- 1Environmental Engineering Program, College of Engineering, University of the Philippines Diliman, Quezon City, Philippines
- 2Institute of Civil Engineering, College of Engineering, University of the Philippines Diliman, Quezon City, Philippines
Microplastics have been increasingly documented globally in numerous environmental compartments. However, little information exists in the Philippines despite the fact that the country is considered to be one of the largest contributors of plastics in oceans. This study, considered as one of the pioneering microplastic research, evaluated the abundance, distribution, and composition of microplastic pollution in the mouths of five rivers, namely Cañas, Meycauayan, Parañaque, Pasig and Tullahan, draining to Manila Bay. Surface water and sediments samples were collected, then passed through a stack of sieves with sizes from 2.36 mm at the top to 0.075 mm at the bottom. These samples were digested to remove organic matter, and salt solutions were added to allow the microplastics to float. Extracted particles were examined under a stereo microscope, and quantified and categorized into shape, size, color, and type. Results show that microplastics were present ubiquitously at all river mouths but with concentrations varying from 1,580 to 57,665 particles/m3 (surface water) and 386 to 1,357 particles/kg (dry sediment). Fragment was the most abundant shape, while white, blue, and transparent were the most prevalent colors. Fourier Transform Infrared Spectroscopy (FTIR) analysis revealed that polypropylene (PP), high and low-density polyethylene (high-density polyethylene and low-density polyethylene) and polystyrene were the main types of microplastics present in the river mouths.
Introduction
Microplastics are recently considered as an emerging pollutant, and these are divided into primary and secondary (Andrady, 2011; Wang et al., 2019). Primary microplastics are found in personal care products, cosmetics, medicines, and textiles as well as those by-products released from the use of plastic-related products such as tyre dust from running cars (Browne et al., 2011; Eriksen et al., 2013; Li et al., 2017; Wang et al., 2019; Suresh et al., 2020). Secondary microplastics are derived from the fragmentation of larger plastic and synthetic materials by processes such as UV degradation or machine washing (Andrady, 2011; GESAMP, 2015; Antunes et al., 2018). Microplastic contamination was first studied by Carpenter et al. (1972). Since then, many studies have documented the occurrence of microplastic contamination among the different bodies of water such as oceans (Anderson et al., 2016; Browne et al., 2011), estuaries (Browne et al., 2010; Lima et al., 2015; Zhao et al., 2014) freshwater bodies (Biginagwa et al. 2016; Free et al., 2014; Sanchez et al, 2014), and even in the remote arctic ice (Zarfle and Matthies, 2010; Hubard et al., 2014).
There is hardly any monitoring of the microplastic contamination in the Philippines despite a study by Jambeck et al. (2015) that in 2010, the country has been identified as the third largest contributor of plastic wastes in the ocean globally and that about 1.01 million tonnes of plastic waste is mismanaged by the country in 2016 (Law, et al., 2020). Within the last 5 years, studies on microplastics contamination in the Philippines have been published which reported the occurrence of microplastics in surface water (Argota et al., 2018; Espiritu et al., 2019; Lumongsod and Tanchuling, 2019), marine sediments (Bucol et al., 2019; Espiritu et al., 2019; Esquinas et al., 2020; Kalnasa et al., 2019), and marine biota such as fishes (Bucol et al., 2019; Espiritu et al., 2019; Paler et al., 2021), mussels (Argamino and Janairo, 2016), and oysters (Espiritu et al., 2019). However, Galarpe et al. (2021) mentioned that there is a need for more macroplastic and microplastic research in the country to develop and support appropriate plastic regulation policies.
This research presents an overview and serves as a benchmark for the initial report on the microplastic contamination in the surface waters and sediments of the Manila Bay particularly in the mouths of its tributary rivers namely Cañas River, Meycauayan River, Parañaque River, Pasig River, and Tullahan River. One of the country’s widely used, semi-closed inlet for various economic, industrial, and domestic activities of more than 16 million Filipinos, Manila Bay, is surrounded by densely populated areas including Metro Manila (PEMSEA and MBEMP TWG RRA, 2004). Due to intensive anthropogenic activities and mismanaged plastic waste and dumping within the watershed of the bay, the surface waters and sediments of the bay may accumulate high concentration of microplastics.
It is important to gather baseline information to minimize health and environmental risks posed by these microplastics in the future, specifically to the water quality of marine and freshwater environment, marine biota and even to human consumption and health.
Materials and Methods
Study Area
Manila Bay (about 220 km coastline and 17,000 km2 drainage area) is a semi-enclosed marine inlet and its coastal water connects to the West Philippine Sea through a 16.7 km wide opening and surrounded by the National Capital Region and the municipalities of Cavite, Bulacan, Bataan, and Pampanga (Chang et al., 2009; Sia et al., 2009). It has 26 catchment areas including the river basins of Pasig and Pampanga, and a volume of approximately 31 km3 with an estimated depth of 17 m on an average (PEMSEA and MBEMP TWG-RRA, 2004). The bay is primarily used for international and local port and harbor, fishing ground, aquaculture, and other maritime activities (Prudente et al., 1994).
At present, it has deteriorating water quality because of intensified disposal and mismanagement of solid and human wastes (Chang et al., 2009; Sia et al., 2009). Despite the fact that a large amount of solid wastes was drained into the bay from the highly populated and industrialized area of Metro Manila and its surrounding provinces, data is lacking on the microplastic pollution in the bay. These solid wastes, specifically the plastic wastes would degrade into smaller pieces due to biological, chemical, and mechanical action, thus contributing to the microplastics in Manila Bay.
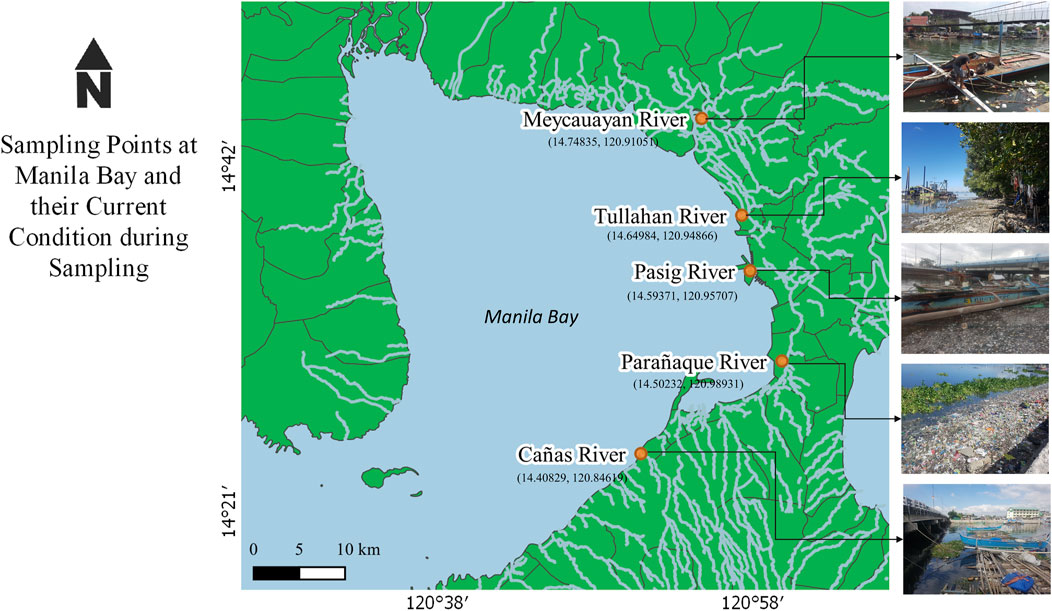
The study mainly focuses on the quantification and characterization of microplastics extracted from the five major river mouths namely Cañas River, Meycauayan River, Parañaque River, Pasig River, and Tullahan River draining to Manila Bay (Figure 1). These sampling areas were selected as these are the major river systems traversing through the National Capital region and the adjacent cities, which drain to Manila Bay. The mouths of these rivers are also accessible. Location and description of the study areas are presented in Table 1.
Collection of Samples
All samples were collected from October 2018 to March 2019. Sampling was conducted once at each site with both surface water and sediments collected on the same day. Surface water samples were collected prior to sediments to avoid collecting suspended solids from the bottom of sampling sites. Water samples of 0.2 m3 with about 5 m away from the riverbanks were collected using a bucket from 0 to 12 cm below the surface of different points in the sampling area. The use of bucket for surface water sampling is also used by Su et al. (2016), Argota et al. (2018), Lumongsod and Tanchuling (2019) and Suresh et al. (2020) as an alternative to the manta net. The use of manta net was not applicable in the study areas due to the presence of macroplastics and large debris along the rivers.
Water from the bucket was poured unto the stacked stainless sieves with mesh sizes of 2.36-mm (No. 8), 1-mm (No. 18), 500-µm (No. 35), 250-µm (No. 60), 125-µm (No. 120) and 75-µm (No. 200). Particles that are greater than 5 mm that were retained from the 2.36-mm sieve were discarded. The used sieves were thoroughly rinsed with distilled water and left to dry for an hour. The retained particles in each sieve were transferred to their respective glass containers using steel tweezers and a brush.
For sediment sampling at each of the five sites, three sediment samples, were gathered using an Ekman grab sampler (∼0.1 m2) and a scoop with minimal disturbance. Two of these were collected from bank deposits about 10 m away from each other while the remaining one sample was taken from the main channel deposits of the river, The topmost 5 cm of the sediments was collected, stored in separate glass containers, and transported to the laboratory for processing together with the surface water samples.
Overall, there were five surface water samples and 15 sediment samples collected from five river mouths, with six subsamples each representing the particles retained in mesh sieves giving a total of 120 subsamples to be processed for digestion, density separation and filtration.
