- 1Key Laboratory of South China Sea Fishery Resources Exploitation & Utilization, Ministry of Agriculture and Rural Affairs, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
- 2Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou, China
- 3Scientific Observation and Research Field Station of Pearl River Estuary Ecosystem, Guangzhou, China
- 4Guangdong Provincial Key Laboratory of Fishery Ecology and Environment, Guangzhou, China
- 5Bio-Transduction Lab, Wuhan, China
Squaliobarbus curriculus, commonly known as red-eye fish, is widely distributed in East Asia. It is one of the important aquatic germplasm resource and economic species in the Pearl River. To give suggestions for better protection and management, we investigated its life history and conducted elasticity analyses. Samples (n = 451) were collected between 2016 and 2017 from the western Pearl River estuary. There were no significant differences between the length-weight relationships of females and males (W = 0.00001SL3.121). The von Bertalanffy growth function was Lt = 553.2 [1 − e−0.111(t+1.009)]. The estimated length at 50% sexual maturity for females was 209.6 and 200.0 mm for males, both at 3 years of age. Oocyte size-frequency distribution suggested batch spawning. Fecundity ranged between 9,407 and 175,086 eggs per fish (mean = 51,040, or 143.9 eggs/g of fish weight). To better understand the ecological phenotypic plasticity of S. curriculus we conducted meta-analyses on all available life history data for this species. Our results showed that the standard lengths at ages 2 and 3 in the estuary were significantly smaller than in the upper reaches of the Pearl River basin, and there were also obvious differences in fecundity and oocyte size. For more, the standard lengths at ages 2 and 3 were correlated negatively with latitude. Elasticity analysis showed that juveniles’ (aged 1–3) survival had the largest contribution to the population growth rate, which suggests that management efforts should focus on the early life stages.
Introduction
Due to its popularity with consumers and resilience to unfavorable environmental conditions, the importance of barbel chub Squaliobarbus curriculus (Richardson, 1846) for freshwater aquaculture and capture fisheries in southern China has been steadily growing during the last few decades (Lei et al., 2012). Belonging to the family Cyprinidae (Leuciscinae subfamily), and commonly known as red-eye fish for its red spots on the superior border of eyes, S. curriculus is mainly distributed in Asia, including China, western Korea, Vietnam, and Amur River drainage in Russia. As it is one of the dominant species in the western Pearl River Estuary (according to our unpublished 2016–2017 survey), it is also an important commercial fishing species in this area. Importantly, the National Aquatic germplasm resources protection area for S. curriculus (and Erythroculter pseudobrevicauda) is also located in the Xijiang River, only 130 km upstream from our studied area.
Life history characteristics such as age, growth, and reproduction of fish populations are crucial for determining the fish recruitment and understanding the fish population dynamics (Quinn and Deriso, 1999; Lowerre-Barbieri et al., 2017). Therefore, life history studies are the most important prerequisite required for the assessment of exploited fish populations (Campana, 2001), and essential for fish population management and conservation (Copeland et al., 2017). By analyzing the life history of S. curriculus, we aim to improve our understanding of its population dynamics and produce data necessary for designing conservation measures to prevent the decline of its aquatic germplasm resources.
Elasticity analysis quantifies the relative importance of vital life history traits (fecundity, growth, and survival) for the overall population growth rate. This, in turn, provides guidance for management efforts, which should generally focus on demographic parameters with the highest elasticity (Caswell, 2000). Elasticity analysis is one of the most widely used tools in demographic studies, evolutionary and population ecology studies, and particularly in studies aimed at the conservation of exploited and endangered species (Heppell et al., 2000; Manlik et al., 2018). It has been used to guide wildlife management (de Kroon et al., 2000; Manlik et al., 2018), not only in vertebrates (Sæther and Bakke, 2000; Heppell et al., 2000, Gerber and Heppell, 2004, Wang et al., 2017), but also in invertebrates (Sommerville et al., 2014) and plants (Crone, 2016).
In order to better protect and manage S. curriculus populations, it is also important to understand their phenotypic plasticity in different environments (Noss 2001). Salinity and temperature (latitude) are two important environmental factors that will affect the variation of the life history characteristics of S. curriculus. In estuaries, freshwater meets seawater, so the salinity in estuary habitats is affected by a number of factors, most notably sea tides and freshwater influx, and therefore it can vary notably over relatively short time periods (Bricheno et al., 2021). Due to the demands of maintaining osmotic balance, this variation causes metabolic stress in fish (Nordlie, 2006). Therefore, these environmental factors (salinity fluctuation) might be producing observable impacts on the life history traits of wild S. curriculus populations inhabiting the estuary of the Pearl River. The body sizes of organisms tend to be inversely correlated with latitude and temperature (Meiri, 2011; Rypel, 2014), but studies in ectotherms (including fish) often produce conflicting results (Bauer, 1992; Mousseau, 1997, Belk and Houston, 2002, Angilletta and Dunham, 2003, Ashton and Feldman, 2003, Heibo et al., 2005, Pincheira-Donoso et al., 2008, Chucholl, 2011, Rypel, 2014). Thus a general explanation for the variation in size-latitude relationships of ectotherms has remained elusive (Angilletta and Dunham, 2003; Chucholl, 2011; Rubalcaba et al., 2019).
To propose appropriate fisheries management measures, we sampled the S. curriculus populations inhabiting the western part of the Pearl River estuary over a 1-year period, inferred their life history traits (age, growth, sex ratio, size at maturity, and fecundity), and then conducted elasticity analyses. Following this, the ecological phenotypic plasticity was studied to allow us to propose better protection and management measures for S. curriculus.
Materials and Methods
Study Site
The Pearl River (length = 2,214 km, drainage basin = 452,000 km2) is the second largest river in China in terms of annual water discharge, with 3.26 × 1011 m3 yr−1. Although they merely share a common delta, Xijiang, Beijiang, and Dongjiang Rivers are considered tributaries of the Pearl River. The largest, Xijiang, accounts for ∼70% of the total Pearl River freshwater discharge (China Bureau of Hydrology, Ministry of Water Resources, http://sqqx.hydroinfo.gov.cn/websq/). All samples for this study were collected in the Xijiang part (or the western part) of the Pearl River estuary, located in the vicinity of Jiangmen City, Guangdong Province, China (Figure 1).
