- 1Graduate School, Chiang Mai University, Chiang Mai, Thailand
- 2Bioprocess Research Cluster (BRC), Faculty of Agro-Industry, Chiang Mai University, Chiang Mai, Thailand
- 3Cluster of Agro Bio-Circular-Green Industry (Agro BCG), Chiang Mai University, Chiang Mai, Thailand
- 4College of Maritime Studies and Management, Chiang Mai University, Samut Sakhon, Thailand
Solid-state fermentation is one of the promising technologies for biogas production because of its low water footprint and solid output which is potentially used in fuel or agricultural applications. Oil palm kernel pulp (OPKP) is a by-product generated from the extraction of palm kernel oil from the mesocarp of the oil palm tree and usually contains a large amount of lignocellulose and moderate protein content, which makes it suitable for use as a mushroom substrate. Cultivation of white rot mushrooms on lignocellulose may enhance its biodegradation by biodelignification. In this study, the incorporation of the cultivation of edible white rot mushrooms, Pluerotus ostreatus and Pleurotus pulmonarius, to enhance biogas production by solid-state digestion was studied. The biological efficiency of mushroom production from the OPKP substrate of P. ostreatus and P. pulmonarius was 49.81% ± 11.28% and 46.94% ± 13.49%, respectively, corresponding to the substrate weight loss of 15.87% and 13.92%. After 30 days, methane yield obtained through the solid-state digestion of P. ostreatus- and P. pulmonarius-treated OPKP substrates was increased to 98.11 mL/gVS (191%) and 101.10 mL/gVS (197%), respectively, compared with the untreated OPKP substrate. In consideration of energy loss during the biological conversion, the calorific values of the OPKP substrate, P. ostreatus-treated OPKP substrate, and P. pulmonarius-treated OPKP substrate were 11.03 ± 0.71 kJ/g, 9.30 ± 0.23 kJ/g, and 8.83 ± 0.70 kJ/g, respectively, while those of the digestion residues of P. ostreatus and P. pulmonarius-treated OPKP substrates were 8.45 ± 0.13 kJ/g and 8.55 ± 0.11 kJ/g, respectively.
1 Introduction
The palm oil industry is one of the largest and most important industries worldwide. Palm oil is used in a wide variety of products, including food, cosmetics, cleaning products, and biofuels. Solid waste from palm oil production is a major environmental issue in areas where palm oil is produced. Normally, lignocellulosic materials from agriculture and agro-industry can be used to produce biofuel, mushroom substrate, and bioethanol, but these processes have not been well developed (Pérez-Chávez et al., 2019; Tanaka et al., 2019). Therefore, the organic solid waste from oil palm can be a valuable resource for producing value-added products if they were properly managed. Palm waste includes empty fruit bunches (EFBs), oil palm kernel pulp (OPKP), and palm shells and sludge (Yamada et al., 2010). Palm shells can be either used directly in a boiler to produce heat or processed into biomass fuels such as briquettes, pallets, and charcoal. Palm sludge is used as animal feed. OPKP, produced in large amounts compared to other wastes, is also potentially used to produce value-added products. OPKP is a fibrous material that contains approximately 46–52% cellulose, 20–25% hemicellulose, and 19–22% lignin. Cellulose and hemicellulose can serve as substrates for methane production through anaerobic digestion which is a process that utilizes microorganisms to break down organic materials in the absence of oxygen, resulting in the production of methane gas. However, the presence of lignin can affect the methane yield during anaerobic digestion. Lignin, a complex and rigid compound, can hinder the accessibility of microorganisms to cellulose and hemicelluloses, thus limiting the efficiency of methane production. The waste from the palm oil extraction process has to be pretreated before it can be used for other purposes (Jungniyom, 2008; Anyaoha et al., 2018). To overcome this limitation, various pretreatment methods, such as steam explosion, acid hydrolysis, or enzymatic treatment, can be utilized to partially remove or modify lignin and enhance the digestibility of lignocellulosic biomass (Kelly–Yong et al., 2007; Yusoff, 2006).
The treatment of the substrate via mushroom cultivation is considered a biological treatment approach, which is environmentally friendly due to its chemical-free process, low energy consumption, low disposal cost, and low production of inhibitors (Mood et al., 2013). The spent mushroom substrate can be a suitable substrate for biogas production because it undergoes biological predigestion processes, which break down organic matter, making it more readily available for bacterial decomposition during anaerobic digestion (Rinker, 2002; Mohd Hanafi et al., 2018). The investigation of the improvement of biodegradability of palm bunches and biogas production by integrating straw mushroom mycelium culture to pretreat the substrate in a solid-state anaerobic degradation process by Mamimin et al. (2021) found that the cultivation of straw mushroom mycelia in conjunction with solid-state anaerobic digestion is likely to be environmentally friendly and economically enhanced by increasing biogas production. The enzymatic reaction of mushrooms on OPKP can increase its digestibility and make it an excellent substrate for biogas production. The process of pretreatment of OPKP by a mushroom cultivation process also results in food security since the mushroom produced is easily accepted by consumers and consists of proteins and essential amino acids. The mechanism of degradation of lignocellulosic components by mushrooms will vary according to the type of each mushroom. The mushrooms in the white rot group have gained significant interest for their decomposition processes. During the degradation of the white rot fungi, three enzymes are produced: lignin peroxidase (LiP), manganese-dependent peroxidase (MnP), and glyoxal oxidase (GLOX) (Kirk et al., 1978). Pleurotus spp. is often considered one of the easiest and most cost-effective mushroom species to cultivate commercially due to several factors. One important factor is the C:N ratio of substrates, which can range from 30 to 300:1, as reported by Zied and Pardo-Giménez (2017). This wide range allows for flexibility in substrate composition, making it easier to find suitable materials for cultivation. Substrate recipes for Pleurotus spp. generally consist of a base material high in lignocellulose, such as agricultural waste or wood chips. These materials provide the necessary carbon source for the mushrooms. To increase mushroom yields, nitrogen supplements are added to the substrate. This ensures a balanced nutrient composition and promotes optimal growth (O'Brien et al., 2019; Zied and Pardo-Giménez, 2017).
