
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 17 March 2021
Sec. Conservation and Restoration Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fenvs.2021.651939
 Muhammad Silmi1
Muhammad Silmi1 Kharisma Putra1
Kharisma Putra1 Ali Amran1
Ali Amran1 Mahfud Huda1
Mahfud Huda1 Aldino Fauzil Fanani1
Aldino Fauzil Fanani1 Birute Mary Galdikas2
Birute Mary Galdikas2 Prima Anggara S2
Prima Anggara S2 Carl Traeholt3*
Carl Traeholt3*The leopard cat (Prionailurus bengalensis) is the most widespread feline in Asia. It has been recorded in a range of habitats, including monoculture landscapes, such as oil palm plantations. Here, we report on a study on the presence, home range, activity patterns and diet of the species in an oil palm landscape to assess their viability as biological pest controller of rats. The study took place in United Plantations/PT SSS estate in Central Kalimantan, Borneo, Indonesia. From July 2014 to March 2018, we captured 11 leopard cats in purpose-built cage-traps and fitted them with VHF radio-transmitters. They were tracked for a 44 months study period, during which we collected a total of 2.031 GPS locations used for estimating the respective cats’ activities and home-ranges. The cats are strictly nocturnal and prefer to hide and rest in thick bush, primarily consisting of sword-fern (Nephrolepis sp.) during day-time, but forage both on the ground and in the palm canopy at night. The average home range (95% FK) for male leopard cats is 1.47 km2 (n = 7; SD = 0.62 km2) with slightly smaller home range for females at 1.29 km2 (n = 4; SD = 0.28 km2). All individuals studied were recorded strictly within the oil palm plantation landscape, although mangrove forest habitat makes up 7% of the greater plantation landscape. In conclusion, leopard cats survive and reproduce well in oil palm habitats and are effective biological controllers of rats that can replace the traditionally used expensive and environmentally polluting chemical rat poisons.
The past decade has seen a significant increase in environmental degradation and loss of ecosystem services in most parts of the world (Sodhi et al., 2004; Dobson et al., 2006; Kettunen and ten Brink, 2006; Malcolm et al., 2006; Greenpeace, 2007; Koh and Wilcove, 2008; Bradshaw et al., 2009; Butler and Laurance, 2009; Craft et al., 2009; Food and Agriculture Organization [FAO], 2009; Hoeinghaus et al., 2009; De Jong et al., 2015; Goodman and Mulik, 2015; Ghazali et al., 2016; Petrenko et al., 2016). In Southeast Asia, the main driver of deforestation is industrial scale agriculture, specifically for palm oil production (Miettinen et al., 2011; Hansen et al., 2013; Stibig et al., 2014), pulp and logging (Abood et al., 2015). This has resulted in habitat loss and fragmentation and, consequently, in the loss of biodiversity (Kinnaird et al., 2003; Canale et al., 2012; Gibson et al., 2013), with rivers, lakes and peat forests suffering enormous siltation, pollution loading and dehydration. Despite the formation of the Round Table for Sustainable Palm Oil in 2004 and increasing attention to environmental issues in operational practices (Laurance et al., 2010; Traeholt and Schriver, 2011; Cattau et al., 2016), southeast Asia’s biodiversity continues to suffer decline (Fuller et al., 2004; Canale et al., 2012; Gibson et al., 2013). While some species such as orangutan have enjoyed increasing conservation attention along with some birds and mammals (Kinnaird et al., 2003; Fuller et al., 2004; Koh, 2008; Phalan et al., 2009; Gibson et al., 2013; Sasidhran et al., 2016), other taxon continue to suffer decline.
Some mammal and bird species have been studied for their use as biological pest-controller of rats in plantation landscapes (Duckett, 1984, 1991; Silmi et al., 2013; Chua et al., 2016) and, while scarcely studied, the leopard cat (Prionailurus bengalensis) is often recorded in oil palm plantations. It is listed as “Least Concern” on the IUCN Red-list and considered common in Asia, where it ranges from southern India to Russia’s far east; to the Indonesian islands of Sumatra, Borneo and Java (Sunquist and Sunquist, 2009; Ross et al., 2015). It is found in a range of habitat types from tropical lowland rain forests to coniferous forests in the Himalayas or Amur region. Leopard cats are not restricted to native habitats only, but have been reported in logged forests, rubber estates and oil palm plantations (Lim, 1999; Rajaratnam et al., 2007; Silmi et al., 2013; Wahyudi and Stuebing, 2013). It is listed as a “protected species” in Indonesia (Indonesian Law #07, 1999).
Radio-tracking studies of leopard cats have been undertaken in national parks, wildlife reserves and sanctuaries in Sabah, Malaysian Borneo (Rajaratnam et al., 2007), Thailand (Rabinowitz, 1990; Grassman, 2000; Grassman et al., 2005; Austin et al., 2007), and Japan (Sakaguchi, 1994). Camera trap studies have focused on commercially used forests (Mohamed et al., 2013) and mixed habitat consisting of forest, grass land, agriculture land, and semi-urban areas, for example, in Taiwan (Chen et al., 2016). Camera trap studies in oil palm plantations on Borneo, reported that leopard cats were common (Rajaratnam et al., 2007; Silmi et al., 2013) and Chua et al. (2016) recorded it on Tekong island, Singapore. No previous studies have focused on the activity and ranging behavior of leopard cats in a palm oil estate, with an assessment of its potential value as biological pest controller of rats.
The study was reviewed and approved by the Ministry of Environment and Forestry, Indonesia, Central Kalimantan Provincial Office for Natural Resources and Conservation under permit No. 191/L/PB/XII/2019 and No. 106/L/PB/VII/2020, respectively.
This study took place in Lada Estate, Central Kalimantan, Indonesia (02°34′ 53″ S, 111°46′ 21″ E). The habitat consists of 5000 ha oil palm estate, surrounded by 1179 ha mangroves, small patches of peat-swamp and dry dipterocarp forests. The landscape contains several small rivers and streams that run into the main Kumai River (Figure 1). The area experiences a wet season from November to April and a dry season from May to October. We used a Vantage Pro 2TM weather station (Davis Instrument, CA, United States) to measure the local climate patterns from 2015 to 2017.
