
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Energy Res. , 21 January 2021
Sec. Electrochemical Energy Storage
Volume 8 - 2020 | https://doi.org/10.3389/fenrg.2020.616665
This article is part of the Research Topic Zn-Based Rechargeable Batteries: Materials, Mechanisms and Applications View all 5 articles
Solid-state zinc-ion batteries (SSZIBs) are receiving much attention as low-cost and safe energy storage technology for emerging applications in flexible and wearable devices, and grid storage. However, the development of SSZIBs faces many challenges from key battery materials development to structure design. Herein, we review the most recent progress in the development of polymer electrolytes, cell chemistry and configuration, and demonstration of SSZIBs. In conclusion, perspectives for future research in materials, interface, and assessment of SSZIBs are discussed.
The expanding flexible electronics market has placed significant demands on flexible batteries (Ma Y et al., 2020; Wang et al., 2020). Lithium-ion batteries (LIBs) have dominated the battery market due to their high operating voltage, long lifetime, and high energy density (Mossali et al., 2020). Unfortunately, LIBs have unsolvable challenges, such as safety issues associated with flammable organic electrolytes, the rarity of their elements (e.g., Li and Co), and the cost of lithium (∼$19.2 US kg−1) (Borah et al., 2020; Mossali et al., 2020), all of which necessitate the need for alternative batteries to suit these flexible designs. Of the next-generation batteries, zinc-ion batteries (ZIBs) show several advantages related to Zn, including high abundance, low cost (∼$2.4 US kg−1), high theoretical capacity (820 mAh g−1), environmental friendliness, ease of manufacture, and high safety (Tang et al., 2020; Zhang N et al., 2020).
Recently, aqueous ZIBs have drawn attention due to their high safety, low cost, and high energy density (Tang et al., 2019). However, the commercialization of aqueous ZIBs has been hindered by challenges associated with aqueous electrolytes, including Zn dendrite formation and parasitic side reactions (Zhang N et al., 2020; Zhang Y et al., 2020). In particular, dendrite formation results from uneven Zn deposition on the anode, altering morphology and inducing ‘dead’ Zn deposition. This leads to low Coulombic efficiency, and with extended cycling, these dendrites may pierce the separator and internally short-circuit the battery. Moreover, Zn corrosion and H2 evolution occur at the anode, resulting in the accumulation of Zn2+ insulating byproducts on the anode surface and battery gassing that degrade the cycling and rate capability of these ZIBs (Ma L et al., 2020; Zhang N et al., 2020; Zhang Y et al., 2020). On the cathode, transition metal dissolution (e.g., Mn from MnO2) has hindered stability and cycle life. Despite many strategies to address these problems, the performance of aqueous ZIBs remains unsatisfactory for practical applications (Dueramae et al., 2020; Zhang N et al., 2020; Zhang Y et al., 2020).
Solid-state Zn-ion batteries (SSZIBs) employ solid-state electrolytes (SSEs) and have drawn increased research attention. Due to the lower quantity or absence of active liquid in solid electrolytes than in aqueous electrolytes, they may fundamentally address Zn corrosion, Zn passivation, dendrite formation, and cathode dissolution common in aqueous ZIBs (Tang et al., 2019; Zhao Q et al., 2020). Additionally, the higher modulus and microporous structure of solid electrolytes may add to dendrite suppression (Zhang N et al., 2020; Zhao Q et al., 2020). Of added convenience, SSEs can act as both the separator and electrolyte, easing manufacture and improving operational stability (Zhao Q et al., 2020; Zhang Y et al., 2020). Moreover, solid electrolytes provide flexibility, making them an ideal candidate for SSZIBs in flexible devices (Yu et al., 2019; Wang et al., 2020). Although solid electrolytes have been broadly investigated in solid-state LIBs (Zhao Q et al., 2020), they have yet to be fully explored in ZIBs.
The development of SSZIBs is in its infancy and requires further research. This mini-review is intended to provide a concise summary of the state-of-the-art progress in the materials and architecture design for SSZIBs and critical analysis on the performance and limitations in current SSZIBs. Challenges in SSZIBs development is discussed, followed by suggested directions of future research. This work is the first focused review on SSZIBs and provides an overview of SSZIBs in hopes to stimulate future research on multivalent solid-state battery chemistry.
During cycling, SSZIB solid electrolytes facilitate Zn2+ transport between the electrodes while remaining electrically insulating. This requires solid electrolytes with high ionic conductivity (0.1 mS cm−1 at room temperature (RT)), low electronic conductivity, wide electrochemical stability window, and high thermal and chemical stability (Bekaert et al., 2017; Zhao Q et al., 2020). In addition, SSEs should have high mechanical strength to suppress Zn dendrite formation (Young’s modulus E = 108 GPa) (Wang M et al., 2019).
