
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 26 May 2017
Sec. Wastewater Management
Volume 5 - 2017 | https://doi.org/10.3389/fenvs.2017.00025
This article is part of the Research Topic Advanced Utilization and Management of Biogas View all 6 articles
 Yusuke Shiratori1*
Yusuke Shiratori1* Takeo Yamakawa2
Takeo Yamakawa2 Mio Sakamoto1
Mio Sakamoto1 Hinomi Yoshida2
Hinomi Yoshida2 Takuya Kitaoka2
Takuya Kitaoka2 Quang Tuyen Tran1
Quang Tuyen Tran1 Duc Chanh Tin Doan3
Duc Chanh Tin Doan3 Mau Chien Dang3
Mau Chien Dang3Fuel-flexible solid oxide fuel cell (SOFC) technologies are presently under study in a Vietnam-Japan international joint research project. The purpose of this project is to develop and demonstrate an SOFC-incorporated energy circulation system for the sustainable development of the Mekong Delta region. Lab-scale methane fermentation experiments in this study with a mixture of biomass feedstock collected in the Mekong Delta (shrimp pond sludge, bagasse, and molasses from sugar production) recorded biogas production yield over 400 L kgVS−1 with H2S concentration below 50 ppm level. This real biogas was directly supplied to an SOFC without any fuel processing such as desulfurization, methane enrichment and pre-reforming, and stable power generation was achieved by applying paper-structured catalyst (PSC) technology.
The rapid economic growth of ASEAN countries is causing (I) environmental pollution due to the increase in volume of organic waste and (II) unstable supply of electricity. The Mekong Delta of Vietnam, located in the southeastern Indochina Peninsula, is one of the largest deltas in Asia with an area of around 40,519 km2 (Cosslett and Cosslett, 2014) and a population of over 17 million as of 2011 (Nguyen and Ye, 2015). As the main agricultural zone in Vietnam, the Mekong Delta accounts for more than 50% of the country's agricultural production (50% of the rice, 70% of the fruit and 52% of the seafood products in 2011), and plays an important role in nationwide economic growth and food security (Garschagen et al., 2012; Nguyen and Ye, 2015).
This region is rich in unutilized biomass resources such as sludge discharged from shrimp and catfish culture and residue of sugar production (bagasse and molasses), which have the potential to satisfy the local energy demand. Although many projects are focused on biofuel production from these biomass resources, not much attention has been paid to developing an efficient utilization technology. Therefore, in April 2015, we started the project “Sustainable Development of Rural Area by Effective Utilization of Bio-wastes with Highly Efficient Fuel Cell Technology” within the framework of the Science and Technology Research Partnership for Sustainable Development (SATREPS) supported by the Japan Science and Technology Agency (JST) and the Japan International Cooperation Agency (JICA).
The project selected a shrimp farm in the Mekong Delta region, where stable power supply is needed and sludge accumulation has become crucial problem, as the demonstration site for the fuel cell-incorporated energy circulation system illustrated in Figure 1. Sludge accumulated at the bottom of the shrimp pond is pumped out and then passed through a membrane filter to increase the concentration of organic substances. Subsequently, the concentrated sludge together with bagasse and molasses collected from a local sugar factory is fed to a methane fermenter to produce biogas, which is used as fuel to generate electricity with a solid oxide fuel cell (SOFC) system having double the efficiency of conventional heat engine systems (Wachsman et al., 2012). The green electricity derived from the local biomass resources is used for shrimp culture, such as for aeration to the shrimp pond, to demonstrate “carbon-neutral energy circulation.” The clarified water obtained by the membrane filter is returned to the pond to maintain the water quality (water circulation). The digested liquid discharged from the fermenter is dried and supplied to the carbonization reactor to produce charcoal, which is used as a soil improvement agent for cultivating local fruits to demonstrate “mineral-neutral material circulation.”
