- 1Laboratory of Biochemistry and Applied Microbiology, Department of Food Engineering and Science, UNESP—University Estadual Paulista—IBILCE, Sao José do Rio Preto, Brazil
- 2Laboratory of Renewable Resources Engineering, Department of Agricultural and Biological Engineering, Purdue University, West Lafayette, IN, United States
- 3Laboratory of Sucrochemistry and Analytical Chemistry, Department of Chemistry and Environmental Sciences, UNESP—University Estadual Paulista—IBILCE, Sao José do Rio Preto, Brazil
- 4Hodson Science and Technology Center, Department of Biology, Hood College, Frederick, Brazil
- 5Laboratory of Enzymology, Department of Cellular Biology, University of Brasília, Brasília, Brazil
In this work, we have tested individual and combination of applications of ozonolysis and liquid hot water (LHW) to pretreat sugarcane bagasse (SCB) for the removal of enzyme and/or microbial inhibitors and generation of potential value-added chemicals. A solid content with 80% cellulose and a liquid phase (liquor) rich in phenolic derived compounds (3 g.L−1) from lignin, sugars (>20 g.L−1), and other compounds, such as furfural and hydroxymethylfurfural (HMF), were generated. Maximal (59%) glucan conversion occurred in the presence of double-pretreated bagasse, which had 32–50% more glucan available than the samples that were individually LHW or ozone-pretreated, resulting in maximal ethanol production (92% after 42 h) from double-pretreated SCB enzyme hydrolyzate. In summary, this work showed that ozone reacts effectively with lignin without the use of any other chemical reagent, and LHW pretreatment, followed by a washing step, was effective in solubilizing and cleaning up the fiber enzyme and microbial inhibitory compounds with ozone being effective against phenolics. Moreover, the generated cellulose-rich substrate is readily fermentable. The acidic liquor fraction removed by sequential washings and containing mainly sugars and phenolic compounds may be evaluated for use in green chemistry bioconversions processes.
Introduction
The high resistance of cellulose to degradation is the main biological barrier to lignocellulosic processing on a large scale (Ximenes et al., 2021). To overcome that, the application of mild and eco-friendly pretreatment techniques have been preferred instead of traditional acid or alkali pretreatments that have been previously proposed for lignocelluloses. Compared to the latter two pretreatments, hydrothermal pretreatment can be performed in a large scale under more gentle conditions (Pedersen and Meyer, 2010; Ruiz et al., 2020).
Liquid hot water (LHW) and to a lesser extent ozonolysis have been tested for the pretreatment of different lignocellulosic materials with LHW pretreatment being one of the leading pretreatments since it improves cellulose digestibility at lower cost, and is carried out once without chemicals (Kim et al., 2009; Kim et al., 2011; Kim et al., 2013; Ximenes et al., 2017; Ruiz et al., 2020; 2021). When choosing operational conditions of pretreatment, it is important to consider the type of biomass as well as the formed lignocellulosic degradation products that are inhibitory to downstream biochemical reactions (Ko et al., 2015a,b,c; Jonsson and Martin, 2016, Ximenes et al., 2017; Ruiz et al., 2021). In this sense, LHW pretreatment of a variety of lignocellulosic materials has included a wide range of operational conditions, including temperature, resident time, particle size, and water-to-solid biomass ratio, among others, and aims to avoid the formation of enzyme and/or microbial inhibitors. Hydrothermal pretreatment is generally performed under conditions of 150–230°C for 10–50 min and pressures corresponding to about 4.9–20 bars (Kim et al., 2009; Rasmussen et al., 2014; Ximenes et al., 2017; Aguilar et al., 2018; Pino et al., 2018; Ruiz et al., 2021). Hydronium ions act as catalysts to hydrolyze and solubilize hemicellulose at an elevated temperature, while acetic acid and other organic acids generated from hemicellulose also facilitate this process (Weil et al., 1998; Kim I. J. et al., 2014; Kim Y. et al., 2014; Ximenes et al., 2017; Ruiz et al., 2020).
Ozonolysis is a less studied pretreatment than LHW and represents another promising approach for lignocellulosic treatment since it has a high specificity of reaction with ozone gas being readily obtained at atmospheric pressure and room temperature. Other benefits are moderate cost of production and no wastewater generation (Barros et al., 2013; Gitifar et al., 2013; Panneerselvam et al., 2013; Travaini et al., 2013; Perrone et al., 2016).
Combined pretreatments of lignocellulosic substrates have recently been proposed for different types of biomass aiming at a more effective result when individual features of the two pretreatments are combined. A more effective recovery of lignin and hemicellulose is possible with the potential of maximizing their application in a biorefinery concept (Sun et al., 2016). The estimated global production of bio-based chemicals and polymers is about 50 million metric tonnes per year (mtpy), but most chemicals and polymers are still produced from petroleum sources (Jong et al., 2012; Rosales-Calderon and Arantes, 2019).
Lignocellulosic materials consist of ∼30% lignin by weight and 40% by energy (Perlack et al., 2005; Beauchet et al., 2012). In this sense, lignin is a valuable resource that merits further study to increase the commercial viability of a biorefinery (Agrawal et al., 2014), although technologies aiming to convert lignin to macromolecules and aromatic chemicals are still under development (Rosales-Calderon and Arantes, 2019). Potential uses of lignin-derived products already include production of activated carbon, binders, carbon fibers, motor fuel, plastic materials, and sorbents (Demuner et al., 2019). The combination of ozonolysis and LHW pretreatments tested here to enhance enzymatic hydrolysis, and alleviate inhibition during saccharification and fermentation of sugarcane bagasse (SCB) is also attractive for generating valued compounds from phenolic compounds derived from lignin.
Material and Methods
Material
SCB from 2013/2014 period of harvesting was supplied by Alta Mogiana sugarcane mill (São Joaquim da Barra, Brazil). The experimental work was developed between 2015 and 2017. The biomass was washed six times with deionized (DI) water, dried in an oven at 45°C to 10% or lower humidity, and milled to a particle size of about 1.0 mm before use. Enzymatic cocktails CellicTM CTec2® and HTec2® were provided by Novozymes Latin America (Araucária, Brazil). All other reagents and chemicals, unless otherwise noted, were purchased from Sigma-Aldrich (St. Louis, MO, United States). The fermentative industrial strain (Saccharomyces cerevisiae JP1) was provided by AEB Latin America (Sao José dos Pinhais, Brazil).
Pretreatments of Biomass
Two types of pretreatments were evaluated individually and/or in a combined sequence. Ozonolysis was performed using O3 gas to chemically oxidize biomass components, and LHW was employed as a physical process using water as the reagent. Combined ozonolysis was performed first to degrade lignin, and then followed by LHW. Operational conditions of each one are described.