To date, ZIB solid electrolyte research has focused on polymer electrolytes due to their high chemical stability, mechanical toughness, flexibility, low cost, ease of synthesis, and scalability (Zhao Q et al., 2020). Polymer electrolytes for SSZIBs contain gel polymer electrolytes (GPEs) and solid polymer electrolytes (SPEs) (shown in Supplementary Figure S1). GPEs—often referred to as quasi-solid-state—consist of a polymer matrix interspersed with a salt(s) and solvent (plasticizer). In contrast, SPEs contain only a salt dispersed throughout the flexible polymer matrix, relying on segmental polymer motion for ionic transport (Bekaert et al., 2017; Zhang Y et al., 2020). Supplementary Table S1 summarizes GPEs and SPEs for SSZIBs, along with their main components (polymer, salt, and plasticizer), ionic conductivity, and other prominent characteristics. It is seen that GPEs provide much higher ionic conductivities (1–40 mS cm−1) than SPEs (10−1–10−6 mS cm−1) at RT due to the higher liquid content of GPEs. The following section highlights the main factors governing the performance of polymer electrolytes.
The selection of a polymer matrix with high polarity and an amorphous structure is preferred for polymer electrolytes of SSZIBs. Polymers with a low glass transition temperature (Tg) have higher amorphosity and ease ion (de)solvation. These amorphous polymers hold higher levels of entropy and free volume space, improving local segmental motion and, in turn, increasing ionic conductivity by 2-3 orders of magnitude (Pucic and Turkovic, 2005; Bekaert et al., 2017). However, amorphous structures fail to provide the mechanical strength necessary to maintain interfacial contact with the electrodes needed in flexible applications.
Homopolymer structures have been evaluated for Zn2+ transport in ZIBs, and the first homopolymers were PEO (Karan et al., 2016; Karan et al., 2017), PVA (Wang J Q et al., 2018; Wang K et al., 2018; Huang et al., 2019; Zeng et al., 2019), and PAM (Li et al., 2018b; Wang Z F et al., 2018; Liu Z X et al., 2019). However, these homopolymers are single-faceted in providing the needed properties. For example, PEO provides good mechanical properties but low ionic conductivity due to its high molecular weight and crystallinity (Ward and Hubbard, 2012; Bekaert et al., 2017).
To better balance ionic conductivity and mechanical properties, copolymers (e.g., PVdF-HFP) and crosslinked polymers (e.g., PEO-PPO-PEO) have been used to decrease crystallinity without sacrificing strength (Zhao Q et al., 2020; Wang et al., 2020). Using a PVdF-HFP SPE, an ionic conductivity of 0.0244 mS cm−1 was achieved while delivering flexibility, thermal and electrochemical stability (∼3.45 V), and dendrite suppression (Liu et al., 2020b). Furthermore, several bio-inspired polymers (e.g., kappa-carrageenan) have emerged, seeking balanced characteristics (Hoang et al., 2017; Wang M et al., 2019). For example, a modified metal-organic framework (MOF) electrolyte provided mechanical characteristics similar to a crystalline structure, an ionic conductivity of 0.21 mS cm−1 at 30°C, and a high transference number of 0.93 (Wang Z Q et al., 2019). The use of a PANa GPE for Zn2+ transport, provided high ionic conductivity (σ = 0.2 S cm−1) in NiCo/Zn batteries, and may prove useful in ZIBs (Liu J et al., 2019).
Beyond the polymer matrix, nanofillers and additives have been investigated (e.g., Al2O3, ZrO2, TiO2, and pyrazole) to further enhance the electrolyte’s properties (Johnsi and Suthanthiraraj, 2015, 2016; Hoang et al., 2017; Nancy and Suthanthiraraj, 2017; Zhao Q et al., 2020). These composite electrolytes provided flexibility, accommodating anode volume change at the anode using fillers, creating a mechanical barrier against dendrite growth (Zhao Q et al., 2020). For example, the combination of fumed silica polymers—a polymer capable of dendrite suppression at the expense of higher Zn corrosion—implements pyrazole as a corrosion inhibitor (Hoang et al., 2017). Suppressing dendrites using solid electrolytes, such as MOFs, poly(ethylene oxide)/aramid, and polyacrylonitrile, has prolonged battery lifespans beyond 300 h (Lee et al., 2018; Wang M et al., 2019; Wang Z Q et al., 2019).
In addition to the polymer and nanofillers/additives, plasticizers are another key GPE component to enhancing the flexibility and ionic conductivity by increasing the free volume. Traditional plasticizers (solvents) used to enhance the GPE polymer network in SSZIBs include water and organic solvents (e.g., ethylene carbonate (EC), propylene carbonate (PC), and tetrahydrofuran (THF)) (Zhao Q et al., 2020). Although water content improves interfacial contact between the electrode and electrolyte, the evaporation of water may accelerate aging alongside parasitic side reactions. Organic solvents have helped address these challenges, retaining interfacial contact and minimizing evaporation and side reactions, contributing to dendrite suppression and improved Coulombic efficiency (Li et al., 2019). For example, a PEGDGE/Zn(CF3SO3)2 SSE using a PC plasticizer addressed evaporation and obtained an ionic conductivity of 0.377 mS cm−1 while maintaining constant surface resistance for over 200 h (Dong et al., 2020). The latest research implements ionic liquids in place of traditional plasticizers, increasing the quantity of ions within the electrolyte (Liu et al., 2020a; Ma L et al., 2020). The addition of these ionic liquids increased the ionic conductivity of polymer electrolytes, widened the electrochemical stability window resolving HER reactions, and provided nonflammability, low volatility, and high thermal and chemical stabilities (Liu et al., 2020a; Ma L et al., 2020; Francis et al., 2020). One example uses 1-ethyl-3-methyl-imidazolium tetrafluoroborate ([EMIM]BF4) and zinc tetrafluoroborate [Zn(BF4)2] salt to form an ILZE electrolyte that reportedly solved both HER side reactions and Zn dendrite issues inherent to ZIBs (Ma L et al., 2020). This electrolyte had an increased electrochemical window (∼3.55 V), had a Coulombic efficiency of ∼100%, retained 90% capacity over 30,000 cycles, and operated from −20 to 70°C. This electrolyte was also much thinner and stronger (5 orders of magnitude stronger modulus) than other GPEs. Using trifluoromethanesulfonate (EMITf), PvDF-HFP and Zn(CF3SO3)2 delivered an ionic conductivity of 0.144 mS cm−1, widened the electrochemical stability window (∼4.14 V), and provided excellent thermal stability up to 305°C (Liu et al., 2020a). These ionic liquids have been found to improve both PVdF-HFP and PEO/PVdF copolymer electrolytes (Tafur et al., 2015; Rathika and Suthanthiraraj, 2018; Zhang Y et al., 2020). In summary, the combination of novel plasticizers, additives/fillers, and copolymers and/or crosslinked polymers holds promise in delivering GPEs with desirable properties for SSZIBs.
