- 1College of Petroleum Engineering, Yangtze University, Wuhan, China
- 2Key Laboratory of Drilling and Production Engineering for Oil and Gas, Wuhan, China
Introduction
Enhanced oil recovery (EOR) technologies are attracting substantial attention worldwide in the last few decades due to the growing gap between energy supply and social demands, as well as the noticeable production decline from the existing reservoirs and the difficulties in discovering new economic reverses (Almahfood and Bai, 2018; Panchal et al., 2021; Wang et al., 2022). Taking the Shengli Oilfield in China as an example, a 1% increase in oil recovery means additional 46 million tons of crude oil can be recovered, which is equivalent to the 2-year output of this oilfield.
A surfactant that possesses multiple EOR mechanisms has attracted a great deal of attention. However, its large-scale applications are generally limited by the high cost (i.e., zwitterionic and Gemini surfactants), the considerable adsorption loss (i.e., cationic surfactants on sandstone and ionic surfactants on carbonates), and the possible formation damage especially at harsh reservoir conditions (i.e., nonionic surfactants at temperatures above their cloud point) (Zhong et al., 2019a; Pal et al., 2019). Nanoparticles (NPs) are small particles (1–100 nm) with high surface energy and are free to enter the tiny pores and channels that might be blocked by macromolecules or other materials. Moreover, their surface properties can be facilely tailored (i.e., hydrophilic or hydrophobic, positively charged or negatively charged) to meet the requirements of different situations. The emergence of nanotechnology has provided some new explications to address some of these head-scratching problems confronted with surfactant applications and thus realize the goal of maintaining sustainable hydrocarbon recovery (Zhong et al., 2021; Chen et al., 2022). Surfactant and NP combinations are widely applied in tight/low-permeability and heavy oil reservoir exploitation, profile control operations, and so on. Integrating surfactant with NPs can induce further interfacial tension (IFT) or oil viscosity reduction, better alter the rock wettability, thicken the displacing fluid, and stabilize the foams or emulsions. Though many satisfying results were obtained, the synergistic mechanisms between NPs and surfactants remain obscure. This study highlights the interactions between NPs and surfactants to clarify the underlying synergistic mechanisms, which may shed light on chemical screening and the manipulation of novel surfactant–NP formulas with greater EOR potential.
Synergistic Mechanisms
IFT Reduction
Sufficient adsorption and accumulation of surface-active agents at the liquid–liquid interface are required to induce a drastic reduction in IFT. The reduction of excess free energy (G = γ. A, where γ is oil/water IFT and A is the oil/water interfacial contact area) is the driving force for surfactants and NPs to stay at the interface. Surfactants can lower the IFT, while NPs can reduce the contact area (Vu and Papavassiliou, 2018). Normally, lower Gibbs free energy of adsorption and lower activation energy of adsorption lead to higher adsorption of active chemicals.
Repulsion-Assist-Diffusion Mechanism
The repulsive Coulomb interactions between surfactants and NPs with a similar charge will promote surfactant diffusion to the liquid–liquid interface. Generally, the higher the surfactant density at the water/oil interface, the lower the IFT. When 1,000 mg/L of negatively charged SiO2 NPs was introduced into an anionic surfactant SDS solution, the kerosene/DI water IFT was reduced by another 30% on the basis of pure SDS (1,500 mg/L) (Zargartalebi et al., 2014). This mechanism also works for the nonionic surfactant dodecyl dimethyl phosphine oxide (C12DMPO, 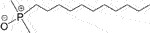 )/silica mixture because of the fractional negative charge of the oxygen atom. When 2.5 wt% SiO2 NPs were added into an 8.5 × 10−6 M C12DMPO solution, a further ∼5 mN/m reduction in heptane/water IFT was reported (Vatanparast et al., 2019). In these cases, the concentration value derived from equilibrium IFT is denoted as equivalent concentration (EC), which increases with increasing NP concentration and decreasing NP size (with higher surface charge density).
)/silica mixture because of the fractional negative charge of the oxygen atom. When 2.5 wt% SiO2 NPs were added into an 8.5 × 10−6 M C12DMPO solution, a further ∼5 mN/m reduction in heptane/water IFT was reported (Vatanparast et al., 2019). In these cases, the concentration value derived from equilibrium IFT is denoted as equivalent concentration (EC), which increases with increasing NP concentration and decreasing NP size (with higher surface charge density).
