- Department of Biotechnology, Delft University of Technology, Delft, Netherlands
Increasing concerns over environmental pollution, climate change and energy security are driving a necessary transition from fossil carbon sources to more sustainable alternatives. Due to lower environmental impact, biochemicals are rapidly gaining significance as a potential renewable solution, particularly of interest in Europe. In this context, process systems engineering (PSE) helps with the decision-making at multiple scales and levels, aiming for optimum use of (renewable) resources. Fermentation using waste biomass or industrial off-gases is a promising way for the production of these products. However, due to the inhibitory effects or low substrate concentrations, relatively low product concentrations can be obtained. Consequently, significant improvements in downstream processing are needed to increase the competitiveness of the overall bioprocesses. This paper supports sustainable development by providing new PSE perspectives on the purification of volatile bioproducts from dilute fermentation broths. Since purification significantly contributes to the total cost of biochemical production processes (20%–40% of the total cost), enhancing this part may substantially improve the competitiveness of the overall bioprocesses. The highly advanced downstream process offers the possibility of recovering high-purity products while enhancing the fermentation step by continuously removing inhibitory products, and recycling microorganisms with most of the present water. Besides higher productivity, the upstream process can be greatly improved by avoiding loss of biomass, enabling closed-loop operation and decreasing the need for fresh water. Applying heat pumping, heat integration and other methods of process intensification (PI) can drastically reduce energy requirements and CO2 emissions. Additionally, the opportunity to use renewable electricity instead of conventional fossil energy presents a significant step toward (green) electrification and decarbonization of the chemical industry.
1 Introduction
Extensive consumption of fossil carbon sources has resulted in significant environmental pollution and climate change. Furthermore, concerns over energy security are increasing due to the rapidly diminishing availability of fossil fuels. Consequently, at the 21st United Nations Climate Change Conference (COP21) in Paris, 196 Parties adopted the Paris Agreement with the goal of effectively responding to climate changes (Adoption of the Paris Agreement, 2015). The overall goal requires finding an alternative for most of the oil, coal and gas used for energy supply, and production of different materials and chemicals (Liew et al., 2016). Due to lower environmental impact, biofuels and biochemicals potentially present an attractive renewable alternative to conventional carbon sources. In this context, the focus of PSE is on multi-scale integration of processing methods for optimal functional performance of (bio) chemical manufacturing processes in a (sustainable) supply chain setting, while absorbing suitable process intensification methods within that setting (Van der Wielen et al., 2021). Thus, PSE supports optimal usage of renewable resources in several ways: integration of various components of sustainable energy systems (e.g., energy from biomass, wind, solar, etc.), optimization of individual process operations, design and optimization of large-scale processes, incorporation of data-driven models for decision making, implementation of optimal scheduling and process control strategies, selection of the feedstock, optimization of supply chains, life cycle assessment and risk analysis, techno-economic evaluation of renewable energy projects, and so on.
Notably, Europe is a major center for chemical engineering production and research, with a strong chemical cluster, being the second largest chemical producer in the world, the second largest chemical exporter; and the third largest energy importer. However, as most of the European resource suppliers and product markets are located elsewhere, the European industries need more energy efficient and cost effective processes, particularly in the context of a bio-based circular economy. Production of various biochemicals, especially platform chemicals and biofuels (e.g., ethanol, isopropanol, butanol, isobutanol, acetone, acetic acid, etc.), has a significant potential to enhance the European energy security, reduce the greenhouse gas emissions (Green Claims Directive, 2023), enable new economic opportunities for development of rural areas and decentralize production of biofuels.
Several key technologies are already available for the production of various biochemicals from renewable sources (Karka et al., 2021). Biomass, which is a significant non-fossil derived energy resource (Mallapragada et al., 2023), can be used for the production of the second-generation biofuels (Adewuyi, 2022). Lignocellulosic fermentation is a way to turn energy-dense lignocellulosic materials, which would otherwise be wasted, into bioethanol that has the potential as sustainable fuel. Due to the nature of lignocellulosic feedstock, this bioethanol production process does not threaten food production (Pham et al., 2022). Alternatively, gas fermentation can be implemented to avoid complications with biomass pretreatment and increase the amount of carbon that is being utilized. In this case, biomass is gasified to syngas that can further be converted by different microorganisms into valuable chemicals such as ethanol, isopropanol, acetone, butanol and others. Additionally, since microorganisms tolerate various impurities and variations in gas composition, syngas from biomass gasification or even waste gasses from different industries (e.g., steel industry) can be used as syngas source for fermentation (Puiman et al., 2022). Moreover, in case of (fossil-based) syngas derived from the steel industry, employing it as a substrate for the production of biochemicals offers considerable environmental benefits. Even though microorganisms are tolerant to a wide range of impurities and do not require strict standard syngas purity, high concentrations of some impurities (especially tars, nitric oxide, ammonia and hydrogen sulfide) might negatively affect microbial growth and product formation. To avoid such effects, a gas clean-up step (e.g., tar cracking, wet cleaning, usage of active carbon, etc.) is commonly implemented prior to the fermentation. Thus, removal of potentially present impurities from the syngas would prevent any possible complications in the fermentation and recovery processes (Daniell et al., 2012).