With the advancement in polymer electrolytes and cathodes, SSZIBs with different configurations have been demonstrated, including traditional planar (Li et al., 2018a; Wan et al., 2018; Wang D H et al., 2018; Zhang H et al., 2019; Zhang N et al., 2019; Mo et al., 2019; Zeng et al., 2019; Chen Y et al., 2020; Ma L et al., 2020; Zhao Y et al., 2020; Dong et al., 2020; Li et al., 2020; Xiao et al., 2020), deformable planar (Wang M et al., 2019), flexible coaxial (Wan et al., 2018; Zhang Q C et al., 2019; Zhao Y et al., 2020), and twisted-pair (He et al., 2019b) or parallel-pair fiber arrangements (Wang K et al., 2018). The key battery components and performance of these SSZIBs are compared in Table 1, and representative examples are illustrated in Figure 1 and discussed below.
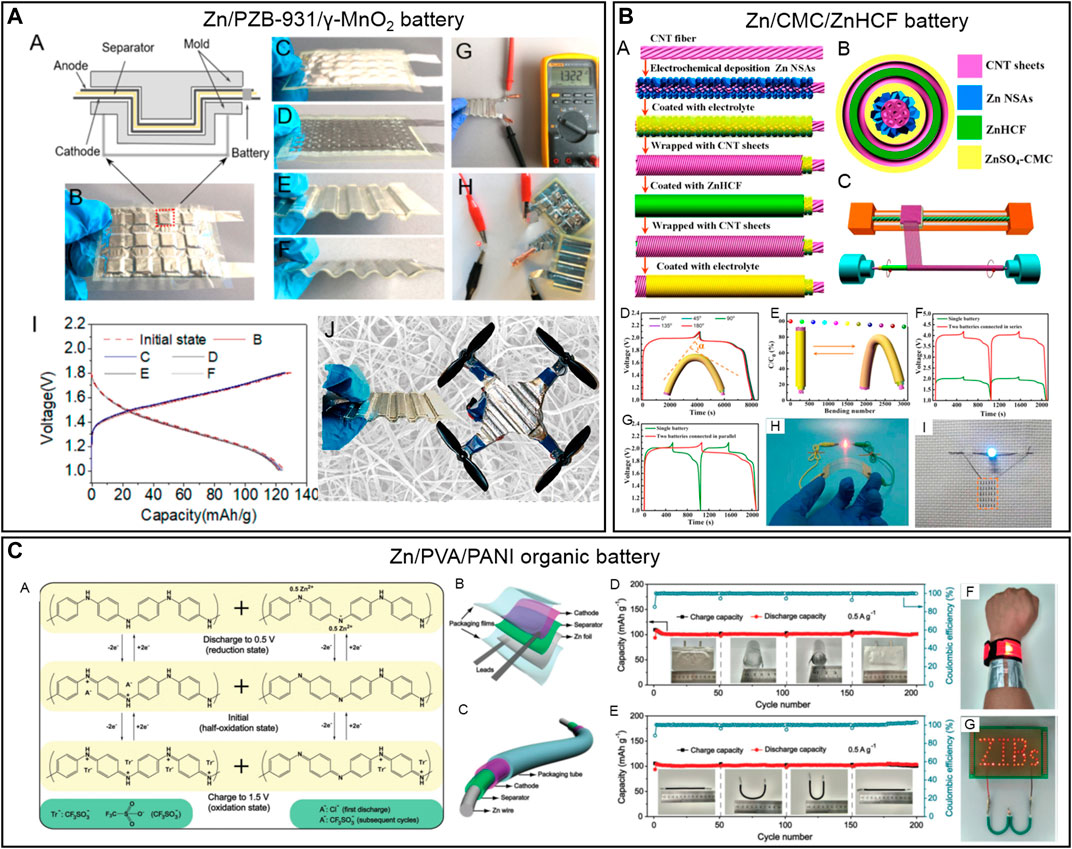
FIGURE 1. (A) Schematics and pictures of different plastically deformed Zn/PZB-931/γ-MnO2 SSZIBs, their galvanostatic charge and discharge curves at 0.2°C, and a drone powered by the Zn/PZB-931/γ-MnO2 planar cells (Wang M et al., 2019). (B) Schematic illustrations showing the fabrication process of coaxial-fiber Zn/CNC/ZnHCF SSZIBs, their charge and discharge curves under different bending (0–180°C), and LED illuminated by the charged coaxial-fiber Zn/CNC/ZnHCF batteries (Zhang Q C et al., 2019). (C) Redox mechanism of PANI cathode, schematic diagrams, cycling performance, and LED demonstration of flexible soft-packaged and cable-type quasi-solid-state Zn/PVA/PANI organic battery (Wan et al., 2018).
