- 1IUCN SSC Species Monitoring Specialist Group, Gingins, Switzerland
- 2Centre for African Wetlands, University of Ghana, Accra, Ghana
- 3Department of Animal Biology and Conservation Science, University of Ghana, Accra, Ghana
- 4Department of Water Resources and Ecosystems, IHE Delft Institute for Water Education, Delft, Netherlands
- 5Department of Soil Science, Stellenbosch University, Stellenbosch, South Africa
Biodiversity is being lost in wetlands at a faster rate than any other biome. Effective conservation and management of wetlands biodiversity requires data on species status and threats to inform decision-making. However, there are key challenges in Africa around the availability, usability and quality of data, willingness to use data, and capacity. We review these challenges, using examples from Ramsar sites and other wetlands across the continent, and propose solutions to help information users access high quality data in the right format at the right time. We assess the relevance of traditional monitoring methods, as well as innovative new tools such as remote sensing and environmental DNA. We conclude by explaining how governments, civil society and the private sector can enhance data collection by applying common, policy-relevant indicators, scaling up the application of traditional and appropriate new tools and protocols, building capacity in key institutions, and using partnerships and credible science-policy interfaces. Only by sharing and upscaling the solutions to data collection and use will we be able to mainstream biodiversity into decision-making and ultimately stop biodiversity loss across African wetlands.
Introduction
The diverse wetlands of Africa, which include some of the longest rivers and some of the largest freshwater bodies in the world, are of immense importance for biodiversity and people (Thieme et al., 2005; Gardner et al., 2015; Okonkwo et al., 2015; IPBES, 2018; Ramsar Convention on Wetlands, 2018). Freshwater ecosystems cover <1% of the Earth yet they are home to more than 10% of known animals and about one-third of known vertebrate species (Balian et al., 2008; WWF, 2018). They also offer a range of ecosystem services, from water purification to hydrological buffering to coastal protection (Mitsch and Gosselink, 2000; Gardner et al., 2015). The importance of wetlands to people in Africa is further reflected by the fact the continent is second only to the Asia-Pacific region in total catch of inland fisheries (Thieme et al., 2005).
However, global wetland area may have declined by as much as 87% since 1700 (Davidson, 2014) and the downward trend for freshwater species is alarming (Vörösmarty et al., 2010). For example, in the last 35 years, the average abundance of freshwater vertebrate species populations declined by 83% (WWF, 2018), and freshwater fishes had the highest extinction rate worldwide among vertebrates in the twentieth century (Burkhead, 2012). Of the freshwater taxa in Africa that have been assessed, the most globally threatened are molluscs (41%), followed by amphibians (31%), crabs (28%), and fish (27%) (Darwall et al., 2011). These declines in aquatic ecosystems have been caused by a suite of threats, including habitat modification, fragmentation and destruction, overfishing, pollution, and climate change (Strayer and Dudgeon, 2010; WWF, 2018).
Effective conservation and management of wetlands biodiversity require data on species status and threats to inform decision-making and adaptive management. However, there are key challenges in Africa around the availability, usability and quality of biodiversity data, willingness to use data, and capacity (Stephenson et al., 2017a). As a result, many decision makers do not have access to the data they need.
We review the challenges of monitoring aquatic biodiversity in Africa, using examples from Ramsar sites and other wetlands across the continent, and propose solutions to help information users access high quality data in the right format at the right time. We assess the relevance of traditional monitoring methods, as well as innovative new tools.
The Need for Biodiversity Data for Wetlands Management
Information on the state of the environment is necessary for informed decision-making at multiple levels across multiple sectors. For example, numerous government decisions relating to wetlands management require biodiversity data, from the development of environmental resource policies and legislation to national and landscape level planning and budgeting for resource management across sectors to the control and licensing of resource use. Biodiversity data are also required for reporting on delivery of multilateral environmental agreements (MEAs) such as the Convention on Biological Diversity (CBD), the Ramsar Convention, the Convention on Migratory Species (CMS), and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) (Stephenson et al., 2017a). The need for biodiversity data for assessing water-related ecosystem services and for decision-making and policy development has also been highlighted (e.g., Russi et al., 2013; Camacho-Valdez et al., 2014).
Across biomes, there is growing demand for more evidence-based conservation, with data informing decisions and evaluating performance (e.g., Segan et al., 2011; Stephenson et al., 2015a). Data needed include: species status (presence, abundance, range), offtake, trade, and threat status; habitat cover (e.g., wetlands extent) and distribution; protected area (PA) coverage and management effectiveness. For wetland sites, additional biome-specific data are often useful for management, such as water depth (Ntiamoa-Baidu et al., 1998) and the abundance and diversity of migratory waders (Ntiamoa-Baidu, 1991a), benthic invertebrates (Basset et al., 2004), and invasive mollusks (Appleton, 2003). However, the demand for data is not always being met and huge gaps remain (Revenga et al., 2005).
Experiences and Challenges of Monitoring Wetlands in Africa
Here we present a review of the experiences and challenges identified with specific wetland monitoring around Ramsar sites and threatened species.
Ramsar Sites in Africa
Fifty African countries are contracting parties of the Ramsar Convention, leaving only four countries (Angola, Eritrea, Ethiopia, and Somalia) that are not signatories of the convention., There are 259 inland wetland sites across Africa, representing 94,777,978 ha of land area, roughly equivalent to the size of the United Republic of Tanzania, or 23 times the size of Switzerland. However, the extent of wetlands varies across Africa: in more than half of the African contracting parties to the Ramsar convention (27), Ramsar sites make up 1% or less of the total national land area. In sixteen countries, Ramsar sites cover 2–9% of the total landmass. The total area of Ramsar sites of six countries cover between 10 and 40% of the each country's total area, these being: Botswana (10%), Chad (10%), Benin (10%), Togo (11), Guinea (22%), and the Republic of the Congo (40%) (Figure 1).
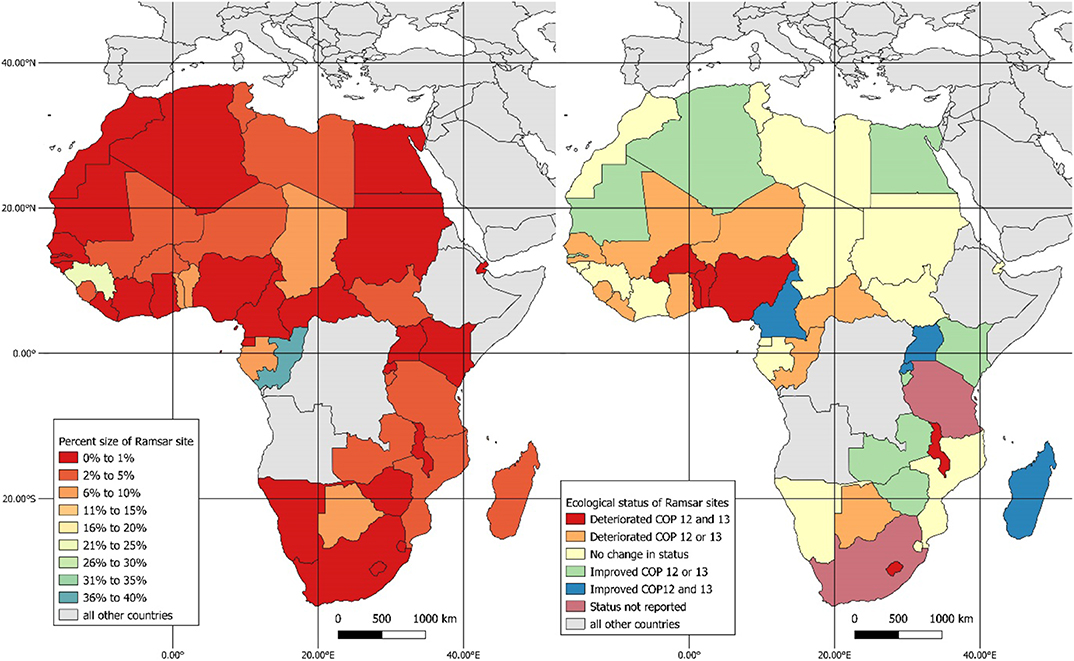
Figure 1. Relative (percentage) size of inland Ramsar wetlands to country size (Left). The change in ecological status of inland Ramsar wetland sites reported from COP 12 and COP13 (Right). Countries where the status was not reported in both reporting periods are shown. For countries that reported in one of the two COPs, that value was included in calculating the change between reporting periods. The change between reporting periods was calculated as COP 12 plus COP 13 divided by two. Maps were produced using QGIS version 3.8 (QGIS Development Team, 2019).
Contracting parties (CPs) must report on the status of Ramsar sites every 3 years. However, not all CPs do so, which makes it difficult to track changes over time, on whether sites have improved or stayed the same or deteriorated. Of the 50 African contracting parties, 44 parties responded with information on status of the Ramsar sites at the COP12 (12th Meeting of the Conference of the Parties, in Uruguay, 2015). The majority of CPs reported no change (17), with slightly more reporting deterioration (14) than improvement (13). Similar to COP12, 44 of 50 African parties responded with information on status of the Ramsar sites at the COP13 (13th Meeting of the Conference of the Parties, in Dubai, 2018). Of these, 11 reported deteriorating condition, 25 reported no change, and 8 reported improving wetland conditions. Overall, between the COP12 and COP 13, most CPs reported no change (17), while 16 reported deterioration and 15 improvement. Of the sites that had deteriorated, almost half (7) deteriorated in consecutive years of reporting. Only four CPs reported sites improving in consecutive reporting periods: Cameroon, Madagascar, Rwanda, and Uganda (Figure 1). Overall, there appears to be a trend toward ecological deterioration of sites over time.
