- School of Science, RMIT University, Bundoora, VIC, Australia
The rapid growth of the aquaculture industry over recent decades, with annual production reaching 94.6 million tonnes in 2018 has resulted in a significant increase in saline wastewater following the use of seawater in both fish and shellfish production and processing. This wastewater contains high concentrations of nutrients, organic compounds, and total nitrogen, resulting in the requirement for significant treatment prior to discharge to meet environmental regulations, which are becoming more stringent. The infrastructure and running costs associated with physico-chemical treatment approaches are generally higher than the implementation of biological approaches; the latter represents both an economic and sustainable technology. However, salinity represents a significant inhibitor to microbial activity, affecting the efficacy of the biological treatment of wastewater. This review aims to 1) identify the major biodegradable components in saline fish wastewater that may result in deleterious effects upon discharge, 2) discuss the current methods used for the treatment of fish processing wastewaters, and 3) identify opportunities for improved processes to be utilised and identify gaps in knowledge that require further research. Total suspended solids (TSS), chemical oxygen demand (COD), biochemical oxygen demand (BOD), and total nitrogen (TN) were found to be the most prevalent components in fish effluent. High concentrations of TSS and TN are likely due to the protein content. One method for reducing the environmental impact of the treated wastewater is to enhance nutrient removal (TSS, TN, BOD) through process modification, leading to an increase in active proteolytic activity. Bioaugmentation using immobilised, saline-tolerant proteases or halophilic, protease-producing microorganisms have both shown significant potential in laboratory studies in reducing both the COD and TN content of fish processing wastewater to below discharge limits and therefore may represent commercial options for future treatment processes.
Highlights
• Current physical and chemical approaches may not adequately treat highly saline fish processing wastewaters to meet current and future environmental legislation.
• Improvements to biological treatment options such as bioaugmentation represent a significant opportunity.
• Bioaugmentation using either saline-tolerant enzymes or moderate halophiles, which are simple to grow and degrade pollutants may offer suitable solutions for biological treatment in the future
Introduction
The increasing global population has contributed to a significant growth in the consumption of fish (FAO, 2020); global fish production reached 96.4 million tonnes in 2018, with aquaculture contributing 47% of the total. In per capita terms, annual fish consumption grew from 9.0 kg in 1961 to 20.2 kg in 2015, at an average annual rate of around 1.5%. The total value of fisheries and aquaculture production in 2016 was estimated at USD 362 billion, of which USD 232 billion originated from aquaculture production (FAO, 2018).
The growth and success of the aquaculture industries have however resulted in a significant increase in waste generation from production systems (Dauda et al., 2019). As more than 90% of fish products are marine (Blaber, 2011), seawater is widely used in the processing of the products from aquaculture (Figure 1). Typical water consumption is about 20 m3 of seawater per tonne of fish. Most water used at fish-processing plants ultimately becomes waste effluent (Xiao and Roberts, 2010). For example, one fish processing plant based in Victoria, Australia produces 2,000 m3 a day of wastewater during tuna processing and canning operations (Construction, 2018) and between 1,500 and 13,000 m3 in salmon processing (Carawan, 1991).
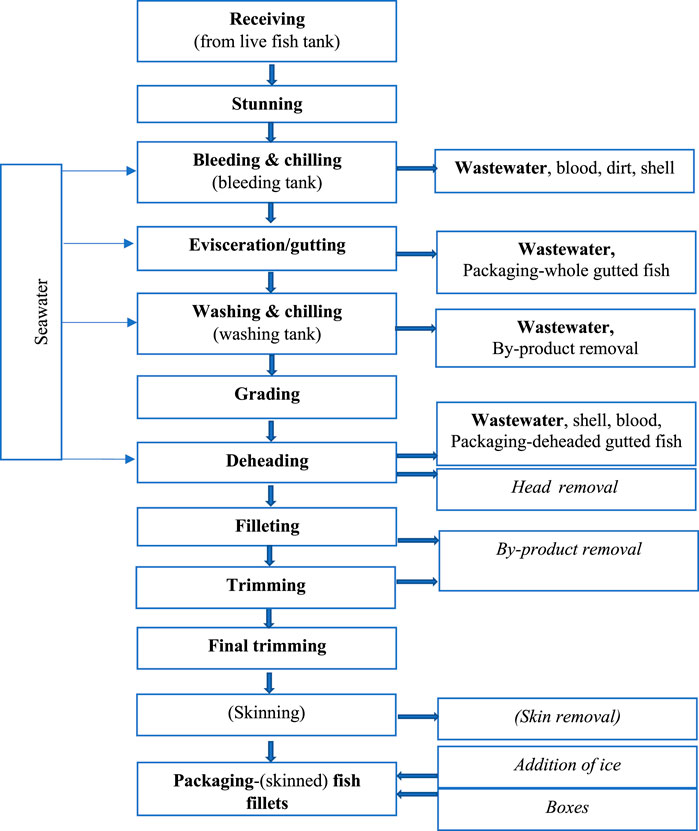
FIGURE 1. Fish processing pathway schematic [adapted from (Vidaček and Bugge, 2016)].
As a consequence, the environmental impact associated with increased fish processing is a subject of increasing concern. Fish processing wastewater generally contains high concentrations of nitrogenous compounds (inorganic and organic) and exhibits a high biochemical oxygen demand (BOD) (Vidya et al., 2020). For example, wastewater from fish processing are characterised by high nutrient concentrations, including high nitrogen content, mainly in the form of ammonia (82 g L−1), high total suspended solids (TSS, 0.15–1.1 g L−1), biological oxygen demand (BOD, 1.4 g L−1) and chemical oxygen demand (COD, 2.9 g L−1) (Muthukumaran and Baskaran, 2013). The nutrients, if released into water untreated can harm fish and other inhabitants of the aquatic ecosystem. A strong positive correlation between BOD, nutrient loads (nitrogen, phosphate, nitrate) of a discharged wastewater, and the development of eutrophic conditions have recently been confirmed (Vidya et al., 2020). The known consequences of eutrophication include blooms of blue-green algae, tainted drinking water supplies, and degradation of recreational opportunities. In the United States, eutrophication has been estimated to cost $2.2 billion annually (Dodds et al., 2009). When these dense algals bloom eventually die, microbial decomposition severely depletes dissolved oxygen, creating a “dead zone,” lacking sufficient oxygen to support most organisms. Dead zones are found in many freshwater lakes including the Laurentian Great Lakes during summer (Arend et al., 2011). Other “dead zones” occur in marine coastal environments surrounding large, nutrient-rich rivers such as the Mississippi River and the Gulf of Mexico, Susquehanna River, and the Chesapeake Bay. They have been shown to affect more than 245,000 square kilometers in over 400 near-shore systems (Diaz and Rosenberg, 2008). Additionally, stress caused by the salinity of fish processing wastewater affects plant growth and restricts the use of surrounding land if the effluent is released (Safdar et al., 2019). About 20% of all irrigated land is affected by salinity, leading to plant osmotic stress and ion toxicity (Kader and Lindberg, 2010).
A number of treatment processes involving physical, chemical, and biological remediation approaches have been applied to treat saline wastewater containing high concentrations of organic material (Kargi and Dinçer, 2000; Paluenzuela-Rollon et al., 2002). While the costs of physical-chemical treatment are generally high due to energy consumption and the likelihood of secondary pollution, biological processes are well suited for fish processing wastewaters and do not result in secondary pollution or residues (Ezeonu et al., 2012). However, fish processing wastewater contains high salinity, similar to or greater than that of seawater (Omil et al., 1995); the inhibitory impact of salinity on the activity of many biological systems results in a significant reduction in the efficacy of current biological approaches.
As a result, there exists an urgent need for the development of cost-effective, efficient solutions for the treatment of fish processing wastewaters. Thus, the aim of this review is to:
1) Identify the major biodegradable components in saline fish wastewater that may result in deleterious effects upon discharge,
2) Discuss the current methods used in aquaculture for the treatment of fish processing wastewater,
3) Identify opportunities for improved processes to be utilised and identify gaps in knowledge that require further research.
The Physico-Chemical Properties of Fish Processing Wastewater
To satisfy environmental regulations, treatment processes are required to remove the majority of the soluble and organic compounds in the wastewater. Although the characteristics of the wastewater depend on the type of protein being processed such as fish, crabs, or shrimp, and the use of any additives such as brine and oil (Chowdhury et al., 2010), the main physical-chemical properties and contaminants present in aquaculture processing wastewater are discussed in the following section.
pH
The pH of fish processing wastewater may be acidic or alkaline due to the composition of the proteinaceous matter in the material. For example, pH in fish cannery processing effluents is around 3.8 (Balslev-Olesen et al., 1990), but six to seven in fish processing effluents (Najafpour et al., 2006); in contrast, fish condensate is produced with a pH range from 9 to 10 (Sandberg and Ahring, 1992). Although not a contaminant, pH is important as a characterisation parameter since it suggests contamination of effluents and relates to the emission of ammonia compounds (Vidya et al., 2020). As pH increases, more ammonia is converted to the un-ionised form, which is extremely toxic to fish.
Solids
Total suspended solids (TSS) in aquaculture effluent are generally high due to the presence of protein and lipids (Paluenzuela-Rollon et al., 2002) which account for approximately 10–30% of total solids. Others reported that in fish processing, TSS varied from 150 to 1,100 mg L−1 (Muthukumaran and Baskaran, 2013) or up to 22,910 mg L−1 in fish processing plant wastewaters (Picos-Benítez et al., 2019). The average TSS was found to be 635 mg L−1 in the final effluent in another report with TSS contributing significantly to COD, BOD, and total nitrogen (TN) levels (Muthukumaran and Baskaran, 2013). Total suspended solid concentrations must be reduced to comply with discharge limits (e.g. in European Union, COD ≤120 mg L−1) (Federal Ministry for the Environment, N. C. A. N. S., Germany, 2004).