The majority of Zn anodes in SSZIBs were fabricated by electroplating Zn (1–5 mg cm−2) onto 3D substrates, such as carbon nanotube (CNT) paper (Li et al., 2018a; Mo et al., 2019), CNT fibers (He et al., 2019a; He et al., 2019b; Zhang Q C et al., 2019), carbon cloth (CC) (Qiu et al., 2017; Zeng et al., 2017; Ma L et al., 2020; Chen Z et al., 2020; Xiao et al., 2020), and graphene foam (Chao et al., 2018). 3D-substrate–supported Zn anodes not only provide the flexibility required for flexible SSZIBs but also reduce Zn passivation and dendrite formation while improving the depth of discharge and cycling life (Parker et al., 2017). The cathodes for SSZIBs explored to date include traditional inorganic materials (MnO2 (Li et al., 2018a; Wang D H et al., 2018; Wang K et al., 2018; Wang M et al., 2019; Dong et al., 2020; Xiao et al., 2020), V2O5, zinc orthovanadate (ZOV) (Chao et al., 2018), zinc hexacyanoferrate (ZnHCF) (Chen Z et al., 2020; Zhang Q C et al., 2019)), organic materials (polyaniline (Xiao et al., 2020)), and novel metal-organic frameworks (MOF, vanadium-based MIL-47 (He et al., 2019a)). Similar to the anode, these cathode materials were also prepared on various 3D substrates to provide the structural flexibility and high rate capability needed for SSZIBs. These 3D cathodes have an increased specific surface area of active material, shortened ionic diffusion distance, and increased channel size and number of interstitial sites, in turn increasing the number of reactive sites and lessening volumetric change during Zn (de)intercalation (He et al., 2019a; Li et al., 2019; Chen Z et al., 2020). It is worth noting that most SSZIBs reported employ GPEs (Table 1), likely due to the higher ionic conductivity and good electrode-electrolyte interface enabled by plasticizers. Strictly speaking, these SSZIBs should be called quasi-solid-state, rather than all-solid-state.
MnO2 is the most popular cathode in SSZIBs, of which the performance could vary significantly with the phase of MnO2, GPEs, and cell configuration. A novel PEO/Zn(CF3SO3)2/branched aramid nanofiber (PZB) provided a high ionic conductivity (2.5 × 10–5 S cm−2 at RT), high tensile strength (58 ± 2.9 MPa), and high Young's modulus (210 ± 11 MPa) (Wang M et al., 2019). Using a corrugated planar design (Figure 1A), a Zn/PZB-931/MnO2 battery was fabricated, and it delivered a stable discharge capacity of 120 mAh g−1 (MnO2) under different elastic and plastic deformations. This cell was integrated into an unmanned aerial vehicle as auxiliary charge storage devices, extending the total flight time. Another arrangement using an α-MnO2/CNT cathode, electroplated Zn/CNT anode, and gelatin and PAM-based hierarchical polymer electrolyte (HPE) provided an extremely safe and wearable SSZIB. The developed SSZIB exhibited a high areal energy and power density of 6.18 mWh cm−2 and 148.2 mW cm−2, respectively (Li et al., 2018a). Moreover, the SSZIBs offered robust structural and property stability under various abuse conditions, such as being cut, bent, hammered, punctured, sewed, and submerged in water or set on fire.
It is essential to develop new cathode materials with high capacity and high voltage to improve the energy density of SSZIBs. Another group developed a new cathode, zinc hexacyanoferrate (ZnHCF), with an open framework structure for the co-insertion/extraction of Zn2+, and applied it to fabricate the first high-voltage coaxial-fiber SSZIB prototype with a ZnSO4-carboxymethyl cellulose sodium (CMC) gel electrolyte and Zn nanosheet arrays on carbon nanotube fiber (CNTF) as the anode (Zhang Q C et al., 2019). The Zn/CMC/ZnHCF coaxial-fiber battery was delicately fabricated by a continuous production process illustrated in Figure 1B. The coaxial-fiber battery delivered a large capacity of 100.2 mAh cm−3 and an energy density of 195.39 mWh cm−3. Moreover, the coaxial-fiber battery underwent negligible degradation of electrochemical performance at various angles from 0° to 180°, proving exceptional flexibility. Furthermore, the battery's capacity remained at 93.2% after bending at 90° for more than 3,000 cycles. High operating voltages and output currents were achieved by connecting the coaxial-fiber battery in series and parallel for high energy and power applications, as illustrated in the 3.3 V blue LED in a flexible textile.
