
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Environ. Sci., 16 November 2020
Sec. Conservation and Restoration Ecology
Volume 8 - 2020 | https://doi.org/10.3389/fenvs.2020.556877
This article is part of the Research TopicProceedings of the 29th International Congress for Conservation Biology (ICCB 2019)View all 9 articles
Natural mineral licks are used by many species throughout the world but information relating to the use of artificial saltlicks for wildlife conservation and management is scant. The Department of Wildlife and National Parks in Peninsular Malaysia has established more than 30 artificial saltlicks intended to enrich the habitat with additional mineral resources for wildlife. We used automated camera traps to assess mammal diversity and visitation rates at 14 artificial saltlicks across three wildlife reserves, and compared these metrics to those from nearby (300 m) forest sites. Cameras were operational for an average of 65.3 trap nights (TN), providing 846 TN at artificial saltlicks and 916 TN in forest areas. At artificial saltlicks we recorded 159.7 independent mammal encounters (100 TN–1), significantly higher (p < 0.0001) than from forest sites at 31.1 100 TN–1. Twenty-three species were recorded at artificial saltlicks while 19 species were detected from forest sites. Of the seven most frequent species to visit the artificial saltlicks, only wild pig (p = 0.012), Malayan tapir (p = 0.033), red muntjac (p = 0.008), and Malayan porcupine (p = 0.007) showed significantly higher encounter rates over forest sites, indicating a specific preference and targeted use of artificial saltlicks for these species. Artificial saltlick sites successfully attracted a wide range of species, possibly because they provided valuable resources that would otherwise be absent in the study area. Such areas may be beneficial in diversifying and enriching habitats, particularly where natural mineral licks have been lost or habitats necessitate restoration or rehabilitation.
Mineral licks are naturally occurring sites found throughout much of the world where the substrate and/or water contains numerous and various mineral elements. Commonly known as salt-licks, these areas are spatially limited, but essential to a variety of wildlife species. Herbivores, in particular, frequently engage in geophagy at these sites, consuming the soil or water from these mineral-rich areas (Kreulen, 1985). While the exact reason for the deliberate ingestion of mineralized water or soil has yet to be fully ascertained, it is likely mineral licks serve multiple functions that may vary for different species, sexes, or at different temporal scales (Kreulen, 1985; Atwood and Weeks, 2002). Natural mineral licks provide essential nutrients that are lacking in the diet (Weeks and Kirkpatrick, 1976; Jones and Hanson, 1985; Heimer, 1988; Ceacero et al., 2009; Flueck et al., 2012) or buffering compounds against dietary toxins, diarrhea, endoparasites or to adjust gut pH (Mahaney et al., 1995; Knezevich, 1998; Krishnamani and Mahaney, 2000; Ayotte et al., 2006).
Mineral licks are considered a crucial micro-habitat and are key to supporting, especially, a high herbivore diversity (Montenegro, 2004). However, these areas must be protected if they are to remain an integral part of a functioning ecosystem (Matsubayashi et al., 2011). With natural forests continuing to decline (Potapov et al., 2017; Watson et al., 2018), the destruction or loss of natural mineral licks continues too. The continuing expansion of road networks, logging areas, agricultural and urban land developments often obliterate these small important micro-habitats.
Whereas natural mineral licks have received considerable scientific attention, the development and use of artificial saltlicks for wildlife conservation and management has received very little attention. Deer and moose are known to visit and consume salt deployed for de-icing roads in north America (Fraser and Thomas, 1982; Grosman et al., 2009), whilst also being attracted to salt or mineral blocks placed by game hunters (DIY hunting, 2019). The use of artificial licks in the United States was also studied by burying commercially available deer mineral blocks at ground level (Schultz, 1990; Schultz and Johnson, 1992). These ‘licks’ were very successful in attracting white-tailed deer which licked and consumed the mineral blocks and granulated mineral. Animal viewing hides can also be baited with salt to attract wildlife for photography and enhance the wildlife viewing experience. Several viewing hides in Malaysia’s Taman Negara National Park have salt and mineral blocks added to attract wildlife.
In Peninsular Malaysia, the Department of Wildlife and National Parks (DWNP) has established more than 30 artificial saltlicks throughout various National Parks and protected areas. These were specifically aimed at enriching the natural forest habitat by providing additional mineral resources for wildlife. In a preliminary investigation Magintan et al. (2015) found 20 mammal species visiting these artificial saltlicks over an 18 months period and concluded that these sites were successful in attracting wildlife. In this study, we used camera traps to quantify and assess species richness, diversity and patterns of use at artificial saltlicks in Malaysia, and compared these metrics to wildlife in nearby forested areas.
Fifteen (15) artificial saltlicks were assessed from six sites across three wildlife reserves in Peninsular Malaysia (Figure 1).
Figure 1. Location of the Sungkai, Sungai Dusun, and Krau wildlife reserves (WR) in Peninsular Malaysia.
The Krau Wildlife Reserve (KWR) in Pahang comprises an area of 62,395 ha of lowland primary forest with the highest peak reaching 2,100 masl. Nine (9) artificial saltlicks were assessed from four areas. At Perlok station (unmanned) one artificial saltlick. At Lompat station (unmanned) two artificial saltlicks. At Jenderak guar conservation center, three artificial saltlicks. At Bukit Rengit operations center, three artificial saltlicks. All artificial saltlicks were situated within 1km of a ranger station or center. Mammal richness in KWR is particularly high within a Peninsular Malaysian context. However, predator diversity is moderate and species abundance for large mammals is low (DWNP, 2001), indicating considerable limiting factors. Indigenous communities live within and around KWR and undertake subsistence hunting. Other nearby local communities are also thought to engage in activities that impact negatively on mammal densities (DWNP, 2001).
