- 1Faculty of Engineering and Natural Sciences, Tampere University, Tampere, Finland
- 2Microbial Ecology Laboratory, School of Natural Sciences, National University of Ireland Galway, Galway, Ireland
- 3Microbial Communities Laboratory, School of Natural Sciences, National University of Ireland Galway, Galway, Ireland
- 4IHE Delft Institute for Water Education, Delft, Netherlands
- 5NVP Energy Ltd., IDA Technology Park, Galway, Ireland
Lipid-containing wastewaters, such as those arising from dairy processing, are frequently discharged at temperatures ≤ 20°C. Their valorization at low ambient temperatures offers opportunities to expand the application of high-rate anaerobic wastewater treatment toward achieving energy neutrality by minimizing the energy demand for heating. Lipid hydrolysis generates long-chain fatty acids (LCFAs), which incur operational challenges and hinder stable bioreactor operation by inducing sludge flotation and washout, coupled with the added challenge of treatment at lower temperature (20°C). These challenges are tackled together uniquely during the treatment of LCFA-rich synthetic dairy wastewater (SDW) (33% COD-LCFA) through de novo formed microbial granular sludge within the dynamic sludge chamber–fixed film (DSC-FF) reactor. The novel reactor design facilitated sludge retention for the entire operational period of 150 days by containing settled, flotating, and LCFA-encapsulated granular sludge and biofilm within a single module. High COD removal efficiencies (87–98%) were achieved in the three replicated DSC-FF reactors, along with complete LCFA removal at 18–72 h HRT (LCFA loading rate of 220–890 mgCOD-LCFA/L⋅day) and partial LCFA removal at 12 h HRT (LCFA loading rate of 1333 mgCOD-LCFA/L⋅day). The high removal efficiencies of unsaturated and saturated LCFAs achieved are reported for the first time during continuous anaerobic wastewater treatment at low temperatures (20°C). Moreover, de novo granulation was achieved within 8 days from a combination of inoculum mixtures at a high LCFA concentration (33% COD-LCFA) in SDW. The results demonstrate the feasibility of the DSC-FF reactor for treating LCFA-rich wastewaters at discharge temperatures and offer potential for expanded and more energetically productive anaerobic valorization of lipid-rich wastewater.
Introduction
The United Nations 2030 Agenda for Sustainable Development necessitates the development of sustainable wastewater treatment and resource recovery systems to successfully attain the goals targeted for the focus areas of clean water and sanitation (SDG 6), renewable energy (SDG 7), sustainable communities (SDG 11), and climate action (SDG 13) (United Nations, 2015). High-rate anaerobic digestion (AD) is a sustainable option for the biological treatment of municipal and industrial wastewaters, and is widely applied in bioreactors under mesophilic (approximately 30–40°C) or thermophilic (approximately 45–60°C) conditions (Batstone and Jensen, 2011). The extension of AD technologies for application at cooler temperatures (≤20°C) is an important innovation in improving the net energy recovery from wastewater treatment, especially in temperate climates where low ambient air temperatures decrease the wastewater temperatures (McHugh et al., 2003). Lipid-containing wastewaters, including a variety of dairy waste streams, are emitted in large quantities at low ambient temperatures and are energy-dense (theoretically, 1.43 L-CH4/g-lipid); thus, opening opportunities for high-potential valorization through bio-methanization (Alves et al., 2009). Anaerobic treatment of such high-volume wastewaters at discharge temperatures would steer the treatment processes toward the achievement of energy neutrality (Martin et al., 2011; Petropoulos et al., 2019b). Thus, it is important to develop high-rate processes for the anaerobic treatment of fat, oil, and grease (FOG)-rich wastewaters at low ambient temperatures.
However, the anaerobic treatment of lipid-rich streams is problematic, since their hydrolysis produces long-chain fatty acids (LCFAs) that can destabilize anaerobic treatment due to physicochemical and microbial inhibition (Lalman and Bagley, 2002; Zheng et al., 2005; Davidsson et al., 2008; Alves et al., 2009; Desbois and Smith, 2010; Sun et al., 2013; Zhou et al., 2013). In high-rate anaerobic reactors, such as upflow anaerobic sludge bed (UASB) reactors, the accumulation of LCFAs produced from lipid degradation has been associated with operational challenges including the sludge flotation and washout, scum layer formation, and substrate diffusion limitation through LCFA-encapsulated sludge (Rinzema et al., 1994; Pereira et al., 2005). Moreover, LCFAs behave as surfactants at neutral pH (Sam-Soon et al., 1991) and disrupt the structure of the anaerobic granules, consequently aggravating the granular sludge washout. Hence, the anaerobic treatment of lipid- and LCFA-rich wastewaters warrants the development of high microbial activity, stable microbial structures, and enhanced sludge retention in reactors, especially at low temperatures with slow microbial growth rates, along with the characterization of LCFA profiles to assess the efficacy of reactor design in the removal of different LCFAs.
Strategies for improved sludge retention for treating lipid-rich wastewaters have included (i) forming biofilms on support material in anaerobic filters, fixed-bed reactors, anaerobic baffled reactors, and moving-bed biofilm reactors (Alves et al., 2001; Pereira et al., 2002; Kim et al., 2004; Biswas and Turner, 2012; Fujihira et al., 2018); (ii) sludge flotation in specialized reactor designs, such as anaerobic flotation reactor (AFRs; Paques, Netherlands) and inverted anaerobic sludge bed (IASB) reactors (Alves et al., 2007); (iii) application of membrane bioreactor (MBR) systems (Ramos et al., 2014; Dereli et al., 2015; Jensen et al., 2015); and (iv) granular sludge reactors (Hwu et al., 1998a; Saatci et al., 2003; Jeganathan et al., 2006; Leal et al., 2006; Passeggi et al., 2009). Of these reactor designs, indeed, various single- and two-stage reactor systems have been employed to treat dairy wastewaters at lower temperatures (5–20°C) (Table 1; Toldrá et al., 1987; Viraraghavan and Kikkeri, 1990; Dague et al., 1998; Ramasamy and Abbasi, 2000; Luostarinen and Rintala, 2005; Park et al., 2012; Buntner et al., 2013; Nikolaeva et al., 2013; Bialek et al., 2014; Zielińska et al., 2018), but these studies used non-fat dry milk substrates (≤3% lipid-COD) which are not representative of the typical dairy wastewaters that have a high lipid content of 0.1–0.5 g/L (28–35% COD basis) (Szabo-Corbacho et al., 2019; Holohan, 2020).
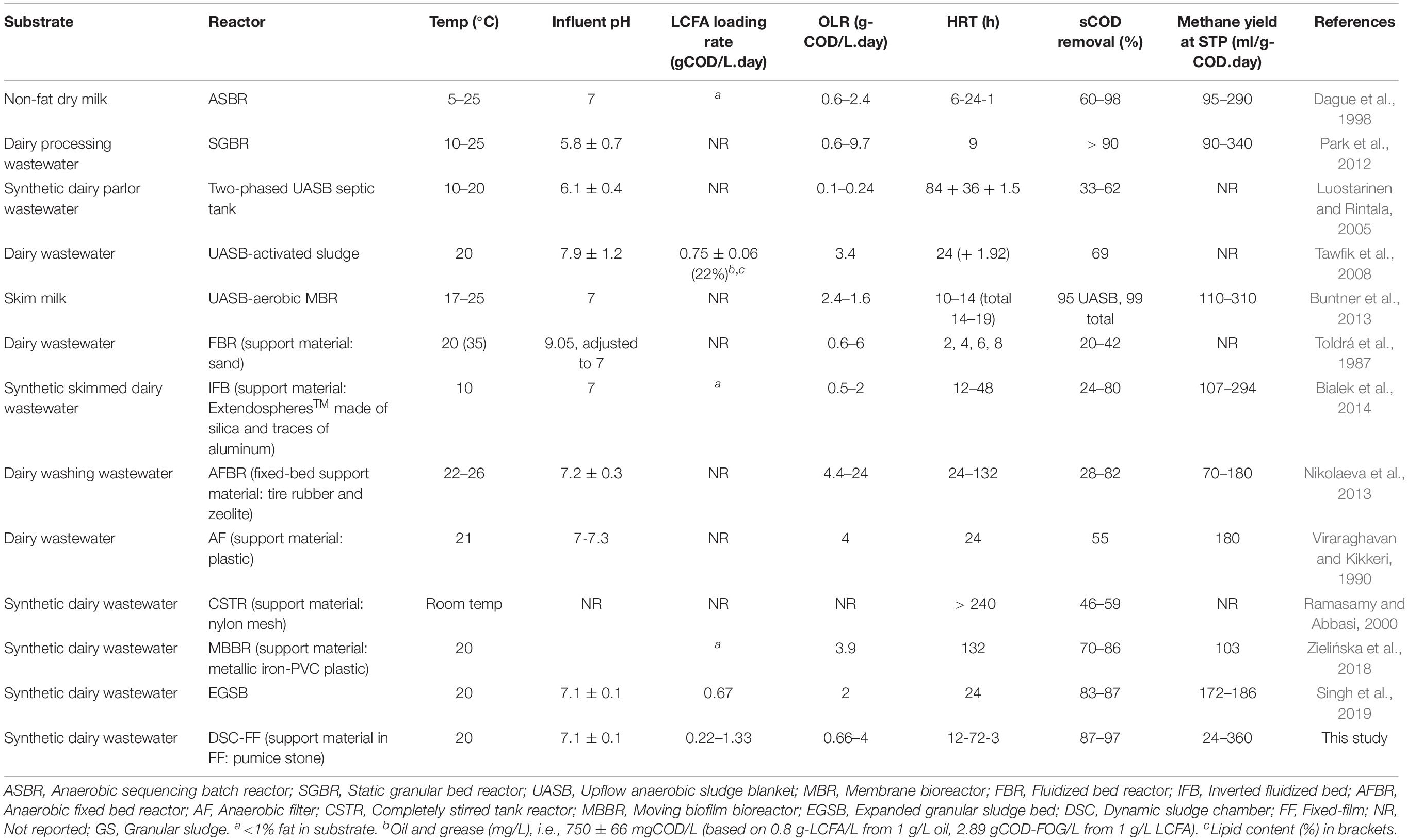
Table 1. Process performance of anaerobic dairy wastewater treatment in adhered-film and granular sludge reactors at psychrophilic and low ambient temperatures.
