- 1Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Republic of Korea
- 2Department of Materials Science and Engineering, University of North Texas, Denton, TX, United States
- 3Department of Mechanical and Energy Engineering, University of North Texas, Denton, TX, United States
Often touted as the most promising next-generation energy storage systems, lithium (Li) metal batteries have drawn extensive interest due to their energy densities beyond those of Li-ion batteries. The use of Li metal, however, presents a major hurdle since it is susceptible to Li dendrite growths, corrosive interfacial reactions, and uncontrolled volume changes. Li-metal protection is an important issue in overcoming those challenges. In particular, studies have shown that molybdenum disulfide (MoS2) can significantly improve the performance and safety of Li metal batteries when used as a protective coating for anodes, separator modification, and stable interfacial layer between solid-electrolytes and Li metal. Herein, we review the successful implementation of MoS2 for improved Li metal batteries including those of the liquid-type and the solid-state cells. We also provide opportunities and prospects of MoS2 applications for safe and practical Li metal batteries.
Introduction
The rising popularity of electric vehicles has attracted great demand for efficient and cost-effective batteries. The development of a lithium-ion (Li-ion) battery is widely considered a success. However, the energy density of Li-ion batteries (∼390 Wh kg−1) cannot keep up with the increasing demands for higher energy storage (Goodenough and Kim, 2010). Thus, lithium metal batteries including lithium–air (Li–air) and lithium–sulfur (Li–S) batteries hold the potential to solve the higher energy demands since they exhibit theoretical energy densities of 3,500 and 2,600 Wh kg−1, respectively (Bruce et al., 2012). However, these rechargeable Li metal batteries have long been considered “impractical” since they utilize pure Li metal as anode materials. As a result, the use of Li metal anodes leaves a lot to be desired due to poor Coulombic efficiency and Li dendrite growths.
There has been an extensive amount of research into Li anodes for both liquid-based and solid-state electrolytes for Li metal batteries. Liquid-based electrolytes have the advantage of fast ion-transport at the electrode–electrolyte interface and the convenience of mass-production capability. However, at least three concerns hamper their development. The first one is the growth of parasitic Li dendrites during the electrochemical process, which could either cause a cell to short-circuit or to accumulate “dead Li” (Fang et al., 2019; Niu et al., 2019). The second concern stems from the intrinsic high-reactivity of Li metal to the organic electrolyte (Zhang, 2018). Thus, corrosive reactions occur on the Li metal, which will consume the electrolyte, increase the resistance of the cell, and lower the cycle life. The third problem is the uncontrollable volume change of the anode during Li deposition/dissolution, which eventually deteriorates the interfacial stability (Li et al., 2015).
Solid-state electrolytes (SSEs) have been considered as the potential solution to the issues of Li anodes in liquid electrolytes by fundamentally changing the deposition behavior of Li (Nagao et al., 2013). These non-liquid systems are designed to be lighter and to block Li dendrites, which enable a safer Li metal battery with a higher energy density. Furthermore, the SSEs can negate the polysulfide shuttling issues that plague the Li–S batteries. Despite these promising features, challenges remain on the interface between SSEs and electrodes due to high ionic-resistance and thermodynamic instability in contact with Li metal (Manthiram et al., 2017).
To address the challenges of any Li metal batteries, researchers have reviewed protective coatings and interlayers to stabilize the reactivity of Li metal (Tikekar et al., 2016; Lin et al., 2017; Gao et al., 2019; ). Previous reviews provide excellent and extensive summaries for various protective coatings and describe how different coatings improve electrochemical performance. For example, techniques to suppress Li dendrites and to enhance Li stability have been accomplished by the following: adopting Li+ conducting polymers (Zhang et al., 2015); utilizing carbon-based coatings such as carbon spheres and graphene (Zheng et al., 2014; Yan et al., 2014); applying a thin layer of alumina (Al2O3) on Li metal (Kozen et al., 2015). Among all the protective coating candidates, the application of molybdenum disulfide (MoS2) in Li metal-based batteries has recently gained considerable success. As schematically illustrated in Figure 1A, strategies to improve both liquid-based and solid-based Li metal cells can be achieved through coating Li surface, modifying separators/cathodes, and engineering a stable interfacial contact with MoS2. MoS2 belongs to a group of two-dimensional transition metal dichalcogenides (2D TMDs). Unlike other 2D materials (e.g., graphene and boron-nitride), MoS2 is known as a semiconductor with a larger interlayer distance of 0.62 nm (∼0.33 nm in graphene), which would facilitate faster lithium-ion diffusion (Li et al., 2020). Additionally, its phase can be transformed into a metallic phase with lithiation (Choi et al., 2017). MoS2 can be coated in a large area with high uniformity and high mechanical stability at an atomic thickness (Cha et al., 2018). Thus, the unique properties of MoS2 are advantageous for cells operating at higher current densities with low polarization. Indeed, the references reporting the results of Li||Li symmetric cells (Figure 1B) show that MoS2 has a distinct advantage over other forms of protective coatings, especially under a large current and capacity. Also, the properties of MoS2 are known to mitigate the shuttle effect in Li–S batteries by suppressing dissolved polysulfides (Stephenson et al., 2014; Lei et al., 2017). When compared with the other coating materials (e.g., carbon, Al2O3, and polymers), MoS2 exhibits higher ionic conductivity (Li et al., 2020), good mechanical stability at the atomic scale, and the highest polysulfide affinity of 4.48 eV (Balach et al., 2018; Dong et al., 2018). These traits are especially useful for the development of a highly reversible Li–S battery.
