
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Energy Res. , 07 August 2020
Sec. Bioenergy and Biofuels
Volume 8 - 2020 | https://doi.org/10.3389/fenrg.2020.00183
This article is part of the Research Topic Advancements in Bio-Based Products from Renewable Feedstocks: Drop-In Replacement and Beyond View all 7 articles
Microbial conversion of lignocellulosic substrates to fuel and platform chemical intermediates offers a sustainable route to establish a viable bioeconomy. However, such approaches face a series of key technical, economic, and sustainability hurdles, including: incomplete substrate utilization, lignocellulosic hydrolysate, and/or end-product toxicity, inefficient product recovery, incompatible cultivation requirements, and insufficient productivity metrics. Development of a production host with native traits suitable for high productivity conversion of lignocellulosic substrates under process-relevant conditions offers a means to bypass the above-described hurdles and accelerate the development of microbial biocatalyst deployment. Clostridium tyrobutyricum, a native producer of short chain fatty acids, displays a series of characteristics that make it an ideal candidate for conversion of lignocellulosic substrates and thus represents a promising host for microbial production of diverse carboxylate-derived product suites. Herein, recent progress and future directions in the development of this bacterium as an industrial microbial cell factory, with emphases on the utilization of lignocellulosic substrates and metabolic engineering approaches, is reviewed.
Bio-derived carboxylic acids have long been identified as particularly promising precursors for biofuels and bioproducts due to their inherent chemical functionality (Guarnieri et al., 2017). They comprise a subset of building blocks identified by the U.S. Department of Energy (DOE) as top value-added chemical production candidates from terrestrial biomass (Werpy and Petersen, 2004). Indeed, recent work has underscored the utility of carboxylic acids as valuable precursors for fuel and polymer production, and demonstrated novel catalytic routes to upgrade carboxylates to jet and diesel blendstocks as well as high-value polymers (Henard et al., 2016; Guarnieri et al., 2017; Karp et al., 2017; Huo et al., 2019; Medoff et al., 2019; Wu et al., 2019). These technologies serve as proof-of-principle for the development of green bioproduction routes that have the potential to substantially displace petroleum-derived fuels and chemicals.
One class of carboxylic acids that is of particular interest for green manufacturing are the volatile fatty acids (VFAs), or short-chain fatty acids (SCFA). These acids are C1–C6 chain length fatty acids that can be distilled at atmospheric pressure and are natively produced by diverse microbial species. While they play key roles in microbial metabolism, they can also be used for numerous biotechnological applications. At present, VFA production is primarily achieved through petrochemical routes (Huang et al., 2002), however, there is a renewed interest in biological routes of VFA production (Akaraonye et al., 2010; Lee et al., 2014). Biological VFA production is generally an anaerobic conversion process involving hydrolysis and acidogenesis of complex organic macromolecules. Such bioproduction schemes typically involve anaerobic digestion-based processes using microbial consortia including, for example, Clostridia, Bacteriocides, Bifidobacteria, Streptococci, and Enterobacteriaceaeare (Weiland, 2010), which have the capacity to generate a suite of mixed VFAs. Additionally, monocultures of select microorganisms are capable of producing narrow ranges or even singular VFAs. One such class of microorganisms includes several species of Clostridium that predominantly produce butyric acid, a promising molecule with chemical functionality suitable for a myriad of direct applications or use as a biological intermediate.
Butyric acid (CH3CH2CH2CO2H) is a short-chain VFA with biotechnological applications in diverse industrial sectors, including: pharmaceutical, chemical, textile, plastic, food, beverage, cosmetics, dairy, and biofuel industries. From a pharmaceutical perspective, butyric acid and numerous derivative molecules have been found to display properties suitable for cancer therapeutics and vasoconstrictor drugs (Leavitt et al., 1978; Chen and Breitman, 1994; Rephaeli et al., 2000; Hamer et al., 2008; Ohara and Suzutani, 2018; Ohara and Mori, 2019). Furthermore, butyric acid demonstrates net positive benefits in the therapy of gastrointestinal diseases (Borycka-Kiciak et al., 2017). While butyric acid itself possesses an unpleasant aroma, derivatives including methyl, ethyl, and amyl butyrate esters are widely used in flavor and fragrance industries to impart fruity or floral aromas (Anton et al., 2014; Ma et al., 2020). In the chemical industry, butyric acid is additionally used as an intermediate in the production of cellulose acetate butyrate (CAB), an amorphous, transparent thermoplastic (El-Sakhawy et al., 2014). For further discussion on butyric acid applications, an extensive review can be found in the following references (Zhang et al., 2009; Jiang et al., 2018).
Industrial-scale production of butyric acid is primarily achieved through chemical synthesis, involving the oxidation of butyraldehyde obtained from crude-oil-derived propylene (Dwidar et al., 2012). However, development of robust biological catalysts present an alternative production path to reduce the carbon footprint and reliance on the petrochemical industry. Numerous bacterial species including Butyrivibrio, Butyribacterium, Sarcina, Eubacterium, Fusobacterium Megasphera, and Clostridium natively produce butyric acid as a primary end product of their principal metabolic strategy (Playne, 1985; Zigova and Sturdik, 2000). From a biotechnological perspective, Clostridium species are the most widely utilized hosts for the production of butyric acid, and are represented by diverse species including C. beijerinckii, C. acetobutylicum, C. populeti, C. thermobutyricum, C. butyricum, and C. tyrobutyricum. A frequent observation of these Clostridium species is their production of mixed product suites, derived from solventogenic and acidogenic metabolic branches. For example, C. acetobutylicum has been used extensively in acetone–butanol–ethanol (ABE) fermentation, leveraging a metabolism favoring solventogenesis. Conversely, C. butyricum and C. tyrobutyricum represent perhaps the two most promising hosts for the production of butyric acid, and other carboxylic acids, owing to a strong acidogenic metabolism and ability to tolerate high concentrations of acidic products.
Despite the promise of microbial biocatalytic strategies for conversion of lignocellulosic substrates to fuels and bioproducts, such approaches currently face a series of key technical and sustainability hurdles. Common hurdles include: incomplete substrate utilization capacity, lignocellulosic hydrolysate and/or end-product toxicity, product recovery-incompatible cultivation requirements, and low yield and productivity metrics (Mills et al., 2009; Wei et al., 2013; Lee et al., 2016; Fu et al., 2017a; Kim et al., 2017; Liu C. L. et al., 2018; Suo et al., 2018c; Xiao et al., 2018). Despite the demonstrated production of multiple product suites in conventional model organisms (e.g., Escherichia coli and Saccharomyces cerevisiae) (Kim et al., 2017; Liu C. L. et al., 2018), few successes have been realized at high-productivity and scale on biomass substrates. In part, this is due to insufficiently robust biocatalysts, inadequate systems design, and limited engineering capabilities in process-relevant, non-model organisms. For example, Escherichia coli, a prototypical biocatalytic host with a robust genetic toolkit has been engineered numerous times for the production of butyric acid, however, productivity metrics have not been able to surpass those of wild-type Clostridium species (Seregina et al., 2010; Baek et al., 2013; Lim et al., 2013; Saini et al., 2014; Volker et al., 2014; Jawed et al., 2016; Kataoka et al., 2017; Wang et al., 2019). Similarly, efforts to engineer S. cerevisiae for the production of volatile fatty acids has generally yielded strains capable of producing product below 1 g/L titers (Leber and Da Silva, 2014). In addition, production of fuel and chemical intermediates from lignocellulose typically entails host-product pairing to biosynthesize a single product in a single host, often necessitating bioprocess redesign for each new target molecule.
Establishment of a production host with native traits suitable for highly productive conversion of lignocellulosic substrates to multiple target products offers a means to bypass the above-described hurdles and accelerate the development of microbial biocatalyst deployment. To this end, C. tyrobutyricum presents a deployment-viable biocatalyst with numerous favorable industrial traits (Figure 1). Successful development of this microbe has the potential to generate a robust host chassis with established acidotolerance and fermentation optimization strategies, suitable for conversion of lignocellulosic substrates to a broad suite of carboxylates and derivatives thereof. Herein, biosynthetic capabilities of C. tyrobutyricum are reviewed, with a focus upon recent metabolic engineering advances to enhance butyric acid biosynthesis. Finally, the potential for expanding the biosynthetic capacity of C. tyrobutyricum via emerging synthetic biology approaches for the production of non-native carboxylates and associated bioproducts is discussed.

Figure 1. Beneficial traits inherent and developed for Clostridium tyrobutyricum which make it an exemplary biocatalyst for the production of suites of fuel and chemical precursors from lignocellulosic biomass.
The Clostridium genus represents a diverse group of industrially-relevant anaerobic bacteria. Clostridium are Gram-positive, spore-forming, and obligate anaerobic firmicutes which can be found in soils, freshwater and marine sediments, and have wide-ranging physiological traits. Some species of Clostridium are known for their negative impact on human society including the spoilage of meat and cheeses (Klijn et al., 1995) and through the biosynthesis of potent toxins. For example, C. botulinum, C. perfringens, and some species of C. butyricum can cause mild-to-fatal food poisoning via botulinum toxins, which are the most lethal toxins identified to date, and 100,000 times more deadly than sarin (McCauley, 2009). Conversely, numerous strains of Clostridium have been used for biotechnologically-relevant advances, in part due to favorable industrially-relevant traits such as cellulosic biomass degradation capacity, autotrophic carbon fixation, and the native production of a myriad of molecules or intermediates targeted by the bioeconomy.
Diverse Clostridium species have been leveraged for the production of chemicals, fuels, and biological intermediates. For example, the production of acetone and butanol was one of the first large-scale industrial fermentative bioprocesses using C. acetobutylicum in the early 1900’s (Sauer, 2016). The resultant acetone-butanol-ethanol (ABE) process has been a focus of biofuel production ever since. Such Clostridium fermentations are biphasic with an initial acidogenic phase, wherein organic acids including acetic and butyric acids are produced, followed by solventogenic phase where these acids are converted to alcohols. Conventionally, alcohols have been the desired product of these fermentations. However, acids have recently been demonstrated to be an equally flexible intermediate that can be coupled with modern catalytic routes to create diverse molecules including aldehydes, alcohols, esters, ketones, olefins, and hydrocarbons (Sjoblom et al., 2016; Huo et al., 2019). With the recent development and optimization of genetic tools in Clostridium species (discussed in more detail below), the ability to redirect metabolism to biosynthesize suites of products in this unique genus is becoming realistically achievable. While many Clostridium species represent viable biocatalytic hosts, C. tyrobutyricum is highlighted in this review due to its numerous beneficial traits.
