- 1Association of Zoos and Aquariums, Silver Spring, MD, United States
- 2Lenfest Ocean Program, Pew Charitable Trusts, Washington, DC, United States
- 3Department of Conservation and Science, Santa Barbara Zoo, Santa Barbara, CA, United States
Conservation should be the higher purpose of any modern zoological facility and has consistently been a required element of accreditation by the Association of Zoos and Aquariums (AZA). Each year, AZA-accredited zoos and aquariums collectively commit considerable resources to conservation around the world, exceeding 150 million USD annually since 2011 and exceeding 231 million USD in 2019. Furthermore, with 195 million people visiting AZA-accredited zoos and aquariums each year, there is enormous opportunity to connect people to nature and engage them as agents of change. As AZA facilities continue to prioritize conservation-driven missions, their participation in field conservation has increased greatly. AZA SAFE: Saving Animals From Extinction (SAFE)® was established in 2014 to encourage greater collaboration of AZA members and their field partners to save species. The SAFE framework is dedicated to species recovery and based on conservation best practices. SAFE species programs develop 3-year action plans that build on established recovery plans, evaluate impact, and combine AZA facilities and visitors to increase resources for research, public engagement, communications, and conservation funding. Here we share preliminary outcomes of the SAFE program as they relate to programmatic measures of success to determine whether the framework 1) is useful for the AZA membership as measured by engagement and participation, and 2) increases conservation activity on behalf of targeted species as measured by the number of facilities supporting a species' conservation and financial investment. In this analysis we utilized data supported by the AZA Annual Report for Conservation and Science (ARCS) to demonstrate benefits of the SAFE framework and provide insights into future strategies to enhance conservation impact.
Introduction
Conservation should be the higher purpose of any modern zoological facility, and collaboration is key to conservation success. Zoos and aquariums are well-positioned to achieve conservation goals, as they have specialized expertise in a breadth of relevant disciplines from small population management, to reproductive endocrinology, animal behavior and welfare, veterinary science, conservation breeding, education, marketing, communications, and related social science fields, while also being embedded in both community and conservation networks.
Conservation has consistently been a required element of accreditation by the Association of Zoos and Aquariums (AZA). Each of the 240 AZA members has met the accreditation standards, which are rigorous, scientifically based, and publicly available (Association of Zoos and Aquariums, 2020a). These standards examine an entire zoo or aquarium’s operation, including animal welfare, veterinary care, conservation, education, guest services, physical facilities, safety, staffing and governing body. The majority of standards are performance-based, which includes assessing the level of achievement considered acceptable to fulfill a performance characteristic, and choice in method for meeting the goal. This approach is in contrast to engineering standards which prescribe the exact, precise steps required to fulfill an engineering characteristic and offer little or no variation in the method for meeting the goal. There is an emphasis on a continual raising of all–including conservation–standards.
Per the accreditation standards, conservation must be a key component of each institution’s mission and messaging. Each institution must follow a written conservation action plan/strategy with defined outcomes that must include: 1) in situ efforts that have a direct and measurable impact on animals and habitat in the wild; 2) natural resource conservation and sustainability/green practices; 3) connecting animals in their care with saving species in the wild; and 4) conservation education and advocacy programs measured against the written conservation goals of the institution.
Many AZA member activities serve the higher conservation purpose within the mission, such as informal learning and awareness-building opportunities and experiences at animal habitats, zoo-based summer camps, staff and volunteer led talks, and investments in green and sustainable business operations and infrastructure. Field conservation (hereby referred to as “conservation”) has been narrowly defined within the AZA as that which directly contributes to the long-term survival of species in natural ecosystems and habitats through: 1) direct action; 2) research; 3) field conservation education; 4) advocacy; and 5) fundraising/direct grants (Association of Zoos and Aquariums, 2019). As such, these are the conservation activities that are the focus of our discussion.
Zoos and aquariums have achieved conservation successes. For instance, the Bronx Zoo began an American bison recovery program in 1913 and led the way to saving the species (Kisling, 2001; Seddon et al., 2007). Conservation breeding programs, often including or led by zoos or aquariums across the globe (Conde et al., 2011a, Conde et al., 2013; Conde et al., 2015), have played an important conservation role for at least thirteen species (Conde et al., 2011b) and over 40 species have been reintroduced into the wild by AZA members through breeding programs. These AZA programs include takhi (Equus ferus przewalskii), California condor (Gymnogyps californianus), Virgin Islands boa (Epicrates monensis granti), lake sturgeon (Acipenser fulvescens), and Kihansi spray toads (Nectophrynoides asperginis; Association of Zoos and Aquariums, 2017).
Every year, AZA members submit their activities related to field conservation, research, education, and green practices to the AZA Annual Report in Conservation and Science (ARCS). For this study, we examine data reported in the “Field Conservation” survey. While AZA has tracked member engagement in conservation since 1990, the scope of the survey and reports were refined in 2010 with respect to field conservation (Association of Zoos and Aquariums, 2020b). Data show that AZA-accredited zoos and aquariums collectively commit considerable resources to conservation around the world, exceeding 150 million USD annually since 2011 and exceeding 231 million USD in both 2018 and 2019. In 2019 alone this benefited 987 species and subspecies in 127 countries–42% of projects were in the United States, while 58% of projects took place in foreign range countries. Though AZA members collaborate with many partners, 28% of those projects were led by AZA member organizations.
Further, 195 million people visit AZA-accredited zoos and aquariums each year, more visitors than NFL, NBA, NHL, and MLB annual attendance combined (Association of Zoos and Aquariums, 2020c). As such, there is enormous opportunity to connect people to nature and engage them as agents of change. The primary causes for species declines are anthropogenic in nature (Lande, 1998) and the growing impact of zoos on visitors’ behaviors, perceptions, and conservation efforts is reviewed in Godinez and Fernandez (2019). Zoos and aquariums are well-known and valued by many in their communities as family institutions and as a local voice for wildlife (Fraser and Sickler, 2008). While some visitors and members are motivated by educational opportunities (Falk et al., 2007; Gusset and Dick, 2011), others are motivated to visit by other factors, such as family bonding and advancement of childhood moral development (Falk et al., 2007; Fraser and Sickler, 2009). Therefore, zoos may uniquely have reach to those who may not be primarily concerned with conservation at the outset of their visit or relationship with zoos and aquariums. A number of studies, conducted at zoos worldwide, have quantified impact to visitor learning during zoo visits or when participating in zoo educational programs that have demonstrated benefits, such as improvement in respondents’ biodiversity understanding (Jensen and MossGusset, 2017; Moss et al., 2015; Moss et al., 2017), increased likelihood that families will engage in nature-based activities after zoo visits while overcoming barriers to spending time in nature (Ernst 2018), and the role of visitor-reported “extra special” zoo experiences (such as observing baby animals) in fostering concern and empathy for wildlife (Luebke, 2018). However, the zoo conservation model is not without controversy, criticism, or calls for additional evaluation and program refinement. Some argue that evidence is still lacking to support the value of zoos’ efficacy in promoting environmental stewardship (Marino et al., 2010), and that additional data are still needed to evaluate this approach (Moss and Esson, 2013), that shortfalls exist in current programming (Buckley et al., 2020; Ojalammi et al., 2018), or that zoos have not yet fully embraced conservation opportunities (Balmford et al., 2011; Brichieri-Colombi et al., 2018; Fa et al., 2014).
