- 1Chemical, Metallurgical and Materials Engineering, Faculty of Engineering and Built Environment, Tshwane University of Technology, Pretoria, South Africa
- 2Center for Energy and Electric power, Electrical Engineering, Faculty of Engineering and Built Environment, Tshwane University of Technology, Pretoria, South Africa
- 3National Laser Center, Council for Scientific and Industrial Research, Pretoria, South Africa
- 4Metallurgical and Materials Engineering, Faculty of Engineering, Air Force Institute of Technology, Kaduna, Nigeria
Energy is a requisite factor for technological advancement and the economic development of any society. Currently, global energy demand and supply largely rely on fossil fuels. The use of fossil fuels as a source of energy has caused severe environmental pollution and global warming. To salvage the dire situation, research effort is geared toward the utilization of clean, renewable and sustainable energy sources and the hydrogen energy economy is among the most preferred choices. Hydrogen energy economy, which includes hydrogen production, storage and conversion has gained wide consideration as an ecofriendly future energy solution with a fuel cell as its conversion device. Fuel cells, especially, the proton exchange membrane category, present a promising technology that converts hydrogen directly into electricity with great efficiency and no hazardous emissions. Unfortunately, the current generation of proton exchange membrane fuel cells faces some drawbacks that prevent them from large-scale market adoption. These challenges include the high costs and durability concerns of catalyst materials. The main source of high cost in fuel cells is the platinum catalyst used in the electrodes, particularly at the cathode where the sluggish oxygen reduction reaction kinetics require high loading of precious metals. Many research efforts on proton exchange membrane fuel cells are directed to reduce the device cost by reducing or completely replacing the platinum metal loading using alternative low-cost materials with “platinum-like” catalytic behaviour while maintaining high power performance and durability. Consequently, this review attempts to highlight recent research efforts to replace platinum and carbon support with other cost-effective and durable materials in proton exchange membrane fuel cell electrocatalysts. Overview of promising materials such as alloy-based (binary, ternary, quaternary and high-entropy alloys), single atom and metal-free electrocatalysts were discussed, as the research areas are still in their infancy and have many open questions that need to be answered to gain insight into their intrinsic requirements that will inform the recommendation for outlook in selecting them as electrocatalysts for oxygen reduction reaction in proton exchange membrane fuel cell.
1 Introduction
The ability of any society to evolve technologically and develop economically depends on the availability and effective utilisation of energy. The current global energy consumption mainly relies on fossil fuels (Abbasi et al., 2022; Shamoon et al., 2022). This state of global energy demand and supply is not sustainable considering the growth in global population, fast exhaustion of fossil fuel deposits and the environmental effects of using fossil fuels on the fragile ecosystem (Bogdanov et al., 2021; Schwanitz and Wierling, 2022). In addition, the increasing rate of fossil fuel utilization threatens to destabilise the environment because of the worsening greenhouse gas emissions and global warming that might result in unprecedented changes to human lives if nothing is done to prevent it (Chen et al., 2022a; Icaza-Alvarez et al., 2022). To address the situation, research effort is focused on utilising clean, renewable and sustainable energy sources and the hydrogen energy economy is among the most preferred choices.
The idea of a hydrogen economy was initiated by John Bockris in the 1970s. It was a dream to generate hydrogen via water electrolysis and channel it through pipelines to factories, homes, and fuelling stations where it would be harnessed and converted to other forms of energy (Oliveira et al., 2021). However, many challenges still need to be overcome in hydrogen production, storage and conversion to realise Bockris’ dream of a global transition to a hydrogen economy. Such challenges include the high cost and poor reliability of the hydrogen energy conversion device known as the fuel cell. A fuel cell is an electrochemical device that converts chemical energy directly into electricity with great efficiency and no hazardous emissions (Wang and Jiang, 2017; Ioroi et al., 2019). It is a thermodynamic system that operates based on electrochemical reactions, which consumes reactants from external source (Rahaman and Islam, 2019). The fuel cell combines the best features of combustion engines and batteries. It can work continuously without any intermediate mechanical energy conversion if fuel is supplied constantly and also shows the characteristics of battery under load condition (Manoharan et al., 2019). Fuel cells are usually classified according to the electrolyte, fuel used, operating conditions, required load or the application for which they are used (Abdelkareem et al., 2021b). Diverse kinds of fuel cells are presented in Table 1 Although these fuel cells use different electrolytes and fuels, they all operate on a similar redox reaction principle and platinum (Pt) is the catalyst widely used for the cathodic and anodic redox reaction in proton exchange membrane fuel cell (PEMFC).
The PEMFC is promising for large-scale commercialisation because of its dynamic response, high efficiency, low operating temperature (60°C—80°C), high power density and quick start-up when compared with other fuel cells (Lucia, 2014; Jiao et al., 2021).
Despite the potential of PEMFC for widespread commercialisation, it needs to meet certain criteria about cost and reliability to gain end-user acceptance and compete favourably with other commercial energy conversion devices such as internal combustion engines and batteries (Wang et al., 2018a). The high cost of active electrocatalyst materials and their poor durability at PEMFC operation conditions are the major challenges facing the large-scale commercialisation of PEMFC (Borup et al., 2020). In addition, it has been reported that the membrane electrode assembly parameters have significant impact on the efficiency of the PEMFC stack (Majlan et al., 2018; Madheswaran and Jayakumar, 2021). Hence, improving the efficiency of PEMFC by rational selection of cost-effective materials is essential to achieve the hydrogen economy potential as an alternative to fossil fuel in electricity generation, fuel for vehicles and industrial processes (Egeland-Eriksen et al., 2021).
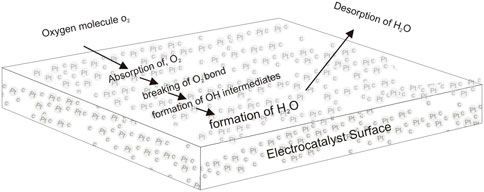
These could be achieved by deliberate attempt to improve the state-of-the-art electrocatalysts in PEMFC. The electrocatalyst is a vital component that performs a significant role in reaction kinetics involved in the effective functioning of PEMFC. It has to do with the surface adsorption of reactants, breaking the reactants bonds and formation of new bonds of intermediates and desorption of products (Figure 1) (Wacławek et al., 2018; Madheswaran and Jayakumar, 2021). The electrocatalyst in the PEMFC works as the cathode and anode, which conventionally uses Pt particles on carbon support (Wang et al., 2020b). Unfortunately, Pt-based electrocatalysts account for about half of the cost of the PEMFC stack and suffer degradation during prolonged PEMFC operation (Xie et al., 2020; Fan et al., 2022). The main degradation mechanisms have been identified as dissolution, migration and agglomeration of metal particles and carbon support corrosion that results in a loss in the electrochemical surface area (ECSA) (Wei et al., 2021b). In addition, Pt-based electrocatalysts are susceptible to carbon monoxide poisoning and requires highly active material loading at the cathode because of the cathodic sluggish oxygen reduction reaction kinetics. To address these challenges, two routes have been widely investigated to achieve low-cost electrocatalysts; which are lowering the loading of Pt and looking for substitutes (Ding et al., 2020b).
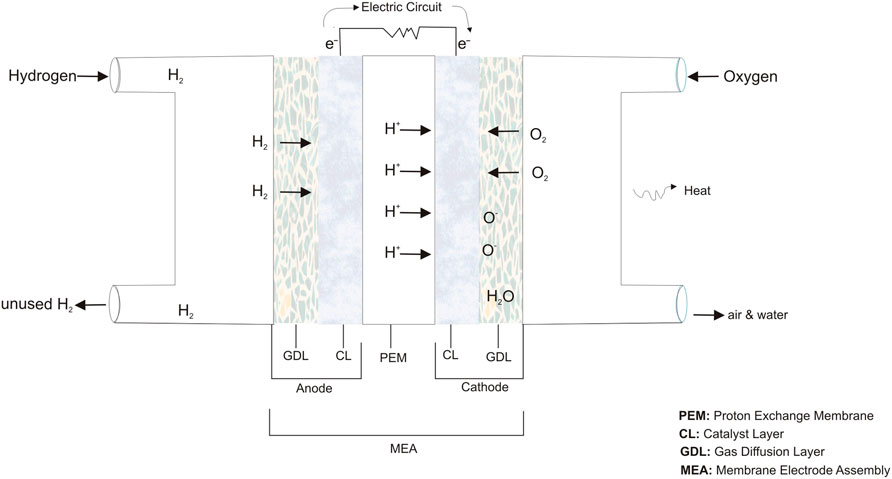
PEMFC energy conversion is achieved through hydrogen oxidation reaction (HOR) and oxygen reduction reaction (ORR) (Figure 2) (Cruz-Martínez et al., 2021). These reactions involve the transfer of charge (electrons) between an electrocatalyst and a chemical species. During the HOR, an external circuit transports electrons to the cathode while protons are transferred across the polymer exchange membrane. Meanwhile, ORR involves oxygen reduction with electrons and hydrogen protons at the cathode, leaving only water and heat as by-products (Cruz-Martínez et al., 2019). Research interest focus more on ORR because of its sluggish kinetics that is 4–6 times slower in order of magnitude when compared with HOR (Wang et al., 2018b; Wan et al., 2020).
The slow rate of ORR remains a major challenge in PEMFC (Kong et al., 2023). Significant progress has been made globally towards improving the-state-of-the-art electrocatalysts for ORR. As a result of these research efforts, commercial Pt/C remains the most widely used electrocatalyst among others because of its comprehensive evaluation and the superior activity of the Pt surface to other oxygen reducing surfaces (Ma et al., 2020a; Liu et al., 2022; Li et al., 2023). However, Pt high cost, scarcity and poor durability during prolong PEMFC operation hinder the widespread commercialisation of PEMFC, as the frequent cost of the replacement of the electrocatalyst significantly affects the overall cost of the fuel cell (Lv et al., 2019b; Meng et al., 2021; Xia, 2021). To this end, addressing the stability challenges, particularly at the cathode where the sluggish ORR kinetics require high loading of precious metal is imperative for its practical employment in PEMFC (Zhao et al., 2022). To overcome these challenges, research efforts are on-going to develop durable electrocatalysts that are highly active and cheap (Batchelor et al., 2019).
1.1 Pt/C electrocatalyst degradation mechanism
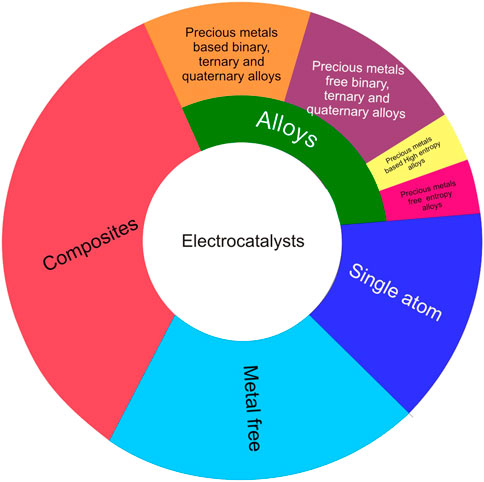
Pt particles dispersed on carbon support remain the most widely used commercial electrocatalyst for PEMFC (He et al., 2022). This is due to the advance conductivity, flexibility and wide range of operable potentials that Pt offers for PEMFC application (Abbas et al., 2020). Unfortunately, under PEMFC operation conditions, Pt dissolves and breaks up and the carbon support also corrodes leading to the deterioration of the performance of the fuel cell (Kregar et al., 2020). The major degradation mechanisms were identified as dissolution, migration and agglomeration of metal particles and carbon support corrosion that results in a loss in the ECSA which causes overall decrease in the performance of PEMFC (Wang et al., 2021a; Wei et al., 2021b). Several studies have been conducted to investigate the degradation mechanism of Pt/C in PEMFC (Weber et al., 2018; Labata et al., 2021; Fan et al., 2022). The studies were aimed at understanding the degradation mechanism of Pt-based electrocatalyst to help researchers in developing mitigation strategies that could significantly reduce the cost of PEMFC (Lopes et al., 2020; Hersbach et al., 2021). Different perspectives exist in the literature on the cause of Pt-based electrocatalyst disintegration. Some researchers argued that Pt disintegrate due to change in cell potential, other researchers asserted that the discrepancy in anodic and cathodic charges is the cause of the disintegration (Okonkwo et al., 2021). With the disparity in the potential ranges at which Pt disintegration occurs in PEMFC operation, factors such as particles size and morphology play important role in the disintegration of Pt (Lopes et al., 2020; Hussain et al., 2022). To address these challenges, promising strategies (Figure 3) such as alloying Pt with transition metals has been suggested as an effective method to reduce the usage of the scarce and expensive precious metal as electrocatalyst without loss in its performance (Bhoyate et al., 2023). In addition, the use of mixed phases has been recommended as an effective strategy to optimise electrocatalysts activity and stability, thereby encouraging the development of electrocatalysts from binary, ternary, quaternary and high entropy alloys (Li et al., 2020c; Prabhu et al., 2020; Li et al., 2021; Du et al., 2022). The desired electrocatalyst activity and stability optimisation in alloy electrocatalysts is achieved by alloy compositional, morphological and particles size control (Zhang et al., 2021a; Loffler et al., 2021).
