- 1Department of Agriculture, Food, Environment and Forestry, Università di Firenze, Firenze, Italy
- 2TERRA Teaching and Research Centre, Gembloux Agro-Bio Tech, University of Liege, Gembloux, Belgium
- 3Dipartimento di Bioscienze e Territorio, Università degli Studi del Molise, Pesche, Italy
- 4Earth and Life Institute–Environmental Sciences, Université Catholique de Louvain, Louvain-la-Neuve, Belgium
- 5Département Agriculture et Milieu Naturel–Unité Fertilité des Sols et Protection des Eaux, Centre Wallon de Recherches Agronomiques, Gembloux, Belgium
There is a lack of long-term field approach investigating biochar impact on soil properties and vegetation, particularly in forest ecosystems. Relic charcoal hearths (RCHs), the result of the historical charcoal production in the forests, preserve a charcoal-enriched topsoil horizon, thus representing a suitable proxy for studying the long-term effect of biochar addition to soil. In this study, we analyzed the chemical properties of a soil as impacted by charcoal accumulation in three RCH plots in southern Wallonia (Belgium) compared to the soil outside RCHs. We further evaluated the effects of RCHs soil properties on the growth performances of silver birch and European beech as well as the leaves' nutrient concentration of the latter. RCHs soil stored much more carbon and nitrogen than the reference ones. Most of the C in RCHs derived from charcoal (70–94% of total organic carbon), which would correspond to a total input of 342 tons of biochar per hectare in these soils. Such an accumulation of charcoal still affects nutrient status of soil even after 150 years since charcoal hearths abandonment: CEC and K, Ca, Mg, Na, Mn, and Zn concentration remained higher in RCHs soil compared to the reference one. In spite of a seemingly higher fertility of RCHs soil, elemental concentrations of European beech leaves grown in RCHs did not show any significant difference compared to the reference plots, except for C and Mn concentration, higher and lower, respectively, in the leaves of European beech trees grown inside than outside RCHs. Overall, RCHs soil chemical properties were not a decisive factor in significantly improving tree growth. On the contrary, tree ring width average values of both tree species was slightly lower in RCH plots, suggesting to better investigate the potential long-term detrimental effect of a large biochar addition to soil on forest trees.
Introduction
The interest and the use of biochar, a pyrolysis derived C-rich material (namely charcoal) used as a soil amendment, increased dramatically in the last decade (Lehmann and Joseph, 2015; Novotny et al., 2015; Tan et al., 2017). In fact, biochar not only improves several soil properties, e.g., water retention capacity and cation exchange capacity (Tan et al., 2017), but it also enhances the function of soil as carbon reservoir because of its high carbon content and chemical recalcitrance to decomposition (Cheng et al., 2008). However, despite it has been reported that there was a relevant historical scientific literature on the use of “charcoal” as fertilizer dated back to Nineteenth century (Thomas and Gale, 2015), biochar as such is a relatively recent research subject and the field studies thereby usually cover short spans, i.e., a few years at most (Santín et al., 2017).
In contrast with annual crops, where a short-term experiment can monitor the complete plant cycle, field experiments of several years are intrinsically required to investigate the effect of biochar on tree growth. Previous studies on this topic generally covered a time period limited to 2 or 3 years (e.g., Pluchon et al., 2014; Glisczynski et al., 2016; Wrobel-Tobiszewska et al., 2016), which allows to investigate at most the response of tree seedlings to biochar addition or the yield of biomass in a short rotation forest plantation. Long-term field trials are therefore needed to consistently investigate the effect of biochar on perennial plant species. Moreover, the residence time of biochar in soils is much longer than uncharred organic matter, persisting for centuries or even millennia (Spokas, 2010). Therefore, it is crucial to examine the long-term fate and impact of biochar on soil properties and vegetation, particularly in forest ecosystems (Li et al., 2018), which have been rarely investigated so far (Gundale et al., 2016; Luo et al., 2016).
In that goal, soils historically enriched with charcoal have the potential to overcome the lack of long-term field experiment on biochar addition to soil. In this respect, the recent interest in biochar was inspired by the study of Amazonian dark earths, whose genesis is closely related to the historical addition of fire residues to soil (Lehmann and Joseph, 2015). Similarly, two recent reviews on biochar application to forest ecosystems included studies dealing with natural charcoal produced during wildfires and accumulated in soil (Luo et al., 2016; Li et al., 2018). However, both models show limitations since Amazonian dark earths were also amended with inorganic and organic materials other than charcoal (Glaser and Birk, 2012), whereas differences in physico-chemical properties between anthropogenically and naturally produced charcoal raise the question if they can be really deemed as interchangeable in field studies (Santín et al., 2017).
Relic or historic charcoal hearths (RCHs, sometimes also called charcoal kilns or charcoal burning platforms) represent the legacy of historic charcoal production, an activity that was particularly widespread in Europe and North America (Hirsch et al., 2017). For centuries charcoal was directly made in forests from wood piled in mounds (earth mounds) or amassed in pits (earth pits) (FAO, 1987; Schenkel et al., 1998). At the sites of these RCHs a thick and dark charcoal-enriched soil layer persists. Thus, RCH soils could be considered as a natural experimental setting, a suitable proxy for studying the long-term effect of biochar addition to soil.
The authors of several recent studies inferred the long-term implications of a large charcoal addition on soil properties from the analysis of the topsoil of RCHs (e.g., Borchard et al., 2014; Criscuoli et al., 2014; Hardy et al., 2016, 2017; Kerré et al., 2016; Mastrolonardo et al., 2018). A few studies focused on the effect of charcoal accumulation at RCHs on tree growth (Mikan and Abrams, 1995, 1996; Young et al., 1996; Carrari et al., 2018). Studies on pot experiments found that seedlings of European beech and oak were apparently not affected in terms of total biomass when growing on charcoal-enriched soil collected from RCHs (Mikan and Abrams, 1995; Carrari et al., 2016, 2018), while plants height was negatively (Mikan and Abrams, 1996) or positively affected (Carrari et al., 2018). Studies on mature trees growth inside RCHs found that tree density and coverage was lower, or even absent, compared to the surrounding vegetation (Mikan and Abrams, 1995; Young et al., 1996; Carrari et al., 2016) and that RCHs soil had a detrimental effect on yellow poplar trees' diameter growth (Mikan and Abrams, 1995). The effects of RCHs soil on trees deserve to be further investigated in order to better define the relationship between the persistence of charcoal in soil and its implications on soil properties and plants growing conditions.
The goal of this study was to evaluate the long-term effect of charcoal addition on tree growth and nutrient cycling in the soil-plant system. To meet this goal, we measured the physical and chemical properties of soil impacted by charcoal accumulation at three RCH plots in southern Wallonia (Belgium). The effect of charcoal accumulation on soil chemical properties was related to growth performances (diameter, height, and tree ring-width) and nutrient concentration in the leaves of two tree species (European beech and silver birch). Our working assumption was that the nutrients concentration in the leaves of the trees might reflect specific soil conditions at RCHs, explaining an increase or a decrease of tree growth.
Materials and Methods
Study Area
The study area is a small forest parcel 2.3 ha wide located at Regniessart, in the municipality of Viroinval in Wallonia, southern Belgium (coordinates N 50,016°E 004,583°; datum WGS84) (Figure 1). The elevation of the study area is 320 m a.s.l., mean annual temperature is 9.8°C and mean annual precipitation is 823 mm. The geological source rock is of Lochkovian age, early Devonian; locally it is comprised by the Oignies formation, consisting of an alternation of siltstones, and green shale. According to the soil map of Wallonia, the soil type is silty with a good drainage. The schist substratum generally appears <1 m deep. According to the World Reference Base for Soil Resources (IUSS Working Group WRB, 2015), the soil can be classified as Dystric Leptic Cambisol.
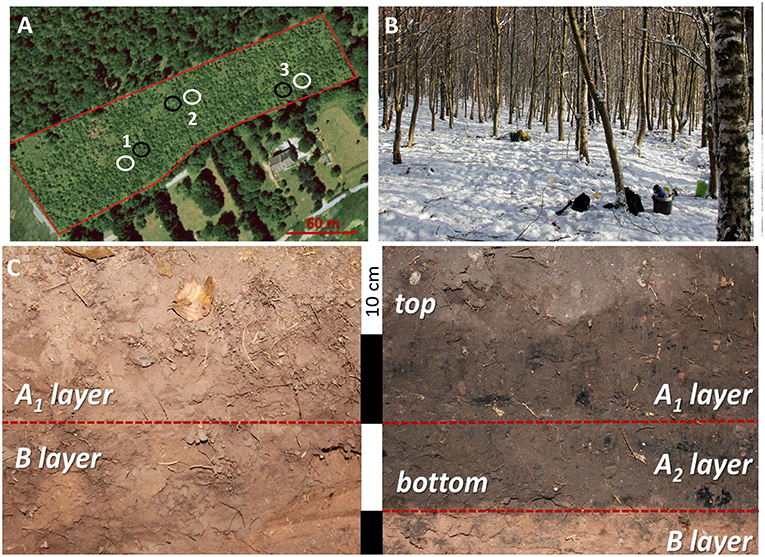
Figure 1. The study area at Regniessart, in Southern Belgium. (A) The investigated parcel how it looked at the beginning of the plantation. Black circles represent the relic charcoal hearth (RCHs) investigated and the relative reference plots. (B) The forest plantation how it looked in January 2017 during the soil sampling campaign. (C) The soil profiles from one RCH (on the right) and the relative reference plot (on the left). The layers in which the soils were divided are marked: in the reference soil the topsoil (A1 layer) and the subsoil (B layer). In the charcoal hearth soil the top and the bottom part of the charcoal-enriched layer (A1 and A2 layer, respectively) and the subsoil (B layer). The so defined soil layers do not coincide necessarily with soil horizons.
