- 1Department of Civil, Construction and Environmental Engineering, Iowa State University, Ames, IA, United States
- 2Department of Agricultural and Biosystems Engineering, Iowa State University, Ames, IA, United States
- 3Department of Food Science and Human Nutrition, Iowa State University, Ames, IA, United States
- 4Black and Veatch Consultants, Overland Park, KS, United States
- 5Livestock Nutrient Management Research Unit, United States Department of Agriculture - Agricultural Research Services (USDA-ARS) Conservation and Production Research Laboratory, Bushland, TX, United States
The 8-billion human population on this planet produces 700,000 m3 wastewater per minute, most of which is treated, usually with a bacterial process, to lower environmental impact. Synthetic substances in wastewater from industry, e.g., agrochemicals, pesticides, and textile dyes are difficult to degrade during such biological treatment. These substances degrade the environment, discolor water, and kill or inhibit aquatic organisms. Removal of synthetic compounds currently involves advanced and more expensive technologies than just biological treatment. The body of work summarized in this article was aimed at improving biological wastewater treatment by breaking down non-biodegradable substances with ozone. What was novel is that the ozone was not introduced conventionally either before or after, but during biological treatment. The work describes how ozone could be used within activated sludge treatment to oxidize undesirable compounds to substances that are either innocuous or biodegradable directly in the same reactor through bacterial action. Research focused on removing dyes, methylene blue and Orange II, cyanide as test substances, and using ozonation within an activated sludge process was found to be quite effective. The choice of test substances was based both on how commonly such substances are contained in industrial wastewater and the ease to identify byproducts. There is growing evidence that a powerful disinfectant such as ozone does not necessarily inhibit beneficial organisms when used directly in a biological process. This is probably due to reaction kinetics. The review showed that it was indeed possible to degrade certain undesirable compounds, such as methylene blue, Orange II, and a range of synthetic organic compounds by combined ozone oxidation and biodegradation within the bioprocess, all without serious inhibition of useful organisms, even protecting these by lowering the toxicity of some target compounds. If an oxidation reaction is fast, the build-up of an ozone residual in solution is minimized, thereby substantially decreasing the disinfecting power the ozone might have had, as disinfection is a function of both disinfectant concentration and exposure time. By cutting both the concentration and shortening the exposure time with rapid competing reactions, the microbes are shielded from ozone.
1 Introduction
The 8-billion humans on Earth produce more than a billion m3 wastewater each day (Jones et al., 2021), most of which is treated, almost always by bacterial degradation to remove organic compounds and sometimes the plant nutrients N and P (Barnard, 1974). Organic substances of synthetic origin, such as agrochemicals, particularly pesticides, and textile dyes are difficult to degrade during biological treatment (McGuinness and Dowling, 2009). These substances persist or bio-accumulate in the environment, inhibit aquatic organisms, discolor water, and degrade the water for downstream users. Synthetic substances are usually recalcitrant and not biodegradable and require advanced and more expensive technologies than just biological treatment. The work in this paper was aimed at improving biological wastewater treatment by breaking down non-biodegradable substances with the powerful oxidant ozone.
Ozonation for improving biodegradability is well-known (Contreras et al., 2003; Collignon et al., 1994; Doré, 1984) and used either before or after bioprocessing. Using ozone before the bioprocess is wasteful as most of the ozone would be consumed in reactions with biodegradables as these are generally more readily oxidized. Ozonation after biotreatment makes more sense to lower ozone demand and thus lower treatment cost. However, since ozonolysis creates biodegradables, sometimes toxic, more biological treatment would be called for. While additional biotreatment could be circumvented with a substantial recycle stream to the bioreactor, the effectiveness of this would be limited because any recycle stream can only be partial. The recycle also effectively shortens the hydraulic retention time in the bioprocess. This would also affect the biomass separation from the effluent, because the increased flow rate impedes settling of the biomass, which has a density only slightly larger than water. Clearly, research into a better, integrated approach as described in this article is warranted.
What if the ozone was not introduced either before or after, but during biological treatment? The liquid inside a completely mixed system has the same composition as the effluent, so the ozone demand for oxidation would be the same as after biotreatment, but all of the liquid will be treated without needing an extra reactor or a liquid recycle. The concern would be that using such a powerful disinfectant in a biological process would negatively affect all beneficial organisms used in this process. Earlier van Leeuwen work Van Leeuwen (1988a), Van Leeuwen (1988b), using ozone within an activated sludge process, was to limit filamentous bacterial growth thereby increasing sludge settleability, and not to improve degradation, although it was also found then that ozonation improved COD removal. The bench-scale work did prove that ozonation inside an activated sludge process would not inhibit the sensitive nutrient removal organisms. Ozonation has been used successfully at the Daspoort wastewater treatment plant, Pretoria, South Africa in one of three 13 ML/d (3.5 MGD) parallel activated sludge treatment systems (Saayman et al., 1996; Saayman et al., 1998).
This particular project targeted dyes, using methylene blue (MeBl), Orange II (O-II) and cyanide (CN-) as test substances in this novel ozonation concept within activated sludge. The one-line hypothesis tested: “Ozonation within a biological treatment process can enhance removal of recalcitrant organic compounds.” The research in this review was aimed at developing a technology for the removal of nonbiodegradable substances from industrial wastewater in a more sustainable and economical manner. The specific objectives were to:
a. Determine ozone dosage rates for improved removal of recalcitrant organic compounds within an activated sludge process without inactivating beneficial microbes.
b. Establish the required conditions for improved removal of recalcitrant compounds exemplified through the use of dyes within an activated sludge system.
c. Identification of the ozonolysis byproducts of the dyes tested, i.e., MeBl and O-II.
d. Identifying pathways for the removal of dyes and ozonolysis byproducts.
2 Literature
Developed nations usually collect wastewater in sewers (Jones et al., 2021). These would usually include both domestic and industrial wastewater. Local authorities typically own and operate wastewater treatment plants to purify these wastewaters to a quality that is suitable for discharge to public streams (Naidoo and Olaniran, 2013). The most common approach to treatment is aerobic bacterial cultures, known as an activated sludge process (Barnard, 1974; Oleszkiewicz and Barnard, 2006). Wastewater can be oxidized into biodegradable substances which can subsequently biologically degrade (Phan et al., 2022). The aerobic process ensures that the readily biodegradables are removed thereby preventing oxygen depletion in receiving public surface waters. Most of the byproducts will be biodegradable; for example, aldehydes, ketones, and organic acids that are generally smaller molecules (Lopez et al., 1998). Wastewater ozonation increases organic removal in subsequent biotreatment (Ikehata et al., 2006; van Leeuwen et al., 1981; van Leeuwen, 1987; Rice and Browning, 1981; Ahmed et al., 2017).
Synthetic substances are typically not readily biodegradable and thus not removed well during conventional wastewater treatment. When these are released into the environment, these could have detrimental effects and degrade the water for downstream users (Susskind, 2011; Fang et al., 2019). The substances may also enter the food chain and pose toxic threats to water life and water users downstream (Muir et al., 2023). Synthetic substances should be removed, but the advanced technologies required are expensive. Current technology needs to be improved to become cost-effective and more feasible. Synthetic substances that occur most frequently are agrochemicals and textile dyes. The focus of the summarized studies was developing a methodology to remove such substances from wastewater. The removal of two synthetic dyes MeBl and O-II was the subject of these studies.