Factors that affect the biogas production process include temperature, pH value, and the carbon-to-nitrogen ratio of organic waste. The temperature range for biogas production is approximately 20–45°C, with the most suitable range being 37–41°C. It is important to maintain an appropriate pH value. Additionally, the suitable pH for biogas production is typically between 7.0 and 7.2, while the optimal C:N ratio is approximately 23 (Tharasawatpipat, 2014). Biogas production at the industrial scale can utilize two processes: liquid fermentation and solid-state fermentation. The anaerobic digestion operated at a substrate solid content level of more than 15–40% usually containing no free water phase is considered solid-state anaerobic digestion (SSAD) (Li et al., 2011; Brown et al., 2012; Chaikitkaew et al., 2015), while liquid fermentation requires significant amounts of water for biogas production (Matheri et al., 2016; Choi et al., 2009). Biogas is produced by fermentation under anaerobic conditions by hydrolytic bacteria, acidogenic bacteria, and methanogenic bacteria (Matheri et al., 2016). The products from the fermentation process under an anaerobic condition consist mainly of methane and carbon dioxide, with approximately 50–70% and 30–50%, respectively, and other gases, such as hydrogen sulfide and ammonia, and some water (Matheri et al., 2016). The main benefit of SSAD is the low water content of the substrate, allowing for high volumetric productivity due to the high solid content of the feed. Moreover, SSAD does not require post-water-treatment, which makes the process simpler and reduces the cost of construction. The effluent of SSAD can be more suitable in fuel application since it contains less water, thus requiring less processing. Therefore, the objective of this study was to investigate the treatment of OPKP by mushroom cultivation and use the treated substrate as feed for solid-state anaerobic digestion to produce biogas. The calorific value of the residual effluent of biogas production was also investigated for its potential use as biofuels.
2 Materials and methods
2.1 Mushroom inoculum
The mushroom mycelia of P. ostreatus and P. pulmonarius were isolated from their pericarp and inoculated into potato dextrose agar (PDA) plates and incubated at 25°C until the mycelium growth covered all over the plates. Afterward, each mycelium-colonized agar was cut into 1.0 × 1.0 cm and inoculated into a bottle containing sterilized sorghum grains and incubated at 25°C until acquiring full colonization, and then the grains were used as sorghum spawn for mushroom cultivation.
2.2 Treatment of oil palm kernel pulp by mushroom cultivation
The OPKP substrate was prepared by mixing 100 kg OPKP with 5 kg rice bran, 5 kg corn dust, 0.25 kg calcium sulfate, and 1 kg calcium oxide. Water was then added to the OPKP substrate to adjust the moisture content to 70%. A measure of 800 g of the OPKP substrate was put into a polyethylene bag and closed with a PVC neck and a cotton pluck. The OPKP substrate was then heated in a mushroom substrate streamer at 80–100°C for 3 h to achieve commercial pasteurization. After cooling down, 15–20 seeds of sorghum spawn were added to the top of the substrate in the cultivation bags. Then, the cultivation bag was incubated at room temperature to promote mycelium growth and simultaneously degrade the OPKP substrate. The fully colonized bag was stimulated for the formation of the mushroom fruiting body by placing it in a mushroom house with a controlled humidity of 80–95%. After the harvest of the first flush of the fruiting body (Figure 1), the spent OPKP substrate was collected and stored at −20°C for subsequent use.

FIGURE 1. Fruiting bodies of Pleurotus ostreatus (A) and Pleurotus pulmonarius (B) obtained from mushroom cultivation on the oil palm kernel substrate.
2.3 Solid-state anaerobic digestion
After mushroom cultivation, the spent OPKP substrate was used in the production of biogas. The biogas inoculum was obtained from an anaerobic digester of the Energy Research and Development Institute of Nakornping, Chiang Mai University, Chiang Mai, Thailand. The anaerobic digester was a covered lagoon used for treating cow manure. The inoculum was prepared by centrifuging the digester sludge at 6,000 rpm for 15 min, and the solid was stored in airtight containers at 4°C and used as the biogas inoculum within 10 days. Solid-state anaerobic digestion (SSAD) was carried out in a 1-L glass bottle in batch style (Figure 2) at three replicates. The spent mushroom substrate from the previous mushroom cultivation was mixed with the inoculum at feed to achieve the inoculum ratio (F/I ratio) of 2 [based on volatile solid (VS)]. The bottles were purged using N2 for 3 min to remove most of the oxygen. To provide the solid-state conditions, the SSAD feed moisture was kept at approximately 75% to ensure that there was no free liquid phase in the setup. Then, the bottles were sealed with a rubber stopper with two sampling ports and incubated at 30°C. The volume of biogas and methane generated was continually monitored for 30 days.