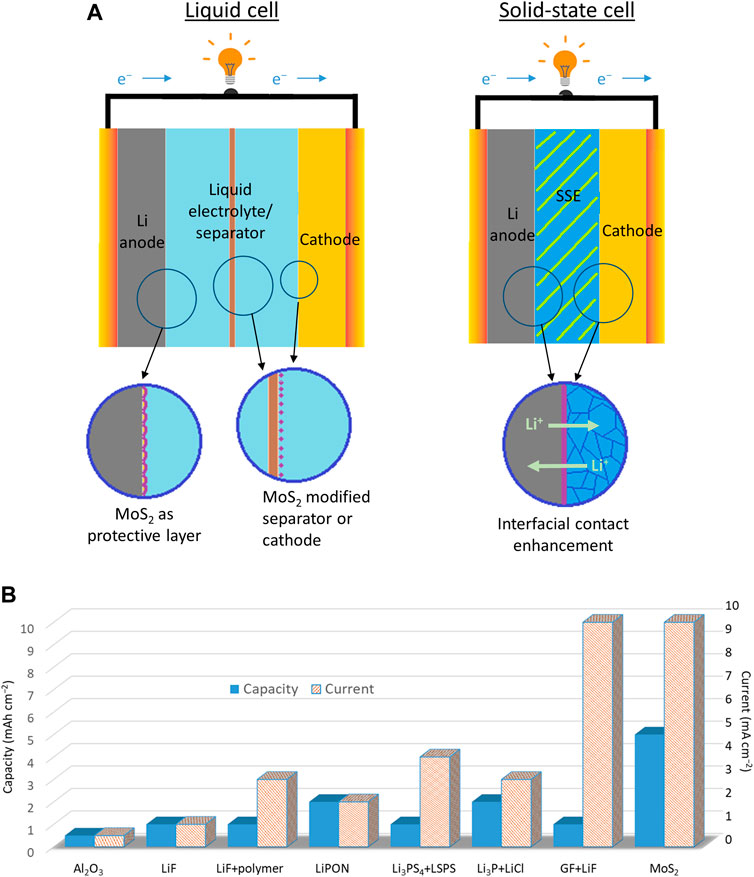
FIGURE 1. (A) Applications of MoS2 for Li metal battery systems from liquid cells to solid-state cells. (B) The statistical analysis of Li||Li symmetric cells with various coatings on the Li metal. References for the reported results: Al2O3 = Wang et al., (2018); LiF = Chen et al. (2018); LiF+polymer = Lang et al. (2019); LiPON = Kozen et al. (2017); Li3PS4+LSPS = Pang et al. (2017); Li3P+LiCl = Lin et al. (2018); GF+LiF = Shen et al. (2019); MoS2 = Cha et al. (2018). Conventional Liquid-Type Cell.
Instead of providing a comprehensive summary of previous literature, this review offers a concise overview of how MoS2 accomplishes both protective and interface-enhancing roles for Li metal batteries. Similar to graphene, MoS2 consists of a layered structure of covalently bonded Mo-S-Mo units into a hexagonal lattice (Choi et al., 2017). However, unlike graphene where its structural defects strongly attract Li-ions, MoS2 defects and grain boundaries are loosely bound to Li+ (Choi et al., 2017; Sun and Wang, 2017). As a result, MoS2 does not hinder Li+ diffusion, which would potentially facilitate a high ionic-conducting interface to circumvent poor interfacial-contact-related issues. The conventional liquid-type section will cover MoS2 as a protective layer for Li anodes and as an interlayer for Li–S batteries. Subsequently, the all-solid-state section will discuss the use of MoS2 as an effective interface for solid-based electrolytes (e.g., sulfide or garnet-type). Lastly, we provide guidelines on the design and propose testing methodologies to assess the performance of the MoS2 for high-energy-density Li metal batteries. We hope this review could attract more attention to MoS2-based protective materials for Li metal batteries.
For cells using a liquid electrolyte, the formation of a solid electrolyte interphase (SEI) layer is an inevitable process. The SEI layer should exhibit high Li-ion conductivity to transfer the ions without exposing the surface of Li to further corrosions. However, the inhomogeneous Li deposition and the rampant volume change with Li dendrites during the cycling process would easily damage the SEI layer (Wood et al., 2016). The repeated exposure of Li metal to the electrolyte due to the growth of Li dendrites and the constant breakdown of the SEI layer would eventually lower the Coulombic efficiency and shorten the cell’s life. Furthermore, Li dendrites are especially problematic for Li–S batteries due to the dissolved polysulfides that cause unwanted side-reactions with the anode (Yu et al., 2015). Therefore, the Li metal-anode must exhibit a homogeneous Li deposition/dissolution to prevent Li-dendrite growth and corrosions associated with polysulfides and Li dendrites.