Up until recently, the high-rate anaerobic treatment of LCFAs has been studied extensively at mesophilic or thermophilic conditions (e.g., Hwu et al., 1998a; Kim et al., 2004; Jeganathan et al., 2006; Ramos et al., 2014; Dereli et al., 2015; Cavaleiro et al., 2016; Duarte et al., 2018; Szabo-Corbacho et al., 2019) but not at low ambient temperatures. An evaluation of the anaerobic LCFA treatment at low temperatures will bring new insights into the treatment of lipid-rich wastewaters. We recently showed the feasibility of anaerobic treatment of LCFA-containing wastewater (with 33% LCFA-COD) at 20°C in expanded granular sludge bed (EGSB) reactors (Singh et al., 2019). The process performance was stable for around 60 days, but prolonged operation involved significant sludge flotation and washout in EGSB reactors influenced by the LCFA loading rates and the LCFA concentrations in complex substrate (Singh et al., 2019). High COD removal (>99%) and methane yield efficiencies (MYE) (89–91%) have been achieved during the treatment of lipid-rich dairy wastewater in MBR (HRT = 53 h) at 35°C (Szabo-Corbacho et al., 2019), demonstrating the suitability of MBR for FOG methanization at mesophilic conditions. Conversely, at psychrophilic conditions (15°C), the MBR has been reported to be unsuitable for lipid treatment due to a high accumulation and the fouling propensity of the lipids (Petropoulos et al., 2019b). The hybrid reactor designs combining the sludge retention principles of UASBs or EGSBs with additional features, such as a biofilm compartment, support improved process performance (e.g., at 20°C for digestion of whey wastewater; McHugh et al., 2006) and may be applied to develop innovative reactor configuration for treating LCFA-rich wastewater at discharge temperature.
The objective of this study was to evaluate for the first time the high-rate anaerobic treatment of synthetic dairy wastewater (SDW) rich in mixed-LCFA at 20°C through the dynamic sludge chamber–fixed film (DSC-FF) reactor configuration. The fate of individual LCFAs at high LCFA loading rates was assessed for the first time at discharge temperatures in FOG-rich wastewaters. The reactors were seeded with a mixture of sludges with distinct capability for LCFA degradation and high acetotrophic and methanogenic activities to engineer a microbial consortium suitable for high-rate low-temperature treatment of SDW in the form of a de novo granular sludge.
Materials and Methods
Inoculum and Synthetic Wastewater
Two anaerobic sludges were sourced, granular sludge from a Lt-AD® reactor (NVP Energy Limited, Galway, Ireland) treating dairy wastewater from Arrabawn Dairies (Kilconnell, Ireland) and flocculent sludge from a ADI-BVF® reactor (ADI Systems, Evoqua) treating FOG-containing dairy effluents from the Dairygold Co-Operative Society (Mitchelstown, Ireland) and stored for 2 weeks at 7°C in a nitrogen-purged atmosphere prior to usage. The sludges were mixed in a 1:1 ratio [by volatile solids (VS)].
SDW (2 gCOD/L) was prepared using skimmed milk powder and a LCFA mixture (palmitate, stearate, oleate, and linoleate in a COD ratio of 3:1.5:4.5:1) in a COD ratio of 2:1. The SDW was supplemented with 2 g/L NaHCO3 and 1 ml/L basal nutrient solution (Singh et al., 2019).
Reactor Design and Experimental Set-Up
Laboratory-scale (7 L) glass reactors (Figure 1) are composed of two operational sections, i.e., a dynamic sludge chamber (DSC) consisting of a granular sludge bed (volume 3.65 L) combined with a flotation zone for sludge retention based on granulation and flotation, and a FF compartment for microbial sludge retention by biofilm. A peristaltic pump (Masterflex) was used to recirculate the reactor liquor through an outlet beneath the flotation compartment to maintain an upflow velocity of 2 m/h for the expansion of the granular sludge bed and to improve sludge-feed contact. The flotation zone was designed to accommodate the LCFA-encapsulated granules. The reactors were set up and run as per Keating et al. (2018) and modified as DSC-FF reactors as per Holohan (2020). Three identical DSC-FF reactors were set up, each inoculated with the sludge mixture (10 gVS/L). SDW stored at 7°C was constantly mixed with a mechanical stirrer placed inside the refrigerator and was connected to nitrogen-filled gas bags to maintain anaerobic conditions. SDW was pumped to the reactors through a multi-channel low-flow peristaltic pump (Masterflex), which were maintained at 20°C for the experimental duration (150 days) by recirculating cooled water through an external water jacket. An outlet located above the FF section discharged effluent to a collection tank. The top of the reactor was connected to a 10 L gas bag to collect biogas. The HRT at start-up (72 h) was decreased gradually to 12 h (stepwise through 42.5, 24, 18, and 12 h on days 9, 25, 59, and 103) on reaching steady state, which was defined based on a similar sCOD removal efficiency for operational durations equivalent to at least three consecutive HRTs, except at 72 h HRT where an operational duration equivalent to two consecutive HRTs was used. Null hypothesis was tested on the sCOD removal efficiency using one-way ANOVA, and statistical significance was nullified at p > 0.01.
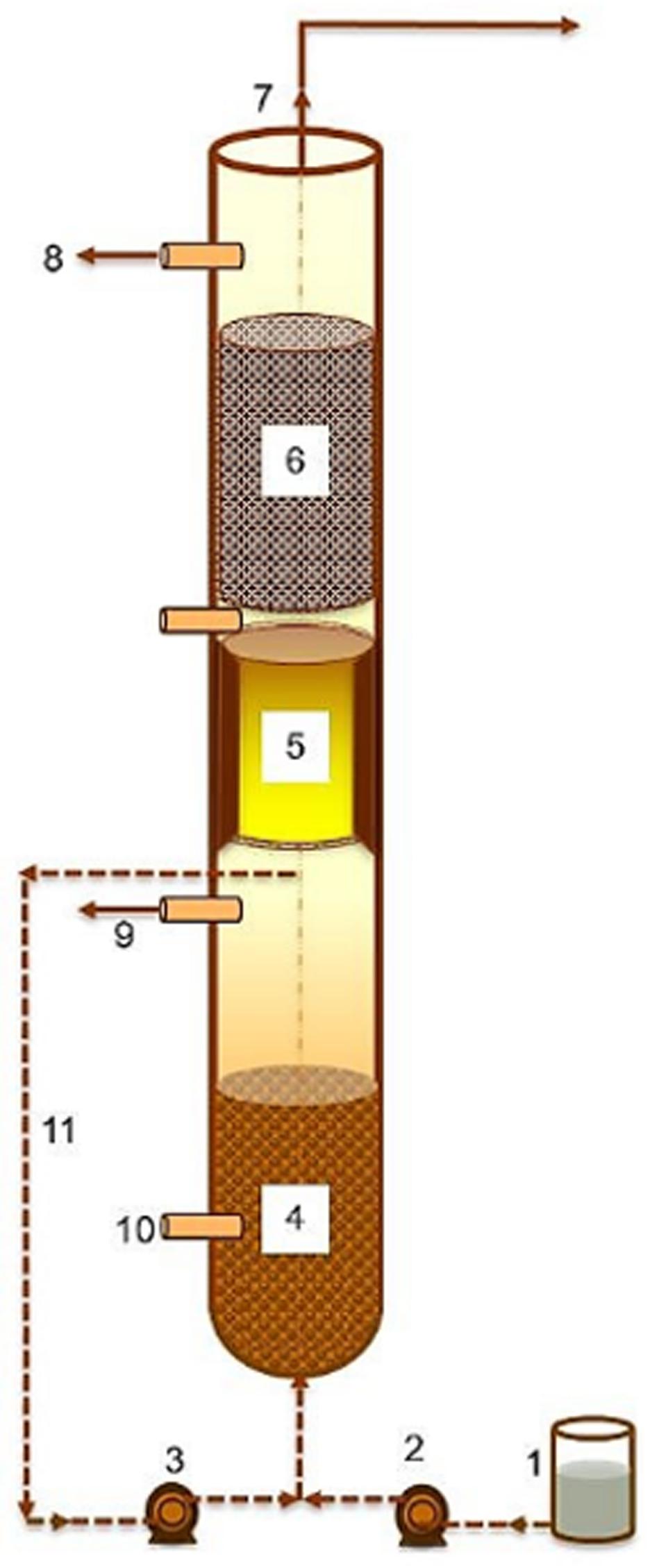
Figure 1. Schematic representation of the components of the dynamic sludge chamber fixed film (DSC-FF) reactor design. (1) Influent tank, (2) peristaltic pump (feed), (3) Peristaltic pump (recirculation), (4) granular sludge bed, (5) anaerobic flotation zone, (6) anaerobic fixed-film (FF) compartment, (7) biogas collection, (8) effluent outlet and sampling, (9) sampling port (for post-granular chamber samples), (10) sampling port (for granular sludge), and (11) liquid recycle.
Liquid samples (20 ml) from a port located above the sludge bed, from a port located above the FF compartment, and from effluent (Figure 1) were taken three to four times weekly to measure pH, total COD (tCOD) and soluble COD (sCOD), volatile fatty acids (VFA), and individual LCFA concentrations.
Analytical Methods
The volume of biogas produced was also determined three to four times weekly, by measuring the biogas volume collected in the gas bag. The methane content of biogas was determined using gas chromatography (Varian), equipped with a glass column and a flame ionization detector. Nitrogen was used as the mobile phase at a flow rate of 25 ml/min. The biogas volume was measured with the water displacement method and reported at standard temperature and pressure (STP). MYE was calculated from the daily methane production based on the COD added (assuming a maximum of 350 ml-CH4 per gram of COD at STP). pH was measured with a HI 2210 pH meter. Total solids (TS) and VS were measured gravimetrically using standard methods (APHA, 2005).
Liquid samples were centrifuged at 8000 rpm for 10 min and the supernatant was used for sCOD measurements using the potassium dichromate colorimetric method in commercially procured Hach Lange HR COD digestion tubes and a Hach Lange DR 5000 TM UV-Vis Spectrophotometer. For VFA measurements, the aliquots of supernatants collected after centrifugation were mixed with 50 μl of 30% orthophosphoric acid and then filtered through 0.22 μm Minisart® syringe filters. The VFAs [acetate (C2), propionate (C3), butyrate (C4), valerate (C5), caproate (C6), and caprylate (C8)] were analyzed by gas chromatography on a Varian Saturn 2000 GC with a BP 21 FFAP capillary column (SGE analytical science) and a flame ionization detector (FID) with helium as the carrier gas at a flow rate of 1 ml/min. Injector and FID detector temperatures were 250 and 300°C, respectively. The oven temperature was programmed to heat as follows: held at 60°C for 10 s, heated from 60 to 110°C at 30°C/min, and then heated up to 200°C at 10°C/min after which the temperature was held at 200°C for 2 min. The even-chained LCFAs [myristate (C14:0), palmitate (C16:0), stearate (C18:0), oleate (C18:1), and linoleate (C18:2)] in the liquid samples were measured according to van Gelder (2017) which was a modification of the protocols of Neves et al. (2009) and Ichihara and Fukubayashi (2010).
The development of granules from the sludge mixture was observed visually and under scanning electron microscopy (SEM). Five milliliters of granules was collected from a port located under the sludge bed using tubing connected to a syringe and were anaerobically transferred to a screw cap tube. The granules were incubated overnight at 4°C and then rinsed with a 0.2 M sodium cacodylate buffer solution. Next, samples were dehydrated by treating through an ethanol gradient [30, 50, 70, and 90% (v/v)] and placed onto aluminum stubs before drying with hexamethyldisiloxane (75 μl). Dehydrated samples were coated with a thin layer of gold and viewed using a SEM (Model S-2600 Hitachi, Japan) at 15 kV.