Extraction of Microplastics
The extraction of microplastics was conducted in accordance with previous studies (Peng et al., 2017; Su et al., 2016; Zhang, 2017), with some minor modifications. All samples from the surface waters and sediments were oven-dried for 72 h with a temperature of 90°C.
After drying, an aliquot of 70 g in each sediment sample was taken and poured through a stacked arrangement of 2.36-mm (No. 8), 1-mm (No. 18), 500-µm (No. 35), 250-µm (No. 60), 125-µm (No. 120) and 75-µm (No. 200) stainless mesh sieves. Sediments that passed through the 75-µm sieve were discarded. The sediments in each sieve were transferred to their respective glass containers.
Wet peroxide oxidation (WPO) method was conducted to remove the organic matter present in all samples. At room temperature, 20 cm3 of both aqueous 0.05 M Fe (II) solution and 30% H2O2 solution were added to the beaker containing collected solids in each size fraction (Yonkos et al., 2014; Masura et al., 2015). The samples were heated to 75°C and agitated at 200 rpm using a magnetic stirring hotplate for at least 45 min (Ling et al., 2017). In instances where natural organic materials were still visible, an additional 20 cm3 of 30% H2O2 was continually poured to the mixture until no gas bubbles were observed.
After all the visible organic matter were removed, density separation method was applied which allowed the high-density polymers to float. High-density sodium chloride (NaCl; 1.202 g/cm3) was added to the mixtures of the samples (Galgani et al., 2013). NaCl solution is an effective medium that allows plastics such as PE (0.91–0.97 g/cm3), PP (0.94 g/cm3), PS (1.05 g/cm3), and PVC (1.14 g/cm3) to float (Van Cauwenberghe et al., 2015). However, for sediment samples of 75 microns in which the floating particles could no longer be seen by the naked eye, zinc chloride (ZnCl2; 1.6 g/cm3) was added to ensure the separation of all common polymers (Van Cauwenberghe et al., 2015). The samples were mixed on a hot plate with a magnetic stirrer at 300 rpm for about 15 min and were kept overnight covered with watch glass to observe clearance level wherein the microplastics floated and the other heavier particles settled down.
The supernatant water from the samples was transferred to another glass container, and then filtered using a Longer medium-high flow rate peristaltic pump with 47-mm Whatman® GF/C filters (pore size of 1 µm) to separate the microplastics.
Identification and Quantification of Microplastics
The extracted microplastics were examined and photographed using a 3.1-Megapixel CMOS camera connected to a ST-7045 Stereo microscope with magnification of 10x to 40x. The length and area of microplastics were analyzed using the ToupView software equipped with the digital microscope calibrated to a standard.
All the particles were sorted according to their color, size, and shape (depends on physical characteristics) using the classification from previous studies (Free et al., 2014; Li et al., 2016) as indicated in Table 2.
Polymer make-up was determined using FTIR spectroscopy (Nicolet 6700 FTIR ATR spectrometer with a diamond accessory). Extracted particles were grouped and representative microplastics in each group of at least 30 particles per site were chosen to test polymer types. This was done as a compromise between labor and cost which was also done by Peng et al., 2017. Due to size constraints of ATR-FTIR Spectroscopy, it should be noted that only particles with sizes of 500 µm and above were analyzed. However, it is assumed that those particles with sizes below 500 µm which were subjected to wet peroxide oxidation and density separation are microplastics.
At a resolution of 4 cm−1, thirty-two scans were collected in each sample, and each generated spectrum was documented in the 4,000–400 cm−1 spectral range. The attained spectra were analyzed using the BioRad KnowItAll® Informatics System (2018) software resourced with KnowItAll FTR spectral library that contained an intensive database of known compounds (Horton et al., 2017). For the reliability of data, only those spectra with matching score of more than 70% with the standard database were accepted (Zhao et al., 2018).
Quality Assurance and Quality Control
Usage of plastic containers for storage samples was avoided since these may cause additional microplastics in the samples, especially those that are not visible to the naked eye. The containers were properly labelled, stored in a box and transported to the laboratory within the same day for processing. Personal protective equipment like laboratory gowns, safety masks and nitrile gloves were always worn by the investigators during the experiment and analysis.
Collected solids in the glass containers were transferred to a clean beaker using minimal rinsing with squirt bottle containing distilled water, and the beaker was immediately covered with a watch glass to avoid spillage and contamination. Samples stored in glass Petri dishes were always covered to lessen the period of exposure.
The beaker and all the transfer apparatus were washed with distilled water multiple times to minimize any sample loss owing to adhesion of microplastics on the walls of the filter apparatus, and all washing solutions were filtered through the same glass-fiber filter. Filters were air dried and subsequently sealed individually in Petri dishes.
The crystal of the ATR-FTIR was cleansed using Kimtech Science Kimwipes and EtOH (96%) before and after analyzing each particle. Before the start of every FTIR analysis, a blank background scan was tested.
Results and Discussion
Abundance of Microplastics
Microplastics were ubiquitously extracted in both surface water and sediment samples. The abundance of these particles, as illustrated in Figure 2, varied from 1,580–57,665 particles/m3 in surface waters and 514–1, 357 particles/kg in dry sediments.
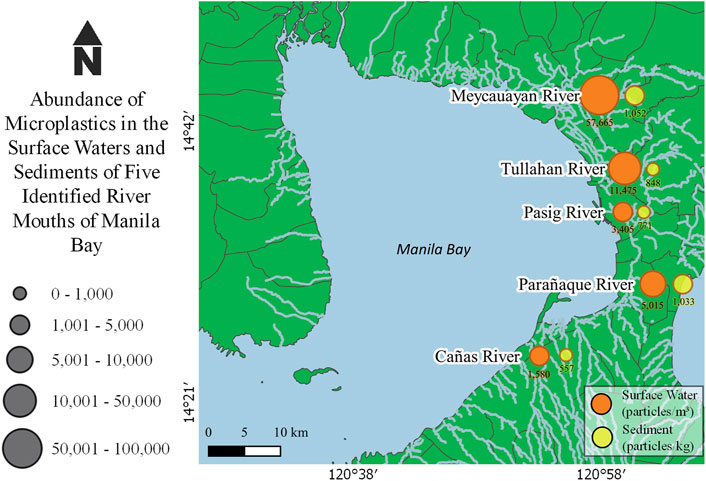
FIGURE 2. Microplastic abundance in surface waters and sediments of five river mouths in Manila Bay.
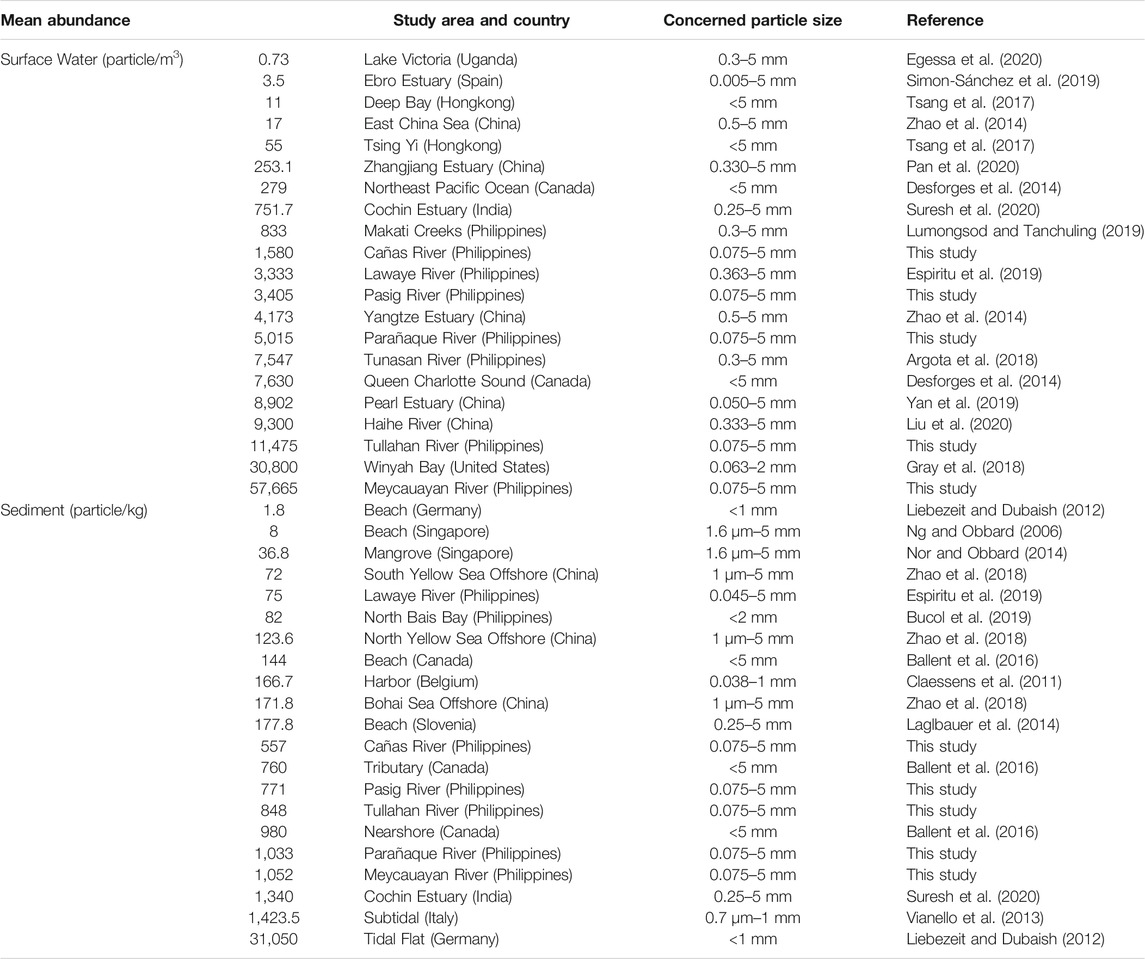
There is no uniform standard for recording the abundance of microplastics in surface water and sediment worldwide, hence not all the results from published studies can be used for comparison. In this case, only related studies that used similar quantification units (particles/m3 for surface water; particles/kg of dry weight for sediment) for the abundance of microplastics were gathered as shown in Table 3. Even though these comparisons might be inaccurate because of the differences in the concerned particle size being studied, sampling methodologies, and time and date of sampling, these studies still provide the general overview of the microplastic pollution worldwide (Tanchuling and Osorio, 2020).