MoS2 as a Protective Layer
An ideal protective layer should be both chemically and electrochemically stable to inhibit the propagation of Li dendrites. MoS2 is regarded as a good Li+ conductor with a calculated energy barrier for Li diffusion of 0.49 eV (Li et al., 2012; Zhang, 2018), which is considerably lower than the diffusion energy barrier of 2.62 eV for nitrogen-doped graphene (Hardikar et al., 2014). Past literature of stable Li metal anodes has stressed the importance of maintaining a uniform Li+ insertion/displacement to inhibit the nucleation of Li dendrites (Zheng et al., 2014; Qian et al., 2015). Thus, passivating Li metal with thin layers of MoS2 is one of the most promising approaches to establish an “artificial SEI” at the Li interface, where a considerable amount of intercalated Li+ contributes a uniform ion transport (Li et al., 2012). Unlike other protective layers (e.g., carbon, polymer, and ceramic-based), the unique structural aspects and the phase-changing characteristics of MoS2 resolve issues associated with high impedance and poor interfacial contact (Wang et al., 2012; Chhowalla et al., 2013; Zhang et al., 2015). As a result, a protective layer of MoS2 could facilitate a fast transference of Li-ions at the interface for a stable deposition/dissolution, which prevents the nucleation of Li dendrites. Cha et al. (2018) have demonstrated this by utilizing 2D MoS2 as a protective layer for lithium metal anodes in liquid-based Li–S batteries. With the enhanced Li+ transport at the interface, the MoS2-passivated Li metal prevented Li dendrites from forming on the surface of the anode while facilitating the reversibility of active materials. Thus, the long-term reversible cycling process for a Li–S full cell is achieved (Figure 2A) with a capacity retention of up to 1,200 cycles at a high sulfur loading of 4 mg cm−2. This also highlights the practical achievement of the Li–S battery, which is attributed to the protective properties of the MoS2 layer.
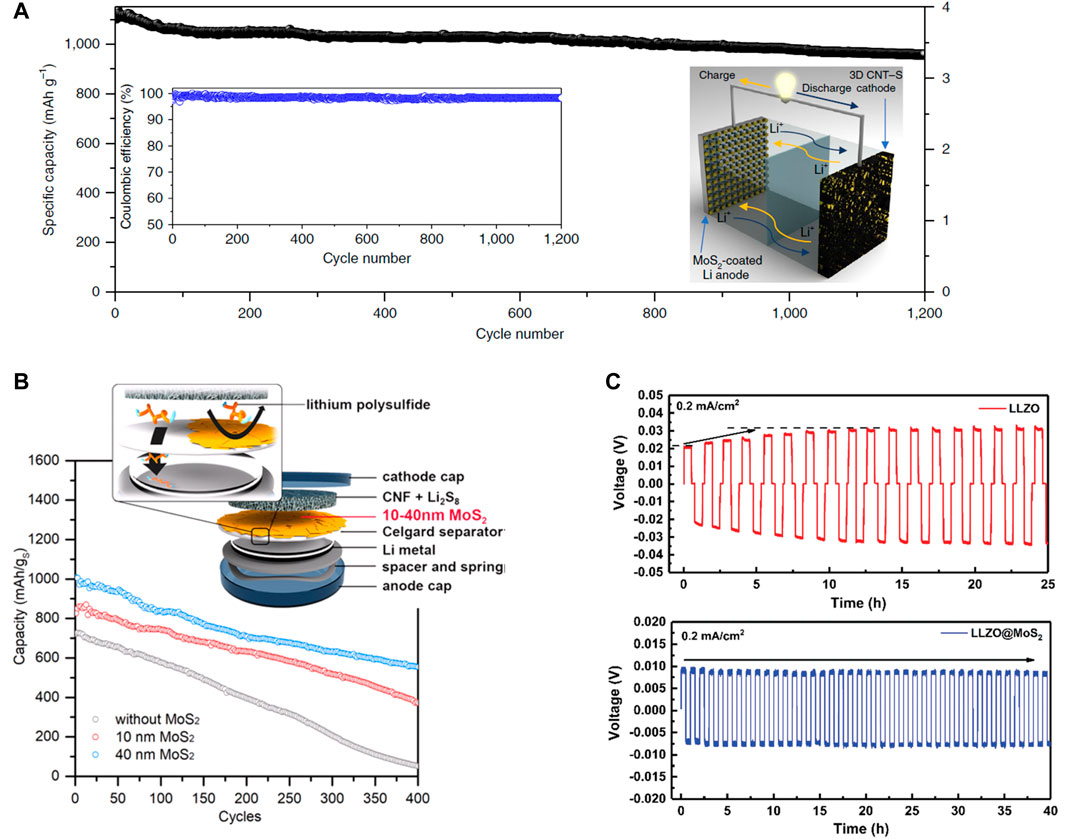
FIGURE 2. (A) The long-term cycling performance of Li–S battery with the MoS2 protected Li anode. (B) Schematic depiction of the MoS2 modified Celgard separator and its application for Li–S batteries. (C) The voltage profiles of the symmetric cells using either the pristine (red) or the MoS2 coated LLZO pellets (blue). (A) Reproduced with permission. Cha et al. (2018) Copyright 2018, Springer Nature. (B) Reproduced with permission. Yu et al. (2019) Copyright 2018, American Chemical Society. (C) Reproduced with permission. Fu et al. (2019) Copyright 2019, Royal Society of Chemistry.