Ozone can be used to oxidize many organic substances to a more biodegradable form. The use of ozone to make organic substances more biodegradable is well-known. However, ozonation byproducts formation (e.g., in drinking water treatment) is of concern (Wang et al., 2022). A large spectrum of pesticides and dyes can be oxidized by ozone while oxidation to CO2 and H2O would be possible, but generally, under practical conditions, intermediates are formed (Glaze et al., 1993).
Treating wastewater prior to biological treatment increased removal of organics in conventional treatment (van Leeuwen et al., 1981; Hu and Yu, 1994; Rivas et al., 2000). However, much of such ozone is used unfruitfully as it oxidizes biodegradables by preference. It would make more sense to subject the wastewater to biotreatment and then ozonate to increase the biodegradability of the remaining organic compounds. This would require more biotreatment after ozonation. Recycling the ozonated effluent to the original bioprocess circumvents having yet another bioreactor, but not without additional challenges. Recycling increases the hydraulic loading through the activated sludge unit, shortening the retention time. Yet a bigger problem encountered by recycling is the much-increased throughput into the settling tanks, which would result in poor settling and loss of biomass with the effluent. Furthermore, recycling is limited to only a fraction of the main flow, and therefore, not all of the new biodegradables can be recycled. This approach is, therefore, severely limited.
Consider this: fully mixed processes have the same liquid concentration throughout and ipso facto the same concentration as the effluent. It is this effluent that needs to be ozonated to degrade the recalcitrants. So why not ozonate this effluent while it is still inside the bioreactor? This would be very convenient as we would achieve ozonolysis of the recalcitrants while the original biodegradables and the newly formed biodegradables will be removed by the bioprocess. This makes a lot of sense, saves ozone needs, and avoids the expense of separate bioreactors and ozonation tanks. This, of course, is only if the disinfectant effect of the ozone can be overcome. We expect to see horror on the faces of many readers using ozone, one of the most powerful disinfectants, directly inside a sensitive bioprocess? This needs to be explained further.
Ozonation within an activated sludge process has already been studied and applied for sludge bulking control for almost 40 years (Doré, 1984; Van Leeuwen, 1988a; Van Leeuwen, 1988b; Van Leeuwen, 1989). It has been clearly demonstrated that low dosages of ozone do not kill all bacteria and improve biomass settling by selectively inactivating filamentous bacteria protruding from the activated sludge flocks. Low rates of applied ozone react rapidly with reducing substances, which would be mainly organic substances, thus avoiding a buildup of dissolved ozone. Any lingering concentrations of ozone would inactivate microbes, including the beneficial bacteria. The filamentous bacteria having a relatively large surface area exposed to any ozone are the most vulnerable and our therefore selectively inhibited, while the flock formers survive unscathed. Ozone dosages had to be enough to inhibit the filamentous bacteria but low enough not to wipe out the beneficial floc-forming bacteria. Ozonation for bulking control was applied at full scale by the city of Pretoria, South Africa (Saayman et al., 1996; Saayman et al., 1998).
Apart from controlling filamentous bacteria, it was observed that the effluent quality was not adversely affected, and indeed, the COD removal was enhanced somewhat. This aspect was not studied in great detail while the main purpose of ozonation was selective disinfection. Furthermore, the research was conducted mainly on domestic wastewater and also on highly degradable industrial wastewater generated during coal gasification and liquid fuel synthesis.
Much attention has been given to using ozone for degrading biomass in Japan (Yasui and Shibata, 1994). This was done not inside the bioprocess but by ozonation of a side stream of the mixed liquor at a high dosage. The authors have been able to reduce the biomass suspended solids concentration to a level where there was no excess sludge for disposal. The concept was improved by Kamiya and Hirotsu (1998) with intermittent ozonation reducing ozone requirements. Biomass growth inhibition as a function of ozone level in the biomass was modeled by Contreras et al. (2003).
3 Methodology
The biological treatment and ozonation studies are described below.
3.1 Ozonated activated sludge (O3AS)
Novel ozone degradation of a recalcitrant substance during biotreatment was studied. Two parallel lab activated sludge units of 6 L each (Figure 1) were continuously fed synthetic wastewater containing either 5 mg/L MeBl or 5 mg/L Orange II (Figure 2), while the second, without ozone, was a control. The molecular structure shows an important key to rapid ozonation: double bonds, C=C and N=N, and sulfur branches are readily oxidized.
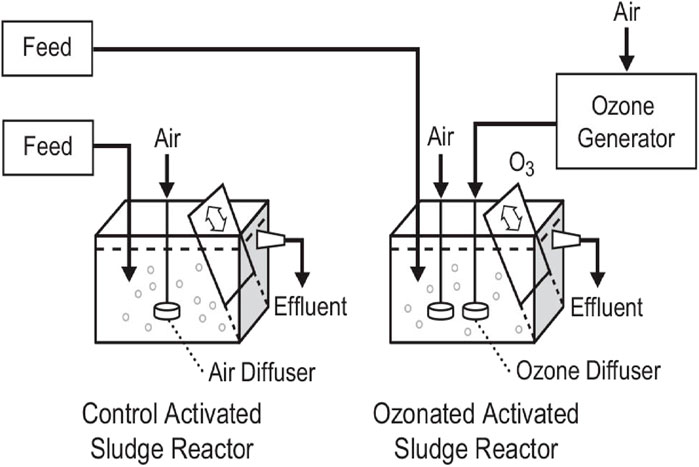
Figure 1. Activated sludge units with ozonation (van Leeuwen et al., 2009b) (Taylor and Francis license 5901930723134).
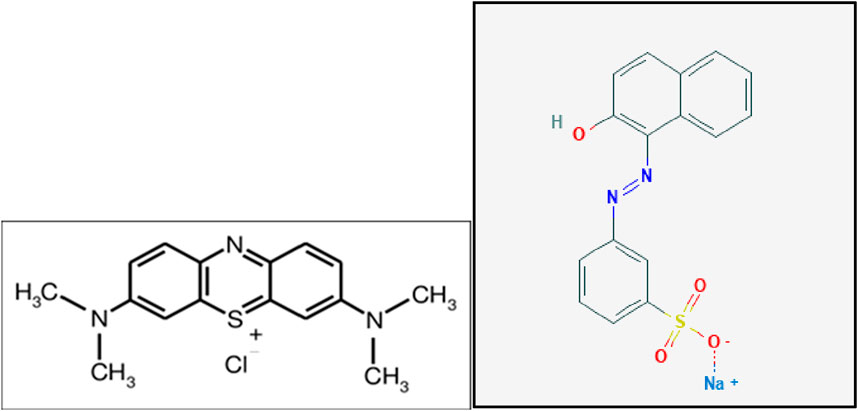
Figure 2. Methylene blue; panel left (van Leeuwen et al., 2009a) (Taylor and Francis license 5902121509894. Orange II: panel right (van Leeuwen et al., 2009b) (Taylor and Francis license 590193072134).
