- 1State Key Laboratory of Fine Chemicals, PSU-DUT Joint Center for Energy Research, School of Chemical Engineering, Dalian University of Technology, Dalian, China
- 2Department of Chemistry, Faculty of Science, The Chinese University of Hong Kong, Hong Kong, China
With the increasing environmental problems caused by carbon dioxide (CO2) emission and the ultimate carbon resources needed for the development of human society, CO2 hydrogenation to methanol with H2 produced with renewable energy represents a promising path forward. Comprehensive analysis shows that the production of methanol by thermal catalytic CO2 hydrogenation is the most promising technology for large-scale industrialization. This review highlights current developments and future perspectives in the production of methanol from CO2, as well as the main existing problems based on a thorough techno-economic analysis. Moreover, the utilization status and future role of methanol as a platform molecule in the energy system is analyzed. Finally, in this review attention is paid to the development of new catalysts, new routes and new technologies for CO2 conversion aiming to clarify the future direction.
Introduction
While absorbing solar radiation, the earth is also losing energy to the space, so that the energy in and out of the earth system is basically the same (Figure 1A). However, human activities are breaking the balance, and the situation is becoming more and more serious. In May 2019, CO2 concentration in the atmosphere exceeded 415 ppm, about 48% higher than that before the industrial revolution. The magnitude and rate of this increase, at least in the earth’s nearly 800,000 years of history, is unprecedented (Figure 1B). The greenhouse effect caused by carbon emission has led to a series of extreme weather and is threatening the future of our living planet (Iizumi et al., 2018). Researchers speculate that the increase of extremely severe cyclonic storms over the Arabian Sea caused by ocean warming may be the ringleader of this unprecedented locust disaster in 2020 (Murakami et al., 2017). Moreover, global warming will continue to increase the risk of a deadly flood outbreak due to the collapse of an ice lake in the Himalayas (Veh et al., 2020). Related researches also pointed out that global warming is making some originally quiet volcanoes restless due to the increase of extremely heavy rainfall (Zhang et al., 2018; Farquharson and Amelung, 2020). Presently, slow GDP growth and rising energy prices have not stopped the rise of energy consumption, and carbon emission exceeded ∼34,000 million tons both in 2018 and 2019, higher than the emission in recent years (Figure 2) (Dudley, 2019).
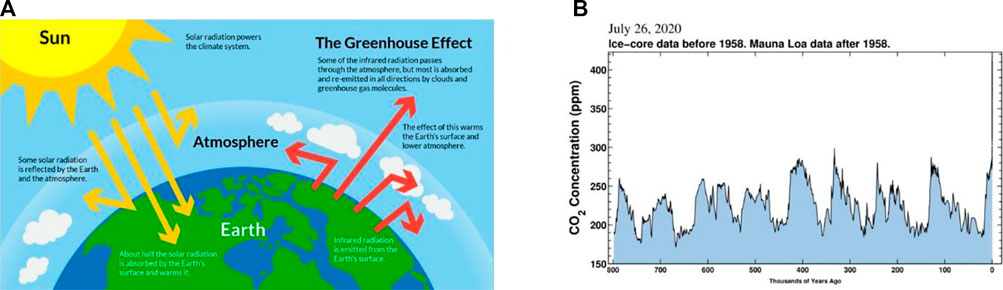
FIGURE 1. (A) Schematic diagram of the energy budget of the earth: the yellow arrows are the short wave radiation reflected and absorbed by the earth; the red arrows are the long wave radiation absorbed by greenhouse gases and released from the earth. Figure from: https://science-u.org/experiments/solar-oven-smores.html. (B) Changes of atmospheric CO2 concentration in the past 800,000 years. Figure from: Scripps Institute of Oceanography, https://sioweb.ucsd.edu/programs/keelingcurve/.
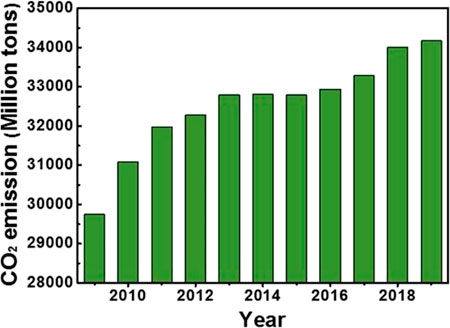
FIGURE 2. Global CO2 emission from the activities related to the combustion of oil, coal and natural gas (Dudley, 2019).
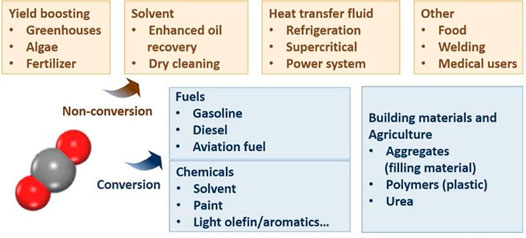
CO2 utilization has been defined as the process of using it as a raw material for products or services with a potential market value. The utilization includes direct approach (International Energy Agency, 2019; Ra et al., 2020), where CO2 is not chemically altered (non-conversion), and the chemical and biological conversion of CO2 to useful products (Figure 3). Most existing commercial applications involve direct utilization, including the production of food and beverages, metals fabrication, dry cleaning, healthcare, fire suppression, and the petroleum industry. Although still under development, the chemical and biological utilization has drawn much attention in recent years, including developing CO2-derived fuels (Satthawong et al., 2013), chemicals and building materials (Jiang et al., 2015; Li et al., 2018; Liu et al., 2018a; Wang et al., 2020a; Zhu et al., 2020). Today, around 230 million tons (Mt) of CO2 are used each year (IEA, 2019a). However, the CO2 utilization is less than 1% of the CO2 released (Figure 4). The largest consumer is agriculture, where around 130 Mt of CO2 per year is used in urea manufacturing, followed by the oil industry, with a consumption of 70 to 80 Mt of CO2 for enhanced oil recovery (IEA, 2019b). More than two-thirds of current global demand for CO2 come from North America (33%), China (21%) and Europe (16%), and the demand for existing uses is expected to grow steadily year-on-year (IEA, 2019a). Until now, the process of CO2 conversion to chemicals is limited by the market scale. Therefore, the development of target product methanol, which can be used as fuels and chemicals (Sakakura et al., 2007; Yu et al., 2010; Cokoja et al., 2011; Peters et al., 2011), is of great significance for achieving a large-scale application.
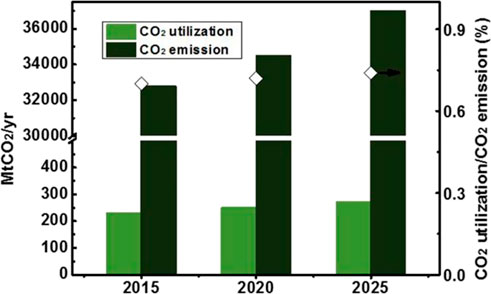
FIGURE 4. Growth in global utilization and emission of CO2. Note: Projections for future global CO2 demand are based on an average year-on-year growth rate of 1.7% (International Energy Agency, 2019). Projections for future global CO2 emission are based on an average year-on-year growth rate of 1.4% (based on the annual average growth rate of 2009–2019) (Dudley, 2019).
Methanol can be integrated into the current energy system and used as 1) a convenient energy-storage material, 2) a fuel, and 3) a feedstock to synthesize hydrocarbons, and an all-around substitute for petroleum (Olah, 2005; He et al., 2013; Araya et al., 2020). Indian government has been promoting clean transportation and the application of fuel-cell vehicles (Reddy et al., 2018). Dor Group began pilot testing in 2012 after the government of Israel determined one of the most favorable way to reduce the reliance on conventional fuels which is the use of methanol as the gasoline replacement, or gasoline-blending component, in internal combustion engines (Dor Group, 2019). China is also speeding up the layout of methanol fuel market. Eight departments including the Ministry of Industry and Information technology of China jointly issued the Guidance on the Application of Methanol Vehicles in Some Regions (2019). Shanxi, Shaanxi, Guizhou, Gansu and other regions are accelerating the application of M100 methanol vehicle and realizing the diversification of vehicle fuel to ensure energy safety, for they have good resource endowment conditions and methanol vehicle operation experience (Ministry of Industry and Information Technology of the People’s Republic of China, 2019). Compared with the top-down development mode of natural gas, ethanol and other clean energy (policy in front, promotion and application in the back), that of methanol is bottom-up, and after long-term exploration, practice and verification, the above policy documents are in place.
Comprehensive reviews were presented about the recent significant advances in CO2 hydrogenation to methanol, focusing on development of catalysts including metals, metal oxides, and bimetallic catalysts, as well as the structure-activity relationship, in situ characterizations on identifying key descriptors and understanding reaction mechanisms (Jiang et al., 2020; Zhong et al., 2020). Researchers also provided an in-depth assessment of core-shell materials for the catalytic conversion of CO2 into chemicals and fuels (Das et al., 2020). ZrO2-containing catalysts are also systematically reviewed to offer insights into the modification of surface properties and bulk structure of catalysts driven by the supports and the resulting effects on the performance for CO2 hydrogenation to methanol (Li and Chen, 2019). Based on the summary of the research status of the catalytic materials in published reviews, this review is organized toward the future development prospects, with an emphasis on the role of methanol in the energy system in the future and technical feasibility. By analyzing the current status of thermocatalytic conversion of CO2 into methanol, the review highlights the development of catalysts regarding precise preparation, large-scale production, high efficiency and low cost.
Analysis of the Whole Process of Thermal Catalysis of CO2 to Methanol
CO2 life cycle assessment is helpful to pick out the main problems existing in the process of CO2 conversion. Researchers have introduced a mathematical formulation to select the promising CO2 capture and utilization (CCU) paths. The results indicate that the optimal solution is greatly influenced by the market demand, scale of CO2 emission source, and H2 availability (Roh et al., 2019). Therefore, target products that can be used as fuels and chemicals are of great significance for the large-scale emission reduction. Moreover, small-molecule products have irreplaceable advantages compared with large-molecule products, due to the high selectivity, simple process, low energy consumption, etc. As a fuel and an important chemical feedstock, methanol is used on a large scale, and has been used as a feedstock for the synthesis of chemicals and fuels (Olsbye et al., 2012). The hydrogenation of CO2 to methanol has attracted much attention as a promising way (Behrens et al., 2012; Kattel et al., 2017a; Wang et al., 2017; Lam et al., 2018; Dang et al., 2019b; Wang et al., 2019b; Li and Chen 2019). Next, we will discuss the role of methanol in the future energy system, technical feasibility and techno-economic analysis for methanol synthesis from CO2. By analyzing the research status and development potential of CO2 hydrogenation to methanol, we aim to find out the existing problems and point out the direction for future research.
The Importance of Methanol in the Field of Energy
In the future we will phase out fossil fuels and switch to sustainable energy, especially hydroelectricity, wind and photovoltaic energy. However, due to the variable nature of the latter sources which depend on time of day, and season of the year, we need to store such energy at peak production times for use in times of low production. Converting such energy into chemical energy and storing it in methanol molecules is regarded as one of the promising methods. Methanol is considered as one of the potential platform molecules because of its available applications in the fields of fuels and chemicals in the future (Figure 5) (Su et al., 2013). At present, methanol-based technologies include methanol synthesis, methanol to olefins, chemicals (formaldehyde, acetic acid, methylamines, glycol, etc.), gasoline, biodiesel, direct combustion and so on (Figure 6). The methanol economy through chemical recycling of CO2 will eventually free human from dependence on fossil fuels (Tountas et al., 2019). In recent years, China has developed a series of clean coal technologies to transform black-dirty coal into clean fuels and chemicals. Clean coal technologies based on methanol platform will play an important role in Chinese energy system in the future (Xu et al., 2017). Shenhua, the largest coal company in China is leading the commercialization of modern clean-coal technologies for value-added chemicals and transportation fuels.