Interfacial Concentrate Mechanism
NPs can concentrate surfactants on the interface and effectively reduce the interfacial area available to the surfactants. There are two action modes. 1) For NPs with no interfacial activity that originally stayed in the water or oil phase, surfactant molecules can adsorb on NPs through electrostatic attraction or hydrophobic interactions. NPs with modified hydrophilic-lipophilic balance can thereafter migrate to the interface. The competitive adsorption between the liquid and the NPs makes surfactant molecules partially desorb from NPs and redistribute on the interface to reduce the IFT. If the NPs can stay at the interface, the reduction in the interfacial area will promote IFT reduction. Otherwise, the impacts of NPs can be dismissed (Vu and Papavassiliou, 2018). Similar results were reported by Moghadam and Azizian (2014) and Vatanparast et al. (2019). In this case, NPs are good candidates as surfactant carriers, but the surfactant/NP concentration ratio should be carefully tailored. NP aggregation or precipitation may cause adverse impacts (Ravera et al., 2006). 2) For original NPs with interfacial activity (such as Janus NPs) that can stably stay at the interface, though there are negligible interactions between NPs and surfactants, this mechanism still works. According to Vu and Papavassiliou (2019), Janus NP alone barely had any impacts on heptadecane/water IFT, and surfactant SDS alone with an interfacial concentration of 0.91 molecule/nm2 could reduce the IFT by ∼25%. When Janus NPs covered 37.5% of the interface, the local SDS concentration would increase from 0.65–0.95 to 1.8–2.4 molecule/nm2, leading to a reduction of ∼75%. In this scenario, NPs that could occupy a larger interfacial area or preferentially be positioned in the middle of the interface are better choices when low IFTs are required at relatively low surfactant concentrations.
Solubility Reduction Mechanism
The interactions between surfactants and NPs can reduce surfactants’ solubility in the water or oil phase and, thereafter, facilitate their migration to the interface. For instance, driven by electrostatic attraction, oil-soluble cationic surfactant dodecylamine (DDA) could adsorb on negatively charged hydrophilic kaolinite NP surfaces. Therefore, its partition in the water phase and its adsorption at the oil–water interface would increase. According to Wang et al. (2004), 1 mM DDA alone could decrease the hexadecane/water IFT by around 10 mN/m from the original 49 mN/m. Introducing 2.0 wt% kaolinite into the system could induce another 10 mN/m reduction. While for palmitic acid (PA, oil-soluble-)–kaolinite system, the repulsion between particle and surfactant would hinder surfactant migration toward the interface and result in an IFT increase.
Adsorption Reduction Mechanism
In real cases, surfactant adsorption loss is a worrying problem that increases the cost. Meanwhile, due to reduced effective concentration, a low IFT can hardly be ensured in middle and deep reservoirs. NPs can effectively reduce surfactant adsorption loss by forming NP shielding (Alonso et al., 2009; Zhong et al., 2019b), increasing the repulsion between surfactant and rocks (Zargartalebi et al., 2015), providing more fierce collision, and friction effect between NPs and sands (Wu et al., 2017), among others. Therefore, the validity of the surfactant in the presence of NPs can be longer.
Wettability Alteration
Surfactant makes rock less oil-wet through ion-pair or adsorption mechanism, while by adsorbing or exerting structural disjoining force, NP addition enhances the likelihood that a more water-wet condition will arise.
Co-Adsorption Mechanism
Surfactant adsorption on NP surfaces through electrostatic attraction or hydrogen bonding can accelerate the co-adsorption of surfactant and NPs by inducing NP aggregation or deposition. Both the formation of nanostructures and the change in surface free energy are conducive to a more noticeable wettability change (Karimi et al., 2012; Songolzadeh and Moghadasi., 2017). In this case, higher salinity and temperature are preferred (Al-Anssari et al., 2018). However, the possible formation damage should be considered, and a suitable NP concentration should be selected.
Dispersity Increase Mechanism
NPs in an aqueous dispersion tend to self-assemble to form a wedge-like structure at the discontinuous phase. Under the drive of Brownian motion or electrostatic repulsion, dispersed NPs push the confined NPs forward and impart a huge force called disjoining pressure (Chengara et al., 2004). Generally, higher disjoining pressure corresponds to a more obvious wettability change. Either increasing NP concentration and stability or decreasing NP size and solution salinity can raise the disjoining pressure (Hendraningrat et al., 2013). The addition of surfactant into NP dispersion can increase NP dispersity and stability in the aqueous phase through hydrophobic interaction or imposing supercharging effect. By generating a more favorable wet–wet condition, a higher recovery can be obtained (Zhao et al., 2018; Zhong et al., 2020).