The biomass in the bench-top reactors was procured from an aeration basin at the Boone, Iowa municipal activated sludge plant. The reactors each had an adjustable underflow weir, which allowed the biomass to settle by shielding the mixed liquor from agitation due to the aeration. The hydraulic level inside the tanks was determined by the height of the outlet and the retention time by the rate of feed. One-tenth of the reactor content was withdrawn daily by siphoning with a hose into a measuring cylinder to maintain the solids residence time.
3.1.1 Synthetic wastewater
Synthetic wastewater was tap water with various organic and inorganic nutrients added (Table 1). The two bench units employed fritted glass porous diffusers to continually aerate the biomass and were operated at a HRT of 10 h by pumping the wastewater in at 0.6 L/h. A solids retention time of 10 days and a solids concentration of 2.1 g/L (MeBl tests) and 3.5 g/L (O-II) was maintained by wasting 0.6 L mixed liquor once every day.
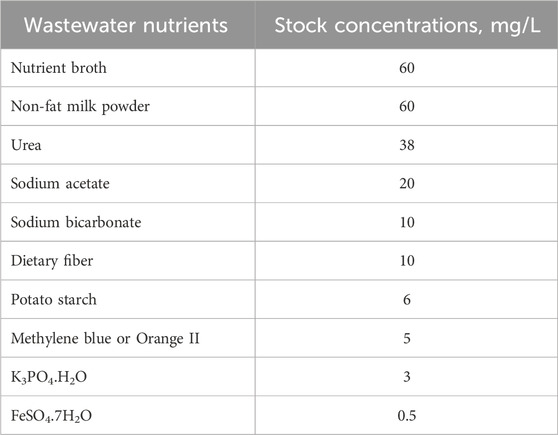
Table 1. Synthetic wastewater composition with dyes (adapted from Ellis and Anselm, 1999).
3.1.2 Ozone dosing
Ozone dosing was done continuously, and directly into one of the two activated sludge units, at 17 mg/L based on inflow rate into the MeBl and 30 mg/L into the O-II unit, this along with the second non-ozonated unit as a control. Initially, an ozone dosage of 45 mg/L was found to detrimentally affect the biomass in the O-II unit. An Ozonology ozone generator (Northbrook, IL, United States) fed with dehumidified air was used. The approach is also described in van Leeuwen et al. (2009a) and Van Leeuwen et al. (2009b). The specific levels of ozone dosed as a function of the biomass in the reactor, were 17 mg × 0.6/(6 × 2.1) = 0.81 mg O3/(g × h) for the MeBl reactor and 30 mg × 0.6/(6 × 3.5) = 0.86 mg O3/g biomass/h for the O-II reactor. These specific dosages were almost the same by design and while not optimized, they seemed to be approaching the maximum allowable to not upset the microbes in the biomass. The higher dosage in the O-II treating activated sludge of 45 mg/L or specifically 1.29 mg O3/(g × h), was clearly inhibiting biomass growth.
3.1.3 Sludge volume index (SVI)
Sludge Volume Index (SVI) is the volume occupied by 1 g of biosolids after settling for 30 min (Jenkins et al., 2003). It was determined from the volume of sludge solids after 30 min settling divided by the mixed liquor concentration. Biomass was characterized further at ×400 magnification to observe floc structure and abundance of filamentous bacteria and protozoa using the manuals of Eikelboom and van Buijsen (1981) and Jenkins et al. (2003).
3.1.4 Water analyses
Effluent dye concentrations were determined using a Milton Roy Spectronic 501 spectrophotometer (Ivyland, PA, United States) in the visible region. The maximum absorbance was at 484 nm for Orange II, as found with a Spectronic Genesys 2 spectrophotometer Pittsburgh, PA, United States).
3.2 Byproducts characterization
3.2.1 Ozone determination
Ozone dosages were determined by measuring the flow rates and the gas-phase concentrations into and out of the reactor by the standard iodometric method described in Standard Methods for the Examination of Water and Wastewater (APHA and WPCF, 1995). Ozone demand tests of feed and effluent were also based on Standard Methods, but with an endpoint determined by establishing a measurable residual or 60% color removal of the Orange II.
3.2.2 Biodegradability
Extant respirometric tests were conducted, using adapted biomass, first oxygenated to saturation, then allowed to consume oxygen by endogenous respiration (Ellis et al., 1996). The effect MeBl or its ozonated byproducts in low concentrations on respiration rate was monitored by the oxygen depletion rate. Increased respiration rate would indicate biodegradability of the substance, decrease toxic effects.
3.2.3 Biosorption
Adsorption without biodegradation was studied as follows. A range of quantities of biomass were added to a 5 mg/L MeBl or O-II solution, shaken for 1.5 h at 2.5 rpm and remaining dye measured spectrophotometrically. The biomass had been inactivated with 5 mg/L of HgCl2 to eliminate possible biodegradation. The dye removal was ascribed to biosorption with no plausible alternative mechanism and the solid-phase concentration of dye was calculated from the removal of dye from solution. These results were plotted on a log-log basis and approximated to a Freundlich isotherm.
3.2.4 Ozonolysis byproducts
5 mg/L Orange II, dissolved in water, was ozonated to 30 mg/L, i.e., about 50 mol O3/mol Orange II. Dye samples were introduced in a 3-L batch reactor provided with a continuous magnetic stirrer and two gas diffusers to improve the contact between the ozone and the target solution (Figure 3). The ozone was produced from oxygen in a Sander Labor ozone generator (Uetze, Eltze, Germany). Ozone at the inlet of the reactor was measured every second by means of an Ozone Analyzer BMT 963 Vent and at the outlet using an Ozone Analyzer BMT 964 BT (both BMT Messtechnik, Berlin). Dissolved ozone in the solution was measured with a dissolved O3 analyzer ATI Q45H (Analytical Technology, Collegeville, PA, United States). All information was collected using a DaqPro 5,300 data-logger (Omega Eng. Norwalk, CT, United States). The ozonated gas flow was maintained at 30 L/h and the dye solutions were led to react until color was visually removed.
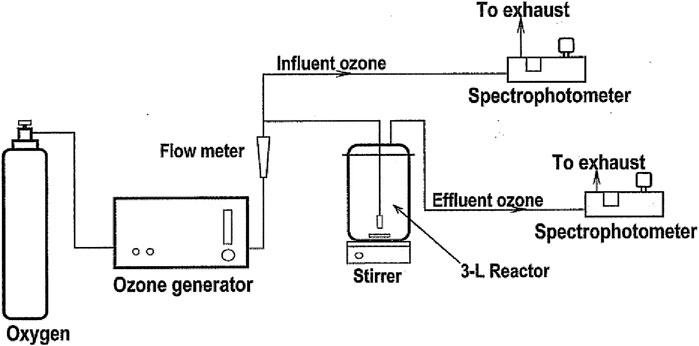
Figure 3. Ozonolysis byproduct generation lab setup (van Leeuwen et al., 2003) (Taylor and Francis License 5092121218057).