In addition to inorganic materials, other novel materials (such as organic materials and MOF) have also been explored as cathodes in SSZIBs. For example, polyaniline (PANI) organic electrode with a supercapacitor-like dual-ion mechanism exhibited a high reversible capacity of 200 mAh g−1 at 0.05 A g−1, high rate capability, and long cycling stability. A Zn/PVA/PANI organic battery in a planar and cable shape was fabricated using a PVA-based GPE, Zn foil/wire, and PANI/carbon fibers as the cathode (Figure 1C). The soft, cable-type quasi-solid-state ZIBs displayed a reversible capacity of 109 and 106 mAh g−1 PANI at 0.5 A g−1, respectively. Moreover, both showed stable capacities under severe bending and maintained high capacity retentions of 91.7% and 91.5% after recovering from bending state to flat, after 200 cycles (Wan et al., 2018).
As in Table 1, current research has proven the feasibility of fabricating flexible SSZIBs, which require dedicated design of the Zn anode, cathode, and polymer electrolytes. The performance of most SSZIBs was assessed using the specific capacity of the cathode. The energy and power densities of SSZIBs varied significantly in the literature, due to various cell chemistry and evaluation methodology. As a result, comparison among SSZIBs and other battery chemistry (such as LIBs) remains difficult and needs to be addressed in future research.
Herein, we discussed progress in the polymer electrolytes and design of SSZIBs. Quasi-solid-state ZIBs of various configurations have demonstrated excellent structural and electrochemical stability under abuse conditions. Therefore, SSZIBs appear as promising energy storage devices for flexible applications. Nevertheless, challenges remain in SSZIBs, necessitating further research into the materials, interface, structure design, and fundamental understanding of interfacial phenomena in SSZIBs:
1)Zn/solid electrolyte interface. The use of solid electrolytes in SSZIBs is motivated by the hypothesis that they may address Zn dendrites and associated side reactions. Despite successful demonstration of SSZIBs, little information has been presented on the Zn/solid electrolyte interface. Most SSZIBs employed electroplated Zn on 3D substrates, making characterization of the Zn/solid electrolyte interface difficult. Therefore, systematic and in-depth studies are needed to clarify the influence of solid electrolytes on the performance of Zn in SSZIBs, such as critical current density, dendrite growth, and H2 evolution. Special attention should be given to the plasticizers in GPEs, which is likely to influence Zn interaction at the Zn/solid electrolyte interface.
2)Polymer electrolytes. Further research is needed to explore new plasticizers that enhance the overall performance of GPEs. Besides ionic conductivity, other key merits of new GPEs must be comprehensively assessed, including transference number, mechanical strength, and electrochemical and chemical stability. In particular, the content of plasticizers in GPEs dramatically influences SSZIB performance and therefore needs to be controlled, quantified, and documented explicitly.
3)Cathode. The use of solid electrolytes makes it possible to couple a high-voltage cathode and Zn anode to further improve the energy and power densities of SSZIBs. Further research needs to be devoted to developing new cathode materials with high voltage, high capacity, and good compatibility with solid electrolytes.
4)Energy and power density of SSZIBs. If reported in the literature, the energy and power densities of SSZIBs were estimated based on two electrodes, with no reports on the additional weights of polymer electrolytes, 3D current collectors, packing, etc. Therefore, the reported values for energy and power densities of SSZIBs might be over optimistic. Future SSZIB work is encouraged to report the cell chemistry and configuration in greater detail, such as the active material loading, the weight of current collectors and polymer electrolytes, and packing (if applicable). This will facilitate meaningful and comparable analysis amongst SSZIB technologies.
EH organized the data and wrote the manuscript. JL conceived the idea, revised the manuscript, and oversaw the project. All authors discussed the topics and contributed to the organization of this paper.
This work was supported by the Nature Sciences and Engineering Research Council of Canada (NSERC), the Canada Foundation for Innovation (CFI), the BC Knowledge Development Fund (BCKDF), and the University of British Columbia (UBC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2020.616665/full#supplementary-material.
Bekaert, E., Buannic, L., Lassi, U., Llordés, A., and Salminen, J. (2017). “Electrolytes for Li- and Na-Ion batteries: concepts, candidates, and the role of nanotechnology,” in Emerging nanotechnologies in rechargeable energy storage systems Editors L.M. Rodriguez-Martinez, and N. Omar (Boston, MA: Elsevier), 1–43.