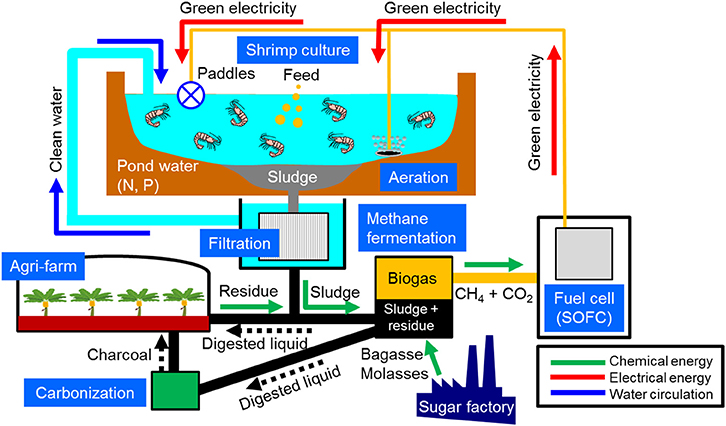
Figure 1. Fuel cell-incorporated energy circulation system to be demonstrated at a shrimp farm located in Ben Tre, Vietnam.
General biogas produced from livestock manure, garbage, sewage sludge etc. contains 500–4,000 ppm H2S (Alves et al., 2013). When the biogas is utilized for power generation or hydrogen production, desulfurization process is indispensable (Abatzoglou and Boivin, 2009; De Arespacochaga et al., 2014) because H2S strongly deactivates catalyst materials for reforming and electrochemical reactions. The present study investigated the optimal conditions for methane fermentation to achieve stable biogas production from the biomass feedstock of the Mekong Delta. This is the first report using methanogenic bacteria in the shrimp pond sludge for the digestion of bagasse and molasses. Furthermore, the produced biogas was used as fuel for the test on SOFC in which direct internal reforming (DIR) technology (Staniforth and Kendall, 1998; Yentekakis, 2006; Janardhanan et al., 2007; Shiratori et al., 2008; Lanzini and Leone, 2010; Leone et al., 2012; Papadam et al., 2012; Lanzini et al., 2013) using a paper-structured catalyst (PSC; Fukahori et al., 2006; Shiratori et al., 2015; Shiratori and Sakamoto, 2016) was applied to achieve SOFC power generation by direct feed of the biogas without any fuel processing.
For the biogas production, sludge discharged from shrimp culture [(S1) shrimp pond sludge] was used as a source of mesophilic methanogenic bacteria, and (S2) bagasse and (S3) molasses discharged during sugar production were used as fermentation substrates. A volume of 350 mL of S1 collected from a shrimp farm in Ben Tre province, Vietnam was sterilized in an autoclave at 121°C for 20 min, followed by drying at 105°C. The necessary quantities of S2 and S3 collected from a sugar factory located in Ben Tre were dried at 105°C.
Raw and dry weights of S1, S2, and S3 were measured to calculate their moisture. Dry samples of S1, S2, and S3 were pulverized in a vibration mill, subsequently fired at 600°C to measure the content of volatile solids (VS) and ash corresponding to organic and inorganic substances, respectively, followed by elemental analysis using energy dispersive X-ray analysis (EDX7000, Shimadzu).
Figure 2 shows the setup for lab-scale methane fermentation (wet-type mesophilic fermentation) composed of a fermenter (4 L), pH meter and gas holder. First of all, acclimatization of bacteria was conducted using 1 kg seed sludge. One kilogram of S1 (VS: 1.86%, Ash: 24.5%, Moisture: 73.6%), 3 L of tap water and 27 g of S2 was added into the fermenter which was thermostated at 35°C, and stirred at 300 rpm. This state was maintained for 120 days for the acclimation of methanogenic bacteria during which 80 g of S2 and designated amounts of minerals (nutrient salts) were added under pH monitoring. The minimum requirement for the nutrient salts to maintain an acetic acid consumption rate of 30 g L−1 d−1 (Takashima and Speece, 1989), and the salts used in this study are summarized in Table 1.
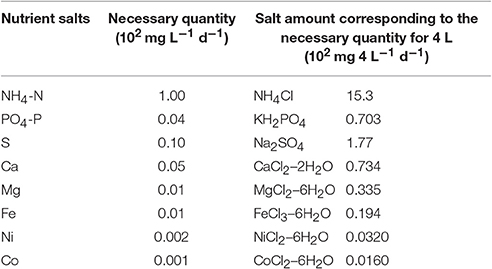
Table 1. Minimum required nutrient salts to maintain acetic acid consumption rate of 30 g L−1 d−1 (Takashima and Speece, 1989).