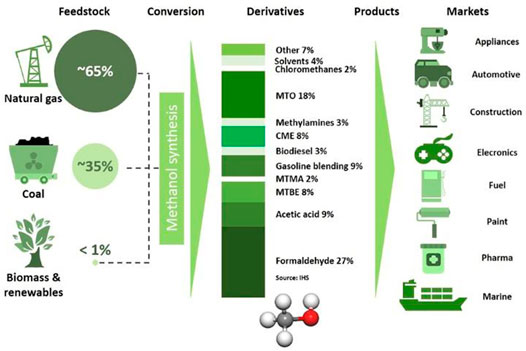
FIGURE 6. Fuels and chemicals based on methanol. Source: Methanol Institute, www.methanol.org.
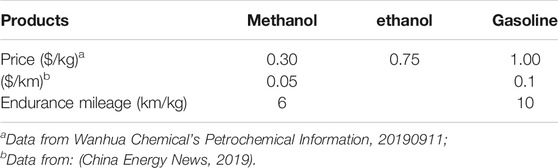
As a potential clean energy carrier, methanol has been widely used in the transportation fields such as methanol vehicles, ships, heavy trucks, industrial boilers, stoves and other industrial and civil fields. The use of methanol as transport power has begun to take shape. There are more than 6,000 well-running methanol vehicles and 20 methanol filling stations in Xi’an, China (China Energy News, 2019). The pilot projects around methanol fuel are also increasing. Methanol gasoline has the characteristics of high-octane value, clean and low energy consumption. Compared with gasoline and diesel, methanol can burn fully, and its application as fuels can effectively reduce the emission of harmful gases. The emission of PM2.5 can be reduced by 80–85%, NOx by 60–80%, and CO by 75–90% (Olah, 2005). According to the economic analysis of methanol vehicles in Xi'an pilot project, the methanol consumption per 100 kilometers is about RMB 35 yuan, and the gasoline consumption is about RMB 70 yuan. The price comparison of conventional oil products is summarized in Table 1.
Dor Group’s pilot tests in Israel suggest that methanol can at least provide a partial alternative to conventional or reformulated gasoline, particularly in regions with abundant but seemingly stranded supplies of natural gas, such as the United States. Based upon methanol-gasoline price ratios as of early January 2015, a reduction of ∼20% in unit energy costs could be achieved depending on the fluctuating cost of crude oil, as well as other market factors (Netzer et al., 2015). Based on the current methanol-gasoline price ratio, at least in China, it is economically feasible to replace conventional gasoline with methanol. Methanol is also used as internal combustion engine fuel for large ships. Diesel methanol dual-fuel ships have entered the marine transportation trade. The first methanol smart industrial park is located at Jiangxi, China. The intelligent industrial park of new energy will be the first specialized industrial cluster in China with “methanol smart industrial chain” as the main body, and the annual output value is expected to reach 10 billion after it is put into operation. ZHONGSHANG GUOXIN is planning to build six distribution centers in China, striving to gradually cover more than 10,000 retail terminals in 3–5 years. As the world’s largest methanol producer and consumer, Chinese methanol production capacity accounts for more than 50% of the world’s total. As of 2016, the annual production capacity has reached 80 million tons, and the production capacity of methanol is still increasing (China Energy News, 2019). Several locations in North America are also considering to convert petroleum coke into methanol. Methanol is becoming an important part of the future energy system.
Technical Feasibility
Thermocatalytic methanol synthesis from CO2 has a solid theoretical basis. Catalysts with different metals like Cu, Zn, Ag, Cr, and Pd have been employed for CO2 hydrogenation to methanol (Kattel et al., 2017a; Dang et al., 2019b; Din et al., 2019). Nevertheless, Cu-based catalysts exhibit high activity and selectivity. Different promoters (ZnO, ZrO2, and LaOx, etc.) have been used to improve the activity of Cu-based catalysts (Ham et al., 2018; Hu et al., 2018; Chen et al., 2019a; Mureddu et al., 2019; Noh et al., 2019). In addition, the reaction conditions (temperature, pressure, and feed gas ratio, etc.) have also been investigated systematically (Arena et al., 2013; Kobl et al., 2016; Din et al., 2019). Due to exothermic nature of the reaction, the process is thermodynamically favorable at low temperature. According to Le Chatelier’s principle, higher pressure will promote the formation of methanol. Therefore, reactors must be able to work at high pressure and moderate temperature. Most importantly, there must be 1) an efficient method to remove the heat released from the reactor, and 2) a recycle facility to send unreacted feedstocks back to the reactor after separating methanol and H2O. The exotherm from the reactor will be used elsewhere, for instance, in preheat of the feedstocks, or distillation of the methanol (Bowker, 2019).
Thermal catalytic methanol production from CO2 has a mature industrial application background (Luu et al., 2015). As early as 1923, methanol was produced at the industrial scale from syngas derived from coal, thanks to the work of Alwin Mittasch and Mathias Pier at BASF (Aresta et al., 2015). Today, more than 90 plants are in operation worldwide, nearly 200,000 tons of methanol is used as a chemical feedstock or a transportation fuel every day (Methanol Institute, 2020). George Olah’s methanol economy is exemplified in a renewable methanol production plant in Reykjavik, Iceland. This industrial facility commissioned in 2007 annually produces 4,000 metric tons of methanol from CO2 and H2 (Tountas et al., 2019). There is another demonstrator plant for methanol synthesis, namely the pan-European MefCO2 project (MefCO2, 2020). It has been constructed very recently at Niederaussem near Cologne, at the RWE coal-fired power station with an annual output of 500 tons of methanol.
Techno-Economic Analysis for Methanol Synthesis From CO2
Techno-economic assessment for CO2 hydrogenation to methanol is helpful to guide decision-making regarding R&D investment and construction of large-scale CCU plants in the future. Therefore, researchers have investigated a solar-based system for methanol synthesis from CO2 and H2O. The entire system (thermochemical reactor, water gas shift reaction system, methanol synthesis reaction system, amine-based CO2 separation system and methanol purification system) is based on mature industrial processes, except for the thermochemical reactor currently under development. Thermochemical reactor is a solar chemical heat engine that allows for the thermochemical splitting of CO2, which is an ultra-high temperature two-step FeO/Fe3O4 cycle process. Detailed sensitivity analysis shows that a breakeven price of methanol produced using this process would be 1.22 $/kg; which is higher than current market price of 0.24 $/kg. Importantly, the analysis here identifies that more than 90% of the capital investment comes from the solar concentrator/reactor system (Kim et al., 2011). Life cycle analysis shows that methanol synthesis from CO2 emitted by coal plant exhaust is predicted to be 1.3–2.6 times higher than that of its fossil-based analogue, which can be estimated to decrease significantly with a drop in electricity cost for H2 production (González-Garay et al., 2019). China will levy carbon tax after 2020 for enterprises whose comprehensive energy consumption is less than 5,000 tons of standard coal (China Energy News, 2019). The implementation of carbon tax policy will further improve the market competitiveness of methanol by reducing the cost of CO2 capture. In addition, significant advances can be achieved by improving separation, combing splitting of H2O and CO2, and process integration and distribution in the future.
Based on a comprehensive economic analysis, the best-case scenario, where electricity price is 0.06 $/kWh with 30 years plant lifetime, 0.02 $/kg CO2 cost and solar-to-hydrogen (STH) efficiency of 10%, has a break-even value of 0.96 $/kg for methanol (Alsayegh et al., 2019). The current price of H2 from natural gas and coal plants varies between 1–3 $/kg, among which the price of H2 by steam methane reforming is about 1.59 $/kg (Roy et al., 2018; Esposito, 2017). The price of H2 from renewable energy sources varies between 4.00–10.00 $/kg (Roy et al., 2018). If the cost of H2 from renewable energy sources can be reduced to 2.75 $/kg, CO2-based fuel becomes cost competitive with gasoline (Smejkal et al., 2014). Fortunately, the cost of H2 varied from 1.60–10.40 $/kg for the photoelectrochemical water splitting by the analysis of all operating costs, capital expenditures for the auxiliaries (compressors, control systems, etc.) and reactors with the particle bed systems (Pinaud et al., 2013), indicating that commercial-scale water splitting could be cost-competitive with fossil-based fuels. CO2 capture incurs costs from capital investment, energy for operating the process, cost of CO2 release, sorbent losses, maintenance of equipment, CO2 compression and transportation. The estimated cost for flue gas capture is between 0.028–0.104 $/kg of CO2 depending on the emission source (Rubin et al., 2015), while estimates for direct air capture costs are still under debate, with reports ranging from 0.030 to 1.000 $/kg of CO2 (Sanz-Pérez et al., 2016). Moreover, the implementation of carbon tax policy will further reduce the cost of CO2 capture. The single pass yield of CO2 hydrogenation to methanol is about 15% (Ham et al., 2018; Chen et al., 2019a; Mureddu et al., 2019; Wang et al., 2019b). Several examples of overall water splitting processes using semiconductor photocatalysts have been reported. The maximum STH efficiency is more than 1%, but still lower than the benchmark STH value of 10% (Chen et al., 2018b). Analysis of the above parameters shows that there is still a big gap between the current technical level and the requirements based on the techno-economic analysis, and detailed parameters are shown in Table 2. However, the efficiency can be improved by adjusting the process route. For example, H2 can be produced by electrolysis of H2O by using clean energy to generate electricity, and the STH value is expected to exceed 20% (silicon-perovskite solar cells: 25.2% efficiency (Service, 2016); Faradaic efficiency of H2 production from electrolytic water: 99% (Dotan et al., 2019). Recently, perovskite/Si dual-absorber cells have been used for the stand-alone solar water splitting. 17.6% STH efficiency was achieved when a Si photocathode was paired in tandem with a perovskite cell (Karuturi et al., 2020). Of course, we must consider its impact on methanol production costs while pursuing energy efficiency. In order to improve the yield of methanol, the production of methanol should be carried out under the conditions of low temperature and high pressure, but the high pressure will inevitably increase the operating cost. Therefore, the development of high efficiency catalysts working at low temperature and pressure for CO2 hydrogenation to methanol is an important research direction in the future. It is essential to combine in situ spectroscopy and theoretical calculation to better understand the mechanism of CO2 conversion, and then develop new catalysts and new reaction paths to improve methanol production efficiency. With the innovation of catalyst preparation technology, new technologies and methods are adopted to achieve precise construction and modification of the active sites, thereby to achieve efficient production of methanol.
Research Direction of Catalysis
A wide variety of heterogeneous catalysts have been evaluated in CO2 hydrogenation to methanol (Figure 7) (Jiang et al., 2015; Dang et al., 2019b; Nie et al., 2019; Das et al., 2020; Jiang et al., 2020; Zhong et al., 2020). The development of high-activity catalysts is conducive to reducing operating pressure and production costs. Simultaneously, the development of multifunctional catalysts to produce value-added products can also pull the equilibrium of the reaction by consuming the methanol intermediate according to the Le Chatelier’s principle, and it can realize the coupling of multiple units such as the capture unit and the different conversion units. The new preparation technology is also a key link in catalyst research and development, which aims to achieve one-step synthesis of catalysts from precursors to industrial shaped catalysts. Therefore, the following four aspects will be discussed: 1) the research progress on active sites for methanol synthesis; 2) development of new catalysts; 3) exploration on new routes, mainly including CO2 capture-transformation and metal oxide-zeolite catalysts; 4) exploration on new technology for catalysts preparation (3D-printing, plasma and atomic layer deposition technologies), emphasizing surface chemistry and engineering, so as to find the direction of future research.