The Sungai Dusun Wildlife Reserve (SDWR) in Selangor covers an area of 4,330 ha of secondary dipterocarp and peat-swamp forest (DWNP, 2004), with an elevation of less than 250 m. At the Sungai Dusun conservation center (which undertakes breeding and rehabilitation of the Malayan tapir), three artificial saltlicks were assessed. All saltlicks were within 1km of the conservation center. Hunting pressure in SDWR is unknown. The mammal diversity is believed to be lower than that of an equal size primary forest, although a comprehensive assessment at the SDWR has not been undertaken.
The Sungkai Wildlife Reserve (SWR) in Perak covers an area of 2,468 ha comprising mostly lowland secondary dipterocarp forest, with an elevation reaching 1,000 masl in the northeast where it adjoins the Titiwangsa mountain range. At the Sungkai conservation center, three artificial saltlicks were assessed and all located within 2 km of the center. The conservation center undertakes wildlife breeding programs for guar, sambar, and various bird species. An assessment of local threats has not been undertaken, although a biodiversity inventory recorded a moderate mammal diversity (DWNP, 2009) including the rare dhole (Magintan et al., 2014).
All Wildlife Reserves share borders with forested areas, local communities and large agricultural developments of oil palm and rubber. Elephants, guar, Sumatran rhino and probably tiger are no longer found in any of these Reserves. All artificial saltlicks were established at elevations from 40 to 140 masl. Most artificial saltlicks were situated further than 1 km apart, but we acknowledge four artificial saltlicks were situated within 500–700 m of another artificial saltlick. As we were not estimating species density, but rather comparative visitation rates between forest and saltlick sites, we feel results should still reflect preferences (or otherwise) for artificial saltlicks.
The DWNP developed the artificial saltlicks used in this study in 2011 and 2012 (Magintan et al., 2015) by digging a shallow pit at an appropriate location in the forest. Typically, these pits were 2–3 m long, 1.5–2 m wide, and 30–50 cm deep. Several artificial lick sites were also formed within natural depressions in the forest. The size and depth of these artificial saltlicks could also change over time due to erosion by animals and weather. An area of 1–3 m around the edge of the pit was cleared of vegetation to form a small clearing of approximately 20–40 m2, including the saltlick itself. Approximately 1,000 kg of common, commercially available course-grain salt (NaCl) (purchased in 40 kg bags) was added to newly dug pits, together with 5–15 domestic-stock mineral blocks (Phos Rich 2% Rockie mineral blocks, Rockies Tithebarn Ltd., United Kingdom). These commercial mineral blocks provide essential macro and micro-nutrients for domestic livestock, and were enriched in sodium, phosphorous, calcium, magnesium, iron, cobalt, iodine, manganese, selenium and zinc. The artificial saltlicks were typically replenished with additional salt (∼200–300 kg) and numerous mineral blocks 2–4 times a year, although such regimes were not regimented. Rainfall would fill the saltlick pits and dissolve the salt, leaving small pools of salty water, from which animals could drink.
As Malaysia is situated close to the equator, it has an ever-wet tropical climate and rain falls throughout the year. The country typically receives rain 10–20 days each month, with a mean monthly rainfall of 259 mm and an annual total exceeding 3,000 mm (for the 1991–2016 period) (World Bank, 2019). Such consistent rainfall usually results in the artificial saltlick pits being saturated with water, resulting in the formation of “wet” lick pools. Many of the natural mineral licks in Malaysia are “wet” licks (where animals drink the pooled or seeping mineral-rich water), as opposed to “dry” licks which are often excavated into the side of a bank by animals consuming the mineral-rich soil.
The wildlife use of artificial saltlicks and surrounding forested area was assessed using automated camera traps (Scoutguard SG 565FV and Bestguarder SG 990V, Guangdong, China). A total of 30 camera traps were deployed in pairs at each site. One camera of each pair was set at each of the 15 artificial saltlick sites, and a further 15 cameras were placed within the surrounding forest. Paired lick-forest cameras were placed at an average distance of 315 m apart. Forest cameras were set on animal trails or at small clearings used by wildlife, while cameras at artificial saltlick sites were typically set 6–10 m from the focal artificial saltlick area. Camera-trapping started on 25 July 2017 and ended in mid-October 2017, with two cameras operational until December 2017.
All cameras were set to record two pictures per trigger event and were operational 24 h/day. The trigger event interval was set at 2 min. Cameras were generally positioned at a height of 50–80 cm above the ground, and all had a small transparent plastic cover attached to the top to help protect against rain. Cameras were deployed to specifically detect medium-large sized terrestrial mammals, although other species were also photographed. All locations were recorded with a 60CSx Garmin GPS.
The camera trapping software CameraSweet (see Sanderson and Harris, 2013) was used to organize and analyze camera trap pictures. All mammals were identified to species where possible. With the exception of the conspicuous moonrat, Echinosorex gymnurus, pictures of smaller mammal species such as rats, mice, treeshrews or squirrels were grouped into “superfamily-type” groups due to difficulties in accurate identification. The Lesser mousedeer (Tragulus kanchil), and the Greater mousedeer (T. napu) were grouped together as mousedeer spp. due to difficulties in distinguishing between the species in some photographs. People, domestic animals, “ghost” shots and animals that could not be identified due to poor image quality or partial shots were excluded.
Pictures of the same species at the same site were considered to be independent after a period of 60 min (an Independent Encounter, IE). The Encounter Rate (ER) for a species (or group) was determined as Independent Encounters per 100 trap nights. The persistence of a species at a camera site was calculated as the time between the first and last picture of an independent encounter, and rounded to 1 min. A single photograph was recorded as a visit of less than 2 min.