C. tyrobutyricum is a mesophilic, Gram-positive, acidogenic, anaerobic bacterium that produces butyrate as a primary organic acid fermentation product, with acetate, hydrogen, and carbon dioxide generated as the major fermentation by-products (Lee et al., 2016; Suo et al., 2018c). C. tyrobutyricum displays a series of characteristics that make it an ideal candidate for conversion of lignocellulosic substrates (Figure 1; Wei et al., 2013; Fu et al., 2017a; Xiao et al., 2018), including:
• C5 and C6 substrate utilization capacity, including glucose, xylose, and oligo- and polysaccharides;
• Robust cultivation and biosynthetic capacity on diverse lignocellulosic hydrolysates (Wei et al., 2013; Fu et al., 2017a; Xiao et al., 2018);
• Rapid C5 and C6 conversion rates (> 1.5 g/L/h) and yield to butyric acid (> 0.45 g/g) (Liu et al., 2006; Jiang et al., 2012);
• High organic acid tolerance and accumulation capacity (butyrate titers exceeding 85 g/L) (Jiang et al., 2011);
• Rapid specific growth rate (> 0.2 h–1);
• Broad pH tolerance, capable of active growth and organic acid biosynthetic capacity at pH < 6.0, which enables facile product separations.
• Genetic tractability, which enables the redirection of metabolic flux to alternative end products using both classical engineering and editing methods (discussed in detail below).
Multiple groups have extensively evaluated this strain using lignocellulosic hydrolysates, achieving rapid conversion of glucose and xylose, and producing butyrate titers exceeding 85 g/L, further underscoring the high-production potential for this bacterium. This represents the highest titer of all known butyrate production organisms reported to date; an in depth interspecies comparison is reviewed by Jiang et al. (2018). An array of alternative fermentation modes (e.g., continuous fermentation and fibrous bed immobilization) have also been established for high-butyrate production in C. tyrobutyricum (Wu and Yang, 2003; Zhu and Yang, 2003; Jiang et al., 2011).
The genome of C. tyrobutyricum Type strain ATCC 25755 was recently sequenced, revealing a 3.07 Mb genome encoding ∼3,040 structurally-annotated open reading frames. The genome is AT-rich, consisting of only ∼31% GC content. Additionally, C. tyrobutyricum harbors a 63 kB extrachromosomal plasmid, pCTK01, which encodes putative proteins for conjugal plasmid transfer as well as DNA topoisomerase, a predicted sigma factor, and a putative arsenite transporter (Lee et al., 2016). The remaining plasmid functionality has yet to be elucidated.
Notably, there are incongruities in the field regarding published genome sequences. Specifically, the genome sequence of the Type strain ATCC 25755 was published in 2013 as a draft genome (Jiang et al., 2013), and subsequently an alternative sequence of the same strain from the Korean Collection for Type Cultures (termed KCTC 5387) was published (Lee et al., 2016). In the case of the former assembly, there are no functionally annotated genes, nor are there any obvious homologs for key xylose regulatory genes xylT or xylR, which are annotated in the KCTC 5387 genome. This discrepancy arises from differential completeness of the draft genomes; genome alignment suggests that there is a significant (>500,000 bp) region in the KCTC 5387 genome that is absent in the draft genome of ATCC 25755. Additionally, it was reported in 2012 that the ptb and buk genes were deleted from ATCC 25755 using molecular genetic approaches (Zhang et al., 2012), yet subsequent genome sequencing and proteomic analyses suggested that these genes were not present in the genome (Lee et al., 2016). These insights are provided not as a commentary on the validity of the prior research, but for completeness to help inform research in this field. Future efforts to ensure that ATCC 25755 and KCTC 5387 are in fact the same strain are encouraged.
Integrated genomic and proteomic analyses have facilitated identification of core metabolic machinery (Lee et al., 2016), indicating C. tyrobutyricum employs a phosphoenolpyruvate-dependent sugar phosphotransferase system (PTS) as a primary carbohydrate transport system including: PTIS, phosphocarrier HPr protein, and two putative glucose-specific PTS enzyme II genes. A series of additional sugar symporters, including a fructose-specific PTS, have been identified as alternative routes for carbohydrate transport. C. tyrobutyricum effectively catabolizes glucose to pyruvate using a canonical glycolytic pathway. Notably, genes encoding Entner-Doudoroff (EDD) and oxidative pentose phosphate pathway (PPP) components are absent from the genome. C. tyrobutyricum also has the capacity to use xylose as a sole carbon and energy source. The genome encodes xylose isomerase and xylulose kinase genes, which are tightly regulated by carbon catabolite repression mechanisms (discussed further below) (Fu et al., 2017b; Luo et al., 2017).
Transcription of catabolic genes subject to CCR is mediated by enzymes of the phosphoenolpyruvate-dependent phosphotransferase system (PTS), which couples glucose uptake to phosphorylation and subsequent entrance into glycolysis. PTS components are enzyme I (EI), histidine-containing phosphorylase protein (HPr), enzyme II (EII) comprised of subunits EIIA, EIIB, and EIIC, and carbon catabolite protein A (CcpA). EIIB and EIIC form a transmembrane complex that specifically uptakes glucose. Transfer of a phosphate group from phosphoenolpyruvate to EI triggers a cascade in which EI, HPr, EIIA, and EIIB are subsequently phosphorylated and dephosphorylated. The result is the uptake and phosphorylation of glucose by the EIIB/EIIC complex (Deutscher et al., 2006). Glycolytic intermediate fructose-1,6-bisphosphate initiates phosphorylation of HPr at a conserved serine residue, which forms a complex with pleiotropic regulator CcpA, a member of the LacI/GalR family of transcriptional repressors/activators (Jault et al., 2000). The CcpA complex binds to sequences called catabolic responsive elements (CRE elements), found in the promoters and coding regions of genes, and prevents transcription from occurring. Expression of catabolic genes of non-preferred sugar substrates like xylose and arabinose is regulated by CcpA (Ren et al., 2012). Furthermore, a putative xylose repressor is encoded in the genome of C. tyrobutyricum, which could contribute to regulation of xylose gene expression in addition to CcpA (Rodionov et al., 2001).
Acetic and butyric acid biosynthesis serve as ATP-generating reactions in C. tyrobutyricum (Figure 2). Phosphotransacetylase (pta) and acetate kinase (ack) mediate acetic acid formation from acetyl-CoA. Butyrate biosynthesis proceeds through downstream acetoacetyl-CoA, 3-hydroxybutyryl-CoA, crotonyl-CoA, and butyryl-CoA intermediates. Ultimately, butyric acid production in C. tyrobutyricum is mediated by butyrate:acetate CoA transferase, cat1. This mechanism notably differs from that found in many related Clostridium species, which exclusively employ the concerted activities of phosphotransbutyrylase and butyrate kinase (Lee et al., 2008; Senger and Papoutsakis, 2008; Xin et al., 2013). However, the ATP yield obtained via the two routes is equivalent due to the coupling of butyrate and ATP-generating acetate formation in cat1-mediated route. For an in-depth analysis of C. tyrobutyricum genome architecture, the reader is directed to Lee et al. (2016).
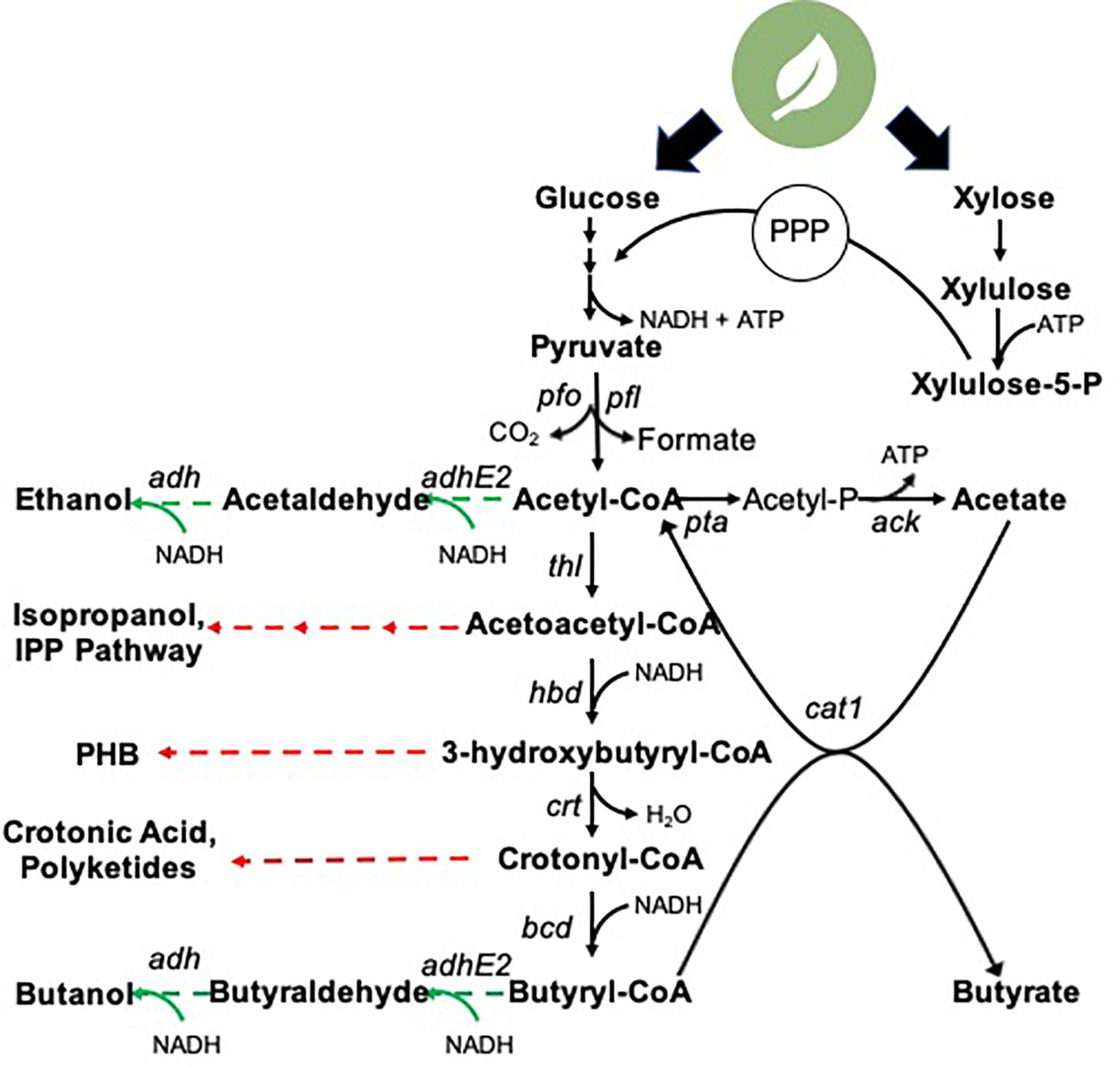
Figure 2. Clostrium tyrobutyricum central carbon metabolism and biosynthetic potential. Black arrows: known biosynthetic pathways native metabolic intermediates. Green arrows: heterologous genes successfully expressed in C. tyrobutyricum for the production of non-native products. Red arrows: unexplored production routes in C. tyrobutyricum. pfo, pyruvate:ferredoxin oxidoreductase; pfl, pyruvate:formate lyase; pta, phosphotransacetylase; ack, acetate kinase; thl, thiolase; hbd, 3-hydroxybutyryl-CoA dehydrogenase; crt, 3-hydroxybutyryl-CoA-dehydratase; bcd, butanoyl-coA dehydrogenase; cat1, butyrate:acetate CoA transferase; thi, thioesterase; adh, alcohol dehydrogenase; adhE2, aldehyde-alcohol dehydrogenase; PHB, polyhydroxybutyrate; IPP, isoprenoid 1.
