
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Health, 19 December 2023
Sec. Occupational Safety and Health Interventions
Volume 2 - 2023 | https://doi.org/10.3389/fenvh.2023.1325197
Background: While it is well-established that occupational exposures significantly contribute to the risk of developing lung cancer, there remains a notable gap in understanding the specific sex differences in lung cancer risk, particularly among female workers. This study aimed to examine sex differences in lung cancer risk across various occupations, with an emphasis on the female workforce.
Methods: A cohort of approximately 2.37 million workers with lost-time compensation claims were linked to the Ontario Cancer Registry and followed until lung cancer diagnosis, age 85, emigration, death, or end of follow-up (Dec 31, 2020). Cox proportional hazards models were used to estimate sex-specific hazard ratios (HRs) and 95% confidence intervals (CIs) for lung cancer by occupational group (division, major and minor levels), adjusted for birth-year and age and indirectly adjusted for cigarette smoking.
Results: A total of 12,216 and 30,291 incident lung cancer cases were identified among females and males, respectively. Several occupations demonstrated stronger associations for lung cancer in females, with at least a 20% increased risk compared to males. These occupations at the major level include food and beverage preparation services (HR = 1.19, 95% CI = 1.13–1.26); materials processing (chemical, petroleum, rubber, plastic) (HR = 1.35, 95% CI = 1.19–1.52); wood processing (HR = 1.87, 95% CI = 1.22–2.87); metal machining (HR = 1.56, 95% CI = 1.21–2.00); metal shaping and forming (HR = 1.46, 95% CI = 1.32–1.62); fabricating and assembling metal products (HR = 1.37, 95% CI = 1.25–1.51), other construction trades (HR = 1.54, 95% CI = 1.16–2.05), motor transport operating (HR = 1.69, 95% CI = 1.48–1.94), mechanic and repair work (HR = 1.39, 95% CI = 1.04–1.85); and printing (HR = 1.51, 95% CI = 1.30–1.75). These patterns were similar across minor level occupations.
Conclusions: This study identified sex differences across various occupations, with some occupational groups demonstrating stronger associations among female workers. However, these findings should be interpreted with caution. The observed differences may be attributed to various factors that influence risk, such as occupational exposures, use and effectiveness of personal protective equipment, and other biological or lifestyle factors.
Lung cancer is the leading cancer and cause of cancer-related deaths in Canada (1). It has been estimated that occupational exposures are responsible for approximately 15% of lung cancer cases in Canada (2). Despite substantial contributions to occupational lung cancer research, there is a significant knowledge gap when it comes to understanding sex differences in occupational lung cancer risk. Research on occupational risk factors for lung cancer have primarily focused on males, lacking sufficient evidence regarding female workers (3).
The International Agency for Research on Cancer (IARC) has identified many occupational exposures, such as asbestos, acheson process, aluminum production, arsenic, cadmium, chromium VI, diesel engine exhaust, iron and steel founding, nickel, outdoor air pollution, painting, radon-222, soot, silica dust, welding fumes, and x- and gamma-radiation, as lung carcinogens in humans (4). There is evidence linking lung cancer risk to specific male-dominated occupations in construction (5), quarries, sand pits and mining (6), transportation (7), painting (8), and welding (9), even after adjusting for cigarette smoking. However, there is limited understanding of sex-specific differences in lung cancer risk (10). Some studies have explored occupations where females may be at a risk, such as hairdressing (11, 12), and nail salons (13, 14), which may involve exposure to chemicals in hair dyes (11), and volatile organic compounds (14), respectively. Another study identified increased risk of lung cancer among female nurses with long duration of rotating night shift work, although this was only observed among smokers (15). Furthermore, environmental tobacco smoke exposure, including second-hand smoke, varies by occupation, with certain industries having the highest exposure (e.g., trades, transport, and equipment operating; sales and services) (16). Exposure to environmental tobacco smoke may also differ by sex, although findings have been limited (17).