Trapping of leopard cats was undertaken intermittently from July 2014 to June 2017 using seven units of Tomahawk steel-mesh metal box trap with dimension 27 × 32 × 82 cm (Tomahawk Live Trap, Hezelhurst, WI, United States). Live rats (Rattus argentiventer) were used as bait and placed in a protected separate compartment attached inside the Tomahawk trap. Traps were set along harvesting paths in the estate fields and activated at night before being recovered in the morning to prevent possible theft. Bait rats were fed daily during the trap sessions. Captured leopard cats were anesthetized using a combination of ketamine hydrochloride (100 mg/ml) and xylazine hydrochloride (20 mg/ml) administered intramuscularly at a dosage of 10 and 1 mg/kg, respectively. We reversed it with atipemazole hydrocloride (5 mg/ml) intramuscularly at a dosage of 0, 1 mg/kg. Sedated leopard cats were aged, sexed and we collected morphometrics and fitted each individual with a radio collar. We used a 45 gr VHF-transmitter (Wildlife Material Inc., Illinois, United States). Photographs were taken to document each individual leopard cat before they were returned to the trap for recovery and release after 3–4 h. After 6–7 months or, when signal from a transmitter began to weaken due to low battery, we recaptured each collared individual and replaced the old radio collar with the new unit for additional 6–8 months of tracking (Table 1). Since our aim was to determine if leopard cats were resident breeders in palm oil estates, we chose to follow fewer individuals over several reproductive cycles rather than more individuals for a shorter period of time. This also allowed us to record annual and seasonal variation. In addition, with conventional radio-telemetry, we did not have staff-resources to track, for example, 15 or more individuals concurrently.
Each Leopard cat was tracked using a directional 3-element Yagi antenna fitted to a receiver (TRX-48S Wildlife Materials Inc.). Cat locations were recorded a minimum four times per night in the period from 18:00 to 05:30 h. To map the cats’ diurnal activity patterns, we also recorded locations randomly from 06:00 to 18:00 h day time. Locations were recorded using a GPS (Garmin Cs62) and tracking was done for six consecutive days per week from July 2014 to March 2018. If direct observation was impossible, the locations of the study objects were determined by triangulation. When direct observation was possible, we used night vision binoculars or spot light at a distance of ≤40 m to record cat behavior, with special focus on food and feeding behavior as well as mating behavior.
We recorded prey selection through direct observation while tracking leopard cats. We used a Nitecore Tiny Monster TM26 with adjustable beam-strength to spot radio-collared cats at night. None of the cat exhibited any sign of being disturbed by the light-beam, allowing the team to record full hunts (stalking, killing, and devouring) from direct observation. We sorted prey into rats, birds, amphibians, snakes, lizards, and “unidentified” categories.
The study-site is almost flat (3–9 m absl), dominated by monocrop oil palms planted in rows of 126 palms ha–1 and with only 3–9 mabsl elevation difference with no obvious physical barriers, we considered the habitat highly homogenous. Therefore, we used Geospatial Modelling Environment (Ver. 0.7.2 RC2) to determine Minimum Convex Polygon (MCP) and 95% Fixed Kernel (FK). We assumed that core areas of 50%FK indicate more frequently used areas in a home range (Powell, 2000). Overlap (% area and size) comparison were calculated pairwise using 95%FK estimator for cumulative ranges following Grassman et al. (2005) and Benavides et al. (2017). We estimated movement by calculating straight-line distance between location points and added these for average monthly movement.
The minimum known population density was estimated using the number of collared leopard cats divided with 95%FK. Habitat use ratio was projected by overlaying home range over habitat type previously mapped from aerial photographs and ground truthing. Independent t-test was used to test for possible seasonal differences in movement patterns as well as possible movement differences between sexes. Mann-Whitney U-test was applied to test for possible differences between male and female home-ranges. Statistical significance was determined at a p-value of ≤0.05. Means are provided with ± SD. Monthly leopard cat movement was analyzed using the open-source GIS OpenJUMP software and correlated with the average monthly rainfall.
Temperatures at the study site ranged from 26 to 34°C with a mean annual precipitation of 2,123 mm.
The 11 radio-collared leopard cats were tracked for 791 independent days during which we recorded 2031 GPS locations. The average male (n = 7) home range size calculated using MCP measured 1.94 km2 (N = 994 locations, SD ± 1.37, range 0.23–3.49 km2) and 1.36 km2 for females (n = 4), (N = 1,037 locations, SD ± 0.52, range 0.73–1.99 km2). The average male home-range size calculated using 95% FK was 1.47 km2 (N = 994, SD ± 0.62, range 0.61–2.04 km2) and 1.29 km2 for females (N = 1,037, SD ± 0.28, range 0.92–1.59 km2). The core area used by males averaged 0.34 km2 (50% FK, SD ± 0.13, range 0.11–0.46 km2) with females using 0.33 km2 (SD ± 0.1, range 0.18–0.42 km2). There was no significant difference in home-range size between males and females (95% FK, U = 9; P > 0.05).
Based on the 95% FK of the 11 study individuals, the total area used was 5.09 km2, which results in a minimum leopard cat density of 2.16 individuals/km2. The average home-range overlap between males averaged 13.96%, (n = 23 pairs, SD ± 12.27 range 0.07–11.89%) and 10.59% between females (n = 4 pairs, SD ± 7.24 range 0.18–15.59%). The average home-range overlap between male and female leopard cat was slightly higher at 15.87% (n = 26 pairs, SD ± 13.36 range 0.27–52.68%) (Table 2).
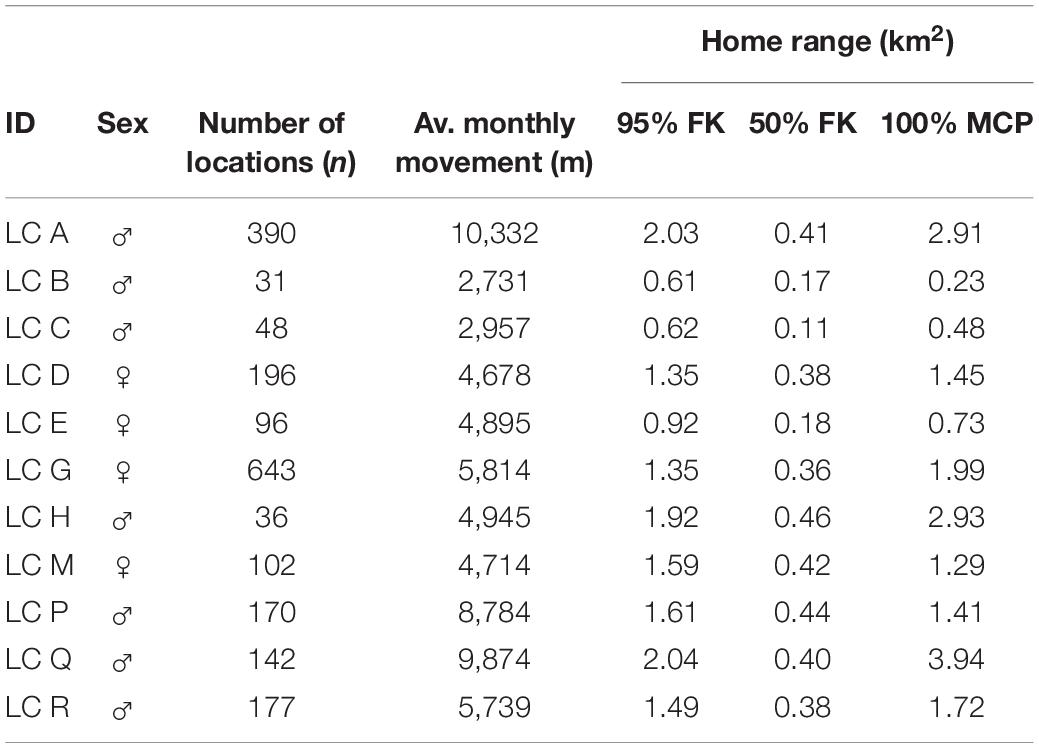
Table 2. The number of recorded location points from each collared leopard cat were used to analyze home range sizes using 100% MCP, 95%FK, 50%FK in the study site, Lada Estate.
