
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Health, 06 May 2024
Sec. Occupational Safety and Health Interventions
Volume 3 - 2024 | https://doi.org/10.3389/fenvh.2024.1381159
Introduction: Firefighters are exposed to polycyclic aromatic hydrocarbons (PAHs) during fire suppression, but the extent of PAH penetration through turnout gear and its impact on blood cell components remains uncertain. The main objective of this study is to investigate the residual levels of PAHs on turnout gear and skin, and to assess their potential effects on blood cell components in firefighters. We hypothesize that firefighting activities lead to increased PAH exposure, which in turn may induce alterations in blood cell composition. We also hypothesize that differences in PAH exposure and corresponding blood cell changes will be observed between volunteer and career firefighters.
Methods: Forty-seven firefighters were recruited from 11 fire departments in Oklahoma. PAH levels on gear and skin were measured, and complete blood count (CBC) parameters were analyzed. Parametric and non-parametric tests were used to examine differences in PAH exposures and hematological profiles between volunteer and career firefighters and possible changes over time. Generalized estimating equation (GEE) models were performed to assess the relationships between PAHs and blood cells.
Results: Our analysis revealed significant hematological differences between volunteer and career firefighters, with volunteers exhibiting lower aerobic capacity and higher systemic inflammation. Increased levels of specific PAHs were found on volunteers' gear post-fire.
Conclusion: Our findings indicate an association between PAH exposure and changes in blood parameters, emphasizing the need for improved decontamination protocols and protective measures, particularly for volunteer firefighters.
Firefighting is a high-risk occupation that exposes firefighters to numerous potential hazards, including toxic chemicals. The risks are attributed to factors such as the inhalation of smoke encountered at fire scenes. The smoke generated during fires contains a mixture of gases and particulates, including polycyclic aromatic hydrocarbons (PAHs), which are of particular concern due to their carcinogenic properties. Extensive research has focused on firefighter exposure to PAHs, as they are prevalent in fire environments and have been associated with an increased cancer risk (1, 2). The International Agency for Research on Cancer (IARC) has classified certain PAHs as probable or possible carcinogens that are linked to cancers in humans, in particular, benzo[a]pyrene, a single analyte of PAH carcinogenic to humans (3).
As firefighters are deployed to random locations, it is challenging to monitor and control their exposure to toxic chemicals. Portable protection is therefore crucial to ensuring their health and safety. Personal protective equipment (PPE), such as turnout gear, is the primary means of protection for firefighters. However, PPE is regarded as the least efficacious measure for mitigating hazards within the framework of industrial hygiene's hierarchy of controls (4). Studies have shown the presence of protective gear (5), findings that underscore the importance of proper PPE practices. For example, when PPE cleaning is not routinely performed following a fire response, PAHs are likely to accumulate on PPE during each fire suppression and could be transferred to the skin (6). These toxic chemicals are absorbed by the skin and subsequently cause a series of immune responses (7). Previous studies have shown that PAHs induce the release of pro-inflammatory cytokines in both immune cells and skin cells (8, 9).
One possible way to assess the effects of PAH exposure on firefighter health is to examine the changes in blood cell parameters. Blood cells are vulnerable to PAH exposure due to their high lipid solubility and can reflect systemic responses to inflammation, oxidative stress, and DNA damage (10–12). As a result, the systemic effects of PAHs may manifest in changes in blood cell parameters. Hematologic parameters obtained from a complete blood count (CBC) are widely used in disease prognosis evaluation, risk stratification, and diagnosis (13–15). A CBC assesses the cell counts of the formed elements in blood, serving as the most extensively used test in clinical practice to evaluate overall health status. For instance, early changes in red blood cell (RBC) morphology may be crucial clinical markers of pathology (16). High red cell distribution width (RDW) has been significantly associated with all-cause mortality, including cancer, cardiovascular disease (CVD), and respiratory disease mortality (17). An increased level of white blood cells (WBC) may be indicative of an ongoing inflammatory response, which is associated with higher rates of cardiovascular and all-cause mortality (18). Thus, blood cells can serve as a valuable tool, as they provide insight into potential health risks from exposure to PAH and allow for early detection and preventative measures.
Existing research investigates CBC parameters in firefighters within simulated settings but does not assess PAH exposure (19). While other studies evaluate firefighters' exposure to PAHs, they often overlook assessing hematological impacts. The focus on controlled environments and/or the absence of comprehensive evaluation of PAH exposure and hematological impacts constrains the understanding of physiological responses to real firefighting situations. Research on the differences in these aspects between volunteer and career firefighters is also limited. This study aims to bridge these gaps by examining the changes in blood cell parameters and PAH levels after firefighting activities on turnout gear and skin in volunteer and career firefighters. Our objectives are to (1) quantify residual PAH levels on gear and skin, (2) assess alterations in blood cell components pre- and post-exposure, and (3) compare these changes between volunteer and career firefighters to evaluate potential health risks. By identifying specific PAH analytes and related impacts, this research seeks to contribute to improving firefighter safety protocols and health outcomes. The hypothesis driving this study is that PAH exposure during firefighting activities leads to significant hematological changes indicative of increased health risks, with potential differences between volunteer and career firefighters.
The recruitment of study participants was a joint effort involving local and regional fire departments in Oklahoma, along with the Oklahoma State University Fire Protection & Safety Engineering Technology program. In addition, we attended routine meetings with volunteer fire chiefs to introduce our research and recruit firefighters. Forty-seven firefighters (28 volunteers and 19 careers) were recruited from 11 fire departments in Oklahoma. All participants gave written informed consent after a detailed explanation was provided about the benefits and risks involved with the investigation. Institutional Review Board approval was obtained for this study. Participants who met the following inclusion criteria were eligible for participation: (1) at least 18 years of age, (2) a volunteer or career firefighter in Oklahoma, and (3) a minimum of one year of firefighting experience. Each participant completed a self-administered questionnaire at baseline, in which they were asked to provide details including demographic information, PPE practices, and firefighting-related practices.
We visited each participating fire department and conducted baseline samplings of all study participants. Prior to baseline sampling, none of the participants had engaged in firefighting activities for a period of seven consecutive days. This approach helped to establish a standardized baseline for each participant. Participants also completed a questionnaire that provided additional information about their PAH exposure. For the post-firefighting samples, we visited each fire department within 24 h of the fire, but before the gear was washed, to collect PAH and blood samples.
PAHs deposited on the surface of each firefighter's turnout gear and skin were collected using a 10 cm × 10 cm polyester fabric wipe (Alpha Wipes, Texwipe, Kernersville, NC) saturated with 1 ml of 99% isopropyl alcohol. We applied the wipe with even firm pressure in a consistent area on both the gear and skin. The selection of sampling sites was based on the existing literature, which highlights the neck as the primary site of dermal exposure among firefighters, as PPE hoods offer limited protection (19, 20). Additionally, the wrist of firefighters have high levels of PAH exposure (21). Gear wipes were taken from the right front sleeve and back neck area on the outside surface of the jacket (Supplementary Figure S1). Skin wipes were collected from each firefighter's right forearm and back of the neck (Supplementary Figure S2).
