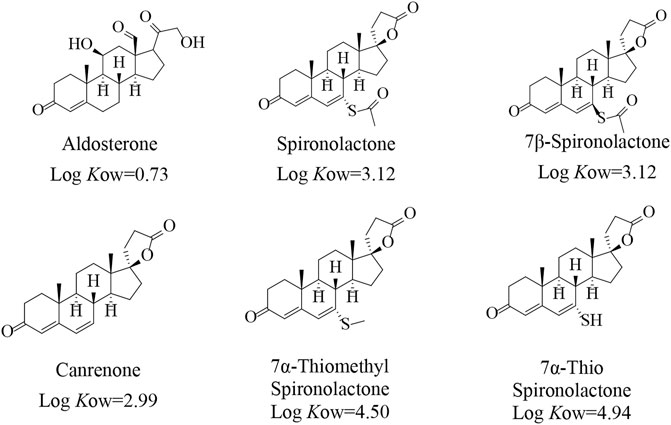
FIGURE 1. Chemical structures and corresponding Log Kow values (ACD/ChemSketch 10.0) of target mineralocorticoids.
Six mineralocorticoids, aldosterone, canrenone, spironolactone, 7β-spironolactone, 7α-thio spironolactone, and 7α-thiomethyl spironolactone were purchased from Steraloids (Newport, United States). Spironolactone-d6 was obtained from Toronto Research Chemicals (Toronto, Canada). All reference materials were of purity greater than 98%. HPLC grade ethyl acetate (EtOAc), acetonitrile (ACN) and methanol were purchased from Fisher Chemicals (Bridgewater, United States). HPLC grade formic acid was supplied by Beijing Chemicals (Beijing, China). Milli-Q water was obtained from Millipore (18.2 MΩ, Bedford, MA, United States).
The sampling sites were shown in Figure 2. At each sampling location, five independent water/sediment samples were collected and pooled together to obtain one composite water/sediment sample. Sediment samples (upper 20–30 cm) were collected using a stainless grab sampler. A total of thirty pairs of composite water and sediment samples were obtained from Taihu Lake in October 2019. The samples were sealed without a headspace and transported to the laboratory within 6 h of collection. Water samples were filtered and extracted immediately, and sediment samples were stored at −20°C until further analyses. The detailed characteristics of collected water samples at each sampling site (pH, dissolved organic carbon (DOC) and temperature) are provided in Supplementary Table S1.
All water samples were filtered by glass fiber pads (GF/F, 0.45 μm, Whatman, Maidstone, United Kingdom) to remove suspended materials. Prior to extraction, isotope-labelled standard (spironolactone-d6) was spiked into water sample at concentrations of 100 ng/L as surrogate to compensate for losses and matrix effects. The water samples passed through the Oasis HLB cartridges (500 mg, 6 ml, Milford, MA, United States) at a flow rate of 5–10 ml/min, which were pre-activated with 6 ml of EtOAc, 6 ml of ACN, and 12 ml of ultrapure water. After water loading, the cartridges were rinsed with 10 ml ultrapure water and dried by nitrogen. Then the cartridges were eluted with 6 ml ethyl acetate/acetonitrile (1:1, v/v). Subsequently, the extracts were evaporated under a gentle stream of nitrogen to dryness and reconstituted with 200 μL methanol for UPLC-ESI-MS/MS analysis.
For sediment samples, they were freeze-dried and grinded to pass through 80 mesh sieve. Prior to extraction, sediment samples were spiked with 20 ng of spironolactone-d6 and were ultrasonically extracted with 20 ml of mixture of methanol and ACN (1:1, v:v). The eluent was evaporated to dryness and reconstituted with 200 ml of ultrapure water. The subsequent purified steps followed the same procedure as for the water samples described above. Each sample was analyzed in duplicate by this method.
The LC apparatus was an ACQUITY Ultra Performance LC system (UPLC; Waters, Milford, MA, United States). Separation was conducted using a UPLC HSS T3 column (100 mm × 2.1 mm, 1.7 µm, Waters). Methanol (A) and 0.1% formic acid in ultrapure water (B) were used as the mobile phases. The gradient condition was as follows: the contribution from A was linearly increased from 60 to 70% in 8 min, to 85% in 5 min, to 100% in 1 min, and held for 3 min, followed by a decrease to 60% in 0.1 min, and held for 3 min to allow re-equilibration. The flow rate was 0.3 ml/min. The column temperature was set to 40°C, and the injected volume of each sample was 2 μL.