In the acclimation process, aiming at multiplication of bacteria that accelerate the decomposition of bagasse, sufficiently moist bagasse that is expected to include cellulase and lignase active bacteria was selected and used for the biogas production experiments.
After the acclimation process mentioned above, molasses (VS 10 g) was added to the digested liquid to start methane fermentation. After 1 week, a mixture of S2 and S3 with a weight ratio of 4:1 (Mixture-I, VS 20 g) was added to the fermenter, and the biogas production amount and pH of the digested liquid were monitored. The composition of the produced biogas was measured with a gas chromatograph (GC-8AT, Shimadzu) equipped with a stainless-steel column (3 mm diameter, 2 m length) packed with ShinCarbon ST (80–100 mesh) and using Ar as a carrier gas (50 ml min−1).
A dense 10ScSZ (10 mol% Sc2O3–1 mol% CeO2-ZrO2) plate with 160 μm thickness and 2 × 2 cm2 area was made from 10ScSZ powder provided by Daiichi Kigenso Kagaku Kogyo, Japan, and used as the electrolyte to fabricate an electrolyte-supported cell as shown in Figure 3A. The paste for the anode functional layer (AFL) [Ni-10ScSZ (NiO:10ScSZ = 56:44 wt%)] was deposited on the 10ScSZ plate via screen printing following by sintering at 1,300°C for 3 h to form a porous anode material with an area of 1.4 × 1.4 cm2. Prior to fabrication of the cathode, paste for the barrier layer [Gd0.10Ce0.90O2 (GDC)] and anode current collector layer (ACCL) (NiO:10ScSZ = 80:20 wt%) was screen printed on the surface of the electrolyte and AFL, respectively, followed by co-sintering at 1,200°C for 5 h. The paste for the cathode functional layer (CFL), which consists of a mixture of (La0.60Sr0.40)0.95(Co0.20Fe0.80)O3−x and Gd0.10Ce0.90O1.95 (LSCF-GDC) (fuelcellmaterials, USA), was screen printed on the sintered GDC layer. Then, the LSCF paste was printed on the dried LSCF-GDC layer as a cathode current collector layer (CCCL). Finally, the deposited pastes were co-sintered at 900°C for 5 h to obtain a porous cathode with an area of 1.4 × 1.4 cm2. Pt wires welded with Pt mesh were applied to the anode and cathode as voltage and current-collecting probes.
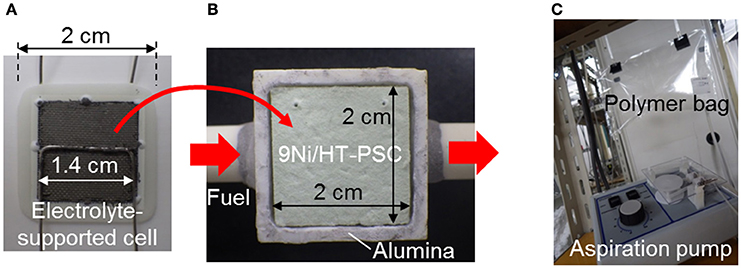
Figure 3. Photographs of (A) SOFC single cell (electrolyte-supported cell) and (B) Ni-loaded hydrotalcite-dispersed PSC (9Ni/HT-PSC) (Shiratori and Sakamoto, 2016). Real biogas stored in a polymer bag was supplied to the PSC/SOFC stack using an aspiration pump (C).
As shown in Figure 3B, 9 wt% Ni-loaded hydrotalcite ([Mg6Al2(OH)16CO3]4H2O, HT)-dispersed PSC (9Ni/HT-PSC) was prepared by a papermaking technique using a dual polyelectrolyte retention system and subsequent impregnation and sintering processes (Shiratori et al., 2015; Shiratori and Sakamoto, 2016). The microstructure of the prepared 9Ni/HT-PSC is shown elsewhere (Shiratori and Sakamoto, 2016). Ceramic fiber (SiO2: 52 wt%, Al2O3: 48 wt%) was welded using a yttria stabilized zirconia (YSZ) sol to form an inorganic fiber network with an average size of macropores and porosity of 16 μm and 89%, respectively. Ni loading was performed via the impregnation process, during which Ni was selectively loaded on the HT-derived oxide phase to form very fine Ni particles (<10 nm).