The study area is in a communal hardwood forest. In 1994 European beech (Fagus sylvatica L.) was planted (original planting pattern 2.5 × 2.5 m) and currently tree vegetation is almost half composed by silver birches (Betula pendula Roth), which were naturally established in 1997 as species typical of the pedoclimatic conditions of the area. The study area included also three RCHs, 100 meters far from each other, with a diameter comprised between 10.4 and 10.8 m, most likely sharing the same history (i.e., period of creation and use) and abandoned more than 150 years ago, like most of the RCHs in Wallonia (Hardy et al., 2016). For each RCH, a reference plot of the same diameter as the corresponding RCH was randomly established at a distance of about 20 m from the RCH. Two RCHs were well-preserved whereas one was noticeably disturbed on about a quarter of its area, apparently because of the uprooting of a tree. This portion of the hearth was not involved in any sampling of soil nor vegetation.
Soil Sampling
Soil sampling was performed in January 2017. In RCH and reference plots, the full soil depth, ranging from a minimum of 72 cm to a maximum of 109 cm, was sampled by a manual auger (dimensions of the soil sampler 15 × 4 cm) at three locations, from 1–2 meters from the center of the hearth site, along N, SW, and SE directions. Soil collected from each sampling point was then bulked according to soil depth, i.e., in topsoil and subsoil samples. In this separation we took into account also the pedogenetic horizons, so avoiding to mix the topsoil horizon with subsoil horizons, which are usually very different in terms of important soil variables like bulk density and SOM content (Palmer et al., 2002). Soil from reference plots showed an A horizon about 20 cm thick and, accordingly, this depth was selected as edge between topsoil and subsoil (A1 and B layer, respectively). This latter included a Bw1 and a Bw2 horizon. Soil of RCHs showed a uniform Auh horizon, i.e., a mineral horizon enriched in charcoal and organic matter (FAO, 2006; Hirsch et al., 2017) thicker than 20 cm. Therefore, we further divided the charcoal enriched topsoil in a top part (A1 layer), 20 cm thick, and a bottom part (A2 layer), so allowing a direct meaningful comparison between topsoil (20 cm; A1 layer) in the RCH and reference plots. Subsoil of RCHs soils (B layer) included a Ab, Bwb, and BCb horizon. In total, 27 and 18 soil samples from RCH and reference plots, respectively, were analyzed.
Soil Chemical and Physical Analysis
Soil samples collected as described above were oven dried at 40°C and gently grounded and sieved to 2 mm, isolating and weighting the fractions larger and smaller than 2 mm. The latter fraction was further analyzed as following.
Soil pH was measured potentiometrically by using a 5:1 1 M KCl solution to soil ratio, and particle size distribution was determined according to the “Robinson's pipette” sedimentation method (AFNOR, NF X31-107).
C and N concentration of soil were determined by dry combustion after having assessed the total absence of carbonates in soil samples using a 1 M HCl solution. Bioavailable elements (P, K, Mg, Ca, Na, Fe, Mn, Cu, Zn) were extracted with ammonium acetate-EDTA 1 M (pH 4.65; Lakanen and Erviö, 1971) and measured by flame atomic absorption spectrometry, with the exception of P that was determined by molybdenum-blue spectrophotometric method. Exchangeable acidity was determined percolating the samples with a not buffered 1 M KCl L−1 solution, which enables extraction of exchangeable acidity (H+ and Al3+) (Pansu and Gautheyrou, 2006). Titration was carried out by volumetry and aluminum was measured by atomic absorption spectrometry. Cation exchange capacity (CEC) was measured by percolating soil columns with 1 M ammonium acetate buffered at pH 7 (Metson, 1956). The excess of ammonium was rinsed with ethanol, ammonium was desorbed with a 1.33 M KCl solution, alkalized with NaOH 50% and then distilled. Finally, solution was titrated with HCl 0.1 M for measuring desorbed . Exchangeable cations were measured by atomic absorption spectrometry. Base saturation (BS) was calculated as the ratio between the sum of exchangeable cations (K, Mg, Ca, Na) and CEC.
Bulk density of topsoil (20 cm) of RCH and reference plots were measured by the irregular hole method (Blake and Hartge, 1986). In fact, the large presence of coarse fragments, namely charcoal, could have invalidated the calculation of bulk soil with other methods (Page-Dumroese et al., 1999). Accordingly, a pit 20 cm deep was excavated, isolated by a nylon film, and filled with sand whose volume was measured in advance by a graduate cylinder, so to measure pit's volume in the field. Finally, all the material excavated from the pits was weighted after drying at 105°C in an oven until constant weight. Bulk density of subsoil was measured just on one RCH and one reference soil digging a trench and collecting tree soil samples at different depths along the subsoil by a steel cylinder of volume 100 cm3. The samples were then weighted after drying at 105°C in an oven until constant weight.
Estimation of Charcoal-C in Soil
For calculating the macroscopic charcoal C content (hereafter called coarse charcoal-C), the charcoal fragments in the soil fraction larger than 2 mm were separated from small roots and stones by hand picking and floatation, dried at 60°C and weighted. Some charcoal particles were randomly collected from each RCH, pulverized, and mixed together, so obtaining a composite sample. This latter was used for the determination of coarse charcoal-C and-N average content by dry combustion on three replicates, after pre-treatment of samples with 6 M HCl at 80°C to eliminate carbonates (Pansu and Gautheyrou, 2006).
The estimation of the charcoal-C content in fine earth (hereafter called fine charcoal-C) was based on the findings of Hardy and Dufey (2017). They found that the Walkley-Black method for the determination of soil organic carbon content partly oxidizes fine charcoal-C, leading to an underestimation of the organic carbon content in RCHs soil. The older–and hence more degraded–is the charcoal, the higher is its degree of oxidation. Hardy and Dufey (2017) calculated the proportion of fine charcoal-C oxidized by dichromate through the Walkley-Black method in 10 RCHs of the approximate same age as the RCHs of this study and under similar soil and environmental conditions. Therefore, we calculated the proportion of charcoal not oxidized by the Walkley-Black method, basing on the data found in Table 1 in Hardy and Dufey (2017). We used only data from forest sites, nine sites in total, excluding the result obtained from a podzol, which is a peculiar soil not comparable to those at Regniessart. Thanks to the content of fine charcoal-C estimated in these soils by differential scanning calorimetry data (Hardy and Dufey, 2017; Hardy et al., 2017), we calculated a value of 0.387 ± 0.067 (mean ± sd) as the fraction of charcoal-C unoxidised by the Walkley and Black procedure. We used this mean value as a constant to determine the content of fine charcoal-C in the soils of this study according to the following equation:
where Fine Charcoal-C (g kg−1) is the fine earth C derived from charcoal, 0.387 is the proportion of fine charcoal-C not oxidized by the Walkley-Black method, as described above, TOC (g kg−1) is the total organic Carbon calculated by dry combustion and SOCWB (g kg−1) is the soil organic Carbon estimated by Walkley-Black procedure, multiplied by the empirical factor of 1.32 accounting for the incomplete oxidation of uncharred SOC (Walkley and Black, 1934).
The total organic carbon (TOC) concentration of the RCHs soil was expressed as grams of C per kg of bulk soil, hence including also the fraction larger than 2 mm so rich in charcoal particles as in Mastrolonardo et al. (2018).
Vegetation Sampling and Analysis
Dendrometric and dendrochronological surveys were performed in October 2017. Dendrometric data, diameter at breast height (DBH) and tree height of European beech (Fagus sylvatica L.), and silver birch (Betula pendula Roth) growing in RCH and reference plots were measured. In total, 15 trees of each species were considered in the reference plots, while 14 and 9 trees in the charcoal hearth plots of Fagus sylvatica and Betula pendula, respectively. Tree volume index was determined with the formula: diamater2 x height (Wilson-Kokes and Skousen, 2014).
In each plot, two woody cores from three trees of each species were collected at breast height (1.3 m) both in RCH and reference plots using a 0.5 cm diameter increment borer.