However, significant improvement is needed to make technologies for production of biofuels and biochemicals from renewable sources competitive with fossil carbon based processes (Usmani et al., 2021). The main challenge for industrial production of volatile biochemicals (liquid bioproducts with boiling points either lower or slightly higher than that of water) is the limit in the concentration that can be reached due to the toxic effects product have on cells (McGregor and Furlong, 2017). Therefore, large bioreactors are needed to achieve an industrially relevant production capacity. Consequently, associated capital and operating costs are substantial, especially for the purification part of the process. To date, significant effort has been put into genetic engineering of microorganisms and optimization of the fermentation part in biochemical production processes (Noorman et al., 2018). However, to the best of our knowledge, downstream processing of volatile biochemicals on large-scale is not that well studied. Since purification costs for bulk biochemicals can significantly add to the total production costs (up to 20%–40%) (Straathof, 2011), this paper focuses on the best ways to enhance the overall economic viability of volatile biochemical production by improving downstream processing. Besides reducing the total production costs, advanced downstream processing can reduce the overall energy requirements, greenhouse gas emissions and water requirements, leading to more sustainable bioprocesses that would facilitate an effective transition from fossil fuels to renewable alternatives.
2 Challenges for purification of volatile fermentation products
The major limitation for wide-spread industrial production of volatile biochemicals by fermentation is the relatively low concentrations of products that can be achieved due to the toxic effect these chemicals often have on the microorganisms (McGregor and Furlong, 2017). When highly dilute aqueous streams are obtained, purification in a cost- and energy-efficient way is challenging. Furthermore, due to low product concentrations, large equipment sizes are needed to achieve production scales that would be comparable to those of conventional petrochemical processes (Köpke et al., 2011). Large equipment is closely associated with large capital and operating costs, both for upstream and downstream processes.
Another challenge for choosing the proper operating conditions for recovery of volatile biochemicals from dilute fermentation broths is the presence of living microorganisms. Besides water and fermentation products, fermentation broth contains some biomass, CO2 and low concentrations of different nonvolatile components (Trevethick et al., 2016). To avoid significant loss of microbial biomass and enhance upstream fermentation process by allowing product recovery in a closed loop, microorganisms should be returned to the fermentation (Daniell et al., 2012). Therefore, operating conditions during the separation of valuable products from the microorganisms should not harm microbial viability. Specifically, temperatures that are much higher than the optimal fermentation temperatures must be avoided for this initial separation. Furthermore, usage of additional chemicals that might be toxic for the cells should be avoided. Product recovery using an external loop allows multi-stage operation, whereas only a single equilibrium stage is used during product recovery directly from the fermenter (Cuellar and Straathof, 2020).
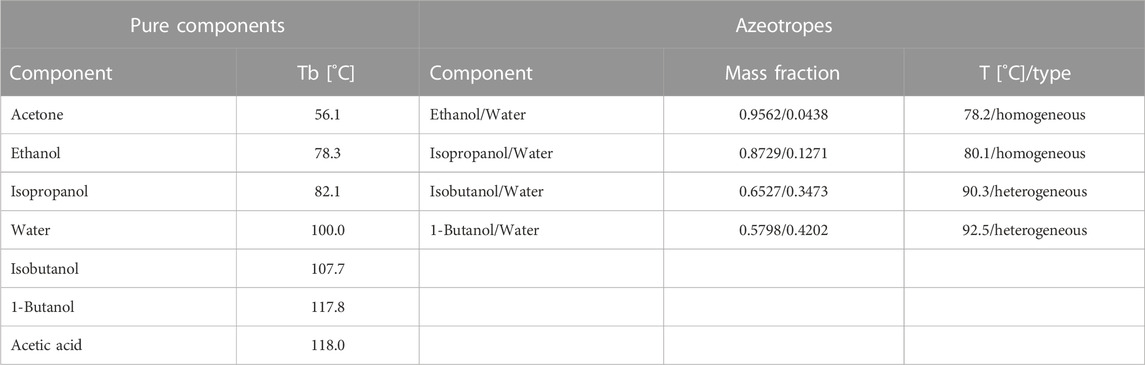
Thermodynamic constraints are frequently another challenge for the recovery volatile biochemicals from fermentation broth. Many well-known bioproducts (ethanol, butanol, isopropanol, etc.) have a boiling points close to that of water (Gmehling et al., 2004). An additional complication is that azeotropes are commonly present (Table 1).