Understanding the Active Sites
One of the main obstacles in developing rational strategies for heterogeneous catalysis is that the complexity of catalysts hinders efforts to characterize their active sites. Deeper insights aid in the design of next-generation catalysts in an optimal manner, which will provide the opportunity to tune the catalytic performance by optimizing the functions of the components. In recent years, surface composition and structure of commercial Cu-ZnO-Al2O3 for methanol synthesis have attracted wide attention from both industry and academia. Active sites such as Zn-Cu bimetallic sites (Li et al., 2016), ZnO-Cu interfacial sites (Kattel et al., 2017b) and Cu steps decorated with Zn atoms have been reported (Figures 8A,B) (Behrens et al., 2012; Kuld et al., 2016). Experimental and theoretical investigation show that ZnCu alloy undergoes surface oxidation under the reaction conditions, which converts surface Zn into ZnO and allows ZnCu to reach the activity of ZnO/Cu with the same Zn coverage (Kattel et al., 2017b). Moreover, researchers found the formation of metastable “graphite-like” ZnO layers during the reductive activation of Cu/ZnO/Al2O3 (Figure 8C). Understanding this metastable layer might help to understand the synergistic effect between the components of Cu/ZnO/Al2O3 (Lunkenbein et al., 2015).
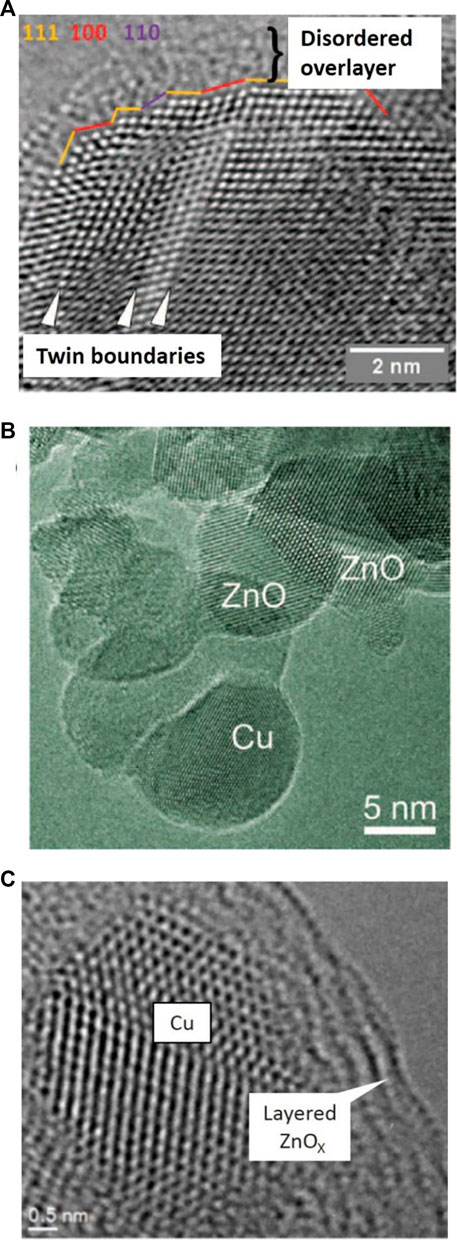
FIGURE 8. (A) Aberration-corrected HRTEM images of Cu particles in the conventionally prepared, most-active Cu/ZnO/Al2O3 catalyst (Behrens et al., 2012). (B) Promoting effect of ZnO on Cu catalysts for methanol synthesis (Kuld et al., 2016). (C) Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal-support interactions (Lunkenbein et al., 2015).
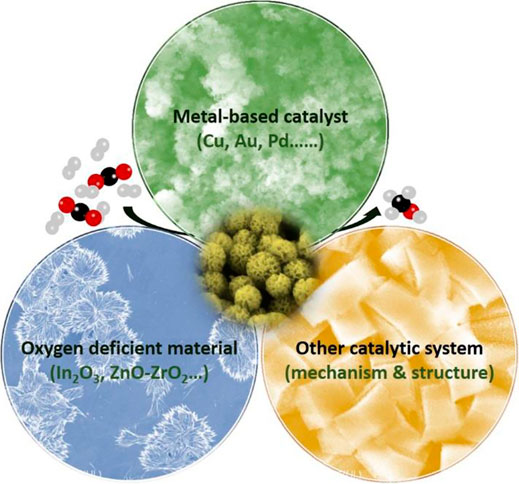
Supported Cu-based catalysts for methanol synthesis display strong support effects. Researchers attribute the difference between oxides to variation in the initial activation of CO2 (Reichenbach et al., 2018). Through DFT (density functional theory) calculations and spectroscopic characterizations, researchers found that the ZrO2-Cu interface is crucial for the conversion of formate to methanol (Larmier et al., 2017). It was also pointed that the beneficial role of the Zn ensemble in the Cu-vacant site of the stepped Cu (211) surface can enhance the reactivity and durability of catalysts for methanol production. The increased activity in the Zn-associated stepped sites is related to the enhancement of the surface affinity toward the adsorbate with the oxygen moiety (especially, HCOO) (Jo et al., 2019). The pre-assembled Zr6(μ3-O)4(μ3-OH)4 and bpy sites in UiO-bpy metal-organic frameworks were used to anchor ultrasmall Cu/ZnOx nanoparticles, thus preventing the agglomeration of Cu nanoparticles and phase separation of Cu/ZnOx nanoparticles (Figure 9A). The Cu/ZnOx@MOF shows high activity with a space-time yield of up to 2.59 gMeOH kgCu−1h−1, 100% methanol selectivity and high stability over 100 hours (An et al., 2017). A CuZnCeOx catalyst with excellent activity, selectivity and stability was prepared by a parallel flow coprecipitation method. Characterization results show that a significant synergistic effect between Cu and metal oxides was observed at the composite catalysts (Hu et al., 2018). CuZnZr catalysts were treated by vapor-phase-treatment (VPT) method. This VPT method with TPABr promotes the formation of the rod-like structure, Zr and Zn enrichments on surface and the presence of more oxygen vacancies. The CuZnZr-TPABr-3 days shows a methanol selectivity above 90% and no significant deactivation within 100 h (Chen et al., 2019b). In short, the conversion of CO2 can also be achieved at the interfacial sites by taking advantage of the synergy between the metals (Au, Cu, Ag, Pt, Pd, etc.) and oxides.
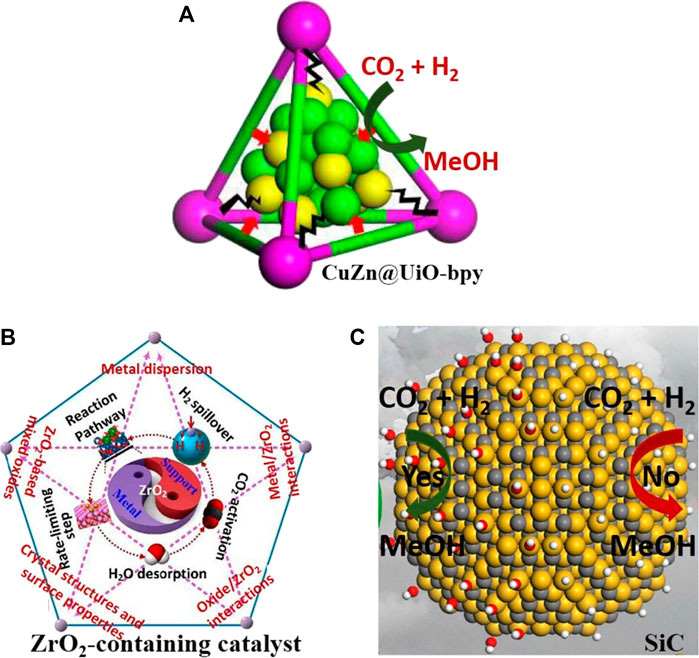
FIGURE 9. (A) CuZn@UiO-bpy for selective methanol synthesis from CO2 hydrogenation (An et al., 2017). (B) CO2 hydrogenation to methanol over ZrO2-containing catalysts (Li and Chen, 2019). (C) Hydroxyl groups of SiC surface boosting catalytic activity in CO2 hydrogenation into methanol (Peng et al., 2018).
The study on metal-oxide interface provides a better understanding of the complex reaction network to identify the key descriptors of the activity and tune reaction performance (Kattel et al., 2017a). Surface organometallic chemistry has been used to tailor active components and oxide supports to understand the structure-activity relationship of catalysts. Cu/Al2O3 catalysts prepared by surface organometallic chemistry display higher activity toward CO2 hydrogenation compared to Cu/SiO2. Researchers found that methanol formation involves formate intermediates and that the increase of rate originates from the metal-oxide interface (Lam et al., 2019). Moreover, Cu nanoparticles supported on isolated Zr(IV) sites modified SiO2 exhibit high methanol selectivity and activity compared to those loaded on SiO2 (Lam et al., 2018). SiO2 decorated with isolated Ti(IV) sites also show significantly improved methanol selectivity and CO2 hydrogenation activity. These isolated Ti(IV) sites stabilize intermediates at the interface between the support and Cu nanoparticles (Noh et al., 2019). However, researchers found that the surface organometallic chemistry approach does not affect the rate of CO formation. Here, further exploration is needed to understand the differences of active sites and reaction paths for methanol and CO formation.
In the study on the support effect of Cu-based catalysts, we need to understand the difference between oxide supports with and without oxygen vacancies. Moreover, the exact structure and composition of the active sites need to be further identified under the reaction conditions, especially for the Cu-ZnO-Al2O3 catalysts. This also requires us to rethink the role of supports in industrial Cu-based catalysts, which is an active component and participating in the construction of interfacial active sites for CO2 and H2 activation or is mainly used to disperse active metals.
New Catalyst Development
Metal oxide (indium-, zinc-based oxide, etc.) catalysts have drawn increasing attention, due to their excellent catalytic performance in the CO2 hydrogenation reaction (Wang et al., 2020a). High activity and stability for 1,000 h on stream of In2O3/ZrO2 has been achieved. Characterization points that the oxygen vacancies are active sites for methanol synthesis (Martin et al., 2016). Pd is applied to enhance the performance of indium-based catalysts. Pd atoms replacing indium atoms in the active In3O5 attract additional Pd atoms to form low-nuclearity clusters, which promotes H2 activation (Frei et al., 2019). Methanol synthesis on the defective In2O3(110) surface was investigated by DFT calculations. The calculation results indicate that the hydrogenation of H2CO* to H3CO* is the rate-limiting step for methanol formation (Ye et al., 2013). A binary metal oxide, ZnO-ZrO2 solid solution catalyst, can achieve high methanol selectivity, high CO2 single-pass conversion and high stability for at least 500 hours. Moreover, no deactivation was observed in the presence of SO2 or H2S in the reactants (Wang et al., 2017). ZrO2 support always plays important roles such as dispersants of active components, promoter and even active component. The interaction between ZrO2 and metals (or oxides) affects the adsorption and activation of CO2 and H2, and changes the reaction pathways and/or the binding of key intermediates (Figure 9B) (Li and Chen, 2019).
Exploring how surface properties regulate catalytic activity is also very important to deepen the mechanistic understanding. A molybdenum phosphide catalyst for methanol synthesis can improve the activity and stability of the catalyst in a wide range of CO/CO2/H2 feeds through weakening the interaction with formate (Duyar et al., 2018). Manganese-cobalt catalysts are also promising for methanol synthesis. A significant improvement in methanol selectivity was observed due to a synergistic effect between cobalt and manganese as well as an increase in surface basicity (Stangeland et al., 2019). Hydrophilic SiC quantum dots (QDs) exhibited higher activity than commercial SiC for CO2 hydrogenation to methanol (Figure 9C). Mechanistic studies show that the surface hydroxyl species directly participate in CO2 hydrogenation through the addition of H atoms in hydroxyl groups into CO2 to form HCOO* intermediate (Peng et al., 2018). Understanding the interactions among different components (active metals, oxide supports and doped ions) and surface properties should help elucidate the governing principles for designing high-performance catalysts with multiple active components.