Comparison of microplastic particle abundance in surface water across the globe with this study (Table 3) indicates a wide variation from as low as 0.73 particles/m3 in Lake Victoria of Uganda (Egessa et al., 2020) to as high as 30, 800 particles/m3 in the Winyah Bay of USA (Gray et al., 2018). Generally, the results in this study were relatively higher compared to other studies (Desforges et al., 2014; Zhao et al., 2014; Tsang et al., 2017; Simon-Sánchez et al., 2019; Egessa et al., 2020; Pan et al., 2020; Suresh et al., 2020). As for the Philippine setting, the abundance of microplastics in the surface waters of Meycauayan River and Tullahan River were at higher levels as compared to Tunasan River in Laguna, Lawaye River in Batangas, and creeks in Makati City (Argota et al., 2018; Espiritu et al., 2019; Lumongsod and Tanchuling, 2019).
With regards to the abundance of microplastics in the sediments, the sampling areas had higher levels than previous studies (Ng and Obbard, 2006; Claessens et al., 2011; Liebezeit and Dubaish, 2012; Van Cauwenberghe et al., 2013; Laglbauer et al., 2014; Nor and Obbard, 2014; Zhao et al., 2018) including those studies from the Philippines (Bucol et al., 2019; Espiritu et al., 2019) (Table 3).
Characteristics of Microplastics
Microplastics are existing in environmental compartments as particles having a variety of colors, shapes, sizes, polymer make-up and even densities (Zhou et al., 2020; Isaac and Kandasubramanian, 2021). These properties were found to be related with the environmental effects and fate of microplastics in the environment (Lambert et al., 2017) and even to the sinking of floating microplastics (Ryan, 2015; Kowalski et al., 2016). Therefore, the extracted microplastics were classified and quantified according to these properties as shown in Figure 3.
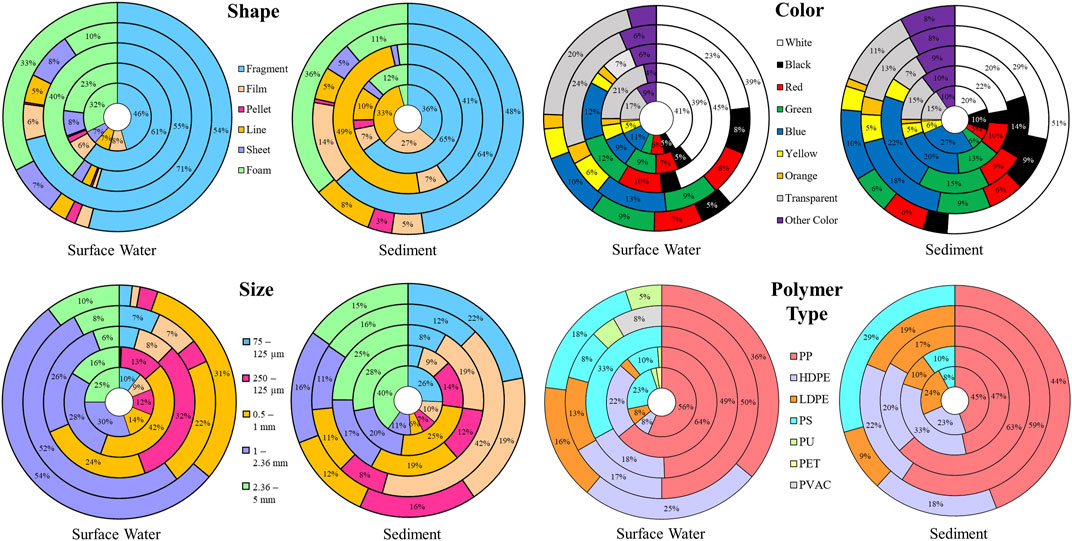
FIGURE 3. Distribution of characteristics of microplastics in surface waters and sediments of five river mouths in Manila Bay (from innermost to outermost circle) – Cañas, Meycauayan, Parañaque, Pasig, and Tullahan.
Shape and Polymer Type
Microplastics were classified according to fragment, film, pellet/granule, line/fiber, sheet, and foam (Figure 4). These shapes could indicate the parent materials of the microplastics (Zhang et al., 2018).
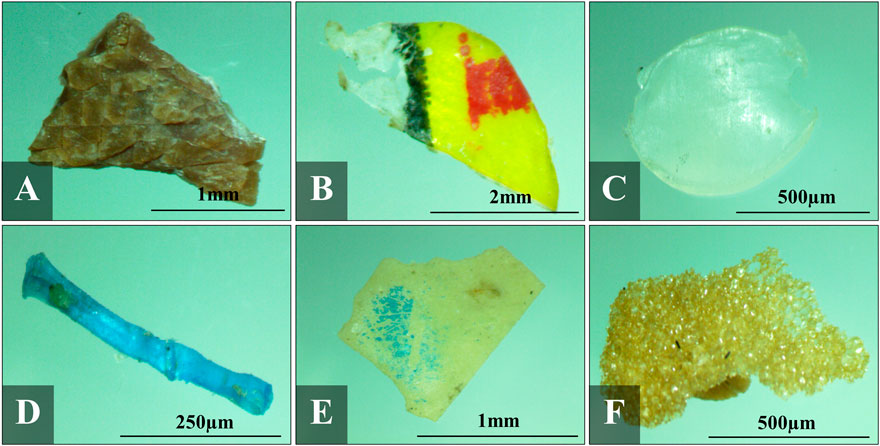
FIGURE 4. Photographs of different shapes of microplastics collected in all samples: (A) fragment, (B) film, (C) pellet, (D) line, (E) sheet, and (F) foam.
Fragment as illustrated in Figure 3 is the most dominant shape across all samples. Possible origins of these particles are from the degraded plastic products which include plastic containers, packaging materials and cleaning media (Derraik, 2002; Thompson et al., 2004; Zhang et al., 2015). Most of these fragments were identified as polypropylene (PP) which was the most abundant polymer type identified in most of the representative samples for both surface water and sediment (Figure 3). The abundance of these fragments maybe attributed to indiscriminate waste dumping and mismanaged plastic wastes as evidenced by the amount of macroplastics observed during sampling.
Films were also significantly abundant in all samples especially in the rivers of Cañas and Pasig as the mouth of these two rivers were surrounded by large residential settlements wherein direct littering and rampant garbage dumping were observed. Most films were detected as low-density polyethylene (LDPE) (Figure 3). These films could possibly originate from plastic wrapping, bags and packing materials (Zhang et al., 2015) including single-use plastics, wrappers and sachets observed in each river mouth.
Primary microplastics such as pellets that are mainly used as raw materials for manufactured plastic products (Hidalgo-Ruz et al., 2012; McDermid and McMullen, 2004), were extracted in most of the samples for both surface waters and sediments, indicating the contribution from the industry. These pellets were identified as high-density polyethylene (HDPE) and could originate from the spillage and personal care products (Fendall and Sewell, 2009; Eerkes-Medrano et al., 2015) (Figure 3). Interestingly, higher number of pellets were extracted from surface waters of Meycauayan River (particle count of 204) compared to the other rivers being studied (particle count of less than 35). Possible origin would be leakage from the plastic manufacturing industries within the watershed of Navotas-Malabon-Tenejeros-Tullahan and Marilao-Meycauayan-Obando River System.
Lines and fibers were also identified in the samples for all sites. These were identified as PP, PE and PET. PET was only identified in the surface water sample of Cañas River while PP and PE were present in all sampling sites (Figure 3). These were probably fragment of plastics originating from fishing nets, ropes, plastic straws and even textiles (Cole et al., 2011; Wang et al., 2017). Aside from garbage dumping and degradation of larger plastics, washing is also considered as an important pathway that releases these lines, fibers, and other airborne particles into the environment (Dris et al., 2016; Salvador Cesa et al., 2017). Numerous fishing activities, aquaculture, and harbors for canoes and bancas present in the sampling areas could be considered as main contributors to the presence of lines and fibers in the samples. The most abundant type in the sediment samples of Parañaque River was line. It is likely due to the existence of large amount of plastic straws, ropes, and fishing nets from the canoes and bancas docked beside the seafood market along the mouth of the river.
Foams mostly polystyrene (PS) were sufficient in amount in the surface waters especially in the rivers of Meycauayan and Tullahan, wherein thousands of foams were extracted (Figure 3). These particles were also identified in the sediments of all study sites in low quantities. It is observed that concentration of PS in surface waters were more abundant than those in the sediments. This is likely due to the air injected into the foam, specifically Styrofoam, making it less dense and allowing it to float. Aside from PS, polyurethane (PU) was identified in some foams in the surface waters of Cañas, Meycauayan, Pasig and Tullahan River, but in low quantities only. Site visit and ocular inspection on the sampling areas determined that the possible origin of the foams present in these sites were from large quantities of disposable plastic containers produced such as food and product containers utilized for food delivery services and takeout food, and even from the foam fishing floats and rafts used by fishermen (Di and Wang, 2018).