Borah, R., Hughson, F. R., Johnston, J., and Nann, T. (2020). On battery materials and methods. Mate. Today Adv 6, 100046. doi:10.1016/j.mtadv.2019.100046
Chao, D. L., Zhu, C., Song, M., Liang, P., Zhang, X., Tiep, N. H., et al. (2018). A high-rate and stable quasi-solid-state zinc-ion battery with novel 2D layered zinc orthovanadate array. Adv. Mater 30 (32), 7. doi:10.1002/adma.201803181
Chen, Y., Zhao, J., and Wang, Y. (2020). Quasi-solid-state zinc ion rechargeable batteries for subzero temperature applications. ACS Appl. Energy Mater 3, 9058–9065. doi:10.1021/acsaem.0c01452
Chen, Z., Wang, P., Ji, Z., Wang, H., Liu, J., Wang, J., et al. (2020). High-voltage flexible aqueous zn-ion battery with extremely low dropout voltage and super-flat platform. Nanomicro Lett 12 (1), 75. doi:10.1007/s40820-020-0414-6
Dong, H., Li, J., Zhao, S., Zhao, F., Xiong, S., Brett, D. J. L., et al. (2020). An anti-aging polymer electrolyte for flexible rechargeable zinc-ion batteries. J. Mater. Chem 8, 22637–22644. doi:10.1039/d0ta07086f
Dueramae, I., Okhawilai, M., Kasemsiri, P., Uyama, H., and Kita, R. (2020). Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery. Sci. Rep 10 (1), 12587. doi:10.1038/s41598-020-69521-x
Francis, C. F. J., Kyratzis, I. L., and Best, A. S. (2020). Lithium‐Ion battery separators for ionic‐liquid electrolytes: a review. Adv. Mater 32 (18), 1904205. doi:10.1002/adma.201904205
He, B., Zhang, Q., Man, P., Zhou, Z., Li, C., Li, Q., et al. (2019a). Self-sacrificed synthesis of conductive vanadium-based metal–organic framework nanowire-bundle arrays as binder-free cathodes for high-rate and high-energy-density wearable zn-ion batteries. Nano. Energy 64, 103935. doi:10.1016/j.nanoen.2019.103935
He, B., Zhou, Z., Man, P., Zhang, Q., Li, C., Xie, L., et al. (2019b). V2O5 nanosheets supported on 3D N-doped carbon nanowall arrays as an advanced cathode for high energy and high power fiber-shaped zinc-ion batteries. J. Mater. Chem 7 (21), 12979–12986. doi:10.1039/c9ta01164a
Hoang, T. K. A., The Nam Long, D., Cho, J. H., Su, J. Y. J., Lee, C., Lu, C., et al. (2017). Sustainable gel electrolyte containing pyrazole as corrosion inhibitor and dendrite suppressor for aqueous Zn/LiMn2O4 battery. Chemsuschem 10 (13), 2816–2822. doi:10.1002/cssc.201700441
Huang, S., Wan, F., Bi, S., Zhu, J., Niu, Z., and Chen, J. (2019). A self-healing integrated all-in-one zinc-ion battery. Angew. Chem. Int. Ed. Engl 58 (13), 4313–4317. doi:10.1002/anie.201814653
Johnsi, M., and Suthanthiraraj, S. A. (2016). Compositional effect of ZrO2 nanofillers on a PVDF-co-HFP based polymer electrolyte system for solid state zinc batteries. Chin. J. Polym. Sci 34 (3), 332–343. doi:10.1007/s10118-016-1750-3
Johnsi, M., and Suthanthiraraj, S. A. (2015). Preparation, zinc ion transport properties, and battery application based on poly (vinilydene fluoride-co-hexa fluoro propylene) polymer electrolyte system containing titanium dioxide nanofiller. High Perform. Polym 27 (7), 877–885. doi:10.1177/0954008314565397
Karan, S., Sahu, T. B., Sahu, M., and Agrawal, R. C. (2016). Investigations on ion transport behaviour in a non-lithium chemical based solid polymer electrolyte (SPE): PEO:ZnA. Mater. Today Proc 3 (2), 109–114. doi:10.1016/j.matpr.2016.01.034
Karan, S., Sahu, T. B., Sahu, M., Mahipal, Y. K., and Agrawal, R. C. (2017). Characterization of ion transport property in hot-press cast solid polymer electrolyte (SPE) films: PEO: Zn(CF3SO3)(2). Ionics 23 (10), 2721–2726. doi:10.1007/s11581-017-2036-7
Lee, B.-S., Cui, S., Xing, X., Liu, H., Yue, X., Petrova, V., et al. (2018). Dendrite suppression membranes for rechargeable zinc batteries. ACS Appl. Mater. Interfaces 10 (45), 38928–38935. doi:10.1021/acsami.8b14022
Li, H. F., Han, C. P., Huang, Y., Huang, Y., Zhu, M. S., Pei, Z. X., et al. (2018a). An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci 11 (4), 941–951. doi:10.1039/c7ee03232c
Li, H. F., Liu, Z., Liang, G., Huang, Y., Huang, Y., Zhu, M., et al. (2018b). Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano 12 (4), 3140–3148. doi:10.1021/acsnano.7b09003
Li, H., Ma, L., Han, C., Wang, Z., Liu, Z., Tang, Z., et al. (2019). Advanced rechargeable zinc-based batteries: recent progress and future perspectives. Nano Energy 62, 550–587. doi:10.1016/j.nanoen.2019.05.059
Li, X., Tang, Y., Zhu, J., Lv, H., Zhao, L., Wang, W., et al. (2020). Boosting the cycling stability of aqueous flexible zn batteries via F doping in nickel-cobalt carbonate hydroxide cathode. Small 16 (31), e2001935. doi:10.1002/smll.202001935
Liu, J., Ahmed, S., Khanam, Z., Wang, T., and Song, S. (2020a). Ionic liquid-incorporated zn-ion conducting polymer electrolyte membranes. Polymers 12 (8), 1755. doi:10.3390/polym12081755
Liu, J., Khanam, Z., Muchakayala, R., and Song, S. (2020b). Fabrication and characterization of zn-ion-conducting solid polymer electrolyte films based on PVdF-HFP/Zn(Tf)2 complex system. J. Mater. Sci. Mater. Electron 31 (8), 6160–6173. doi:10.1007/s10854-020-03169-1
Liu, J., Hu, M., Wang, J., Nie, N., Wang, Y., Wang, Y., et al. (2019). An intrinsically 400% stretchable and 50% compressible NiCo//Zn battery. Nano Energy 58, 338–346. doi:10.1016/j.nanoen.2019.01.028