Leaves of 18 European beeches were collected in May 2017 on the same trees involved in the dendrochronological survey. A large number of leaves were pooled from various randomly-oriented shade branches producing a single sample per tree. Leaves of silver birch were not sampled because the branches were higher than 10 m and the plants did not have any low branch.
Determination of Leaf Elemental Composition
The harvested leaves were oven-dried at 40° to constant weight and then finely ground. Two grams of each sample were digested in a 50/50 mix of HNO3 (60%) and perchloric HClO4 (70%) acid for 16 h. The extract was then heated till complete evaporation. The residue was recovered with HCl (10%). After being dissolved in distilled water the samples were filtered through Whatman No. 42 filter paper and analyzed for elements quantification. A total of 18 samples (3 plants each RCH and reference plot) were analyzed by a flame atomic absorption spectrometry for K, Mn, Ca, Al, Cu, Fe, Mg, Na, and Zn concentrations. P was determined by molybdenum-blue spectrophotometric method. C and N were determined by dry combustion.
Dendrochronological Analysis
The collected tree cores were cut with a microtome to obtain plane surface to easily detect tree rings. The surface of tree cores was covered with white chalk powder to underline the vessels distribution and the ring boundaries (Gärtner and Nievergelt, 2010).
Tree-ring widths (TRW) were measured with a 0.01-mm resolution with LINTAB measurement equipment, coupled to a stereomicroscope (60x magnification; Leica, Germany) and time series analysis program TSAP Win (Frank Rinn, Heidelberg, Germany). Raw TRW chronologies of each dated tree were cross-dated statistically using COFECHA (Holmes, 1983; Grissino-Mayer, 2001). The individual chronologies were standardized in R environment and tree-growth index (TRI) chronologies of each tree and standardized mean chronologies of trees in the reference and charcoal hearth plots were obtained.
In order to remove age trends and non-climatic noise in TRW raw series and to amplify the climatic signal, individual-based detrending model was applied using a spline curve function with a 50% frequency variability cut-off at 5 years. The detrended series of the tree rings were averaged by year using a bi-weighted robust mean to develop a chronology that represented the common high-frequency variation of the individual series (Fritts, 1976; Cook, 1985).
Dendroclimatological analysis was performed using climatic data, temperature and precipitation on a monthly base as recorded by the meteorological stations of Dourbes, about 8 km far from Regniessart, for the period 1992–2016, provided by the Institut Royal Météorologique de Belgique. The climate-growth relationships between tree-ring chronologies and monthly climatic data were examined using the correlation function (CF) analysis (Fritts, 1976), which is ordered in time from the previous year (t−1) to the current year (t) of each growing season with respect to the ring formation (Biondi and Waikul, 2004). The current tree growth, namely each diameter increment, is affected by the climatic variables of the year previous the growing season (Fritts, 1976). Therefore, mean values of monthly air temperature and total precipitation are independent variables in the climate-growth relationships. Thus, temperature and precipitation were considered in the period between March of the previous year and October of the current year relative to ring formation.
Statistical Analysis
Soil data were analyzed comparing: A1 and A2 layers of RCHs; A1 layers of RCH and reference soils; B layers of RCH and reference soils. Elemental composition of European beeches' leaves from RCH and reference plots were compared as well. Differences in soil layers and leaves' elemental composition between RCH and reference soils were tested using t-test at 95% confidence level (SigmaPlot 12.0). Mann-Whitney Rank Sum Test was used for data non-parametrically distributed [not significant normality test (Shapiro-Wilk) and the equal variance test].
Descriptive statistics were used to compare key properties of chronology from each tree's core: (i) mean ring width (MRW); (ii) first order serial autocorrelation (AR1, which quantifies the temporal persistence in growth among consecutive years and measures the persistence retained before and after standardization); (iii) mean inter-series correlation (rbar, the average pairwise correlation between series), that is determined as the average correlation among all data pairs during time t, computed over their maximum period of overlap; (iv) expressed population signal (EPS, which estimates the signal strength, i.e., the climatic information in the developed chronologies). The EPS considers the inter-series correlation and the sample size and it estimates the theoretical population mean by a finite number of trees. A value of EPS > 0.85 is the threshold of statistical quality in reliable chronologies (Wigley et al., 1984). The EPS mean value was obtained for 9 years with an overlapping of 5 years in 3 time spans (1997–2006, 2002–2011, 2007–2016) and the mean was obtained as the average of all values ≥ 0.85.
Climate and tree-growth relationship was assessed using the treeclim package (Zang and Biondi, 2013) in R (R Foundation for Statistical Computing, Vienna, Austria), and a linear model was fitted to monthly temperature and summer precipitation.
Principal component analysis (PCA) were performed using IBM SPSS statistics 22. The considered variables were: total nitrogen; uncharred C; fine charcoal-C; coarse charcoal-C; pH (KCl); available P; available K; available Mg; available Na; available Ca; available Fe; available Mn; available Cu; available Zn; available Al; soil conductivity; CEC; and base saturation (BS).
Results
Soil General Physical Features
Texture of soil was quite uniform in RCH and reference plots, between loam and silt loam (Table 1). Bulk density did not differ significantly between RCHs top horizon and the reference soil despite the high content of macroscopic charcoal, a very low density material, in the RCHs soil (Table 1). On the contrary, bulk density of subsoil (B layer) seemed to be higher in the reference soils.
The content of macroscopic charcoal particles (>2 mm), a fraction often ignored in RCHs' studies (Mastrolonardo et al., 2018), showed a decreasing trend with increasing depth within RCHs soil, from 8.9% of total soil mass in the first 20 cm of soil profile (A1 layer) to 3.7% at 20–35 cm of depth (A2 layer), to 0.2% in the deep soil (>35 cm; B layer) (Table 1). Charcoal particles content was negligible in the reference soils that, conversely, contained much more stones.
Charcoal-C, Uncharred Carbon, and Nitrogen
Charcoal-C content in the fine earth was estimated according to Equation (1). In most of the samples from reference soils subtracting SOCWB from TOC resulted in a negative value, meaning that all C was oxidizable by the Walkley-Black method. In those samples, fine charcoal-C content was assumed to be zero. In the RCHs soil, conversely, we obtained a positive value by subtracting SOCWB from TOC for every sample except for two samples from the subsoil (B layer). On the other hand, on four of the samples from RCHs the estimated fine charcoal-C content exceeded the TOC content. In these latter cases we approximated the fine charcoal-C content of the samples to be equal to the highest result obtained from all samples, i.e., 98% of TOC. Overall, taking into account the relatively low accuracy of the Walkley-Black method, the selected approach seemed to be suitable for our samples, providing realistic results (Table 2).
In fact, fine charcoal-C content was low in the reference soil, on average 3.9 and 0.4 g C kg−1 fine earth for topsoil and subsoil, respectively. Conversely, fine charcoal-C content in RCHs soil was very high, even exceeding the content of uncharred C in the charcoal-enriched layer (Table 2). The bottom part of this latter, the A2 layer, seemed to be even richer in fine charcoal-C compared to the A1 layer (105.4 and 76 g C kg−1 of fine earth in the A2 and A1 layer, respectively), while it was depleted in uncharred C (7.6 and 69.5 g C kg−1 of fine earth in the A2 and A1 layer, respectively). Finally, also the B layer in RCHs soil showed an enrichment of fine charcoal-C, but not of uncharred C. As a term of comparison, RCHs soil from the nine forest sites investigated by Hardy and Dufey (2017) contained between 38 and 125 g charcoal-C kg−1 fine earth, while in an Italian forest Mastrolonardo et al. (2018) found a content of 22–45 g charcoal-C kg−1 fine earth.
Coarse charcoal-C content ranged from 23 to 34% of TOC in the bottom and the top part of the charcoal-enriched horizon (Table 2). Overall, TOC of bulk soil in RCHs, hence including both fine earth and the >2 mm fraction, was made up of 70 and 94% of charcoal-derived C in the A1 and the A2 layer, respectively.
Charcoal hearths topsoil stored twice the N content than the reference topsoil (6.7 and 3.2 g N kg−1 fine earth, respectively) (Table 2).
Soil Chemistry
Values of soil pH(KCl) at Regniessart ranged between 3.5 and 4.1 (Table 3). pH values in topsoil and subsoil between RCH and reference soils did not significantly differ.