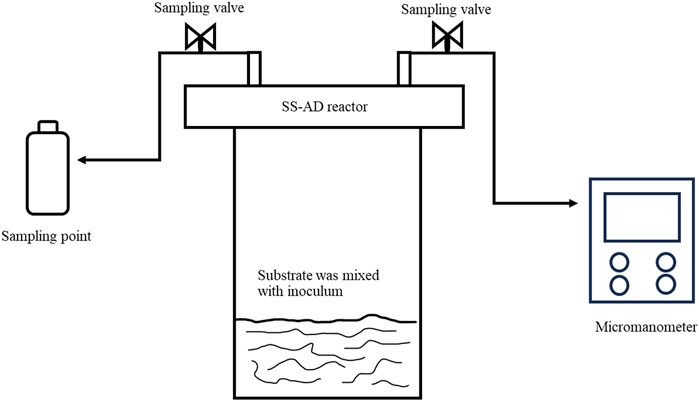
FIGURE 2. Schematic diagram of solid-state anaerobic digestion. The reactor consists of a 1-L glass bottle with an air-tight seal and two sampling valves to facilitate nitrogen flushing of the sample and pressure measurement.
2.4 Analytical methods
The yield of mushrooms was calculated as the sum of all harvests during the experiment. Mushroom biological efficiency (%) was calculated using the following formula: total harvest yield per kg of dry substrate × 100%. The content of lignin, cellulose, and hemicellulose in raw EFB and S-mEFB was determined using the procedures described by Van Soest et al. (1991). The biogas production for each experiment was measured as pressure difference using a micromanometer (MP112, SNDway®) and calculated to the volume of biogas generated. The methane content was determined by gas chromatography (GC 2010; Agilent Technologies) using nitrogen as the gas carrier at a constant pressure of 100 kpa and a flow rate of 20 mL/min, equipped with an Agilent column HP-5MS capillary column (30 m × 0.25 mm × 0.25 m ID) and a thermal conductivity detector (TCD). The temperature of the injector, column oven, and detector was 120°C, 120°C, and 160°C, respectively, increased at a rate of 10°C/min. The injection port temperature was 250°C. The specific methane yield was calculated as the total volume of methane produced per mass of VS added. The pH, TS, and VS were analyzed according to the standard method (APHAAWWA and WEF, 1998). The calorific value of each sample was analyzed in a bomb calorimeter (PARR model 1356 Isoperibol calorimeter, United States) at three replicates.
3 Results and discussion
3.1 Change in OPKP substrate content and yields of Pleurotus cultivation
The properties of the OPKP substrate and spent OPKP substrate after P. ostreatus and P. pulmonarius treatment are shown in Table 1. In P. ostreatus cultivation, the contents of cellulose, hemicellulose, and lignin were decreased from 31.92% ± 1.98%, 23.38% ± 1.88%, and 30.70% ± 1.11% to 25.23% ± 3.48%, 19.06% ± 2.59%, and 25.29% ± 0.95%, while in P. pulmonarius cultivation, the contents were reduced to 25.72% ± 1.35%, 18.61% ± 0.41%, and 26.16% ± 0.43%, respectively. Both P. ostreatus and P. pulmonarius are white rot fungi that degrade polymer in the OPKP substrate by producing a group of enzymes that can change the structure of lignin and other lignocellulosic biomass including laccase, lignin peroxidase, manganese peroxidase, and versatile peroxidase. The main difference between laccases and peroxidase enzymes is that laccases use oxygen as an electron acceptor, while peroxidase enzymes use hydrogen peroxide (H2O2) to receive electrons (Blanchette, 1995; Mir-Tutusaus et at., 2018).
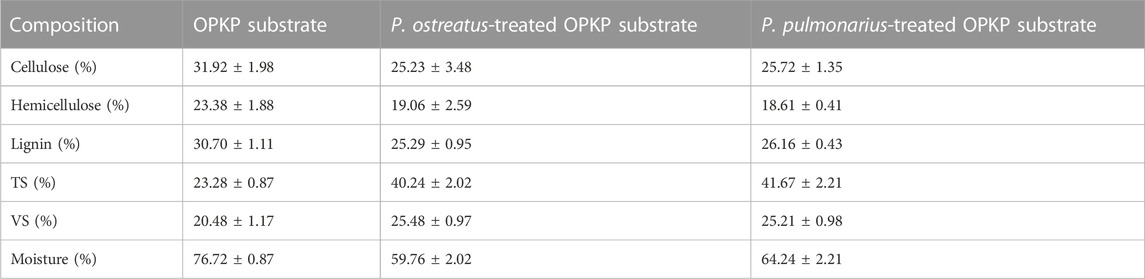
TABLE 1. Composition of OPKP substrate, P. ostreatus-treated OPKP substrate, and P. pulmonarius-treated OPKP substrate.