From a PSE viewpoint, there are also challenges related to, e.g., bioprocess synthesis and design, process flowsheet optimization, economic viability and improvements of sustainability metrics.
3 Large-scale purification of common fermentation products
3.1 Methodology for designing purification processes
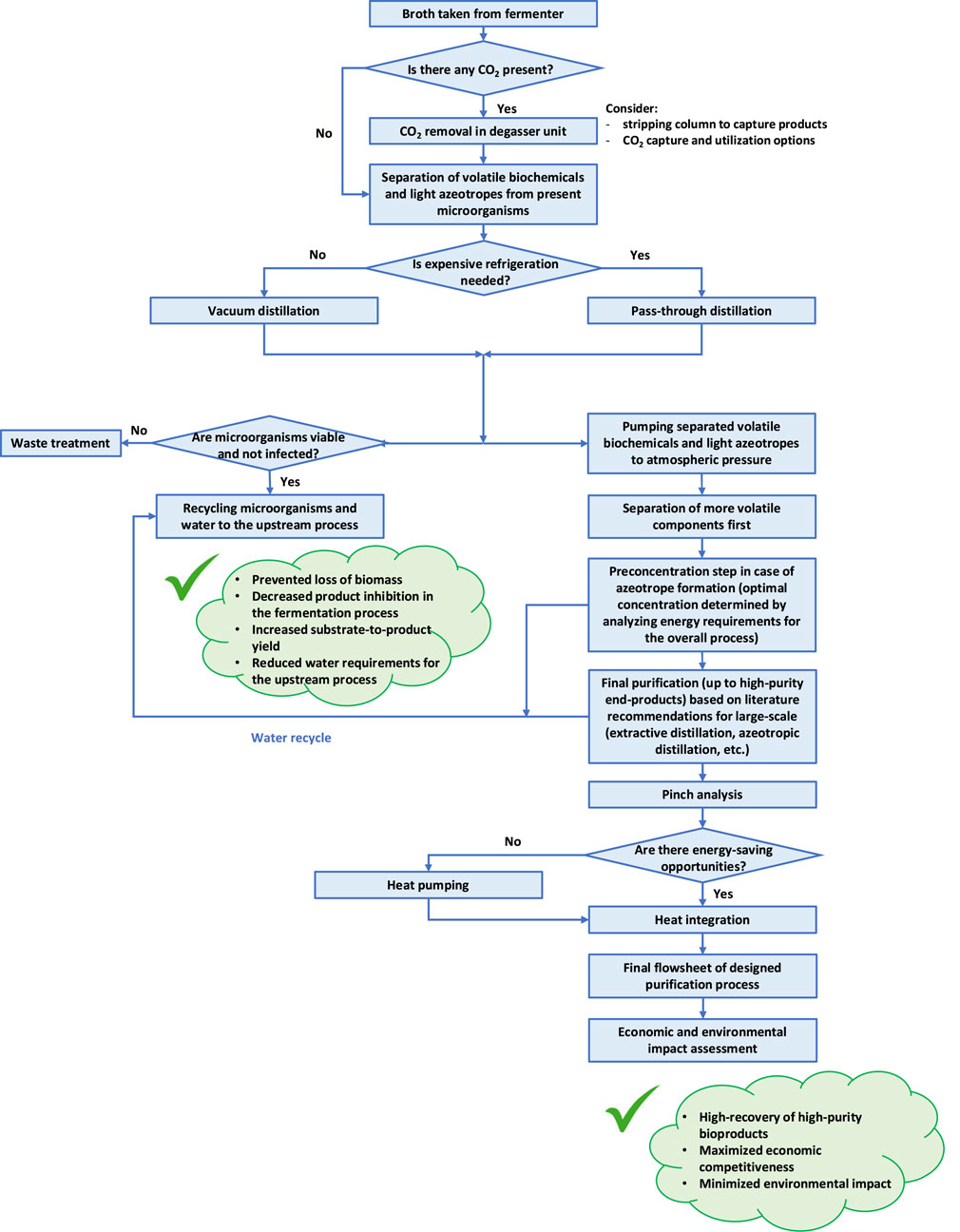
To efficiently mitigate all mentioned challenges and recover fermentation products in a cost-effective and energy-efficient way, we propose several steps to be followed when designing downstream processing sequences (Figure 1).
Different separation techniques (precipitation, extractive distillation, chromatography, membrane separations) have been studied for purification of common volatile fermentation products from diluted solutions (Huang et al., 2008; Vane, 2008). However, the reported yields of the recovered components are usually insufficiently high or usage of additional chemicals that are toxic for microorganisms is required. Additionally, most of the documented data refer to lab-scale without considering scaling-up to an industrial level plant, which might be impractical due to high cost for equipment and/or additional chemicals (Ramaswamy et al., 2013; Li et al., 2016). On the contrary, distillation is most commonly used separation technique for large-scale purification (Kiss, 2013; Singh and Rangaiah, 2017). Besides being already mature on an industrial level, distillation does not require usage of additional chemicals that might harm living cells and can be designed to obtain high-purity products with high recoveries. Therefore, this perspective paper further develops usage of distillation as the main fluid separation method for the purification of volatile biochemicals from fermentation broths.