Significant number of sheets was detected, and these particles were also associated to the degradation of larger plastic products especially for those macroplastics existing in the sampling areas (Cole et al., 2011). Analyzed sheet particles were mostly PP and HDPE. One of the sheets in the surface water sample of Pasig River was identified as polyvinyl acetate (PVAC) which is primarily used as adhesives for porous materials such as white glue. It is possible that the adhesive material stuck on the particle was identified by ATR-FTIR instead of the polymer make-up of the sheet itself.
Low-density polymers including PP, PE, PS and PU particles are expected to float in surface water because their densities are lower as compared to freshwater. However, the polymer types of PP and PE were the most identified types of plastic among the analyzed samples in the sediments, implying that density is not the only factor affecting the microplastic distribution in the surface waters and sediments. These low-density particles could also be conveyed through current and deposited on the sediments through the following mechanisms: 1) increase in net density of microplastics through biofouling (Andrady, 2011), 2) natural substances adsorption to the surface (Frias et al., 2010), 3) inclusion of inorganic fillers during manufacturing (Corcoran et al., 2015), and 4) fecal express (Cole et al., 2015).
Interestingly, PET whose density is higher than the freshwater was detected in the surface waters of Cañas River even though it is expected to settle down, although in relatively smaller amounts. This might be due to environmental factors such as temperature, wind driven turbulence, tides and waves, these denser plastic particles could have been resuspended from the deeper waters to the surface (Zhao et al., 2014). PET bottles were commonly collected and sold to junkshops; hence this might be one of the reasons why PET particles were barely identified in the samples.
Spectra were generated for all the analyzed particles. Some of these are shown in Figure 5, and the additional spectral band observed may be associated to the plastic additives or the adsorption of persistent organic pollutants or organic materials (Cole et al., 2011). Further investigation is recommended to identify these specific substances adsorbed to the microplastic samples.
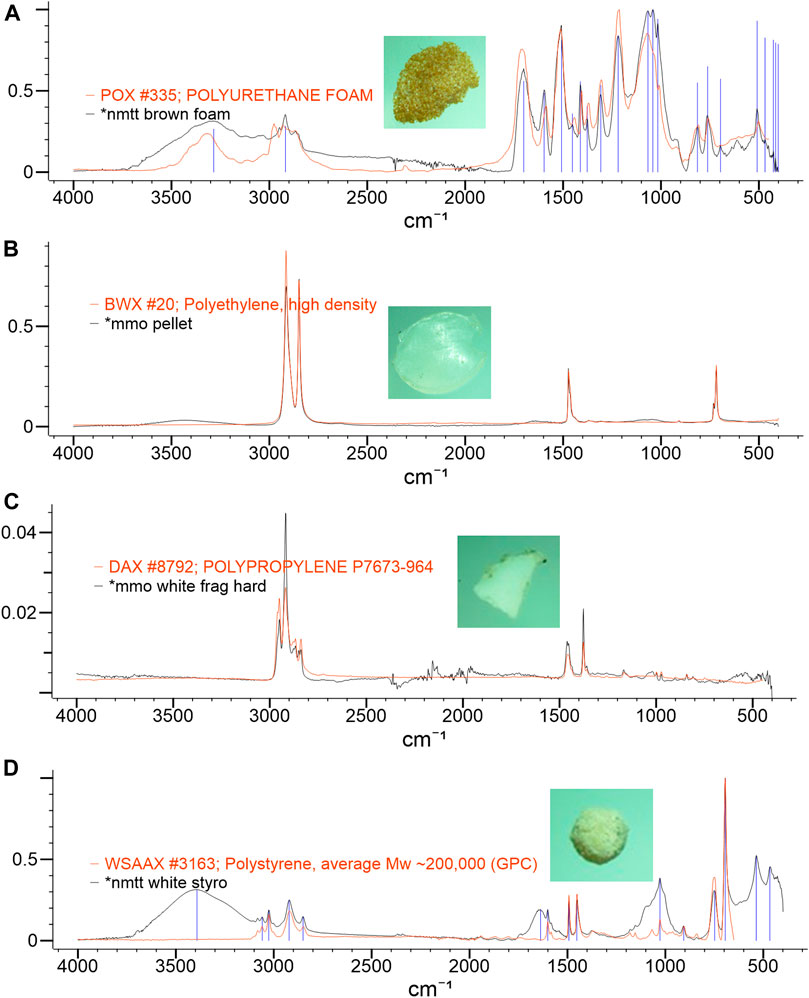
FIGURE 5. Spectrum for (A) brown foam identified as PU, (B) white pellet identified as HDPE, (C) white fragment identified as PP, and (D) white foam identified as PS.
The abundance of the fragments, films, lines and fibers, foams and sheets suggested that majority of the collected microplastics in the mouths of the rivers being studied were locally derived secondary microplastics (Wessel et al., 2016; Yonkos, et al., 2014).
The observed morphological patterns such as scratches, creases, micro-pores, and cracks, among others in the extracted microplastic particles indicate that their source from the degradation of products such as plastic containers, packaging materials, and ropes (Zhang et al., 2016). Moreover, the shape of microplastics were modified by different forces such as the actions from tides and waves (Karthik et al., 2018). Several factors including origins, debris quality, breakdown of plastics, and plastic sinking rate could control the plastic distribution, hence diverse shape distributions in the sampling areas were identified (Critchell and Lambrechts, 2016).
Color
The microplastic particles were also classified into colors such as red, orange, yellow, green, blue, black, white, transparent, and other colors including violet, brown, and multi-colored particles. Photographs of typical microplastic samples categorized into different colors of microplastics were presented in Figure 6.

FIGURE 6. Photographs of different colors of microplastics collected in all samples – (A) white, (B) black, (C) red, (D) green, (E) blue, (F) yellow, (G) orange, (H) transparent, and (I) violet.
White colored particles may represent the color of plastics such as Styrofoam™, cap bottles, disposable spoon and fork, and ice cream cups while transparent colored microplastics may come from the virgin pellets, cellophanes, single-use plastics, plastic packing bags and boxes used as food containers. Other identified color particles such as red, green, blue, yellow, orange, violet, and brown were possibly fragmented from commonly used products such as packaging, toys, household products, sachets, plastic straw ropes, and food wrappers. Colored microplastics detected in the environment could be related to the high consumption of the colored plastic products in everyday living (Zhang et al., 2015; Wang et al., 2017).
Color could also be one of the factors for the ingestion of microplastics by marine biota due to resemblance of their prey (Wright et al., 2013). Based on previous studies, it was found out that fish may mistakenly feed on microplastics with colors of white, brown, and yellow that most closely resemble their zooplankton prey (Shaw and Day, 1994; Boerger et al., 2010). Moreover, it was discovered that translucent and light-colored plastics were commonly ingested by sea turtles (Bugoni et al., 2001) and some shapes and colors of plastics were accidentally ingested by some seabirds (Barboza and Gimenez, 2015).
As illustrated in Figure 3, white was the most prevalent color among all surface and sediments samples. This color was also the most observed in Guanabara Bay (Castro et al., 2016) and Northeast Atlantic Ocean (Lusher et al., 2013). These white particles primarily consisted of foams, pellets, and fragments.
Most of the plastic pellets extracted from the surface waters and sediments were white and transparent, like previous studies conducted (Turner and Holmes, 2011; Corcoran et al., 2015). This finding is expected since white and transparent pellets are the most common color manufactured (Redford et al., 1997). However, there were very few amounts of black, blue, and orange pellets extracted in some samples, which was also observed by Young and Elliott, 2016.
Transparent and blue were also identified abundantly in all samples. These transparent particles were recognized to be dominant in surface water samples and possibly originated from the short-lifetime and disposable packaging plastic products such as containers, bottles, cups and bags. Furthermore, blue microplastics were mainly comprised of fragments and lines (most probably fishing lines and plastic straws). High prevalence of blue-colored microplastics in the aquatic environment could increase the likelihood of ingestion since some marine fishes reportedly ingested blue microplastics as food (Güven et al., 2017; Ory et al., 2017). Moreover, blue microplastics were also frequently found in clams (Shi, 2018).
Other colored microplastics such as red, green, and yellow were also found in significant amount in the samples as these could be derived from different plastic packaging materials (Zhang et al., 2018). High abundance of colored plastics, especially colored fragments, attributed to the broad usage of coloration in the manufacturing, since coloration is used to attract consumers of plastic products (Thetford et al., 2003).
Size
It has been reported that microplastic ingestion is primarily determined by size and abundance as compared to color and it occurs by chance (Rodríguez-Sejio and Pereira, 2017). The microplastics could pose risks to the marine biota as demonstrated in fish larvae wherein organisms likely to consume their prey with the smallest dimension (Hunter, 1981). These particles are now considered available for ingestion to aquatic organisms since the sizes of these approximate the size of some planktons (Cole et al., 2011; Wright et al., 2013; Cole et al., 2015). However, due to the differences in the results from published research, further studies are needed to verify which among the attributes of microplastics aid the ingestion.
The extracted particles in this study were classified into six size categories as shown in Figure 7. The lower size limit of microplastics in this study is 75 µm. Among these different size categories of microplastic particles, the class 1–2.36 mm, contributed the highest quantity in the surface water samples in most of the study sites, except in the rivers of Meycauayan and Parañaque wherein the most abundant size category was 500–1,000 μm and 250–500 μm, respectively (Figure 3).