Ozonolysis
Ozonolysis was performed at room temperature and atmospheric pressure, according to Travaini et al. (2013), with few modifications. In total, 25.0 g of dry SCB were humidified at 50% (w/v) with DI water, filled in a fixed bed glass column (2.7 × 50.0 cm), and kept under saturated gas O3 (flux of 32.0 mg min−1) for 60 min. Ozone was produced from atmospheric air by the corona process (Radast 10C, Ozoxi-Ozonio). Ozone flux was monitored according to the Standard Methods for the Examination of Water and Wastewater (APHA, 1998). After pretreatment, SCB was air-dried at room temperature prior to cold water washing, performed by a five-step sequential procedure. Each step was carried out mixing 5.0% (w/v) of pretreated SCB with DI water under 30 min of agitation at room temperature, followed by filtration to separate washed solids, which were dried at 45°C for 24 h before enzymatic hydrolysis. The supernatants of each washing were stored at 4°C protected from light until analysis of total phenolics.
Liquid Hot Water
LHW pretreatment was conducted as described previously (Kim et al., 2009; Ko et al., 2015a) with few modifications. Each batch was performed by mixing 3.5 g of dry SCB (untreated or ozonized SCB) with DI water for a final concentration of 10% solids (w/v). The resulting material was placed in a metal column (2.2 × 13.5 cm) for heating in a sand bath at 190°C for 15 min. It was heated for 5 min. The tube was quenched in water for 20 s and then placed in an ice bucket for 25 min to stop the reaction. The pretreated material was then vacuum filtered using Whatman® no 1 filter paper to separate the solids from liquor (suspension). Same five washing steps at room temperature described before were performed in the LHW-pretreated solids. The liquor from pretreatment and supernatants of each washing were stored at 4°C protected from light until analysis of total phenolics.
Enzymatic Hydrolysis
Hydrolysis experiments were conducted using 10% (w/v) of pretreated SCB as a substrate. SCB was suspended in 0.05M sodium citrate buffer at pH 5.0. The reaction was conducted in an incubator at 50°C and 150 rpm, using a 4:1 mixture of Cellic CTec2 and HTec2 (180 FPU mL−1 FPase activity; 13,213 UI mL−1 xylanase activity; 7,240 UI mL−1 β-glucosidase activity) diluted into two enzyme loadings. Loadings were calculated based on the chemical characterization of samples, that is, 9.1 and 17.5 mg protein g−1of glucan. At 96 h of hydrolysis, aliquots from supernatant were taken every 24 h in duplicate and analyzed for soluble carbohydrates, reported here as an average with an indicated standard deviation. The final volume of hydrolyzate from each replicate unit was recovered after the separation from solid residues by centrifugation (12,096 × g, 10 min), combined in a single unit, corresponding to individual pretreatments, and kept frozen until the fermentative step.
Alcoholic Fermentation
The fermentability of selected hydrolyzate obtained after the hydrolysis of double-pretreated SCB was tested using two Saccharomyces cerevisiae strains. The first (JP1) is an industrial strain selected by its roughness and adaptability to perform alcoholic fermentation under adverse conditions in the first-generation production of ethanol, which is also already in use in Brazilian mills. The second yeast, Y150, is a strain obtained by the adaptative laboratory evolution method following similar protocol described by Vasconcellos et al. (2019). Yeast reactivation was made by pre-cultivation of freeze-stored cells in the YEPD medium (10 g L−1 yeast extract; 10 g L−1 peptone; 20 g.L−1 glucose) in an incubator at 28°C, 200 rpm, and 24 h prior use.
Three hydrolyzates were obtained from each pretreated material (ozonolysis; LHW; and combined ozonolysis + LHW, respectively) as substrates for alcoholic fermentations. Substrates were sterilized by vacuum filtering through a 0.22-µm membrane, and 0.5 g fresh cells was added per liter as inoculum. The inoculum standardization was carried out through measuring the cell density of precultured suspension in a colorimeter, followed by two steps of centrifugation (12,096 × g, 10 min), washing and resuspension in sterilized DI water. The washed cells were inoculated in 40 ml of each hydrolyzate substrate. The fermentation experiments were carried out in an incubator at 32°C and 150 rpm in duplicate at a semi-anaerobic condition. The concentration of cells, ethanol production, and glucose consumption were systematically monitored by sampling at 6, 12, 20, 24, 30, and 40 h of fermentation, respectively. Glucose to ethanol conversion yields (%) were calculated at the endpoint of each fermentation experiment by assuming: [(g.L−1 of ethanol produced)/(g.L−1 of initial glucose * 0.511)] * 100.
Analytical Methods
The chemical composition of SCB was determined according to LAP-010—determination of extractives in biomass (Sluiter et al., 2012) and the LAP for the determination of structural carbohydrates and lignin in biomass (Sluiter et al., 2012). Glucose and ethanol concentrations were measured by HPLC analysis as described previously (Cao et al., 2013) using an Aminex HPX-87H ion exchange column (300 × 7.8 mm, Bio-Rad Laboratories Inc., Hercules, CA). The column was connected with a Milton Roy mini pump (Milton Roy Co., Ivyland, PA), a WatersTM 717 plus autosampler, and a WatersTM 2,414 refractive index detector (Waters Corp., Milford, MA). The procedure for total phenolic analyses was adapted from the study by Singleton et al. (1999) to a micro-scale analysis using Folin–Ciocalteau reagent.
The results were expressed in milligrams per liter (mg L−1) of gallic acid equivalent (GAE). The protein content of commercial enzymes used in hydrolysis assays was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Filter paper activity was measured according to Mandels et al. (1976). Cell concentration was estimated by optical density in a spectrophotometer at 630 nm, using 1:10 dilution with sterile DI water, in comparison to the dry weight curve of cellular growth, previously determined to JP1 strain.
Infrared spectroscopy data (FTIR-ATR) were collected using a Perkin Elmer FTIR Spectrum Two. Both FTIR-ATR and powder X-ray diffraction (XRD) patterns of SCB were obtained on a Model 300 miniFlex Rigaku® diffractometer according to Perrone et al. (2016). The crystallinity index was calculated by the method proposed by Segal et al. (1959).
Scanning electron microscopy (SEM) was carried out using a FEI Quanta 200 scanning electron microscope (FEI Company, Eindhoven, Netherlands) with an accelerating voltage of 12.5 kV. Sample preparation comprised 1) mixing bagasse in 2.5% (v/v) glutaraldehyde in 0.1 M phosphate buffer (pH 7.3) for 48 h at room temperature; and 2) washing the sample in distilled water and after fixing it in 1% (v/v) osmium tetroxide diluted in distilled water for 30 min at room temperature. Bagasse was dehydrated by a series of ethanol washes and then critical point-dried with CO2, and sputter-coated with gold (Bal-Tec SCD 050) (Perrone et al., 2016).