Organic Content
Fish processing wastewater contains high BOD and COD, which originate primarily from carbonaceous compounds and nitrogen-containing compounds. Biological Oxygen Demand and COD are a measure of how much dissolved oxygen is being consumed as microbes break down organic matter and oxidise all pollutants (Li and Liu, 2019). The organic content directly influences the demand for dissolved oxygen in rivers and streams, with serious implications for the river’s biodiversity. The consequences of high BOD and COD include increases stress on aquatic organisms with the potential for death by suffocation. For example, a strong positive correlation exists between the BOD concentration of wastewater discharged into rivers and subsequent eutrophication, which results in negative impacts on aquatic organisms in the receiving water body, including fish (Vidya et al., 2020). In the fish processing industry, the COD of the effluent is usually higher than the 5-days biochemical oxygen demand (BOD5): the ratio of the two varies according to the type of fish processing plants (from 1.1: 1 to 3:1) (Technical Report series FREMP, 1994). One report showed that the BOD5 in tuna waste varies between 500–1,500 mg L−1, only 40% of the COD value (1,300–3,250 mg L−1) (Carawan, 1979). In another study by Muthukumaran et al., the BOD5:COD ratio for the fish wastewater generated was almost 1:1 (Muthukumaran and Baskaran, 2013). They reported that the average BOD in the final effluent was 3,000 mg L−1 and COD was 3,500 mg L−1. The current discharge limits stated in European Union guidelines, for BOD and COD in the final effluent are 50 and 120 mg L−1, respectively; in this case, BOD and COD levels must be reduced by 83 and 66%, respectively (Federal Ministry for the Environment, N. C. A. N. S., Germany, 2004).
Total Nitrogen and Phosphorus
The concentration of nitrogen in fish processing wastewater varies based on the type and amount of fish processed; high concentrations are likely due to the protein content (15–20% of wet weight) of fish and marine invertebrates (Picos-Benítez et al., 2019). High ammonia concentrations are often observed due to high blood and slime content in wastewater streams. The total nitrogen in fish processing was reported to be 1,200 mg L−1 (Picos-Benítez et al., 2019), while the average total nitrogen was found to be 347 mg L−1 in the final fish wastewater (Muthukumaran and Baskaran, 2013). Phosphorus also partly originates from the fish but can be introduced with processing and cleaning agents, with concentrations reported in the range 13–47 mg L−1 (Cristóvão et al., 2014a). Excess quantities of nitrogen and phosphorus may cause the proliferation of algae and affect aquatic life in a water body. Large algal growth, algal blooms severely reduce or eliminate oxygen in the water, leading to the death of large numbers of fish (EPA, 2019).
Fat, Oil, and Grease (FOG)
Fat, oil, and grease (FOG) concentrations vary depending on the type of material. One study reported that the FOG concentration in fish canning wastewater varied from 156–2,808 mg L−1 depending on the season (Cristóvão et al., 2014a). Fats oil and grease are readily removed from wastewater through skimming as FOG float on the water’s surface; however, if they remain then this will affect oxygen transfer into the wastewater below.
Salinity
In the fish processing industry, the sources of waste are initially related to the raw materials and seawater used in various processes. For example, the wastewater generated from fish processing contains in the range of 3.5–20 g L−1 NaCl (Val del Rio et al., 2018; Picos-Benítez et al., 2019), while wastewater from dried salted fish plant contains NaCl ranging from 17 to 46 g L−1 (Yun Chen and Ghufran, 2017). High salinity can cause high osmotic stress and the inhibition of microorganism activity, which results in a significant decrease in biological treatment efficiency.
There is therefore a requirement for the aquaculture industry to ensure that fish processing has minimum impact on the environment. This is reflected in government policies, where pollution control regulations in terms of wastewater discharge have become more stringent to manage the effluent discharge (Table 1).
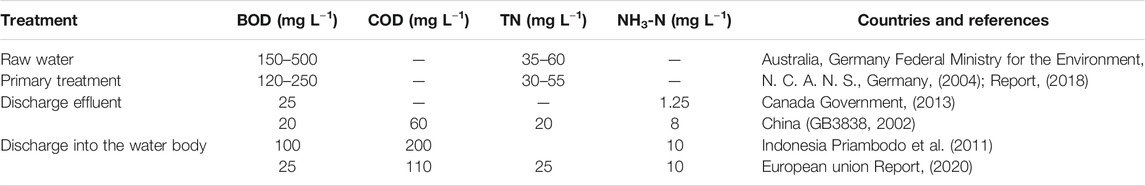
TABLE 1. Example of guidelines for the discharge of wastewaters from fish processing in various countries.
Another issue with the effective treatment of waste from the aquaculture industry lies in the variability of the wastewater in terms of composition which makes it difficult for standard approaches for aquaculture wastewater treatment to be developed and implemented.
Current Treatment Approaches
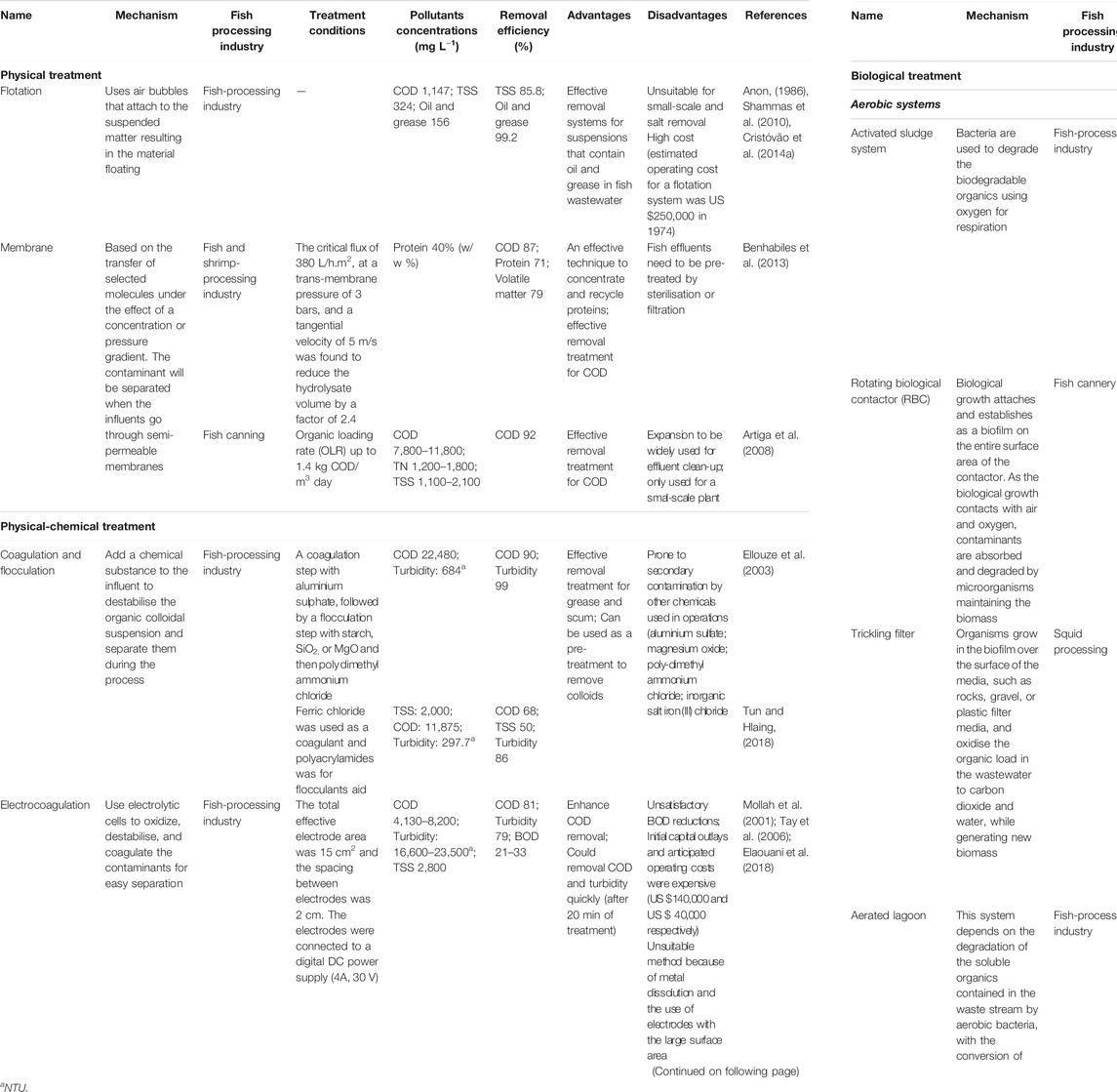
Various treatment processes including non-biological (physical, chemical treatment) and biological approaches (aerobic and anaerobic treatment) have been applied to treat saline aquaculture containing high concentrations of organic material (Paluenzuela-Rollon et al., 2002). With the availability of different treatment techniques, the question of which treatment is most effective must be considered. The answer to this question is not simple because of the broad range of characteristics of aquaculture wastewater. Table 2 compares the advantages and disadvantages of the current approaches.
Non-biological approaches are considered a high-cost approach since they require significant initial capital and operating costs. The efficacy of non-biological approaches is also often limited due to the presence of chemical residuals and secondary pollutants; as a consequence, these technologies are only applied in certain conditions or used for pre-treatment due perhaps to the presence of high concentrations of suspended matter (Dinçer and Kargi, 2000; Neilly et al., 2009; Fan et al., 2011). For example, one study found that non-biological approaches such as flotation were not a suitable primary treatment method for the treatment of fish canning wastewater (Cristóvão et al., 2014a). However, any integrated bioprocess such as microalgae, chemical treatment, and membrane microfiltration are complex to install and run and are often expensive in terms of the costs for equipment, maintenance, and operations.