Genetic tools have been established for C. tyrobutyricum, including conventional integrative and replicative plasmids for gene knockout and overexpression (Liu et al., 2006; Fu et al., 2017b), a fluorescent gene reporter system for promoter strength quantification (Cheng et al., 2019), and an array of CRISPR/Cas systems, enabling multiplex in vivo genome editing capacity (Zhang et al., 2018, 2020). Until the recent deployment of CRISPR/Cas systems (Zhang et al., 2018, 2020), the majority of gene manipulation in C. tyrobutyricum was performed via ClosTron, a tool developed from Group II intron mutagenesis specifically for Clostridium spp (Heap et al., 2007), and a tandem modular shuttle-plasmid system, the pMTL8000-series (Heap et al., 2009). The modular nature of the pMTL-series allows for interchangeable components such as selection markers and gram positive/negative replication origins. Many of the genetic engineering strategies pursued in C. tyrobutyricum to date have deployed pMTL82151 as a starting point for their gene overexpression systems (Yu et al., 2015b; Suo et al., 2017, Suo et al., 2018c; Zhang J. et al., 2017; He et al., 2020). The plasmid has the gram-negative, high copy-number ColE1 origin, the pBP1 gram-positive Clostridial origin from a plasmid native to C. botulinum NCTC 2916, and the genes catP and traJ for selection and conjugal function, respectively (Heap et al., 2009).
While systems like ClosTron and the modular pMTL8000-series plasmids canonically fulfill the needs of biotechnological research, the advent of the CRISPR/Cas system in 2012 offered a novel way to edit and replace genes in biological systems in a more precise and targeted fashion. Originally a bacterial immune system, these RNA-guided endonucleases have been repurposed for an array of genome editing capabilities in a multitude of microbial hosts due to their high specificity and flexibility (Adli, 2018). Several well-established CRISPR/Cas systems, Cas9, nCas9, and asCpf1, were cloned directly into wild type C. tyrobutyricum (Zhang et al., 2018). After analyzing the genome, it was revealed that C. tyrobutyricum had an endogenous native type I-B CRISPR/Cas system, which was leveraged to establish CRISPR-mediated editing. The host plasmid for this system utilized the pBP1 origin and traJ from the pMTL8000-series, and the core cas gene operon (cas6-cas8b-cas7-cas5-cas3-cas4-cas1-cas2) found via genome analysis. The system achieved its highest transformation and editing efficiency with 30–38 nt spacers at the 5′-end of the PAM sites, TCA or TCG. The optimized system deleted the spo0A and pyrF genes and replaced the cat1 gene with adhE2 from C. acetobutylicum, demonstrating the system’s flexibility and editing capacity (Zhang et al., 2018). Recently, it was revealed that with the combined deletion of a type I restriction endonuclease and the native pCTK01 plasmid, which contains genes limiting conjugative transfer, the transformation efficiency of C. tyrobutyricum can be enhanced 15.3-fold. By leveraging this more malleable mutant, successful transformation of the Cas9 and the Cpf1 system were achieved, and both demonstrated successful spo0A deletion (Zhang et al., 2020). Overall, this collection of genetic tools has been deployed to probe the essentiality of above-described central carbon metabolism and biosynthetic genes and ultimately metabolically engineer this strain for altered flux to butyric acid and non-native biosynthetic capacity.
A series of metabolic engineering efforts have successfully been pursued in C. tyrobutyricum to increase butyrate biosynthesis, expand substrate utilization capacity, and rewire metabolism for the production of non-native metabolic intermediates (Zhu et al., 2005; Zhang et al., 2012; Yu et al., 2015b, c; Fu et al., 2017b; Figure 2 and Table 1). Such studies importantly demonstrate the potential non-essentiality of native end-products, underscore the metabolic flexibility of C. tyrobutyricum to divert carbon flux away from butyric acid, and serve as proof-of-principle for rewiring this strain for enhanced and/or altered flux.
Due to the required expense to separate acetate and butyrate, multiple strain development strategies in C. tyrobutyricum focus on shifting carbon flux predominantly to butyrate. In an early study, a mutant strain with a deleted phosphotransacetylase gene, pta, showed lower PTA and AK activities. While the specific growth rate was diminished, likely due to the decreased generation of ATP by the acetate formation pathway, the resulting strain showed a 68% increase in final butyrate concentration while the acetate decreased by 14% (Table 1; Zhu et al., 2005). Despite ack being determined an essential gene as a result of genomic analysis, and failure to replicate a similar gene deletion with the endogenous CRISPR system, a study employing homologous recombination via suicide vector reported successful deletion and ∼50% decrease in AK activity (Liu et al., 2006; Lee et al., 2016; Zhang et al., 2018). Lui et al. (Liu et al., 2006) observed 23.5% increase in butyrate, and additionally they reported no acetate or lactate production when the strain was cultured in a fibrous bed bioreactor (FBB) on xylose as a sole carbon source, at a pH of 5 (Table 1). Deletion of the aforementioned putative phosphotransbutyrylase (ptb) gene was also evaluated (Figure 2). Despite quantifying only 24% activity for PTB, the mutant and wild type had yields of butyrate that were essentially the same, but with an increase in hydrogen, acetate, and subsequently the growth rate (Zhang et al., 2012).
Suo et al. (2018a) performed a series of studies focusing on limiting enzymes in the Embden-Meyerhof-Parnas pathway and fundamental genes in the butyrate pathway. First, they overexpressed genes for phosphofructokinase and pyruvate kinase, pfkA and pykA. Up-regulation of these genes increased available NADH and ATP, and the resulting strain had enhanced butyric acid productivity and final concentration, 38.9 and 38.1% in fed-batch trials, respectively (Table 1). They also reported that the strain was more resistant to butyric acid and glucose toxicity (Suo et al., 2018a). In a second study, the same group upregulated crotonase, crt, and butyryl:acetate CoA transferase, cat1, to try to increase the butyrate/acetate ratio. When the two genes were co-overexpressed, there was a 123.5 and 11% increase of butyrate/acetate ratio and butyric acid yield, respectively (Table 1). Combining overexpression genes for crt, cat1, pfkA and pykA, Suo et al. (2018c) achieved increased butyrate/acetate ratio by 94.6% and a final butyric acid concentration increase of 35.2% in a fed-batch fermentation of glucose (Table 1; Suo et al., 2018c).
Rather than focusing on individual gene manipulation, Jiang et al. (2012) examined adaptive evolution in repeated-batch FBB. Over the course of 130 days, they selected for enhanced butyric acid production and high glucose concentration tolerance, and a mutant strain resulted with 2.25 g/(L h) butyric acid productivity (Table 1). A metabolic analysis on the mutant revealed a 30% decrease in the phosphoenolpyruvate-dependent phosphotransferase (PEP-PTS) system activity; it was proposed that the mutant was able to bypass the high substrate inhibition leading to the higher yield and productivity (Jiang et al., 2012).
In addition to enhancing existing substrate capacity, researchers are also expanding into more diverse substrate capabilities. One study used adaptive evolution techniques to generate butyrate from pretreated and hydrolyzed wheat straw (PHWS). Starting with an initial tolerance of only 10% v/v for the substrate, the research team was able to adapt their strain to grow on 80% v/v PHWS with a butyric acid selectivity of 92.5% at pH 7 (Baroi et al., 2015a). Another study applied adaptive evolution to pretreated biomass from Saccharina japonica, which contain high concentrations of mannitol. Initial investigation of the wild type indicated that C. tyrobutyricum did not consume mannitol and stopped growing when the glucose was gone, but in the presence of the retarding agent NaHCO3 at 200 mM, a strain that utilized mannitol evolved and ultimately demonstrated heightened butyric acid titer of ∼154% (Ra et al., 2019).