Comparisons between artificial saltlicks and forest sites were analyzed using pooled data from the two sampling groups with a T-test for unequal variance. Paired T-tests were used for species comparisons from paired cameras. Means (± SD) were expressed for pooled data, while statistical significance levels were set at p ≤ 0.05. Species diversities were assessed using Shannon diversity indices (H′), Shannon Evenness metric (E) and the Simpson dominance index (D) for pooled data from all cameras from saltlick and forest sites, and assessed with T-tests. Sampling completeness and the equivalent number of species (or “true diversity”) for sites were based on Hill numbers described by Chao et al. (2016). Species similarity was assessed with Jaccard’s similarity coefficient.
From the 30 cameras deployed (15 at artificial lick sites, 15 in forests), two were stolen from the Jenderak site, while another malfunctioned completely. The 27 operational cameras (14 at licks and 13 at forest sites, i.e., 13 paired sites) where active for an average of 65.3 trap nights (TN) per camera. Trap effort was not equal across sites with 846 TN recorded at the artificial saltlicks, while 916 TN were recorded in the forest.
More than 11,000 pictures were recorded in all, of which 6,431 were identified visitors to the camera site locations. From these, 1,775 independent encounters were made of various species. The vast majority of encounters were of medium and large-sized mammals (93.1%), followed by birds (3.0%), small mammals (2.3%), people (1.0%), and reptiles (0.6%).
Capture rates for all mammals were significantly higher (T-test, p < 0.0001) at artificial saltlick sites (159.7 ± 60.6 encounters 100 TN–1) than from forest sites (31.1 ± 18.9 encounters 100 TN–1), indicating a targeted use of the artificial saltlicks by some (or all) mammal species.
Overall, 29 mammal species (or mammal groups) were recorded during the study; 23 species from artificial lick sites, and 19 species from forest areas (Table 1). Thirteen species were common to both artificial lick and forest sites, resulting in a Jaccard similarity coefficient of 0.45 (or 45%). Ten species were only recorded at artificial lick sites while six species were only recorded at forest sites (Table 1). Species richness was not significantly different for pooled site mammal data (T-test, p = 0.076) between artificial lick sites ( = 7.2 ± 2.5 species, n = 14) and forest sites ( = 5.4 ± 2.7 species, n = 13). Sampling completeness at each site (artificial licks 77%; forest sites 95%) however, indicates that further camera trapping should result in the detection of more species.
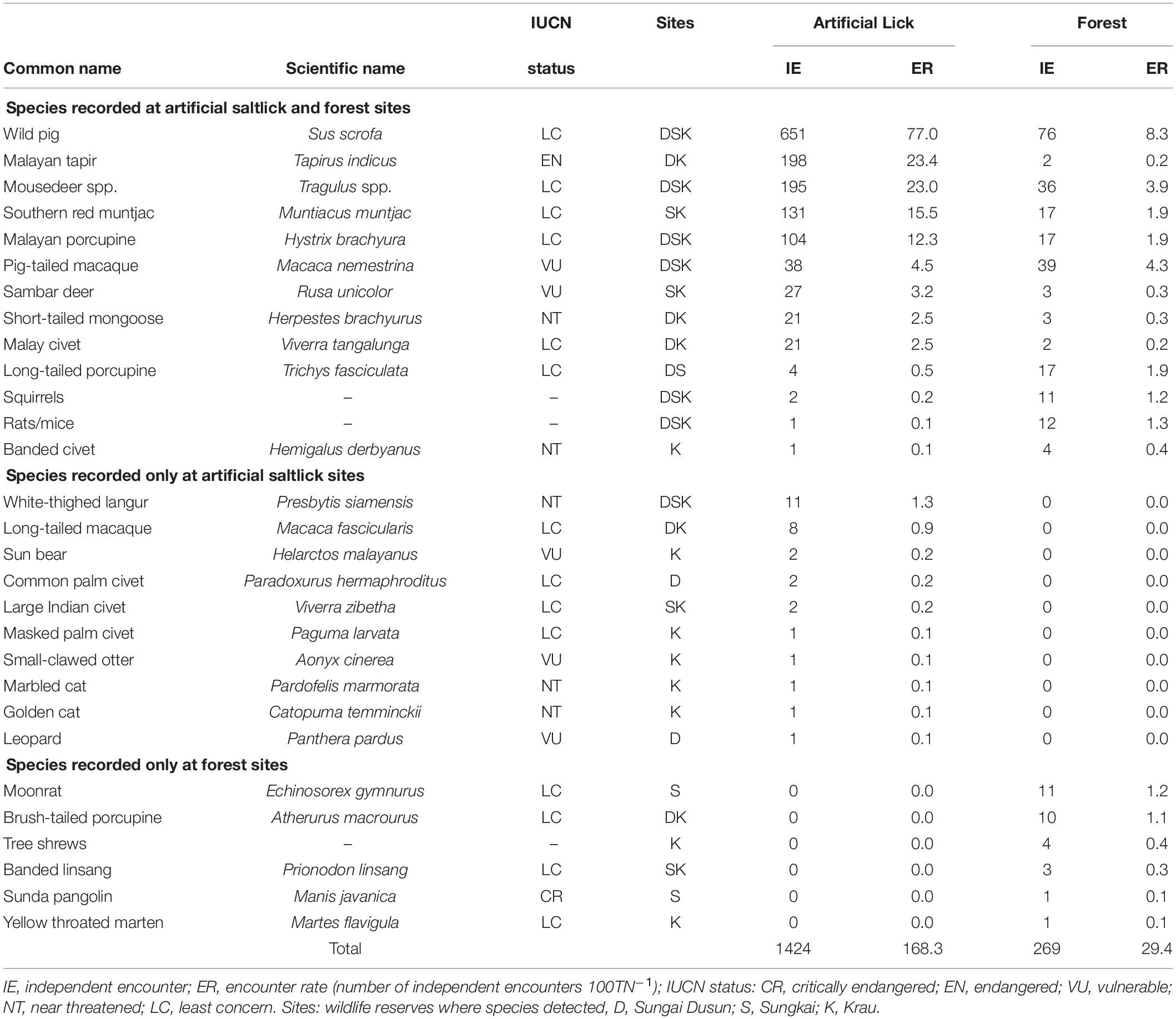
Table 1. Mammal species (or groups) detected visiting artificial saltlicks and forest sites with camera traps.