Results and Discussion
Chemical Characterization of Pretreated Sugarcane Bagasse
Three different samples of pretreated SCB were obtained from individual ozonolysis and LHW pretreatments or a combination. Chemical characterization shows that ozonolysis and LHW have distinct effects on SCB, as summarized in Table 1. Ozone acted mostly on delignification, causing about 37% reduction of acid insoluble lignin (AIL) and partially decreasing the hemicellulose portion (components determined as xylose, arabinose, and acetyl groups) as a secondary effect (20% solubilization). Delignification by ozonolysis was relevant to overcome the recalcitrant character of lignin through its separation and the breakdown of lignin (Santos et al., 2019; Ázar et al., 2019). It also generated a rich fraction of phenolics and other compounds with potential use in green chemistry bioconversions, while also removing their enzyme and the microbial potential inhibitory effect in subsequent steps of enzyme hydrolysis and microbial fermentation (Kim et al., 2011, 2016; Ximenes et al., 2011; Michelin et al., 2016). Although the individual LHW pretreatment reduced the hemicellulose content (22% of solubilization), it had little effect on lignin (about 5% reduction of AIL).
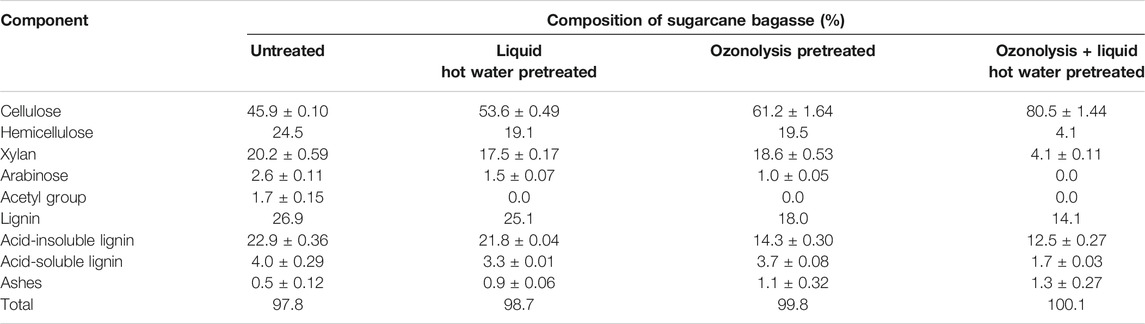
TABLE 1. Compositional analysis of sugarcane bagasse generated by different pretreatment approaches and the untreated sample. Solid composition is presented as dry weight on a free extractive basis (%).
The double-pretreated SCB (ozonolysis and LHW combined) generated the highest cellulose (80.5%) and the lowest hemicellulose (4.1%) contents among all samples, indicating an intensive solubilization (up to 80%) of hemicellulose into liquor and reaching a maximal delignification rate (45% reduction of AIL). In that sense, even taking into consideration all the pretreatments increased the glucan availability, its availability in the ozone + LHW-pretreated biomass was 75, 50, and 32% higher than that of the initial content (comparing only untreated LHW and ozone-pretreated SCB), respectively. The action on the lignin barrier observed in double-pretreated SCB helps to reduce enzyme adsorption on lignin and increase the accessibility of cellulose to enzyme (Zanchetta et al., 2018), which can also lead to a reduction of enzyme loading and cost.
We observed an increase of the crystallinity index (CrI) according to the intensity of pretreatments in the following order: untreated < ozonolysis < LHW < Oz + LHW (Figure 1). We observed the highest CrI (73.1%) in the most intensive pretreatment (combined ozonolysis + LHW), possibly associated with lignin and hemicellulose degradation. In agreement with that reported in the literature (Gabhane et al., 2015; Pereira et al., 2016; Perrone et al., 2016), it seems that the increase in CrI observed here is more a function of removal of non-crystalline components from the biomass.
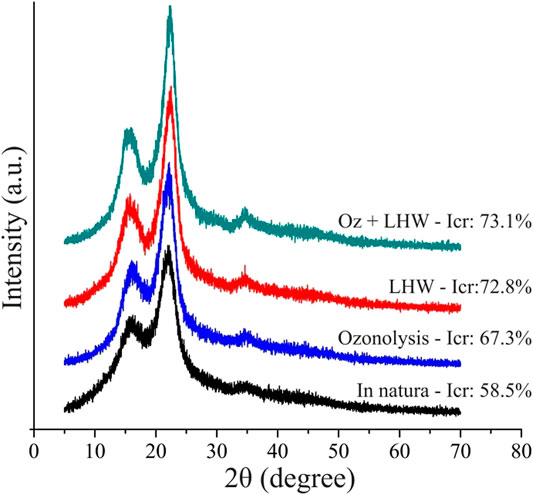
FIGURE 1. XRD patterns and the corresponding results of crystallinity index (Icr) for in nature sugarcane bagasse, ozonolysis, LHW, and ozonolysis + LHW pretreatments.
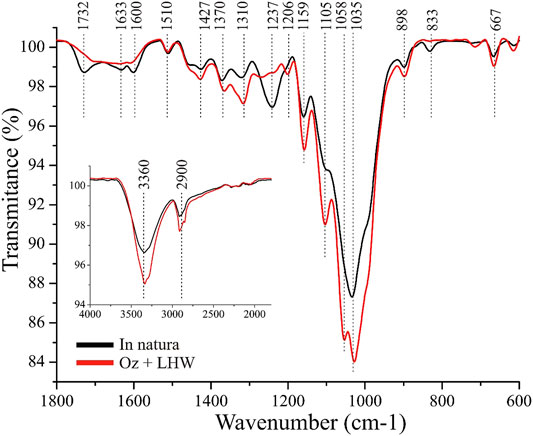
FTIR Analysis
Infrared spectrometry (FTIR-ATR) was used to analyze changes in functional groups that compose the fibers of SCB after pretreatment, indicating possible targets of reaction in the material (Figure 2). A pronounced reduction in the intensity of infrared absorption bands found at 1,732 cm−1 and 1,237 cm−1 in the pretreated sample confirmed the strong removal of hemicellulose (Liu et al., 2007), which is also detected by compositional characterization analysis (Table 1). The increase of the cellulose content is also shown by FTIR-ATR, especially by the increase of infrared absorption bands at 1,427 cm−1 and 1,370 cm−1 (assigned to the crystalline cellulose structure), and amorphous cellulose at 898 cm−1 (Pereira et al., 2016).
A decrease in the lignin content in the double-pretreated sample was observed based on the presence of bands related to functional groups or specific lignin linkages, such as aromatic rings at 1,600 cm−1 and 1,510 cm−1 (Pereira et al., 2016), and carbonyl groups conjugated with aromatic rings at 1,633 cm−1 (Zhou et al., 2016). Also, the band at 833 cm−1 is associated to [C-H] vibrations out of a plane in p-hydroxyphenyl units (Hoareau et al., 2004). The presence of these bands indicates a clear decrease of the intensities in the spectra corresponding to the combined pretreatments, which is again in agreement with the large extent of lignin removal (compositional characterization, Table 1).