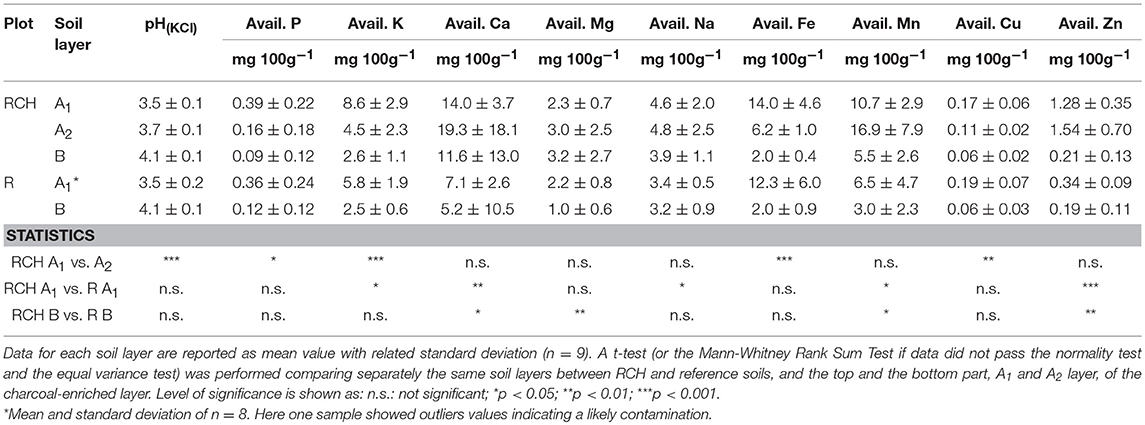
Table 3. pH and available elements of relic charcoal hearths (RCH) and reference (R) soils divided into different soil layers.
Overall, the sum of all available bases in both the topsoil and subsoil was significantly higher in terms of concentration in RCH compared to the reference soils. In particular, the concentration of bioavailable K and Na was higher in the topsoil and Mg in the subsoil, while the concentration of bioavailable Ca, Mn, and Zn was higher in the whole soil profile of RCH plots.
CEC was much higher in the RCHs topsoil while base saturation did not differ significantly compared to reference topsoil (Table 4). Exchangeable acidity was also higher in the hearth topsoil (Table 4) as well as Al cations adsorbed on CEC sites. The percentage of CEC saturated by the single base cations did not change significantly between hearth and reference soils, with the exception of Ca and Mg, whose percentage was higher in the hearth subsoil (Table 4). The percentage of CEC saturated by Mg in the topsoil, on the contrary, was higher in the reference soil.
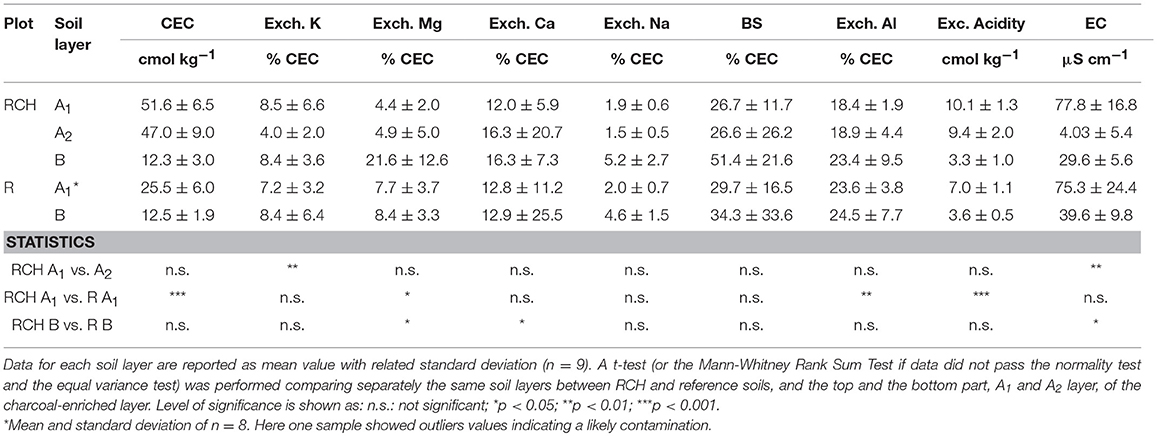
Table 4. Cation exchange capacity (CEC), exchangeable acidity and Al, base saturation (BS), exchangeable cations, and soil electrical conductivity (EC) of relic charcoal hearths (RCH), and reference (R) soils divided into different soil layers.
Comparing the top, A1 layer, and the bottom part, A2 layer, of charcoal enriched horizon the pH value was slightly but significantly higher in the A2 compared to the A1 layer. The concentration of bioavailable P, K, and Fe halved from A1 to A2 layer, whereas Cu concentration also decreased but to a lesser extent.
Principal Component Analysis of Soil Data
PCA performed combining soil variables from both RCH and reference plots highlighted that data were clustered according to the soil layer and position inside or outside RCH plots (Figure 2). Factor 1 explained 51% of total variation and was strongly (loadings > 0.8 and <−0.8) related to N, K, Fe, Cu, CEC, and Al (positively) and to pH (negatively); it mostly separated layers from different soil depth. Factor 2 explained 19% of total variation and was quite strongly positively (loadings > 0.6) related to Mg, Ca, and Mn; it separated soil from RCH and reference plots. All data about PCA are shown in the Supplementary Material.
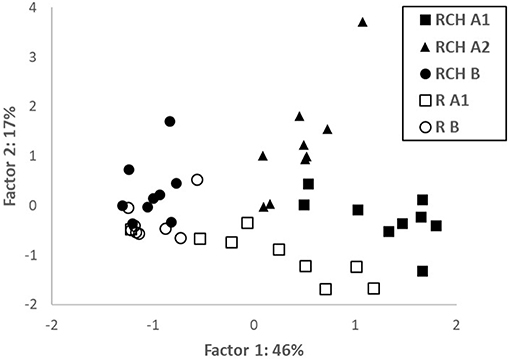
Figure 2. Score plot of principal component analysis (PCA), Factors 1 and 2, with the percentage of the total variance explained, for the variables examined relative to soil layers from relic charcoal hearths (RCHs) and reference soils (R).
Leaf Composition
The elemental composition of European beech leaves did not show significant differences in RCH and reference plots, except from Mn concentration that was significantly higher in the leaves of trees in reference than RCH plots, 1.4 against 0.8 g Mn kg−1, respectively (Figure 3). The C concentration in European beech leaves was significantly higher in RCH than reference plots, 460 and 453 g C kg−1, respectively (Figure 3). Nonetheless, stoichiometric ratios between the main elements (i.e., C, N, and P) were not statistically different between RCH and reference plots (data not shown).
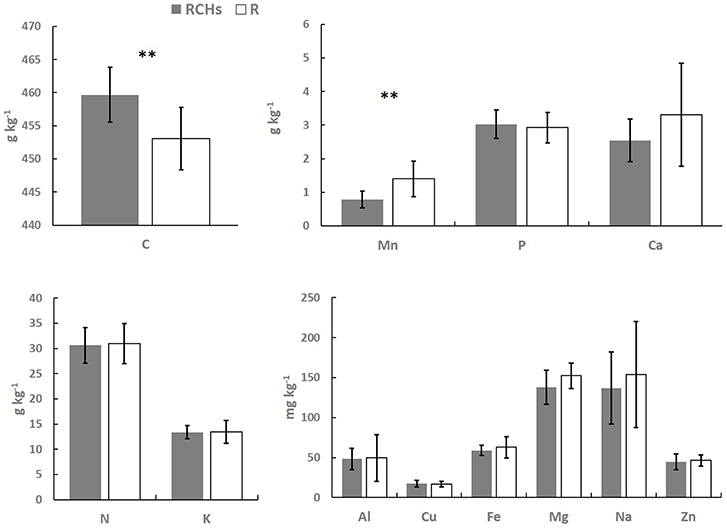
Figure 3. Elements concentration in European beech leaves (dry mass) from relic charcoal hearths (RCHs) and reference (R) plots. Bars represent mean values and error bars represent standard deviation (n = 9). Differences between nutrients concentration from trees inside and outside RCH plots were tested by t-test (**p < 0.01).
Dendrometric and Dendrochronological Analysis
DBH and height of European beech did not differ between trees grown in RCH and reference plots (Table 5). Conversely, the height of silver birch trees was significantly higher in reference than RCH plots, 17.3 and 15.9 m, respectively. However, DBH and tree volume index of silver birch did not change between RCH and reference plots.
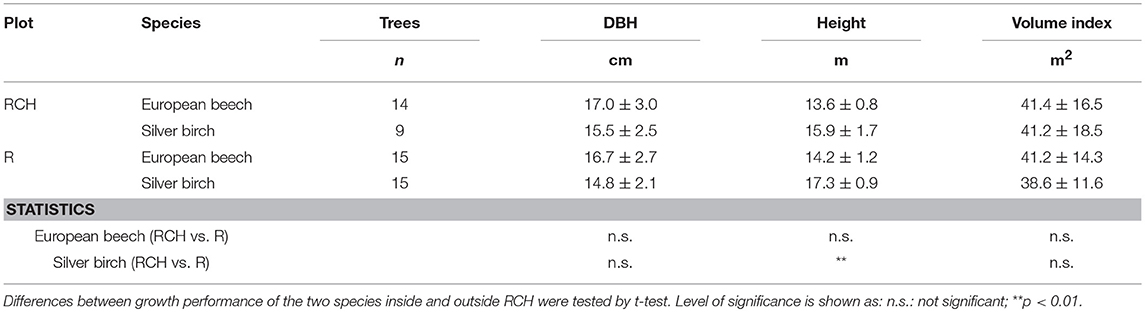
Table 5. Growth performance, expressed as diameter at breast height (DBH), height, and a volume index (diamater2 x height), of European beech and silver birch trees (Fagus sylvatica L. and Betula pendula Roth) growth in the relic charcoal hearth (RCH) and in the reference (R) plots.