Mass spectrometry was performed using a Waters Xevo TQ-S Triple Quadrupole Mass Spectrometer equipped with an electrospray ionization source (Waters, United States). The mass spectrometer was operated in positive electrospray ionization pattern using a multi-reaction monitoring (MRM) mode. The optimal parameters adopted for the ESI source as follows: capillary voltage was set to 3.0 kV, ion source temperature was 150°C, the desolvation gas was set at 450°C with desolvation gas flow of 900 L/h, and collision gas flow was 150 L/h.
The recovery experiment was spiking the mixed standard solution to the pooled samples (n = 5) from several water and sediment samples of Taihu Lake before extraction. Analyte addition was at least three times the original concentration, and was 5 ng/L in filtered water (except for aldosterone at 100 ng/L) and 1 ng/g dry weight (dw; except for aldosterone at 20 ng/g dw) in sediment samples. The relative recoveries in the spiked water and sediment samples were estimated by correcting the absolute recoveries of target analytes to that of the surrogate.
The six natural and synthetic mineralocorticoids, including aldosterone, canrenone, spironolactone, 7β-spironolactone, 7α-thio spironolactone, and 7α-thiomethyl spironolactone, were simultaneously analyzed by MS/MS in the MRM mode. The two abundant MRM transitions, cone voltage and collision energies, were optimized for each analyte by infusing the standard solutions into the mass spectrometer (Table 1). All of the precursor ions were protonated molecular ions ([M + H]+), except for spironolactone and 7β-spironolactone ([M-SOCH3+H]+) which readily lose the 7α-acetylthio group being transformed to canrenone. The signals of canrenone, spironolactone, and 7β-spironolactone can be easily distinguished by their different retention times (Figure 3). Under MS/MS conditions, natural aldosterone generated abundant product ions at m/z 315 and 343, which can be formed due to the lose sequences of H2O and COCH2OH at C-17. For canrenone, spironolactone, and 7β-spironolactone, the most abundant product ions were at m/z 107 and 187 due to the cleavages in the A and B rings. For 7α-thiomethyl spironolactone, the most abundant product ions were at m/z 341 and 323 due to the sequential loss of SCH3 and H2O. The fragmentation of 7α-thio spironolactone showed a loss of 283 and 199 Da, corresponding to the loss of SH and ester group and the cleavage in the C ring.
The mineralocorticoids in the samples were identified by comparing the retention time (within 2%) and the ratio (within 20%) of the two selected MRM ion transition with those of standards. A recovery experiment was conducted to evaluate the method accuracy by spiking at least three times the original concentration of target analytes into water and sediment samples. The recoveries of the present method were in the range of 72–108% for water and 81–110% for sediment samples. Two procedural blanks were carried out with each batch to check the interfering peaks and potential background values, and no analytes were found in the blank analysis. The limit of quantifications (LOQs) for each analyte in the field samples were calculated based on the signal-to-noise (S/N) ratio near the target peak. The LOQs (S/N = 10) of the analytes were 0.002–0.02 ng/g dw in the sediment and 0.01–0.03 ng/L in the water, respectively.