Ramsar Sites in Africa: Ghana as a Case Study
Ghana offers some insights into how biodiversity data are used in wetland conservation (Stephenson et al., in press). In response to a notable decline in abundance of roseate terns (Sterna dougallii) in the 1980s, an inventory of wetlands and water birds was conducted along the Ghanaian coast (Ntiamoa-Baidu and Hepburn, 1988). The survey covered around 50 lagoons, estuaries, marshlands, flood plains, and salt pans and was followed by monthly surveys on selected sites. The survey data were used to identify priority sites for water birds, marine turtles and fish and a range of threats from extensive human exploitation of wetland resources (Ntiamoa-Baidu and Hepburn, 1988; Ntiamoa-Baidu, 1991b; Koranteng et al., 2000).
In turn, the bird counts provided strong justification for designating internationally important wetlands under the Ramsar Convention (Ntiamoa-Baidu and Gordon, 1991) and, in 1992, Ghana designated five coastal wetland Ramsar sites (Keta, Songor, Sakumo, Densu Delta, and Muni-Pomadze). The Ramsar designation requires monitoring of the changes in the ecological character of the site and a monitoring protocol was designed and advocated for the sites. However, the protocol required significant resourcing which was not forthcoming.
In spite of the capacity challenges, counts of water birds have continued for over three decades, initially by the local NGO, Ghana Wildlife Society, and subsequently by the Centre for African Wetlands, University of Ghana. The counts show declining populations of certain species and a reduction in the international importance of some sites. Evidence of habitat loss from encroachment by human settlement, industrial development and erosion, as well as decreases in habitat quality from pollution, has been documented through single-site studies (e.g., Osei et al., 2010; Appeaning Addo and Adeyemi, 2013). However, there are no data to feed into management interventions addressing the declining ecological status of the sites. The lack of a comprehensive long-term monitoring program, compounded by the lack of resources to manage the sites effectively and the ongoing negative impacts of climate change on coastal ecosystems, pose tangible threats to the existence of Ghana's coastal Ramsar sites.
The Ghana case is one of several examples where monitoring of birds has resulted in the creation of more protected areas and better conservation of water birds (Nagy et al., 2015). However, this study underlines trends seen elsewhere in Africa (Stephenson et al., in press), where data are often used to designate protected areas but then lack of capacity means ongoing monitoring—so vital for effective management—is lacking. While Ramsar sites are sometimes monitored through satellite-based remote-sensing to track the extent of the wetland (e.g., Dixon et al., 2016), there is a paucity of quantitative information on species and habitats and the threats they face.
Aquatic Species Diversity and Importance
An estimated 126,000 freshwater species have been described worldwide, which represents 9.5% of all described species (Balian et al., 2008). Considering that only 0.01% of the earth's surface is taken up by freshwater, aquatic ecosystems harbor a disproportionately large amount of the planet's genetic diversity. About 60.4% of described freshwater taxa are insects, 14.5% vertebrates, 10% crustaceans, 5% arachnids, and 4% mollusks; the remaining major taxa are rotifers (1.6%), annelids (1.4%), nematodes (1.4%), and platyhelminths (1%) (Balian et al., 2008).
The above proportions show that the vast majority of described freshwater taxa (85.5%) are invertebrates. These taxa are far more diverse and phylogenetically separated than the vertebrates. Being small and numerous, they are not as well-studied, and only recently have some taxa gained recognition for their conservation importance, especially aquatic insects (dragonflies), crustaceans, and mollusks. Freshwater invertebrates live nearly everywhere there is surface or groundwater, being absent perhaps only from highly polluted surface waters and deep underground groundwaters. Densities of freshwater invertebrates can be up to 106/m2 in sediments and 106/m3 in open waters (Wetzel, 2001).
Invertebrates are functionally vital to aquatic ecosystems and can indirectly or directly affect human health and well-being. As Strayer (2006) summarizes, “invertebrates regulate rates of primary production, decomposition, water clarity, thermal stratification, and nutrient cycling in lakes, streams and rivers.” Aquatic invertebrates are the primary food source for most fish species, and many vertebrate species that live in or around the water. Some species, particularly in the Mollusca and Decapoda, are harvested from the wild or farmed, supporting regionally important fisheries. Finally, some are intermediate hosts or vectors of disease, such as malaria, schistosomiasis, and river blindness, to name a few.
Dragonflies in Southern Africa
Invertebrates are rarely monitored. Standardized monitoring protocols are rare, and data are lacking on the abundance of species and changes in space and time (Cardoso et al., 2011). The situation is especially acute in freshwater systems in Africa (Stephenson et al., in press). However, some efforts are underway to monitor invertebrates and even use some insects—especially mayflies (Ephemeroptera), caddisflies (Trichoptera), stoneflies (Plecoptera), and dragonflies (Odonata)—as indicators of broader wetland health. In southern Africa, a rapid bioassessment method for rivers uses aquatic invertebrates as indicators of ecological health (Dickens and Graham, 2002). This works well, as the relative diversity of taxa is low, and there are taxonomic keys available and sufficient capacity to train technicians. The Dragonfly Biotic Index, developed in South Africa (Simaika and Samways, 2008, 2011), based on the use of adult dragonflies, has been adapted for use in rivers and wetlands throughout Africa (Vorster et al., 2020). The success of this method is largely due to the relatively low diversity of dragonflies compared with other insect taxa, their large size, their well-resolved taxonomy, public interest in these insects, and the burgeoning numbers of freshwater assessment handbooks (Samways and Simaika, 2016), field guides (Tarboton and Tarboton, 2015), and taxonomic texts (Dijkstra and Clausnitzer, 2014). The Dragonfly Biotic Index has been used to inform the ecological status of rivers (Diedericks et al., 2013), to assess the restoration of rivers (Samways et al., 2011) and succession in wetland habitats (Harabiš et al., 2013) and is therefore a model for how invertebrate data can be used for monitoring and decision-making for wetlands.
Aquatic Mammals
Small mammals are largely overlooked in biodiversity monitoring programs across Africa and Madagascar, and basic data are lacking on species abundance and distribution (Stephenson et al., in press), especially for aquatic species (Kennerley et al., 2018; Stephenson et al., 2018, 2019b). Otter shrews (a family of aquatic small mammals closely allied to the tenrecs of Madagascar) are a good example of an African taxon that is overlooked. Otter shrews inhabit waterways in the forests of Central and West Africa. They are known to feed on aquatic invertebrates, fish and amphibians yet their ecology, abundance and distribution are poorly understood (Stephenson et al., 2018).
The Nimba otter shrew (Micropotamogale lamottei) is endemic to a small, mountainous region of West Africa where it inhabits streams in an area <15,000 km2 (Stephenson et al., 2018). In spite of clearly identified threats to the species from mining (causing habitat loss and siltation) and fishing (where animals are killed in fish traps), and the importance of threat monitoring for the success of mammal conservation projects (Crees et al., 2016), there are no available data on the rate of habitat loss and the impact of hunting on this species. Its conservation status in the IUCN Red List of Threatened Species changes regularly (Stephenson et al., 2018) reflecting how scant data make it hard to assess the status of the species and plan conservation action. Therefore, further research is required to assess the distribution, abundance, habitat needs and threats of this threatened species to determine any additional conservation actions that might be needed.
Other African mammals inhabiting rivers, lagoons, and wetlands are also relatively poorly known and data deficient when compared with terrestrial mammals, examples including the African manatee Trichechus senegalensis (Keith Diagne, 2015), and the aquatic tenrec Microgale mergulus (Stephenson et al., 2019b). A training workshop organized by Wetlands International in 2010 pulled together available information on the West African manatees in Mauritania, Senegal, The Gambia, Guinea-Bissau, Guinea, and Sierra Leone and outlined threats and needed conservation action. However, information on population status is scanty and dated, and there is currently no systematic monitoring in place. The population status and trends of the African clawless otter (Aonyx capensis) are unknown, in spite of its continent-wide distribution (Jacques et al., 2015).
Africa and Asia are the most understudied regions for conservation research (Velasco et al., 2015). Therefore, dedicated research projects targeting key information gaps are essential. Since biodiversity monitoring in Africa is unlikely to ever focus primarily on small mammals, it may be prudent to integrate small mammal monitoring into schemes focused on larger, more charismatic species (Stephenson et al., 2019b).
Summary of Challenges
In spite of the importance of wetlands, monitoring of habitats and species is limited. If any monitoring occurs, it is usually in relation to the extent of wetlands or bird populations. These problems reflect a broader issue across Africa where challenges block access to, and use of, biodiversity data.
Barriers to using biodiversity information in decision-making in Africa include lack of availability of data, poor quality and usability of data, limited political will among key actors to collect and share data, a lack of capacity and limited resources for biodiversity research (Stephenson et al., 2017a, in press). Only 10% of recent conservation science studies were carried out in Africa, reflecting the fact that research is poorly aligned with biodiversity distribution and conservation priorities (Di Marco et al., 2017). As a result, many global data sets have taxonomic, temporal, and geographic gaps in coverage (Stephenson et al., 2015a; McRae et al., 2017). Monitoring of freshwater habitats is also hindered by resource and logistical implications and lack of data sharing (Turak et al., 2017; Hill et al., 2018). As a result, data sets on wetlands biodiversity are rarely available and often inadequate, hindering conservation planning (Van Deventer et al., 2016). Many African countries regularly census wildlife populations, yet the survey data are rarely analyzed and presented in a format that could be of direct use to decision makers (Bubb et al., 2011). In some cases, data presentation and use are influenced by a donor placing conditions on sharing.
