- 1Beihang University, Beijing, China
- 2College of Automotive Engineering, Jilin University, Changchun, China
- 3Centre for E-Mobility and Clean Growth, Coventry University, Coventry, United Kingdom
- 4Dyson School of Design Engineering, Imperial College London, London, United Kingdom
Lithium–sulfur batteries have received increasing research interest due to their superior theoretical capacity, cost-effectiveness, and eco-friendliness. However, the commercial realization of lithium–sulfur batteries faces critical obstacles, such as the significant volume change of sulfur cathodes over the de/lithiation processes, uncontrollable shuttle effects of polysulfides, and the lithium dendrite issue. On this basis, the lithium–sulfur battery based on solid-state electrolytes was developed to alleviate the previously mentioned problems. This article aims to provide an overview of the recent progress of solid-state lithium–sulfur batteries related to various kinds of solid-state electrolytes, which mainly include three aspects: the fundamentals and current status of lithium–sulfur solid-state batteries and several adopted solid-state electrolytes involving polymer electrolyte, inorganic solid electrolyte, and hybrid electrolyte. Furthermore, the future perspective for lithium–sulfur solid-state batteries is presented. Finally, this article proposed an initiation for new and practical research activities and paved the way for the design of usable lithium–sulfur solid-state batteries.
1 Introduction
With the growing demands for global energy, high-energy-density and long-cycling batteries are broadly developed and play a growing role in the global energy system (Wu et al., 2021). A rechargeable Li battery based on the Li chemistry is considered a promising candidate for battery systems and related functions. Typically, lithium–sulfur batteries (LSBs) are selected as ideal choices for energy storage systems due to their high theoretical-specific capacity (1,672 mA h/g) and theoretical-specific energy density (2,600 W h/kg), which is five times higher than traditional lithium-ion batteries (LIBs) (Dai et al., 2021; Zhou et al., 2021; Zhu et al., 2022). Meanwhile, compared to the lithium-ion battery, elemental sulfur, the main active material in LSBs, has the advantages of being abundantly stored, low-cost, simple to prepare, and environmentally friendly (Li et al., 2019; Gong and Wang, 2020; Liu X.-Z. et al., 2021; Pang et al., 2021). Therefore, significant research effort into LSB has potential advantages for future energy storage (Wang H. et al., 2017; Fan et al., 2021; Zheng et al., 2021; Phuc et al., 2022).
However, several issues still inhibit the development of Li-S batteries, such as the shuttle effect that dissolution and unwanted crossover between the anode and cathode of long-chain polysulfide, undesirably causing a capacity loss and a reduced roundtrip efficiency (Bonnick et al., 2019). The continued growth of lithium dendrites can easily lead to internal short-circuit and even thermal runaway failure (Wei et al., 2019; Zhang R. et al., 2022). To alleviate the aforementioned issues, numerous strategies have been applied, such as using host matrices (Wang M. et al., 2017) and electrolyte additives (Ding et al., 2020). Researchers (Gong et al., 2022) demonstrated that by modulating the multiple interactions between the functional groups through copolymerization the binder was able to coordinate the LiPSs with higher binding energy for shuttle effect alleviation and cycling performance improvement. Significant advances were achieved by designing sulfur cathodes with nanostructure (Li et al., 2021) and developing polysulfide-affinitive metal catalysts (De Luna et al., 2021; Liu X.-M. et al., 2021). Lei et al. (2018) reviewed the recent research progress of solid-state Li-S batteries, mainly including gel, solid-state polymer, ceramic, and composite electrolytes, and strategies for overcoming the deficiencies of solid-state electrolytes such as low room-temperature ionic conductivity and high interfacial resistance.
Recently, solid-state electrolytes (SSEs) have received much attention from academics in the energy field due to many advantages of being applied to LSBs. SSE could function as a physical barrier to block or hinder polysulfide migration (Yang S. et al., 2020). The mechanically robust solid electrolyte could also withstand the puncture of Li dendrites, thereby lowering the risk of internal short-circuiting and thermal runaway of batteries caused by dendrites (Cheng et al., 2019; Ding et al., 2022). Moreover, diverse SSEs and combinations can be exploited with lithium–sulfur chemistries, conveying various lithium–sulfur solid-state batteries (Miura et al., 2019; Bi et al., 2022). However, its practical applications are restricted by the sluggish electrochemical activities due to the high interfacial resistance and limited utilization of actives (Li et al., 2019; Chen Y. et al., 2021). The problem can be improved by modifying the solid electrolyte with various additives and preparing different types of SSEs. Generally, lithium–sulfur SSEs can be divided into polymer electrolytes, inorganic solid electrolytes, and hybrid electrolytes. A polymer electrolyte involves solid polymer electrolytes and gel electrolytes. Solid Polymer electrolytes attract more research attention due to their excellent processability, but this kind of electrolyte faces three critical issues: 1) insufficient ionic conductivity at an ambient temperature; 2) soft nature that can barely survive in the penetration of dendrites; and 3) dissolution of polysulfides in electrolytes. Gel electrolytes feature the advantages of the liquid electrolyte, such as high ionic conductivity of above 1.0 mS/cm and good interfacial compatibility to electrodes. Shuttle effects of polymer sulfides and dendrites are two significant issues challenging their applications. In contrast to gel electrolytes, inorganic solid electrolytes possess better mechanical strength and electrochemical/chemical stability. However, the apparent resistance of this electrolyte is exceptionally high due to the resistance of involved grain boundaries. Poor processability and shapeability of inorganic electrolytes limit the tangible applications of this inorganic electrolyte. For example, sulfide-based electrolytes possess a high bulky ionic conductivity of 10 mS/cm. Fabricating this electrolyte into a battery-usable membrane requires the addition of organic polymers. This approach inevitably sacrificed the ionic conductivity. In comparison, hybrid electrolytes combine the advantages of various components of electrolyte and alleviate the aforementioned issues. This article comprehensively reviewed the lithium–sulfur SSE current development status and proposed the future development direction. It aimed to provide guidance to the lithium–sulfur SSE design.
This review is organized as follows: the details of lithium–sulfur solid-state batteries (SSBs) are presented in Section 2. Lithium–Sulfur SSB working principle, charging/discharging curves, ion conduction mechanism, and current challenges are given in Sections 2.1–2.4. Designing electrolyte material strategies are reviewed in Section 3. Polymer electrolyte application for the lithium–sulfur SSB is provided in Section 4. Inorganic solid electrolytes and hybrid electrolytes are discussed separately in Section 5 and Section 6, respectively. Finally, the conclusions and future perspectives are provided in Section 7.
2 Solid-State Lithium–Sulfur Battery
2.1 Working Principle
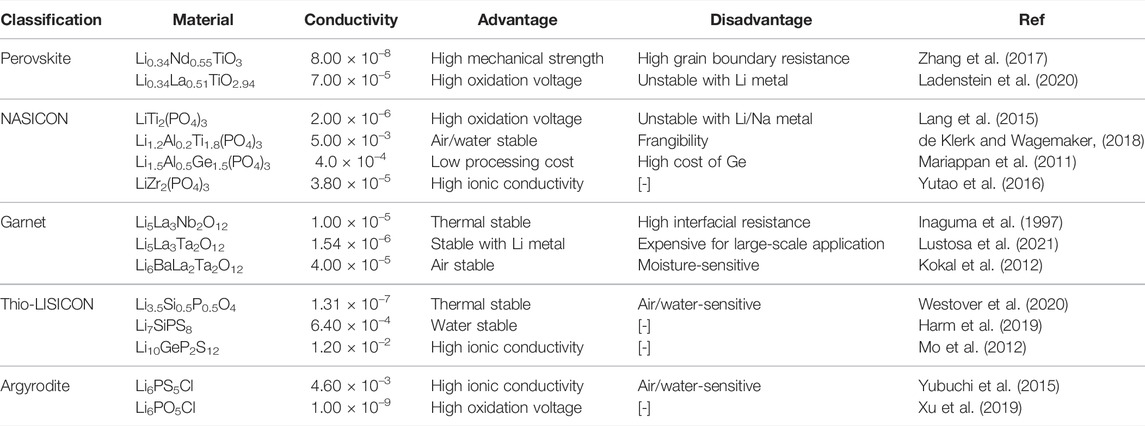
A lithium–sulfur SSB usually included the Li metal anode, SSE, and sulfur-based cathode. Figure 1A presents the typical lithium–sulfur configurations (Yang X. et al., 2020). In terms of the anode, the metallic Li is a suitable material choice because of its outstanding theoretical capacity (3,860 mA hg−1) and lowest negative electrochemical potential (−3.040 V) (Cheng et al., 2017). Yet, lithium–sulfur SSB faces Li dendrite growth issues during the battery charging/discharging cycle due to the existing Li metal anode/unstable SSE interface. To alleviate the Li dendrite issues, the Li-M alloys (M = In, Sn, or Ge) and metal are usually conducted as anodes to solve the problems (Hikima et al., 2020; Zhang et al., 2021).