Six natural and synthetic mineralocorticoids were analyzed in the water and sediment samples collected from Taihu Lake in 2019. As shown in Figure 4, all six mineralocorticoids were detected in sediment and water samples, with the detection frequencies as high as 96–100%. In sediment samples, the detected concentrations were 0.12–6.7 ng/g dry weight (dw) for aldosterone, 0.004–0.43 ng/g dw for spironolactone, 0.002–0.02 ng/g dw for 7β-spironolactone, 0.002–0.03 ng/g dw for canrenone, <LOQ–0.02 ng/g dw for 7α-thiomethyl spironolactone, and 0.01–0.20 ng/g dw for 7α-thio spironolactone, with a median value of 1.44, 0.10, 0.01, 0.01, 0.06, and 0.044 ng/g dw, respectively. In water samples, the detected concentrations were 2.8–45 ng/L for aldosterone, 0.02–1.0 ng/L for spironolactone, 0.04–0.38 ng/L for 7β-spironolactone, 0.02–0.60 ng/L for canrenone, <LOQ–0.08 ng/L for 7α-thiomethyl spironolactone, and 0.08–3.3 ng/L for 7α-thio spironolactone, with a median value of 14, 0.32, 0.15, 0.12, 0.04, and 0.78 ng/L, respectively. Of the six mineralocorticoids, endogenous aldosterone was always the dominant compound, indicating the mineralocorticoid pollution in Taihu Lake was mainly derived from human and/or animal excrement rather than pharmaceutical industry and usage. Spironolactone is a renal competitive aldosterone antagonist in a class of pharmaceuticals called potassium-sparing diuretics, used primarily to treat ascites in patients with liver disease, low-renin hypertension, hypokalemia, and Conn’s syndrome.
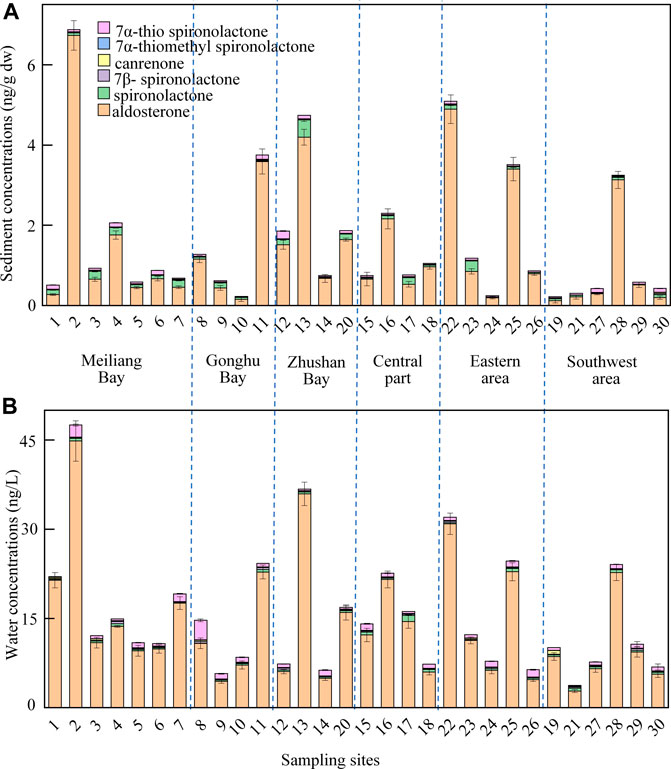
FIGURE 4. Concentration distributions of the target mineralocorticoids detected in sediment (A) and water (B) samples from Taihu Lake. Error bars represent SD values of two duplicate composite samples.
Canrenone, 7α-thiomethyl spironolactone, 7α-thio spironolactone, and 7β-spironolactone were the metabolites of spironolactone. In this study, the concentrations of spironolactone and its four metabolites were relatively low, but their ubiquitous present in water and sediment samples indicate that they should be included in further monitoring campaigns of steroid hormones. Few studies have reported the concentrations of mineralocorticoids in aquatic environments. To our knowledge, the present study is the first one that reports 7α-thio spironolactone and 7β-spironolactone, the two metabolites of spironolactone in aquatic environments. In contrast to Taihu Lake in this study, Gong et al. (2019) reported lower measured aldosterone concentrations of 1.1–9.6 ng/L in Zhujiang River, and Ammann et al. (2014) found higher spironolactone concentrations of 1–4 ng/L in Swedish and Czechic rivers. It is interesting that two metabolites of canrenone (0.2–8.3 ng/L) and 7α-thiomethyl spironolactone (0.05–1.3 ng/L) were detected in the rivers and streams containing an elevated percentage of treated wastewater (Weizel et al., 2018). In contrast to its metabolites, spironolactone was not detected at all in that study. The phenomenon could be explained that spironolactone could be rapidly metabolized in humans to canrenone and 7α-thiomethyl spironolactone (Vlase et al., 2011) and fast degrade in contact with activated sludge and in aqueous solutions (Sulaiman et al., 2015). This pattern is different from that found in the river waters downstream from pharmaceutical manufacture discharges where spironolactone (402–14,681 ng/L) was measured with detected with higher levels of canrenone (226–7,336 ng/L) (Creusot et al., 2014). Thus, the frequent detection of spironolactone in Taihu Lake in this study may suggest the industrial discharge and/or the different degradation behaviors although it was not a major pollution source to Taihu Lake.