Shannon diversity indices, however, indicate significantly higher mammal diversity at forest sites (H′ = 2.342 forest vs. 1.767 at lick sites, p < 0.0001). This translates to an effective number of species (ENS) of 10.4 mammal species at forest sites while only 5.9 mammal species at artificial lick sites. The lower ENS of mammals was a result of the targeted use of the artificial saltlicks by some species. This results in a highly skewed species abundance visitation profile, and a lower species evenness index at artificial saltlick sites (Shannon E = 0.564), when compared to forested sites (Shannon E = 0.796). Simpson dominance indices (D) also support skewed visitation toward artificial lick sites by mammals (D = 0.262 artificial licks vs. D = 0.135 forest)—that is, some mammal species were targeting artificial saltlicks, resulting in a higher species dominance index and a lower diversity index; as diversity is the function of species richness and evenness.
The majority of the 23 mammal species detected at the 14 artificial lick sites and forest sites were seen infrequently (Table 1). Due to such low encounter rates with large variations between sites, comparisons between the two sampling groups would not be statistically meaningful. The most common mammals to visit the artificial saltlicks were wild pigs (45.7% of all lick visits), Malayan tapirs (13.9%), mousedeer spp. (13.7%), red muntjac (9.2%), and Malayan porcupines (7.3%). These five species alone represented 90% of all encounters at artificial saltlick sites. By comparison the five most common species photographed in forest areas were wild pigs (28.3% of forest photographs), pig-tailed macaques (14.5%), mousedeer spp., (13.4%), red muntjac (6.3%), and Malayan porcupine (6.3%), representing 69% of forest encounters.
Only seven species visiting the artificial saltlicks had encounter rates of ≥ 3.0 100 TN–1, which we consider as “frequent” visitors. Only four of these species showed significantly higher mean encounter rates at the artificial saltlicks over forest sites, when assessed with paired T-tests. The wild pig (p = 0.012), Malayan tapir (p = 0.033), red muntjac (p = 0.008), and Malayan porcupine (p = 0.007) all had significantly higher visit rates at artificial saltlicks, indicating a specific preference and targeted use of artificial saltlicks over forest sites (Table 1). Mean encounter rates for mousedeer spp. (p = 0.253), sambar deer (p = 0.136) and pig-tailed macaque (p = 0.318), which were also “frequent” visitors at the artificial saltlicks, were not significantly different from encounter rates at forest sites (Figure 2).
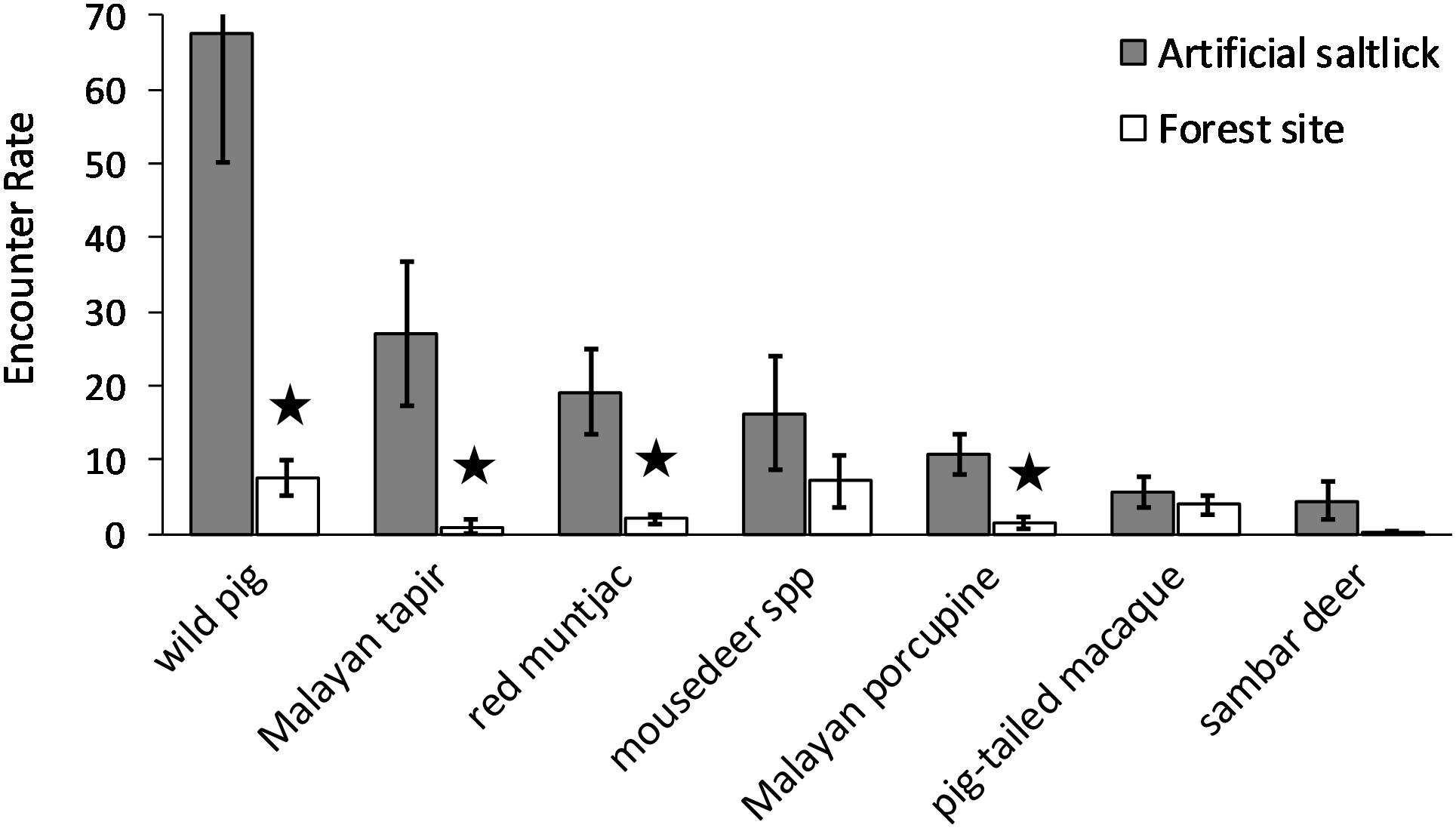
Figure 2. Mean encounter rates for frequent species visiting artificial saltlicks and forest sites. Frequent species were considered those with artificial lick-site encounter rates of ≥ 3 visits 100TN–1. Error bars are standard errors. Encounter rates are independent pictures 100TN–1. Star denotes significantly different encounter rates (p ≤ 0.05) assessed with paired T-tests.