It is noteworthy that other electrocatalyst design strategies such as Pt-free alloys, single atom and metal-free electrocatalysts have been investigated (Bhatt and Lee, 2020; Han et al., 2020; Zhang and Guan, 2020; Wei et al., 2021a; Muhyuddin et al., 2021). Efforts have also been directed toward the development of other durable catalyst-support materials. Consequently, this review summarises recent research efforts to enhance or replace Pt and carbon support with other cost-effective and durable materials in PEMFC electrocatalysts application (Table 2). Overview of design strategies in developing alternative electrocatalyst using other cost-effective and durable materials to replace platinum and carbon support are highlighted with the activity and stability of various electrocatalysts for oxygen reduction reaction in PEMFC.
2 An overview of materials used as electrocatalysts in PEMFC
Herein research progress on electrocatalyst design strategies to address the drawbacks associated with the state-of-the-art Pt/C electrocatalyst for PEMFC were summarised. Pt-based electrocatalyst were discussed followed by alloy electrocatalysts. Emerging electrocatalysts such as high-entropy alloys, single atom and metal-free electrocatalysts were also highlighted.
2.1 Platinum-based electrocatalysts
Pt/C electrocatalyst remains the state-of-the-art catalyst for ORR in PEMFC because of its effectiveness in ORR kinetics (Tian et al., 2017; Huang et al., 2021; Kong et al., 2021). However, due to its high cost and durability concerns, research to enhance its performance and reduce its high Pt particle loading are on-going (Shao et al., 2018; Lopes et al., 2020; Kishida et al., 2021). According to Etesami et al. (2021), there are three major research areas for Pt-based catalysts, which are 1) reducing the particle size of Pt and increasing its dispersion in the carbon support in order to increase its active sites surface area 2) producing Pt-based catalysts with particles surface oriented in a specific direction and 3) dispersing Pt into other metals especially transition metals or modified carbon supports to form alloys or mixtures. Efforts have also been directed toward the development of other durable catalyst-support materials such as transition metal-based compounds (carbides, nitrides, oxides and chalcogenides) (Shang et al., 2020), carbon-based materials (carbon nanotube and graphene) (Liu et al., 2018), nitrogen doped carbon (Chen et al., 2021) and metal-organic-framework to address the carbon support corrosion challenge. On the other hand, Mukherjee et al. (2022) asserted that electrocatalysts that use only micro-sized Pt particles as their actives sites are no longer the state-of-the-art catalysts for fuel cell application. The authors claimed that high cost of electrocatalysts due to unrealistic Pt loading requirements and Pt particle susceptibility to carbon monoxide poisoning are the key reasons for the assertion. Along this line, research have evolved from using micro sized Pt on carbon supports to using Pt nanoparticles, Pt nanowires, introduction of porosity to increase catalysts surface area and bimetallic Pt electrocatalysts with other metals (Table 3) (Gilroy et al., 2016; Li and Sun, 2019; Shao et al., 2019; Xiao et al., 2021a).
2.2 Platinum-free electrocatalysts
To reduce the overall cost of PEMFC, researchers focus on the reduction or substitution of Pt in Pt-based electrocatalyst. Thus, research efforts have been directed towards the development of non-Pt-based electrocatalysts. Other Pt group metal-based electrocatalysts such as Pd-C, Ir-C, Rh-C electrocatalysts have been reported (Wang et al., 2022). Several Pt-free electrocatalysts have been developed using non-precious metals because of their low-cost, availability and abundance in nature (Etesami et al., 2021). Non-precious metal-based electrocatalysts have been widely investigated because of their excellent catalytic performance, durability and cost-effectiveness (Etesami et al., 2020). Such electrocatalysts include transition metal-nitrogen-carbon electrocatalysts, nitrides, carbides, chalcogenides and transition metal oxides (Gewirth et al., 2018; Xiao et al., 2021a). To achieve a Pt metal group-free electrocatalyst for PEMFC application Sanad et al. (2021) synthesised a non-precious metals Co-Cu metal organic framework bimetallic electrocatalyst by low-temperature hydrothermal strategy. The catalytic activity of the electrocatalyst was tuned by varying Co/Cu molar ratios. It was observed that the metal organic framework bimetallic electrocatalyst outperformed the electrocatalytic activity of commercial Pt–C catalyst for ORR in alkaline environment. It exhibited onset potential of 1.06V, half wave potential of 0.95 V and electrochemical stability of 30 mV after 1000 ORR cycles in 1.0 M sodium hydroxide solution. Chandran et al. (2018) adopted a single-step synthesis approach to develop Pd-Co alloy supported on reduced graphene oxide doped with nitrogen. The ORR activity of the electrocatalyst proceeded through the four-electron reaction pathway. The electrocatalyst showed 1.6 times enhanced mass activity when compared to the Pd-reduced graphene oxide doped with nitrogen. The electrocatalyst also recorded of power density of 68 mWcm– 2 with metal loading of 0.5 mg cm– 2 at 60°C without any back pressure.
2.3 Alloy-based electrocatalysts
2.3.1 Bimetallic alloy electrocatalysts
Research findings have shown that alloying Pt with the d-block metals to form a bimetallic electrocatalyst could positively influence the stability and activity of Pt-C electrocatalyst in ORR of PEMFC (Seh et al., 2017; Sui et al., 2017). Literature also showed that the addition of a second metal to Pt-based electrocatalyst can prevent the adsorption of carbon-monoxide on the electrocatalyst’s surface (Molochas and Tsiakaras, 2021; Lee et al., 2022b). In addition, such secondary metal addition could re-expose blocked sites on a poisoned Pt surface by oxidising the carbon monoxide thereby speeding up ORR kinetics (Zhang et al., 2021b; Lu and Elam, 2022). Owing to these characteristics, bimetallic electrocatalysts have been reported to exhibit comparatively better efficiency than Pt-C electrocatalyst due to their highly exposed surfaces (Bai, 2018; Huang et al., 2018; Wang and Spendelow, 2021). Guterman et al. (2018) prepared CuxPt-C electrocatalyst by successive deposition of copper and Pt on carbon support. The electrochemical performance of the CuxPt-C electrocatalyst was studied at ambient temperature in a three-electrode electrochemical cell with the help of CV using a rotating-disk as the working electrode. The results obtained were compared with that of commercial Pt-C catalyst. The study showed that the bimetallic electrocatalyst exhibited a combination of higher stability and mass activity values when compared with the commercial Pt-C catalyst with the same Pt loading. Ying et al. (2018) also developed a metal-organic framework Pt-Co bimetallic nanoparticles supported by hollow porous carbon capsules that were doped with nitrogen as highly active and durable electrocatalyst for ORR. The bimetallic catalyst was synthesised by wet chemistry method and was found to demonstrate outstanding ORR performance, with a mass activity that was 5.5 and 13.5 times better than those of commercial Pt/C and Pt/black catalysts respectively. Moreover, the catalyst exhibited better durability in terms of ECSA and mass activity when compared with commercial catalysts. The exceptional ORR performance of the catalyst was ascribed to the large surface area of bimetallic Pt-Co nanoparticles and hollow porous structure of nitrogen-doped carbon capsules. Although Pt has been widely alloyed with many transition metals and of recent lanthanides to form bimetallic electrocatalysts, they have not gain wide adoption for commercial application in fuel cell (Martínez-Hincapié and Čolić, 2022). However, it was reported that Pt-Co bimetallic electrocatalyst was recently use in commercial electric vehicle (Toyota Mirai) (Wang and Spendelow, 2021). The poor commercial patronage of Pt-based bimetallic electrocatalysts could be attributed to their susceptibility to poisoning by formic acid electrooxidation and dissolution into strong acids in working electrolytes, which lead to the loss of integrity of electrocatalysts and result in deterioration of catalytic performance. As a result, different electrochemical reaction pathways have different electronic structure requirements (Zhang et al., 2018; Mukherjee et al., 2022).
2.3.2 Trimetallic/ternary alloy electrocatalysts
To benefit from synergistic effect that can result from using three elements in trimetallic alloys, researchers have attempted to develop ternary alloy electrocatalysts for ORR in PEMFC (Wang et al., 2019c; Wang et al., 2020a; Zhu et al., 2020a). The introduction of a third element has proven to improve the lifespan of the parent bimetallic electrocatalyst at PEMFC operating conditions (Zhang et al., 2017). Hu et al. (2021) synthesised ternary platinum-based electrocatalyst using co-doping strategy. Cu and Co were co-doped into Pt-C to modulate the electronic structure of Pt in order to weaken the adsorption of deoxygenated species by Pt thereby enhancing the ORR kinetics. It was found that the trimetallic electrocatalyst exhibited better mass activity of 0.52 A mgpt−1 and power density 1.15 Wcm−2. Moreover, the mass activity of the ternary electrocatalyst only reduced by 8.3% after 30,000 cycles indicating comparatively good durability. Xiao et al. (2021b) reported a facile synthesis of carbon supported Pt-Cu-Fe nanoparticles trimetallic electrocatalyst for ORR in PEMFC. The authors found that the trimetallic electrocatalyst recorded a significant 0.99 A mgpt−1 ORR activity at 0.9V potential and enhanced stability, losing only 7.6% of mass activity after 5000 durability cycles. Ma et al. (2022) developed a Pt-Ru-Te ternary electrocatalyst with only 11 at% of Pt for HOR in fuel cell. The electrocatalyst showed a current density of 30.6 mAcm– 2 at 50 mV and exchange current density of 0.426 mAcm– 2. Furthermore, the electrocatalyst exhibited excellent stability with 5% loss in activity after 2000 durability circle.
The literature showed improvement in performance of ternary electrocatalysts in fuel cell application over their bimetallic counterparts, establishing the significance of ternary platinum based electrocatalyst in improving electrocatalytic performance. However, ternary electrocatalysts are also faced with the challenges of metal dissolution, agglomeration and carbon support corrosion. In addition, it is uncertain if the electrocatalyst will be able to replicate their excellent experimental activities in actual fuel cell operating conditions (Mukherjee et al., 2022).
2.3.3 Quaternary alloy electrocatalysts
Quaternary alloy has the potential to offer additional options that could improve electrocatalytic performance and durability than binary and ternary alloy electrocatalysts. However, it remains the least reported alloy-based electrocatalysts in literature (Du et al., 2022). Wang et al. (2019a) developed a quaternary structurally ordered Pt(Fe,Co,Ni) alloy electrocatalyst with equal proportion of constituent elements via a facile synthesis method that requires spray dehydration on a solid surface followed by annealing procedure. The electrocatalyst showed improved electrocatalytic performance towards ORR with mass activity 6.6 fold higher than that of commercial Pt/C with only 17% attenuation after 10,000 potential cycles at 0.9 V. Hornberger et al. (2022) synthesised an octahedral quaternary PtNi(RhMo) alloy electrocatalyst. The alloy electrocatalyst was prepared to control the shape of PtNi by co-doping it with Rh and Mo using solvothermal process. The electrocatalysts exhibited remarkable mass activity of 1.53 mgpt – 1.
2.3.4 High-entropy alloy electrocatalysts
For centuries, alloying has been employed to impart desired material properties. It usually entails the addition of comparatively small quantities of secondary elements to the parent metal. However, for the past two decades, a new alloying approach has been in vogue that entails the combination of several key elements in high concentrations to create novel materials called high entropy alloys (HEAs) (George et al., 2019). HEAs have two definitions that are based on composition and configuration entropy, respectively. They are alloys that contain a minimum of five primary elements having respective atomic weight percentage between 5 and 35 based on composition. The entropic definition states that HEAs are alloys having configurational entropies at a random state greater than 1.5R, whether they are single or multiphase at ambient temperature; where R is gas constant (Yeh and Lin, 2018). HEAs have been recently used in catalysis because of their exceptional benefits, which include high tolerance, complex surface and adjustable composition (Li and Chen, 2021). They have gained wide acceptance as catalysts in electrochemical reactions due to their ability to catalyse different reactions. Such reactions include but are not limited to nitrogen reduction reaction (NRR), alcohol oxidation reaction (AOR), oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), oxygen evolution reaction (OER) and carbon dioxide reduction reaction (CO2 RR) (Zhang et al., 2022). HEA catalysts characteristics that distinguished them from other catalysts have been highlighted as their multiple combinations of elements located next to each other, which results in tailorable active sites. In addition, it was emphasised that their non-typical electrochemical behaviour made them a distinctive class of catalyst materials that are promising as a better alternative to other catalysts (Löffler et al., 2019). The application of HEAs in ORR is being researched to improve the cathodic sluggish kinetics that limits the reliability of PEMFC. This is because HEAs emerged as materials that are capable of overcoming the limitation of Pt and Pt group metals (PGM) catalysts (Martínez-Hincapié and Čolić, 2022). Wu et al. (2020) studied the catalytic behaviour of the six PGM as self-supported HEA catalyst synthesised by wet chemistry method for ethanol oxidation reaction and found that the HEA exhibited variety of adsorption sites on its surface, which can catalyse many complex reactions. The PGM HEA catalyst’s intrinsic mass activity was also compared with those of commercial palladium-C, palladium-black and Pt-C catalysts and found to be 2.5, 6.1 and 12.8 times higher than the intrinsic mass activity of the commercial catalysts, respectively. Lee et al. (2022a) synthesised IrPtPdRuRh HEA electrocatalyst by plasma ionic liquid reduction and showed excellent hydrogen evolution reaction catalytic performance with overpotential of 60 mV at a current density of 10 mAcm−2. The electrocatalyst also recorded a Tafel slope of 42 mV dec−1 in alkaline electrolyte and demonstrated good stability for 6 h at a high constant current density of 100 mAcm−2 without appreciable decay. The HEA nanoparticles were deposited on a carbon support. Qiu et al. (2019) synthesised senary AlNiCuPtPdAu octonary AlNiCuPtPdAuCoFe and senary all-non-noble metal AlNiCuMoCoFe nanoporous HEAs using a route that combined bulk melting and fast cooling followed by dealloying. The nanoporous HEA with low Pt loading exhibited 10 times mass activity when compared with commercial Pt-C catalyst for ORR and maintained 92.5% of its initial activity after 100,000 electrochemical cycles. It was also discovered that, unlike binary alloy system, HEAs exhibited high stability under electrochemical cycling conditions. An attribute that made them promising electrocatalysts for PEMFC. Furthermore, to explore catalytic potentials of PGM–free HEAs in ORR, Nandan et al. (2022) reported a facile method that combines wet chemistry and pyrolysis to synthesise FeCoNiMnCr HEA nanoparticles grafted on nitrogen-doped carbon nanotubes. The electrocatalyst designed for electrochemical ORR utilised the synergy between nitrogen-doped carbon nanotubes and HEA nanoparticles to promote improved performance in the ohmic polarization region of fuel cells when compared with commercial Pt-C electrocatalyst. Moreover, the FeCoNiMnCr-based catalysts exhibited better ORR performance compared to various newly reported transition metal-based conventional catalysts. In another research, Liu et al. (2021) synthesised CoCrFeMnNi HEA nanoparticle-activated carbon nanocomposites electrocatalyst. The electrocatalyst was fabricated by impregnation–adsorption method of precursor salt solution followed by calcination. The HEA-activated carbon electrocatalyst performed excellently in the degradation of methylene blue at a comparable rate with those of other catalysts. The exceptional efficiency was because of the coupling effects of the solid-solution structure of HEA nanoparticles and the large specific surface area and considerable reaction channels of the activated carbon. In addition, HEA nanoparticles embedded in distinctive porous structure accelerated the mass transport and the electron transfer as nanoscale galvanic cells in the active bond splitting of methylene blue.