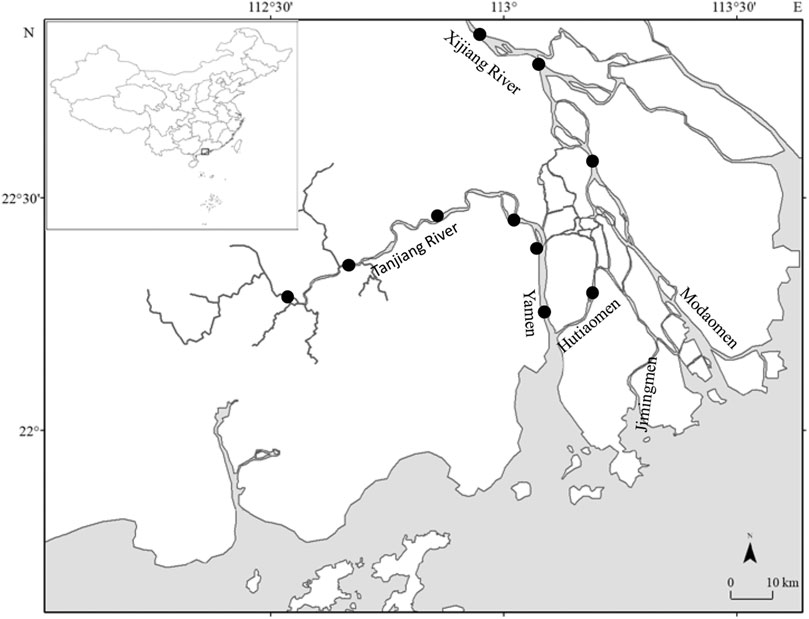
FIGURE 1. Sampling locations of S. curriculus in the western Pearl River estuary. Black dots represent sampled sites.
Sample Collection and Processing
Sampling was conducted on 10 sites between December 2016 and September 2017. Each site was sampled thrice in each season: 22–24th December 2016, 24–26th March, 12–14th July, and 19–21st September 2017 (Figure 1). The sampling was conducted using 5 drift gillnets (100 m long × 1.5–2.3 m high with mesh sizes ranging from 4 to 7 cm) and 2 trap nets (20 m long × 0.6 m high, with mesh size 0.2 cm) set in the evening for 5 h. In total, 451 specimens were collected. The standard length (SL 1 mm) and weight (W 0.01 g) were recorded. The sexual characteristics were macroscopically analysed on 358 specimens (after discarding too small and damaged samples). Gonads were surgically removed, weighed (precision = 0.01 g), and preserved in 10% formalin for subsequent determination of the fecundity and oocyte size-frequency distributions. Gonad maturity stages, determined using a dissection microscope, were identified as stage I - inactive stage, stage II - recovering stage, stage III - early maturing stage, stage IV - late maturing stage, stage V - mature stage, and stage VI - spent stage (Bancroft and Stevens, 1996). Oocyte diameter was calculated as the average of the major and minor axes. Oocytes that did not contain yolk were not measured.
Length-Weight Relationships
We examined the difference in SL distributions between sexes using the Kolmogorov–Smirnov test (K–S test). The standard length and weight relationship (W = aSLb) was converted into the logarithmic form lnW = lna + blnL (Ricker, 1975), where a and b parameters were calculated using least-squares regression. SL–W relationships between sexes were compared using the analysis of covariance (ANCOVA). To test whether the growth of fish was isometric, the student’s T-test was applied to assess whether the b parameter significantly differed from the expected value of 3 (Pauly, 1984).
Age Estimates and Growth
The annual growth of scales was used to estimate the age of fish, needed for subsequent growth estimates. Scales were taken from the end of the pectoral fin and the beginning of the dorsal fin of 437 specimens and analysed under a dissecting microscope as described before (Wang et al., 2015). Each scale was interpreted twice by one reader, with an interval of at least 2 weeks between the two counts. The reader did not have any prior information on the sex, length, or capture time of specimens. Counts were accepted as correct if they were in agreement. If the two counts differed, a recount was conducted and accepted as correct only if it matched any of the previous two counts. If the count did not match any of the previous two, the specimen was not used for downstream analyses. Specimens in different age groups were all assigned a birth date of the 1st of May. The von Bertalanffy growth function (VBGF) based on length-at-age from all age readings were fitted by non-linear regression: Lt = L∞ [1 − e−k(t−t0)]. Lt is the length at age t, L∞ is the asymptotic length, k is the growth coefficient, and t0 is the age at length 0.
Reproduction
Chi-squared (χ2) test was used to determine whether the sex ratio deviated from 1:1 (Zar, 1999). Sexually mature specimens for both sexes were defined as possessing stage III to stage VI gonads during the spawning season. The SL at 50% maturity (L50) was calculated by fitting the logistic curves to the proportion of fish designated as mature in each 10 mm length class using a non-linear least-squares procedure (the Marquardt method). The logistic equation used is P = 100/{1 + exp[−a × (SL-L50)]}, where P is the percentage of mature specimens at SL, SL is the standard length (mm), and a is the slope of the curve.
Phenotypic Plasticity
To better understand the ecological phenotypic plasticity of S. curriculus, we tested the impacts of varying salinity on life history traits and correlation between size and latitude by conducting a meta-analysis using all available life history data for this species, across a broad range of latitudes and habitat types (Table 1). We obtained all published literature on the life history of S. curriculus, and calculated the length at the ages of 2 and 3 according to VBGF. If there was data available for the length at the ages of 2 and 3 in the literature, we directly used the data from the literature. We obtained latitude data from Google Maps. In the analysis of length and latitude, we excluded the relevant data for the estuary.