If the four “close-proximity” artificial saltlicks were removed from analysis (see Study site), the results for “frequent” visitors remained unchanged. The same seven species were identified as “frequent” visitors with encounter rates of ≥ 3.0 100 TN–1, with only the wild pig, Malayan tapir, red muntjac and Malayan porcupine maintaining significantly higher visit rates at artificial saltlick sites.
The four “frequent” visitors showing significantly higher visit rates at the artificial saltlicks (wild pig, Malayan tapir, red muntjac and Malayan porcupine) all varied in the time spent at the artificial saltlick sites. Wild pigs (with group sizes up to 23 individuals) were the most frequent visitors to artificial lick sites, but more than half of the time (55%) they were simply passing by, or spending less than 2 min at the site (a single photograph only). Pigs spent an average of 7.4 (± 13.2) min at the artificial lick site, often wallowing at some sites, or rooting in the surrounds. This was significantly longer (T-test, p < 0.0001) than the 2.6 (± 5.2) min spent at forest cameras. Tapirs only spent about a quarter of their time (27%) on short (< 2 min) visits to artificial lick sites, with an average site persistence of 16.9 (± 18.3) min/visit. This was significantly (p < 0.0001) longer than at forest sites (1 min). Tapirs would spend considerable time drinking the mineralized water, and, at one deeper saltlick, swimming. Red muntjac spent an average of 14.1 (± 21.1) min at the artificial saltlicks, significantly longer (p < 0.0001) than at forest sites (1.5 ± 2.2 min). The time spent at artificial saltlicks (8.4 ± 15.0 min) by the Malayan porcupine was not significantly longer (p = 0.219) than at forest sites (4.3 ± 11.4 min). However, this was exclusively due to a single forest visit represented by two photographs 49 min apart, which considerably increased average persistence time and standard deviation for forest cameras. Whether this was the same individual remaining at the forest site or two different individuals is unknown.
It is clear from this study that mammals were using the artificial saltlicks, with 23 mammal species (groups) detected over the approximate 2-month sampling period. This was slightly higher than the 19 species detected in nearby forest areas, albeit with a somewhat different species composition. Sampling completeness, however, indicates that both artificial lick and forest sites were yet to reach species saturation. Tobler et al. (2005) suggests that trap effort is more important than trap placement for recording a larger number of species, and thus further trapping would no doubt result in more species being detected at both sites.
Not all species detected at the artificial saltlicks were specifically targeting the area, with many species also found in forest sites at similar encounter rates. As these small artificial sites form an integral part of the larger forested landscape, many species seen at the saltlicks will simply be passing through another micro-habitat within their habitat or range. The effectiveness of artificial saltlicks to attract wildlife therefore cannot be evaluated by species richness alone, as indicated by Magintan et al. (2015), but through additional indicators which may assess comparative frequencies, persistence at site or activities undertaken. Such indicators may also be affected by biotic, abiotic, behavioral or biological factors or be impacted at different temporal scales. White-tailed deer in the United States, for example, were seen to utilize artificial licks to varying extents, depending on location, month and season (Schultz and Johnson, 1992).
It is clear that some species were deliberately targeting the artificial saltlicks. Wild pigs, tapirs, muntjac and Malayan porcupines all showed significantly higher visit rates to artificial saltlicks than to forest areas, and were targeting these sites to engage in geophagy, or wallowing as was the case for the wild pig. Forest clearings such as these may also provide useful hunting, basking, feeding or browsing areas, and thus prove attractive to a suite of species seeking the more open terrestrial habitat of the artificial saltlick site itself. Small patches of cleared forest such as these may be beneficial in helping to diversify the forest habitat. Further research will be beneficial for comparing species visiting artificial saltlicks to those of forest clearings, wallows and natural licks. The results may help further clarify the attractiveness of artificial lick sites for some species.