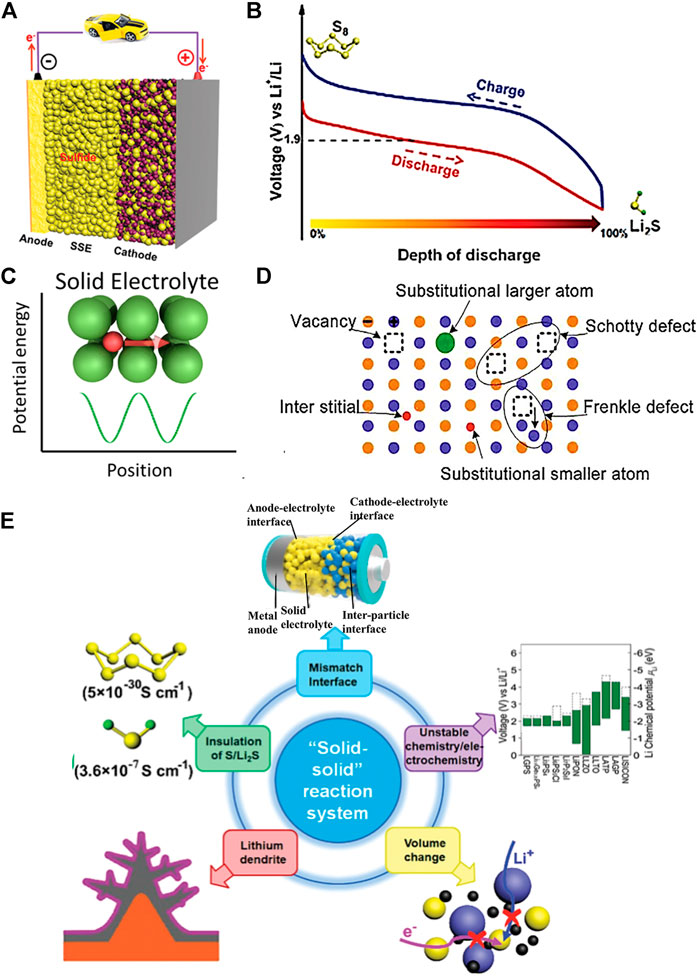
FIGURE 1. Scheme mechanism and challenges of lithium–sulfur SSB. (A) Schematic illustration of a lithium–sulfur SSB structure diagram (Yang X. et al., 2020). (B) Molecular structure and charging/discharging platforms of Li2S/S (Yang X. et al., 2020). (C) Mobile ion in a crystalline solid (Ding et al., 2020). (D) Schematic representation of different defects in the inorganic solid electrolytes (Ding et al., 2020). (E) Existing challenges of lithium–sulfur SSB (Yang X. et al., 2020).
2.2 Charge/Discharge Curves
When LiPSs are not dissolved into the solvent, lithium–sulfur SSBs follow a solid–solid reaction process. Wherein, there is direct interconversion between S and LiS, with no intermediate LiPSs. This reaction is common in sulfide-based SSE systems, as shown in Figure 1B. The discharge process is known as a one-step discharge process (Yang X. et al., 2020), which is likely attributed to the sluggish electrochemical kinetics of solid sulfur electrodes. High output energy could be achieved in this system. The theoretical discharge capacities of sulfur and lithium metal are 1,675 and 3,860 mAh g−1, respectively (Guo and Zheng, 2020). However, liquid carbonate-based LSB with bonded S or an intact coating on the sulfur cathode can effectively suppress LiPS dissolution and be observed to exhibit a single discharge plateau at around 2.0v during the reaction (Luo et al., 2020). Sometimes, in polymer electrolytes, where a small amount of liquid or polymer electrolyte is present in a mixed SSE, the solid–liquid and solid–solid reactions occur simultaneously, so that the mixed discharge profile exhibits multiple discharge plateaus. The reaction process is known as a quasi-solid phase reaction process (Li et al., 2018).
2.3 Ion Conduction Mechanism
As shown in Figure 1C, ions are transported through a periodic barrier (Goodenough, 2003), the energy barrier in a crystalline solid in a lithium–sulfur SSB. In amorphous polymers, the transport of lithium ions can generally be described by a hopping transport model. As shown in Figure 1D, lithium ions travel along the polymer chain, jumping from one coordination site to another or migrating from one coordination site to another (Sing et al., 2014). In some cases, the crystalline polymer phase’s ionic conductivity in the SSE may be higher than that in the non-crystalline phase. This is because the polymer chains in the crystalline phase can form a cylindrical tunnel in which the lithium ions can be transported rapidly without the aid of segmental movement of the polymer chains. However, most studies have not shown a clear correlation between structure and properties of solid polymer electrolytes, so the ion transport mechanism is still not clear (Ding et al., 2020). The ion transport mechanism of inorganic solid-state electrolytes is achieved by employing various defects in the crystal structure (Zhou et al., 2020). As shown in Figure 1D, different defects determine the type and concentration of carriers, such as point defects (i.e., Schottky defects and Frenkel defects), thus directly impacting the ion conductivity of inorganic SSEs (Varzi et al., 2016). In addition, the spatial distribution, composition, and structure of the fixed framework within the inorganic SSE and its interaction with Li-ions can affect the energy barriers and thus should be overcome during Li-ion transport. (Motavalli, 2015).
2.4 Current Challenges
Despite the significant advantages and advances in developing lithium–sulfur SSBs, there are still fundamental challenges to the current state of research. As shown in Figure 1E, these challenges are currently the ones that need to be overcome to improve the electrochemical performance and ultimately bring lithium–sulfur solid-state batteries into practical applications. In solid-phase reaction systems, the main issues are interfacial problems (Zhong et al., 2021) and chemical/electrochemical instabilities (Han et al., 2015). In contrast, shuttle effects (Liu et al., 2010) and gas emissions (Yang et al., 2016) (in systems containing liquids) remain the main drawbacks of solid–liquid dual-phase reaction systems. Problems such as insulating properties of S/Li2S, Li dendrite growth, and volume changes during cycling are common to both solid and liquid LSBs (Wang D. et al., 2017). In future research, the optimal design of lithium–sulfur LSBs should be carried out to address the aforementioned issues and to make an essential contribution to promoting the commercialization of lithium–sulfur SSBs.
3 General Strategies for Designing Electrolyte Materials
Lithium–sulfur SSEs are important to achieve high performance of lithium–sulfur SSBs. Generally, lithium–sulfur SSEs can be classified into polymer electrolytes, inorganic solid electrolytes, and hybrid electrolytes. Therein, polymer electrolytes include solid polymer electrolytes and gel electrolytes. The content will be comprehensively reviewed in the next section. In some cases, it greatly declined the lithium–sulfur SSB performance due to the shuttling effect of polysulfides. Therefore, different types of cathode-bonded S in polymers have been developed to alleviate the problem of shuttle effects. For instance, polyacrylonitrile (PAN)-s (Peng et al., 2017), 1,3-diisopropenylbenzene (DIB)-s, and trithiocyanuric acid (TTCA)-s (Kim et al., 2015) by polymerizing the sulfur chains into the scaffold of polymers can suppress the LiPS dissolution and shuttling effects.
Quasi/solid polymer electrolytes are among the promising SSEs and being received extensive attention due to their excellent flexibility and diversity (Huo et al., 2019; Lu et al., 2020). Critical issues restrict their applications with lithium–sulfur chemistries, such as shuttle effects, dendrite problems, and liquid leakage in some specific quasi-solid electrolytes (Pan et al., 2020). As polymer electrolytes are soft materials with thermal instability, temperature variation within polymer electrolytes can lead to uneven ionic conductivity. This is very favorable for the local overcharge and the formation of Li-dendrites (Yin et al., 2020). Regarding the quasi-solid electrolyte, suitable matrices are highly required, which should meet the following requirements: first, high porosity and good wettability so that it can quickly impregnate enough liquid electrolytes to obtain high ionic conductivity; then, high Li transfer number to achieve homogenous Li-ion migration and reduce ion concentration gradient; additionally, influential functional groups to limit the shuttle of polysulfide and obtain excellent long-term cycling stability (Chen S. et al., 2021; You et al., 2022); moreover, good mechanical properties to inhibit dendrite growth; finally, high thermal stability (Han et al., 2019).
At present, polyvinylidene fluoride (PVDF) and polyethylene oxide (PEO), polyvinylidene fluoride-hexafluoropropylene (PVDF-HIFP), and polypropylene cyanide (PAN) have been demonstrated as polymeric matrices to fabricate quasi-solid electrolytes (Qiu et al., 2019). However, an SEI layer would form in the systems using quasi-solid electrolytes. Although this may result in the loss of some active substances, it can effectively inhibit the shuttle effect, ensuring the high utilization rate of active substances. Additionally, incorporating an organic redox mediator within a quasi-solid electrolyte facilitates the redox kinetics of sulfur, mitigates the dissolution of sulfur, and promotes battery power (Zhao et al., 2021). Although it is challenging to design an utterly insoluble electrolyte for polysulfides, the negative impact caused by the dissolution of multi-lithium sulfide on the battery can be minimized.
Among inorganic solid electrolytes, solid sulfide electrolytes are the critical material of lithium–sulfur all-solid-state batteries (Miura et al., 2019; Deng et al., 2021) because of its high ion conductivity and deformability. LSBs are not sufficient to completely break down Li2S (Wang et al., 2020). Employing this SSE could effectively alleviate the shuttle effects and mitigate the dendrite issue. Some strategies are applied to improve the performance of lithium–sulfur SSBs such as surface modification to improve the ionic conductivity, defect engineering, incorporating liquid with solid, and enhancing the stability of SEI (Chen P. et al., 2021). However, the application of inorganic solid electrolytes introduces new challenges, such as increased interfacial resistance. The significant volume expansion of about 76% in solid cells will result in mechanical degradation and poor contact between electrodes and electrolytes (Wang et al., 2020). A proper design of the inorganic materials is also required.
4 Polymer Electrolyte Application for the Lithium–Sulfur SSB
4.1 Gel/Quasi-Solid Polymer Electrolyte
4.1.1 Metal–Organic Framework-Modified Gel Polymer Electrolyte
A MOF-based gel polymer electrolyte (GPE) has been used for LSBs to stabilize the lithium anode (Ren et al., 2020). Due to abundant micropores in the MOF skeleton, the as-prepared GPE can block the large-sized polysulfide ions in the MOF skeleton and immobilize the relatively large anions. This unique property significantly enables a uniform transport of Li-ions and achieves a homogenous lithium stripping/plating (Gao et al., 2021). In addition to the size-exclusion of MOF matrices, the impregnated liquid electrolyte would help to form a dense SEI passive layer, which further improves the cycling performance of batteries. As shown in Figures 2A,B, Gao et al. proposed a novel GPE with immobilized anions is applied in LSBs, stabilizing the lithium anode. Wherein, Mg-MOF-74 material is used to modify the PVDF-based GPE to attain a MOF-modified GPE. The MOF material has a suitable pore size of 10.2 Å and abundant Lewis acidic sites. Additionally, the Li-ion transportation by the skeleton of Mg-MOF-74 material is shown in Figure 2C. The experimental result presented that the MOF-PVDF electrolyte has the highest by comparing Celgard and PVDF GPE. It indicated that the MOF-PVDF has excellent ionic diffusivity. Meanwhile, as shown in Figures 2D–F, scanning electron microscopy (SEM) analysis found that the Mg-MOF-74-based PVDF electrolyte has a smooth lithium surface, which indicates that the Mg-MOF-74 material has an even lithium plating/stripping process (Huo et al., 2019).