Foam/Emulsion Stabilization
Surfactants are traditional foam and emulsion stabilizers. Using surfactants and NPs together can construct three-phase foam and Pickering emulsions with higher stability. To generate the synergisms, NPs that are capable to stably adsorb on the air/water or oil/water interface is a precondition. Using surfactant with NPs can in situ modify the hydrophilicity-lipophilicity balance and surface charge of NPs to stabilize the foams and the emulsions better.
Emulsion Stabilization
De-emulsion occurs in two successive steps: coagulation and coalescence. Adsorbed charged NPs can increase the electrostatic or steric repulsion between oil droplets and control coagulation. However, NPs can form an obstacle to restrict the coalescence by forming a rigid coating around the liquid droplets to prevent coalescence. Meanwhile, using surfactant and NPs together can also thicken the continuous phase by forming network structures (Ortiz et al., 2020). It is also worth noting that for Pickering emulsion preparation, a reduction in IFT is not an essential precondition. A lower IFT can reduce the required external energy input. To stabilize the emulsions, NPs should be able to go and stay at the oil/water interface. According to Lian et al. (2020), for mono-layer NP stabilized emulsions, NPs with contact angles of 15°–90° and 90°–165° should be selected for O/W and W/O emulsion, respectively. While for multiple-layer NP stabilized emulsions, the ranges are 15°–129.3° and 50.7°–165°. The adsorption of surfactants on NPs can in situ change NP wettability and favor their migration to the interface.
Foam Stabilization
NPs have higher adhesion energy compared to surfactants. By accumulating at the interfaces, minimizing the contact area between air and water, increasing the film strength and lamella elasticity, and decreasing the gas diffusion, NPs are good foam stabilizers. However, the impacts of NP hydrophilicity, size, and surface charge are significant. NPs should be sufficiently hydrophilic to disperse in the aqueous phase and also sufficiently lipophilic to stay stably at the interface (Majeed et al., 2021). Properly taking full advantage of surfactant–NP interactions can partially alleviate this contradiction.
Surfactant Adsorption on NPs
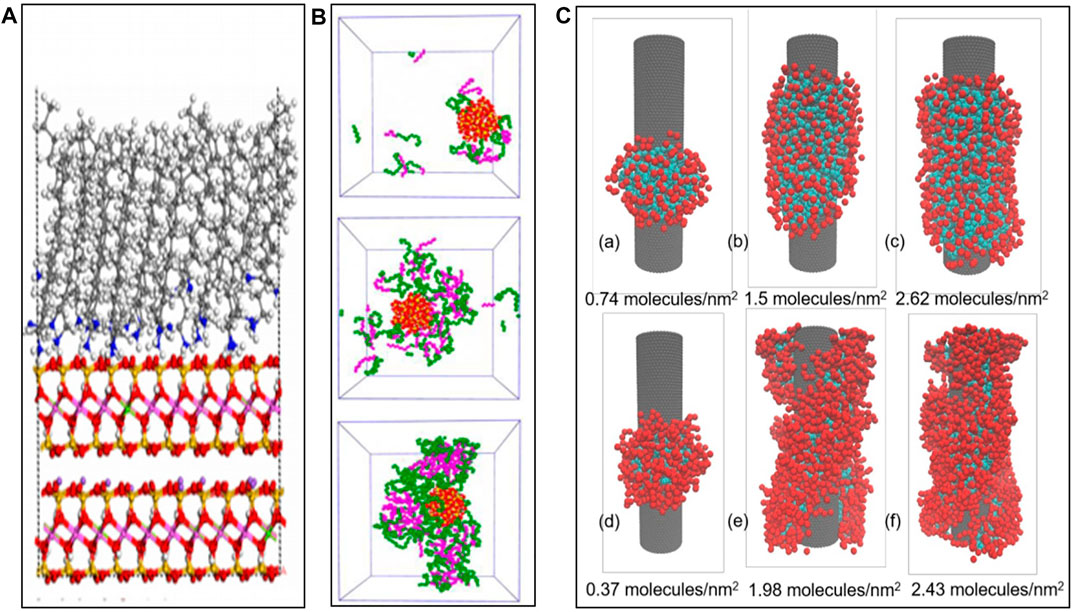
The synergisms between NPs and surfactants highly rely on surfactant–NP interactions, and surfactant adsorption on NP surfaces is an important embodiment. The driving forces that push surfactant to adsorb on NPs are electrostatic attraction (Figure 1A, Peng et al., 2017), hydrogen bonding (Figure 1B, Zhong et al., 2019b), hydrophobic interactions (Figure 1C, Vu and Papavassiliou, 2018), and so on.