From our observations, male leopard cat LC-A exhibited clear dominant behavioral characteristics. At 95% FK, LC-A’s home-range overlapped with four breeding females (n = 4 pairs, SD ± 22.45 range 5.11–52.68%) (Figure 2), while only overlapping sporadically with other males (n = 3 pairs, range 6.55–7.67%). LC-A was frequently observed scent marking, which we considered to be part of territory defense. A rival male, LC-H, overlapped LC-A’s home-range a modest 7.67% until they had a fierce altercation. After the fight, LC-H established a new home-range far from LC-A with no more home-range overlap (Figure 3).
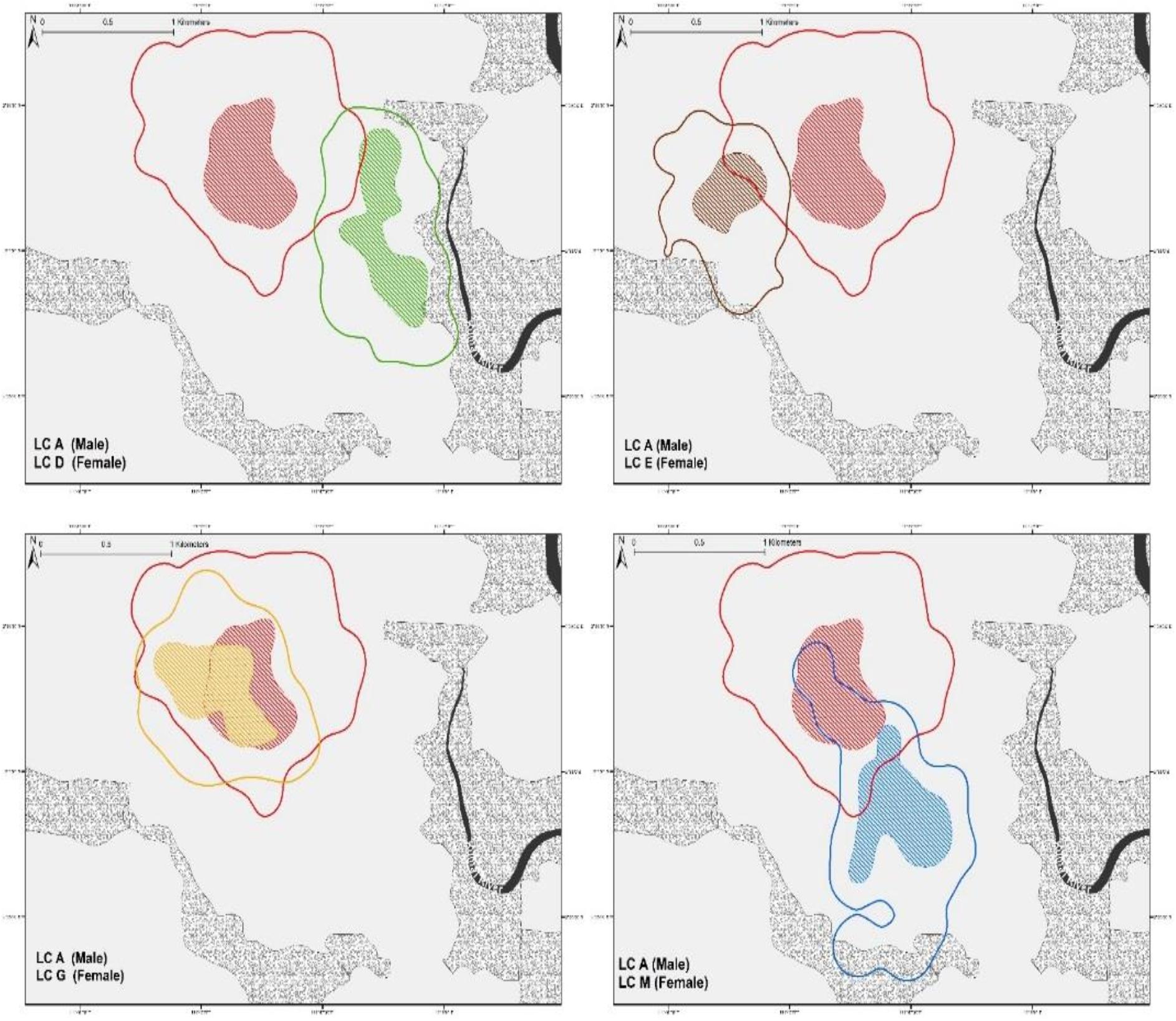
Figure 2. Home range of male LC-A with overlapping females. Solid line illustrates 95%FK and color shaded indicates 50%FK. The proportion of LC-A overlap with sympatric females is subject to individual variation.