Capacity for biological research is particularly challenging in Africa (e.g., Yevide et al., 2016; Cresswell, 2017). Integrated approaches to water and wetlands management are key, since many water issues in Africa are linked to food and energy issues (Simpson et al., 2019). However, the implementation of integrated water resource management has been difficult in parts of Africa, mostly due to a lack of experience, capacity, and resources (Claassen, 2013). Limited capacity and expertise for data sharing and use are often compounded not only by more limited resources to pay for raw images and/or data processing, but by limited internet capacity (Roy et al., 2010; Stephenson et al., in press). Many of the recent assessments of African biodiversity data have been led and conducted by scientists who are predominantly based outside the region (Beresford et al., 2013; Waeber et al., 2016). These trends reflect the fact that most of the global data sets, and most of the scientists with access and capacity to analyze them, are housed in Europe or North America. Many local communities use indigenous knowledge to make local decisions on farming and resource use (e.g., Mapfumo et al., 2016), yet this capacity is rarely tapped for more formal decision-making processes in Africa.
Solutions
In order to tackle the challenges with monitoring wetland biodiversity in Africa, we propose that governments, civil society, the private sector, and wetland scientists should enhance data collection by applying common, policy-relevant indicators, scaling up the application of traditional and appropriate new tools and protocols, building capacity in key institutions, and using partnerships and credible science-policy interfaces (Table 1).
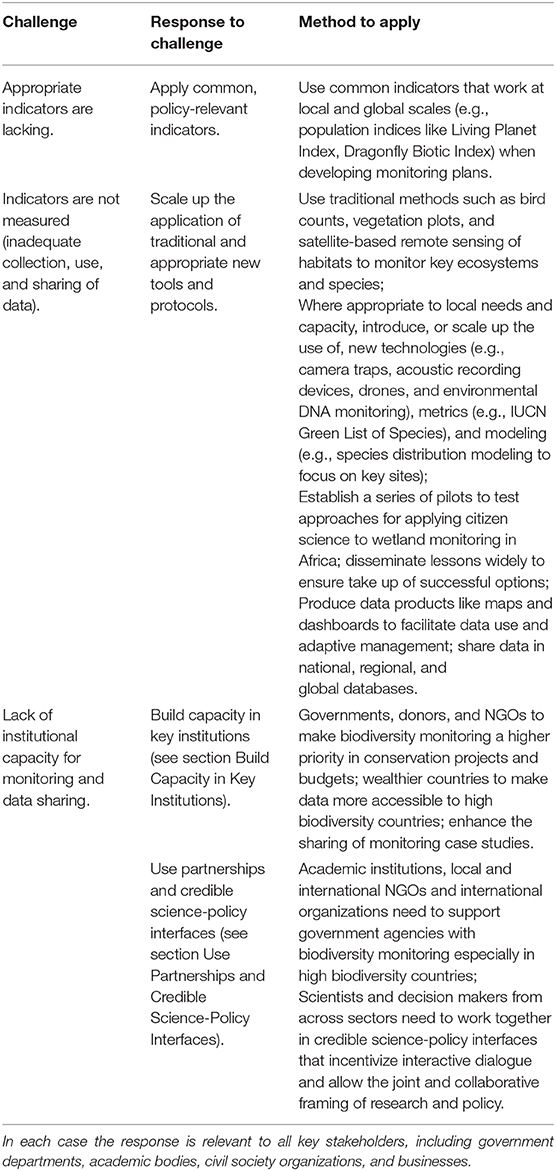
Table 1. Summary of responses needed to tackle the challenges with wetlands biodiversity monitoring in Africa.
Apply Common, Policy-Relevant Indicators
Government departments, academic institutions, civil society organizations, and businesses monitoring biodiversity should use the same core indicators across sites to facilitate the aggregation of results at national level, which enhances data sharing and allows managers to compare sites and also link results to higher-level global policy goals, such as the Aichi Targets and Sustainable Development Goals (Stephenson et al., 2015a; Stephenson, 2019a,b).
Species abundance is still one of the most pertinent indicators for conservation (Gotelli and Colwell, 2001), and population trends should be monitored in key species. Tools available include, for example, the Living Planet Index for freshwater species (Collen et al., 2009), the wetlands extent trends index (Dixon et al., 2016), water bird counts (Sayoud et al., 2017), and the Dragonfly Biotic Index (Simaika and Samways, 2008). Sampling methods should always reflect the monitoring question being asked (Radinger et al., 2019). Wetland habitats should also be monitored with indicators such as environmental flows (King et al., 2015) and water quality (see e.g., Wronski et al., 2015). Protected area coverage and management effectiveness measurements (e.g., Knights et al., 2014; UNEP-WCMC, 2018) will provide managers with data on the level of protection offered to key wetland habitats. Threats that require research and monitoring include pollution (e.g., Olujimi et al., 2010) and invasive species (e.g., Villamagna and Murphy, 2010). In wetlands that are relied on by people for ecosystem services, other relevant indicators will be required, such as fisheries catch data (Bartley et al., 2015).
Scale Up the Application of Traditional and Appropriate New Tools and Protocols
“Observations of species and threats are most valuable when generated from systematic protocols so that data can be collected in common formats, shared, and scaled up” (Stephenson, 2019a).
Traditional Methods
Several traditional methods are working well in Africa and need to be scaled up by site managers. Where biodiversity monitoring exists at wetland sites of conservation interest it is often focused on water birds. The International Waterbird Census co-ordinated by Wetlands International (Delany, 2005) has been promoting regular surveys for many years, and bird counts offer insights into broader wetland health. Water quality measurements and benthos sampling for invertebrates have been done as single site, fixed time period studies. In terms of habitat monitoring, satellite-based remote sensing has been used to track wetlands extent and habitat over time for many years (e.g., Ozesmi and Bauer, 2002; Rebelo et al., 2018) and, although it involves technology, it can be considered a traditional technique. The tool has been used to monitor wetlands across Africa from the Niger Delta in Nigeria (Ayanlade and Proske, 2016) and Lake Bam in Burkina Faso (Moser et al., 2016) to Lake Victoria (e.g., Fuller et al., 1998) and the Akagera wetland complex (Ndayisaba et al., 2017) of East Africa. Ground truthing with vegetation plots is often useful (Ndayisaba et al., 2017; Seki et al., 2018).
Modern Methods
Satellite-based remote sensing technology is being complemented by a new generation of Earth-based sensors including camera traps (Rovero and Zimmermann, 2016), acoustic recording devices (e.g., Alvarez-Berríos et al., 2016) and unmanned aerial vehicles or drones (Wich and Koh, 2018). These sensors can enhance the quality and volume of monitoring data, reduce the fieldwork involved in data collection and, if used in systematic ways (e.g., Beaudrot et al., 2016), help fill data gaps in high biodiversity tropical countries (McRae et al., 2017). Successful uses of such devices in Africa include the use of camera traps in monitoring cryptic waterbird species (Colyn et al., 2017) and acoustic monitoring of frogs (Measey et al., 2017). Environmental DNA monitoring is another evolving technique, especially useful for tracking community composition in freshwater systems (e.g., Biggs et al., 2015; Valentini et al., 2016).
Cameras fitted to blimps (non-rigid airship systems; Hodgson, 2007) have been used to monitor river dolphins (e.g., Oliveira et al., 2017), and drones have been used to detect Sirenia (Martin et al., 2012; Hodgson et al., 2013) and monitor coastal habitats in MPAs (Castellanos-Galindo et al., 2019). However, technology alone cannot be used to monitor all species (Stephenson, 2019a), and in many cases more specialized methods are needed. For example, tests using environmental DNA to detect manatees suggests the technique “may be effective for population monitoring,” especially in sites where they are in low densities or difficult to spot (Hunter et al., 2018). Valentini et al. (2016) used eDNA metabarcoding techniques to detect amphibians and bony fish and found that, “when compared with traditional surveys or historical data, eDNA metabarcoding showed a much better detection probability overall.”
Other techniques that have been tested for monitoring aquatic species, including fecal DNA (Fernández-García and Cedillo, 2017) and artificial shelters (tested on desmans; González-Esteban et al., 2018), also need to be used more widely for smaller species. Techniques for lesser known species need to be integrated into standardized protocols, as has been done recently for taxa such as invertebrates and plants (e.g., van Swaay et al., 2015; Borges et al., 2018) that are often neglected.
The IUCN Green List of Species (Akçakaya et al., 2018) is a new tool to assess species recovery and conservation success that may also be relevant for many freshwater species. Early pilot testing has provided useful results for fish and amphibians, as well as aquatic mammals and birds (Stephenson et al., 2020). One advantage of the tool is that it encourages conservation planning and status monitoring across the historic indigenous range (see Stephenson et al., 2019a).