The mean standardized TRW chronologies of European beech showed average values slightly lower in charcoal hearths (0.994 mm) than reference plots (0.999 mm). Mean standardized TRW chronologies of silver birch showed the same trend, but the difference was even lower, i.e., 0.002 mm (Figure 4). Values of mean inter-series correlations were lower for European beech than silver birch and for silver birch they showed similarity of the growth patterns for RCH and reference plots. The cross-dating of TRW chronologies was statistically significant (EPS values > 0.85) in each species and plot, except for European beech in RCH plots (EPS = 0.784, value close to threshold limit to assess a reasonable signal strength) (Table 6).
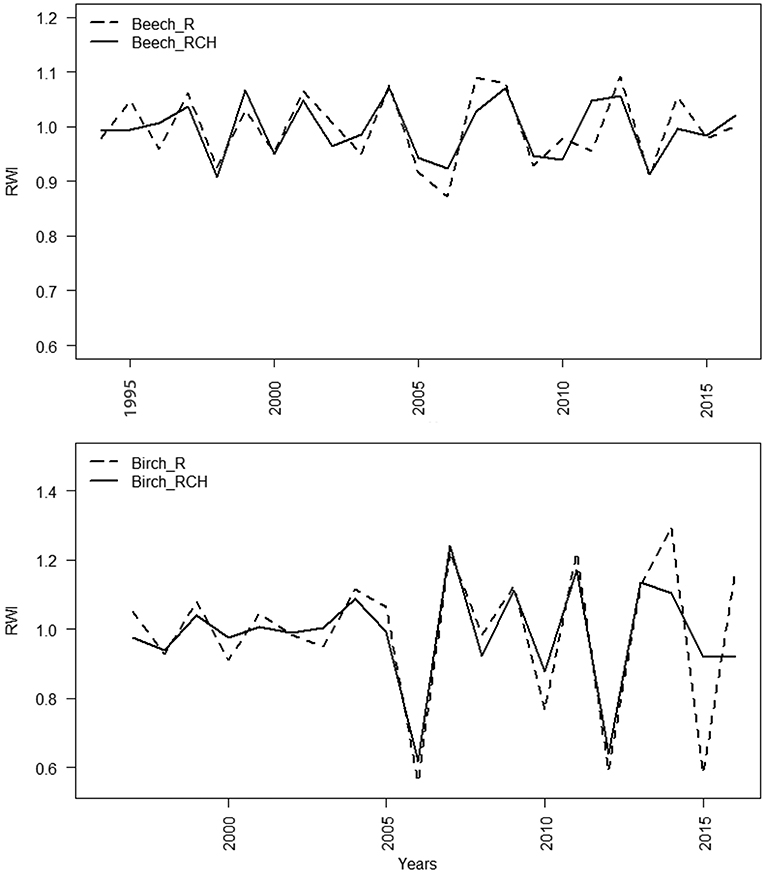
Figure 4. Mean standardized TRW chronologies in reference and RCH plots for European beech (above) and silver birch (below). The “x” axis refers to the years. Dashed lines refer to European beech and silver birch trees grown on the reference plots (R) and the solid lines on the relic charcoal hearth (RCH) plots.
Figure 5 shows mean monthly temperature and precipitation for the period 1992–2016. The mean annually precipitation was 68.9 ± 7.6 mm. The distribution of precipitation ranged from 53.5 ± 6.4 mm in April to 84.5 ± 9.0 mm in December. The mean annually temperature was 9.79 ± 0.33°C in the period 1992–2016, with low monthly values in January (2.6 ± 0.4°C) and high monthly values in July (17.7 ± 0.4°C).
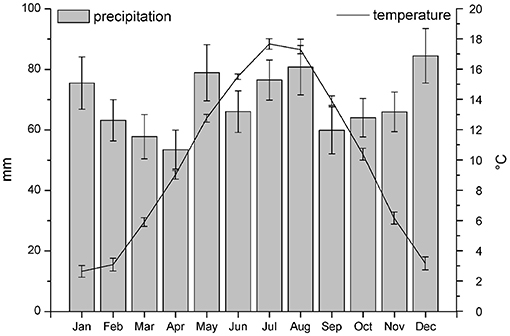
Figure 5. Mean monthly temperature and precipitation for the period 1992–2016 as recorded by the meteorological stations of Dourbes, about 8 km far from Regniessart.
The effects of temperature and precipitation on tree growth were shown by bootstrapped correlation values of mean standardized chronologies of the two species (Figure 6). The growth of European beech in the reference plots was positively correlated with precipitation of January and June of the current year, and with temperature of June of the previous year and February and March of the current year, while it was negatively correlated with temperature of June of current year. In RCH plots the growth of European beech was positively correlated to temperature in April and June of the previous year and August of the current year (Figure 6A). In reference plots the growth of silver birch was positively correlated to precipitation in March, April, October, and November of previous year, and June of the current year. It was also positively correlated with temperature of January, February, and April of current year and negatively correlated with March, April, and October of previous year. In RCH plots the growth of silver birch was positively correlated to precipitation of March, April, October, and November of previous year and negatively correlated with April and October of current year (Figure 6B). Tree growth of silver birch in RCH plots was also positively correlated to temperature of July of previous year and April of current year, while it was negatively correlated with April of previous year.
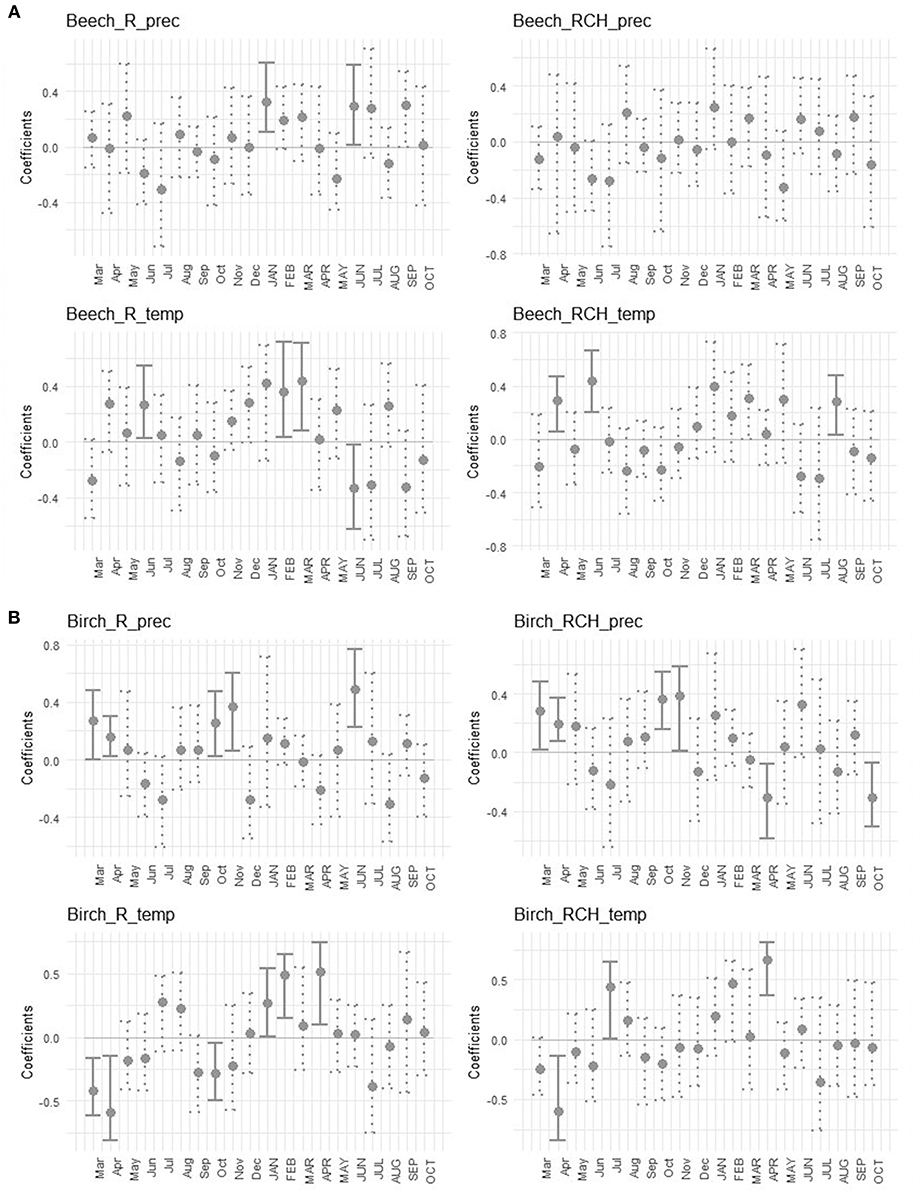
Figure 6. Bootstrapped correlation values of mean standardized chronologies for (A, above) European beech and (B, below) silver birch, growth in reference (R) and relic charcoal hearths (RCH) plots, and total monthly precipitation (prec) and mean temperature (temp). Correlations were separately calculated for each month for the period from March of the previous year (lowercase letters) to October of the current year (uppercase letters). The bars indicate a significant coefficient at p < 0.05, lines represent 95% confidence interval. Solid lines underline significant correlation values and dotted lines identify not significant correlation values.