Thermodynamic Calculations
The feasibility of anaerobic conversion of LCFAs to acetate was evaluated at standard condition (25°C), and at temperatures of 20°C, and 37°C based on the standard Gibbs free energy changes for reactions (ΔG°’). Hydrogenation of the unsaturated LCFAs (linoleate and oleate) and β-oxidation of the saturated (stearate, palmitate) and unsaturated LCFAs (linoleate, oleate) were evaluated using the relationship ΔG°’ = ΣΔGf°(products) - ΣΔGf°(substrates), where ΔGf° refers to the standard free energy of formation. Standard Gibbs free energy of formation of LCFA (ΔGf°) at standard conditions (25°C) was estimated by using the group contribution method (Mavrovouniotis, 1991), and of the other compounds were obtained from Thauer et al. (1977). Standard change in enthalpy for reactions (ΔH°’) was calculated based on the relationship ΔH°’ = ΣΔHf°(products) - ΣΔHf°(substrates), where ΔHf° refers to the standard enthalpy of formation of compounds, obtained from NIST (Linstrom and Mallard, 2014). Standard Gibbs free energy change of reactions (ΔG°’T°C) were calculated at 20 and 37°C based on temperature corrections according to the Gibbs-Helmholtz equation (Eq. 1):
where ΔGf°’T°C is the standard Gibbs free energy change of reaction at temperature of interest, ΔGf°’25°C is the standard Gibbs free energy change for reaction at standard conditions (25°C), T is the temperature of interest in Kelvin, and ΔH°’T°C is the standard change in enthalpy for reaction. All calculation procedures were followed as described by Dolfing (2015).
Results
COD Removal and Methane Production
The sCOD removal efficiency achieved by all three DSC-FF bioreactors was similar during the steady-state periods (p > 0.01) (Supplementary Table S1) throughout the experiment. The results for only one DSC-FF bioreactor are presented, but the data from all three DSC-FF reactors are provided as supplementary data (Supplementary Figures S1–S3). During the first 8 days of operation, when the HRT was 72 h, the tCOD and sCOD removal efficiencies were 88–94% and 96–98%, respectively (Figure 2), and the MYE was 5–9%. As the HRT was reduced to 42.5 h, tCOD and sCOD removal efficiencies remained high (94–98% and 97–98%, respectively) and the MYE increased to 26–28% (Table 2). After the HRT was reduced further to 24 h, the tCOD removal efficiency fluctuated (82–94%) but the sCOD removal efficiency remained high (90–98%), while the MYE increased further to 49–57% (Table 2). After further reducing the HRT to 18 h, the tCOD removal efficiency was 88–91%, the sCOD removal efficiency was 96–98%, and the MYE was 48–62%. Finally, as the HRT was reduced from 18 to 12 h, the removal efficiencies of tCOD and sCOD decreased to 63–72% and 84–89%, respectively (Figure 2), but the MYE further improved (up to 103%) (Table 2).
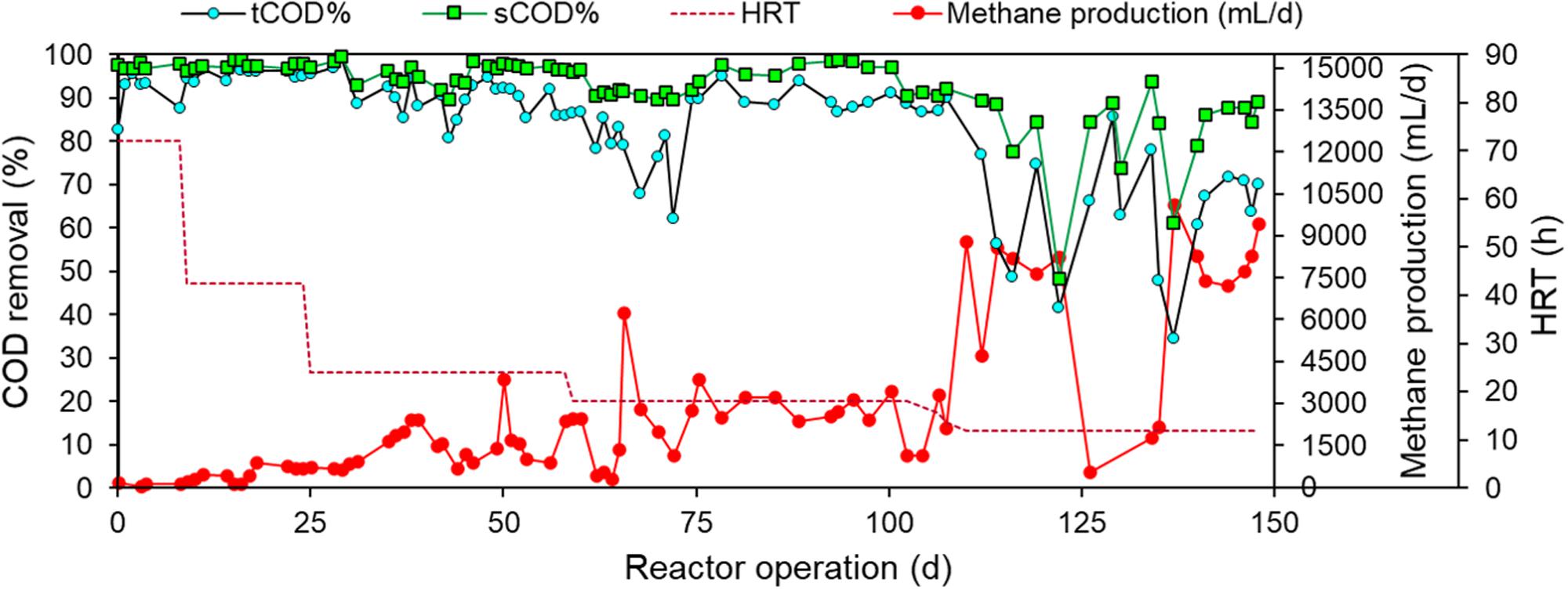
Figure 2. Total COD (tCOD) and soluble COD (sCOD) removals and daily methane production from DSC-FF reactor at the different HRTs of 72, 42.5, 24, 18, and 12 h.
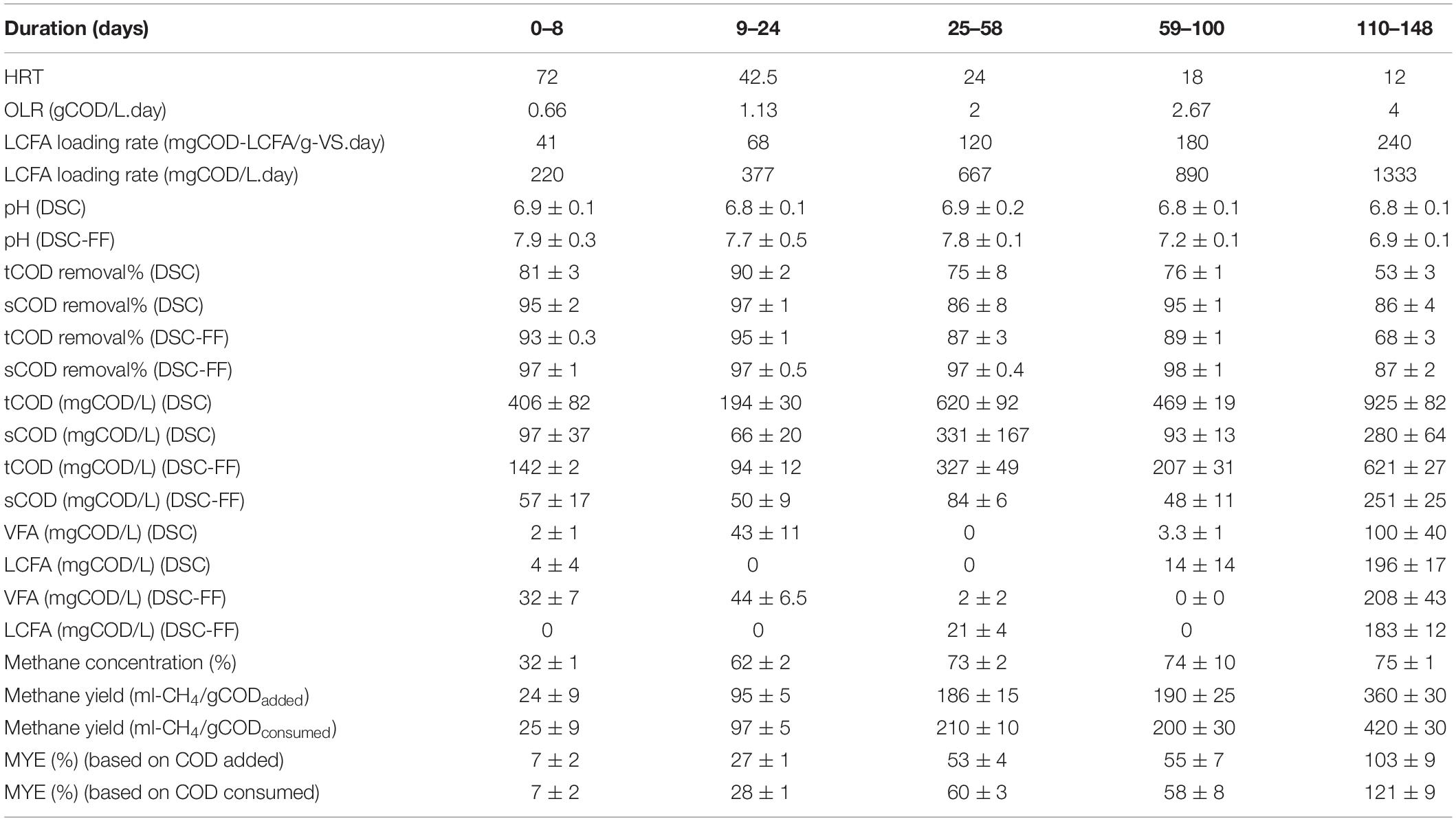
Table 2. Operational conditions and process performance of the DSC-FF reactor and separately of DSC at the different HRTs of 72, 42.5, 24, 18, and 12 h.