The average yields per bag of P. ostreatus and P. pulmonarius were 79.70 ± 18.05 g and 75.13 ± 21.58 g, respectively, which corresponded to the average number of fruiting bodies of 6.50 ± 4.31 and 6.18 ± 2.58 (Table 2). The biological efficiency of the OPKP substrate for P. ostreatus and P. pulmonarius was 49.81% ± 11.28% and 46.94% ± 13.49%, respectively, which implied that P. ostreatus grow better on the OPKP substrate than P. pulmonarius. The weight loss of the OPKP substrate during P. ostreatus cultivation was 15.87% ± 1.98% compared with 13.92% ± 1.74% of P. pulmonarius. The weight loss indicated the enzyme activities to break down the composition of the substrate for use as nutrients for growth. The degradation of lignocellulosic material in mushroom growth resulted in a decrease in volatile solid (VS), which was mostly organic matter. The cultivation was considered a pretreatment of OPKP since lignin was removed while other lignocellulosic biomass was partially degraded, making it easier for the conversion into volatile acids, which are important substrates for methanogenic microorganisms.
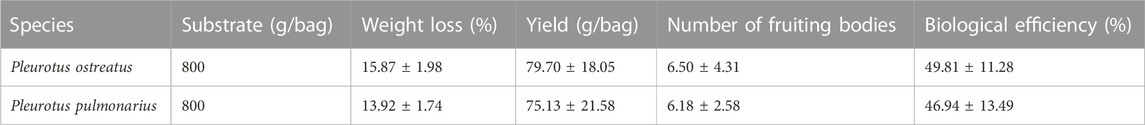
TABLE 2. Yield, weight loss, and biological efficiency of Pleurotus ostreatus and Pleurotus pulmonarius cultivation on the OPKP substrate.
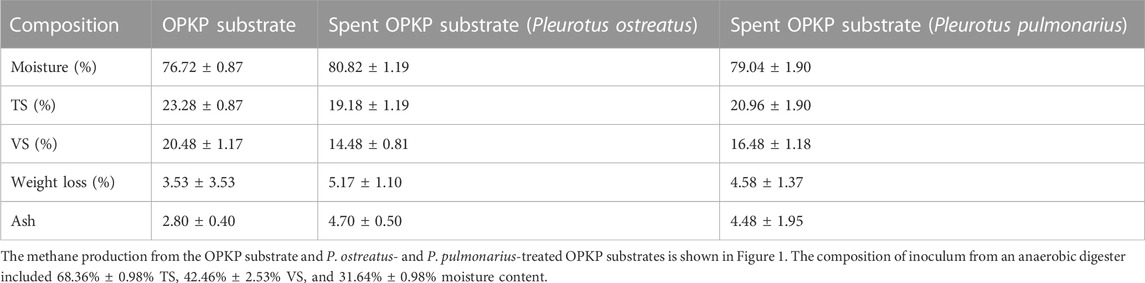
3.2 Biogas and methane yield of the OPKP substrate and mushroom-treated OPKP substrate by SSAD
During the cultivation and harvesting of mushrooms, the substrate was treated by Pleurotus biodegradation activity, converting it into better feed for anaerobic digestion. The composition of OPKP substrate and P. ostreatus- and P. pulmonarius-treated substrates is shown in Table 3. The reduced percentage of TS and VS reflected the utilization of the substrate for methanogenic and biosynthesis activities, while the weight loss of the OPKP substrate and P. ostreatus- and P. pulmonarius-treated substrates after the SSAD process were 3.53% ± 3.53%, 5.17% ± 1.10%, and 4.58% ± 1.37%, respectively. In methanogenesis, methane is synthesized via two pathways: conversion of acetic acid into methane or conversion of carbon dioxide and hydrogen into methane in which hydrogen provides the electron between reactions. Both reactions produce approximately 65–70% and 27–30% of methane, respectively (Matheri et al., 2016). The biogas generation led to the decrease in mass and simultaneously increased the moisture content of the system. This can be observed from the slight increase in moisture content which was within the solid-state conditions of the experiment.
Figure 3 shows the specific biogas yield per day of the OPKP substrate which initially increased after the SSAD process started. However, after the third day, the amount of biogas decreased from 4.93 ± 0.22 mL/gVS to 1.30 ± 0.11 mL/gVS at day 30. In case of P. ostreatus- and P. pulmonarius-treated OPKP substrates, biogas generation was quite similar, which implies that both mushrooms belong to the same genus and may have similar biodegradation activity. Both Pluerotus-treated OPKP substrates generated the highest biogas at day 13 before the volume gradually decreased. From day 13 to day 30, the biogas yield of P. ostreatus- and P. pulmonarius-treated OPKP substrates was decreased from 6.19 ± 0.02 mL/gVS to 4.82 ± 0.11 mL/gVS and 6.27 ± 0.07 mL/gVS to 4.87 ± 0.16 mL/gVS, respectively.
