- 1Crop Production Division, ICAR- Indian Institute of Oil Palm Research, Ellore, Andhra Pradesh, India
- 2Department of Crop Chemistry and Soil Science, ICAR- Central Tobacco Research Institute, Rajahmundry, Andhra Pradesh, India
- 3School of Agriculture and Animal Sciences, The Gandhigram Rural Institute, Dindigul, Tamil Nadu, India
- 4Department of Soil Science and Agricultural Chemistry, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India
- 5Department of Environmental Science, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India
Intensive cultivation of agricultural soils causes soil degradation which emphasizes the need for sustainable soil management. Biochar, a pyrolysed carbon rich material has gained great interests among the researchers because of its eco-friendly benefits in addition to soil quality enhancement. Reviews on biochar, mainly confined to its environmental benefits like carbon sequestration and climate change. In this review, we summarize i) the effect of biochar application on soil properties (physical, chemical, biological), ii) remediation potential of biochar in heavy metal contaminated soils and iii) its impact on crop productivity. The properties of biochar like pH, greater surface area, cation exchange capacity, and nutrient content positively influences the soil properties and ultimately improves the soil fertility. Their effectiveness depends on biochar type, its dosage, soil type, etc. General trends from this review indicated that biochar as an effective amendment in acid soils than the alkaline or calcareous soils. Furthermore, the biochar effects are studied mostly under controlled conditions in laboratory, which needs to be validated under field conditions having varied soil types and agro-climatic zones.
Introduction
Intensive cultivation of agricultural soils could end in soil degradation, involving fertility decline, soil acidity or alkalinity, loss of organic matter, soil erosion, and soil pollution (De Meyer et al., 2011). Furthermore, the annihilation or diminution of soil properties like soil organic carbon decreases the stability of soil aggregates and causes soil degradation (Nunes et al., 2020). Therefore, it is necessary to come up with sustainable soil management practices to remediate the degraded soils. Various organic and inorganic wastes such as agricultural crop residues, forest waste, industrial waste, municipal solid waste, etc. were generated in massive quantities (Gabhane et al., 2020), from which, a majority are disposed directly through burning or dumping in fields polluting the air, soil and water. Several studies (Raut et al., 2008; Gabhane et al., 2016) have proposed that composting of these wastes as promising technique. However, composting is not found to be attractive because of its slow degradation rate (Gabhane et al., 2012). Also, utilization of composts and manures result in potential emissions of methane and ammonia, aggravating the global warming (Ding et al., 2016). In recent years, the interest towards the soil application of biochar has grownup considering the twin benefits of biochar on climate change mitigation and as an amendment to improve soil quality (Liu et al., 2014). Biochar is the recalcitrant carbon rich product (Mukherjee et al., 2014) obtained through a thermo-chemical process at the temperature range of 350°C-600°C in an oxygen-free environment and in some cases with abysmally low/limited oxygen (Amonette and Joseph, 2009). In the early phase, biochar was viewed as a source of energy, material for water purification, etc. The physical (surface morphology, surface area, porosity) and chemical (composition and functional groups) properties makes biochar an amicable adsorption material to remove the pollutants from aqueous medium (Tan et al., 2015).
This renewable resource is found to be efficient in soil fertility management. For instance, several studies (Spokas et al., 2012; Xu G. et al., 2014; Kammann et al., 2015; Schmidt et al., 2015) proposed that the nutrient loaded biochar acts as slow-release fertilizer for soil fertility enhancement. The chemical and physical properties of the biochar depend on the raw material utilized, pyrolysis temperature, furnace temperature, and residence time (Yaashikaa et al., 2020). Wide range of feed stocks were utilized for biochar production with their pH ranging from 8.2–13.0, total carbon content ranging between 33.0%–82.4%, N content of 0.18%–2.0% and with the C:N ratio of 19–221: 1 (DeLuca et al., 2009). Nutrient retention in the biochar depends on pyrolysis temperature. The volatilizing temperature varies with nutrients, i.e., nitrogen (200 °C), phosphorus and potassium (700°C–800 °C) and sulphur (375 °C). The biochar produced at high pyrolysis temperature of about 800 °C recorded higher pH, salt concentration, and extractable NO3−, whereas the biochars produced at low-temperature of 350 °C showed higher extractable P, NH4+, and phenols (DeLuca et al., 2009). The calcium and magnesium composition of biochar makes it an amendment to reclaim acidic soils.
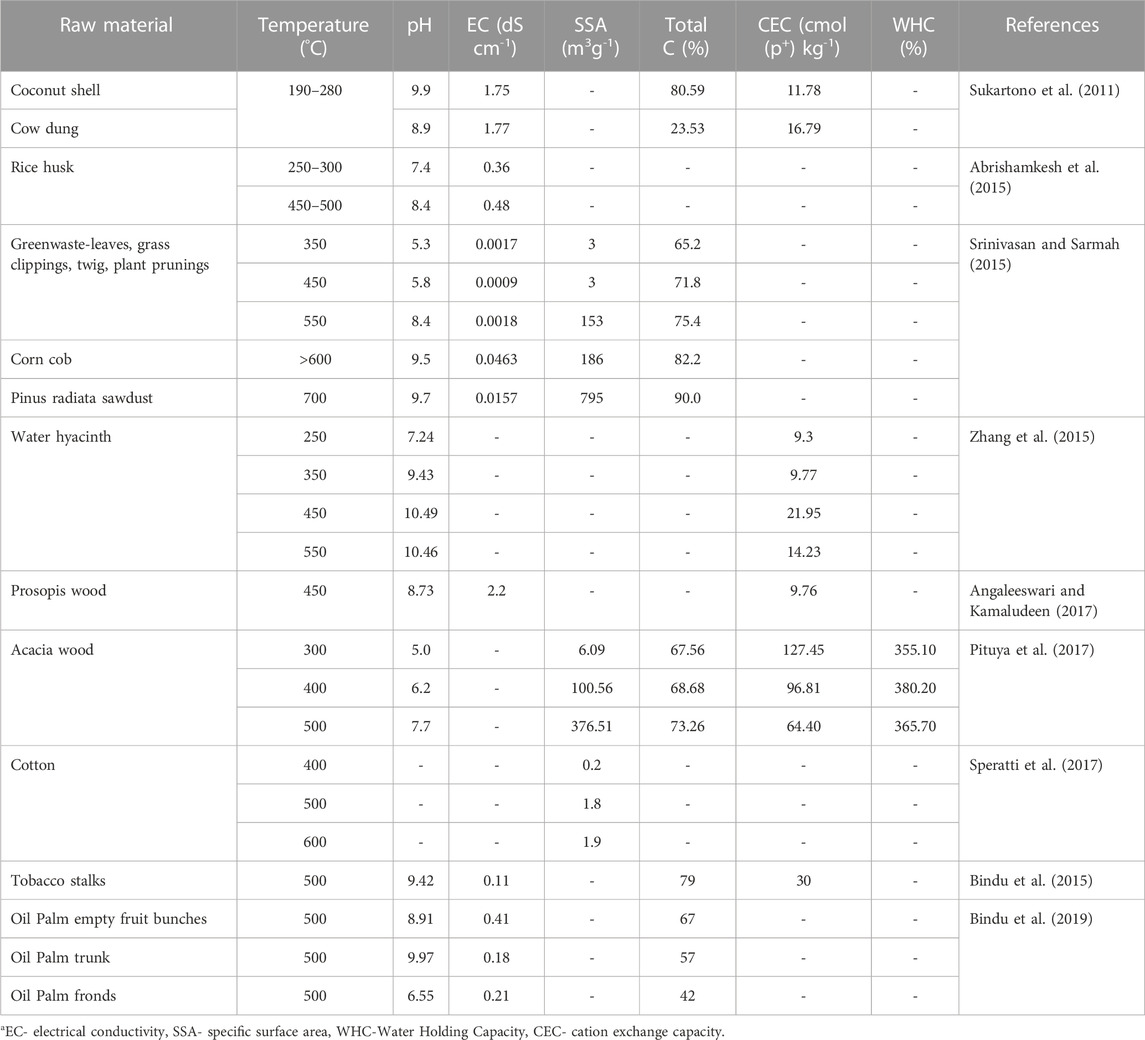
Biochar, the pyrolysed residues have more residence time than unpyrolysed wastes and improves the soil fertility through their impacts on physico-chemical and biological properties of soil (Lehmann et al., 2011; Kuzyakov et al., 2014). Several studies have suggested the positive impact of biochar additions on soil fertility, heavy metal remediation and crop productivity (Pandian et al., 2016; Novak et al., 2018b). However, there are studies stating the negative impact of biochar under certain conditions (Warnock et al., 2010; Revell et al., 2012). Different methods of application like surface application, and soil incorporation are followed. Li et al. (2020) recommended that surface application of biochar mixed with nitrogenous fertilizers as effective strategy to reduce nitrogen (N) losses. The biochar application rate is highly varied and is decided by the feedstock from which the biochar is produced, its composition and soil type to which it is to be applied. The characteristics of some biochar are furnished in Table 1. The biochar of about 5–50 t ha-1 have been utilized in the field experiments and there are no specific recommendations on application rates. It has been reported that biochar dosage of about 1% by weight or even lower than that are used in field crops (Major, 2013). If the biochar is applied to soil in higher doses, it may increase the carbon storage but it could cause reduction in crop productivity in soil by limiting N and widening the C: N ratio of soil (Lehmann et al., 2006). Application of higher doses of biochar to soils with substantial amounts of N and soils cropped with legumes or legume-based cropping system had returned greater profits.
Soil contamination is a global issue which threatens the human health and food security. The major toxic elements generated through anthropogenic activities are Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn, which often accumulates in the soil. About 5.0 million sites worldwide faces the soil contamination by toxic elements (Khan et al., 2021). The Ministry of Environment, Forest and Climate Change (MoEF&CC) has identified 320 locations of high probability of contamination with heavy metals (Cr, Pb, Hg, As, and Cu) and pesticides in India. Most of the reviews on biochar, mainly confined to its environmental benefits (Kumar et al., 2022), rather than its effect on agriculture. In this review, we summarize i) the effect of biochar application on soil properties (physical, chemical, biological), ii) remediation potential of biochar in heavy metal contaminated soils and iii) its impact on crop productivity.
Effect of biochar on soil properties
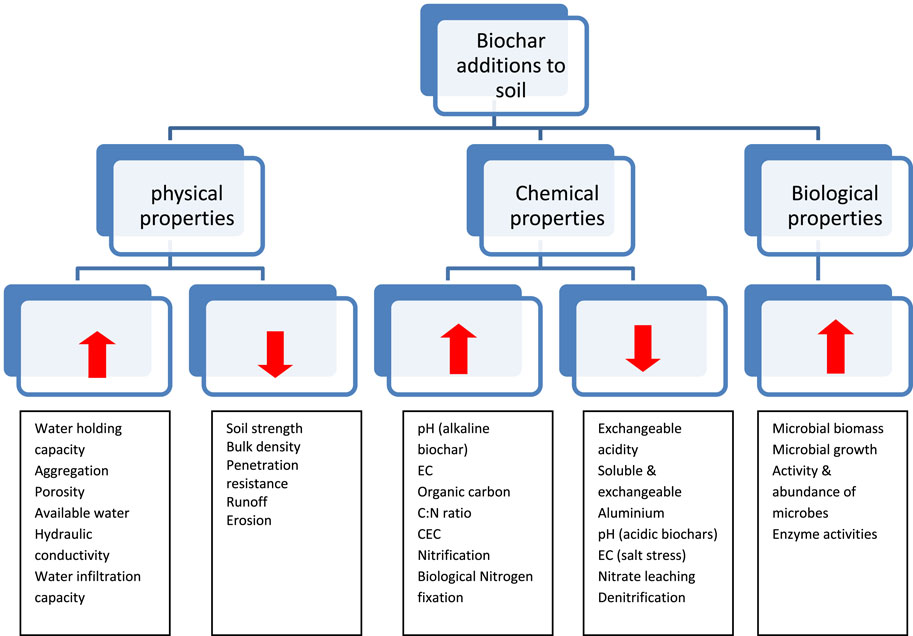
The biochar’s effect on soil properties depends on the characteristics of both, soil to which it is to be applied and biochar’s composition. The effect of biochar on soil properties is depicted in Figure 1 and the quantitative impact of biochar on different soil properties are furnished in Table 2.
Soil physical properties
The extent of changes in soil properties depends on characteristics of both biochar and soil to which it is to be applied (Joseph et al., 2021). Biochar application to soil has its influence on wettability of soil, water infiltration, water retention, aggregation and stability thereby helping in combating erosion, mitigating drought and nutrient loss and enhancing groundwater quality.
Total porosity and bulk density
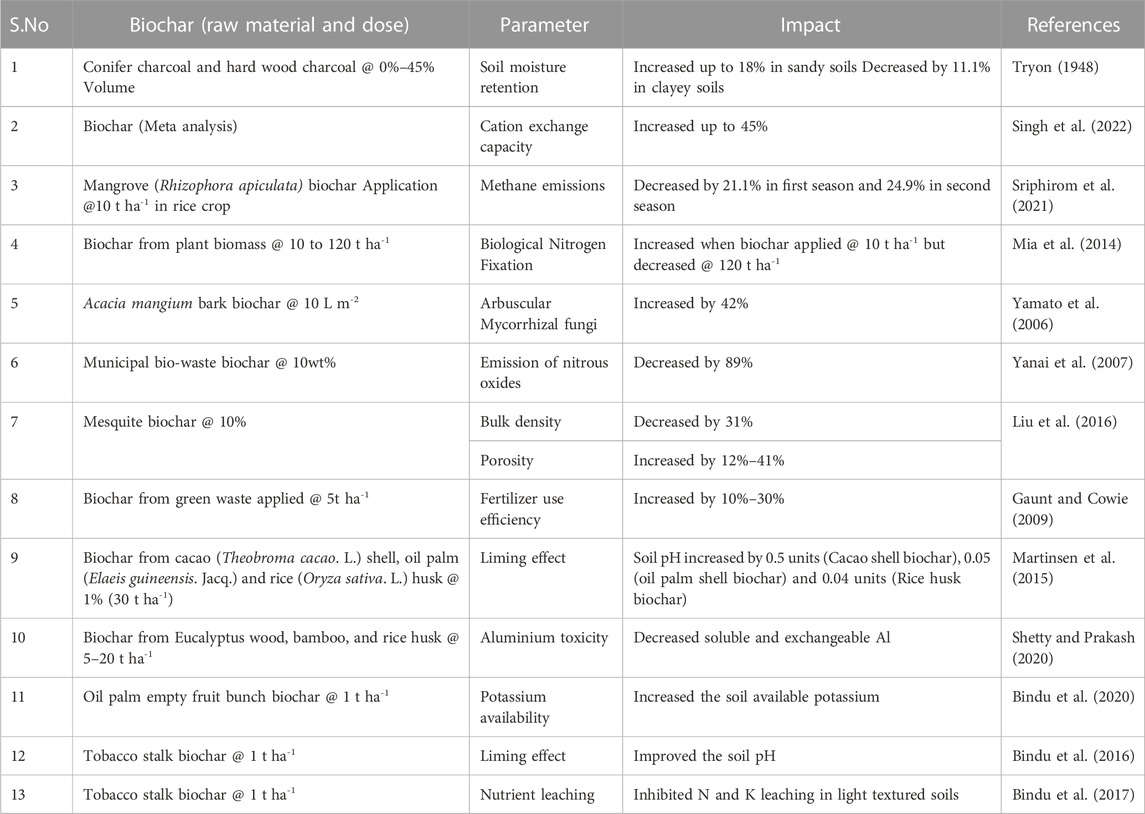
Biochar additions improved the porosity and thereby reduction in density of the soils (McElligott, 2011). The total porosity of the soil increases proportionately with increment in the biochar doses (Liu et al., 2020). The study conducted by Masulili et al. (2010) had showed an increase in total pores, available water and decrease in penetration resistance on application of rice husk biochar. Application of biochar increase the total porosity of the soil, which results in reduction of soil bulk density and increase in the infiltration rate (Herath et al., 2013). This reduction in soil bulk density has a positive effect on other soil physical (water holding capacity, aggregation, texture and structure), chemical and biological properties.