For economic and environmental sustainability, fish processing wastewater needs to be considered in terms of compliance with quality requirements before discharge or recycling. Biological treatment is one of the best options for the disposal of organic matter-rich wastewater by fish processing (Parvathy et al., 2017). With biological treatment, wastewater pollutant reduction efficiencies of greater than 90% can be attained (Kiepper, 2001). The microorganisms used are responsible for the degradation of organic matter and organic waste stabilisation (Arvanitoyannis and Kassaveti, 2008); both BOD and COD significantly decrease as a result of microbiological activity (Cristóvão et al., 2012). For this reason, significant potential lies in improving existing biological systems based on their low cost and their sustainability. However, current biological processes can be inefficient due to the environmental conditions faced by the microbial community present in aquaculture wastewater. These are discussed further below.
Factors Affecting Biological Processes Involved in the Treatment of Fish Processing Wastewater
Factors Affecting Anaerobic Treatment
Effect of pH and Ammonia Content on Anaerobic Treatment
At present, several types of anaerobic digesters (AD) are used to treat saline fish processing wastewater; these include anaerobic fixed-bed and fluidised bed reactors (AFB), up-flow anaerobic sludge blanket reactors (UASB), and up-flow microbial fuel cells (MFC) (Table 3). Since AD leads to the formation of biogas (a mixture of methane and carbon dioxide) and relatively low volumes of microbial biomass, it offers numerous advantages, including low sludge production, low energy requirement, and green energy recovery (Massé et al., 2010; Xia et al., 2012). This technology has also a positive net energy production as the biogas produced can replace fossil fuel, resulting in a direct positive effect on greenhouse gas reduction. Despite these benefits, however, poor operational stability still prevents the AD process from being widely employed for the treatment of fish processing wastewater (Shanmugam and Horan, 2009). Several factors affect the AD process performance and stability, including the initial concentration of organic compounds, pH, and ammonia concentrations (NH3+-N). The optimal pH for biogas reactors in terms of methane production is 6.8–7.3 (Liu et al., 2019); most methanogenic bacteria have optima for growth between pH 7 and 8, whereas volatile fatty acid-degrading bacteria have lower pH optima, 5.5–9 (Jun et al., 2012). However, the reported pH of fish wastewater effluents ranges from 7.2 to 7.8 (Vidya et al., 2020) or 6.1 to 7.1 (Cristóvão et al., 2014a) depending on the level of total soluble and suspended COD, which vary between processing and fish type (Chowdhury et al., 2010). In one study, Sandberg and Ahring (1992) demonstrated that a 15–17% reduction in COD removal during AD occurred when the pH was increased slowly to 8.0 or more (Sandberg and Ahring, 1992).
Additionally, ammonia is one of the main intermediate products of AD, as a result of the biodegradation of proteins, urea, and nucleic acids (González-Fernández and García-Encina, 2009). The high concentrations of protein often associated with fish processing effluents are readily converted into ammonia, an inhibitor of methanogenesis (Aspé et al., 2001). Ammonia affects methanogenic bacteria in two ways: 1) the ammonium ion may inhibit the methane-producing enzyme directly and/or 2) hydrophobic ammonia molecules may diffuse passively into bacterial cells, causing proton imbalance or potassium deficiency (Gallert et al., 1998). In one study, during anaerobic treatment, methanogenic activity was shown to be significantly reduced by the presence of high concentrations of ammonia (Chen et al., 2008; Hejnfelt and Angelidaki, 2009); the results showed that an NH3-N concentration greater than 100 mg L−1 inhibited methanogenesis (Aspé et al., 2001). It was also reported that free ammonia inhibitory concentrations for mesophilic treatment were 25–140 mg N-NH3 L−1 (Guerrero et al., 1997), but in one case, ammonia was reported to reach approximately 600 mg N L−1 (Sarnaik et al., 2015). Numerous possible remediation techniques have been reported for the control of ammonia inhibition in the AD process including anaerobic ammonium oxidation (anammox) (Egli, 2003), the use of zeolite, and carbon fiber textiles (Sasaki et al., 2011). These materials work as ion exchange elements for NH4-N and the adsorption of NH3 (Kimura et al., 2010). However, all these approaches bear the disadvantage that the materials are expensive to implement at a large scale and toxic for the microorganisms at high levels.
Effect of Salinity on Anaerobic Treatment
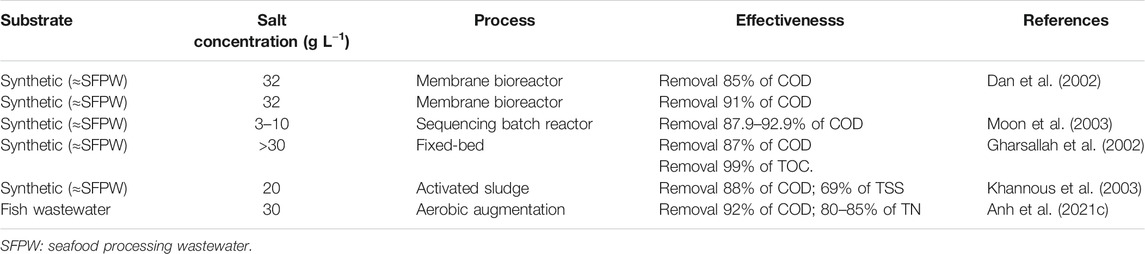
The performance of AD is often reduced to different degrees by sodium concentrations within the range 3.5–28 g L−1 (Chen et al., 2008). This is because the physical and biological properties of microorganisms are changed at high osmotic pressure, which affects the production of hydrogen in anaerobic fermentation (Zhang et al., 2017). At high salinity (3.0–3.5%) and alkaline condition (pH 8.0–10.0), hydrogen production was reported to be significantly reduced, while a sodium concentration exceeding 10 g L−1 strongly inhibited methanogenesis (Picos-Benítez et al., 2019). Additionally, high salinity is reported to inhibit the metabolism of nitrifying bacteria, resulting in the reduction of the nitrogen removal rate (Shen et al., 2015). In one study the total nitrogen removal was reduced from 85 to 70% when the salt concentration increased from 20–30 g L−1, while COD removal was reduced from 90 to 71% when the salt content in fish wastewater increased from 5–30 g L−1; similarly, phosphorus removal decreased from 38 to 10% with increases in salt content from 0 to 30 g L−1 (Panswad and Anan, 1999). In addition, biogas yield was reduced to 64% when salinity increased from 0 to 20 g L−1; COD removal also decreased to between 83 and 90% (Picos-Benítez et al., 2019).
Factor Affecting Aerobic Treatment
The efficacy of the aerobic wastewater treatment processes is also known to be adversely affected by high salinity. High salt concentrations increase osmotic pressure, which results in a reduction in particle size and density, causing cell plasmolysis and cell death (Medveďová et al., 2018). In addition, reports suggest that the size and fractal dimension of flocs decrease as the salt concentration increases (Moon et al., 2003), resulting in reduced settling; Moussa et al. also stated that most microorganisms were almost completely inhibited at a salinity of 20–30 g L−1 (Medveďová et al., 2018). Furthermore, other reports suggest that when the chloride concentration exceeds 40 g L−1, a reduction in pollutant removal was observed (Aloui et al., 2009). In one report, the COD removal efficiency of the effluent fell from 80 to 48.5% when salinity increased from 0.5 to 1.5% (Salmanikhas et al., 2016). This experiment investigated the effect of salt concentration on the efficacy of the aerobic treatment of synthetic effluents using a fed-batch biological reactor with activated sludge. In other research using a sequencing batch reactor to treat a synthetic saline effluent, the COD removal efficiency was reduced from 90 to 32% when salinity increased from 0 to 6% (Uygur and Kargi, 2004). Yun et al. also concluded that the total nitrogen removal of the system was reduced from 85 to 70%, and COD removal decreased from 90 to 71% when the salt level in wastewater increased from 5–30 g L−1 (Yun Chen and Ghufran, 2017).
In summary, research suggests that while the efficacy of aerobic wastewater systems is largely dependent on salinity, anaerobic approaches require adaptation to several factors (salinity, pH, and ammonia concentrations). Fish wastewaters are rich in protein, which generates high concentrations of ammonia; both nitrogen and salt can inhibit anaerobic digestion; this is the reason why the use of anaerobic treatment of fish processing wastewater has resulted in poor results in terms of treatment efficacy (Picos-Benítez et al., 2019). In summary, aerobic systems appear to be generally represent the most promising method to treat fish processing wastewaters. However, the salinity of the wastewater remains an important issue, often resulting in reduced treatment efficiency. Therefore, the cost-effective and efficient treatment of nitrogen-rich saline fish processing wastewater remains a challenge. The next section will look at possible solutions to deal with high salinity and alternative methods of treatment.
Options for Pre-Treatment of Highly Saline Wastewaters
A number of technologies are available which reduce the salinity of the wastewater before aerobic biological treatment (Table 4). These options are further discussed below.