Thus, methane production from the DSC-FF reactor increased with the decrease in HRTs. At a HRT of 72 h (OLR of 0.67 gCOD/⋅day), the methane production was low (24 ± 9 ml-CH4/gCODadded). However, the methane production increased to 95 (± 5), 186 (± 15), and 190 (± 25) ml-CH4/gCODadded at HRTs of 42.5, 24, and 18 h, respectively, corresponding to the increasing OLRs of 1.13, 2, and 2.67 gCOD/L⋅day (Table 2). A further decrease in the HRT to 12 h (OLR of 4 gCOD/L⋅day) resulted in a higher methane production of 360 (± 30) ml-CH4/gCODadded and a MYE of 103%. Although the OLR and LCFA loading rate at the different HRTs were applied consistently, substrate accumulated in the DSC-FF reactor as revealed by the higher tCOD removal than could be accounted by the methane production and effluent tCOD concentrations (Figure 3). The substrate accumulation, as shown by the unaccounted COD, was highest at the HRT of 72 h, and, decreased at the HRTs of 24 and 18 h (Figure 3). However, at 12 h HRT, the cumulative COD from the methane production and effluent tCOD concentrations was higher than the influent COD concentration (of 2 gCOD/L equivalent to 100%) (Figure 3). This suggests methanization of the accumulated substrates at the 12 h HRT. Moreover, at particular durations observed at 12 h HRT, tCOD removal was low but with a high methane production (e.g., days 113–119), which was followed by higher tCOD removal but with low methane production (e.g., days 126–134), and subsequently again had a lower tCOD removal but increased VFA and methane production (e.g., days 135–140) (Figure 2). This trend could be due to the sorption of the substrate during the high COD removal (exerting a substrate overload beyond the intended OLR), followed by methanization of the accumulated substrate in the subsequent duration. Thus, the DSC experienced alternating cycles of organic overloads, particularly when the HRT was 12 h.
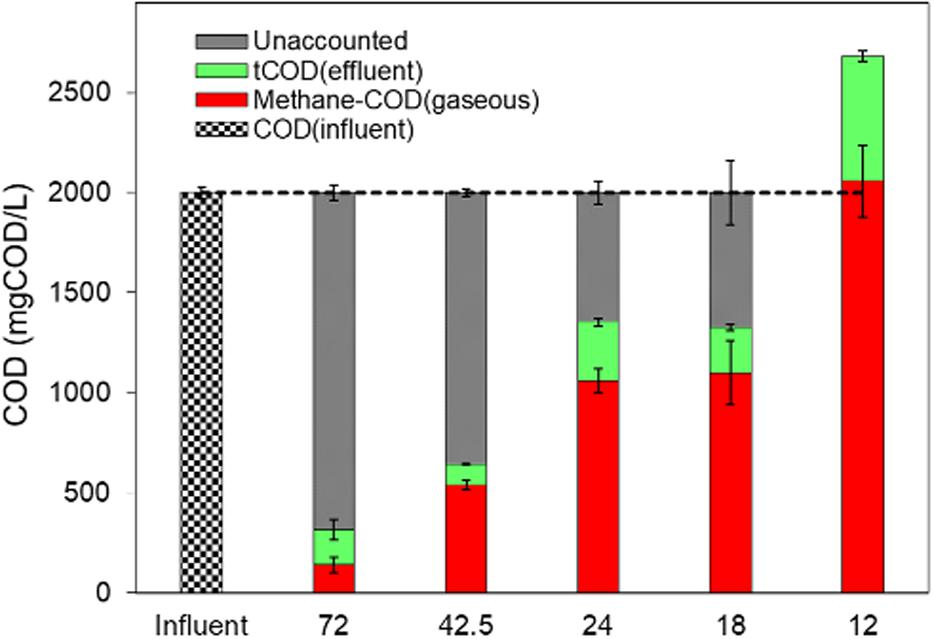
Figure 3. COD balance for the DSC-FF reactor at the different HRTs of 72, 42.5, 24, 18, and 12 h along with the influent COD (equivalent to 2 gCOD/L) shown as reference.
Metabolic Intermediates From LCFA Oxidation
VFAs (C2–C6) and the even-chained LCFAs (C14–C18) in the DSC-FF effluent were analyzed periodically. With HRTs of 72 and 42.5 h, the total VFA concentration of the DSC-FF effluent was 20–120 mgCOD/L, comprising mainly of acetate (C2), propionate (C3), butyrate (C4), and low concentrations of valerate (C5). However, at HRTs of 24 and 18 h, the VFA concentrations were negligible, with only low concentrations of acetate detected (Figure 4A). At HRTs of 18–72.5 h, the LCFAs fed to the reactor (palmitate, oleate, and linoleate) were removed completely, and stearate was found at 24 h HRT in low concentrations (>10 mg/L) (Figure 4B), though, with the decrease in HRT from 18 to 12 h, the effluent concentration of VFAs increased (up to 190 mg/L) due to marked increases in the concentrations of acetate (60 mg/L) and propionate (130 mg/L). Toward the end of the trial, the VFA concentrations decreased to 97 mg/L (Figure 4A). Caproate (C6) that is produced from the β-oxidation of even-chained LCFAs was found only at the 12 h HRT (26–45 mg/L) (Figure 4A), along with the saturated LCFAs palmitate (14–40 mg/L) and stearate (20–2 mg/L) (Figure 4B), resulting in a LCFA removal efficiency of 71–74%.
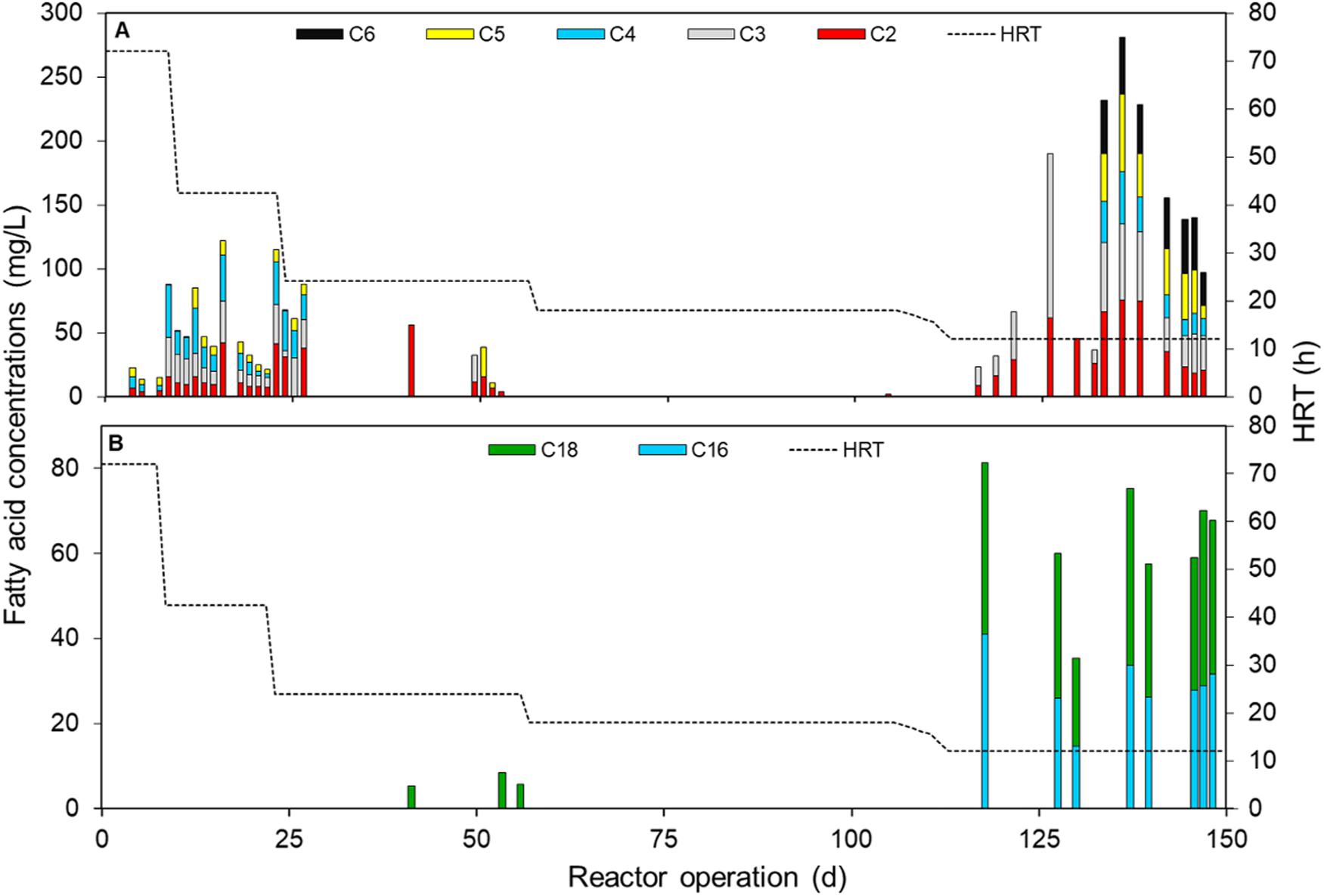
Figure 4. Profiles of (A) volatile fatty acids (VFA), i.e. acetate (C2), propionate (C3), butyrate and iso-butyrate (C4), valerate (C5) and caproate (C6), and (B) long chain fatty acids (LCFA), i.e. palmitate (C16:0), and stearate (C18:0), in DSC-FF reactor effluent at the different HRTs of 72, 42.5, 24, 18, and 12 h. LCFAs myristate (C14:0), oleate (C18:1), and linoleate (C18:2) were not found in the liquid samples.
Role of FF Compartment in Organics Removal
At 72 and 42.5 HRTs, compared to DSC, the FF contributed to an additional 4–12% tCOD removal (93–96% by DSC-FF vs. 78–92% by DSC alone), and 1% LCFA removal (100% by DSC-FF vs. 99% by DSC alone) (Figures 5A,B), whereas the sCOD removal efficiency after treatment by FF remained relatively unchanged (93–98%) (Table 2). At the HRTs of 24 and 18 h, compared to DSC, the FF removed an additional 7–17% tCOD (84–90% by DSC-FF vs. 67–83% by DSC alone), and reduced LCFA concentrations to 25 mgCOD/L (Supplementary Figure S4). FF contributed to an additional 3–19% sCOD removal compared to DSC (Figures 5A,B), and resulted in overall sCOD removal efficiency of 97–99% by the DSC-FF reactor at 24 and 18 h HRTs (Table 2). At 12 h HRT, the tCOD removal by DSC had decreased (50–56%), and FF contributed appreciably (15%) to the tCOD removal, resulting in a tCOD removal efficiency of 65–71% by DSC-FF. Moreover, at 12 h HRT, the LCFA removal efficiency by DSC was 68–73%, wherein FF contributed to an additional 3–4% removal resulting in 71–74% LCFA removal by DSC-FF (Supplementary Figure S4). This suggests that FF had an important role in the removal of particulate COD, contingent to the incoming tCOD concentrations from DSC. The tCOD removal by FF was likely due to the entrapment of particulates by the support matrix.