Soil aggregation
Aggregation of soil particles is greatly influenced by biochar incorporation in soils. Application of maize straw and peanut hull biochar at 7.8 t ha-1 in combination with inorganic fertilizers increased the proportion of macro-aggregates and mean weight diameter of the aggregates (Ma et al., 2016). The effect of biochar on stability of aggregates depends on soil texture. Soil aggregation in coarse textured soils (sandy) are more sensitive than fine textured on biochar additions (Ouyang et al., 2013). Liu et al. (2012) studied four soil types of loess plateau, China and reported an improvement in water stable aggregates in silty loam soils whereas no significant effect on sandy loam soils. But contradictorily, Hardie et al. (2014) reported a decrement in aggregate stability on biochar application. Biochar addition on soil aggregation and its stability depends on the biochar interaction with soil organic matter, microorganisms and minerals. (Domingo-Olive et al., 2016). The biochar provides habitat to the microorganisms and protects them from desiccation and predators. The microorganisms secrete polysaccharides that increase the soil aggregation (Aslam et al., 2014).
Available water content and hydraulic conductivity
Biochar when applied as a soil amendment improved the water quality, soil moisture retention and its availability to plants (Steiner et al., 2007). Their effect on available water depends on soil texture. Application of biochar increased plant available water content in sandy soil, whereas it decreased in clayey soils (Glaser et al., 2002). The hydraulic conductivity of the sandy soils reduced with biochar additions (Uzoma et al., 2011), whereas the biochar had no significant effect on soil moisture content in loamy sand (Hardie et al., 2014). Major et al. (2010) reported that biochar addition increased saturated hydraulic conductivity from 2.70 to 13.4 cm h-1.
Water infiltration, run-off and soil erosion
Application of biochar increased the water infiltration, reduced the water runoff and thereby erosion of soil particles (Doan et al., 2015; Sun et al., 2018). This reduced soil loss might be due to increased mean weight of the soil aggregates. In sandy soils, application of biochar declined the diffusion distance of the horizontal wetting front whereas, the diffusion distance of the vertical wetting front declined initially followed by a increase (Pu et al., 2019). The infiltration rate improved by 1.7 times in non-calcareous loamy sand with application of 2% biochar produced at 620°C from mixed wood sieving whereas the calcareous loamy soils had no significant effect on this biochar application (Abrol et al., 2016). Contradiction to this, there are studies stating reduced water infiltration due to hydrophobicity of the biochar (Jeffery et al., 2015). Supporting this, Jien and Wang (2013) reported reduced soil loss by 50% and 60% on biochar application at 2.5% and 5% w/w, respectively in Typic Paleudults. Contradictory results of effect of biochar on soil erosion were also observed (Peng et al., 2016). Erosion of silty loam loess increased with higher rates of biochar application, whereas the erosion reduced with application of biochar particles of larger size (Li et al., 2019). It is clear that, biochar of different particle sizes is required for different soil textures to reduce the water runoff and erosion. The biochar produced under slow pyrolysis from the mangrove tree (Rhizophora apiculata) in combination with alternate wetting and drying practices in the field reduced the irrigation water usage of rice crop by 12.7% (Sriphirom et al., 2020).
Soil structure and texture
Biochar application improves platy soil to granular/crumb structures that are highly suitable for agriculture. Application of biochar had significant effect on soil texture and this effect was found to be short termed (Brodowski et al., 2007). The possible mechanisms defining the improvement in soil physical properties on biochar application are high porosity, its adsorptive nature, provision of microbial habitat and contents of total organic carbon content (Aslam et al., 2014).
Soil chemical properties
Soil pH
The simple measure of soil dynamics is the soil reaction. Soil pH is greatly influenced by biochar application (Xu et al., 2006). The biochar of alkaline nature (pH of leachates >7.0) resulted from higher pyrolysis temperature, lower heating rate and longer residence time whereas, the acidic biochars were produced at lower temperature, higher heating rate and shorter residence time (Uronic Stefanko and Leszczynska, 2020). Biochar had a liming effect on acid soils (Yuan et al., 2013). The presence of oxides, hydroxides and carbonates of alkaline metals in the biochar resulted in soil pH increase (Dai et al., 2017. The negatively charged functional groups like carboxyl, hydroxyl and phenolic groups bind the H+ ions from soil solution, this in turn reduced the activity of H+ ions and increased soil pH (Chintala et al., 2014). Conversely, Zhang et al. (2019) found reduction in pH of alkaline soils, which could be due to the production of acids on oxidation of biochars. The contents of NaHCO3 and Na2CO3 present in alkaline soils got converted to Ca (HCO3)2 and CaCO3 and had caused the reduction in soil pH (Liu et al., 2020. Several authors (Senesi and Plaza, 2007; Dias et al., 2010) stated that acidic compounds produced from decomposition of soil organic matter lowered the pH of alkaline soils. For calcareous soils also, biochar additions increased the soil pH (Alazzaz et al., 2020). However, application of acidic biochars produced through slow pyrolysis and steam activation reduced the soil pH between 0.2 and 0.4 units in calcareous soils (Ippolito et al., 2016). Even at higher rates of biochar application, the buffering capacity of the soils prevented the major changes in soil reaction (Ippolito et al., 2014). Application of poultry litter biochar of alkaline nature raised the soil pH above 8.0, where the availability of nutrients for the plants got declined (Novak et al., 2014). Under salt stress conditions, application of water hyacinth derived biochar increased the soil pH and the increase was directly proportional to the biochar dose (Premalatha et al., 2023).
Electrical conductivity
The electrical conductivity (EC) of the biochar ranges from 0.04 to 54.2 dSm-1 (Rajkovich et al., 2012; Smider and Singh, 2014). The EC of the soil increased by14 times at 120 tha-1 biochar application rates (Mia et al., 2014). The soluble compounds (organic and mineral) released on reaction of biochar with water, resulted in increase of soil EC (Joseph et al., 2021). Pandian et al. (2016) observed an increase in EC of the acidic red soil on biochar addition. The physical entrapment of salts in the pores of biochar resulted in the reduction of EC in salt affected soils (Thomas et al., 2013). The biochar was found to mitigate the salinity stress. Under salt stress conditions, EC decreased with increased rate of biochar application (Premalatha et al., 2023).
Soil organic carbon
Biochar additions improved the organic carbon of the soil due to its high carbon content of recalcitrant nature (Zhang et al., 2012). Supporting this Shenbagavalli and Mahimairaja, (2012) observed a 33%–35% higher SOC content on incorporation of different levels of biochar. The increase in SOC on biochar addition is the additive effect of carbon from biochar, microorganisms, rhizosphere decomposition and root exudates (Pandian et al., 2016). Application of biochar from corn stover with high ash content and low volatile matter decreased the C mineralization in mollisols (Purakayastha et al., 2016).
Cation exchange capacity
Higher surface area, hydroxyl and carboxy functional groups, and variable charges of biochar improve the cation exchange capacity (CEC) of the soils (Van Zwieten et al., 2010). Van Zwieten et al. (2009) evidenced a positive linear correlation between biochar application rates and CEC. Fresh biochars had little capacity to retain cations in soil but with the passage of time, biochar on undergoing surface oxidation had increased carboxyl groups thereby had increased negative charges and ultimately had resulted in sorption of cations. Conversely, application of 4 months aged wood biochar reduced the CEC by 10% in 70 days incubation compared to fresh biochar (Zhao et al., 2015). In a laboratory incubation study, addition of 3% and 6% w/w hardwood biochar had no change in CEC of the sandy soils (Basso et al., 2013). This confirmed that the effects of biochar application on soil properties are dynamic.
Soil available nutrients
The processes like adsorption and immobilization are promoted in soil after biochar application, which in turn causes decline in leaching of nutrients. Biochar has the capacity to adsorb and retain cations in exchangeable forms than other forms as they have greater surface area and surface negative charge (Clough et al., 2013. Through this mechanism, nutrients and chemicals are retained in soil improving the fertilizer use efficiency with sustainable crop yields. As the biochar prevents the washing out of nutrients, enrichment of an ecosystem with chemical nutrients and impairment of water ecosystem can be controlled. By preventing the losses, more nutrients can be retained in the soil and external dependence of synthetics can be reduced. Nutrient concentration of biochar varied widely and depends on the feedstock, pyrolysis temperature, residence time and heating rate (Adhikari et al., 2019). The functional groups such as hydroxyl, carboxylic, ketone, chromene and lactone groups present in the biochar strongly adsorb the nutrient ions and reduce the losses (Schmidt et al., 2015). Supporting this, several studies (Qian et al., 2014; Yao et al., 2015; Mandal et al., 2016; Wen et al., 2017; Chen et al., 2018; Cao et al., 2019) had reported higher nutrient use efficiency on utilization of biochar-based fertilizers. Lehmann (2007) reported that biochar is not a fertilizer but it reduces the fertilizer use and improves the soil quality. Application of biochar at 15% w/w was optimal for the growth of Indian mustard (Park et al., 2011) whereas 5% w/w biochar promoted the maize growth (Zheng et al., 2013). In a study conducted by Suppadit et al. (2012), application of 15% w/w biochar affected the nut growth. The suppressive effect on excessive use of biochar was due to highly porous nature of the biochar which accelerated the loss of water and nutrients from the soil (Xu et al., 2016). Therefore, study on the appropriate dose of biochar for the particular soil and crop is required. The effect of biochar on nutrient availability and crop growth was found to be variable (Fang et al., 2016 which depends on chemical composition of biochar, application dose, soil pH, nutrient status of soils and microbial interaction with biochar (Singh et al., 2019. Biochar contributes significant quantities of macro and micro nutrients. They are considered as slow-release fertilizers (Schneider and Haderlein, 2016).
Nitrogen
Biochar addition reduced the concentration of NH4+ in soil due to higher assimilation of NH4+ or oxidation of NH4+ to NO3− (Harter et al., 2014). Sorption of NH4+ to the oxygenated carbonyl and carboxyl functional groups reduced the NH4+ availability for nitrification. This was supported by the higher concentration of nitrates in soil on incremental doses of biochar (Abbruzzini et al., 2019). Contradictorily, Thomazini et al. (2015), through their incubation study reported increased concentration of NH4+ in forest soils. Biochar application declined the NO3 leaching (Laird et al., 2010), which is attributed by the hydroxyl and alkyl functional groups of biochar Supporting this, Mukherjee et al. (2014) reported a 33% reduction in leaching of nitrates on biochar application @ 0.01 kg per kg of silty loam soil. This was also supported by Sika and Hardie (2014) in sandy soils. Many studies (Jeffery et al., 2011; Jones et al., 2012; Abbruzzini et al., 2019) reported that biochar incorporation increased the soil N availability, N uptake and nitrogen use efficiency by the crops. Higher doses of biochar stimulate the nitrification process (Edwards et al., 2018). Incorporation of biochar to the tropical wheat growing soils reduced the N2O emissions by 71% (Abbruzzini et al., 2019). Meta-analysis by Gao Y. et al. (2020) reported 12% and 11% reduction in nitrate and ammonium contents of top soil, respectively.
Biochar sorbed the ammonia more efficiently and reduced the volatilization of ammonia (Iyobe et al., 2004). Conversely, several studies (Liu Z. et al., 2017; Nguyen et al., 2017) reported that application of biochar stimulated ammonia volatilization and N immobilization and ultimately reduced mineral N concentration of soil. Nguyen et al. (2017) observed reduced nitrification and abundance of genes encoding ammonia monooxygenase (amoA gene) on co-application of urea with high CEC biochar whereas the reduction was less in low CEC biochar. N immobilization on biochar application is short -lived (Abbruzzini et al., 2019). Supporting this, Martin et al. (2015) opinioned that biochar addition stimulated the activity of N immobilizing heterotrophs limiting the nitrification. Biochar based controlled release nitrogen fertilizers reduced denitrification and abundance of nirS and nirK genes, whereas it increased soil nitrification and amoA gene abundance (Liao et al., 2020). Conversely, stimulation of denitrification process on biochar addition was reported which decreased soil N (Singh et al., 2010; Harter et al., 2014). Supporting this, Cayuela et al. (2013) stated that biochar facilitates the electron transfer to denitrifiers in soil which on combination with liming effect of biochar accelerate the denitrification process. The nosZ gene encoding nitrous oxide reductase, a key enzyme for dentrification increased on biochar application (Harter et al., 2014).
Application of biochar 5–20 t ha-1 increased the nitrogen use efficiency (NUE) by wheat cultivated in saline soils of China, whereas overuse (>20tha-1) had negative effect on NUE (Sun et al., 2019). Application of maize stalk biochar for Zucchini plants in calcareous sandy soils, improved partial factor productivity and agronomic efficiency for N whereas the effects were found to be insignificant for P (Amin and Eissa, 2017). Tian et al. (2021) reported that application of rice straw biochar at 10 t ha-1 replaced 20% nitrogen fertilizer and NUE and yield of rapeseed in calcareous soils of China. Wang et al. (2015) found that addition of biochar in acidic soils reduced the population of ammonia oxidizing bacteria. Utilization of biochar based controlled release fertilizers improved the population of Cyanobacteria and Saccharibacteria involved in N fixation and organic compounds degradation, respectively and ultimately had improved soil fertility (Liao et al., 2020.
Higher soil moisture retention and root growth on biochar addition favored maximum nitrogen fixation by groundnut crop (Pandian et al., 2016). Higher biological nitrogen fixation (BNF) in common beans were observed at biochar dose of 78 t ha-1 (Rondon et al., 2007) whereas biochar at100 tha-1 recorded higher BNF in soybean (Tagoe et al., 2008). Variation between the above results was due to biochar properties, response of the crop species to biochar or soil nutrient status (Mia et al., 2014). BNF and nodulation in clover was higher at biochar rates of 10 t ha-1 and recorded a significant reduction beyond that dose (Mia et al., 2014). The synergistic effect on BNF was due to greater bioavailability of essential nutrients like boron (B) and Molybdenum (Mo) (Rondon et al., 2007) on experimenting in an acidic tropical soil with common beans. Increased soil salinity on biochar incorporation reduced the BNF (Revell et al., 2012).
Phosphorus
The pyrolysis temperature has its effect on the availability of P in the biochar. A high concentration of labile calcium phosphates is found in low temperature biochars whereas stable P forms are dominant in temperature above 600°C (Bruun et al., 2017). The concentration of total and water-soluble nutrients (P &K) was higher in animal-based biochar (poultry manure) compared to lignocellulosic (harwood and softwood) biochars (Novak et al., 2013; Novak et al., 2018b). Novak and Busscher (2012) stated that the nutrients which were unassimilated by the animals would be released in excreta and it attributed to higher nutrient contents.