Exposures to carcinogens within the same industry or occupation may vary by sex. Personal protective equipment (PPE) and safety measures may not be equally effective for females as they are for males, given that the equipment is not designed with female-specific fit in mind (18). Additionally, females may have less training in proper PPE use and experience higher energy costs when using PPE (18). Gender roles may also influence specific tasks assigned to workers within a particular industry or occupation, leading to sexual division of labour (18, 19). Studies have shown that females and males with the same job title often perform different tasks (20, 21). For example, in railway cleaning occupations, females were more likely to be assigned tasks involving greater exposure and inhalation of chemical products (21).
The objective of this study was to examine potential sex differences in lung cancer risk within the Occupational Disease Surveillance System (ODSS), a large cohort of Ontario workers. This study aimed to explore whether females may have a higher risk of lung cancer compared to males, across the same occupational groups.
The Occupational Disease Surveillance System (ODSS) is a unique cohort that was developed by linking Ontario workers to large administrative health databases to monitor occupational disease (22, 23). The ODSS was established using accepted lost-time compensations claimants from the Workers Safety Insurance Board (WSIB) from 1983 to 2019 (n = 2,387,756). The WSIB claims data included the workers' occupation and industry at the time of claim. Occupation was coded according to the 1971 Canadian Classification Dictionary of Occupation (CCDO) and industry was coded using the 1970 and 1980 Standard Industrial Classification (SIC). Both coding systems consist of three levels of classification: division (broadest), major (intermediate), and minor (most specific). Workers with missing sex, birthdate, claim date, occupation/industry, or under the age of 15 years were excluded (n = 17,557). Workers were then linked using name, sex, and birthdate to the Registered Persons' Database (RPDB) which provides information on death data, residence in the province (emigration), and unique health insurance number (HIN). A small number of workers could not be linked to the RPDB due to missing information (n = 1,979). This linkage resulted in a total of 2,368,220 workers in the cohort (Figure 1).

Figure 1. Description of the linkage process used to identify incident cases of lung cancer among female and male workers in the occupational disease surveillance system (ODSS).
Workers were then linked to the Ontario Cancer Registry (OCR) (1964–2020) through deterministic (by use of HIN) and probabilistic (without HIN, by use of name, sex, birth date, and death date) linkages. Workers with a cancer diagnosis prior to cohort entry (January 1, 1983) were excluded as these were recognized as prevalent cases (n = 197,927) (Figure 1). Workers entering the cohort with a cancer claim were also excluded from this analysis. Incident cases were coded using the International Classification of Diseases 10th Revision.
Entry into the cohort was based on the first claim and workers were censored at date of cancer diagnosis, death, emigration, age 85, and the end of the study period (December 31, 2020). Lung cancer risk was estimated using a Cox proportional hazard model to compare the risk within one occupation to all other groups within the cohort.
All models were adjusted for age at start of follow-up and birth year and indirectly adjusted for current and former cigarette smoking. Indirect adjustment for cigarette smoking was achieved using another provincial data source known as the Canadian Community Health Survey (CCHS). The CCHS is a cross-sectional national survey that collects information on health determinants, health status, among other characteristics. Prevalence estimates (i.e., the proportion of current and former smokers) were calculated from division level industry groups obtained from eight pooled cycles of the CCHS (2007–2014) for Ontario respondents only (age 15 and older). Stratums were created by grouping CCHS participants by their North American Industry Classification System (NAICS) code, sex, and birth year (as five-year age groups). The prevalence of current and former smokers was calculated for each stratum. As the ODSS uses the Standard Industrial Classification (SIC), a different coding system from what is used in the CCHS data; a crosswalk was required and successfully applied between the two datasets to help transition from one coding system to the other. This is to ensure that industry groups presented in the ODSS were represented by the industry groups in the CCHS data. If workers had multiple claims for different industries, smoking proportion was assigned based on their first accepted claim.
Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for each occupation group at division, major, and minor levels. Hazard ratios for occupational groups in which there were less than 6 cases are suppressed due to reporting guidelines. Statistical analysis was performed using SAS V.9.4 (SAS Institute). This study was approved by the University of Toronto Health Sciences Research Ethics Board (#39013).
A total of 12,216 (29%) and 30,291 (71%) incident lung cancers were identified among females and males, respectively. Follow up-time was generally shorter for lung cancer cases but similar for both sexes (Table 1).