The first step in a recovery process should be the separation of target products from microorganisms, preferably in a way that does not threaten viability of cells and avoids infection. This separation should be performed without additional chemicals, at moderate temperatures and with relatively short residence time so that cells can survive without additional nutrients. Commonly, microorganisms can be separated from the rest of the broth using a solid-liquid separation such as centrifugation, flocculation, or microfiltration. However, for the recovery of volatile biochemicals, vacuum distillation is a potential option to skip the solid-liquid separation step (Maiorella et al., 1984; Sundquist et al., 1990). During vacuum distillation of fermentation broth, microorganisms will flow directly to the aqueous bottom stream, resulting in a short residence time which is important to keep cells alive without additional nutrients. Furthermore, exposing microorganisms to reduced pressure might require additional experimental validation, but it has already been proven on the pilot-scale that fermentation broth can be exposed to vacuum (Taylor et al., 1997) as cells remain in liquid phase. The operating pressure for this step should be chosen such that the reboiler temperature does not exceed the temperature appropriate for the used microorganism. Volatile biochemicals can be obtained as aqueous top stream from this distillation column. The temperature at the top of this column is determined by the selected operating pressure. Consequently, depending on the allowed reboiler temperature, the temperature at the top might be low and condensation might require expensive refrigeration.
This potential constraint of vacuum distillation can be avoided by implementing pass-through distillation, which implies decoupling the evaporation and condensation steps by inserting an adsorption-desorption loop using an electrolyte absorption fluid. Part of the fermentation broth, containing valuable volatile products, is firstly evaporated from the rest of the feed stream including the microorganisms. The vapor is absorbed by LiBr absorption fluid in an exothermic reaction (Kiss et al., 2014a). The heat released by the absorption can be used for the evaporation step in a unique process equipment unit called stripping-absorption module (McGregor and Belchers, 2014). The target products are later desorbed from the dilute LiBr brine and condensed. Therefore, the working principle of pass-through distillation allows evaporation to be performed at lower pressure and temperature, while condensation can be performed at higher pressure and temperature. Consequently, the temperature limit for heat-sensitive components will not be exceeded and cheaper cooling utilities can be used (McGregor and Furlong, 2017). The operating conditions can be kept appropriate for the microorganisms since they are separated during the first evaporation step and never contact the absorption fluid.
Due to mild temperatures and moderate process conditions, both in case of conventional vacuum distillation and novel pass-through distillation, microorganisms with most of water from the fermentation broth may be recycled to the fermentation. As a result, fermentation might operate in a closed loop without loss of biomass and with decreased requirements for fresh water. Recycling microorganisms to the fermentation might also increase substrate to product yield, as less substrate needs to be spent for microbial growth. Reusing the separated water in the fermentation step can significantly reduce the environmental impact of the overall bioprocess. Furthermore, continuously removing fermentation products from the broth may decrease inhibition effects and increase fermentation yield. From this initial separation step, the solution of volatile biochemicals that is obtained is more concentrated than the fermentation broth. Since this stream with products is under reduced pressure, simple pumping can be used to increase its pressure to the atmospheric one for further treatment. Note that keeping vacuum for the next purification steps would result in unnecessary large equipment and high capital and operating costs.
Since CO2 will be present in the fermentation broth as metabolic co-product, its separation in a degasser unit might be added prior the described steps (Batista and Meirelles, 2009; Marriaga, 2009). This can be done in a simple flash vessel by reducing pressure. Operating conditions should be chosen such that most of the CO2 is evaporated, while the remaining fermentation broth remains liquid. In case a product such as acetone or ethanol is partly removed with CO2, it can be captured with water in a stripping column and returned to the recovery process.
Depending on the composition of volatile biochemicals in the separated aqueous stream, an additional preconcentration step might be needed prior to final purification. The optimal concentration after this preconcentration step should be determined considering the total energy requirements for the overall purification process. Water separated from this step can be cooled and recycled to reduce fresh water requirements upstream.