Similarly, 5 mg/L solutions of MeBl were ozonated to 30 mg/L. Samples of both ozonated dyes were collected and subjected to headspace analysis with solid-phase microextraction (SPME). The SPME sampler was then fully desorbed into gas chromatography (GC) injector. The GC oven program started from 40°C at a rate of 7°C/min to 220°C and the sorbed substances that were separated and analyzed by mass spectrometry. The total ion chromatograms were interpreted electronically and matched to most likely compounds as ozonolysis byproducts.
3.2.5 Byproduct identification
Water samples with MeBl or O-II were ozonated and analyzed for byproducts. Headspace of samples was sampled with solid-phase microextraction (SPME) and analyzed by gas chromatography–mass spectrometry (GC-MS) (Pawliszyn, 1997; Lo et al., 2008; Xiong et al., 2004). MeBl byproducts were also determined with electro spray ionization (Fenn et al., 1990). Ozonolysis was also performed on wastewater samples from coking operations, in this case from the plant of BlueScope Steel, Port Kembla, NSW, Australia to determine relative reaction rates in the removal of cyanide and thiocyanate. Such testing was also done on solutions of MeBl and O-II.
4 Summary of findings
4.1 MeBl removal mechanism
MeBl removal averaged 95% in the ozonated activated sludge (O3AS) against 40% in the control. The mechanism of removal was analyzed as follows. Biosorption of dye can only take place on new biomass as the older biomass is already in equilibrium with the dye in solution, provided the MeBl concentration does not change. O3AS effluent with typical 0.25 mg/L MeBl (5% of the original 5 mg/L) would be in equilibrium with 0.5 mg/g on the biomass, according to the extrapolated Freundlich isotherm (Figure 4). For verification, log 0.25 = −0.6, which corresponds to a log solid-phase concentration of −3.3 or 10−3.3 = 5 × 10−4 g/g or 0.5 mg/g.
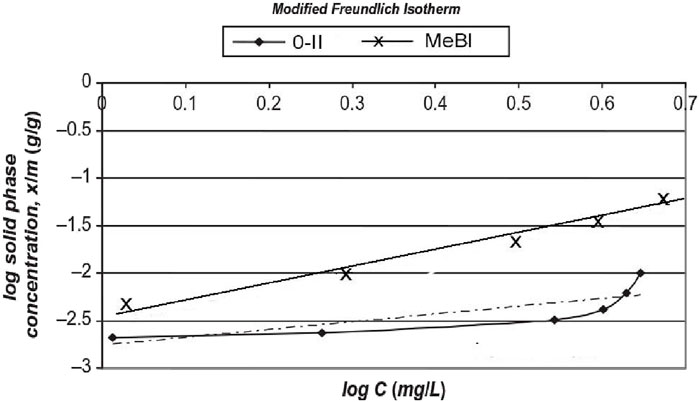
Figure 4. Freundlich isotherms of MeBl and O-II for adsorption on biomass (van Leeuwen et al., 2009a; 2009b).
Each unit, with 12 g biomass per 6-L unit, had a net biomass production of 0.1 g × g−1 × d−1) at a sludge age of 10 days, i.e., 1.2 g biomass/d/unit. This would result in biosorptive MeBl removal in the ozonated biomass of 1.2 × 0.5 = 0.6 mg/d from a total feed of 5 mg/L × 0.6 L/h × 24 h/d = 72 mg/d, i.e., 0.83% removal by biosorption. The rest of the 95% MeBl removal was therefore >99% by ozonation and biodegradation.
Similarly, the control, with average effluent MeBl 3 mg/L, would have 20 mg/g at adsorption equilibrium on biomass and, consequently, biosorptive removal of 20 × 1.2 = 24 mg/d. As the actual removal was 40% of 72 mg, i.e., 29 mg/d, only 5% was removed by other mechanisms, possibly just inaccuracy in the evaluation. The adsorption isotherm was developed with clean water, for instance, while the organic material might alter the biosorption equilibrium. Otherwise, maybe there was some biodegradation.
4.2 O-II removal mechanism
More than three times as much O-II removal was achieved in the O3AS (ca. 65%) than in the control (ca. 20%). The mixed liquor suspended solids averaged about 3.5 g/L, i.e., 21 g biomass per 6-L activated sludge unit. Since the solids retention time was maintained at 10 days, 10% of biomass production amounted to 2.1 g/L per day. The dye concentration was 1.75 mg/L dye after 65% removal in the ozonated activated sludge, and the equilibrium concentration on the biomass would be 2.8 mg/g biomass according to the Freundlich isotherm. The daily adsorptive removal could be predicted to amount to 5.9 mg on the 2.1 g new growth, which, from 72 mg/d of Orange II dosed = 8%, which is substantially less than the actual removal. Biosorption is therefore a minor factor (<1/8 removal) in the integrated process, and the main removal mechanism must have been ozonolysis and biodegradation.
The control reactor, with an effluent concentration of 4 mg/L of O-II after 20% total removal, would have approximately 5.6 mg/g biomass according to the Freundlich isotherm. This would result in a daily removal of 11.8 mg dye based on 2.1 g new biomass/d. This is 16% of the total feed of 72 mg/d. Even though the actual removal amounted to 20%, adsorption was clearly the main removal mechanism of O-II in the non-ozonated control.
4.3 Toxicity of dyes
Extant respirometry was the procedure used. The dissolved oxygen (DO) profiles were monitored to detect changes in oxygen uptake by an active biomass sample, following dye addition. The term “extant” means “currently existing” and refers to the fact that the procedure reflects conditions immediately before the assay and what happens subsequently (Grady et al., 1996). If the assay compound was easily biodegradable, the DO respirometric line would trend downwards; if the compound has no tangible effect on the biomass, the line would remain level. An upwards trend would indicate that the biomass is under stress, indicating toxicity. The extant respirometric test indicated minimal MeBl biodegradability in acclimatized culture with a non-significant change in the oxygen depletion rate slope. O-II caused decreased respiration relative to a control, indicating some inhibitory effect that could be ascribed to toxicity.
The fact that the control activated sludge performed as well as the ozonated unit may indicate that the biomass could adapt to the relatively low concentrations as used in these continuous experiments. A concern that ozone, a powerful disinfectant, could inhibit beneficial microbes in the activated sludge process was proven incorrect.
4.4 Biomass characteristics
The average SVI of the unozonated sludge treating MeBl was 150 mL/g against 120 mL/g of ozonated sludge. The non-ozonated activated sludge had a much deeper blue color, due to more dye adsorbed. Microscope photos confirmed O3AS biomass was more compact with fewer filamentous bacteria than in the control (Figure 5). This confirms that ozonation discouraged the growth of the more exposed filaments and can be expected to improve biomass settleability. The reaction rate with organic substances depleted the dissolved ozone so that the inactivation of treatment bacteria and protozoa was minimal, mostly affecting filamentous bacteria only. Filamentous bacteria appeared to be Type 0041 or 0675 mainly, both characterized by dense attached growths.