In addition to direct release of P from biochar through ligand exchange, desorption or dissolution (Chathurika et al., 2016), biochar also improves the P availability through P adsorption against leaching (Madiba et al., 2016), mineralization of organic P through enhanced microbial growth (Dume et al., 2017. For instance, biochar addition to acidic soils resembles the liming effect and improves the P availability through rise in soil pH (Biederman and Harpole, 2013 Biochar derived from biosolid sludge was a P rich material (Li et al., 2019). Biochar produced at pyrolysis temperature between 300°C and 500°C had high P contents whereas the biochar produced at >500°C had significantly less labile P due to formation of insoluble and stable forms of P (Adhikari et al., 2019; Li et al., 2019). Similarly, biochar from blady grass (Imperata cylindrical) had extractable p > 700 μgP g-1 biochar (Zhang H. et al., 2016) whereas it was 152 g kg-1 in animal bone chips biochar (Siebers and Leinweber, 2013). Uzoma et al. (2011) reported that woody biochar produced at 500°C had extractable P of about 23 g kg-1 whereas it was only 1.2 gkg-1 in same biochar produced at 300°C. Organic, inorganic, available P and total P increased on application of biochar derived from biosolid sludge in acid soils (Figueiredo et al., 2020). Biochar improved the arbuscular mycorrhizae colonization by 6% and enhanced the uptake of sparingly soluble P (FePO4) and increased root access to soluble P (NaH2PO4) by beans (Vanek and Lehmann, 2015).
Meta-analysis of Glaser and Lehr, (2019) stated that addition of biochars improved the soil P availability in acidic and neutral soils by a factor 5.1 whereas non-significant effect was found in alkaline soils. Similar findings were reported by Xu et al. (2018). Contradictory to this, increased soil P availability on biochar addition in calcareous sandy soils was reported (Amin, 2018). DeLuca et al. (2015) reported higher P availability on biochar addition in alkaline soils. Application of rice straw biochar at 10 g kg-1 improved the soil pH and P availability whereas it reduced exchangeable Al3+ in acidic red soils. The liming effect of biochar helped in alleviating P deficiency and Al toxicity (Zhu et al., 2014). In acid soils, P binds to Al or Fe oxides/hydroxides which on biochar addition got solubilized and was made available to the crops (Borno et al., 2018. In contrast to higher availability of P, addition of biochar with low P concentration in acid soils reduced the P availability (Schneider and Haderlein, 2016) through sorption (Trazzi et al., 2016; Ngatia et al., 2017), immobilization (Mitchell et al., 2016; Xu et al., 2018) and precipitation (Gao Y. et al., 2020). The organic acids released from biochar compete with phosphate ions and increase the soil P availability (Schneider and Haderlein, 2016). For maize, sewage sludge biochar at 30tha-1 enhanced root growth which inturn increased rhizodeposition and microbial activity and ultimately P availability in acidic Ultisols (Gonzaga et al., 2022). Borno et al. (2018) reported higher P fixation in alkaline soils amended with biochar.
Effect of biochar additions on P availability of the soil depends on soil texture. Zhang H. et al. (2016) reported a 25% higher P availability in heavy textured soils than coarse textured sandy soils on biochar addition. This effect was contradictory to Liu et al. (2013), who reported greater P availability in sandy soils. A meta-analysis showed that application of biochar upto 10 t ha-1 enhanced the soil P availability and P uptake especially in heavy textured, acidic P deficient soils (Tesfaye et al., 2021). Incorporation of biochar was found to be an effective strategy in enhancing the availability of legacy P in acid soils and not applicable for other alkaline soils with low legacy P (Alotaibi et al., 2021). Phosphorus laden maize stalk biochar releases the P slowly, acting as a slow-release fertilizer and hence improves phosphorus use efficiency (Li et al., 2020). Angst and Sohi, (2013) reported the potential substitution of certain proportion of inorganic P fertilizers by biochar.
Potassium
Unlike other nutrients, potassium present in the raw material would be converted to their salts of higher solubility (Karim et al., 2017). Biochar amendment increased the soluble and exchangeable fractions of K (Oram et al., 2014). The poultry manure contains the salts like KCl, KNO3, and Ca3(PO4)2, of which potassic salts were highly soluble compared to calcium (Sigua et al., 2016. This contributed to higher concentration of potassium from leachates of biochar amended soils; Wang et al. (2018) reported on the prolonged effects of biochar application on K uptake prolonged in soils dominated with 2:1 K bearing minerals. Biochar application enhanced soil K availability and growth of K- dissolving bacteria in alfisols and entisols. Biochar improved the soil K availability and supplemented the inorganic K fertilizers (Singh et al., 2019). Addition of K from the ash fraction of biochar increases the exchangeable K levels in soil and reduces the leaching losses (Qayyum et al., 2020. Biochar itself contain significant quantities of cations like K, Ca, Mg and application of that improved their levels in the soil (Jien and Wang, 2013). Alazzaz et al. (2020) reported that biochar derived from the olive mill solid waste should be restricted in calcareous loamy sand soils as it reduced the concentration of P, Ca, Mg and Mn in maize shoots. In calcareous loamy sand soils, biochar application increased the available K, Na, Ca, Mg, AB- DTPA extractable Fe and Mn whereas it reduced AB- DTPA extractable Zn. Though application of biochar improved the soil nutrient status, care should be taken before amending the soils with biochar considering the negative effects like EC and exchangeable Na. It increases the risk of salinity particularly in alkaline soils.
Aluminium
Biochar plays a vital role in detoxification of Al toxicity. There was a significant reduction in the soluble and exchangeable forms of aluminium (Al) on biochar addition (wood biochar at 20 t ha-1) and plays a vital role in Al detoxification (Shetty et al., 2021). Contradictorily, a negligible effect of biochar on reduction of Al toxicity in Al enriched acidic mine spoils was reported by Novak et al. (2018a). The efficiency of biochar on Al amelioration depends on calcium carbonate equivalent of the biochar.
Soil biological properties
Microbial population
Addition of biochar to the soil has the capability to modify the physical and chemical properties thereby providing a habitat conducive for microorganisms (Xu H. J. et al., 2014). Microbial attraction to biochar depends on surface hydrophobicity. Optimal amount of biochar in combination with fertilizers helps in improving the microbial population thereby increasing the nutrient release (chemical properties). Supporting the above statement, Warnock et al. (2007) evidenced an increase in microbial activity on biochar application due to the alteration of soil physico-chemical properties, detoxification of allelochemicals on biochar and refugia for smaller organisms. Improved microbial activity on biochar application is due to their higher water holding potentials (Steiner et al., 2008). The pores present in the biochar serves as habitat for soil microorganisms and protects them from other predatory micro athropods present in the soil (Jaafar et al., 2014; Quilliam et al. (2013) reported that the macropores of size greater than 200 nm serves as an idle habitat for soil bacteria. The micro and meso pores of biochar stores water and dissolved substances required for the microbial metabolism (Brewer and Brown, 2012). Higher surface area of biochar improved the microbial colonization. The black colour of the biochar absorbs more heat, which improved the rate of microbial growth and enzymatic activity (Gul et al., 2015). Biochar’s alkaline nature favored the growth of gram-positive soil bacteria than the gram-negative bacteria (Farrell et al., 2013). Zimmermann et al. (2012) reported that with increase in the age of biochar, the pH declined and had supported the growth of fungi. Addition of pine wood biochar-bacterial mixture (Enterobacter cloacae UW5 strains) in the sandy loam soils increased the population density of bacteria by 16% after 4 weeks incubation period (Hale et al., 2015). Quilliam et al. (2013) reported poor microbial colonization on biochar application to the soils, which might due to the lower nutrient content in biochar compared to the bulk soil and higher sorption of substances with lower molecular weight.
The activity and abundance of microbes got enhanced by biochar additions, which ultimately had affected the nutrient cycling (Grossman et al., 2010). A meta-analysis review by Schmidt et al. (2021) stated that microbial biomass in acidic and neutral soils was improved by biochar incorporation but not in alkaline soils. Bollmann et al. (2005) reported that biochar application to acid soils increases soil pH suitable for nitrifying organisms like Nitrosospira amoA, Nitrosospira briensis thereby increased the nitrification rate and nutrients immobilization. Addition of 0.6 g of corn stover biochar to 100 mL nutrient broth had showed fivefold increase in growth of Bacillus mucilaginosus (K- dissolving bacteria) and 80% higher K- dissolving activity (Liu S. et al, 2017). Supporting this, Wang et al. (2018) reported enhanced growth of K- dissolving bacteria on biochar incorporation. The allelochemicals were sorbed by biochar and increased the AM (mycorrhizal fungi) colonization (Elmer and Pignatello, 2011). Conversely, biochar on sorption of toxic volatile organic compounds affected the microbial growth (Gurtler et al., 2014). Application of biochar had increased AM population by 40% (Ishii and Kadoya, 1994) and it would be helpful in sustained crop production, restoration of ecosystem, carbon sequestration and mitigation of climate change. Contradictorily, Warnock et al. (2010) reported that application of pine biochar @ 2.0% and 4.0%w/w declined the arbuscular mycorrhizal fungal abundance in both root and soil.
Microbial biomass carbon
Application of biochar influences the microbial biomass carbon significantly. Rutigliano et al. (2011) observed no changes in microbial biomass in biochar amended soils. Similarly, Mitchell et al. (2015) reported no changes in MBC (microbial biomass carbon) on short term (20–30 days) whereas the biochar additions improved the MBC after 1 year. Application of high temperature wood biochar (≥600°C) derived from eucalyptus reduced the MBC by 28% in sandy soils (Dempster et al., 2012). Conversely, Ameloot et al. (2013) reported a 29% increase in MBC in sandy loam soils on application of biochar produced from willow wood at 700°C. Similarly, Luo et al. (2013) reported greater MBC in clay loam soils amended with biochar produced at 700°C. There is a need of applying organic amendments in conjugation with biochar to offset the short-term reduction effect on MBC.
Enzymes
Enzymes are the substances secreted by plant roots or soil microorganisms which influence the nutrient bioavailability (Gianfreda, 2015). As biochar addition affect the microbial response (activity, abundance and community structure), enzymatic activity is also influenced by their addition. The interaction of biochar with the substrate and enzyme strongly influences the soil enzymatic activity (Lammirato et al., 2011). The enzymatic activity can either be promoted or limited, when the substrate and enzymes had sorbed to the functional groups present in the biochar (Czimczik and Masiello, 2007). The positive effect of biochar on soil enzymes were observed in some studies (Park et al., 2011; Kumar et al., 2013), whereas Lehmann et al. (2011) reported conversely. This increase in enzymatic activities on biochar addition was reported to be induced by higher soil organic carbon, microbial biomass carbon and nitrogen pools which serve as the organic substrates for such enzymes (Wang et al., 2015). The application of rice husk biochar at 12tha-1 significantly improved the activities of soil urease, catalase, alkaline phosphatase and invertase (Oladele, 2019). Soil invertase activity was positively correlated with soil organic carbon, available nitrogen and phosphorus (Zhang et al., 2005). Shahzad et al. (2014) reported an increased activity of phosphatase on biochar addition, which signify greater proportion of bio-available phosphorus. Sakin et al. (2021) reported that addition of almond shell biochar had significant positive effect on dehydrogenase and urease activities. Jing et al. (2020) reported no significant correlation between enzyme activity and soil organic carbon. The invertase activity was correlated with NH4+-N and NO3−-N, whereas the urease activity was positively correlated with available nitrogen and phosphorus. This improved enzymatic activity on biochar additions was due to the stimulated root growth of the plants to exude these enzymes (Kotroczo et al., 2014). Activity of dehydrogenase in soils that were incubated with high temperature biochars (700°C) decreased by 47% whereas, addition of low temperature biochar (350°C) improved the dehydrogenase activity by 73% (Ameloot et al., 2013). Irrespective of soil types, biochar addition increased the activity of alkaline phosphomonoesterase whereas acidic phosphomonoesterase activity inhibited (Neble et al., 2007; Jin et al., 2016). The study by Masto et al. (2013) showed the increased activity of phosphomonoesterase (both acidic and alkaline) on biochar addition in red soils. Application of biochar mixed with compost increased the enzymatic activities (dehydrogenase and phosphatase) and nutrient availability in saline soils (Liu et al., 2021). Biochar addition to the vermicomposting of sewage sludge reduces the toxicity and bioavailability of heavy metals, which in turn improved the growth, and reproduction of earth worms (Khan et al., 2020). However, some studies showed reduction in earthworm biomass on biochar addition (Malinska et al., 2016).