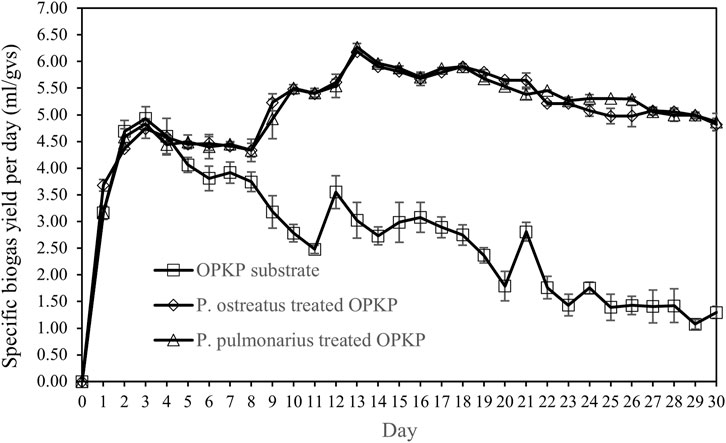
FIGURE 3. Specific biogas yield (ml/gVS) of the OPKP substrate, P. pulmonarius-treated OPKP substrate, and P. ostreatus-treated OPKP substrate in solid-state anaerobic digestion at 30°C.
Figure 4 shows that the specific methane yield per day of OPKP substrate was also initially increased after the SSAD process started and reached 2.69 ± 0.12 mL/gVS on the third day. After the third day, the amount of daily methane yield remained relatively constant until day 21. After day 21, the amount of methane yield decreased from 1.85 ± 0.15 mL/gVS to 0.09 ± 0.01 mL/gVS at 30 days. The highest methane yield of the OPKP substrate was 2.70 ± 0.22 mL/gVS at day 12. The methane yield of the OPKP substrate treated with Pleurotus ostreatus was initially increased to 2.84 ± 0.08 mL/gVS at day 8. After day 8, the amount of methane yield was increased from 2.84 ± 0.08 mL/gVS to the highest yield of 4.30 ± 0.04 mL/gVS at day 13. Likewise, P. pulmonarius-treated OPKP was initially increased after the fermentation process started at 0.39 ± 0.02 mL/gVS on the first day and reached its highest yield of 4.34 ± 0.06 mL/gVS at day 13. Afterward, the amount of methane produced each day decreased slightly until the end of the experiment on day 30, with the methane concentration of 60%–70% in biogas.
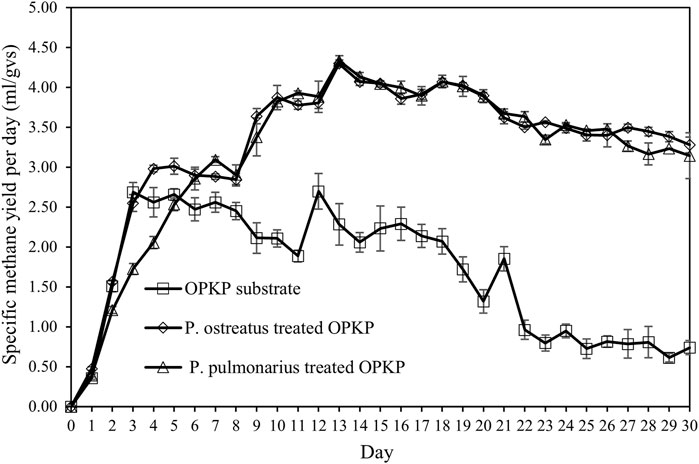
FIGURE 4. Specific methane yield (m1/gVS) of the OPKP substrate, P. pulmonarius-treated OPKP substrate, and P. ostreatus-treated OPKP substrate in solid-state anaerobic digestion at 30°C.
The results suggested that during the first fermentation period of the substrate (days 1–3), the microorganisms in SSAD used readily available biodegradable substrates in OPKP to produce biogas and methane. However, OPKP contains a large amount of lignin, which prevents the microorganisms to digest cellulose, because most bacterial enzymes are unable to digest lignin (Pawongrat, 2015). Therefore, biogas generated from the OPKP substrate has a lower yield. In the other way, biogas generated from both Pleurotus-treated OPKP substrates showed significantly higher yields. When treated with both mushrooms, the amount of lignin was reduced resulting in an increase in the bioavailability of readily degradable energy sources for microorganisms. In the early stages of digestion, carbohydrate is converted into acetic acid and carbon dioxide, so the methane content is not very high initially. Afterward, methanogenic bacteria convert acetic acid into methane, resulting in higher methane yields in the later stages (Matheri et al., 2016).
Overall, the biogas yield generated from the OPKP substrate and P. ostreatus-treated and P. pulmonarius-treated substrates in SSAD was 82.29 ± 2.22 mL/gVS, 154.53 ± 0.43 mL/gVS, and 154.80 ± 0.40 mL/gVS, respectively. Moreover, the highest specific methane yield accumulation was obtained from the P. ostreatus-treated substrate (101.10 ± 0.14 mL/gVS), which was close to that obtained from the P. pulmonarius-treated substrate (98.11 ± 0.25 mL/gVS). The lowest methane yield accumulation was found in the untreated OPKP substrate, which was 51.26 ± 1.15 mL/gVS, as shown in Figure 5. Therefore, the treatment with mushroom cultivation on the OPKP substrate may improve its biodegradability by either removing lignin or converting cellulose and hemicellulose to the substrate of fermentative microorganisms. When comparing the methane yield in this study with that of others, as shown in Table 4, methane accumulation from the OPKP substrate treated by P. pulmonarius and P. ostreatus was lower. Several factors influence the methane yield in anaerobic digesters, including operating temperature, fermentation process, retention time, and the nature of the substrate.