Most conventional alloys are made up of a single element with various alloying elements added to improve the properties of the principal element, forming an alloy family based on the principal element (Tsai and Yeh, 2014). HEAs were thought to be complex because they contained more than four primary elements and complex phase diagram systems that are often unavailable. As a result, the majority of HEAs earlier reported were created using the traditional trial-and-error method (Zhang et al., 2012). However, as the design of high entropy alloys progresses, several design routes have emerged which include the use of alloys design principles of materials science, building on promising binary or ternary alloy systems, the use of combinatorial material synthesis technique, Taguchi optimisation method and material science computational methods such as finite element, molecular dynamics, simulation, phase computation (PHACOMP) and calculation of phase diagram (CALPHAD) (Yeh, 2013). Wen et al. (2019) used material design strategy that combines machine learning model and experimental algorithms to determine high entropy alloys with high hardness in AlCoCrCuFeNi alloy system. Several alloys with hardness values that are 10% higher than the best original data set were fabricated using only seven experiments. In another study, Floriano et al. (2020) designed equiatomic TiZrNbFeNi and non-equiatomic Ti20Zr20Nb5Fe40Ni15 high entropy alloys via thermodynamic calculations using CALPHAD. The alloys were fabricated by arc melting technique and showed small amount of cubic phases and C14 laves phases with (Zr,Ti)1, (Fe,Ni,Nb, Ti)2 constitution.
2.3.4.1 High-entropy alloy-based composites
HEA composites are emerging advanced materials that are produced by dispersing reinforcing phases (usually ceramics, whiskers or fibers) in multi-principal element metal matrix to improve their desired properties from the standpoint of dispersion strengthening mechanism (Liu et al., 2019; Zhu et al., 2020b; Wang et al., 2021b; Wang et al., 2021c). The reinforcing phase may be coated to prevent its chemical reaction with the matrix (Sharma et al., 2020). It is noteworthy that the use of HEAs as matrix in metal-based composites is still in the early stage (Sun et al., 2022). HEAs usually contain multi-principal elements in high concentration, thereby hindering their industrial application as bulk materials because of their higher cost compared to the conventional alloys (Fu et al., 2017; Wang et al., 2021b). However, their application as coatings have received considerable research interest because of the wide field of application and relatively cheaper cost of thin films (Li et al., 2018c). However, the hardness of HEAs coatings is usually relatively low, hence, their application as coatings requires enhanced hardness and wear resistance, which could be achieved by the addition of hard phases (Wang et al., 2021b; Guan et al., 2021). Wang et al. (2021b) prepared a HEA composite coating from titanium powder, C3N4 powder and Cr20Cu20Fe20Ni20Al20 by plasma transfer arc cladding technique and studied the hardness, wear properties and microstructural features of the HEA composite coating using microhardness tester, dry wear test multifunctional tribometer, SEM, TEM and EDS. The results showed that the microhardness of the composite coating was 3.33 and 127.4 times higher than those of the Q235 steel substrate and the unreinforced HEA. The wear resistance of the composite coatings was 3.03 and 8.06 times greater than the monolithic HEA and the Q235 steel substrate. The coating exhibited good interfacial bonding with the substrate. Microstructural examination revealed that the coating contains body-centred cubic matrix grains with intergranular cuboidal nanoprecipitates that have face-centred cubic Ti(C, N) particles distributed along its grain boundary and a small quantity of face-centred cubic Cu–rich phase around the intergranular nanoprecipitate. A core-shell structure was observed in the nanoprecipitates, with the core rich in nitrogen and the shell found to be carbon-rich. Along the same line, Zhu et al. (2020b) developed TiN and Al2O3 reinforced CoCrFeNiMn HEA composite through the plasma cladding method. The reinforcements were prepared from high purity commercial Al, TiO2 and BN precursors. The microstructure, chemical composition, hardness and wear properties were determined using field emission scanning electron microscopy, EDS, XRD, Vicker hardness tester and multi-functional tribometer. The microstructural examination revealed that the as-developed coating has a single face-centred cubic phase with TiN–Al2O3 as crystal dendrites associated with the Cr-B rich interdendritic phase. The hardness and wear resistance of the HEA composite coating were compared with those of the pure HEA and found to be 17.6% and 12.5% better. It was found that the reinforcing phases play significant roles in restricting the adhesive wear and encouraged the steady-state friction of the HEA composite coating throughout the sliding process. Cui et al. (2022) produced a corrosion resistance CeO2 reinforced FeCoNiCrMo HEA composite coating by laser cladding technique. The composite coating was developed for TC4 titanium alloy surface coating. The morphology, microhardness and corrosion resistance of the composite coating were investigated using XRD, EDS, SEM, Vickers hardness tester and electrochemical workstation. The result revealed that the introduction of the CeO2 powder to the HEA matrix reduced the coating sensitivity to crack. In addition, the introduction of the reinforcing phase to the HEA matrix did not change its body-centred cubic phase structure. Moreover, the microstructural examination showed that the CeO2 was evenly distributed at the grain boundary, thereby refining the grains. This phenomenon improved the strength and toughness of the HEA composite coating. Due to this improvement, the microhardness of the coating increased 2.7 times than that of the TC4 titanium alloy substrate. The corrosion behaviour of the substrate, pure HEA and composite coating were examined, and it was found that corrosion products were deposited on the TC4 titanium substrate. Pitting corrosion was observed in the pure HEA, however, the addition of CeO2 enhanced the formation of higher density of stable passive film that significantly inhibited the pitting corrosion.
The use of HEA composites as coatings has been considerably explored, however, their application for catalytic purposes which are equally a surface phenomenon have not been well investigated. Hence, the authors of this review are currently investigating the potentials of HEA composites as self-supported electrocatalysts for ORR in PEMFC.
Table 4 summarises alloy-based electrocatalysts and their composites.
2.4 Single-atom electrocatalysts
This is a novel brand of electrocatalyst represented by the acronym M-N-C, where M is usually a transition metal and N-C is carbonaceous material doped with nitrogen (Xu et al., 2018). The electrocatalyst has shown promising optimum atomic efficiency, outstanding intrinsic activity, high electrical conductivity, large surface area, well-defined structure with composition and configuration that can serve as an alternative substitute to the pricy precious metal-based electrocatalyst for ORR in PEMFC (Han et al., 2020; Zhang et al., 2020; Zhang and Guan, 2020; Chen et al., 2021). Single-atom electrocatalyst is synthesised by the pyrolysis of transition metal with doped-carbonaceous materials, a process that covalently anchors well dispersed metal particles at an atomic scale on suitable support. The resulting electrocatalyst thereby exhibit exceptional activity and stability for specific reaction (Hou et al., 2020a). The multiscale tunability of a single atom electrocatalyst facilitates increasing active sites density with enhanced activity, stability, anti-poisoning properties and ultra-high affinity for oxygen (Zhu et al., 2018). (Yang et al., 2019) synthesised two-dimensional conjugated single atom electrocatalyst consisting of 10 wt% iron and 0.73 wt% cobalt atoms anchored on phthalocyanine macrocycles. The electrocatalyst was synthesised by pyrolysis-free one step ball milling of polyphthalocyanine and showed excellent ORR mass activity of 47 mAmg – 1 that was 6.4 times superior to that of commercial Pt/C with no appreciable loss in stability after 100 h of operation. Ding et al. (2020a) designed and synthesised an atomically dispersed Fe-N4/C single atom electrocatalyst via an organic solution esterification process followed by melamine treatment to introduce nitrogen and restrict the migration of metal particles. ORR test was conducted on the single atom electrocatalyst and revealed a half-wave potential of 0.78 V. The electrocatalyst also showed a 17% loss in current density after 7 h of continuous polarization in acidic condition. (Xu et al., 2021). developed a single atom copper anchored on graphite foam doped with sulphur and nitrogen synthesised through underpotential deposition strategy. The single atom electrocatalyst showed outstanding ORR activity recording half wave potential of 0.862 V with calculated mass activity of 5.71 AmgCu – 1. The stability test revealed that the electrocatalyst retains 98% of its initial current density after 20,000s under continuous potential of 0.85 V. Chen et al. (2020) reported a single atom tungsten dispersed on nitrogen doped carbon nanosheet produced by modulated pyrolysis method. The electrocatalyst showed notable electrocatalytic activity with half wave potential of 0.88 V and mass activity of 0.63 Amg−1 at 0.9 V. The electrocatalyst only loss 13.9% of its mass activity after 10,000 sweeping cycles of cyclic voltammetry durability test. Wan et al. (2019) developed a concave Fe–N–C single-atom catalyst possessing an enhanced external surface area and porosity. The Concave-shaped Fe–N–C electrocatalyst was synthesised by preheating mesoporous SiO2-coated ZIF-8 (Z8) metal-organic framework at 650°C under argon-controlled atmosphere, the silica layer was etched off to form a concave-shaped host having a negative zeta potential and larger micropores. The electrocatalysts displayed high current density of 0.047 A cm – 2 at 0.88 V under 1.0 bar H2–O2 that stems from the high density of active sites, which was achieved by exposing Fe–N4 moieties and enhanced mass transportation of the mesoporous electrocatalyst. Furthermore, Zhu et al. (2021) reported a nitrogen doped carbon embedded rare-earth single-cerium-atom metal–organic framework electrocatalyst. The electrocatalyst was synthesised via hard–template approach and showed comparable ORR activity but inadequate stability to that of commercial Pt–C catalyst in PEMFC application. The result of the study revealed half-wave potential of 0.862 V in ORR and 0.525 W cm – 2 power density under 2.0 bar hydrogen–oxygen in fuel cell test.
2.5 Metal-free electrocatalysts
Heteroatom-doped carbon materials have demonstrated excellent ORR activity that is comparable or even better than that of commercial Pt-C electrocatalyst because of their extraordinary large specific surface area, good electrical conductivity and excellent durability under unfavourable conditions (Hu and Dai, 2019; Ma et al., 2019). Research progress have been made in developing highly stable and durable heteroatom-doped advanced carbon electrocatalysts such as iodine-doped graphene (Marinoiu et al., 2018), boron-doped carbon nanotube or graphene (Sawant et al., 2022), sulphur-doped graphene (Garino et al., 2021) and phosphorus-doped graphite layers (Shimoyama et al., 2015). It was also observed that doping advanced carbon materials with more than one heteroatom showed better ORR activity (Gao et al., 2019). For instance, Sun et al. (2018) co-doped carbon nanomaterial with boron and nitrogen to produce a very active, stable and inexpensive metal-free bifunctional electrocatalyst for ORR and OER. The catalyst was prepared by pyrolysis of precursors under ammonia atmosphere and exhibited excellent activity and stability for both ORR and OER. The catalyst showed an onset potential of 0.98 V for ORR in alkaline medium, which is similar to that of Pt-C catalysts. On the other hand, the onset potential recorded in acidic medium was 0.81 V for a 4–electron transfer process. The outstanding performance of the catalyst was attributed to the joint positive effect of rich carbon defects and the heteroatomic co-dopants. Duraisamy et al. (2022) synthesised a nitrogen-sulphur dual heteroatom doped mesoporous carbon electrocatalyst using 2D amorphous silica as support material and l-cysteine as nitrogen and sulphur precursor. The electrocatalyst showed high concentration of defective sites on the surface of the mesoporous carbon that favours improved ORR performance with onset potential of 0.78 V, half-wave potential of 0.68 V and current density of 2.8 mAcm– 2. Li et al. (2020a) co-doped porous carbon with nitrogen and sulphur for ORR and CO2RR catalytic applications. The electrocatalyst was prepared by pyrolysis of glucosamine hydrochloride and thiocyanuric acid precursors using hard silica dioxide templates. The co-doped carbon electrocatalyst showed enhanced activity and selectivity with the porous structure exposing abundant active sites to reaction species which resulted in increased activity of the electrocatalyst. The authors reported that the electrocatalyst exhibited excellent ORR activities in both acidic and alkaline media and is suitable for PEMFC application.