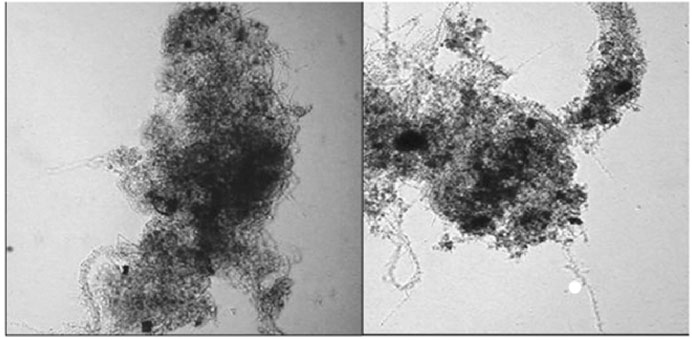
Figure 5. The effect of MeBl on biomass (left); with ozonation (right) (van Leeuwen et al., 2009a) (Taylor and Francis license 5902121509894).
The microscope photos showed another interesting phenomenon. The non-ozonated biomass treating MeBl did not include any protozoa, but the ozonated biomass had quite a few protozoa feeding on the bacteria (Figure 6). The protozoa were of the genera Chilodonella and Paramecium. This was confirmed by comparing the microscope photos with these in the manual by Eikelboom and van Buijsen (1981). The viability of the protozoa was clear as they were moving around, and their cilia were moving rapidly as observed under a light microscope with a magnification of ×200 (see Figure 6 right). Protozoa are considered a sign of healthy activated sludge. It could be concluded that the higher concentration of MeBl was toxic to protozoa, but not the lowered MeBl concentration as a consequence of ozonation. It should be pointed out that both ozone and MeBl would be toxic to protozoa when exceeding tolerance levels.
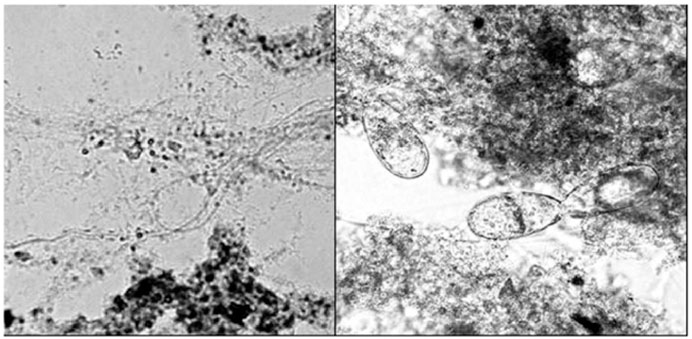
Figure 6. The effect of ozonation (right) on protozoa in the MeBl activated sludge biomass at ×200 magnification (van Leeuwen et al., 2009a) Left depicts non-ozonated biomass from the control (Taylor and Francis license 5902121509894).
Due to the redox reaction between MeBl and ozone, both these protozoan inhibitors had been lowered to tolerable levels within the activated sludge biomass.
4.5 Ozonolysis byproducts from MeBl and O-II
The identified products from oxidation of MeBl and O-II in pure solutions (van Leeuwen et al., 2009a; van Leeuwen et al., 2009b) were found to be biodegradable. MeBl MeBl has as byproducts mainly benzene derivatives and all, including dichloromethane, are biodegradable (Freedman and Gossett, 1991). O-II byproducts are mainly aldehydes, and also a ketone and an alcohol; all of which are known to be biodegradable.
Ozonation during biotreatment. The COD of the feed was 450 mg/L and that of the effluent was about 80 mg/L. The ozone demand of the feed was shown to be around 300 mg/L before any measurable ozone residual was established lasting for at least 10 s. An ozone dosage of 145 mg/L was required to obtain a 60% color removal. Similarly, the ozone demand of the effluent was 21–25 mg/L to establish a residual. This would indicate that the ozone demand for color removal would be much lower during activated sludge treatment than before treatment.
4.6 Coking wastewater treatment
The removal of cyanide and thiocyanate by ozonation (van Leeuwen et al., 2003) is shown in Figure 7. A closer analysis of SCN− removal as a function of ozone dosage is shown in Figure 8. Slope analyses showed that the rate constants of CN− and SCN− were 6.25 × 10−3 1/s and 0.57 × 10−3 L/s initially, increasing to 2.2 × 10−3 L/s, respectively.
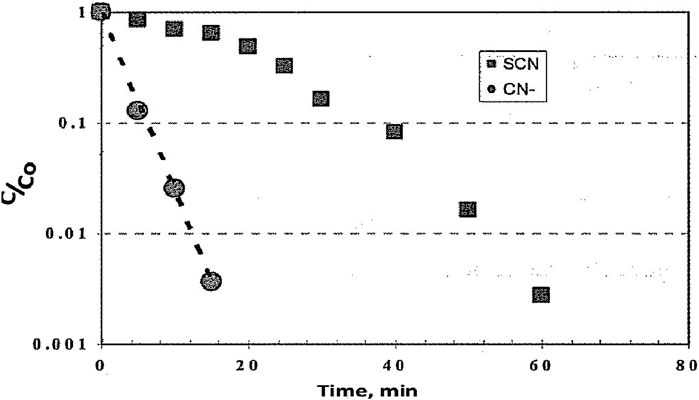
Figure 7. CN− and SCN− remaining after ozonolysis as ratio of the initial concentration (van Leeuwen et al., 2003) (Taylor and Francis license 5902121218057).
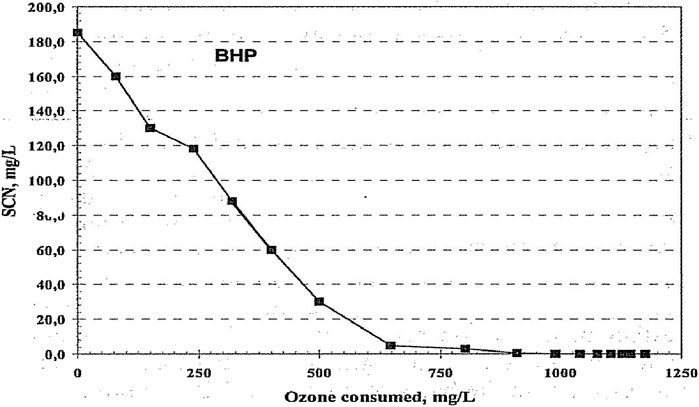
Figure 8. SCN− concentration remaining as a function of ozone consumed (van Leeuwen et al., 2003) (Taylor and Francis license 5902121218057).
Figure 9 shows the effect of ozonation on total organic carbon (TOC) and color removal (van Leeuwen et al., 2003). The total organic compounds concentration was only gradually affected by ozonation. This would indicate that it would be easy to remove CN− in the presence of TOC, but SCN− removal would be at a low rate. CN− is also removed biologically unless the concentration reaches a toxic limit, so ozonation could be used, when needed, to ensure that the system does not reach toxic levels. Cyanide is readily oxidized and more rapidly than TOC, so ozone is not expected to build up a disinfecting residual. However, SCN− can also reach toxic limits, and is oxidized much slower, so it would require much higher ozone dosages to control this substance at the risk of the ozone becoming toxic.