In addition to sulfur, Li metal anodes paired with nickel-rich LiNixMnyCo1-x-yO2 (NMC) cathodes can exhibit specific energy densities of up to 500 Wh kg−1 (Cano et al., 2018). Indeed, recent studies have demonstrated that practical Li metal batteries should possess a low electrolyte weight per cell capacity (E/C) ratio to minimize the inactive weight, which maximizes the energy density (Liu et al., 2018; Nagpure et al., 2018). Since a cell designed with a low E/C ratio leads to an immediate dry-out and premature failure, it is usually considered a technical trade-off where obtaining higher energy density may compromise cycle-life. To address this trade-off, Choi and co-workers have employed the protective properties of the MoS2 layer to mitigate both electrolyte and Li metal consumption, which also extends the cycle-life of the Li metal batteries (Cha et al., 2020). As shown in Supplementary Figure S1A, the MoS2-coated Li anode exhibits stable cycling for up to 170 cycles with the cathode loading of 4.2 mAh cm−2 and the E/C ratio of 3 g Ah−1. Unprotected Li, on the other hand, suffers from severe capacity fading after the 20th cycle due to the deteriorative side-reactions with the electrolyte. Thus, the MoS2 layer on Li anodes plays a key role in preventing Li metal from interacting with electrolytes, which effectively enhances the cycle-life under the minimum E/C ratio. Even with the reduced Li thickness of ∼50 μm, the MoS2 layer still outperformed the other polymeric-based protective films (Supplementary Figure S1B). Kim et al. (2019) also reported a Langmuir-Blodgett artificial SEI (LBASEI) composed of MoS2 to protect the surface of the lithium–aluminum anodes. As illustrated in Supplementary Figure S2A, this MoS2 coated anode further enhanced the Li migration than its unprotected counterparts, which thereby enhanced the cycle life of the full-cell for up to 250 cycles at 0.5 C-rate with more than 80% capacity retention. The use of MoS2 protective layer not only improves Li migration at the interfacial contact of the anode but also promotes compact deposition of Li throughout a large surface of the anode.
MoS2 as an Interlayer
In addition to protecting Li anodes with MoS2 layer, another approach is to apply MoS2 as an interlayer material for the separator in Li–S batteries. Separators modified with MoS2 are reported to have the features of physical/chemical absorption for polysulfides and act as the selective sieve for Li+ diffusion. Another attractive feature about MoS2 is that it exhibits a large area to thickness ratio (i.e., aspect ratio) when exfoliated into chemically stable flakes (Chen et al., 2017). This would be advantageous for designing an efficient film to reduce the polysulfide shuttling effect without relying on complicated and bulky host materials for sulfur cathodes. Ghazi et al. (2017) incorporated MoS2 nanosheets to trap polysulfides without reducing ion-conductivity. By obtaining a flexible composite separator of MoS2/Celgard, the group demonstrated an enhanced cycle life with the corresponding capacity decay of 0.083% and the Coulombic efficiency of 99.5% throughout the 600 cycles. Similarly, Yu et al. (2019) employed a solution-based method to coat a Celgard separator with MoS2 film (Figure 2B). When tested with Li–S batteries, the cell with the separator coated with a sufficient amount of MoS2 displayed the lowest capacity decay with the specific capacity of ∼1,000 mAh g−1 at 0.5 C-rate. Moreover, the MoS2 coating merely contributed <1% weight of the cathode material (22 µg cm−2 of inactive weight), which goes to show the effectiveness of the 2D structures in MoS2 that self-assemble into a lightweight interlayer.
There have been instances where MoS2 was used in conjunction with carbon materials to design the interlayer. Such a hybrid approach typically features the merit of providing electron transfer to insulating polysulfides for enhanced reversible redox-reactions. This would therefore prevent further accumulation of irreversible sulfur species throughout the separator during cycling (Rana et al., 2019). In this regard, MoS2 is usually combined with either carbon nanotubes (CNTs) or graphene. For instance, Guo et al. (2017) developed a dual functional MoS2/graphene interlayer where graphene provided a conducting network to accommodate electron transfer for polysulfides; MoS2, on the other hand, served as an additional chemical barrier to prevent polysulfides from shuttling. The full-cell with MoS2/graphene interlayer delivered the specific capacities of 850, 770, 701, and 600 mAh g−1 at current densities of 0.5, 1.0, 2.0, and 3.0 A g−1, respectively. Furthermore, the cell retained the reversible capacity of 718 mAh g−1 even after the 200th cycle. Another example can be attributed to Jeong et al. (2017), where the group designed a separator composed of 1T MoS2 nanosheets and CNTs to improve polysulfide trapping and ion transfer. As shown in Supplementary Figure S2B, the separator configured with MoS2/CNTs enhanced the electrochemical performance noticeably by retaining the capacity of 670 mAh g−1 for 500 cycles at a high current density of 1 C-rate. Very recently, the hybrid approach has been expanded to 2D TMDs alloys. For example, Bhoyate et al. (2020) presented a novel 2D Mo0.5W0.5S2 alloy with 2H (semiconducting)-1T (metallic) mixed-phase synthesized on CNTs. The approach exhibited the synergistic effect of an accelerated electron transfer, higher LiPSs binding effect, and catalytic performance. Thus, the Li–S full cell assembled with the 2D Mo0.5W0.5S2/CNT/S cathode shows a high specific capacity of 1,228 mAh g−1 at 0.1 C-rate and much higher cyclic stability than the other pristine cathodes (up to 400 cycles). The versatility of configuring the interlayers/cathodes and the consistent performance prove that modifying inactive and active materials with MoS2 is one of the most promising approaches for obtaining stable Li–S batteries.