As AZA facilities have continued to prioritize conservation-driven missions their participation in field conservation has continued to increase. However, ARCS data show that while members contributed to or supported similar field conservation initiatives, coordination among these institutions was not apparent. Social network analysis has similarly demonstrated relatively low connectivity to one another (Maynard et al., 2020). Interdisciplinary and inter-organizational collaborations are necessary and continue to grow in the field of conservation and when utilized can enhance conservation impacts (Goring et al., 2014; Fan et al., 2020). With access to a large community with diverse experience and visitor background, collaboration across AZA members has the potential for even greater conservation impact. AZA SAFE: Saving Animals From ExtinctionⓇ was established in 2014 to focus the collective expertise within AZA-accredited zoos and aquariums and to leverage the community’s massive audiences to save species. The SAFE framework is dedicated to species recovery and is based heavily in principles of the Open Standards for the Practice of Conservation (Conservation Measures Partnership 2013) and the IUCN One Plan Approach to Conservation (Byers et al., 2013).
SAFE targets animals that have historically benefited from AZA facility commitments, and that are at risk of extinction with the intent to increase the conservation impact of the AZA community by strategic partnerships and resource stewardship. SAFE is effectively split into species- or taxon-specific groups proposed by AZA members and their field partners. These groups are called “SAFE species programs”. A SAFE species program is led by a SAFE Program Leader- and in some cases a Vice Program Leader- and a Steering Committee made up of SAFE Program Partners. Field partners join the program plan as advisors and collaborators for various projects. Each SAFE species program creates a 3-year program plan that aligns with and seeks to implement aspects of a current recovery or action plan for that species. SAFE program plans outline a 3-year goal, objectives to reach the goal, and actions to reach their objectives. Goals, objectives, and actions are time bound.
Several metrics for SAFE were developed to monitor and evaluate progress and achievements. Because AZA’s conservation-related standards are performance-based, there is no required method for how those standards are met. As such, AZA member participation in SAFE is voluntary and not required for accreditation. The benchmarks developed for SAFE were based off previous long-term data collection including conservation spending and member engagement in field conservation projects as reported through the Field Conservation ARCS survey and seek to determine whether the SAFE framework was deemed attractive enough for voluntary participation by AZA members. In addition to tracking spending and member engagement to support field conservation projects, the number of SAFE species proposed by the AZA community, and the percent of members participating in SAFE are also included to evaluate the uptake of SAFE by AZA facilities over time.
Expected outcomes as part of the SAFE framework include: demonstrated conservation impact for species in the wild, positive impact on public perception of zoos and aquariums, increased favorability of AZA zoos and aquariums, increased awareness of the role of zoos and aquariums in conservation, and the development of a culture of collaborative conservation across the AZA community. As many of these are long-term outcomes that require measurement across multiple datasets, both qualitative and quantitative, we seek to understand in the short-term whether this framework is useful to the AZA community and whether or not it has potential to facilitate increasing conservation engagement and commitment among members.
The ARCS survey and recorded data provide an initial opportunity to understand these preliminary outcomes. Here we share results from data analysis of the ARCS dataset as it relates to SAFE programmatic measures of success to determine whether the framework 1) is useful for the AZA membership as measured by engagement and conservation spending, and 2) increases conservation activity on behalf of targeted species as measured by the number of facilities supporting a species' conservation and financial investment. As SAFE progresses, additional data are being collected and will be analyzed to determine progress towards other SAFE goals mentioned above.
Materials and Methods
Annual Report in Conservation and Science and Data Collection
AZA members complete an annual survey to track their contributions in field conservation, research, green business operations, and education. The collected results are recorded in a proprietary online database and published in AZA’s Annual Report in Conservation and Science (ARCS). The ARCS survey began in 1990 with a focus on understanding AZA member operations. In 2010, AZA more clearly defined field conservation to enhance consistency in reporting, focusing specifically on those activities that have direct impacts on animals and habitats in the wild. Submissions focus on activities which directly contribute to the long-term survival of species in natural ecosystems and habitats through, 1) direct action; 2) research; 3) field conservation education; 4) advocacy; and 5) fundraising/direct grants. Members record their engagement in field conservation by submitting project-specific data such as project name, description, target species, country in which a project occurs, and the amount spent each year. To enhance consistency across the data, submissions are reviewed by between three and five members of AZA’s Wildlife Conservation Committee for adherence to the standardized definition of field conservation (Association of Zoos and Aquariums, 2019) who indicate whether the submission meets the definition, does not meet it, or whether there is insufficient information to determine adherence. AZA staff provide the reviewers’ feedback for those with insufficient information to the submitting organization for additional clarification and to confirm whether the project should be included in the database. AZA staff perform an additional editorial review and add the target species or country to the appropriate data field if that information is explicitly provided in either the title or description but was not entered.
Completing the ARCS survey is not required for accreditation but is encouraged and lists of programs and projects submitted to ARCS may serve as evidence during the accreditation process that the institution is following its conservation action plan/strategy. Field conservation survey response rates are on average 85.4 ± 2.4%. We used data from the field conservation section of ARCS to analyze SAFE’s impact on AZA members’ conservation engagement. Data concerning report year, organization name, project description, focal species, amount spent, and keywords for projects were exported from the online database into Excel. While some of the AZA survey data are publicly available on AZA’s website, additional data may be made available upon requests for research collaborations.
Evaluating Conservation Engagement Through SAFE
The raw data file was split into separate data sheets to evaluate questions. “Amount spent” and “number of members engaged” have been recorded in ARCS since its inception, providing a consistent and long-term (>5 years) data series to evaluate member participation and commitment to conservation over time. We used “amount spent” and “number of members engaged” as proxies to evaluate conservation engagement for field conservation and then species-specific conservation before and after SAFE was established. To understand conservation engagement and species-specific conservation engagement, we refined the dataset to exclude duplicates that resulted from multiple keyword entries to ARCS. For field conservation engagement we added a column to assign a category for the years before SAFE began (“before SAFE”; ≤2014) and after SAFE was established (“after SAFE”; ≥2015).