Remediation of heavy metal contaminated soils
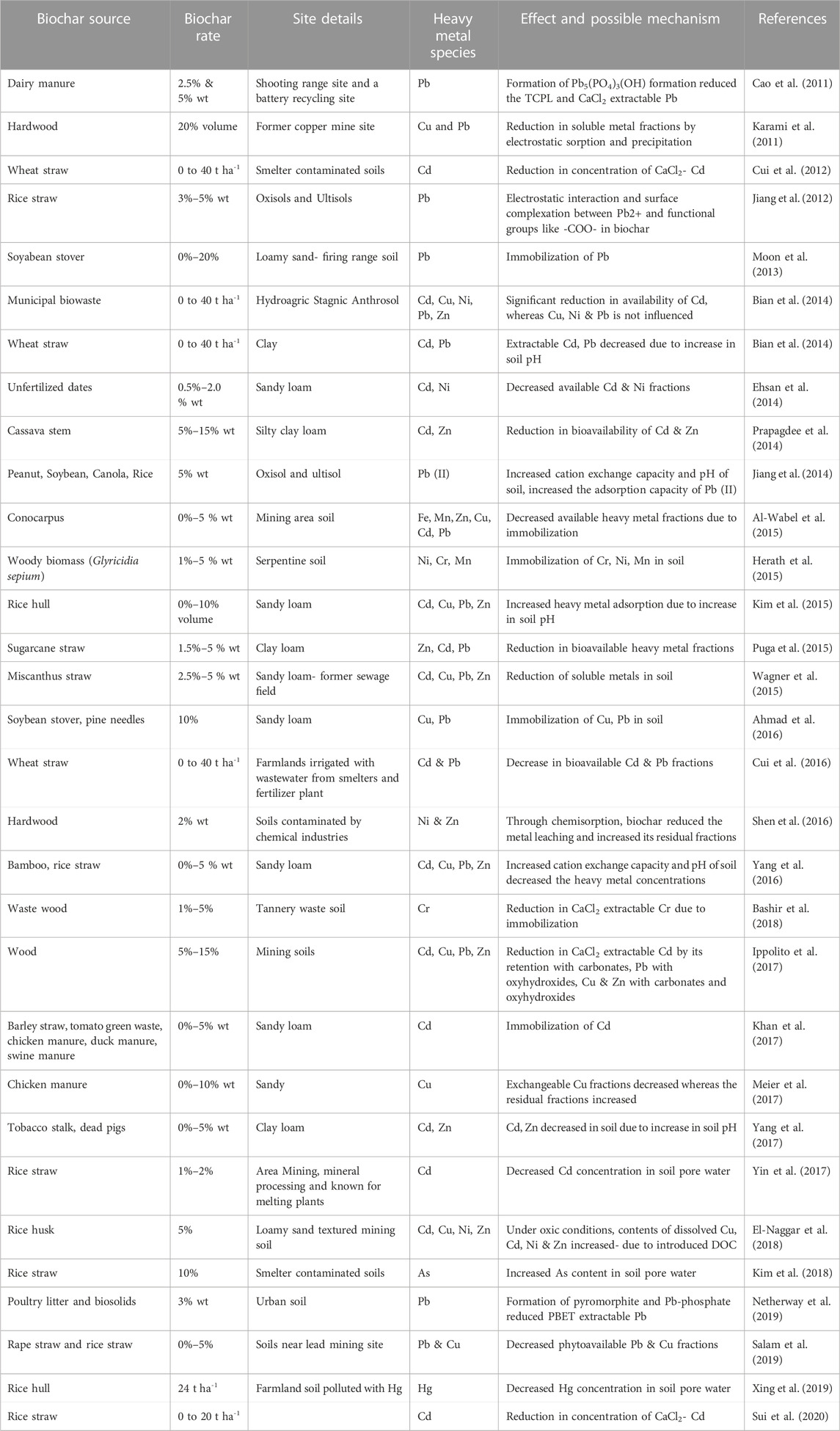
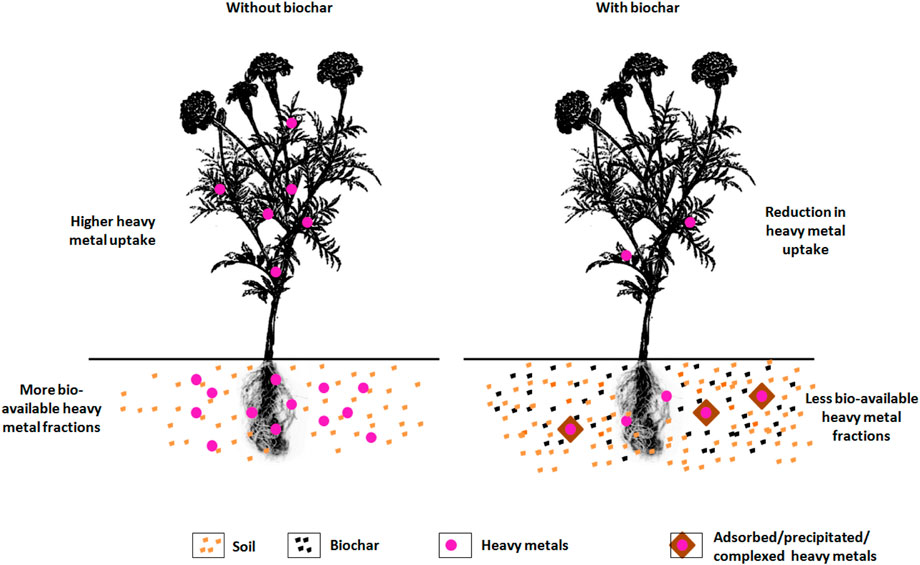
Environmental sustainability is under threat by the worldwide problem of soil contamination. Various in situ and ex situ remediation techniques are developed in order to restore the ecosystem services in the contaminated soils. Biochar as an amendment in remediation of heavy metal contaminated soils has been investigated. It is not an eradication technique but it transforms the soluble toxic heavy metals to less soluble and unavailable form. Varied capacities and efficiencies of the biochar on remediation of heavy metal contaminated soils have been documented and a detailed information on the effect of biochar on heavy metals is furnished in Table 3. The speciation of the heavy metals and its transformation is greatly influenced by biochar (Wu et al., 2017). Biochar reduced the acid extractable heavy metal fractions and increased the organic bound and residual fractions (Dai et al., 2018) whereas, the bioavailable fractions especially As increased on biochar additions in some studies (Qiao et al., 2018). The feedstock for biochar, its dosage, application method, soil properties and heavy metal species are the factors influencing the biochar’s effectiveness on soil remediation (Wang et al., 2021). A considerable reduction in bioavailability of heavy metals on biochar additions is due to the mechanisms of immobilization (Venegas et al., 2016), cation exchange and electrostatic interaction (Uchimiya et al., 2011), surface complexation (Karami et al., 2011), and chemical precipitation (metal hydroxide formation, precipitates of carbonate, or phosphate). The porous structure and surface functional groups of the biochar enables the immobilization of the heavy metals (Kavitha et al., 2018) and thereby reduce its availability. The proportion of reduction in available heavy metal fractions depends on water management. Under intermittent irrigation, biochar potentially alleviates the adverse heavy metal pollution (Chen et al., 2021). Biochar reduces the metal ions (Cr) leaching by its action on redox reactions of metals (Choppala et al., 2012). Contradictorily, mobilization of arsenic due to enhanced pH on biochar application reported by Hartley et al. (2009). The effect of biochar on heavy metal availability and its uptake by plants is depicted in Figure 2.
Crop yield
As the soil properties (physical, chemical and biological) get improves on biochar application, this in turn enhances crop yield. Quantitative effect of biochar on crop yield is given in Table 4. Biochar application improved the seed germination, root density and crop yield. Application of biochar at higher doses in combination with chemical fertilizers (NPK) improved the crop yield. In acid soils (pH < 5.2), application of biochar enhanced the yield of carrot and bean (Rondon et al., 2004). According to Lehmann et al. (2006), application of biochar upto 140 t ha-1on highly weathered soils of humid tropical regions increased the crop yield, but this was not suitable for all situations and crops. Accordingly, Rondon et al. (2004) found that biomass yield in beans increased with application of biochar upto 60 t ha-1 but when dose increased to 90 t ha-1, it reached the same value as that of control plots. Masulili et al. (2010) conducted an experiment with rice husk biochar to study its reclamating capacity in acid soils. Among the different treatments, the rice plants in the biochar amended soils recorded greater yield attributes and yield.
In a study conducted at Colombia in isohyperthermic kaolinitic Typic Haplustox, application of biochar (at 0, 8 and 20 t ha−1) for 4 years showed no significant effect on maize yield in first year but in subsequent years, there was an improvement in yield (Major et al., 2010). Supporting this, the meta-analyses by Jeffery et al. (2017) stated that biochar application at the rate of 30 tha-1, decreased the crop yields by 3% in temperate areas. Higher rates of biochar application reduced the crop growth and yield (Kammann et al., 2011). Application of biochar at higher rates (50% w/w) reduces the plant growth and flowering in Viola cornuta (Regmi et al., 2022).
Conclusion and future prospects
The following conclusions are drawn from this review.
• Benefits of biochar application on soil fertility
Application of biochar increase the total porosity of the soils, which reduces the soil bulk density, improves the soil aggregation and moisture content. The biochar itself is having substantial quantities of plant nutrients, the effect on soil fertility varies with properties of biochar and the characteristics of the soil. Since most of the biochar produced are alkaline in nature, application to acidic soils improves the nutrient availability, but enhanced salt concentration on biochar addition increases the risk of salinity in alkaline soils. So, care has to be taken before amending the soil with biochar. Biochar provides habitat conducive for the soil micro-organisms, thereby improve the soil biological properties.
• Effectiveness of biochar
The effect of biochar on soil properties, heavy metal remediation or crop yield depends on biochar type, its dose, soil type, agroclimatic condition, crop species, etc. The effects hence are always soil and site-specific.
• Need for long term field trials to study the effectiveness of biochar
The effect of biochar on soil properties are studied mostly under laboratory conditions on short term basis, the long term or aging effect of biochar in field conditions is needed in detail to develop valid recommendation under varied soil and climatic conditions. The economic feasibility of biochar additions needs to be addressed in future.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbruzzini, T. F., Davies, C. A., Toledo, F. H., and Cerri, C. E. P. C. (2019). Dynamic biochar effects on nitrogen use efficiency, crop yield and soil nitrous oxide emissions during a tropical wheat-growing season. J. Environ. Manage. 252, 109638. doi:10.1016/j.jenvman.2019.109638
Abrishamkesh, S., Gorji, M., Asadi, H., Bagheri-Marandi, G. H., and Pourbabaee, A. A. (2015). Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ. 61 (11), 475–482. doi:10.17221/117/2015-PSE
Abrol, V., Ben- Hur, M., Verheijen, F. G. A., Keizer, J. J., Martins, M. A. S., Tenaw, H., et al. (2016). Biochar effects on soil water infiltration and erosion under seal formation conditions: Rainfall simulation experiment. J. Soils Sediments 16, 2709–2719. doi:10.1007/s11368-016-1448-8
Adekiya, A. O., Agbede, T. M., Olayanju, A., Ejue, W. S., Adekanye, T. A., Adenusi, T. T., et al. (2020). Effect of biochar on soil properties, soil loss, and cocoyam yield on a tropical sandy loam alfisol. Sci. World J. 2020, 1–9. doi:10.1155/2020/9391630
Adhikari, S., Gasco, G., Mendez, A., Surapaneni, A., Jegatheesan, V., Shah, K., et al. (2019). Influence of pyrolysis parameters on phosphorus fractions of biosolids derived biochar. Sci. Total Environ. 695, 133846. doi:10.1016/j.scitotenv.2019.133846
Ahmad, M., Ok, Y. S., Rajapaksha, A. U., Lim, J., Kim, B. Y., Ahn, J. H., et al. (2016). Lead and copper immobilization in a shooting range soil using soybean stover-and pine needle-derived biochars: Chemical, microbial and spectroscopic assessments. J. Hazard. Mater. 301, 179–186. doi:10.1016/j.jhazmat.2015.08.029
Akhtar, S., Andersen, M. N., and Liu, F. (2015a). Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 201, 368–378. doi:10.1111/jac.12132
Akhtar, S. S., Andersen, M. N., and Liu, F. (2015b). Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 158, 61–68. doi:10.1016/j.agwat.2015.04.010
Al-Wabel, M. I., Usman, A. R. A., El-Naggar, A. H., Aly, A. A., Ibrahim, H. M., Elmaghraby, S., et al. (2015). Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 22, 503–511. doi:10.1016/j.sjbs.2014.12.003
Alazzaz, A., Usman, A. R. A., Ahmad, M., Ibrahim, H. M., Elfaki, J., Sallam, A. S., et al. (2020). Potential short-term negative versus positive effects of olive mill-derived biochar on nutrient availability in a calcareous loamy sand soil. PLOS one 15, e0232811. doi:10.1371/journal.pone.0232811
Alotaibi, K. D., Arcand, M., and Ziadi, N. (2021). Effect of biochar addition on legacy phosphorus availability in long-term cultivated arid soil. Chem. Biol. Technol. Agric. 8, 47–11. doi:10.1186/s40538-021-00249-0
Ameloot, N., Neve, S. D., Jegajeevagan, K., Yildiz, G., Buchan, D., Funkuin, Y. N., et al. (2013). Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. biochem. 57, 401–410. doi:10.1016/j.soilbio.2012.10.025
Amer, M. M. (2016). Effect of biochar, compost tea and magnetic iron ore application on some soil properties and productivity of some field crops under saline soils conditions at North Nile Delta. Egypt. J. Soil Sci. 56, 1–17. doi:10.21608/EJSS.2017.1097
Amin, A. E. A. Z., and Eissa, M. A. (2017). Biochar effects on nitrogen and phosphorus use efficiencies of zucchini plants grown in a calcareous sandy soil. J. Soil Sci. Plant Nutr. 17, 912–921. doi:10.4067/S0718-95162017000400006
Amin, A. E. A. Z. (2018). Phosphorus dynamics and corn growth under applications of corn stalks biochar in a clay soil. Arab. J. Geosci. 11, 379. doi:10.1007/s12517-018-3719-8
Amonette, J. E., and Joseph, S. (2009). “Characteristics of biochar: Microchemical properties,” in Biochar for environmental management- science and technology. Editors J. Lehmann, and S. Joseph (London: Earthscan), 65–84.
Angalaeeswari, K., and Kamaludeen, S. P. B. (2017). Production and characterization of coconut shell and mesquite wood biochar. Int. J. Chem. Stud. 5, 442–446.
Angst, T. E., and Sohi, S. P. (2013). Establishing release dynamics for plant nutrients from biochar. GCB Bioenergy 5, 221–226. doi:10.1111/gcbb.12023
Asai, H., Samson, B. K., Stephan, H. M., Songyikhangsuthor, K., Homma, K., Kiyono, Y., et al. (2009). Biochar amendment techniques for upland rice production in northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crop Res. 111, 81–84. doi:10.1016/j.fcr.2008.10.008
Aslam, Z., Khalid, M., and Aon, M. (2014). Impact of biochar on soil physical properties. Sch. J. Agric. Sci. 4 (5), 280–284.
Bashir, M. A., Khalid, M., Naveed, M., Ahmad, R., and Gao, B. (2018). Influence of feedstock and pyrolytic temperature of biochar on physico-chemical characteristics and sorption of chromium in tannery polluted soil. Int. J. Agric. Biol. 20, 2823–2834. doi:10.17957/IJAB/15.0841
Basso, A. S., Miguez, F. E., Laird, D. A., Horton, R., and Westgate, M. (2013). Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 5, 132–143. doi:10.1111/gcbb.12026
Bian, R. J., Joseph, S., Cui, L. Q., Pan, G. X., Li, L. Q., Liu, X. Y., et al. (2014). A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater. 272, 121–128. doi:10.1016/j.jhazmat.2014.03.017
Biederman, L. A., and Harpole, W. S. (2013). Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 5, 202–214. doi:10.1111/gcbb.12037
Bindu, J. P., Reddy, D. D., Rao, C. C. S., Manorama, K., Prasad, L., and Mathur, R. (2020). Effect of oil palm waste biochars on FCV tobacco productivity in light textured Alfisols. Tob. Res. 46 (2), 47–52.
Bindu, J. P., Reddy, D. D., Rao, C. C. S., Manorama, K., Prasad, L., Mathur, R., et al. (2019). Production and characterization of oil palm biomass waste biochar. Tob. Res. 45 (2), 70–74.
Bindu, J. P., Reddy, D. D., Santhy, P., Rao, C. C., and Prasad, L. K. (2016). Comparative study on tobacco stalks based organic soil amendments and inorganic soil amendment (zeolite) on soil reaction and nutrient (N, K) availability of an Alfisol. Int. J. Trop. Agric. 34 (6), 17–22.
Bindu, J. P., Reddy, D. D., Santhy, P., Sellamuthu, K. M., Yassin, M. M., and Naik, R. (2017). Nutrient leaching behaviour of an alfisol as affected by tobacco stalk biochar and synthetic zeolite. Tob. Res. 43 (1), 10–14.
Bindu, J. P., Reddy, D. D., Santhy, P., Sellamuthu, K. M., Yassin, M. M., and Naik, R. (2015). Production and characterization of tobacco stalk biochar. Tob. Res. 41 (2), 91–96.
Bollmann, A., Schmidt, I., Saunders, A. M., and Nicolaisen, M. H. (2005). Influence of starvation on potential ammonia-oxidizing activity and amoA mRNA levels of Nitrosospira briensis. Appl. Environ. Microbiol. 71, 1276–1282. doi:10.1128/AEM.71.3.1276-1282.2005
Borno, M. L., Muller-Stover, D. S., and Liu, F. (2018). Contrasting effects of biochar on phosphorus dynamics and bioavailability in different soil types. Sci. Total Environ. 627, 963–974. doi:10.1016/j.scitotenv.2018.01.283
Brewer, C. E., and Brown, R. C. (2012). “Biochar,” in Comprehensive renewable energy. Editor A. Sayigh (Oxford, UK: Elsevier), 357–384.