The concentration variations of the six natural and synthetic mineralocorticoids were investigated in water and sediment samples collected from Taihu Lake. Their total concentrations were 3.7–48 ng/L in water and 0.22–6.9 ng/g dw in sediment, and had similar spatial distributions for the target mineralocorticoids in water and sediment of Taihu Lake. The total concentrations of mineralocorticoids in the eastern area (sites 22 and 25), Meiliang Bay (site 2), Gonghu Bay (site 11), and Zhushan Bay (Site 13) were relatively higher than those in other areas of Taihu Lake. In the central part (sites 15–18), the concentrations of mineralocorticoids showed a similar level. In the southwest area (sites 19, 21, and 27–30), relatively lower levels of mineralocorticoids were found. The occurrence and distribution of mineralocorticoids in Taihu Lake were influenced by human/animal wastewater discharges, lake currents, inflow/outflow rivers, and sediment properties (Zhou et al., 2016). Relatively high concentrations of mineralocorticoids in the northwestern (Meiliang Bay, Gonghu Bay, and Zhushan Bay) and eastern area were probably due to human/animal wastewater discharges and fewer disturbances of lake currents in the areas. The similar levels of mineralocorticoids in the central area might be influenced by strong lake currents in the area.
The compositions of each analyte relative to the total mean concentrations were also investigated in the water and sediment samples collected from Taihu Lake (Figure 5). Natural aldosterone was the predominant mineralocorticoid and accounted for 91 and 89% of the total concentrations in the water and sediment samples. Synthetic spironolactone and its metabolites only accounted for 9 and 11% in water and sediment. It suggests that compared to pharmaceutical production and usage, human/animal excrement probably the main source of mineralocorticoid pollution in Taihu Lake.
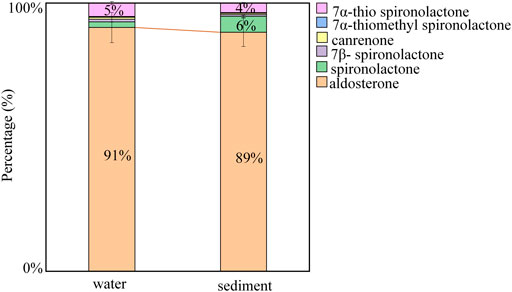
FIGURE 5. Profiles of the six mineralocorticoids in water and sediment samples from Taihu Lake. Error bars represent SD values of two duplicate composite samples.
Despite the relatively low levels of spironolactone in Taihu Lake, the detection of its four metabolites, of which 7β-spironolactone and 7α-thio spironolactone were detected for the first time, afforded the opportunity to assess the compound-specific transformation of the important synthetic mineralocorticoid in the environment (Figure 6). Canrenone and 7α-thiomethyl spironolactone were reported to be two metabolites of spironolactone in humans and also two degradation products in contact with activated sludge and in aqueous solutions (Vlase et al., 2011; Sulaiman et al., 2015), and their ratios relative to spironolactone were 0.36 and 0.12 in water. Other two metabolites 7β-spironolactone and 7α-thio spironolactone were first reported in this study, and their ratios relative to spironolactone were 0.47 and 2.26 in water. The metabolite 7β-spironolactone is a 7β-isomer of spironolactone, and it has been reported to be able to bind effectively to human plasma proteins. In comparison, low ratios of metabolites to spironolactone were observed in sediment, and the ratios of canrenone/spironolactone, 7α-thiomethyl spironolactone/spironolactone, 7β-spironolactone/spironolactone, and 7α-thio spironolactone/spironolactone were 0.10, 0.05, 0.14, and 0.75, respectively. The results indicate that the mineralocorticoids are prone to degradation in water phase compared to sediment environment.