In addition to the wipe samples, a phlebotomist drew 3 ml of blood from each firefighter, storing the samples in EDTA-containing tubes (BD Vacutainer, Franklin Lakes, NJ) to avoid coagulation. The collected blood samples were analyzed at the University of Oklahoma Medical Center laboratory, while the collected wipe samples were analyzed on a Varian 450 Gas Chromatography (GC) equipped with a 200 Mass Spectrometry (MS) detector (Agilent Technologies, Santa Clara, CA), as described previously (22).
For the wipe samples, we excluded a PAH analyte from the analysis if its concentration fell below the limit of detection (LOD) in more than 70% of the samples. For PAHs with concentrations below the LOD, but in less than 70% of the samples, we addressed the issue of missing data by employing regression imputation to estimate each missing value. We employed the IARC group classifications to predict missing values for PAHs detected on gear. For example, phenanthrene exhibited no missing data, while pyrene had 15% missing data. Leveraging the available data from phenanthrene, we imputed the missing values for pyrene using firefighter groups and fire departments as independent variables in pre- and post-firefighting scenarios, respectively. We applied the same approach to impute the missing values for the remaining PAHs.
We compared baseline demographic characteristics between volunteer and career firefighters using Student's t-tests or Mann-Whitney U tests for continuous variables and Pearson Chi-squared tests or Fisher's exact tests for categorical variables. For the analysis of PAHs and CBC parameters, we initially assessed the normality of the data. Based on the data's normality, we selected either parametric or non-parametric statistical methods for data analysis. To compare differences before and after firefighting, we employed paired sample t-tests or Wilcoxon signed-rank tests. For comparing intergroup differences between volunteer and career firefighters, we utilized Student's t-tests or Mann-Whitney U tests.
For the blood samples, a heatmap was created to analyze correlations between 10 PAHs on gear and 16 CBC parameters using the “pheatmap” package (version 1.0.12) in R (version 4.3.0). Spearman correlation coefficients were calculated to measure correlations. The heatmap used a color gradient (red for positive, blue for negative) to depict these correlations, with asterisks indicating statistical significance (p < 0.05). Generalized estimating equations (GEE) models, which can account for the correlation between repeated measurements made on the same subject over time, were used to estimate the association between the CBC parameters and the ∑PAHs deposited on gear or skin. ∑PAHs refers to the sum of the individual PAH concentrations measured separately on the skin or gear samples for each firefighter. Note that in the ∑PAH on skin model, none of the variables exhibited statistical significance. CBC parameters were log-transformed and GEE models were fitted for each CBC parameter with the covariates, which included age, career/volunteer firefighters, pre/post firefighting, BMI, smoking status, years served as a firefighter, and hours per month on a fire scene. Covariates were selected into the models in a forward approach, and any covariate that had a significance level of p < 0.05 was retained. Then, the final models were obtained by adding ∑PAHs to these selected models. The quasi-likelihood information criterion (QIC) was used to compare the final model with the null model, and the model with the smaller QIC was the best working model. The correlations between each type of blood cell from the CBC and each individual PAH were evaluated by the Spearman rank correlation coefficient. Statistical significance was taken at 2-sided P < 0.05. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
In this study, 28 of the firefighters were volunteers, 19 were career, and a majority (94.7%) of the participants were male (Table 1). The average number of years served as a firefighter was 11.6 for volunteers and 10.8 for careers (p = 0.64). All participating firefighters reported engaging in fire-related activities an average of four times per month. However, participation in non-fire-related activities varied significantly between the volunteer and career groups, with volunteers engaging in an average of 1.5 non-fire-related activities per week and career firefighters engaging in an average of eight per week (p < 0.05). Most of the volunteer firefighters did not have a typical work shift; rather, they were always on call (p < 0.05). Regarding health status, body mass index (BMI) showed notable differences between career and volunteer firefighters (p < 0.05). Among career firefighters, 36.84% were overweight and 26.32% were obese category. In contrast, among volunteer firefighters, 21.43% were overweight and a higher percentage, 64.29%, were obese category. Volunteer firefighters reported higher rates of respiratory diseases compared to career firefighters (Supplementary Table S1). Specifically, 14.29% reported asthma, 7.14% reported chronic bronchitis, 10.71% reported coughing, and 3.57% reported seasonal allergies.
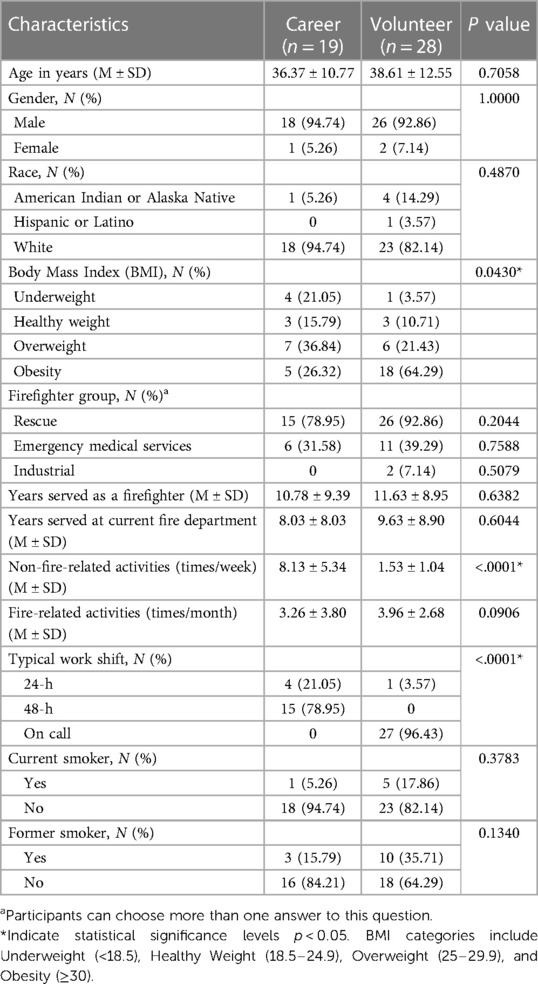
Table 1. Demographic characteristics of career and volunteer firefighters: means and standard deviations (M ± SD) or counts and percentages [N (%)].
Tables 2, 3 present the results of the quantitative analysis of PAHs on the gear and skin, respectively, of firefighters before and after firefighting activities. One post-test gear sample from a career firefighter was not available due to issues with the laboratory analytical extraction. The majority of the studied PAHs showed increased concentration on gear after firefighting, with significant increases observed in acenaphthylene, fluoranthene, fluorene, 1-methyl-naphthalene, 2-methyl-naphthalene, and pyrene. However, there were no significant differences in any PAH levels on the gear between the two groups of firefighters, volunteer and career. Regarding PAH deposition on the skin, we found a significant difference in fluorene after firefighting, while acenaphthene was significantly higher on the skin of volunteer firefighters than on career firefighters.