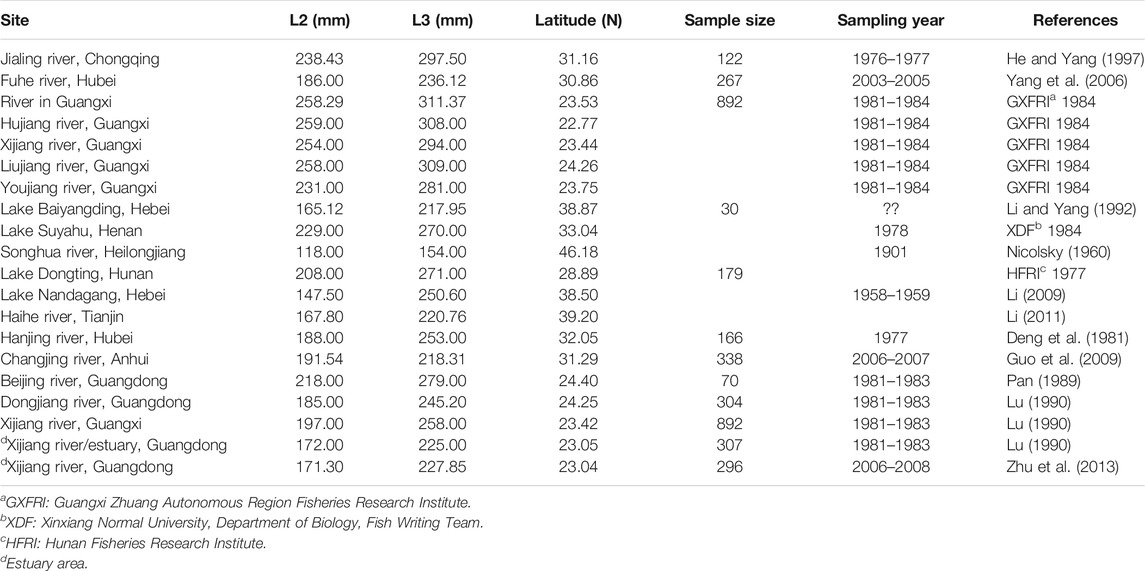
TABLE 1. Length at age and sampling site for S. curriculus populations from China analysed in this study. L2 and L3 indicate length at the ages of 2 and 3 respectively.
Survivorship, Mortality Rates, and Elasticity Analysis
The population catch curve was established from the age-frequency distribution to estimate the total mortality rate (Z), corresponding to the sum of natural mortality (M) and fishing mortality (F). The natural log of the number of specimens (ln N) was plotted against the age. The slope (b) of the descending right limb of the age-frequency distribution curve was found through least-squares linear regression, with b estimating the Z (Ricker, 1975).
The methodology described by Chen and Watanabe (1989) was used to estimate the M (natural mortality) for each age-class t:
and
in which tmat is the age at 50% sexual maturity, tmax is the maximum age in a population (life span), k and t0 are the von Bertalanffy growth curve parameters.
Survival rates of the age zero (S0) can be calculated according to the population growth rate (λ).
Survival values (S) for each mortality rate were estimated using the formula described by Ricker (1975): S = e-z. For the cases when t ≤ tmat, age-specific survivorship and mortality rates were estimated using the methodology described by Chen and Watanabe (1989); for tmat < t, age-specific survivorship and mortality rates were estimated using the population catch curve.
The mean number of female offspring per female was multiplied by 1/2 of the fecundity. Fecundity was then converted to age-specific fecundity, according to the relationship between fecundity and the age of the fish species.
According to the above data, elasticity analyses were calculated using the projection matrix A, or Leslie matrix (Caswell 1989):
Fi is age-specific fecundity multiply by S0, Pi is age-specific survivorship, and λ is the dominant eigenvalue of A.
We calculated the elasticity matrices E from the eigenvectors (λ) of each projection matrix A (de Kroon et al., 1986):
where aij is the (i, j) element of the matrix A, v and w are the left and right eigenvectors of the projection matrix A, and <w, v> is the scalar product of the two vectors {v1 × w1 + v2 × w2 ...}. From there, fertility elasticity, juvenile survival elasticity and adult survival elasticity were calculated according to Heppell et al. (2000) and Wang et al. (2017). Elasticity analyses were performed using “Popbio” R package (http://cran.r-project.org/web/packages/popbio/).
In the present paper, we defined juvenile as age-1 to age at maturity, and adult as age after maturation.
Results
Length-Weight Relationships
The standard length ranged from 40 to 350 mm (mean ± SD = 185.1 ± 53.4 mm), and weight from 1.00 to 986.21 g (mean ± SD = 147.3 ± 127.0 g). The K–S test found a significant difference between the SL distributions of females and males (H = 1.448, p = 0.030), but the difference was not significant after removing 11 individuals larger than 290 mm (H = 1.121, p = 0.162; among these 11 individuals, there was only one male specimen, which skewed the data distribution).
Length–weight relationships were calculated separately for females and males. The regression equations were W = 0.00001SL3.095 (r2 = 0.966, n = 174) for females and W = 0.00001SL3.215 (r2 = 0.974, n = 147) for males. No statistically significant differences were detected for SL–W relationships between sexes (ANCOVA after log-transformation, n = 321, F = 3.641, p = 0.057). The regression equation derived from pooled data was W = 0.00001SL3.121 (r2 = 0.986, n = 451). The allometric index value (b = 3.121) obtained from the function was significantly larger than 3 (t-test, df = 450, t = 6.94, p < 0.01), which indicates a positive allometric growth (Figure 2).
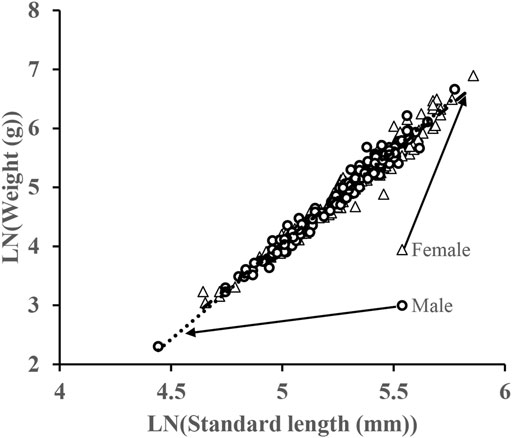
FIGURE 2. Log-scale visual representation of the length-weight relationship of S. curriculus in the western Pearl River estuary, China.
Age Structure and Growth
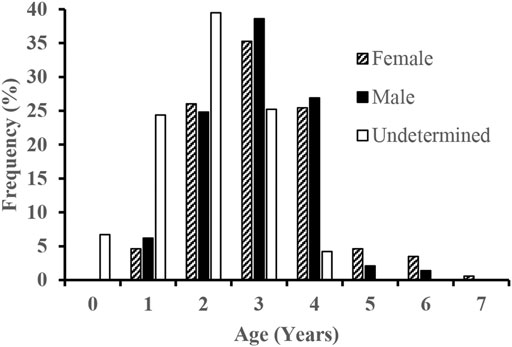
The age varied from 1 to 7 years for females and 1–6 years for males (Figure 3). The VBGFs fitted to length-at-age data were described as Lt = 552.2 [1 − e −0.111(t+1.123)] for females (n = 174) and Lt = 616.5 [1 − e −0.091(t+1.21)] for males (n = 147). As there were no significant differences in SL–W relationships between sexes, the VBGFs fitted to all of the length-at-age data together was: Lt = 553.2 [1 − e−0.111(t+1.009)] (Figure 4).