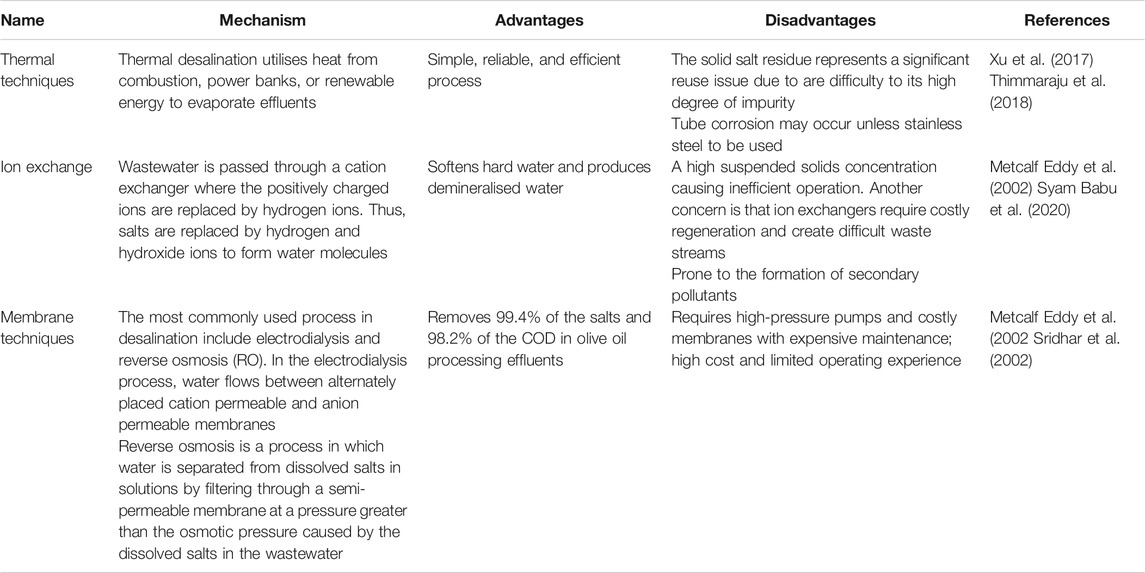
TABLE 4. Current options for the treatment of salinity in wastewaters; advantages and disadvantages of current techniques.
Due to the requirement of significant energy and its related costs in terms of energy and maintenance, overall treatment costs rise significantly with the addition of a desalination step. In 2013, desalination, removal of salt from seawater, brackish water, and wastewater was calculated to cost between United States $0.45 to United States $1.00 per m3 (Xu et al., 2017).
To overcome the costs associated with desalination an emerging environmental biotechnology opportunity exists, through bioremediation of saline wastewater by bioaugmentation using microorganisms that can tolerate or adapt to high salinity and degrade nutrients, resulting in enhanced growth and degradative activities. The options are discussed further below.
The Potential Solutions to Treat Saline Fish Wastewater Effluents
Bioaugmentation Using Salt-Tolerant Bacteria
In this approach, wastewater is bioaugmented with salt-adapted bacteria capable of withstanding high salinities and at the same time degrading the pollutants that are contained in wastewater (Marsh et al., 2021). In one study, a salt-tolerant bacterial consortium present in sludge was adapted to high salt concentrations (Lefebvre and Moletta, 2006). The novel characteristics and capacity for large-scale culturing make halophilic bacteria potentially valuable for biotechnology (Ventosa et al., 2011). However, most halophiles are inactivated when the NaCl or KCl concentration of the solution decreases to less than 2.4% (Madern et al., 2000). Therefore although these organisms thrive in high salt concentration environments (at least 3%) (Kushner, 1988), as their proteins require the presence of high concentrations of salt for optimum stability and activity (Madern et al., 2000) they may not be versatile enough for application in all fish processing wastewater applications, limiting their commercial potential.
However, halophiles can be divided into two groups, namely moderate and extreme halophiles. Moderate halophiles are microorganisms that grow best in a medium containing 3–15% NaCl, yet moderate halophiles are capable of growth at concentrations less than 1%. In contrast, extreme halophiles exhibit optimum growth in media containing 15–30% NaCl (Oren, 2010). For example, the moderate halophiles Vibrio costicola (Smith, 1936), Micrococcus halobios (Onishi and Kamekura, 1972), Spirochaeta halophila (Greenberg and Canale-Parola, 1976), Marinirhabdus sp. and Marinorbacter hydrocarbonoclasticus (Anh et al., 2021c) grew well from 3 to 15%. Comparing moderate and extreme halophilic bacteria, a survey demonstrated moderate halophiles have diverse metabolic requirements and capabilities. They may compete well with extreme halophilic bacteria in some hypersaline environments because they have relatively high growth rates at ambient temperatures (Rodriguez-Valera et al., 1981). For example, the moderate halophilic bacteria Salinivibrio costicola and Halomonas halodenitrificans are able to grow over a range of water activities between 0.98 (close to freshwater) to 0.86 (close to saturated NaCl) (Kushner, 1978).
Aside from their salinity tolerance, these strains must be able to release protease in order to improve the efficiency of COD and TN removal. This is because fish processing wastewater contain high loads of organic nutrients that originate primarily from carbonaceous compounds and nitrogen-containing compounds such as protein, peptide, and volatile amines (Ching and Redzwan, 2017). Increasing nitrogen removal through process modification, resulting in an increase in active proteolytic activity represents one possible option to reduce the environmental impact of the wastewater. In a recent study, two moderately halophilic, protease-producing bacteria Marinirhabdus sp. and Marinobacter hydrocarbonoclasticus were used to bioaugment non-sterile fish processing wastewater which led to COD and TN removal of 92%, and 80–85% respectively (Anh et al., 2021c). This study confirmed the effectiveness of bioaugmentation in removing COD and TN in saline fish wastewater. Additionally, Marinobacter hydrocarbonclasticus was found dominate the bacterial community suggesting the commercial potential of this organism for bioaugmentation of fish processing wastewater without the need for further bioaugmentation (Anh et al., 2021b).
Halophilic bacteria are metabolically more versatile than the archaea, exhibiting more diverse enzymatic activities (Oren, 2010). However, there are, to date limited reports of these bacteria being the basis of commercial products, although extensive research has been undertaken to elucidate the properties of the enzymes from halophilic and halotolerant bacteria. Due to the stability and properties of halophilic enzymes, they are good candidates for use in industrial processes but are yet to be fully exploited.
The application of salt-tolerant bacteria to biological saline wastewater treatment has been previously reported (Breugelmans et al., 2008; Oren et al., 1992). Other reports suggest that hypersaline water polluted with organic compounds such as petroleum hydrocarbons and aromatic compounds can be remediated using halophiles (Fathepure, 2014). Furthermore, a profile of different halophiles capable of degrading organic pollutants was presented by Chen et al. (Chen et al., 2018). Similarly, Li et al. showed that the halophilic bacterium NY-4 was capable of efficient denitrification (94.2% of nitrate removal and 80.9% of total nitrogen removal in 48 h) (Li et al., 2013). Another study reported that the utilisation of halophilic microorganisms along with the activated sludge culture resulted in enhanced treatment performance, with 85% of COD removal within 9 h of fed-batch operation (Kargi and Dinçer, 2000).
Other industrial processes that generate highly saline wastewater have used halophilic microorganisms to treat saline wastewater such as pickling plants and tanneries (Kargi, 2002). For example, the single-celled, photosynthetic green alga, Dunaliella were studied in terms of their efficacy to remove organic carbon and toxic compounds in several processes (Santos et al., 2001). In another study, the addition of a Halobacter sp. resulted in significant improvement in the performance of an activated sludge plant to treat synthetic wastewater (Kargi and Uygur, 1996). The results showed high COD removal efficiencies at salt concentrations as high as 5% in the influent. Similarly, this augmented system was shown to be successful in treating effluent generated by the pickling industry with more than 95% COD removal (Kargi and Dinçer, 2000). The same technique (inoculation of the halotolerant bacteria Staphylococus sp. and Bacillus cereus) was applied to hypersaline effluent (15% NaCl) generated by the production of plum pickles and achieved COD removal efficiency of 90% in a sequencing batch system (Kubo et al., 2001).
However, most of the studies carried out and reported were conducted at a laboratory-scale model. No commercial applications have yet been developed for such enzymes although research examined the properties of the enzymes from halotolerant bacteria and their possible application.
As a consequence, there appears to be a commercial opportunity for the development of moderately halophilic bacteria with efficient degradative abilities as bioaugmentation agents. Despite the fact that the use of a salt-tolerant inoculum for the treatment of wastewater from fish processing has only be explored in a few studies, the benefits of their application appear significant. The use of microbial additives during composting is considered highly efficient, likely to enhance the production of different enzymes resulting in a better rate of waste degradation (Rastogi et al., 2020). Successful augmentation requires the appropriate selection of microbial strains or microbial consortia, which involves consideration of a few, key features of the added microorganisms, including rapid growth, readily culturable, ability to withstand high concentrations of contaminants, and survival in a wide range of environmental conditions.
Bioaugmentation Using Enzymes as Biodegrading Agents
The use of enzymes may represent a good alternative for overcoming most issues related to saline fish wastewater. Enzymes are recognised as highly efficient and green biocatalysts with the additional characteristics of high regioselectivity, chemoselectivity, and stereoselectivity (Bilal et al., 2018; Khan et al., 2018). Enzymes are also not affected by inhibitors of microbial metabolism and they can be used to remediate many compounds under extreme conditions limiting microbial activity (Rao et al., 2010). Ojuederia and Babalola (2017) found enzymes having a great potential to effectively transform pollutants at a detectable rate and were potentially suitable to restore polluted environments (Ojuederie and Babalola, 2017). The most representative enzymatic classes in the remediation of polluted environments are hydrolases, dehalogenases, transferases, and oxidoreductases. Their main producers are bacteria, fungi, mainly white-rot fungi, plants, and microbe-plant associations (Karigar and Rao, 2011).
In terms of the treatment of fish processing wastewater, which has high loads of organic nutrients that originate primarily from carbonaceous compounds and nitrogen-containing compounds such as protein, peptide, and volatile amines (Ching and Redzwan, 2017), proteases would appear to be offer significant potential. Proteases hydrolyse the breakdown of the peptide bonds of proteinaceous substance to components absorbed by bacteria and converted to biomass. For example, protease breaks down proteins and provides free amino acids to lactic acid bacteria, which are the final hydrolysis products and a rich source of protein needed for their growth (Kieliszek et al., 2021).