Species distribution modeling (SDM) may also be able to assist monitoring efforts by, for example predicting range shifts of species due to climate and land use change (e.g., Pauls et al., 2013) or predicting the advance of alien invasive species, and monitoring those hotspots identified in models (e.g., Bazzichetto et al., 2018). SDM has the potential to focus monitoring efforts on key sites or species, saving time and effort. WET-Health is a method developed in southern Africa for assessing the current and projected ecological condition of a wetland by measuring hydrology, geomorphology and vegetation (Kotze et al., 2018). The scope to use this tool for monitoring trends over time should be explored.
Citizen Science
Data collection requires local inputs. Equitable participation of data providers and users, including local communities, can lead to better results and sustainability (Danielsen et al., 2014). Citizen science initiatives offer an opportunity to enhance data collection and efforts should be expanded across Africa's wetlands. While citizen science schemes are most prevalent in Europe and North America, the approach is being adopted increasingly in Africa (Pocock et al., 2019). Dragonflies have been successfully monitored by school children as part of environmental education projects in Tanzania and South Africa (Clausnitzer et al., 2017). FreshWater Watch (2020) encourages people to collect data on freshwater ecosystem health and has several collection hubs in Africa. Citizen science methods developed in Europe have also been used to collect data on bird populations in Botswana, Kenya and Uganda, demonstrating that, with technical support and modest investment (c. US$ 30,000 per scheme per year), meaningful biodiversity indicators could be measured (Wotton et al., 2020). Indigenous knowledge on biodiversity can be of relevance to conservation in Africa (e.g., Sitati and Ipara, 2012; Larson et al., 2016) so more effort needs to be made to integrate local indigenous communities into citizen science schemes where appropriate.
While progress has been made with citizen science in Africa, for the approach to be scaled up, issues such as the sustainability of recruiting, training, and retaining volunteers need to be resolved (Stephenson et al., in press). In addition, data generated by citizen science need to be curated in secure, neutrally governed institutional homes—the function fulfilled in South Africa by SANBI—and converted into forms of use for decision makers, such as reports, graphs, and maps (Barnard et al., 2017).
Citizen science may be less expensive than traditional science and may offer opportunities to monitor less charismatic species, but it also requires resources to support relevant associations, online toolkits, and network portals (Chandler et al., 2017). Establishing monitoring systems, particularly in Africa, generally tend to require more investment at the outset to support training and awareness creation and to pay for equipment and materials (Bennun et al., 2005). Therefore, more pilot projects should be established by site managers to build on existing case studies to test the validity, scope, and suitable approaches for using citizen science for monitoring African wetlands. Lessons learned should be published and disseminated widely to ensure the take up of successful options.
Use and Share Data
The different individuals and organizations monitoring biodiversity need to ensure the data they collect are used for decision-making and adaptive management. Data-derived products such as maps and dashboards can simplify information and make it easier for decision makers to analyze and interpret, ultimately facilitating data use for adaptive management (Han et al., 2014; Stephenson et al., 2015a; Nel et al., 2016). “The focus should be on ensuring simplicity and on open access to underlying data and methodologies to encourage transparency and easy replication” (Stephenson et al., 2017a). Data should also be fed into relevant management systems and discussed regularly to facilitate action and lesson learning.
Data also need to be shared as widely as possible to enhance national, regional, and global data sets. Several global databases are of use to national decision makers in planning and monitoring (see https://www.speciesmonitoring.org/data--databases.html). Databases especially relevant to sharing wetlands data include:
• Aquastat (FAO; http://www.fao.org/nr/water/aquastat/data/query/index.html?lang=en)
• Global Environmental Flow Information System (International Water Management Institute; http://gef.iwmi.org/)
• International Waterbird Census Database (Wetlands International; http://wpe.wetlands.org/)
• Water Information Network System—IHP-WINS (UNESCO; http://ihp-wins.unesco.org/)
• Water Quality Index for Biodiversity (United Nation's Environment Programme's) Global Environment Monitoring System for Water (GEMS/Water; https://www.bipindicators.net/indicators/water-quality-index-for-biodiversity)
• Water-related Ecosystems (UN Environment; https://www.sdg661.app/)
• Wetland Extent Trends (WET) Index database (UNEP-WCMC; https://www.bipindicators.net/indicators/wetland-extent-trends-index).
There are also a number of biodiversity databases focused on Africa, such as FishBase for Africa (http://www.fishbaseforafrica.org/) and the ARCOS (Albertine Rift Conservation Society) Biodiversity Management Information System (http://arbmis.arcosnetwork.org/), which has data on African Great Lakes. These databases can supplement data collated by national biodiversity centers (e.g., Egypt's National Biodiversity Unit, South Africa's SANBI, Uganda's National Biodiversity Data Bank).
Build Capacity in Key Institutions
Capacity building for monitoring in relevant national institutions is essential (Stephenson et al., 2015a,b) and needs to become a higher priority in conservation projects and budgets for governments, donors, and NGOs. Bubb et al. (2011) demonstrated that, in most east and southern African countries, at least some biodiversity indicators of national relevance can be produced from existing data. In South Africa, biodiversity measures are tracked by the SANBI information system (Huntley, 2014); similar institutional structures may be useful in other African countries.
Capacity issues are often linked to resources, but “not all knowledge needs for wetland management and policy-making require cost-intensive and sophisticated monitoring” (Ramsar Convention on Wetlands, 2018). Initiating integrated biodiversity monitoring programs in sub-Saharan Africa could require as little as US$ 30–50,000 per country per year (Pereira et al., 2010; Wotton et al., 2020), and taxa for which monitoring capacity exists could be prioritized.
Wealthier countries and large conservation organizations should support African governments in realizing their data collection needs and make existing data more available. An example of such support is provided by the GlobWetland Africa Project (http://globwetland-africa.org/), a European Space Agency led initiative with the Africa team of the Ramsar Secretariat. The project will “help African authorities to make the best use of satellite-based information on wetland extent and condition for better measuring the ecological state of wetlands and hence their capacity to support biodiversity and provide ecosystem services to human communities” (Gardner et al., 2015).
Capacity building would be further enhanced if the conservation community would learn and adapt, and “document and share examples of monitoring, with case studies of what works well and less well” (Stephenson et al., 2015b).
Use Partnerships and Credible Science-Policy Interfaces
Partnerships facilitating improved co-ordination and collaboration are key for the improvement of biodiversity monitoring (Secades et al., 2014; Stephenson et al., 2017a; Vimal, 2017). Academic institutions, local and international NGOs and international organizations have a significant role to play in supporting government agencies and it is encouraging to see that several global efforts to improve biodiversity monitoring explicitly target high biodiversity countries (Stephenson et al., 2017b; Stephenson, 2018). Some of the large biodiversity databases could be useful tools for businesses throughout project planning and implementation (see Bennun et al., 2018), so the private sector could in turn share data relevant to resource-strapped governments.
Scientists and decision makers from across sectors need to work together in credible science-policy interfaces that incentivize interactive dialogue and allow the joint and collaborative framing of research and policy (Young et al., 2014). In turn, scientists and other data collectors need to understand decision makers' priorities and information requirements and co-develop relevant tools and information products (Cowling et al., 2008). Stephenson et al. (2017a) recommend that African governments, NGOs and academic bodies test different science-policy interfaces in pilot countries or regions to see what works best, building on existing methods and support systems (e.g., Dicks et al., 2014). Existing examples of platforms, networks and partnerships to build on for African wetlands might include the AfriBES network of scientific and technical information for Africa (which focuses on south-south collaboration) and the African-Eurasian Waterbird Monitoring Partnership coordinated by Wetlands International, as well as the Ramsar-led Global Wetlands Observing System which is being piloted in Africa by the aforementioned GlobWetland Africa Project.
There may not be one common solution across Africa for improving partnerships for biodiversity monitoring. Government ministries may facilitate and build structures for dialogue around data in some countries; in others, MEA secretariats or NGOs may facilitate national-level dialogues of actors from different sectors and help mobilize resources for their functioning (such as the Nairobi Convention and its science-policy platform for regional marine environmental issues). The Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) could play a role, as could communities of practice, such as the NBSAP Forum (http://nbsapforum.net/) and Biodiversity Observation Networks (Wetzel et al., 2015).
Conclusions
Wetlands are vital for nature conservation and human well-being, yet our knowledge is limited on the state of biodiversity and its threats. In order to enhance monitoring, governments, civil society, academia and the private sector can enhance data collection in several ways by: (a) applying common, policy-relevant indicators; (b) scaling up the application of traditional and appropriate new tools and protocols; (c) building capacity in key institutions; and (d) using partnerships and credible science-policy interfaces.
Looking to the future, the SDGs should help stimulate an increasing number of governments to use monitoring data across sectors and encourage inter-disciplinary research and collaboration (Stephenson et al., in press). In African countries where biodiversity goals have been closely aligned with governmental development priorities, such as in Namibia (especially around communal conservancies) and in South Africa, biodiversity indicators have been used more widely and outcomes have often been positive (Tallis et al., 2008; Brown et al., 2014).
Building on successful examples of wetland biodiversity monitoring across Africa will require a concerted, collaborative effort. Governments will need to be open to collaboration with other states, with NGOs and with academia, within strong, open and transparent partnerships and credible science-policy fora. Only by sharing and upscaling the solutions to data collection and use will we be able to mainstream biodiversity into decision-making and ultimately minimize biodiversity loss across African wetlands.
Author Contributions
PS and YN-B drafted the manuscript with support from JS. Spatial analyses and production of figure done by JS. All authors contributed to the final version of the manuscript.