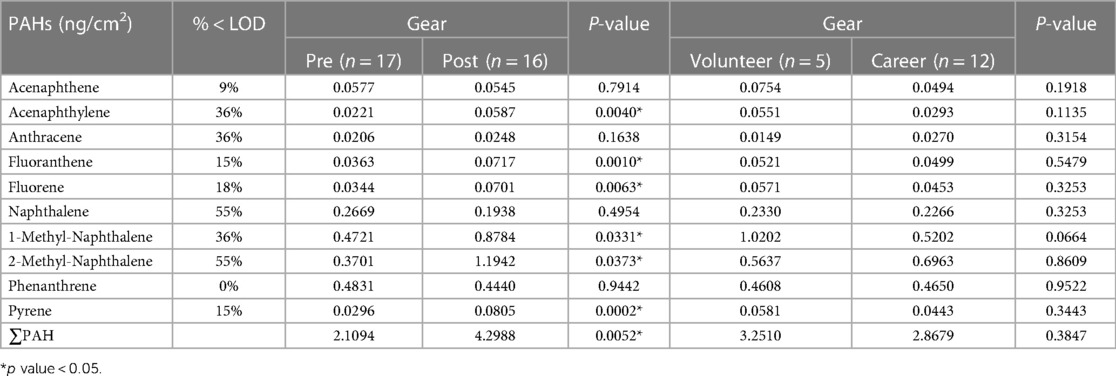
Table 2. Geometric mean concentrations of PAHs on firefighters’ gear at baseline and post-firefighting.
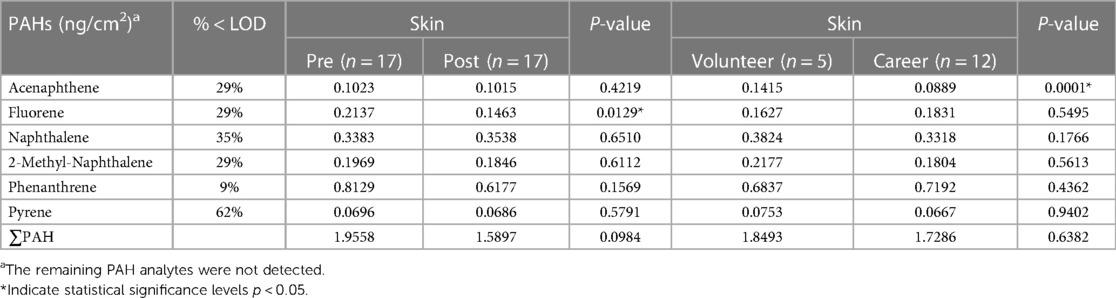
Table 3. Geometric mean concentrations of PAHs on firefighters’ skin at baseline and post-firefighting.
Table 4 compares the six common PAHs found on the firefighters' gear and skin. At baseline, the gear had significantly lower levels of acenaphthene, fluorene, phenanthrene, and pyrene compared to skin. 2-methyl-naphthalene was significantly higher on gear vs. skin at baseline. After firefighting, the gear continued to have lower levels of acenaphthene, fluorene, and phenanthrene vs. skin (p < 0.05). Again, 2-methyl-naphthalene was significantly higher on gear after firefighting compared to skin. There was no significant difference in pyrene levels between gear and skin after firefighting.
Table 5 displays the PAH levels on gear and skin before and after firefighting for both career and volunteer firefighters. For total PAH on gear, there was a statistically significant difference for career firefighters, with an increase from 1.87 ng/cm2 to 4.56 ng/cm2, while the increase for volunteers from 2.80 ng/cm2 to 3.76 ng/cm2 was not statistically significant. Regarding total PAH on skin, the amount on volunteers increased slightly after firefighting, whereas career firefighters showed a decrease from 2.05 ng/cm2 to 1.45 ng/cm2 (p < 0.05).
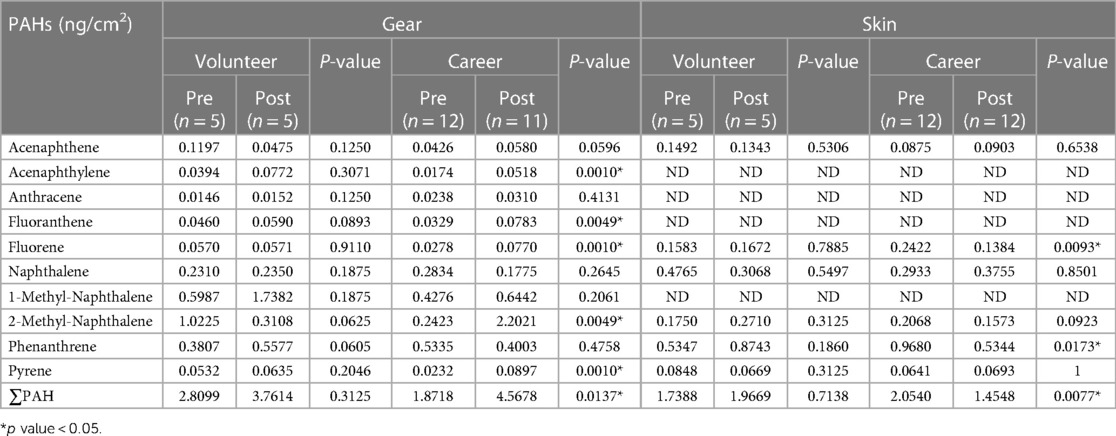
Table 5. Comparison of geometric means of pre- and post-fire levels of PAHs by volunteer and career firefighters.
Table 6 illustrates the response of CBC parameters to firefighting activities for both volunteer and career firefighters. Significant differences were observed in career firefighters for mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), white blood cell (WBC), granulocyte (GRAN) count, and lymphocyte (LYMPH) count (p < 0.05) after firefighting compared to baseline, whereas no significant changes were observed in any CBC parameters for volunteer firefighters.
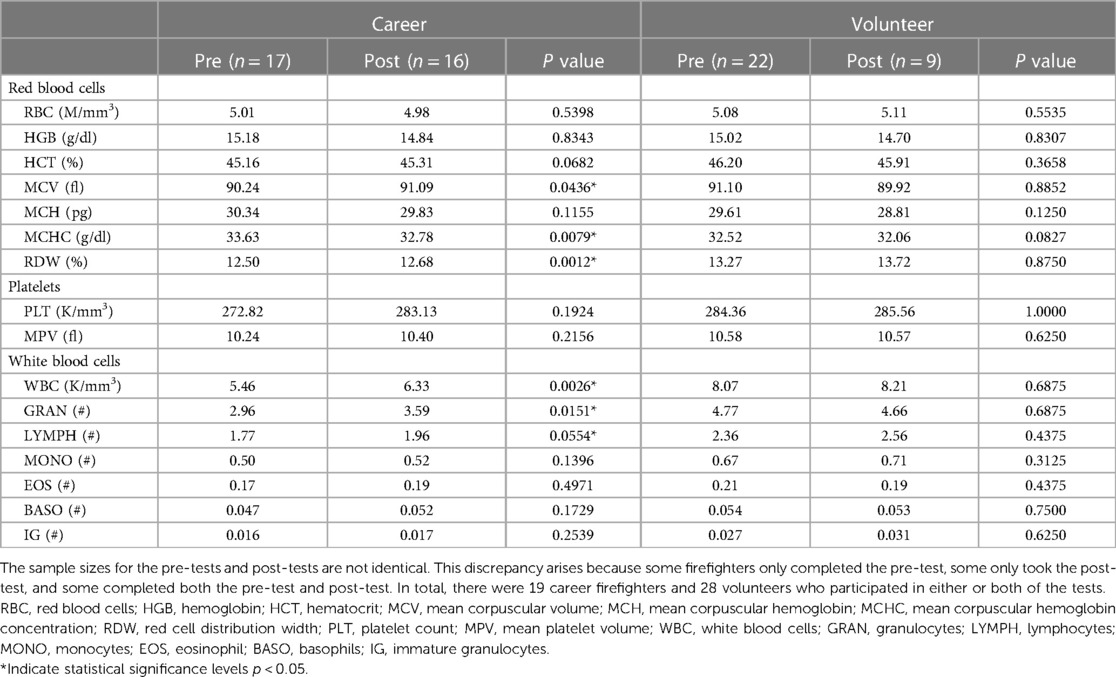
Table 6. Comparison of complete blood count (CBC) parameters before and after firefighting within volunteer and career firefighter groups, with mean values reported.