By occupation at the division level, sex differences in lung cancer risk were observed with adjustment for age at start of follow-up, birth year, and indirect adjustment for cigarette smoking (Table 2). Eleven of the 20 division level groups, including a wide range of occupations, observed hazard ratios for lung cancer greater than 10% higher among females compared to males. Females in mining and quarrying, machining, forestry and logging, construction, services, transport equipment operating, and other crafts and equipment operating had approximately 20% or higher increased risk of lung cancer, compared to males.

Table 2. Sex-specific risk of lung cancer by division level occupation groups compared to all other workers in the ODSS (1983–2020).
Table 3 presents selected occupational major groups where females or males had elevated risks of lung cancer, with adjustment for age at start of follow-up, birth year, and cigarette smoking. Within these major groups, selected minor groups are shown where there were distinct differences or similarities in lung cancer risk in females or males. It is clear that many major level groups had higher risks among female workers, in comparison to male workers (e.g., food and beverage preparation services; metal machining; printing) and patterns remain consistent when examining minor level groups. Lung cancer risk in workers across all major and minor level groups are shown in Supplementary Table S1.
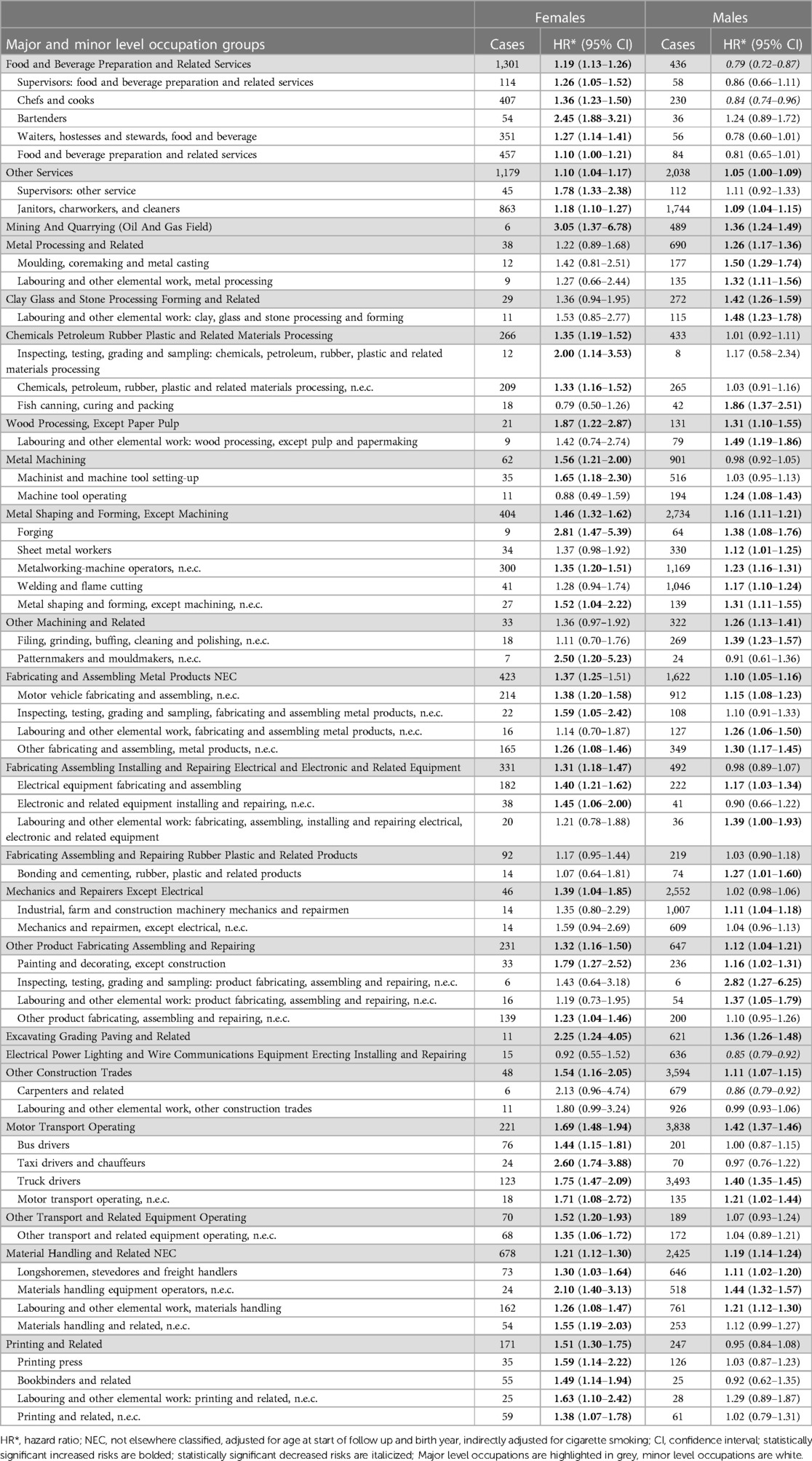
Table 3. Sex-specific risk of lung cancer in selected major and minor level occupation groups compared to all other workers in the ODSS (1983–2020).