Brodowski, S., Amelung, W., Haumaier, L., and Zech, W. (2007). Black carbon contribution to stable humus in German arable soils. Geoderma 139, 220–228. doi:10.1016/j.geoderma.2007.02.004
Bruun, S., Harmer, S. L., Bekiaris, G., Christel, W., Zuin, L., Hu, Y., et al. (2017). The effect of different pyrolysis temperatures on the speciation and availability in soil of P in biochar produced from the solid fraction of manure. Chemosphere 169, 377–386. doi:10.1016/j.chemosphere.2016.11.058
Calys-Tagoe, E., Sadick, A., Yeboah, E., and Amoah, B. (2019). Biochar effect on maize yield in selected farmers fields in the northern and upper east regions of Ghana. J. Exp. Agric. Int. 30, 1–9. doi:10.9734/jeai/2019/44168
Cao, H., Ning, L., Xun, M., Feng, F., Li, P., Yue, S., et al. (2019). Biochar can increase nitrogen use efficiency of Malus hupehensis by modulating nitrate reduction of soil and root. Appl. Soil Ecol. 135, 25–32. doi:10.1016/j.apsoil.2018.11.002
Cao, X., Ma, L., Liang, Y., Gao, B., and Harris, W. (2011). Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ. Sci. Technol. 45, 4884–4889. doi:10.1021/es103752u
Cayuela, M. L., Sanchez-Monedero, M. A., Roig, A., Hanley, K., Enders, A., and Lehmann, J. (2013). Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 3, 1732. doi:10.1038/srep01732
Chan, K. Y., Meszaros, I., Downie, A., and Joseph, S. (2007). Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 45, 629–634. doi:10.1071/SR07109
Chan, K. Y., Van Zwieten, L., Meszaros, I., Downie, A., and Joseph, S. (2008). Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 46, 437–444. doi:10.1071/SR08036
Chathurika, J. A. S., Kumaragamage, D., Zvomuya, F., Akinremi, O. O., Flaten, D. N., Indraratne, S. P., et al. (2016). Woodchip biochar with or without synthetic fertilizers affects soil properties and available phosphorus in two alkaline, chernozemic soils. Can. J. Soil Sci. 96, 472–484. doi:10.1139/cjss-2015-0094
Chen, L., Chen, Q., Rao, P., Yan, L., Shakib, A., and liu, G. (2018). Formulating and optimizing a novel biochar-based fertilizer for simultaneous slow-release of nitrogen and immobilization of cadmium. Sustainability 10, 2740. doi:10.3390/su10082740
Chen, L., Guo, L., Ali, A., Zhou, Q., Liu, M., Zhan, S., et al. (2021). Effect of biochar on the form transformation of heavy metals in paddy soil under different water regimes. Arch. Agron. Soil Sci. 69, 387–398. doi:10.1080/03650340.2021.2000966
Chintala, R., Mollinedo, J., Schumacher, T. E., Malo, D. D., and Julson, J. L. (2014). Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 60, 393–404. doi:10.1080/03650340.2013.789870
Choppala, G. K., Bolan, N., Megharaj, M., Chen, Z., and Naidu, R. (2012). The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. J. Environ. Qual. 41, 1175–1184. doi:10.2134/jeq2011.0145
Clough, T. J., Condron, L. M., Kammann, C., and Muller, C. (2013). A review of biochar and soil nitrogen dynamics. Agronomy 3, 275–293. doi:10.3390/agronomy3020275
Cui, H., Fan, Y., Fang, G., Zhang, H., Su, B., and Zhou, J. (2016). Leachability, availability and bioaccessibility of Cu and Cd in a contaminated soil treated with apatite, lime and charcoal: A five-year field experiment. Ecotoxicol. Environ. Saf. 134, 148–155. doi:10.1016/j.ecoenv.2016.07.005
Cui, L. Q., Pan, G. X., Li, L. Q., Yan, J. L., Zhang, A., Bian, R. J., et al. (2012). The reduction of wheat Cd uptake in contaminated soil via biochar amendment: A two-year field experiment. Bioresources 7, 5666–5676. doi:10.15376/biores.7.4.5666-5676
Czimczik, C. I., and Masiello, C. A. (2007). Controls on black carbon storage in soils: Black carbon in soils. Glob. Biogeochem. Cycles 21, 1–11. doi:10.1029/2006GB002798
Dai, S., Li, H., Dai, M., Dong, X., Ge, X., Sun, M., et al. (2018). Effects of biochar amendments on speciation and bioavailability of heavy metals in coal-mine-contaminated soil. Hum. Ecol. Risk Assess. 24, 1887–1900. doi:10.1080/10807039.2018.1429250
Dai, Z., Zhang, X., Tang, C., Muhammad, N., Wu, J., Brookes, P. C., et al. (2017). Potential role of biochars in decreasing soil acidification - a critical review. Sci. Total Environ. 581–582, 601–611. doi:10.1016/j.scitotenv.2016.12.169
De Meyer, A., Poesen, J., Isabirye, M., Deckers, J., and Rates, D. (2011). Soil erosion rates in tropical villages: A case study from lake victoria basin, Uganda. Catena 84, 89–98. doi:10.1016/j.catena.2010.10.001
DeLuca, T. H., MacKenzie, M. D., and Gundale, M. J. (2015). “Biochar effects on soil nutrient transformation,” in Biochar for environment management: Science and technology. Editors J. Lehmann, and S. Joseph (London: Earthscan), 419–425.
DeLuca, T. H., Mackenzie, M. D., and Gundale, M. J. (2009). Biochar effects on soil nutrient transformations. Biochar Environ. Manag. Sci. Technol., 251–270.
Dempster, D., Gleeson, D., Solaiman, Z. I., Jones, D., and Murphy, D. (2012). Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 354, 311–324. doi:10.1007/s11104-011-1067-5
Deng, B., Tammeorg, P., Luukkanen, O., Helenius, J., and Starr, M. (2017). Effects of Acacia seyal and biochar on soil properties and sorghum yield in agroforestry systems in South Sudan. Agrofor. Syst. 91, 137–148. doi:10.1007/s10457-016-9914-2
Dias, B. O., Silva, C. A., Higashikawa, F. S., Roig, A., and Sanchez-Monedero, M. A. (2010). Use of biochar as bulking agent for the composting of poultry manure: Effect on organic matter degradation and humification. Bioresour. Technol. 101, 1239–1246. doi:10.1016/j.biortech.2009.09.024
Ding, Y., Liu, Y., Liu, S., Li, Z., Tan, X., Huang, X., et al. (2016). Biochar to improve soil fertility - a review. Agron. Sustain. Dev. 36, 36–18. doi:10.1007/s13593-016-0372-z
Doan, T. T., Henry-des-Tureaux, T., Rumpel, C., Janeau, J. L., and Jouquet, P. (2015). Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in northern vietnam: A three year mesocosm experiment. Sci. Total Environ. 514, 147–154. doi:10.1016/j.scitotenv.2015.02.005
Domingo-Olive, F., Bosch-Serra, A. D., Yague, M. R., Poch, R. M., and Boixadera, J. (2016). Long term application of dairy cattle manure and pig slurry to winter cereals improves soil quality. Nutr. Cycl. Agroecosyst. 104, 39–51. doi:10.1007/s10705-015-9757-7
Dume, B., Ayele, D., Regassa, A., and Berecha, G. (2017). Improving available phosphorus in acidic soil using biochar. J. Soil Sci. Environ. Manage. 8, 87–94. doi:10.5897/JSSEM2015.0540
Edwards, J. D., Pittelkow, C. M., Kent, A. D., and andYang, W. H. (2018). Dynamic biochar effects on soil nitrous oxide emissions and underlying microbial processes during the maize growing season. Soil Biol. biochem. 122, 81–90. doi:10.1016/j.soilbio.2018.04.008
Ehsan, M., Barakat, M. A., Husein, D. Z., and Ismail, S. M. (2014). Immobilization of Ni and Cd in soil by biochar derived from unfertilized dates. Water Air Soil Poll. 225, 2123. doi:10.1007/s11270-014-2123-6
El-Naggar, A., Shaheen, S. M., Ok, Y. S., and Rinklebe, J. (2018). Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci. Total Environ. 624, 1059–1071. doi:10.1016/j.scitotenv.2017.12.190
Elmer, W. H., and Pignatello, J. J. (2011). Effect of biochar amendments on mycorrhizal associations and fusarium crown and root rot of asparagus in replant soils. Plant Dis. 95, 960–966. doi:10.1094/PDIS-10-10-0741
Fang, B., Lee, X., Zhang, J., Li, Y., Zhang, L., Cheng, J., et al. (2016). Impacts of straw biochar additions on agricultural soil quality and greenhouse gas fluxes in karst area, Southwest China. Soil Sci. Plant Nutr. 62, 526–533. doi:10.1080/00380768.2016.1202734
Farrell, M., Kuhn, T. K., Macdonald, L. M., Maddern, T. M., Murphy, D. V., Hall, P. A., et al. (2013). Microbial utilisation of biochar-derived carbon. Sci. Total Environ. 465, 288–297. doi:10.1016/j.scitotenv.2013.03.090
Figueiredo, C. C., Pinheiro, T. D., Oliveira, L. E. Z., Araujo, A. S., Coser, T. R., and Paz-Ferreiro, J. (2020). Direct and residual effect of biochar derived from biosolids on soil phosphorus pools: A four-year field assessment. Sci. Total Environ. 739, 140013. doi:10.1016/j.scitotenv.2020.140013
Frimpong, K. A., Phares, C. A., Boateng, I., Abban-Baidoo, E., and Apuri, L. (2021). One-time application of biochar influenced crop yield across three cropping cycles on tropical sandy loam soil in Ghana. Heliyon 7 (2), e06267. doi:10.1016/j.heliyon.2021.e06267
Gabhane, J., Tripathi, A., Athar, S., William, S. P. M. P., Vaidya, A. N., and Wate, S. R. (2016). Assessment of bioenergy potential of agricultural wastes: A case study cum template. J. Biofuels Bioenergy 2, 122–131. doi:10.5958/2454-8618.2016.00011.0
Gabhane, J. W., Bhange, V. P., Patil, P. D., Bankar, S. T., and Kumar, S. (2020). Recent trends in biochar production methods and its application as a soil health conditioner: A review. SN Appl. Sci. 2, 1307. doi:10.1007/s42452-020-3121-5
Gabhane, J., William, S. P., Bidyadhar, R., Bhilawe, P., Anand, D., Vaidya, A. N., et al. (2012). Additives aided composting of green waste: Effects on organic matter degradation, compost maturity, and quality of the finished compost. Bioresour. Technol. 114, 382–388. doi:10.1016/j.biortech.2012.02.040
Gao, S., Wang, D., Dangi, S. R., Duan, Y., Pflaum, T., Gartung, J., et al. (2020b). Nitrogen dynamics affected by biochar and irrigation level in an onion field. Sci. Total Environ. 714, 136432. doi:10.1016/j.scitotenv.2019.136432
Gao, Y., Shao, G., Lu, J., Zhang, K., Wu, S., and Wang, Z. (2020a). Effects of biochar application on crop water use efficiency depend on experimental conditions: A meta-analysis. Field Crops Res. 249, 107763. doi:10.1016/j.fcr.2020.107763
Gaskin, J. W., Speir, R. A., Harris, K., Das, K. C., Lee, R. D., Morris, L. A., et al. (2010). Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 102, 623–633. doi:10.2134/agronj2009.0083
Gaunt, J., and Cowie, A. (2009). “Biochar, greenhouse gas accounting and emissions trading,” in Biochar for environmental management: Science and technology. Editors J. Lehmann, and S. Joseph (London: Earthscan), 317–340.
Gianfreda, L. (2015). Enzymes of importance to rhizosphere processes. J. Soil Sci. Plant Nutr. 15, 0–306. doi:10.4067/S0718-95162015005000022
Glaser, B., Lehmann, J., and Zech, W. (2002). Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal–a review. Biol. Fertil. Soils 35, 219–230. doi:10.1007/s00374-002-0466-4
Glaser, B., and Lehr, V. I. (2019). Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 9, 9338–9339. doi:10.1038/s41598-019-45693-z
Gonzaga, M. I. S., Santos, J. C. J., Almeida, A. Q., Ros, K., and Santos, W. M. (2022). Nitrogen and phosphorus availability in the rhizosphere of maize plants cultivated in biochar amended soil. Arch. Agron. Soil Sci. 68, 1–13. doi:10.1080/03650340.2020.1869215
Grossman, J. M., O’Neill, B. E., Tsai, S. M., Liang, B. Q., Neves, E., Lehmann, J., et al. (2010). Amazonian anthrosols support similar microbial communities that differ distinctly from those extant in adjacent, unmodified soils of the same mineralogy. Microb. Ecol. 60, 192–205. doi:10.1007/s00248-010-9689-3
Gul, S., Whalen, J. K., Thomas, B. W., Sachdeva, V., and Deng, H. (2015). Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 206, 46–59. doi:10.1016/j.agee.2015.03.015
Gurtler, J. B., Boateng, A. A., Han, Y., and Douds, D. D. (2014). Inactivation of E. coli O157:H7 in cultivable soil by fast and slow pyrolysis-generated biochar. Food borne Pathog. Dis. 11, 215–223. doi:10.1089/fpd.2013.1631
Haefele, S. M., Konboon, Y., Wongboon, W., Amarante, S., Maarifat, A. A., Pfeiffer, E. M., et al. (2011). Effects and fate of biochar from rice residues in rice-based systems. Field Crops Res. 121, 430–440. doi:10.1016/j.fcr.2011.01.014
Hale, L., Luth, M., and Crowley, D. (2015). Biochar characteristics relate to its utility as an alternative soil inoculum carrier to peat and vermiculite. Soil Biol. biochem. 81, 228–235. doi:10.1016/j.soilbio.2014.11.023
Hardie, M., Clothier, B., Bound, S., Oliver, G., and Close, D. (2014). Does biochar influence soil physical properties and soil water availability? Plant Soil 376, 347–361. doi:10.1007/s11104-013-1980-x
Harter, J., Krause, H-M., Schuettler, S., Ruser, R., Fromme, M., Scholten, T., et al. (2014). Linking N2O emissions from biochar-amended soil to the structure and function of the N- cycling microbial community. ISME J. 8, 660–674. doi:10.1038/ismej.2013.160
Hartley, W., Dickinson, N. M., Riby, P., and Lepp, N. W. (2009). Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ. Pollut. 157, 2654–2662. doi:10.1016/j.envpol.2009.05.011
Herath, H. M. S. K., Arbestain, M. C., and Hedley, M. (2013). Effect of biochar on soil physical properties in two contrasting soils: An alfisol and an andisol. Geoderma 209–210, 188–197. doi:10.1016/j.geoderma.2013.06.016
Herath, I., Kumarathilaka, P., Navaratne, A., Rajakaruna, N., and Vithanage, M. (2015). Immobilization and phytotoxicity reduction of heavy metals in serpentine soil using biochar. J. Soils Sediments 15, 126–138. doi:10.1007/s11368-014-0967-4
Hossain, M. K., Strezov, V., Yin Chan, K., and Nelson, P. F. (2010). Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 78, 1167–1171. doi:10.1016/j.chemosphere.2010.01.009
Ippolito, J. A., Berry, C. M., Strawn, D. G., Novak, J. M., Levine, J., and Harley, A. (2017). Biochars reduce mine land soil bioavailable metals. J. Environ. Qual. 46, 411–419. doi:10.2134/jeq2016.10.0388
Ippolito, J. A., Ducey, T. F., Cantrell, K. B., Novak, J. M., and Lentz, R. D. (2016). Designer, acidic biochar influences calcareous soil characteristics. Chemosphere 142, 184–191. doi:10.1016/j.chemosphere.2015.05.092
Ippolito, J., Stromberger, M. E., Lentz, R. D., and Dungan, R. S. (2014). Hardwood biochar influences calcareous soil physicochemical and microbiological status. J. Environ. Qual. 43, 681–689. doi:10.2134/jeq2013.08.0324
Ishii, T., and Kadoya, K. (1994). Effects of charcoal as a soil conditioner on citrus growth and vesicular-arbuscular mycorrhizal development. J. Japn. Soc. Hortic. Sci. 63, 529–535. doi:10.2503/jjshs.63.529
Iyobe, T., Asada, T., Kawata, K., and Oikawa, K. (2004). Comparison of removal efficiencies for ammonia and amine gases between woody charcoal and activated carbon. J. Health Sci. 50, 148–153. doi:10.1248/jhs.50.148
Jaafar, N. M., Clode, P. L., and Abbott, L. K. (2014). Microscopy observations of habitable space in biochar for colonization by fungal hyphae from soil. J. Integr. Agric. 13, 483–490. doi:10.1016/S2095-3119(13)60703-0
Jeffery, S., Abalos, D., Prodana, M., Bastos, A. C., van Groenigen, J. W., Hungate, B. A., et al. (2017). Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 12, 053001. doi:10.1088/1748-9326/aa67bd