For the success of saline fish wastewater treatment, the proteases added needs to be stable and exhibit efficient activity in a saline environment. Therefore, the enzymes applied may be immobilised onto a suitable matrix to be an effective catalyst for the degradation in saline environments (Pounsamy et al., 2017). An immobilised enzyme is physically confined to a certain region of space, retaining its catalytic activity and the capacity to be used repeatedly or continuously (Mohamad et al., 2015). Immobilised enzymes have usually long-term operational stability, being very stable toward physical, chemical, and biological denaturing agents. For example, an immobilised protease enzyme previously isolated and extracted from Enterococcus faecalis, exhibited enhanced activity (104%) compared with that of the free purified enzyme; furthermore, this activity was not affected by the presence of either organic matter or the salt concentration (Pounsamy et al., 2017).
Halotolerant enzymes are commonly produced by halotolerant microorganisms (Graziano and Merlino, 2014). For example, Gao et al. (2019) reported that the proteases secreted by Aspergillus oryzae was more stable than non-salt-tolerant proteases at high salinity, remaining 20% active even in the presence of 3.0 mol L−1 NaCl after 7 days (Gao et al., 2019). An protease was also isolated from the halotolerant organism Lycinibacillus macrolides, with activity of 304 U mL−1 in highly saline conditions (>4%) (Pounsamy et al., 2017). Protease of co-cultured Marinirhabdus sp. and Marinobacter hydrocarbonoclasticus showed activity and stability over a broader range of environmental conditions (temperature 25–60°C, pH 4–12, and 10–30% salinity, respectively) (Anh et al., 2021a).
Although salt-tolerant enzymes already play a key role in numerous processes at low to high NaCl concentrations, including various applications in detergent formulation, fish and meat processing (Graziano and Merlino, 2014), only a few reports on their application have been reported. In one study, halotolerant proteases from halophilic bacteria were used to treat marine waste (crab shell, shrimp shell, and squid pen powder) (Annamalai et al., 2014; Mokashe et al., 2018). Similarly, Maruthiah et al. reported protease production using marine shell waste from a marine Bacillus sp. APCMST-RS3 (Maruthiah et al., 2015). A salt-stable protease from the halophilic bacteria Lysinibacillus macroides, was found to efficiently degrade proteins in saline tannery wastewater, with a complete fragmentation time of 90 min at pH 6 and 30°C (Pounsamy et al., 2019). Another extracellular halophilic proteases from the halophile Alkalibacillus sp. NM-Da2 could remove 50% protein in synthetic saline wastewater after 5 h (Abdel-Hamed et al., 2016).
These initial results suggest that further research on the identification and development of halophilic bacteria, immobilised enzymes, be carried out in terms of their commercial potential.
Conclusion
The main components of fish effluent are high organic carbon concentrations, total nitrogen, and salinity. As a result, the fish processing industry faces significant challenges in terms of the effective treatment of these wastewaters to meet increasingly stringent discharge limits. Current treatment methods include physical, chemical, and biological approaches. While the major limitation associated with physico-chemical treatment is the production of secondary contaminants and elevated treatment costs, biological approaches, represent cost-effective and sustainable approaches. However, the efficacy of biological treatments is hampered by high concentrations of salinity in the wastewater, resulting in a need to develop new improved approaches. The use of RO and membranes are considered efficient for the removal of salts, but suspended solids reduce the efficiency and increase costs. Enhanced biological treatment using bioaugmentation using halophilic microorganisms with high protease activity and immobilised protease enzymes from halophilic bacteria represent potential cost-effective approaches to ensure that aerobic biological treatment of fish processing wastewaters can attain discharge target concentrations. Certainly, this review suggests that lab-scale studies indicate that bioaugmentation of biological systems, particularly aerobic systems such as activated sludge, lagoons, trickling filter, and rotating biological contactors using halophilic bacteria capable of both growth and degradative activity in the wastewater represents a simple yet effective solution to improve aquaculture wastewater treatment. Moderate halophiles are simple to grow in the salinity range 3-15% and may offer a potential commercial solution to the effective treatment of saline wastewaters. In addition, the application of immobilised, halophilic enzymes such as proteases represents a promising in situ remediation technique.
Author Contributions
HA, ES, NB, and AB conceived and designed the structure of the review. HA conducted the draft. ES and NB contributed the resources. HA collected the data with help of ES. HA wrote the manuscript. ES and AB revised the manuscript. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdel-Hamed, A. R., Abo-Elmatty, D. M., Wiegel, J., and Mesbah, N. M. (2016). Biochemical Characterization of a Halophilic, Alkalithermophilic Protease from Alkalibacillus Sp. NM-Da2. Extremophiles. 20, 885–894. doi:10.1007/s00792-016-0879-x
Achour, M., Khelifi, O., Bouazizi, I., and Hamdi, M. (2000). Design of an Integrated Bioprocess for the Treatment of Tuna Processing Liquid Effluents. Process Biochem. (1991). 35, 1013–1017. doi:10.1016/s0032-9592(00)00133-3
Aloui, F., Khoufi, S., Loukil, S., and Sayadi, S. (2009). Performances of an Activated Sludge Process for the Treatment of Fish Processing saline Wastewater. Desalination 246, 389–396. doi:10.1016/j.desal.2008.03.062
Anh, H. T. H., Shahsavari, E., Bott, N. J., and Ball, A. S. (2021a). Application of Co-culture Technology to Enhance Protease Production by Two Halophilic Bacteria, Marinirhabdus Sp. And Marinobacter Hydrocarbonoclasticus. Molecules 26, 3141. doi:10.3390/molecules26113141
Anh, H. T. H., Shahsavari, E., Bott, N. J., and Ball, A. S. (2021b). The Application of Marinobacter Hydrocarbonoclasticus as a Bioaugmentation Agent for the Enhanced Treatment of Non-sterile Fish Wastewater. J. Environ. Manage 291, 112658. doi:10.1016/j.jenvman.2021.112658
Anh, H. T. H., Shahsavari, E., Bott, N. J., and Ball, A. S. (2021c). Bioaugmentation of Seafood Processing Wastewater Enhances the Removal of Inorganic Nitrogen and Chemical Oxygen Demand. Aquaculture 542, 736818. doi:10.1016/j.aquaculture.2021.736818
Annamalai, N., Rajeswari, M. V., Sahu, S. K., and Balasubramanian, T. (2014). Purification and Characterization of Solvent Stable, Alkaline Protease from Bacillus Firmus CAS 7 by Microbial Conversion of marine Wastes and Molecular Mechanism Underlying Solvent Stability. Process Biochem. 49, 1012–1019. doi:10.1016/j.procbio.2014.03.007
Anon, (1986). Environmental Assessment and Management of the Fish Processing Industry. Sectoral Studies Series No. 28, 1986. Vienna, Austria: UNIDO. doi:10.1179/014788886794641234
Arend, K. K., Beletsky, D., Depinto, J. V., Ludsin, S. A., Roberts, J. J., Rucinski, D. K., et al. (2011). Seasonal and Interannual Effects of Hypoxia on Fish Habitat Quality in central Lake Erie. Freshw. Biol. 56, 366–383. doi:10.1111/j.1365-2427.2010.02504.x
Artiga, P., García-Toriello, G., Méndez, R., and Garrido, J. M. (2008). Use of a Hybrid Membrane Bioreactor for the Treatment of saline Wastewater from a Fish Canning Factory. Desalination. 221, 518–525. doi:10.1016/j.desal.2007.01.112
Arvanitoyannis, I. S., and Kassaveti, A. (2008). Fish Industry Waste: Treatments, Environmental Impacts, Current and Potential Uses. Int. J. Food Sci. Technol. 43, 726–745. doi:10.1111/j.1365-2621.2006.01513.x
Aspé, E., Martí, M. C., Jara, A., and Roeckel, M. (2001). Ammonia Inhibition in the Anaerobic Treatment of Fishery Effluents. Water Environ. Res. 73, 154–164. doi:10.2175/106143001x138813
Balslev-Olesen, P., Lynggaard-Jensen, A., and Nickelsen, C. (1990). Pilot-scale Experiments on Anaerobic Treatment of Wastewater from a Fish Processing Plant. Water Sci. Technology 22, 463–474. doi:10.2166/wst.1990.0170
Benhabiles, M. S., Abdi, N., Drouiche, N., Lounici, H., Pauss, A., Goosen, M. F. A., et al. (2013). Protein Recovery by Ultrafiltration during Isolation of Chitin from Shrimp Shells Parapenaeus Longirostris. Food Hydrocolloids 32, 28–34. doi:10.1016/j.foodhyd.2012.11.035
Bilal, M., Rasheed, T., Zhao, Y., Iqbal, H. M. N., and Cui, J. (2018). “Smart” Chemistry and its Application in Peroxidase Immobilization Using Different Support Materials. Int. J. Biol. macromolecules 119, 278–290. doi:10.1016/j.ijbiomac.2018.07.134
Blaber, S. J. M. (2011). “8.09 - Removals (Wild Harvesting) of the Biological Resources from Systems,” in Treatise on Estuarine and Coastal Science Editors E. Wolanski, and D. Mclusky (Waltham: Academic Press).
Breugelmans, P., Barken, K. B., Tolker-Nielsen, T., Hofkens, J., Dejonghe, W., and Springael, D. (2008). Architecture and Spatial Organization in a Triple-Species Bacterial Biofilm Synergistically Degrading the Phenylurea Herbicide Linuron. FEMS Microbiol. Ecol. 64, 271–282. doi:10.1111/j.1574-6941.2008.00470.x
Carawan, R. E. (1991). Processing Plant Waste Management Guidelines for Aquatic Fishery Products. Seafood and the Environment - 1991. Newport, Oregon: Pollution Prevention Short Course.