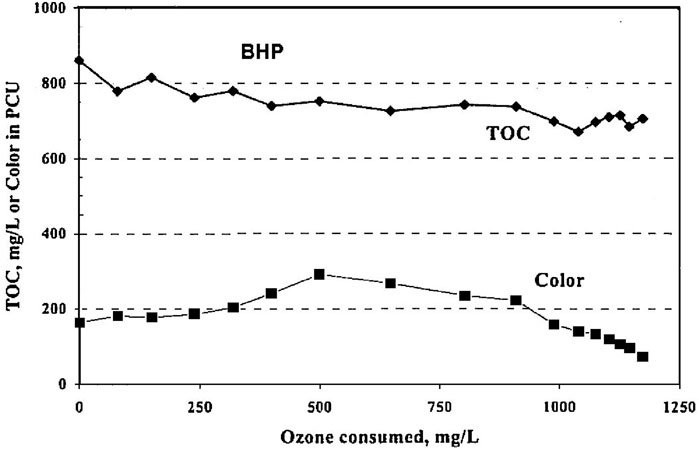
Figure 9. Ozonation effect on TOC and color in coal coking wastewater (van Leeuwen et al., 2003) (Taylor and Francis license 5902121218057).
4.7 Economic viability of ozonation
Ozone has to be generated on-site and thus requires quite some equipment for pretreatment of the feed gas, ozone generation and contacting reactors. There may also be the need for more power and a building to house the generation equipment. Furthermore, there would be operational cost to run and maintain the equipment. A recent study by van Leeuwen et al. (2024) investigated the different requirements and cost to generate ozone for the purpose of extending the shelf life of various crop and food products. The costs were all combined and expressed as a cost of ozone per kg. Obviously, the cost of any equipment is a function of the size of the equipment. The cost of ozone plus delivering it into water or wastewater would amount to $5/kg ozone at a size ranging 3–10 kg/h ozone generated. This amount of ozone could treat about 3,000–10,000 m3/d or roughly 1–2.5 MGD to a dosage of 25 mg/L. Of course, even within this range, the unit cost of ozonation would be lower at higher dosages.
Costs of ozone would obviously decrease more on a larger scale. Assuming ozonation were to be used on a mid-sized industrial wastewater treatment plant, the cost would amount to ca. $5 × 10 × 24 = $1,200 to treat 10 k m3/d or $0.12/m3. Compare this with an estimated cost of 108–125 Euro/m3 as predicted by Mousset et al. (2021) for the most economical technique: electro-Fenton oxidation. Of course, the cost of the biological treatment would have to be added still. Cost estimates on this differ substantially, ranging from ca. $0.60 to $1.5 m−3 (Sekandari, 2019; Mousset et al., 2021; Arif and Sorour, 2020). Although this is a very rough approach to costing, it is clear that ozonation is an economical approach to improving wastewater treatment and offer many additional benefits.
5 Discussion
A method was developed for selective oxidation integrated with biodegradation of recalcitrant compounds in a single reactor. The bold concept of ozonation to oxidize non-degradable organic substances to biodegradables within a biological process, with biomass largely unharmed, opens up opportunities to new technological development.
However, most recent studies were all two-stage processes, and ozonation was usually conducted first to improve the biodegradability of raw wastewater (Chen et al., 2021; Zhang et al., 2014; Zhuang et al., 2014). It showed that the combination of ozonation and biodegradation could achieve COD removal of 49.7%–61.8%, limited COD removal was achieved (Chen et al., 2021; Zhang et al., 2014). Furthermore, the two-stage process needed the higher ozone dose to oxidize refractory organics and reduce toxicity, and the larger foot-print was also required. A few researchers have used ozone directly in biological treatment, using a solid phase for attached biogrowth, and using the solid-phase media to protect the biofilm against ozone (Su et al., 2020; Cui et al., 2023; Yang et al., 2024).
The only research conducted with ozonation directly inside a suspended growth system was the work by van Leeuwen et al. (2009a) and van Leeuwen et al. (2009b). Ozone applied directly in activated sludge can oxidize recalcitrant organic compounds to biodegradable products. While ozonation leads to some bacterial selection, a healthy and effective biomass can be maintained for the removal of a variety of organic pollutants, including ozonolysis byproducts. The removal of recalcitrant dyes in a biological process was shown to be a combination of ozonolysis and biodegradation and to a much lesser extent biosorption. Without ozonation, color removal in activated sludge was due mainly to biosorption. Higher dye concentrations without ozonation might have toxic effects on protozoa because of toxicity of the dyes.
Selective oxidation within a biotreatment system depends on the relative kinetics. Fast ozonolysis kinetics of a target compound means that it can be removed by addition of ozone at rates below the rate of consumption in the desired reaction. This avoids build-up of an ozone residual that would affect the active biomass.
Simultaneous ozonation and biodegradation of the ozonolysis byproducts offers the benefit of removal of toxic byproducts as these are formed to avoid possible downstream toxicity issues. The simultaneous oxidation and biodegradation can also ensure that toxic substances in the wastewater influent are kept within safe limits.
This research developed a more effective and economical integrated treatment process for wastewater from industries with a high proportion of non-biodegradables, such as dyes. The ozone demand, and subsequently energy requirements, in an integrated process will be lower than in separated biological and ozone treatment. Capital costs will be substantially lower as smaller ozone generation facilities will be required and no separate ozonation reactor necessary. The integrated process will lead to greater sustainability in textile and paper mills, agrichemicals production, pharmaceutical production, coke production for steel smelting, or combined industrial wastewater treatment.
Various researchers have studied the combination of ozonation and biodegradation, albeit with the biomass protected within porous media to treat coking wastewater (Cui et al., 2023; Chen et al., 2021; Olak-Kucharczyk et al., 2023).
Emerging contaminants are of much interest in public and scientific discourse. One such class of compounds is per- and polyfluoroalkyl substances (PFAS) (a group of man-made chemicals that are resistant to heat, water, and oil). These compounds are one of the latest concerns in wastewater treatment. These substances cannot be degraded by ozone and require a stronger oxidant such as OH. radicals. This could be formed by reacting ozone with UV or H2O2, but the reaction conditions would be too extreme to be performed within activated sludge. There are a range of hormonal products such as progesterone, medroxyprogesterone, norethindrone and levonorgestrel that are easily oxidized with molecular ozone (Broséus et al., 2009) and thus potentially removed with the combination of ozonation and activated sludge. Pharmaceuticals and personal care products such as methylparaben, propylparaben, paracetamol (acetaminophen), carbamazepine and sulfamethoxazole are also readily oxidized with ozone (Jesus et al., 2022). Most of these hormones and personal care products are not removed much during normal biological wastewater treatment. Even low concentrations can be quite disruptive to various organisms in water, so more attention should be given to their destruction. It is very likely that the combination of ozonation with biological treatment — in a combined reactor — would be an effective way to remove many of these environmental hazards and certainly would warrant closer investigation.
Data availability statement
Relevant data is included in the manuscript. Requests to access the datasets should be directed to ZHJoYW5zdmxAZ21haWwuY29t.
Author contributions
JHL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. JB: Conceptualization, Methodology, Resources, Writing–original draft, Writing–review and editing. JK: Writing - review and editing. TE: Conceptualization, Methodology, Resources, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Author JB was employed by Black and Veatch Consultants.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, M. B., Zhou, J. L., Ngo, H. H., Guo, W., Thomaidis, N. S., and Xu, J. (2017). Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: a critical review. J. Haz. Mat. 323, 274–298. doi:10.1016/j.jhazmat.2016.04.045
Apha, AWWAWPCF (1995). Standard methods for the examination of water and wastewater. Washington, DC, USA. 19th Edition.