Three pieces of 9Ni/HT-PSCs with an area of 2 × 2 cm2 and a thickness of 1.1 mm were stacked and mounted in an alumina cell housing as shown in Figure 3B. Then, the electrolyte-supported SOFC was placed on the PSC to form a PSC/SOFC stack. A porous Pt electrode was attached to the electrolyte surface of the cathode side as a reference electrode. Ceramic adhesive (Aron Ceramic, Toagosei Co., Ltd., Japan) was pasted around the outer perimeter of the cell for sealing, followed by heating to 800°C in 5 h. Then, 50 ml min−1 of pure N2 was fed to the anode side in-plane direction to completely purge the anode compartment, after which the anode gas was switched from pure N2 to 50 ml min−1 of pure H2 (dry) for reduction treatment of the anode and 9Ni/HT-PSC. After 15 h, testing of the DIR-SOFC was started at 800°C.
The anode gas was switched from pure H2 to 40 ml min−1 of simulated biogas [equimolar mixture of CH4 and CO2 (CH4/CO2 = 1)]. For the cathode side, 1 L min−1 of air was supplied. Electrochemical measurements were performed using an automatic SOFC testing system (Toyo Corporation, Japan). H2S poisoning test was conducted during a galvanostatic test at 0.2 A cm−2 in the direct feed of CH4/CO2 = 1 (CH4: 20 ml min−1, CO2: 20 ml min−1) by adding 5 or 10 ppm H2S to the fuel simulating a real biogas produced from the biomass feedstock in Mekong Delta. Electrochemical impedance spectroscopy (EIS) was carried out before and during H2S poisoning under open-circuit conditions using a frequency response analyzer (VersaSTAT 3, Princeton Applied Research, USA) in a frequency range between 0.1 and 100 kHz with a voltage amplitude of 10 mV.
For the test with real biogas derived from the mixture of S1, S2, and S3, as shown in Figure 3C, the gas stored in a polymer bag (Smart Bag PA, GL Sciences, Japan) was supplied to the PSC/SOFC stack using an aspiration pump at 20 ml min−1 without any fuel processing such as desulfurization and reforming.
Mass content of VS and ash (dry basis) and moisture, and elemental composition of the biomass feedstock collected in the Mekong Delta are listed in Table 2. Shrimp pond sludge contained only 7.4% VS. The high amount of Si and Al indicates that clay is a main component of the sludge. When sludge is collected from unlined ponds like those in Ben Tre, materials that are unsuitable for methane fermentation such as inorganic substances must be separated using a precipitation technique.
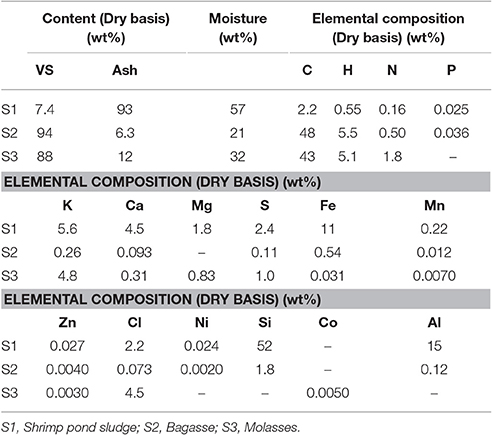
Table 2. Mass content of VS, ash and moisture, and elemental composition in the biomass feedstock collected in the Mekong Delta, Vietnam.
When only molasses was added, as shown in Figure 4, a rather high gas production rate was measured; however, this was accompanied by a large drop in pH down to 5.5, and the generation of CO2 and H2 was pronounced (see Figure 5A). The CH4/CO2 ratio was 0.66 at 3 days, which was considerably lower than the normal ratio of 1.5. For Mixture-I subsequently added to the fermenter, CH4 production was steady at pH 6.5 without H2 production, and biogas with CH4/CO2 = 1.7 was obtained (see Figure 5B).
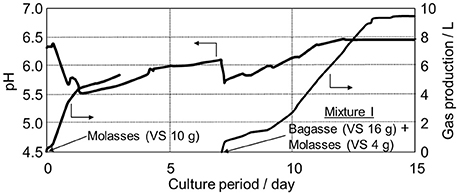
Figure 4. Monitoring of gas production amount and pH during the fermentation of molasses only and bagasse:molasses = 4:1 mixture (Mixture-I) added to the digested liquid based on the shrimp pond sludge after the acclimation process.