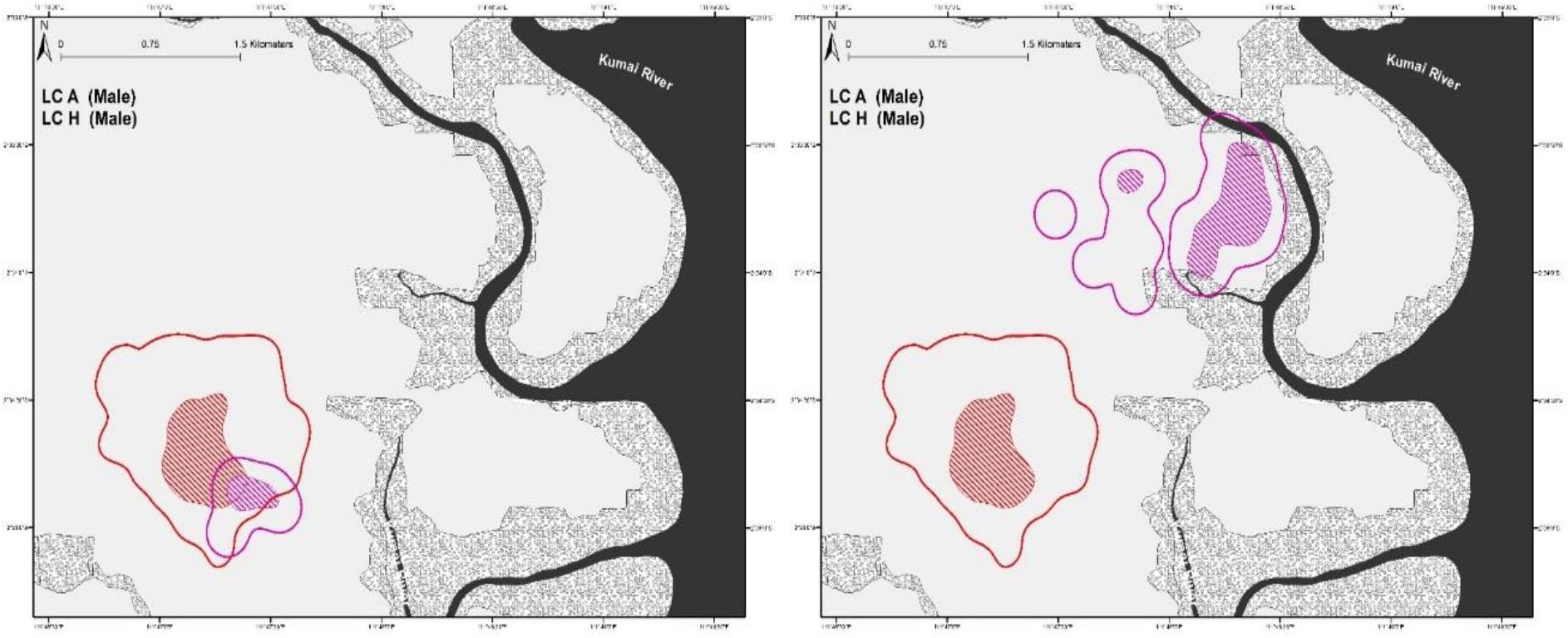
Figure 3. Home-ranges of two male leopard cats, LC-A and LC-H before and after an antagonistic encounter. Considering the small pre-fight home-range size that is almost entirely within LC-A’s home-range, it is likely that LC-H was one of LC-A’s male offspring that was chased out when it began to rival LC-A.
Male leopard cats (n = 7) moved an average 7,421 m per month (SD ± 6,974, range 717–39,184 m) and females (n = 4) 5,286 m per month (SD ± 4,315, range 120–18,149 m). There was no significant difference in monthly distance moved between males and females (t = 1.746, df = 88). There was also no significant seasonal movement difference (t = 0.202, df = 10), despite substantial difference in the amount of average rainfall between the rainy season (November–April, 221.13 mm) and the dry season (May–October, 132.79 mm).
Our study site consisted of two main types of habitat, oil palm plantation and mangrove forest. During the study period 100% of all recorded leopard cat GPS locations were from inside the oil palm plantation. Using 95% FK home-range polygon estimates a 93% use of plantation habitat and 7% use of mangrove habitat (Figure 4).
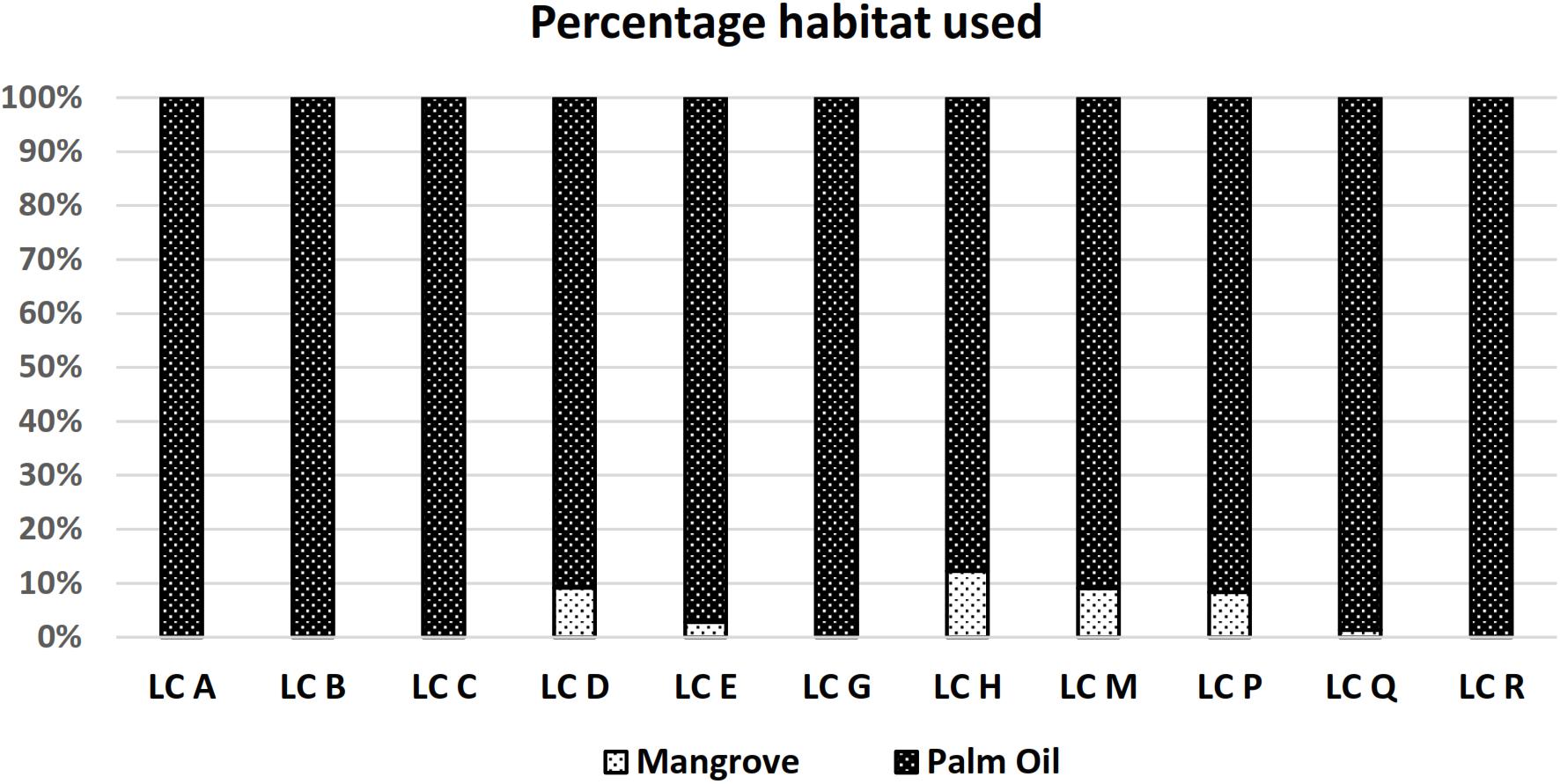
Figure 4. Leopard cat habitat use at the study site. Whereas there were plenty of mangrove habitat available in the larger plantation landscape, only a small percentage of it was ever used.