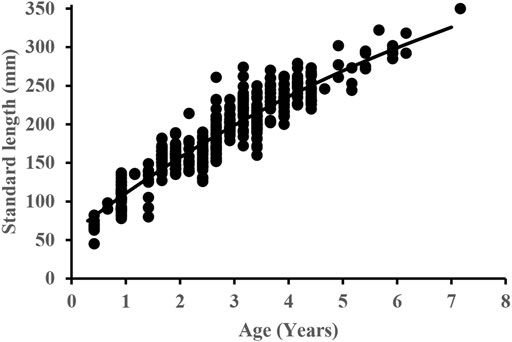
FIGURE 4. The von Bertalanffy growth curve of S. curriculus based on the observed standard length at age in the western Pearl River estuary, China.
Reproduction
Among the 321 successfully sexed specimens, 174 were females and 147 were males. This puts the overall sex ratio (F/M) at 1.18, not significantly different from the expected 1:1 ratio (χ2 = 2.271, df = 1, p = 0.132). Analysis of seasonal gonadal maturation stages variation showed that maturity stage IV of females and males was found between March and September, with the highest frequency in July (summer), whereas stage III was found throughout the year (Figure 5).
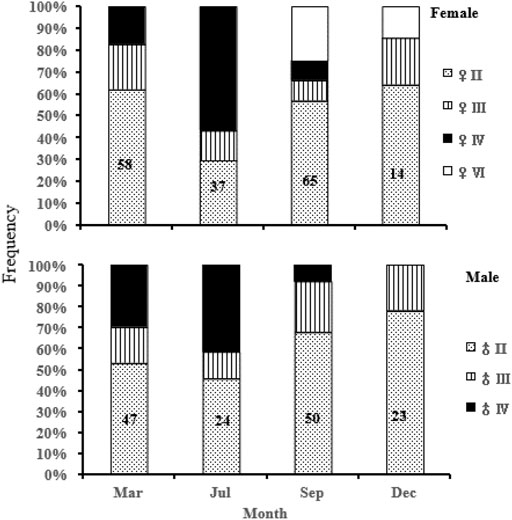
FIGURE 5. Seasonal gonad stages for female and male S. curriculus in the western Pearl River estuary, China.
Size-at-maturity estimates were based on the examination of the 282 specimens collected during the spawning period (March to September). Logistic curves describing the proportion of mature specimens in each 10 mm interval of SL were expressed as P = 100/[1 + e(−0.047(SL−209.6))] (r2 = 0.934, n = 160) for females and P = 100/[1 + e(−0.050(SL−200.0))] (r2 = 0.841, n = 122) for males (Figure 6). The estimated length at 50% sexual maturity (SL50) was 209.6 mm for females and 200.0 mm for males, both at 3 years of age according to the VBGFs. The SLs of the smallest sexually mature female and male were 175 mm (aged 2) and 172 mm (aged 3), respectively.
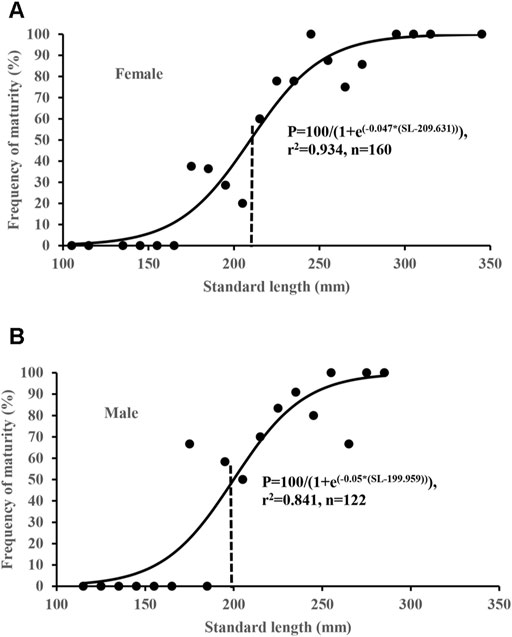
FIGURE 6. Logistic curve fitted to the percentage contributions of S. curriculus to determine the L50 for both sexes in the western Pearl River estuary, China.
Oocyte size-frequency distribution in stage IV consisted of up to two cohorts of oocytes, which indicates two batches in the spawning period (Figure 7). Fecundity was estimated from 30 mature females that ranged from 213 to 350 mm SL. The estimated values of fecundity ranged between 9,407 and 175,086 eggs per fish, and the mean was 51,040 eggs (SD 37,945). The relative fecundity was between 25.4 and 291.8 eggs/g of fish body weight, with the mean of 143.9 eggs/g (SD 75.4). The fecundity of S. curriculus increased linearly with increasing standard length and total weight (Figure 8), and the fitted regression equation was F = 592.89SL–100561, r2 = 0.2573 and F = 138.17W + 1622.7, r2 = 0.4198 respectively. The fecundity also increased linearly with increasing age (Figure 8), and the best function was described as F = 21527A – 42780, r2 = 0.3949.
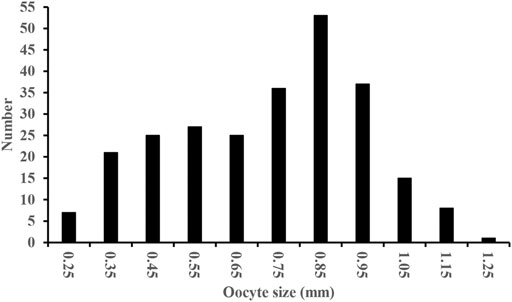
FIGURE 7. Oocyte size-frequency distribution in a randomly chosen S. curriculus specimen with stage IV gonads (period: July, SL = 252 mm, weight = 379.03 g, No. of eggs = 255).