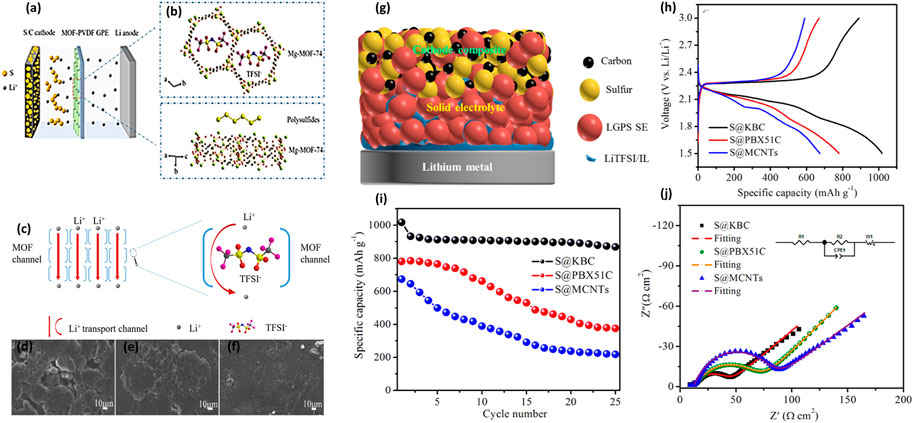
FIGURE 2. (A,B) Schematic illustration of MOF−PVDF GPE with anions immobilized for the lithium−sulfur battery. (C) Transport of Li ions in the Mg-MOF-74 material (Huo et al., 2019). (D–F) SEM pictures of Li in the Li/Li symmetrical cells using a commercial separator. (G) Schematic diagram of the quasi-solid-state LSB. Comparison of the (H) galvanostatic discharge/charge profiles. (I) Cycling performances and (J) Nyquist plots for different sulfur/carbon composite electrodes in quasi-solid state LSBs (Umeshbabu et al., 2019).
4.1.2 Ionic Liquid Interface-Modified Gel Polymer Electrolyte
A straightforward approach to stabilize the interface stability is employing electro-chemically stable ionic liquid. As a typical example, lithium-based ionic liquid, i.e., 1 M LiTFSI/PYR13TFSI was exploited as a surface modifier between the Li10GeP2S12 (LGPS) solid electrolyte and lithium metal anode. As depicted in Figure 2G, due to ionic-liquid-modified LGPS being considered the quasi-solid or gel electrolyte, the resulting battery featured a considerably decreased interfacial resistance of 142 Ω cm2 (Figure 2J). Paired with the sulfur-based cathode within S@KBC in this case, the battery delivered a high discharge capacity of 1,017 mAh g−1 (Figure 2H). The researchers explored a facile and straightforward approach to enhance the interface stability between the lithium superionic-conducting Li10GeP2S12 solid electrolyte and Li metal (Umeshbabu et al., 2019).
4.1.3 Polymer Matrix-Based Quasi-Solid/Gel Electrolyte
PVDF-HFP is a proper polymer matrix, demonstrated as a functional host in quasi-solid electrolytes (Yubuchi et al., 2019). For example, propylene polycarbonate was blended with PVDF-HFP to fabricate a flexible membrane separator via a solution casting method (Bi et al., 2021; Wang et al., 2022). The as-prepared gel polymer electrolyte features a high ionic conductivity of 1.18 × 10–3 S cm−1 and a wide electrochemical window of 4.8 V vs. Li/Li + at an ambient temperature. The increased ionic conductivity could be attributed to the matrix’s lower polymer crystalline and excellent ion conduction of impregnated propylene polycarbonate. With flexible polymer chains in PVDF-HFP, this polymer possesses good chain mobility and could potentially achieve an excellent ionic conductivity (Chen et al., 2019; Xiao et al., 2021). However, the PVDF-HFP electrolyte membrane has low mechanical strength, which can hardly hinder the dendrite growth and withstand the puncture of dendrites. Meanwhile, there are no influential functional groups within the structure of PVDF-HFP. Post-modification or tuning its functional structure to achieve negatively charged constituents might be a promising approach to prevent the shuttle effect of polysulfides. Donnan-exclusion will be introduced to repulse the negatively charged polysulfides.
4.2 Solid Polymer Electrolyte
4.2.1 Solid Polymer Electrolyte-Based Natural Halloysite Nano-Tube
A new type of a solid polymer electrolyte (SPE) membrane was prepared using a natural halloysite nano-tube (HNT), and it is applied to the flexible solid electrolyte membrane of the HNT lithium–sulfur SSB for the first time. Wherein, the lithium–sulfur SSB can work in a broad temperature range of 25–100°C with the HNT electrolyte. The Gamry electrochemical instrument measured the electrochemical properties of the PEO/LiTFSI/HNT electrolyte. The experimental data indicated that the highest ionic conductivity was attained with the presence of 10% HNT and an EO: Li molar ratio of 15:1 (Figure 3A). As shown in Figure 3B, at 100°C, the highest ionic conductivity is 2.14 × 10–3 S cm−1, whereas the ionic conductivity of SPE at room temperature is in a range from 10–8 to 10–4 S cm−1. Therefore, the PEO/LiTFSI/HNT electrolyte presents excellent ion conduction ability. The reason for the electrolyte improving the ionic conductivity was further studied. It is found that the Lewis acid–base interaction between HNT, LiTFSI, and PEO arranges lithium ions into 3D channels (Figure 3G). These interactions will curtail the free Li+ ion transfer distance, reduce ionic coupling, interfere with PEO crystallinity, and provide a high-speed channel for lithium-ion transmission. They also make SPE film electrolytes more even and have better mechanical strength. Additionally, the rate characteristics of the LSB with the PEO/LiTFSI electrolyte containing HNT were compared with those without HNT at 100°C, and the results are illustrated in Figure 3C. A battery based on PEO/LiTFSI/HNT indicated a higher capacity than batteries without HNT (Lin et al., 2017).
FIGURE 3. (A) Ionic conductivities of the PEO + LiTFSI + HNT films with different HNT contents. (B) Optical photos of PEO, PEO + LiTFSI, and PEO + LiTFSI (EO:Li = 15:1)+HNT (10%) at a magnification of 50 and ionic conductivity table of PEO + LiTFSI and PEO + LiTFSI (EO:Li = 15:1)+HNT (10%) at 25, 60, and 100°C; (C) Rate performance of PEO + LiTFSI + HNT (green) and PEO + LiTFSI (purple) electrolyte-based lithium–sulfur batteries at 100°C. (D) Tensile strengths of SPEs with different iCP@TFSI amounts. (E) TGA curves of SPEs. (F) Arrhenius plots of the conductivity of SPEs with different iCP@TFSI amounts. (G) Mechanism of HNT addition for enhanced ionic conductivity. (H) TEM and elemental mapping images of iCP@TFSI.
4.2.2 Polymer Electrolyte Based on PEO
PEO/LiTFSI polymer electrolytes with different (5%–20%) cyclopropenium cationic-based covalent organic polymers (iCP@TFSI) have been prepared. The effect of iCP@TFSI on the electrochemical performance of polymer LSBs was investigated. The iCP@TFSI-incorporated PEO/LiTFSI matric has a positive influence on ionic conductivity, and the mechanical capacity of SPEs, additionally, increases the stability of lithium metal anode. As shown in Figure 3D, the ultimate tensile strength of PEO-10%iCP@TFSI electrolyte can reach 1.9 MPa, and the elongation at break augments to 3,557.15%. Additionally, LSBs with PEO-10%iCP@TFSI electrolyte have the high Coulombic efficiency, excellent cycling stability, and low capacity fade of 0.032% per cycle after 500 cycles at 1°C (Wang et al., 2021). As shown in Figure 3E, the PEO/iCP@TFSI electrolyte shows high thermal stability at above 300°C, and it meets the basic requirements of energy storage. Meanwhile, it is also found that the PEO-10%iCP@TFSI electrolyte has a higher ion on conductivity, as shown in Figure 3F. The ionic conductivity of PEO-10%iCP@TFSI at a room temperature of 80°C is up to 1.2 × 10–3 Scm−1. However, the PEO-20%iCP@TFSI polymer electrolyte presents lower ion conductivity, possibly due to the uneven distribution of iCP@TFSI in the PEO matric. The surplus iCP@TFSI may lead to aggregation, phase separation, and bubbles and finally decrease the ionic conductivity of SPEs. In addition to the discussion mentioned earlier, SPE membranes’ capacity to resist high stress and high strain is vital for realizing the stable cycling of lithium–sulfur SSB. As shown in Figure 3H, the transmission electron microscope (TEM) combined with (energy dispersion spectrometer) EDS elemental mapping was employed to obtain the element distribution of iCP@TFSI. The EDS spectra proved that the TFSI-ions are evenly dispersed in the polymer without aggregation, which is essential for improving the ionic conductivity of the PEO/iCP@TFSI electrolyte.