Despite the significant research progress in developing advanced carbon based electrocatalysts, the fundamental understanding of the doping effects and structural diversity of such catalysts remain unclear, particularly in terms of the molecular structures of active sites and the specific doping effects that control electrocatalytic reactions (Zhu et al., 2020c; Wu et al., 2021; Chattopadhyay et al., 2022). Many factors hinder the understanding of these phenomena, which include the sophistication of reactions at electrochemical interfaces; a lack of efficient strategies for precisely controlling the structures of active sites for directing catalytic reactions; and troubles inspecting and understanding the electrode-electrolyte interfaces through direct in situ observations. (Hu and Dai, 2019; Lai et al., 2022).
2.6 Catalyst supports
Most electrocatalysts used in fuel cell rely on support materials for mass transfer and water management (Ziv et al., 2018). Loading the electrocatalyst on high surface area support enhanced its catalytic activity and durability by increasing its electrochemical surface area (Hou et al., 2020b). Many carbon-based materials such as graphene, carbon nanotube, carbon black and mesoporous carbon have been used as catalyst supports due to their high surface area and electrical conductivity (Qiao et al., 2019). However, due to carbon corrosion that leads to the loss of ECSA, other electrocatalysts support materials such as transition metal oxides, carbides and nitrides have been adopted because of their excellent stability in harsh conditions (Samad et al., 2018). The presence of surface functional groups that promote catalyst support interaction, corrosion resistance, low surface poisoning, electrochemical stability, high electrical conductivity, a large surface area and a porous structure that improves the triple phase boundary are the characteristics of a good catalyst support material (Fashedemi et al., 2022). Asset et al. (2018) attempted to improve the ORR kinetics by investigating the effect of carbon supports' structure, texture and chemistry on the morphological properties, stability, and ORR activity of porous hollow Pt-Ni nanoparticles carbon black, carbon nanotubes, graphene nanosheets, and carbon xerogel. The nanomaterials had varying degrees of graphitisation, surface areas, and surface functionalisation. The inner and outer diameters of the supported porous hollow Pt-Ni/C nanoparticles were found to decrease as the surface area of the carbon mesopore increased. Despite their differences, the nanomaterials demonstrated comparable morphological properties and electrocatalytic activities for the ORR. The simulated electrochemical potential of PEMFC was used to evaluate the stability of the synthesised electrocatalysts. Identical location transition electron microscopy (IL-TEM) and electrochemical method showed that degradation in carbon nanotube, carbon xerogel and graphene nanosheet were by carbon corrosion to carbon dioxide while carbon black showed carbon surface oxidation to carbon monoxide. Souza et al. (2018) added niobium pentoxide (Nb2O5) and tungsten carbide (WC) separately to Pt/C electrocatalyst by impregnation method followed by heat treatment to modify its carbon content for improved stability. Both modified electrocatalysts were analysed by X-ray diffraction (XRD), energy dispersive X-ray spectroscopy (EDS), IL-TEM and cyclic voltammetry (CV). Results of the analyses were compared with those of commercial Pt/C electrocatalyst. The authors found two phenomena that led to ECSA loss in the electrocatalysts; which are Pt particle agglomeration and the loss of catalyst materials due to the degradation of carbon support. It was observed that WC addition increased the electrocatalyst particles' corrosion and detachment, however, the addition of Nb2O5 was more effective in improving the Pt/C electrocatalyst stability. More so, Yuan et al. (2022) synthesised a dandelion-like structured titanium nitride nanospheres as a non-carbon-based catalyst support for Pt nanoparticles, which were deposited on the dendritic structured titanium nitride by an electrochemical pulse deposition method. The catalyst recorded ORR mass and specific activity of 0.44 mA g – 1 and 0.33 mA cm – 2 at 0.9 V. At similar test conditions, the catalysts demonstrated superior stability to the commercial Pt/C catalysts recording 61% of the initial activity after 3000 repeated runs. In another study, Islam et al. (2019) attempted to address the carbon corrosion problem by developing a silica-coated carbon nanofibers catalyst support for Pt particles. Platelet-type carbon nanofibers were uniformly coated with silica using the hydrolysis method. Thereafter, the Pt was then deposited on the silica coating rather than carbon nanofibers. Accelerated degradation test was conducted on the silica-coated carbon nanofibers Pt catalyst and the result showed that the silica coated catalyst was more durable than Pt/C nanofibers catalyst under potential cycling. After 30,000 potential cycles, it was observed that silica coated carbon nanofiber catalyst lost 11% of its ECSA, while the loss of ECSA recorded by its uncoated Pt/C nanofiber counterpart was 21%.
3 Conclusion and recommendation
This review clearly showed that various authors asserted that the state-of-the-art Pt/C electrocatalyst typically impose cost ineffectiveness on PEMFC. In addition, Pt/C electrocatalyst suffers from rapid degradation during prolonged PEMFC operation, thereby, posing significant reliability challenge of the device. The rapid degradation is attributed to the dissolution, migration or aggregation of Pt as well as the carbon support corrosion leading to ECSA loss of the electrocatalysts and the eventual deterioration of electrocatalyst’s activity during long-term operation. Many studies have been conducted to unveil other low-cost materials that can serve as active catalyst constituents to augment or replace the precious metal loading in the state-of-the-art electrocatalyst used in PEMFC. Efforts have also been made to modify or replace the carbon support with other corrosion resistance and highly stable materials. In the light of these, the following recommendations are made for further research in various emerging electrocatalysts:
1. Bimetallic, ternary and quarterly alloys electrocatalysts serve a pivotal role as emerging electrocatalysts for ORR in PEMFC. Significant progress has been made in the fabrication and characterisation of these alloys. However, the effective control of their composition, morphology and particle sizes could pave a way for their commercial application in PEMFC. An understanding of the tunability of elemental composition, morphology and particles size could be used to control these alloys’ microstructures thereby positively influencing their activity and stability towards ORR in PEMFC. Tuning alloy electrocatalyst composition has significant effect on its ORR activity and stability. Consequently, effect of elemental composition and ratios on various alloy electrocatalyst could be investigated. Some researchers have asserted that the ORR mass activity of alloy-based electrocatalyst is inversely proportional to its particle size, therefore, advancing design strategy to fabricate nanostructured alloy electrocatalysts could expose more active sites thereby improving catalytic mass activity. To investigate the assertion that alloy electrocatalyst activity has direct correlation to its particles shape, the effect of different particle shapes on various alloy electrocatalyst could be studied. In addition, considering a preferred crystallographic orientation for alloy particles in electrocatalyst can be a promising strategy for achieving its high performance for ORR in PEMFC.
2. HEA electrocatalysts have been suggested to be promising candidates that will address the existing limitations in the state-of-the-art electrocatalyst. However, their complex catalysts' structure-activity relationship still needs to be unravelled. In addition, only a small portion of the compositional space of HEAs has been investigated, therefore, there exist a vast possibility of HEA electrocatalysts to be explored. Understanding elemental interactions in HEAs is also necessary in order to predict suitable elemental combinations for PEMFC catalytic application. Research is required to determine the compositional limits of elemental choices needed for specific phase formation in HEA electrocatalysts. The effect of each element on the stability of HEA electrocatalysts need to be studied, as well as how to tune each element to achieve physical or chemical stability. Furthermore, the synergistic interactions between HEA and emerging electrocatalyst support materials can be investigated by developing their self-supported HEA-based composite electrocatalysts. More so, heteroatoms such as nitrogen, boron, sulphur and phosphorus could be introduced into the interstices of transition metals based high entropy alloys to offer the advantages of a self-supported electrocatalysts with surface that enhances accelerated electron transfer, high activity and stability that can meet PEMFC performance requirements at low cost.
3. Single atom electrocatalysts have the potential of offering high density of active sites that could speed up the sluggish kinetics of ORR in PEMFC. However, the research progress of this emerging electrocatalyst still needs to be consolidated in the areas of its synthesis and characterisation to achieve its commercial application in PEMFC. Synthesis of single atom electrocatalysts require expensive precursors and equipment, hence, there is a need to find alternative low-cost precursors and facile synthesis method for the large-scale commercialisation of the electrocatalyst. In addition, developing porous support materials that will enhance accessibility to single atom actives sites could greatly enhance the electrocatalysts activity and stability. More so, the support materials should possess high conductivity and corrosion resistance to address the undesired Fenton reaction that characterises single atom electrocatalyst in acidic medium. Designing and synthesising single atom electrocatalyst with dual adjacent metal atoms could result in synergistic effect that significantly improve the ORR activity and stability. Lastly, there is a need for in situ characterisation techniques for single atom electrocatalyst to identify and understand the central metal interactions with oxygen containing species at electrocatalyst working potential conditions.
4. The catalytic properties of metal-free electrocatalyst is based on doping carbonaceous materials with heteroatoms which serve as defects that enhance promising catalytic performance. Developing metal-free electrocatalyst with hierarchical porous structure could be beneficial in providing multiple active sites, however, there is a need for facile fabrication techniques for this structure due to the uncontrollability of the pyrolysis process currently used in its synthesis. Further research is required to understand the nature of the active sites in the emerging electrocatalyst to facilitate its future large-scale commercialisation and catalytic application in PEMFC. Sophisticated operando characterisation techniques such as the use of in situ equipment or on-line monitoring are required to unravel the nature of the active sites in this electrocatalyst in order to gain insight on the requirements for its structure-activity and stability correlations and the improvement strategies that will engender its commercial application in PEMFC.
Author contributions
AA contributed to the conceptualization and writing of the review draft. AP, OP, NM, and MA contributed to the writing of the review, editing and funding.