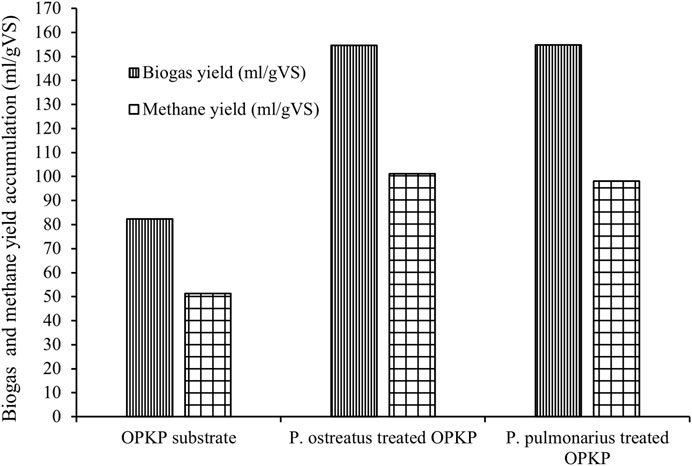
FIGURE 5. Specific biogas yield accumulation (m1/gVS) and specific methane yield accumulation (m1/gVS) of the OPKP substrate, P. pulmonarius-treated OPKP substrate, and P. ostreatus-treated OPKP substrate in solid-state anaerobic digestion at 30°C.
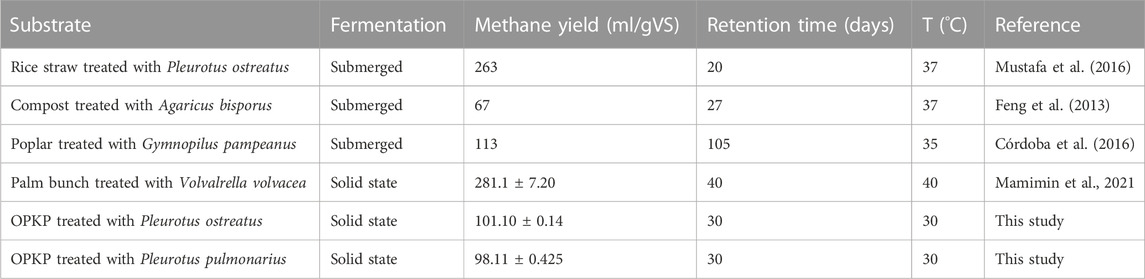
TABLE 4. Comparison of studies for the pretreatment of the substrate by mushroom cultivation to enhance biogas and methane production.
Mamimin et al. (2021) used a higher temperature of 40°C, which likely boosted the activity of methanogenic enzymes, resulting in a higher methane production compared to our study. However, it is essential to consider that raising the temperature above ambient levels incurs additional heating costs, which may not be practical for all anaerobic digester facilities.
Although submerged fermentation, which provides better mixing, may be preferred for methane production, in some cases, solid-state fermentation yields more methane yield with a lower water footprint. This highlights the trade-offs between different fermentation approaches and their respective methane yields.
Overall, the methane yield observed in this study was lower compared with that in other studies, and various factors, including temperature, fermentation process, and water footprint, can influence the efficiency of methane production in anaerobic digesters. Each approach has its advantages and disadvantages, and selecting the most appropriate method depends on the specific conditions and objectives of the biogas production facility.
The total amount of methane gas generated was derived as reported by Raksri et al. (2020). The considerable amount of lignocellulose-degrading enzyme produced during mushroom growth may somewhat affect the biodegradation in the SSAD process as well.
Table 5 shows the calorific value of the substrates in each step. The calorific value of OPKP decreased after passing each process. Factors affecting the calorific value include TS, fixed carbon, and ash. After the cultivation and SSAD of P. ostreatus and P. pulmonarius, the TS value decreased as a result of microbial consumption. The calorific values of the OPKP substrate and P. ostreatus- and P. pulmonarius-treated OPKP substrates was 11.03 ± 0.71 kJ/g, 9.30 ± 0.23 kJ/g, and 8.83 ± 0.70 kJ/g, respectively. After SSAD, the calorific values of effluent of the OPKP substrate and P. ostreatus- and P. pulmonarius-treated substrates were 9.70 + 0.43 kJ/g, 8.45 ± 0.13 kJ/g, and 8.55 ± 0.11 kJ/g, respectively. Although the calorific value output is quite much lower than that of the palm shell, which has a calorific value of 16.90, the calorific value of this biomass is close to that of rice husk, which is regularly used as a biomass fuel to produce energy.