5 Inorganic Solid-State Electrolytes
In contrast to polymer-based electrolytes, inorganic solid electrolytes exhibit better thermal stability and mechanical strength. Moreover, inorganic solid electrolytes feature a wide electrochemical window and insolubility to polysulfides. Ideally, they are the best separator to realize the lithium–sulfur SSBs with a good electrochemical performance. Currently, inorganic solid electrolytes include two widely studied families, oxide-based SSEs and sulfide-based SSEs. Sulfide-based SSEs include glass and glass-ceramic ionic conductors. Oxide-based SSEs include LISICON, NASICON, thio-LISICON, perovskite, and garnet-type solid electrolytes. The ionic conductivity, advantages, and disadvantages of inorganic SSEs are summarized in Table 1. However, all these electrolytes generally have a high contact resistance to electrodes due to the rigid contact of solids. The grain boundaries involved in these electrolytes also increase the interfacial resistance and reduce the overall ionic conductivity. In lithium–sulfur SSBs, sparse inorganic electrolytes were explored.
5.1 Sulfide Solid-State Electrolytes
5.1.1 Mixed Electrolyte of Li10GeP2S12
Recently, Yao et al. (2017) reported double-layer Li10GeP2S12 (LGPS) 24%/75% Li2P2S5 1% P2O5 as the electrolyte and cathode for RGo-modified S of all-solid-state LSB. As shown in Figure 4, the reduction of graphene oxide (rGO) nano S layer on the interface between the cathode and the electrolyte resistance decreased significantly, as proved by the atomic force microscope (AFM) results. The resultant lithium–sulfur SSB exhibited an initial discharge capacity of 1,629 mAh g−1 and good rate ability (0.05–5°C). The remarkable output capacity is attributed to the restricted shuttle effects and limited volume change of sulfur in de/lithiation. Later, a nanocomposite S@CNTs cathode was prepared and investigated in lithium–sulfur SSBs. This battery demonstrated a high-rate performance (60°C) and long cycle life. In addition, CNTs@S conveyed a stable capacity of 660.3 mAh g−1 at 1°C over 400 cycles. The electron-conductive nano-tubes could effectively accommodate sulfur and tolerate its volume change over electrochemical processes (Wu J. et al., 2020).
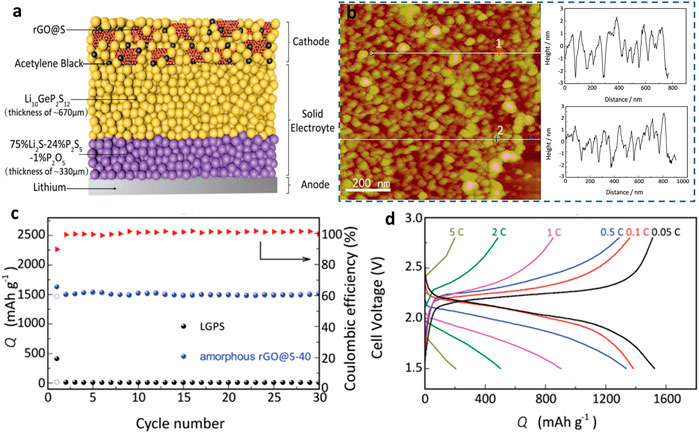
FIGURE 4. (A) Schematic illustration of a lithium–sulfur solid-state battery (Wang M. et al., 2017; Wu F. et al., 2020). (B) AFM images of amorphous rGO@S composite and the surface roughness. (C) Cycling performances of the amorphous rGO@S composite. (D) Galvanostatic discharge–charge curves for the amorphous rGO@S composite in lithium–sulfur SSBs.
5.1.2 High-Conductivity Argyrodite Li6PS5Cl Solid Electrolytes
Argyrodite Li6PS5Cl was regarded as a promising electrolyte with high ionic conductivity in the range of 10−3–10−2 S cm−1 at room temperature (Deiseroth et al., 2008). The typical preparation method for Li6PS5Cl SSEs is ball milling. Wang S. et al. (2018) prepared highly Li-ion-conductive Li6PS5Cl solid-state electrolytes (SSEs) by sintering at 550°C for 10 min, which was more efficient compared with other reported methods to synthesize Li6PS5Cl SSEs. All-solid-state lithium sulfur batteries (ASSLSBs) with Li6PS5Cl SSE were assembled using nano-sulfur/multiwall carbon nano-tube composite materials and combined with Li6PS5Cl as the cathode and Li-In alloy as the anode. The cell delivered a high discharge capacity of 1,850 mAh g−1 at room temperature for the first full cycle at 0.176 mA cm−2. In addition, the Coulombic efficiency remained nearly 100% during galvanostatic cycling. The experiment results indicated that Li6PS5Cl was a good candidate for SSE used in ASSLSBs.
5.2 Oxide Solid-State Electrolytes
Oxide-SSEs, another major inorganic SSEs, have recently received extensive attention from researchers due to their high electrical conductivity properties compared to polymer-SSEs. Developed oxide-based SSEs primarily involve LISICON, NASICON, thio-LISICON, perovskite, and garnet-type solid electrolytes. The perovskite-type Li3xLa(2/3)-x□(1/3)-2x TiO3 (LLTO), which has RT ionic conductivities of over 10–4 S cm−1 (Kwon et al., 2017; Yang D. et al., 2021). Li et al. (2016) have first developed NASICON-structured Na3V2(PO4)3 as the cathode material for Zn-ion batteries. With Na3V2(PO4)3/C as a cathode, Zn metal as both counter and reference electrodes, and 0.5 mol L−1 Zn(CH3COO)2 solution as an electrolyte, the aqueous Zn-ion battery delivers a reversible capacity of 97 mA h g−1 at 0.5°C and retains 74% capacity after 100 cycles. Usually, oxide-based SSEs are coupled with polymer-based SSEs, liquid electrolytes, or ionic liquids to reduce the interfacial resistance. These classic hybrid SSEs will be discussed in Section 6.
6 Hybrid Electrolytes
Though inorganic solid electrolytes have relatively high ionic conductivity, they endure instability with high interfacial resistance and instability of the surrounding atmosphere (Pan et al., 2020). To alleviate the shortcomings of single-component SSEs, in recent years, hybrid electrolytes have drawn increasing research interest due to their advantages, such as ionic conductivity and easy processability (Ding et al., 2020). Hybrid electrolytes (inorganic–organic composite electrolytes (IOCEs) and inorganic solid–liquid composite electrolytes (ISLCEs)) containing two or more components have been considered potential electrolytes for LSBs (Wang L. et al., 2018). Hybrid electrolytes are expected to combine each component’s advantages to improve the electrochemical performance of the LSBs. However, the disadvantages between the components cannot be completely avoided. This section mainly discusses the effect of hybrid electrolytes on improving the electrochemical performance of LSBs.
6.1 Inorganic–Organic Composite Electrolytes
Composite polymer electrolytes (CPEs) with solid-state fillers are widely researched to improve the Li-ion conductivity of SPEs. There are two kinds of fillers in the polymer matrix (Pan et al., 2020), which involve nonionically conductive fillers, such as TiO2, Al2O3, and ceramic nanowires (Liu et al., 2017) and Li ionically conductive fillers (such as Li7La3Zr2O12 and Li10SnP2S12). For nonionically conductive fillers, the use of alumina nanoparticles can improve the mechanical properties and electrochemical stability of CEPs due to the formation of a more stable SEI. Nevertheless, it is not suitable to apply Al2O3-CPEs directly in Li-S cells, as Judez et al. (2017) show that the discharge capacity of LSB with Al2O3-CPEs is only 300 mAh gs−1. In addition to oxides, the Li ionically conductive fillers are another strategy to ameliorate Li-ion conductivity for SPEs. A novel three-dimensional nanostructured Li6.28La3Zr2Al0.24O12 framework as a filler in the PEO matrix was developed in (Bae et al., 2018) to study the effect of Li-ion conductivity. As illustrated in Figures 5G–R, the three-dimensional garnet frame provides a continuous Li-ion conduction path, and the simulation results showed that the ionic conductivity was improved up to ∼10–3 S cm−1 at 60°C (Bae et al., 2018).
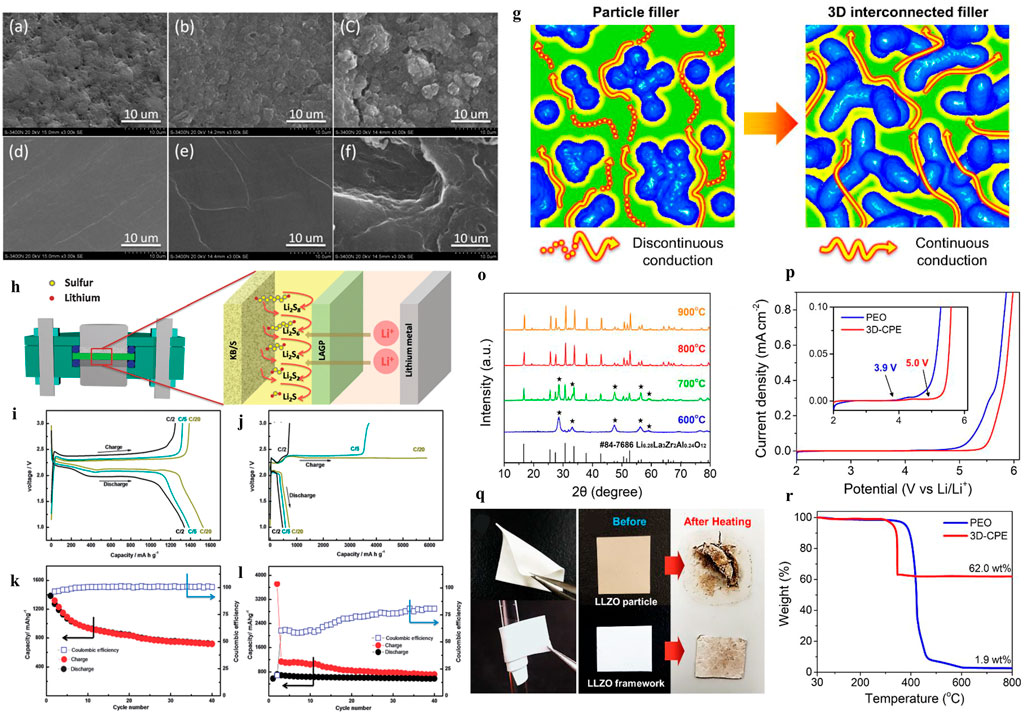
FIGURE 5. SEM images of sulfur cathodes and the lithium anode at the end of the 10th charge cycle. (A) Initial cathode, (B) cathode cycled in the HE cell, (C) cathode cycled in the LE cell, (D) fresh lithium anode, (E) the lithium anode cycled in the HE cell, and (F) lithium anode cycled in the LE cell (Wang et al., 2014). (G) Schematic of the ionic conduction mechanism in composite polymer electrolytes (Pan et al., 2020). (H) Inorganic solid–liquid hybrid electrolytes. Voltage versus specific charge–discharge capacity profiles of initial galvanostatic cycles of (I) HE Li-S cells and (J) LE Li-S cells at C/2, C/5, and C/20 rates. Cycling performance and Coulombic efficiency of (K) the HE Li-S cell and (L) the LE Li-S cell at the C/5 rate. (O) XRD patterns of garnet frameworks heat-treated at 600, 700, 800, and 900°C La2Zr2O7 impurities. (P) LSV curve of Li/PEO/SS and Li/3D-CPE/SS from 2 to 6 V at 60°C. (Q) Photographs of 3D-CPE demonstrating its mechanical flexibility (bending and rolling). (R) TGA plot of pure PEO and 3D-CPE from 30 to 800°C.