Liu, Z. X., Wang, D. H., Tang, Z. J., Liang, G. J., Yang, Q., Li, H. F., et al. (2019). A mechanically durable and device-level tough Zn-MnO2 battery with high flexibility. Energy Storage Mater 23, 636–645. doi:10.1016/j.ensm.2019.03.007
Ma, L., Chen, S., Li, N., Liu, Z., Tang, Z., Zapien, J. A., et al. (2020). Hydrogen-free and dendrite-free all-solid-state zn-ion batteries. Adv. Mater 32 (14), e1908121. doi:10.1002/adma.201908121
Ma, Y., Zhang, Y., Cai, S., Han, Z., Liu, X., Wang, F., et al. (2020). Flexible hybrid electronics for digital healthcare. Adv. Mater 32 (15), 1902062. doi:10.1002/adma.201902062
Mo, F. N., Liang, G. J., Meng, Q. Q., Liu, Z. X., Li, H. F., Fan, J., et al. (2019). A flexible rechargeable aqueous zinc manganese-dioxide battery working at-20 degrees C. Energy Environ. Sci 12 (2), 706–715. doi:10.1039/c8ee02892c
Mossali, E., Picone, N., Gentilini, L., Rodrìguez, O., Pérez, J. M., and Colledani, M. (2020). Lithium-ion batteries towards circular economy: a literature review of opportunities and issues of recycling treatments. J. Environ. Manag 264, 110500. doi:10.1016/j.jenvman.2020.110500
Nancy, A. C., and Suthanthiraraj, S. A. (2017). Effect of Al2O3 nanofiller on the electrical, thermal and structural properties of PEO:PPG based nanocomposite polymer electrolyte. Ionics 23 (6), 1439–1449. doi:10.1007/s11581-017-1976-2
Parker, J. F., Chervin, C. N., Pala, I. R., Machler, M., Burz, M. F., Long, J. W., et al. (2017). Rechargeable nickel-3D zinc batteries: an energy-dense, safer alternative to lithium-ion. Science 356 (6336), 415–418. doi:10.1126/science.aak9991
Pucic, I., and Turkovic, A. (2005). Radiation modification of (PEO)(8)ZnCl2 polyelectrolyte and nanocomposite. Solid State Ion 176 (19-22), 1797–1800. doi:10.1016/j.ssi.2005.04.042
Qiu, W. D., Li, Y., You, A., Zhang, Z. M., Li, G. F., Lu, X. H., et al. (2017). High-performance flexible quasi-solid-state Zn-MnO2 battery based on MnO2 nanorod arrays coated 3D porous nitrogen-doped carbon cloth. J. Mater. Chem 5 (28), 14838–14846. doi:10.1039/c7ta03274a
Rathika, R., and Suthanthiraraj, S. A. (2018). Influence of 1-ethyl-3-methylimidazolium bis (trifluoromethyl sulfonyl) imide plasticization on zinc-ion conducting PEO/PVdF blend gel polymer electrolyte. J. Mater. Sci. Mater. Electron 29 (23), 19632–19643. doi:10.1007/s10854-018-0024-y
Tafur, J. P., Santos, F., and Romero, A. J. (2015). Influence of the ionic liquid type on the gel polymer electrolytes properties. Membranes 5 (4), 752–771. doi:10.3390/membranes5040752
Tang, B. Y., Shan, L. T., Liang, S. Q., and Zhou, J. (2019). Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci 12 (11), 3288–3304. doi:10.1039/c9ee02526j
Tang, Y., Li, X., Lv, H., Xie, D., Wang, W., Zhi, C., et al. (2020). Stabilized Co3+/Co4+ redox pair in in situ produced CoSe2−x-derived cobalt oxides for alkaline Zn batteries with 10 000-cycle lifespan and 1.9-V voltage plateau. Adv. Energy Mater 10, 2000892. doi:10.1002/aenm.202000892
Wan, F., Zhang, L., Wang, X., Bi, S., Niu, Z., and Chen, J. (2018). An aqueous rechargeable Zinc-organic battery with hybrid mechanism. Adv. Funct. Mater 28 (45), 1804975. doi:10.1002/adfm.201804975
Wang, D., Han, C., Mo, F., Yang, Q., Zhao, Y., Li, Q., et al. (2020). Energy density issues of flexible energy storage devices. Energy Storage Mater 28, 264–292. doi:10.1016/j.ensm.2020.03.006
Wang, D. H., Li, H. F., Liu, Z. X., Tang, Z. J., Liang, G. J., Mo, F. N., et al. (2018). A nanofibrillated cellulose/polyacrylamide electrolyte-based flexible and sewable high-performance Zn-MnO2 battery with superior shear resistance. Small 14 (51), e1803978. doi:10.1002/smll.201803978
Wang, J. Q., Liu, J., Hu, M. M., Zeng, J., Mu, Y. B., Guo, Y., et al. (2018). A flexible, electrochromic, rechargeable Zn//PPy battery with a short circuit chromatic warning function. J. Mater. Chem 6 (24), 11113–11118. doi:10.1039/c8ta03143f
Wang, K., Zhang, X. H., Hang, J. W., Zhang, X., Sun, X. Z., Li, C., et al. (2018). High-performance cable-type flexible rechargeable Zn battery based on MnO2@CNT fiber microelectrode. ACS Appl. Mater. Interfaces 10 (29), 24573–24582. doi:10.1021/acsami.8b07756
Wang, M., Emre, A., Tung, S., Gerber, A., Wang, D., Huang, Y., et al. (2019). Biomimetic solid-state Zn2+ electrolyte for corrugated structural batteries. ACS Nano 13 (2), 1107–1115. doi:10.1021/acsnano.8b05068
Wang, Z. F., Mo, F. N., Ma, L. T., Yang, Q., Liang, G. J., Liu, Z. X., et al. (2018). Highly compressible cross-linked polyacrylamide hydrogel-enabled compressible Zn-MnO2 battery and a flexible battery-sensor system. ACS Appl. Mater. Interfaces 10 (51), 44527–44534. doi:10.1021/acsami.8b17607
Wang, Z. Q., Hu, J. T., Han, L., Wang, Z. J., Wang, H. B., Zhao, Q. H., et al. (2019). A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy 56, 92–99. doi:10.1016/j.nanoen.2018.11.038
Ward, I. M., and Hubbard, H. V. S. A. (2012). “Polymer gel electrolytes: conduction mechanism and battery applications,” in Ionic interactions in natural and synthetic macromolecules Editors A. P. Alberto Ciferri, and A. Perico. (Hoboken, NJ: Wiley). 817–840.