Eight of the current 28 SAFE species programs, as of April 2020, were selected as case studies to evaluate the impact of the SAFE program on species-specific conservation engagement. Five of the selected species programs were established in 2015, including African penguin, cheetah, sharks and rays, vaquita, and western pond turtle. SAFE was initially launched by AZA with ten species and these five were the first to develop 3-year program plans. AZA members were invited to propose additional SAFE species programs in 2017 and the three established that year were for African vultures, giraffe, and radiated tortoise. Therefore, the primary reason for the selection of these eight SAFE species programs was their longevity. For this dataset, we added a variable to assign a category for when the SAFE species program was established (“before SAFE” and “after SAFE”). For projects that benefited multiple species or taxa (e.g., projects in Africa focused on conservation of both African lions and African vultures), the total amount spent was divided by the number of species or taxon that were supported so that the total amount spent reflected spending for the specific SAFE species or taxon of concern.
We evaluated overall AZA member engagement in field conservation through conservation spending (USD) and the number of members engaged in field conservation before and after the inception of SAFE using individual generalized linear mixed models (GLMM) each with a gamma-distributed response variable built in the lme4 package (Bates et al., 2012) in the R software platform (R Core Team 2014). Year served as the random effect to account for repeated measures and the ordinal nature of the data, while timeline (before vs after SAFE initiation) served as the fixed effect. Estimates, 95% confidence intervals, and odds ratios were back transformed to the original response scale through natural exponent.
In addition to overall member involvement in field conservation, we wanted to examine the benefits for creating species-specific programs within the overall SAFE framework. First, we utilized a GLMM with a gamma-distributed response variable [conservation spending (USD) and the number of members engaged] across all the case study species combined. Species served as the random effect to account for repeated measures within each species, while timeline (before vs after SAFE species program initiation) served as the fixed effect. Next, in order to determine the individual effect of the SAFE program for each of the case species, we developed individual GLMMs for each of the eight case study species to test for significant differences in member engagement (amount spent and number of members engaged) before and after the individual SAFE species programs were approved. Year served as the random effect for each of these models with timeline as the fixed effect. Following analysis estimates, odd ratios, and 95% confidence intervals were back transformed to the original response scale through natural exponent.
Results
Our first research question sought to determine if implementation of SAFE increased AZA member engagement in in situ field conservation on a broad scale. Thus, we analyzed the reported total amount spent and the number of members participating in any field conservation projects from 2010 to 2019, with 2010–2014 serving as before SAFE and 2015–2019 serving as after SAFE in the overall timeline. For general conservation engagement, we found a positive increase for both the amount spent and total number of members engaged in field conservation after the establishment of SAFE (Table 1). Amount spent in field conservation by AZA members showed a 1.4-fold increase in expenditures while the number of member institutions reporting participation showed a 1.2-fold increase. While this increase could be attributed to a gradual increase over time and inflation, the variance and standard error of Year, which was used as the random effect, are both small (0.0064 and 0.08 respectively) and so we are confident that there was a positive and potentially causative influence of SAFE on dollars spent and AZA members involved in in situ conservation.
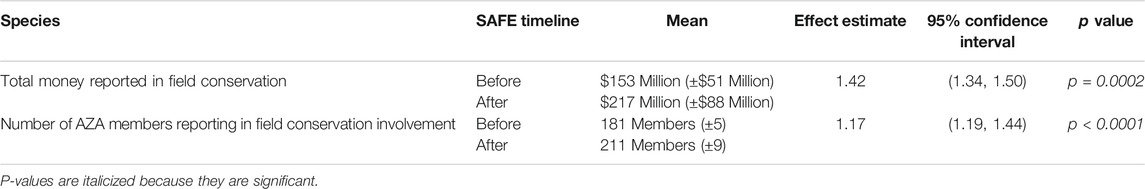
TABLE 1. AZA Member contribution and engagement in in situ conservation projects before and after initiation of SAFE conservation programs in 2015. Model estimated means are provided for each variable level, and regression coefficient (Effect Estimate), 95% confidence interval, and p-value are provided for each pairwise comparison.
Our second research question addressed conservation engagement for species-specific conservation. When we look specifically at the selected SAFE species case studies we see that the overall effect of SAFE has a highly positive effect on amount spent (p < 0.0001) and on the number of members reporting engagement (p < 0.0001) for in situ conservation projects within the targets species programs with a 3-fold and 2-fold increase respectively following initiation of SAFE species programs (Table 2). While we saw strong positive effects overall for both variables and the confidence intervals around these effects were both small in size, we knew from observing the high variance of the random effect of “species” and the greatly differing slopes when species was included as a fixed effect, that there was a large difference in how the individual species were impacted by the SAFE species program initiation. The results from running an individual analysis on the effect or benefits of SAFE for each species program for amount spent and number of AZA members engaged are presented in Table 2. In most cases, both the amount of funding spent and the number of members engaged increased at similar rates, but there were some cases, such as with the giraffe program, that while both trajectories were positive, the amount of funds spent increased at a higher rate than the number of AZA members participating. All species, other than the vaquita, have a high effect estimate and a tight 95% confidence interval which does not overlap zero, indicating a highly significant relationship in member donations to field conservation projects for that target species following induction of individual SAFE species programs and a general trend on increasing amounts of funds donated by AZA member facilities. Spending for African vulture conservation increased the most with nearly a 7-fold increase in spending after becoming a SAFE species program, followed by radiated tortoise and giraffe species nearing 5-fold increases. The vaquita displays a larger confidence interval, although it does not overlap zero and so could be classified as significant, due to extreme fluctuations in spending from 1 year to another even after the initiation of the SAFE species program.
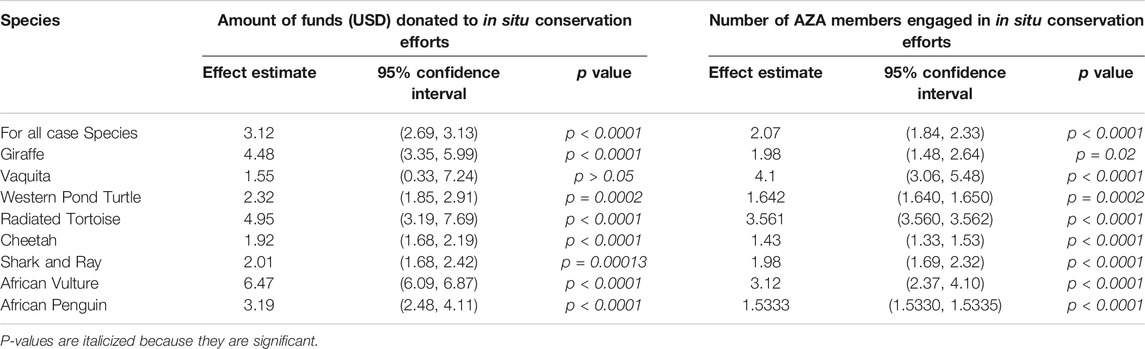
TABLE 2. Effect of SAFE program initiation on amount of money donated to and number of AZA member facilities engaged in in situ conservation efforts across the eight selected case species. Regression coefficient (Effect Estimate), 95% confidence interval, and p-value produced by individual models are provided for each species.