6.2 Inorganic Solid–Liquid Electrolytes
As illustrated in Figures 5A–F, X-ray photoelectron spectroscopy (XPS) and EDS spectra analysis indicated side reactions between lithium metal and polysulfides for LSBs with the solid–liquid electrolyte. The polysulfide shuttling effects can be suppressed by applying the solid–liquid hybrid electrolyte (Wang et al., 2014). To address the shuttle effect of the Li2Sx problem and interface contact problem (Liu G. et al., 2021), as shown in Figure 5H, Wang and co-workers proposed a room temperature hybrid electrolyte-based Li-s battery, which combined solid and liquid electrolytes. In this work, Li1.5Al0.5Ge1.5(PO4)3 (LAGP) as a separator to hinder the soluble polysulfides for a hybrid electrolyte (HE) Li-S battery was proposed. The NASICON-type structured LAGP electrolyte is a choice due to its favorable chemical stability against lithium and wide electrochemical window up to 6V. Meanwhile, the electrode and solid electrolyte are connected by 1 M LiN(CF3SO2)2 in the 1,3-dioxolane and 1,2-dimethoxyethane (1:1, v/v) (LiTFSI/DOL/DME) liquid electrolyte. As shown in Figures 5I–L, the discharge-specific capacity reached 1,386 mAh g−1 and 1,341 mAh g−1 at C/20 and C/5, respectively, while the initial discharge-specific capacity was 1,528 mAh g−1. Additionally, the reversible specific capacity remains at 720 mAh g−1 after 40 cycles at the C/5 rate, indicating an outstanding columbic efficiency.
7 Conclusion and Outlook
In this review, we have systematically reviewed the recent progress of lithium–sulfur SSBs. The structure and principle of the lithium–sulfur SSBs, electrolyte material design, and specific work are summarized, and Figure 6 gives a summary of the conductivity of various electrolytes. The electrolytes of lithium–sulfur SSBs can generally be divided into three types: polymers, inorganics, and hybrid SSEs. GPEs are able to trap a large fraction of liquids (organic or ionic liquids), and the conductivity could almost reach that of the free solvent, which is up to almost 10–2 S cm−1 at room temperature. In addition, gels together with dry polymers could tether negative charge to the backbone, which results in transference numbers close to unity. Eliminating liquid electrolytes for safety consideration, SPEs are attractive alternatives but limited by their low ionic conductivities (typically 10−8–10–6 S cm−1 at RT). Inorganics (ceramics) are often considered the ultimate solution to address the weaknesses of the liquid electrolyte systems. However, sintering processes cannot prepare solid electrolytes with thicknesses below 30 µm, and a high temperature is required for garnet electrolytes. The composite materials prepared by the deposition techniques that involve a vacuum process are normally expensive and have low production efficiency. In this respect, sulfide-based electrolytes are considered a promising candidate because they can be easily pressed and shaped in a low-cost manner. The interfacial contact is often established by the insertion of a layer of polymer/gel or liquid. Hybrid SSEs intend to balance the merits and drawbacks of each component. The most common hybrid SSEs are composed of a soft component and rigid inorganic SSEs, in which the inorganic SSEs contribute to high ionic conductivity/mechanical strength and the flexible component ensures a good interface.
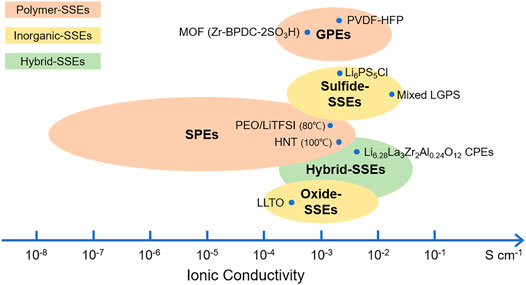
FIGURE 6. Ionic conductivity of various electrolyte materials. Unless otherwise specified, the electrical conductivity is measured at room temperature (RT).
Despite the tremendous efforts to promote performances of the lithium–sulfur SSBs, the following aspects remain challenging:
1) As the ultimate solution of a lithium metal battery, SSB could tackle the shuttle effect and the pulverization of lithium metal at its root, which could manipulate the irreversible capacity loss and low coulomb efficiency. Nevertheless, the SSE may debase the advantage of high specific energy because of the low ion and electron conductivity. Moreover, because the electrochemical reaction of SSB generates on the tri-phase-boundary, the crucial element of the stability and the regulation between each boundary is urgent for research.
2) The flexible electrode will also be a critical challenge to the lithium–sulfur SSBs. Initially, the flexible sulfur cathode has the characteristics of synergistic effort of both chemical and physical adsorptions, which could excellently restrain the shuttle effect. Moreover, the flexible design could buffer the volume expansion of the sulfur cathode. All the aforementioned methods can significantly improve the electrochemical performances of lithium–sulfur SSBs. Unfortunately, as another crucial role, the flexible lithium metal anode is facing more challenges but less studied than the flexible sulfur cathode. Developing flexible lithium metal anode is worthy of further research.
3) Big data is a promising solution to the full-lifespan management of lithium–sulfur SSBs, including material synthesis, manufacturing of batteries, operating process, battery recycling (Hu et al., 2016), and cascade utilization. Our group proposed a cyber hierarchy and interaction network (CHAIN) to solve this problem (Yang S. et al., 2020). A series of multi-scale models, the “digital twin” technology, and the “digital thread” technology can be applied to virtual out a battery completely from a multi-dimension containing molecular scale, morphology scale, electrode and electrolyte scale, and battery and system scale, which could provide guidance for the design and optimization process of the batteries (Yang S. et al., 2021; Zhang L.-S. et al., 2022). With related data shared and stored in the cloud, we could attain an optimized control strategy via data processing and artificial intelligence to enhance the full-lifespan management of lithium–sulfur SSBs.
In general, the future work of the lithium–sulfur SSBs will be focused not only on the material development and battery electrochemical performances but also on how to realize full-lifespan management.
Author Contributions
SY: conceptualization, methodology, and writing—original draft. ZZ: methodology, software, and visualization. JL: investigation and validation. LZ: data curation. LW: supervision and visualization. SC: supervision and writing—review and editing. CZ: supervision. XL: supervision.