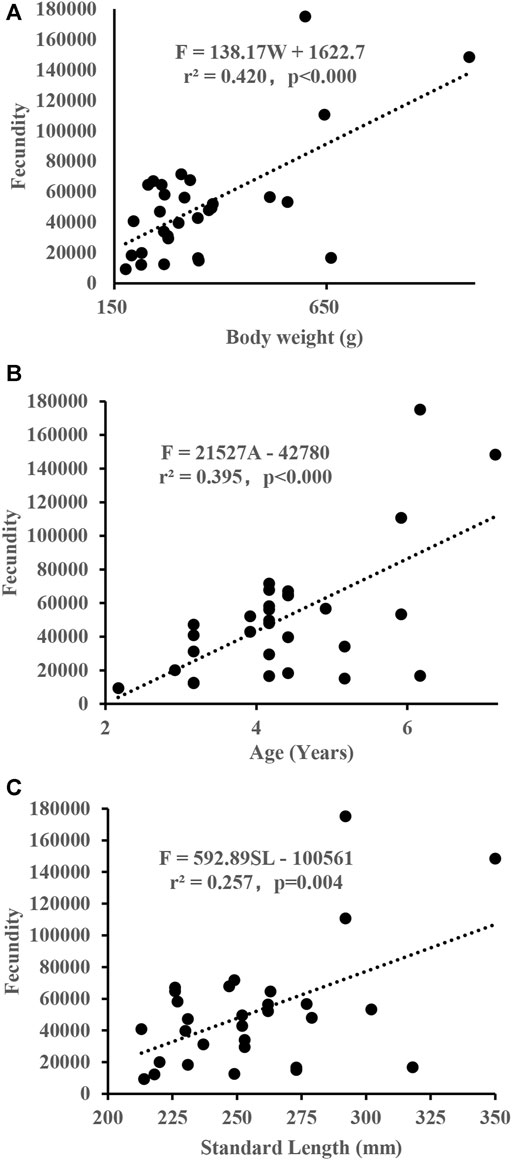
FIGURE 8. The relationships between body weight (A), age (B), standard length (C), and fecundity of S. curriculus in the western Pearl River estuary, China.
Phenotypic Plasticity
The standard lengths at ages 2 (157.00 mm) and 3 (199.00 mm) determined in the present study are significantly smaller than in the upper reaches of the Pearl River basin (Table 1): 254.00 and 294.00 mm in the Xijiang River, 258.00 and 309.00 mm in the Liujiang River, 231.00 and 281.00 mm in the Youjiang River (all three in the Guangxi province, China, and all are tributaries of the Pearl River). The corresponding two values were also somewhat larger in a study of this species conducted in the years 1981–1983 in the Xijiang River/estuary (Guangdong province): 172.00 and 225.00 mm. The fecundity of 3- (21,801 eggs) and 4-year-olds (43,328 eggs) is also significantly lower than in the upper reaches of the river (54,238 and 126,639 eggs, respectively) (Pearl River Fishery Resources Survey Editorial Committee, 1985). As opposed to this, the oocyte size in stage IV (0.70–1.20 mm Figure 7) is larger than in the upper reaches of the river (0.37–1.09 mm) (Pearl River Fishery Resources Survey Editorial Committee, 1985).
Our meta-analysis of S. curriculus populations in China indicates that standard lengths at ages 2 (L2 = −4.7809N + 348.93, r2 = 0.6643, p < 0.01) and 3 (L3 = −4.6586N + 399.43, r2 = 0.6422, p < 0.01) were correlated negatively with latitude (the latitude ranged from 23.04 to 46.18; Table 1).
Elasticity Analyses
According to the relationship between fecundity and age of S. curriculus in this study, and the fact that it breeds twice a year, the age-specific fecundities of 3–7 year-old females were 21,801, 43,328, 64,855, 86,382, and 107,909 eggs per fish, respectively. We divided the number of specimens aged 2 and 3 in September 2017 by the number of specimens aged 2 and 3 in December 2016, thereby calculating the finite rate of population increase: λ = 1.069. Using this number, the survival rate of the age zero (S0) was 0.000035 year−1. Thus, Fi values of 3–7 year-old females were 0.766, 1.521, 2.277, 3.033, 3.789 offsprings per fish, respectively. Age-specific survival rates of 1–6 year-olds were 0.499, 0.838, 0.944, 0.290, 0.290, 0.290 year−1, respectively. Based on the above results, the inferred Leslie matrix (age-based projection matrix model) is:
Elasticity analyses based on the age-based projection matrix model showed that: fertility elasticity (age 0–1 elasticity) of S. curriculus was 0.242, juvenile (specimens aged 1–3) survival elasticity was 0.485, and adult (specimens aged > 3) survival elasticity was 0.273.
Discussion
S. curriculus is a medium-sized fish (its maximum total length is 48.8cm according to the FishBase) with a short lifespan (the maximum age in this study was 7 years old). It has early sexual maturity (the earliest sexual maturity was 2 years old), high fecundity, long breeding period, and it can reproduce twice a year. This life history characteristics underlie its strong adaptability to environmental and human disturbances. This study showed through meta-analysis that the life history characteristics of S. curriculus were affected by fluctuations in environmental factors (such as salinity and temperature), and elasticity analysis proved that the early life history stage was the most critical life history stage to maintain its population growth.
Estuarine Fish Life History Characteristics
The estuary is a region that is affected both by the sea tides and widely varying freshwater influx, which can result in a strong variation in salinity (Bricheno et al., 2021). In such an environment, maintaining homeostasis can be energetically very costly, and affect the amount of energy available for growth and reproduction (Schreck, 2010). Therefore, fluctuating salinity often has highly significant effects on life history traits, such as growth and reproduction (Stearns, 1992; Martin et al., 2009). The S. curriculus populations studied in this research also demonstrate this effect, for the individuals of the same age in the estuary were significantly smaller in size and fecundity than individuals in the upper reaches, but the oocyte size in stage IV was larger. Although there could be a large number of variables synergistically producing these observed phenomena, we hypothesise that they may be at least partially attributed to increased metabolic costs of osmoregulation in a fluctuating-salinity environment (Gan et al., 2016). For example, a similar decrease in growth and reproduction parameters with increasing salinity has been reported in Oreochromis niloticus and Colossoma macropomum (Lowe et al., 2012; Fiúza et al., 2015). Intriguingly, the “environmental stability hypothesis” predicts that more environmentally variable habitats select for organisms that have higher reproductive allotment (per unit body mass), larger brood sizes, and smaller offspring size relative to organisms from more stable habitats (Stearns, 1983, 1992). Examples of species fitting this pattern are the sailfin molly Poecilia latipinna, the western mosquitofish Gambusia affinis, and the least killifish Heterandria formosa (Martin et al., 2009). The discrepancy between our results and this hypothesis may be caused by different adaptation characteristics of the fish to the environment. We should also mention that some of the differences in fecundity and oocyte size may be partially caused by the comparison of studies spanning several decades, so changes in climate and water environment may also have an impact.