The main users targeting these artificial sites showed typical encounter rates of ∼10–70 visits per 100 trap nights. These high visitation rates were comparable to frequent visitors utilizing natural mineral licks in tropical forests. Bearded pigs from Borneo were recorded at rates of 14–20 visits 100 TN–1 (Matsubayashi et al., 2007a; Matsuda et al., 2015), sambar deer 9–40 visits 100 TN–1 (Matsubayashi et al., 2007b; Matsuda et al., 2015), lesser mouse deer 8 visits 100TN–1 (Matsubayashi et al., 2007a), red muntjac 13 visits 100TN–1 (King et al., 2016) and lowland tapirs 55–98 visits 100TN–1 (Blake et al., 2012; Link et al., 2012). Natural mineral licks from Peninsular Malaysia showed encounter rates (100 TN–1) of 6–47 for Malayan tapir, 3–25 for wild pig, 4–46 for red muntjac and 2–6 for Malayan porcupine (Simpson unpublished data). Artificial saltlicks are therefore attracting a similar suite of species at rates similar to those of natural mineral licks, and may be seen as an alternative to natural licks, at least in terms of behavioral activities. How artificial saltlicks compare to natural mineral licks in terms of their chemical properties or their implied health benefits has yet to be ascertained, however, several studies have shown that natural mineral licks provide a wide variety of macro and micro minerals and gut buffering properties beneficial to wildlife health (Jones and Hanson, 1985; Kreulen, 1985; Mahaney et al., 1995; Ayotte et al., 2006).
Comparisons of visit rates for a particular species between the different artificial saltlicks sites can be compounded by numerous sampling biases. Mean encounter rates were sometimes accompanied by large standard deviations. Not all camera sites were subjected to the same disturbance levels, forest cover, structure, substrate or species densities, with the effects of such factors being highlighted in studies on species distributions and modeling (Blom et al., 2005; Fahrig and Rytwinski, 2009; Vanthomme et al., 2013; Whitworth et al., 2019). While some artificial saltlicks were located in dense forest, others were situated in proximity to built-up areas, which may then affect species encounter rates, especially for more timid species. Hunting pressures and general human disturbance, which were unknown for all areas, could have an effect on mean encounter rates, resulting in large standard deviations for species-specific means. Underlying these factors are also species densities and distributions, which are no doubt heterogeneous across wildlife reserves. Schultz and Johnson (1992) also found large variations in artificial saltlick use by white-tailed deer, and suggest dietary needs may influence artificial saltlick use. It is possible that species like the sambar deer may actually target artificial saltlicks under favorable conditions. Although not borne out in this study, sambar deer were the second most common visitor to artificial saltlicks in an earlier study in Malaysia (Magintan et al., 2015).
In the Krau wildlife reserve, for example, sambar deer were recorded at both areas in the Jenderak and Lompat sites, yet not at all around the more-disturbed Bukit Rengit site. Proximity to noise and other disturbance at Bukit Rengit may have precluded the species from cameras in this area. Wild pigs spent considerable time wallowing at some of the artificial saltlicks where deep mud was formed. At other lick sites, feeding (rooting in the soil) was a major activity. A small pond-type artificial saltlick which attracted a large number of tapirs for drinking and swimming, had few wild pig visits. It is also interesting to note that tapir was recorded in all areas except for the six camera sites (3 lick and 3 forest) at the Sungkai wildlife reserve—and this may relate to tapir distribution, rather than any association with artificial saltlicks. Magintan et al. (2014) did however, record tapir in this Wildlife Reserve. The white thighed langur is known to utilize natural mineral licks in the Krau wildlife reserve (pers. obs., BS), but was only recorded drinking from three artificial saltlicks in this study. Whether this was due to the unsuitability of the other artificial saltlicks, or because artificial saltlick sites were unknown to langurs, or due to other biological or behavioral aspects is unknown. Typically, primate species utilize natural mineral licks with specific physical or chemical characteristics, and incorporate anti-predator and ranging behaviors when descending to mineral lick sites (Link et al., 2011; Matsubayashi et al., 2011; Ampeng et al., 2016).
With natural forest estates declining due to increasing forestry and agricultural practices (Potapov et al., 2017), natural mineral licks will also come under increasing risk of annihilation. Natural mineral licks are known to attract numerous wildlife species that often engage in geophagy, or drink the mineralised water (Jones and Hanson, 1985). Ungulates seem particularly attracted, although other herbivores (primates, sloths) and birds have also been recorded using natural mineral licks (Krishnamani and Mahaney, 2000; Brightsmith et al., 2008; Mosquera et al., 2019). Such natural licks, which are often small, non-descript and spatially limited, are considered crucial micro-habitats (Montenegro, 2004). They are key to supporting healthy biodiversities and are integral to functioning ecosystems (Matsubayashi et al., 2011). The effect on ecological functions from the loss of natural mineral licks is unknown, but may impact on certain species’ population distribution, health or vigor. Although the effectiveness of artificial saltlicks to supplement the nutrition of wild species on native ranges has yet to be fully ascertained (Schultz and Johnson, 1992), artificial saltlicks may deliver an avenue to rehabilitate degraded landscapes by providing a range of species access to essential dietary needs. Developing artificial saltlicks to support wildlife populations should be considered where natural sites have been degraded or where areas will benefit from additional mineral resources and diversified micro-habitats.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethical review and approval was not required for the study. The manuscript presents results of an observational study of forest and forest resources. Cameras were used to record forest activities, which included images of forest animals. Animals were not captured, handled, restrained or disturbed. No animal or animal products were collected, handled or sampled. Activities comply with the legal and ethical requirements of Department of Wildlife and National Parks, Malaysia.
BS and SN conceived and designed the research. BS and NN conducted the fieldwork. BS conducted the data analysis and all authors contributed to writing and editing the manuscript.
Fieldwork was undertaken with funding from Copenhagen Zoo’s Southeast Asia Programme. Universiti Kebangsaan Malaysia provided funds for open access publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was carried out under a Memorandum of Understanding between Copenhagen Zoo, Denmark and the Department of Wildlife and National Parks (DWNP), Malaysia. All activities comply with the legal and ethical requirements of DWNP. We thank the Director General of DWNP, YBhg. Dato’ Abdul Kadir bin Abu Hashim and Dr. Pazil bin Abdul Patah, DWNP Director of Biodiversity Conservation for facilitating this work. We thank Adrian Jawing for assisting with fieldwork and also DWNP staff at Bukit Rengit, Jenderak, Kuala Lompat, Perlok, Sungai Dusun and Sungkai for their assistance. Three reviewers and provided comments that improved earlier drafts of the manuscript.