Funding
The authors appreciate the kind support from the National Key R&D Program of China (No. 2021YFB2400200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bae, J., Li, Y., Zhao, F., Zhou, X., Ding, Y., and Yu, G. (2018). Designing 3D Nanostructured Garnet Frameworks for Enhancing Ionic Conductivity and Flexibility in Composite Polymer Electrolytes for Lithium Batteries. Energy Storage Mater. 15, 46–52. doi:10.1016/j.ensm.2018.03.016
Bi, C. X., Zhao, M., Hou, L. P., Chen, Z. X., Zhang, X. Q., Li, B. Q., et al. (2022). Anode Material Options toward 500 Wh Kg −1 Lithium-Sulfur Batteries. Adv. Sci. 9, 2103910. doi:10.1002/advs.202103910
Bi, S., Wan, F., Wang, S., Jia, S., Tian, J., and Niu, Z. (2021). Flexible and Tailorable Quasi‐solid‐state Rechargeable Ag/Zn Microbatteries with High Performance. Carbon Energy 3, 167–175. doi:10.1002/cey2.64
Bonnick, P., Niitani, K., Nose, M., Suto, K., Arthur, T. S., and Muldoon, J. (2019). A High Performance All Solid State Lithium Sulfur Battery with Lithium Thiophosphate Solid Electrolyte. J. Mat. Chem. A 7, 24173–24179. doi:10.1039/c9ta06971b
Chen, H., Xiao, Y., Chen, C., Yang, J., Gao, C., Chen, Y., et al. (2019). Conductive MOF-Modified Separator for Mitigating the Shuttle Effect of Lithium-Sulfur Battery through a Filtration Method. ACS Appl. Mat. Interfaces 11, 11459–11465. doi:10.1021/acsami.8b22564
Chen, P., Wu, Z., Guo, T., Zhou, Y., Liu, M., Xia, X., et al. (2021). Strong Chemical Interaction between Lithium Polysulfides and Flame‐Retardant Polyphosphazene for Lithium-Sulfur Batteries with Enhanced Safety and Electrochemical Performance. Adv. Mat. 33, 2007549. doi:10.1002/adma.202007549
Chen, S., Gao, Z., and Sun, T. (2021). Safety Challenges and Safety Measures of Li‐ion Batteries. Energy Sci. Eng. 9, 1647–1672. doi:10.1002/ese3.895
Chen, Y., Wang, T., Tian, H., Su, D., Zhang, Q., and Wang, G. (2021). Advances in Lithium-Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mat. 33, 2003666. doi:10.1002/adma.202003666
Cheng, Q., Li, A., Li, N., Li, S., Zangiabadi, A., Li, T.-D., et al. (2019). Stabilizing Solid Electrolyte-Anode Interface in Li-Metal Batteries by Boron Nitride-Based Nanocomposite Coating. Joule 3, 1510–1522. doi:10.1016/j.joule.2019.03.022
Cheng, X.-B., Zhang, R., Zhao, C.-Z., and Zhang, Q. (2017). Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 117, 10403–10473. doi:10.1021/acs.chemrev.7b00115
Dai, Y. Y., Xu, C. M., Liu, X. H., He, X. X., Yang, Z., Lai, W. H., et al. (2021). Manipulating Metal-Sulfur Interactions for Achieving High‐performance S Cathodes for Room Temperature Li/Na-Sulfur Batteries. Carbon Energy 3, 253–270. doi:10.1002/cey2.101
de Klerk, N. J. J., and Wagemaker, M. (2018). Space-Charge Layers in All-Solid-State Batteries; Important or Negligible? ACS Appl. Energy Mat. 1, 5609–5618. doi:10.1021/acsaem.8b01141
De Luna, Y., Abdullah, M., Dimassi, S. N., and Bensalah, N. (2021). All-solid Lithium-Sulfur Batteries: Present Situation and Future Progress. IONICS 27, 4937–4960. doi:10.1007/s11581-021-04284-7
Deiseroth, H.-J., Kong, S.-T., Eckert, H., Vannahme, J., Reiner, C., Zaiß, T., et al. (2008). Li6PS5X: A Class of Crystalline Li-Rich Solids with an Unusually High Li+ Mobility. Angew. Chem. Int. Ed. 47, 755–758. doi:10.1002/anie.200703900
Deng, R., Wang, M., Yu, H., Luo, S., Li, J., Chu, F., et al. (2021). Recent Advances and Applications toward Emerging Lithium-Sulfur Batteries: Working Principles and Opportunities. Energy Environ Mater. doi:10.1002/eem2.12257
Ding, B., Wang, J., Fan, Z., Chen, S., Lin, Q., Lu, X., et al. (2020). Solid-state Lithium-Sulfur Batteries: Advances, Challenges and Perspectives. Mater. Today 40, 114–131. doi:10.1016/j.mattod.2020.05.020
Ding, J. F., Xu, R., Ma, X. X., Xiao, Y., Yao, Y. X., Yan, C., et al. (2022). Quantification of the Dynamic Interface Evolution in High‐Efficiency Working Li‐Metal Batteries. Angew. Chem. Int. Ed. 61, e202115602. doi:10.1002/anie.202115602
Fan, B., Guan, Z., Wang, H., Wu, L., Li, W., Zhang, S., et al. (2021). Electrochemical Processes in All-Solid-State Li-S Batteries Studied by Electrochemical Impedance Spectroscopy. SOLID STATE IONICS 368, 115680. doi:10.1016/j.ssi.2021.115680
Gao, X.-L., Liu, X.-H., Xie, W.-L., Zhang, L.-S., and Yang, S.-C. (2021). Multiscale Observation of Li Plating for Lithium-Ion Batteries. Rare Metals 40, 3038–3048. doi:10.1007/s12598-021-01730-3
Gong, Q., Hou, L., Li, T., Jiao, Y., and Wu, P. (2022). Regulating the Molecular Interactions in Polymer Binder for High-Performance Lithium-Sulfur Batteries. ACS Nano 16, 8449–8460. doi:10.1021/acsnano.2c03059
Gong, Y.-X., and Wang, J.-J. (2020). Solid-state Batteries: From Fundamental Interface Characterization to Realize Sustainable Promise. Rare Metals 39, 743–744. doi:10.1007/s12598-020-01429-x
Goodenough, J. B. (2003). Oxide-Ion Electrolytes. Annu. Rev. Mat. Res. 33, 91–128. doi:10.1146/annurev.matsci.33.022802.091651
Guo, Q., and Zheng, Z. (2020). Rational Design of Binders for Stable Li‐S and Na‐S Batteries. Adv. Funct. Mat. 30, 1907931. doi:10.1002/adfm.201907931
Han, D.-D., Wang, Z.-Y., Pan, G.-L., and Gao, X.-P. (2019). Metal-Organic-Framework-Based Gel Polymer Electrolyte with Immobilized Anions to Stabilize a Lithium Anode for a Quasi-Solid-State Lithium-Sulfur Battery. ACS Appl. Mat. Interfaces 11, 18427–18435. doi:10.1021/acsami.9b03682
Han, F., Gao, T., Zhu, Y., Gaskell, K. J., and Wang, C. (2015). A Battery Made from a Single Material. Adv. Mater 27 (23), 3473–3483. doi:10.1002/adma.201500180
Harm, S., Hatz, A.-K., Moudrakovski, I., Eger, R., Kuhn, A., Hoch, C., et al. (2019). Lesson Learned from NMR: Characterization and Ionic Conductivity of LGPS-like Li7SiPS8. Chem. Mat. 31, 1280–1288. doi:10.1021/acs.chemmater.8b04051
Hikima, K., Yamamoto, T., Phuc, N. H. H., Matsuda, R., Muto, H., and Matsuda, A. (2020). Improved Ionic Conductivity of Li2S-P2s5-LiI Solid Electrolytes Synthesized by Liquid-phase Synthesis. Solid State Ionics 354, 115403. doi:10.1016/j.ssi.2020.115403
Hu, P., Wang, H., Yang, Y., Yang, J., Lin, J., and Guo, L. (2016). Renewable-Biomolecule-Based Full Lithium-Ion Batteries. Adv. Mat. 28, 3486–3492. doi:10.1002/adma.201505917
Huo, H., Chen, Y., Luo, J., Yang, X., Guo, X., and Sun, X. (2019). Rational Design of Hierarchical "Ceramic‐in‐Polymer" and "Polymer‐in‐Ceramic" Electrolytes for Dendrite‐Free Solid‐State Batteries. Adv. Energy Mat. 9, 1804004. doi:10.1002/aenm.201804004
Inaguma, Y., Matsui, Y., Yu, J., Shan, Y.-J., Nakamura, T., and Itoh, M. (1997). Effect of Substitution and Pressure on Lithium Ion Conductivity in Perovskites Ln 12 Li 12 TiO 3 (Ln = La, Pr, Nd AND Sm). J. Phys. Chem. Solids 58, 843–852. doi:10.1016/S0022-3697(96)00226-0
Judez, X., Zhang, H., Li, C., Eshetu, G. G., Zhang, Y., González-Marcos, J. A., et al. (2017). Polymer-Rich Composite Electrolytes for All-Solid-State Li-S Cells. J. Phys. Chem. Lett. 8, 3473–3477. doi:10.1021/acs.jpclett.7b01321
Kim, H., Lee, J., Ahn, H., Kim, O., and Park, M. J. (2015). Synthesis of Three-Dimensionally Interconnected Sulfur-Rich Polymers for Cathode Materials of High-Rate Lithium-Sulfur Batteries. Nat. Commun. 6, 7278. doi:10.1038/ncomms8278
Kokal, I., Ramanujachary, K. V., Notten, P. H. L., and Hintzen, H. T. (2012). Sol-gel Synthesis and Lithium Ion Conduction Properties of Garnet-type Li6BaLa2Ta2O12. Mater. Res. Bull. 47, 1932–1935. doi:10.1016/j.materresbull.2012.04.032
Kwon, W. J., Kim, H., Jung, K.-N., Cho, W., Kim, S. H., Lee, J.-W., et al. (2017). Enhanced Li+ Conduction in Perovskite Li3xLa2/3−x□1/3−2xTiO3 Solid-Electrolytes via Microstructural Engineering. J. Mat. Chem. A 5, 6257–6262. doi:10.1039/C7TA00196G
Ladenstein, L., Simic, S., Kothleitner, G., Rettenwander, D., and Wilkening, H. M. R. (2020). Anomalies in Bulk Ion Transport in the Solid Solutions of Li7La3M2O12 (M = Hf, Sn) and Li5La3Ta2O12. J. Phys. Chem. C 124, 16796–16805. doi:10.1021/acs.jpcc.0c03558