Size-Latitude Relationships
Variation in life-history traits with latitude (temperature) can be explained by phenotypic plasticity (Roff, 2002; Ren et al., 2020), and a ubiquitous rule is that body sizes of organisms tend to be smaller at high temperatures and low latitudes, and larger at low temperatures and high latitudes (Meiri, 2011; Rypel, 2014). Our meta-analysis of S. curriculus populations in China indicates exactly the opposite. This rule was also disproven in many other freshwater fishes (Belk and Houston, 2002; Heibo et al., 2005, Rypel, 2014), as well as other ectotherm animals, such as insects (Mousseau, 1997), snakes (Ashton and Feldman, 2003), and lizards (Pincheira-Donoso et al., 2008). However, many ectotherms are known to exhibit a larger size in colder environments (Angilletta and Dunham, 2003), such as the freshwater pearl mussel Margaritifera (Bauer, 1992), chelonian turtles (Ashton and Feldman, 2003), some crayfish (Procambarus clarkii) (Chucholl, 2011). A general explanation for the variation in size-latitude relationships of ectotherms remains elusive (Angilletta and Dunham, 2003; Chucholl, 2011; Rubalcaba et al., 2019).
Intriguingly, among the freshwater fishes on the North American continent, cool- or cold-water species were in agreement with the rule, whereas the opposite was found for warm-water species (Rypel, 2014). Temperature is perhaps the most important environmental factor for fish growth, as fish have a thermal optimum (Topt, Zarco-Perello et al., 2012): at lower temperatures, metabolic rate declines and growth rate decreases (Reynolds, 2002; Chung et al., 2021), but temperatures higher than Topt are likely to lead to declines in growth (Zarco-Perello et al., 2012). Since the environmental temperature decreases with latitude, our meta-analysis of S. curriculus populations in China shows that size is positively correlated with the average temperature. As our data comprised only localities north of the 23 degrees north latitude, they don’t comprise the Topt of S. curriculus; this explains the positive association between growth and temperature found in this study.
Elasticity Analysis
Elasticity analysis quantifies the contributions of different variables to the population growth rate (Heppell et al., 2000), so it can help us predict the response of populations to disturbance and decide which life stages should be protected (Heppell et al., 2000; Manlik et al., 2018). Our results indicate that management efforts should focus on protecting the juvenile S. curriculus, as this life stage had the highest contribution to the population growth rate (0.485). In the context of mostly overfished fishery resources in China (Chen et al., 2009), these life history characteristics of S. curriculus might be advantageous. This may be the explanation for the increase in the relative abundance of this species in recent decades (Wang et al., 2017), with the finite rate of population increase of 1.069 inferred in our study. Although this indicates that S. curriculus should be able to withstand the current fishing pressure, excessive overfishing would still be likely to produce negative impacts on this population. At present, a yearly fishing ban from March to June is the main protection and management measure employed in the Pearl River Basin. This measure is primarily aimed at protecting the fish during the reproductive season and early life history stages. According to our results, the contribution of the 0–1-year-old life stage of S. curriculus to population growth is merely 0.242, whereas the contribution of the 1–3-year-old stage is much higher (0.485). This indicates that protection (or fishing intensity regulation) measures aimed at juveniles are more likely to produce positive outcomes on population growth and stability than the measures currently employed. Thus, implementing a minimum catch size and/or minimum mesh size is particularly important. In the study area, the minimum catch size should not be less than 200 mm (3 years old), and the minimum mesh size of the fishing net should also be adjusted accordingly.
According to our research, the spawning period of S. curriculus is from March to September, which may actually be longer than the fishing ban period mentioned above. Therefore, if this species is to be better protected and managed, the fishing ban period needs to be extended. The unique environment of the estuary has a significant impact on the growth of this species, which directly shows that changes in the aquatic habitat, such as environmental pollution, are likely to affect its growth. In summary, only by formulating reasonable management methods based on the life history characteristics of S. curriculus can we ensure the permanent sustainability of this resource.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Key Laboratory of South China Sea Fishery Resources Exploitation & Utilization, Ministry of Agriculture and Rural Affairs, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China.
Author Contributions
Conceptualization, TW, YL, and C-HL; investigation, TW, Y-YX, LL; methodology, TW, Y-YX; formal analysis, TW; writing original draft, TW, IJ, and LL; writing-review & editing, TW, YL, IJ, C-HL, and PW; data curation, YL, IJ, and LL; funding acquisition, C-HL and PW.
Funding
This research was supported by the National Key R&D Program of China (2019YFD0901201, 2019YFD0901204), Guangdong Basic and Applied Basic Research Foundation (2019B1515120065), Science and Technology Planning Project of Guangdong Province (2019B121201001), Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (GML2019ZD0605), Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2016TS35), Central Public-interest Scientific Institution Basal Research Fund, CAFS (No. 2020TD16), Science and Technology Program of Guangzhou, China (202102080509).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Sun T., Chen D. F., Wei F. S., and Huang H. Y. for their help in collecting the survey data.
References
Angilletta, Jr., M. J., and Dunham, A. E. (2003). The Temperature‐Size Rule in Ectotherms: Simple Evolutionary Explanations May Not Be General. The Am. Naturalist 162, 332–342. doi:10.1086/377187
Ashton, K. G., and Feldman, C. R. (2003). Bergmann's Rule in Nonavian Reptiles: Turtles Follow it, Lizards and Snakes Reverse it. Evolution 57, 1151–1163. doi:10.2307/344881410.1111/j.0014-3820.2003.tb00324.x
Bancroft, J. D., and Stevens, A. (1996). Theory and Practice of Histological Techniques. 4th edn. New York: Churchill Livingstone.
Bauer, G. (1992). Variation in the Life Span and Size of the Freshwater Pearl Mussel. J. Anim. Ecol. 61, 425–436. doi:10.2307/5333
Belk, M. C., and Houston, D. D. (2002). Bergmann's Rule in Ectotherms: A Test Using Freshwater Fishes. Am. Naturalist 160, 803–808. doi:10.1086/343880
Bricheno, L. M., Wolf, J., and Sun, Y. (2021). Saline Intrusion in the Ganges-Brahmaputra-Meghna Megadelta. Estuarine, Coastal Shelf Sci. 252, 107246. doi:10.1016/j.ecss.2021.107246
Campana, S. E. (2001). Accuracy, Precision and Quality Control in Age Determination, Including a Review of the Use and Abuse of Age Validation Methods. J. Fish Biol. 59, 197–242. doi:10.1111/j.1095-8649.2001.tb00127.x
Caswell, H. (1989). Matrix Population Models: Construction, Analysis and Interpretation. First edition. Sunderland, MA, USA: Sinauer Associates Inc.