The leopard cats predated on a variety of species from amphibians, birds, reptiles and small mammals, with rats making up by far the biggest diet component in terms of numbers as well as biomass (Figure 5).
The average leopard cat home-range recorded in our study (∼140 ha FK95%/∼173 ha MCP) is much smaller than previous studies in other locations. In Thailand, leopard cat home-ranges were recorded to be 1,270 ha (Grassman et al., 2005) and 360 ha (Grassman, 2000), with 319 ha in Malaysia (Rajaratnam et al., 2007) and 350 ha in Japan (Schmidt et al., 2003). This is a clear indication of the species’ adaptability under different ecological circumstances with the home-range size likely influenced by the food availability and breeding opportunity (Sandell, 1989; Bailey, 1993; Davies et al., 2012). In an oil palm plantation, rat populations are usually very high, to the extent that plantation managers consider it a serious pest and are forced to combat it with chemical application (Wood and Liau, 1984; Hafidzi and Saayon, 2001; Wood, 2001; Wood and Chung, 2003; Puan et al., 2011; Phua et al., 2017). In our study site, the team estimated 7.29 rats per hectare (Silmi et al., 2013). This is considered low in a plantation, where numbers can often reach 50 rats per hectare in areas without leopard cats, barn owls (Tyto alba) or regular chemical application. However, in a logged forest Rajaratnam et al. (2007) recorded only 5.16 rats per hectare, or approx. 30% lower than in our study site. Whereas leopard cats predate on a range of prey species, murids are considered the main prey base for leopard cats (Yasuma, 1981; Sakaguchi and Ono, 1994; Grassman et al., 2005). This trend was also observed in our study site where there was a strong reverse correlation between the number of rats and the number of leopard cats (Silmi et al., 2013) and 73.60% (N = 72) of the recorded prey consisted of rats (unpubl. obs). The relatively small home-range sizes recorded in this study are likely due to the abundance of food and extensive shrub growth ideal for shelter and hiding kittens. With such ideal ecological conditions, there is no need for large home-ranges. The very high population density recorded (2.16 individuals/km2) supports this as well. The leopard cat population density in an oil palm plantation on Tekong island, Singapore, was also very high (2.86 individual/km2) (Chua et al., 2016), whereas the leopard cat population density recorded in a forest habitat Sabah, Malaysia varied from only 0.09–0.16 individual/km2 (Mohamed et al., 2013).
Although there was no significant difference in average home-range size between males and females, there were clear individual variation with males’ home-ranges larger (MCP = 1.94 km2 or 95% FK = 1.47 km2) than females’ home-ranges (MCP = 1.36 km2 or 95% FK = 1.29 km2). This trend was also observed in studies in Sabah, Malaysia (Rajaratnam et al., 2007), Thailand (Grassman, 2000; Grassman et al., 2005), Japan (Nakanishi et al., 2005), and Taiwan (Chen et al., 2016) and is consistent with other felid species (Tewes, 1986; Weisbein and Mendelssohn, 1990; Ferreras et al., 1997; Goodrich, 2010). Female dispersal is likely limited during the nursing period where she has to stay close to young kittens and/or are unable to move larger distances (Sandell, 1989; Nakanishi et al., 2005; Chen et al., 2016). Weighing 2.3 kg, LC-A is a large dominant male that maintained an extensive home-range overlapping 3–4 female home-ranges similar to that recorded for other cat species (Stander et al., 1997; Sliwa, 2004; Simcharoen et al., 2008; Goodrich et al., 2010; Anile et al., 2017; Nuñez-Perez and Miller, 2019) suggesting that overlapping home-range results from males attempting to monopolize access to females. In some cases, LC-A overlapped a breeding female home-range 52.65% and fought off a smaller male, LC-H (1.8 kg), at a time when LC-H attempted to mate with a female inside LC-A’s home-range.
Leopard cats have been reported as strictly nocturnal (Silmi et al., 2013; Chen et al., 2016). In this study, radio-tracking 11 individuals confirmed that none were active during daytime but remained sheltered in debris rows, mainly consisting of stacked palm fronds overgrown with sword-ferns (Nephrolepis sp.) and other plants. Individuals that sheltered in such areas were not even disturbed when plantation harvesters collected fruits and stacked new fresh palm fronds on top of the debris in which a leopard cat was resting. On several occasions we observed females with 2–4 kittens in this type of bush and since there were no seasonal differences in home-range size and activity patterns, we assume that the debris rows provide excellent shelter during monsoon rains too.
Although leopard cats have been reported to be common in oil palm plantations (Lim, 1999; Rajaratnam et al., 2007; Lorica and Heaney, 2013; Mohamed et al., 2013; Silmi et al., 2013; Chua et al., 2016), some have speculated that leopard cats only use oil palm plantations for foraging and has to return to adjacent forest seeking refuge and for breeding (Rajaratnam et al., 2007; Mohamed et al., 2013). In this study the home-range of five leopard cats (4 males and 1 female) consisted exclusively of oil palm plantation and six leopard cats (3 males and 3 females) used both oil palm plantation and mangrove forest. However, the adjacent mangrove forest only made up an average of 7.10% of these individuals’ home-ranges and, due to its relatively inaccessibility, is less important foraging grounds. Within the oil palm plantation, we recorded leopard cats most frequently in thick undergrowth consisting primarily of sword-ferns (Nephrolepis sp.) that proliferate in stacks of palm fronds, where they found refuge for kittens too.
The high population densities in our study site suggest that the leopard cat is very adaptable to a variety of habitat types, which stands in contrast to a number of other small felids such as the ocelot, Leopardus pardalis (Di Bitteti et al., 2008) and flat headed cat, Priornailurus planiceps (Wilting et al., 2010). The combination of prey abundance and limited predator pressure provides for ideal conditions for the species, although we recorded a few adults falling prey to pythons (Malayopython reticulatus) and expect snakes and monitor lizards (Varanus salvator) regularly prey on kittens. Our results clearly reveal that an oil palm habitat can sustain a high leopard cat population and that, with active micro-habitat management, the species can be used as an effective biological pest controller of rats. It offers an opportunity that oil palm growers should embrace as complementary to barn owls (Tyto alba) in an effort to reduce the dependency of toxic chemicals to combat rats.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was reviewed and approved by the Ministry of Environment and Forestry, Indonesia, Central Kalimantan Provincial Office for Natural Resources and Conservation under permit Nos. 191/L/PB/XII/2019 and 106/L/PB/VII/2020, respectively.