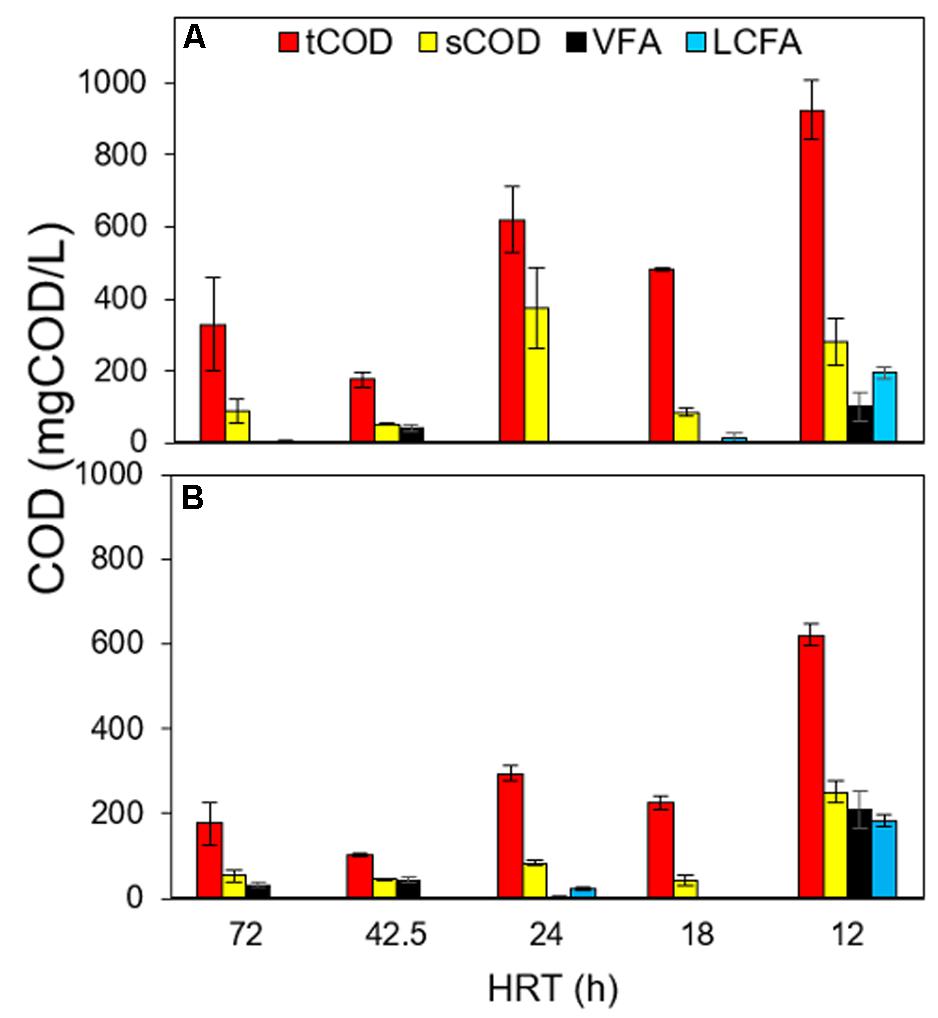
Figure 5. COD fractionation as tCOD, sCOD, VFA-COD and LCFA-COD after treatment by (A) the DSC and further by (B) the FF at the different HRTs of 72, 42.5, 24, 18, and 12 h.
At steady periods of 24 h and 18 h HRT, the VFA concentrations after treatment by DSC were low (<10 mgCOD/L) (Supplementary Figure S5). However, at the HRTs of 72 and 12 h, VFA concentrations were higher after treatment by FF (higher by 16- and 2-fold at 72 and 12 h HRT, respectively) than the VFA concentrations after treatment by DSC (Supplementary Figure S5), which suggests that acidification occurred in the FF compartment. During the steady state at 12 h HRT, the VFAs propionate, valerate, and caproate were found, whereas after treatment by FF, acetate and butyrate were additionally detected (Supplementary Figure S5). This production of acetate and butyrate at 12 h HRT in FF (Supplementary Figure S5), along with the simultaneous decrease in palmitate and stearate concentrations in FF (Supplementary Figure S4), signifies partial β-oxidation of the saturated LCFAs (stearate and palmitate) in the FF compartment at 12 h HRT.
Overall, the removal of COD and LCFAs by the DSC indicated its importance for the overall anaerobic treatment of SDW, and especially for the removal of saturated and unsaturated LCFAs. Up to the HRT of 18 h (LCFA loading rate of 890 mgCOD/L⋅day, specific LCFA loading rate 180 mgCOD/gVS⋅day), DSC achieved a COD removal efficiency exceeding 75% (both tCOD and sCOD). However, the COD removal by DSC decreased upon shortening the HRT to 12 h (LCFA loading rate of 1333 mgCOD/⋅day, specific LCFA loading rate 240 mgCOD-LCFA/gVS⋅day), leading to a higher inflow of particulates and saturated LCFAs (stearate and palmitate) into the FF compartment, and consequently a more prominent role of the FF compartment in entrapment and acidification of the SDW.
Sludge Washout and Flotation
No sludge washout was observed at HRTs of 72, 42.5, or 24 h. However, as the HRT was further shortened to 18 and 12 h, the effluent became more turbid (visual observation), though subsequently clarifying after prolonged operation at that particular HRT. The average effluent VS was 0.6 (± 0.1) and 4.5 (± 0.1) gVS/L at HRTs of 18 and 12 h, respectively. The higher sludge washout observed at 12 h HRT presumably resulted from the sloughing from the biofilm at the increased effluent flow rate. Flotation of small broken granules was observed (less than 10% of sludge in the DSC) at HRTs from 42.5 to 12 h. However, the sludge flotation at these different HRTs did not vary, despite the increase in specific LCFA loading rate, and was accommodated by the DSC.
De novo Granulation in DSC
Initially, the two sludges comprising the inoculum mixture, i.e. the flocculent and granular sludge, were distinguishable visually due to their different colorings in physical formations (Figures 6a,c) and under SEM (Figures 6b,d). However, over time in the DSC, the two sludges were not visually distinguishable due to continuous mixing of the two inocula and they developed an overall grayish appearance, in contrast to the earlier distinct yellow (Figure 6a) and black (Figure 6c) respective colors of the inocula. During the continuous mixing, the mixed sludges began to form de novo sludge granules. By the end of reactor operation at 72 h HRT (day 8), appearance of distinct rounded granules was observed in the granular sludge bed in the DSC (Figures 6e,f).
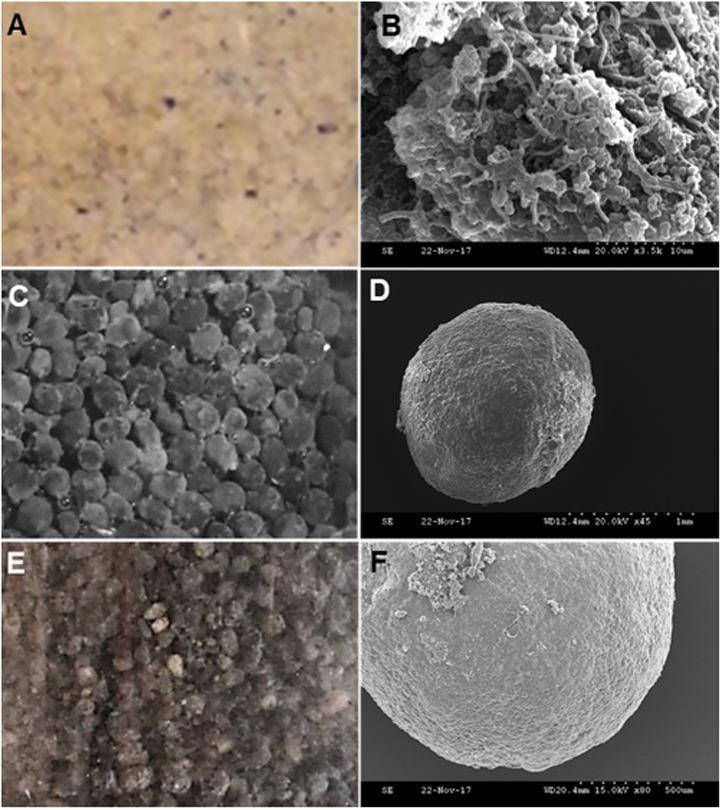
Figure 6. Schematic representation of rapid granulation from inoculum mixture. Sludges prior to inoculation, i.e. flocculent sludge on (A) visual observation and (B) under Scanning Electron Microscopy (SEM) at 10 μm, and granular sludge on (C) visual observation and (D) under SEM at 1 mm. De novo granulation in the dynamic sludge compartment (DSC) after 8 days on (E) visual observation and (F) under SEM at 500 μm.
Thermodynamic Feasibility of Degradation of Saturated and Unsaturated LCFAs
The standard Gibbs free energy changes of reaction (ΔG°’) for the degradation of LCFAs (linoleate, oleate, stearate, and palmitate) present in the feed (SDW) were calculated at 20°C (ΔG°’20°C) (discharge temperature used in this study) and 37°C (ΔG°’37°C) (Table 3), as numerous studies evaluating lipid or LCFA degradation at mesophilic conditions have used 37°C as the operational temperature (Ramos et al., 2014; Dereli et al., 2015; Jensen et al., 2015; Cavaleiro et al., 2016).
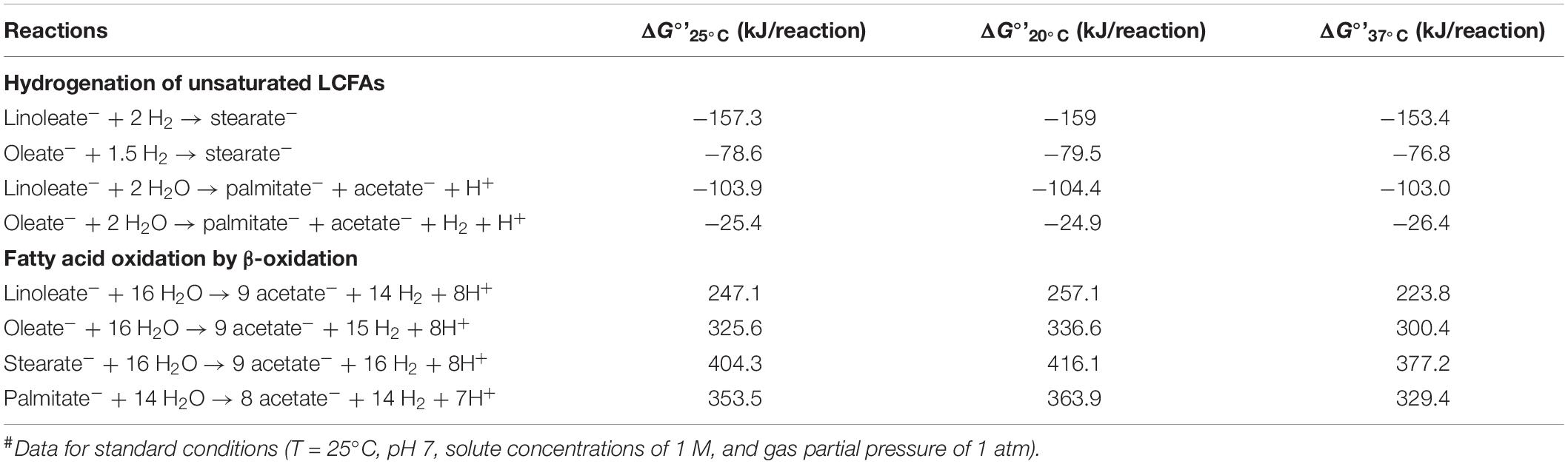
Table 3. Change in Gibbs free energy values of reactions (ΔG°’) involved in hydrogenation and oxidation of selected LCFAs at standard conditions#.