If, following the initial separation step, the resulting stream contains multiple volatile products, the composition of this stream determines the purification to end-products that satisfy market conditions, whereby more volatile components are typically separated first. Some biochemical products, such as acetone and acetic acid, do not form azeotropes with water and simple distillation can be used to obtain final products. However, azeotropes (Table 1) will often complicate separation and lead to multiple steps. Depending on the nature of azeotrope, different purification techniques have been proven to be optimal in terms of investment and operating costs for the large-scale plant and should be followed. Bioethanol, which is already widely used as renewable fuel, forms a homogeneous azeotrope with water at around 95.6 wt%. Consequently, additional dehydration is needed to obtain final product that satisfies required standards: 99.8 vol% in EU, 99.0 vol% in United States and 99.6 vol% in Brazil (Kiss et al., 2014b). Several studies have proven that for large-scale dehydration, extractive distillation with ethylene glycol is preferred to azeotropic distillation or use of molecular sieves, due to lower operating costs, lower energy requirements, smaller solvent flowrates, a more flexible process sequence, and a less complicated control system (Meirelles et al., 1992; Bastidas et al., 2010; Singh and Rangaiah, 2017). Similarly, extractive distillation with ethylene glycol has also been recommended for large-scale isopropanol dehydration (Liu et al., 2023). 1-Butanol and isobutanol, which are more energy dense and less hygroscopic than ethanol, form heterogeneous azeotropes with water. Conventional processes for separation of biobutanol from acetone—butanol—ethanol fermentation (ABE fermentation) are based on different sequences of distillation columns. However, hybrid heat pump assisted azeotropic dividing-wall column distillation has recently been proposed as novel and less energy intensive alternative (Patrascu et al., 2017; Patrascu et al., 2018). Therefore, reliable literature recommendations for large-scale purification up to high-purity end-product should be followed when choosing final purification methods.
Due to relatively low product concentrations and large flowrates and high water concentrations, some of the described steps might require high heating duties. Therefore, additional process intensification and integration opportunities should be considered to improve competitiveness of the overall biochemical production process by reducing overall costs, energy requirements and CO2 emissions.
3.2 Process intensification opportunities
Process Intensification (PI) is defined as improvements of a process at unit operational, functional and/or phenomena levels that can be obtained by integration of unit operations, integration of functions and phenomena’s or targeted enhancement of the phenomena for a set of target operations. PI uses a set of innovative techniques in process engineering, integration and equipment design to offer breakthrough solutions for making chemical processes with smaller environmental footprint, cost-effective, energy efficient, more controllable and safer, and smaller processing volume. In that respect, different PI techniques can be implemented to enhance downstream processing of volatile biochemicals from the fermentation broth, and some of them are discussed in more detail.
As this perspective paper is considering recovery of volatile fermentation products, most of the feed stream for downstream process is composed of water and relatively close boiling components. Due to very similar temperatures of hot and cold process streams, the system’s pinch temperature is around 100°C and not much energy can be recovered within the process due to insufficient temperature difference (Smith, 2016). Additionally, since process throughputs are large for an industrial-scale plant, significant amounts of water (>90 wt% of the feed stream) need to be evaporated in reboilers of distillation columns. This is particularly the case for bioproducts that have a higher boiling point than water. Consequently, the heating requirements for these columns can easily be very high, on the order of tens of MW. However, since these distillation columns are separating relatively close boiling components, the temperature difference between top and bottom of the column is not large (usually less than 30°C). This feature can be used to apply heat pumps. More precisely, mechanical vapor recompression (MVR) can be implemented to enhance lower grade energy in the condenser and use it to power the reboiler (Kiss, 2013). In MVR, vapor that is coming from the top of distillation column is compressed and used to evaporate liquid from the bottom of the column. Consequently, the need for external heating can be completely avoided, while the need for external cooling is significantly reduced (Kiss and Infante Ferreira, 2016).
This phenomenon can be understood using composite curves before and after intensifying a process with MVR. Analysis of temperature—enthalpy diagram of hot composite curve (presenting all hot process streams) and cold composite curve (presenting all cold process streams) is useful to determine maximum amount of energy that can be recovered in a system (overlapping area between composite curves). As temperatures of all process streams are really close before applying heat pumping, the overlapping area of hot and cold composite curve is relatively small (usually less than 15%). Therefore, the amount of heat that can be recovered within the system is small, while the need for both external heating and external cooling is significant. Implementing MVR shifts composite curves and substantially increases the overlapping area. As a result, much more energy can be recovered by using hot process streams to heat up cold process streams. This significantly reduces the need for both external heating and cooling (Kiss and Infante Ferreira, 2016), resulting in decreased energy requirements (by about 60%–80%) and total recovery costs (by about 40%–80%). Furthermore, since CO2 emissions are directly related to the energy usage, implementation of advance heat pump systems can significantly increase sustainability of the overall biochemical production process by lowering these emissions (reduction in CO2 emissions for the recovery process by about 60%–75% in case electricity from fossil fuels has to be used, or even up to 100% if green electricity is available) (Janković et al., 2023a; Janković et al., 2023b).