MS, KP, AA, MH, and AF undertook most of the field work. MS did most of the data analysis. MS and CT wrote the manuscript and undertook most of the editing. PA was responsible for the veterinarian issues. BG provided advice. All authors contributed to the article and approved the submitted version.
This study was funded by the following organizations; United Plantations Bhd/PT Suria Sawit Sejati (subsidiary of UP) provided the necessary funding to operate a biodiversity unit and its research activities. All staff affiliated with this are permanent staff of UP/PTSSS, all equipment used including radio-transmitters, vehicles, computers and software was funded by UP/PTSSS as part of their commitment to sustainable palm oil production. Copenhagen Zoo, Denmark, provided the necessary staff time of the corresponding author, expenses associated with international travel and accommodation. Orangutan Foundation International provided staff support in the form of a veterinarian.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank United Plantations Bhd/PT Suria Sawit Sejati for the ongoing support for this study; to Copenhagen Zoo for their support; to Balai Konservasi Sumber Daya Alam (BKSDA) Kalimantan Tengah; for their support and permission given to conduct the research; BKSDA SKW II Pangkalan Bun for support and field coordination; to Pak Adib Gunawan, Handi Nasoka, Dendi Setiadi, Agung Widodo, and Pak Hartono for their conservation commitment support; to the entire Biodiversity Division (Sakti Anggara, Hernandea Forestriko, Fajri, Muhammad Ikhsan, Fila Istina Machid, Suryadi, Misalan) for their dedication and fruitful team work; to volunteer Wandanil Putra for his dedication and to Orangutan Foundation International for providing veterinarian support to the project.
Abood, S. A., Lee, J. S. H., Burivalova, Z., Garcia-Ulloa, J., and Koh, L. P. (2015). Relative contributions of the logging, fiber, oil palm, and mining industries to forest loss in Indonesia. Conserv. Lett. 8, 58–67. doi: 10.1111/conl.12103
Anile, S., Bizzarri, L., Lacrimini, M., Sforzi, A., Ragni, B., and Devillard, S. (2017). Home-range size of the European wildcat (Felis silvestris silvestris): a report from two areas in Central Italy. Mammalia 82, 1–11. doi: 10.1515/mammalia-2016-0045
Austin, S. C., Tewes, M. E., Grasaman, L. I. Jr., and Silvy, N. J. (2007). Road ecology of the leopard cat prionailurus bengalensis in Khao Yai National Park, Thailand. Acta Zool. Sinica 53, 373–377.
Bailey, T. N. (1993). The African Leopard: Ecology and Behavior of a Solitary Felid. New York, NY: Columbia University Press.
Benavides, C., Arce, A., and Pacheco, L. (2017). Home range and habitat use by pacas in a montane tropical forest in Bolivia. Acta Amazonica 47, 227–236. doi: 10.1590/1809-4392201603163
Bradshaw, C. J. A., Sodhi, N. S., and Brook, B. (2009). Tropical turmoil: a biodiversity tragedy in Progress. Front. Ecol. Environ. 7, 79–87. doi: 10.1890/070193
Butler, R. A., and Laurance, W. F. (2009). Is oil palm the next emerging threat to the Amazon? Trop. Conserv. Sci. 2, 1–10. doi: 10.1177/194008290900200102
Canale, G. R., Peres, C. A., Guidorizzi, C. E., Gatto, C. A. F., and Kierulff, M. C. M. (2012). Pervasive defaunation of forest remnants in a tropical biodiversity hotspot. PLoS One 7:e41671. doi: 10.1371/journal.pone.0041671
Cattau, M. E., Marlier, M. E., and DeFries, R. (2016). Effectiveness of roundtable on sustainable palm oil (RSPO) for reducing fires on oil palm concessions in Indonesia from 2012 to 2015. Environ. Res. Lett. 11:105007. doi: 10.1088/1748-9326/11/10/105007
Chen, M.-T., Liang, Y.-J., Kuo, C.-C., and Pei, K. J.-C. (2016). Home ranges, movements and activity patterns of leopard cats (Prionailurus bengalensis) and threats to them in Taiwan. Mammal Study 41, 77–86. doi: 10.3106/041.041.0205
Chua, M. A. H., Sivasothi, N., and Meier, R. (2016). Population density, spatiotemporal use and diet of the leopard cat (Prionailurus bengalensis) in a human-modified succession forest landscape of Singapore. Mammal Res. 61, 99–108. doi: 10.1007/s13364-015-0259-4
Craft, C., Clough, J., Ehman, J., Joye, S., Park, R., Penning, S., et al. (2009). Forecasting the effect of accelerated sea-level rise on tidal marsh ecosystem services. Front. Ecol. Environ. 7:73–78. doi: 10.1890/070219
Davies, N. B., Krebs, J. R., and West, S. A. (2012). An Introduction to Behavioral Ecology, 4th Edn. Hoboken, NJ: John Wiley & Sons Ltd.
De Jong, E. B. P., Ragas, A. M. J., Nooteboom, G., and Mursidi, M. (2015). Changing water quality in the Middle Mahakam Lakes: water quality trends in a context of rapid deforestation, mining and palm oil plantation development in Indonesia’s Middle Mahakam Wetlands. Wetlands 35, 733–744. doi: 10.1007/s13157-015-0665-z
Di Bitteti, M. S., Paviolo, A., De Angelo, C. D., and Di Blanco, Y. E. (2008). Local and continental correlates of the abundance of a Neotropical cat, the ocelot (Leopardus pardalis). J. Tropical Ecol. 24, 189–200. doi: 10.1017/s0266467408004847
Dobson, A., Lodge, D., Alder, J., Cumming, G. S., Keymer, J., McGlade, J., et al. (2006). Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87, 1915–1924. doi: 10.1890/0012-9658(2006)87[1915:hltcat]2.0.co;2
Duckett, J. E. (1984). Barn Owls (Tyto alba) and the ‘second generation’ rat-baits utilised in oil palm in Malaysia. Planter 60, 3–11.
Duckett, J. E. (1991). Management of the barn owl (Tyto alba javanica) as a predator of rats in oil palm (Elaeis guineensis) plantations in Malaysia. Birds Prey Bul. 4, 11–24.
Ferreras, P., Beltran, J. F., Aldama, J. J., and Delibes, M. (1997). Spatial organization and land tenure system of the endangered Iberian lynx (Lynx pardinus). J. Zool. 243, 163–189. doi: 10.1111/j.1469-7998.1997.tb05762.x
Food and Agriculture Organization [FAO] (2009). The State of the World’s Forest. Rome: Food and Agriculture Organization, 168.
Fuller, D. O., Jessup, T. C., and Salim, A. (2004). Deforestation trends in a tropical landscape and implications for endangered large mammals. Conserv. Biol. 18, 249–254.