All-Solid-State Cell
Solid-state electrolytes (SSEs) have the obvious advantage of mechanical rigidity when compared to conventional liquid electrolytes. Thus, SSEs are expected to prevent Li dendrites with the added benefits of being safe and non-flammable (Janek and Zeier, 2016). So far, sulfide and oxide garnet-type electrolytes are the two promising SSEs for Li metal batteries. Sulfide SSEs are regarded for their high ion-conductivity at room temperature; garnet-type SSEs, on the other hand, are often touted for their excellent thermal/chemical stability (Seino et al., 2014). However, obstacles remain for a successful implementation of SSEs due to the large interfacial resistance between Li metal and the electrolyte. This large interfacial resistance is ascribed to two traits: (1) the formation of a decomposition layer due to the unstable reaction between Li metal and SSEs; (2) the uneven contact between Li metal and SSEs, which limits the ion transport (Wang et al., 2017; Xu et al., 2018). In addition to the issues of low ion-conductivity, Li dendrites grow through the grain boundaries of an SSE, which defeats their practical applications. Therefore, it is crucially important to introduce a kinetically stable interlayer that not only facilitates fast ion-transport but also improves the interfacial contact (i.e., wettability) between Li metal and SSEs.
The feasibility of developing an effective interlayer for SSEs is realized with the use of MoS2. Recently, Kizilaslan et al. (2020) utilized the MoS2 interlayer to prevent the degradation of both SSE and Li anode and to maintain fast Li+ transport across the interface. As displayed by the cycling profiles in Supplementary Figure S2C, the Li–S full cell with the MoS2 interlayer for Li7P3S11 sulfide electrolyte maintained the discharge capacity of ∼590 mAh g−1 after 200 cycles, which corresponds to about 13.58% capacity loss. The full cell without the interlayer, on the other hand, exhibited a capacity loss of 27.3% after 200 cycles. The results indicate how the MoS2 interlayer prevents the decomposition between the SSE and Li anode to reduce the total impedance of the cell.
MoS2 is known to undergo a conversion reaction with Li below 1.1 V vs. (Li+/Li); as a result, the MoS2 interlayer facilitates better wettability of Li to enhance the interfacial contact between SSE and Li anode (Zhang et al., 2018). However, the conversion reaction often results in the formation of Li2S/Mo film, which would irreversibly transform the MoS2 interlayer into an electronic insulator (Stephenson et al., 2014). As an artificial SEI layer, the electronic insulation of the Li2S/Mo ensures a sufficient ionic conductivity at the interface and prevents further Li dendrite growths. Fu et al. (2019) have employed this feature by coating MoS2 layers to Li6.5La3Zr1.5Ta0.5O12 (LLZO) pellet. As indicated by the galvanostatic cycling for the two symmetric cells (Figure 2C), the bare LLZO pellet exhibited an initial polarization of 21 mV before increasing up to 30 mV. The MoS2 coated LLZO pellet maintained a flat voltage profile with a reduced polarization of 8 mV throughout the test. The group also discovered how the conversion of the MoS2 layers suppressed the local growth of Li dendrites, which effectively prevented further dendrite propagation within the grain boundaries of the SSE. Therefore, the MoS2 interlayer not only reduces the interfacial resistance between Li anode and SSE but also forms a homogeneous contact to promote a uniform deposition/dissolution of Li.
Summary and Perspectives
To summarize, we reviewed the application of 2D MoS2 for the enhanced electrochemical performance in Li metal batteries. The integration of MoS2 not only applies to Li metal batteries with liquid-based electrolytes but also for cells employing all-solid-state electrolytes. As we have observed, the applications of MoS2 encompass Li metal coating, separator modification, and stable interface engineering. Thus, MoS2 provides a wide range of benefits for Li metal batteries by preventing Li dendrites, protecting Li metal against corrosion, and facilitating high ionic-conducting and stable interface. Despite the advancements in electrochemical properties of Li metal batteries with MoS2, aspects of the MoS2 protection mechanism remain poorly understood. Also, strategies to maximize the cell’s energy density are still in great demand. Therefore, we advocate the following perspectives that merit attention from the research community:
1. Despite the potentials of the MoS2 application, there are concerns about the oxidation of MoS2 when it is used in the air, which would complicate its long-term storage and its protective quality (Budania et al., 2017). Also, MoS2 could undergo a conversion reaction when it comes in direct contact with Li metal (Stephenson et al., 2014). Thus, researchers are recommended to assess these phenomena and determine if they have a negative effect on the practical application.
2. The mechanisms of MoS2 layers for immobilization of polysulfides, high ion transport, lithiophilicity, and their phase transformation during cycling are not investigated. Thus, in-operando techniques, such as in situ electron microscopy, X-ray technique, and X-ray-based Synchron techniques, are potentially useful to obtain time-dependent information.
3. The excessive amount of Li on the anode diminishes the useful capacity of Li metal batteries. For instance, the claimed specific capacity of 3,860 mAh g−1 for Li metal dramatically drops to 640 mAh g−1 when 500% of excess Li metal is used (Niu et al., 2019). Thus, the effect of MoS2 coating on reducing the excessive Li-metal needs to be thoroughly explored to maximize the energy density.