Besides improving energy efficiency of the overall recovery process, implementation of heat pumps is a significant step towards (green) electrification of the chemical industry and reduction of greenhouse gas emissions. Using heat pumps provides renewable heat by upgrading waste heat streams. In MVR, electrical energy needed to power the compressor replaces a significantly higher amount of thermal energy. This is especially important since chemical industry is the third largest industrial source of greenhouse gas emissions, after steel and cement industry (Mallapragada et al., 2023). Using renewable electricity to power compressors in heat pump systems instead of fossil fuels as the primary energy source would significantly reduce overall carbon footprint of biochemical production processes. In that respect, PSE can significantly contribute to leveraging the opportunity to use green electricity by ensuring availability of environmentally friendly energy sources (e.g., wind, solar, hydro or geothermal energy), optimizing the energy supply, storage and management, integrating smart technologies, conducting life cycle assessment of the entire bioprocess, optimizing entire supply chain, ensuring compliance with environmental regulations, providing relevant training for the employees and raising awareness of broader community. Besides substantial environmental benefits, the use of green electricity can lead to significant economic and social advantages.
After implementing heat pump systems, additional heat integration opportunities should be considered to maximize energy savings and improve overall process efficiency. Lastly, different process intensification opportunities can be used for final dehydration of commonly produced bioproducts. For example, if separation of multi-component mixture is needed (e.g., purification after ABE fermentation), dividing-wall column (DWC) can be used. In this novel design, sequence of two or more conventional distillation columns is integrated into one shell (Kiss, 2013). Depending on the components that need to be separated, the integrated design can result in significant energy savings compared to the sequence of conventional distillation columns. Furthermore, the more compact design can lead to lower capital costs (Patrascu et al., 2018). However, the distillation column sequence and DWC should be compared per system, since DWC is not always the optimal solution (Janković et al., 2023a).
Thus, the described PI methods lead to more efficient separation processes. By maximizing the amount of energy that can be recovered within the process (e.g., by applying heat pump or heat integration systems), need for external energy supply is minimized. Reduced energy requirements can potentially result in more stable operation in times of uncertain energy supply. Moreover, given the correlation between CO2 emissions and energy usage, implementation of PI methods will decrease these emissions, resulting in more sustainable bioprocesses.
The final flowsheet of the recovery process is developed after considering opportunities for heat pumping, heat integration and additional process intensification. Lastly, in order to evaluate the overall performance of the designed process, economic and environmental impact should be analyzed. The complete assessment of economic factors, including both total capital (CAPEX) and total operating (OPEX) costs, can be conducted following the NREL procedure (Humbird et al., 2011), for example. Environmental performance can be assessed calculating several sustainability metrics: energy intensity, water consumption, material intensity, greenhouse gas emissions, pollutants and toxic materials. The smaller values of these metrics indicate better process performance in terms of sustainability (Schwarz et al., 2002). Following the proposed methodology for the design of advanced downstream processes may significantly contribute to a more sustainable development by minimizing the overall environmental impact of bioprocesses. Furthermore, the significant reduction in the total production costs will make fermentative production of chemicals more economically attractive to stakeholders.
4 Conclusion
This perspective paper provides novel insights into the purification of volatile biochemicals from highly diluted fermentation broths and a generic framework for the design of downstream processes. Significant loss of biomass may be avoided by implementing vacuum distillation or pass-through distillation for initial separation of valuable products from most of the broth. The operating conditions in this step should be designed to maintain viability of present microorganisms that can later be recycled to the upstream fermentation process. Furthermore, literature recommendations for large-scale purification should be followed to maximize downstream process performance. Due to highly diluted aqueous solutions of volatile components, closeness of temperatures of hot and cold process streams does not offer a lot of possibilities for heat integration. This challenge can be overcome by implementing heat pumping to shift the system’s pinch temperature. Consequently, the amount of energy that can be recovered within a process can be substantially increased, considerably reducing the need for external heating and cooling, fresh water requirements, and greenhouse gas emissions. Additionally, applying heat pump systems significantly contributes to electrification of chemical industry as it may replace high amounts of thermal energy by drastically smaller amounts of renewable electricity. Therefore, the proposed methodology for the purification of volatile fermentation products enhances the competitiveness of bioindustry by providing a possibility to recover high-purity biochemicals in a cost-effective and energy efficient manner, while simultaneously enabling enhanced fermentation.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
TJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing–original draft, Writing–review and editing. AS: Formal Analysis, Methodology, Supervision, Validation, Writing–review and editing. AK: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adewuyi, A. (2022). Underutilized lignocellulosic waste as sources of feedstock for biofuel production in developing countries. Front. Energy Res. 10, 1–21. doi:10.3389/fenrg.2022.741570
Adoption of the Paris Agreement (2015). Adoption of the Paris agreement. Available at: https://unfccc.int/sites/default/files/english_paris_agreement.pdf.
Bastidas, P. A., Gil, I. D., and Rodriguez, G. (2010) ‘Comparison of the main ethanol dehydration technologies through process simulation’, in 20th European Symposium on Computer Aided Process Engineering – ESCAPE20.