The analysis of the effect of temperature on the energetic feasibility of hydrogenation revealed that the hydrogenation of linoleate and oleate to stearate was feasible both at 20 and 37°C under standard conditions, however, the hydrogenation reactions had higher free energy at 20°C (−159 and −79.5 kJ/mol) than at 37°C (−153.4 and −76.8 kJ/mol) (Table 3). Moreover, the coupling of hydrogenation to one cycle of β-oxidation (producing palmitate) yielded a higher free energy for linoleate (−104.4 kJ/mol) than for oleate (-24.9 kJ/mol). In contrast, the fatty acid oxidation of LCFAs (producing equivalent moles of acetate by β-oxidation) was not energetically feasible at either 20 or 37°C, and was more unfavorable at 20°C than at 37°C.
Discussion
Treatment of FOG-Rich Wastewaters at Low-Temperature Through DSC-FF Configuration
This study shows for the first time that high-rate anaerobic treatment is a feasible option for LCFA-rich wastewaters at 20°C, at HRTs as low as 12 h. To the best of the authors’ knowledge, many previous studies on anaerobic treatment of dairy wastewaters at lower temperatures (5–20°C) used feed with 1.8–10 times lower lipid or LCFA content (e.g., 3% COD-LCFA) (Table 1). While the anaerobic treatment of a similar synthetic dairy wastewater (OLR of 2 gCOD/L⋅day, 33% COD-LCFA) in lab-scale EGSB reactors at 20°C was feasible for operational durations of 60 days and achieved COD removal efficiencies of 83–87%, the treatment performance deteriorated on prolonged SDW feeding at 24 h HRT and did not recover even after the feeding was stopped (Singh et al., 2019). Contrarily, in this study the DSC-FF reactors achieved stable COD removal (87–97%), despite the application of higher LCFA loading rates. Previous studies reported a lower COD removal efficiency; for example, COD removal efficiency of 46–69% was reported in the anaerobic treatment of lipid-containing dairy wastewater (22% COD-lipid) at 24 h HRT at 20°C (Tawfik et al., 2008), whereas a COD removal efficiency of 38–47% was achieved in the batch treatment of lipid-containing municipal wastewater at 4–15°C at an OLR of 0.29 gCOD/L⋅day (lipid loading rates of 0.1–0.13 gCOD/L⋅day, 38–45% lipids) (Petropoulos et al., 2018).
Recently, real municipal wastewater containing 70% lipids (FOG loading rate of 224 mgCOD/L⋅day) at 15°C was treated in UASB and MBR reactors at short HRTs of 7.7 h with COD removal efficiencies of 79 and 86% and MYE of 17 and 23%, respectively (Petropoulos et al., 2019b). The presence of unhydrolyzed COD (Petropoulos et al., 2018, 2019b) suggested a low anaerobic conversion of the accumulated lipids. Real wastewaters are rich in particulate COD, which often is challenging to hydrolyze at lower temperatures, more so for lipids than carbohydrates and proteins (Pavlostathis and Giraldo-Gomez, 1991; Perle et al., 1995; Vidal et al., 2000). There is a lack of consensus regarding the main bottleneck in lipid methanization, with both lipolysis and LCFA degradation being reported as the rate-limiting step (Hanaki et al., 1981; Pavlostathis and Giraldo-Gomez, 1991; Petropoulos et al., 2019a). In this study, the accumulated substrates were methanized at 12 h HRT in DSC-FF reactors (Figure 3), demonstrating the anaerobic degradation of mixed LCFAs at 20°C at LCFA loading rates up to 890 mgCOD-LCFA/L⋅day (Figure 4B and Supplementary Figures S3, S4). It is recommended for future studies discerning the rate-limiting step in low-temperature anaerobic lipid degradation to evaluate lipolysis and LCFA degradation at LCFA loading rates ≥ 1333 mgCOD-LCFA/L⋅day.
Furthermore, in this study, the MYE increased from 7 to 103% over the 150-day operational duration (Table 2), whereas a MYE of ∼80% is typical of high-performing mesophilic reactors (van Lier et al., 2015). The MYE at 20°C in the DSC-FF reactor at 72–18 h HRT was low (7–55%), likely due to accumulation of substrate (in DSC and FF). At 12 h HRT, the high MYE (>100%) resulted from the conversion of substrate that had accumulated in the DSC or in the FF section. The cyclical alternating organic loads experienced by the DSC at 12 h HRT was presumably due to the LCFA accumulation on granular sludge, as LCFAs have a high sorption propensity. Cavaleiro et al. (2009) overloaded sludge with a LCFA-rich feed at 37°C in feed cycles (20–30 days) followed by react cycles (no feeding) for methanization of the accumulated substrates. This strategy of alternating organic loads had enhanced the MYE from 67 to 91% wherein LCFA accumulation increased in the reactor up to 60 days (2 feed cycles), but was subsequently methanized due to specialization of the microbial community (Cavaleiro et al., 2009). In our study, the alternating organic loads likely enriched the LCFA degraders and could be employed as a strategy to improve methanization of LCFA-rich wastewater at 20°C, as previously demonstrated at 35–37°C for oleate treatment (Cavaleiro et al., 2009; Ziels et al., 2017). Tawfik et al. (2008) prevented sludge washout by maintaining a regular sludge discharge (20% of total influent COD) at a LCFA loading rate of 0.75 gCOD-LCFA/L⋅day (specific LCFA loading rate 95.4 mgCOD-LCFA/gVS⋅day) while treating dairy wastewater in a UASB reactor at 20°C, though such daily sludge disposal means a loss of the energy-rich organic fraction from the reactor. In comparison, the non-requirement of sludge disposal from the DSC-FF reactors resulted in an efficient containment of the energy-rich LCFAs, which were subsequently converted to methane in the reactors. Overall, the process performance of the DSC-FF reactors during the 150-day trials demonstrates the suitability of this reactor design for the methanization of LCFA-rich dairy wastewater at discharge temperature.
The DSC-FF reactor design facilitated a high contact between the sludge and substrate, and yet prevented suction of the floating granules by the recycle pump (Figure 1), which sometimes is the reason for process failure in laboratory scale studies due to increased sludge washout (Yoda and Nishimura, 1997). The upflow velocity of 2 m/h was high enough to effectuate the separation of gas bubbles from the surface of anaerobic granules, thus preventing an incidental lifting of the sludge bed. The anaerobic flotation compartment in the DSC could accommodate the flotation of LCFA-encapsulated granules, although minimal flotation (<10%) was observed even at the LCFA loading rate of 1333 mgCOD-LCFA/L⋅day (specific LCFA loading rate of 240 mgCOD-LCFA/gVS⋅day). Indeed, sludge flotation has previously been reported at lower LCFA loading rate of 86–203 mgCOD-LCFA/gVS⋅day (Hwu et al., 1998b) or 80 mgCOD-FOG/gTS⋅day (Macarie et al., 2018), wherein flotation of the entire sludge bed resulted in reactor failure. The anaerobic flotation compartment enabled the slow degradation of LCFA from the floating LCFA-encapsulated granules, followed by settling of the granules to the sludge bed. This dynamic behavior allowed for an increased microbial activity in the DSC, while ensuring continuous treatment of the LCFA-rich wastewater at low ambient temperature, and distinguishes the reactor design from the well-known reactor configurations, viz., UASB, EGSB, AFR, and anaerobic filter. Due to the anaerobic treatment achieved in the DSC (by tCOD and LCFA removal), the FF received wastewater with relatively low concentrations of particulate matter and LCFA. Consequently, the potential challenges associated with high lipid concentrations, such as biofilm-thinning or filter clogging, previously observed in anaerobic filters treating oleate (Alves et al., 2001) were prevented in this study. However, the application of LCFA loading rates higher than 1333 mgCOD-LCFA/L⋅day in the DSC-FF reactors needs to be further evaluated at low or psychrophilic temperatures.
Anaerobic Degradation of Saturated and Unsaturated LCFAs at 20°C
The DSC-FF reactors consistently removed the saturated and unsaturated LCFAs in SDW to concentrations below 50 mg/L in the effluent, at HRTs as short as 18 h. The saturated LCFAs, palmitate and stearate, were partially removed at 12 h. During treatment of wastewaters with high lipid loads, LCFA accumulation constituting of palmitate or stearate has often been encountered in various reactor types (Pereira et al., 2005; Cavaleiro et al., 2009; Dereli et al., 2015; Ziels et al., 2015, 2017; Duarte et al., 2018) due to the fast conversion of unsaturated LCFAs (linoleate and oleate) to palmitate (Cavaleiro et al., 2016).
The degradation of LCFA proceeds sequentially, with an initial sorption to the cell surface, followed by the activation of saturated and unsaturated LCFAs, facilitating their transport into the cytosol of bacteria. The unsaturated LCFAs are hydrogenated to their saturated counterpart, and are subsequently degraded by β-oxidation (Sousa et al., 2009). Subsequently, during each cycle of β-oxidation, the LCFAs are shortened by two carbons in chain length, producing one fatty acid molecule with smaller chain length and one acetate molecule, wherein the LCFA degradation to lower molecular weight Cn–2 fatty acid proceeds cyclically up until the production of an equivalent number of acetate or propionate molecules from the LCFA is achieved (Alves et al., 2009). The change in Gibb’s free energy for the hydrogenation reactions are favorable at 20°C, whereas the β-oxidation reactions are not (Table 3). At HRTs of 18–72 h, both hydrogenation and β-oxidation reactions proceeded in the DSC and FF, resulting in complete removal of the unsaturated LCFAs and high removal of the saturated LCFAs (Table 3). In comparison, at 12 h HRT, the complete removal of the unsaturated LCFAs, oleate and linoleate, proceeded due to the increased energetic favorability of the hydrogenation reactions at 20°C than at mesophilic conditions. However, the saturated LCFAs, palmitate and stearate were only removed partially at 12 h HRT, presumably due to the limitations in LCFA uptake at the higher LCFA loading rate (1333 mgCOD-LCFA/L⋅day). Further studies evaluating the uptake and degradation of individual LCFAs, saturated as well as unsaturated, are needed at temperatures below 20°C to comprehend the temperature dependence of mechanisms involved in the methanization of LCFAs.