Ultrastructural Changes in Pretreated Sugarcane Bagasse
Scanning electron microscopy (SEM) was performed to analyze possible tissue damage and ultrastructural changes on the bagasse surface after each pretreatment (ozone, LHW, and ozone + LHW) in comparison to the untreated sample (Figures 3A,B). The initial smooth and intact structure of fibers was strongly affected by both ozone and hot water pretreatments (Figures 3C–F). Cell walls were ruptured by the ozone gas, generating opened cells with increased porosity and surface area (Figures 3C,D). Similar effects were observed with hot water. A cracked surface characterized by holes formed in the cell wall is noted; the holes may be caused by the high pressure experienced during LHW processing (Figures 3E,F).
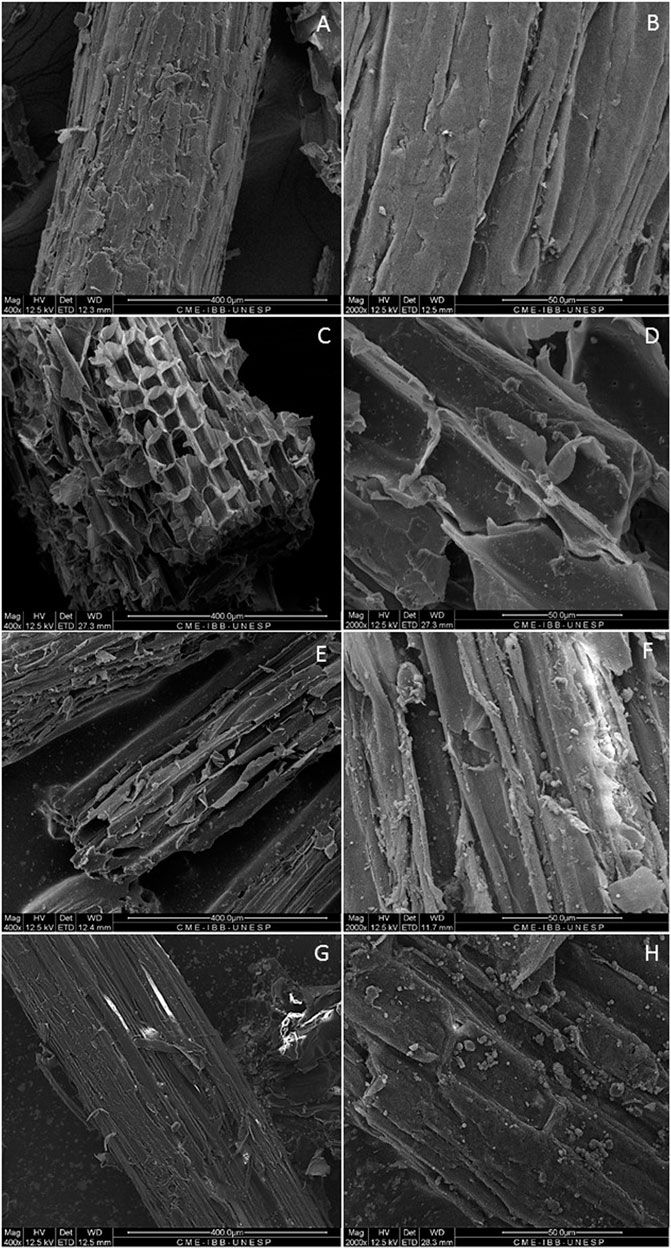
FIGURE 3. Scanning electron microscopy of untreated bagasse (A,B), ozone-treated bagasse (C,D), LHW-treated bagasse (E,F), and combined pretreatment ozone + LHW (G,H).
A disorganized structure with greater exposure of fibers was also present in SCB pretreated with SO2 and CO2 steam (Corrales et al., 2012). The presence of globular structures on the surface of samples exposed to high temperature is probably related to the formation and accumulation of globular lignin at 190°C. It is known that lignin softens and agglomerates at a relatively low temperature (<200°C) (Hamdan et al., 2000; Zhang et al., 2015). All these observations also apply to the double-pretreated sample, which showed a random breaking along the fibers, and a total collapse of the cellular structure due to the combined process (Figures 3G,H). These morphological changes of SCB obtained after combined pretreatment enhance the accessibility of cellulose-degrading enzymes and facilitate the hydrolysis of cellulose.
Effect of Pretreatments on Phenolic Compound Releasing
When LHW pretreatment alone was performed, 1,462 mg L−1 of phenolics were solubilized through liquor, and 857 mg L−1 remained on solids, reaching a total of 2,320 mg L−1 of released phenolics. The lowest concentration was observed when ozonolysis was performed as a single pretreatment. Here, 936 mg L−1 of total phenolic compounds were released. Although delignification is stronger in ozonolysis than in LHW pretreatment, as previously demonstrated by biomass compositional and FTIR-ART analysis, the lower release of phenols may be explained by the conversion of acid-insoluble lignin (AIL) preferentially into acids with low molecular weight, such as formic, acetic, oxalic, and carboxylic acids (Travaini et al., 2016). This hypothesis is further supported by the intense acidification of the ozonized solids, which regularly had a pH near to 2.0 after ozonolysis. The highest concentration of total phenolics released was almost 3,000 mg.L−1 when combining ozonolysis and LHW pretreatments, with 2,300 mg.L−1 solubilized in the liquor and 681 mg.L−1 remaining in solids. Thus, the use of this combined pretreatment approach resulted in a maximal release of phenolic compounds.
Effect of Phenolic Compound Removal on 96-h Enzymatic Hydrolysis
The low molecular weight phenolic compounds derived from lignin depolymerization had a negative impact on enzyme performance, possibly due to both non-productive adsorption and inhibitory effects during saccharification and microbial fermentation (Ximenes et al., 2010; Nakagame et al., 2011; Ximenes et al., 2011; Jönsson and Martín, 2016). Therefore, phenolic compounds must be removed from pretreated solids prior to enzymatic hydrolysis to enhance the yields of both the saccharification and the fermentation steps (Kim et al., 2013; Xiros and Olsson, 2014).