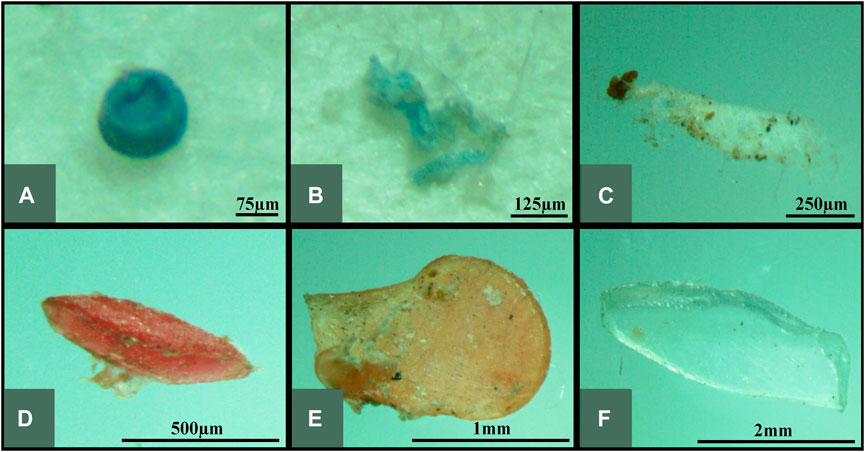
FIGURE 7. Photographs of different sizes of microplastics collected from the surface water and sediment samples – (A) 75 – <125 µm, (B) 125 – <250 µm, (C) 250 – <500 µm, (D) 500 – <1000 µm, (E) 1 – <2.36 mm, and (F) 2.36 – <5 mm.
In general, about 80% of total microplastics recovered in the samples from surface water were within the size class of 250 μm–5 mm. The size range of these microplastics were still considered visible to the naked eye.
In terms of the microplastic size extracted from the sediment samples, it is observed in Figure 3 that the sizes were relatively evenly distributed in each study site as compared to the distribution of sizes of microplastics collected in the surface water samples. Microplastic particles with size class of 2.36–5 mm, contributed the highest quantity in the sediment samples in most of the study sites, except in the rivers of Pasig and Tullahan wherein the most abundant size category was 125–250 μm and 75–125 μm, respectively. Despite the differences in the percentage distribution of sizes, a significant amount of microplastics was extracted from each size class in each study site.
Source, Fate and Transport of Microplastics
The highest concentration of microplastics for both surface waters and sediments were found to be in Meycauayan River while the lowest was detected in Cañas River (Figure 2). All sampling was done during ebb tide wherein axial convergence occurs near the bottom of the river mouths and axial divergence prevails at the surface making floating matters such as microplastics are pushed towards the banks (Wolanski and Elliot, 2015). This could explain the occurrence of microplastic particles collected in the surface water and sediment along the banks in the mouths of the river sampling.
It could be observed that from south (Cañas River) to north (Meycauayan River), the concentration of microplastics in surface waters tend to increase (Figure 2). The abundance of microplastics in the rivers of Pasig, Tullahan and Meycauayan could be further correlated with the calculated plastic emissions of these rivers from the study of Meijer et al. (2021). This could also be associated with the presence of several plastic manufacturing industries within the watershed of Marilao-Meycauayan-Obando and Navotas-Malabon-Tenejeros-Tullahan River Systems. This is supported by the presence of pellets which are considered as primary microplastics.
With regards to the wind transport in the bay, individual average wind blow at specific period of the year-controlled bay’s circulated gyres differently as simulated by De Las Alas and Sodusta (1985). There are Northeasterly winds, with speeds averaging about 5 m/s from October to March and Southwesterly winds, with speeds of 5–7 m/s from April to September. Since all sampling were conducted during Northeast monsoon, the effect of winds could possibly contribute to the high abundance of microplastics collected in the rivers of Meycauayan and Tullahan as the direction of the flow of the winds is in counterclockwise direction. While the direction of winds at the southeastern part of the bay where the rivers of Parañaque and Cañas are located is in clockwise direction, the microplastics at their river mouths might be flushed out to the bay.
However, it is recommended to conduct more detailed studies on the combined influence of river runoff, tidal input, coastal currents, and wind transport to the abundance of microplastics in Manila Bay.
In terms of the concentration of microplastics in the sediments, the microplastic pollution levels in sediments from the bay were more closely related to the distance from the contamination source (Su et al., 2016) and these microplastic most observed in Guanabara B particles could only be highly disturbed, collected and transported during dredging activities. For example, the amount of microplastics extracted in the sediments in Parañaque River could have possibly originated from the seafood market beside its river mouth.
Based on the study of Siringan and Ringor (1998), the transport direction of surface sediment is southward off Pampanga and northeastward off Cavite. The bottom sediments are always transported from the bay mouth into the bay, and the sediments do not flow from Manila Bay. This would imply that the sediments were more stable as compared to surface water, and the suspended microplastics in the sediments will be transported slower than those floating in surface water (Su et al., 2016).
Manila Bay has a narrow mouth and a relatively weak tidal current, hence the residence time of water inside the bay is relatively longer (Pokavanich and Nadaoka, 2006). It is possible that the microplastics floating in the surface water in each river mouth will be drained off into the bay and will be retained in the bay for a long period of time. These floating microplastics could retain and undergo degradation and be biofouled, making them dense enough to sink (Andrady, 2011; Van Cauwenberghe et al., 2013). This scenario would likely increase not just the concentration of microplastics in the surface waters, but also in the bottom sediments of Manila Bay.
Conclusion
The widespread occurrence in different environmental compartments of microplastics has been recorded worldwide, however, few studies are available on the microplastics present in the aquatic systems in the Philippines. This is the first extensive microplastic study in Manila Bay. This bay serves as an important area for fishing ground and aquaculture activities, hence the occurrence of microplastics in this bay will likely impact on marine organisms and the entire food web through bioaccumulation.
Surface water and sediment samples were collected in the river mouths of Cañas, Meycauayan, Parañaque, Pasig and Tullahan which are draining to Manila Bay. The abundance of microplastics ranges from 580 to 57,665 particles/m3 in surface water and 386 to 1,357 particles/kg in dry sediment. The levels of microplastic pollution found in these rivers are currently at higher levels, compared to the other studies. Secondary microplastics were found to be the most abundant as fragments indicating that these originate from plastic wastes which were improperly disposed. Therefore, prevention of leakage of both macroplastics and microplastics in the tributary rivers and the bay itself is very important to preserve the marine environment in Manila Bay.
More extensive studies in different waterbodies, and other environmental compartments with sampling in both wet and dry periods to account the effect of seasonal aberration in the abundance and distribution of microplastics are recommended to provide a clear overview on the extent of microplastic pollution in the country. The rehabilitated areas and other tributaries draining to Manila Bay are suggested to be used as sampling areas for further studies to provide more assessment regarding the microplastic contamination in the bay.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
EO, MT and MD conceived of the idea, developed the methods, and edited the manuscript. EO conducted the experiment and ran analyses. All authors approved the submitted version of the manuscript and agreed to be listed.
Funding
This work was supported by the Engineering Research and Development for Technology (ERDT) grant of the Department of Science and Technology (DOST) and the Office of the Vice Chancellor for Research and Development Outright Research Grant of the University of the Philippines Diliman.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson, J. C., Park, B. J., and Palace, V. P. (2016). Microplastics in Aquatic Environments: Implications for Canadian Ecosystems. Environ. Pollut. 218, 269–280. doi:10.1016/j.envpol.2016.06.074
Andrady, A. L. (2011). Microplastics in the marine Environment. Mar. Pollut. Bull. 62 (8), 1596–1605. doi:10.1016/j.marpolbul.2011.05.030
Antunes, J., Frias, J., and Sobral, P. (2018). Microplastics on the Portuguese Coast. Mar. Pollut. Bull. 131, 294–302. doi:10.1016/j.marpolbul.2018.04.025
Argamino, C. R., and Janairo, J. I. B. (2016). Qualitative Assessment and Management of Microplastics in Asian Green Mussels (Perna Viridis) Cultured in Bacoor Bay, Cavite, Phillipines. Environ. Asia 9 (2), 48–54. doi:10.14456/ea.2016.7
Argota, H. L., Bajado, J. A., Diola, M. D., and Tanchuling, M. A. N. (2018). “Macro- and Microplastic Pollution in Tunasan River, Metro Manila, Philippines,” in Proceedings of the 4th symposium of the Asian regional branch of international waste working group (IWWG-ARB) 2019, Bangkok, Thailand, 20–22 February 2019.