Jeffery, S., Meinders, M., Stoof, C., Bezemer, T. M., Voorde, T., Mommer, L., et al. (2015). Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma 251–252, 47–54. doi:10.1016/j.geoderma.2015.03.022
Jeffery, S., Verheijen, F. G. A., Van Der Velde, M., and Bastos, A. C. (2011). A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 144, 175–187. doi:10.1016/j.agee.2011.08.015
Jiang, S., Liu, J., Wu, J., Dai, G., Wei, D., and Shu, Y. (2020). Assessing biochar application to immobilize Cd and Pb in a contaminated soil: A field experiment under a cucumber-sweet potato-rape rotation. Environ. Geochem. Health 42, 4233–4244. doi:10.1007/s10653-020-00564-9
Jiang, T. Y., Jiang, J., Xu, R. K., and Li, Z. (2012). Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 89, 249–256. doi:10.1016/j.chemosphere.2012.04.028
Jiang, T. Y., Xu, R. K., Gu, T. X., and Jiang, J. (2014). Effect of crop-straw derived biochars on Pb(II) adsorption in two variable charge soils. J. Integr. Agric. 13, 507–516. doi:10.1016/S2095-3119(13)60706-6
Jien, S. H., and Wang, C. S. (2013). Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 110, 225–233. doi:10.1016/j.catena.2013.06.021
Jin, Y., Liang, X., He, M., Liu, Y., Tian, G., and Shi, J. (2016). Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: A microcosm incubation study. Chemosphere 142, 128–135. doi:10.1016/j.chemosphere.2015.07.015
Jing, Y., Zhang, Y., Han, I., Wang, P., Mei, Q., and Huang, Y. (2020). Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 10, 8837. doi:10.1038/s41598-020-65796-2
Jones, D. L., Rousk, J., Edwards-Jones, G., DeLuca, T. H., and Murphy, D. V. (2012). Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. biochem. 45, 113–124. doi:10.1016/j.soilbio.2011.10.012
Joseph, S., Cowie, A. L., Zwieten, L. V., Bolan, N., Budai, A., Buss, W., et al. (2021). How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 13, 1731–1764. doi:10.1111/gcbb.12885
Kammann, C. I., Linsel, S., Gobling, J. W., and Koyro, H. W. (2011). Influence of biochar on drought tolerance of Chenopodium quinoa willd and on soil-plant relations. Plant Soil 345, 195–210. doi:10.1007/s11104-011-0771-5
Kammann, C. I., Schmidt, H. P., Messerschmidt, N., Linsel, S., Steffens, D., Muller, C., et al. (2015). Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 5, 11080. doi:10.1038/srep11080
Karami, N., Clemente, R., Moreno-Jiménez, E., Lepp, N. W., and Beesley, L. (2011). Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 191 (1-3), 41–48. doi:10.1016/j.jhazmat.2011.04.025
Karim, A. A., Kumar, M., Singh, S. K., Panda, C. R., and Mishra, B. K. (2017). Potassium enriched biochar production by thermal plasma processing of banana peduncle for soil application. J. Anal. Appl. Pyrolysis 123, 165–172. doi:10.1016/j.jaap.2016.12.009
Kavitha, B., Reddy, P. V. L., Kim, B., Lee, S. S., Pandey, S. K., and Kim, K. (2018). Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manage. 227, 146–154. doi:10.1016/j.jenvman.2018.08.082
Khan, K. Y., Ali, B., Cui, X. Q., Feng, Y., Yang, X. E., and Stoffella, P. J. (2017). Impact of different feedstocks derived biochar amendment with cadmium low uptake affinity cultivar of pak choi (Brassica rapa ssb. chinensis L) on phytoavoidation of Cd to reduce potential dietary toxicity. Ecotoxicol. Environ. Saf. 141, 129–138. doi:10.1016/j.ecoenv.2017.03.020
Khan, M. B., Cui, X., Jilani, G., Tang, L., Lu, M., Cao, X., et al. (2020). New insight into the impact of biochar during vermi-stabilization of divergent biowastes: Literature synthesis and research pursuits. Chemosphere 238, 124679. doi:10.1016/j.chemosphere.2019.124679
Khan, S., Naushad, Mu., Lima, E. C., Zhang, S., Shaheen, S. M., and Rinklebe, J. (2021). Global soil pollution by toxic elements: Current status and future perspectives on the risk assessment and remediation strategies-a review. J. Hazard. Mater. 417, 126039. doi:10.1016/j.jhazmat.2021.126039
Kim, H-S., Kim, K-R., Yang, J. E., Ok, Y. S., Owens, G., Nehls, T., et al. (2016). Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L) response. Chemosphere 142, 153–159. doi:10.1016/j.chemosphere.2015.06.041
Kim, H. B., Kim, S. H., Jeon, E. K., Kim, D. H., Tsang, D. C. W., Alessi, D. S., et al. (2018). Effect of dissolved organic carbon from sludge, rice straw and spent coffee ground biochar on the mobility of arsenic in soil. Sci. Total Environ. 636, 1241–1248. doi:10.1016/j.scitotenv.2018.04.406
Kim, H. S., Kim, K. R., Kim, H. J., Yoon, J. H., Yang, J. E., Ok, Y. S., et al. (2015). Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L) in agricultural soil. Environ. Earth Sci. 74, 1249–1259. doi:10.1007/s12665-015-4116-1
Kotroczo, Z., Veres, Z., Biro, B., Toth, J. A., and Fekete, I. (2014). Influence of temperature and organic matter content on soil respiration in a deciduous oak forest. Eurasian J. Soil Sci. 3, 303–310. doi:10.18393/ejss.87903
Kumar, A., Singh, E., Mishra, R., and Kumar, S. (2022). Biochar as environmental armour and its diverse role towards protecting soil, water and air. Sci. Total Environ. 806, 150444. doi:10.1016/j.scitotenv.2021.150444
Kumar, S., Mastro, R., Ram, L., Sarkar, P., George, J., and Selvi, V. (2013). Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecol. Eng. 55, 67–72. doi:10.1016/j.ecoleng.2013.02.011
Kuzyakov, Y., Bogomolova, I., and Glaser, B. (2014). Biochar stability in soil: Decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol. biochem. 70, 229–236. doi:10.1016/j.soilbio.2013.12.021
Laird, D. A., Fleming, P., Davis, D. D., Horton, R., Wang, B., and Karlen, D. L. (2010). Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158, 443–449. doi:10.1016/j.geoderma.2010.05.013
Lammirato, C., Miltner, A., and Kaestner, M. (2011). Effects of wood char and activated carbon on the hydrolysis of cellobiose by b-glucosidase from Aspergillus niger. Soil Biol. biochem. 43, 1936–1942. doi:10.1016/j.soilbio.2011.05.021
Lee, S., Shah, H. S., Igalavitkana, A. D., Awad, Y. M., and Ok, Y. (2013). Enhancement of C3 and C4 plants productivity in soils amended with biochar and polyacrylamide. Tech. Bull. - Food Fertilizer Technol. Cent. Glob. Asia (East Pac). 12.
Lehmann, J., Gaunt, J., and Rondon, M. (2006). Biochar sequestration in terrestrial ecosystems: A review. Mitig. Adapt. Strateg. Glob. Change 11, 403–427. doi:10.1007/s11027-005-9006-5
Lehmann, J., Pereira da Silva, J., Steiner, C., Nehls, T., Zech, W., and Glaser, B. (2003). Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the central amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 249, 343–357. doi:10.1023/A:1022833116184
Lehmann, J., Rillig, M. C., Thies, J., Masiello, C. A., Hockaday, W. C., and Crowley, D. (2011). Biochar effects on soil biota-a review. Soil Biol. biochem. 43, 1812–1836. doi:10.1016/j.soilbio.2011.04.022
Li, H., Li, Y., Xu, Y., and Lu, X. (2020). Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere 244, 125471. doi:10.1016/j.chemosphere.2019.125471
Li, Y. Y., Zhang, F. B., Yang, M. Y., Zhang, J. Q., and Xie, Y. G. (2019). Impacts of biochar application rates and particle sizes on runoff and soil loss in small cultivated loess plots under simulated rainfall. Sci. Total Environ. 649, 1403–1413. doi:10.1016/j.scitotenv.2018.08.415
Liao, J., Liu, X., Hu, A., Song, H., Chen, X., and Zhang, Z. (2020). Effects of biochar-based controlled release nitrogen fertilizer on nitrogen-use efficiency of oilseed rape (Brassica napus L). Sci. Rep. 10, 11063. doi:10.1038/s41598-020-67528-y
Liu, D., Ding, Z., Ali, E. F., Kheir, A. M. S., Eissa, M. A., and Ibrahim, O. H. M. (2021). Biochar and compost enhance soil quality and growth of roselle (Hibiscus sabdariffa L) under saline conditions. Sci. Rep. 11, 8739. doi:10.1038/s41598-021-88293-6
Liu, D., Feng, Z., Zhu, H., Yu, L., Yang, K., Yu, S., et al. (2020). Effects of corn straw biochar application on soybean growth and alkaline soil properties. Bioresources 15, 1463–1481. doi:10.15376/biores.15.1.1463-1481
Liu, J., Shen, J., Li, Y., Su, Y., Ge, T., Jones, D. L., et al. (2014). Effects of biochar amendment on the net greenhouse gas emission and greenhouse gas intensity in a Chinese double rice cropping system. Eur. J. Soil Biol. 65, 30–39. doi:10.1016/j.ejsobi.2014.09.001
Liu, S., Tang, W., Yang, F., Meng, J., Chen, W., and Li, X. (2017a). Influence of biochar application on potassium-solubilizing Bacillus mucilaginosus as potential biofertilizer. Prep. Biochem. Biotechnol. 47, 32–37. doi:10.1080/10826068.2016.1155062
Liu, X. H., Han, F. P., and Zhang, X. C. (2012). Effect of biochar on soil aggregates in the loess plateau: Results from incubation experiments. Int. J. Agric. Biol. 14, 975–979.
Liu, X., Zhang, A., Ji, C., Joseph, S., Bian, R., Li, L., et al. (2013). Biochar's effect on crop productivity and the dependence on experimental conditions: A meta-analysis of literature data. Plant Soil 373, 583–594. doi:10.1007/s11104-013-1806-x
Liu, Z., Dugan, B., Masiello, C. A., Barnes, R. T., Gallagher, M. E., and Gonnermann, H. (2016). Impacts of biochar concentration and particle size on hydraulic conductivity and DOC leaching of biochar–sand mixtures. J. Hydrol. 533, 461–472. doi:10.1016/j.jhydrol.2015.12.007
Liu, Z., He, T., Lan, Y., Yang, X., Meng, J., and Chen, W. (2017b). Maize stover biochar accelerated urea hydrolysis and short-term nitrogen turnover in soil. BioResources 12, 6024–6039. doi:10.15376/biores.12.3.6024-6039
Luo, Y., Durenkamp, M., Nobili, M. D., Lin, Q., Devonshire, B. J., and Brookes, P. C. (2013). Microbial biomass growth following incorporation of biochars produced at 350ºC or 700ºC, in a silty-clay loam soil of high and low pH. Soil Biol. biochem. 57, 513–523. doi:10.1016/j.soilbio.2012.10.033
Ma, N., Zhang, L., Zhang, Y., Yang, L., Yu, C., Yin, G., et al. (2016). Biochar improves soil aggregate stability and water availability in a mollisol after three years of field application. PLOS one 11, e0154091. doi:10.1371/journal.pone.0154091
Madiba, O. F., Solaiman, Z. M., Carson, J. K., and Murphy, D. V. (2016). Biochar increases availability and uptake of phosphorus to wheat under leaching conditions. Biol. Fertil. Soils 52, 439–446. doi:10.1007/s00374-016-1099-3
Major, J., Rondon, M., Molina, D., Riha, S. J., and Lehmann, J. (2010). Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333, 117–128. doi:10.1007/s11104-010-0327-0
Malinska, K., Zabochnicka-Swiatek, M., Caceres, R., and Marfa, O. (2016). The effect of precomposted sewage sludge mixture amended with biochar on the growth and reproduction of Eisenia fetida during laboratory vermicomposting. Ecol. Eng. 90, 35–41. doi:10.1016/j.ecoleng.2016.01.042
Mandal, S., Thangarajan, R., Bolan, N. S., Sarkar, B., Khan, N., Ok, Y. S., et al. (2016). Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 142, 120–127. doi:10.1016/j.chemosphere.2015.04.086
Martin, S. L., Clarke, M. L., Othman, M., Ramsden, S. J., and West, H. M. (2015). Biochar-mediated reductions in greenhouse gas emissions from soil amended with anaerobic digestates. Biomass Bioenergy 79, 39–49. doi:10.1016/j.biombioe.2015.04.030
Martinsen, V., Alling, V., Nurida, N. L., Mulder, J., Hale, S. E., Ritz, C., et al. (2015). pH effects of the addition of three biochars to acidic Indonesian mineral soils. Soil Sci. Plant Nutr. 61, 821–834. doi:10.1080/00380768.2015.1052985
Masto, R. E., Kumar, S., Rout, T. K., Sarkar, P., George, J., and Ram, L. C. (2013). Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 111, 64–71. doi:10.1016/j.catena.2013.06.025
Masulili, A., Utomo, W. H., and Syechfani, M. (2010). Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia. J. Agric. Sci. 2, 39. doi:10.5539/jas.v2n1p39
McElligott, K. M. (2011). “Biochar amendments to forest soils: Effects on soil properties and tree growth,”. Master’s Thesis (Moscow, ID, USA: College of Graduate studies, University of Idaho).
Meier, S., Curaqueo, G., Khan, N., Bolan, N., Cea, M., Eugenia, G. M., et al. (2017). Chicken-manure-derived biochar reduced bioavailability of copper in a contaminated soil. J. Soils Sediments. 17, 741–750. doi:10.1007/s11368-015-1256-6
Mia, S., van Groenigen, J. W., van de Voorde, T. F. J., Oram, N. J., Bezemer, T. M., Mommer, L., et al. (2014). Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 191, 83–91. doi:10.1016/j.agee.2014.03.011
Mitchell, P. J., Simpson, A. J., Soong, R., Schurman, J. S., Thomas, S. C., and Simpson, M. J. (2016). Biochar amendment and phosphorus fertilization altered forest soil microbial community and native soil organic matter molecular composition. Biogeochemistry 130, 227–245. doi:10.1007/s10533-016-0254-0
Mitchell, P. J., Simpson, A. J., Soong, R., and Simpson, M. J. (2015). Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol. biochem. 81, 244–254. doi:10.1016/j.soilbio.2014.11.017
Moon, D. H., Park, J. W., Chang, Y. Y., Ok, Y. S., Lee, S. S., Ahmad, M., et al. (2013). Immobilization of lead in contaminated firing range soil using biochar. Environ. Sci. Pollut. Res. 20 (12), 8464–8471. doi:10.1007/s11356-013-1964-7
Mousa, A. A. (2017). Effect of using some soil conditioners on salt affected soil properties and its productivity at El-Tina Plain area, North Sinai, Egypt. Egypt. J. Soil Sci. 57, 0–111. doi:10.21608/EJSS.2017.1526
Mukherjee, A., Zimmerman, A., Hamdan, R., and Cooper, W. (2014). Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid earth. 5, 693–704. doi:10.5194/se-5-693-2014
Nabavinia, F., Emami, H., Astaraee, A., and Lakzian, A. (2015). Effect of tannery wastes and biochar on soil chemical and physicochemical properties and growth traits of radish. Int. Agrophys. 29, 333–339. doi:10.1515/intag-2015-0040
Neble, S., Calvert, V., Petit Le, J., and Criquet, S. (2007). Dynamics of phosphatase activities in a cork oak litter (Quercus suber L) following sewage sludge application. Soil Biol. biochem. 39, 2735–2742. doi:10.1016/j.soilbio.2007.05.015
Netherway, P., Reichman, S. M., Laidlaw, M., Scheckel, K., Pingitore, N., Gascó, G., et al. (2019). Phosphorus-rich biochars can transform lead in an urban contaminated soil. J. Environ. Qual. 48, 1091–1099. doi:10.2134/jeq2018.09.0324
Ngatia, L. W., Hsieh, Y. P., Nemours, D., Fu, R., and Taylor, R. W. (2017). Potential phosphorus eutrophication mitigation strategy: Biochar carbon composition, thermal stability and pH influence phosphorus sorption. Chemosphere 180, 201–211. doi:10.1016/j.chemosphere.2017.04.012
Nguyen, T. T. N., Xu, C. Y., Tahmasbian, I., Che, R., Xu, Z., Zhou, X., et al. (2017). Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 288, 79–96. doi:10.1016/j.geoderma.2016.11.004
Novak, J. M., and Busscher, W. J. (2012). “Selection and use of designer biochars to improve characteristics of south eastern USA Coastal Plain degraded soils,” in Advanced biofuels and bioproducts. Editor J. E. Lee (New York: Springer Science).