Funding
IHE Delft Institute for Water Education covered the balance for the publication fee.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to our colleagues, including those in the IUCN SSC Species Monitoring Specialist Group and the Centre for African Wetlands, for ongoing discussions on how best to improve biodiversity monitoring for conservation in Africa and beyond. Comments from three helped improve the manuscript.
References
Akçakaya, H. R., Bennett, E. L., Brooks, T. M., Grace, M. K., Heath, A., Hedges, S., et al. (2018). Quantifying species recovery and conservation success to develop an IUCN Green List of species. Conserv. Biol. 32, 1128–1138. doi: 10.1111/cobi.13112
Alvarez-Berríos, N., Campos-Cerqueira, M., Hernández-Serna, A., Delgado, C. J. A., Román-Dañobeytia, F., and Aide, T. M. (2016). Impacts of small-scale gold mining on birds and anurans near the Tambopata Natural Reserve, Peru, assessed using passive acoustic monitoring. Trop. Conserv. Sci. 9, 832–851. doi: 10.1177/194008291600900216
Appeaning Addo, K., and Adeyemi, M. (2013). Assessing the impact of sea-level rise on a vulnerable coastal community in Accra, Ghana. Jàmbá: J. Disaster Risk Stud. 5, 1–8. doi: 10.4102/jamba.v5i1.60
Appleton, C. C. (2003). Alien and invasive fresh water Gastropoda in South Africa. Afr. J. Aquatic Sci. 28, 69–81. doi: 10.2989/16085914.2003.9626602
Ayanlade, A., and Proske, U. (2016). Assessing wetland degradation and loss of ecosystem services in the Niger Delta, Nigeria. Mar. Freshw. Res. 67, 828–836. doi: 10.1071/MF15066
Balian, E. V., Segers, H., Lévèque, C., and Martens, K. (2008). The Freshwater Animal Diversity Assessment: an overview of the results. Hydrobiologia 595, 627–637. doi: 10.1007/s10750-007-9246-3
Barnard, P., Altwegg, R., Ebrahim, I., and Underhill, L. G. (2017). Early warning systems for biodiversity in southern Africa – how much can citizen science mitigate imperfect data? Biol. Conserv. 208, 183–188. doi: 10.1016/j.biocon.2016.09.011
Bartley, D. M., De Graaf, G. J., Valbo-Jørgensen, J., and Marmulla, G. (2015). Inland capture fisheries: status and data issues. Fish. Manag. Ecol. 22, 71–77. doi: 10.1111/fme.12104
Basset, A., Sangiorgio, F., and Pinna, M. (2004). Monitoring with benthic macroinvertebrates: advantages and disadvantages of body size descriptors. Aquat. Conserv. 14, S43–S58. doi: 10.1002/aqc.649
Bazzichetto, M., Malavasi, M., Bartak, V., Acosta, A. T. R., Rocchini, D., and Carranza, M. L. (2018). Plant invasion risk: a quest for invasive species distribution modelling in managing protected areas. Ecol. Indic. 95, 311–319. doi: 10.1016/j.ecolind.2018.07.046
Beaudrot, L., Ahumada, J. A., O'Brien, T., Alvarez-Loayza, P., Boekee, K., Campos-Arceiz, A., et al. (2016). Standardized Assessment of Biodiversity Trends in Tropical Forest Protected Areas: The End Is Not in Sight. PLOS Biol. 14:e1002357. doi: 10.1371/journal.pbio.1002357
Bennun, L., Matiku, P., Mulwa, R., Mwangi, S., and Buckley, P. (2005). Monitoring Important Bird Areas in Africa: towards a sustainable and scaleable system. Biodiv. Conserv. 14, 2575–2590. doi: 10.1007/s10531-005-8389-7
Bennun, L., Regan, E. C., Bird, J., van Bochove, J. W., Katariya, V., Livingstone, S., et al. (2018). The value of the IUCN red list for business decision-making. Conserv. Lett. 11:e12353. doi: 10.1111/conl.12353
Beresford, A. E., Eshiamwata, G. W., Donald, P. F., Balmford, A., Bertzky, B., Brink, A. B., et al. (2013). Protection reduces loss of natural land-cover at sites of conservation importance across Africa. PLoS ONE 8:e65370. doi: 10.1371/journal.pone.0065370
Biggs, J., Ewald, N., Valentini, A., Gaboriaud, C., Dejean, T., Griffiths, R. A., et al. (2015). Using eDNA to develop a national citizen science-based monitoring programme for the great crested newt (Triturus cristatus). Biol. Conserv. 183, 19–28. doi: 10.1016/j.biocon.2014.11.029
Borges, P. A. V., Cardoso, P., Kreft, H., Whittaker, R. J., Fattorini, S., Emerson, B. C., et al. (2018). Global Island Monitoring Scheme (GIMS): a proposal for the long-term coordinated survey and monitoring of native island forest biota. Biodiv. Conserv. 27, 2567–2586. doi: 10.1007/s10531-018-1553-7
Brown, C., Reyers, B., Ingwall-King, L., Mapendembe, A., Nel, J., O'Farrell, P., et al. (2014). Measuring Ecosystem Services: Guidance on Developing Ecosystem Service Indicators. Cambridge: UNEP-WCMC.
Bubb, P. P., Chenery, A., Herkenrath, P., Kapos, V., Mapendembe, A., and Walpole, M. (2011). National Indicators, Monitoring and Reporting for the Strategic Plan for Biodiversity 2011–2020: A Review Of Experience and Recommendations In Support of the CBD AD Hoc Technical Expert Group (AHTEG) on Indicators For The Strategic Plan 2011-2020. Cambridge: UNEP-WCMC.
Burkhead, N. M. (2012). Extinction rates in North American freshwater fishes, 1900–2010. Bioscience 62, 798–808. doi: 10.1525/bio.2012.62.9.5
Camacho-Valdez, V., Ruiz-Luna, A., Ghermandi, A., Berlanga-Robles, C. A., and Nunes, P. A. L. D. (2014). Effects of land use changes on the ecosystem service values of coastal wetlands. Environ. Manage. 54, 852–864. doi: 10.1007/s00267-014-0332-9
Cardoso, P., Erwin, T. L., Borges, P. A. V., and New, T. R. (2011). The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 144, 2647–2655. doi: 10.1016/j.biocon.2011.07.024
Castellanos-Galindo, G. A., Casella, E., Mejía-Rentería, J. C., and Rovere, A. (2019). Habitat mapping of remote coasts: evaluating the usefulness of lightweight unmanned aerial vehicles for conservation and monitoring. Biol. Conserv. 239:108282. doi: 10.1016/j.biocon.2019.108282
Chandler, M., See, L., Copas, K., Bonde, A. M., López, B. C., Danielsen, F., et al. (2017). Contribution of citizen science towards international biodiversity monitoring. Biol. Conserv. 213, 280–294. doi: 10.1016/j.biocon.2016.09.004
Claassen, M. (2013). Integrated water resource management in South Africa. Int. J. Water Gov. 1, 323–338. doi: 10.7564/13-IJWG12
Clausnitzer, V., Simaika, J. P., Samways, M. J., and Daniel, B. A. (2017). Dragonflies as flagships for sustainable use of water resources in environmental education. Appl. Environ. Educ. Commun. 16, 196–209. doi: 10.1080/1533015X.2017.1333050
Collen, B., Loh, J., Whitmee, S., McRae, L., Amin, R., and Baillie, J. E. (2009). Monitoring change in vertebrate abundance: the living planet index. Conserv. Biol. 23, 317–327. doi: 10.1111/j.1523-1739.2008.01117.x
Colyn, R. B., Campbell, A. M., and Smit-Robinson, H. A. (2017). The application of camera trapping to assess Rallidae species richness within palustrine wetland habitat in South Africa, Ostrich 88, 235–245. doi: 10.2989/00306525.2017.1292562
Cowling, R. M., Egoh, B., Knight, A. T., O'Farrell, P. J., Reyers, B., Rouget, M., et al. (2008). An operational model for mainstreaming ecosystem services for implementation. Proc. Natl. Acad. Sci. U.S.A. 105, 9483–9488. doi: 10.1073/pnas.0706559105
Crees, J. J., Collins, A. C., Stephenson, P. J., Meredith, H. M. R., Young, R. P., Howe, C., et al. (2016). A comparative approach to assess drivers of success in mammalian conservation recovery programs. Conserv. Biol. 30, 694–705. doi: 10.1111/cobi.12652
Cresswell, W. (2017). The continuing lack of ornithological research capacity in almost all of West Africa. Ostrich 2017, 1–7. doi: 10.2989/00306525.2017.1388301
Danielsen, F., Jensen, P. M., Burgess, N. D., Altamirano, R., Alviola, P. A., Andrianandrasana, H., et al. (2014). A multicountry assessment of tropical resource monitoring by local communities. BioScience 64, 236–251. doi: 10.1093/biosci/biu001
Darwall, W. R. T., Smith, K. G., Allen, D. J., Holland, R. A., Harrison, I. J., and Brooks, E. G. E. (2011). The Diversity of Life in African Freshwaters: Under Water, Under Threat. An Analysis of the Status and Distribution of Freshwater Species throughout Mainland Africa. Cambridge; Gland, Switzerland: IUCN.
Davidson, N. C. (2014). How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshwater Res. 65, 934–941. doi: 10.1071/MF14173
Delany, S. (2005). Guidelines for Participants in the International Waterbird Census (IWC). Wageningen: Wetlands International.