4. Recently, the shape of a current collector is modified from a conventional 2D structure to a 3D porous framework to accommodate the volume change of Li metal and to reduce the amount of “dead” Li (Cheng et al., 2018; Gao et al., 2019). Most of the MoS2 applications for Li metal batteries are limited to a flat 2D structure. Thus, the use of MoS2 coatings on the 3D framework is highly projected to deliver a synergetic improvement.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Asian Office of Aerospace R&D (FA2386-18-4075).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2021.645403/full#supplementary-material.
References
Balach, J., Linnemann, J., Jaumann, T., and Giebeler, L. (2018). Metal-based nanostructured materials for advanced lithium–sulfur batteries. J. Mater. Chem. A 6, 23127–23168. doi:10.1039/c8ta07220e
Bhoyate, S., Kim, J., Lee, E., Park, B., Lee, E., Park, J., et al. (2020). Mixed phase 2D Mo0.5W0.5S2 alloy as a multi-functional electrocatalyst for a high-performance cathode in Li–S batteries. J. Mater. Chem. A 8, 12436–12445. doi:10.1039/D0TA04354K
Bruce, P. G., Freunberger, S. A., Hardwick, L. J., and Tarascon, J. M. (2012). Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29. doi:10.1038/nmat3191
Budania, P., Baine, P., Montgomery, J., McGeough, C., Cafolla, T., Modreanu, M., et al. (2017). Long-term stability of mechanically exfoliated MoS2 flakes. MRS Commun. 7, 813‒818. doi:10.1557/mrc.2017.105
Cano, Z. P., Banham, D., Ye, S., Hintennach, A., Lu, J., Fowler, M., et al. (2018). Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3, 279–289. doi:10.1038/s41560-018-0108-1
Cha, E., Lee, H., and Choi, W. (2020). Improving lithium-metal battery performance under the conditions of lean electrolyte through MoS2 coating. ChemElectroChem 7, 890–892. doi:10.1002/celc.201901735
Cha, E., Patel, M. D., Park, J., Hwang, J., Prasad, V., Cho, K., et al. (2018). 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li–S batteries. Nat. Nanotechnol. 13, 337–343. doi:10.1038/s41565-018-0061-y
Chen, K.-S., Balla, I., Luu, N. S., and Hersam, M. C. (2017). Emerging opportunities for two-dimensional materials in lithium-ion batteries. ACS Energy Lett. 2, 2026–2034. doi:10.1021/acsenergylett.7b00476
Chen, L., Chen, K. S., Chen, X., Ramirez, G., Huang, Z., Geise, N. R., et al. (2018). Novel ALD chemistry enabled low-temperature synthesis of lithium fluoride coatings for durable lithium anodes. ACS Appl. Mater. Interf. 10, 26972–26981. doi:10.1021/acsami.8b04573
Cheng, Q., Wei, L., Liu, Z., Ni, N., Sang, Z., Zhu, B., et al. (2018). Operando and three-dimensional visualization of anion depletion and lithium growth by stimulated Raman scattering microscopy. Nat. Commun. 9, 2942. doi:10.1038/s41467-018-05289-z
Chhowalla, M., Shin, H. S., Eda, G., Li, L. J., Loh, K. P., and Zhang, H. (2013). The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275. doi:10.1038/nchem.1589
Choi, W., Choudhary, N., Park, J., Akinwande, D., and Lee, Y. (2017). Recent development of 2D materials and their applications. Mater. Today 20, 116–130. doi:10.1016/j.mattod.2016.10.002
Dong, C., Gao, W., Jin, B., and Jiang, Q. (2018). Advances in cathode materials for high-performance lithium–sulfur batteries. iScience 6, 151–198. doi:10.1016/j.isci.2018.07.021
Fang, C., Li, J., Zhang, M., Zhang, Y., Yang, F., Lee, J. Z., et al. (2019). Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515. doi:10.1038/s41586-019-1481-z
Fu, J., Yu, P., Zhang, N., Ren, G., Zheng, S., Huang, W., et al. (2019). In situ formation of a bifunctional interlayer enabled by a conversion reaction to initiatively prevent lithium dendrites in a garnet solid electrolyte. Energy Environ. Sci. 12, 1404–1412. doi:10.1039/C8EE03390K
Gao, Y., Yan, Z., Gray, J. L., He, X., Wang, D., Chen, T., et al. (2019). Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389. doi:10.1038/s41563-019-0305-8
Ghazi, Z. A., He, X., Khattak, A. M., Khan, N. A., Liang, B., Iqbal, A., et al. (2017). MoS2/Celgard separator as efficient polysulfide barrier for long-life lithium–sulfur batteries. Adv. Mater. 29, 1606817. doi:10.1002/adma.201606817
Goodenough, J. B., and Kim, Y. (2010). Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603. doi:10.1021/cm901452z
Guo, P., Liu, D., Liu, Z., Shang, X., Liu, Q., and He, D. (2017). Dual functional MoS2/graphene interlayer as an efficient polysulfide barrier for advanced lithium–sulfur batteries. Electrochim. Acta 256, 28–36. doi:10.1016/j.electacta.2017.10.003