Ballent, A., Corcoran, P. L., Madden, O., Helm, P. A., and Longstaffe, F. J. (2016). Sources and Sinks of Microplastics in Canadian Lake Ontario Nearshore, Tributary and beach Sediments. Mar. Pollut. Bull. 110 (1), 383–395. doi:10.1016/j.marpolbul.2016.06.037
Barboza, L. G. A., and Gimenez, B. C. G. (2015). Microplastics in the marine Environment: Current Trends and Future Perspectives. Mar. Pollut. Bull. 97, 5–12. doi:10.1016/j.marpolbul.2015.06.008
Biginagwa, F. J., Mayoma, B. S., Shashoua, Y., Syberg, K., and Khan, F. R. (2016). First Evidence of Microplastics in the African Great Lakes: Recovery from Lake Victoria Nile Perch and Nile tilapia. J. Great Lakes Res. 42 (1), 146–149. doi:10.1016/j.jglr.2015.10.012
Boerger, C. M., Lattin, G. L., Moore, S. L., and Moore, C. J. (2010). Plastic Ingestion by Planktivorous Fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 60, 2275–2278. doi:10.1016/j.marpolbul.2010.08.007
Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., et al. (2011). Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 45 (21), 9175–9179. doi:10.1021/es201811s
Browne, M. A., Galloway, T. S., and Thompson, R. C. (2010). Spatial Patterns of Plastic Debris along Estuarine Shorelines. Environ. Sci. Technol. 44, 3404–3409. doi:10.1021/es903784e
Bucol, L. A., Romano, E. F., Cabcaban, S. M., Siplon, L. M. D., Madrid, G. C., Bucol, A. A., et al. (2020). Microplastics in marine Sediments and Rabbitfish (Siganus fuscescens) from Selected Coastal Areas of Negros Oriental, Philippines. Mar. Pollut. Bull. 150, 110685–110694. doi:10.1016/j.marpolbul.2019.110685
Bugoni, L., Krause, L., and Virgı́nia Petry, M. (2001). Marine Debris and Human Impacts on Sea Turtles in Southern Brazil. Mar. Pollut. Bull. 42, 1330–1334. doi:10.1016/s0025-326x(01)00147-3
Carpenter, E. J., Anderson, S. J., Harvey, G. R., Miklas, H. P., and Peck, B. B. (1972). Polystyrene Spherules in Coastal Waters. Science 178, 749–750. doi:10.1126/science.178.4062.749
Castro, R. O., Silva, M. L., Marques, M. R. C., and de Araújo, F. V. (2016). Evaluation of Microplastics in Jurujuba Cove, Niterói, RJ, Brazil, an Area of Mussels Farming. Mar. Pollut. Bull. 110, 555–558. doi:10.1016/j.marpolbul.2016.05.037
Chang, K. H., Amano, A., Miller, T. W., Isobe, T., Maneja, R., Siringan, F. P., et al. (2009). Pollution Study in Manila Bay: Eutrophication and its Impact on Plankton Community. Interdiscip. Stud. Environ. Chemistry—Environmental Res. Asia, 261–267.
Claessens, M., Meester, S. D., Landuyt, L. V., Clerck, K. D., and Janssen, C. R. (2011). Occurrence and Distribution of Microplastics in marine Sediments along the Belgian Coast. Mar. Pollut. Bull. 62, 2199–2204. doi:10.1016/j.marpolbul.2011.06.030
Cole, M., Lindeque, P., Fileman, E., Halsband, C., and Galloway, T. S. (2015). The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine CopepodCalanus Helgolandicus. Environ. Sci. Technol. 49, 1130–1137. doi:10.1021/es504525u
Cole, M., Lindeque, P., Halsband, C., and Galloway, T. S. (2011). Microplastics as Contaminants in the marine Environment: a Review. Mar. Pollut. Bull. 62 (12), 2588–2597. doi:10.1016/j.marpolbul.2011.09.025
Corcoran, P. L., Norris, T., Ceccanese, T., Walzak, M. J., Helm, P. A., and Marvin, C. H. (2015). Hidden Plastics of Lake Ontario, Canada and Their Potential Preservation in the Sediment Record. Environ. Pollut. 204, 17–25. doi:10.1016/j.envpol.2015.04.009
Critchell, K., and Lambrechts, J. (2016). Modelling Accumulation of marine Plastics in the Coastal Zone; what Are the Dominant Physical Processes?. Estuarine, Coastal Shelf Sci. 171, 111–122. doi:10.1016/j.ecss.2016.01.036
De Las Alas, J. G., and Sodusta, J. A. (1985). A Model for the Wind Driven Circulation of Manila Bay. Nat. Appl. Sci. Bull. 37 (2), 159–170.
Derraik, J. G. B. (2002). The Pollution of the marine Environment by Plastic Debris: a Review. Mar. Pollut. Bull. 44, 842–852. doi:10.1016/s0025-326x(02)00220-5
Desforges, J.-P. W., Galbraith, M., Dangerfield, N., and Ross, P. S. (2014). Widespread Distribution of Microplastics in Subsurface Seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 79, 94–99. doi:10.1016/j.marpolbul.2013.12.035
Di, M., and Wang, J. (2018). Microplastics in Surface Waters and Sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 616-617, 1620–1627. doi:10.1016/j.marpolbul.2016.01.00610.1016/j.scitotenv.2017.10.150
Dris, R., Gasperi, J., Saad, M., Mirande, C., and Tassin, B. (2016). Synthetic Fibers in Atmospheric Fallout: a Source of Microplastics in the Environment?. Mar. Pollut. Bull. 104, 290–293. doi:10.1016/j.marpolbul.2016.01.006
Eerkes-Medrano, D., Thompson, R. C., and Aldridge, D. C. (2015). Microplastics in Freshwater Systems: a Review of the Emerging Threats, Identification of Knowledge Gaps and Prioritisation of Research Needs. Water Res. 75, 63–82. doi:10.1016/j.watres.2015.02.012
Egessa, R., Nankabirwa, A., Ocaya, H., and Pabire, W. G. (2020). Microplastic Pollution in Surface Water of Lake Victoria. Sci. Total Environ. 741, 140201. doi:10.1016/j.scitotenv.2020.140201
Eriksen, M., Mason, S., Wilson, S., Box, C., Zellers, A., Edwards, W., et al. (2013). Microplastic Pollution in the Surface Waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 77, 177–182. doi:10.1016/j.marpolbul.2013.10.007
Espiritu, E. Q., Dayrit, S. A. S., Coronel, A. S. O., Paz, N. S. C., Ronquillo, P. I. L., Castillo, V. C. G., et al. (2019). Assessment of Quantity and Quality of Microplastics in the Sediments, Waters, Oysters, and Selected Fish Species in Key Sites along the Bombong Estuary and the Coastal Waters of Ticalan in San Juan, Batangas. Philippine J. Sci. 148 (4), 789–801.
Esquinas, G. G. M. S., Mantala, A. P., Atilano, M. G., Apugan, R. P., and Galarpe, V. R. K. R. (2020). Physical characterization of litter and microplastic along the urban coast of Cagayan de Oro in Macajalar Bay, Philippines. Mar. Pollut. Bull. 154, 111083–111086. doi:10.1016/j.marpolbul.2020.111083
Fendall, L. S., and Sewell, M. A. (2009). Contributing to marine Pollution by Washing Your Face: Microplastics in Facial Cleansers. Mar. Pollut. Bull. 58, 1225–1228. doi:10.1016/j.marpolbul.2009.04.025
Free, C. M., Jensen, O. P., Mason, S. A., Eriksen, M., Williamson, N. J., and Boldgiv, B. (2014). High-levels of Microplastic Pollution in a Large, Remote, mountain lake. Mar. Pollut. Bull. 85, 156–163. doi:10.1016/j.marpolbul.2014.06.001
Frias, J. P. G. L., Sobral, P., and Ferreira, A. M. (2010). Organic Pollutants in Microplastics from Two Beaches of the Portuguese Coast. Mar. Pollut. Bull. 60, 1988–1992. doi:10.1016/j.marpolbul.2010.07.030
Galarpe, V. R. K. R., Jaraula, C. M. B., and Paler, M. K. O. (2021). The Nexus of Macroplastic and Microplastic Research and Plastic Regulation Policies in the Philippines marine Coastal Environments. Mar. Pollut. Bull. 167, 112343. doi:10.1016/j.marpolbul.2021.112343
Galgani, F., Hanke, G., Werner, S., and De Vrees, L. (2013). Marine Litter within the European Marine Strategy Framework Directive. ICES J. Mar. Sci. 70, 1055–1064. doi:10.1093/icesjms/fst122
Gesamp, (2015). “Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment,” in IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (Rep. Stud. GESAMP No.. London. International Maritime Organization Editor P. J. Kershaw, 90, 96.
Gray, A. D., Wertz, H., Leads, R. R., and Weinstein, J. E. (2018). Microplastic in Two South Carolina Estuaries: Occurrence, Distribution, and Composition. Mar. Pollut. Bull. 128, 223–233. doi:10.1016/j.marpolbul.2018.01.030
Güven, O., Gökdağ, K., Jovanović, B., and Kıdeyş, A. E. (2017). Microplastic Litter Composition of the Turkish Territorial Waters of the Mediterranean Sea, and its Occurrence in the Gastrointestinal Tract of Fish. Environ. Pollut. 223, 286–294. doi:10.1016/j.envpol.2017.01.025
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C., and Thiel, M. (2012). Microplastics in the marine Environment: a Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 46 (6), 3060–3075. doi:10.1021/es2031505
Horton, A. A., Svendsen, C., Williams, R. J., Spurgeon, D. J., and Lahive, E. (2017). Large Microplastic Particles in Sediments of Tributaries of the River Thames, UK - Abundance, Sources and Methods for Effective Quantification. Mar. Pollut. Bull. 114 (1), 218–226. doi:10.1016/j.marpolbul.2016.09.004
Hunter, J. R. (1981). Feeding Ecology and Predation of marine Fish Larvae. Morphol. Ecol. Relation Fish., 34–77.