Electrostatic attraction dominates in systems when the surfactant and NP possess the opposite charge, for example, cationic surfactant CTAB/hydrophilic SiO2 system (Songolzadeh and Moghadasi., 2017) and zwitterionic carboxyl betaine/hydrophilic SiO2 system. Liu et al. (2017) reported that the saturated adsorption density of C12B on silica was 8.5 × 10−3 mmol/m2 at pH = 6.1.
Hydrogen bonding is the main driving force that facilitates the adsorption of oligooxyethylene-based nonionic surfactants on hydrophilic NPs with hydroxyl groups on the surfaces and mainly adsorbs in the form of micellar structures. In this case, NP stability is relevant to the extent of micelles coverage. According to Sharma et al. (2010), a maximum of 14 C12E9 micelles could be adsorbed (on average) on each silica particle (diameter = 15 nm).
Hydrophobic interaction is the main driving force that promotes the adsorption of water-soluble surfactant on hydrophobic NPs. By forming nano-complex and exposing the hydrophilic head of surfactant molecules to the aqueous phase, the dispersity of hydrophobic NPs in water significantly increases. According to Vu and Papavassiliou (2018), the maximum adsorption density of SDS and C12E8 on hydrophobic nanotubes (length = 20 nm, diameter = 4 nm) was 2.62 and 2.43 molecules/nm2, respectively. The loading of short- and straight-molecule surfactants is higher than long and branched ones.
Conclusion
Integrating NPs with a surfactant can produce many synergisms: 1) inducing further IFT reduction through the repulsion-assist-diffusion mechanism, interfacial concentrate mechanism, solubility reduction mechanism, and adsorption reduction mechanism; 2) better modifying rock wettability through the Co-adsorption mechanism or dispersity increase mechanism; and 3) increasing the stability of emulsions and foams by forming a rigid coating around the liquid droplets, thickening the continuous phase or increasing the lamella elasticity. However, all the synergisms are closely related to surfactant adsorption on NP surfaces, which can be affected by surfactant type and structure, NP size and surface charge, and solution conditions. In order to exert the synergisms adequately, NP hydrophilicity should be carefully tailored, and the adsorption behaviors of surfactants on NPs or at the interface and the other interactions between NPs and surfactants should be further studied.
Author Contributions
FX: investigation and writing—original draft. XZ: conceptualization, supervision, funding acquisition, and writing—review and editing. ZL, WC, YY, and ML: investigation and editing.
Funding
This work was supported by the Natural Science Foundation of China (NSFC) (Grant no. 52104019), the Scientific Research Development Fund of Yangtze University, and the Innovation and Entrepreneurship Training program for College Students of Yangtze University (Yz2020287).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Anssari, S., Arif, M., Wang, S., Barifcani, A., Lebedev, M., and Iglauer, S. (2018). Wettability of Nanofluid-Modified Oil-Wet Calcite at Reservoir Conditions. Fuel 211, 405–414. doi:10.1016/j.fuel.2017.08.111
Almahfood, M., and Bai, B. (2018). The Synergistic Effects of Nanoparticle-Surfactant Nanofluids in EOR Applications. J. Petroleum Sci. Eng. 171, 196–210. doi:10.1016/j.petrol.2018.07.030
Alonso, U., Missana, T., and Patelli, A. (2009). Quantification of Au Nanoparticles Retention on a Heterogeneous Rock Surface. Colloids Surf. A 347 (1), 230–238. doi:10.1016/j.colsurfa.2009.04.046