Conclusion
This study evaluated the anaerobic treatment of mixed LCFA-containing dairy wastewater at low temperature (20°C) by using a novel DSC-FF reactor design. High sCOD removal efficiencies (85–89%) and methane production (360 ± 30 ml-CH4/gCODadded) were achieved with mixed LCFA-containing dairy wastewater at 20°C up to an OLR of 4 gCOD/L⋅day (LCFA loading rate 1333 mgCOD-LCFA/L⋅day, HRT 12 h). The complete removal of the unsaturated LCFAs (oleate and linoleate) was achieved due to the thermodynamic feasibility of hydrogenation of these LCFAs at 20°C, whereas the saturated LCFAs (palmitate and stearate) were removed partially due to the thermodynamic limitations in the β-oxidation of palmitate and stearate at 20°C. Rapid sludge granulation from an inoculum mixture of granular and flocculent sludges in DSC, and the formation of biofilm in FF were achieved during the treatment of mixed-LCFA wastewater even with high LCFA concentration (33% COD basis) and LCFA loading rates of 220–1333 mgCOD-LCFA/⋅day to allow successful treatment of LCFA-containing wastewater at 20°C. The results from this study demonstrate that the high-rate treatment of LCFA-containing industrial wastewater is feasible at discharge temperature due to sludge retention by granulation, flotation, and biofilm formation in the novel DSC-FF reactor configuration.
Data Availability Statement
All datasets analyzed for this study are included in the article/Supplementary Material.
Author Contributions
SS and VO’F were involved in the planning of experiments and designing of reactors. SS performed the experiments, the related physico-chemical and data analysis, and wrote the manuscript. BH and JC-A helped in the setup of reactors. SM helped in reactor operation. VO’F, JR, MK, PL, BH, and GC participated in the preparation and correction of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska–Curie European Joint Doctorate (EJD) in Advanced Biological Waste-To-Energy Technologies (ABWET), under grant agreement No. 643071. This research was also supported through VO’F through the Irish Dairy Processing Technology Centre through the Enterprise Ireland Technology Centres Programme (TC/2014/0016) and Science Foundation Ireland (14/IA/2371 and 16/RC/3889). Financial supported to BH through Irish Research Council Employment Based Postgraduation Scheme with NVP Energy Ltd. is gratefully acknowledged. Financial supported to SM through College of Science Scholarship, NUI Galway, and to GC through Science Foundation Ireland Career Development Award is also gratefully acknowledged.
Conflict of Interest
BH was employed by the company NVP Energy Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Arrabawn Dairies (Kilconnell, Ireland) and NVP Energy Limited (Galway, Ireland) for providing the granular sludge. We would also like to thank Dairygold Co-Operative Society (Mitchelstown, Ireland) and ADI Systems (Evoqua Water Technologies, Ireland) for providing the flocculent sludge.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2020.00166/full#supplementary-material
References
Alves, M. M., Pereira, M. A., Sousa, D. Z., Cavaleiro, A. J., Picavet, M., Smidt, H., et al. (2009). Waste lipids to energy: how to optimize methane production from long-chain fatty acids (LCFA). Microb. Biotechnol. 2, 538–550. doi: 10.1111/j.1751-7915.2009.00100.x
Alves, M. M., Picavet, M. A., Pereira, M. A., Cavaleiro, A. J., and Sousa, D. Z. (2007). Novel anaerobic reactor for the removal of long chain fatty acids from fat containing wastewater. WO Patent No. WO/2007/058,557. Braga: University of Minho.
Alves, M. M., Vieira, M. J. A., Pereira, Á. R. M., Pereira, M. A., and Mota, M. E. (2001). Effects of lipids and oleic acid on biomass development in anaerobic fixed bed reactors. Part I: biofilm growth and activity. Water Res. 35, 1264–1270.
APHA (2005). Standard Methods for the Examination of Water and Wastewater, Vol. 79. (Washington, DC: APHA), 453–456.
Batstone, D. J., and Jensen, P. D. (2011). “Anaerobic processes,” in Treatise on Water Science, ed. P. Wilderer (Oxford: Academic Press), 615–639.
Bialek, K., Cysneiros, D., and O’Flaherty, V. (2014). Hydrolysis, acidification and methanogenesis during low-temperature anaerobic digestion of dilute dairy wastewater in an inverted fluidised bioreactor. Appl. Microbiol. Biotechnol. 98, 8737–8750. doi: 10.1007/s00253-014-5864-7
Biswas, K., and Turner, S. J. (2012). Microbial community composition and dynamics of moving bed biofilm reactor systems treating municipal sewage. Appl. Environ. Microbiol. 78, 855–864. doi: 10.1128/AEM.06570-11
Buntner, D., Sanchez, A., and Garrido, J. M. (2013). Feasibility of combined UASB and MBR system in dairy wastewater treatment at ambient temperatures. Chem. Eng. J. 230, 475–481. doi: 10.1016/j.cej.2013.06.043
Cavaleiro, A. J., Pereira, M. A., Guedes, A. P., Stams, A. J. M., Alves, M. M., and Sousa, D. Z. (2016). Conversion of Cn-Unsaturated into Cn-2-Saturated LCFA Can Occur Uncoupled from Methanogenesis in Anaerobic Bioreactors. Environ. Sci. Technol. 50, 3082–3090. doi: 10.1021/acs.est.5b03204
Cavaleiro, A. J., Salvador, A. F., Alves, J. I., and Alves, M. (2009). Continuous high rate anaerobic treatment of oleic acid based wastewater is possible after a step feeding start-up. Environ. Sci. Technol. 43, 2931–2936. doi: 10.1021/es8031264
Dague, R. R., Banik, G. C., and Ellis, T. G. (1998). Anaerobic sequencing batch reactor treatment of dilute wastewater at psychrophilic temperatures. Water Environ. Res. 70, 155–160.
Davidsson, Å., Lövstedt, C., la Cour Jansen, J., Gruvberger, C., and Aspegren, H. (2008). Co-digestion of grease trap sludge and sewage sludge. Waste Manag. 28, 986–992. doi: 10.1016/j.wasman.2007.03.024
Dereli, R. K., Heffernan, B., Grelot, A., van der Zee, F. P., and van Lier, J. B. (2015). Influence of high lipid containing wastewater on filtration performance and fouling in AnMBRs operated at different solids retention times. Sep. Purif. Technol. 139, 43–52. doi: 10.1016/j.seppur.2014.10.029
Desbois, A. P., and Smith, V. J. (2010). Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 85, 1629–1642. doi: 10.1007/s00253-009-2355-3
Dolfing, J. (2015). “Protocols for calculating reaction kinetics and thermodynamics,” in Hydrocarbon and Lipid Microbiology Protocols: Springer Protocols Handbooks, eds T. J. McGenity K. N. Timmis B. Nogales (Berlin: Springer-Verlag), 155–163. doi: 10.1007/8623
Duarte, M. S., Silva, S. A., Salvador, A. F., Cavaleiro, A. J., Stams, A. J. M., Alves, M. M., et al. (2018). Insight into the role of facultative bacteria stimulated by micro-aeration in continuous bioreactors converting LCFA to methane. Environ. Sci. Technol. 52, 6947–6507. doi: 10.1021/acs.est.8b00894
Fujihira, T., Seo, S., Yamaguchi, T., Hatamoto, M., and Tanikawa, D. (2018). High-rate anaerobic treatment system for solid/lipid-rich wastewater using anaerobic baffled reactor with scum recovery. Bioresour. Technol. 263, 145–152. doi: 10.1016/j.biortech.2018.04.091
Hanaki, K., Matsuo, T., and Nagase, M. (1981). Mechanism of inhibition caused by long-chain fatty acids in anaerobic digestion process. Biotechnol. Bioeng. 23, 1591–1610. doi: 10.1002/bit.260230717
Holohan, B. C. (2020). High-Rate Anaerobic Treatment of Lipid-Rich Wastewater. Ph.D. thesis, National University of Ireland Galway, Galway.
Hwu, C. S., Lier, J. B., and Lettinga, G. (1998a). Physicochemical and biological performance of expanded granular sludge bed reactors treating long-chain fatty acids. Process Biochem. 33, 75–81.
Hwu, C. S., Tseng, S. K., Yuan, C. Y., Kulik, Z., and Lettinga, G. (1998b). Biosorption of long-chain fatty acids in UASB treatment process. Water Res. 32, 1571–1579. doi: 10.1016/S0043-1354(97)00352-7
Ichihara, K., and Fukubayashi, Y. (2010). Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 51, 635–640. doi: 10.1194/jlr.d001065
Jeganathan, J., Nakhla, G., and Bassi, A. (2006). Long-term performance of high-rate anaerobic reactors for the treatment of oily wastewater. Environ. Sci. Technol. 40, 6466–6472. doi: 10.1021/es061071m
Jensen, P. D., Yap, S. D., Boyle-Gotla, A., Janoschka, J., Carney, C., Pidou, M., et al. (2015). Anaerobic membrane bioreactors enable high rate treatment of slaughterhouse wastewater. Biochem. Eng. J. 97, 132–141. doi: 10.1016/j.bej.2015.02.009
Keating, C., Hughes, D., Mahony, T., Cysneiros, D., Ijaz, U. Z., Smith, C. J., et al. (2018). Cold adaptation and replicable microbial community development during long-term low-temperature anaerobic digestion treatment of synthetic sewage. FEMS Microbiol. Ecol. 94:fiy095. doi: 10.1093/femsec/fiy095
Kim, S. H., Han, S. K., and Shin, H. S. (2004). Two-phase anaerobic treatment system for fat-containing wastewater. J. Chem. Technol. Biotechnol. 79, 63–71. doi: 10.1002/jctb.939
Lalman, J., and Bagley, D. M. (2002). Effects of C18 long chain fatty acids on glucose, butyrate and hydrogen degradation. Water Res. 36, 3307–3313. doi: 10.1016/S0043-1354(02)00014-3
Leal, C. M. R. M., Freire, D. M. G., Cammarota, M. C., and Sant’Anna, G. L. Jr. (2006). Effect of enzymatic hydrolysis on anaerobic treatment of dairy wastewater. Process Biochem. 41, 1173–1178. doi: 10.1016/j.procbio.2005.12.014
Linstrom, P. J., and Mallard, W. G. (2014). NIST Chemistry webBook, NIST Standard Reference Database Number 69. Gaithersburg, MD: National Institute of Standards and Technology.
Luostarinen, S. A., and Rintala, J. A. (2005). Anaerobic on-site treatment of black water and dairy parlour wastewater in UASB-septic tanks at low temperatures. Water Res. 39, 436–448. doi: 10.1016/j.watres.2004.10.006
Macarie, H., Esquivel, M., Laguna, A., Baron, O., El Mamouni, R., Guiot, S. R., et al. (2018). Strategy to identify the causes and to solve a sludge granulation problem in methanogenic reactors: application to a full-scale plant treating cheese wastewater. Environ. Sci. Pollut. Res. 25, 21318–21331. doi: 10.1007/s11356-017-9818-3
Martin, I., Pidou, M., Soares, A., Judd, S., and Jefferson, B. (2011). Modelling the energy demands of aerobic and anaerobic membrane bioreactors for wastewater treatment. Environ. Technol. 32, 921–932. doi: 10.1080/09593330.2011.565806
Mavrovouniotis, M. L. (1991). Estimation of standard Gibbs energy changes of biotransformations. J. Biol. Chem. 266, 14440–14445.