Figure 1 presents the CBC parameters measured for each group at baseline and after firefighting. At baseline, there were significant differences between volunteer firefighters and career firefighters in MCHC, RDW, WBC, LYMPH count, GRAN count, mononucleosis (MONO) count, and immature granulocytes (IG) count (p < 0.05). Among these, volunteer firefighters had higher levels of all significantly different indicators except for MCHC. However, no significant group difference in CBC parameters was detected after firefighting.
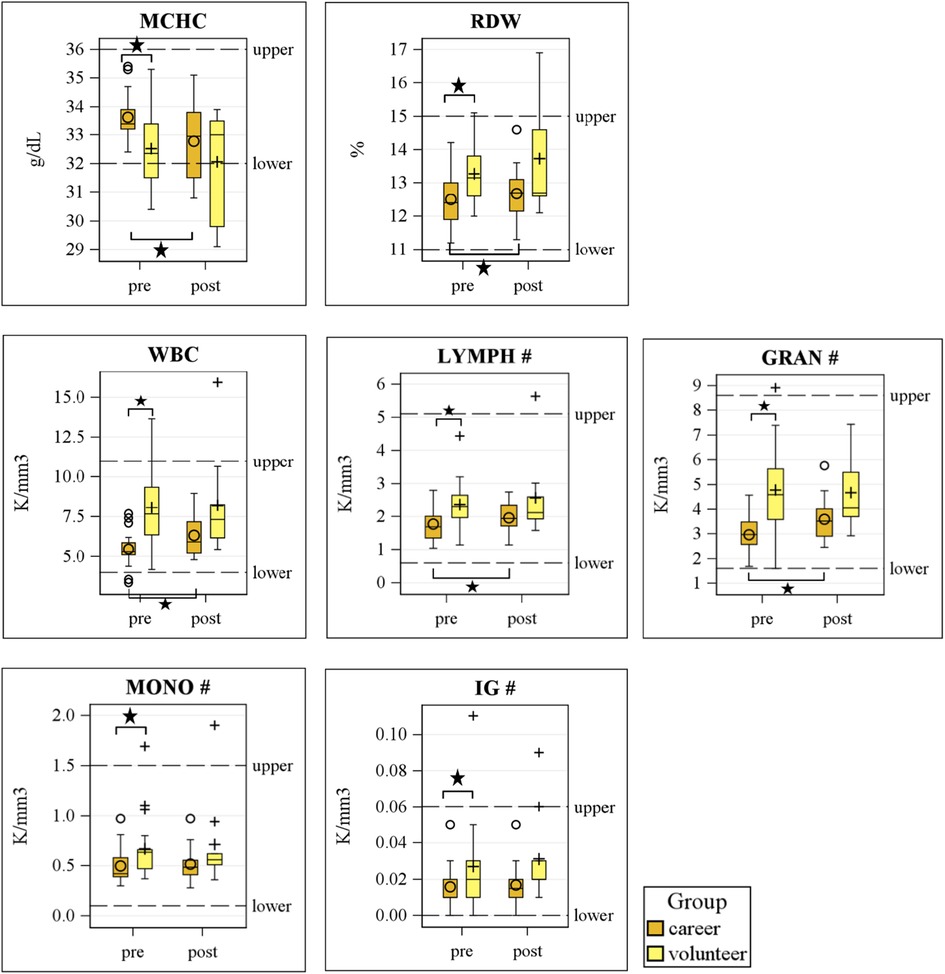
Figure 1. Differences of blood cells between volunteer and career firefighters pre- and post-firefighting. MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; WBC, white blood cells; GRAN, granulocytes; LYMPH, lymphocytes; MONO, monocytes; EOS, eosinophil; BASO, basophils; IG, immature granulocytes.
Table 7 shows the results regarding generalized estimating equations (GEE) modeling between each CBC parameter and the ∑PAHs deposited on gear. Among all firefighters, a 10-unit (ng/cm2) increase in ∑PAHs was associated with a 0.013 (95% CI: 0.002–0.024; p = 0.0155) and 0.028 (95% CI: 0.000–0.057; p = 0.0470) increase in log(MCH) and log(RDW), respectively. A 10-unit increase in ∑PAHs was associated with a 0.096 (95% CI: 0.012–0.180; p = 0.0248) and 0.285 (95% CI: 0.109–0.462; p = 0.0015) increase in log(WBC) and log(IG), respectively. This data suggests that increased ∑PAHs deposition on firefighters’ gear was associated with alterations in hematological and immune parameters. While the changes in MCH and RDW were relatively modest, the notable increases in WBC and IG levels warrant further attention and investigation, as they might be indicative of a significant immune response or inflammation due to PAH exposure.
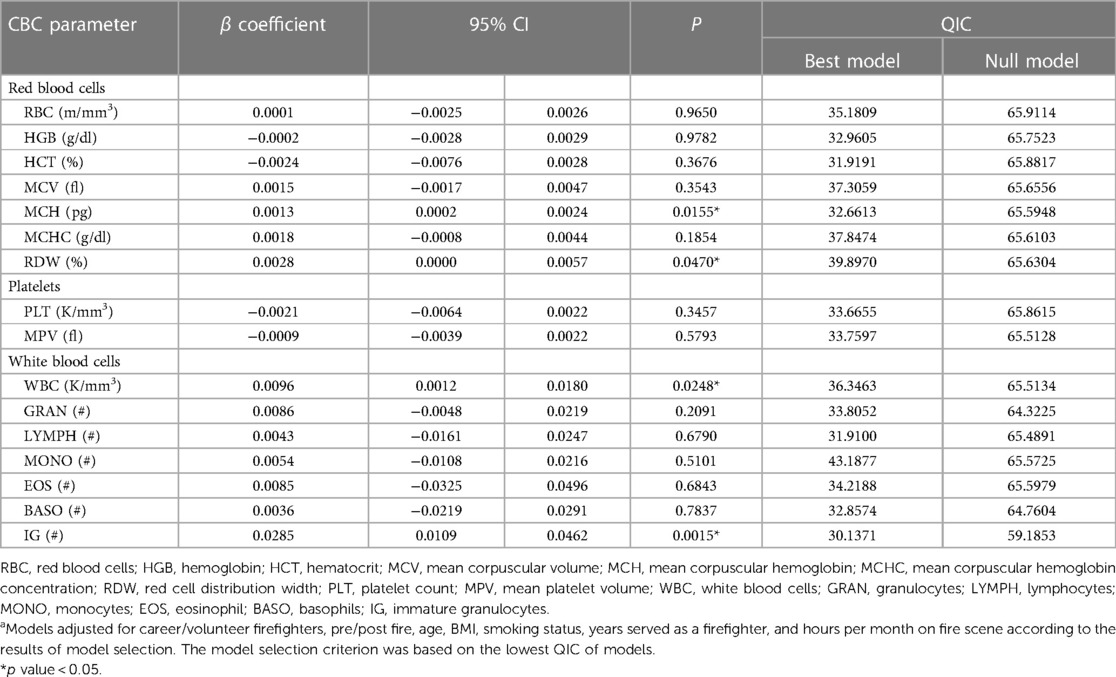
Table 7. Changes of log CBCs (95% CI) and ∑PAHs concentration on turnout gear among firefighters from generalized estimating equations (GEE)a.