Novak, J. M., Cantrell, K. B., Watts, D. W., Busscher, W. J., and Johnson, M. G. (2014). Designing relevant biochars as soil amendments using lignocellulosic-based and manure-based feedstocks. J. Soils Sediments 14, 330–343. doi:10.1007/s11368-013-0680-8
Novak, J. M., Cantrell, K. B., and Watts, D. W. (2013). Compositional and thermal evaluation of lignocellulosic and poultry litter chars via high and low temperature pyrolysis. Bioenergy Res. 6, 114–130. doi:10.1007/s12155-012-9228-9
Novak, J. M., Ippolito, J. A., Ducey, T. F., Watts, D. W., Spokas, K. A., Trippe, K. M., et al. (2018a). Remediation of an acidic mine spoil: Miscanthus biochar and lime amendment affects metal availability, plant growth, and soil enzyme activity. Chemosphere 205, 709–718. doi:10.1016/j.chemosphere.2018.04.107
Novak, J. M., Johnson, M. G., and Spokas, K. A. (2018b). Concentration and release of phosphorus and potassium from lignocellulosic- and manure-based biochars for fertilizer reuse. Front. Sustain. Food Syst. 2, 54. doi:10.3389/fsufs.2018.00054
Nunes, M. R., van Es, H. M., Veum, K. S., Amsili, J. P., and Karlen, D. L. (2020). Anthropogenic and inherent effects on soil organic carbon across the U.S. Sustainability 12, 5695. doi:10.3390/su12145695
Nzanza, B., Marais, D., and Soundy, P. (2012). Effect of arbuscular mycorrhizal fungal inoculation and biochar amendment on growth and yield of tomato. Int. J. Agric. Biol. 14, 965–969.
Oladele, S. O. (2019). Effect of biochar amendment on soil enzymatic activities, carboxylate secretions and upland rice performance in a sandy clay loam Alfisol of Southwest Nigeria. Sci. Afr. 4, e00107. doi:10.1016/j.sciaf.2019.e00107
Oram, N. J., van de Voorde, T. F. J., Ouwehand, G. J., Bezemer, T. M., Mommer, L., Jeffery, S., et al. (2014). Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 191, 92–98. doi:10.1016/j.agee.2014.03.031
Ouyang, L., Wang, F., Tang, J., Yu, L., and Zhang, R. (2013). Effects of biochar amendment on soil aggregates and hydraulic properties. J. Soil Sci. Plant Nutr. 13, 0–1002. doi:10.4067/S0718-95162013005000078
Pandian, K., Subramaniayan, P., Gnasekaran, P., and Chitraputhirapillai, S. (2016). Effect of biochar amendment on soil physical, chemical and biological properties and groundnut yield in rainfed Alfisol of semi-arid tropics. Arch. Agron. Soil Sci. 62, 1293–1310. doi:10.1080/03650340.2016.1139086
Park, J. H., Choppala, G., Bolan, N., Chung, J. W., and Chuasavathi, T. (2011). Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348, 439–451. doi:10.1007/s11104-011-0948-y
Peng, X., Zhu, Q. H., Xie, Z. B., Darboux, F., and Holden, N. M. (2016). The impact of manure, straw and biochar amendments on aggregation and erosion in a hillslope Ultisol. Catena 138, 30–37. doi:10.1016/j.catena.2015.11.008
Pituya, P., Sriburi, T., and Wijitkosum, S. (2017). Optimization of biochar preparation from Acacia wood for soil amendment. Eng. J. 21, 99–105. doi:10.4186/ej.2017.21.2.99
Prapagdee, S., Piyatiratitivorakul, S., Petsom, A., and Tawinteung, N. (2014). Application of biochar for enhancing cadmium and zinc phytostabilization in Vignaradiata L. cultivation. Water Air Soil Pollut. 225, 2233. doi:10.1007/s11270-014-2233-1
Premalatha, R. P., Malarvizhi, P., and Parameswari, E. (2023). Effect of biochar doses under various levels of salt stress on soil nutrient availability, soil enzyme activities and plant growth in a marigold crop. Crop Pasture Sci. 74, 66–78. doi:10.1071/cp21542
Pu, S., Li, G., Tang, G., Zhang, Y., Xu, W., Li, P., et al. (2019). Effects of biochar on water movement characteristics in sandy soil under drip irrigation. J. Arid. Land 11, 740–753. doi:10.1007/s40333-019-0106-6
Puga, A. P., De Abreu, C. A., Melo, L. C., Pazferreiro, J., and Beesley, L. (2015). Cadmium, lead, and zinc mobility and plant uptake in a mine soil amended with sugarcane straw biochar. Environ. Sci. Pollut. Res. 22, 17606–17614. doi:10.1007/s11356-015-4977-6
Purakayastha, T. J., Das, K. C., Gaskin, J., Harris, K., Smith, J. L., and Kumari, S. (2016). Effect of pyrolysis temperatures on stability and priming effects of C3 and C4 biochars applied to two different soils. Soil Tillage Res. 155, 107–115. doi:10.1016/j.still.2015.07.011
Qayyum, M. F., Haider, G., Raza, M. A., Mohamed, A. K. S. H., Rizwan, M., El-Sheikh, M. A., et al. (2020). Straw-based biochar mediated potassium availability and increased growth and yield of cotton (Gossypium hirsutum L). J. Saudi Chem. Soc. 24, 963–973. doi:10.1016/j.jscs.2020.10.004
Qian, L., Chen, L., Joseph, S., Pan, G., Li, L., Zheng, J., et al. (2014). Biochar compound fertilizer as an option to reach high productivity but low carbon intensity in rice agriculture of China. Carbon Manag. 5, 145–154. doi:10.1080/17583004.2014.912866
Qiao, J. T., Liu, T. X., Wang, X. Q., Li, F. B., Lv, Y. H., Cui, J. H., et al. (2018). Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 195, 260–271. doi:10.1016/j.chemosphere.2017.12.081
Quilliam, R. S., Glanville, H. C., Wade, S. C., and Jones, D. L. (2013). Life in the ‘charosphere’ – does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. biochem. 65, 287–293. doi:10.1016/j.soilbio.2013.06.004
Rab, A., Khan, M. R., Haq, S. U., Zahid, S., Asim, M., Afridi, M. Z., et al. (2016). Impact of biochar on mungbean yield and yield components. Pure Appl. Biol. 5, 632. doi:10.19045/bspab.2016.50082
Raboin, L. M., Razafimahafaly, A. H. D., Rabenjarisoa, M. B., Rabary, B., Dusserre, J., and Becquer, T. (2016). Improving the fertility of tropical acid soils: Liming versus biochar application? A long term comparison in the highlands of Madagascar. Field Crops Res. 199, 99–108. doi:10.1016/j.fcr.2016.09.005
Rajkovich, S., Enders, A., Hanley, K., Hyland, C., Zimmerman, A. R., and Lehmann, J. (2012). Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 48, 271–284. doi:10.1007/s00374-011-0624-7
Raut, M., Princewilliam, S., Bhattacharyya, J., Chakrabarti, T., and Devotta, S. (2008). Microbial dynamics and enzyme activities during rapid composting of municipal solid waste—A compost maturity analysis perspective. Bioresour. Technol. 99, 6512–6519. doi:10.1016/j.biortech.2007.11.030
Regmi, A., Singh, S., Moustaid-Moussa, N., Coldren, C., and Simpson, C. (2022). The negative effects of high rates of biochar on Violas can be counteracted with fertilizer. Plants 11, 491. doi:10.3390/plants11040491
Revell, K. T., Maguire, R. O., and Agblevor, F. A. (2012). Field trials with poultry litter biochar and its effect on forages, green peppers, and soil properties. Soil Sci. 177, 573–579. doi:10.1097/SS.0b013e3182741050
Rondon, M., Lehmann, J., Ramirez, J., and Hurtado, M. (2007). Biological nitrogen fixation by common beans (Phaseolus vulgaris L) increases with bio-char additions. Biol. Fertil. Soils 43, 699–708. doi:10.1007/s00374-006-0152-z
Rondon, M., Ramirez, A., and Hurtado, M. (2004). Charcoal additions to high fertility ditches enhance yields and quality of cash crops in Andean hillsides of Colombia. Colombia: CIAT Annual Report Cali.
Rutigliano, F. A., Romano, M., Marzaioli, R., Baglivo, I., Baronti, S., Miglietta, F., et al. (2011). Effect of biochar addition on soil microbial community in a wheat crop. Eur. J. Soil Biol. 60, 9–15. doi:10.1016/j.ejsobi.2013.10.007
Sakin, E., Ramazanoglu, E., and Seyrek, A. (2021). Effects of different biochar amendments on soil enzyme activities and carbon dioxide emission. Commun. Soil Sci. Plant Anal. 52, 2933–2944. doi:10.1080/00103624.2021.1971694
Salam, A., Bashir, S., Khan, I., and Hu, H. (2019). Two years impacts of rapeseed residue and rice straw biochar on Pb and Cu immobilization and revegetation of naturally co-contaminated soil. Appl. Geochem. 105, 97–104. doi:10.1016/j.apgeochem.2019.04.011
Schmidt, H. P., Kammann, C., Hagemann, N., Leifeld, J., Bucheli, T. D., Monedero, M. A. S., et al. (2021). Biochar in agriculture- A systematic review of 26 global meta-analyses. GCB Bioenergy 13, 1708–1730. doi:10.1111/gcbb.12889
Schmidt, H. P., Pandit, B. H., Martinsen, V., Cornelissen, G., Conte, P., and Kammann, C. I. (2015). Fourfold increase in pumpkin yield in response to low-dosage root zone application of urine-enhanced biochar to a fertile tropical soil. Agriculture 5, 723–741. doi:10.3390/agriculture5030723
Schneider, F., and Haderlein, S. B. (2016). Potential effects of biochar on the availability of phosphorus—Mechanistic insights. Geoderma 277, 83–90. doi:10.1016/j.geoderma.2016.05.007
Senesi, N., and Plaza, C. (2007). Role of humification processes in recycling organic wastes of various nature and sources as soil amendments. Clean. Soil Air Water 35, 26–41. doi:10.1002/clen.200600018
Shahzad, S. M., Khalid, A., Arif, M. S., Riaz, M., Ashraf, M., Iqbal, Z., et al. (2014). Co-inoculation integrated with P- enriched compost improved nodulation and growth of Chickpea (Cicer arietinum L) under irrigated and rainfed farming systems. Biol. Fertil. Soils 50, 1–12. doi:10.1007/s00374-013-0826-2
Shen, Z., Som, A. M., Wang, F., Jin, F., McMillan, O., and Al-Tabbaa, A. (2016). Long-term impact of biochar on the immobilisation of nickel (II) and zinc (II) and the revegetation of a contaminated site. Sci. Total Environ. 542, 771–776. doi:10.1016/j.scitotenv.2015.10.057
Shenbagavalli, S., and Mahimairaja, S. (2012). Characterization and effect of biochar on nitrogen and carbon dynamics in soil. Int. J. Adv. Bio. Res. 2, 249–255.
Shetty, R., and Prakash, N. B. (2020). Effect of different biochars on acid soil and growth parameters of rice plants under aluminium toxicity. Sci. Rep. 10, 12249. doi:10.1038/s41598-020-69262-x
Shetty, R., Vidya, C. S. N., Prakash, N. B., Lux, A., and Vaculik, M. (2021). Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 765, 142744. doi:10.1016/j.scitotenv.2020.142744
Siebers, N., and Leinweber, P. (2013). Bone char: A clean and renewable phosphorus fertilizer with cadmium immobilization capability. J. Environ. Qual. 42, 405–411. doi:10.2134/jeq2012.0363
Sigua, G. C., Novak, J. M., Watts, D. W., Johnson, M. G., and Spokas, K. (2016). Efficacies of designer biochars in improving biomass and nutrient uptake of winter wheat grown in a hard setting subsoil layer. Chemosphere 142, 176–183. doi:10.1016/j.chemosphere.2015.06.015
Sika, M., and Hardie, A. (2014). Effect of pine wood biochar on ammonium nitrate leaching and availability in a South African sandy soil. Eur. J. Soil Sci. 65, 113–119. doi:10.1111/ejss.12082
Singh, A., Singh, A. P., and Purakayastha, T. J. (2019). Characterization of biochar and their influence on microbial activities and potassium availability in an acid soil. Arch. Agron. Soil Sci. 65, 1302–1315. doi:10.1080/03650340.2018.1563291
Singh, B. P., Hatton, B. J., Singh, B., Cowie, A. L., and Kathuria, A. (2010). Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J. Environ. Qual. 39, 1224–1235. doi:10.2134/jeq2009.0138
Singh, H., Northup, B. K., Rice, C. W., and Vara Prasad, P. V. (2022). Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 4, 8. doi:10.1007/s42773-022-00138-1
Smider, B., and Singh, B. (2014). Agronomic performance of a high ash biochar in two contrasting soils. Agric. Ecosyst. Environ. 191, 99–107. doi:10.1016/j.agee.2014.01.024
Speratti, A. B., Johnson, M. S., Sousa, H. M., Torres, G. N., and Couto, E. G. (2017). Impact of different agricultural waste biochars on maize biomass and soil water content in a Brazilian Cerrado Arenosol. Agronomy 7, 1–19. doi:10.3390/agronomy7030049
Spokas, K. A., Cantrell, K. B., Novak, J. M., Archer, D. W., Ippolito, J. A., Collins, H. P., et al. (2012). Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 41, 973–989. doi:10.2134/jeq2011.0069
Srinivasan, P., and Sarmah, A. K. (2015). Characterisation of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: A spectroscopic investigation. Sci. Total Environ. 502, 471–480. doi:10.1016/j.scitotenv.2014.09.048
Sriphirom, P., Chidthaisong, A., Yagi, K., Tripetchkul, S., Boonapatcharoen, N., and Towprayoon, S. (2021). Effects of biochar on methane emission, grain yield, and soil in rice cultivation in Thailand. Carbon Manage 12, 109–121. doi:10.1080/17583004.2021.1885257
Sriphirom, P., Chidthaisong, A., Yagi, K., Tripetchkul, S., and Towpravoon, S. (2020). Evaluation of biochar applications combined with alternate wetting and drying (AWD) water management in rice field as a methane mitigation option for farmers’ adoption. Soil Sci. Plant Nutr. 66, 235–246. doi:10.1080/00380768.2019.1706431
Steiner, C., Glaser, B., Geraldes Teixeira, W., Lehmann, J., Blum, W. E., and Zech, W. (2008). Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 171, 893–899. doi:10.1002/jpln.200625199
Steiner, C., Teixeira, W. G., Lehmann, J., Nehls, T., De Macedo, J. L. V., Blum, W. E., et al. (2007). Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 291, 275–290. doi:10.1007/s11104-007-9193-9
Sui, F., Wang, J., Zuo, J., Joseph, S., Munroe, P., Drosos, M., et al. (2020). Effect of amendment of biochar supplemented with Si on Cd mobility and rice uptake over three rice growing seasons in an acidic Cd-tainted paddy from central South China. Sci. Total Environ. 709, 136101. doi:10.1016/j.scitotenv.2019.136101
Sukartono, , Utomo, W. H., Kusuma, W. Z., and Nugroho, W. H. (2011). Soil fertility status, nutrient uptake, and maize (Zea mays L) yield following biochar and cattle manure application on sandy soils of Lombok, Indonesia. J. Trop. Agric. 49, 47–52.