Discussion
Contribution of Charcoal to Soil Organic Carbon Content
Most of TOC content in RCHs derived from charcoal, between 70 and 94% of TOC in the charcoal-enriched horizon (Table 2). These values are consistent with some studies (e.g., Criscuoli et al., 2014; Hardy and Dufey, 2017), but quite higher than others (e.g., Borchard et al., 2014; Kerré et al., 2016; Mastrolonardo et al., 2018) reporting contribution to TOC from charcoal-C <50%. On average, the stock of charcoal-C in the RCHs soil was 15.6 and 9.8 kg C m−2 in the A1 and A2 layer, respectively. This extremely high value would represent a total input of 342 tons of biochar per hectare, assuming 74% as C content of wood biochar (Ippolito et al., 2015). Such a charcoal content is quite close to the value of 390 tons per hectare estimated by Criscuoli et al. (2014) as the initial charcoal input of three charcoal hearths in Northern Italy abandoned in 1864. Even the subsoil at RCH plots was enriched of charcoal-C, on average 42% of TOC (Table 2), while uncharred C content was slightly lower.
Along with TOC content, five times higher in RCHs soil, total N content in soil was twice higher in the RCH than reference plots (Table 2). In the literature, there is not a clear trend on N content in charcoal hearth soils. Hardy et al. (2016) did not find such a large difference between hearth and reference soils at several hearth sites in Wallonia, like other authors did in other locations (e.g., Mikan and Abrams, 1996; Criscuoli et al., 2014). Hirsch et al. (2017) found even a lower N content in hearth soils in Connecticut. However, an increase of N is consistent with the accumulation of uncharred SOM often found in hearth soils (Borchard et al., 2014; Kerré et al., 2016; Hardy et al., 2017; Mastrolonardo et al., 2018). Furthermore, charcoal would still retain a portion of total N, although in a heterocyclic form (Mastrolonardo et al., 2015) virtually unavailable to plants. Indeed, N content in coarse charcoal was 10 g N kg (C content was 646 g C kg).
Soil in RCH plots showed also a clear distinction between the top part and the bottom part of the charcoal-enriched horizon, i.e., the A1 and A2 layer. In fact, the A1 layer was enriched in coarse charcoal-C, total N, uncharred C, and TOC compared to the A2 horizon (Table 2). This result was partly due to the quite stony nature of the bottom part of charcoal-enriched horizon (Table 1). Furthermore, the concentration of some bioavailable nutrients in soil, such as P, K, Fe, and Cu, significantly decreased from the A1 to the A2 layer of RCHs, because strictly related to the content of SOM and the biological recycling of elements through SOM decomposition (Jobbagy and Jackson, 2000). Overall, our finding would suggest to separate the charcoal-enriched horizon in two or more layers according to its thickness, which usually ranges from 10 to 80 cm (Mastrolonardo et al., 2018). Indeed, analyzing a thick charcoal-enriched layer as a whole could lead to biased results or, at best, to loss of information.
Charcoal Effects on Nutrient Cycling
Charcoal accumulation in RCH plots strongly affected the nutrient status of soil. Even after > 150 years of leaching, the concentration of bioavailable nutrients like K, Ca, Mg, Na, Mn, and Zn remain higher than in the reference soil. In acidic contexts, the pH value of soil rises soon after charring because of the presence of ashes, which are produced along with charcoal (Hardy et al., 2016). However, soil pH did not differ between RCHs and reference soil, which confirms re-acidification of soil due to vertical leaching of cations from topsoil to subsoil (Hardy et al., 2016). As proof of this, in the RCHs soil the A2 layer of char enriched horizon showed a slightly higher pH than the A1 layer of the same horizon. Furthermore, also base saturation in subsoil was higher than in topsoil. Nevertheless, CEC was much higher in the RCHs topsoil, slowing down the loss of nutrients through leaching. The higher CEC of soil in RCHs can be attributed to the negatively charged sites on the (aged) charcoal surface or on the organic matter absorbed on charcoal surface (Cheng et al., 2006).
Despite total exchangeable acidity was higher in the RCHs soil, a higher proportion of reference soil's cation exchange capacity was saturated by Al3+ because of the lower CEC (Table 4). A high proportion of Al on the exchange complex can limit the fertility of acidic soils, causing negative effect on plant growth, However, Cronan and Grigal (1995), proposed a BS level lower than 15% as an additional index for Al toxicity, a value not observed for soils at Regniessart (Table 4).
Regardless of total content of bioavailable nutrients, increasing concentration of nutrients with depth might be related to leaching from topsoil, while the opposite to absorption and recycling by plants (Jobbágy and Jackson, 2001). P, Fe, and Cu concentration did not change between RCHs and reference soil and showed a shallow distribution, suggesting a high plant uptake and cycling of these elements, whose concentration, thus, might be a limiting factor for vegetation (Table 3). The same trend was found for K, Ca, Mn, and Zn, whose concentration, however, was higher in the RCHs than reference soil, suggesting an improved soil nutrient status of RCHs soil with respect to those nutrients promptly absorbed by plants. Only Mg increased with depth in the RCHs soil, which much contributed to the high BS of subsoil (Table 4). According to Marschner (1995), the rate of Mg uptake can be strongly depressed by cations, such as K+, Ca2+, and Mn2+, which were actually more concentrated in RCHs soil. However, Mg deficiency was not found in European beech leaves in RCH plots (Figure 3).
Despite the differences in the nutrient concentration between RCHs and reference soil, our results showed almost no difference in the nutrient concentration of European beech leaves grown inside and outside RCHs (Figure 3) and not even a relationship between soil and leaf nutrient concentration. Only C and Mn concentration differed in European beech leaves. C concentration of leaves was significantly higher in RCH than reference plots; however, the stoichiometric ratios between C, N, and P in the leaves did not change, and therefore a limitation of N and P in the reference soil can be excluded (Wang et al., 2018). Carbon allocation, as carbon investment in leaves, is controlled by water and light conditions (Xia et al., 2017). In the present investigation, carbon allocation in leaves might be related to high water availability in soil. This result would be consistent with the general finding that charcoal can increase water absorbance and water-holding capacity of soil because of high porosity and surface area of charcoal (Novotny et al., 2015).
The Mn concentration of European beech leaves was higher in reference plots than RCH, an unexpected result considering that Mn concentration in reference soil was lower compared to RCHs soil. Mn in European beech leaves is highly dependent on soil pH (Flückiger and Braun, 1998), but this latter did not change between RCHs and reference soil. However, usually Mn concentration in leaves shows the greatest concentration variation, its uptake fluctuating more rapidly and distinctly than any other nutrient (Marschner, 1995). Although Mn accumulation in plant tissues might lead to toxicity, foliar symptoms were absent and leaves of European beech in the reference plots did not show any elemental deficiency compared to European beech growth in RCHs.
Dendrometric and Dendrochronological Analysis
Although silver birch height was significantly lower in the RCH than reference plots, charcoal accumulated in soil apparently did not affect the overall growth of European beech and silver birch trees (Table 5). Nonetheless, the specific growing conditions of RCHs soil affected tree ring width defining a negative growth development for both tree species, being TRW lower in the RCH than reference plots, with different species-specific TRW values (Figure 4). This finding clashes with the apparently improved soil nutrient conditions we found in RCH plots. However, several studies on RCHs showed an overall negative response on trees growth in temperate regions (Mikan and Abrams, 1996; Young et al., 1996; Carrari et al., 2016, 2018). 1995, Mikan and Abrams (1996) hypothesized a cumulative detrimental effect in the long-term led by the peculiar soil chemistry conditions of RCHs, like deficiencies in some nutrients, changes in nutrient availability due to pH change and osmotic problems in the rhizosphere, due to an excess in soluble salts in soil solution, which would lead to physiological drought. At Regniessart, however, pH did not change in RCHs compared to the reference soil and soil conductivity, related to soluble salts, did not change between the two soil types (Table 4). Still, it is possible that a not balanced elements stoichiometry in RCHs soil could lead to nutrient deficiency in plants, because of positive or negative interaction between nutrients (Marschner, 1995), although European beech foliar chemical composition does not seem to support such a hypothesis. Finally, Schneider et al. (2018), found that charcoal hearths soil in an eastern German forest is affected by a high preferential infiltration of water, which imply that large parts of soil remain dry during and after a dry period, thus worsening conditions for plant growth.