We employed heatmap to illustrate the correlations between CBC parameters and individual PAH (Figure 2). Despite the use of multiple testing corrections, our limited sample size had low statistical power to detect certain correlations. Therefore, we present the unadjusted correlation results, with a warning against potential Type I errors. Among the PAHs deposited on gear, we found that the concentration of acenaphthylene, a PAH that increased in firefighters after firefighting, was positively correlated with WBC, GRAN count, LYMPH count, MONO count, and IG count (p < 0.05). Phenanthrene was positively correlated with PLT, LYMPH count, and MONO count. Fluorene was found to be positively correlated with RBC and HCT (p < 0.05). Notably, 1-methyl-naphthalene was positively correlated with RDW (p < 0.05) while negatively correlated with MCH (p < 0.05). However, for PAHs deposited on the skin, we found a different correlation pattern with CBC parameters (Supplementary Figure S3). Acenaphthene was positively correlated with RBC, RDW, WBC, and GRAN count (p < 0.05). Phenanthrene and pyrene were positively correlated with MCHC (p < 0.05), while negatively correlated with MPV, although the latter correlation was not statistically significant.
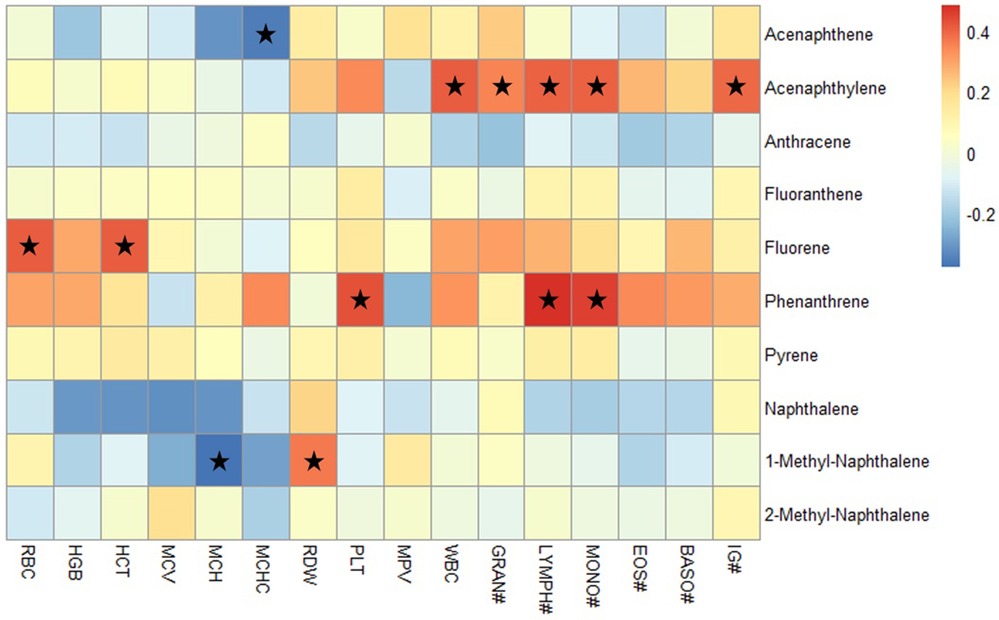
Figure 2. Heatmap analysis of the correlation between PAHs on gear and CBC parameters. The heatmap displays the Spearman correlation coefficients ranging from −0.5 to 0.5. The red spots indicate a positive correlation, while the blue spots represent a negative correlation between PAHs and CBC parameters; the stars indicate significant correlation coefficients with p < 0.05. RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; PLT, platelet count; MPV, mean platelet volume; WBC, white blood cells; GRAN, granulocytes; LYMPH, lymphocytes; MONO, monocytes; EOS, eosinophil; BASO, basophils; IG, immature granulocytes.
We quantified residual PAH levels on gear and skin, highlighting persistent exposure despite protective measures. We assessed alterations in blood cell components pre- and post-exposure, revealing systemic inflammation. By comparing these changes between volunteer and career firefighters, we uncovered differing susceptibility levels, contributing to a better understanding of occupational health risks. Our study is among the first to identify significant differences in CBC components between volunteer and career firefighters prior to engaging in fire suppression activities. This finding is noteworthy because it suggests that even before exposure to the acute stressors and hazards associated with firefighting, these two groups may already differ in ways that could impact their health. However, the literature is scant when it comes to exploring baseline differences in CBC parameters between volunteer and career firefighters, making our study a significant contribution to this area of research. Our study also found that volunteer firefighters had higher total PAH concentrations on their skin after fire suppression compared to baseline levels. This observation is concerning, given that PAH exposure has been associated with a myriad of adverse health outcomes, including increased risks of cancer (7). These findings identify a need to enhance gear cleaning procedures and to implement rigorous decontamination protocols, especially among volunteer firefighters (22). The use of advanced protective clothing designed to minimize PAH skin absorption could also be beneficial (1). Our study thus serves as a call to action for further research and intervention strategies aimed at reducing the health risks associated with firefighting.
MCHC is a measure of the concentration of hemoglobin in cells. A low concentration of hemoglobin within a given volume of red blood cells results in a reduced capacity to carry oxygen to the tissues and affects the cardiovascular system through oxygen supply (23). In our study, we observed a decrease in MCHC levels in both career and volunteer firefighters following firefighting activities. Notably, prior to firefighting, volunteer firefighters exhibited a significantly lower MCHC compared to career firefighters, suggesting potential pre-existing hematological differences that could have implications for their overall health for firefighting duties. Moreover, we identified a negative correlation between MCHC levels and acenaphthene concentrations in the gear wipe samples (p < 0.05). Acenaphthene, a type of PAH, albeit present in low concentrations, may still play a role in reducing MCHC levels in firefighters. This area warrants further investigation, especially considering that acenaphthene is known to follow various metabolic pathways, including oxidation to acenaphthenequinone (24). On the other hand, MCHC levels have been shown to be affected in various hematological conditions (25, 26). Understanding the specific mechanisms through which PAH affects hematological parameters like MCHC could have significant implications for firefighter health.