Sequential washing of the material at room temperature (see Material and Methods section 2.2.1, section 2.2.2) significantly reduced phenolics embedded in the pretreated fibers (Figure 3). A maximum of 33 g.L−1 of glucose was reached in 24 h during hydrolysis of washed ozone-pretreated SCB (Figure 4A), which represented a conversion of about 75% of cellulose into glucose vs. 44% for the enzymatic hydrolysis of the non-washed pretreated sample. Similarly, high cellulose conversion was observed for washed LHW-pretreated SCB samples (Figure 4B), although lower yields were obtained than washed ozone-pretreated samples (compare Figures 4A,B). This suggests better accessibility of substrate to enzyme hydrolysis in the ozone-pretreated sample and the presence of more enzyme inhibitors, including remaining phenolic compounds in the LHW-pretreated samples, or both.
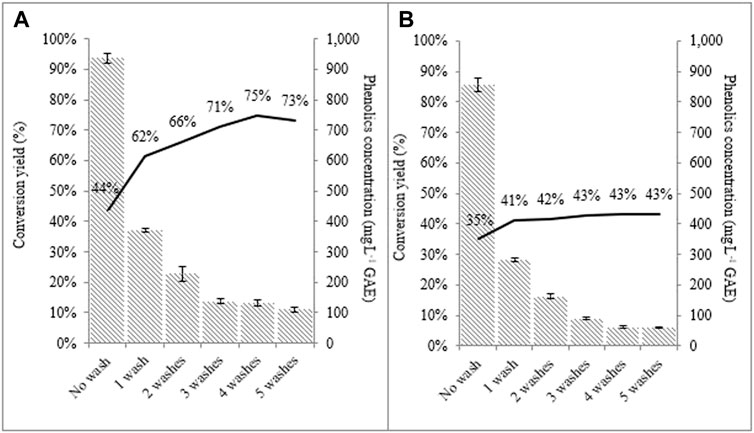
FIGURE 4. Concentration of phenolic compounds (bars) and cellulose conversion yield (lines) in enzymatic hydrolysis (17.5 mg protein × g−1 glucan; 10% total solids loading, 96 h) related to sequential room temperature washes. (A) ozone-pretreated sugarcane bagasse; (B) LHW-pretreated sugarcane bagasse.
Kinetics of Enzymatic Hydrolysis of Pretreated Sugarcane Bagasse
A kinetic study was performed using reduced enzyme loading (9 mg of total protein per gram of glucan) for hydrolysis of untreated and pretreated SCB under different conditions for 96 h. The double pretreated SCB was also tested under non- and washed conditions.
The kinetics of hydrolysis of different SCB samples showed that the yield increase was consistent with the increase of the cellulose content after pretreatment and washes, relative to the decline of hemicellulose removal (in LWH-pretreated bagasse), or lignin reduction (in ozone-pretreated bagasse), or associated to both effects (in combined pretreatments). For the combined pretreatments, 43.0 g L−1 of glucose was generated after enzymatic hydrolysis of double-pretreated SCB, corresponding to 59% of conversion from initial glucan vs. 20, 47, 37, and 10% for untreated, ozone, LHW, and double pretreated samples, respectively. The hydrolyzate from double pretreated samples was tested by fermentation experiments that are reported and discussed in the next section. A strong inhibitory effect of pretreatment by-products was observed over cellulolytic and hemicellulolytic enzymes when the hydrolysis was performed in the presence of liquor derived from combined pretreatment, reducing cellulose conversion to about 10%, probably due to potential enzyme inhibitors mentioned before (Ximenes et al., 2010, 2011; Kim et al., 2011; Gabhane et al., 2015).
Alcoholic Fermentation of Hydrolyzate
JP1 and Y150 yeast strains were able to ferment the selected hydrolyzate without nutrient supplementation, with all glucose available exhausted after 42 h (Figure 5), while xylose concentration remained constant. This latter result was expected since these yeast strains cannot ferment xylose to ethanol. The final yield of glucose to ethanol was similar for both strains, 87% for JP1 vs. 92% for Y150 (Figure 5). However, Y150 strain was faster on the fermentation (conversion yield of 67% for Y150 after 24 h vs. 43% for JP1), indicating some possible adaptation advantage to the microbial inhibitory compounds still present in the hydrolyzate, including phenolics (Larsson et al., 2000; Palmqvist and Hahn-Hägerdal, 2000a, 2000b). Since the cellular density in all replicates of fermentations was similar, Y150 cells were found to be more efficient on ethanol production.
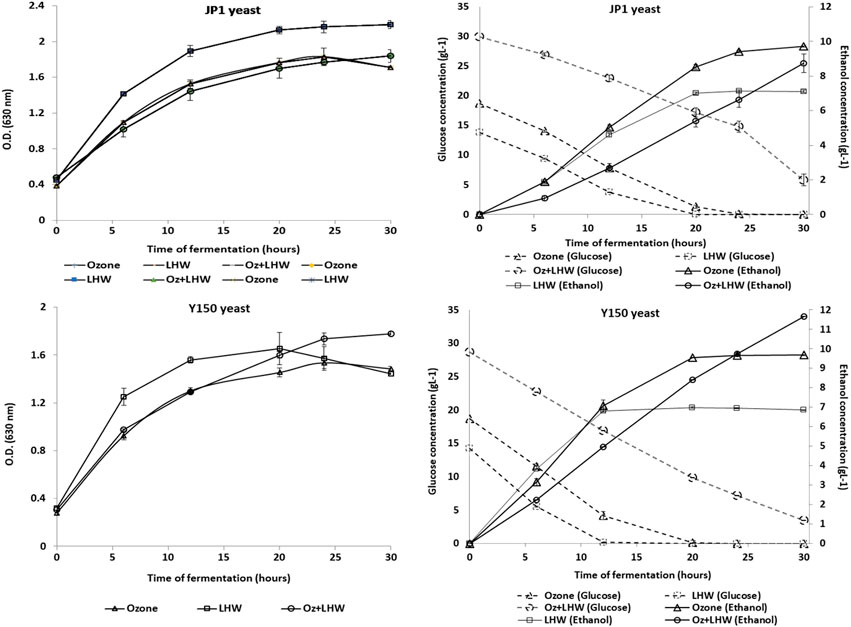
FIGURE 5. Kinetics of yeasts JP1 and Y150 in alcoholic fermentation of sugarcane bagasse hydrolysate pretreated by individual and combined Ozonolysis and LHW approaches.