The amount spent before and after SAFE programs is presented graphically for four of the eight test species [Figure 1: A) Western pond turtle; B) Radiated tortoise; C) African vulture; D) African penguin; expressed as mean ± SEM generated from the GLMM]. Each of these four cases show interesting but different patterns within the overall increase in funding following the SAFE program based on the magnitude of the effect and the size of the after-timeline error bars.
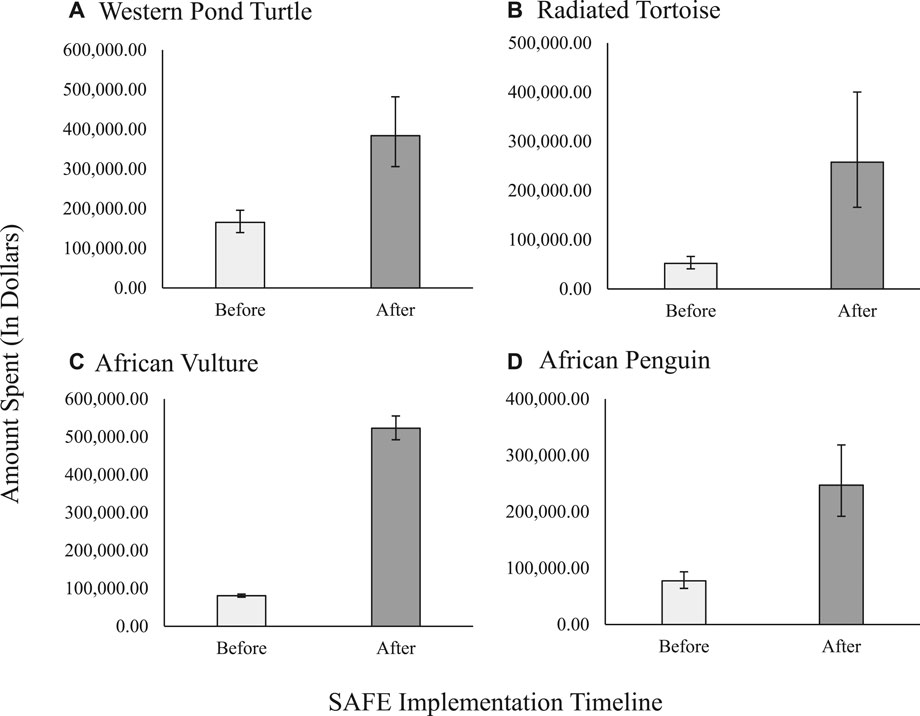
FIGURE 1. Evaluation of the effect of SAFE project implementation on amount of funding contributed to in situ conservation projects for individual species programs. Programs include (A) Western Pond Turtle, (B) Radiated Tortoise, (C) African Vulture, and (D) African Penguin. Data is expressed as model generated means ± SEM. For (A) and (C) SAFE programs were implemented in 2015 (Before: 2011–2014, n = 4 years; After: 2015–2019, n = 5 years), while for (B) and (D) SAFE was implemented in 2017 (Before: 2011–2016 n = 6 years; After: 2017–2019 n = 3 years). Variation in SEM is indicative of variable effect of SAFE programs and variation between different years.
Along with an increase in the amount of funding donated to in situ conservation, all the test case species displayed a positive increase (effect estimate) in reported number of members engaged following the SAFE species program initiation compared with before (Table 2). A graphical representation of member engagement increase is presented in Figure 2 based on model predicted means ± SEM. Overall, the trend for increased member engagement is similar across species but there are noted differences, such as the large error bars surrounding the “After” timeline for giraffe, indicating that member engagement in projects has had larger variation over time. However, with a tight 95% confidence interval, it is reasonable to trust this positive trend. Other species, such as the radiated tortoise or the African penguin have much smaller error bars, as well as tight 95% confidence intervals, which depicts a consistent trend or increase over time. Member engagement increase was highest for the vaquita with about a 4-fold increase, was followed closely by African vulture and radiated tortoise member engagement with effect estimates at 3.12 and 3.561, respectively. While giraffe saw one of the greatest increases in conservation spending, member engagement saw a lower effect at around a 2-fold increase.
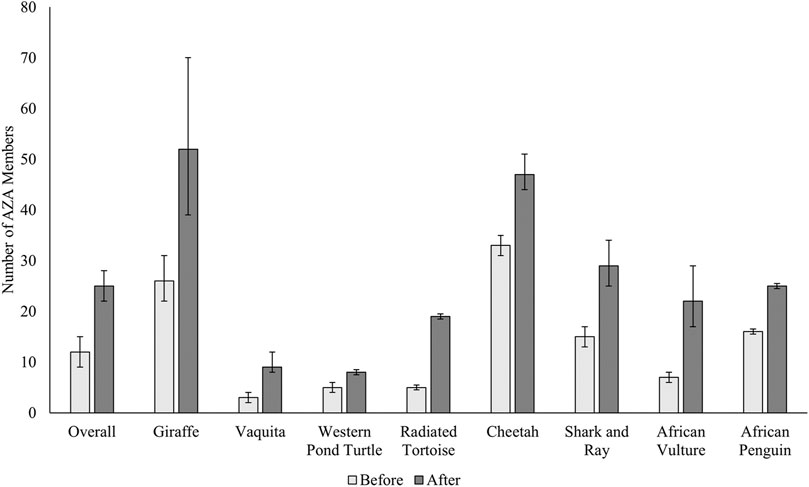
FIGURE 2. Evaluation of the effect of SAFE project implementation on number of AZA member facilities participating in in situ conservation projects for individual species programs. Data is expressed as model generated means ± SEM. Variation in SEM is indicative of variable effect of SAFE programs and variation between different years. Overall effect and each species individually showed significantly more member engagement following SAFE program initiation.
Discussion
SAFE’s Effect on Field Conservation Engagement
Zoos and aquariums worldwide are uniquely positioned to make lasting contributions to conservation outcomes. Their professional expertise in conservation breeding programs, education and advocacy, as well as the capacity to reach large audiences, positions them to be an important force for effective conservation strategies (Gusset and Dick, 2011). We recognize that this study evaluates solely the contributions of the AZA community of zoos and aquariums. 235 of the 241 current AZA-accredited zoos and aquariums are located in the Americas, and 217 of those are located in the United States. As such, this analysis is focused on conservation efforts by institutions that are located in a narrow region of the world. These contributions must be contextualized within the rich contributions of the broader global zoo and aquarium community, including regional zoo and aquarium associations from other areas of the world, such as the European Association of Zoos and Aquaria (EAZA), the Central Zoo Authority (CZA), the Pan-African Association of Zoos & Aquaria (PAAZA), and the Zoo and Aquarium Association Australasia (ZAA). The World Association of Zoos and Aquariums, which has over 300 members worldwide, including national and regional associations as well as zoos and aquariums, has developed a comprehensive conservation strategy (Barongi et al., 2015) with a global perspective. More recently, Gusset (2019) details a holistic approach to integrated species conservation by the global zoo and aquarium community.