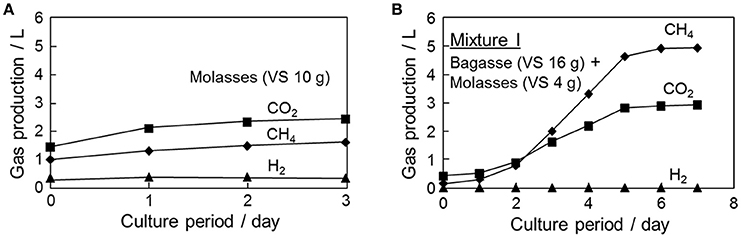
Figure 5. Production amounts of CH4, CO2, and H2 during the fermentation of (A) molasses only and (B) bagasse:molasses = 4:1 (Mixture-I) added to the digested liquid based on the shrimp pond sludge after the acclimation process.
After the termination of gas production for Mixture-I in the experiment of Figure 5B, 1-week methane fermentation experiments were performed for the different mixtures of bagasse and molasses (total VS 20 g) with the weight ratios of (II) 4:1, (III) 3:2, and (IV) 2:3 in series. When a fermentation substrate was supplied to the fermenter, 500 mL of digested liquid was discharged, and then double the necessary quantities of the nutrient salts listed in Table 1 were added with 400 mL of tap water. Finally, 100 mL of tap water was poured into the fermenter while washing the supply port so that the total volume of slurry became 4 L. The results of the fermentation experiments are summarized in Table 3.
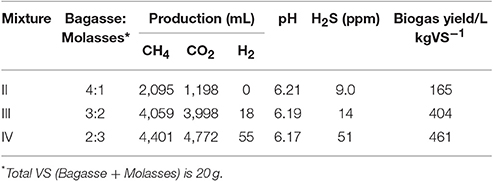
Table 3. Cumulative biogas production at 7 days (Results of the fermentation experiments performed after biogas production for Mixture-I shown in Figure 5B).
In the lab-scale methane fermentation experiments with the mixtures of biomass feedstock collected in the Mekong Delta, biogas production yields of 165, 404 and 461 L kgVS−1 were recorded for the Mixture-II, III and IV, respectively. As shown in Figure 4 and Figure 5A, molasses leads to a considerable drop in slurry pH accompanied by H2 production, and therefore, pH of the digested liquid decreased and the production amount of H2 increased in the order of Mixture-II, III and IV. The addition of the minerals listed in Table 1 promoted fermentation of the biomass feedstock of the Mekong Delta. Furthermore, in this study, partially fermented bagasse collected from Ben Tre was used as a substrate, and therefore, cellulose- and lignin-assimilating bacteria are considered to be activated to form more acetic acid for assimilation by methanogenic bacteria, resulting in a rather high biogas production rate comparable to that of alkali-treated bagasse (540 L kgVS−1) (Ishihara et al., 1988).
Stable biogas production from the biomass feedstock of the Mekong Delta, bagasse and molasses, was achieved by using the shrimp pond sludge as the source of methanogenic bacteria. An attractive feature of the present biogas is its very low H2S concentration of 9.0, 14 and 51 ppm for Mixture-II, III and IV, respectively, in comparison to general biogas containing 500–4,000 ppm (Alves et al., 2013). Using SOFC technology, the real biogas produced in this study is considered to be converted directly into electricity without any fuel processing, provided that PSC is applied on the anode material (Shiratori and Sakamoto, 2016).