To date, few studies have explored the relationship between occupation and lung cancer risk in females compared to males in the same occupations. In this large worker cohort, we found that females in various traditional high-risk occupations had elevated risks for lung cancer consistent with males in the same occupations. Our study also observed higher lung cancer risks among females than males in certain occupations, emphasizing sex-based differences in lung cancer risk. Previously, Pukkala et al. (24) also reported similar findings for female construction workers, transport workers, printers, mechanics, chemical process workers, and drivers, among other occupations (24).
In this study we observed a greater risk of lung cancer among females relative to males in some occupational groups with well-established exposure to workplace lung carcinogens (25). This includes certain construction occupations, particularly in excavating, grading, and paving, as well as among carpenters and glaziers, with exposure to asbestos, diesel engine exhaust, and crystalline silica (26, 27). Asbestos exposure is likely when construction workers are engaged in the maintenance, renovation, or demolition of older buildings that once used asbestos materials. They can be exposed to diesel engine exhaust when operating diesel powered machines, especially in enclosed spaces. Exposure to silica is also likely as many construction activities generate dust from silica-containing materials (e.g., chipping, sawing). Workers may also be exposed to bitumens used in asphalt and roofing, which is classified as possibly carcinogenic to humans (4). Females in chemical, petroleum, rubber, plastic and related processing had a higher risk of lung cancer and rubber manufacturing work has been associated to lung cancer (4). Female transport equipment operators, including truck, bus, and taxi drivers, with exposure to diesel engine and other motor vehicle exhaust were also at increased risk (28). A very large gap, though based on very small numbers, was also seen for mining, with exposure to diesel engine exhaust, crystalline silica, radon, and metals (25). Females in printing occupations also showed an increased risk of lung cancer, which has been linked to lung cancer with limited evidence (4). In almost all cases, males were also at increased risk, but the relative risk among females was higher.
We also observed a greater risk of lung cancer females relative to males in some other “blue collar” occupational groups with plausible, but less established exposure to workplace lung carcinogens. These include machining; forestry and logging; wood processing; many fabricating and assembling involving metal; and electrical/electronic products. Risk of lung cancer varied in processing occupations, with stronger associations among females in specific materials processing and wood processing, while males had stronger associations in metal processing. Females in metal-related occupations, including metal machining, demonstrated a stronger association with lung cancer risk compared to males. These metal workers are likely exposed to dusts and fumes containing carcinogenic metals and their compounds (2, 29–32). We also observed a stronger association among female mechanics/repairers (excluding electrical) and those in other occupations involving fabrication, assembly, and repair.
We identified greater risk of lung cancer among females in service occupations, specifically among food and beverage preparation services (e.g., chefs/cooks, bartenders) and other services (e.g., cleaning). Service occupations includes a wide range of jobs, making it challenging to pinpoint potential exposures. However, a common exposure among these workers, particularly those in restaurant settings, is second-hand smoke, prior to the ban on smoking in restaurants and patios (16). Some research suggests that polycyclic aromatic hydrocarbons (PAHs found in cooking fumes) may be related to lung cancer risk (32, 33) but there also may be other unaccounted-for exposures. There is also evidence that lung cancer may be associated to occupational cleaning activities, particularly among females with increased duration of employment (34).