Researchers reported that supported Pt nanoparticles on MoOx/TiO2 promote selective hydrogenation of CO2 to methanol under mild conditions (Toyao et al., 2019). Another kind of catalyst (NiaInbAl/SiO2) for methanol synthesis at ambient pressure was prepared by a phyllosilicate precursor, which can form well-dispersed metallic particles. The performances of NiaInbAl/SiO2 is better than that of conventional Cu/ZnO/Al2O3 catalyst at ambient pressure (Richard and Fan, 2017). A Ni-Ga catalyst can reduce CO2 to methanol at ambient pressure. Ni5Ga3 is particularly active and selective among a series of tested catalysts (Studt et al., 2014). Moreover, SiO2, acting as a ligand and support, can also modify cobalt species via Co-O-Si linkages, which favors the reactivity of *CH3O intermediates and hydrogenation to methanol rather than the C-O dissociation to produce methane (Wang et al., 2020b).
At present, the harsh operation conditions of industrial process restrict the development of the methanol industry, and also bring large energy consumption. In the future, the research and development of new catalysts should proceed toward mild operating conditions, with the purpose of reducing energy consumption. Simultaneously, if H2 production is decentralized, small-scale CO2 reduction devices that can be operated at low pressures and low temperature are required. Moreover, the development of catalysts with high activity at low temperature is also conducive to coupling the active components of CO2 capture, and realizing the integrated operation of CO2 capture and conversion. Transition metals (Ni, Co, etc.) often have high hydrogenation activity. How to realize the synthesis of methanol with high selectivity by transition metal catalysts is a fascinating research direction in the future.
New Route Exploration
Integrative CO2 Capture and Conversion
The integration of CO2 capture and conversion can simplify the CO2 cycle process and reduce energy consumption. An air-stable and well-defined Mn-PNP pincer complex catalyzed one-pot homogeneous CO2 hydrogenation to methanol is demonstrated. The hydrogenation consists of two steps, N-formylation of an amine utilizing CO2 and H2, and subsequent formamide reduction to methanol, regenerating the amine. Methanol yields up to 71% and 84% (w.r.t amine) were obtained, when morpholine and benzylamine were used, respectively; and a TON (turn over number) of 36 was observed (Kar et al., 2017). CO2 can also be captured in amine aqueous solution and then hydrogenated to methanol (>90% yield) in a biphasic 2-Methyltetrahydrofuran/water system, which allows for easy separation and recycling of the amine. CO2 from air can also be converted to methanol using this route (Kar et al., 2018). Amines were also immobilized onto silica support and employed for tandem CO2 capture and methanol synthesis. Covalently attached amine functionalities on solid supports displayed high recycling potential with almost no leaching under the reaction conditions (Kar et al., 2019). CZA-HT catalyst was prepared by physically mixing copper-based catalyst for methanol synthesis with hydrotalcite for high temperature CO2 adsorption. The catalytic performance of the CZA-HT catalyst was clearly promoted by CO2 adsorption on hydrotalcite. The sample containing 40 wt% hydrotalcite and 60 wt% CZA shows the highest methanol selectivity of 73.4% (Fang et al., 2019).
In the current demonstration project, CO2 capture and conversion are two independent operation units, which may also involve CO2 transportation, storage and different downstream conversion processes. We postulate that tandem CO2 capture and hydrogenation to methanol system presented here could be an important step toward the implementation of the carbon neutral and methanol economy concept, which can reduce the energy consumption and simplify the production process. However, matching the working conditions of CO2 conversion and capture is a key issue that must be solved, and the adsorbent also faces the problems of decomposition and poisoning of nitrogen oxides and sulfur oxides. Although this process is still in the embryonic stage of research and facing many problems, it has an attractive prospect.
Metal Oxide-zeolite Catalysts
To improve the efficiency of CO2 conversion, researchers are trying to transform the methanol and other intermediates into fuels and chemicals in situ (Wang et al., 2018a; Ye et al., 2019). Therefore, metal oxide-zeolite bifunctional catalysts have been developed, which can catalyze CO2 to gasoline. Metal oxide is responsible for CO2 hydrogenation to oxygenates, and zeolite accounts for the subsequent C-C coupling reaction (Figure 10A). A bifunctional catalyst composed of reducible In2O3 and ZSM-5 has shown high selectivity to gasoline (78.6%) with a very low methane selectivity (1%) (Gao et al., 2017). Moreover, the catalysts exhibit a better performance during an industry-relevant test, which indicates promising prospects of its industrial application.
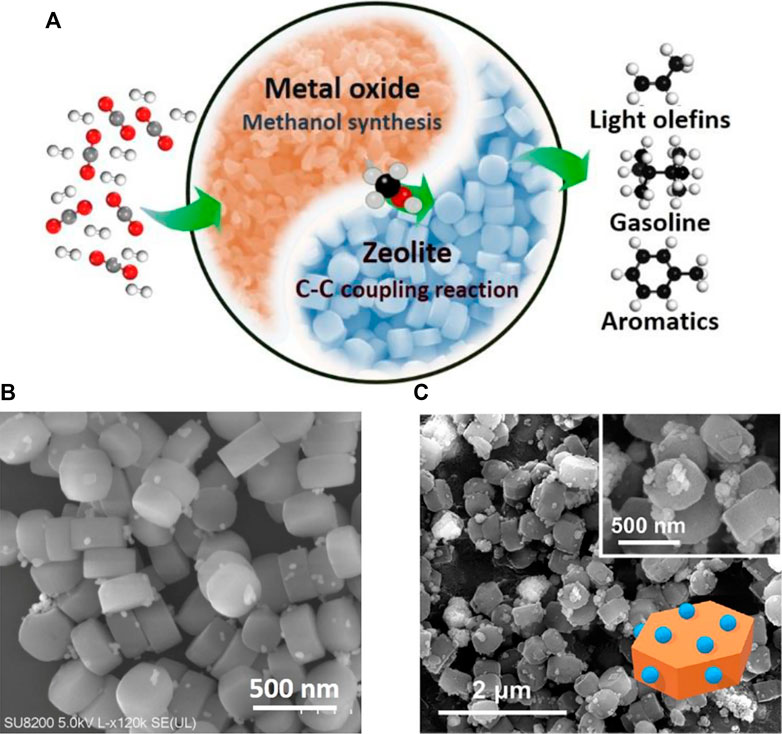
FIGURE 10. (A) Schematic for CO2 conversion on metal oxide-zeolite bifunctional catalysts. (B) ZnO/ZrO2-ZSM-5 bifunctional catalyst was designed for the direct conversion of CO2 to aromatics. (C) Highly selective conversion of CO2 to aromatics over ZnZrO/ZSM-5 (Li et al., 2019).
Metal oxide-zeolite bifunctional catalysts can also catalyze CO2 to lower olefins with high selectivity. For example, a series of bifunctional catalysts containing In2O3-based or ZnO-based oxides and various SAPO (Silicoaluminophosphate) zeolites with different crystal sizes, pore structures and amount of acid sites were developed for the production of lower olefins by CO2 hydrogenation (Dang et al., 2019a; Tan et al., 2019). It can remarkably realize highly selective synthesis of lower olefins and inhibit the formation of methane. In-Zr oxide and SAPO-34 bifunctional catalyst exhibits an excellent C2=-C4= selectivity of up to 80% at more than 35% CO2 conversion, and no significant deactivation was observed within 150 h (Gao et al., 2017). ZnZrO/SAPO tandem catalyst fabricated with ZnO-ZrO2 solid solution and Zn-modified SAPO-34 zeolite can also achieve a selectivity for C2=-C4= as high as 80–90% among hydrocarbons through CO2 hydrogenation (Li et al., 2017). Similarly, a ZnGa2O4 and SAPO-34 bifunctional catalyst can also catalyze the direct conversion of CO2 to C2=-C4= with a selectivity of 86% (Liu et al., 2018b).
Researchers have also developed metal oxide-zeolite bifunctional catalysts to convert CO2 to aromatics in a single path with methanol and other oxygenates as the intermediates. A composite catalyst of ZnAlOx and H-ZSM-5 has high aromatics selectivity (73.9%) with low CH4 selectivity (0.4%) among hydrocarbons. Furthermore, The selectivity of p-xylene in xylenes is 58.1% on the composite catalyst containing Si-H-ZSM-5 (Ni et al., 2018). Cr2O3/H-ZSM-5 bifunctional catalyst can also realize the one-step conversion of CO2 to aromatics. Due to the synergistic effect between the two components, aromatics selectivity of ∼76% was achieved, and there was no deactivation after 100 h on stream (Wang et al., 2019). ZnO/ZrO2-ZSM-5 tandem catalyst was prepared for direct CO2 conversion to aromatics with a selectivity of 70%, and the selectivity of CH4 is greatly suppressed to lower than 1% (Figure 10B) (Zhang et al., 2019). Similarly, CO2 is converted into aromatics with selectivity up to 73% over ZnZrO/ZSM-5 tandem catalyst (Figure 10C). The presence of H2O and CO2 suppresses the formation of polycyclic aromatics and enhances the stability of the catalyst (Li et al., 2019). Moreover, the conversion of CO2 into para-xylene was also reported, in one-pass by combining Zn-ZSM-5@SiO2 and Cr2O3. Through regulation of the acidity of Zn-ZSM-5@SiO2, high p-xylene selectivity (38.7% in the total products) at a CO2 conversion of 22.1% was achieved (Wang et al., 2019a).
In brief, the direct transformation of CO2 into high value-added hydrocarbons (i.e., olefins and aromatics) has obtained important fruits (Ye et al., 2019). The development of multifunctional catalysts can often achieve high-value and complex chemicals synthesis. Moreover, the multifunctional catalyst has become a research direction favored by the industry due to its simple operation and low energy consumption. However, there is a lack of in-depth understanding of its reaction paths, intermediate species, and the synergistic effect among multiple components. The bifunctional catalyst has shown obvious advantages in terms of anti-carbon deposition deactivation compared with the single methanol conversion process. The permanent deactivation of the acid sites of the zeolite, due to the migration of metal oxides during the reaction, restricts the regeneration and utilization of the catalyst. At present, we still lack powerful tools to control the distance between two components to prevent the acidic sites from being covered by metal oxides. The development of multi-functional catalysts also requires more efficient technology for precise control of the distance between different components. Although the current understanding is limited, its excellent performance attracts the unremitting efforts of researchers.
New Technology Exploration
3D-Printing Technology
3D-printing technology has been considered for numerous research fields, ranging from medicine, mechanical engineering, and materials science to chemistry. For example, 3D-printing technologies pave the way for the design and manufacture of higher performing and cheaper electrochemical devices (Ambrosi and Pumera, 2016). It is believed that some concepts of supramolecular chemistry can be directly transferred to the bio-ink developments in future. A 3D printing method has been developed for rapid, programmable, and scalable manufacturing of artificial micro-leaves with 3D architectures ranging from nanometer to centimeter. Thus, a TiO2-based ink was developed to construct hierarchical 3D architectures with high surface area (∼259 m2 g−1) (Figure 11) (Chen et al., 2018a). 3D printing has unique advantages on the modification of catalytic surfaces and the fabrication of catalysts (Jungst et al., 2016). It provides a convenient and economical way to prepare 3D architectures with well-designed patterns. With the reduction of operation cost in the future, 3D printing will be more widely used in the preparation of catalysts with controllable structure and highly dispersed active components (Zhou and Liu, 2017), as well as integration of the whole process from the design of active sites to the shaping of industrial catalysts.
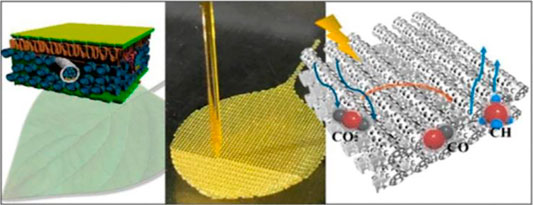
FIGURE 11. 3D printing of artificial leaf with tunable hierarchical porosity for CO2 photoreduction (Chen et al., 2018a).