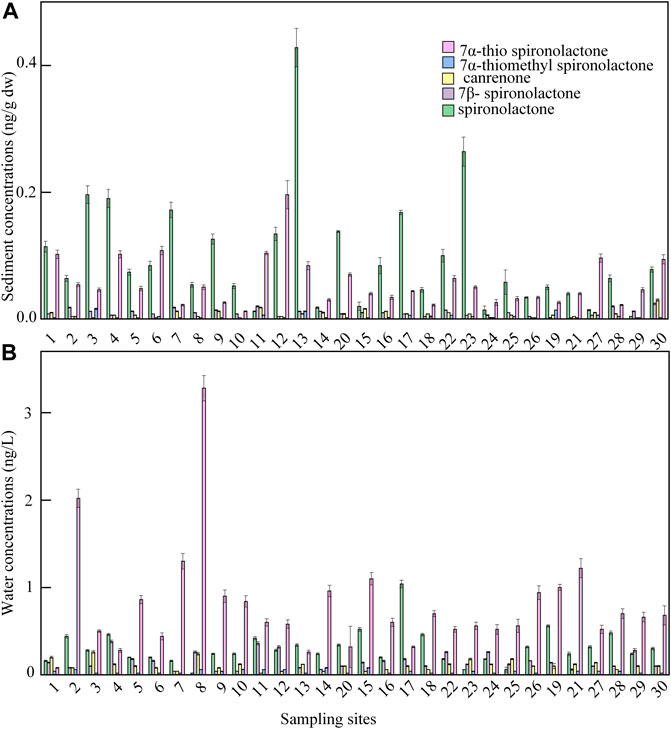
FIGURE 6. Concentration variations of spironolactone and its four metabolites in sediment (A) and water (B) samples from Taihu Lake. Error bars represent SD values of two duplicate composite samples.
An UPLC-MS/MS method with a high sensitivity was established for simultaneous determination of six natural and synthetic mineralocorticoids in water and sediment matrices. All the target mineralocorticoids were widely detected in Taihu Lake. Natural aldosterone was the predominant mineralocorticoid detected in both water and sediment samples, and thus human/animal excrement was probably the main pollution source for Taihu Lake. Synthetic spironolactone and its metabolites were also ubiquitous in Taihu Lake despite lower concentrations compared to natural aldosterone. Two metabolites 7β-spironolactone and 7α-thio spironolactone were first found in this study. Low ratios of metabolites to spironolactone were observed in sediment in contrast to water, indicating the mineralocorticoids prone to degradation in water phase compared to sediment environment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
HC was involved in writing the article with the help of LZ, FS, and HS. FS and HS helped in field sample collection and reviewing the article. LZ was involved in method development and data analysis. All authors read and approved the final article.
The authors are grateful for financial support by the Major Science and Technology Program for Water Pollution Control and Treatment (2018ZX07208001), the National Natural Science Foundation of China (42077306), the Fundamental Research Funds for the Central Universities (JC 2015-02), and the State Key Laboratory of Environmental Criteria and Risk Assessment (SKLECRA2019OFP07). The authors also thank the Beijing Municipal Education Commission for its financial support through Innovative Transdisciplinary Program “Ecological Restoration Engineering”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2021.727645/full#supplementary-material
Ammann, A. A., Macikova, P., Groh, K. J., Schirmer, K., and Suter, M. J. F. (2014). LC-MS/MS Determination of Potential Endocrine Disruptors of Cortico Signalling in Rivers and Wastewaters. Anal. Bioanal. Chem. 406, 7653–7665. doi:10.1007/s00216-014-8206-9
Creusot, N., Aït-Aïssa, S., Tapie, N., Pardon, P., Brion, F., Sanchez, W., et al. (2014). Identification of Synthetic Steroids in River Water Downstream from Pharmaceutical Manufacture Discharges Based on a Bioanalytical Approach and Passive Sampling. Environ. Sci. Technol. 48, 3649–3657. doi:10.1021/es405313r
Davis, J. M., Ekman, D. R., Skelton, D. M., Lalone, C. A., Ankley, G. T., Cavallin, J. E., et al. (2017). Metabolomics for Informing Adverse Outcome Pathways: Androgen Receptor Activation and the Pharmaceutical Spironolactone. Aquat. Toxicol. 184, 103–115. doi:10.1016/j.aquatox.2017.01.001
Fent, K. (2015). Progestins as Endocrine Disrupters in Aquatic Ecosystems: Concentrations, Effects and Risk Assessment. Environ. Int. 84, 115–130. doi:10.1016/j.envint.2015.06.012