Figure 6 shows the voltage of the SOFCs in the direct feed of simulated biogas mixture (CH4/CO2 = 1) at 800°C with and without 9Ni/HT-PSC monitored during H2S poisoning under galvanostatic conditions at 0.2 A cm−2. The result without 9Ni/HT-PSC (Shiratori and Sakamoto, 2016) supports the previous reports (Shiratori et al., 2008; Hagen et al., 2014; Papurello et al., 2016) that DIR-SOFCs are quite susceptible to fuel impurities (H2S), and their electrochemical performances rapidly deteriorate due to the chemisorption of sulfur species derived from H2S on the surface of the Ni particles used in the anode material, which blocks the active sites for methane reforming and electrochemical oxidation, leading to a large increase in anode overvoltage. The drastic cell voltage drop by the addition of 5 ppm H2S indicates the strong deactivation of dry reforming of methane (CH4 + CO2 → 2H2 + 2CO) within the porous anode volume but not by the deactivation of electrochemical oxidation of H2 and CO. Because the real biogas produced from Mixture-III had almost identical composition to that of the simulated biogas mixture (CH4/CO2 = 1) except for the presence of 14 ppm H2S, in this study, 10 ppm H2S poisoning test was performed during the galvanostatic measurement at 0.2 A cm−2 in the direct feed of the simulated biogas. In this 10 ppm poisoning test, 9Ni/HT-PSC was applied on the anode material. Although DIR-SOFC fueled by biogas was intolerant to the 5 ppm H2S contamination of the fuel, as shown in Figure 6, significant improvement of sulfur tolerance could be achieved by the application of 9Ni/HT-PSC even in the presence of H2S at twice the concentration (10 ppm).
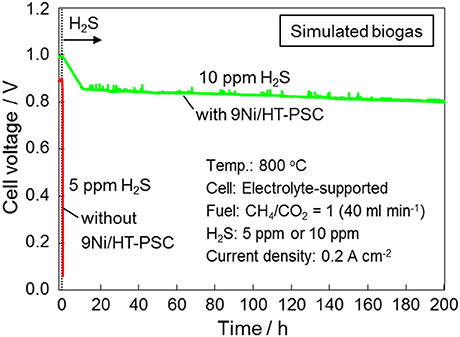
Figure 6. H2S poisoning test in the direct feed of simulated biogas mixture (CH4/CO2 = 1) at 800°C under 0.2 A cm−2 with and without 9Ni/HT-PSC.
Electrochemical behavior at the anode/electrolyte interface was investigated with EIS. Figure 7 shows the anode-side impedance spectra for the direct feed of the simulated biogas mixture (CH4/CO2 = 1) with and without 9Ni/HT-PSC measured under open-circuit voltage (OCV) conditions (Shiratori and Sakamoto, 2016), showing the influence of the addition of 5 ppm H2S to the fuel. For both cases with and without 9Ni/HT-PSC, the trace H2S retarded the electrochemical processes. In the case without 9Ni/HT-PSC, impedances corresponding to charge transfer processes (>500 Hz) and mass transfer processes (<100 Hz) were both increased; especially the diffusion of reactant gases in the porous anode (100–10 Hz) and the gas conversion related to fuel reforming (<5 Hz) were strongly affected (Hagen et al., 2014). Application of 9Ni/HT-PSC on the anode material was confirmed to be highly effective for suppressing the increase in mass transfer resistance by sulfur introduction, indicating that H2 transport to the anode functional layer adjacent to the electrolyte/anode interface was uninterrupted even under 5 ppm H2S contamination of the CH4-CO2 mixture.
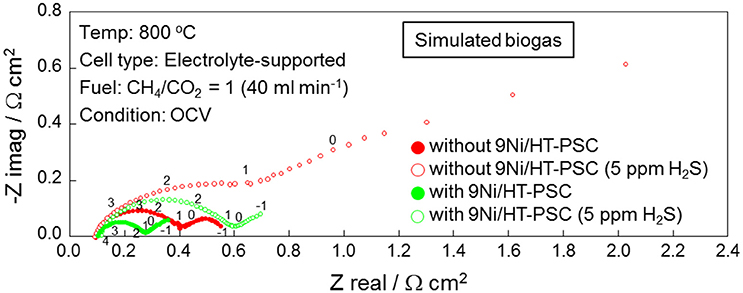
Figure 7. Anode-side electrochemical impedance spectra (EIS) of the SOFC with the direct feed of simulated biogas measured under OCV conditions, showing the positive impact of PSC on the enhancement of H2S tolerance.
Judging from the results of the simulated biogas (CH4/CO2 = 1), if 9Ni/HT-PSC is applied, SOFC operation with direct feed of the real biogas derived from Mixture-III (CH4/CO2 ≈ 1, H2S concentration of 14 ppm) is considered to be possible. Figure 8 shows the result of the galvanostatic test of DIR-SOFC fueled by the real biogas without any fuel processing such as desulfurization, CH4 enrichment, and pre-reforming. Stable voltage over 0.9 V was obtained until the biogas stored in the polymer bag was used up. The duration of the test with the real biogas was limited within 4 h due to the capacity of the polymer bag, however the performance stability obtained with the simulated biogas under the same testing condition shown in Figure 6 supports that direct conversion of the biogas derived from the biomass feedstock in Mekong Delta into electricity is feasible by the SOFC-PSC combined technology. In future, this technology can contribute to cost reduction and downsizing of the SOFC system because fuel purification unit and external reformer can be simplified or removed.