Tree growth was differently affected by climate in European beech and silver birch and in the two soil conditions. Precipitation did not affect European beech tree growth in RCH plots, although it did in the reference plots, as shown by the positive significant correlation between European beech growth and precipitation in a couple of months (Figure 6). Rather, (low) precipitation produced negative effects on the growth of silver birch at the beginning of vegetative and dormancy period (in April and October). Thus, apparently neither charcoal nor a high soil organic matter content at RCH soils did positively affect soil water capacity. Nonetheless, seemingly growing conditions of both European beech and silver birch in the RCH plots were ameliorated in relation to air temperature (Figure 6). In fact, the negative effect of air temperature on trees' growth were reduced in the RCH compared to the reference plots as observed in June of the current year relative to ring formation for European beech and in March and October of the previous year for silver birch. On the other hand, it was missing in RCH plots the positive effect of air temperature on tree growth of both species found in the reference plots during the winter season (January and February of the current year).
Overall, although the two species showed different sensitivity to precipitation and temperature, the RCHs soil conditions were not a decisive factor in significantly improving or declining tree growth. We cannot exclude, however, that the effects of RCHs soil on vegetation growth were more evident in the early stage of trees development.
Conclusions
Large charcoal addition to soil affects most of the physico-chemical soil properties even after 150 years since last charcoal accumulation. N in RCHs topsoil was twice and TOC five times larger than in reference topsoil. This large difference was mostly due to charcoal-C, which represents between 70 and 94% of TOC. This quantity would amount to a total input of 342 tons of biochar per hectare. To this respect, RCHs represent a quite extreme natural experiment of biochar addition to soil in terms of both quantities of biochar applied and time since its application.
The extended period since charcoal incorporation into soil was most likely the driving factor of the vertical differentiation, not apparent in the field, of the charcoal-enriched horizon in the RCHs soil. The top and the bottom part of the latter, in fact, showed a different nature in terms of several soil properties, like charcoal-C and uncharred C content, N content, pH, available P, K, Fe, and Cu.
In general, soil of RCHs seemed more fertile than reference soil and, hence, a more favorable site to vegetation: the concentration of many nutrients, as well as CEC, was higher in the topsoil of RCHs, namely N, K, Ca, Mn, Zn. However, no significant effect has been noticed on the overall growth of both European beech and silver birch and in the foliar nutrient concentration of European beech in the RCHs sites, with the only exception of C and Mn concentration. Even, the analysis of the tree ring width defined a slightly negative growth development for both European beech and silver birch trees inside RCH sites, also highlighting a subtle sensitivity of the silver birch growing in RCHs to scarce precipitation at the beginning of the vegetative and dormancy period.
Overall, although RCHs soil would represent an extreme case in terms of both quantities of biochar applied and time since its application to soil, they had not represented a decisive factor on tree growth. Nonetheless, the results we obtained suggest to better investigate the potential long-term detrimental effect of a large biochar addition to soil for different tree species, at different life-stages and exposure, in order to promote specific management practices for biochar use in forest ecosystems.
Author Contributions
GM, BH, JD, and J-TC conceived the study. GM, ChC, BH, JD, and J-TC carried out the field work. GM conducted the analysis on soil and leafs, ChC those on tree cores. GM and ChC organized the database and performed the statistical analysis. GM wrote the first draft of the manuscript. ChC, ClC, and JD wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
GM has benefited a Marie-Curie COFUND postdoctoral fellowship, co-founded by the European Commission and the University of Liege (ref. number: 600405).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Félix de Tombeur, Demis Andrade, Ryosuke Nakamura, and Wissem Hamdi for their help with field work, and Raphaël Tarantino, and Sébastien Ligot for their help with laboratory work. We thank Paolo Cherubini who hosted the dendroclimatological analysis in the lab of WSL (Birmensdorf, Switzerland).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2019.00051/full#supplementary-material
References
Biondi, F., and Waikul, K. (2004). DENDROCLIM2002: a C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput. Geosci. 30, 303–311. doi: 10.1016/j.cageo.2003.11.004
Blake, G. R., and Hartge, K. H. (1986). “Bulk density,” in Methods of Soil Analysis. Part I. Physical and Mineralogical Methods, ed A. Klute (Madison: Soil Science Society of America, Inc.), 363–376.
Borchard, N., Ladd, B., Eschemann, S., Hegenberg, D., Möseler, B. M., and Amelung, W. (2014). Black carbon and soil properties at historical charcoal production sites in Germany. Geoderma 232–234, 236–242. doi: 10.1016/j.geoderma.2014.05.007
Carrari, E., Ampoorter, E., Bussotti, F., Coppi, A., Garcia Nogales, A., Pollastrini, M., et al. (2018). Effects of charcoal hearth soil on forest regeneration: evidence from a two-year experiment on tree seedlings. For. Ecol. Manage. 427, 37–44. doi: 10.1016/j.foreco.2018.05.038
Carrari, E., Ampoorter, E., Verheyen, K., Coppi, A., Selvi, F., and Ewald, J. (2016). Former charcoal kiln platforms as microhabitats affecting understorey vegetation in Mediterranean forests. Appl. Veget. Sci. 19, 486–497. doi: 10.1111/avsc.12238
Cheng, C. H., Lehmann, J., Thies, J. E., and Burton, S. D. (2008). Stability of black carbon in soils across a climatic gradient. J. Geophys. Res. Biogeosci. 113, 1–10. doi: 10.1029/2007JG000642
Cheng, C. H., Lehmann, J., Thies, J. E., Burton, S. D., and Engelhard, M. (2006). Oxidation of black carbon by biotic and abiotic. Org. Geochem. 37, 1477–1488. doi: 10.1016/j.orggeochem.2006.06.022
Cook, E. R. (1985). A Time Series Analysis Approach to Tree Ring Standardization. Ph.D. Dissertation, University of Arizona (Tucson), 185.
Criscuoli, I., Alberti, G., Baronti, S., Favilli, F., Martinez, C., Calzolari, C., et al. (2014). Carbon sequestration and fertility after centennial time scale incorporation of charcoal into soil. PLoS ONE 9:e91114. doi: 10.1371/journal.pone.0091114
Cronan, C. S., and Grigal, D. F. (1995). Use of calcium/aluminum ratios as indicators of stress in forest ecosystems. J. Environ. Qual. 24, 209–226. doi: 10.2134/jeq1995.00472425002400020002x
FAO (1987). Simple Technologies for Charcoal Making. Mechanical Wood Products Branch, Forest Industries Division, FAO Forestry paper 41, Rome.
Flückiger, W., and Braun, S. (1998). Nitrogen deposition in Swiss forests and its possible relevance for leaf nutrient status, parasite attacks and soil acidification. Environ. Pollut. 102, 69–76. doi: 10.1016/S0269-7491(98)80017-1
Gärtner, H., and Nievergelt, D. (2010). The core-microtome: a new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 28, 85–92. doi: 10.1016/j.dendro.2009.09.002
Glaser, B., and Birk, J. J. (2012). State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de índio). Geochim. Cosmochim. Acta 82, 39–51. doi: 10.1016/j.gca.2010.11.029
Glisczynski, F., von Pude, R., Amelung, W., and Sandhage-Hofmann, A. (2016). Biochar-compost substrates in short-rotation coppice: effects on soil and trees in a three-year field experiment. J. Plant Nutr. Soil Sci. 179, 574–583. doi: 10.1002/jpln.201500545
Grissino-Mayer, H. D. (2001). Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree Ring Res. 57, 5–21. Available online at: http://hdl.handle.net/10150/251654
Gundale, M. J., Nilsson, M. C., Pluchon, N., and Wardle, D. A. (2016). The effect of biochar management on soil and plant community properties in a boreal forest. GCB Bioenergy 8, 777–789. doi: 10.1111/gcbb.12274
Hardy, B., Cornelis, J.-T., Houben, D., Leifeld, J., Lambert, R., and Dufey, J. E. (2017). Evaluation of the long-term effect of biochar on properties of temperate agricultural soil at pre-industrial charcoal kiln sites in Wallonia, Belgium. Eur. J. Soil Sci. 68, 80–89. doi: 10.1111/ejss.12395
Hardy, B., Cornelis, J. T., Houben, D., Lambert, R., and Dufey, J. E. (2016). The effect of pre-industrial charcoal kilns on chemical properties of forest soil of Wallonia, Belgium. Eur. J. Soil Sci. 67, 206–216. doi: 10.1111/ejss.12324
Hardy, B., and Dufey, J. E. (2017). The resistance of centennial soil charcoal to the “Walkley-Black” oxidation. Geoderma 303, 37–43. doi: 10.1016/j.geoderma.2017.05.001
Hirsch, F., Raab, T., Ouimet, W., Dethier, D., Schneider, A., and Raab, A. (2017). Soils on historic charcoal hearths: terminology and chemical properties. Soil Sci. Soc. Am. J. 81, 1427–1435. doi: 10.2136/sssaj2017.02.0067
Holmes, R. L. (1983). Program COFECHA User's Manual. Laboratory of Tree-Ring Research, The University of Arizona, Tucson.