Sun, H., Zhang, H., Shi, W., Zhou, M., and Ma, X. (2019). Effect of biochar on nitrogen use efficiency, grain yield and amino acid content of wheat cultivated on saline soil. Plant Soil Environ. 65, 83–89. doi:10.17221/525/2018-PSE
Sun, J., Yang, R., Li, W., Pan, Y., Zheng, M., and Zhang, Z. (2018). Effect of biochar amendment on water infiltration in a coastal saline soil. J. Soils Sediments 18, 3271–3279. doi:10.1007/s11368-018-2001-8
Suppadit, T., Kitikoon, V., Phubphol, A., and Neumnoi, P. (2012). Effect of quail litter biochar on productivity of four new physic nut varieties planted in cadmium-contaminated soil. Chil. J. Agric. Res. 72, 125–132. doi:10.4067/S0718-58392012000100020
Tagoe, S., Horiuchi, T., and Matsui, T. (2008). Effects of carbonized and dried chicken manures on the growth, yield, and N content of soybean. Plant Soil 306, 211–220. doi:10.1007/s11104-008-9573-9
Tan, X., Liu, Y., Zeng, G., Wang, X., Hu, X., Gu, Y., et al. (2015). Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125, 70–85. doi:10.1016/j.chemosphere.2014.12.058
Tesfaye, F., Liu, X., Zheng, J., Cheng, K., Bian, R., Zhang, X., et al. (2021). Could biochar amendment be a tool to improve soil availability and plant uptake of phosphorus? A meta-analysis of published experiments. Environ. Sci. Pollut. Res. 28, 34108–34120. doi:10.1007/s11356-021-14119-7
Thomas, S. C., Frye, S., Gale, N., Garmon, M., Launchbury, R., Machado, N., et al. (2013). Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manage. 129, 62–68. doi:10.1016/j.jenvman.2013.05.057
Thomazini, A., Spokas, K., Hall, K., Ippolito, J., Lentz, R., and Novak, J. (2015). GHG impacts of biochar: Predictability for the same biochar. Agric. Ecosyst. Environ. 207, 183–191. doi:10.1016/j.agee.2015.04.012
Tian, X., Li, Z., Liu, Z., Wang, Y., Li, B., Zhang, K., et al. (2021). Combined effect of biochar and nitrogen fertilizer reduction on rapeseed productivity and nitrogen use efficiency. Arch. Agron. Soil Sci. 68, 1159–1174. doi:10.1080/03650340.2021.1872782
Trazzi, P. A., Leahy, J. J., Hayes, M. H. B., and Kwapinski, W. (2016). Adsorption and desorption of phosphate on biochars. J. Environ. Chem. Eng. 4, 37–46. doi:10.1016/j.jece.2015.11.005
Tryon, E. H. (1948). Effect of charcoal on certain physical, chemical, and biological properties of forest soils. Ecol. Monogr. 18 (1), 81–115. doi:10.2307/1948629
Uchimiya, M., Klasson, K. T., Wartelle, L. H., and Lima, I. M. (2011). Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper sorption isotherms and the release of cations. Chemosphere 82, 1431–1437. doi:10.1016/j.chemosphere.2010.11.050
Uroic Stefanko, A., and Leszczynska, D. (2020). Impact of biomass source and pyrolysis parameters on physicochemical properties of biochar manufactured for innovative applications. Front. Energy Res. 8, 138. doi:10.3389/fenrg.2020.00138
Usman, A. R. A., Al-Wabel, M. I., Abdulaziz, A-H., Mahmoud, W-A., El-Naggar, A. H., Ahmad, M., et al. (2016). Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 26, 27–38. doi:10.1016/S1002-0160(15)60019-4
Uzoma, K. C., Inoue, M., Andry, H., Zahoor, A., and Nishihara, E. (2011). Influence of biochar application on sandy soil hydraulic properties and nutrient retention. J. Food Agric. Environ. 9, 1137–1143. doi:10.2136/vzj2018.05.0101
Vaccari, F. P., Baronti, S., Lugato, E., Genesio, L., Castaldi, S., Fornasier, F., et al. (2011). Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur. J. Agron. 34, 231–238. doi:10.1016/j.eja.2011.01.006
Van Zwieten, L., Kimber, S., Morris, S., Ky, C., Downie, A., Rust, J., et al. (2010). Effects of biochar from slow pyrolysis of paper mill waste on agronomic performance and soil fertility. Plant Soil 327, 235–246. doi:10.1007/s11104-009-0050-x
Van Zwieten, L., Singh, B., Joseph, S., Kimber, S., Cowie, A., and Chan, Y. (2009). “Biochar and emissions of non-CO2 greenhouse gases from soil,” in Biochar for environmental management: Science and technology. Editors J. Lehmann, and S. Joseph (London: Earthscan), 227–249.
Vanek, S. J., and Lehmann, J. (2015). Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil 395, 105–123. doi:10.1007/s11104-014-2246-y
Venegas, A., Rigol, A., and Vidal, M. (2016). Changes in heavy metal extractability from contaminated soils remediated with organic waste or biochar. Geoderma 279, 132–140. doi:10.1016/j.geoderma.2016.06.010
Wagner, A., Kaupenjohann, M., Hu, Y., Kruse, J., and Leinweber, P. (2015). Biochar-induced formation of Zn-P-phases in former sewage field soils studied by P K-edge XANES spectroscopy. J. Plant Nutr. Soil Sci. 178, 582–585. doi:10.1002/jpln.201400601
Wang, B., Lehmann, J., Hanley, K., Hestrin, R., and Enders, A. (2015). Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 138, 120–126. doi:10.1016/j.chemosphere.2015.05.062
Wang, J., Shi, L., Zhai, L., Zhnag, H., Wang, S., Zou, J., et al. (2021). Analysis of the long term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: A review. Ecotoxicol. Environ. Saf. 207, 111261. doi:10.1016/j.ecoenv.2020.111261
Wang, L., Xue, C., Nie, X., Liu, Y., and Chen, F. (2018). Effects of biochar application on soil potassium dynamics and crop uptake. J. Plant Nutr. Soil Sci. 181 (5), 635–643. doi:10.1002/jpln.201700528
Warnock, D. D., Lehmann, J., Kuyper, T. W., and Rillig, M. C. (2007). Mycorrhizal responses to biochar in soil–concepts and mechanisms. Plant Soil 300, 9–20. doi:10.1007/s11104-007-9391-5
Warnock, D. D., Mummey, D. L., Mcbride, B., Major, J., Lehmann, J., and Rillig, M. C. (2010). Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: Results from growth-chamber and field experiments. Appl. Soil Ecol. 46, 450–456. doi:10.1016/j.apsoil.2010.09.002
Wen, P., Wu, Z., Han, Y., Cravotto, G., Wang, J., and Ye, B. (2017). Microwave-assisted synthesis of a novel biochar-based slow-release nitrogen fertilizer with enhanced water-retention capacity. ACS Sustain. Chem. Eng. 5, 7374–7382. doi:10.1021/acssuschemeng.7b01721
Wu, P. P., Li, L. J., Wang, J. J., and Min, L. (2017). Effects of application of straw derived biochar on forms of heavy metals in mining contaminated soil. J. Ecol. Rural. Environ. 33, 453–459. doi:10.11934/j.issn.1673-4831.2017.05.010
Xiao, Q., Zhu, L-X., Zhang, H-P., Li, X-Y., Shen, Y-F., and Li, S-Q. (2016). Soil amendment with biochar increases maize yields in a semi-arid region by improving soil quality and root growth. Crop Pasture Sci. 67, 495–507. doi:10.1071/CP15351
Xing, Y., Wang, J. X., Xia, J. C., Liu, Z. M., Zhang, Y. H., Du, Y., et al. (2019). A pilot study on using biochars as sustainable amendments to inhibit rice uptake of Hg from a historically polluted soil in a karst region of China. Ecotoxicol. Environ. Saf. 170, 18–24. doi:10.1016/j.ecoenv.2018.11.111
Xu, G., Shao, H., Zhang, Y., and Junna, S. (2018). Non additive effects of biochar amendments on soil phosphorus fractions in two contrasting soils. Land Degrad. Dev. 29, 2720–2727. doi:10.1002/ldr.3029
Xu, G., Sun, J. N., Shao, H. B., and Chang, S. X. (2014a). Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 62, 54–60. doi:10.1016/j.ecoleng.2013.10.027
Xu, H. J., Wang, X. H., Li, H., Yao, H. Y., Su, J. Q., and Zhu, Y. G. (2014b). Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 48, 9391–9399. doi:10.1021/es5021058
Xu, J. M., Tang, C., and Chen, Z. L. (2006). The role of plant residues in pH change of acid soils differing in initial pH. Soil Biol. biochem. 38, 709–719. doi:10.1016/j.soilbio.2005.06.022
Xu, J., Niu, W. Q., Zhang, M. Z., Li, Y., Lv, W., Li, K. Y., et al. (2016). Effect of biochar addition on soil evaporation. Chin. J. Appl. Ecol. 27, 3505–3513. doi:10.13287/j.1001-9332.201611.018
Yaashikaa, P. R., Senthil Kumar, P., Varjani, S., and Saravanan, A. (2020). A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 28, e00570. doi:10.1016/j.btre.2020.e00570
Yamato, M., Okimori, Y., Wibowo, I. F., Anshori, S., and Ogawa, M. (2006). Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 52, 489–495. doi:10.1111/j.1747-0765.2006.00065.x
Yanai, Y., Toyota, K., and Okazaki, M. (2007). Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci. Plant Nutr. 53 (2), 181–188. doi:10.1111/j.1747-0765.2007.00123.x
Yang, D., Yunguo, L., Shaobo, L., Xixian, H., Zhongwu, L., Xiaofei, T., et al. (2017). Potential benefits of biochar in agricultural soils: A review. Pedosphere 27, 645–661. doi:10.1016/S1002-0160(17)60375-8
Yang, S., Xiao, Y. N., Sun, X., Ding, J., Jiang, Z., and Xu, J. (2019). Biochar improved rice yield and mitigated CH4 and N2O emissions from paddy field under controlled irrigation in the taihu lake region of China. Atmos. Environ. 200, 69–77. doi:10.1016/j.atmosenv.2018.12.003
Yang, X., Liu, J. H., McGrouther, K., Huang, H. G., Lu, K. P., Guo, X., et al. (2016). Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 23, 974–984. doi:10.1007/s11356-015-4233-0
Yao, C. X., Joseph, S., Li, L. Q., Pan, G. X., Lin, Y., Munroe, P., et al. (2015). Developing more effective enhanced biochar fertilisers for improvement of pepper yield and quality. Pedosphere 25, 703–712. doi:10.1016/S1002-0160(15)30051-5
Yin, D., Wang, X., Peng, B., Tan, C., and Ma, L. Q. (2017). Effect of biochar and Fe-biochar on Cd and as mobility and transfer in soil-rice system. Chemosphere 186, 928–937. doi:10.1016/j.chemosphere.2017.07.126
Yuan, H., Lu, T., Zhao, D., Huang, H., Noriyuki, K., and Chen, Y. (2013). Influence of temperature on product distribution and biochar properties by municipal sludge pyrolysis. J. Mater. Cycles Waste Manag. 15, 357–361. doi:10.1007/s10163-013-0126-9
Zhang, A., Liu, Y., Pan, G., Hussain, Q., Li, L., Zheng, J., et al. (2012). Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 351, 263–275. doi:10.1007/s11104-011-0957-x
Zhang, D., Pan, G., Wu, G., Kibue, G. W., Li, L., Zhang, X., et al. (2016b). Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rainfed low fertility inceptisol. Chemosphere 142, 106–113. doi:10.1016/j.chemosphere.2015.04.088
Zhang, H., Chen, C., Gray, E. M., Boyd, S. E., Yang, H., and Zhang, D. (2016a). Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 276, 1–6. doi:10.1016/j.geoderma.2016.04.020
Zhang, M., Riaz, M., Zhang, L., El-desouki, Z., and Jiang, C. (2019). Biochar induces changes to basic soil properties and bacterial communities of different soils to varying degrees at 25 mm rainfall: More effective on acidic soils. Front. Microbiol. 10, 1321. doi:10.3389/fmicb.2019.01321
Zhang, Q., Du, Z., Lou, Y., and He, X. (2015). A one-year short-term biochar application improved carbon accumulation in large macroaggregate fractions. Catena 127, 26–31. doi:10.1016/j.catena.2014.12.009
Zhang, Y. M., Wu, N., Zhou, G. Y., and Bao, W. K. (2005). Changes in enzyme activities of spruce (Picea balfouriana) forest soil as related to burning in the eastern Qinghai- Tibetan plateau. Appl. Soil Ecol. 30, 215–225. doi:10.1016/j.apsoil.2005.01.005
Zhao, R., Coles, N., Kong, Z., and Wu, J. (2015). Effects of aged and fresh biochars on soil acidity under different incubation conditions. Soil Tillage Res. 146, 133–138. doi:10.1016/j.still.2014.10.014
Zheng, H., Wang, Z., Deng, X., Herbert, S., and Xing, B. (2013). Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 206, 32–39. doi:10.1016/j.geoderma.2013.04.018
Zhu, Q. H., Peng, X. H., Huang, T. Q., Xie, Z. B., and Holden, N. M. (2014). Effect of biochar addition on maize growth and nitrogen use efficiency in acidic red soils. Pedosphere 24, 699–708. doi:10.1016/S1002-0160(14)60057-6
Keywords: biochar, soil fertility, crop productivity, heavy metal remediation, pyrolysed carbon
Citation: Premalatha RP, Poorna Bindu J, Nivetha E, Malarvizhi P, Manorama K, Parameswari E and Davamani V (2023) A review on biochar’s effect on soil properties and crop growth. Front. Energy Res. 11:1092637. doi: 10.3389/fenrg.2023.1092637
Received: 09 November 2022; Accepted: 20 June 2023;
Published: 30 June 2023.
Edited by:
Sunita K. Meena, Dr. Rajendra Prasad Central Agricultural University, IndiaReviewed by:
Muhammad Bilal Khan, Ayub Agriculture Research Institute, PakistanAndrés Calderín García, Federal Rural University of Rio de Janeiro, Brazil
Copyright © 2023 Premalatha, Poorna Bindu, Nivetha, Malarvizhi, Manorama, Parameswari and Davamani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. P. Premalatha, cHJlbWFsYXRoYS5ycEBpY2FyLmdvdi5pbg==
 R. P. Premalatha
R. P. Premalatha J. Poorna Bindu
J. Poorna Bindu E. Nivetha
E. Nivetha P. Malarvizhi
P. Malarvizhi K. Manorama
K. Manorama E. Parameswari5
E. Parameswari5 V. Davamani
V. Davamani