Conclusions
The effects of ozonolysis and LHW sugarcane bagasse pretreatment were observed in individual and combined pretreatment processes that resulted in a new approach for achieving a high amount of cellulose for hydrolysis purposes while generating an acid liquor fraction rich in sugars and phenolic compounds. The double pretreatment removed enzyme and microbial inhibitors, and generated water-soluble products that can be explored in green chemistry bioconversions processes. The combined ozonolysis and LHW pretreatment also generated, after enzyme hydrolysis, a hydrolyzate rapidly fermented by S. cerevisiae without the need for detoxification steps or nutrient supplementation. The maximal conversion yield by strain Y150 in fermenting glucose to ethanol was 92% in 42 h. This approach and results obtained are in agreement with a proposed model of the lignocellulosic biorefinery (Silva et al., 2018), in which sugars from cellulose and hemicellulose are used to generate biofuels and bioproducts, while lignin components are utilized in the synthesis of other bioproducts and act as an alternative heat and energy source.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
SB: experimental design, performance and manuscript writing; EX: experimental design, manuscript writing, review, and formatting; OP: experimental design, performance, and manuscript writing; CC: experimental design and performance; DK: experimental design and performance; MB: experimental design and manuscript writing; EG: experimental design and manuscript writing; EF: manuscript writing, review, and formatting; RS: experimental design and manuscript writing; ML: experimental design, manuscript writing, and review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with the authors EX and ML.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge support from the United States Department of Energy Bioenergy Technologies Office (DOE-BETO) under the contract number DEEE0008256. We also thank the companies Usina Alta Mogiana, AEB Latin America, and Novozymes Latin America for the donation of materials; to the Brazilian government for financial support through the Coordination for the Improvement of Higher Education Personnel (CAPES Print program, process 88887.364337/2019-00) and “Ciência Sem Fronteiras” scholarship) and the Brazilian National Council for Scientific and Technological Development (CNPq); to FAPESP for supporting projects 2008/58077-0, 2010/12624-0, 2014/02080-4; to INCT-Bioetanol, Electron Microscopy Center of the Biosciences Institute (Unesp, Botucatu-SP, Brazil); to undergraduate students Márcio Justi Laranja (Unesp, São José do Rio Preto-SP, Brazil) and Haley Roos (Purdue University, West Lafayette-IN, United States) for their contribution in preparing samples and performing previous analytical assays; and to Xingya Liu for her technical support.
References
Agrawal, A., Kaushik, N., and Biswas, S. (2014). Derivatives and Applications of Lignin— an Insight. Scitech. J. 1, 30–36.
Aguilar, D. L., Rodríguez-Jasso, R. M., Zanuso, E., Lara-Flores, A. A., Aguilar, C. N., Sanchez, A., et al. (2018). “Operational Strategies for Enzymatic Hydrolysis in a Biorefinery,” in Biorefining Biomass to Biofuels -Opportunities and Perception. Editors S. Kumar, and R. Sani (Cham: Springer), 223–248. doi:10.1007/978-3-319-67678-4_10
APHA (1998). Standard Methods for the Examination of Water and Wastewater. Washington: American Public Health Association, 202–242.
Ázar, R. I. S. L., Morgan, T., Barbosa, M. H. P., Guimarães, V. M., Ximenes, E., and Ladisch, M. (2019). Impact of Protein Blocking on Enzymatic Saccharification of Bagasse from Sugarcane Clones. Biotechnol. Bioeng. 116 (7), 1584–1593. doi:10.1002/bit.26962
Barros, R. d. R. O. d., Paredes, R. d. S., Endo, T., Bon, E. P. d. S., and Lee, S.-H. (2013). Association of Wet Disk Milling and Ozonolysis as Pretreatment for Enzymatic Saccharification of Sugarcane Bagasse and Straw. Bioresour. Tech. 136, 288–294. doi:10.1016/j.biortech.2013.03.009
Beauchet, R., Monteil-Rivera, F., and Lavoie, J. M. (2012). Conversion of Lignin to Aromatic-Based Chemicals (L-Chems) and Biofuels (L-Fuels). Bioresour. Tech. 121, 328–334. doi:10.1016/j.biortech.2012.06.061
Cao, G., Ximenes, E., Nichols, N. N., Zhang, L., and Ladisch, M. (2013). Biological Abatement of Cellulase Inhibitors. Bioresour. Tech. 146, 604–610. doi:10.1016/j.biortech.2013.07.112
Corrales, R. C. N. R., Mendes, F. M. T., Perrone, C. C., Sant’Anna, C., de Souza, W., Abud, Y., et al. (2012). Structural Evaluation of Sugar Cane Bagasse Steam Pretreated in the Presence of CO2 and SO2. Biotechnol. Biofuels 5, 36. doi:10.1186/1754-6834-5-36
Demuner, I. F., Colodette, J. L., Demuner, A. J., and Jardim, C. M. (2019). Biorefinery Review: Wide-Reaching Products through Kraft Lignin. BioRes. 14, 7543–7581. doi:10.15376/biores.14.3.demuner
dos Santos, A. C., Ximenes, E., Kim, Y., and Ladisch, M. R. (2019). Lignin-Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass. Trends Biotechnol. 37, 518–531. doi:10.1016/j.tibtech.2018.10.010
Gabhane, J., William, S. P. M. P., Vaidya, A. N., Das, S., and Wate, S. R. (2015). Solar Assisted Alkali Pretreatment of Garden Biomass: Effects on Lignocellulose Degradation, Enzymatic Hydrolysis, Crystallinity and Ultra-structural Changes in Lignocellulose. Waste Manag. 40, 92–99. doi:10.1016/j.wasman.2015.03.002
Gitifar, V., Eslamloueyan, R., and Sarshar, M. (2013). Experimental Study and Neural Network Modeling of Sugarcane Bagasse Pretreatment with H2SO4 and O3 for Cellulosic Material Conversion to Sugar. Bioresour. Tech. 148, 47–52. doi:10.1016/j.biortech.2013.08.060
Hamdan, S., Dwianto, W., Morooka, T., and Norimoto, M. (2000). Softening Characteristics of Wet wood under Quasi Static Loading. Holzforschung 54, 557–560. doi:10.1515/hf.2000.094
Hoareau, W., Trindade, W. G., SiegmundCastellana, B., Castellan, A., and Frollini, E. (2004). Sugar Cane Bagasse and Curaua Lignins Oxidized by Chlorine Dioxide and Reacted with Furfuryl Alcohol: Characterization and Stability. Polym. Degrad. Stab. 86, 567–576. doi:10.1016/j.polymdegradstab.2004.07.005
Jong, E., Higson, A., Walsh, P., and Wellisch, M. (2012). Biobased Chemicals—Value Added Products from Biorefineries, 42. Wageningen, Netherlands: IEA Bioenergy Task. www.iea-bioenergy.taks42-biorefineries.com.