In our research, RDW was significantly higher in volunteer firefighters after firefighting, while there was no significant difference in career firefighters. Elevated RDW levels have been associated with various cardiovascular conditions (27) and increased risk of cancer mortality (28). A comprehensive meta-analysis has underscored RDW's potential as a prognostic marker in cancer patients, linking high RDW levels to poor outcomes (29). The concentration of 1-methyl-naphthalene on gear had a positive correlation with RDW (p < 0.05). Our wipe sample results showed that the concentration of 1-methyl-naphthalene increased after firefighting compared to the baseline for both career and volunteer firefighters. While 1-methyl-naphthalene is a derivative of naphthalene, which has been studied for its potential chronic effects (30), its specific impact on RDW levels remains unclear. The observed correlation between 1-methyl-naphthalene and RDW may also be helpful in estimating heart disease or cancer risk (14, 31, 32) and warrants further investigation. One study revealed a linear association between RDW and the risk of myocardial infarction (MI), indicating that individuals with a higher RDW were more likely to experience MI, with each 1% increase in RDW associated with a 13% elevated risk (33).
WBC is associated with infection and inflammation in the body. This association highlights the significance of regularly monitoring WBC levels as a valuable marker for overall health assessment and the early detection of potential health risks. A previous study reported a substantial increase in WBC counts, rising from 4.94 K/mm3 to 9.15 K/mm3 immediately after firefighting activities and subsequently stabilizing at 8.12 K/mm3 after 90 min, as serving as markers of systemic inflammation and physiological stress (34). In our study, we observed an increase in WBC counts among career firefighters, rising from 5.46 K/mm3 to 6.33 K/mm3, a statistically significant finding. In contrast, volunteer firefighters experienced a more modest increase, going from 8.07 K/mm3 to 8.21 K/mm3. It is noteworthy that volunteer firefighters exhibited significantly higher baseline WBC counts compared to career firefighters.
These findings collectively contribute to our understanding of the dynamic changes in leukocyte parameters between career and volunteer firefighters in their response to firefighting-related stressors. Positive correlation coefficients were observed between the concentration of acenaphthylene on gear and WBC, GRAN count, LYMPH count, and IG count in firefighters. High LYMPH and GRAN counts could indicate several issues, including infection, as elevated leukocyte counts have been associated with possible infections (35); blood cell cancer, as lymph node counts have been linked to the stage of illness and patient outcome in cancers like pancreatic ductal adenocarcinoma (36); or some type of autoimmune disease, as lymph node alterations have been observed in conditions like chronic kidney disease (37). The increase in leukocytes in firefighters after firefighting may be associated with an elevated level of 1-methyl-naphthalene. The lymphocytes have been proposed as an inflammatory biomarker potential predictor of risk and prognosis in CVD (38). Therefore, 1-methyl-naphthalene exposure may increase the risk of CVD, cancer, and other diseases in firefighters, warranting further investigation.
Our results from the GEE model indicate that the elevated MCH and RDW levels could signify underlying hematological changes that may have long-term health implications for firefighters. A modest 10-unit increase in the sum of PAHs that led to these significant changes is a cause for concern, particularly given the cumulative nature of occupational hazards faced by firefighters. Even low-level chronic exposure to toxic chemicals may result in adverse health outcomes, emphasizing the need for ongoing vigilance. Earlier research has indicated that changes in erythrocyte physiology, such as alterations in MCH, could serve as early markers for systemic issues like anemia or other blood disorders. For instance, the erythrocyte zinc-protoporphyrin/heme (ZPP/H) ratio has been identified as a valid point-of-care biomarker to diagnose iron deficiency anemia (39). Additionally, erythrocyte destruction has been noted as a major factor in the early stages of anemia or inflammation (40). As mentioned earlier, elevated RDW has been identified as an independent prognostic marker for systemic inflammatory responses (41) and specific cardiovascular conditions (42). A stronger increased in sum of PAHs was associated with an elevated WBC and IG. Elevated WBC and IG levels have been associated with an increased risk for infection (43). Such elevations in WBC and IG may not only signify an ongoing inflammatory process but could also be indicative of compromised immune functioning over time.
Most PAHs on gear, including acenaphthylene, fluoranthene, fluorene, 1-methyl-naphthalene, 2-methyl-naphthalene, and pyrene, exhibited significant increases after firefighting, potentially attributable to variations in firefighters' turnout gear practices. While turnout gear is designed to serve as a critical barrier against immediate hazards, our study adds to the existing literature by revealing that it may not provide comprehensive protection against the dermal absorption of PAHs. Specifically, we found compelling evidence of significant disparities in PAH levels between firefighter gear and skin, both at baseline and following firefighting activities. Previous research has shown that PAHs most likely enter firefighters' bodies through their skin, with the neck being a primary site of exposure due to lower dermal protection (20). Moreover, dermal absorption has been identified as a significant contributor to biological levels of PAH metabolites in firefighters (44). The turnout gear, which is treated with per- and polyfluoroalkyl substances (PFAS) chemicals for water and oil resistance, undergoes a shedding process in which PFAS migrate from the outer shell to the thermal liner, the layer in direct contact with the skin (45). This migration could potentially compromise the gear's protective barrier, facilitating the absorption of PAHs through the skin. As PFAS degradation occurs, the chemicals could be absorbed through skin contact with the thermal liner, thereby creating a pathway for PAHs to penetrate as well. Also, the composition of turnout gear has also been found to affect moisture and thermal management, which could influence the protection offered to firefighters (46, 47). Therefore, while turnout gear is designed to protect firefighters, its material properties and degradation over time may inadvertently facilitate the skin absorption of PAHs. Given these findings, our results underscore the need for further research to elucidate the mechanisms leading to differential PAH absorption on gear and skin, as well as to optimize the design and material composition of turnout gear to minimize such risks.
Our study investigating the exposure of firefighters to PAHs has provided valuable insights into the potential health risks associated with firefighting activities. However, it is important to acknowledge several limitations that should be considered when interpreting the findings. Firstly, the study design involved collecting baseline samples from firefighters who had not engaged in firefighting activities for seven consecutive days. While this approach aimed to establish a standardized baseline, it may not fully capture the day-to-day variations in PAH exposure that firefighters experience during their active duty. Additionally, the post-firefighting sampling was conducted within 24 h before the gear was washed. This timing constraint, coupled with the need to coordinate with different fire departments, resulted in multiple visits and potential variations in the post-firefighting data collection process. Secondly, the sample size in this study was relatively small. While efforts were made to recruit a diverse group of participants, the limited sample size may impact the generalizability of the results to a broader population of firefighters, especially considering the potential variations in firefighting practices and PAH exposure levels across different regions. Although PAHs are the primary combustion byproducts in fire smoke, their presence in ambient air is widespread. Our detection of PAHs was unable to disentangle the effects of other confounding factors. Thirdly, the study used self-administered questionnaires to collect information on demographic information, PPE practices, and firefighting-related practices. Self-reported data can be subject to recall bias and may not always accurately reflect participants' actual behaviors and exposures. Finally, the study primarily focused on the association between PAH exposure and CBC parameters, which provide important insights into potential health effects. However, other relevant health indicators or long-term health outcomes were not assessed, limiting the comprehensive understanding of the health implications of PAH exposure among firefighters.