Batista, F. R. M., and Meirelles, A. J. A. (2009). A strategy for controlling acetaldehyde content in an industrial plant of bioethanol. IFAC Proc. Vol. 42 (7), 928–933. doi:10.3182/20090712-4-tr-2008.00152
Cuellar, M. C., and Straathof, A. J. (2020). Downstream of the bioreactor: advancements in recovering fuels and commodity chemicals. Curr. Opin. Biotechnol. 62, 189–195. doi:10.1016/j.copbio.2019.11.012
Daniell, J., Köpke, M., and Simpson, S. D. (2012). Commercial biomass syngas fermentation. Energies 5, 5372–5417. doi:10.3390/en5125372
Huang, H.-J., Ramaswamy, S., Tschirner, U., and Ramarao, B. (2008). A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 62, 1–21. doi:10.1016/j.seppur.2007.12.011
Humbird, D., Ryan, D., and Nicholas, G. (2011). Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol. Golden, Colorado: National Renewable Energy Laboratory. http://www.nrel.gov/biomass/pdfs/47764.pdf.
Janković, T., Straathof, A. J. J., and Kiss, A. A. (2023a). Advanced downstream processing of bioethanol from syngas fermentation. Sep. Purif. Technol. 322, 124320. doi:10.1016/j.seppur.2023.124320
Janković, T., Straathof, A. J. J., and Kiss, A. A. (2023b). Turning waste into value: eco-efficient recovery of by-products from biomass pretreatment in lignocellulosic biorefineries. Biofuels, Bioprod. Biorefining, 1–14. doi:10.1002/bbb.2532
Karka, P., Johnsson, F., and Papadokonstantakis, S. (2021). Perspectives for greening European fossil-fuel infrastructures through use of biomass: the case of liquid biofuels based on lignocellulosic resources. Front. Energy Res. 9, 636782. doi:10.3389/fenrg.2021.636782
Kiss, A. A. (2013). “Design, control and economics of distillation,” in Advanced distillation technologies: design, control and applications (Chichester: Wiley), 37–66. doi:10.1002/9781118543702
Kiss, A. A., Ignat, R. M., and Bildea, C. S. (2014a). Optimal extractive distillation process for bioethanol dehydration. Comput. Aided Chem. Eng. 33, 1333–1338. doi:10.1016/B978-0-444-63455-9.50057-X
Kiss, A. A., and Infante Ferreira, C. A. (2016). “Mechanically driven heat pumps,” in Heat pumps in chemical process industry (Boca Raton: CRC Press), 189–251. doi:10.1201/9781315371030
Kiss, A. A., McGregor, I. R., and Furlong, S. (2014b). Pass-through distillation - a new player in separation technology. Belgium: NPT Procestechnologie, 1404.
Köpke, M., Mihalcea, C., Bromley, J. C., and Simpson, S. D. (2011). Fermentative production of ethanol from carbon monoxide. Curr. Opin. Biotechnol. 22, 320–325. doi:10.1016/j.copbio.2011.01.005
Li, Q.-Z., Jiang, X. L., Feng, X. J., Wang, J. M., Sun, C., Zhang, H. B., et al. (2016). Recovery processes of organic acids from fermentation broths in the biomass-based industry. J. Microbiol. Biotechnol. 26, 1–8. doi:10.4014/jmb.1505.05049
Liew, F. M., Martin, M. E., Tappel, R. C., Heijstra, B. D., Mihalcea, C., and Köpke, M. (2016). Gas fermentation - a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front. Microbiol. 7, 694. doi:10.3389/fmicb.2016.00694
Liu, X., Wu, J., Lei, Y., Wu, X., Man, Y., Luo, H., et al. (2023). Data-driven surrogate optimized and intensified extractive distillation process for clean separation of isopropanol from water: a sustainable alternative. J. Clean. Prod. 383, 135475. doi:10.1016/j.jclepro.2022.135475
Maiorella, B. L., Blanch, H. W., and Wilke, C. R. (1984). Economic evaluation of alternative ethanol fermentation processes. Econ. Eval. Altern. Ethnaol Ferment. Processes’ Biotechnol. Bioeng. 26, 1003–1025. doi:10.1002/bit.260260902
Mallapragada, D. S., Dvorkin, Y., Modestino, M. A., Esposito, D. V., Smith, W. A., Hodge, B. M., et al. (2023). Decarbonization of the chemical industry through electrification: barriers and opportunities. Joule 7, 23–41. doi:10.1016/j.joule.2022.12.008
McGregor, I., and Furlong, S. (2017). Concurrent alcohol recovery and fermentation using pass-through distillation. Ind. Biotechnol. 13, 107–112. doi:10.1089/ind.2017.29081.imc