While xylose is a known substrate for C. tyrobutyricum, xylose consumption is impeded by CCR, as indicated above (Fu et al., 2017b; Luo et al., 2017). In order to bypass host CCR, Fu et al. (Fu et al., 2017b) heterologously overexpressed xylose catabolism genes xylT, xylA, and xylB from C. acetobutylicum. With the use of heterologous xylose utilization machinery, the strain achieved simultaneous glucose and xylose catabolism, and the butyric acid productivity and yield was enhanced by 104 and 14%, respectively. Additionally, when benzyl viologen, an artificial electron carrier, was optimized at 3.75 uM, the results showed better flux from acetate formation to butyric acid (Table 1; Fu et al., 2017b). Another research group showed that the overexpression of C. acetobutylicum genes, galK, galE, and galT, C. tyrobutyricum was able metabolize up to 97.2% of galactose in the spent coffee grounds. Similar CCR complications were seen with the new substrate, but the addition of the heterologous non-phosphorylation transporter galP from C. acetobutylicum eliminated the substrate repression (He et al., 2020). All of the above substrates required some degree of pretreatment, however, untreated cane molasses can be simultaneously used as a carbon, nitrogen, and essential nutrient source for C. tyrobutyricum by overexpressing scrK, scrA, and scrB from C. acetyobutylicum. As a result of needing no treatment, cost analysis revealed a 47% cost saving by the process and comparable butyrate yields to other research (Table 1; Guo et al., 2020).
Due to the enhanced energy density of biobutanol as opposed to other native fermentation products, there has been a strong interest in equipping C. tyrobutyricum with appropriate metabolic features to convert butyryl-CoA into n-butanol, rather than only focusing on enhanced butyrate production. As C. tyrobutyricum does not natively encode the aldehyde/alcohol dehydrogenases for this conversion, an initial study explored the overexpression of the adhE2 gene from Clostridium acetobutylicum in a variety of C. tyrobutyricum strains. While n-butanol synthesis was achieved with only the heterologous expression of adhE2, coupling this overexpression with disruption of ack, Ct(Δack), increased the carbon flux toward butyryl-CoA, resulting in a butanol production of 10.0 and 16.0 g/L for substrates of glucose and mannitol, respectively, while simultaneously driving down the production of acetate by 600% as compared to the WT and adhE2 strain with an intact ack gene (Table 1; Yu et al., 2011). Additionally, methyl viologen (MV) has been used in multiple studies to promote C4 biosynthesis due to its capacity increase the flow of NADH to the production of butyrate or butanol instead acetate formation. A fed-batch fermentation trial with the Ct(Δack-adhE2) strain observed that as concentrations of MV increased to 500 and 1000 μM, the toxicity of the artificial electron carrier began crippling cell growth, but also increased the n-butanol by 40% and drove down organic acid production by 80–90% (Du et al., 2015).
n-butanol production from alternative feedstocks has also been extensively evaluated. Using a double mutant, Ct(Δack, adhE2), Yu et al. (2015a) investigated the addition of α-glucosidase, agluI, from C. acetobutylicum to catalyze α-1,4-glycosidic bonds in maltose and soluble starch for increased n-butanol production via batch fermentation. After optimizing the pH to accommodate both the butanol machinery and α-glucosidase activity, the strain with agluI expression showed improved performance to the Ct(Δack, adhE2) and the novel substrate had efficient selective pressure to eliminate the need for antibiotics (Table 1; Yu et al., 2015a). Continued variations of the Ct(Δack, adhE2) strain revealed overexpressing scrA, scrB, and scrK from C. acetobutylicum allowed for successful sucrose catabolism. When sugarcane juice (primarily glucose, fructose, and sucrose) was used as a feedstock with MV, the overexpression of the sucrose metabolic cassette was not susceptible CCR inhibition and the strain had better butanol yield than the Ct(Δack, adhE2) strain on glucose (Table 1; Zhang J. et al., 2017). Another study with this double mutant used a repeated batch FBB system with cassava bagasse hydrolysate as a substrate, indicating another viable low-cost feedstock for biobutanol production. A techno-economic analysis reveals that in a 50,000 metric ton/year plant with this strain the cost of the n-butanol would be $0.91/kg (Table 1; Huang et al., 2019).
Genomic analysis indicated that C. tyrobutyricum lacks several key enzymes that other Clostridium spp. use in their solventogenic pathways including a variation of CoA transferase. The CoA transferases in C. acetobutylicum, ctfA, and ctfB, promote a high recycling of acetate and butyrate back into acetyl-/butyryl-CoA, which allows for carbon flux to other products. Giving C. tyrobutyricum access to acetate and butyrate re-assimilation via the overexpression of the ctfAB genes increased the butanol titer by 21–31% and butanol yield and productivity by 100% with the strain’s efficiency likely curtailed both by butanol toxicity and insufficient supply of requisite NADH for the pathway to stay active (Yu et al., 2015c). Since reducing agent availability limits the production of biobutanol, a study demonstrated that it was possible to rebalance the redox by introducing an NADH pathway via the heterologous fdh gene from Moorella thermoacetica ATCC 39073. The result was an increase in butanol titer by 2.15 fold in serum vials and 2.72-fold in bioreactors (Table 1; Ma et al., 2016). While C. tyrobutyricum may lack solventogenic CoA transferases, its native butyrate:acetate CoA transferase, cat1, is essential for butyrate biosynthesis. With the aid of the endogenous Type I-B CRISPR-Cas, deletion and replacement of the cat1 gene with adhE2 resulted in successful carbon flux redirection toward butanol production, which was heightened when the batch fermentation temperature was tuned to 20°C (Table 1; Zhang et al., 2018).
Despite high emphasis on C4 products, other studies have explored the potential to biosynthesize alternative metabolic intermediates and chain-length products (Cheng et al., 2019). Jo et al. (2010) examined the impact of native [FeFe]-hydrogenase overexpression, hydA, upon hydrogen evolution in C. tyrobutyricum JM1. The enzyme activity increased 1.7 times and resulted in 150% increase in hydrogen production relative to wild type. The metabolic implications of increased hydrogenase activity resulted in a mild decrease of acetic acid, notable decrease of lactic acid, and flux toward butyric acid production (Jo et al., 2010). Another study developed a strain of C. tyrobutyricum capable of utilizing inulin in Jerusalem artichokes without pretreatment for enhanced H2 production capacity. They overexpressed the exo-inulinase gene, inu, from Paenibacillus polymyxa SC-2, and in a 500 L simultaneous saccharification batch fermentation, the mutant achieved 3.5 ± 0.23 mol of H2 with little sugar inhibition on growth due to gradual inulin breakdown (Jiang et al., 2017). A study examining gene expression reporter system quantification via the Bs2 fluorescent protein performed a proof-of-concept trial comparing two separate promoters driving an isopropanol pathway in the Ct(Δack) strain. With the addition of ctfA, ctfB, and adc genes from C. acetobutylicum and the adh gene from C. beijerinckii, isopropanol quantities from each strain approximately mirrored the fluorescence ratios of each promoter, indicating both capacity for expression quantification and isopropanol biosynthesis in C. tyrobutyricum (Cheng et al., 2019). Finally, Biosynthesis of ethyl acetate has also been achieved with a Δcat1:adhE1 strain of C. tyrobutyricum overexpressing lipaseB to facilitate the esterification process (Table 1; Feng et al., 2020).
In addition to diversification of substrate and product capacity, multiple studies have examined providing C. tyrobutyricum with better capabilities to cope with external stress, both from typical hydrolysate inhibitory molecules as well with desirable end products. While still inhibitory in high concentrations, C. tyrobutyricum is capable of metabolizing three primary inhibitors including acetate, furfural and hydroxymethylfurfural (Liu et al., 2013), as well as lignin-derived phenolics (e.g., coumaric acid). A series of studies examined the effects of overexpression of native Class I heat shock genes to help offset inhibition by external stress. Heat shock proteins, also known as chaperonins, reassemble improperly folded proteins and several other Clostridium spp. had heightened butanol tolerance by upregulating these genes, likely from decreased disruption to proteins in metabolic pathways. The first such study on C. tyrobutyricum explored resistance to the butyric acid via overexpression of native groESL genes. The strain achieved a higher tolerance to the organic acid demonstrated by a 28.2% increased final concentration (Suo et al., 2017). Suo et al. (2018b) next explored the Ct(groESL) strain’s resistance to the more toxic residues from hydrolyzed lignocellulosic biomass, with phenolic compounds being of key interest. The addition of these genes decreased the culture’s lag time by 24 h, and in batch fermentation of corn and rice straw hydrolysates containing known phenolic inhibitors, including syringaldehyde, coumarate, ferrulate), production of butyric acid outperformed the wild type by 26.5 and 19.4%, respectively (Suo et al., 2018b). A separate series of studies explored unconventional 12C6+ irradiation to achieve a phenotype with a higher resistance to butyrate for enhanced fermentation efficiency. After optimizing the process for a single round of radiation, mutants demonstrated similar acetate levels as the wild type but an overall metabolic shift toward butyrate production and toxicity resistance indicated by a 45.9% increase in the butyrate/acetate ratio and a 68% higher final concentration of butyrate (Zhou et al., 2014a). A 12C6+ irradiated strain was grown on Eucommia ulmoides Oliv hydrolysate indicating the capacity of this type of mutants to grow on renewable feedstock (Table 1; Zhou et al., 2014b). The group performed another study to explore the impact of two rounds of 12C6+ irradiation on acid tolerance. The strain they developed indicated higher resilience in lower pH environments of 4.5 and 5, and maintained increased butyrate production of ∼180% at pH 5 (Zhou et al., 2016).
Additional efforts to develop enhanced butyrate tolerance in C. tyrobutyricum have employed immobilized adaptation in a fed-batch, fibrous bed bioreactor (Jiang et al., 2011; Huang et al., 2016a, b; Fu et al., 2017a). Using this strategy, Jiang et al. (2011) were able to develop a strain capable of producing 86.9 g/L butyric acid. At 40 g/L butyric acid concentration, the wild-type strain stopped growing completely, while the adapted strain could still grow at 50% of its maximum growth rate. In the adapted strain, differences in growth, enzyme activities of butyrate-producing enzymes, intracellular pH, and cell morphology were observed in the presence of butyric acid as compared to the wild-type. Additionally, Zhou et al. (2014a, 2016) report that mutagenesis of cells by irradiation and subsequent selection of mutants for higher tolerance to butyric acid is another viable strategy for enhancing acid tolerance in C. tyrobutyricum (discussed further below).