Plasma Technology
Plasma technology is increasingly attracting interest in the preparation of catalysts. Nucleation and crystal growth of materials under the influence of plasma is different from those in the conventional thermal method. Plasma is also an effective tool for oxidation, reduction, etching, doping, coating and surface treatment. It can operate at room temperature and allows the catalyst preparation on temperature-sensitive supporting materials. A method using plasma to remove template has been established for zeolites synthesis (Liu et al., 2015). In addition, transition-metal catalysts prepared by plasma technology show enhanced activity at low-temperature (Yan et al., 2015; Wang et al., 2018b). Moreover, plasma-assisted CO2 conversion is attracting more and more attention (Zhang et al., 2010; Shirazi et al., 2017). However, the process is highly complex due to the interaction between plasma and catalysts, and little is known about the factors leading to the observed synergy. Catalytic mechanisms relevant to the specific application should be extensively studied (Neyts et al., 2015). Plasma has been introduced as a promising technology for modification of carbon materials, and modification of surface can often provide sites that can anchor active components. Hydrophobic/hydrophilic properties can also be tuned via plasma technology (Zhang et al., 2017). The water produced in the process of CO2 hydrogenation can accelerate the sintering of metal catalysts and occupy the active sites through competitive adsorption. The stability of catalysts used for the hydrogenation of CO2 to methanol can be enhanced by replacing oxide supports (i.e., Al2O3, SiO2, TiO2, etc.) with hydrophobic carbon supports (Furimsky, 2020). Therefore, plasma technology can further optimize the performance of catalysts by adjusting the hydrophobic/hydrophilic properties. Cold plasma is also used to improve the dispersion of active components and enhance metal-support interaction. Plasma reduction under room temperature was developed for the reduction of metal oxides, where no hazardous reducing agent or H2 were needed. This provides many opportunities for the preparation of supported catalysts with heat sensitive supports (high surface area carbon, metal organic frameworks (MOFs), covalent organic framework (COFs), peptide, proteins and others) (Liu et al., 2016). As an example, β-Mo2C nanorods were coupled with non-thermal plasma to catalyze CO2 reduction to CO by H2. In the absence of additional thermal input, the turnover frequency was an order of magnitude higher than that obtained during thermal catalysis (Zhang et al., 2020). The combination of the plasma with Pt/γ-Al2O3 or Cu/γ-Al2O3 enhanced the methanol yield compared to the plasma hydrogenation of CO2 without catalyst. The methanol selectivity of 53.7% and methanol yield of 11.3% were achieved over Cu/γ-Al2O3 with a CO2 conversion of 21.2% in the plasma process (Wang et al., 2018). Chemicals (e.g., acetic acid, methanol, ethanol, and formaldehyde) were synthesized in a one-step process from CO2 and CH4 at 30 °C and atmospheric pressure by using a plasma reactor with a water electrode. The total selectivity to oxygenates was approximately 50–60%, with acetic acid being the major component at 40.2% selectivity (Wang et al., 2017). The use of plasma with the catalytic bed enhanced the CO2 conversion (∼20 times) relative with thermal catalysis, whereas CH4 selectivity increased around 5 times by introducing nickel catalyst into plasma discharge compared to plasma only at 150 °C (Ahmad et al., 2020). In the future, plasma technology will play an important role in the preparation of catalysts and subsequent CO2 conversion.
Atomic Layer Deposition Technology
Atomic layer deposition (ALD) technology is expanding into new areas and discovering other applications that benefit from its precise control capability (George, 2010). The design of catalysts for CO2 conversion requires high selectivity, activity and stability. ALD is a promising technology to address the main problems of CO2 reduction, since it can construct catalysts with atomic precision in a highly controllable manner. Researchers have been focusing on the designs of nanomaterials via ALD technology and its applications in CO2 capture and conversion (Chen et al., 2019c). The preparation of CaO-based sorbents assisted via ALD technology has shown high and cyclically stable CO2 uptake (Armutlulu et al., 2017). Arrays of parallel CuO nanowires were surface decorated with dense ZnO islands via a few pulsed cycles of ALD. A mechanism of CO2 reduction and H2O oxidation occurred simultaneously in the active region between CuO nanowires and ZnO islands is proposed to elaborate the photocatalysis of CO2 into CO (Wang et al., 2015). Pore mouth of 5 Å zeolite was decorated by depositing an ultrathin TiO2 layer on its external surface. The composite sorbents show an ideal CO2/N2 adsorption selectivity, which is 4-fold higher than uncoated zeolite sorbents, while maintaining a fast CO2 adsorption rate and a high capacity (Song et al., 2018). Porous TiO2 from a metal-organic framework MIL-125 was surface engineered using ALD method to deposit an ultrathin MgO layer. The CO2 photoreduction activity increased more than 4 times compared with that of the commercial P25 (Feng et al., 2018). Surface atomic-layer modification technology has provided an effective strategy to control the performance of nanomaterials. In the future, development of low-temperature precursor presents unique opportunities, because it is easy to perform ALD at low temperatures and it can avoid the aggregation of particles at high temperature (Adhikari et al., 2018).
Summary and Future Perspective
Capturing CO2 from flue gas and the atmosphere and its catalytic conversion to fuel and chemicals using H2 from renewable energy can lead to a sustainable future for humankind. Methanol can be used as fuel-substitute and raw material for hydrocarbon and chemicals with many industrial applications. The hydrogenation of CO2 to methanol not only effectively alleviates the greenhouse effect, but also produces fuel and value-added chemicals. Carbon capture and storage efforts are expected to reduce CO2 emissions by about 8 Gt in the IEA 2010 Energy technology perspectives by year 2050 (Styring et al., 2015). At present, the production capacity of methanol in the world is about 0.20 Gt (China Energy News, 2019). If methanol is produced entirely from CO2, it will consume 0.27 Gt of CO2. Therefore, the energy system with methanol as the platform molecule requires further expansion of methanol production scale. One of the main obstacles in developing rational strategies for methanol synthesis is the complexity of the catalysts, which hinders characterization of the active sites. Therefore, an in-depth understanding of active sites and reaction mechanism is significant for the rational design of high-performance catalysts. Furthermore, operando characterization of catalysts under working conditions is highly recommended to correlate the structure-activity relationship. In situ techniques with high sensitivity of surface species (e.g., in situ IR, in situ XPS) and active sites (e.g., in situ TEM, in situ X-ray absorption techniques) should be widely employed.
Various strategies have been explored for thermocatalytic CO2 hydrogenation into methanol via heterogeneous catalysis, spanning from new catalyst development (transition metals/metal oxides to main group metal/metal oxides) to new route exploration (metal oxide-zeolite catalysts and integrative CO2 capture and hydrogenation). The development of new catalysts is toward the direction of lower energy consumption (e.g., low-pressure hydrogenation process) and higher methanol yield (e.g., low temperature methanol synthesis). Here, we need to take into account the problem that highly active catalysts are more likely to be poisoned by impurities. On the other hand, the design of membrane reactor is of great practical significance for delaying water-induced catalyst deactivation and for the shift of thermodynamic equilibrium after the in situ removal of the water byproduct. Highly efficient in situ by-product H2O removal through water-conduction membrane has led to a drastic increase in ethanol yield in CO2 hydrogenation to `methanol (Li et al., 2020). Moreover, nano-reactor with hydrophilic/hydrophobic surfaces will act as powerful supports for metal nanoparticles, and the molecular-fence concept should open a promising route to more-efficient catalysts for methanol synthesis (Jin et al., 2020). The construction of multifunctional catalysts to realize the further conversion of product methanol to high value-added chemicals can also drive the shift of thermodynamic equilibrium. What we must explore is how to achieve controllable coupling between different components while avoiding cross-contamination issues. Moreover, more sensitive spectroscopy techniques should be used to characterize key intermediate species, and combined with kinetic simulation to explore the influence of reaction atmosphere and intermediates concentration on the yield of the target products. It is expected to guide the development of high-performance composite catalysts based on a deep understanding of the reaction paths.
The research and development of catalysts will also go hand in hand in high-throughput screening mode and precision construction mode. Because the function of heterogeneous catalysts is defined by a mixture of molecular and mesoscopic components, atomistic simulations cannot fully capture this multi-length-scale complexity in present, and the design of such catalyst from first principles is still rare (Woodley and Catlow, 2008). At present, the integration of machine learning and high-throughput technology have been emerging to improve the development of new materials and performance of catalysts (Cole et al., 2017; Damith et al., 2018; Hartrampf et al., 2020). High-throughput synthesis and evaluation devices are speeding up the development of new catalysts. Moreover, robots will become one of researchers in the future laboratory, and the heavy and repetitive work will be completed by robots (Burger et al., 2020; Epps et al., 2020). High-throughput technology will also be the mainstream means of catalyst research and development in the months and years to come. With the development of new technology (3D, Plasmas and ALD), efforts are also being made to engineer catalytic materials with desired structure in nanoscale or even at the atomic level. New technologies are applied to design of catalysts toward precise construction of active sites and environmental protection in the preparation process. Moreover, the preparation of the catalyst and the molding technology are no longer independent, but the synthesis of catalyst from the precursor to the industrial catalyst is realized in one step to eliminate the current amplification effect of the catalyst in industrial applications. In the future, the reactor integrates the functions of CO2 capture from air, methanol synthesis, in situ by-product H2O removal and further conversion of methanol (Figure 12). With the development of distributed wind and solar energy, energy generation will gradually decentralize. Fragmented forms of energy utilization also require development of CO2 capture units, separation units and conversion units toward the direction of assembly and modularization. Promoting the development of CO2 conversion process toward high integration, green and efficient process.
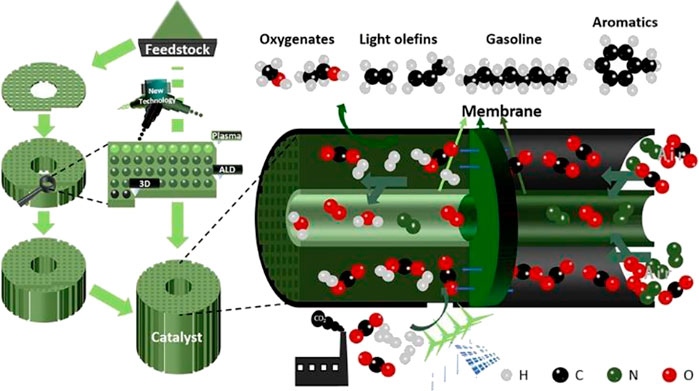
FIGURE 12. Schematic diagram of CO2 capture and conversion process and industrialization process of catalysts assisted by new technologies.
Author Contributions
XZ summarized the literature and wrote the paper. GZ made the manuscript design, writing-reviewing and funding acquisition. CS made the writing-reviewing, editing and supervision. XG made the writing-reviewing, editing, supervision and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge funding from the National Key Research and Development Program of China (2016YFB0600902-4), National Natural Science Foundation of China (21902019), and the Fundamental Research Funds for the Central Universities (DUT20RC(5)002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adhikari, S., Selvaraj, S., and Kim, D.-H. (2018). Progress in powder coating technology using atomic layer deposition. Adv. Mater. Interfaces 5, 1800581. doi:10.1002/admi.201800581
Ahmad, F., Lovell, E. C., Masood, H., Cullen, P. J., Ostrikov, K. K., Scott, J. A., et al. (2020). Low-temperature CO2 methanation: synergistic effects in plasma-Ni hybrid catalytic system. ACS Sustain. Chem. Eng. 8, 1888–1898. doi:10.1021/acssuschemeng.9b06180
Alsayegh, S., Johnson, J. R., Ohs, B., and Wessling, M. (2019). Methanol production via direct carbon dioxide hydrogenation using hydrogen from photocatalytic water splitting: process development and techno-economic analysis. J. Clean. Prod. 208, 1446–1458. doi:10.1016/j.jclepro.2018.10.132
Ambrosi, A., and Martin, P. (2016). 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 45, 2740–2755. doi:10.1039/C5CS00714C
An, B., Zhang, J., Cheng, K., Ji, P., Wan, C., Lin, W., et al. (2017). Confinement of ultrasmall Cu/ZnO. J. Am. Chem. Soc. 139, 3834–3840. doi:10.1021/jacs.7b00058
Araya, S. S., Liso, V., Cui, X.-T., Li, N., Zhu, J.-M., Sahlin, S. L., et al. (2020). A review of the methanol economy: the fuel cell route. Energies 13, 596. doi:10.3390/en13030596
Arena, F., Mezzatesta, G., Zafarana, G., Trunfio, G., Frusteri, F., Spadaro, L., et al. (2013). Effects of oxide carriers on surface functionality and process performance of the Cu-ZnO system in the synthesis of methanol via CO2 hydrogenation. J. Catal. 300, 141–151. doi:10.1016/j.jcat.2012.12.019
Aresta, M., Karimi, I., and Kawi, S. (2015). Methanol: the basic chemical and energy feedstock of the future, Geneva, Switzerland: Springer Nature Switzerland AG.