Gong, J., Lin, C., Xiong, X., Chen, D., Chen, Y., Zhou, Y., et al. (2019). Occurrence, Distribution, and Potential Risks of Environmental Corticosteroids in Surface Waters from the Pearl River Delta, South China. Environ. Pollut. 251, 102–109. doi:10.1016/j.envpol.2019.04.110
Kolodziej, E. P., Gray, J. L., and Sedlak, D. L. (2003). Quantification of Steroid Hormones with Pheromonal Properties in Municipal Wastewater Effluent. Environ. Toxicol. Chem. 22, 2622–2629. doi:10.1897/03-42
Kuhnle, U., Lewicka, S., and Fuller, P. J. (2004). Endocrine Disorders of Sodium Regulation. Horm. Res. Paediatr. 61, 68–83. doi:10.1159/000075242
Lalone, C. A., Villeneuve, D. L., Cavallin, J. E., Kahl, M. D., Durhan, E. J., Makynen, E. A., et al. (2013). Cross-species Sensitivity to a Novel Androgen Receptor Agonist of Potential Environmental Concern, Spironolactone. Environ. Toxicol. Chem. 32, 2528–2541. doi:10.1002/etc.2330
Larsson, D. G. J., Hallman, H., and Forlin, L. (2000). More Male Fish Embryos Near a Pulp Mill. Environ. Toxicol. Chem. 19 (12), 2911–2917. doi:10.1002/etc.5620191210
Li, Y., Chen, Y., Song, B., Olson, D., Yu, N., and Chen, L. (2009). Ecosystem Structure and Functioning of Lake Taihu (China) and the Impacts of Fishing. Fish. Res. 95, 309–324. doi:10.1016/j.fishres.2008.09.039
Liang, Y.-Q., Huang, G.-Y., Liu, S.-S., Zhao, J.-L., Yang, Y.-Y., Chen, X.-W., et al. (2015). Long-term Exposure to Environmentally Relevant Concentrations of Progesterone and Norgestrel Affects Sex Differentiation in Zebrafish (Danio rerio). Aquat. Toxicol. 160, 172–179. doi:10.1016/j.aquatox.2015.01.006
Lifton, R. P., Gharavi, A. G., and Geller, D. S. (2001). Molecular Mechanisms of Human Hypertension. Cell 104, 545–556. doi:10.1016/s0092-8674(01)00241-0
Orlando, E. F., Kolok, A. S., Binzcik, G. A., Gates, J. L., Horton, M. K., Lambright, C. S., et al. (2004). Endocrine-disrupting Effects of Cattle Feedlot Effluent on an Aquatic sentinel Species, the Fathead Minnow. Environ. Health Perspect. 112, 353–358. doi:10.1289/ehp.6591
Parks, L. G., Lambright, C. S., Orlando, E. F., Guillette, L. J., Ankley, G. T., and Gray, L. E. (2001). Masculinization of Female Mosquitofish in Kraft Mill Effluent-Contaminated Fenholloway River Water Is Associated with Androgen Receptor Agonist Activity. Toxicol. Sci. 62, 257–267. doi:10.1093/toxsci/62.2.257
Qin, B., Xu, P., Wu, Q., Luo, L., and Zhang, Y. (2007). Environmental Issues of Lake Taihu, China. Hydrobiologia 581, 3–14. doi:10.1007/s10750-006-0521-5
Raut, S. A., Howell, W. M., and Angus, R. A. (2011). Endocrine-disrupting Effects of Spironolactone in Female Western Mosquitofish, Gambusia affinis. Environ. Toxicol. Chem. 30, 1376–1382. doi:10.1002/etc.504
Runnalls, T. J., Beresford, N., Losty, E., Scott, A. P., and Sumpter, J. P. (2013). Several Synthetic Progestins with Different Potencies Adversely Affect Reproduction of Fish. Environ. Sci. Technol. 47, 2077–2084. doi:10.1021/es3048834
Sadée, W., Dagcioglu, M., and Schröder, R. (1973). Pharmacokinetics of Spironolactone, Canrenone and Canrenoate-K in Humans. J. Pharmacol. Exp. Ther. 185, 686–695.