Ghazali, A., Asmah, S., Syafiq, M., Yahya, M., Aziz, N., Peng, T., et al. (2016). Effects of monoculture and polyculture farming in oil palm smallholdings on terrestrial arthropod diversity. J. Asia Pac. Entomol. 19, 415–421. doi: 10.1016/j.aspen.2016.04.016
Gibson, L., Lynam, A. J., Bradshaw, C. J. A., He, F., Bickford, D. P., Woodruff, D. S., et al. (2013). Near-complete extinction of native small mammal fauna 25 years after forest fragmentation. Science 341, 1508–1510. doi: 10.1126/science.1240495
Goodman, L. K., and Mulik, K. (2015). Clearing the Air: Palm Oil, Peat Destruction, and Air Pollution. Cambridge: Union of Concerned Scientists.
Goodrich, J. M. (2010). Human-tiger conflict: a review and call for comprehensive plans. Integr. Zool. 5, 300–312. doi: 10.1111/j.1749-4877.2010.00218.x
Goodrich, J. M., Miquelle, D. G., Smirnov, E. N., Kerley, L. L., Quigley, H. B., and Hornocker, M. G. (2010). Spatial structure of Amur (Siberian) tigers (Phantera tigris altatica) on sikhote-alin biosphere Zapovednik. Rusia. J. Mammal. 91, 737–748. doi: 10.1644/09-mamm-a-293.1
Grassman, L. I, Tewes, M. E., Silvy, N. J., and Kreetiyutanont, K. (2005). Spatial organization and diet of the leopard cat (Prionailurus bengalensis) in north-central Thailand. J. Zool. 266, 45–54. doi: 10.1017/s095283690500659x
Grassman, L. I. Jr. (2000). Movements and diet of leopard cat (Prionailurus bengalensis) in a seasonal evergreen forest in south-central Thailand. Acta Theriol. 45, 421–426. doi: 10.4098/at.arch.00-41
Greenpeace (2007). How the Oil Palm Industry is Cooking the Climate. Amsterdam: Greenpeace International.
Hafidzi, M. N., and Saayon, M. K. (2001). Status of rat infestation and recent control strategies in oil palm plantations in Peninsular Malaysia. J. Trop. Agric. Sci. 24, 109–114.
Hansen, M. C., Potapov, P. V., Moore, R., Hancher, M., Turubanova, S. A., Tyukavina, A., et al. (2013). High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. doi: 10.1126/science.1244693
Hoeinghaus, D. J., Agostinho, A. A., Gomes, L. C., Pelicice, F. M., Okada, E. K., Latini, J. D., et al. (2009). Effects of river impoundment on ecosystem services of large tropical rivers: embodied energy and market value of artisanal fisheries. Conserv. Biol. 23, 1222–1231. doi: 10.1111/j.1523-1739.2009.01248.x
Kettunen, M., and ten Brink, P. (2006). Value of Biodiversity-Documenting EU Examples Where Biodiversity Loss has Led to the Loss of Ecosystem Services. Final Report for the European Commission (Brussels, Belgium: Institute for European Environmental Policy (IEEP)).
Kinnaird, M. F., Sanderson, E. W., O’Brien, T. G., Wibisono, H. T., and Woolmer, G. (2003). Deforestation trends in a tropical landscape and implications for endangered large mammals. Conserv. Biol. 17, 245–257. doi: 10.1046/j.1523-1739.2003.02040.x
Koh, L. P. (2008). Can oil palm plantations be made more hospitable for forest butterflies and birds? J. Appl. Ecol. 45, 1002–1009. doi: 10.1111/j.1365-2664.2008.01491.x
Koh, L. P., and Wilcove, D. S. (2008). Is oil palm agriculture really destroying tropical biodiversity? Conserv. Lett. 2, 1–5. doi: 10.1016/j.agee.2018.04.011
Laurance, W. F., Koh, L. P., Butler, R., Sodhi, N. S., Bradshaw, C. J. A., Neidel, J. D., et al. (2010). Improving the performance of the round table on sustainable palm oil for nature conservation. Conserv. Biol. 24, 377–381. doi: 10.1111/j.1523-1739.2010.01448.x
Lim, B. L. (1999). The distribution, food habits and parasite patterns of the leopard cat (Prionailurus bengalensis) in peninsular Malaysia. J. Wildlife Parks 19, 17–27.
Lorica, M. R. P., and Heaney, L. R. (2013) Survival of a native mammalian carnivore, the leopard cat Prionailurus bengalensis Kerr, 1792 (Carnivore: Felidae), in an agricultural landscape on an oceanic Philippine island. J. Threatened Taxa 5, 4451–4460. doi: 10.11609/JoTT.o3352.4451-60
Malcolm, J. R., Liu, C., Neilson, R. P., Hansen, L., and Hannah, L. (2006). Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 20, 538–548. doi: 10.1111/j.1523-1739.2006.00364.x
Miettinen, J., Shi, C., and Liew, S. C. (2011). Deforestation rates in insular Southeast Asia between 2000 and 2010. Global Change Biol. 17:2261. doi: 10.1111/j.1365-2486.2011.02398.x
Mohamed, A., Sollmann, R., Bernard, H., Ambu, L. N., Lagan, P., Mannan, S., et al. (2013). Density and habitat use of the leopard cat (Prionailurus bengalensis) in three commercial forest reserves in Sabah, Malaysian Borneo. J. Mammol. 94, 82–89. doi: 10.1644/11-mamm-a-394.1
Nakanishi, N., Okamura, M., Watanabe, S., Izawa, M., and Doi, T. (2005). The effect of habitat on home range size in the Iriomote cat Prionailurus bengalensis iriomotensis. Mammal Study 30, 1–10. doi: 10.3106/1348-6160(2005)30[1:teohoh]2.0.co;2
Nuñez-Perez, R., and Miller, B. (2019). “Movements and home range of jaguars (Panthera onca) and mountain lions (Puma concolor) in a tropical dry forest of Western Mexico,” in Movement Ecology of Neotropical Forest Mammals, eds R. Reyna-Hurtado and C. Chapman (Berlin: Springer).
Petrenko, C., Paltseva, J., and Searle, S. (2016). Ecological Impacts of Palm Oil Expansion in Indonesia. Washington, DC: ICCT.
Phalan, B., Fitzherbet, E. B., Rafflegeau, S., Struebig, M. J., and Verwilghen, A. (2009). Conservation in oil-palm landscapes. Conserv. Biol. 23, 244–245. doi: 10.1111/j.1523-1739.2008.01151.x
Phua, M.-H., Chong, C. W., Ahmad, A. H., and Hafidzi, M. N. (2017). Understanding rat occurrences in oil palm plantation using high-resolution satellite image and GIS data. Precis. Agric. 19, 42–54. doi: 10.1007/s11119-016-9496-z
Powell, R. A. (2000). “Animal home ranges and territories and home range estimators,” in Research Techniques in Animal Ecology: Controversies and Consequences, eds L. Boitani and T. Fuller (New York, NY: Columbia University Press), 65–110.