Hardikar, R. P., Das, D., Han, S. S., Lee, K.-R., and Singh, A. K. (2014). Boron doped defective graphene as a potential anode material for Li-ion batteries. Phys. Chem. Chem. Phys. 16, 16502–16508. doi:10.1039/C4CP01412J
Janek, J., and Zeier, W. G. (2016). A solid future for battery development. Nat. Energy 1, 16141. doi:10.1038/nenergy.2016.141
Jeong, Y. C., Kim, J. H., Kwon, S. H., Oh, J. Y., Park, J., Jung, Y., et al. (2017). Rational design of exfoliated 1T MoS2@CNT-based bifunctional separators for lithium sulfur batteries. J. Mater. Chem. A 5, 23909–23918. doi:10.1039/c7ta08153g
Kim, M. S., Deepika, , Lee, S. H., Kim, M. ‐S., Ryu, J. ‐H., Lee, K. ‐R., et al. (2019). Enabling reversible redox reactions in electrochemical cells using protected LiAl intermetallics as lithium metal anodes. Sci. Adv. 5, eaax5587. doi:10.1126/sciadv.aax5587
Kozen, A. C., Lin, C. F., Zhao, O., Lee, S. B., Rubloff, G. W., and Noked, M. (2017). Stabilization of lithium metal anodes by hybrid artificial solid electrolyte interphase. Chem. Mater. 29, 6298–6307. doi:10.1021/acs.chemmater.7b01496
Kozen, A. C., Lin, C., Pearse, A. J., Schroeder, M. A., Han, X., Hu, L., et al. (2015). Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 9, 5884–5892. doi:10.1021/acsnano.5b02166
Kızılaslan, A., Çetinkaya, T., and Akbulut, H. (2020). 2H‐MoS2 as an artificial solid electrolyte interface in all‐solid‐state lithium–sulfur batteries. Adv. Mater. Interf. 7, 2001020. doi:10.1002/admi.202001020
Lang, J., Long, Y., Qu, J., Luo, X., Wei, H., Huang, K., et al. (2019). One-pot solution coating of high quality LiF layer to stabilize Li metal anode. Energy Storage Mater. 16, 85–90. doi:10.1016/j.ensm.2018.04.024
Lei, T., Chen, W., Huang, J., Yan, C., Sun, H., Wang, C., et al. (2017). Multi-functional layered WS2 nanosheets for enhancing the performance of Lithium−Sulfur batteries. Adv. Energy Mater. 7, 1601843. doi:10.1002/aenm.201601843
Li, W., Yao, H., Yan, K., Zheng, G., Liang, Z., Chiang, Y. M., et al. (2015). The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 6, 7436. doi:10.1038/ncomms8436
Li, Y., Chang, K., Sun, Z., Shangguan, E., Tang, H., Li, B., et al. (2020). Selective preparation of 1T- and 2H-phase MoS2 nanosheets with abundant monolayer structure and their applications in energy storage devices. ACS Appl. Energy Mater. 3, 998‒1009. doi:10.1021/acsaem.9b02043
Li, Y., Wu, D., Zhou, Z., Cabrera, C. R., and Chen, Z. (2012). Enhanced Li adsorption and diffusion on MoS2 zigzag nanoribbons by edge effects: a computational study. J. Phys. Chem. Lett. 3, 2221–2227. doi:10.1021/jz300792n
Lin, D., Liu, Y., and Cui, Y. (2017). Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206. doi:10.1038/nnano.2017.16
Lin, L., Liang, F., Zhang, K., Mao, H., Yang, J., and Qian, Y. (2018). Lithium phosphide/lithium chloride coating on lithium for advanced lithium metal anode. J. Mater. Chem. A 6, 15859–15867. doi:10.1039/c8ta05102j
Liu, B., Zhang, J. G., and Xu, W. (2018). Advancing lithium metal batteries. Joule 2, 833–845. doi:10.1016/j.joule.2018.03.008
Manthiram, A., Yu, X., and Wang, S. (2017). Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103. doi:10.1038/natrevmats.2016.103
Nagao, M., Hayashi, A., Tatsumisago, M., Kanetsuku, T., Tsuda, T., and Kuwabata, S. (2013). In situ SEM study of a lithium deposition and dissolution mechanism in a bulk-type solid-state cell with a Li2S-P2S5 solid electrolyte. Phys. Chem. Chem. Phys. 15, 18600–18606. doi:10.1039/c3cp51059j
Nagpure, S. C., Tanim, T. R., Dufek, E. J., Viswanathan, V. V., Crawford, A. J., Wood, S. M., et al. (2018). Impacts of lean electrolyte on cycle life for rechargeable Li metal batteries. J. Power Source 407, 53–62. doi:10.1016/j.jpowsour.2018.10.060
Niu, C., Lee, H., Chen, S., Li, Q., Du, J., Xu, W., et al. (2019). High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 4, 551–559. doi:10.1038/s41560-019-0390-6
Niu, C., Pan, H., Xu, W., Xiao, J., Zhang, J. G., Luo, L., et al. (2019). Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 14, 594–601. doi:10.1038/s41565-019-0427-9
Pang, Q., Liang, X., Shyamsunder, A., and Nazar, L. F. (2017). An in vivo formed solid electrolyte surface layer enables stable plating of Li metal. Joule 1, 871–886. doi:10.1016/j.joule.2017.11.009