Acknowledgments
The authors acknowledge the funding support of the Department of Chemical, Metallurgical and Materials Engineering, Faculty of Engineering and Built Environment. Tshwane University of Technology, Pretoria. South Africa.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, Q., Mirzaeian, M., Hunt, M. R., Hall, P., and Raza, R. (2020). Current state and future prospects for electrochemical energy storage and conversion systems. Energies 13, 5847. doi:10.3390/en13215847
Abbasi, K. R., Shahbaz, M., Zhang, J., Irfan, M., and Alvarado, R. (2022). Analyze the environmental sustainability factors of China: The role of fossil fuel energy and renewable energy. Renew. Energy 187, 390–402. doi:10.1016/j.renene.2022.01.066
Abdelkareem, M. A., Elsaid, K., Wilberforce, T., Kamil, M., Sayed, E. T., and Olabi, A. (2021a). Environmental aspects of fuel cells: A review. Sci. Total Environ. 752, 141803. doi:10.1016/j.scitotenv.2020.141803
Abdelkareem, M. A., Wilberforce, T., Elsaid, K., Sayed, E. T., Abdelghani, E. A. M., and Olabi, A. G. (2021b). Transition metal carbides and nitrides as oxygen reduction reaction catalyst or catalyst support in proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrogen Energy 46, 23529–23547. doi:10.1016/j.ijhydene.2020.08.250
Alias, M., Kamarudin, S., Zainoodin, A., and Masdar, M. (2020). Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 45, 19620–19641. doi:10.1016/j.ijhydene.2020.04.202
Antolini, E. (2011). The stability of molten carbonate fuel cell electrodes: A review of recent improvements. Appl. energy 88, 4274–4293. doi:10.1016/j.apenergy.2011.07.009
Asset, T., Job, N., Busby, Y., Crisci, A., Martin, V., Stergiopoulos, V., et al. (2018). Porous hollow PtNi/C electrocatalysts: Carbon support considerations to meet performance and stability requirements. ACS Catal. 8, 893–903. doi:10.1021/acscatal.7b03539
Bai, L. (2018). Synthesis of PtRu/Ru heterostructure for efficient methanol electrooxidation: The role of extra Ru. Appl. Surf. Sci. 433, 279–284. doi:10.1016/j.apsusc.2017.10.026
Batchelor, T. A., Pedersen, J. K., Winther, S. H., Castelli, I. E., Jacobsen, K. W., and Rossmeisl, J. (2019). High-entropy alloys as a discovery platform for electrocatalysis. Joule 3, 834–845. doi:10.1016/j.joule.2018.12.015
Bhatt, M. D., and Lee, J. Y. (2020). Advancement of platinum (Pt)-free (non-Pt precious metals) and/or metal-free (non-precious-metals) electrocatalysts in energy applications: A review and perspectives. Energy & Fuels 34, 6634–6695. doi:10.1021/acs.energyfuels.0c00953
Bhoyate, S. D., Kim, J., De Souza, F. M., Lin, J., Lee, E., Kumar, A., et al. (2023). Science and engineering for non-noble-metal-based electrocatalysts to boost their ORR performance: A critical review. Coord. Chem. Rev. 474, 214854. doi:10.1016/j.ccr.2022.214854
Bogdanov, D., Ram, M., Aghahosseini, A., Gulagi, A., Oyewo, A. S., Child, M., et al. (2021). Low-cost renewable electricity as the key driver of the global energy transition towards sustainability. Energy 227, 120467. doi:10.1016/j.energy.2021.120467
Borup, R. L., Kusoglu, A., Neyerlin, K. C., Mukundan, R., Ahluwalia, R. K., Cullen, D. A., et al. (2020). Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 21, 192–200. doi:10.1016/j.coelec.2020.02.007
Cao, J., Cao, H., Shen, J., Wang, F., and Zhu, H. (2020). Impact of CuFe bimetallic core on the electrocatalytic activity and stability of Pt shell for oxygen reduction reaction. Electrochimica Acta 350, 136205. doi:10.1016/j.electacta.2020.136205
Chandran, P., Ghosh, A., and Ramaprabhu, S. (2018). High-performance Platinum-free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep. 8, 3591. doi:10.1038/s41598-018-22001-9
Chattopadhyay, J., Pathak, T. S., and Pak, D. (2022). Heteroatom-doped metal-free carbon nanomaterials as potential electrocatalysts. Molecules 27, 670. doi:10.3390/molecules27030670
Chen, X., Si, C., Gao, Y., Frenzel, J., Sun, J., Eggeler, G., et al. (2015). Multi-component nanoporous platinum–ruthenium–copper–osmium–iridium alloy with enhanced electrocatalytic activity towards methanol oxidation and oxygen reduction. J. Power Sources 273, 324–332. doi:10.1016/j.jpowsour.2014.09.076
Chen, Z., Gong, W., Liu, Z., Cong, S., Zheng, Z., Wang, Z., et al. (2019). Coordination-controlled single-atom tungsten as a non-3d-metal oxygen reduction reaction electrocatalyst with ultrahigh mass activity. Nano Energy 60, 394–403. doi:10.1016/j.nanoen.2019.03.045
Chen, D., Zhu, J., Mu, X., Cheng, R., Li, W., Liu, S., et al. (2020). Nitrogen-Doped carbon coupled FeNi3 intermetallic compound as advanced bifunctional electrocatalyst for OER, ORR and zn-air batteries. Appl. Catal. B Environ. 268, 118729. doi:10.1016/j.apcatb.2020.118729
Chen, M. X., Tong, L., and Liang, H. W. (2021). Understanding the catalytic sites of metal–nitrogen–carbon oxygen reduction electrocatalysts. Chemistry–A Eur. J. 27, 145–157. doi:10.1002/chem.202002427
Chen, L., Msigwa, G., Yang, M., Osman, A. I., Fawzy, S., Rooney, D. W., et al. (2022a). Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 20, 2277–2310. doi:10.1007/s10311-022-01435-8
Chen, X., Niu, K., Xue, Z., Liu, X., Liu, B., Zhang, B., et al. (2022b). Ultrafine platinum nanoparticles supported on N, S-codoped porous carbon nanofibers as efficient multifunctional materials for noticeable oxygen reduction reaction and water splitting performance. Nanoscale Adv. 4, 1639–1648. doi:10.1039/d2na00014h
Chung, D. Y., Park, S., Lee, H., Kim, H., Chung, Y.-H., Yoo, J. M., et al. (2020). Activity–stability relationship in Au@ Pt nanoparticles for electrocatalysis. ACS Energy Lett. 5, 2827–2834. doi:10.1021/acsenergylett.0c01507
Cruz-Martínez, H., Tellez-Cruz, M., Guerrero-Gutiérrez, O., Ramírez-Herrera, C., Salinas-Juárez, M., Velázquez-Osorio, A., et al. (2019). Mexican contributions for the improvement of electrocatalytic properties for the oxygen reduction reaction in PEM fuel cells. Int. J. hydrogen energy 44, 12477–12491. doi:10.1016/j.ijhydene.2018.05.168
Cruz-Martínez, H., Rojas-Chávez, H., Matadamas-Ortiz, P., Ortiz-Herrera, J., López-Chávez, E., Solorza-Feria, O., et al. (2021). Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 19, 100406. doi:10.1016/j.mtphys.2021.100406
Cui, C., Wu, M., Miao, X., Zhao, Z., and Gong, Y. (2022). Microstructure and corrosion behavior of CeO2/FeCoNiCrMo high-entropy alloy coating prepared by laser cladding. J. Alloys Compd. 890, 161826. doi:10.1016/j.jallcom.2021.161826
Deng, C., Wu, K.-H., Scott, J., Zhu, S., Amal, R., and Wang, D.-W. (2019). Ternary MnO/CoMn alloy@ N-doped graphitic composites derived from a bi-metallic pigment as bi-functional electrocatalysts. J. Mater. Chem. A 7, 20649–20657. doi:10.1039/c9ta08016c
Ding, R., Liu, Y., Rui, Z., Li, J., Liu, J., and Zou, Z. (2020a). Facile grafting strategy synthesis of single-atom electrocatalyst with enhanced ORR performance. Nano Res. 13, 1519–1526. doi:10.1007/s12274-020-2768-y
Ding, Z., Bian, J., Shuang, S., Liu, X., Hu, Y., Sun, C., et al. (2020b). High entropy intermetallic–oxide core–shell nanostructure as superb oxygen evolution reaction catalyst. Adv. Sustain. Syst. 4, 1900105. doi:10.1002/adsu.201900105
Deng, Z., Pang, W., Gong, M., Jin, Z., and Wang, X. (2022). Revealing the role of mo doping in promoting oxygen reduction reaction performance of Pt3Co nanowires. J. Energy Chem. 66, 16–23. doi:10.1016/j.jechem.2021.06.018
Do, M., Ngo, H., Guo, W., Liu, Y., Chang, S., Nguyen, D., et al. (2018). Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci. Total Environ. 639, 910–920. doi:10.1016/j.scitotenv.2018.05.136
Du, M., Li, X., Pang, H., and Xu, Q. (2022). Alloy electrocatalysts. EnergyChem, 100083. doi:10.1016/j.enchem.2022.100083
Duraisamy, V., Venkateshwaran, S., Thangamuthu, R., and Kumar, S. M. S. (2022). Hard template derived N, S dual heteroatom doped ordered mesoporous carbon as an efficient electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 47, 40327–40339. doi:10.1016/j.ijhydene.2022.03.250
Egeland-Eriksen, T., Hajizadeh, A., and Sartori, S. (2021). Hydrogen-based systems for integration of renewable energy in power systems: Achievements and perspectives. Int. J. hydrogen energy 46, 31963–31983. doi:10.1016/j.ijhydene.2021.06.218
Erikson, H., Antoniassi, R. M., Solla-Gullón, J., Torresi, R. M., Tammeveski, K., and Feliu, J. M. (2022). Oxygen electroreduction on small (<10 nm) and {100}-oriented Pt nanoparticles. Electrochimica Acta 403, 139631. doi:10.1016/j.electacta.2021.139631
Esfahani, R. A. M., Gavidia, L. M. R., García, G., Pastor, E., and Specchia, S. (2018). Highly active platinum supported on Mo-doped titanium nanotubes suboxide (Pt/TNTS-Mo) electrocatalyst for oxygen reduction reaction in PEMFC. Renew. Energy 120, 209–219. doi:10.1016/j.renene.2017.12.077
Etesami, M., Oroujzadeh, M., and Mehdipour-Ataei, S. (2020). 3D nitrogen-doped carbon supported non-precious metals electrocatalyst for oxygen reduction reaction. Mol. Catal. 485, 110834. doi:10.1016/j.mcat.2020.110834
Etesami, M., Mehdipour-Ataei, S., Somwangthanaroj, A., and Kheawhom, S. (2021). Recent progress of electrocatalysts for hydrogen proton exchange membrane fuel cells. Int. J. Hydrogen Energy 47, 41956. doi:10.1016/j.ijhydene.2021.09.133
Fan, L., Zhao, J., Luo, X., and Tu, Z. (2022). Comparison of the performance and degradation mechanism of PEMFC with Pt/C and Pt black catalyst. Int. J. Hydrogen Energy 47, 5418–5428. doi:10.1016/j.ijhydene.2021.11.135
Fashedemi, O. O., Bello, A., Adebusuyi, T., and Bindir, S. (2022). Recent trends in Carbon support for improved performance of Alkaline Fuel cells. Curr. Opin. Electrochem. 36, 101132. doi:10.1016/j.coelec.2022.101132
Ferriday, T. B., and Middleton, P. H. (2021). Alkaline fuel cell technology-A review. Int. J. hydrogen energy 46, 18489–18510. doi:10.1016/j.ijhydene.2021.02.203
Floriano, R., Zepon, G., Edalati, K., Fontana, G. L., Mohammadi, A., Ma, Z., et al. (2020). Hydrogen storage in TiZrNbFeNi high entropy alloys, designed by thermodynamic calculations. Int. J. Hydrogen Energy 45, 33759–33770. doi:10.1016/j.ijhydene.2020.09.047
Fu, X., Schuh, C. A., and Olivetti, E. A. (2017). Materials selection considerations for high entropy alloys. Scr. Mater. 138, 145–150. doi:10.1016/j.scriptamat.2017.03.014
Gao, K., Wang, B., Tao, L., Cunning, B. V., Zhang, Z., Wang, S., et al. (2019). Efficient metal-free electrocatalysts from N-doped carbon nanomaterials: Mono-doping and co-doping. Adv. Mater. 31, 1805121. doi:10.1002/adma.201805121
Gao, P., Zhao, S., Qu, X., Qian, X., Duan, F., Lu, S., et al. (2022). Bifunctional high-entropy alloys for sensitive nitrite detection and oxygen reduction reaction. Electrochimica Acta 432, 141160. doi:10.1016/j.electacta.2022.141160
Garino, N., Sacco, A., Chiodoni, A., Pirri, C. F., and Castellino, M. (2021). Microwave-Assisted synthesis of nitrogen and sulphur doped graphene decorated with antimony oxide: An effective catalyst for oxygen reduction reaction. Materials 15, 10. doi:10.3390/ma15010010
Garlyyev, B., Kratzl, K., Rück, M., Michalička, J., Fichtner, J., Macak, J. M., et al. (2019). Optimizing the size of platinum nanoparticles for enhanced mass activity in the electrochemical oxygen reduction reaction. Angew. Chem. Int. Ed. 58, 9596–9600. doi:10.1002/anie.201904492
George, E. P., Raabe, D., and Ritchie, R. O. (2019). High-entropy alloys. Nat. Rev. Mater. 4, 515–534. doi:10.1038/s41578-019-0121-4
Gewirth, A. A., Varnell, J. A., and Diascro, A. M. (2018). Nonprecious metal catalysts for oxygen reduction in heterogeneous aqueous systems. Chem. Rev. 118, 2313–2339. doi:10.1021/acs.chemrev.7b00335
Gilroy, K. D., Ruditskiy, A., Peng, H.-C., Qin, D., and Xia, Y. (2016). Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 116, 10414–10472. doi:10.1021/acs.chemrev.6b00211
Gong, M., Deng, Z., Xiao, D., Han, L., Zhao, T., Lu, Y., et al. (2019). One-nanometer-thick Pt3Ni bimetallic alloy nanowires advanced oxygen reduction reaction: Integrating multiple advantages into one catalyst. Acs Catal. 9, 4488–4494. doi:10.1021/acscatal.9b00603