This study reveals noteworthy findings regarding the impact of firefighting activities on both career and volunteer firefighters. Baseline disparities in key blood parameters between these two groups were observed even before engaging in firefighting duties. Specifically, our research identified associations between the concentration of PAHs on gear and changes in blood parameters, including MCHC, RDW, WBC, GRAN count, LYMPH count, MONO count, and IG count. Notably, volunteer firefighters exhibited more adverse baseline levels of these blood parameters compared to their career counterparts. The research highlights an increase in PAH contamination on both gear and skin among volunteer firefighters following firefighting activities, emphasizing the urgent need for enhanced decontamination procedures and improved protective gear. Moving forward, future research should focus on elucidating the mechanisms underlying PAH absorption on gear and skin. This need suggests potential limitations in the protective capabilities of firefighting gear. Overall, these findings emphasize the importance of prioritizing the health and safety of firefighters through better cleaning practices, gear design enhancements, and continued research to understand and mitigate the risks associated with firefighting activities.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Institutional Review Board at the University of Oklahoma Health Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MZ: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. RA: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. CX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Software, Validation, Writing – review & editing. TV: Funding acquisition, Project administration, Resources, Validation, Writing – review & editing. XX: Investigation, Resources, Validation, Writing – review & editing. JH: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported in part by the National Institute for Occupational Safety and Health (NIOSH K01OH011891), the Oklahoma Shared Clinical and Translational Resources (NIGMS U54GM104938), and an Institutional Research Grant from the American Cancer Society (134128-IRG-19-142-01). JH was partially supported by the Southwest Center for Occupational and Environmental Health (NIOSH T42OH008421).
We sincerely thank all the firefighters who voluntarily participated in this study. Their commitment was fundamental to the progress of our research. Thanks to Brittany Karfonta and Gina Herbert for their role in blood sample collection for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvh.2024.1381159/full#supplementary-material
1. Fernando S, Shaw L, Shaw D, Gallea M, VandenEnden L, House R, et al. Evaluation of firefighter exposure to wood smoke during training exercises at burn houses. Environ Sci Technol. (2016) 50(3):1536–43. doi: 10.1021/acs.est.5b04752
2. Guidotti TL. Health Risks and Fair Compensation in the Fire Service. Cham, Switzerland: Springer (2016).
3. International Agency for Research on Cancer. Painting, Firefighting, and Shiftwork. Lyon, France: IARC Press, International Agency for Research on Cancer (2010).
4. Plog B, Quinlan P. Fundamentals of Industrial Hygiene. 6th ed Itasca, IL: National Safety Council (2012).
5. Hakkarainen T, Tillander K, Järnström H, Paloposki T, Laitinen J, Oksa P. Chemical exposure and protection of fire site workers. In Interflam 2010, 5.5–7.7. Nottingham. (2010). p. 937–48.
6. Fent KW, Alexander B, Roberts J, Robertson S, Toennis C, Sammons D, et al. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J Occup Environ Hyg. (2017) 14(10):801–14. doi: 10.1080/15459624.2017.1334904
7. Stec AA, Dickens KE, Salden M, Hewitt FE, Watts DP, Houldsworth PE, et al. Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Sci Rep. (2018) 8(1):2476. doi: 10.1038/s41598-018-20616-6
8. Bahri R, Saidane-Mosbahi D, Rouabhia M. Cytokine release and cytotoxicity in human keratinocytes induced by polycyclic aromatic hydrocarbons (1-methylpyrene and perylene). J Toxicol Environ Health, Part A. (2010) 73(8):552–64. doi: 10.1080/15287390903566617
9. Lauer FT, Parvez F, Factor-Litvak P, Liu X, Santella RM, Islam T, et al. Changes in human peripheral blood mononuclear cell (HPBMC) populations and T-cell subsets associated with arsenic and polycyclic aromatic hydrocarbon exposures in a Bangladesh cohort. PLoS One. (2019) 14(7):e0220451. doi: 10.1371/journal.pone.0220451
10. Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci. (2015) 145(1):5–15. doi: 10.1093/toxsci/kfv040
11. Zhang H, Han Y, Qiu X, Wang Y, Li W, Liu J, et al. Association of internal exposure to polycyclic aromatic hydrocarbons with inflammation and oxidative stress in prediabetic and healthy individuals. Chemosphere. (2020) 253:126748. doi: 10.1016/j.chemosphere.2020.126748
12. Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. (2005) 206(1):73–93. doi: 10.1016/j.taap.2004.11.006
13. Kounis NG, Soufras GD, Tsigkas G, Hahalis G. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost. (2015) 21(2):139–43. doi: 10.1177/1076029614531449
14. Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis. (2015) 7(10):E402. doi: 10.3978/j.issn.2072-1439.2015.10.04
15. Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Tex Heart Inst J. (2013) 40(1):17.23467296
16. Bogdanova A, Kaestner L, Simionato G, Wickrema A, Makhro A. Heterogeneity of red blood cells: causes and consequences. Front Physiol. (2020) 11:392. doi: 10.3389/fphys.2020.00392
17. Pan J, Borné Y, Engström G. The relationship between red cell distribution width and all-cause and cause-specific mortality in a general population. Sci Rep. (2019) 9(1):16208. doi: 10.1038/s41598-019-52708-2
18. Shankar A, Mitchell P, Rochtchina E, Wang JJ. The association between circulating white blood cell count, triglyceride level and cardiovascular and all-cause mortality: population-based cohort study. Atherosclerosis. (2007) 192(1):177–83. doi: 10.1016/j.atherosclerosis.2006.04.029
19. Fent KW, Eisenberg J, Evans D, Sammons D, Robertson S, Striley C, et al. (2013). Report No.: 2010-0156-3196. Evaluation of dermal exposure to polycyclic aromatic hydrocarbons in fire fighters. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, NIOSH HETA.