Xiao, X., Liu, W., Wang, K., Li, C., Sun, X., Zhang, X., et al. (2020). High-performance solid-state Zn batteries based on a free-standing organic cathode and metal Zn anode with an ordered nano-architecture. Nanoscale Adv 2 (1), 296–303. doi:10.1039/c9na00562e
Yu, P., Zeng, Y., Zhang, H., Yu, M., Tong, Y., and Lu, X. (2019). Flexible Zn-Ion batteries: recent progresses and challenges. Small 15 (7), e1804760. doi:10.1002/smll.201804760
Zeng, Y. X., Zhang, X. Y., Meng, Y., Yu, M. H., Yi, J. N., Wu, Y. Q., et al. (2017). Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn-MnO2 battery. Adv. Mater 29 (26), 1700274. doi:10.1002/adma.201700274
Zeng, Y. X., Zhang, X. Y., Qin, R. F., Liu, X. Q., Fang, P. P., Zheng, D. Z., et al. (2019). Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-Ion batteries. Adv. Mater 31 (36), e1903675. doi:10.1002/adma.201903675
Zhang, H., Wang, J., Liu, Q., He, W., Lai, Z., Zhang, X., et al. (2019). Extracting oxygen anions from ZnMn2O4: robust cathode for flexible all-solid-state Zn-ion batteries. Energy Storage Mater 21, 154–161. doi:10.1016/j.ensm.2018.12.019
Zhang, N., Jia, M., Dong, Y., Wang, Y., Xu, J., Liu, Y., et al. (2019). Hydrated layered vanadium oxide as a highly reversible cathode for rechargeable aqueous zinc batteries. Adv. Funct. Mater 29 (10), 1807331. doi:10.1002/adfm.201807331
Zhang, Q. C., Li, C. W., Li, Q. L., Pan, Z. H., Sun, J., Zhou, Z. Y., et al. (2019). Flexible and high-voltage coaxial-fiber aqueous rechargeable zinc-ion battery. Nano Lett 19 (6), 4035–4042. doi:10.1021/acs.nanolett.9b01403
Zhang, N., Chen, X., Yu, M., Niu, Z., Cheng, F., and Chen, J. (2020). Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev 49 (13), 4203–4219. doi:10.1039/c9cs00349e
Zhang, Y., Chen, Z., Qiu, H., Yang, W., Zhao, Z., Zhao, J., et al. (2020). Pursuit of reversible Zn electrochemistry: a time-honored challenge towards low-cost and green energy storage. NPG Asia Mater 12 (1), 1–24. doi:10.1038/s41427-019-0167-1
Zhao, J., Ren, H., Liang, Q. H., Yuan, D., Xi, S. B., Wu, C., et al. (2019). High-performance flexible quasi-solid-state zinc-ion batteries with layer-expanded vanadium oxide cathode and zinc/stainless steel mesh composite anode. Nano Energy 62, 94–102. doi:10.1016/j.nanoen.2019.05.010
Zhao, Q., Stalin, S., Zhao, C.-Z., and Archer, L. A. (2020). Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater 5 (3), 229–252. doi:10.1038/s41578-019-0165-5
Keywords: Polymer electrolyte, ionic conductivity, solid-solid interface, Zn dendrite, Zn-ion battery, solid-state battery
Citation: Hansen EJ and Liu J (2021) Materials and Structure Design for Solid-State Zinc-Ion Batteries: A Mini-Review. Front. Energy Res. 8:616665. doi: 10.3389/fenrg.2020.616665
Received: 12 October 2020; Accepted: 25 November 2020;
Published: 21 January 2021.
Edited by:
Cheng Zhong, Tianjin University, ChinaReviewed by:
Hongfei Li, City University of Hong Kong, Hong KongCopyright © 2021 Hansen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Liu, Smlhbi5saXVAdWJjLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.