Armutlulu, A., Naeem, M. A., Liu, H.-J., Kim, S. M., Kierzkowska, A., Fedorov, A., et al. (2017). Multishelled CaO microspheres stabilized by atomic layer deposition of Al2O3 for enhanced CO2 capture performance. Adv. Mater. 29, 1702896. doi:10.1002/adma.201702896
Behrens, M., Studt, F., Kasatkin, I., Kühl, S., Hävecker, M., Abild-Pedersen, F., et al. (2012). The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897. doi:10.1126/science.1219831
Bowker, M. (2019). Methanol synthesis from CO2 hydrogenation. ChemCatChem 11, 4238–4246. doi:10.1002/cctc.201900401
Burger, B., Maffettone, P. M., Gusev, V. V., Aitchison, C. M., Bai, Y., Wang, X.-Y., et al. (2020). A mobile robotic chemist. Nature 583, 237–241. doi:10.1038/s41586-020-2442-2
Chen, K., Fang, H.-H., Wu, S., Liu, X., Zheng, J.-W., Zhou, S., et al. (2019a). CO2 hydrogenation to methanol over Cu catalysts supported on La-modified SBA-15: the crucial role of Cu-LaOx interfaces. Appl. Catal. B Environ. 251, 119–129. doi:10.1016/j.apcatb.2019.03.059
Chen, L., Tang, X.-W., Xie, P.-W., Xu, J., Chen, Z.-H., Cai, Z.-C., et al. (2018a). 3D printing of artificial leaf with tunable hierarchical porosity for CO2 photoreduction. Chem. Mater. 30, 799–806. doi:10.1021/acs.chemmater.7b04313
Chen, S.-S., Qi, Y., Li, C., Domen, K., and Zhang, F.-X. (2018b). Surface strategies for particulate photocatalysts toward artificial photosynthesis. Joule 2, 2260–2288. doi:10.1016/j.joule.2018.07.030
Chen, S.-Y., Zhang, J.-F., Wang, P., Wang, X.-X., Song, F.-E., Bai, Y.-X., et al. (2019b). Effect of vapor-phase-treatment to CuZnZr catalyst on the reaction behaviors in CO2 hydrogenation into methanol. ChemCatChem. 11, 1448–1457. doi:10.1002/cctc.201801988
Chen, Z.-S., Zhang, G.-X., Prakash, J., Zheng, Y., and Sun, S.-H. (2019c). Rational design of novel catalysts with atomic layer deposition for the reduction of carbon dioxide. Adv. Energy Mater. 1900889 9 (37), 1900889. doi:10.1002/aenm.201900889
China Energy News (2019). E. coli. Availble at: http://www.cnenergynews.cn/ (Accessed May 6, 2020).
Cokoja, M., Bruckmeier, C., Rieger, B., Herrmann, W. A., and Kühn, F. E. (2011). Transformation of carbon dioxide with homogeneous transition-metal catalysts: a molecular solution to a global challenge? Angew. Chem. Int. Ed. 50, 8510–8537. doi:10.1002/anie.201102010
Cole, K. P., Groh, M., Johnson, M. D., Burcham, C. L., Campbell, B. M., et al. (2017). Kilogram-scale prexasertib monolactate monohydrate synthesis under continuous-flow CGMP conditions. Science 356, 1144–1150. doi:10.1126/science.aan0745
Damith, P., Tucker, J. W., Brahmbhatt, S., Helal, C. J., Chong, A., Farrell, W., et al. (2018). A platform for automated nanomolescale reaction screening and micromole-scale synthesis in flow. Science 359, 429–434. doi:10.1126/science.aap9112
Dang, S.-S., Li, S.-G., Yang, C.-G., Chen, X.-Q., Li, X.-P., Zhong, L.-S., et al. (2019a). Selective transformation of CO2 and H2 into lower olefins over In2O3-ZnZrOx/SAPO-34 bifunctional catalysts. ChemSusChem 12, 3582–3591. doi:10.1002/cssc.201900958
Dang, S.-S., Yang, H.-Y., Gao, P., Wang, H., Li, X.-P., Wei, W., et al. (2019b). A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 330, 61–75. doi:10.1016/j.cattod.2018.04.021
Das, S., Pe´rez-Ramı´rez, J., Gong, J.-L., Dewangan, N., Hidajat, K., Gates, B. C., et al. (2020). Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 49, 2937–3004. doi:10.1039/C9CS00713J
Din, I. U., Shaharun, M. S., Alotaibi, M. A., Alharthi, A. I., and Naeem, A. (2019). Recent developments on heterogeneous catalytic CO2 reduction to methanol. J. CO2 Util. 34, 20–33. doi:10.1016/j.jcou.2019.05.036
Dor Group (2019). E. coli. Available at: http://www.dorchemicals.com/eng/family/Methanol?catid=Dor-Chem_Methanol. (Accessed March 19, 2020).
Dotan, H., Landman, A., Sheehan, S. W., Malviya, K. D., Shter, G. E., Grave, D. A., et al. (2019). Decoupled hydrogen and oxygen evolution by a two-step electrochemical-chemical cycle for efficient overall water splitting. Nat. Energy 4, 786–795. doi:10.1038/s41560-019-0462-7
Dudley, B. (2019). BP statistical review of world energy. London, United Kingdom; BP Statistical Review.
Duyar, M. S., Tsai, C., Snider, J. L., Singh, J. A., Gallo, A., Yoo, J. S., et al. (2018). A highly active molybdenum phosphide catalyst for methanol synthesis from CO and CO2. Angew. Chem. Int. Ed. 57, 15045–15050. doi:10.1002/anie.201806583
Epps, R. W., Bowen, M. S., Volk, A. A., Abdel-Latif, K., Han, S., Reyes, K. G., et al. (2020). Artificial chemist: an autonomous quantum dot synthesis bot. Adv. Mater. 32, 2001626. doi:10.1002/adma.202001626
Esposito, D. V. (2017). Membraneless electrolyzers for low-cost hydrogen production in a renewable energy future. Joule 1, 651–658. doi:10.1016/j.joule.2017.07.003
Fang, X., Men, Y.-H., Wu, F., Zhao, Q.-H., Singh, R., Xiao, P., et al. (2019). Promoting CO2 hydrogenation to methanol by incorporating adsorbents into catalysts: effects of hydrotalcite. Chem. Eng. J. 378, 122052. doi:10.1016/j.cej.2019.122052
Farquharson, J. I., and Amelung, F. (2020). Extreme rainfall triggered the 2018 rift eruption at Kilauea Volcano. Nature 580, 491–495. doi:10.1038/s41586-020-2172-5
Feng, X.-H., Pan, F.-P., Zhao, H.-L., Deng, W., Zhang, P., Zhou, H.-C., et al. (2018). Atomic layer deposition enabled MgO surface coating on porous TiO2 for improved CO2 photoreduction. Appl. Catal. B Environ. 238, 274–283. doi:10.1016/j.apcatb.2018.07.027
Frei, M. S., Mondelli, C., García-Muelas, R., Kley, K. S., Puertolas, B., López, N., et al. (2019). Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 10, 3377. doi:10.1038/s41467-019-11349-9
Furimsky, E. (2020). CO2 hydrogenation to methanol and methane over carbon-supported catalysts. Ind. Eng. Chem. Res. 59, 15393–15423. doi:10.1021/acs.iecr.0c02250
Gao, P., Dang, S.-S., Li, S.-G., Bu, X.-N., Liu, Z.-Y., Qiu, M.-H., et al. (2017). Direct production of lower olefins from CO2 conversion via bifunctional catalysis. ACS Catal. 8, 571–578. doi:10.1021/acscatal.7b02649
Gao, P., Li, S.-G., Bu, X.-N., Dang, S.-S., Liu, Z.-Y., Wang, H., et al. (2017). Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024. doi:10.1038/nchem.2794
George, S. M. (2010). Atomic layer deposition: an overview. Chem. Rev. 110, 111–131. doi:10.1021/cr900056b
González-Garay, A., Frei, M. S., Al-Qahtani, A., Mondelli, C., Guillén-Gosálbez, G., Pérez-Ramírez, J., et al. (2019). Plant-to-planet analysis of CO2-based methanol processes. Energy Environ. Sci. 12, 3425–3436. doi:10.1039/c9ee01673b
Ham, H., Baek, S. W., Shin, C.-H., and Bae, J. W. (2018). Roles of structural promoters for direct CO2 hydrogenation to dimethyl ether over ordered mesoporous bifunctional Cu/M-Al2O3 (M = Ga or Zn). ACS Catal. 9, 679–690. doi:10.1021/acscatal.8b04060
Hartrampf, N., Saebi, A., Poskus, M., Gates, Z. P., Callahan, A. J., Cowfer, A. E., et al. (2020). Synthesis of proteins by automated flow chemistry. Science 368, 980–987. doi:10.1126/science.abb2491
He, M.-Y., Sun, Y.-H., and Han, B.-X. (2013). Green carbon science: scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. 52, 9620–9633. doi:10.1002/anie.201209384
Hu, X.-S., Qin, W., Guan, Q.-X., and Li, W. (2018). The synergistic effect of CuZnCeOx in controlling the formation of methanol and CO from CO2 hydrogenation. ChemCatChem 10, 4438–4449. doi:10.1002/cctc.201800668
Iizumi, T., Shiogama, H., Imada, Y., Hanasaki, N., Takikawa, H., and Nishimori, M. (2018). Crop production losses associated with anthropogenic climate change for 1981-2010 compared with preindustrial levels. Int. J. Climatol. 38, 5405–5417. doi:10.1002/joc.5818
International Energy Agency (2019b). Exploring clean energy pathways: the role of CO2 storage, IEA, Paris, France; IEA.