20. Fent KW, Eisenberg J, Snawder J, Sammons D, Pleil JD, Stiegel MA, et al. Systemic exposure to PAHs and benzene in firefighters suppressing controlled structure fires. Ann Occup Hyg. (2014) 58(7):830–45. doi: 10.1093/annhyg/meu036
21. Levasseur JL, Hoffman K, Herkert NJ, Cooper E, Hay D, Stapleton HM. Characterizing firefighter’s exposure to over 130 SVOCs using silicone wristbands: a pilot study comparing on-duty and off-duty exposures. Sci Total Environ. (2022) 834:155237. doi: 10.1016/j.scitotenv.2022.155237
22. Hwang J, Taylor R, Cann C, Norris P, Golla V. Evaluation of accumulated polycyclic aromatic hydrocarbons and asbestiform fibers on firefighter vehicles: pilot study. Fire Technol. (2019) 55:2195–213. doi: 10.1007/s10694-019-00851-7
23. Finch CA. Oxygen transport in man. Chest. (1972) 61(2):12S–3S. doi: 10.1016/S0012-3692(15)32680-5
24. Schocken MJ, Gibson DT. Bacterial oxidation of the polycyclic aromatic hydrocarbons acenaphthene and acenaphthylene. Appl Environ Microbiol. (1984) 48(1):10–6. doi: 10.1128/aem.48.1.10-16.1984
25. Steinberg MH, Rosenstock W, Coleman MB, Adams JG, Platica O, Cedeno M, et al. Effects of thalassemia and microcytosis on the hematologic and vasoocclusive severity of sickle cell anemia. Blood. (1984) 63(6):1353–60. doi: 10.1182/blood.V63.6.1353.1353
26. Cheng L, Li L, Liu C, Yan S, Chen H, Li H, et al. Variation of red blood cell parameters in behcet’s disease: association with disease severity and vascular involvement. Clin Rheumatol. (2021) 40:1457–64. doi: 10.1007/s10067-020-05397-6
27. Bujak K, Wasilewski J, Osadnik T, Jonczyk S, Kołodziejska A, Gierlotka M, et al. The prognostic role of red blood cell distribution width in coronary artery disease: a review of the pathophysiology. Dis Markers. (2015) 2015:824624. doi: 10.1155/2015/824624
28. Li J, Yang X, Ma J, Gong F, Chen Q. Relationship of red blood cell distribution width with cancer mortality in hospital. BioMed Res Int. (2018) 2018:8914617. doi: 10.1155/2018/8914617
29. Hu L, Li M, Ding Y, Pu L, Liu J, Xie J, et al. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget. (2017) 8(9):16027. doi: 10.18632/oncotarget.13784
30. Adedayo Adesina O, Ademola Sonibare J, Diagboya PN, Adejuwon A, Famubode T, Bello JO. Periodic characterization of alkyl-naphthalenes in stack gas and ambient air around a medical waste incinerator. Environ Sci Pollut Res. (2017) 24:21770–7. doi: 10.1007/s11356-017-9828-1
31. Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all cause mortality in critically ill patients. Crit Care Med. (2011) 39(8):1913. doi: 10.1097/CCM.0b013e31821b85c6
32. Xanthopoulos A, Giamouzis G, Melidonis A, Kitai T, Paraskevopoulou E, Paraskevopoulou P, et al. Red blood cell distribution width as a prognostic marker in patients with heart failure and diabetes mellitus. Cardiovasc Diabetol. (2017) 16(1):1–9. doi: 10.1186/s12933-017-0563-1
33. Skjelbakken T, LappegAard J, Ellingsen TS, Barrett-Connor E, Brox J, Løchen ML, et al. Red cell distribution width is associated with incident myocardial infarction in a general population: the Tromsø study. J Am Heart Assoc. (2014) 3(4):e001109. doi: 10.1161/JAHA.114.001109
34. Smith DL, Petruzzello S, Chludzinski M, Reed J, Woods J. Selected hormonal and immunological responses to strenuous live-fire firefighting drills. Ergonomics. (2005) 48(1):55–65. doi: 10.1080/00140130412331303911
35. McClain MT, Park LP, Nicholson B, Veldman T, Zaas AK, Turner R, et al. Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J Clin Virol. (2013) 58(4):689–95. doi: 10.1016/j.jcv.2013.09.015
36. Lahat G, Lubezky N, Gerstenhaber F, Nizri E, Gysi M, Rozenek M, et al. Number of evaluated lymph nodes and positive lymph nodes, lymph node ratio, and log odds evaluation in early-stage pancreatic ductal adenocarcinoma: numerology or valid indicators of patient outcome? World J Surg Oncol. (2016) 14:1–8. doi: 10.1186/s12957-016-0983-5
37. Karlsen TV, Nikpey E, Han J, Reikvam T, Rakova N, Castorena-Gonzalez JA, et al. High-salt diet causes expansion of the lymphatic network and increased lymph flow in skin and muscle of rats. Arterioscler, Thromb, Vasc Biol. (2018) 38(9):2054–64. doi: 10.1161/ATVBAHA.118.311149
38. Venkatraghavan L, Tan TP, Mehta J, Arekapudi A, Govindarajulu A, Siu E. Neutrophil lymphocyte ratio as a predictor of systemic inflammation-A cross-sectional study in a pre-admission setting. F1000Res. (2015) 4:123. doi: 10.12688/f1000research.6474.1
39. Kanuri G, Chichula D, Sawhney R, Kuriakose K, De’Souza S, Pais F, et al. Optimizing diagnostic biomarkers of iron deficiency anemia in community-dwelling Indian women and preschool children. Haematologica. (2018) 103(12):1991. doi: 10.3324/haematol.2018.193243
40. Weiss D, Krehbiel J. Studies of the pathogenesis of anemia of inflammation: erythrocyte survival. Am J Vet Res. (1983) 44(10):1830–1.6638642
41. Li B, You Z, Xiong X-Z, Zhou Y, Wu S-J, Zhou R-X, et al. Elevated red blood cell distribution width predicts poor prognosis in hilar cholangiocarcinoma. Oncotarget. (2017) 8(65):109468. doi: 10.18632/oncotarget.22694
42. Yang T, Sun Y-J, Xiong C-M, Zeng W-J, Ni X-H, Zhao Z-H, et al. Red blood cell distribution width predicts survival in patients with eisenmenger syndrome. Clin Chem Lab Med. (2014) 52(5):743–50. doi: 10.1515/cclm-2013-0747
43. van der Geest PJ, Mohseni M, Brouwer R, van der Hoven B, Steyerberg EW, Groeneveld AJ. Immature granulocytes predict microbial infection and its adverse sequelae in the intensive care unit. J Crit Care. (2014) 29(4):523–7. doi: 10.1016/j.jcrc.2014.03.033
44. Fent KW, Toennis C, Sammons D, Robertson S, Bertke S, Calafat AM, et al. Firefighters’ and instructors’ absorption of PAHs and benzene during training exercises. Int J Hyg Environ Health. (2019) 222(7):991–1000. doi: 10.1016/j.ijheh.2019.06.006
45. Peaslee GF, Wilkinson JT, McGuinness SR, Tighe M, Caterisano N, Lee S, et al. Another pathway for firefighter exposure to per-and polyfluoroalkyl substances: firefighter textiles. Environ Sci Technol Lett. (2020) 7(8):594–9. doi: 10.1021/acs.estlett.0c00410
46. Carballo-Leyenda B, Villa JG, López-Satué J, Rodríguez-Marroyo JA. Impact of different personal protective clothing on wildland firefighters’ physiological strain. Front Physiol. (2017) 8:618. doi: 10.3389/fphys.2017.00618
Keywords: firefighters, polycyclic aromatic hydrocarbons (PAHs), occupational exposure, blood cells, fire smoke, turnout gear
Citation: Zhu M, Agnew RJ, Xu C, VanWagoner T, Xu X and Hwang J (2024) Residual polycyclic aromatic hydrocarbons and firefighters' hematological profile. Front. Environ. Health 3:1381159. doi: 10.3389/fenvh.2024.1381159
Received: 2 February 2024; Accepted: 23 April 2024;
Published: 6 May 2024.
Edited by:
Antonino Maniaci, Kore University of Enna, ItalyReviewed by:
Salvatore Lavalle, San Raffaele Hospital (IRCCS), Italy© 2024 Zhu, Agnew, Xu, VanWagoner, Xu and Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jooyeon Hwang am9veWVvbi5od2FuZ0B1dGgudG1jLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.