McHugh, S., Collins, G., and O’Flaherty, V. (2006). Long-term, high-rate anaerobic biological treatment of whey wastewaters at psychrophilic temperatures. Bioresour. Technol. 97, 1669–1678. doi: 10.1016/j.biortech.2005.07.020
McHugh, S., O’Reilly, C., Mahony, T., Colleran, E., and O’Flaherty, V. (2003). Anaerobic granular sludge bioreactor technology. Rev. Environ. Sci. Biotechnol. 2, 225–245. doi: 10.1023/B:RESB.0000040465.45300.97
Neves, L., Pereira, M. A., Mota, M., and Alves, M. M. (2009). Detection and quantification of long chain fatty acids in liquid and solid samples and its relevance to understand anaerobic digestion of lipids. Bioresour. Technol. 100, 91–96. doi: 10.1016/j.biortech.2008.06.018
Nikolaeva, S., Sanchez, E., and Borja, R. (2013). Dairy wastewater treatment by anaerobic fixed bed reactors from laboratory to pilot-scale plant:a case study in Costa Rica operating at ambient temperature. Int. J. Environ. Res. 7, 759–766. doi: 10.22059/IJER.2013.655
Park, J., Oh, J. H., Evans, E. A., Lally, M. F., Hobson, K. L., and Ellis, T. G. (2012). Industrial wastewater treatment by on-site pilot static granular bed reactor (SGBR). Water Pract. Technol. 7:2166. doi: 10.2166/wpt.2012.006
Passeggi, M., López, I., and Borzacconi, L. (2009). Integrated anaerobic treatment of dairy industrial wastewater and sludge. Water Sci. Technol. 59, 501–506. doi: 10.2166/wst.2009.010
Pavlostathis, S. G., and Giraldo-Gomez, E. (1991). Kinetics of anaerobic treatment – a critical review. CRC Crit. Rev. Environ. Control 21, 411–490. doi: 10.1080/10643389109388424
Pereira, M. A., Pires, O. C., Mota, M., and Alves, M. M. (2002). Anaerobic degradation of oleic acid by suspended and granular sludge: identification of palmitic acid as a key intermediate. Water Sci. Technolgy 45, 139–144.
Pereira, M. A., Pires, O. C., Mota, M., and Alves, M. M. (2005). Anaerobic biodegradation of oleic and palmitic acids: evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol. Bioeng. 92, 15–23. doi: 10.1002/bit.20548
Perle, M., Kimchie, S., and Shelef, G. (1995). Some biochemical aspects of the anaerobic degradation of dairy wastewater. Water Res. 29, 1549–1554. doi: 10.1016/0043-1354(94)00248-6
Petropoulos, E., Dolfing, J., Yu, Y., Wade, M. J., Bowen, E. J., Davenport, R. J., et al. (2018). Lipolysis of domestic wastewater in anaerobic reactors operating at low temperatures. Environ. Sci. Water Res. Technol. 4, 1002–1013. doi: 10.1039/c8ew00156a
Petropoulos, E., Shamurad, B., Acharya, K., and Tabraiz, S. (2019a). Domestic wastewater hydrolysis and lipolysis during start-up in anaerobic digesters and microbial fuel cells at moderate temperatures. Int. J. Environ. Sci. Technol. 17, 27–38. doi: 10.1007/s13762-019-02426-z
Petropoulos, E., Yu, Y., Tabraiz, S., Yakubu, A., Curtis, T. P., and Dolfing, J. (2019b). High rate domestic wastewater treatment at 15°C using anaerobic reactors inoculated with cold-adapted sediments/soils-shaping robust methanogenic communities. Environ. Sci. Water Res. Technol. 5, 70–82. doi: 10.1039/c8ew00410b
Ramasamy, E. V., and Abbasi, S. A. (2000). Energy recovery from dairy waste-waters: impacts of biofilm support systems on anaerobic CST reactors. Appl. Energy 65, 91–98. doi: 10.1016/S0306-2619(99)00079-3
Ramos, C., García, A., and Diez, V. (2014). Performance of an AnMBR pilot plant treating high-strength lipid wastewater: biological and filtration processes. Water Res. 67, 203–215. doi: 10.1016/j.watres.2014.09.021
Rinzema, A., Boone, M., van Knippenberg, K., and Lettinga, G. (1994). Bactericidal effect of long chain fatty acids in anaerobic digestion. Water Environ. Res. 66, 40–49. doi: 10.2175/WER.66.1.7
Saatci, Y., Arslan, E. I., and Konar, V. (2003). Removal of total lipids and fatty acids from sunflower oil factory effluent by UASB reactor. Bioresour. Technol. 87, 269–272. doi: 10.1016/S0960-8524(02)00255-9
Sam-Soon, P., Loewenthal, R. E., Wentzel, M. C., and Marais, G. V. R. (1991). A long-chain fatty acid, oleate, as sole substrate in upflow anaerobic sludge bed (UASB) reactor systems. Water S. Afr. 17, 31–36.
Singh, S., Rinta-Kanto, J. M., Kettunen, R., Tolvanen, H., Lens, P., Collins, G., et al. (2019). Anaerobic treatment of LCFA-containing synthetic dairy wastewater at 20°C: process performance and microbial community dynamics. Sci. Total Environ. 691, 960–968. doi: 10.1016/j.scitotenv.2019.07.136
Sousa, D. Z., Smidt, H., Alves, M. M., and Stams, A. J. M. (2009). Ecophysiology of syntrophic communities that degrade saturated and unsaturated long-chain fatty acids. FEMS Microbiol. Ecol. 68, 257–272. doi: 10.1111/j.1574-6941.2009.00680.x
Sun, Y., Wang, D., Qiao, W., Wang, W., and Zhu, T. (2013). Anaerobic co-digestion of municipal biomass wastes and waste activated sludge: dynamic model and material balances. J. Environ. Sci. 25, 2112–2122. doi: 10.1016/s1001-0742(12)60236-8
Szabo-Corbacho, M. A., Pacheco-Ruiz, S., Míguez, D., Hooijmans, C. M., García, H. A., Brdjanovic, D., et al. (2019). Impact of solids retention time on the biological performance of an AnMBR treating lipid-rich synthetic dairy wastewater. Environ. Technol. 1–12. doi: 10.1080/09593330.2019.1639829 [Epub ahead of print].
Tawfik, A., Sobhey, M., and Badawy, M. (2008). Treatment of a combined dairy and domestic wastewater in an up-flow anaerobic sludge blanket (UASB) reactor followed by activated sludge (AS system). Desalination 227, 167–177. doi: 10.1016/j.desal.2007.06.023
Thauer, R. K., Jungermann, K., and Decker, K. (1977). Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 40, 100–181. doi: 10.1108/eb027807
Toldrá, F., Flors, A., Lequerica, J. L., and Vallés, S. (1987). Fluidized bed anaerobic biodegradation of food industry wastewaters. Biol. Wastes 21, 55–61. doi: 10.1016/0269-7483(87)90146-7
United Nations (2015). United Nations General Assembly Resolution A/Res/70/1: Transforming Our World: the 2030 Agenda for Sustainable Development. New York, NY: United Nations.
van Gelder, T. (2017). Quantification of LCFA Profiles. Wageningen: Wageningen University & Research.
van Lier, J. B., van der Zee, F. P., Frijters, C. T. M. J., and Ersahin, M. E. (2015). Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev. Environ. Sci. Biotechnol. 14, 681–702. doi: 10.1007/s11157-015-9375-5
Vidal, G., Carvalho, A., Méndez, R., and Lema, J. M. (2000). Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Bioresour. Technol. 74, 231–239. doi: 10.1016/S0960-8524(00)00015-8
Viraraghavan, T., and Kikkeri, S. R. (1990). Dairy wastewater treatment using anaerobic filters. Can. Agric. Eng. 33, 143–149.
Yoda, M., and Nishimura, S. (1997). Controlling granular sludge floatation in UASB reactors. Water Sci. Technol. 36, 165–173. doi: 10.1016/S0273-1223(97)00520-9
Zheng, C. J., Yoo, J. S., Lee, T. G., Cho, H. Y., Kim, Y. H., and Kim, W. G. (2005). Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 579, 5157–5162. doi: 10.1016/j.febslet.2005.08.028
Zhou, X., Meile, L., Kreuzer, M., and Zeitz, J. O. (2013). The effect of saturated fatty acids on methanogenesis and cell viability of Methanobrevibacter ruminantium. Archaea 2103:106916. doi: 10.1155/2013/106916
Zielińska, M., Zieliński, M., and Dębowski, M. (2018). Organic compounds and phosphorus removal from dairy wastewater by biofilm on iron-containing supports. J. Environ. Eng. 144:04017087. doi: 10.1061/(ASCE)EE.1943-7870.0001309
Ziels, R. M., Beck, D. A. C., Marti, M., Gough, H. L., Stensel, H. D., and Svensson, B. H. (2015). Monitoring the dynamics of syntrophic β-oxidizing bacteria during anaerobic degradation of oleic acid by quantitative PCR. FEMS Microbiol. Ecol. 91:fiv028. doi: 10.1093/femsec/fiv028
Keywords: dynamic sludge chamber fixed film reactor, dairy wastewater, long-chain fatty acid mixture, anaerobic sludge granulation, biofilm formation
Citation: Singh S, Holohan BC, Mills S, Castilla-Archilla J, Kokko M, Rintala J, Lens PNL, Collins G and O’Flaherty V (2020) Enhanced Methanization of Long-Chain Fatty Acid Wastewater at 20°C in the Novel Dynamic Sludge Chamber–Fixed Film Bioreactor. Front. Energy Res. 8:166. doi: 10.3389/fenrg.2020.00166
Received: 05 February 2020; Accepted: 30 June 2020;
Published: 04 August 2020.
Edited by:
Su Shiung Lam, University of Malaysia Terengganu, MalaysiaReviewed by:
Qaisar Mahmood, COMSATS University Islamabad, PakistanFrancisco Jesus Fernandez Morales, University of Castilla-La Mancha, Spain
Copyright © 2020 Singh, Holohan, Mills, Castilla-Archilla, Kokko, Rintala, Lens, Collins and O’Flaherty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marika Kokko, bWFyaWthLmtva2tvQHR1bmkuZmk=
 Suniti Singh
Suniti Singh B. Conall Holohan
B. Conall Holohan Simon Mills3
Simon Mills3 Marika Kokko
Marika Kokko Gavin Collins
Gavin Collins Vincent O’Flaherty
Vincent O’Flaherty