In this paper, we emphasize associations showing an increased risk among females because we believe that highlights a significant gap in the literature. There notably fewer associations indicating a greater risk among males (e.g., other machining). It is likely that lung cancer risk is misrepresented among female workers due to inadequate occupational history-taking, lack of recognition of environmental exposures, and limited and biased bibliographies by conflicted authors, as previously demonstrated in the incidence of mesothelioma (35).
Some of the sex differences in this study may be due to true differences in exposure. Two studies by Messing and colleagues identified that the tasks assigned to workers within a given occupation differed by gender and could in turn influence hazardous exposures (20, 21). Gender biases may also influence the tasks assigned to workers within the same occupation (19). Sex differences in PPE effectiveness may contribute to study findings. Many of the blue-collar occupations are male dominated where PPE was primarily designed for a male-specific fit and this may impact female worker occupational exposures to carcinogenic agents (19). Han (18) found that respirators had decreased fit among females which would result in increased exposure to dusts and fumes when respirators are used as a form of protection against contaminants in the air (18).
Sex differences in occupational lung cancer may be confounded by lifestyle factors that vary by sex and occupation. In our study, we indirectly adjusted for cigarette smoking using group industry smoking prevalence estimates from another Ontario population data source and observed little change in associations (<10%). This may suggest that there are occupational exposures or other factors driving the increased risk of lung cancer in identified occupations. It is important to note we do not have information on individual cigarette smoking habits and this may still impact the associations we observe in this study. Syamlal and colleagues found that even with adjustments for age, race, education and income, the odds of smoking were higher among females in manual labour occupations than males (36). Education, income, diet, and physical activity are also important factors to consider when assessing risk of lung cancer by sex and occupation. We could not adjust for these factors which may impact our findings by sex and occupation, particularly among occupations where few occupational exposures are established.
There may be biological and physiological differences between males and females which may impact lung cancer susceptibility and the hazardous effects of certain occupational exposures. A systematic review by Kiyohara and Ohno (37) suggests that, for a given number of cigarettes smoked, females may be at greater risk of lung cancer than males (37). Female lungs may also be more susceptible to developing cancer following the same occupational exposure to a carcinogenic agent as a male. Furthermore, on average, females have smaller lung capacity and higher airway flow rates (38) which could result in greater aerosol deposition in females (10). Sex differences in genetic and hormonal factors may also influence susceptibility (39).
There are a number of limitations with this study. Information on workers' occupation was collected at a single point in time through claims data. This may not be reflective of a worker's complete employment history or duration of employment. This could result in non-differential misclassification of exposure, biasing the result towards the null. Some workers had multiple accepted claims between 1983 and 2019 and when restricting to those who did not change occupation based on claim information, the results changed minimally (<5%). Where there were minor changes in risk estimates, the direction of association and significance did not change. We also lack information on occupational exposures related to lung cancer. We were unable to adjust for lifestyle factors such as diet or physical activity as there is no information on these characteristics in the cohort. Although we attempted to adjust for cigarette smoking using population group estimates, this data is limited as it pertains to the period from 2007 to 2014, overlapping only a portion of the follow-up period in our study. We were restricted to utilizing data from these years as it encompassed the maximum number of years available—eight years of cigarette smoking data. We could not incorporate data from more recent years because cigarette smoking estimates could not be combined with data predating 2015 due to a significant re-design in the national survey used to obtain cigarette smoking data. These group estimates do not accurately represent individual cigarette smoking habits among workers in the ODSS. There also may be residual confounding from smoking which may have an impact on findings but is challenging to minimize or account for in this study.
Another potential limitation is selection bias; workers who are injured at work and receive lost-time compensation may differ systematically from those whose claims were rejected or not compensation for, or where workers did not submit a claim at all. For example, females report lower injury rates at work and are more likely to experience injuries of repetitive motion such as carpel tunnel syndrome which are less likely to receive compensation (40). Workers' compensation data may under-represent injury, particularly among females and precariously employed workers (41). Reflecting Ontario's overall workforce, in our cohort, females are over-represented in service and administrative occupations while male workers are over-represented in manufacturing occupations where workers are generally at greater risk of carcinogenic exposures (42, 43).
The key strength of this study is the large sample size of male and female Ontario workers, made possible by the linkage of very large administrative datasets. This allowed for sex-specific risks to be analyzed among a very large number of occupational groups, even among male-dominated occupations where females are often excluded from analyses. In addition, by analyzing lung cancer risk within the ODSS, we were able to compare workers to other workers, minimizing the healthy worker effect and providing a comparison group that is similar.