In the Mekong Delta, large amounts of residual biomass are aggregated during shrimp culture and sugar production, which are key industries of this region. On-site power generation systems independent of the power grid are indispensable due to insufficiency of the power grid and its vulnerability especially in the dry season when there is an increase in electricity demand. Therefore, social receptivity of biogas-fueled SOFC is very high.
We have taken on the challenge of innovation in fuel cell technology through agriculture-engineering collaboration, and this study found a novel SOFC power generation technology combined with fermentation technology to propose a simple and highly efficient power generation technology using local bio-waste as energy resources suitable for rural areas abundant in biomass resources, such as the Mekong Delta.
In the ongoing international joint research project under the SATREPS program, based on the concept of Figure 1, we have constructed a pilot plant of the 1 kW SOFC-incorporated energy circulation systems in Dec. 2016 in a shrimp farm located at Ben Tre, Vietnam. The DIR-SOFC technology presented in this paper will contribute to the simplification or the downsizing of the SOFC system and resulting cost reduction.
YS: corresponding author, study on biogas fueled SOFC, and development of paper-structured catalyst for DIR-SOFC. TY: study on the biogas production from local biomass feedstock in the Mekong Delta. MS: preparations of SOFC and paper-structured catalyst. HY: methane fermentation experiments. TK: catalyst development for fuel reforming. QT: electrochemical measurements. DD: sampling of local biomass feedstock in the Mekong Delta. MD: catalyst development for DIR-SOFC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MG and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
This study was supported by JST-JICA SATREPS.
Abatzoglou, N., and Boivin, S. (2009). A review of biogas purification processes. Biofuels Bioproducts Biorefining 3, 42–71. doi: 10.1002/bbb.117
Alves, H. J., Junior, C. B., Niklevicz, R. R., Frigo, E. P., Frigo, M. S., and Coimbra-Araujo, C. H. (2013). Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrogen Energy 38, 5215–5225. doi: 10.1016/j.ijhydene.2013.02.057
Cosslett, T. L., and Cosslett, P. D. (2014). Water Resources and Food Security in the Vietnam Mekong Delta. Switzerland: Springer International Publishing.
De Arespacochaga, N., Valderrama, C., Mesa, C., Bouchy, L., and Cortina, J. L. (2014). Biogas deep clean-up based on adsorption technologies for solid oxide fuel cell applications. Chem. Eng. J. 255, 593–603. doi: 10.1016/j.cej.2014.06.072
Fukahori, S., Kitaoka, T., Tomoda, A., Suzuki, R., and Wariishi, H. (2006). Methanol steam reforming over paper-like composites of Cu/ZnO catalyst and ceramic fiber. Appl. Catal. A 300, 155–161. doi: 10.1016/j.apcata.2005.11.008
Garschagen, M., Diez, J. R., Nhan, D. K., and Kraas, F. (2012). “Socio-economic development in the Mekong Delta: between the prospects for progress and the realms of reality,” in The Mekong Delta System, Interdisciplinary Analyses of a River Delta, Springer Environmental Science and Engineering, eds F.G. Renaud and C. Kuenzer (Dordrecht: Springer), 83–132.
Hagen, A., Johnson, G. B., and Hjalmarsson, P. (2014). Electrochemical evaluation of sulfur poisoning in a methane-fueled solid oxide fuel cell: effect of current density and sulfur concentration. J. Power Sources 272, 776–785. doi: 10.1016/j.jpowsour.2014.08.125
Ishihara, M., Toyama, S., and Yonaha, K. (1988). Biogas production from methane fermentation of sugarcane bagasse. Sci. Bull. Fac. Agric. Univ. Ryukyus 35, 45–51.