Issac, M. N., and Kandasubramanian, B. (2021). Effect of Microplastics in Water and Aquatic Systems. Environ. Sci. Pollut. Res. 28, 19544–19562. doi:10.1007/s11356-021-13184-2
Ivar do Sul, J. A., Spengler, Â., and Costa, M. F. (2009). Here, there and everywhere. Small plastic fragments and pellets on beaches of Fernando de Noronha (Equatorial Western Atlantic). Mar. Pollut. Bull. 58 (8), 1236–1238. doi:10.1016/j.marpolbul.2009.05.004
Jambeck, J. R., Geyer, R., Wilcox, C., Siegler, T. R., Perryman, M., Andrady, A., et al. (2015). Plastic Waste Inputs from Land into the Ocean. Science 347, 768–771. doi:10.1126/science.1260352
Kalnasa, M. L., Lantaca, S. M. O., Boter, L. C., Flores, G. J. T., and Galarpe, V. R. K. R. (2019). Occurrence of Surface Sand Microplastic and Litter in Macajalar Bay, Philippines. Mar. Pollut. Bull. 149, 110521–110524. doi:10.1016/j.marpolbul.2019.110521
Karthik, R., Robin, R. S., Purvaja, R., Ganguly, D., Anandavelu, I., Raghuraman, R., et al. (2018). Microplastics along the Beaches of Southeast Coast of India. Sci. Total Environ. 645, 1388–1399. doi:10.1016/j.scitotenv.2018.07.242
Kowalski, N., Reichardt, A. M., and Waniek, J. J. (2016). Sinking Rates of Microplastics and Potential Implications of Their Alteration by Physical, Biological, and Chemical Factors. Mar. Pollut. Bull. 109, 310–319. doi:10.1016/j.marpolbul.2016.05.064
Laglbauer, B. J. L., Franco-Santos, R. M., Andreu-Cazenave, M., Brunelli, L., Papadatou, M., Palatinus, A., et al. (2014). Macrodebris and Microplastics from Beaches in Slovenia. Mar. Pollut. Bull. 89, 356–366. doi:10.1016/j.marpolbul.2014.09.036
Lambert, S., Scherer, C., and Wagner, M. (2017). Ecotoxicity Testing of Microplastics: Considering the Heterogeneity of Physicochemical Properties. Integr. Environ. Assess. Manag. 13, 470–475. doi:10.1002/ieam.1901
Law, K. L., Starr, N., Siegler, T. R., Jambeck, J. R., Mallos, N. J., and Leonard, G. H. (2020). The United States' Contribution of Plastic Waste to Land and Ocean. Sci. Adv. 6 (44), 1–7. doi:10.1126/sciadv.abd0288
Li, D., Xu, Y., Li, Y., Wang, J., Yin, X., Ye, X., et al. (2018). Sedimentary Records of Human Activity and Natural Environmental Evolution in Sensitive Ecosystems: a Case Study of a Coral Nature reserve in Dongshan Bay and a Mangrove forest Nature reserve in Zhangjiang River Estuary, Southeast China. Org. Geochem. 121, 22–35. doi:10.1016/j.orggeochem.2018.02.011
Li, J., Liu, H., and Paul Chen, J. (2018). Microplastics in Freshwater Systems: A Review on Occurrence, Environmental Effects, and Methods for Microplastics Detection. Water Res. 137, 362–374. doi:10.1016/j.watres.2017.12.056
Li, J., Qu, X., Su, L., Zhang, W., Yang, D., Kolandhasamy, P., et al. (2016). Microplastics in Mussels along the Coastal Waters of China. Environ. Pollut. 214, 177–184. doi:10.1016/j.envpol.2016.04.012
Liebezeit, G., and Dubaish, F. (2012). Microplastics in Beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull. Environ. Contam. Toxicol. 89 (1), 213–217. doi:10.1007/s00128-012-0642-7
Lima, A. R. A., Barletta, M., and Costa, M. F. (2015). Seasonal Distribution and Interactions between Plankton and Microplastics in a Tropical Estuary. Estuarine, Coastal Shelf Sci. 165, 213–225. doi:10.1016/j.ecss.2015.05.018
Ling, S. D., Sinclair, M., Levi, C. J., Reeves, S. E., and Edgar, G. J. (2017). Ubiquity of Microplastics in Coastal Seafloor Sediments. Mar. Pollut. Bull. 121 (5), 104–110. doi:10.1016/j.marpolbul.2017.05.038
Liu, Y., Zhang, J., Cai, C., He, Y., Chen, L., Xiong, X., et al. (2020). Occurrence and Characteristics of Microplastics in the Haihe River: An Investigation of a Seagoing River Flowing through a Megacity in Northern China. Environ. Pollut. 262, 114261. doi:10.1016/j.envpol.2020.114261
Lumongsod, S. L. R., and Tanchuling, M. A. N. (2019). “Microplastic Characterization and Analysis in Three Makati City Creeks,” in Proceedings on the 4th symposium of the international waste working group-Asian regional board (IWWG-ARB) 2019, Bangkok, Thailand, 20–22 February 2019.
Lusher, A. L., McHugh, M., and Thompson, R. C. (2013). Occurrence of Microplastics in the Gastrointestinal Tract of Pelagic and Demersal Fish from the English Channel. Mar. Pollut. Bull. 67, 94–99. doi:10.1016/j.marpolbul.2012.11.028
Masura, J., Baker, J., Foster, G., and Arthur, C. (2015). Laboratory Methods for Analysis of Microplastics in the marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. Maryland: NOAA Marine Debris Division.
McDermid, K. J., and McMullen, T. L. (2004). Quantitative Analysis of Small-Plastic Debris on Beaches in the Hawaiian Archipelago. Mar. Pollut. Bull. 48, 790–794. doi:10.1016/j.marpolbul.2003.10.017
Meijer, L. J. J., van Emmerik, T., van der Ent, R., Schmidt, C., and Lebreton, L. (2021). More Than 1000 Rivers Account for 80% of Global Riverine Plastic Emissions into the Ocean. Sci. Adv. 7 (18), 1–13. doi:10.1126/sciadv.aaz5803
Mohamed Nor, N. H., and Obbard, J. P. (2014). Microplastics in Singapore's Coastal Mangrove Ecosystems. Mar. Pollut. Bull. 79, 278–283. doi:10.1016/j.marpolbul.2013.11.025
Ng, K. L., and Obbard, J. P. (2006). Prevalence of Microplastics in Singapore's Coastal marine Environment. Mar. Pollut. Bull. 52, 761–767. doi:10.1016/j.marpolbul.2005.11.017
Obbard, R. W., Sadri, S., Wong, Y. Q., Khitun, A. A., Baker, I., and Thompson, R. C. (2014). Global Warming Releases Microplastic Legacy Frozen in Arctic Sea Ice. Earth's Future 2, 315–320. doi:10.1002/2014EF000240
Ory, N. C., Sobral, P., Ferreira, J. L., and Thiel, M. (2017). Amberstripe Scad Decapterus Muroadsi (Carangidae) Fish Ingest Blue Microplastics Resembling Their Copepod Prey along the Coast of Rapa Nui (Easter Island) in the South Pacific Subtropical Gyre. Sci. Total Environ. 586, 430–437. doi:10.1016/j.scitotenv.2017.01.175
Paler, M. K. O., Leistenschneider, C., Migo, V., and Burkhardt-Holm, P. (2021). Low Microplastic Abundance in Siganus Spp. From the Tañon Strait, Central Philippines. Environ. Pollut. 284, 117166–117169. doi:10.1016/j.envpol.2021.117166
Pan, Z., Sun, Y., Liu, Q., Lin, C., Sun, X., He, Q., et al. (2020). Riverine Microplastic Pollution Matters: A Case Study in the Zhangjiang River of Southeastern China. Mar. Pollut. Bull. 159, 111516. doi:10.1016/j.marpolbul.2020.111516
PEMSEA, and MBEMP TWG-RRA (2004). “Manila Bay: Refined Risk Assessment. PEMSEA Technical Report No. 9. Global Environment Facility/United Nations Development Programme/International Maritime Organization Regional Programme on Building Partnerships in Environmental Management for the Seas of East Asia (PEMSEA), and Manila Bay Environmental Management Project (MBEMP), Technical Working Group for Refined Risk Assessment (TWG- RRA),” in Quezon City, Philippines: GEF/UNDP/IMO Regional Programme on Building Partnerships in Environmental Management for the Seas of East Asia. Quezon City. GEF/UNDP/IMO PEMSEA and MBEMP, 69.
Peng, G., Zhu, B., Yang, D., Su, L., Shi, H., and Li, D. (2017). Microplastics in Sediments of the Changjiang Estuary, China. Environ. Pollut. 225, 283–290. doi:10.1016/j.envpol.2016.12.064
Pokavanich, T., and Nadaoka, K. (2006). Three-Dimensional Hydrodynamics Simulation of Manila Bay. Symposium on Infrastructure Development and the Environment [Preprint]. Available at: http://www.wv.mei.titech.ac.jp/IMSWES07/files/pokavanich_MB.pdf (Accessed February 23, 2019).
Prudente, M. S., Ichihashi, H., and Tatsukawa, R. (1994). Heavy Metal Concentrations in Sediments from Manila Bay, Philippines and Inflowing Rivers. Environ. Pollut. 86, 83–88. doi:10.1016/0269-7491(94)90009-4
Redford, D. P., Trulli, H. K., and Trulli, W. R. (1997). Sources of Plastic Pellets in the Aquatic Environment. Springer-Verlag 25, 335–343. doi:10.1007/978-1-4613-8486-1_30
Rodríguez-Seijo, A., and Pereira, R. (2017). “Morphological and Physical Characterization of Microplastics”, in Comprehensive Analytical Chemistry Series (Amsterdam: Elsevier), 49–66. doi:10.1016/bs.coac.2016.10.007
Ryan, P. G. (2015). Does Size and Buoyancy Affect the Long-Distance Transport of Floating Debris? Environ. Res. Lett. 10, 6. doi:10.1088/1748-9326/10/8/084019
Salvador Cesa, F., Turra, A., and Baruque-Ramos, J. (2017). Synthetic Fibers as Microplastics in the marine Environment: a Review from Textile Perspective with a Focus on Domestic Washings. Sci. Total Environ. 598, 1116–1129. doi:10.1016/j.scitotenv.2017.04.172
Sanchez, W., Bender, C., and Porcher, J.-M. (2014). Wild Gudgeons (Gobio gobio) from French Rivers Are Contaminated by Microplastics: Preliminary Study and First Evidence. Environ. Res. 128, 98–100. doi:10.1016/j.envres.2013.11.004
Shaw, D. G., and Day, R. H. (1994). Colour- and Form-dependent Loss of Plastic Micro-debris from the North Pacific Ocean. Mar. Pollut. Bull. 28 (1), 39–43. doi:10.1016/0025-326X(94)90184-8
Sia, S. G. L., Martillano, K. J., Alcantara, T. P., Ragragio, E., De Jesus, J., Hallare, A., et al. (2009). Assessing Heavy Metals in the Waters, Fish, and Macroinvertebrates in Manila Bay, Philippines. J. Appl. Sci. Environ. Sanitation 4 (3), 187–195.