Sanchez, W., Sremski, W., Piccini, B., Palluel, O., Maillot-Maréchal, E., Betoulle, S., et al. (2011). Adverse Effects in Wild Fish Living Downstream from Pharmaceutical Manufacture Discharges. Environ. Int. 37, 1342–1348. doi:10.1016/j.envint.2011.06.002
Shen, X., Chang, H., Sun, Y., and Wan, Y. (2020). Determination and Occurrence of Natural and Synthetic Glucocorticoids in Surface Waters. Environ. Int. 134, 105278. doi:10.1016/j.envint.2019.105278
Sorensen, P. W., Pinillos, M., and Scott, A. P. (2005). Sexually Mature Male Goldfish Release Large Quantities of Androstenedione into the Water where it Functions as a Pheromone. Gen. Comp. Endocrinol. 140, 164–175. doi:10.1016/j.ygcen.2004.11.006
Sulaiman, S., Khamis, M., Nir, S., Lelario, F., Scrano, L., Bufo, S. A., et al. (2015). Stability and Removal of Spironolactone from Wastewater. J. Environ. Sci. Health A 50, 1127–1135. doi:10.1080/10934529.2015.1047668
U.S.E.P.A (1997). Wastewater Treatment Manuals – Primary, Secondary and Tertiary Treatment. Washington DC: USEPA.
Vlase, L., Imre, S., Muntean, D., Achim, M., and Muntean, D.-L. (2011). Determination of Spironolactone and Canrenone in Human Plasma by High-Performance Liquid Chromatography with Mass Spectrometry Detection. Croat. Chem. Acta. 84, 361–366. doi:10.5562/cca1761
Weizel, A., Schlüsener, M. P., Dierkes, G., and Ternes, T. A. (2018). Occurrence of Glucocorticoids, Mineralocorticoids, and Progestogens in Various Treated Wastewater, Rivers, and Streams. Environ. Sci. Technol. 52, 5296–5307. doi:10.1021/acs.est.7b06147
Zhou, L.-J., Zhang, B.-B., Zhao, Y.-G., and Wu, Q. L. (2016). Occurrence, Spatiotemporal Distribution, and Ecological Risks of Steroids in a Large Shallow Chinese lake, Lake Taihu. Sci. Total Environ. 557-558, 68–79. doi:10.1016/j.scitotenv.2016.03.059
Zucchi, S., Castiglioni, S., and Fent, K. (2013). Progesterone Alters Global Transcription Profiles at Environmental Concentrations in Brain and Ovary of Female Zebrafish (Danio rerio). Environ. Sci. Technol. 47, 12548–12556. doi:10.1021/es403800y
Zucchi, S., Castiglioni, S., and Fent, K. (2012). Progestins and Antiprogestin s Affect Gene Expression in Early Development in Zebrafish (Danio rerio) at Environmental Concentrations. Environ. Sci. Technol. 46, 5183–5192. doi:10.1021/es300231y
Keywords: mineralocorticoids, sediment, water, metabolites, degradation, concentration profiles 3
Citation: Zhao L, Chang H, Sun F and Su H (2021) Determination and Occurrence of Mineralocorticoids in Taihu Lake of China. Front. Environ. Sci. 9:727645. doi: 10.3389/fenvs.2021.727645
Received: 19 June 2021; Accepted: 23 July 2021;
Published: 02 August 2021.
Edited by:
Shuwen Yan, Fudan University, ChinaCopyright © 2021 Zhao, Chang, Sun and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Chang, Y2hhbmdoQGJqZnUuZWR1LmNu; Fuhong Sun, c3VuZmhpYWVAMTI2LmNvbQ==; Hailei Su, c3VoYWlsZWk2NjZAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.