Di Marco, M., Chapman, S., Althor, G., Kearney, S., Besancon, C., Butt, N., et al. (2017). Changing trends and persisting biases in three decades of conservation science. Global Ecol. Conserv. 10, 32–42. doi: 10.1016/j.gecco.2017.01.008
Dickens, C. W. S., and Graham, P. M. (2002). The South African Scoring System (SASS) version 5 rapid bioassessment method for rivers. Afr. J. Aquat. Sci. 27, 1–10. doi: 10.2989/16085914.2002.9626569
Dicks, L. V., Walsh, J. C., and Sutherland, W. J. (2014). Organising evidence for environmental management decisions: a ‘4S' hierarchy. Trends Ecol. Evol. 29, 607–613. doi: 10.1016/j.tree.2014.09.004
Diedericks, G., Simaika, J., and Roux, F. (2013). A Survey of Adult Odonata Along the Crocodile-Inkomati River Main Stem from Source to Ocean. Report to the Mpumalanga parks and tourism Agency, 56.
Dijkstra, K. D., and Clausnitzer, V. (2014). The Dragonflies and Damselflies of Eastern Africa: Handbook for all Odonata from Sudan to Zimbabwe. Tervuren: Royal Museum for Central Africa.
Dixon, M. J. R., Loh, J., Davidson, N. C., Beltrame, C., Freeman, R., and Walpole, M. (2016). Tracking global change in ecosystem area: the Wetland extent trends index. Biol. Conserv. 193, 27–35. doi: 10.1016/j.biocon.2015.10.023
Fernández-García, J. L., and Cedillo, M. D. P. V. (2017). Faecal DNA template as non-invasive tools in order to distinguish the endangered Pyrenean desman (Galemys pyrenaicus, Eulipotyphla, Talpidae) from Mediterranean water shrews (Neomys anomalus, Soricomorpha, Soricidae). Hystrix 28, 92–97. doi: 10.4404/hystrix-28.1-12307
FreshWater Watch (2020). FreshWater Watch. Avaliable online at: https://freshwaterwatch.thewaterhub.org/ (accessed March 16, 2020).
Fuller, R. M., Groom, G. B., Mugisha, S., Ipulet, P., Pomeroy, D., Katende, A., et al. (1998). The integration of field survey and remote sensing for biodiversity assessment: a case study in the tropical forests and wetlands of Sango Bay, Uganda. Biol. Conserv. 86, 379–391. doi: 10.1016/S0006-3207(98)00005-6
Gardner, R. C., Barchiesi, S., Beltrame, C., Finlayson, C. M., Galewski, T., Harrison, I., et al. (2015). State of the World's Wetlands and their Services to People: A compilation of Recent Analyses. Ramsar Briefing Note No. 7. Gland: Ramsar Convention Secretariat. doi: 10.2139/ssrn.2589447
González-Esteban, J., Esnaola, A., and Aihartza, J. (2018). A new sampling method to detect the Pyrenean desman (Galemys pyrenaicus). Hystrix 29, 190–194. doi: 10.4404/hystrix-00078-2018
Gotelli, N. J., and Colwell, R. K. (2001). Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391. doi: 10.1046/j.1461-0248.2001.00230.x
Han, X., Smyth, R. L., Young, B. E., Brooks, T. M., de Lozada, A. S., Bubb, P., et al. (2014). A biodiversity indicators dashboard: addressing challenges to monitoring progress towards the Aichi biodiversity targets using disaggregated global data. PLoS ONE 9:e112046. doi: 10.1371/journal.pone.0112046
Harabiš, F., Tichanek, F., and Tropek, R. (2013). Dragonflies of freshwater pools in lignite spoil heaps: restoration management, habitat structure and conservation value. Ecol. Eng. 55, 51–61 doi: 10.1016/j.ecoleng.2013.02.007
Hill, M. J., Hassall, C., Oertli, B., Fahrig, L., Robson, B. J., Biggs, J., et al. (2018). New policy directions for global pond conservation. Conserv. Lett. 11:e12447. doi: 10.1111/conl.12447
Hodgson, A. (2007). “BLIMP-CAM”: aerial video observations of marine animals. Mar. Tech. Soc. J. 41, 39–43. doi: 10.4031/002533207787442169
Hodgson, A., Kelly, N., and Peel, D. (2013). Unmanned aerial vehicles (UAVs) for surveying marine fauna: a dugong case study. PLoS ONE 8:e79556. doi: 10.1371/journal.pone.0079556
Hunter, M. E., Meigs-Friend, G., Ferrante, J. A., Kamla, A. T., Dorazio, R. M., Diagne, L. K., et al. (2018). Surveys of environmental DNA (eDNA): a new approach to estimate occurrence in vulnerable manatee populations. Endanger. Species Res. 35, 101–111. doi: 10.3354/esr00880
Huntley, B. J. (2014). Good news from the South: biodiversity mainstreaming – a paradigm shift in conservation? S. Afr. J. Sci. 110, 1–4. doi: 10.1590/sajs.2014/a0080
IPBES (2018). The IPBES Regional Assessment Report on Biodiversity and Ecosystem Services for Africa, eds E. Archer, L. Dziba, K. J. Mulongoy, M. A. Maoela, and M. Walters. Bonn: Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services.
Jacques, H., Reed-Smith, J., and Somers, M. J. (2015). Aonyx capensis. The IUCN Red List of Threatened Species. International Union for the Conservation of Nature (IUCN). doi: 10.2305/IUCN.UK.2015-2.RLTS.T1793A21938767.en
Keith Diagne, L. (2015). Trichechus senegalensis. The IUCN Red List of Threatened Species. International Union for the Conservation of Nature (IUCN). doi: 10.2305/IUCN.UK.2015-4.RLTS.T22104A81904980.en
Kennerley, R. J., Lacher, T. E. Jr., Mason, V., McCay, S., Roach, N., Stephenson, P. J., et al. (2018). “Conservation priorities and actions for the orders Cingulata, Pilosa, Afrosoricida, Macroscelidea, Eulipotyphla, Dermoptera and Scandentia,” in Handbook of the Mammals of the World - Volume 8. Insectivores, Sloths and Colugos, eds D. E. Wilson, and R. A. Mittermeier (Barcelona: Lynx Edicions), 15–27.
King, A. J., Gawne, B., Beesley, L., Koehn, J. D., Nielsen, D. L., and Price, A. (2015). Improving ecological response monitoring of environmental flows. Environ. Manage. 55, 991–1005. doi: 10.1007/s00267-015-0456-6
Knights, K., Cuadros, I., Zamora, C., Coad, L., Leverington, F., O'Connor, B., et al. (2014). A preliminary assessment of protected area management within the WWF ‘Coastal East Africa' priority place, eastern Africa. Parks 20, 77–88. doi: 10.2305/IUCN.CH.2014.PARKS-20-2.KK.en
Koranteng, K., Ofori-Danson, P., and Entsua-Mensah, M. (2000). Fish and fisheries of the Muni lagoon in Ghana, West Africa. Biodivers. Conserv. 9, 487–499. doi: 10.1023/A:1008903813222
Kotze, D. C., Macfarlane, D. M., and Ollis, D. J. (2018). “WET-Health, a method for rapidly assessing the ecological condition of wetlands in Southern Africa,” in Wetland and Stream Rapid Assessments: Development, Validation, and Applications, eds J. Dorney, R. Savage, R. W. Tiner and P. Adamus (London: Academic Press), 545–550.
Larson, L. R., Conway, A. L., Hernandez, S. M., and Carroll, J. P. (2016). Human-wildlife conflict, conservation attitudes, and a potential role for citizen science in Sierra Leone, Africa. Conserv. Soc. 14, 205–217. doi: 10.4103/0972-4923.191159
Mapfumo, P., Mtambanengwe, F., and Chikowo, R. (2016). Building on indigenous knowledge to strengthen the capacity of smallholder farming communities to adapt to climate change and variability in southern Africa. Clim. Dev. 8, 72–82. doi: 10.1080/17565529.2014.998604
Martin, J., Edwards, H. H., Burgess, M. A., Percival, H. F., Fagan, D. E., Gardner, B. E., et al. (2012). Estimating distribution of hidden objects with drones: from tennis balls to Manatees. PLoS ONE 7:e38882. doi: 10.1371/journal.pone.0038882
McRae, L., Deinet, S., and Freeman, R. (2017). The diversity-weighted Living Planet Index: controlling for taxonomic bias in a global biodiversity indicator. PLoS ONE 12:e0169156. doi: 10.1371/journal.pone.0169156
Measey, G. J., Stevenson, B. C., Scott, T., Altwegg, R., and Borchers, D. L. (2017). Counting chirps: acoustic monitoring of cryptic frogs. J. Appl. Ecol. 54, 894–902. doi: 10.1111/1365-2664.12810
Mitsch, W. J., and Gosselink, J. G. (2000). The value of wetlands: importance of scale and landscape setting. Ecol. Econ. 35, 25–33. doi: 10.1016/S0921-8009(00)00165-8
Moser, L., Schmitt, A., Wendleder, A., and Roth, A. (2016). Monitoring of the Lac Bam wetland extent using dual-polarized X-band SAR data. Remote Sens. 8:302. doi: 10.3390/rs8040302
Nagy, S., Flink, S., and Langendoen, T. (2015). Report on the Conservation Status of Migratory Waterbirds in the Agreement Area. 6th Edn. Avaliable online at: http://www.unep-aewa.org/sites/default/files/document/mop6_14_csr6_including%20annexes.pdf (accessed September 10, 2019).