Puan, C. L., Goldizen, A. W., Zakaria, M., Hafidzi, M. N., and Baxter, G. S. (2011). Relationships among rat numbers, abundance of oil palm fruit and damage levels to fruit in an oil palm plantation. Integr. Zool. 6, 130–139. doi: 10.1111/j.1749-4877.2010.00231.x
Rabinowitz, A. (1990). Notes on the behavior and movements of leopard cats, Felis bengalensis, in a dry tropical forest mosaic in Thailand. Biotropica 22, 397–403. doi: 10.2307/2388557
Rajaratnam, R., Sunquist, M., Rajaratnam, L., and Ambu, L. (2007). Diet and habitat selection of the leopard cat (Prionailurus bengalensis borneoensis) in an agricultural landscape in Sabah, Malaysian Borneo. J. Tropical Ecol. 23, 209–217. doi: 10.1017/s0266467406003841
Ross, J., Brodie, J., Cheyne, S., Hearn, A., Izawa, M., Loken, B., et al. (2015). Prionailurus bengalensis. The IUCN Red List of Threatened Species. Report number: e.T18146A50661611.
Sakaguchi, N. (1994). Ecological Aspects and Social System of the Iriomote Cat Felis iriomotensis (Carnivora; Felidae). Ph.D. Thesis, Kyushu University, Fukuoka, Japan, 67.
Sakaguchi, N., and Ono, Y. (1994). Seasonal change in the food habits of the Iriomote cat Felis iriomotensis. Ecol. Res. 9, 167–174. doi: 10.1007/bf02347492
Sandell, M. (1989). “The mating tactics and spacing patterns of solitary carnivores,” in Carnivore Behavior, Ecology, and Evolution, ed. J. L. Gittleman (New York, NY: Cornell University Press), 164–182. doi: 10.7591/9781501745812-011
Sasidhran, S., Adila, N., Hamdan, M. S., Samantha, L. D., Aziz, N., Kamarudin, N., et al. (2016). Habitat occupancy patterns and activity rate of native mammals in tropical fragmented peat swamp reserves in Peninsular Malaysia. For. Ecol. Manage. 363, 140–148. doi: 10.1016/j.foreco.2015.12.037
Schmidt, K., Nakanishi, N., Okamura, M., Doi, T., and Izawa, M. (2003). Movements and use of home range in the Iriomote cat (Prionailurus bengalensis iriomotensis). J. Zool. 261, 273–283. doi: 10.1017/s0952836903004205
Silmi, M., Mislan, Anggara, S., and Dahlen, B. (2013). Using leopard cats (Prionailurus bengalensis) as a biological pest control of rats in a palm oil plantation. J. Indones. Nat. Hist. 1, 31–36.
Simcharoen, S., Barlow, A. C. D., Simcharoen, A., and Smith, J. L. D. (2008). Home range size and daytime habitat selection of leopards in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Biol. Conserv. 141, 2242–2250. doi: 10.1016/j.biocon.2008.06.015
Sliwa, A. (2004). Home range size and social organisation of black-footed cats. Mammalian Biol. 69, 96–107. doi: 10.1078/1616-5047-00124
Sodhi, N. S., Koh, L. P., Brook, B. W., and Ng, P. K. L. (2004). Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 19, 654–660. doi: 10.1016/j.tree.2004.09.006
Stander, P. E., Haden, P. J., Kaqece, I. L., and Ghau, I. L. (1997). The ecology of a sociality in Namibian leopards. J. Zool. 242, 343–364. doi: 10.1111/j.1469-7998.1997.tb05806.x
Stibig, H. J., Achard, F., Carboni, S., Rasi, R., and Miettinen, J. (2014). Change in tropical forest cover of Southeast Asia from 1990 to 2010. Biogeosciences 11, 247–258. doi: 10.5194/bg-11-247-2014
Sunquist, M. E., and Sunquist, F. C. (2009). “Family Felidae (cats),” in Handbook of the Mammals of the World. Carnivores, Vol. 1, eds D. E. Wilson and R. A. Mittermeier (Barcelona: Lynx Edicions), 54–169.
Tewes, M. E. (1986). Ecological and Behavioral Correlates of Ocelot Spatial Patterns. Ph. D. thesis, University of Idaho, Moscow, 128 pp.
Traeholt, C., and Schriver, C. (2011). RSPO P&C – The challenge of making the principles and criteria operational. Oil Palm Ind. Econ. J. 11, 1–11.
Wahyudi, D., and Stuebing, R. (2013). Camera trapping as a conservation tool in a mixed-use landscape in East Kalimantan. J. Indones. Nat. Hist. 1, 37–45.
Weisbein, Y., and Mendelssohn, H. (1990). The biology and ecology of caracal Felis caracal in the northern Aravah valley of Israel. Cat News 12, 20–22.
Wilting, A., Cord, A., Hearn, A. J., Hesse, D., and Mohamed, A. (2010). Modelling the species distribution of flatheaded cats (Prionailurus planiceps), an endangered south-east Asian small felid. PLoS One 5:e9612. doi: 10.1371/journal.pone.0009612
Wood, B. (2001). Rat control in oil palms and rice fields. Pesticide Outlook 12, 71–74. doi: 10.1039/b102665a
Wood, B. J., and Chung, G. F. (2003). A critical review of the development of rat control in Malaysian agriculture since the 1960s. Crop Prot. 22, 445–461.
Wood, B. J., and Liau, S. S. (1984). A long-term study of Rattus tiomanicus populations in an oil palm plantation in Johore, Malaysia: II. Recovery from control and economic aspects. J. Appl. Ecol. 21, 465–472. doi: 10.2307/2403422
Keywords: leopard cat (Prionailurus bengalensis), oil palm plantation, home-range, biological pest control, activity
Citation: Silmi M, Putra K, Amran A, Huda M, Fanani AF, Galdikas BM, Anggara S P and Traeholt C (2021) Activity and Ranging Behavior of Leopard Cats (Prionailurus bengalensis) in an Oil Palm Landscape. Front. Environ. Sci. 9:651939. doi: 10.3389/fenvs.2021.651939
Received: 11 January 2021; Accepted: 24 February 2021;
Published: 17 March 2021.
Edited by:
Enrique Martínez-Meyer, National Autonomous University of Mexico, MexicoReviewed by:
Benoit Goossens, Cardiff University, United KingdomCopyright © 2021 Silmi, Putra, Amran, Huda, Fanani, Galdikas, Anggara S and Traeholt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carl Traeholt, Y3RyYWVob2x0QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.