Caswell, H. (2000). Prospective and Retrospective Perturbation Analyses: Their Roles in Conservation Biology. Ecology 81, 619–627. doi:10.1890/0012-9658(2000)081[0619:parpat]2.0.co;2
Chen, D., Xiong, F., Wang, K., and Chang, Y. (2009). Status of Research on Yangtze Fish Biology and Fisheries. Environ. Biol. Fish. 85, 337–357. doi:10.1007/s10641-009-9517-0
Chen, S., and Watanabe, S. (1989). Age Dependence of Natural Mortality Coefficient in Fish Population Dynamics. Nippon Suisan Gakkaishi 55, 205–208. doi:10.2331/suisan.55.205
Chucholl, C. (2011). Population Ecology of an Alien “Warm Water” Crayfish (Procambarus clarkii) in a New Cold Habitat. Knowl. Managt. Aquat. Ecosyst. 401, 29. doi:10.1051/kmae/2011053
Chung, M. T., Jørgensen, K. E. M., Trueman, C. N., Knutsen, H., Jorde, P. E., and Grønkjær, P. (2021). First Measurements of Field Metabolic Rate in Wild Juvenile Fishes Show strong thermal Sensitivity but Variations between Sympatric Ecotypes. Oikos 130, 287–299. doi:10.1111/oik.07647
Copeland, T., Ackerman, M. W., Wright, K. K., and Byrne, A. (2017). Life History Diversity of Snake River Steelhead Populations between and within Management Categories. North Am. J. Fish. Manag. 37 (2), 395–404. doi:10.1080/02755947.2016.1264506
Crone, E. E. (2016). Contrasting Effects of Spatial Heterogeneity and Environmental Stochasticity on Population Dynamics of a Perennial Wildflower. J. Ecol. 104, 281–291. doi:10.1111/1365-2745.12500
de Kroon, H., van Groenendael, J., and Ehrlén, J. (2000). Elasticities: A Review of Methods and Model Limitations. Ecology 81, 607–618. doi:10.1890/0012-9658(2000)081[0607:earoma]2.0.co;2
Deng, Z. L., Yu, Z. T., Xu, Y. X., Wei, X. J., and Zhao, Y. (1981). “On the Age and Growth of Main Commercial Fishes Collected from Hanshui River,” in Chinese Ichthyological SocietyTransactions of the Chinese Ichthyologic Society (I) (Beijing: Science Press), 97–116. (in Chinese).
Eberhardt, L. L., and Ricker, W. E. (1977). Computation and Interpretation of Biological Statistics of Fish Populations. J. Wildl. Manag. 41, 154. doi:10.2307/3800109
Fiúza, L. S., Aragão, N. M., Ribeiro Junior, H. P., de Moraes, M. G., Rocha, Í. R. C. B., Lustosa Neto, A. D., et al. (2015). Effects of Salinity on the Growth, Survival, Haematological Parameters and Osmoregulation of tambaquiColossoma Macropomumjuveniles. Aquac. Res. 46, 1–9. doi:10.1111/are.12224
Gan, L., Xu, Z. X., Ma, J. J., Xu, C., Wang, X. D., Chen, K., et al. (2016). Effects of Salinity on Growth, Body Composition, Muscle Fatty Acid Composition, and Antioxidant Status of Juvenile Nile tilapia Oreochromis niloticus (Linnaeus, 1758). J. Appl. Ichthyol. 32, 372–374. doi:10.1111/jai.12997
Gerber, L. R., and Heppell, S. S. (2004). The Use of Demographic Sensitivity Analysis in marine Species Conservation Planning. Biol. Conservation 120, 121–128. doi:10.1016/j.biocon.2004.01.029
Guangxi Zhuang Autonomous Region Fisheries Research Institute (1984). Investigation Report on Fishery Natural Resources in Inland Waters of Guangxi Zhuang Autonomous Region. Guangxi: Guangxi Zhuang Autonomous Region Fisheries Research Institute press. (in Chinese).
Guo, L.-L., Yan, Y.-Z., and Xi, Y.-L. (2009). Age and Growth of Squaliobarbus Curriculus (Richiardoson)in Wuhu Reach of Yangtze River. Acta Hydr Sin 33, 130–135. (in Chinese with English abstract). doi:10.3724/sp.j.1035.2009.00130
He, X. F., and Yang, Q. F. (1997). A Study on the Growth of Squaliobarbus Curriculus (Richardson) in West Stream of Jialingjiang. J. Southwest China Normal Univ. (Natural Science) 22, 680–685. (in Chinese with English abstract).
Heibo, E., Magnhagen, C., and Vøllestad, L. A. (2005). Latitudinal Variation in Life-History Traits in Eurasian Perch. Ecology 86, 3377–3386. doi:10.1890/04-1620
Heppell, S. S., Caswell, H., and Crowder, L. B. (2000). Life Histories and Elasticity Patterns: Perturbation Analysis for Species with Minimal Demographic Data. Ecology 81, 654–665. doi:10.1890/0012-9658(2000)081[0654:lhaepp]2.0.co;2
Hilborn, R., and Walters, C. J. (1992). Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty. New York: Chapman & Hall.
Hunan Fisheries Research Institute (1977). Fishes in Hunan Province. Changsha: Hunan People's Press. (in Chinese).
Lei, L., Li, J., Li, G.-Y., Hu, J.-N., Tang, L., Liu, R., et al. (2012). Stereospecific Analysis of Triacylglycerol and Phospholipid Fractions of Five Wild Freshwater Fish from Poyang Lake. J. Agric. Food Chem. 60, 1857–1864. doi:10.1021/jf204584t
Li, M. D. (2009). Selected Papers of Prof. Li Mingde. Beijing: China Science and Technology Press. (in Chinese).
Lowe, M. R., Wu, W., Peterson, M. S., Brown-Peterson, N. J., Slack, W. T., and Schofield, P. J. (2012). Survival, Growth and Reproduction of Non-native Nile tilapia II: Fundamental Niche Projections and Invasion Potential in the Northern Gulf of Mexico. PLoS One 7, e41580. doi:10.1371/journal.pone.0041580
Lowerre-Barbieri, S., DeCelles, G., Pepin, P., Catalán, I. A., Muhling, B., Erisman, B., et al. (2017). Reproductive Resilience: a Paradigm Shift in Understanding Spawner-Recruit Systems in Exploited marine Fish. Fish Fish 18 (2), 285–312. doi:10.1111/faf.12180
Manlik, O., Lacy, R. C., and Sherwin, W. B. (2018). Applicability and Limitations of Sensitivity Analyses for Wildlife Management. J. Appl. Ecol. 55, 1430–1440. doi:10.1111/1365-2664.13044
Martin, S., Hitch, A., Purcell, K., Klerks, P., and Leberg, P. (2009). Life History Variation along a Salinity Gradient in Coastal Marshes. Aquat. Biol. 8, 15–28. doi:10.3354/ab00203
Meiri, S. (2011). Bergmann's Rule - What's in a Name. Glob. Ecol Biogeogr 20, 203–207. doi:10.1111/j.1466-8238.2010.00577.x
Mousseau, T. A. (1997). Ectotherms Follow the Converse to Bergmann's Rule. Evolution 51, 630–632. doi:10.1111/j.1558-5646.1997.tb02453.x
Nordlie, F. G. (2006). Physicochemical Environments and Tolerances of Cyprinodontoid Fishes Found in Estuaries and Salt Marshes of Eastern North America. Rev. Fish. Biol. Fish. 16, 51–106. doi:10.1007/s11160-006-9003-0
Noss, R. F. (2001). Beyond Kyoto: forest Management in a Time of Rapid Climate Change. Conservation Biol. 15, 578–590. doi:10.1046/j.1523-1739.2001.015003578.x
Pan, X. H. (1989). Pearl River Water System Beijiang Fishery Resources. Guangzhou: Guangdong Science and Technology Press.