In conclusion, this study identified sex differences in lung cancer risk by occupation among Ontario workers. This study expanded on previous lung cancer findings in the ODSS to demonstrate that females experienced similar or higher risks of lung cancer compared to males in many traditional blue-collar as well as other occupations. Given the limitations of this study, the findings should be interpreted carefully. This study emphasizes the need to examine risk among female workers in other large cohorts or where the sample size of female workers is much greater to compare findings from this study. Further understanding on sex differences among occupational exposures involved in identified occupations is needed. This study highlights the important role that work plays in cancer, particularly among female workers.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the University of Toronto Health Sciences Research Ethics Board (#39013). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because because this study uses administrative health records that adhere to provincial and federal privacy regulations.
JS: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. GC: Formal analysis, Software, Writing – original draft. FE: Formal analysis, Investigation, Methodology, Writing – review & editing. PD: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work is supported by the Ontario Ministry of Labour, Immigration, Training, and Skills Development (14-R-029). The Occupational Cancer Research Centre is supported by the Ontario Ministry of Labour, Immigration, Training, and Skills Development, the Ontario Ministry of Health, and the Ontario Health agency.
We would like to acknowledge Nelson Chong for his support with the Occupational Disease Surveillance System (ODSS) linkage.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvh.2023.1325197/full#supplementary-material
1. Brenner DR, Poirier A, Woods RR, Ellison LF, Billette JM, Demers AA, et al. Projected estimates of cancer in Canada in 2022. Can Med Assoc J. (2022) 194(17):E601–7. doi: 10.1503/cmaj.212097
2. Labrèche F, Kim J, Song C, Pahwa M, Ge CB, Arrandale VH, et al. The current burden of cancer attributable to occupational exposures in Canada. Prev Med. (2019) 122:128–39. doi: 10.1016/j.ypmed.2019.03.016
3. Xu M, Ho V, Siemiatycki J. Role of occupational exposures in lung cancer risk among women. Occup Environ Med. (2021) 78:98–104. doi: 10.1136/oemed-2020-106470
4. International Agency for Research on Cancer. List of classifications by cancer site: Sufficient or limited evidence in humans, IARC monographs volumes 1–132. Lyon, France: International Agency for Research on Cancer (2022). Available at: https://monographs.iarc.who.int/wp-content/uploads/2019/07/Classifications_by_cancer_site.pdf
5. Consonni D, De Matteis S, Pesatori AC, Bertazzi PA, Olsson AC, Kromhaut H, et al. Lung cancer risk among bricklayers in a pooled analysis of case-control studies. Int J Cancer. (2015) 136:360–71. doi: 10.1002/ijc.28986
6. Taeger D, Pesch B, Kendzia B, Behrens T, Jockel KH, Dahmann D, et al. Lung cancer among coal miners, ore miners and quarrymen: smoking-adjusted risk estimates from the synergy pooled analysis of case-control studies. Scand J Work Environ Health. (2015) 41:467–77. doi: 10.5271/sjweh.3513
7. Garshick E, Laden F, Hart JE, Rosney B, Davis ME, Eisen EA, et al. Lung cancer and vehicle exhaust in trucking industry workers. Environ Health Perspect. (2008) 116:1327–32. doi: 10.1289/ehp.11293
8. Ramanakumar AV, Parent MÉ, Richardson L, Siemiatycki J. Exposures in painting-related occupations and risk of lung cancer among men: results from two case-control studies in Montreal. Occup Environ Med. (2011) 68:44–51. doi: 10.1136/oem.2009.04995