Arif, A. U. A., and Sorour, M. T. (2020). Cost analysis of activated sludge and membrane bioreactor WWTPs using CapdetWorks simulation program: case study of Tikrit WWTP (middle Iraq). Alex. Engng J. 59 (6), 4659–4667. doi:10.1016/j.aej.2020.08.023
Broséus, R., Vincent, S., Aboulfadl, K., Daneshvar, A., Sauvé, S., Barbeau, B., et al. (2009). Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment. Water Res. 43 (18), 4707–4717. doi:10.1016/j.watres.2009.07.031
Chen, X., Wang, C., Jiang, L., Li, H., Wang, J., and Xuwen, H. (2021). Pilot-scale catalytic ozonation pre-treatment for improving the biodegradability of fixed-bed coal gasification wastewater. Process Saf. Environ. Prot. 148 (April), 13–19. doi:10.1016/j.psep.2020.09.056
Collignon, A., Martin, G., Martin, N., and Laplanche, A. (1994). Bulking reduced with the use of ozone – study of the mechanism of action versus bacteria. Ozone Sci. Eng. 16 (4), 385–402. doi:10.1080/01919512.1994.10555748
Contreras, S., Ollis, D. F., and Esplugas, S. (2003). Sequential ozonation and biological oxidation of wastewaters: a model including biomass inhibition by residual oxidant, Ozone Sci. Eng. 25(2), 95–105. doi:10.1080/713610664
Cui, B., Fu, S., Hao, X., and Zhou, D. (2023). Synergistic effects of simultaneous coupling ozonation and biodegradation for coking wastewater treatment: advances in COD removal, toxic elimination, and microbial regulation. Chemosphere 318, 137956. doi:10.1016/j.chemosphere.2023.137956
Doré, M. (1984). “Action de l’ozone sur les constituents cellulaires in vitro,” in Symposium Ozone et Biologie. Rennes, 17–19.
Eikelboom, D. H., and van Buijsen, H. J. J. (1981). Microscopic sludge investigation manual, report A 94a, tno research institute for environmental hygiene. Delft, Netherlands.
Ellis, T. G., and Anselm, C. V. (1999). Effect of batch discharges on extant biodegradation kinetics in activated sludge systems. Wat. Env. Res. 71, 290–298. doi:10.2175/106143098x121860
Ellis, T. G., Barbeau, D. S., Smets, B. F., and Grady, C. P. L. J. (1996). Respirometric technique for determination of extant kinetic parameters describing biodegradation. Water Environ. Res. 68, 917–926. doi:10.2175/106143096x127929
Fang, W., Peng, Y., Muir, D., Lin, J., and Zhang, X. (2019). A critical review of synthetic chemicals in surface waters of the US, the EU and China. Environ. Int. 131, 104994. doi:10.1016/j.envint.2019.104994
Fenn, J. B., Mann, M., Meng, C. K., Wong, S. F., and Whitehouse, C. M. (1990). Electrospray ionization - principles and practice. Mass Spectr. Rev. 9 (1), 37–70. doi:10.1002/mas.1280090103
Freedman, D. L., and Gossett, J. M. (1991). Biodegradation of dichloromethane and its utilization as a growth substrate under methanogenic conditions. Appl. Environ. Microbiol. 57 (10), 2847–2857. doi:10.1128/aem.57.10.2847-2857.1991
Glaze, W. H., Andelman, J. B., Bull, R. J., Conolly, R. B., Hertz, C. D., Hood, R. D., et al. (1993). Determining health risks associated with disinfectants and disinfection by-products: research needs. J. Am. Water Works Assoc. 85, 53–56. doi:10.1002/j.1551-8833.1993.tb05955.x
Grady, C. P. L., Smets, B. F., and Barbeau, D. S. (1996). Variability in kinetic parameter estimates: a review of possible causes and a proposed terminology. Wat. Res. 30, 742–748. doi:10.1016/0043-1354(95)00199-9
Hu, S., and Yu, Y. (1994). Preozonation of chlorophenolic wastewater for subsequent biological treatment. Ozone Sci. Eng. 16, 1613–1628. doi:10.1080/01919519408552377
Ikehata, K., Jodeiri Naghashkar, N., and Gamal El-Din, M. (2006). Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation review. Ozone Sci. Eng. 28 (6), 353–414. doi:10.1080/01919510600985937
Jenkins, D., Richard, M. G., and Daigger, G. T. (2003). Manual on the causes and control of activated sludge bulking, foaming, and other solids separation problems. 3rd Ed. CRC Press, Boca, FL, USA
Jesus, F., Domingues, E., Bernardo, C. J. L., Martins, R. C., and Gomes, J. (2022). Ozonation of selected pharmaceutical and personal care products in secondary effluent—degradation kinetics and environmental assessment. Toxics 10 (12), 765. doi:10.3390/toxics10120765
Jones, E. R., van Vliet, M. T. H., Qadir, M., and Bierkens, M. F. P. (2021). Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst. Sci. Data 13, 237–254. doi:10.5194/essd-13-237-2021
Kamiya, T., and Hirotsu, J. (1998). New combined system of biological process and intermittent ozonation for advanced wastewater treatment. Water Sci. Technol. 38 (8/9), 145–153. doi:10.2166/wst.1998.0801
Lo, Y. C., Koziel, J. A., Cai, L., Hoff, S. J., Jenks, W. S., and Xin, H. (2008). Simultaneous chemical and sensory characterization of volatile organic compounds and semi-volatile organic compounds emitted from swine manure using solid phase microextraction and multidimensional gas chromatography–mass spectrometry–olfactometry. J. Environ. Qual. 37 (2), 521–534. doi:10.2134/jeq2006.0382
Lopez, A., Ricco, G., Mascolo, G., Tiravanti, G., di Pinto, A. C., and Passino, R. (1998). Biodegradability enhancement of refractory pollutants by ozonation: a laboratory investigation on an azo-dyes intermediate. Wat. Sci. Technol. 38 (4/5), 239–245. doi:10.2166/wst.1998.0634
McGuinness, M., and Dowling, D. (2009). Plant-associated bacterial degradation of toxic organic compounds in soil. Int. J. Environ. Res. Pub. Health 6 (8), 2226–2247. doi:10.3390/ijerph6082226
Mousset, E., Loh, W. H., Lim, W. S., Jarry, L., Wang, Z., and Lefebvre, O. (2021). Cost comparison of advanced oxidation processes for wastewater treatment using accumulated oxygen-equivalent criteria. Water Res. 200, 117234. doi:10.1016/j.watres.2021.117234
Muir, D. C. G., Getzinger, G. J., McBride, M., and Ferguson, P. L. (2023). How many chemicals in commerce have been analyzed in environmental media? Environ. Sci. and Technol. 57 (25), 9119–9129. doi:10.1021/acs.est.2c09353
Naidoo, S., and Olaniran, A. O. (2013). Treated wastewater effluent as a source of microbial pollution of surface water resources. Int. J. Environ. Res. Pub. Health. 11 (1), 249–270. doi:10.3390/ijerph110100249
Olak-Kucharczyk, M., Festinger, N., and Smułek, W. (2023). Application of ozonation-biodegradation hybrid system for polycyclic aromatic hydrocarbons degradation. Int. J. Environ. Res. Public Hlth 20 (7), 5347. doi:10.3390/ijerph20075347
Oleszkiewicz, J., and Barnard, J. L. (2006). Nutrient removal technology in North America and the European Union: a review. Water Qual. Res. J. 41 (4), 449–462. doi:10.2166/wqrj.2006.048
Phan, L. T., Schaar, H., Saracevic, E., Krampe, J., and Kreuzinger, N. (2022). Effect of ozonation on the biodegradability of urban wastewater treatment plant effluent. Sci. Tot. Environ. 812, 152466. doi:10.1016/j.scitotenv.2021.152466
Rice, R. G., and Browning, M. E. (1981). Ozone treatment of industrial wastewater. Park Ridge, NJ: Noyes Data Corp.