Qian, J., Henderson, W. A., Xu, W., Bhattacharya, P., Engelhard, M., Borodin, O., et al. (2015). High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362. doi:10.1038/ncomms7362
Rana, M., Li, M., Huang, X., Luo, B., Gentle, I., and Knibbe, R. (2019). Recent advances in separators to mitigate technical challenges associated with re-chargeable lithium sulfur batteries. J. Mater. Chem. A 7, 6596–6615. doi:10.1039/C8TA12066H
Seino, Y., Ota, T., Takada, K., Hayashi, A., and Tatsumisago, M. (2014). A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 7, 627–631. doi:10.1039/C3EE41655K
Shen, X., Li, Y., Qian, T., Liu, J., Zhou, J., Yan, C., et al. (2019). Lithium anode stable in air for low-cost fabrication of a dendrite-free lithium battery. Nat. Commun. 10, 900. doi:10.1038/s41467-019-08767-0.900
Stephenson, T., Li, Z., Olsen, B., and Mitlin, D. (2014). Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 7, 209–231. doi:10.1039/C3EE42591F
Sun, X., and Wang, Z. (2017). Ab initio study of adsorption and diffusion of lithium on transition metal dichalcogenide monolayers. Beilstein J. Nanotechnol. 8, 2711–2718. doi:10.3762/bjnano.8.270
Tikekar, M. D., Choudhury, S., Tu, Z., and Archer, L. A. (2016). Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114. doi:10.1038/nenergy.2016.114
Wang, C., Gong, Y., Liu, B., Fu, K., Yao, Y., Hitz, E., et al. (2017). Conformal, nanoscale ZnO surface modification of garnet-based solid-state electrolyte for lithium metal anodes. Nano Lett. 17, 565–571. doi:10.1021/acs.nanolett.6b04695
Wang, L., Zhang, L., Wang, Q., Li, W., Wu, B., Jia, W., et al. (2018). Long lifespan lithium metal anodes enabled by Al2O3 sputter coating. Energy Storage Mater. 10, 16–23. doi:10.1016/j.ensm.2017.08.001
Wang, Q. H., Kalantar-Zadeh, K., Kis, A., Coleman, J. N., and Strano, M. S. (2012). Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712. doi:10.1038/nnano.2012.193
Wood, K. N., Kazyak, E., Chadwick, A. F., Chen, K.-H., Zhang, J.-G., Thornton, K., et al. (2016). Dendrites and Pits: untangling the complex behavior of lithium metal anodes through operando video microscopy. ACS Cent. Sci. 2, 790–801. doi:10.1021/acscentsci.6b00260
Xu, L., Tang, S., Cheng, Y., Wang, K. Y., Liang, J. Y., Liu, C., et al. (2018). Interfaces in solid-state lithium batteries. Joule 2, 1991–2015. doi:10.1016/j.joule.2018.07.009
Yan, K., Lee, H., Gao, T., Zheng, G., Yao, H., Wang, H., et al. (2014). Ultrathin two-dimensional atomic crystals as stable interfacial layer for improvement of lithium metal anode. Nano Lett. 14, 6016–6022. doi:10.1021/nl503125u
Yu, M., Li, R., Wu, M., and Shi, G. (2015). Graphene materials for lithium–sulfur batteries. Energy Storage Mater. 1, 51–73. doi:10.1016/j.ensm.2015.08.004
Yu, X., Zhou, G., and Cui, Y. (2019). Mitigation of shuttle effect in Li–S battery using a self-assembled ultrathin molybdenum disulfide interlayer. ACS Appl. Mater. Interf. 11, 3080–3086. doi:10.1021/acsami.8b19354
Zhang, J., Bai, Y., Sun, X.-G., Li, Y., Guo, B., Chen, J., et al. (2015). Superior conductive solid-like electrolytes: nanoconfining liquids within the hollow structures. Nano Lett. 15, 3398–3402. doi:10.1021/acs.nanolett.5b00739
Zhang, L., Sun, D., Kang, J., Feng, J., Bechtel, H. A., Wang, L. W., et al. (2018). Electrochemical reaction mechanism of the MoS2 electrode in a lithium-ion cell revealed by in Situ and operando X-ray absorption spectroscopy. Nano Lett. 18, 1466–1475. doi:10.1021/acs.nanolett.7b05246
Zhang, S. S. (2018). Problem, status, and possible solutions for lithium metal anode of rechargeable batteries. ACS Appl. Energy Mater. 1, 910–920. doi:10.1021/acsaem.8b00055
Keywords: transition metal dicalcogenide, molybdenum disulfide, lithium metal battery, Li–S battery, anode protection, solid state electrolyte
Citation: Cha E, Kim DK and Choi W (2021) Advances of 2D MoS2 for High-Energy Lithium Metal Batteries. Front. Energy Res. 9:645403. doi: 10.3389/fenrg.2021.645403
Received: 23 December 2020; Accepted: 25 January 2021;
Published: 05 March 2021.
Edited by:
Fu Sun, Qingdao Institute of Bioenergy and Bioprocess Technology (CAS), ChinaReviewed by:
Tang Wei, Xi’an Jiaotong University, ChinaLiu Shan, North China Institute of Science and Technology, China
Copyright © 2021 Cha, Kim and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wonbong Choi, V29uYm9uZy5DaG9pQHVudC5lZHU=
 Eunho Cha1
Eunho Cha1 Wonbong Choi
Wonbong Choi