9. Kendzia B, Behrens T, Jöckel KH, Siemiatycki J, Kromhout H, Vermeulen R, et al. Welding and lung cancer in a pooled analysis of case-control studies. Am J Epidemiol. (2013) 178:1513–25. doi: 10.1093/aje/kwt201
10. Camp PG, Dimich-Ward H, Kennedy SM. Women and occupational lung disease: sex differences and gender influences on research and disease outcomes. Clin Chest Med. (2004) 25:269–79. doi: 10.1016/j.ccm.2004.01.004
11. Takkouche B, Regueira-Méndez C, Montes-Martínez A. Risk of cancer among hairdressers and related workers: a meta-analysis. Int J Epidemiol. (2009) 38:1512–31. doi: 10.1093/ije/dyp283
12. Heibati B, Jaakkola MS, Lajunen TK, Ducatman A, Bamshad Z, Eslamizad S, et al. Occupational exposures and respiratory symptoms and lung function among hairdressers in Iran: a cross-sectional study. Int Arch Occup Environ Health. (2021) 94:877–87. doi: 10.1007/s00420-020-01645-z
13. Quach T, Doan-Billing PA, Layefsky M, Nelson D, Nguyen KD, Okahara L, et al. Cancer incidence in female cosmetologists and manicurists in California, 1988–2005. Am J Epidemiol. (2010) 172:691–9. doi: 10.1093/aje/kwq190
14. Ma GX, Wei Z, Husni R, Do P, Zhou K, Rhee J, et al. Characterizing occupational health risks and chemical exposures among Asian nail salon workers on the east coast of the United States. J Community Health. (2019) 44:1168–79. doi: 10.1007/s10900-019-00702-0
15. Schernhammer ES, Feskanich D, Liang G, Han J. Rotating night-shift work and lung cancer risk among female nurses in the United States. Am J Epidemiol. (2013) 178:1434–41. doi: 10.1093/aje/kwt155
16. Rydz E, Arrandale VH, Peters CE. Population-level estimates of workplace exposure to secondhand smoke in Canada. Can J Public Health. (2019) 111:125–33. doi: 10.17269/s41997-019-00252-x
17. Levesque J, Mischki T. Exposure to tobacco smoke among Canadian nonsmokers based on questionnaire and biomonitoring data. Govt Canada Stats Canada. (2021) 32(2):16–26. doi: 10.25318/82-003-x202100200002-eng
18. Han DH. Fit factors for quarter masks and facial size categories. Ann Occup Hyg. (2000) 44:227–34. doi: 10.1016/S0003-4878(99)00087-3
19. Biswas A, Harbin S, Irvin E, Johnston H, Begum M, Tiong M, et al. Sex and gender differences in occupational hazard exposures: a scoping review of the recent literature. Curr Envir Health Rpt. (2021) 8:267–80. doi: 10.1007/s40572-021-00330-8
20. Messing K, Dumais L, Courville J, Seifert AM, Boucher M. Evaluation of exposure data from men and women with the same job title. J Occup Med. (1994) 36:913–7.7807275
21. Messing K, Doniol-Shaw G, Haëntjens C. Sugar and spice and everything nice: health effects of the sexual division of labor among train cleaners. Int J Health Serv. (1993) 23:133–46. doi: 10.2190/AAAF-4XWM-XULT-WCTE
22. Jung JKH, Feinstein SG, Palma Lazgare L, Macleod J, Arrandale VH, McLeod CB, et al. Examining lung cancer risks across different industries and occupations in Ontario, Canada: the establishment of the occupational disease surveillance system. Occup Environ Med. (2018) 75:545–52. doi: 10.1136/oemed-2017-104926
23. Sritharan J, Kirkham TL, MacLeod J, Marjerrison N, Lau A, Dakouo M, et al. Cancer risk among firefighters and police in the Ontario workforce. Occup Environ Med. (2022) 79(8):533–9. doi: 10.1136/oemed-2021-108146
24. Pukkala E, Martinsen JI, Lynge E, Gunnarsdottir HK, Sparén P, Tryggvadottir L, et al. Occupation and cancer—follow-up of 15 million people in five nordic countries. Acta Oncol. (2009) 48(5):646–790. doi: 10.1080/02841860902913546
25. Occupational Cancer Research Centre. Burden of occupational cancer in Canada: major workplace carcinogens and prevention of exposure. (2019). Toronto, ON.