Finally, to reduce impacts of lethal oxidative stress, the treS gene was expressed in C tyrobutyricum W428. Trehalose is a 1-1 alpha-alpha glycosidicly bonded glucose pair which is common in an array of organisms. Emerging research indicates that, in addition to energy storage, it provides organisms with heightened bandwidth against stressors, in this case low pH and hypoxic/aerobic conditions. The strain overexpressing the treS showed heightened ability to grow in both of these conditions while producing burytic acid (Table 1; Wu et al., 2017).
While metabolic engineering and adaptive evolution pose as glamorous mechanisms to enhance biofuel capacity in biological systems, there is considerable value in understanding how fermentation systems and media composition influence the productivity of target organisms. A variety of fermentation strategies have been installed with C. tyrobutyricum, ranging from a bioelectrical reactor (Choi et al., 2012), cells immobilized in a PVA gel (Rebroš et al., 2016), and stationary and moving fiborous bed bioreactors (Table 1; Jiang et al., 2011; Wei et al., 2013; Shi et al., 2014). A few notable studies include: a consolidated hydrolysis/fermentation bioprocessing system utilizing rice straw and achieving a 60.7% selectivity for butyric acid (Chi et al., 2018), and systems with contiuous acid extraction to reduce the inhibitory effect of the organic acid products (Table 1; Baroi et al., 2015b; Nelson et al., 2019). Co-fermentations with other bacteria have also been leveraged to capitalize on different metabolic machinery without cloning. Using C. tyrobutyricum to supply C beijerinckii with organic acids for re-uptake has had success with several different hydrolysates, yielding target biochemicals like n-butanol, isopropanol, and butyl-butyrate (Table 1; Li et al., 2013; Zhang et al., 2016; Cui et al., 2020). Additionally, C tyrobutyricum has benefited from the levansucrase enzyme in the Bacillus SGP1 strain, which has the capacity to hydrolyze sucrose and provides convertible monomers for C tyrobutyricum to upgrade into butyric acid (Table 1; Dwidar et al., 2013).
Other research has examined the impact that media optimization, additives, or novel renewable feedstock on C tyrobutyricum. Diverse strategies include optimizing growth with varying concentrations of glucose or xylose (Table 1; Luo et al., 2017), the enhancing capabilities of small arabinose concentrations on metabolism in sugars media and corncob hydrolysate (Chen et al., 2017), and the optimal ratio of rice straw and brown algae to enhance productivity as both components supplement different sugar needs for optimal growth (Oh et al., 2019b). The addition of additives have also shown some promise in enhancing fermentation yields. One strategy revealed that by supplementing media with 20 g/L glycerol, the selectivity for butyric acid could be enhanced to 98.3% as compared to 69.1% on just sugars (Oh et al., 2019a). The production of butyl-butyrate with an added lipase and 10 g/L butanol was also a success (Zhang Z. T. et al., 2017). Furthermore, the exploration of C. tyrobutyricum’s ability to grow on various renwable substrates has been documented here (Table 1; Lee et al., 2015; Huang et al., 2016a, b; Xiao et al., 2018; Liu S. et al., 2018).
Given the multitude of favorable production traits natively displayed by C. tyrobutyricum (Figure 1), the potential use as a microbial chassis for industrial production of butyrate and additional fuel and chemical intermediates is high. However, to bring to bear the full potential of C. tyrobutyricum as a production host, a series of outstanding hurdles must be addressed. Of particular relevance to lignocellulosic conversion, complete biomass sugar utilization capacity is essential to enable economic and sustainable bioproduction. As noted above, CCR represents a key hurdle in this regard. Repression of xylose utilization has successfully been bypassed via heterologous expression of C. acetobutylicum xylose utilization machinery. However, utilization of arabinose, the second most abundant pentose sugar in plant biomass, as a carbon and/or energy source remains largely unexplored. Heterologous expression of araBAD operon, required for the breakdown of L-arabinose in E. coli, presents a potential means to enable complete arabinose utilization (Chou et al., 2015). Similarly, hydrolysis and utilization of residual oligomeric sugars in lignocellulosic hydrolysates via the expression of bacterial hydrolases presents a potential mechanism to maximize lignocellulosic carbon conversion capacity.
Though some efforts have been made to achieve homo-fermentative production of butyrate, as described above, residual production of alternative fermentative products remains a key issue that must be addressed to avoid complications in downstream purification processes. In particular, acetate, lactate, and formate represent intermediates that must be targeted for deletion in order to achieve higher purity fermentation. These efforts, in turn, must be balanced with cellular energetics to maintain the favorable substrate conversion rates displayed by the wild-type organisms. Related, though the acidotolerance of C. tyrobutyricum is superior to model production hosts, efforts to achieve low pH production (pH < 5) of butyric acid must still be evaluated. Indeed, end product toxicity is currently a limiting factor in wild type C. tyrobutyricum, necessitating the employ of extractive fermentation configurations to achieve high-titer production of butyrate at low pH. As described above, some efforts to enhance end product tolerance have been successfully deployed, however, additional efforts to confer lower pH production capacity and high butyrate tolerance, which would in turn enable more facile product recovery, would further promote C. tyrobutyricum as a favorable production host.
Biosynthesis of butyric acid proceeds through a series of metabolic intermediates that serve as “production nodes” for an array of additional non-native target molecules (Figure 2). Notably, acetoacetyl-CoA presents an entry point for production of diverse industrial molecules. As noted above, C. tyrobutyricum has been rewired to produce acetone (and ultimately, isopropanol) from this node. Further, acetoacetyl-CoA serves as a key intermediate in the mevalonate isoprenoid pathway, which has been leveraged to produce a series of isoprenoid derivatives in model hosts. These molecules present a multitude of applications in fields including medicine, agriculture, cosmetics, and nutrition (Chatzivasileiou et al., 2019). Notably, however, this pathway is both reductant (NADPH)- and ATP-intensive. Downregulation and/or redistribution of flux from the reductant-intensive butyrate biosynthesis pathway (Figure 2) could provide access to the NADPH pool for such metabolic rewiring. However, the butyrate biosynthetic pathway is a key ATP-generating route in C. tyrobutyricum; thus, flux rewiring could be ATP-limiting. Acetoacetyl CoA, and the downstream conversion intermediate 3-hydroxybutyryl-CoA, also present precursors for the biosynthesis of (poly)hydroxybutyrate (PHB), itself a precursor for a range of polymer and biodegradable plastics (Katayama et al., 2018). For example, an alternative biosynthetic route to 3-hydroxybutyrate (3HB) entails incorporation of phosphor-transbutyrylase (ptb) and butyrate kinase (buk) genes, which diverts flux from 3-hydroxybutyryl-CoA to enable two-step conversion to 3HB via a 3HB-P intermediate, notably generating ATP en route to 3HB. Such strategies have led to > 10 g/L titers and biosynthetic rates exceeding 0.25 g/L/h in E. coli (Gao et al., 2002). Alternatively, heterologous expression of acetyl-CoA acetyltransferase and acetoacetyl-CoA reductase present a route to biosynthesis of PHB. This pathway requires a single reducing equivalent, but notably has no ATP requirements, however, at present, evaluation of C. tyrobutyricum intracellular carbon storage in the form of PHB has yet to be explored. Lastly, the crotonyl-CoA metabolic intermediate presents the opportunity for direct production of crotonic acid via heterologous eexpression of a single thioesterase (Garg et al., 2018), as well as a series ethyl of malonyl-CoA derivatives, including a myriad of polyketides (Song et al., 2006; Wilson and Moore, 2012). Thus, the research community has merely begun to explore the potential for C. tyrobutyricum as a production chassis for diverse fuel and chemical intermediates.
All authors contributed equivalently to manuscript writing and editing.
This research was supported by the Department of Energy, Office of Energy Efficiency and Renewable Energy (EERE) under Agreements No. 28598. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akaraonye, E., Keshavarz, T., and Roy, I. (2010). Production of polyhydroxyalkanoates: the future green materials of choice. J. Chem. Technol. Biotechnol. 85, 732–743. doi: 10.1002/jctb.2392
Anton, M. J., Suarez Valles, B., Garcia Hevia, A., and Picinelli Lobo, A. (2014). Aromatic profile of ciders by chemical quantitative, gas chromatography-olfactometry, and sensory analysis. J. Food Sci. 79, S92–S99.
Baek, J. M., Mazumdar, S., Lee, S. W., Jung, M. Y., Lim, J. H., Seo, S. W., et al. (2013). Butyrate production in engineered Escherichia coli with synthetic scaffolds. Biotechnol. Bioeng. 110, 2790–2794.
Baroi, G. N., Baumann, I., Westermann, P., and Gavala, H. N. (2015a). Butyric acid fermentation from pretreated and hydrolysed wheat straw by an adapted Clostridium tyrobutyricum strain. Microb. Biotechnol. 8, 874–882. doi: 10.1111/1751-7915.12304
Baroi, G. N., Skiadas, I. V., Westermann, P., and Gavala, H. N. (2015b). Effect of in situ acids removal on mixed glucose and xylose fermentation by Clostridium tyrobutyricum. AMB Express 5:67.
Borycka-Kiciak, K., Banasiewicz, T., and Rydzewska, G. (2017). Butyric acid - a well-known molecule revisited. Prz. Gastroenterol. 12, 83–89. doi: 10.5114/pg.2017.68342
Chatzivasileiou, A. O., Ward, V., Edgar, S. M., and Stephanopoulos, G. (2019). Two-step pathway for isoprenoid synthesis. Proc. Natl. Acad. Sci. U.S.A. 116, 506–511. doi: 10.1073/pnas.1812935116
Chen, T., Zhang, L., Luo, G., and Yuan, W. (2017). Butyric acid fermentation by Clostridium tyrobutyricum in sugar mixtures and corncob hydrolysate containing arabinose. Bioresources 12, 7931–7942.
Chen, Z. X., and Breitman, T. R. (1994). Tributyrin: a prodrug of butyric acid for potential clinical application in differentiation therapy. Cancer Res. 54, 3494–3499.