Rivas, J., F. Beltran, F., Acedo, B., and Gimeno, O. (2000). Two-step wastewater treatment: sequential ozonation – aerobic biodegradation. Ozone Sci. Eng. 22, 617–636. doi:10.1080/01919510009408803
Saayman, G. B., Schutte, C. F., and van Leeuwen, J. (1996). The effect of chemical bulking control on biological nutrient removal in a full-scale activated sludge plant. Water Sci. Technol. 34 (3–4), 275–282. doi:10.2166/wst.1996.0441
Saayman, G. B., Schutte, C. F., and van Leeuwen, J. (1998). Chemical control of filamentous sludge bulking in A full-scale biological nutrient removal activated sludge plant. Ozone Sci. Eng. 20 (1), 1–15. doi:10.1080/01919519808547286
Sekandari, A. W. (2019). Cost comparison analysis of wastewater. Int. J. Sci. Techn. and Engng. 6(1) 2019 ISSN (online): 2349-784X Treatment Plants
Su, Y., Wang, X., Dong, S., Fu, S., Zhou, D., and Rittmann, B. E. (2020). Towards a simultaneous combination of ozonation and biodegradation for enhancing tetracycline decomposition and toxicity elimination. Bioresour. Technol. 304, 123009. doi:10.1016/j.biortech.2020.123009
Susskind, L. (2011). “Strategies for sustainability,” in Studies of strategies for sustainability, 274. Chittaranjan, R. and Jain, R.
Van Leeuwen, J. (1987). Preliminary investigation into the improvement of the biodegradability of organic substances in surface waters and effluents through ozonation. Water Sci. Technol. 19, 931–937. doi:10.2166/wst.1987.0271
Van Leeuwen, J. (1988a). Improved sewage treatment with ozonated activated sludge. J. Inst. Water Environ. Manage. 2, 493–499. doi:10.1111/j.1747-6593.1988.tb01330.x
Van Leeuwen, J. (1988b). Domestic and industrial wastewater treatment with ozonated activated sludge. Ozone Sci. Eng. 10 (3), 291–307. doi:10.1080/01919518808552259
Van Leeuwen, J. (1989). Ozonation for non-filamentous bulking control in an activated sludge plant treating fuel synthesis waste water. Water sa. 15, 127–132.
Van Leeuwen, J., Badriyha, B., and Vaczi, S. (2003). Investigation into ozonation of coal coking processing wastewater for cyanide, thiocyanate and organic removal. Ozone Sci. Eng. 25 (4), 273–283. doi:10.1080/01919510390481595
Van Leeuwen, J., Prinsloo, J., van Steenderen, R. A., and Melekus, W. (1981). The effect of various oxidants on the performance of activated carbon used in water reclamatoin. Ozone Sci. Eng. 3, 225–237. doi:10.1080/01919518108550928
Van Leeuwen, J., Sridhar, A., Esplugas, M., Onuki, S., Cai, L., and Koziel, J. (2009b). Ozonation within an activated sludge system for azo dye removal by partial oxidation and biodegradation. Ozone Sci. Eng. 31 (4), 279–286. doi:10.1080/01919510902907720
Van Leeuwen, J., Sridhar, A., Harrata, A. K., Esplugas, M., Onuki, S., L. Cai, L., et al. (2009a). Improving the biodegradation of organic pollutants with ozonation during biological wastewater treatment. Ozone Sci. Eng. 31 (2), 63–70. doi:10.1080/01919510802668380
van Leeuwen, J. (H. ), Pandiselvam, R., and Jeevarathinam, G. (2024). Cost estimation for the preservation of selected food/crop products with ozone. J. Food Proc. Engng. 47, 1e14772. doi:10.1111/jfpe.14772
Wang, Y., Wang, S., Li, J., Yan, X., Li, C., Zhang, M., et al. (2022). The formation and control of ozonation by-products during drinking water advanced treatment in a pilot-scale study. Sci. Tot. Environ. 808, 151921. doi:10.1016/j.scitotenv.2021.151921
Xiong, G., Koziel, J. A., and Pawliszyn, J. (2004). Air sampling of aromatic hydrocarbons in the presence of ozone by solid-phase microextraction. J. Chromatogr. A 1025, 57–62. doi:10.1016/j.chroma.2003.10.078
Yang, X., Liu, Z., Chen, C., Zhang, T., Wang, Q., Zhang, R., et al. (2024). Hybrid packed bed bioreactor using combined biodegradation and ozonation to enhance nitrogen and micropollutants removal from landfill leachate. Biores. Technol. 412, 131413. doi:10.1016/j.biortech.2024.131413
Yasui, H., and Shibata, M. (1994). An innovative approach to reduce excess sludge production in the activated sludge process. Water Sci. Technol. 30 (9), 11–20. doi:10.2166/wst.1994.0434
Zhang, S., Zheng, J., and Chen, Z. (2014). Combination of ozonation and biological aerated filter (BAF) for bio-treated coking wastewater. Sep. Purif. Technol. 132, 610–615. doi:10.1016/j.seppur.2014.06.019
Keywords: ozone, selective oxidation, disinfection, sewage, waste management, municipal wastewater, industrial wastewater, environmental technology
Citation: van Leeuwen JH, Barnard JL, Koziel JA and Ellis TG (2025) Reflection on ozonation within a wastewater biotreatment process for synthetics degradation. Front. Environ. Chem. 6:1534405. doi: 10.3389/fenvc.2025.1534405
Received: 25 November 2024; Accepted: 19 February 2025;
Published: 13 March 2025.
Edited by:
Muhammad Usman Khan, Washington State University, United StatesReviewed by:
Budi Harahap, Washington State University Tri-Cities, United StatesAlnour Bokhary, Washington State University Tri-Cities, United States
Usman Amin, North Carolina State University, United States
Copyright © 2025 van Leeuwen, Barnard, Koziel and Ellis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. (Hans) van Leeuwen, ZHJoYW5zdmxAZ21haWwuY29t; Timothy G. Ellis, dGdlQGlhc3RhdGUuZWR1
 J. (Hans) van Leeuwen1,2,3*
J. (Hans) van Leeuwen1,2,3* Jacek A. Koziel
Jacek A. Koziel Timothy G. Ellis
Timothy G. Ellis