Chen, J., Zhong, X., and Xu, F. (2022). Nanoparticles as Depressurization and Augmented Injection Agents to Facilitate Low Permeability Reservoir Exploitation: Potentials and Risks. Front. Energy Res. 10, 830742. doi:10.3389/fenrg.2022.830742
Chengara, A., Nikolov, A. D., Wasan, D. T., Trokhymchuk, A., and Henderson, D. (2004). Spreading of Nanofluids Driven by the Structural Disjoining Pressure Gradient. J. Colloid Interface Sci. 280 (1), 192–201. doi:10.1016/j.jcis.2004.07.005
Fereidooni Moghadam, T., and Azizian, S. (2014). Effect of ZnO Nanoparticle and Hexadecyltrimethylammonium Bromide on the Dynamic and Equilibrium Oil-Water Interfacial Tension. J. Phys. Chem. B 118, 1527–1534. doi:10.1021/jp4106986
Gonzalez Ortiz, D., Pochat-Bohatier, C., Cambedouzou, J., Bechelany, M., and Miele, P. (2020). Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 6 (4), 468–482. doi:10.1016/j.eng.2019.08.017
Hendraningrat, L., Li, S., and Torsater, O. (2013). “Effect of Some Parameters Influencing Enhanced Oil Recovery Process Using Silica Nanoparticles: an Experimental Investigation,” in Proceedings of SPE Reservoir Characterization and Simulation Conference and Exhibition, Abu Dhabi, UAE, September 16-18. SPE Paper 165955. doi:10.2118/165955-ms
Karimi, A., Fakhroueian, Z., Bahramian, A., Pour Khiabani, N., Darabad, J. B., Azin, R., et al. (2012). Wettability Alteration in Carbonates Using Zirconium Oxide Nanofluids: EOR Implications. Energy fuels. 26 (2), 1028–1036. doi:10.1021/ef201475u
Lian, E. L., Sangeetaprivya, P. S., and Yong, K. H. (2020). Recent Advances of Characterization Techniques for the Formation, Physical Properties and Stability of Pickering Emulsion. Adv. Colloid Interface Sci. 277, 102117. doi:10.1016/j.cis.2020.102117
Liu, K., Jiang, J., Cui, Z., and Binks, B. P. (2017). pH-Responsive Pickering Emulsions Stabilized by Silica Nanoparticles in Combination with a Conventional Zwitterionic Surfactant. Langmuir 33 (9), 2296–2305. doi:10.1021/acs.langmuir.6b04459
Majeed, T., Kamal, M. S., Zhou, X., and Solling, T. (2021). A Review on Foam Stabilizers for Enhanced Oil Recovery. Energy fuels. 35 (7), 5594–5612. doi:10.1021/acs.energyfuels.1c00035
Pal, N., Kumar, N., Saw, R. K., and Mandal, A. (2019). Gemini Surfactant/polymer/silica Stabilized Oil-In-Water Nanoemulsions: Design and Physicochemical Characterization for Enhanced Oil Recovery. J. Petroleum Sci. Eng. 183, 106464. doi:10.1016/j.petrol.2019.106464
Panchal, H., Patel, H., Patel, J., and Shah, M. (2021). A Systematic Review on Nanotechnology in Enhanced Oil Recovery. Petroleum Res. 6 (3), 204–212. doi:10.1016/j.ptlrs.2021.03.003
Peng, C., Min, F., and Liu, L. (2017). Effect of pH on the Adsorption of Dodecylamine on Montmorillonite: Insights from Experiments and Molecular Dynamics Simulations. Appl. Surf. Sci. 425, 996–1005. doi:10.1016/j.apsusc.2017.07.085
Ravera, F., Santini, E., Loglio, G., Ferrari, M., and Liggieri, L. (2006). Effect of Nanoparticles on the Interfacial Properties of Liquid/liquid and Liquid/air Surface Layers. J. Phys. Chem. B 110, 19543–19551. doi:10.1021/jp0636468
Sharma, K. P., Aswal, V. K., and Kumaraswamy, G. (2010). Adsorption of Nonionic Surfactant on Silica Nanoparticles: Structure and Resultant Interparticle Interactions. J. Phys. Chem. B 114 (34), 10986–10994. doi:10.1021/jp1033799
Songolzadeh, R., and Moghadasi, J. (2017). Stabilizing Silica Nanoparticles in High Saline Water by Using Ionic Surfactants for Wettability Alteration Application. Colloid. Polym. Sci. 295, 145–155. doi:10.1007/s00396-016-3987-3