Cheng, C., Lin, M., Jiang, W., Zhao, J., Li, W., Yang, S.-T., et al. (2019). Development of an in vivo fluorescence based gene expression reporter system for Clostridium tyrobutyricum. J. Biotechnol. 305, 18–22. doi: 10.1016/j.jbiotec.2019.08.019
Chi, X., Li, J., Wang, X., Zhang, Y., Leu, S. Y., and Wang, Y. (2018). Bioaugmentation with Clostridium tyrobutyricum to improve butyric acid production through direct rice straw bioconversion. Bioresour. Technol. 263, 562–568. doi: 10.1016/j.biortech.2018.04.120
Choi, O., Um, Y., and Sang, B. I. (2012). Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol. Bioeng. 109, 2494–2502. doi: 10.1002/bit.24520
Chou, Y. C., Linger, J., Yang, S., and Zhang, M. (2015). Genetic engineering and improvement of a zymomonas mobilis for arabinose utilization and its performance on pretreated corn stover hydrolyzate. J. Biotechnol. Biomater. 5:179.
Cui, Y., He, J., Yang, K. L., and Zhou, K. (2020). Production of isopropyl and butyl esters by clostridium mono-culture and co-culture. J. Indust. Microbiol. Biotechnol. doi: 10.1007/s10295-020-02279-3
Deutscher, J., Francke, C., and Postma, P. W. (2006). How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70, 939–1031. doi: 10.1128/mmbr.00024-06
Du, Y., Jiang, W., Yu, M., Tang, I. C., and Yang, S. T. (2015). Metabolic process engineering of Clostridium tyrobutyricum Δack-adhE2 for enhanced n-butanol production from glucose: effects of methyl viologen on NADH availability, flux distribution, and fermentation kinetics. Biotechnol. Bioeng. 112, 705–715. doi: 10.1002/bit.25489
Dwidar, M., Kim, S., Jeon, B. S., Um, Y., Mitchell, R. J., and Sang, B. I. (2013). Co-culturing a novel Bacillus strain with Clostridium tyrobutyricum ATCC 25755 to produce butyric acid from sucrose. Biotechnol. Biofuels 6:35. doi: 10.1186/1754-6834-6-35
Dwidar, M., Park, J. Y., Mitchell, R. J., and Sang, B. I. (2012). The future of butyric acid in industry. Sci. World J. 2012:471417.
El-Sakhawy, M., Kamel, S., Salama, A., and Sarhan, H. A. (2014). Carboxymethyl cellulose acetate butyrate: a review of the preparations, properties, and applications. J. Drug Deliv. 2014:575969.
Feng, J., Zhang, J., Wang, P., Jimenez-Bonilla, P., Gu, Y., Zhou, J., et al. (2020). Renewable fatty acid ester production in Clostridium. bioRxiv [Preprint], doi: 10.1101/2020.03.29.014746
Fu, H., Yang, S. T., Wang, M., Wang, J., and Tang, I. C. (2017a). Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing xylose catabolism genes for glucose and xylose co-utilization. Bioresour. Technol. 234, 389–396. doi: 10.1016/j.biortech.2017.03.073
Fu, H., Yu, L., Lin, M., Wang, J., Xiu, Z., Yang, S.-T., et al. (2017b). Metabolic engineering of Clostridium tyrobutyricum for enhanced butyric acid production from glucose and xylose. Metab. Eng. 40, 50–58. doi: 10.1016/j.ymben.2016.12.014
Gao, H. J., Wu, Q., and Chen, G. Q. (2002). Enhanced production of D-(-)-3-hydroxybutyric acid by recombinant Escherichia coli. FEMS Microbiol. Lett. 213, 59–65. doi: 10.1016/s0378-1097(02)00788-7
Garg, S., Wu, H., Clomburg, J. M., and Bennett, G. N. (2018). Bioconversion of methane to C-4 carboxylic acids using carbon flux through acetyl-CoA engineered Methylomicrobium buryatense 5GB1C. Metab. Eng. 48, 175–183. doi: 10.1016/j.ymben.2018.06.001
Guarnieri, M. T., Chou, Y. C., Salvachúa, T., Mohagheghi, A., John, P. C. S., Peterson, D. J., et al. (2017). Metabolic engineering of Actinobacillus succinogenes provides insights into succinic acid biosynthesis. Appl. Environ. Microbiol. 83:AEM.00996-17.
Guo, X., Fu, H., Feng, J., Hu, J., and Wang, J. (2020). Direct conversion of untreated cane molasses into butyric acid by engineered Clostridium tyrobutyricum. Bioresour. Technol. 301:122764. doi: 10.1016/j.biortech.2020.122764
Hamer, H. M., Jonkers, D., Venema, K., Vanhoutvin, S., Troost, F. J., Brummer, R. J., et al. (2008). Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther. 27, 104–119. doi: 10.1111/j.1365-2036.2007.03562.x
He, F., Qin, S., Yang, Z., Bai, X., Suo, Y., Wang, J., et al. (2020). Butyric acid production from spent coffee grounds by engineered Clostridium tyrobutyricum overexpressing galactose catabolism genes. Bioresour. Technol. 304:122977. doi: 10.1016/j.biortech.2020.122977
Heap, J. T., Pennington, O. J., Cartman, S. T., Carter, G. P., and Minton, N. P. (2007). The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70, 452–464. doi: 10.1016/j.mimet.2007.05.021
Heap, J. T., Pennington, O. J., Cartman, S. T., and Minton, N. P. (2009). A modular system for Clostridium shuttle plasmids. J. Microbiol. Methods 78, 79–85. doi: 10.1016/j.mimet.2009.05.004
Henard, C. A., Smith, H., Dowe, N., Kalyuzhnaya, M. G., Pienkos, P. T., Guarieri, M. T., et al. (2016). Bioconversion of methane to lactate by an obligate methanotrophic bacterium. Sci. Rep. 6:21585.
Huang, J., Dai, H., Yan, R., and Wang, P. (2016a). Butyric acid production from recycled waste paper by immobilized Clostridium tyrobutyricum in a fibrous-bed bioreactor. J. Chem. Technol. Biotechnol. 91, 1048–1054. doi: 10.1002/jctb.4680
Huang, J., Zhu, H., Tang, W., Wang, P., and Yang, S. T. (2016b). Butyric acid production from oilseed rape straw by Clostridium tyrobutyricum immobilized in a fibrous bed bioreactor. Process Biochem. 51, 1930–1934. doi: 10.1016/j.procbio.2016.08.019
Huang, J., Du, Y., Bao, T., Lin, M., Wang, J., and Yang, S. T. (2019). Production of n-butanol from cassava bagasse hydrolysate by engineered Clostridium tyrobutyricum overexpressing adhE2: kinetics and cost analysis. Bioresour. Technol. 292:121969. doi: 10.1016/j.biortech.2019.121969
Huang, Y. L., Wu, Z., Zhang, L., Cheung, C. M., and Yang, S. T. (2002). Production of carboxylic acids from hydrolyzed corn meal by immobilized cell fermentation in a fibrous-bed bioreactor. Bioresour. Technol. 82, 51–59. doi: 10.1016/s0960-8524(01)00151-1
Huo, X., Nabila, H., James, J., Nicholas, S., Anne, S., Amy, S., et al. (2019). Tailoring diesel bioblendstock from integrated catalytic upgrading of carboxylic acids: a “fuel property first” approach. Green Chem. 21, 5813–5827. doi: 10.1039/c9gc01820d
Jault, J. M., Fieulaine, S., Nessler, S., Gonzalo, P., Di Pietro, A., Deutscher, J., et al. (2000). The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose 1,6-bisphosphate binding. J. Biol. Chem. 275, 1773–1780. doi: 10.1074/jbc.275.3.1773
Jawed, K., Mattam, A. J., Fatma, Z., Wajid, S., Abdin, M. Z., Yazdani, S. S., et al. (2016). Engineered production of short chain fatty acid in Escherichia coli using fatty acid synthesis pathway. PLoS One 11:e0160035. doi: 10.1371/journal.pone.0160035
Jiang, L., Fu, H., Yang, H. K., Xu, W., Wang, J., Yang, S. T., et al. (2018). Butyric acid: applications and recent advances in its bioproduction. Biotechnol. Adv. 36, 2101–2117. doi: 10.1016/j.biotechadv.2018.09.005
Jiang, L., Li, S., Hu, Y., Xu, Q., and Huang, H. (2012). Adaptive evolution for fast growth on glucose and the effects on the regulation of glucose transport system in Clostridium tyrobutyricum. Biotechnol. Bioeng. 109, 708–718. doi: 10.1002/bit.23346
Jiang, L., Wang, J., Liang, S., Cai, J., Xu, Z., Cen, P., et al. (2011). Enhanced butyric acid tolerance and bioproduction by Clostridium tyrobutyricum immobilized in a fibrous bed bioreactor. Biotechnol. Bioeng. 108, 31–40. doi: 10.1002/bit.22927
Jiang, L., Wu, Q., Xu, Q., Zhu, L., and Huang, H. (2017). Fermentative hydrogen production from Jerusalem artichoke by Clostridium tyrobutyricum expressing exo-inulinase gene. Sci. Rep. 7:7940.
Jiang, L., Zhu, L., Xu, X., Li, Y., Li, S., and Huang, H. (2013). Genome sequence of Clostridium tyrobutyricum ATCC 25755, a butyric acid-overproducing strain. Genome Announc. 1:e00308-13.
Jo, J. H., Jeon, C. O., Lee, S. Y., Lee, D. S., and Park, J. M. (2010). Molecular characterization and homologous overexpression of [FeFe]-hydrogenase in Clostridium tyrobutyricum JM1. Intern. J. Hydro. Energy 35, 1065–1073. doi: 10.1016/j.ijhydene.2009.11.102
Karp, E. M., Eaton, T. R., Sànchez, I., Nogué, V., Vorotnikov, V., Biddy, M. J., et al. (2017). Renewable acrylonitrile production. Science 358, 1307–1310.
Kataoka, N., Vangnai, A. S., Pongtharangkul, T., Yakushi, T., and Matsushita, K. (2017). Butyrate production under aerobic growth conditions by engineered Escherichia coli. J. Biosci. Bioeng. 123, 562–568. doi: 10.1016/j.jbiosc.2016.12.008
Katayama, N., Iijima, H., and Osanai, T. (2018). Production of bioplastic compounds by genetically manipulated and metabolic engineered cyanobacteria. Adv. Exp. Med. Biol. 1080, 155–169. doi: 10.1007/978-981-13-0854-3_7
Kim, S. J., Kim, J. W., Lee, Y. G., Park, Y. C., and Seo, J. H. (2017). Metabolic engineering of Saccharomyces cerevisiae for 2,3-butanediol production. Appl. Microbiol. Biotechnol. 101, 2241–2250.