Lang, B., Ziebarth, B., and Elsässer, C. (2015). Lithium Ion Conduction in LiTi2(PO4)3 and Related Compounds Based on the NASICON Structure: A First-Principles Study. Chem. Mat. 27, 5040–5048. doi:10.1021/acs.chemmater.5b01582
Lei, D., Shi, K., Ye, H., Wan, Z., Wang, Y., Shen, L., et al. (2018). Progress and Perspective of Solid-State Lithium-Sulfur Batteries. Adv. Funct. Mat. 28, 1707570. doi:10.1002/adfm.201707570
Li, G., Yang, Z., Jiang, Y., Jin, C., Huang, W., Ding, X., et al. (2016). Towards Polyvalent Ion Batteries: A Zinc-Ion Battery Based on NASICON Structured Na3V2(PO4)3. Nano Energy 25, 211–217. doi:10.1016/j.nanoen.2016.04.051
Li, S., Zhang, W., Zheng, J., Lv, M., Song, H., and Du, L. (2021). Inhibition of Polysulfide Shuttles in Li-S Batteries: Modified Separators and Solid‐State Electrolytes. Adv. Energy Mat. 11, 2000779. doi:10.1002/aenm.202000779
Li, X., Banis, M., Lushington, A., Yang, X., Sun, Q., Zhao, Y., et al. (2018). A High-Energy Sulfur Cathode in Carbonate Electrolyte by Eliminating Polysulfides via Solid-phase Lithium-Sulfur Transformation. Nat. Commun. 9, 4509. doi:10.1038/s41467-018-06877-9
Li, X., Liang, J., Luo, J., Wang, C., Li, X., Sun, Q., et al. (2019). High‐Performance Li-SeS X All‐Solid‐State Lithium Batteries. Adv. Mat. 31, 1808100. doi:10.1002/adma.201808100
Li, Y., Zhou, W., Chen, X., Lü, X., Cui, Z., Xin, S., et al. (2016). Mastering the Interface for Advanced All-Solid-State Lithium Rechargeable Batteries. Proc. Natl. Acad. Sci. U.S.A. 113, 13313–13317. doi:10.1073/pnas.1615912113
Lin, Y., Wang, X., Liu, J., and Miller, J. D. (2017). Natural Halloysite Nano-Clay Electrolyte for Advanced All-Solid-State Lithium-Sulfur Batteries. Nano Energy 31, 478–485. doi:10.1016/j.nanoen.2016.11.045
Liu, G., Sun, Q., Li, Q., Zhang, J., and Ming, J. (2021). Electrolyte Issues in Lithium-Sulfur Batteries: Development, Prospect, and Challenges. Energy fuels. 35, 10405–10427. doi:10.1021/acs.energyfuels.1c00990
Liu, S., Imanishi, N., Zhang, T., Hirano, A., Takeda, Y., Yamamoto, O., et al. (2010). Lithium Dendrite Formation in Li/Poly(ethylene Oxide)–Lithium Bis(trifluoromethanesulfonyl)imide and N-Methyl-N-Propylpiperidinium Bis(trifluoromethanesulfonyl)imide/Li Cells. J. Electrochem Soc. 157 (10), A1092. doi:10.1149/1.3473790
Liu, W., Lee, S. W., Lin, D., Shi, F., Wang, S., Sendek, A. D., et al. (2017). Enhancing Ionic Conductivity in Composite Polymer Electrolytes with Well-Aligned Ceramic Nanowires. Nat. Energy 2, 17035. doi:10.1038/nenergy.2017.35
Liu, X.-M., Cui, X., Dastafkan, K., Wang, H.-F., Tang, C., Zhao, C., et al. (2021). Recent Advances in Spinel-type Electrocatalysts for Bifunctional Oxygen Reduction and Oxygen Evolution Reactions. J. Energy Chem. 53, 290–302. doi:10.1016/j.jechem.2020.04.012
Liu, X.-Z., Ding, L., Liu, Y.-Z., Xiong, L.-P., Chen, J., and Luo, X.-L. (2021). Room-temperature Ionic Conductivity of Ba, Y, Al Co-doped Li7La3Zr2O12 Solid Electrolyte after Sintering. Rare Metals 40, 2301–2306. doi:10.1007/s12598-020-01526-x
Lu, Q., Zou, X., Liao, K., Ran, R., Zhou, W., Ni, M., et al. (2020). Direct Growth of Ordered N‐doped Carbon Nanotube Arrays on Carbon Fiber Cloth as a Free‐standing and Binder‐free Air Electrode for Flexible Quasi‐solid‐state Rechargeable Zn‐Air Batteries. Carbon Energy 2, 461–471. doi:10.1002/cey2.50
Luo, C., Hu, E., Gaskell, K. J., Fan, X., Gao, T., Cui, C., et al. (2020). A Chemically Stabilized Sulfur Cathode for Lean Electrolyte Lithium Sulfur Batteries. Proc. Natl. Acad. Sci. U.S.A. 117, 14712–14720. doi:10.1073/pnas.2006301117
Lustosa, G. M. M. M., Franchetti, M. G. S., de Souza, A., Goulart, F. A. B., da Conceição, L., and Berton, M. A. C. (2021). Fast Synthesis and Sintering of Li5La3Nb2O12 Garnet Ceramic. Mater. Chem. Phys. 257, 123848. doi:10.1016/j.matchemphys.2020.123848
Mariappan, C. R., Yada, C., Rosciano, F., and Roling, B. (2011). Correlation between Micro-structural Properties and Ionic Conductivity of Li1.5Al0.5Ge1.5(PO4)3 Ceramics. J. Power Sources 196, 6456–6464. doi:10.1016/j.jpowsour.2011.03.065
Miura, A., Rosero-Navarro, N. C., Sakuda, A., Tadanaga, K., Phuc, N. H. H., Matsuda, A., et al. (2019). Liquid-phase Syntheses of Sulfide Electrolytes for All-Solid-State Lithium Battery. Nat. Rev. Chem. 3, 189–198. doi:10.1038/s41570-019-0078-2
Mo, Y., Ong, S. P., and Ceder, G. (2012). First Principles Study of the Li10GeP2S12 Lithium Super Ionic Conductor Material. Chem. Mat. 24, 15–17. doi:10.1021/cm203303y
Pan, H., Cheng, Z., He, P., and Zhou, H. (2020). A Review of Solid-State Lithium-Sulfur Battery: Ion Transport and Polysulfide Chemistry. Energy fuels. 34, 11942–11961. doi:10.1021/acs.energyfuels.0c02647
Pang, M.-C., Yang, K., Brugge, R., Zhang, T., Liu, X., Pan, F., et al. (2021). Interactions Are Important: Linking Multi-Physics Mechanisms to the Performance and Degradation of Solid-State Batteries. Mater. Today 49, 145–183. doi:10.1016/j.mattod.2021.02.011
Peng, H., Wang, X., Zhao, Y., Tan, T., Mentbayeva, A., Bakenov, Z., et al. (2017). Enhanced Electrochemical Performance of Sulfur/polyacrylonitrile Composite by Carbon Coating for Lithium/sulfur Batteries. J. Nanoparticle Res. 19 (10), 348. doi:10.1007/s11051-017-4049-6
Phuc, N. H. H., Hikima, K., Muto, H., and Matsuda, A. (2022). Recent Developments in Materials Design for All-Solid-State Li-S Batteries. Crit. Rev. SOLID STATE Mater. Sci. 47, 283–308. doi:10.1080/10408436.2021.1886045
Qiu, Z., Shi, L., Wang, Z., Mindemark, J., Zhu, J., Edström, K., et al. (2019). Surface Activated Polyethylene Separator Promoting Li+ Ion Transport in Gel Polymer Electrolytes and Cycling Stability of Li-Metal Anode. Chem. Eng. J. 368, 321–330. doi:10.1016/j.cej.2019.02.107
Ren, J., Huang, Y., Zhu, H., Zhang, B., Zhu, H., Shen, S., et al. (2020). Recent Progress on MOF‐derived Carbon Materials for Energy Storage. Carbon Energy 2, 176–202. doi:10.1002/cey2.44
Sing, C. E., Zwanikken, J. W., and de la Cruz, M. O. (2014). Electrostatic Control of Block Copolymer Morphology. Nat. Mater. 13 (7), 694–698. doi:10.1038/nmat4001
Umeshbabu, E., Zheng, B., Zhu, J., Wang, H., Li, Y., and Yang, Y. (2019). Stable Cycling Lithium-Sulfur Solid Batteries with Enhanced Li/Li10GeP2S12 Solid Electrolyte Interface Stability. ACS Appl. Mat. Interfaces 11, 18436–18447. doi:10.1021/acsami.9b03726
Varzi, A., Raccichini, R., Passerini, S., and Scrosati, B. (2016). Challenges and Prospects of the Role of Solid Electrolytes in the Revitalization of Lithium Metal Batteries. J. Mater. Chem. A 101039, C6TA07384K. doi:10.1039/c6ta07384k
Wang, D., Zhang, W., Zheng, W., Cui, X., Rojo, T., and Zhang, Q. (2017). Towards High-Safe Lithium Metal Anodes: Suppressing Lithium Dendrites via Tuning Surface Energy. Adv. Sci. 4, 1600168. doi:10.1002/advs.201600168
Wang, H., Yang, Y., and Guo, L. (2017). Nature-Inspired Electrochemical Energy-Storage Materials and Devices. Adv. Energy Mat. 7, 1601709. doi:10.1002/aenm.201601709
Wang, L., Ye, Y., Chen, N., Huang, Y., Li, L., Wu, F., et al. (2018). Development and Challenges of Functional Electrolytes for High-Performance Lithium-Sulfur Batteries. Adv. Funct. Mat. 28, 1800919. doi:10.1002/adfm.201800919
Wang, M., Fan, L., Wu, X., Tian, D., Cheng, J., Qiu, Y., et al. (2017). Hierarchical Mesoporous SnO2 Nanosheets on Carbon Cloth toward Enhancing the Polysulfides Redox for Lithium-Sulfur Batteries. J. Mat. Chem. A 5, 19613–19618. doi:10.1039/c7ta04937d
Wang, Q., Jin, J., Wu, X., Ma, G., Yang, J., and Wen, Z. (2014). A Shuttle Effect Free Lithium Sulfur Battery Based on a Hybrid Electrolyte. Phys. Chem. Chem. Phys. 16, 21225–21229. doi:10.1039/c4cp03694h
Wang, S., Zhang, Y., Zhang, X., Liu, T., Lin, Y.-H., Shen, Y., et al. (2018). High-Conductivity Argyrodite Li6PS5Cl Solid Electrolytes Prepared via Optimized Sintering Processes for All-Solid-State Lithium-Sulfur Batteries. ACS Appl. Mat. Interfaces 10, 42279–42285. doi:10.1021/acsami.8b15121