Ndayisaba, F., Nahayo, L., Guo, H., Bao, A., Kayiranga, A., Karamage, F., et al. (2017). Mapping and monitoring the Akagera Wetland in Rwanda. Sustainability 9:174. doi: 10.3390/su9020174
Nel, J. L., Roux, D. J., Driver, A., Hill, L., Maherry, A. C., Snaddon, K., et al. (2016). Knowledge co-production and boundary work to promote implementation of conservation plans. Conserv. Biol. 30, 176–188. doi: 10.1111/cobi.12560
Ntiamoa-Baidu, Y. (1991a). Seasonal changes in the importance of coastal wetlands in Ghana for wading birds. Biol. Conserv. 57, 139–158. doi: 10.1016/0006-3207(91)90135-V
Ntiamoa-Baidu, Y. (1991b). Conservation of coastal lagoons in Ghana: the traditional approach. Landsc. Urban Plan 20, 41–46. doi: 10.1016/0169-2046(91)90089-5
Ntiamoa-Baidu, Y., and Gordon, C. (1991). Coastal Wetlands Management Plans: Report prepared for the World Bank and Environmental Protection Council, Ghana, 131.
Ntiamoa-Baidu, Y., and Hepburn, I. (1988). Wintering waders in coastal Ghana. RSPB Conserv. Rev. 2, 85–88.
Ntiamoa-Baidu, Y., Piersma, T., Wiersma, P., Poot, M., Battley, P., and Gordon, C. (1998). Water depth selection, daily feeding routines and diets of waterbirds in coastal lagoons in Ghana. Ibis 140, 89–103. doi: 10.1111/j.1474-919X.1998.tb04545.x
Okonkwo, C. N. P., Kumar, L., and Taylor, S. (2015). The Niger Delta wetland ecosystem: what threatens it and why should we protect it? Afr. J. Environ. Sci. Technol. 9, 451–463. doi: 10.5897/AJEST2014.1841
Oliveira, J. S. F., Georgiadis, G., Campello, S., Brandão, R. A., and Ciuti, S. (2017). Improving river dolphin monitoring using aerial surveys. Ecosphere 8:e01912. doi: 10.1002/ecs2.1912
Olujimi, O. O., Fatoki, O. S., Odendaal, J. P., and Okonkwo, J. O. (2010). Endocrine disrupting chemicals (phenol and phthalates) in the South African environment: a need for more monitoring. Water S. Africa 36, 671–682. doi: 10.4314/wsa.v36i5.62001
Osei, J., Nyame, F. K., Armah, T. K., Osae, S. K., Dampare, S. B., Fianko, J. R., et al. (2010). Application of multivariate analysis for identification of pollution sources in the Densu delta wetland in the vicinity of a landfill site in Ghana. J. Water Res. Prot. 2, 1020–1029. doi: 10.4236/jwarp.2010.212122
Ozesmi, S. L., and Bauer, M. E. (2002). Satellite remote sensing of wetlands. Wetlands Ecol. Manage. 10, 381–402. doi: 10.1023/A:1020908432489
Pauls, S. U., Nowak, C., Balint, M., and Pfenninger, M. (2013). The impact of global climate change on genetic diversity within populations and species. Mol. Ecol. 22, 925–946. doi: 10.1111/mec.12152
Pereira, H. M., Belnap, J., Brummitt, N., Collen, B., Ding, H., Gonzalez-Espinosa, M., et al. (2010). Global biodiversity monitoring. Front. Ecol. Environ. 8, 459–460. doi: 10.1890/10.WB.23
Pocock, M. J., Roy, H. E., August, T., Kuria, A., Barasa, F., Bett, J., et al. (2019). Developing the global potential of citizen science: assessing opportunities that benefit people, society and the environment in East Africa. J. Appl. Ecol. 56, 274–281. doi: 10.1111/1365-2664.13279
QGIS Development Team (2019). QGIS Geographic Information System. Open Source Geospatial Foundation Project. Avaliable online at: http://qgis.osgeo.org (accessed September 10, 2019).
Radinger, J., Britton, J. R., Carlson, S. M., Magurran, A. E., Alcaraz-Hernández, J. D., Almodóvar, A., et al. (2019). Effective monitoring of freshwater fish. Fish Fish. 20, 729–747. doi: 10.1111/faf.12373
Ramsar Convention on Wetlands (2018). Global Wetland Outlook: State of the World's Wetlands and their Services to People. Gland: Ramsar Convention Secretariat.
Rebelo, L. M., Finlayson, C. M., Strauch, A., Rosenqvist, A., Perennou, C., Tottrup, C., et al. (2018). The use of Earth Observation for wetland Inventory, Assessment and Monitoring: An Information Source for the Ramsar Convention on Wetlands. Ramsar Technical Report No.10. Gland: Ramsar Convention Secretariat. doi: 10.1007/978-90-481-9659-3_339
Revenga, C., Campbell, I., Abell, R., De Villiers, P., and Bryer, M. (2005). Prospects for monitoring freshwater ecosystems towards the 2010 targets. Philos. T. R. Soc. B 360, 397–413. doi: 10.1098/rstb.2004.1595
Rovero, F., and Zimmermann, F. (2016). Camera Trapping for Wildlife Research. Exeter: Pelagic Publishing.
Roy, D. P., Ju, J., Mbow, C., Frost, P., and Loveland, T. (2010). Accessing free Landsat data via the Internet: Africa's challenge. Remote Sens Lett. 1, 111–117. doi: 10.1080/01431160903486693
Russi, D., ten Brink, P., Farmer, A., Badura, T., Coates, D., Förster, J., et al. (2013). The Economics of Ecosystems and Biodiversity for Water and Wetlands. London; Brussels: Institute for European Environmental Policy; Gland: Ramsar Secretariat.
Samways, M. J., Sharratt, N. J., and Simaika, J. P. (2011). Effect of alien riparian vegetation and its removal on a highly endemic river macroinvertebrate community. Biol. Invasions 13, 1305–1324. doi: 10.1007/s10530-010-9891-8
Samways, M. J., and Simaika, J. P. (2016). Manual of Freshwater Assessment for South Africa: Dragonfly Biotic Index. Pretoria: South African National Biodiversity Institute (SANBI).
Sayoud, M. S., Salhi, H., Chalabi, B., Allali, A., Dakki, M., Qninba, A., et al. (2017). The first coordinated trans-North African mid-winter waterbird census: the contribution of the International Waterbird Census to the conservation of waterbirds and wetlands at a biogeographical level. Biol. Conserv. 206, 11–20. doi: 10.1016/j.biocon.2016.12.005
Secades, C., O'Connor, B., Brown, C., and Walpole, M. (2014). Earth Observation for Biodiversity Monitoring: A Review of Current Approaches and Future Opportunities for Tracking Progress Towards the Aichi Biodiversity Targets. CBD Technical Series No. 72. Montreal, QC: Secretariat of the Convention on Biological Diversity.
Segan, D. B., Bottrill, M. C., Baxter, P. W. J., and Possingham, H. P. (2011). Using conservation evidence to guide management. Conserv. Biol. 25, 200–202. doi: 10.1111/j.1523-1739.2010.01582.x
Seki, H. A., Shirima, D. D., Courtney Mustaphi, C. J., Marchant, R., and Munishi, P. K. (2018). The impact of land use and land cover change on biodiversity within and adjacent to Kibasira Swamp in Kilombero Valley, Tanzania. Afr. J. Ecol. 56, 518–527. doi: 10.1111/aje.12488
Simaika, J. P., and Samways, M. J. (2008). An easy-to-use index of ecological integrity for prioritizing freshwater sites and for assessing habitat quality. Biodivers. Conserv. 18, 1171–1185. doi: 10.1007/s10531-008-9484-3
Simaika, J. P., and Samways, M. J. (2011). Comparative assessment of indices of freshwater habitat conditions using different invertebrate taxon sets. Ecol. Indic. 11, 370–378. doi: 10.1016/j.ecolind.2010.06.005
Simpson, G. B., Badenhorst, J., Jewitt, G. P. W., Berchner, M., and Davies, E. (2019). Competition for land: the water-energy-food nexus and coal mining in Mpumalanga Province, South Africa. Front. Environ. Sci. 7:86. doi: 10.3389/fenvs.2019.00086
Sitati, N. W., and Ipara, H. (2012). Indigenous ecological knowledge of a human-elephant interaction in Transmara District, Kenya: implications for research and management. Adv. Anthropol. 2, 107–111 doi: 10.4236/aa.2012.23012
Stephenson, P. J. (2018). A global effort to improve species monitoring for conservation. Oryx 52, 412–413. doi: 10.1017/S0030605318000509
Stephenson, P. J. (2019a). Integrating remote sensing into wildlife monitoring for conservation. Environ. Conserv. 46:181–183. doi: 10.1017/S0376892919000092
Stephenson, P. J. (2019b). The Holy Grail of biodiversity conservation management: monitoring impact in projects and project portfolios. Perspect. Ecol. Conserv. 17, 182–192. doi: 10.1016/j.pecon.2019.11.003
Stephenson, P. J., Bakarr, M., Bowles-Newark, N., Kleinschroth, F., Mapendembe, A., and Ntiamoa-Baidu, Y. (in press). “Conservation science in Africa: mainstreaming biodiversity information into decision-making” in Closing the Knowledge-Implementation Gap in Conservation Science - Evidence Transfer Across Spatiotemporal Scales Different Stakeholders, eds C. Ferreira, C. F. C. Klütsch (New York, NY: Springer).