Guan, H., Chai, L., Wang, Y., Xiang, K., Wu, L., Pan, H., et al. (2021). Microstructure and hardness of NbTiZr and NbTaTiZr refractory medium-entropy alloy coatings on Zr alloy by laser cladding. Appl. Surf. Sci. 549, 149338. doi:10.1016/j.apsusc.2021.149338
Guterman, V. E., Belenov, S. V., Alekseenko, A. A., Lin, R., Tabachkova, N. Y., and Safronenko, O. I. (2018). Activity and stability of Pt/C and Pt-Cu/C electrocatalysts. Electrocatalysis 9, 550–562. doi:10.1007/s12678-017-0451-1
Han, J., Bian, J., and Sun, C. (2020). Recent advances in single-atom electrocatalysts for oxygen reduction reaction. Research 2020, 9512763. doi:10.34133/2020/9512763
He, W., Xiang, Y., Xin, M., Qiu, L., Dong, W., Zhao, W., et al. (2022). Investigation of multiple commercial electrocatalysts and electrocatalyst degradation for fuel cells in real vehicles. RSC Adv. 12, 32374–32382. doi:10.1039/d2ra05682h
Hersbach, T. J., Garcia, A. C., Kroll, T., Sokaras, D., Koper, M. T., and Garcia-Esparza, A. T. (2021). Base-accelerated degradation of nanosized platinum electrocatalysts. ACS Catal. 11, 9904–9915. doi:10.1021/acscatal.1c02468
Hornberger, E., Klingenhof, M., Polani, S., Paciok, P., Kormányos, A., Chattot, R., et al. (2022). On the electrocatalytical oxygen reduction reaction activity and stability of quaternary RhMo-doped PtNi/C octahedral nanocrystals. Chem. Sci. 13, 9295–9304. doi:10.1039/d2sc01585d
Hou, C.-C., Wang, H.-F., Li, C., and Xu, Q. (2020a). From metal–organic frameworks to single/dual-atom and cluster metal catalysts for energy applications. Energy & Environ. Sci. 13, 1658–1693. doi:10.1039/c9ee04040d
Hou, J., Yang, M., Ke, C., Wei, G., Priest, C., Qiao, Z., et al. (2020b). Platinum-group-metal catalysts for proton exchange membrane fuel cells: From catalyst design to electrode structure optimization. EnergyChem 2, 100023. doi:10.1016/j.enchem.2019.100023
Hu, B., Yuan, J., Zhang, J., Shu, Q., Guan, D., Yang, G., et al. (2021). High activity and durability of a Pt–Cu–Co ternary alloy electrocatalyst and its large-scale preparation for practical proton exchange membrane fuel cells. Compos. Part B Eng. 222, 109082. doi:10.1016/j.compositesb.2021.109082
Hu, C., and Dai, L. (2019). Doping of carbon materials for metal-free electrocatalysis. Adv. Mater. 31, 1804672. doi:10.1002/adma.201804672
Huang, L., Zhang, X., Wang, Q., Han, Y., Fang, Y., and Dong, S. (2018). Shape-control of Pt–Ru nanocrystals: Tuning surface structure for enhanced electrocatalytic methanol oxidation. J. Am. Chem. Soc. 140, 1142–1147. doi:10.1021/jacs.7b12353
Huang, X.-Y., You, L.-X., Zhang, X.-F., Feng, J.-J., Zhang, L., and Wang, A.-J. (2019). -proline assisted solvothermal preparation of Cu-rich rhombic dodecahedral PtCu nanoframes as advanced electrocatalysts for oxygen reduction and hydrogen evolution reactions. Electrochimica Acta 299, 89–97. doi:10.1016/j.electacta.2019.01.002
Huang, L., Zaman, S., Tian, X., Wang, Z., Fang, W., and Xia, B. Y. (2021). Advanced platinum-based oxygen reduction electrocatalysts for fuel cells. Accounts Chem. Res. 54, 311–322. doi:10.1021/acs.accounts.0c00488
Hussain, S., Kongi, N., Erikson, H., Rähn, M., Merisalu, M., Matisen, L., et al. (2019). Platinum nanoparticles photo-deposited on SnO2-C composites: An active and durable electrocatalyst for the oxygen reduction reaction. Electrochimica Acta 316, 162–172. doi:10.1016/j.electacta.2019.05.104
Hussain, I., Lamiel, C., Sahoo, S., Ahmad, M., Chen, X., Javed, M. S., et al. (2022). Factors affecting the growth formation of nanostructures and their impact on electrode materials: A systematic review. Mater. Today Phys. 27, 100844. doi:10.1016/j.mtphys.2022.100844
Icaza-Alvarez, D., Jurado, F., Tostado-Véliz, M., and Arevalo, P. (2022). Decarbonization of the Galapagos Islands. Proposal to transform the energy system into 100% renewable by 2050. Renew. Energy 189, 199–220. doi:10.1016/j.renene.2022.03.008
Ioroi, T., Siroma, Z., Yamazaki, S., and Yasuda, K. (2019). Electrocatalysts for PEM fuel cells. Adv. Energy Mater. 9, 1801284. doi:10.1002/aenm.201801284
Islam, J., Kim, S.-K., Lee, E., and Park, G.-G. (2019). Durability enhancement of a Pt/C electrocatalyst using silica-coated carbon nanofiber as a corrosion-resistant support. Int. J. Hydrogen Energy 44, 4177–4187. doi:10.1016/j.ijhydene.2018.12.138
Jeerh, G., Zhang, M., and Tao, S. (2021). Recent progress in ammonia fuel cells and their potential applications. J. Mater. Chem. A 9, 727–752. doi:10.1039/d0ta08810b
Jiang, A., Chen, J., Liu, S., Wang, Z., Li, Q., Xia, D., et al. (2021). Intermetallic rhodium alloy nanoparticles for electrocatalysis. ACS Appl. Nano Mater. 4, 13716–13723. doi:10.1021/acsanm.1c03128
Jiao, K., Xuan, J., Du, Q., Bao, Z., Xie, B., Wang, B., et al. (2021). Designing the next generation of proton-exchange membrane fuel cells. Nature 595, 361–369. doi:10.1038/s41586-021-03482-7
Kishida, K., Harigai, T., Takikawa, H., and Hashimoto, T. (2021). Performance improvement of platinum-based electrocatalyst layer for PEFC by mixing single walled carbon nanotubes. Denki Gakkai Ronbunshi, B (Online) 141, 316–321. doi:10.1541/ieejpes.141.316
Kong, J., Qin, Y.-H., Wang, T.-L., and Wang, C.-W. (2020). Photodeposition of Pt nanoparticles onto TiO2@ CNT as high-performance electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 45, 1991–1997. doi:10.1016/j.ijhydene.2019.11.016
Kong, Z., Zhang, D., Lu, Y., Yang, C., Du, S., Li, W., et al. (2021). Advanced cathode electrocatalysts for fuel cells: Understanding, construction, and application of carbon-based and platinum-based nanomaterials. ACS Mater. Lett. 3, 1610–1634. doi:10.1021/acsmaterialslett.1c00450
Kong, F., Wang, M., Huang, Y., Meng, G., Chen, M., Tian, H., et al. (2023). Cu-N-bridged Fe-3d electron state regulations for boosted oxygen reduction in flexible battery and PEMFC. Energy Storage Mater. 54, 533–542. doi:10.1016/j.ensm.2022.11.003
Kregar, A., Tavčar, G., Kravos, A., and Katrašnik, T. (2020). Predictive system-level modeling framework for transient operation and cathode platinum degradation of high temperature proton exchange membrane fuel cells. Appl. energy 263, 114547. doi:10.1016/j.apenergy.2020.114547
Labata, M. F., Li, G., Ocon, J., and Chuang, P.-Y. A. (2021). Insights on platinum-carbon catalyst degradation mechanism for oxygen reduction reaction in acidic and alkaline media. J. Power Sources 487, 229356. doi:10.1016/j.jpowsour.2020.229356
Lai, W., Ma, Z., Zhang, J., Yuan, Y., Qiao, Y., and Huang, H. (2022). Dynamic evolution of active sites in electrocatalytic CO2 reduction reaction: Fundamental understanding and recent progress. Adv. Funct. Mater. 32, 2111193. doi:10.1002/adfm.202111193
Lee, G., Nguyen, N.-A., Nguyen, V.-T., Larina, L. L., Chuluunbat, E., Park, E., et al. (2022a). High entropy alloy electrocatalyst synthesized using plasma ionic liquid reduction. J. Solid State Chem. 314, 123388. doi:10.1016/j.jssc.2022.123388
Lee, S., Gwon, K., Kim, H., Park, B. J., and Shin, J. H. (2022b). High-performance amperometric carbon monoxide sensor based on a xerogel-modified PtCr/C microelectrode. Sensors Actuators B Chem. 369, 132275. doi:10.1016/j.snb.2022.132275
Li, J., and Sun, S. (2019). Intermetallic nanoparticles: Synthetic control and their enhanced electrocatalysis. Accounts Chem. Res. 52, 2015–2025. doi:10.1021/acs.accounts.9b00172
Li, K., and Chen, W. (2021). Recent progress in high-entropy alloys for catalysts: Synthesis, applications, and prospects. Mater. Today Energy 20, 100638. doi:10.1016/j.mtener.2021.100638
Li, C., Yuan, Q., Ni, B., He, T., Zhang, S., Long, Y., et al. (2018a). Dendritic defect-rich palladium-copper-cobalt nanoalloys as robust multifunctional non-platinum electrocatalysts for fuel cells. Nat. Commun. 9, 3702. doi:10.1038/s41467-018-06043-1
Li, K., Li, X., Huang, H., Luo, L., Li, X., Yan, X., et al. (2018b). One-nanometer-thick PtNiRh trimetallic nanowires with enhanced oxygen reduction electrocatalysis in acid media: Integrating multiple advantages into one catalyst. J. Am. Chem. Soc. 140, 16159–16167. doi:10.1021/jacs.8b08836
Li, W., Liu, P., and Liaw, P. K. (2018c). Microstructures and properties of high-entropy alloy films and coatings: A review. Mater. Res. Lett. 6, 199–229. doi:10.1080/21663831.2018.1434248
Li, R., Liu, F., Zhang, Y., Guo, M., and Liu, D. (2020a). Nitrogen, sulfur co-doped hierarchically porous carbon as a metal-free electrocatalyst for oxygen reduction and carbon dioxide reduction reaction. ACS Appl. Mater. Interfaces 12, 44578–44587. doi:10.1021/acsami.0c06506
Li, S., Tang, X., Jia, H., Li, H., Xie, G., Liu, X., et al. (2020b). Nanoporous high-entropy alloys with low Pt loadings for high-performance electrochemical oxygen reduction. J. Catal. 383, 164–171. doi:10.1016/j.jcat.2020.01.024
Li, Y., Sun, Y., Qin, Y., Zhang, W., Wang, L., Luo, M., et al. (2020c). Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 10, 1903120. doi:10.1002/aenm.201903120
Li, H., Lai, J., Li, Z., and Wang, L. (2021). Multi-sites electrocatalysis in high-entropy alloys. Adv. Funct. Mater. 31, 2106715. doi:10.1002/adfm.202106715
Li, W., Bhuvanendran, N., Liu, H., Xu, Q., Hooshyari, K., and Su, H. (2023). Ternary PtPdCo mesoporous nanospheres with superior electrocatalytic performance towards methanol oxidation reaction. J. Alloys Compd. 933, 167706. doi:10.1016/j.jallcom.2022.167706
Liang, J., Li, N., Zhao, Z., Ma, L., Wang, X., Li, S., et al. (2019). Tungsten-doped L10-PtCo ultrasmall nanoparticles as a high-performance fuel cell cathode. Angew. Chem. Int. Ed. 58, 15617–15623. doi:10.1002/ange.201908824
Lim, J., Shin, H., Kim, M., Lee, H., Lee, K.-S., Kwon, Y., et al. (2018). Ga–doped Pt–Ni octahedral nanoparticles as a highly active and durable electrocatalyst for oxygen reduction reaction. Nano Lett. 18, 2450–2458. doi:10.1021/acs.nanolett.8b00028
Lim, A., Lee, J. S., Lee, S., Lee, S. Y., Kim, H.-J., Yoo, S. J., et al. (2021). Polymer electrolyte membrane unitized regenerative fuel cells: Operational considerations for achieving high round trip efficiency at low catalyst loading. Appl. Catal. B Environ. 297, 120458. doi:10.1016/j.apcatb.2021.120458
Liu, D., Tao, L., Yan, D., Zou, Y., and Wang, S. (2018). Recent advances on non-precious metal porous carbon-based electrocatalysts for oxygen reduction reaction. ChemElectroChem 5, 1775–1785. doi:10.1002/celc.201800086
Liu, H., Liu, J., Chen, P., and Yang, H. (2019). Microstructure and high temperature wear behaviour of in-situ TiC reinforced AlCoCrFeNi-based high-entropy alloy composite coatings fabricated by laser cladding. Opt. Laser Technol. 118, 140–150. doi:10.1016/j.optlastec.2019.05.006
Liu, Y., Chen, Z., Yang, X., Zhang, J., Sun, Z., Chen, Y., et al. (2021). A facile synthesis of high entropy alloy nanoparticle–activated carbon nanocomposites for synergetic degradation of methylene blue. RSC Adv. 11, 24636–24646. doi:10.1039/d1ra03661k
Liu, B., Feng, R., Busch, M., Wang, S., Wu, H., Liu, P., et al. (2022). Synergistic hybrid electrocatalysts of platinum alloy and single-atom platinum for an efficient and durable oxygen reduction reaction. ACS Nano 16, 14121–14133. doi:10.1021/acsnano.2c04077
Loffler, T., Ludwig, A., Rossmeisl, J., and Schuhmann, W. (2021). What makes high-entropy alloys exceptional electrocatalysts? Angew. Chem. Int. Ed. Engl. 60, 26894–26903. doi:10.1002/anie.202109212
Lopes, P. P., Li, D., Lv, H., Wang, C., Tripkovic, D., Zhu, Y., et al. (2020). Eliminating dissolution of platinum-based electrocatalysts at the atomic scale. Nat. Mater. 19, 1207–1214. doi:10.1038/s41563-020-0735-3
LöFfler, T., Savan, A., Garzón-Manjón, A., Meischein, M., Scheu, C., Ludwig, A., et al. (2019). Toward a paradigm shift in electrocatalysis using complex solid solution nanoparticles. ACS Energy Lett. 4, 1206–1214. doi:10.1021/acsenergylett.9b00531
Lu, Z., and Elam, J. W. (2022). Synthesis techniques for ultrathin oxide layers of heterogeneous catalysts. Ultrathin Oxide Layers Sol. Electrocatalytic Syst. 30, 210. doi:10.1039/9781839163708-00210