Vatanparast, H., Eftekhari, M., Javadi, A., Miller, R., and Bahramian, A. (2019). Influence of Hydrophilic Silica Nanoparticles on the Adsorption Layer Properties of Non-ionic Surfactants at Water/heptane Interface. J. Colloid Interface Sci. 545, 242–250. doi:10.1016/j.jcis.2019.03.047
Vu, T. V., and Papavassiliou, D. V. (2018). Modification of Oil-Water Interfaces by Surfactant-Stabilized Carbon Nanotubes. J. Phys. Chem. C 122 (48), 27734–27744. doi:10.1021/acs.jpcc.8b08735
Vu, T. V., and Papavassiliou, D. V. (2019). Synergistic Effects of Surfactants and Heterogeneous Nanoparticles at Oil-Water Interface: Insights from Computations. J. Colloid Interface Sci. 553, 50–58. doi:10.1016/j.jcis.2019.05.102
Wang, W., Zhou, Z., Nandakumar, K., Xu, Z., and Masliyah, J. H. (2004). Effect of Charged Colloidal Particles on Adsorption of Surfactants at Oil-Water Interface. J. Colloid Interface Sci. 274 (2), 625–630. doi:10.1016/j.jcis.2004.03.049
Wang, X., Liu, Y., and Hou, J. (2022). The Relationship between Synsedimentary Fault Activity and Reservoir Quality — A Case Study of the Ek1 Formation in the Wang Guantun Area, China. Interpretation 8, sm15–sm24. doi:10.1190/int-2019-0131.1
Wu, Y., Chen, W., Dai, C., Huang, Y., Li, H., Zhao, M., et al. (2017). Reducing Surfactant Adsorption on Rock by Silica Nanoparticles for Enhanced Oil Recovery. J. Petroleum Sci. Eng. 153, 283–287. doi:10.1016/j.petrol.2017.04.015
Zargartalebi, M., Barati, N., and Kharrat, R. (2014). Influences of Hydrophilic and Hydrophobic Silica Nanoparticles on Anionic Surfactant Properties: Interfacial and Adsorption Behaviors. J. Petroleum Sci. Eng. 119, 36–43. doi:10.1016/j.petrol.2014.04.010
Zargartalebi, M., Kharrat, R., and Barati, N. (2015). Enhancement of Surfactant Flooding Performance by the Use of Silica Nanoparticles. Fuel 143, 21–27. doi:10.1016/j.fuel.2014.11.040
Zhao, M., Lv, W., Li, Y., Dai, C., Wang, X., Zhou, H., et al. (2018). Study on the Synergy between Silica Nanoparticles and Surfactants for Enhanced Oil Recovery during Spontaneous Imbibition. J. Mol. Liq. 261, 373–378. doi:10.1016/j.molliq.2018.04.034
Zhong, X., Chen, J., An, R., Li, K., and Chen, M. (2021). A State-Of-The-Art Review of Nanoparticle Applications with a Focus on Heavy Oil Viscosity Reduction. J. Mol. Liq. 344, 117845. doi:10.1016/j.molliq.2021.117845
Zhong, X., Li, C., Li, Y., Pu, H., Zhou, Y., and Zhao, J. X. (2020). Enhanced Oil Recovery in High Salinity and Elevated Temperature Conditions with a Zwitterionic Surfactant and Silica Nanoparticles Acting in Synergy. Energy fuels. 34, 2893–2902. doi:10.1021/acs.energyfuels.9b04067
Zhong, X., Li, C., Pu, H., Zhou, Y., and Zhao, J. X. (2019b). Increased Nonionic Surfactant Efficiency in Oil Recovery by Integrating with Hydrophilic Silica Nanoparticle. Energy fuels. 33, 8522–8529. doi:10.1021/acs.energyfuels.9b02245
Keywords: synergistic, mechanisms, nanoparticles, surfactant, interactions
Citation: Xu F, Zhong X, Li Z, Cao W, Yang Y and Liu M (2022) Synergistic Mechanisms Between Nanoparticles and Surfactants: Insight Into NP–Surfactant Interactions. Front. Energy Res. 10:913360. doi: 10.3389/fenrg.2022.913360
Received: 05 April 2022; Accepted: 25 April 2022;
Published: 20 May 2022.
Edited by:
Fuyong Wang, China University of Petroleum, ChinaReviewed by:
Minglu Shao, Changzhou University, ChinaYing Sun, Suzhou Institute of Nano-tech and Nano-bionics (CAS), China
Copyright © 2022 Xu, Zhong, Li, Cao, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xun Zhong, ODE0NDIyNTc4QHFxLmNvbQ==
 Fangzhou Xu
Fangzhou Xu Xun Zhong
Xun Zhong Zhiqi Li1
Zhiqi Li1