Meirelles, A., Weiss, S., and Herfurth, H. (1992). Ethanol dehydration by extractive distillation. J. Chem. Technol. Biotechnol. 53, 181–188. doi:10.1002/jctb.280530213
Noorman, H. J., van Winden, W., Heijnen, J. J., and van der Lans, R. G. J. M. (2018). “Intensified fermentation processes and equipment,” in Intensification of Biobased Processes. Editor A. Górak, and A. Stankiewicz (Green Chemistry Series, RSC). doi:10.1039/9781788010320-00001
Patraşcu, I., Bîldea, C. S., and Kiss, A. A. (2017). Eco-efficient butanol separation in the ABE fermentation process. Sep. Purif. Technol. 177, 49–61. doi:10.1016/j.seppur.2016.12.008
Patrascu, I., Bîldea, C. S., and Kiss, A. A. (2018). Enhanced biobutanol separation by a heat pump assisted azeotropic dividing-wall column. Chem. Eng. Trans. 69, 205–210. doi:10.3303/CET1869035
Pham, L. T. M., Choudhary, H., Gauttam, R., Singer, S. W., Gladden, J. M., Simmons, B. A., et al. (2022). Revisiting theoretical tools and approaches for the valorization of recalcitrant lignocellulosic biomass to value-added chemicals. Front. Energy Res. 10 (July), 1–18. doi:10.3389/fenrg.2022.863153
Puiman, L., Elisiário, M. P., Crasborn, L. M., Wagenaar, L. E., Straathof, A. J., and Haringa, C. (2022). Gas mass transfer in syngas fermentation broths is enhanced by ethanol. Biochem. Eng. J. 185, 108505–108507. doi:10.1016/j.bej.2022.108505
Ramaswamy, S., Huang, H.-J., and Ramarao, B. R. (2013). “Overview of biomass conversion processes and separation and purification technologies in biorefineries,” in Separation and purification technologies in biorefineries (John Wiley and Sons), 3–36. doi:10.1002/9781118493441
Schwarz, J., Beloff, B., and Beaver, E. (2002). Use sustainability metrics to guide decision-making. Chem. Eng. Prog. 98, 58–63.
Singh, A., and Rangaiah, G. P. (2017). Review of technological advances in bioethanol recovery and dehydration. Industrial Eng. Chem. Res. 56, 5147–5163. doi:10.1021/acs.iecr.7b00273
Smith, R. (2016). Chemical process design and integration. 2nd edn. Chichester: John Wiley and Sons Ltd.
Straathof, A. J. J. (2011). “The proportion of downstream costs in fermentative production processes,” in Comprehensive biotechnology (Elsevier B.V.), 811–213. doi:10.1016/B978-0-08-088504-9.00492-X
Sundquist, J., Blanch, H. W., and Wilke, C. R. (1990). “Vacuum fermentation,” in Extractive bioconversions (Boca Raton: CRC Press), 237–258.
Taylor, F., Kurantz, M. J., Goldberg, N., and Craig Jr., J. C. (1997). Effects of ethanol concentration and stripping temperature on continuous fermentation rate. Appl. Microbiol. Biotechnol. 48, 311–316. doi:10.1007/s002530051055
Trevethick, S. R., Bromley, J. C., Waters, G. W., Koepke, M., Tran, L. R., and Jensen Overgaard, R. (2016). Multi-stage bioreactor processes.
Usmani, Z., Sharma, M., Awasthi, A. K., Lukk, T., Tuohy, M. G., Gong, L., et al. (2021). Lignocellulosic biorefineries: the current state of challenges and strategies for efficient commercialization. Renew. Sustain. Energy Rev. 148, 111258. doi:10.1016/j.rser.2021.111258
Van der Wielen, L. A. M., Mussatto, S. I., and van Breugel, J. (2021). Bioprocess intensification: Cases that (don’t) work. New Biotechnology 61, 108–115. doi:10.1016/j.nbt.2020.11.007
Keywords: biochemical production, downstream processing, distillation, heat pumps, heat integration, process intensification, electrification
Citation: Janković T, Straathof AJJ and Kiss AA (2024) Process systems engineering perspectives on eco-efficient downstream processing of volatile biochemicals from fermentation. Front. Energy Res. 11:1340612. doi: 10.3389/fenrg.2023.1340612
Received: 18 November 2023; Accepted: 12 December 2023;
Published: 04 January 2024.
Edited by:
Antonio Espuña, Universitat Politecnica de Catalunya, SpainReviewed by:
Juan Gabriel Segovia Hernandez, University of Guanajuato, MexicoCopyright © 2024 Janković, Straathof and Kiss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anton A. Kiss, dG9ueWtpc3NAZ21haWwuY29t
 Tamara Janković
Tamara Janković Adrie J. J. Straathof
Adrie J. J. Straathof Anton A. Kiss
Anton A. Kiss