When evaluating the overall impact that global zoos and aquariums have on in situ conservation projects, Gusset & Dick found that members of the World Association of Zoos and Aquariums (WAZA) significantly contributed to the success of those projects, both monetarily and with support of resources (2010). They also suggested that coordinated efforts within the zoo and aquarium community has potential for even greater contributions, and thus impact. The experience of the AZA-accredited zoo and aquarium community and AZA SAFE: Saving Animals From Extinction suggests that this may be true. We found that after SAFE was established in 2014, AZA member conservation engagement increased significantly. There had been an increasing trend in field conservation spending and member engagement before SAFE was established, however, after the launch of SAFE in 2014, there was a significant increase in the amount of field conservation spending (1.4 fold increase) and member engagement (1.2 fold increase), where spending and engagement were significantly greater after SAFE. With a small variance and standard error between years (included as the random effect), there is strong evidence that SAFE had a positive and potentially causative effect on field conservation engagement overall. It is possible SAFE may have encouraged member organizations to have greater participation in field conservation projects as a result of shifting priorities in the AZA community (e.g., increasing communication around and promotion of field conservation). Another explanation could be that SAFE encouraged members who were already participating in field conservation programs to report their contributions more consistently and fully, thus SAFE may be a catalyst for creating greater transparency and accountability for field conservation engagement. Future analysis and surveys to understand the perception of AZA members are needed to fully understand the impact of the SAFE program.
Engagement in Species-specific Conservation
When we analyzed how SAFE affected member engagement for species-specific conservation, we found that conservation spending and the number of members engaged in that species' conservation were significantly higher for all case species. However, the estimated effect varied across species. African vultures, giraffe, and radiated tortoise saw the greatest increase in conservation spending, each with at least a 4-fold increase. Vaquita, radiated tortoise, and African vultures had the greatest increase in member engagement. This suggests that SAFE can increase resources for species conservation, but the definition of “resources” may be different for some species. Several factors may be associated with these trends. AZA members range in budget size from small (≤$1,999,999) to extra-large (≥$26,000,000) which may affect their level of involvement. SAFE species program plans include objectives related to field conservation support, public engagement, public awareness, and funding. AZA members can choose to help further these programs by signing on as “program partners” and selecting areas of the program plan that they can support, either financially, or in-kind benefits such as dedicated conservation education staff that create shared messaging for the program for public engagement objectives; or dedicated field and animal care staff that monitor an amphibian head start. Some species, such as the SAFE giraffe program, historically already engaged multiple members, so may not see as much of an increase in member participation but may see an increase in conservation spending.
Some species may see large variance in spending from year to year due to current events and shifts in priorities. For example, the large error bars on the radiated tortoise and African penguin conservation spending chart are explained by increased spending in 1 year after the SAFE species program was established because of concerted efforts by the AZA community to rally around a certain event (Figures 1B,D). In 2017, the SAFE African penguin program launched a Kickstarter campaign to develop and build artificial nests for installation at critical nesting sites in South Africa. This campaign generated a $193,560 spike for African penguin conservation (Kickstarter, 2017). The radiated tortoise conservation spending shows a spike in 2018 when more than 10,000 radiated tortoises were confiscated from illegal trade (Gray, 2018) in April of that year and the SAFE radiated tortoise program made a plea for emergency funds to support the confiscation and care of these tortoises (Association of Zoos and Aquariums, 2018). A subsequent confiscation of another 7,000 radiated tortoises that October drove additional need and engagement (TRAFFIC, 2019). The AZA community responded to these pleas immediately, providing funds and sending veterinarians and animal care staff to care for these animals and building infrastructure to support their long-term rehabilitation and ultimate reintroduction in Madagascar. Member engagement saw similar trends, such as the 4-fold increase for the vaquita, due to a push to engage members in a vaquita rescue effort in 2017, resulting in a spike that year for member engagement (Taylor et al., 2020). These each suggest that the spotlight added to a species when they become a SAFE species may bring added benefits when emergencies rise; however, this relationship would need to be further explored. Even with these dramatic examples and emergencies, we still see an overall increase in member engagement following the initiation of a species-specific SAFE program.
While increased member engagement in field conservation is one desired outcome of SAFE implementation, and reflects scalability of AZA member conservation efforts this outcome may also be integral to achieving and enabling more directly targeted species-specific conservation efforts and achieve strategic species-specific conservation outcomes as established in SAFE program plans. Data regarding this more direct measure will become available over time as the SAFE program matures.
Regarding species-specific conservation, the debate on whether to use a species-specific versus an ecosystem-based approach when planning for strategic conservation activities is still relevant today. Along a management continuum, species managers may employ a single or multi-species approach when developing strategies to promote protection and conservation of ecosystems and landscapes (Barrows et al., 2005; Rinne and Stefferud 1999; Runge et al., 2019), while in other contexts they may employ an alternative -- protecting species through ecosystem-level conservation planning (Barrows et al., 2005; Block et al., 1995; Simberloff 1998). Each approach has its strengths and limitations. There are certainly risks in focusing too heavily on single species to the exclusion of a more ecosystem-based approach. For instance, food webs may seem healthy when assessing constituent species, but resilience of these communities may be affected by trophic cascades (Gilarranz et al., 2016). Likewise, there can be a danger in focusing on an ecosystem or landscape to the exclusion of constituent species. For instance, Tracy and Brussard (1994) argue that, by using an ecosystem approach, individual species may be lost while preserving many ecosystem-level processes. It has been convincingly argued (Lindemayer et al., 2007) that, where possible–and this may be resource-dependent–the simultaneous implementation of both types of approaches is ideal and can be complementary and even synergistic.
In the case of zoos and aquariums, a careful species-specific approach has the benefit of reflecting recovery work that is being accomplished in partnership with USFWS and other stakeholders under the species-driven focus of the Endangered Species Act (ESA), though ecosystem- and habitat-based goals are also considered under the ESA (Barrows et al., 2005). Further, many zoos and aquariums rely on “flagship species” to communicate complex conservation issues to visitors and how the public can engage in conservation solutions (Skibins et al., 2017), building on the connection that people make with specific animals they see during their visit. By experiencing a sense of connection, empathy, or instilled values that can help protect a species, the zoo and aquarium visiting public may become more likely to express intentions or prosocial behaviors to protect that species, thus helping to protect the habitat in which it resides (Bexell et al., 2007; Clayton et al., 2014; Grajal et al., 2017; Young et al., 2016).