Simon-Sánchez, L., Grelaud, M., Garcia-Orellana, J., and Ziveri, P. (2019). River Deltas as Hotspots of Microplastic Accumulation: the Case Study of the Ebro River (NW Mediterranean). Sci. Total Environ. 687, 1186–1196. doi:10.1016/j.scitotenv.2019.06.168
Siringan, F., and Ringor, C. (1998). Changes in Bathymetry and Their Implications to Sediment Dispersal and Rates of Sedimentation in Manila Bay. Sci. Diliman 10 (2), 12–26.
Su, L., Cai, H., Kolandhasamy, P., Wu, C., Rochman, C. M., and Shi, H. (2018). Using the Asian Clam as an Indicator of Microplastic Pollution in Freshwater Ecosystems. Environ. Pollut. 234, 347–355. doi:10.1016/j.envpol.2017.11.075
Su, L., Xue, Y., Li, L., Yang, D., Kolandhasamy, P., Li, D., et al. (2016). Microplastics in Taihu Lake, China. Environ. Pollut. 216, 711–719. doi:10.1016/j.envpol.2016.06.036
Suresh, A., Vijayaraghavan, G., K.S., S., K.V., N., B., A., and S., B. N. (2020). Microplastics Distribution and Contamination from the Cochin Coastal Zone, India. Reg. Stud. Mar. Sci. 40, 101533. doi:10.1016/j.rsma.2020.101533
Tanchuling, M. A. N., and Osorio, E. D. (2020). “The Microplastics in Metro Manila Rivers: Characteristics, Sources, and Abatement,” in The Handbook of Environmental Chemistry. Editors F. Stock, G. Reifferscheid, N. Brennholt, and E. Kostianaia (Quezon City: Springer), 1–22. doi:10.1007/698_2020_659
Thetford, D., Chorlton, A., and Hardman, J. (2003). Synthesis and Properties of Some Polycyclic Barbiturate Pigments. Dyes Pigm. 59, 185–191. doi:10.1016/S0143-7208(03)00104-9
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., et al. (2004). Lost at Sea: where Is All the Plastic? Science 304, 838. doi:10.1126/science.1094559
Tsang, Y. Y., Mak, C. W., Liebich, C., Lam, S. W., Sze, E. T.-P., and Chan, K. M. (2017). Microplastic Pollution in the marine Waters and Sediments of Hong Kong. Mar. Pollut. Bull. 115 (1–2), 20–28. doi:10.1016/j.marpolbul.2016.11.003
Turner, A., and Holmes, L. (2011). Occurrence, Distribution and Characteristics of Beached Plastic Production Pellets on the Island of Malta (central Mediterranean). Mar. Pollut. Bull. 62, 377–381. doi:10.1016/j.marpolbul.2010.09.027
Van Cauwenberghe, L., Claessens, M., Vandegehuchte, M. B., Mees, J., and Janssen, C. R. (2013). Assessment of marine Debris on the Belgian Continental Shelf. Mar. Pollut. Bull. 73, 161–169. doi:10.1016/j.marpolbul.2013.05.026
Van Cauwenberghe, L., Devriese, L., Galgani, F., Robbens, J., and Janssen, C. R. (2015). Microplastics in Sediments: a Review of Techniques, Occurrence and Effects. Mar. Environ. Res. 111, 5–17. doi:10.1016/j.marenvres.2015.06.007
Vianello, A., Boldrin, A., Guerriero, P., Moschino, V., Rella, R., Sturaro, A., et al. (2013). Microplastic Particles in Sediments of Lagoon of Venice, Italy: First Observations on Occurrence, Spatial Patterns and Identification. Estuarine, Coastal Shelf Sci. 130, 54–61. doi:10.1016/j.ecss.2013.03.022
Wang, T., Li, B., Zou, X., Wang, Y., Li, Y., Xu, Y., et al. (2019). Emission of Primary Microplastics in mainland China: Invisible but Not Negligible. Water Res. 162, 214–224. doi:10.1016/j.watres.2019.06.042
Wang, W., Ndungu, A. W., Li, Z., and Wang, J. (2017). Microplastics Pollution in Inland Freshwaters of China: a Case Study in Urban Surface Waters of Wuhan, China. Sci. Total Environ. 575, 1369–1374. doi:10.1016/j.scitotenv.2016.09.213
Wessel, C. C., Lockridge, G. R., Battiste, D., and Cebrian, J. (2016). Abundance and Characteristics of Microplastics in beach Sediments: Insights into Microplastic Accumulation in Northern Gulf of Mexico Estuaries. Mar. Pollut. Bull. 109, 178–183. doi:10.1016/j.marpolbul.2016.06.002
Wolanski, E., and Elliott, M. (2015). “Estuarine Water Circulation,” in In Estuarine Ecohydrology. Editors E. Wolanski, and M. Elliott. 2nd edition (Amsterdam: Elsevier), 17–39. doi:10.1016/B978-044453066-0.50003-9
Wright, S. L., Thompson, R. C., and Galloway, T. S. (2013). The Physical Impacts of Microplastics on marine Organisms: A Review. Environ. Pollut. 178, 483–492. doi:10.1016/j.envpol.2013.02.031
Yan, M., Nie, H., Xu, K., He, Y., Hu, Y., Huang, Y., et al. (2019). Microplastic Abundance, Distribution and Composition in the pearl river along Guangzhou City and pearl river Estuary, China. Chemosphere 217, 879–886. doi:10.1016/j.chemosphere.2018.11.093
Yonkos, L. T., Friedel, E. A., Perez-Reyes, A. C., Ghosal, S., and Arthur, C. D. (2014). Microplastics in Four Estuarine Rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 48, 14195–14202. doi:10.1021/es5036317
Young, A. M., and Elliott, J. A. (2016). Characterization of Microplastic and Mesoplastic Debris in Sediments from Kamilo Beach and Kahuku Beach, Hawai'i. Mar. Pollut. Bull. 113 (1–2), 477–482. doi:10.1016/j.marpolbul.2016.11.009
Zarfl, C., and Matthies, M. (2010). Are marine Plastic Particles Transport Vectors for Organic Pollutants to the Arctic?. Mar. Pollut. Bull. 60 (10), 1810–1814. doi:10.1016/j.marpolbul.2010.05.026
Zhang, H. (2017). Transport of Microplastics in Coastal Seas. Estuarine, Coastal Shelf Sci. 199, 74–86. doi:10.1016/j.ecss.2017.09.032
Zhang, K., Gong, W., Lv, J., Xiong, X., and Wu, C. (2015). Accumulation of Floating Microplastics behind the Three Gorges Dam. Environ. Pollut. 204, 117–123. doi:10.1016/j.envpol.2015.04.023
Zhang, K., Shi, H., Peng, J., Wang, Y., Xiong, X., Wu, C., et al. (2018). Microplastic Pollution in China's Inland Water Systems: A Review of Findings, Methods, Characteristics, Effects, and Management. Sci. Total Environ. 630, 1641–1653. doi:10.1016/j.scitotenv.2018.02.300
Zhang, K., Su, J., Xiong, X., Wu, X., Wu, C., and Liu, J. (2016). Microplastic Pollution of Lakeshore Sediments from Remote Lakes in Tibet Plateau, China. Environ. Pollut. 219, 450–455. doi:10.1016/j.envpol.2016.05.048
Zhao, J., Ran, W., Teng, J., Liu, Y., Liu, H., Yin, X., et al. (2018). Microplastic Pollution in Sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 640-641, 637–645. doi:10.1016/j.scitotenv.2018.05.346
Zhao, S., Zhu, L., Wang, T., and Li, D. (2014). Suspended Microplastics in the Surface Water of the Yangtze Estuary System, China: First Observations on Occurrence, Distribution. Mar. Pollut. Bull. 86 (1–2), 562–568. doi:10.1016/j.marpolbul.2014.06.032
Keywords: Manila bay, microplastics, plastic waste, sediment, surface water
Citation: Osorio ED, Tanchuling MAN and Diola MBLD (2021) Microplastics Occurrence in Surface Waters and Sediments in Five River Mouths of Manila Bay. Front. Environ. Sci. 9:719274. doi: 10.3389/fenvs.2021.719274
Received: 03 June 2021; Accepted: 16 August 2021;
Published: 03 September 2021.
Edited by:
Jennifer Drummond, University of Birmingham, United KingdomCopyright © 2021 Osorio, Tanchuling and Diola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ezra D. Osorio, ZXpyYS5vc29yaW9AdXBkLmVkdS5waA==
 Ezra D. Osorio
Ezra D. Osorio Maria Antonia N. Tanchuling
Maria Antonia N. Tanchuling Ma. Brida Lea D. Diola
Ma. Brida Lea D. Diola