Parra, I., Almodóvar, A., Nicola, G. G., and Elvira, B. (2009). Latitudinal and Altitudinal Growth Patterns of Brown troutSalmo Truttaat Different Spatial Scales. J. Fish. Biol. 74, 2355–2373. doi:10.1111/j.1095-8649.2009.02249.x
Pearl River Fishery Resources Survey Editorial Committee (1985). Pearl River Fishery Resources Investigation Report II. Guangzhou: Pearl River Fishery Resources Survey Editorial Committee.
Pincheira-Donoso, D., Hodgson, D. J., and Tregenza, T. (2008). The Evolution of Body Size under Environmental Gradients in Ectotherms: Why Should Bergmann's Rule Apply to Lizards. BMC Evol. Biol. 8, 68. doi:10.1186/1471-2148-8-68
Quinn, J. T. I. I., and Deriso, R. B. (1999). Quantitative Fish Dynamics. New York: Oxford University Press.
Ren, L., Guo, X., Liu, S., Yu, T., Guo, W., Wang, R., et al. (2020). Intraspecific Variation in Phragmites Australis : Clinal Adaption of Functional Traits and Phenotypic Plasticity Vary with Latitude of Origin. J. Ecol. 108 (6), 2531–2543. doi:10.1111/1365-2745.13401
Reynolds, J. D. (2002). “Growth and Reproduction,” in Biology of Freshwater Crayfish. Editor DM Holdich (Oxford: Blackwell Scientific Press), 152–191.
Rubalcaba, J. G., Gouveia, S. F., and Olalla‐Tárraga, M. A. (2019). A Mechanistic Model to Scale up Biophysical Processes into Geographical Size Gradients in Ectotherms. Glob. Ecol Biogeogr 28 (6), 793–803. doi:10.1111/geb.12893
Rypel, A. L. (2014). The Cold-Water Connection: Bergmann's Rule in North American Freshwater Fishes. Am. Naturalist 183, 147–156. doi:10.1086/674094
Saether, B.-E., and Bakke, O. (2000). Avian Life History Variation and Contribution of Demographic Traits to the Population Growth Rate. Ecology 81, 642–653. doi:10.2307/177366
Schreck, C. B. (2010). Stress and Fish Reproduction: the Roles of Allostasis and Hormesis. Gen. Comp. Endocrinol. 165 (3), 549–556. doi:10.1016/j.ygcen.2009.07.004
Somerville, G. J., Krkosek, M., and Hepburn, C. D. (2014). A Matrix Model and Elasticity Analysis for New Zealand's Blackfoot Pāua Haliotis Iris. Fish. Res. 151, 158–168. doi:10.1016/j.fishres.2013.11.008
Stearns, S. C. (1983). The Evolution of Life-History Traits in Mosquitofish since Their Introduction to Hawaii in 1905: Rates of Evolution, Heritabilities, and Developmental Plasticity. Am. Zool 23, 65–75. doi:10.1093/icb/23.1.65
Wang, T., Gao, X., Jakovlić, I., and Liu, H.-Z. (2017). Life Tables and Elasticity Analyses of Yangtze River Fish Species with Implications for Conservation and Management. Rev. Fish. Biol. Fish. 27, 255–266. doi:10.1007/s11160-016-9464-8
Wang, T., Gao, X., Wang, J., Jakovlić, I., Dan, S.-G., and Liu, H.-Z. (2015). Life History Traits and Implications for Conservation of Rock Carp Procypris Rabaudi Tchang, an Endemic Fish in the Upper Yangtze River, China. Fish. Sci. 81, 515–523. doi:10.1007/s12562-015-0872-9
Xinxiang Normal University, Department of Biology, Fish Writing Team (1984). Fishes in Henan Province. Zhengzhou: Henan Science and Technology Press. (in Chinese).
Yang, M. S., Chen, J. A., Huang, X. X., and Li, J. H. (2006). Growth and Population Structure Characteristics of Squaliobarbus Curriculus (Richardson) in Fuhe River. Reservoir Fish. 26, 59–63. (in Chinese with English abstract).
Zarco-Perello, S., Pratchett, M., and Liao, V. (2012). Temperature-growth Performance Curves for a Coral Reef Fish, Acanthochromis polyacanthus. Galaxea, J. Coral Reef Stud. 14, 97–103. doi:10.3755/galaxea.14.97
Keywords: growth, reproductive biology, salinity, ectotherms, latitude
Citation: Wang T, Lin L, Liu Y, Jakovlić I, Li C-h, Xiao Y-y and Wu P (2021) Life History Traits, Elasticity Analyses, and Phenotypic Plasticity of Squaliobarbus curriculus in the Pearl River Estuary, China. Front. Environ. Sci. 9:707130. doi: 10.3389/fenvs.2021.707130
Received: 09 May 2021; Accepted: 14 September 2021;
Published: 30 September 2021.
Edited by:
Chao Song, Chinese Academy of Fishery Sciences, ChinaCopyright © 2021 Wang, Lin, Liu, Jakovlić, Li, Xiao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Liu, bGl1eW9uZ0BzY3NmcmkuYWMuY24=; Chun-hou Li, c2NzbGNoQHZpcC4xNjMuY29t
 Teng Wang
Teng Wang Lin Lin
Lin Lin Yong Liu
Yong Liu Ivan Jakovlić5
Ivan Jakovlić5 Chun-hou Li
Chun-hou Li Ya-yuan Xiao
Ya-yuan Xiao Peng Wu
Peng Wu