Wang, Y., Ji, H., Zhang, X., Shi, J., Li, X., Jiang, X., et al. (2021). Cyclopropenium Cationic-Based Covalent Organic Polymer-Enhanced Poly(ethylene Oxide) Composite Polymer Electrolyte for All-Solid-State Li-S Battery. ACS Appl. Mat. Interfaces 13, 16469–16477. doi:10.1021/acsami.1c02309
Wang, Z., Li, Y., Wang, J., Ji, R., Yuan, H., Wang, Y., et al. (2022). Recent Progress of Flexible Aqueous Multivalent Ion Batteries. Carbon Energy 4, 411–445. doi:10.1002/cey2.178
Wang, Z., Tang, Y., Zhang, L., Li, M., Shan, Z., and Huang, J. (2020). In Situ TEM Observations of Discharging/Charging of Solid‐State Lithium‐Sulfur Batteries at High Temperatures. Small 16, 2001899. doi:10.1002/smll.202001899
Wei, B., Shang, C., Pan, X., Chen, Z., Shui, L., Wang, X., et al. (2019). Lotus Root-like Nitrogen-Doped Carbon Nanofiber Structure Assembled with VN Catalysts as a Multifunctional Host for Superior Lithium-Sulfur Batteries. Nanomaterials 9, 1724. doi:10.3390/nano9121724
Westover, A. S., Kercher, A. K., Kornbluth, M., Naguib, M., Palmer, M. J., Cullen, D. A., et al. (2020). Plasma Synthesis of Spherical Crystalline and Amorphous Electrolyte Nanopowders for Solid-State Batteries. ACS Appl. Mat. Interfaces 12, 11570–11578. doi:10.1021/acsami.9b20812
Wu, F., Chu, F., Ferrero, G. A., Sevilla, M., Fuertes, A. B., Borodin, O., et al. (2020). Boosting High-Performance in Lithium-Sulfur Batteries via Dilute Electrolyte. Nano Lett. 20, 5391–5399. doi:10.1021/acs.nanolett.0c01778
Wu, F., Srot, V., Chen, S., Zhang, M., van Aken, P. A., Wang, Y., et al. (2021). Metal-Organic Framework-Derived Nanoconfinements of CoF2 and Mixed-Conducting Wiring for High-Performance Metal Fluoride-Lithium Battery. ACS Nano 15, 1509–1518. doi:10.1021/acsnano.0c08918
Wu, J., Liu, S., Han, F., Yao, X., and Wang, C. (2020). Lithium/Sulfide All‐Solid‐State Batteries Using Sulfide Electrolytes. Adv. Mat. 33, 2000751. doi:10.1002/adma.202000751
Xiao, Q., Yang, J., Wang, X., Deng, Y., Han, P., Yuan, N., et al. (2021). Carbon‐based Flexible Self‐supporting Cathode for Lithium‐sulfur Batteries: Progress and Perspective. Carbon Energy 3, 271–302. doi:10.1002/cey2.96
Xu, H., Yu, Y., Wang, Z., and Shao, G. (2019). A Theoretical Approach to Address Interfacial Problems in All-Solid-State Lithium Ion Batteries: Tuning Materials Chemistry for Electrolyte and Buffer Coatings Based on Li6PA5Cl Hali-Chalcogenides. J. Mat. Chem. A 7, 5239–5247. doi:10.1039/C8TA11151K
Yang, D., Chen, D., Jiang, Y., Ang, E. H., Feng, Y., Rui, X., et al. (2021). Carbon‐based Materials for All‐solid‐state Zinc-Air Batteries. Carbon Energy 3, 50–65. doi:10.1002/cey2.88
Yang, S., He, R., Zhang, Z., Cao, Y., Gao, X., and Liu, X. (2020). CHAIN: Cyber Hierarchy and Interactional Network Enabling Digital Solution for Battery Full-Lifespan Management. Matter 3, 27–41. doi:10.1016/j.matt.2020.04.015
Yang, S., Zhang, Z., Cao, R., Wang, M., Cheng, H., Zhang, L., et al. (2021). Implementation for a Cloud Battery Management System Based on the CHAIN Framework - ScienceDirect. Energy AI 5, 100088. doi:10.1016/j.egyai.2021.100088
Yang, X., Chen, Y., Wang, M., Zhang, H., Li, X., and Zhang, H. (2016). Phase Inversion: A Universal Method to Create High-Performance Porous Electrodes for Nanoparticle-Based Energy Storage Devices. Adv. Funct. Mat. 26, 8427–8434. doi:10.1002/adfm.201604229
Yang, X., Luo, J., and Sun, X. (2020). Towards High-Performance Solid-State Li-S Batteries: From Fundamental Understanding to Engineering Design. Chem. Soc. Rev. 49, 2140–2195. doi:10.1039/c9cs00635d
Yao, X., Huang, N., Han, F., Zhang, Q., Wan, H., Mwizerwa, J. P., et al. (2017). High‐Performance All‐Solid‐State Lithium-Sulfur Batteries Enabled by Amorphous Sulfur‐Coated Reduced Graphene Oxide Cathodes. Adv. Energy Mat. 7, 1602923. doi:10.1002/aenm.201602923
Yin, X., Wang, L., Kim, Y., Ding, N., Kong, J., Safanama, D., et al. (2020). Thermal Conductive 2D Boron Nitride for High‐Performance All‐Solid‐State Lithium-Sulfur Batteries. Adv. Sci. 7, 2001303. doi:10.1002/advs.202001303
You, H., Zhu, J., Wang, X., Jiang, B., Sun, H., Liu, X., et al. (2022). Nonlinear Health Evaluation for Lithium-Ion Battery within Full-Lifespan. J. Energy Chem. 72, 333–341. doi:10.1016/j.jechem.2022.04.013
Yubuchi, S., Teragawa, S., Aso, K., Tadanaga, K., Hayashi, A., and Tatsumisago, M. (2015). Preparation of High Lithium-Ion Conducting Li6PS5Cl Solid Electrolyte from Ethanol Solution for All-Solid-State Lithium Batteries. J. Power Sources 293, 941–945. doi:10.1016/j.jpowsour.2015.05.093
Yubuchi, S., Uematsu, M., Hotehama, C., Sakuda, A., Hayashi, A., and Tatsumisago, M. (2019). An Argyrodite Sulfide-Based Superionic Conductor Synthesized by a Liquid-phase Technique with Tetrahydrofuran and Ethanol. J. Mat. Chem. A 7, 558–566. doi:10.1039/c8ta09477b
Zhang, H., Hao, S., and Lin, J. (2017). Influence of Li2O-B2o3 Glass on Ionic Migration and Interfacial Properties of La2/3−xLi3xTiO3 Solid Electrolyte. J. Alloys Compd. 704, 109–116. doi:10.1016/j.jallcom.2017.02.059
Zhang, L.-S., Gao, X.-L., Liu, X.-H., Zhang, Z.-J., Cao, R., Cheng, H.-C., et al. (2022). CHAIN: Unlocking Informatics-Aided Design of Li Metal Anode from Materials to Applications. Rare Metals 41, 1477–1489. doi:10.1007/s12598-021-01925-8
Zhang, R., Shen, X., Zhang, Y.-T., Zhong, X.-L., Ju, H.-T., Huang, T.-X., et al. (2022). Dead Lithium Formation in Lithium Metal Batteries: A Phase Field Model. J. Energy Chem. 71, 29–35. doi:10.1016/j.jechem.2021.12.020
Zhang, Y., Chen, P., Wang, Q., Wang, Q., Zhu, K., Ye, K., et al. (2021). High‐Capacity and Kinetically Accelerated Lithium Storage in MoO 3 Enabled by Oxygen Vacancies and Heterostructure. Adv. Energy Mat. 11, 2101712. doi:10.1002/aenm.202101712
Zhao, M., Chen, X., Li, X. Y., Li, B. Q., and Huang, J. Q. (2021). An Organodiselenide Comediator to Facilitate Sulfur Redox Kinetics in Lithium-Sulfur Batteries. Adv. Mat. 33, 2007298. doi:10.1002/adma.202007298
Zheng, C., Wang, K., Li, L., Huang, H., Liang, C., Gan, Y., et al. (2021). High-Performance All-Solid-State Lithium-Sulfur Batteries Enabled by Slurry-Coated Li6PS5Cl/S/C Composite Electrodes. Front. Energy Res. 8, 606494. doi:10.3389/fenrg.2020.606494
Zhong, L., Wang, S., Xiao, M., Liu, W., Han, D., Li, Z., et al. (2021). Addressing Interface Elimination: Boosting Comprehensive Performance of All-Solid-State Li-S Battery. ENERGY STORAGE Mater. 41, 563–570. doi:10.1016/j.ensm.2021.06.035
Zhou, C.-C., Su, Z., Gao, X.-L., Cao, R., Yang, S.-C., and Liu, X.-H. (2021). Ultra-high-energy Lithium-Ion Batteries Enabled by Aligned Structured Thick Electrode Design. Rare Metals 41, 14–20. doi:10.1007/s12598-021-01785-2
Zhou, S., Fang, C., Song, X., and Liu, G. (2020). The Influence of Compact and Ordered Carbon Coating on Solid‐state Behaviors of Silicon during Electrochemical Processes. Carbon Energy 2, 143–150. doi:10.1002/cey2.28
Keywords: solid-state lithium-sulfur batteries, solid-state electrolyte, shuttle effect, lithium dendrites, interface
Citation: Yang S, Zhang Z, Lin J, Zhang L, Wang L, Chen S, Zhang C and Liu X (2022) Recent Progress in Quasi/All-Solid-State Electrolytes for Lithium–Sulfur Batteries. Front. Energy Res. 10:945003. doi: 10.3389/fenrg.2022.945003
Received: 16 May 2022; Accepted: 30 May 2022;
Published: 06 July 2022.
Edited by:
Hongtao Sun, The Pennsylvania State University (PSU), United StatesReviewed by:
Bo Nie, The Pennsylvania State University, United StatesGongkai Wang, Hebei University of Technology, China
Copyright © 2022 Yang, Zhang, Lin, Zhang, Wang, Chen, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijing Wang, d2FuZ2xpamluZ0BidWFhLmVkdS5jbg==; Siyan Chen, Y2hlbnNpeWFuMTk4N0BqbHUuZWR1LmNu; Xinhua Liu, bGl1eGluaHVhMTlAYnVhYS5lZHUuY24=
 Shichun Yang
Shichun Yang Zhengjie Zhang1
Zhengjie Zhang1 Siyan Chen
Siyan Chen Xinhua Liu
Xinhua Liu