Ippolito, J. A., Spokas, K. A., Novak, J. M., Lentz, R. D., and Cantrell, K. B. (2015). “Biochar elemental composition and factors influencing nutrient retention,” in Biochar for Environmental Management: Science, Technology and Implementation. London; New York, NY: Earthscan from Routledge, 139–164.
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014. Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106. FAO Rome.
Jobbagy, E. G., and Jackson, R. B. (2000). The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 10, 423–436. doi: 10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2
Jobbágy, E. G., and Jackson, R. B. (2001). The distribution of soil nutrients with depth: global patterns and the imprint of plants. Biogeochemistry 53, 51–77. doi: 10.1023/A:1010760720215
Kerré, B., Bravo, C. T., Leifeld, J., Cornelissen, G., and Smolders, E. (2016). Historical soil amendment with charcoal increases sequestration of non-charcoal carbon: a comparison among methods of black carbon quantification. Eur. J. Soil Sci. 67, 324–331. doi: 10.1111/ejss.12338
Lakanen, E., and Erviö, R. (1971). A comparison of eight extractans for the determination of plant available micronutrients in soils. Acta Agral. Fenn. 123, 223–232.
Lehmann, J., and Joseph, S. (2015). Biochar for Environmental Management: Science, Technology and Implementation. London; New York, NY: Earthscan from Routledge. doi: 10.4324/9780203762264
Li, Y., Hu, S., Chen, J., Müller, K., Li, Y., Fu, W., et al. (2018). Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: a review. J. Soils Sediments 18, 546–563. doi: 10.1007/s11368-017-1906-y
Luo, Y., Yu, Z., Zhang, K., Xu, J., and Brookes, P. C. (2016). The properties and functions of biochars in forest ecosystems. J. Soils Sediments 16, 2005–2020. doi: 10.1007/s11368-016-1483-5
Mastrolonardo, G., Francioso, O., and Certini, G. (2018). Relic charcoal hearth soils: a neglected carbon reservoir. Case study at Marsiliana forest, Central Italy. Geoderma 315, 88–95. doi: 10.1016/j.geoderma.2017.11.036
Mastrolonardo, G., Francioso, O., Di Foggia, M., Bonora, S., Forte, C., and Certini, G. (2015). Soil pyrogenic organic matter characterisation by spectroscopic analysis: a study on combustion and pyrolysis residues. J. Soils Sediments 15, 769–780. doi: 10.1007/s11368-014-1034-x
Metson, A. J. (1956). Methods of Chemical Analysis for Soil Survey Samples, Bulletin 12. Wellington: New Zealand Soil Bureau.
Mikan, C. J., and Abrams, M. D. (1995). Altered forest composition and soil properties of historic charcoal hearths in southeastern Pennsylvania. Can. J. For. Res. 25, 687–696. doi: 10.1139/x95-076
Mikan, C. J., and Abrams, M. D. (1996). Mechanism inhibiting thr forest development of historic charcoal hearths in southeastern Pennsylvania. Can. J. For. Res. 26, 1893–1898. doi: 10.1139/x26-213
Novotny, E. H., Maia, C. M. B., Carvalho, M., and Madari, B. E. (2015). Biochar: pyrogenic carbon for agricultural use - a critical review. Rev. Brasil. Ciênc. Do Solo 39, 321–344. doi: 10.1590/01000683rbcs20140818
Page-Dumroese, D. S., Brown, R. E., Jurgensen, M. F., and Mroz, G. D. (1999). Comparison of methods for determining bulk densities of rocky forest soils. Soil Sci. Soc. Am. J. 63, 379–383. doi: 10.2136/sssaj1999.03615995006300020016x
Palmer, C. J., Smith, W. D., and Conkling, B. L. (2002). Development of a protocol for monitoring status and trends in forest soil carbon at a national level. Environ. Pollut. 116, S209–S219. doi: 10.1016/S0269-7491(01)00253-6
Pansu, M., and Gautheyrou, J. (2006). Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods. New York, NY: Springer. doi: 10.1007/978-3-540-31211-6
Pluchon, N., Gundale, M. J., Nilsson, M. C., Kardol, P., and Wardle, D. A. (2014). Stimulation of boreal tree seedling growth by wood-derived charcoal: effects of charcoal properties, seedling species and soil fertility. Funct. Ecol. 28, 766–775. doi: 10.1111/1365-2435.12221
Santín, C., Doerr, S. H., Merino, A., Bucheli, T. D., Bryant, R., Ascough, P., et al. (2017). Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Sci. Rep. 7:11233. doi: 10.1038/s41598-017-10455-2
Schenkel, Y., Bertaux, P., Vanwijnbserghe, S., and Carre, J. (1998). An evaluation of the mound kiln carbonization technique. Biomass Bioenergy 14, 505–516. doi: 10.1016/S0961-9534(97)10033-2
Schneider, A., Hirsch, F., Raab, A., and Raab, T. (2018). Dye tracer visualization of infiltration patterns in soils on relict charcoal hearths. Front. Environ. Sci. 6:143. doi: 10.3389/fenvs.2018.00143
Spokas, K. A. (2010). Review of the stability of biochar in soils: predictability of O:C molar ratios. Carbon Manage. 1, 289–303. doi: 10.4155/cmt.10.32
Tan, Z., Lin, C. S. K., Ji, X., and Rainey, T. J. (2017). Returning biochar to fields: a review. Appl. Soil Ecol. 116, 1–11. doi: 10.1016/j.apsoil.2017.03.017
Thomas, S. C., and Gale, N. (2015). Biochar and forest restoration: a review and meta-analysis of tree growth responses. New For. 46, 931–946. doi: 10.1007/s11056-015-9491-7
Walkley, A., and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Wang, N., Fu, F., Wang, B., and Wang, R. (2018). Carbon, nitrogen and phosphorus stoichiometry in Pinus tabulaeformis forest ecosystems in warm temperate Shanxi Province, north China. J. For. Res. 29, 1665–1673. doi: 10.1007/s11676-017-0571-8
Wigley, T. M., Briffa, K. R., and Jones, P. D. (1984). On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 23, 201–213. doi: 10.1175/1520-0450(1984)023<0201:OTAVOC>2.0.CO;2
Wilson-Kokes, L., and Skousen, J. (2014). Nutrient concentrations in tree leaves on brown and gray reclaimed mine soils in West Virginia. Sci. Total Environ. 481, 418–424. doi: 10.1016/j.scitotenv.2014.02.015
Wrobel-Tobiszewska, A., Boersma, M., Adams, P., Singh, B., Franks, S., and Sargison, J. (2016). Biochar for eucalyptus forestry plantations. Acta Hortic. 1108, 55–62. doi: 10.17660/ActaHortic.2016.1108.7
Xia, J., Yuan, W., Wang, Y. P., and Zhang, Q. (2017). Adaptive carbon allocation by plants enhances the terrestrial carbon sink. Sci. Rep. 7:3341. doi: 10.1038/s41598-017-03574-3
Young, M. J., Johnson, J. E., and Abrams, M. D. (1996). Vegetative and edaphic characteristics on relic charcoal hearths in the Appalachian mountains. Vegetatio 125, 43–50. doi: 10.1007/BF00045203
Keywords: relic charcoal hearths, biochar, charcoal kiln soils, soil nutrients concentration, leaf nutrients concentration, Fagus sylvatica, Betula pendula, dendroclimatology
Citation: Mastrolonardo G, Calderaro C, Cocozza C, Hardy B, Dufey J and Cornelis J-T (2019) Long-Term Effect of Charcoal Accumulation in Hearth Soils on Tree Growth and Nutrient Cycling. Front. Environ. Sci. 7:51. doi: 10.3389/fenvs.2019.00051
Received: 15 October 2018; Accepted: 02 April 2019;
Published: 24 April 2019.
Edited by:
Dionisios Gasparatos, Aristotle University of Thessaloniki, GreeceReviewed by:
Peter A. Roussos, Agricultural University of Athens, GreeceMiguel Angel Sanchez-Monedero, Spanish National Research Council (CSIC), Spain
Gerrit Angst, Institute of Soil Biology (ASCR), Czechia
Copyright © 2019 Mastrolonardo, Calderaro, Cocozza, Hardy, Dufey and Cornelis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Mastrolonardo, Z2lvdmFubmkubWFzdHJvbG9uYXJkb0B1bmlmaS5pdA==
 Giovanni Mastrolonardo
Giovanni Mastrolonardo Chiara Calderaro3
Chiara Calderaro3 Claudia Cocozza
Claudia Cocozza Brieuc Hardy
Brieuc Hardy Joseph Dufey
Joseph Dufey Jean-Thomas Cornelis
Jean-Thomas Cornelis