Single species, or even specific animals within a species, are often used by AZA zoos and aquariums to tell the larger story of conservation efforts and to also effect social change for conservation impact. For example, some aquariums may use vulnerable freshwater species such as lake sturgeon to engage visitors and community stakeholders in reintroduction efforts (George et al., 2013), marine species such as corals or Magellanic penguins to explain climate change to their guests (Katz-Kimchi and Atkinson 2014), or zoos may share the story of confiscated animals rescued and rehomed by law enforcement to illustrate the impact of illegal wildlife trade on individual animals and species (Raghavan et al., 2015). Along this vein, SAFE species programs target specific species or taxonomic groups and engage other members and their field partners in strategies that can promote conservation and public engagement both for those species and for the ecosystems they inhabit.
Priority Alignment for Conservation
Miller et al. (2004) outlines questions for organizations to consider when they have a conservation mission, particularly those of collection-based organizations such as zoos, aquariums, museums, and botanical gardens (e.g., how does the institution define institutional policies, how do they allocate their resources and staff time, what are the appropriate target audiences in education programs, what are the direct contributions to conservation and protection, and how can exhibits portray the correct conservation messages?). The objectives outlined in SAFE species program plans can provide a “menu” of options for zoo and aquarium directors seeking to understand where their resources can have the greatest impact.
SAFE species program plan requirements such as defining a 3-year goal, conservation target, threats, and objectives to address these threats, align with the well-accepted standards for conservation planning from the Conservation Measurement Partnership (CMP) as well as principles of IUCN’s One Plan Approach. Similar best practices have been shown to be effective in tracking and monitoring conservation impact overtime (Margoluis et al., 2009; Washington et al., 2015). With increased support for SAFE species and greater transparency of field conservation projects that AZA members are supporting, it is possible that SAFE may help to influence decision-making at AZA member organizations searching for the most impactful allocation of resources.
After mapping alignment of reported keywords from projects reported in ARCS for species-specific conservation efforts, we found initial evidence that SAFE species programs are helping to align priorities for field conservation support through conservation spending. However, this is still anecdotal and will need to be tracked and evaluated after a longer period to show influence over decision-making and management at AZA facilities.
We acknowledge that while conservation spending and member engagement are relevant proxies to evaluate member participation in field conservation at the outset of SAFE, other variables will need to be tracked over time to evaluate the actual conservation impact of AZA member efforts on the target species. AZA has worked with members to develop a study that will examine the “conservation culture” of AZA members over time to encourage and assess how SAFE affects and promotes organizational priorities for conservation engagement. Other initiatives and collaboration of AZA committees, such as the Wildlife Conservation, Conservation Education, Public Relations, and Marketing committees can assist in making efforts of SAFE species programs stronger and more effective. As the number of SAFE species programs increase, other factors that provide potential for greater collaboration also increase. For example, many SAFE species programs are addressing threats that overlap with one another (i.e. climate change, habitat loss/degradation, wildlife trafficking). Building on the species-specific work already in place, AZA can work with members to develop initiatives that help bolster and connect public engagement sections of SAFE species program plans to social change movements and wildlife conservation. Knowing that SAFE has already impacted the AZA community positively allows for the opportunity to continue growth and collaboration.
When revisiting our initial questions as to whether or not the SAFE framework is useful for the AZA membership for increasing their engagement in field conservation and, subsequently, increasing engagement for species-specific conservation, the early analysis provides a resounding “yes” to both questions. While it remains unclear if SAFE is the causative driving force behind these increases, analysis has suggested that SAFE has impacted members and is propelling them in a direction of more collaborative and focused conservation. The ultimate test will be with respect to impact on saving animals from extinction and it appears the community is on track to play a role in making a difference.
Data Availability Statement
The AZA survey data, except financial information, are available on AZA's website (http://www.aza.org/field-conservation; http://www.aza.org/research-and-science). Additional data are available from the corresponding author on reasonable request.
Author Contributions
KR, ES, and SG conceived of the presented idea. KR and ES presented this work at the 29th International Congress for Conservation Biology (ICCB 2019). KR and MB processed and analyzed the data and MB derived the models and designed the figures. All authors contributed to the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express an appreciation to all of the members that have submitted their data to AZA each year and to the Wildlife Conservation Committee for their efforts to define field conservation for the community and to shepherd the annual surveys. We would also like to acknowledge the immense and great work of all SAFE species program leaders to coordinate these programs and keep them moving forward. They, and their partners, are making a difference for our community and for the animals they care so much about. We would also like to thank Gillian Cannataro, who, as an AZA SAFE Intern, helped analyze relevant literature for this research. We would like to thank the Reviewers for providing helpful feedback on earlier versions of this manuscript. Thank you to the Society for Conservation Biology for the opportunity to present these findings at the International Congress for Conservation Biology in 2019, and to all AZA directors that make it possible to carry out the conservation mission of their facilities and SAFE.
References
Association of ZoosAquariums (2019). Defining Field Conservation for the AZA Community. Available at: https://assets.speakcdn.com/assets/2332/fcc_fieldconservationdefinition_approved2013.pdf. (Accessed November 21, 2020).
Association of ZoosAquariums (2017). My AZA Online Conservation Database. Silver Spring. Available at: https://www.aza.org/(Accessed March 14, 2017).
Association of ZoosAquariums (2018). Radiated Tortoise SAFE Program Plan 2018. Available at: https://assets.speakcdn.com/assets/2332/radiated_tortoise_safe_species_program_plan_16_september_2018.pdf (Accessed November 21, 2020).
Association of ZoosAquariums (2020b). Resources for the ARCS Surveys. Available at: https://www.aza.org/resources-for-the-arcs-surveys (Accessed July 26, 2020).
Association of ZoosAquariums (2020a). The Accreditation Standards & Related Policies. Available at: https://assets.speakcdn.com/assets/2332/aza-accreditation-standards.pdf (Accessed July 26, 2020).
Association of ZoosAquariums (2020c). Visitor Demographics. Available at: https://www.aza.org/partnerships-visitor-demographics (Accessed July 26, 2020).
Balmford, A., Kroshko, J., Leader-Williams, N., and Mason, G. (2011). Zoos and Captive Breeding. Science 332, 1149–1150. doi:10.1126/science.332.6034.1149-k
Barrows, C. W., Swartz, M. B., Hodges, W. L., Allen, M. F., Rotenberry, J. T., Li, B.‐L., et al. (2005). A Framework for Monitoring Multiple‐species Conservation Plans. J. Wildl. Manag. 69, 1333–1345. doi:10.2193/0022-541X(2005)69[1333:AFFMMC]2.0.CO;2
Bates, D., Maechler, M., and Bolker, B. (2012). lme4, Linear Mixed‐effects Models Using S4 Classes. Available at: https://CRAN.R‐project.org/package=lme4 (Accessed April 14, 2020).