Chen, X., Zhuang, J., and Bester, K. (2018). Degradation of Triclosan by Environmental Microbial Consortia and by Axenic Cultures of Microorganisms with Concerns to Wastewater Treatment. Appl. Microbiol. Biotechnol. 102, 5403–5417. doi:10.1007/s00253-018-9029-y
Chen, Y., Cheng, J. J., and Creamer, K. S. (2008). Inhibition of Anaerobic Digestion Process: a Review. Bioresour. Technol. 99, 4044–4064. doi:10.1016/j.biortech.2007.01.057
Ching, Y. C., and Redzwan, G. (2017). Biological Treatment of Fish Processing Saline Wastewater for Reuse as Liquid Fertilizer. Sustainability 9, 1062. doi:10.3390/su9071062
Chowdhury, P., Viraraghavan, T., and Srinivasan, A. (2010). Biological Treatment Processes for Fish Processing Wastewater - A Review. Bioresour. Technology. 101, 439–449. doi:10.1016/j.biortech.2009.08.065
Construction, C. (2018). GWE Sets Environmental Standards with New Wastewater Treatment Plant. [Online]. Available at: https://constructionreviewonline.com/corporate-news/gwe-sets-environmental-standards-with-new-wastewater-treatment-plant/ (Accessed May12, 2021).
Cortez, S., Teixeira, P., Oliveira, R., and Mota, M. (2008). Rotating Biological Contactors: a Review on Main Factors Affecting Performance. Rev. Environ. Sci. Biotechnol. 7, 155–172. doi:10.1007/s11157-008-9127-x
Cristóvão, R. O., Botelho, C. M. S., Martins, R. J. E., and Boaventura, R. A. R. (2012). Chemical and Biological Treatment of Fish Canning Wastewaters. Ijbbb. 2, 237–242. doi:10.7763/ijbbb.2012.v2.108
Cristóvão, R. O., Botelho, C. M., Martins, R. J. E., Loureiro, J. M., and Boaventura, R. A. R. (2014a). Primary Treatment Optimization of a Fish Canning Wastewater from a Portuguese Plant. Water Resour. Industry 6, 51–63. doi:10.1016/j.wri.2014.07.002
Cristóvão, R. O., Gonçalves, C., Botelho, C. M., Martins, R. J. E., and Boaventura, R. A. R. (2014b). Chemical Oxidation of Fish Canning Wastewater by Fenton's Reagent. J. Environ. Chem. Eng. 2, 2372–2376. doi:10.1016/j.jece.2013.12.023
Cristóvão, R. O., Pinto, V. M. S., Martins, R. J. E., Loureiro, J. M., and Boaventura, R. A. R. (2016). Assessing the Influence of Oil and Grease and Salt Content on Fish Canning Wastewater Biodegradation through Respirometric Tests. J. Clean. Prod. 127, 343–351. doi:10.1016/j.jclepro.2016.04.057
Dan, N. P., Visvanathan, C., Polprasert, C., and Ben Aim, R. (2002). High Salinity Wastewater Treatment Using Yeast and Bacterial Membrane Bioreactors. Water Sci. Technol. : a J. Int. Assoc. Water Pollut. Res. 46, 201–209. doi:10.2166/wst.2002.0239
Dauda, A. B., Ajadi, A., Tola-Fabunmi, A. S., and Akinwole, A. O. (2019). Waste Production in Aquaculture: Sources, Components and Managements in Different Culture Systems. Aquaculture Fish. 4, 81–88. doi:10.1016/j.aaf.2018.10.002
Diaz, R. J., and Rosenberg, R. (2008). Spreading Dead Zones and Consequences for Marine Ecosystems. Science 321, 926–929. doi:10.1126/science.1156401
Dinçer, A. R., and Kargi, F. (2000). Effects of Operating Parameters on Performances of Nitrification and Denitrification Processes. Bioproc. Eng. 23, 75–80. doi:10.1007/s004499900126
Dodds, W. K., Bouska, W. W., Eitzmann, J. L., Pilger, T. J., Pitts, K. L., Riley, A. J., et al. (2009). Eutrophication of U.S. Freshwaters: Analysis of Potential Economic Damages. Environ. Sci. Technol. 43, 12–19. doi:10.1021/es801217q
Egli, K. R. (2003). On the Use of Anammox in Treating Ammonium-Rich Wastewater Corvallis, Oregon: ETH Zurich. doi:10.1002/0471263397.env274
Elaouani, H., Jaafari, K., Benkhouja, K., Elbada, N., and Haffad, H. (2018). Industrial Fish Wastewater Treatment by Electrocoagulation Processes Powered by Solar Energy Piscataway, New Jersey: IEEE. doi:10.1109/repsgie.2018.8488845
Ellouze, E., Amar, R. B., Boufi, S., and Salah, A. B. (2003). Performances de la coagulation ‐ floculation dans le traitement des effluents de seiche. Environ. Technology. 24, 1357–1366. doi:10.1080/09593330309385680
Ezeonu, C. S., Tagbo, R., Anike, E. N., Oje, O. A., and Onwurah, I. N. (2012). Biotechnological Tools for Environmental Sustainability: Prospects and Challenges for Environments in Nigeria-a Standard Review. Biotechnol. Res. Int. 2012, 450802. doi:10.1155/2012/450802
Fan, J., Zhang, J., Zhang, C., Ren, L., and Shi, Q. (2011). Adsorption of 2,4,6-trichlorophenol from Aqueous Solution onto Activated Carbon Derived from Loosestrife. Desalination 267, 139–146. doi:10.1016/j.desal.2010.09.016
FAO (2018). The State of World Fisheries and Aquaculture. [Online]. Available at: http://www.fao.org/3/i9540en/i9540en.pdf (Accessed 20 January, 2021).
FAO (2020). The State of World Fisheries and Aquaculture [Online]. Available at: http://www.fao.org/publications/sofia/en/ (Accessed May11, 2021).
Fathepure, B. Z. (2014). Recent Studies in Microbial Degradation of Petroleum Hydrocarbons in Hypersaline Environments. Front. Microbiol. 5, 173. doi:10.3389/fmicb.2014.00173
Federal Ministry for the Environment, N. C. A. N. S., Germany (2004). The Ordinance on Requirements for the DIscharge of Waste Water into Waters. [Online] (Accessed 17 May, 2021).
Gallert, C., Bauer, S., and Winter, J. (1998). Effect of Ammonia on the Anaerobic Degradation of Protein by a Mesophilic and Thermophilic Biowaste Population. Appl. Microbiol. Biotechnol. 50, 495–501. doi:10.1007/s002530051326
Gao, X., Yin, Y., Yan, J., Zhang, J., Ma, H., and Zhou, C. (2019). Separation, Biochemical Characterization and Salt‐tolerant Mechanisms of Alkaline Protease from Aspergillus oryzae. J. Sci. Food Agric. 99, 3359–3366. doi:10.1002/jsfa.9553
Gharsallah, N., Khannous, L., Souissi, N., and Nasri, M. (2002). Biological Treatment of saline Wastewaters from marine-products Processing Factories by a Fixed-Bed Reactor. J. Chem. Technol. Biotechnol. 77, 865–870. doi:10.1002/jctb.647
González, J. F.Food & Nations, and , A. O. O. T. U. (1996). Wastewater Treatment in the Fishery Industry. Rome, Italy: Food and Agriculture Organization of the United Nations.
González-Fernández, C., and García-Encina, P. A. (2009). Impact of Substrate to Inoculum Ratio in Anaerobic Digestion of Swine Slurry. Biomass and Bioenergy 33, 1065–1069. doi:10.1016/j.biombioe.2009.03.008
Government, C. (2013). <Canadaregulation.pdf> [Online]. Available at: https://www.canada.ca/en/environment-climate-change/services/wastewater/system-effluent-regulations-reporting/owners-operators-continuously-discharging.html (Accessed December 15, 2020).
Gray, N. F. (2005). Water Technology: An Introduction for Environmental Scientists and Engineers. Oxford, England: Elsevier Butterworth-Heinemann.
Graziano, G., and Merlino, A. (2014). Molecular Bases of Protein Halotolerance. Biochim. Biophys. Acta (Bba) - Proteins Proteomics. 1844, 850–858. doi:10.1016/j.bbapap.2014.02.018
Greenberg, E. P., and Canale-Parola, E. (1976). Spirochaeta Halophila Sp. n., a Facultative Anaerobe from a High-Salinity Pond. Arch. Microbiol. 110, 185–194. doi:10.1007/bf00690227
Guerrero, L., Omil, F., Méndez, R., and Lema, J. M. (1997). Treatment of saline Wastewaters from Fish Meal Factories in an Anaerobic Filter under Extreme Ammonia Concentrations. Bioresour. Technology 61, 69–78. doi:10.1016/s0960-8524(97)84701-3
He, H., Chen, Y., Li, X., Cheng, Y., Yang, C., and Zeng, G. (2017). Influence of Salinity on Microorganisms in Activated Sludge Processes: A Review. Int. Biodeterioration Biodegradation. 119, 520–527. doi:10.1016/j.ibiod.2016.10.007
Hejnfelt, A., and Angelidaki, I. (2009). Anaerobic Digestion of Slaughterhouse By-Products. Biomass and bioenergy 33, 1046–1054. doi:10.1016/j.biombioe.2009.03.004
Kader, M. A., and Lindberg, S. (2010). Cytosolic Calcium and pH Signaling in Plants under Salinity Stress. Plant Signaling Behav. 5, 233–238. doi:10.4161/psb.5.3.10740
Kargi, F., and Dinçer, A. R. (2000). Use of Halophilic Bacteria in Biological Treatment of Saline Wastewater by Fed-Batch Operation. Water Environ. Res. 72, 170–174. doi:10.2175/106143000x137248
Kargi, F. (2002). Enhanced Biological Treatment of saline Wastewater by Using Halophilic Bacteria. Biotechnol. Lett. 24, 1569–1572. doi:10.1023/a:1020379421917
Kargi, F., and Uygur, A. (1996). Biological Treatment of Saline Wastewater in an Aerated Percolator Unit Utilizing Halophilic Bacteria. Environ. Technology. 17, 325–330. doi:10.1080/09593331708616391
Karigar, C. S., and Rao, S. S. (2011). Role of Microbial Enzymes in the Bioremediation of Pollutants: a Review. Enzyme Res. 2011, 805187. doi:10.4061/2011/805187
Khan, A. Z., Bilal, M., Rasheed, T., and Iqbal, H. M. N. (2018). Advancements in Biocatalysis: From Computational to Metabolic Engineering. Chin. J. Catal. 39, 1861–1868. doi:10.1016/s1872-2067(18)63144-4
Khanal, S. K., Giri, B., Nitayavardhana, S., and Gadhamshetty, V. (2017). “10 - Anaerobic Bioreactors/Digesters: Design and Development,” in Current Developments in Biotechnology and Bioengineering Editors D.-J. Lee, V. Jegatheesan, H. H. Ngo, P. C. hallenbeck, and A. Pandey (Elsevier).