Jönsson, L. J., and Martín, C. (2016). Pretreatment of Lignocellulose: Formation of Inhibitory By-Products and Strategies for Minimizing Their Effects. Bioresour. Tech. 199, 103–112. doi:10.1016/j.biortech.2015.10.009
Kim, D., Ximenes, E. A., Nichols, N. N., Cao, G., Frazer, S. E., and Ladisch, M. R. (2016). Maleic Acid Treatment of Biologically Detoxified Corn stover Liquor. Bioresour. Tech. 216, 437–445. doi:10.1016/j.biortech.2016.05.086
Kim, I. J., Lee, H. J., Choi, I.-G., and Kim, K. H. (2014a). Synergistic Proteins for the Enhanced Enzymatic Hydrolysis of Cellulose by Cellulase. Appl. Microbiol. Biotechnol. 98 (20), 8469–8480. doi:10.1007/s00253-014-6001-3
Kim, Y., Kreke, T., Hendrickson, R., Parenti, J., and Ladisch, M. R. (2013). Fractionation of Cellulase and Fermentation Inhibitors from Steam Pretreated Mixed Hardwood. Bioresour. Tech. 135, 30–38. doi:10.1016/j.biortech.2012.10.130
Kim, Y., Kreke, T., Mosier, N. S., and Ladisch, M. R. (2014b). Severity Factor Coefficients for Subcritical Liquid Hot Water Pretreatment of Hardwood Chips. Biotechnol. Bioeng. 111 (2), 254–263. doi:10.1002/bit.25009
Kim, Y., Mosier, N. S., and Ladisch, M. R. (2009). Enzymatic Digestion of Liquid Hot Water Pretreated Hybrid poplar. Biotechnol. Prog. 25, 340–348. doi:10.1002/btpr.137
Kim, Y., Ximenes, E., Mosier, N. S., and Ladisch, M. R. (2011). Soluble Inhibitors/deactivators of Cellulase Enzymes from Lignocellulosic Biomass. Enzyme Microb. Tech. 48, 408–415. doi:10.1016/j.enzmictec.2011.01.007
Ko, J. K., Kim, Y., Ximenes, E., and Ladisch, M. R. (2015a). Effect of Liquid Hot Water Pretreatment Severity on Properties of Hardwood Lignin and Enzymatic Hydrolysis of Cellulose. Biotechnol. Bioeng. 112 (2), 252–262. doi:10.1002/bit.25349
Ko, J. K., Um, Y., Park, Y.-C., Seo, J.-H., and Kim, K. H. (2015b). Compounds Inhibiting the Bioconversion of Hydrothermally Pretreated Lignocellulose. Appl. Microbiol. Biotechnol. 99 (10), 4201–4212. doi:10.1007/s00253-015-6595-0
Ko, J. K., Ximenes, E., Kim, Y., and Ladisch, M. R. (2015c). Adsorption of Enzyme onto Lignins of Liquid Hot Water Pretreated Hardwoods. Biotechnol. Bioeng. 112 (3), 447–456. doi:10.1002/bit.25359
Larsson, S., Quintana-Sáinz, A., Reimann, A., Nilvebrant, N.-O., and Jönsson, L. J. (2000). Influence of Lignocellulose-Derived Aromatic Compounds on Oxygen-Limited Growth and Ethanolic Fermentation by Saccharomyces cerevisiae. Abab 84-86, 617–632. doi:10.1385/abab:84-86:1-9:617
Mandels, M., Andreotti, R., and Roche, C. (1976). Measurement of Saccharifying Cellulase. Biotechnol. Bioeng. Symp. 6, 21–33.
Michelin, M., Ximenes, E., de Lourdes Teixeira de Moraes Polizeli, M., and Ladisch, M. R. (2016). Effect of Phenolic Compounds from Pretreated Sugarcane Bagasse on Cellulolytic and Hemicellulolytic Activities. Bioresour. Tech. 199, 275–278. doi:10.1016/j.biortech.2015.08.120
Nakagame, S., Chandra, R. P., Kadla, J. F., and Saddler, J. N. (2011). The Isolation, Characterization and Effect of Lignin Isolated from Steam Pretreated Douglas-fir on the Enzymatic Hydrolysis of Cellulose. Bioresour. Tech. 102, 4507–4517. doi:10.1016/j.biortech.2010.12.082
Palmqvist, E., and Hahn-Hägerdal, B. (2000a). Fermentation of Lignocellulosic Hydrolysates. I: Inhibition and Detoxification. Bioresour. Tech. 74, 17–24. doi:10.1016/s0960-8524(99)00160-1
Palmqvist, E., and Hahn-Hägerdal, B. (2000b). Fermentation of Lignocellulosic Hydrolysates. II: Inhibitors and Mechanisms of Inhibition. Bioresour. Tech. 74, 25–33. doi:10.1016/s0960-8524(99)00161-3
Panneerselvam, A., Sharma-Shivappa, R. R., Kolar, P., Ranney, T., and Peretti, S. (2013). Potential of Ozonolysis as a Pretreatment for Energy Grasses. Bioresour. Tech. 148, 242–248. doi:10.1016/j.biortech.2013.08.129
Pedersen, M., and Meyer, A. S. (2010). Lignocellulose Pretreatment Severity - Relating pH to Biomatrix Opening. New Biotechnol. 27, 739–750. doi:10.1016/j.nbt.2010.05.003
Pereira, S. C., Maehara, L., Machado, C. M. M., and Farinas, C. S. (2016). Physical-chemical-morphological Characterization of the Whole Sugarcane Lignocellulosic Biomass Used for 2G Ethanol Production by Spectroscopy and Microscopy Techniques. Renew. Energ. 87, 607–617. doi:10.1016/j.renene.2015.10.054
Perlack, R. D., Wright, L. L., Turhollow, A. F., and Grahm, L. L. (2005). Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply. Oak Ridge, TN: U.S. Department of Energy (DOE), U.S. Department of Agriculture (USDA). http://www.osti.gov./bridge.