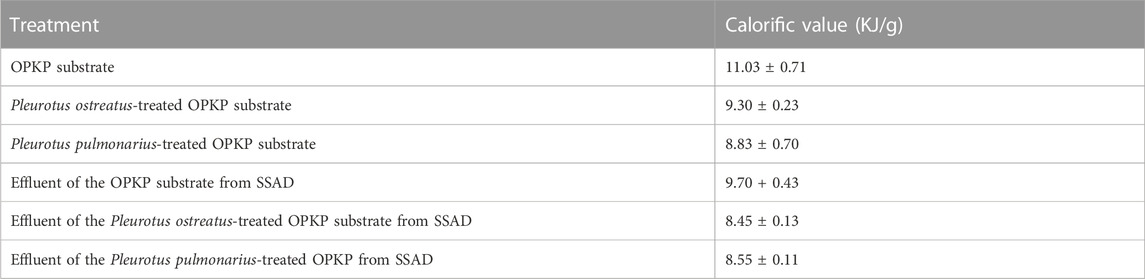
TABLE 5. Calorific value of the OPKP substrate, Pleurotus ostreatus-treated OPKP substrate, and Pleurotus pulmonarius-treated OPKP substrate and the calorific value of effluent of the OPKP substrate, Pleurotus ostreatus-treated OPKP substrate, and Pleurotus pulmonarius-treated OPKP substrate from the solid-state anaerobic digester.
4 Conclusion
OPKP is considered waste generated during the processing of palm oil from the oil palm fruit. This research presents a way to fully utilize OPKP to produce different products. First, it is used as a substituted substrate to produce commercial mushrooms. From our results, the bioconversion of both P. ostreatus and P. pulmonarius during mushroom cultivation improved the quality of OPKP as the substrate for anaerobic digestion to produce biogas. Although some biomass was lost during cultivation, the observed methane production was approximately two times compared with untreated OPKP due to the removal of lignin, which makes cellulose and hemicellulose available for breaking down by a variety of microorganisms that reside in a solid-state anaerobic digester. Finally, the spent substrate effluent from the solid-state anaerobic digester can be a valuable biomass fuel since it contains residual biomass that has not been fully converted into biogas. This residual biomass still has high energy content (approximately 8.50 kJ/g) and less water content compared with that in the traditional submerged anaerobic digestion, which facilitates various applications. One possible application of the spent substrate is that it can be used as feedstock for combustion or gasification to produce electricity or heat. The spent substrate is also possible to be processed into pallets or briquettes for use as fuels in boilers or stoves. Overall, the full utilization of OPKP can provide economic and environmental benefits by reducing waste and creating value-added products. In addition, the use of OPKP as feedstock for biogas production can help reduce greenhouse gas emissions, as it provides an alternative to the disposal of the pulp through burning or landfilling.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SJ and PP contributed to the conception and design of the study. PP performed most of the experiments. NL, PR, and YC facilitated the analysis. All authors contributed to the article and approved the submitted version.
Funding
This research project was supported by the National Research Council of Thailand (NRCT): NRCT5-RRI63004-M10.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anyaoha, K. E., Sakrabani, R., Patchigolla, K., and Mouazen, A. M. (2018). Critical evaluation of oil palm fresh fruit bunch solid wastes as soil amendments: prospects and challenges. Resour. Conservation Recycl. 136, 399–409. doi:10.1016/j.resconrec.2018.04.022
APHA, AWWAWEF (1998). Standard methods for the examination of water and wastewater. Maryland: American Public Health Association. Washington D.C. U.S.A.
Brown, D., Shi, J., and Li, Y. (2012). Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour. Technol. 124, 379–386. doi:10.1016/j.biortech.2012.08.051
Chaikitkaew, S., Kongjan, P., and O-Thong, S. (2015). Biogas production from biomass residues of palm oil mill by solid state anaerobic digestion. Energy Procedia 79, 838–844. doi:10.1016/j.egypro.2015.11.575
Choi, Y.-S., Kim, G.-H., Lim, Y. W., Kim, S. H., Imamura, Y., Yoshimura, T., et al. (2009). Characterization of a strong CCA-treated wood degrader. Unkn. Crustoderma species Ant. Van Leeuwenhoek 95 (3), 285–293. doi:10.1007/s10482-009-9311-1
Córdoba, V., Colavolpe, M. B., Fernández, M., Santalla, E., and Albertó, E. (2016). Potential methane production of spent sawdust used in the cultivation of Gymnopilus pampeanus. J. Environ. Chem. Eng. 4 (4), 4418–4425. doi:10.1016/j.jece.2016.10.009
Energy for Environment Foundation (2004). Biomass general information. available at: http://biomass.dede.go.th/biomass_web/index (November 5, 2022).
Feng, X., Pilar Castillo, M. D., and Schnürer, A. (2013). Fungal pretreatment of straw for enhanced biogas yield. Malmö Sweden: Svenskt Gastekniskt Center AB.
Jungniyom, T. (2008). Zero-waste process in oil palm extraction industries. Haiyai J. 6 (2), 160–164.