Ampeng, A., Shukor, M. N., Sahibin, A. R., Idris, W. M. R., Ahmad, S., Mohammad, H., et al. (2016). Patterns of mineral lick use by Northwest Bornean orangutans (Pongo pygmaeus pygmaeus) in the Lanjak Entimau Wildlife Sanctuary, Sarawak, Malaysia. Eur. J. Wildlife Res. 62, 147–150. doi: 10.1007/s10344-015-0983-8
Atwood, T., and Weeks, H. P. Jr. (2002). Sex-and age-specific patterns of mineral lick use by white-tailed deer (Odocoileus virginianus), American Midland. Naturalist 148, 289–296. doi: 10.1674/0003-0031(2002)148[0289:saaspo]2.0.co;2
Ayotte, J. B., Parker, K. L., Arocena, J. M., and Gillingham, M. P. (2006). Chemical composition of lick soils: functions of soil ingestion by four ungulate species. J. Mammal. 87, 878–888. doi: 10.1644/06-mamm-a-055r1.1
Blake, J. G., Mosquera, D., Loiselle, B. A., Swing, K., Guerra, J., and Romo, D. (2012). Temporal activity patterns of terrestrial mammals in lowland rainforest of eastern Ecuador. Ecotropica 18, 137–146.
Blom, A., Van Zalinge, R., Heitkönig, I. M., and Prins, H. H. (2005). Factors influencing the distribution of large mammals within a protected central, African forest. Oryx 39, 381–388. doi: 10.1017/s0030605305001080
Brightsmith, D. J., Taylor, J., and Phillips, T. D. (2008). The roles of soil characteristics and toxin adsorption in avian geophagy. Biotropica 40, 766–774. doi: 10.1111/j.1744-7429.2008.00429.x
Ceacero, F., Landete-Castillejos, T., García, A. J., Estévez, J. A., Martinez, A., Calatayud, A., et al. (2009). Free-choice mineral consumption in Iberian red deer (Cervus elaphus hispanicus) response to diet deficiencies. Livestock Sci. 122, 345–348. doi: 10.1016/j.livsci.2008.08.002
Chao, A., Ma, K. H., and Hsieh, T. C. (2016). iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity. Program and User’s Guide. Available online at: http://chao.stat.nthu.edu.tw/wordpress/software_download/ (accessed August 18, 2018).
DIY hunting (2019). DIY Make Your Own Artificial Salt Lick. Available online at: http://www.diy-hunting-and-outdoors.com/salt-lick.html (accessed November 3, 2019).
DWNP (2001). Krau Wildlife Reserve Management Plan. Kuala Lumpur: Department of Wildlife and National Parks, and Danish Cooperation for Environment.
DWNP (2004). Sungai Dusun Wildlife Reserve: Development and Management Plan. Kuala Lumpur: Department of Wildlife and National Parks and University Putra Malaysia.
DWNP (2009). Laporan Inventori Biodiversiti Rezab Hidupan Liar Sungkai. Kuala Lumpur: Department of Wildlife & National Parks (unpublished report).
Fahrig, L., and Rytwinski, T. (2009). Effects of roads on animal abundance: an empirical review and synthesis. Ecol. Soc. 14:21.
Flueck, W. T., Smith-Flueck, J. M., Mionczynski, J., and Mincher, B. J. (2012). The implications of selenium deficiency for wild herbivore conservation: a review. Eur. J. Wildlife Res. 58, 761–780. doi: 10.1007/s10344-012-0645-z
Fraser, D., and Thomas, E. R. (1982). Moose-vehicle accidents in Ontario: relation to highway salt. Wildlife Soc. Bull. (1973-2006) 10, 261–265.
Grosman, P. D., Jaeger, J. A., Biron, P. M., Dussault, C., and Ouellet, J. P. (2009). Reducing moose–vehicle collisions through salt pool removal and displacement: an agent-based modelling approach. Ecol. Soc. 14:17.
Heimer, W. E. (1988). “A magnesium-driven hypothesis of Dall sheep mineral lick use: preliminary tests and management relevance,” in Proceedings of the Biennial Symposium of the Northern Wild Sheep and Goat Council, Banff, Vol. 6, 269–278.
Jones, R. L., and Hanson, H. C. (1985). Mineral Licks, Geophagy, and Biochemistry of North American Ungulates. Ames: The Iowa State University Press.
King, A., Behie, A. M., Hon, N., and Rawson, B. M. (2016). Patterns of salt lick use by mammals and birds in northeastern Cambodia. Cambodian J. Natural History 1, 40–50.
Knezevich, M. (1998). Geophagy as a therapeutic mediator of endoparasitism in a free−ranging group of rhesus macaques (Macaca mulatta). Am. J. Primatol. 44, 71–82. doi: 10.1002/(sici)1098-2345(1998)44:1<71::aid-ajp6>3.0.co;2-u
Kreulen, D. A. (1985). Lick use by large herbivores: a review of benefits and banes of soil consumption. Mammalian. Rev. 15, 107–123. doi: 10.1111/j.1365-2907.1985.tb00391.x
Krishnamani, R., and Mahaney, W. C. (2000). Geophagy among primates: adaptive significance and ecological consequences. Anim. Behav. 59, 899–915. doi: 10.1006/anbe.1999.1376
Link, A., Di Fiore, A., Galvis, N., and Fleming, E. (2012). Patterns of mineral lick visitation by lowland tapir (Tapirus terrestris) and lowland paca (Cuniculus paca) in a western Amazonian rainforest in Ecuador. Mastozool. Neotropical 19, 63–70.