Khannous, L., Souissi, N., Ghorbel, B., Jarboui, R., Kallel, M., Nasri, M., et al. (2003). Treatment of saline Wastewaters from marine‐products Processing Factories by Activated Sludge Reactor. Environ. Technology 24, 1261–1268. doi:10.1080/09593330309385668
Kieliszek, M., Pobiega, K., Piwowarek, K., and Kot, A. M. (2021). Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules. 26, 1858. doi:10.3390/molecules26071858
Kiepper, B. (2001). A Survey of Wastewater Treatment Practices in the Broiler Industry. Proc. Water Environ. Fed. 2001, 12–25. doi:10.2175/193864701790864854
Kimura, Y., Isaka, K., Kazama, F., and Sumino, T. (2010). Effects of Nitrite Inhibition on Anaerobic Ammonium Oxidation. Appl. Microbiol. Biotechnol. 86, 359–365. doi:10.1007/s00253-009-2359-z
Kubo, M., Hiroe, J., Murakami, M., Fukami, H., and Tachiki, T. (2001). Treatment of Hypersaline-Containing Wastewater with Salt-Tolerant Microorganisms. J. Biosci. Bioeng. 91, 222–224. doi:10.1263/jbb.91.222
Lefebvre, O., and Moletta, R. (2006). Treatment of Organic Pollution in Industrial saline Wastewater: a Literature Review. Water Res. 40, 3671–3682. doi:10.1016/j.watres.2006.08.027
Li, D., and Liu, S. (2019). “Chapter 12 - Water Quality Monitoring in Aquaculture,” in Water Quality Monitoring and Management. Editors D. Li, and S. Liu (Academic Press).
Li, R., Zi, X., Wang, X., Zhang, X., Gao, H., and Hu, N. (2013). Marinobacter Hydrocarbonoclasticus NY-4, a Novel Denitrifying, Moderately Halophilic marine Bacterium. SpringerPlus. 2, 1–9. doi:10.1186/2193-1801-2-346
Liu, X., Li, R., and Ji, M. (2019). Effects of Two-Stage Operation on Stability and Efficiency in Co-digestion of Food Waste and Waste Activated Sludge. Energies 12, 2748. doi:10.3390/en12142748
Moon, B.-H., Seo, G.-T., Lee, T.-S., Kim, S.-S., and Yoon, C.-H. (2003). Effects of Salt Concentration on Floc Characteristics and Pollutants Removal Efficiencies in Treatment of Seafood Wastewater by SBR. Water Sci Technol. 47, 65–70. doi:10.2166/wst.2003.0017
Madern, D., Ebel, C., and Zaccai, G. (2000). Halophilic Adaptation of Enzymes. Extremophiles. 4, 91–98. doi:10.1007/s007920050142
Marsh, W. S., Heise, B. W., Krzmarzick, M. J., Murdoch, R. W., and Fathepure, B. Z. (2021). Isolation and Characterization of a Halophilic Modicisalibacter Sp. Strain Wilcox from Produced Water. Scientific Rep. 11, 6943. doi:10.1038/s41598-021-86196-0
Maruthiah, T., Somanath, B., Immanuel, G., and Palavesam, A. (2015). Deproteinization Potential and Antioxidant Property of Haloalkalophilic Organic Solvent Tolerant Protease from marine Bacillus Sp. APCMST-RS3 Using marine Shell Wastes. Biotechnol. Rep. 8, 124–132. doi:10.1016/j.btre.2015.10.009
Massé, D. I., Masse, L., Xia, Y., and Gilbert, Y. (2010). Potential of Low-Temperature Anaerobic Digestion to Address Current Environmental Concerns on Swine Production1. J. Anim. Sci. 88, E112–E120. doi:10.2527/jas.2009-2432
Medveďová, A., Šipošová, P., Mančušková, T., and Valík, Ľ. (2018). The Effect of Salt and Temperature on the Growth of Fresco Culture. Fermentation 5, 2. doi:10.3390/fermentation5010002
Metcalf Eddy, I., Tchobanoglous, G., Burton, F., and Stensel, H. D. (2002). Wastewater Engineering: Treatment and Reuse. New York: McGraw-Hill Education.
Mohamad, N. R., Marzuki, N. H. C., Buang, N. A., Huyop, F., and Wahab, R. A. (2015). An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnological Equipment. 29, 205–220. doi:10.1080/13102818.2015.1008192
Mokashe, N., Chaudhari, B., and Patil, U. (2018). Operative Utility of Salt-Stable Proteases of Halophilic and Halotolerant Bacteria in the Biotechnology Sector. Int. J. Biol. Macromolecules 117, 493–522. doi:10.1016/j.ijbiomac.2018.05.217
Mollah, M. Y. A., Schennach, R., Parga, J. R., and Cocke, D. L. (2001). Electrocoagulation (EC) - Science and Applications. J. Hazard. Mater. 84, 29–41. doi:10.1016/s0304-3894(01)00176-5
Muthukumaran, S., and Baskaran, K. (2013). Organic and Nutrient Reduction in a Fish Processing Facility - A Case Study. Int. Biodeterioration Biodegradation 85, 563–570. doi:10.1016/j.ibiod.2013.03.023
Najafpour, G. D., Zinatizadeh, A. A. L., and Lee, L. K. (2006). Performance of a Three-Stage Aerobic RBC Reactor in Food Canning Wastewater Treatment. Biochem. Eng. J. 30, 297–302. doi:10.1016/j.bej.2006.05.013
Neilly, A., Jegatheesan, V., and Shu, L. (2009). Evaluating the Potential for Zero Discharge from Reverse Osmosis Desalination Using Integrated Processes - A Review. Desalination Water Treat 11, 58–65. doi:10.5004/dwt.2009.843
Ojuederie, O., and Babalola, O. (2017). Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Ijerph. 14, 1504. doi:10.3390/ijerph14121504
Omil, F., Méndez, R., and Lema, J. M. (1995). Anaerobic Treatment of saline Wastewaters under High Sulphide and Ammonia Content. Bioresour. Technology. 54, 269–278. doi:10.1016/0960-8524(95)00143-3
Onishi, H., and Kamekura, M. (1972). Micrococcus Halobius Sp. N. Int. J. Syst. Bacteriol. 22, 233–236. doi:10.1099/00207713-22-4-233
Oren, A., Gurevich, P., Azachi, M., and Henis, Y. (1992). Microbial Degradation of Pollutants at High Salt Concentrations. Biodegradation 3, 387–398. doi:10.1007/bf00129095
Oren, A. (2010). Industrial and Environmental Applications of Halophilic Microorganisms. Environ. Technology 31, 825–834. doi:10.1080/09593330903370026
Palenzuela-Rollon, A., Zeeman, G., Lubberding, H. J., Lettinga, G., and Alaerts, G. J. (2002). Treatment of Fish Processing Wastewater in a One- or Two-step Upflow Anaerobic Sludge Blanket (UASB) Reactor. Water Sci. Technol. 45, 207–212.
Panswad, T., and Anan, C. (1999). Specific Oxygen, Ammonia, and Nitrate Uptake Rates of a Biological Nutrient Removal Process Treating Elevated Salinity Wastewater. Bioresour. Technology. 70, 237–243. doi:10.1016/s0960-8524(99)00041-3
Park, E., Enander, R., Barnett, S. M., and Lee, C. (2001). Pollution Prevention and Biochemical Oxygen Demand Reduction in a Squid Processing Facility. J. Clean. Prod. 9, 341–349. doi:10.1016/s0959-6526(00)00074-3
Parvathy, U., Rao, K., Jeyakumari, A., and Zynudheen, A. (2017). Biological Treatment Systems for Fish Processing Wastewater-A Review.