Perrone, O. M., Colombari, F. M., Rossi, J. S., Moretti, M. M. S., Bordignon, S. E., Nunes, C. d. C. C., et al. (2016). Ozonolysis Combined with Ultrasound as a Pretreatment of Sugarcane Bagasse: Effect on the Enzymatic Saccharification and the Physical and Chemical Characteristics of the Substrate. Bioresour. Tech. 218, 69–76. doi:10.1016/j.biortech.2016.06.072
Pino, M. S., Rodríguez-Jasso, R. M., Michelin, M., Flores-Gallegos, A. C., Morales-Rodriguez, R., Teixeira, J. A., et al. (2018). Bioreactor Design for Enzymatic Hydrolysis of Biomass under the Biorefinery Concept. Chem. Eng. J. 347, 119–136. doi:10.1016/j.cej.2018.04.057
Rasmussen, H., Sørensen, H. R., and Meyer, A. S. (2014). Formation of Degradation Compounds from Lignocellulosic Biomass in the Biorefinery: Sugar Reaction Mechanisms. Carbohydr. Res. 385, 45–57. doi:10.1016/j.carres.2013.08.029
Rosales-Calderon, O., and Arantes, V. (2019). A Review on Commercial-Scale High-Value Products that Can Be Produced Alongside Cellulosic Ethanol. Biotechnol. Biofuels 12, 240. doi:10.1186/s13068-019-1529-1
Ruiz, H. A., Conrad, M., Sun, S.-N., Sanchez, A., Rocha, G. J. M., Romaní, A., et al. (2020). Engineering Aspects of Hydrothermal Pretreatment: From Batch to Continuous Operation, Scale-Up and Pilot Reactor under Biorefinery Concept. Bioresour. Tech. 299, 122685. doi:10.1016/j.biortech.2019.122685
Ruiz, H. A., Galbe, M., Garrote, G., Ramirez-Gutierrez, D. M., Ximenes, E., Sun, S.-N., et al. (2021). Severity Factor Kinetic Model as a Strategic Parameter of Hydrothermal Processing (Steam Explosion and Liquid Hot Water) for Biomass Fractionation under Biorefinery Concept. Bioresour. Tech. 342, 125961. doi:10.1016/j.biortech.2021.125961
Segal, L., Creely, J. J., Martin, A. E., and Conrad, C. M. (1959). An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Textile Res. J. 29, 786–794. doi:10.1177/004051755902901003
Silva, C. O. G., Vaz, R. P., and Filho, E. X. F. (2018). Bringing Plant Cell wall-degrading Enzymes into the Lignocellulosic Biorefinery Concept. Biofuels, Bioprod. Bioref. 12, 277–289. doi:10.1002/bbb.1832
Singleton, V. L., Orthofer, R., and Lamuela-Raventós, R. M. (1999). [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 299, 152–178. doi:10.1016/s0076-6879(99)99017-1
Sluiter, A., Hames, A. B., RuizScarlata, R. C., Sluiter, J., Templeton, D., and Crocker, D. (2012). Determination of Structural Carbohydrates and Lignin in Biomass. Golden, CO: Laboratory Analytical Procedure, National Renewable Energy Laboratory (NREL). Version 08-03-2012.
Sun, S., Sun, S., Cao, X., and Sun, R. (2016). The Role of Pretreatment in Improving the Enzymatic Hydrolysis of Lignocellulosic Materials. Bioresour. Tech. 199, 49–58. doi:10.1016/j.biortech.2015.08.061
Travaini, R., Barrado, E., and Bolado-Rodríguez, S. (2016). Effect of Ozonolysis Pretreatment Parameters on the Sugar Release, Ozone Consumption and Ethanol Production from Sugarcane Bagasse. Bioresour. Tech. 214, 150–158. doi:10.1016/j.biortech.2016.04.102
Travaini, R., Otero, M. D. M., Coca, M., Da-Silva, R., and Bolado, S. (2013). Sugarcane Bagasse Ozonolysis Pretreatment: Effect on Enzymatic Digestibility and Inhibitory Compound Formation. Bioresour. Tech. 133, 332–339. doi:10.1016/j.biortech.2013.01.133
Vasconcellos, V. M., Farinas, C. S., Ximenes, E., Slininger, P., and Ladisch, M. (2019). Adaptive Laboratory Evolution of Nanocellulose‐producing Bacterium. Biotechnol. Bioeng. 116, 1923–1933. doi:10.1002/bit.26997
Weil, J. R., Sarikaya, A., Rau, S.-L., Goetz, J., Ladisch, C. M., Brewer, M., et al. (1998). Pretreatment of Corn Fiber by Pressure Cooking in Water. Appl. Biochem. Biotechnol. 73, 1–17. doi:10.1007/bf02788829
Ximenes, E., Farinas, C., Baldino, A., and Ladisch, M. (2021). Moving from Residual Lignocellulosic Biomass into High-Value Products: Outcomes from a Long-Term International Cooperation. Biofuels, Bioprod. Biorefin. 15 (2), 563–573. doi:10.1002/bbb.2179
Ximenes, E., Farinas, C. S., and Kim, Y., and (2017) Hydrothermal Processing in Biorefineries - Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass. Editors, H. L. Trajano, and M. H. Thomsen (Springer International Publishing Switzerland), 181–206. 978-3-319-56456-2. doi:10.1007/978-3-319-56457-9_7
Ximenes, E., Kim, Y., Mosier, N., Dien, B., and Ladisch, M. (2011). Deactivation of Cellulases by Phenols. Enzyme Microb. Tech. 48, 54–60. doi:10.1016/j.enzmictec.2010.09.006
Ximenes, E., Kim, Y., Mosier, N., Dien, B., and Ladisch, M. (2010). Inhibition of Cellulases by Phenols. Enzyme Microb. Tech. 46, 170–176. doi:10.1016/j.enzmictec.2009.11.001
Xiros, C., and Olsson, L. (2014). Comparison of Strategies to Overcome the Inhibitory Effects in High-Gravity Fermentation of Lignocellulosic Hydrolysates. Biomass and Bioenergy 65, 79–90. doi:10.1016/j.biombioe.2014.03.060
Zanchetta, A., Santos, A. C. F., Ximenes, E., Nunes, C. C. C., BoscoloGomes, M. E., and Ladisch, M. R. (2018). Temperature Dependent Cellulase Adsorption on Lignin from Sugarcane Bagasse. Bioresour. Technol. 252, 143–149. doi:10.1016/j.biortech.2017.12.061
Zhang, Q., Chen, Q., Chen, J., Wang, K., Yuan, S., and Sun, R. (2015). Morphological Variation of Lignin Biomacromolecules during Acid-Pretreatment and Biorefinery-Based Fractionation. Ind. Crop Prod. 77, 527–534. doi:10.1016/j.indcrop.2015.09.021
Keywords: ozone, liquid hot water, pretreatment, enzyme hydrolysis, ethanol fermentation, value-added chemicals, inhibitors
Citation: Bordignon SE, Ximenes E, Perrone OM, Carreira Nunes CdC, Kim D, Boscolo M, Gomes E, Filho EXF, da Silva R and Ladisch MR (2022) Combined Sugarcane Pretreatment for the Generation of Ethanol and Value-Added Products. Front. Energy Res. 10:834966. doi: 10.3389/fenrg.2022.834966
Received: 14 December 2021; Accepted: 27 January 2022;
Published: 17 February 2022.
Edited by:
Allison E. Ray, Idaho National Laboratory (DOE), United StatesReviewed by:
Zhi-Hua Liu, Texas A&M University, United StatesHéctor A. Ruiz, Universidad Autónoma de Coahuila, Mexico
Copyright © 2022 Bordignon, Ximenes, Perrone, Carreira Nunes, Kim, Boscolo, Gomes, Filho, da Silva and Ladisch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael R. Ladisch, bGFkaXNjaEBwdXJkdWUuZWR1
 Sidnei Emilio Bordignon1
Sidnei Emilio Bordignon1 Eduardo Ximenes
Eduardo Ximenes Daehwan Kim
Daehwan Kim Eleni Gomes
Eleni Gomes Edivaldo Ximenes Ferreira Filho
Edivaldo Ximenes Ferreira Filho