Janardhanan, V. M., Heuveline, V. V., and Deutschmann, O. (2007). Performance analysis of a SOFC under direct internal reforming conditions. J. Power Sources 172, 296–307. doi: 10.1016/j.jpowsour.2007.07.008
Lanzini, A., and Leone, P. (2010). Experimental investigation of direct internal reforming of biogas in solid oxide fuel cells. J. Power Sources 35, 2463–2476. doi: 10.1016/j.ijhydene.2009.12.146
Lanzini, A., Leone, P., Guerra, C., Smeacetto, F., Brandon, N. P., and Santarelli, M. (2013). Durability of anode supported Solid Oxide Fuel Cells (SOFC) under direct dry-reforming of methane. Chem. Eng. J. 220, 254–263. doi: 10.1016/j.cej.2013.01.003
Leone, P., Lanzini, A., Ortigoza-Villalba, G. A., and Borchiellini, R. (2012). Operation of a solid oxide fuel cell under direct internal reforming of liquid fuels. Chem. Eng. J. 191, 349–355. doi: 10.1016/j.cej.2012.03.030
Nguyen, Q. C., and Ye, F. (2015). Study and evaluation on sustainable industrial development in the Mekong Delta of Vietnam. J. Clean. Prod. 86, 389–402. doi: 10.1016/j.jclepro.2014.08.087
Papadam, T., Goula, G., and Yentekakis, I. V. (2012). Long-term operation stability tests of intermediate and high temperature Ni-based anodes' SOFCs directly fueled with simulated biogas mixtures. Int. J. Hydrogen Energy 37, 16680–16685. doi: 10.1016/j.ijhydene.2012.02.147
Papurello, D., Lanzini, A., Fiorilli, S., Smeacetto, F., Singh, R., and Santarelli, M. (2016). Sulfur poisoning in Ni-anode solid oxide fuel cells (SOFCs): deactivation in single cells and a stack. Chem. Eng. J. 283, 1224–1233. doi: 10.1016/j.cej.2015.08.091
Shiratori, Y., Oshima, T., and Sasaki, K. (2008). Feasibility of direct-biogas SOFC. Int. J. Hydrogen Energy 33, 6316–6321. doi: 10.1016/j.ijhydene.2008.07.101
Shiratori, Y., and Sakamoto, M. (2016). Performance improvement of direct internal reforming solid oxide fuel cell fueled by H2S-contaminated biogas with paper-structured catalyst technology. J. Power Sources 332, 170–179. doi: 10.1016/j.jpowsour.2016.09.095
Shiratori, Y., Sakamoto, M., Uchida, T., Le, H., Quang-Tuyen, T., and Sasaki, K. (2015). Hydrotalcite-dispersed paper-structured catalyst for the dry reforming of methane. Int. J. Hydrogen Energy 40, 10807–10815. doi: 10.1016/j.ijhydene.2015.07.016
Staniforth, J., and Kendall, K. (1998). Biogas powering a small tubular solid oxide fuel cell. J. Power Sources 71, 275–277. doi: 10.1016/S0378-7753(97)02762-6
Takashima, M., and Speece, R. E. (1989). Mineral nutrient requirements for high-rate methane fermentation of acetate at low SRT. Res. J. WPCF 61(11/12), 1645–1650.
Wachsman, E. D., Marlowe, C. A., and Lee, K. T. (2012). Role of solid oxide fuel cells in a balanced energy strategy. Energy Environ. Sci. 5, 5498–5509. doi: 10.1039/C1EE02445K
Keywords: biogas, SOFC, direct internal reforming, methane fermentation, bagasse, molasses, shrimp culture
Citation: Shiratori Y, Yamakawa T, Sakamoto M, Yoshida H, Kitaoka T, Tran QT, Doan DCT and Dang MC (2017) Biogas Production from Local Biomass Feedstock in the Mekong Delta and Its Utilization for a Direct Internal Reforming Solid Oxide Fuel Cell. Front. Environ. Sci. 5:25. doi: 10.3389/fenvs.2017.00025
Received: 23 February 2017; Accepted: 10 May 2017;
Published: 26 May 2017.
Edited by:
Pierluigi Leone, Politecnico di Torino, ItalyReviewed by:
Marta Gandiglio, Politecnico di Torino, ItalyCopyright © 2017 Shiratori, Yamakawa, Sakamoto, Yoshida, Kitaoka, Tran, Doan and Dang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusuke Shiratori, c2hpcmF0b3JpLnl1c3VrZS41MDBAbS5reXVzaHUtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.