Jiang, X., Koizumi, N., Guo, X.-W., and Song, C.-S. (2015). Bimetallic Pd-Cu catalysts for selective CO2 hydrogenation to methanol. Appl. Catal. B Environ. 170, 173–185. doi:10.1016/j.apcatb.2015.01.010
Jiang, X., Nie, X.-W., Guo, X.-W., Song, C.-S., and Chen, J.-G. G. (2020). Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem. Rev. 120, 7984–8034. doi:10.1021/acs.chemrev.9b00723
Jin, Z., Wang, L., Zuidema, E., Mondal, K., Zhang, M., and Zhang, J. (2020). Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367, 193–197. doi:10.1126/science.aaw1108
Jo, D. Y., Lee, M. W., Ham, H. C., and Lee, K.-Y. (2019). Role of the Zn atomic arrangements in enhancing the activity and stability of the kinked Cu(2 1 1) site in CH3OH production by CO2 hydrogenation and dissociation: first-principles microkinetic modeling study. J. Catal. 373, 336–350. doi:10.1016/j.jcat.2019.04.00
Jungst, T., Smolan, W., Schacht, K., Scheibel, T., and Groll, J. (2016). Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 116, 1496–1539. doi:10.1021/acs.chemrev.5b00303
Kar, S., Goeppert, A., Kothandaraman, J., and Prakash, G. K. S. (2017). Manganese-catalyzed sequential hydrogenation of CO2 to methanol via formamide. ACS Catal. 7, 6347–6351. doi:10.1021/acscatal.7b02066
Kar, S., Goeppert, A., and Prakash, G. K. S. (2019). Combined CO2 capture and hydrogenation to methanol: amine immobilization enables easy recycling of active elements. ChemSusChem. 12, 3172–3177. doi:10.1002/cssc.201900324
Kar, S., Sen, R., Goeppert, A., and Prakash, G. K. S. (2018). Integrative CO2 capture and hydrogenation to methanol with reusable catalyst and amine: toward a carbon neutral methanol economy. J. Am. Chem. Soc. 140, 1580–1583. doi:10.1021/jacs.7b12183
Karuturi, S. K., Shen, H.-P., Sharma, A., Beck, F. J., Varadhan, P., Duong, T., et al. (2020). Over 17% efficiency stand-alone solar water splitting enabled by perovskite-silicon tandem absorbers. Adv. Energy Mater. 10, 2000772. doi:10.1002/aenm.202000772
Kattel, S., Liu, P., and Chen, J.-G. G. (2017a). Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J. Am. Chem. Soc. 139, 9739–9754. doi:10.1021/jacs.7b05362
Kattel, S., Ramírez, P. J., Chen, J.-G., Rodriguez, J. A., and Liu, P. (2017b). Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 355, 1296–1299. doi:10.1126/science.aal3573
Kim, J., Henao, C. A., Johnson, T. A., Dedrick, D. E., Miller, J. E., and Stecheld, E. B. (2011). Methanol production from CO2 using solar-thermal energy: process development and techno-economic analysis. Energy Environ. Sci. 4, 3122–3132. doi:10.1039/c1ee01311d
Kobl, K., Thomas, S., Zimmermann, Y., Parkhomenko, K., and Roger, A.-C. (2016). Power-law kinetics of methanol synthesis from carbon dioxide and hydrogen on copper-zinc oxide catalysts with alumina or zirconia supports. Catal. Today 270, 31–42. doi:10.1016/j.cattod.2015.11.020
Kuld, S., Thorhauge, M., Falsig, H., Elkjær, C. F., Helveg, S., Chorkendorff, I., et al. (2016). Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 352, 969–974. doi:10.1126/science.aaf0718
Lam, E., Corral-Pérez, J. J., Larmier, K., Noh, G., Wolf, P., Comas-Vives, A., et al. (2019). CO2 hydrogenation on Cu/Al2O3: role of metal/support interface in driving activity and selectivity of a bifunctional catalyst. Angew. Chem. Int. Ed. 58, 13989–13996. doi:10.1002/anie.201908060
Lam, E., Larmier, K., Wolf, P., Tada, S., Safonova, O. V., and Copéret, C. (2018). Isolated Zr surface sites on silica promote hydrogenation of CO2 to CH3OH in supported Cu catalysts. J. Am. Chem. Soc. 140, 10530–10535. doi:10.1021/jacs.8b05595
Larmier, K., Liao, W.-C., Tada, S., Lam, E., Verel, R., Bansode, A., et al. (2017). CO2-to-methanol hydrogenation on Zirconia-supported copper nanoparticles: reaction intermediates and the role of the metal-support interface. Angew. Chem. Int. Ed. 56, 2318–2323. doi:10.1002/anie.201610166
Li, H.-Z., Qiu, C.-L., Ren, S.-J., Dong, Q.-B., Zhang, S.-X., Zhou, F.-L., et al. (2020). Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 367, 667–671. doi:10.1126/science.aaz6053
Li, K.-Z., and Chen, J.-G. G. (2019). CO2 hydrogenation to methanol over ZrO2-containing catalysts: insights into ZrO2 induced synergy. ACS Catal. 9, 7840–7861. doi:10.1021/acscatal.9b01943
Li, M. M.-J., Zeng, Z.-Y., Liao, F.-L., Hong, X.-L., and Tsang, S. C. E. (2016). Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts. J. Catal. 343, 157–167. doi:10.1016/j.jcat.2016.03.020
Li, W.-H., Nie, X.-W., Jiang, X., Zhang, A.-F., Ding, F.-S., Liu, M., et al. (2018). ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl. Catal. B Environ. 220, 397–408. doi:10.1016/j.apcatb.2017.08.048
Li, Z.-L., Qu, Y.-Z., Wang, J.-J., Liu, H.-L., Li, M.-R., Miao, S., et al. (2019). Highly selective conversion of carbon dioxide to aromatics over tandem catalysts. Joule 3, 570–583. doi:10.1016/j.joule.2018.10.027
Li, Z.-L., Wang, J.-J., Qu, Y.-Z., Liu, H.-L., Tang, C.-Z., Miao, S., et al. (2017). Highly selective conversion of carbon dioxide to lower olefins. ACS Catal. 7, 8544–8548. doi:10.1021/acscatal.7b03251
Liu, C.-J., Li, M.-Y., Wang, J.-Q., Zhou, X.-T., Guo, Q.-T., Yan, J.-M., et al. (2016). Plasma methods for preparing green catalysts: current status and perspective. Chin. J. Catal. 37, 340–348. doi:10.1016/S1872-2067(15)61020-8
Liu, J.-H., Zhang, A.-F., Jiang, X., Liu, M., Sun, Y.-W., Song, C.-S., et al. (2018a). Selective CO2 hydrogenation to hydrocarbons on Cu-promoted Fe-based catalysts: dependence on Cu-Fe interaction. ACS Sustain. Chem. Eng. 6, 10182–10190. doi:10.1021/acssuschemeng.8b01491
Liu, X.-L., Wang, M.-H., Zhou, C., Wei, Z., Cheng, K., Kang, J.-C., et al. (2018b). Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34. Chem.Commun. 54, 140–143. doi:10.1039/C7CC08642C
Liu, Y., Wang, Z., and Liu, C.-J. (2015). Mechanism of template removal for the synthesis of molecular sieves using dielectric barrier discharge. Catal. Today 256, 137–141. doi:10.1016/j.cattod.2015.03.009
Lunkenbein, T., Schumann, J., Behrens, M., Schlogl, R., and Willinger, M. G. (2015). Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal-support interactions. Angew. Chem. Int. Ed. 54, 4544–4548. doi:10.1002/anie.201411581
Luu, M.-T., Milani, D., Bahadori, A., and Abbas, A. (2015). A comparative study of CO2 utilization in methanol synthesis with various syngas production technologies. J. CO2 Util. 12, 62–76. doi:10.1016/j.jcou.2015.07.001
Martin, O., Martín, A. J., Mondelli, C., Mitchell, S., Segawa, T. F., Hauert, R., et al. (2016). Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 55, 6261–6265. doi:10.1002/anie.201600943
MefCO2 (Methanol fuel from CO2) (2020). E. coli. Available at: https://www.spire2030.eu/mefco2 (Accessed October 21, 2020).
Methanol Institute (2020). E. coli. Available at: https://www.methanol.org/the-methanol-industry/ (Accessed October 21, 2020).
Ministry of Industry and Information Technology of the People’s Republic of China (2019). E. coli. Available at: http://www.miit.gov.cn/index.html (Accessed March 19, 2020).
Murakami, H., Vecchi, G. A., and Underwood, S. (2017). Increasing frequency of extremely severe cyclonic storms over the Arabian Sea. Nat. Clim. Change 7, 885–889. doi:10.1038/s41558-017-0008-6
Mureddu, M., Ferrara, F., and Pettinau, A. (2019). Highly efficient CuO/ZnO/ZrO2@SBA-15 nanocatalysts for methanol synthesis from the catalytic hydrogenation of CO2. Appl. Catal. B Environ. 258, 117941. doi:10.1016/j.apcatb.2019.117941
Netzer, D., Antverg, J., and Goldwine, G. (2015). Methanol proves low-cost, sustainable option for gasoline blending. Oil Gas J. 113, 82–87.
Neyts, E. C., Ostrikov, K. K., Sunkara, M. K., and Bogaerts, A. (2015). Synergistic effects at the nanoscale. Chem. Rev. 115, 13408–13446. doi:10.1021/acs.chemrev.5b00362
Ni, Y.-M., Chen, Z.-Y., Fu, Y., Liu, Y., Zhu, W.-L., and Liu, Z.-M. (2018). Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 9, 3457. doi:10.1038/s41467-018-05880-4
Nie, X.-W., Li, W.-H., Jiang, X., Guo, X.-W., and Song, C.-S. (2019). Recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons. Adv. Catal. 65, 121–233. doi:10.1016/bs.acat.2019.10.002
Noh, G., Lam, E., Alfke, J. L., Larmier, K., Searles, K., Wolf, P., et al. (2019). Selective hydrogenation of CO2 to CH3OH on supported Cu nanoparticles promoted by isolated Ti (IV) surface sites on SiO2. ChemSusChem 12, 968–972. doi:10.1002/cssc.201900134
Olah, G. A. (2005). Beyond oil and gas: the methanol economy. Angew. Chem. Int. Ed. 44, 2636–2639. doi:10.1002/anie.200462121
Olsbye, U., Svelle, S., Bjørgen, M., Beato, P., Janssens, T. V. W., Joensen, F., et al. (2012). Conversion of methanol to hydrocarbons: how zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 51, 5810–5831. doi:10.1002/anie.201103657
Peng, Y.-H., Wang, L.-B., Luo, Q.-Q., Cao, Y., Dai, Y.-Z., Li, Z.-L., et al. (2018). Molecular-level insight into how hydroxyl groups boost catalytic activity in CO2 hydrogenation into methanol. Chem. 4, 613–625. doi:10.1016/j.chempr.2018.01.019
Peters, M., Köhler, B., Kuckshinrichs, W., Leitner, W., Markewitz, P., and Müller, T. E. (2011). Chemical technologies for exploiting and recycling carbon dioxide into the value chain. ChemSusChem. 4, 1216–1240. doi:10.1002/cssc.201000447
Pinaud, B. A., Benck, J. D., Seitz, L. C., Forman, A. J., Chen, Z.-B., Deutsch, T. G., et al. (2013). Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002. doi:10.1039/c3ee40831k
Ra, E. C., Kim, K. Y., Kim, E. H., Lee, H., An, K., and Lee, J. S. (2020). Recycling carbon dioxide through catalytic hydrogenation: recent key developments and perspectives. ACS Catal. 10, 11318–11345. doi:10.1021/acscatal.0c02930
Reddy, B. M., Samuel, P., and Reddy, N. S. M. (2018). Government policies help promote clean transportation in India: proton-exchange membrane fuel cells for vehicles. IEEE Electrification Magazine 6, 26–36. doi:10.1109/mele.2017.2784633
Reichenbach, T., Mondal, K., Jäger, M., Vent-Schmidt, T., Himmel, D., Dybbert, V., et al. (2018). Ab initio study of CO2 hydrogenation mechanisms on inverse ZnO/Cu catalysts. J. Catal. 360, 168–174. doi:10.1016/j.jcat.2018.01.035
Richard, A. R., and Fan, M. (2017). Low-pressure hydrogenation of CO2 to CH3OH using Ni-In-Al/SiO2 catalyst synthesized via a phyllosilicate precursor. ACS Catal. 7, 5679–5692. doi:10.1021/acscatal.7b00848
Roh, K., Al-Hunaidy, A. S., Imran, H., and Lee, J.-H. (2019). Optimization-based identification of CO2 capture and utilization processing paths for life cycle greenhouse gas reduction and economic benefits. AIChE J. 65, e16580. doi:10.1002/aic.16580
Roy, S., Cherevotan, A., and Peter, S. C. (2018). Thermochemical CO2 hydrogenation to single carbon products: scientific and technological challenges. ACS Energy Lett. 3, 1938–1966. doi:10.1021/acsenergylett.8b00740
Rubin, E. S., Davison, J. E., and Herzog, H. J. (2015). The cost of CO2 capture and storage. Int. J. Greenh. Gas. Con. 40, 378–400. doi:10.1016/j.ijggc.2015.05.018
Sakakura, T., Choi, J.-C., and Yasuda, H. (2007). Transformation of carbon dioxide. Chem. Rev. 107, 2365–2387. doi:10.1021/cr068357u
Sanz-Pérez, E. S., Murdock, C. R., Didas, S. A., and Jones, C. W. (2016). Direct capture of CO2 from ambient air. Chem. Rev. 116, 11840–11876. doi:10.1021/acs.chemrev.6b00173
Satthawong, R., Koizumi, N., Song, C.-S., and Prasassarakich, P. (2013). Bimetallic Fe-Co catalysts for CO2 hydrogenation to higher hydrocarbons. J. CO2 Util. 3-4, 102–106. doi:10.1016/j.jcou.2013.10.002
Service, R. F. (2016). Perovskite solar cells gear up to go commercial. Science 354, 1214–1215. doi:10.1126/science.354.6317.1214
Shirazi, M., Neyts, E. C., and Bogaerts, A. (2017). DFT study of Ni-catalyzed plasma dry reforming of methane. Appl. Catal. B Environ. 205, 605–614. doi:10.1016/j.apcatb.2017.01.004
Smejkal, Q., Rodemerck, U., Wagner, E., and Baerns, M. (2014). Economic assessment of the hydrogenation of CO2 to liquid fuels and petrochemical feedstock. Chem. Ing. Tech. 86, 679–686. doi:10.1002/cite.201300180
Song, Z.-N., Dong, Q.-B., Xu, W.-W. L., Zhou, F.-L., Liang, X.-H., and Yu, M. (2018). Molecular layer seposition-modified 5A zeolite for highly efficient CO2 capture. ACS Appl. Mater. Interfaces 10, 769–775. doi:10.1021/acsami.7b16574
Stangeland, K., Kalai, D. Y., Ding, Y., and Yu, Z.-X. (2019). Mesoporous manganese-cobalt oxide spinel catalysts for CO2 hydrogenation to methanol. J. CO2 Util. 32, 146–154. doi:10.1016/j.jcou.2019.04.018
Studt, F., Sharafutdinov, I., Abild-Pedersen, F., Elkjaer, C. F., Hummelshøj, J. S., Dahl, S., et al. (2014). Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 6, 320–324. doi:10.1038/nchem.1873
Styring, P., Quadrelli, E. A., and Armstrong, K. (2015). Carbon dioxide utilisation: closing the carbon cycle, Holand, Europe; Elsevier: Academic Press.