26. Tompa E, Kalcevich C, McLeod C, Lebeau M, Song C, McLeod K. The economic burden of lung cancer and mesothelioma due to occupational and para-occupational asbestos exposure. Occup Environ Med. (2017) 74:816–22. doi: 10.1136/oemed-2016-104173
27. Carex Canada. Construction sector: occupational exposure summary. British Columbia, Canada: Carex Canada (2021). Available at: https://www.carexcanada.ca/CAREX-exposure-summary-construction.pdf
28. International Agency for Research on Cancer. Diesel and gasoline engine exhausts and some nitroarenes. IARC Monogr eval carcinog risks to humans. Lyon, France: International Agency for Research on Cancer (2014). Vol. 105
29. Peters CE, Ge CB, Hall AL, Davies HW, Demers PA. CAREX Canada: an enhanced model for assessing occupational carcinogen exposure. Occup Environ Med. (2015) 72:64–71. doi: 10.1136/oemed-2014-102286
30. International Agency for Research on Cancer. Arsenic, metals, fibres and dusts. IARC monogr eval carcinog risks to humans. Lyon, France: International Agency for Research on Cancer (2012). Vol. 100C.
31. International Agency for Research on Cancer. Welding, molybdenum trioxide, and indium tin oxide. IARC monogr eval carcinog risks to humans. Lyon, France: International Agency for Research on Cancer (2018). Vol. 118.
32. International Agency for Research on Cancer. Chemical agents and related occupations. IARC monogr eval carcinog risks to humans. Lyon, France: International Agency for Research on Cancer (2012). Vol. 100F.
33. International Agency for Research on Cancer. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC monogr eval carcinog risks to humans. Lyon, France: International Agency for Research on Cancer (2010). Vol. 92.
34. Atramont A, Guida F, Mattei F, Matrat M, Cenee S, Sanchez M, et al. Professional cleaning activities and lung cancer risk among women, results from the ICARE study. J Occup Environ Med. (2016) 58(6):610–6. doi: 10.1097/JOM.0000000000000722
35. Baur X, Frank AL, Magnani C, Oliver CL, Soskolne CL. Malignant mesothelioma in females: the institutional failture by WHO and IARC to protect public health. J Sci Pract Integr. (2023). doi: 10.35122/001c.75390, Commentary. Published on August 11, 2023.
36. Syamlal G, Mazurek JM, Dube SR. Gender differences in smoking among U.S. working adults. Am J Prev Med. (2014) 47:467–75. doi: 10.1016/j.amepre.2014.06.013
37. Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med. (2010) 7:381–401. doi: 10.1016/j.genm.2010.10.002
38. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. (1991) 144:1202–18. doi: 10.1164/ajrccm/144.5.1202
39. Mederos N, Friedlaender A, Peters S, Addeo A. Gender-specific aspects of epidemiology, molecular genetics and outcome: lung cancer. ESMO Open. (2020) 5:e000796. doi: 10.1136/esmoopen-2020-000796
40. Barnetson B, Foster J, Matsunaga-Turnbull J. Estimating under-claiming of compensable workplace injuries in Alberta, Canada. Canadian Public Policy. (2018) 44:400–10. doi: 10.3138/cpp.2018-014
41. Cox R, Lippel K. Falling through the legal cracks: the pitfalls of using workers compensation data as indicators of work-related injuries and illnesses. Policy Pract Health Saf. (2008) 6:9–30. doi: 10.1080/14774003.2008.11667721
42. Moyser M. (2017). Women in Canada: A Gender-based Statistical Report—Women and Paid Work. Statistics Canada, Minister of Industry. Available at: https://www150.statcan.gc.ca/n1/pub/89-503-x/2015001/article/14694-eng.htm
Keywords: sex differences, female workers, lung cancer, occupation, surveillance
Citation: Sritharan J, Christopher G, Eros FR and Demers PA (2023) Exploring sex differences in lung cancer risk among workers in Ontario, Canada's Occupational Disease Surveillance System. Front. Environ. Health 2:1325197. doi: 10.3389/fenvh.2023.1325197
Received: 20 October 2023; Accepted: 24 November 2023;
Published: 19 December 2023.
Edited by:
Corrado Magnani, University of Eastern Piedmont, ItalyReviewed by:
Andrea Kaifie-Pechmann, University Hospital RWTH Aachen, Germany© 2023 Sritharan, Christopher, Eros and Demers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeavana Sritharan amVhdmFuYS5zcml0aGFyYW5Ab250YXJpb2hlYWx0aC5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.