Klijn, N., Nieuwenhof, F. F., Hoolwerf, J. D., van der Waals, C. B., and Weerkamp, A. H. (1995). Identification of Clostridium tyrobutyricum as the causative agent of late blowing in cheese by species-specific PCR amplification. Appl. Environ. Microbiol. 61, 2919–2924. doi: 10.1128/aem.61.8.2919-2924.1995
Leavitt, J., Barrett, J. C., Crawford, B. D., and Ts’o, P. O. (1978). Butyric acid suppression of the in vitro neoplastic state of Syrian hamster cells. Nature 271, 262–265. doi: 10.1038/271262a0
Leber, C., and Da Silva, N. A. (2014). Engineering of Saccharomyces cerevisiae for the synthesis of short chain fatty acids. Biotechnol. Bioeng. 111, 347–358. doi: 10.1002/bit.25021
Lee, J., Jang, Y. S., Han, M. J., Kim, J. Y., and Lee, S. Y. (2016). Deciphering Clostridium tyrobutyricum metabolism based on the whole-genome sequence and proteome analyses. mBio 7:e00743-16.
Lee, J., Yun, H., Feist, A. M., Palsson, B. O., and Lee, S. Y. (2008). Genome-scale reconstruction and in silico analysis of the Clostridium acetobutylicum ATCC 824 metabolic network. Appl. Microbiol. Biotechnol. 80, 849–862. doi: 10.1007/s00253-008-1654-4
Lee, K. M., Kim, K. Y., Choi, O., Woo, H. M., Kim, Y., Han, S. O., et al. (2015). In situ detoxification of lignocellulosic hydrolysate using a surfactant for butyric acid production by Clostridium tyrobutyricum ATCC 25755. Process Biochem. 50, 630–635. doi: 10.1016/j.procbio.2015.01.020
Lee, W. S., Chua, A. S. M., Yeoh, H. K., and Ngoh, G. C. (2014). A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 235, 83–99. doi: 10.1016/j.cej.2013.09.002
Li, L., Ai, H., Zhang, S., Li, S., Liang, Z., Wu, Z. Q., et al. (2013). Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour. Technol. 143, 397–404. doi: 10.1016/j.biortech.2013.06.023
Lim, J. H., Seo, S. W., Kim, S. Y., and Jung, G. Y. (2013). Refactoring redox cofactor regeneration for high-yield biocatalysis of glucose to butyric acid in Escherichia coli. Bioresour. Technol. 135, 568–573. doi: 10.1016/j.biortech.2012.09.091
Liu, C. L., Tian, T., Alonso-Gutierrez, J., Garabedian, B., Wang, S., Baidoo, E. E. K., et al. (2018). Renewable production of high density jet fuel precursor sesquiterpenes from Escherichia coli. Biotechnol. Biofuels 11:285.
Liu, S., Duncan, S., Qureshi, N., and Rich, J. (2018). Fermentative production of butyric acid from paper mill sludge hydrolysates using Clostridium tyrobutyricum NRRL B-67062/RPT 4213. Biocatal. Agric. Biotechnol. 14, 48–51. doi: 10.1016/j.bcab.2018.02.002
Liu, X., Zhu, Y., and Yang, S. T. (2006). Construction and characterization of ack deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid and hydrogen production. Biotechnol. Prog. 22, 1265–1275. doi: 10.1021/bp060082g
Liu, Y., Geng, Y., Zhou, Q., and Yuan, W. (2013). The effect of furfural and 5-hydroxymethyl furfural on butyric acid fermentation by Clostridium tyrobutyricum. J. Chem. Technol. Biotechnol. 93, 849–854. doi: 10.1002/jctb.5439
Luo, G., Zhang, L., Chen, T., Wuan, W., and Geng, Y. (2017). Butyric acid fermentation in xylose and glucose by Clostridium tyrobutyricum. Bioresources 12, 2930–2940.
Ma, C., Ou, J., Xu, N., Fierst, J. L., Yang, S. T., and Liu, X. (2016). Rebalancing redox to improve biobutanol production by Clostridium tyrobutyricum. Bioengineering 3:2. doi: 10.3390/bioengineering3010002
Ma, Y., Deng, Q., Du, Y., Ren, J., Chen, Y., Liu, X., et al. (2020). Biosynthetic pathway for ethyl butyrate production in Saccharomyces cerevisiae. J. Agric. Food Chem. 68, 4252–4260. doi: 10.1021/acs.jafc.0c00750
McCauley, L. A. (2009). Epidemiology of chemical warfare agents. Handb. Toxicol. Chem. Warfare Agents 2009, 47–54. doi: 10.1016/b978-0-12-800159-2.00006-3
Medoff, M., Masterman, T. C., and Paradis, R. (2019). Processing Hydroxy-Carboxylic Acids To Polymers. Wakefield, MA: Xyleco Inc.
Mills, T. Y., Sandoval, N. R., and Gill, R. T. (2009). Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol. Biofuels 2:26. doi: 10.1186/1754-6834-2-26
Nelson, R. S., Peterson, D. J., Saboe, P., Karp, E. M., Beckham, G. T., Linger, J. G., et al. (2019). Production of a Biofuel Intermediate from Biomass Sugars by Clostridium tyrobutyricum (No. NREL/PO-5100-73786). Golden, CO: National Renewable Energy Lab.
Oh, H. J., Kim, K. Y., Lee, K. M., Lee, S. M., Gong, G., Oh, M. K., et al. (2019a). Butyric acid production with high selectivity coupled with acetic acid consumption in sugar-glycerol mixture fermentation by Clostridium tyrobutyricum ATCC25755. J. Industr. Eng. Chem. 75, 44–51. doi: 10.1016/j.jiec.2019.01.047
Oh, H. J., Kim, K. Y., Lee, K. M., Lee, S. M., Gong, G., Oh, M. K., et al. (2019b). Enhanced butyric acid production using mixed biomass of brown algae and rice straw by Clostridium tyrobutyricum ATCC25755. Bioresour. Technol. 273, 446–453. doi: 10.1016/j.biortech.2018.11.037
Ohara, T., and Mori, T. (2019). Antiproliferative effects of short-chain fatty acids on human colorectal cancer cells via gene expression inhibition. Anticancer. Res. 39, 4659–4666. doi: 10.21873/anticanres.13647
Ohara, T., and Suzutani, T. (2018). Intake of Bifidobacterium longum and fructo-oligosaccharides prevents colorectal carcinogenesis. Euroas. J. Hepatogastroenterol. 8, 11–17. doi: 10.5005/jp-journals-10018-1251
Playne, M. J. (1985). “Propionic and butyric acids,” in Comprehensive Biotechnology, ed. M. Moo-Young, (London: Pergamon), 731–759.
Ra, C. H., Sunwoo, I. Y., Nguyen, T. H., Sukwong, P., Sirisuk, P., Jeong, G. T., et al. (2019). Butanol and butyric acid production from Saccharina japonica by Clostridium acetobutylicum and Clostridium tyrobutyricum with adaptive evolution. Bioprocess Biosyst. Eng. 42, 583–592. doi: 10.1007/s00449-018-02063-9
Rebroš, M., Dolejš, I., Stloukal, R., and Rosenberg, M. (2016). Butyric acid production with Clostridium tyrobutyricum immobilised to PVA gel. Process Biochem. 51, 704–708. doi: 10.1016/j.procbio.2016.03.003
Ren, C., Gu, Y., Wu, Y., Zhang, W., Yang, C., Yang, S., et al. (2012). Pleiotropic functions of catabolite control protein CcpA in butanol-producing Clostridium acetobutylicum. BMC Genomics 13:349. doi: 10.1186/1471-2164-13-349
Rephaeli, A., Zhuk, R., and Nudelman, A. (2000). Prodrugs of butyric acid from bench to bedside: synthetic design, mechanisms of action, and clinical applications. Drug Dev. Res. 50, 379–391. doi: 10.1002/1098-2299(200007/08)50:3/4<379::aid-ddr20>3.0.co;2-8
Rodionov, D. A., Mironov, A. A., and Gelfand, M. S. (2001). Transcriptional regulation of pentose utilisation systems in the Bacillus/Clostridium group of bacteria. FEMS Microbiol. Lett. 205, 305–314. doi: 10.1111/j.1574-6968.2001.tb10965.x
Saini, M., Wang, Z. W., Chiang, C. J., and Chao, Y. P. (2014). Metabolic engineering of Escherichia coli for production of butyric acid. J. Agric. Food Chem. 62, 4342–4348.