Su, L.-W., Li, X.-R., and Sun, Z.-Y. (2013). The consumption, production and transportation of methanol in China: a review. Energy Pol. 63, 130–138. doi:10.1016/j.enpol.2013.08.031
Tan, L., Zhang, P.-P., Cui, Y., Suzuki, Y., Li, H.-J., Guo, L.-S., et al. (2019). Direct CO2 hydrogenation to light olefins by suppressing CO by-product formation. Fuel Process.Technol. 196, 106174, doi:10.1016/j.fuproc.2019.106174
Tountas, A. A., Peng, X.-Y., Tavasoli, A. V., Duchesne, P. N., Dingle, T. L., Dong, Y.-C., et al. (2019). Towards solar methanol: past, present, and future. Adv. Sci. 6, 1801903. doi:10.1002/advs.201801903
Toyao, T., Kayamori, S., Maeno, Z., Siddiki, S. M. A. H., and Shimizu, K.-I. (2019). Heterogeneous Pt and MoOx Co-loaded TiO2 catalysts for low-temperature CO2 hydrogenation to form CH3OH. ACS Catal. 9, 8187–8196. doi:10.1021/acscatal.9b01225
Veh, G., Korup, O., and Walz, A. (2020). Hazard from Himalayan glacier lake outburst floods. PNA 117, 907–912. doi:10.1073/pnas.1914898117
Wang, J.-J., Li, G.-N., Li, Z.-L., Tang, C.-Z., Feng, Z.-C., An, H.-Y., et al. (2017). A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 3, e1701290. doi:10.1126/sciadv.1701290
Wang, J.-Y., Liu, C.-Y., Senftle, T. P., Zhu, J., Zhang, G.-H., Guo, X.-W., et al. (2020a). Variation in In2O3 crystal phase alters catalytic performance toward the reverse water gas shift reaction. ACS Catal. 10, 3264–3273. doi:10.1021/acscatal.9b04239
Wang, J.-Y., Zhang, A.-F., Jiang, X., Song, C.-S., and Guo, X.-W. (2018a). Highly selective conversion of CO2 to lower hydrocarbons (C2-C4) over bifunctional catalysts composed of In2O3-ZrO2 and zeolite. J. CO2 Util. 27, 81–88. doi:10.1016/j.jcou.2018.07.006
Wang, L.-X., Guan, E.-J., Wang, Y.-Q., Wang, L., Gong, Z.-M., Cui, Y., et al. (2020b). Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nat. Commun. 11, 1033. doi:10.1038/s41467-020-14817-9
Wang, L., Yi, Y.-H., Guo, H.-C., and Tu, X. (2018). Atmospheric pressure and room temperature synthesis of methanol through plasma-catalytic hydrogenation of CO2. ACS Catal. 8, 90–100. doi:10.1021/acscatal.7b02733
Wang, L., Yi, Y.-H., Wu, C.-F., Guo, H.-C., and Tu, X. (2017). One-step reforming of CO2 and CH4 into high-value liquid chemicals and fuels at room temperature by plasma-driven catalysis. Angew. Chem. Int. Ed. 56, 13679–13683. doi:10.1002/anie.201707131
Wang, W.-N., Wu, F., Myung, Y., Niedzwiedzki, D. M., Im, H. S., Park, J., et al. (2015). Surface engineered CuO nanowires with ZnO islands for CO2 photoreduction. ACS Appl. Mater. Interfaces 7, 5685–5692. doi:10.1021/am508590j
Wang, Y.-H., Kattel, S., Gao, W.-G., Li, K.-Z., Liu, P., Chen, J.-G. G., et al. (2019b). Exploring the ternary interactions in Cu-ZnO-ZrO2 catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 10, 1166. doi:10.1038/s41467-019-09072-6
Wang, Y., Gao, W.-Z., Kazumi, S., Li, H.-J., Yang, G.-H., and Tsubaki, N. (2019a). Direct and oriented conversion of CO2 into value-added aromatics. Chem. Eur J. 25, 5149–5153. doi:10.1002/chem.201806165
Wang, Y., Tan, L., Tan, M.-H., Zhang, P.-P., Fang, Y., Yoneyama, Y., et al. (2019). Rationally designing bifunctional catalysts as an efficient strategy to boost CO2 hydrogenation producing value-added aromatics. ACS Catal. 9, 895–901. doi:10.1021/acscatal.8b01344
Wang, Z., Zhang, Y., Neyts, E. C., Cao, X.-X., Zhang, X.-S., Jang, B. W.-L., et al. (2018b). Catalyst preparation with plasmas: how does it work?. ACS Catal. 8, 2093–2110. doi:10.1021/acscatal.7b03723
Woodley, S. M., and Catlow, R. (2008). Crystal structure prediction from first principles. Nat. Mater. 7, 937–946. doi:10.1038/nmat2321
Xu, X.-Y., Liu, Y., Zhang, F., Di, W., and Zhang, Y.-L. (2017). Clean coal technologies in China based on methanol platform. Catal. Today 298, 61–68. doi:10.1016/j.cattod.2017.05.070
Yan, X.-L., Zhao, B.-R., Liu, Y.-L., and Li, Y.-N. (2015). Dielectric barrier discharge plasma for preparation of Ni-based catalysts with enhanced coke resistance: current status and perspective. Catal. Today 256, 29–40. doi:10.1016/j.cattod.2015.04.045
Ye, J.-Y., Liu, C.-J., Mei, D.-H., and Ge, Q.-F. (2013). Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): a DFT study. ACS Catal. 3, 1296–1306. doi:10.1021/cs400132a
Ye, R.-P., Ding, J., Gong, W.-B., Argyle, M. D., Zhong, Q., Wang, Y.-J., et al. (2019). CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 10, 5698. doi:10.1038/s41467-019-13638-9
Yu, T., Cristiano, R., and Weiss, R. G. (2010). From simple, neutral triatomic molecules to complex chemistry. Chem. Soc. Rev. 39, 1435–1447. doi:10.1039/b821320h
Zhang, A.-J., Zhu, A.-M., Guo, J., Xu, Y., and Shi, C. (2010). Conversion of greenhouse gases into syngas via combined effects of discharge activation and catalysis. Chem. Eng. J. 156, 601–606. doi:10.1016/j.cej.2009.04.069
Zhang, L.-F., Sadanandam, G., Liu, X.-Y., and Scurrell, M. S. (2017). Carbon surface modifications by plasma for catalyst support and electrode materials applications. Top. Catal. 60, 823–830. doi:10.1007/s11244-017-0747-7
Zhang, W.-X., Zhou, T.-J., Zou, L.-W., Zhang, L.-X., and Chen, X.-L. (2018). Reduced exposure to extreme precipitation from 0.5 °C less warming in global land monsoon regions. Nat. Commun. 9, 3153. doi:10.1038/s41467-018-05633-3
Zhang, X.-B., Zhang, A.-F., Jiang, X., Zhu, J., Liu, J.-H., Li, J.-J., et al. (2019). Utilization of CO2 for aromatics production over ZnO/ZrO2-ZSM-5 tandem catalyst. J. CO2 Util. 29, 140–145. doi:10.1016/j.jcou.2018.12.002
Zhang, X., Liu, Y., Zhang, M.-T., Yu, T., Chen, B.-B., Xu, Y., et al. (2020). Synergy between β-Mo2C nanorods and non-thermal plasma for selective CO2 reduction to CO. Chem. 6, 3312–3328. doi:10.1016/j.chempr.2020.09.016
Zhong, J.-W., Yang, X.-F., Wu, Z.-L., Liang, B.-L., Huang, Y.-Q., and Zhang, T. (2020). State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 49, 1385–1413. doi:10.1039/C9CS00614A
Zhou, X.-T., and Liu, C.-J. (2017). Three-dimensional printing for catalytic applications: current status and perspectives. Adv. Funct. Mater. 27, 1701134. doi:10.1002/adfm.201701134
Keywords: CO2 conversion, methanol synthesis, new catalysts, new routes, new technologies
Citation: Zhang X, Zhang G, Song C and Guo X (2021) Catalytic Conversion of Carbon Dioxide to Methanol: Current Status and Future Perspective. Front. Energy Res. 8:621119. doi: 10.3389/fenrg.2020.621119
Received: 25 October 2020; Accepted: 29 December 2020;
Published: 09 February 2021.
Edited by:
Michele Aresta, IC2R srl, ItalyReviewed by:
Eugenio Quaranta, University of Bari Aldo Moro, ItalyStefano Stendardo, Energy and Sustainable Economic Development (ENEA), Italy
Copyright © 2021 Zhang, Zhang, Song and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghui Zhang, Z3poYW5nQGRsdXQuZWR1LmNu; Chunshan Song, Y2h1bnNoYW5zb25nQGN1aGsuZWR1Lmhr; Xinwen Guo, Z3VveHdAZGx1dC5lZHUuY24=
 Xinbao Zhang
Xinbao Zhang Guanghui Zhang
Guanghui Zhang Chunshan Song1,2*
Chunshan Song1,2* Xinwen Guo
Xinwen Guo