Stephenson, P. J., Bowles-Newark, N., Regan, E., Stanwell-Smith, D., Diagana, M., Hoft, R., et al. (2017a). Unblocking the flow of biodiversity data for decision-making in Africa. Biol. Conserv. 213, 335–340. doi: 10.1016/j.biocon.2016.09.003
Stephenson, P. J., Brooks, T. M., Butchart, S. H. M., Fegraus, E., Geller, G. N., Hoft, R., et al. (2017b). Priorities for big biodiversity data. Front. Ecol. Environ. 15, 124–125. doi: 10.1002/fee.1473
Stephenson, P. J., Burgess, N. D., Jungmann, L., Loh, J., O'Connor, S., Oldfield, T., et al. (2015a). Overcoming the challenges to conservation monitoring: integrating data from in situ reporting and global data sets to measure impact and performance. Biodiversity 16, 68–85. doi: 10.1080/14888386.2015.1070373
Stephenson, P. J., Grace, M. K., Akçakaya, H. R., Rodrigues, A. S. L., Long, B., Mallon, D. P., et al. (2019a). Defining the indigenous ranges of species to account for geographic and taxonomic variation in the history of human impacts. Conserv. Biol. 33, 1211–1213. doi: 10.1111/cobi.13400
Stephenson, P. J., Monadjem, A., and Decher, J. (2018). Uplisting a threatened small mammal: the Nimba otter-shrew of West Africa. Oryx 59:606. doi: 10.1017/S0030605318000753
Stephenson, P. J., O'Connor, S., Reidhead, W., and Loh, J. (2015b). Using biodiversity indicators for conservation. Oryx 49:396. doi: 10.1017/S0030605315000460
Stephenson, P. J., Workman, C., Grace, M. K., and Long, B. (2020). Testing the IUCN Green List of Species. Oryx 54, 10–11. doi: 10.1017/S0030605319001200
Stephenson, P. J., Soarimalala, V., Goodman, S. M., Nicoll, M. E., Andrianjakarivelo, V., Everson, K. M., et al. (2019b). Review of the status and conservation of tenrecs (Mammalia: Afrotheria: Tenrecidae). Oryx. 1–10. doi: 10.1017/S0030605318001205
Strayer, D. L. (2006). Challenges for freshwater invertebrate conservation. J. N. Am. Benthol. Soc. 25, 271–287. doi: 10.1899/0887-3593(2006)25[271:CFFIC]2.0.CO;2
Strayer, D. L., and Dudgeon, D. (2010). Freshwater biodiversity conservation: recent progress and future challenges. J. N. Am. Benthol. Soc. 29, 344–358. doi: 10.1899/08-171.1
Tallis, H., Kareiva, P., Marvier, M., and Chang, A. (2008). An ecosystem services framework to support both practical conservation and economic development. Proc. Natl. Acad. Sci. U.S.A. 105, 9457–9464. doi: 10.1073/pnas.0705797105
Tarboton, W., and Tarboton, M. (2015). Guide to the Dragonflies and Damselflies of South Africa. Struik: Random House.
Thieme, M. L., Abell, R., Stiassny, M. L. J., Skelton, P., Lehner, B., Tuegels, G. G., et al. (2005). Freshwater Ecoregions of Africa and Madagascar: A Conservation Assessment. Washington, DC: Island Press.
Turak, E., Dudgeon, D., Harrison, I. J., Freyhof, J., De Wever, A., Revenga, C., et al. (2017). “Observations of inland water biodiversity: progress, needs and priorities” in The GEO Handbook On Biodiversity Observation Networks, eds M. Walters, and R. J. Scholes (Cham: Springer), 165–186.
UNEP-WCMC IUCN, and NGS. (2018). Protected Planet Report 2018. Cambridge; Gland and Washington, DC: UNEP-WCMC, IUCN and NGS.
Valentini, A., Taberlet, P., Miaud, C., Civade, R., Herder, J., Thomsen, P. F., et al. (2016). Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 25, 929–942. doi: 10.1111/mec.13428
Van Deventer, H., Nel, J., Mbona, N., Job, N., Ewart-Smith, J., Snaddon, K., et al. (2016). Desktop classification of inland wetlands for systematic conservation planning in data-scarce countries: mapping wetland ecosystem types, disturbance indices and threatened species associations at country-wide scale. Aquat. Conserv. 26, 57–75. doi: 10.1002/aqc.2605
van Swaay, C., Regan, E., Ling, M., Bozhinovska, E., Fernandez, M., Marini-Filho, O. J., et al. (2015). Guidelines for Standardised Global Butterfly Monitoring. GEO BON Technical Series 1. Lepizig: Group on Earth Observations Biodiversity Observation Network.
Velasco, D., García-Llorente, M., Alonso, B., Dolera, A., Palomo, I., Iniesta-Arandia, I., et al. (2015). Biodiversity conservation research challenges in the 21st century: a review of publishing trends in 2000 and 2011. Environ. Sci. Policy 54, 90–96. doi: 10.1016/j.envsci.2015.06.008
Villamagna, A. M., and Murphy, B. R. (2010). Ecological and socioeconomic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biol. 55, 282–298. doi: 10.1111/j.1365-2427.2009.02294.x
Vimal, R. (2017). Monitoring for conservation in African tropical national parks: an agenda towards policy-relevant science. Biol. Conserv. 214, 27–135 doi: 10.1016/j.biocon.2017.07.014
Vörösmarty, C. J., McIntyre, P. B., Gessner, M. O., Dudgeon, D., Prusevich, A., Green, P., et al. (2010). Global threats to human water security and river biodiversity. Nature 467:555. doi: 10.1038/nature09440
Vorster, C., Samways, M. J., Simaika, J. P., Kipping, J., Clausnitzer, V., Suhling, F., et al. (2020). Development of a new continental-scale index for freshwater assessment based on dragonfly assemblages. Ecol. Indic. 109:105819. doi: 10.1016/j.ecolind.2019.105819
Waeber, P. O., Wilmé, L., Mercier, J. R., Camara, C., and Lowry, P. P. II. (2016). How effective have thirty years of internationally driven conservation and development efforts been in Madagascar? PLoS ONE 11:e0161115. doi: 10.1371/journal.pone.0161115
Wetzel, F. T., Saarenmaa, H., Regan, E., Martin, C. S., Mergen, P., Smirnova, L., et al. (2015). The roles and contributions of Biodiversity Observation Networks (BONs) in better tracking progress to 2020 biodiversity targets: a European case study. Biodiversity 16, 137–149. doi: 10.1080/14888386.2015.1075902
Wich, S. A., and Koh, L. P. (2018). Conservation Drones: Mapping and Monitoring Biodiversity. Oxford: Oxford University Press.
Wotton, S. R., Eaton, M. A., Sheehan, D., Munyekenye, F. B., Burfield, I. J., Butchart, S. H. M., et al. (2020). Developing biodiversity indicators for African birds. Oryx 54, 62–73. doi: 10.1017/S0030605317001181
Wronski, T., Dusabe, M. C., Apio, A., Hausdorf, B., and Albrecht, C. (2015). Biological assessment of water quality and biodiversity in Rwandan rivers draining into Lake Kivu. Aquatic Ecol. 49, 309–320. doi: 10.1007/s10452-015-9525-4
WWF (2018). Living Planet Report - 2018: Aiming Higher. eds M. Grooten and R. E. A. Almond. Gland: WWF.
Yevide, A. S., Wu, B., Khan, A. S., Zeng, Y., and Liu, J. (2016). Bibliometric analysis of ecosystem monitoring-related research in Africa: implications for ecological stewardship and scientific collaboration. Int. J. Sust. Dev. World Ecol. 23, 412–422. doi: 10.1080/13504509.2015.1129998
Young, J. C., Waylen, K. A., Sarkki, S., Albon, S., Bainbridge, I., Balian, E., et al. (2014). Improving the science-policy dialogue to meet the challenges of biodiversity conservation: having conversations rather than talking at one-another. Biodiv. Conserv. 23, 387–404. doi: 10.1007/s10531-013-0607-0
Keywords: capacity building, conservation, data collection, indicators, protected areas, Ramsar sites
Citation: Stephenson PJ, Ntiamoa-Baidu Y and Simaika JP (2020) The Use of Traditional and Modern Tools for Monitoring Wetlands Biodiversity in Africa: Challenges and Opportunities. Front. Environ. Sci. 8:61. doi: 10.3389/fenvs.2020.00061
Received: 10 September 2019; Accepted: 29 April 2020;
Published: 02 June 2020.
Edited by:
Hong Yang, Swiss Federal Institute of Aquatic Science and Technology, SwitzerlandReviewed by:
Renato Tavares Martins, Universidade Federal de Goiás, BrazilSharad Kumar Jain, National Institute of Hydrology (Roorkee), India
Copyright © 2020 Stephenson, Ntiamoa-Baidu and Simaika. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaa Ntiamoa-Baidu, eW5iYWlkdUB1Zy5lZHUuZ2g=
 P. J. Stephenson
P. J. Stephenson Yaa Ntiamoa-Baidu
Yaa Ntiamoa-Baidu John P. Simaika
John P. Simaika