Lucia, U. (2014). Overview on fuel cells. Renew. Sustain. Energy Rev. 30, 164–169. doi:10.1016/j.rser.2013.09.025
Lv, H., Xu, D., Sun, L., Henzie, J., Suib, S. L., Yamauchi, Y., et al. (2019a). Ternary palladium–boron–phosphorus alloy mesoporous nanospheres for highly efficient electrocatalysis. ACS Nano 13, 12052–12061. doi:10.1021/acsnano.9b06339
Lv, Y., Yang, L., and Cao, D. (2019b). Sulfur, nitrogen and fluorine triple-doped metal-free carbon electrocatalysts for the oxygen reduction reaction. ChemElectroChem 6, 741–747. doi:10.1002/celc.201801433
Ma, R., Lin, G., Zhou, Y., Liu, Q., Zhang, T., Shan, G., et al. (2019). A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 5, 78–15. doi:10.1038/s41524-019-0210-3
Ma, Z., Cano, Z. P., Yu, A., Chen, Z., Jiang, G., Fu, X., et al. (2020a). Enhancing oxygen reduction activity of Pt-based electrocatalysts: From theoretical mechanisms to practical methods. Angew. Chem. 132, 18490–18504. doi:10.1002/ange.202003654
Ma, Z., Tian, H., Meng, G., Peng, L., Chen, Y., Chen, C., et al. (2020b). Size effects of platinum particles@ CNT on HER and ORR performance. Sci. China Mater. 63, 2517–2529. doi:10.1007/s40843-020-1449-2
Ma, H., Zheng, Z., Zhao, H., Shen, C., Chen, H., Li, H., et al. (2021). Trimetallic PtNiCo branched nanocages as efficient and durable bifunctional electrocatalysts towards oxygen reduction and methanol oxidation reactions. J. Mater. Chem. A 9, 23444–23450. doi:10.1039/d1ta07488a
Ma, S.-Y., Ma, T., Hu, Q., Yang, H.-P., and He, C.-X. (2022). Ternary PtRuTe alloy nanofibers as an efficient and durable electrocatalyst for hydrogen oxidation reaction in alkaline media. Sci. China Mater. 65, 3462–3469. doi:10.1007/s40843-022-2089-6
Madakannu, I., Patil, I., Kakade, B., and Datta, K. K. R. (2022). Electrocatalytic oxygen reduction activity of AgCoCu oxides on reduced graphene oxide in alkaline media. Beilstein J. Nanotechnol. 13, 1020–1029. doi:10.3762/bjnano.13.89
Madheswaran, D. K., and Jayakumar, A. (2021). Recent advancements on non-platinum based catalyst electrode material for polymer electrolyte membrane fuel cells: A mini techno-economic review. Bull. Mater. Sci. 44, 1–12. doi:10.1007/s12034-021-02572-6
Majlan, E. H., Rohendi, D., Daud, W. R. W., Husaini, T., and Haque, M. A. (2018). Electrode for proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 89, 117–134. doi:10.1016/j.rser.2018.03.007
Manoharan, Y., Hosseini, S. E., Butler, B., Alzhahrani, H., Senior, B. T. F., Ashuri, T., et al. (2019). Hydrogen fuel cell vehicles; current status and future prospect. Appl. Sci. 9, 2296. doi:10.3390/app9112296
Marinoiu, A., Raceanu, M., Carcadea, E., and Varlam, M. (2018). Iodine-doped graphene–Catalyst layer in PEM fuel cells. Appl. Surf. Sci. 456, 238–245. doi:10.1016/j.apsusc.2018.06.100
Martínez-Hincapié, R., and Čolić, V. (2022). Electrocatalysts for the oxygen reduction reaction: From bimetallic platinum alloys to complex solid solutions. ChemEngineering 6, 19. doi:10.3390/chemengineering6010019
Meng, H.-L., Lin, S.-Y., Cao, Y., Wang, A.-J., Zhang, L., and Feng, J.-J. (2021). CoFe alloy embedded in N-doped carbon nanotubes derived from triamterene as a highly efficient and durable electrocatalyst beyond commercial Pt/C for oxygen reduction. J. Colloid Interface Sci. 604, 856–865. doi:10.1016/j.jcis.2021.07.061
Mohammed, H., Al-Othman, A., Nancarrow, P., Tawalbeh, M., and Assad, M. E. H. (2019). Direct hydrocarbon fuel cells: A promising technology for improving energy efficiency. Energy 172, 207–219. doi:10.1016/j.energy.2019.01.105
Molochas, C., and Tsiakaras, P. (2021). Carbon monoxide tolerant Pt-based electrocatalysts for H2-PEMFC applications: Current progress and challenges. Catalysts 11, 1127. doi:10.3390/catal11091127
Muhyuddin, M., Mustarelli, P., and Santoro, C. (2021). Recent advances in waste plastic transformation into valuable platinum-group metal-free electrocatalysts for oxygen reduction reaction. ChemSusChem 14, 3785–3800. doi:10.1002/cssc.202101252
Mukherjee, P., Kakade, B., and Swami, A. (2022). Current trends in platinum-based ternary alloys as promising electrocatalysts for the oxygen reduction reaction: A mini review. Energy & Fuels 36, 2306–2322. doi:10.1021/acs.energyfuels.1c03667
Nandan, R., Raj, G., and Nanda, K. K. (2022). FeCoNiMnCr high-entropy alloy nanoparticle-grafted NCNTs with promising performance in the ohmic polarization region of fuel cells. ACS Appl. Mater. Interfaces 14, 16108–16116. doi:10.1021/acsami.1c21336
Nguyen, A. T. N., and Shim, J. H. (2018). Facile one-step synthesis of Ir-Pd bimetallic alloy networks as efficient bifunctional catalysts for oxygen reduction and oxygen evolution reactions. J. Electroanal. Chem. 827, 120–127. doi:10.1016/j.jelechem.2018.09.012
Niu, L., Liu, G., Li, Y., An, J., Zhao, B., Yang, J., et al. (2021). CoNi alloy nanoparticles encapsulated in N-doped graphite carbon nanotubes as an efficient electrocatalyst for oxygen reduction reaction in an alkaline medium. ACS Sustain. Chem. Eng. 9, 8207–8213. doi:10.1021/acssuschemeng.1c02098
Okonkwo, P. C., Ige, O. O., Uzoma, P. C., Emori, W., Benamor, A., Abdullah, A. M., et al. (2021). Platinum degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrogen Energy 46, 15850–15865. doi:10.1016/j.ijhydene.2021.02.078
Oliveira, A. M., Beswick, R. R., and Yan, Y. (2021). A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 33, 100701. doi:10.1016/j.coche.2021.100701
Prabhu, P., Jose, V., and Lee, J.-M. (2020). Design strategies for development of TMD-based heterostructures in electrochemical energy systems. Matter 2, 526–553. doi:10.1016/j.matt.2020.01.001
Qiao, Z., Hwang, S., Li, X., Wang, C., Samarakoon, W., Karakalos, S., et al. (2019). 3D porous graphitic nanocarbon for enhancing the performance and durability of Pt catalysts: A balance between graphitization and hierarchical porosity. Energy & Environ. Sci. 12, 2830–2841. doi:10.1039/c9ee01899a
Qiu, H.-J., Fang, G., Wen, Y., Liu, P., Xie, G., Liu, X., et al. (2019). Nanoporous high-entropy alloys for highly stable and efficient catalysts. J. Mater. Chem. A 7, 6499–6506. doi:10.1039/c9ta00505f
Rahaman, M. Z., and Islam, M. M. (2019). Comparative study of different fuel cell technologies. Asian J. con. Sci. Technol. 1, 29–32. doi:10.5281/zenodo.3384260
Sahoo, L., Garg, R., Kaur, K., Vinod, C., and Gautam, U. K. (2022). Ultrathin twisty PdNi alloy nanowires as highly active ORR electrocatalysts exhibiting morphology-induced durability over 200 K cycles. Nano Lett. 22, 246–254. doi:10.1021/acs.nanolett.1c03704
Samad, S., Loh, K. S., Wong, W. Y., Lee, T. K., Sunarso, J., Chong, S. T., et al. (2018). Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int. J. hydrogen energy 43, 7823–7854. doi:10.1016/j.ijhydene.2018.02.154
Sanad, M. F., Puente Santiago, A. R., Tolba, S. A., Ahsan, M. A., Fernandez-Delgado, O., Shawky Adly, M., et al. (2021). Co–Cu bimetallic metal organic framework catalyst outperforms the Pt/C benchmark for oxygen reduction. J. Am. Chem. Soc. 143, 4064–4073. doi:10.1021/jacs.1c01096
Sawant, S. V., Patwardhan, A. W., Joshi, J. B., and Dasgupta, K. (2022). Boron doped carbon nanotubes: Synthesis, characterization and emerging applications–A review. Chem. Eng. J. 427, 131616. doi:10.1016/j.cej.2021.131616
Schwanitz, V. J., and Wierling, A. (2022). “Toward sustainable global energy production and consumption,” in Responsible consumption and production (Springer).
Seh, Z. W., Kibsgaard, J., Dickens, C. F., Chorkendorff, I., Nørskov, J. K., and Jaramillo, T. F. (2017). Combining theory and experiment in electrocatalysis: Insights into materials design. Science 355, eaad4998. doi:10.1126/science.aad4998
Shamoon, A., Haleem, A., Bahl, S., Javaid, M., Garg, S. B., Sharma, R. C., et al. (2022). Environmental impact of energy production and extraction of materials-a review. Mater. Today Proc. 57, 936–941. doi:10.1016/j.matpr.2022.03.159
Shang, X., Tang, J.-H., Dong, B., and Sun, Y. (2020). Recent advances of nonprecious and bifunctional electrocatalysts for overall water splitting. Sustain. Energy & Fuels 4, 3211–3228. doi:10.1039/d0se00466a
Shao, Q., Li, F., Chen, Y., and Huang, X. (2018). The advanced designs of high-performance platinum-based electrocatalysts: Recent progresses and challenges. Adv. Mater. Interfaces 5, 1800486. doi:10.1002/admi.201800486
Shao, Q., Wang, P., Zhu, T., and Huang, X. (2019). Low dimensional platinum-based bimetallic nanostructures for advanced catalysis. Accounts Chem. Res. 52, 3384–3396. doi:10.1021/acs.accounts.9b00262
Sharma, D. K., Mahant, D., and Upadhyay, G. (2020). Manufacturing of metal matrix composites: A state of review. Mater. Today Proc. 26, 506–519. doi:10.1016/j.matpr.2019.12.128
Shimoyama, I., Hakoda, T., Shimada, A., and Baba, Y. (2015). Influence of configuration at dopant sites on catalytic activity of phosphorus-doped graphite. Carbon 81, 260–271. doi:10.1016/j.carbon.2014.09.057
Song, T. W., Chen, M. X., Yin, P., Tong, L., Zuo, M., Chu, S. Q., et al. (2022). Intermetallic PtFe electrocatalysts for the oxygen reduction reaction: Ordering degree-dependent performance. Small 18, 2202916. doi:10.1002/smll.202202916
Souza, N. E., Bott-Neto, J. L., Rocha, T. A., Da Silva, G. C., Teixeira-Neto, E., Gonzalez, E. R., et al. (2018). Support modification in Pt/C electrocatalysts for durability increase: A degradation study assisted by identical location transmission electron microscopy. Electrochimica Acta 265, 523–531. doi:10.1016/j.electacta.2018.01.180
Stonehart, P., and Wheeler, D. (2005). “Phosphoric acid fuel cells (PAFCs) for utilities: Electrocatalyst crystallite design, carbon support, and matrix materials challenges,” in Modern aspects of electrochemistry. Editors B. E. Conway, C. G. Vayenas, R. E. White, and M. E. Gamboa-Adelco (Boston, MA: Springer) Vol. 38, 373–424. doi:10.1007/0-387-25838-8_4
Sui, S., Wang, X., Zhou, X., Su, Y., Riffat, S., and Liu, C.-J. (2017). A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 5, 1808–1825. doi:10.1039/c6ta08580f
Sun, T., Wang, J., Qiu, C., Ling, X., Tian, B., Chen, W., et al. (2018). B, N codoped and defect-rich nanocarbon material as a metal-free bifunctional electrocatalyst for oxygen reduction and evolution reactions. Adv. Sci. 5, 1800036. doi:10.1002/advs.201800036
Sun, D., Cai, Y., Zhu, L., Gao, F., Shan, M., Manladan, S. M., et al. (2022). High-temperature oxidation and wear properties of TiC-reinforced CrMnFeCoNi high entropy alloy composite coatings produced by laser cladding. Surf. Coatings Technol. 438, 128407. doi:10.1016/j.surfcoat.2022.128407
Tian, X. L., Xu, Y. Y., Zhang, W., Wu, T., Xia, B. Y., and Wang, X. (2017). Unsupported platinum-based electrocatalysts for oxygen reduction reaction. ACS Energy Lett. 2, 2035–2043. doi:10.1021/acsenergylett.7b00593
Tsai, M.-H., and Yeh, J.-W. (2014). High-entropy alloys: A critical review. Mater. Res. Lett. 2, 107–123. doi:10.1080/21663831.2014.912690
Wacławek, S., Padil, V. V., and Černík, M. (2018). Major advances and challenges in heterogeneous catalysis for environmental applications: A review. Ecol. Chem. Eng. S 25, 9–34. doi:10.1515/eces-2018-0001
Wan, X., Liu, X., Li, Y., Yu, R., Zheng, L., Yan, W., et al. (2019). Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268. doi:10.1038/s41929-019-0237-3
Wan, C., Duan, X., and Huang, Y. (2020). Molecular design of single-atom catalysts for oxygen reduction reaction. Adv. Energy Mater. 10, 1903815. doi:10.1002/aenm.201903815
Wang, C., and Spendelow, J. S. (2021). Recent developments in Pt–Co catalysts for proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 28, 100715. doi:10.1016/j.coelec.2021.100715
Wang, S., and Jiang, S. P. (2017). Prospects of fuel cell technologies. Natl. Sci. Rev. 4, 163–166. doi:10.1093/nsr/nww099
Wang, Y., Leung, D. Y., Xuan, J., and Wang, H. (2016). A review on unitized regenerative fuel cell technologies, part-A: Unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 65, 961–977. doi:10.1016/j.rser.2016.07.046