Link, A., Galvis, N., Fleming, E., and Di Fiore, A. (2011). Patterns of mineral lick visitation by spider monkeys and howler monkeys in Amazonia: are licks perceived as risky areas? Am. J. Primatol. 73, 386–396. doi: 10.1002/ajp.20910
Magintan, D., Ahmad, M. A., Ismail, A., and Rasdi, I. (2014). Observation of dhole (Cuon alpinus) at Sungkai wildlife reserve, Perak, Malaysia. J. Wildlife Parks 29, 69–72.
Magintan, D., Rahmah, I., Adnan, I., Adrian, J., Rasdi, I., and Sanusi, M. (2015). A preliminary observation of mammals and other species visiting artificial salt licks in Peninsular Malaysia. J. Wildlife Parks 30, 59–74.
Mahaney, W. C., Aufreiter, S., and Hancock, R. G. (1995). Mountain gorilla geophagy: a possible seasonal behavior for dealing with the effects of dietary changes. Int. J. Primatol. 16:475. doi: 10.1007/bf02735798
Matsubayashi, H., Ahmad, A. H., Wakamatsu, N., Nakazono, E., Takyu, M., Majalap, N., et al. (2011). Natural-licks use by orangutans and conservation of their habitats in Bornean tropical production forest. Raffles Bull. Zool. 59, 109–115.
Matsubayashi, H., Lagan, P., Majalap, N., Tangah, J., Sukor, J. R. A., and Kitayama, K. (2007a). Importance of natural licks for the mammals in Bornean inland tropical rain forests. Ecol. Res. 22, 742–748. doi: 10.1007/s11284-006-0313-4
Matsubayashi, H., Lagan, P., Sukor, J. R. A., and Kitayama, K. (2007b). Seasonal and daily use of natural licks by sambar deer (Cervus unicolor) in a Bornean tropical rain forest. Tropics 17, 81–86. doi: 10.3759/tropics.17.81
Matsuda, I., Ancrenaz, M., Akiyama, Y., Tuuga, A., Majalap, N., and Bernard, H. (2015). Natural licks are required for large terrestrial mammals in a degraded riparian forest, Sabah, Borneo, Malaysia. Ecol. Res. 30, 191–195. doi: 10.1007/s11284-014-1219-1
Montenegro, O. L. (2004). Natural Licks as Keystone Resources for Wildlife and People in Amazonia. Ph.D. thesis, University of Florida, Florida.
Mosquera, D., Vinueza-Hidalgo, G., and Blake, J. G. (2019). Patterns of mineral lick visitation by Linnaeus’s two-toed sloth Choloepus didactylus (Pilosa. Megalonychidae) in eastern Ecuador. Notes South Am. Mammals 2019, 2–11. doi: 10.31687/saremNMS.19.0.07
Potapov, P., Hansen, M. C., Laestadius, L., Turubanova, S., Yaroshenko, A., Thies, C., et al. (2017). The last frontiers of wilderness: tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 3:e1600821. doi: 10.1126/sciadv.1600821
Sanderson, J., and Harris, G. (2013). Automatic data organization, storage, and analysis of camera trap pictures. J. Indonesian Nat. Hist. 1, 11–19.
Schultz, S. R. (1990). Effects of Artificial Mineral Licks on White-Tailed Deer. Ph.D. thesis, Louisiana State Univ, Baton Rouge, LA.
Schultz, S. R., and Johnson, M. K. (1992). Use of artificial mineral licks by white-tailed deer in Louisiana. Rangeland Ecol. Manage. J. Range Manage. Arch. 45, 546–548. doi: 10.2307/4002569
Tobler, M. W., Carrillo-Percastegui, S. E., Leite Pitman, R., Mares, R., and Powell, G. (2005). An evaluation of camera traps for inventorying large and medium-sized terrestrial rainforest mammals. Anim. Conserv. 11, 169–178. doi: 10.1111/j.1469-1795.2008.00169.x
Vanthomme, H., Kolowski, J., Korte, L., and Alonso, A. (2013). Distribution of a community of mammals in relation to roads and other human disturbances in Gabon, Central Africa. Conserv. Biol. 27, 281–291. doi: 10.1111/cobi.12017
Watson, J. E., Evans, T., Venter, O., Williams, B., Tulloch, A., Stewart, C., et al. (2018). The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610.
Weeks, H. P., and Kirkpatrick, C. M. (1976). Adaptations of white-tailed deer to naturally occurring sodium deficiencies. J. Wildlife Manage. 40, 610–625. doi: 10.2307/3800555
Whitworth, A., Beirne, C., Pillco Huarcaya, R., Whittaker, L., Serrano Rojas, S. J., Tobler, M. W., et al. (2019). Human disturbance impacts on rainforest mammals are most notable in the canopy, especially for larger−bodied species. Divers. Distributions 25, 1166–1178. doi: 10.1111/ddi.12930
World Bank (2019). Malaysia Climate Data. Available online at: https://climateknowledgeportal.worldbank.org/ country/malaysia/climate-data-historical (accessed August 14, 2019).
Keywords: mineral lick, camera trap, biodiversity, rehabilitation, mammal, natural lick, man-made saltlick, conservation
Citation: Simpson BK, Nasaruddin N, Traeholt C and Nor SM (2020) Mammal Diversity at Artificial Saltlicks in Malaysia: A Targeted Use. Front. Environ. Sci. 8:556877. doi: 10.3389/fenvs.2020.556877
Received: 29 April 2020; Accepted: 16 October 2020;
Published: 16 November 2020.
Edited by:
Tsitsi McPherson, State University of New York at Oneonta, United StatesReviewed by:
Emiliano Mori, University of Siena, ItalyCopyright © 2020 Simpson, Nasaruddin, Traeholt and Nor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Boyd K. Simpson, Ym95ZHNpbXBzb25AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.