Sauer, M. (2016). Industrial production of acetone and butanol by fermentation-100 years later. FEMS Microbiol. Lett. 363:fnw134. doi: 10.1093/femsle/fnw134
Senger, R. S., and Papoutsakis, E. T. (2008). Papoutsakis, genome-scale model for Clostridium acetobutylicum: part I. Metabolic network resolution and analysis. Biotechnol. Bioeng. 101, 1036–1052. doi: 10.1002/bit.22010
Seregina, T. A., Shakulov, R. S., Debanov, V. G., and Mironov, A. S. (2010). Construction of a butyrate-producing E. coli strain without use of heterologous genes. Appl. Biochem. Microbiol. 46, 745–754. doi: 10.1134/s000368381008003x
Shi, Z., Huang, L., Wu, X., Luo, L., Xiao, K., Cai, J., et al. (2014). Long-term production of butyric acid through immobilization of Clostridium tyrobutyricum in a moving fibrous-bed bioreactor (MFBB). J. Chem. Technol. Biotechnol. 89, 1883–1889. doi: 10.1002/jctb.4271
Sjoblom, M., Matsakas, L., Christakopoulos, P., and Rova, U. (2016). Catalytic upgrading of butyric acid towards fine chemicals and biofuels. FEMS Microbiol. Lett. 363:fnw064. doi: 10.1093/femsle/fnw064
Song, L., Barona-Gomez, F., Corree, C., Xiang, L., Udwary, D. W., Austin, M. B., et al. (2006). Type II Polyketide synthase beta-ketoacyl-ACP starter unit and ethylmalonyl-CoA extender unit selectivity discovered by Streptomyces coelicolor genome mining. J. Am. Chem. Soc. 128, 14754–14755. doi: 10.1021/ja065247w
Suo, Y., Fu, H., Ren, M., Liao, Z., Ma, Y., Wang, J., et al. (2018a). Enhanced butyric acid production in Clostridium tyrobutyricum by overexpression of rate-limiting enzymes in the embden-meyerhof-parnas pathway. J. Biotechnol. 272–273, 14–21. doi: 10.1016/j.jbiotec.2018.02.012
Suo, Y., Fu, H., Ren, M., Yang, X., Liao, Z., Wang, J., et al. (2018b). Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing Class I heat shock protein GroESL. Bioresour. Technol. 250, 691–698. doi: 10.1016/j.biortech.2017.11.059
Suo, Y., Ren, M., Yang, X., Liao, Z., Fu, H., Wang, J., et al. (2018c). Metabolic engineering of Clostridium tyrobutyricum for enhanced butyric acid production with high butyrate/acetate ratio. Appl. Microbiol. Biotechnol. 102, 4511–4522. doi: 10.1007/s00253-018-8954-0
Suo, Y., Luo, S., Zhang, Y., Liao, Z., and Wang, J. (2017). Enhanced butyric acid tolerance and production by Class I heat shock protein-overproducing Clostridium tyrobutyricum ATCC 25755. J. Ind. Microbiol. Biotechnol. 44, 1145–1156. doi: 10.1007/s10295-017-1939-7
Volker, A. R., Gogerty, D. S., Bartholomay, C., Hennen-Bierwagen, T., and Zhu, H. (2014). Fermentative production of short-chain fatty acids in Escherichia coli. Microbiology 160, 1513–1522. doi: 10.1099/mic.0.078329-0
Wang, L., Chauliac, D., Moritz, B. E., Zhang, G., Ingram, L. O., Shanmugam, K. T., et al. (2019). Metabolic engineering of Escherichia coli for the production of butyric acid at high titer and productivity. Biotechnol. Biofuels 12:62.
Wei, D., Liu, X., and Yang, S. T. (2013). Butyric acid production from sugarcane bagasse hydrolysate by Clostridium tyrobutyricum immobilized in a fibrous-bed bioreactor. Bioresour. Technol. 129, 553–560. doi: 10.1016/j.biortech.2012.11.065
Weiland, P. (2010). Biogas production: current state and perspectives. Appl. Microbiol. Biotechnol. 85, 849–860. doi: 10.1007/s00253-009-2246-7
Werpy, T., and Petersen, G. (2004). Top Value Added Chemicals from Biomass: Volume I - Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Washington, DC: United States Department of Energy.
Wilson, M. C., and Moore, B. S. (2012). Beyond ethylmalonyl-CoA: the functional role of crotonyl-CoA carboxylase/reductase homologs in expanding polyketide diversity. Nat. Prod. Rep. 29, 72–86. doi: 10.1039/c1np00082a
Wu, Q., Xian, B., Guo, W.-Q., Wang, B., Li, Y., Luo, H., et al. (2019). Medium chain carboxylic acids production from waste biomass: current advances and perspectives. Biotechnol. Adv. 37, 599–615. doi: 10.1016/j.biotechadv.2019.03.003
Wu, Q., Zhu, L., Xu, Q., Huang, H., Jiang, L., and Yang, S. T. (2017). Tailoring the oxidative stress tolerance of Clostridium tyrobutyricum CCTCC W428 by introducing trehalose biosynthetic capability. J. Agric. Food Chem. 65, 8892–8901. doi: 10.1021/acs.jafc.7b03172
Wu, Z., and Yang, S. T. (2003). Extractive fermentation for butyric acid production from glucose by Clostridium tyrobutyricum. Biotechnol. Bioeng. 82, 93–102. doi: 10.1002/bit.10542
Xiao, Z. Z., Cheng, C., Bao, T., Liu, L., Wang, B., Tao, W., et al. (2018). Production of butyric acid from acid hydrolysate of corn husk in fermentation by Clostridium tyrobutyricum: kinetics and process economic analysis. Biotechnol. Biofuels 11:164.
Xin, B., Tao, F., Wang, Y., Gao, C., Ma, C., Xu, P., et al. (2013). Genome sequence of Clostridium butyricum strain DSM 10702, a promising producer of biofuels and biochemicals. Genome Announc. 1:e00563-13.
Yu, L., Xu, M., Tang, I. C., and Yang, S. T. (2015a). Metabolic engineering of Clostridium tyrobutyricum for n-butanol production from maltose and soluble starch by overexpressing alpha-glucosidase. Appl. Microbiol. Biotechnol. 99, 6155–6165. doi: 10.1007/s00253-015-6680-4
Yu, L., Xu, M., Tang, I. C., and Yang, S. T. (2015b). Metabolic engineering of Clostridium tyrobutyricum for n-butanol production through co-utilization of glucose and xylose. Biotechnol. Bioeng. 112, 2134–2141. doi: 10.1002/bit.25613
Yu, L., Zhao, J., Xu, M., Dong, J., Varghese, S., Yu, M., et al. (2015c). Metabolic engineering of Clostridium tyrobutyricum for n-butanol production: effects of CoA transferase. Appl. Microbiol. Biotechnol. 99, 4917–4930. doi: 10.1007/s00253-015-6566-5
Yu, M., Zhang, Y., Tang, I. C., and Yang, S. T. (2011). Metabolic engineering of Clostridium tyrobutyricum for n-butanol production. Metab. Eng. 13, 373–382. doi: 10.1016/j.ymben.2011.04.002
Zhang, C., Yang, H., Yang, F., and Ma, Y. (2009). Current progress on butyric acid production by fermentation. Curr. Microbiol. 59, 656–663. doi: 10.1007/s00284-009-9491-y
Zhang, J., Hong, W., Guo, L., Wang, Y., and Wang, Y. (2020). Enhancing plasmid transformation efficiency and enabling CRISPR-Cas9/Cpf1-based genome editing in Clostridium tyrobutyricum. Biotechnol. Bioeng. doi: 10.1002/bit.27435
Zhang, J., Yu, L., Xu, M., Yang, S. T., Yan, Q., Lin, M., et al. (2017). Metabolic engineering of Clostridium tyrobutyricum for n-butanol production from sugarcane juice. Appl. Microbiol. Biotechnol. 101, 4327–4337. doi: 10.1007/s00253-017-8200-1
Zhang, J., Zong, W., Hong, W., Zhang, Z. T., and Wang, Y. (2018). Exploiting endogenous CRISPR-Cas system for multiplex genome editing in Clostridium tyrobutyricum and engineer the strain for high-level butanol production. Metab. Eng. 47, 49–59. doi: 10.1016/j.ymben.2018.03.007
Zhang, S., Qu, C., Huang, X., Suo, Y., Liao, Z., and Wang, J. (2016). Enhanced isopropanol and n-butanol production by supplying exogenous acetic acid via co-culturing two clostridium strains from cassava bagasse hydrolysate. J. Indust. Microbiol. Biotechnol. 43, 915–925. doi: 10.1007/s10295-016-1775-1
Zhang, Y., Yu, M., and Yang, S. T. (2012). Effects of ptb knockout on butyric acid fermentation by Clostridium tyrobutyricum. Biotechnol. Prog. 28, 52–59. doi: 10.1002/btpr.730
Zhang, Z. T., Taylor, S., and Wang, Y. (2017). In situ esterification and extractive fermentation for butyl butyrate production with Clostridium tyrobutyricum. Biotechnol. Bioeng. 114, 1428–1437. doi: 10.1002/bit.26289
Zhou, X., Lu, X. H., Li, X. H., Xin, Z. J., Xie, J. R., Zhao, M. R., et al. (2014a). Radiation induces acid tolerance of Clostridium tyrobutyricum and enhances bioproduction of butyric acid through a metabolic switch. Biotechnol. Biofuels 7:22. doi: 10.1186/1754-6834-7-22
Zhou, X., Wang, S. Y., Lu, X. H., and Liang, J. P. (2014b). Comparison of the effects of high energy carbon heavy ion irradiation and Eucommia ulmoides Oliv. on biosynthesis butyric acid efficiency in Clostridium tyrobutyricum. Bioresour. Technol. 161, 221–229. doi: 10.1016/j.biortech.2014.03.039
Zhou, X., Yang, Z., Jiang, T.-T., Wang, S.-Y., Liang, J.-P., Lu, X.-H., et al. (2016). The acquisition of Clostridium tyrobutyricum mutants with improved bioproduction under acidic conditions after two rounds of heavy-ion beam irradiation. Sci. Rep. 6:29968.
Zhu, Y., Liu, X., and Yang, S. T. (2005). Construction and characterization of pta gene-deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid fermentation. Biotechnol. Bioeng. 90, 154–166. doi: 10.1002/bit.20354
Zhu, Y., and Yang, S. T. (2003). Adaptation of Clostridium tyrobutyricum for enhanced tolerance to butyric acid in a fibrous-bed bioreactor. Biotechnol. Prog. 19, 365–372. doi: 10.1021/bp025647x
Keywords: bioenergy, carboxylic acid, metabolic engineering, biocatalysis, butyric acid, Clostridium tyrobutyricum ATCC 25755
Citation: Linger JG, Ford LR, Ramnath K and Guarnieri MT (2020) Development of Clostridium tyrobutyricum as a Microbial Cell Factory for the Production of Fuel and Chemical Intermediates From Lignocellulosic Feedstocks. Front. Energy Res. 8:183. doi: 10.3389/fenrg.2020.00183
Received: 26 April 2020; Accepted: 10 July 2020;
Published: 07 August 2020.
Edited by:
Sachin Kumar, Sardar Swaran Singh National Institute of Renewable Energy, IndiaReviewed by:
Keikhosro Karimi, Isfahan University of Technology, IranCopyright © 2020 Linger, Ford, Ramnath and Guarnieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael T. Guarnieri, bWljaGFlbC5ndWFybmllcmlAbnJlbC5nb3Y=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.