Bexell, S. M., Jarrett, O. S., Lan, L., Yan, H., Sandhaus, E. A., Zhihe, Z., et al. (2007). Observing Panda Play: Implications for Zoo Programming and Conservation Efforts. Curator: Mus. J. 50, 287–297. doi:10.1111/j.2151-6952.2007.tb00273.x
Block, W. M., Finch, D. M., and Brennan, L. A. (1995). “Single-species versus Multiple-Species Approaches for Management,” in In Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues. Editors T. E. Martin, and D. M. Finch (New York: Oxford University Press), 461–476.
Brichieri-Colombi, T. A., Lloyd, N. A., McPherson, J. M., and Moehrenschlager, A. (2018). Limited Contributions of Released Animals from Zoos to North American Conservation Translocations. Conserv Biol. 33 (1), 33–39. doi:10.1111/cobi.13160
Buckley, K. A., Smith, L. D. G., Crook, D. A., Pillans, R. D., and Kyne, P. M. (2020). Conservation Impact Scores Identify Shortfalls in Demonstrating the Benefits of Threatened Wildlife Displays in Zoos and Aquaria. J. Sustain. Tourism 28 (7), 978–1002. doi:10.1080/09669582.2020.1715992
Byers, O., Lees, C., Wilcken, J., and Schwitzer, C. (2013). The One Plan Approach: the Philosophy and Implementation of CBSG's Approach to Integrated Species Conservation Planning. WAZA Mag. 14, 2–5.
Clayton, S., Luebke, J., Saunders, C., Matiasek, J., and Grajal, A. (2014). Connecting to Nature at the Zoo: Implications for Responding to Climate Change. Environ. Educ. Res. 20 (4), 460–475. doi:10.1080/13504622.2013.816267
Conde, D. A., Colchero, F., Güneralp, B., Gusset, M., Skolnik, B., Parr, M., et al. (2015). Opportunities and Costs for Preventing Vertebrate Extinctions. Curr. Biol. 25, R219–R221. doi:10.1016/j.cub.2015.01.048
Conde, D. A., Colchero, F., Gusset, M., Pearce-Kelly, P., Byers, O., Flesness, N., et al. (2013). Zoos through the Lens of the IUCN Red List: A Global Metapopulation Approach to Support Conservation Breeding Programs. PLOS ONE 8 (12), e80311. doi:10.1371/journal.pone.0080311
Conde, D. A., Flesness, N., Colchero, F., Jones, O. R., and Scheuerlein, A. (2011a). An Emerging Role of Zoos to Conserve Biodiversity. Science 331 (6023), 1390–1391. doi:10.1126/science.1200674
Conde, D. A., Flesness, N., Colchero, F., Jones, O. R., and Scheuerlein, A. (2011b). Zoos and Captive Breeding--Response. Science 332 (6034), 1150–1151. doi:10.1126/science.332.6034.1150
Conservation Measures Partnership (2013). Open Standards for the Practice of Conservation. Version 3.0.
Ernst, J. (2018). Zoos' and Aquariums' Impact and Influence on Connecting Families to Nature: An Evaluation of the Nature Play Begins at Your Zoo & Aquarium Program. Visitor Stud. 21 (2), 232–259. doi:10.1080/10645578.2018.1554094
Fa, J. E., Gusset, M., Flesness, N., and Conde, D. A. (2014). Zoos Have yet to Unveil Their Full Conservation Potential. Anim. Conserv 17, 97–100. doi:10.1111/acv.12115
Falk, J. H., Reinhard, E. M., Vernon, C. L., Bronnenkant, K., Deans, N. L., and Heimlich, J. E. (2007). Why Zoos and Aquariums Matter: Assessing the Impact of a Visit. MD: Association of Zoos and Aquariums, Silver Spring.
Fan, P.-F., Yang, L., Liu, Y., and Lee, T. M. (2020). Build up Conservation Research Capacity in China for Biodiversity Governance. Nat. Ecol. Evol. 4, 1162–1167. doi:10.1038/s41559-020-1253-z
Fraser, J., and Sickler, J. (2009). Measuring the Cultural Impact of Zoos and Aquariums. Int. Zoo Yearb. 43, 103–112. doi:10.1111/j.1748-1090.2008.00064.x
Fraser, J., and Sickler, J. (2008). Why Zoos & Aquariums Handbook: Handbook of Research Key Findings and Results from National Audience Survey. MD: Association of Zoos & Aquariums, Silver Spring.
George, A. L., Hamilton, M. T., and Alford, K. F. (2013). We All Live Downstream: Engaging Partners and Visitors in Freshwater Fish Reintroduction Programmes. Int. Zoo Yb. 47, 140–150. doi:10.1111/j.1748-1090.2012.00189.x
Gilarranz, L. J., Mora, C., and Bascompte, J. (2016). Anthropogenic Effects Are Associated with a Lower Persistence of marine Food Webs. Nat. Commun. 7 (1), 10737. doi:10.1038/ncomms10737
Godinez, A. M., and Fernandez, E. J. (2019). What Is the Zoo Experience? How Zoos Impact a Visitor's Behaviors, Perceptions, and Conservation Efforts. Front. Psychol. 10, 1–8. doi:10.3389/fpsyg.2019.01746
Goring, S. J., Weathers, K. C., Dodds, W. K., Soranno, P. A., Sweet, L. C., Cheruvelil, K. S., et al. (2014). Improving the Culture of Interdisciplinary Collaboration in Ecology by Expanding Measures of success. Front. Ecol. Environ. 12, 39–47. doi:10.1890/120370
Grajal, A., Luebke, J. F., Kelly, L. A. D., Matiasek, J., Clayton, S., Karazsia, B. T., et al. (2017). The Complex Relationship between Personal Sense of Connection to Animals and Self‐reported Proenvironmental Behaviors by Zoo Visitors. Conservation Biol. 31, 322–330. doi:10.1111/cobi.12780
Gray, J. (2018). Monumental Radiated Tortoise Seizure!. Available at: https://turtlesurvival.org/monumental-radiated-tortoise-seizure/. (Accessed November 21, 2020).
Gusset, M., and Dick, G. (2011). The Global Reach of Zoos and Aquariums in Visitor Numbers and Conservation Expenditures. Zoo Biol. 30, 566–569. doi:10.1002/zoo.20369
Gusset, M. (2019). “Zoos and Aquariums Committing to Integrated Species Conservation,” in The Routledge Handbook of Animal Ethics 2 Ed. Editors B. Fischer (New York: Routledge), 357–366. doi:10.4324/9781315105840
Jensen, E. A., MossGusset, A. M., and Gusset, M. (2017). Quantifying Long-Term Impact of Zoo and Aquarium Visits on Biodiversity-Related Learning Outcomes. Zoo Biol. 36, 294–297. doi:10.1002/zoo.21372
Katz-Kimchi, M., and Atkinson, L. (2014). Popular Climate Science and Painless Consumer Choices. Sci. Commun. 36 (6), 754–777. doi:10.1177/1075547014555998
Kickstarter (2017). Invest in the Nest: Save Penguins from Extinction. Available at: https://www.kickstarter.com/projects/aza/invest-in-the-nest-save-penguins-from-extinction (Accessed November 21, 2020).