Wang, J., Wang, H., and Fan, Y. (2018a). Techno-economic challenges of fuel cell commercialization. Engineering 4, 352–360. doi:10.1016/j.eng.2018.05.007
Wang, Y.-J., Long, W., Wang, L., Yuan, R., Ignaszak, A., Fang, B., et al. (2018b). Unlocking the door to highly active ORR catalysts for PEMFC applications: Polyhedron-engineered Pt-based nanocrystals. Energy & Environ. Sci. 11, 258–275. doi:10.1039/c7ee02444d
Wang, S., Luo, Q., Zhu, Y., Tang, S., and Du, Y. (2019a). Facile synthesis of quaternary structurally ordered L12-Pt (Fe, Co, Ni) 3 nanoparticles with low content of platinum as efficient oxygen reduction reaction electrocatalysts. ACS omega 4, 17894–17902. doi:10.1021/acsomega.9b02918
Wang, Z., Yao, X., Kang, Y., Miao, L., Xia, D., and Gan, L. (2019b). Structurally ordered low-Pt intermetallic electrocatalysts toward durably high oxygen reduction reaction activity. Adv. Funct. Mater. 29, 1902987. doi:10.1002/adfm.201902987
Wang, Z., Yao, X., Kang, Y., Xia, D., and Gan, L. (2019c). Rational development of structurally ordered platinum ternary intermetallic electrocatalysts for oxygen reduction reaction. Catalysts 9, 569. doi:10.3390/catal9070569
Wang, X., Zhang, L., Wang, F., Yu, J., and Zhu, H. (2020a). Nickel-introduced structurally ordered PtCuNi/C as high performance electrocatalyst for oxygen reduction reaction. Prog. Nat. Sci. Mater. Int. 30, 905–911. doi:10.1016/j.pnsc.2020.10.017
Wang, Y., Ruiz Diaz, D. F., Chen, K. S., Wang, Z., and Adroher, X. C. (2020b). Materials, technological status, and fundamentals of PEM fuel cells – a review. Mater. Today 32, 178–203. doi:10.1016/j.mattod.2019.06.005
Wang, K., Li, N., Yang, Y., Ke, S., Zhang, Z., Dou, M., et al. (2021a). Effect of load-cycling amplitude on performance degradation for proton exchange membrane fuel cell. Chin. Chem. Lett. 32, 3159–3163. doi:10.1016/j.cclet.2021.02.045
Wang, M., Lu, Y., Zhang, G., Cui, H., Xu, D., Wei, N., et al. (2021b). A novel high-entropy alloy composite coating with core-shell structures prepared by plasma cladding. Vacuum 184, 109905. doi:10.1016/j.vacuum.2020.109905
Wang, Y., Zhao, L., Wan, D., Guan, S., and Chan, K. (2021c). Additive manufacturing of TiB2-containing CoCrFeMnNi high-entropy alloy matrix composites with high density and enhanced mechanical properties. Mater. Sci. Eng. A 825, 141871. doi:10.1016/j.msea.2021.141871
Wang, Z., Cheng, L., Zhang, R., Lv, W., and Wang, W. (2021d). Surface-oxidized Fe–Co–Ni alloys anchored to N-doped carbon nanotubes as efficient catalysts for oxygen reduction reaction. J. Alloys Compd. 857, 158249. doi:10.1016/j.jallcom.2020.158249
Wang, X., Li, X., Kong, D., Zhao, L., Cui, Y., Wang, Y., et al. (2022). Platinum-free electrocatalysts for hydrogen oxidation reaction in alkaline media. Nano Energy 104, 107877. doi:10.1016/j.nanoen.2022.107877
Weber, P., Werheid, M., Janssen, M., and Oezaslan, M. (2018). Fundamental insights in degradation mechanisms of Pt/C nanoparticles for the ORR. ECS Trans. 86, 433–445. doi:10.1149/08613.0433ecst
Wei, P., Li, X., He, Z., Sun, X., Liang, Q., Wang, Z., et al. (2021a). Porous N, B co-doped carbon nanotubes as efficient metal-free electrocatalysts for ORR and Zn-air batteries. Chem. Eng. J. 422, 130134. doi:10.1016/j.cej.2021.130134
Wei, X., Wang, R.-Z., Zhao, W., Chen, G., Chai, M.-R., Zhang, L., et al. (2021b). Recent research progress in PEM fuel cell electrocatalyst degradation and mitigation strategies. EnergyChem 3, 100061. doi:10.1016/j.enchem.2021.100061
Wei, Y., Wu, D., Yong, C., Wang, Z., Zhong, P., Qiu, J., et al. (2023). Robust and highly conductive Ti4O7/MXene nanocomposites as high-performance and long cyclic stability oxygen reduction electrocatalysts. Appl. Surf. Sci. 607, 154929. doi:10.1016/j.apsusc.2022.154929
Wen, C., Zhang, Y., Wang, C., Xue, D., Bai, Y., Antonov, S., et al. (2019). Machine learning assisted design of high entropy alloys with desired property. Acta Mater. 170, 109–117. doi:10.1016/j.actamat.2019.03.010
Wu, R., Li, Y., Gong, W., and Shen, P. K. (2019). One-pot synthesis of Pt–Pd bimetallic nanodendrites with enhanced electrocatalytic activity for oxygen reduction reaction. ACS Sustain. Chem. Eng. 7, 8419–8428. doi:10.1021/acssuschemeng.9b00056
Wu, D., Kusada, K., Yamamoto, T., Toriyama, T., Matsumura, S., Kawaguchi, S., et al. (2020). Platinum-group-metal high-entropy-alloy nanoparticles. J. Am. Chem. Soc. 142, 13833–13838. doi:10.1021/jacs.0c04807
Wu, Q., Yan, X., Jia, Y., and Yao, X. (2021). Defective carbon-based materials: Controllable synthesis and electrochemical applications. EnergyChem 3, 100059. doi:10.1016/j.enchem.2021.100059
Xia, B. (2021). Progress and perspective on oxygen reduction electrocatalysts toward practical fuel cells. Angewandte Chemie. (International ed. in English).
Xiao, F., Wang, Y. C., Wu, Z. P., Chen, G., Yang, F., Zhu, S., et al. (2021a). Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells. Adv. Mater. 33, 2006292. doi:10.1002/adma.202006292
Xiao, Z., Jiang, Y., Wu, H., Zhong, H., Song, H., Abdelhafiz, A., et al. (2021b). De-alloyed ternary electrocatalysts with high activity and stability for oxygen reduction reaction. J. Alloys Compd. 877, 160221. doi:10.1016/j.jallcom.2021.160221
Xie, X., He, C., Li, B., He, Y., Cullen, D. A., Wegener, E. C., et al. (2020). Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells. Nat. Catal. 3, 1044–1054. doi:10.1038/s41929-020-00546-1
Xie, M., Chu, T., Wang, X., Li, B., Yang, D., Ming, P., et al. (2022). Effect of mesoporous carbon on oxygen reduction reaction activity as cathode catalyst support for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 47, 28074–28085. doi:10.1016/j.ijhydene.2022.06.131
Xu, H., Cheng, D., Cao, D., and Zeng, X. C. (2018). A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 1, 339–348. doi:10.1038/s41929-018-0063-z
Xu, J., Li, R., Xu, C.-Q., Zeng, R., Jiang, Z., Mei, B., et al. (2021). Underpotential-deposition synthesis and in-line electrochemical analysis of single-atom copper electrocatalysts. Appl. Catal. B Environ. 289, 120028. doi:10.1016/j.apcatb.2021.120028
Yang, S., Yu, Y., Dou, M., Zhang, Z., Dai, L., and Wang, F. (2019). Two-dimensional conjugated aromatic networks as high-site-density and single-atom electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 58, 14866–14872. doi:10.1002/ange.201908023
Yang, B., Wang, J., Zhang, M., Shu, H., Yu, T., Zhang, X., et al. (2020). A state-of-the-art survey of solid oxide fuel cell parameter identification: Modelling, methodology, and perspectives. Energy Convers. Manag. 213, 112856. doi:10.1016/j.enconman.2020.112856
Yeh, J.-W. (2013). Alloy design strategies and future trends in high-entropy alloys. Jom 65, 1759–1771. doi:10.1007/s11837-013-0761-6
Yeh, J.-W., and Lin, S.-J. (2018). Breakthrough applications of high-entropy materials. J. Mater. Res. 33, 3129–3137. doi:10.1557/jmr.2018.283
Ying, J., Li, J., Jiang, G., Cano, Z. P., Ma, Z., Zhong, C., et al. (2018). Metal-organic frameworks derived platinum-cobalt bimetallic nanoparticles in nitrogen-doped hollow porous carbon capsules as a highly active and durable catalyst for oxygen reduction reaction. Appl. Catal. B Environ. 225, 496–503. doi:10.1016/j.apcatb.2017.11.077
Yuan, Z., Cao, Y., Zhang, Z., Fang, Y., Liu, Q., Dang, D., et al. (2022). Dandelion-like titanium nitride supported platinum as an efficient oxygen reduction catalyst in acidic media. Int. J. Hydrogen Energy 47, 15035–15043. doi:10.1016/j.ijhydene.2022.03.010
Zhai, L., Yang, S., Yang, X., Ye, W., Wang, J., Chen, W., et al. (2020). Conjugated covalent organic frameworks as platinum nanoparticle supports for catalyzing the oxygen reduction reaction. Chem. Mater. 32, 9747–9752. doi:10.1021/acs.chemmater.0c03614
Zhang, Q., and Guan, J. (2020). Single-atom catalysts for electrocatalytic applications. Adv. Funct. Mater. 30, 2000768. doi:10.1002/adfm.202000768
Zhang, Y., Yang, X., and Liaw, P. (2012). Alloy design and properties optimization of high-entropy alloys. Jom 64, 830–838. doi:10.1007/s11837-012-0366-5
Zhang, C., Shen, X., Pan, Y., and Peng, Z. (2017). A review of Pt-based electrocatalysts for oxygen reduction reaction. Front. Energy 11, 268–285. doi:10.1007/s11708-017-0466-6
Zhang, B. W., Yang, H. L., Wang, Y. X., Dou, S. X., and Liu, H. K. (2018). A comprehensive review on controlling surface composition of Pt-based bimetallic electrocatalysts. Adv. Energy Mater. 8, 1703597. doi:10.1002/aenm.201703597
Zhang, C., Wang, S., Li, J., Zhu, Y., Peng, T., and Yang, H. (2020). Additive manufacturing of products with functional fluid channels: A review. Addit. Manuf. 36, 101490. doi:10.1016/j.addma.2020.101490
Zhang, J., Yuan, Y., Gao, L., Zeng, G., Li, M., and Huang, H. (2021a). Stabilizing Pt-based electrocatalysts for oxygen reduction reaction: Fundamental understanding and design strategies. Adv. Mater. 33, 2006494. doi:10.1002/adma.202006494
Zhang, S., Xia, Z., Zhang, M., Zou, Y., Shen, H., Li, J., et al. (2021b). Boosting selective hydrogenation through hydrogen spillover on supported-metal catalysts at room temperature. Appl. Catal. B Environ. 297, 120418. doi:10.1016/j.apcatb.2021.120418
Zhang, Y., Wang, D., and Wang, S. (2022). High-entropy alloys for electrocatalysis: Design, characterization, and applications. Small 18, 2104339. doi:10.1002/smll.202104339
Zhao, L., Zhu, J., Zheng, Y., Xiao, M., Gao, R., Zhang, Z., et al. (2022). Materials engineering toward durable electrocatalysts for proton exchange membrane fuel cells. Adv. Energy Mater. 12, 2102665. doi:10.1002/aenm.202102665
Zhu, C., Shi, Q., Xu, B. Z., Fu, S., Wan, G., Yang, C., et al. (2018). Hierarchically porous M–N–C (M = Co and Fe) single-atom electrocatalysts with robust MN x active moieties enable enhanced ORR performance. Adv. Energy Mater. 8, 1801956. doi:10.1002/aenm.201801956
Zhu, J., Xie, M., Chen, Z., Lyu, Z., Chi, M., Jin, W., et al. (2020a). Pt-Ir-Pd trimetallic nanocages as a dual catalyst for efficient oxygen reduction and evolution reactions in acidic media. Adv. Energy Mater. 10, 1904114. doi:10.1002/aenm.201904114
Zhu, S., Yu, Y., Zhang, B., Zhang, Z., Yan, X., and Wang, Z. (2020b). Microstructure and wear behaviour of in-situ TiN-Al2O3 reinforced CoCrFeNiMn high-entropy alloys composite coatings fabricated by plasma cladding. Mater. Lett. 272, 127870. doi:10.1016/j.matlet.2020.127870
Zhu, X., Hu, C., Amal, R., Dai, L., and Lu, X. (2020c). Heteroatom-doped carbon catalysts for zinc–air batteries: Progress, mechanism, and opportunities. Energy & Environ. Sci. 13, 4536–4563. doi:10.1039/d0ee02800b
Zhu, M., Zhao, C., Liu, X., Wang, X., Zhou, F., Wang, J., et al. (2021). Single atomic cerium sites with a high coordination number for efficient oxygen reduction in proton-exchange membrane fuel cells. ACS Catal. 11, 3923–3929. doi:10.1021/acscatal.0c05503
Zhu, S., Yang, L., Bai, J., Chu, Y., Liu, J., Jin, Z., et al. (2022). Ultra-stable Pt5La intermetallic compound towards highly efficient oxygen reduction reaction. Nano Res., 1–6. doi:10.1007/s12274-022-4868-3
Keywords: proton exchange membrane fuel cell, oxygen reduction reaction, platinum-based electrocatalysts, platinum-free electrocatalysts, alloy-based electrocatalysts, single atom electrocatalysts, metal-free electrocatalysts
Citation: Alabi AS, Popoola API, Popoola OM, Mathe NR and Abdulwahab M (2023) Materials for electrocatalysts in proton exchange membrane fuel cell: A brief review. Front. Energy Res. 11:1091105. doi: 10.3389/fenrg.2023.1091105
Received: 06 November 2022; Accepted: 03 February 2023;
Published: 14 February 2023.
Edited by:
Xiaohu Yang, Xi’an Jiaotong University, ChinaReviewed by:
Gouse Peera Shaik, Keimyung University, Republic of KoreaLindiwe Eudora Khotseng, University of the Western Cape, South Africa
Copyright © 2023 Alabi, Popoola, Popoola, Mathe and Abdulwahab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. S. Alabi, YXlvLmFsYWJpQHltYWlsLmNvbQ==
 A. S. Alabi
A. S. Alabi A. P. I. Popoola
A. P. I. Popoola O. M. Popoola2
O. M. Popoola2 N. R. Mathe
N. R. Mathe