Patwardhan, A. W. (2003). Rotating Biological Contactors: A Review. Ind. Eng. Chem. Res. 42, 2035–2051. doi:10.1021/ie0200104
Picos-Benítez, A. R., Peralta-Hernández, J. M., López-Hincapié, J. D., and Rodríguez-García, A. (2019). Biogas Production from saline Wastewater of the Evisceration Process of the Fish Processing Industry. J. Water Process Eng. 32, 100933. doi:10.1016/j.jwpe.2019.100933
Pounsamy, M., Magthalin, J., Mannacharaju, M., Sunkapur, L., Swarnalatha, S., and Sekaran, G. (2017). Treatment of Tannery saline Wastewater by Using Effective Immobilized Protease Catalyst Produced from Salt Tolerant Enterococcus Feacalis. J. Environ. Chem. Eng. 5, 2042–2055. doi:10.1016/j.jece.2017.04.014
Pounsamy, M., Somasundaram, S., Palanivel, S., Balasubramani, R., Chang, S. W., Nguyen, D. D., et al. (2019). A Novel Protease-Immobilized Carbon Catalyst for the Effective Fragmentation of Proteins in High-TDS Wastewater Generated in Tanneries: Spectral and Electrochemical Studies. Environ. Res. 172, 408–419. doi:10.1016/j.envres.2019.01.062
Prasertsan, P., Jung, S., and Buckle, K. A. (1994). Anaerobic Filter Treatment of Fishery Wastewater. World J. Microbiol. Biotechnol. 10, 11–13. doi:10.1007/bf00357553
Priambodo, G., Mangkoedihardjo, S., Hadi, W., and Soedjono, E. S. (2011). Wastewater Treatment Strategy for Fish Processing Industry in Kota Pantai Muncar of Indonesia. Int. J. Acad. Res. 3, 93–97.
Queiroz, M. I., Hornes, M. O., Gonçalves da Silva Manetti, A., Zepka, L. Q., and Jacob-Lopes, E. (2013). Fish Processing Wastewater as a Platform of the Microalgal Biorefineries. Biosyst. Eng. 115, 195–202. doi:10.1016/j.biosystemseng.2012.12.013
Rao, M. A., Scelza, R., Scotti, R., and Gianfreda, L. (2010). Role of Enzymes in the Remediation of Polluted Environments. J. Soil Sci. Plant Nutr. 10, 333–353. doi:10.4067/s0718-95162010000100008
Rastogi, M., Nandal, M., and Khosla, B. (2020). Microbes as Vital Additives for Solid Waste Composting. Heliyon. 6, e03343. doi:10.1016/j.heliyon.2020.e03343
Report, M. (2018). Federal Ministry for the Environment, Nature Conservation and Nuclear Safety - Building Automation Msr Incl. London: Maintenance for 4 Years - 43/18 [Tender Documents : T434374112].
Report, M. (2020). Extension of the Federal Ministry for the Environment, Nature Conservation and Nuclear Safety Section 41 Ff Hoai, Phases 2 to 8. Berlin, Germany: Waste And Pollutant Management (143/20). [Tender documents : T451865318].
Rodriguez-Valera, F., Ruiz-Berraquero, F., and Ramos-Cormenzana, A. (1981). Characteristics of the Heterotrophic Bacterial Populations in Hypersaline Environments of Different Salt Concentrations. Microb. Ecol. 7, 235–243. doi:10.1007/bf02010306
Safdar, H., Amin, A., Shafiq, Y., Ali, A., Yasin, R., Shoukat, A., et al. (2019). A Review: Impact of Salinity on Plant Growth. Nat. Sci. 17, 34–40. doi:10.7537/marsnsj170119.06
Salmanikhas, N., Tizghadam, M., and Rashidi Mehrabadi, A. (2016). Treatment of saline Municipal Wastewater Using Hybrid Growth System. J. Biol. Eng. 10, 9–19. doi:10.1186/s13036-016-0030-7
Sandberg, M., and Ahring, B. K. (1992). Anaerobic Treatment of Fish Meal Process Waste-Water in a UASB Reactor at High pH. Appl. Microbiol. Biotechnol. 36, 800–804. doi:10.1007/bf00172198
Santos, C. A., Vieira, A. M., Fernandes, H. L., Empis, J., and Novais, J. M. (2001). Optimisation of the Biological Treatment of Hypersaline Wastewater from Dunaliella salina Carotenogenesis. J. Chem. Technology Biotechnol. 76, 1147–1153. doi:10.1002/jctb.497
Sarnaik, S. S., Phalke, V. V., and Kanekar, P. (2015). Removal of Ammoniacal Nitrogen from Fish Processing Wastewater Using Bioaugmentation Technique. Int. J. Pharma Bio Sci. 6, B1021–B1029.
Sasaki, K., Morita, M., Hirano, S.-I., Ohmura, N., and Igarashi, Y. (2011). Decreasing Ammonia Inhibition in Thermophilic Methanogenic Bioreactors Using Carbon Fiber Textiles. Appl. Microbiol. Biotechnol. 90, 1555–1561. doi:10.1007/s00253-011-3215-5
Shammas, N. K., Wang, L. K., and Hahn, H. H. (2010). “Fundamentals of Wastewater Flotation,”. Flotation Technology. Editors L. K. Wang, N. K. Shammas, W. A. Selke, and D. B. Aulenbach (Totowa, NJ: Humana Press), Vol. 12.
Shanmugam, P., and Horan, N. J. (2009). Optimising the Biogas Production from Leather Fleshing Waste by Co-digestion with MSW. Bioresour. Technology. 100, 4117–4120. doi:10.1016/j.biortech.2009.03.052
Shen, Q.-H., Gong, Y.-P., Fang, W.-Z., Bi, Z.-C., Cheng, L.-H., Xu, X.-H., et al. (2015). Saline Wastewater Treatment by Chlorella Vulgaris with Simultaneous Algal Lipid Accumulation Triggered by Nitrate Deficiency. Bioresour. Technology. 193, 68–75. doi:10.1016/j.biortech.2015.06.050
Smith, F. B. (1936). Investigation of a Taint in Rib Bones of Bacon: The Determination of Halophilic Vibrios. Adelaide, Australia: N. Spp.. [Adel.].
Sridhar, S., Kale, A., and Khan, A. A. (2002). Reverse Osmosis of Edible Vegetable Oil Industry Effluent. J. Membr. Sci. 205, 83–90. doi:10.1016/s0376-7388(02)00065-0
Syam Babu, D., Anantha Singh, T. S., Nidheesh, P. V., and Suresh Kumar, M. (2020). Industrial Wastewater Treatment by Electrocoagulation Process. Separation Sci. Technology 55, 3195–3227. doi:10.1080/01496395.2019.1671866
Tay, J.-H., Show, K.-Y., and Hung, Y.-T. (2006). Seafood Processing Wastewater Treatment. Cheminform. 37, 29–66. doi:10.1002/chin.200613273
Thimmaraju, M., Sreepada, D., Babu, G. S., Dasari, B. K., Velpula, S. K., and Vallepu, N. (2018). Desalination of Water. Desalination Water Treat., 333–347. doi:10.5772/intechopen.78659
Uygur, A., and Kargi, F. (2004). Salt Inhibition on Biological Nutrient Removal from saline Wastewater in a Sequencing Batch Reactor. Enzyme Microb. Technology. 34, 313–318. doi:10.1016/j.enzmictec.2003.11.010
Val del Rio, A., Pichel, A., Fernandez-Gonzalez, N., Pedrouso, A., Fra-Vázquez, A., Morales, N., et al. (2018). Performance and Microbial Features of the Partial Nitritation-Anammox Process Treating Fish Canning Wastewater with Variable Salt Concentrations. J. Environ. Manag. 208, 112–121. doi:10.1016/j.jenvman.2017.12.007
Ventosa, A., Oren, A., and Ma, Y. (2011). Halophiles and Hypersaline Environments: Current Research and Future Trends. 1st Edition. Berlin, HeidelbergBerlin, Heidelberg: Springer.
Vidaček, S., and Bugge, E. (2016). Chapter 26 - Hygienic Design of Fish Processing Equipment. Amsterdam, Netherlands: Elsevier
Vidya, V., Prasad, G., and Sheela, A. M. (2020). Assessment of Threats to a Ramsar Site from Seafood Processing Operation Effluents. Lakes & Reserv 25, 196–213. doi:10.1111/lre.12321
Xia, Y., Massé, D. I., Mcallister, T. A., Beaulieu, C., and Ungerfeld, E. (2012). Anaerobic Digestion of Chicken Feather with Swine Manure or Slaughterhouse Sludge for Biogas Production. Waste Manag. 32, 404–409. doi:10.1016/j.wasman.2011.10.024
Xiao, Y., and Roberts, D. J. (2010). A Review of Anaerobic Treatment of saline Wastewater. Environ. Technol. 31, 1025–1043. doi:10.1080/09593331003734202
Xu, B., Li, P., and Guo, P. (2017). Solar Thermal‐Driven Desalination Pursuing Products of Pure Water and Salts and Leaving Minimum Impact to Environment. doi:10.5772/intechopen.68702
Yun Chen, C., and Ghufran, R. (2017). Biological Treatment of Fish Processing Saline Wastewater for Reuse as Liquid Fertilizer. Sustainability (Basel, Switzerland). 9, 1062. doi:10.3390/su9071062
Keywords: aquaculture, bioaugmentation, halophilic bacteria, protease activity, fish processing
Citation: Anh HTH, Shahsavari E, Bott NJ and Ball AS (2021) Options for Improved Treatment of Saline Wastewater From Fish and Shellfish Processing. Front. Environ. Sci. 9:689580. doi: 10.3389/fenvs.2021.689580
Received: 01 April 2021; Accepted: 11 June 2021;
Published: 25 June 2021.
Edited by:
Xiaofang Li, Institute of Genetics and Developmental Biology (CAS), ChinaReviewed by:
Betina Lukwambe, Ningbo University, ChinaKonstantinos Plakas, Centre for Research and Technology Hellas (CERTH), Greece
Vikash Kumar, Central Inland Fisheries Research Institute (ICAR), India
Copyright © 2021 Anh, Shahsavari, Bott and Ball. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hoang Thi Hong Anh, UzM2MzQxOTBAc3R1ZGVudC5ybWl0LmVkdS5hdS1pdA==
 Hoang Thi Hong Anh
Hoang Thi Hong Anh Esmaeil Shahsavari
Esmaeil Shahsavari Nathan J. Bott
Nathan J. Bott Andrew S. Ball
Andrew S. Ball