Kisling, V. N. (2001). “Zoological Gardens of the United States,” in Zoo and Aquarium History: Ancient Animal Collections to Zoological Gardens. Editor V. N. KislingJr. (Boca Raton, FL: CRC Press), 147–180.
Lande, R. (1998). Anthropogenic, Ecological and Genetic Factors in Extinction and Conservation. Res. Popul. Ecol. 40 (3), 259–269. doi:10.1007/BF02763457
Lindemayer, D., Fischer, J., Felton, A., Montague-Drake, R., Manning, A. D., Simberloff, D., et al. (2007). The Complementarity of Single-Species and Ecosystem-Oriented Research in Conservation Research. Oikos 116 (7), 1220–1226. Available at: www.jstor.org/stable/40235167.
Luebke, J. F. (2018). Zoo Exhibit Experiences and Visitors' Affective Reactions: A Preliminary Study. Curator The Museum Journal 61 (2), 345–352. doi:10.1111/Cura.12253
Margoluis, R., Stem, C., Salafsky, N., and Brown, M. (2009). Design Alternatives for Evaluating the Impact of Conservation Projects. New Dir. Eval. 2009, 85–96. doi:10.1002/ev.298
Marino, L., Lilienfeld, S. O., Malamud, R., Nobis, N., and Broglio, R. (2010). Do zoos and Aquariums Promote Attitude Change in Visitors? A Critical Evaluation of the American Zoo and Aquarium Study. Soc. Anim. 18 (2), 126–138. doi:10.1163/156853010X491980
Maynard, L., McCarty, C., Jacobson, S. K., and Monroe, M. C. (2020). Conservation Networks: Are Zoos and Aquariums Collaborating or Competing through Partnerships? Envir. Conserv. 47, 166–173. doi:10.1017/S0376892920000168
Miller, B., Conway, W., Reading, R. P., Wemmer, C., Wildt, D., Kleiman, D., et al. (2004). Evaluating the Conservation Mission of Zoos, Aquariums, Botanical Gardens, and Natural History Museums. Conservation Biol. 18 (1), 86–93. doi:10.1111/j.1523-1739.2004.00181.x
Moss, A., and Esson, M. (2013). The Educational Claims of Zoos: Where Do We Go from Here? Zoo Biol. 32, 13–18. doi:10.1002/zoo.21025
Moss, A., Jensen, E., and Gusset, M. (2015). Evaluating the Contribution of Zoos and Aquariums to Aichi Biodiversity Target 1. Conservation Biol. 29 (2), 537–544. doi:10.1111/cobi.12383
Moss, A., Jensen, E., and Gusset, M. (2017). Impact of a Global Biodiversity Education Campaign on Zoo and Aquarium Visitors. Front. Ecol. Environ. 15 (5), 243–247. doi:10.1002/fee.1493
Ojalammi, S., and Nygren, N. V. (2018). Visitor Perceptions of Nature Conservation at Helsinki Zoo. Anthrozoös 31 (2), 233–246. doi:10.1080/08927936.2018.1434063
R Core Team (2014). R, a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Raghavan, R., Luz, L., Shepherd, C., Lewis, R., Gibbons, P., and Goode, E. (2015). A Case Study of the Ploughshare Tortoise Astrochelys Yniphora and the Role Zoos Can Play in Conservation. TRAFFIC Bull. 27, 79–84.
Runge, C. A., Withey, J. C., Naugle, D. E., Fargione, J. E., Helmstedt, K. J., Larsen, A. E., et al. (2019). Single Species Conservation as an Umbrella for Management of Landscape Threats. PLoS ONE 14 (1), e0209619. doi:10.1371/journal.pone.0209619
Seddon, P. J., Armstrong, D. P., and Maloney, R. F. (2007). Developing the Science of Reintroduction Biology. Conservation Biol. 21, 303–312. doi:10.1111/j.1523-1739.2006.00627.x
Rinne, J., and Stefferud, J. (1999). Single Versus Multiple Species Management: Native Fishes in Arizona. Forest and Ecology Management 114 (2–3), 357–365. doi:10.1111/j.1523-1739.2006.00627.x
Simberloff, D. (1998). Flagships, Umbrellas, and Keystones: Is Single-Species Management Passé in the Landscape Era? Biol. Conservation. 83 (3), 247–257. doi:10.1016/S0006-3207(97)00081-5
Skibins, J. C., Dunstan, E., and Pahlow, K. (2017). Exploring the Influence of Charismatic Characteristics on Flagship Outcomes in Zoo Visitors. Hum. Dimensions Wildl. 22 (2), 157–171. doi:10.1080/10871209.2016.1276233
Taylor, B. L., Abel, G., Miller, P., Gomez, F., Fersen, L. V., DeMaster, D., et al. (2020). “Ex Situ Options for Cetacean Conservation. Report of the 2018 Workshop, Nuremberg, Germany,” Occasional Paper of the IUCN Species Survival Commission No. 66 (Gland, Switzerland: IUCN).
Tracy, C., and Brussard, P. (1994). Preserving Biodiversity: Species in Landscapes. Ecol. Appl. 4 (2), 206–207. Available at: www.jstor.org/stable/1941924.
TRAFFIC (2019). TRAFFIC Bulletin: Seizures and Prosecutions March 1997–October 2019. Available at: https://www.traffic.org/publications/reports/latest-seizures/. (Accessed November 21, 2020).
Washington, H., Baillie, J., Waterman, C., and Milner-Gulland, E. J. (2015). A Framework for Evaluating the Effectiveness of Conservation Attention at the Species Level. Oryx 49 (3), 481–491. doi:10.1017/S0030605314000763
Keywords: wildlife conservation, collaboration, evaluation, zoos and aquariums, endangered species, threatened species, safe, species recovery
Citation: Ripple KJ, Sandhaus EA, Brown ME and Grow S (2021) Increasing AZA-Accredited Zoo and Aquarium Engagement in Conservation. Front. Environ. Sci. 9:594333. doi: 10.3389/fenvs.2021.594333
Received: 13 August 2020; Accepted: 02 September 2021;
Published: 24 September 2021.
Edited by:
Leslie Cornick, University of Washington Bothell, United StatesReviewed by:
Susan Clayton, College of Wooster, United StatesMarkus Gusset, Federal Office for Agriculture, Switzerland
Copyright © 2021 Ripple, Sandhaus, Brown and Grow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Estelle A. Sandhaus, ZXNhbmRoYXVzQHNiem9vLm9yZw==
 Kayla J. Ripple
Kayla J. Ripple Estelle A. Sandhaus
Estelle A. Sandhaus Megan E. Brown
Megan E. Brown Shelly Grow
Shelly Grow