Kelly-Yong, T. L., Lee, K. T., Mohamed, A. R., and Bhatia, S. (2007). Potential of hydrogen from oil palm biomass as a source of renewable energy worldwide. Energy Policy 35 (11), 5692–5701. doi:10.1016/j.enpol.2007.06.017
Kirk, T. K., Schultz, E., Connors, W. J., Lorenz, L. F., and Zeikus, J. G. (1978). Influence of culture parameters on lignin metabolism byPhanerochaete chrysosporium. Arch. Microbiol. 117, 277–285. doi:10.1007/bf00738547
Li, Y., Park, S. Y., and Zhu, J. (2011). Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 15 (1), 821–826. doi:10.1016/j.rser.2010.07.042
Mamimin, C., Chanthong, S., Leamdum, C., O-Thong, O., and Prasertsan, P. (2021). Improvement of empty palm fruit bunches biodegradability and biogas production by integrating the straw mushroom cultivation as a pretreatment in the solid-state anaerobic digestion. Bioresour. Technol. 319, 124227. doi:10.1016/j.biortech.2020.124227
Matheri, A. N., Belaid, M., Seodigeng, T., and Ngila, C. J. (2016). Modelling the kinetic of biogas production from co-digestion of pig waste and grass clippings. Proceedings of the World Congress on Engineering 2016 Vol II, 8.
Mohd Hanafi, F. H., Rezania, S., Mat Taib, S., Md Din, M. F., Yamauchi, M., Sakamoto, M., Hara, S. S., Park, J., and Ebrahimi, S. S. (2018). Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): an overview. J. Mater Cycles Waste Manag. 20, 1383–1396. doi:10.1007/s10163-018-0739-0
Mood, S. H., Golfeshan, A. H., Tabatabaei, M., Jouzani, G. S., Najafi, G. H., Gholami, M., et al. (2013). Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 27, 77–93. doi:10.1016/j.rser.2013.06.033
Mustafa, A. M., Poulsen, T. G., and Sheng, K. (2016). Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl. Energy 180, 661–671. doi:10.1016/j.apenergy.2016.07.135
O'Brien, B. J., Milligan, E., Carver, J., and Roy, E. D. (2019). Integrating anaerobic co-digestion of dairy manure and food waste with cultivation of edible mushrooms for nutrient recovery. Bioresour. Technol. 285, 121312. doi:10.1016/j.biortech.2019.121312
Pawongrat, R. (2015). Pretreatment processes for enhancing the efficiency of ethanol production from lignocellulosic-agricultural wastes. Veridian E-journal Sci. Technol. Silpakorn Univ. 2 (1), 143–157.
Pérez-Chávez, A. M., Mayer, L., and Albertó, E. (2019). Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy for Sustain. Dev. 50, 50–60.
Raksri, W., Gaewyana, N., and Aggarangsi, p. (2020). Development of methane production from starch industry wastewater by addition of ion metal. Thai Sci. Technol. J. 28 (4), 705–716.
Rinker, D. L. (2002). Mushroom biology and mushroom products. Cuernavaca: UAEM.Handling and using “spent” mushroom substrate around the world
Tanaka, T., Onuma, H., Shigihara, T., Kimura, E., Fukuta, Y., Shirasaka, N., et al. (2019). Anti-osteoporotic effects of syringic acid and vanilic acid in the extracts of waste beds after mushroom cultivation. J. Biosci. Bioeng. 128 (5), 622–629.
Tharasawatpipat, C. (2014). Biogas production from agricultural waste. Available at: http://www.thai-explore.net/file_upload/submitter/file_doc/572379aeb3254703dc0f64daa03fff3e.pdf May 7, 2023).
Van Soest, P. V., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. dairy Sci. 74 (10), 3583–3597. doi:10.3168/jds.s0022-0302(91)78551-2
Yamada, H., Tanaka, R., Sulaiman, O., Hashim, R., Hamid, Z. A. A., Yahya, M. K. A., Kosugi, Y., Arai, T., Murata, Y., and Nirasawa, S. (2010). Old oil palm trunk: a promising source of sugars for bioethanol production. Biomass Bioenergy 34 (11), 1608–1613. doi:10.1016/j.biombioe.2010.06.011
Yusoff, S. (2006). Renewable energy from palm oil - innovation on effective utilization of waste. J. Clean. Prod. 14 (1), 87–93. doi:10.1016/j.jclepro.2004.07.005
Keywords: agricultural waste, anaerobic digestion, bioenergy, methane, mushroom cultivation, palm oil, palm kernel, solid-state anaerobic digestion
Citation: Panngoen P, Leksawasdi N, Rachtanapun P, Chakrabandhu Y and Jinsiriwanit S (2023) Integration of white rot mushroom cultivation to enhance biogas production from oil palm kernel pulp by solid-state digestion. Front. Energy Res. 11:1204825. doi: 10.3389/fenrg.2023.1204825
Received: 12 April 2023; Accepted: 01 August 2023;
Published: 07 September 2023.
Edited by:
Chinnathan Areeprasert, Kasetsart University, ThailandReviewed by:
Yu Zhang, Zhejiang University City College, ChinaDachao Ma, Guangxi University, China
Marcin Debowski, University of Warmia and Mazury in Olsztyn, Poland
Copyright © 2023 Panngoen, Leksawasdi, Rachtanapun, Chakrabandhu and Jinsiriwanit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siriwat Jinsiriwanit, c2lyaXdhdC5qaW5AY211LmFjLnRo
 Pathompong Panngoen
Pathompong Panngoen Noppol Leksawasdi
Noppol Leksawasdi Pornchai Rachtanapun
Pornchai Rachtanapun Yasinee Chakrabandhu
Yasinee Chakrabandhu Siriwat Jinsiriwanit
Siriwat Jinsiriwanit